Abstract
Gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus play a key role in the regulation of reproductive function. In this study, we sought an efficient method for generating GnRH neurons from human embryonic and induced pluripotent stem cells (hESC and hiPSC, respectively). First, we found that exposure of primitive neuroepithelial cells, rather than neuroprogenitor cells, to fibroblast growth factor 8 (FGF8), was more effective in generating GnRH neurons. Second, addition of kisspeptin to FGF8 further increased the efficiency rates of GnRH neurogeneration. Third, we generated a fluorescent marker mCherry labeled human embryonic GnRH cell line (mCh-hESC) using a CRISPR-Cas9 targeting approach. Fourth, we examined physiological characteristics of GnRH (mCh-hESC) neurons: similar to GnRH neurons in vivo, they released the GnRH peptide in a pulsatile manner at ~60 min intervals; GnRH release increased in response to high potassium, kisspeptin, estradiol, and neurokinin B challenges; and injection of depolarizing current induced action potentials. Finally, we characterized developmental changes in transcriptomes of GnRH neurons using hESC, hiPSC, and mCh-hESC. The developmental pattern of transcriptomes was remarkably similar among the 3 cell lines. Collectively, human stem cell–derived GnRH neurons will be an important tool for establishing disease models to understand diseases, such as idiopathic hypothalamic hypogonadism, and testing contraceptive drugs.
Keywords: GnRH, human stem cell, induced pluripotent stem cell, kisspeptin
The gonadotropin-releasing hormone (GnRH) neuron plays a central role in reproductive function (note that GnRH in this article is synonymous to GnRH-1). GnRH neurons in the hypothalamus release the decapeptide hormone from the median eminence into the portal circulation in a pulsatile manner and stimulate pituitary luteinizing hormone (LH) and follicle-stimulating hormone release, which controls gamete production and gonadal steroid secretion in the gonads. Consequently, patients with idiopathic hypogonadotropic hypogonadism (IHH) due to carrying defective genes for the generation or migration of GnRH neurons or for upstream regulatory neurons of normal GnRH function suffer from abnormal GnRH release and therefore an absence of reproductive function. For example, patients with Kallmann syndrome (no GnRH neurons in the hypothalamus due to failure of GnRH neurons to migrate from the olfactory placode) do not undergo puberty and exhibit IHH, and therefore they are infertile (1-4). Presently, symptomatic improvement in Kallmann syndrome patients can be achieved by treatments with a GnRH agonist and gonadal steroids, but lifelong treatments are required, as a tool for permanent cure has not been found (5,6). If GnRH neurons were generated from IHH patient’s somatic cells, this may not only provide a new tool for understanding the underlying pathophysiology of this disease but also provide a treatment tool for individual IHH patients, such as a cell replacement therapy. Overall, there is an urgent need to create a model to study GnRH neuronal biology, particularly as it relates to both rare and common diseases of human reproduction that occur across our reproductive life.
For many years we and others have been studying primary GnRH neurons derived from the monkey and mouse embryonic nasal placode (7,8). Similar to neurons in vivo (9,10), they release GnRH peptide in a pulsatile manner (11,12), exhibit periodical intracellular calcium [Ca2+]i oscillations (13,14), and respond to external signals such as adenosine 5′-triphosphate, estradiol (E2), and kisspeptin (14-18). However, there are some species differences in physiological characteristics of GnRH neurons from rodents to monkeys and even monkeys to humans, and examination of primary GnRH neurons derived from human fetuses is ethically not possible in the United States. In fact, to date, molecular and cellular studies of human GnRH neurons have not been conducted, and therefore many human disease states have not been easily studied. Accordingly, several years ago, we started to generate GnRH neurons from human embryonic stem cells (hESC) and induced pluripotent stem cells (hiPSC). Unlike most neurons in the central nervous system, which arise from the neural tube, GnRH neurons originate from the olfactory placode (19,20). Genetic analysis of Kallmann syndrome patients (21) and transgenic mice (22) indicate that the presence of fibroblast growth factor 8 (FGF8) and its receptor, FGFR1, are critical for GnRH neurogeneration. Moreover, during embryonic development, neurogenesis of the GnRH niche is regulated by bone morphogenetic protein 4, noggin, and FGF8 (23), and neural crest cell signaling to the olfactory placode is a key for GnRH neurogeneration (24). Based on these developmental characteristics, we have developed a reliable method to generate GnRH neurons from human hESC and hiPSC, generated an ESC line carrying the mCherry-labeled human GnRH gene from which we can identify GnRH neurons in live culture and compared physiological characteristics between stem cell–derived human GnRH neurons and olfactory placode–derived monkey primary GnRH neurons. Additionally, we characterized developmental changes in GnRH neurons transcriptomes using hESC, hiPSC, and mCh-hESC.
This work provides a tool to study human GnRH neurons in vitro, by which we can understand the normal state of human GnRH neurons including the differentiation process, migration patterns, and neurocircuitry formation and then compare these processes to disease states, such as IHH.
Materials and Methods
Cell Cultures
In the present study, pluripotent H9 hESC (WA09, WiCell Research Institute, Madison, WI, USA) and hiPSC (IMR90 clone #4, WiCell) were used. All work with H9 was reviewed and approved by the Stem Cell Oversight Committee, University of Wisconsin.
H9 and IMR90 cells were maintained in the presence of 5% CO2 at 37°C on 6-well tissue culture plates (Greiner Bio-One Cellstar, Monroe, NC, USA) coated with matrigel (WiCell) in mTeSR1 media (Stem Cell Technologies). The culture medium was changed daily, and cells were passaged with versene (Fisher Scientific, Hampton, NH, USA) every 4 to 6 days.
Generation of GnRH Neurons: Exposure to FGF8 at 2 different Developmental Stages
Protocol 1
In general, treating neuroepithelial (NE) cells with small molecules results in a specific type of neurons (25). Three to four days after passaging ESC, adherent cells were exposed to dual SMAD inhibitors (10 µM SB431542 and 100 nM LDN-193189) in cell differentiation medium (CDM) [see Figshare Data Repository Table 1 in (26)] for 10 days. This procedure led to stem cells transforming predominantly to NE cells. Subsequently, cells were lifted from the culture plate with 2 mg/mL dispase (17105-041, Fisher) and left to become predominantly neural progenitor cells (ie, neurospheres) in the presence of neural induction medium [see Figshare Data Repository Table 1 in (26)] in suspension for 7 to 10 days. Next, cells were dispersed with accutase (SCR005, Sigma), plated on Matrigel, and exposed to FGF8 (10 ng/mL) in neural differentiation medium (NDM) [see Figshare Data Repository Table 1 in (26)] for 3 to 6 weeks to induce GnRH neurons. The first day of exposure to FGF8 was designated as day 0 (Fig. 1A, top).
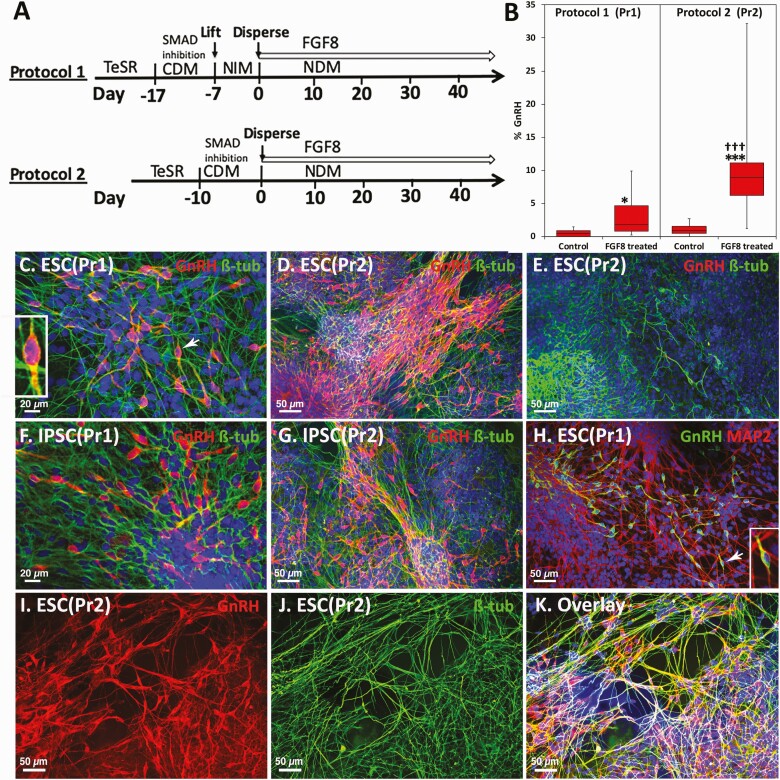
Figure 1.
Comparison of 2 protocols for FGF8-induced GnRH neurogeneration from hESC or hiPSC. Schematic illustrations of 2 protocols that we examined for the efficiency rates of GnRH neurogeneration (A). Comparison of the efficiency rate between immunohistochemically stained GnRH neurons generated by the 2 protocols (B) and examples of immunopositive GnRH neurons from the 2 protocols (C-K) are shown. (A) The first day of FGF8 treatment is designated day 0. In Protocol 1, after dual SMAD inhibition in cell differentiation media cells were exposed to neural induction media for at least 7 days resulting in neurospheres (neuroprogenitor cells). Then neuroprogenitor cells were exposed to FGF8 in neural differentiation media (NDM). In Protocol 2, after dual SMAD inhibition NE cells were exposed to FGF8 in NDM. These cultures contain multiple types of cells at multiple developmental stages. (B) Whisker plot shows Protocol 2 yielded a significantly higher efficiency rate of GnRH neurogeneration than Protocol 1 (Left). *** P < 0.001 vs control (NDM alone); ††† P < 0.001 vs Protocol 1. n = 5-20. Exposure of hESC (C and D) or hiPSC (F and G) to FGF8 resulted in GnRH neurogeneration. Comparison between Protocol 1 (C and F) and Protocol 2 (D and G) by immunohistochemical staining with the polyclonal antibody GF6 (red, C-G) indicates that Protocol 2 yielded a larger number of GnRH neurons in both hESC and hiPSC. (E) Without FGF8 (control) only a few GnRH neurons were occasionally seen in control hESC culture. For C to G, green = β-tubulin. (H) GnRH neurogeneration by FGF8 is also shown with immunostaining using the monoclonal antibody LRH13 (green) and the neural marker MAP2 (red). Insets in C and H are a high power (2.5×) magnification of GnRH neurons with arrows. (I-K) GnRH neurons (red) derived from hESC by FGF8 treatment, immunostained by polyclonal antibody SW1 (I), double stained with β-tubulin (green, J) and overlay (K). For all, Blue = 4′,6-diamidino-2-phenylindole nuclear staining.
Protocol 2
As stated earlier, GnRH neurons originate from nasal placode, yet need a signal from neural crest cells (24). As such, exposure of primitive NE cells in the presence of various types of undifferentiated cells to FGF8 would be helpful for GnRH neurogeneration. After stem cells were exposed to dual SMAD inhibitors in CDM for 10 days resulting in primitive NE cells, they were dispersed with accutase, replated on Matrigel, and exposed to FGF8 (10 ng/mL) in NDM for a similar period to Protocol 1. Again, the first day of exposure to FGF8 was designated as day 0 (Fig. 1A, bottom)
Differentiation of hESC and human hiPSC and molecules that facilitate GnRH neurogenesis
We first examined FGF8 at different doses (2, 10, 20, and 100 ng/mL) and time periods, and FGF21 (10 ng/mL, AF10042; PeproTech). Next, the additive effect of various small molecules including kisspeptin (0.1 µM; Phoenix Pharm., Burlingame, CA, USA), Sonic Hedgehog (SHH, 20 ng/mL; Fisher), and retinoic acid (RA; 3 ng/mL; Fisher), with or without FGF8, was examined. Additionally, the effects of 2 notch inhibitors were examined: compound E (0.5 ng/mL; Fisher), DAPT (20µM; Sigma), and the antimitotic drug fluorodeoxyuridine (FdUR; 30 µM; Sigma). The doses of DAPT and FdUR were the same or 50% lower as those described for stem cell–derived GnRH cultures (27,28) and for primary cultures of GnRH neurons (8,17), respectively.
Reverse transcription-polymerase chain reaction
hESC at days 0, 5, 10, and 24 of FGF8 treatment (n = 4 for all) from Protocol 2 were frozen and isolated using RNA STAT-60 (Tel-Test, Friendswood, TX, USA) according to the manufacturer’s instructions. Individual RNA samples were then analyzed for the presence of KISS1 using reverse transcription-polymerase chain reaction (RT-PCR) with primer sequences [see Figshare Data Repository Table 2 in (26)]. RT-PCR was conducted as described previously (15). For RT 1 μg of total RNA and random hexamer primers were used with the GeneAmp RNA PCR Core Kit (Applied Biosystems, Branchburg, NJ, USA) according to manufacturer’s specifications. For PCR, primers were synthesized by the University of Wisconsin Biotechnology Center. Aliquots (3 µL) of each RT reaction were used for PCR and combined with 2.5 mm deoxynucleotide triphosphates (Amersham Biosciences), 1 × PCR Buffer II with MgCl, 1.25 U AmpliTaq (Applied Biosystems), and 12.5 pmol of primer in a final volume of 50 L water mixed with each PCR/primer cocktail served as a negative control. PCR amplification conditions consisted of 1 cycle at 95°C for 2 min; 45 cycles at 95°C for 45 sec, and 58°C for 45 sec and an incubation at 72°C for 7 min. After PCR amplification, 20-μL aliquots of each reaction were loaded on a 1% or 2% agarose tris-borate-EDTA gel, and products were visualized using ethidium bromide staining.
Immunohistochemistry
Neurons grown on glass coverslips were fixed with 4% paraformaldehyde for 20 min. Coverslips were washed with phosphate-buffered saline (PBS) for 5 min 3 times and blocked for 1 h in 0.5% normal goat serum before incubation with the primary antibody in 0.1% Triton X-100 at 4°C overnight. Cells were subsequently washed with PBS and stained with Alexa Fluor–conjugated secondary antibodies. Coverslips were washed and mounted onto glass slides using Vectashield mounting media with 4′,6-diamidino-2-phenylindole (DAPI; Vector Labs, Burlingame, CA, USA). To ensure the consistency of the GnRH staining, we used 3 primary antibodies (the polyclonal antibodies GF6 and SW1 and the monoclonal antibody LRH13). A complete list of antibodies is available in Figshare Data Repository Table 3 in (26). Images were taken using a Nikon TiE2 fluorescence microscope or a Nikon Confocal TiE microscope (Nikon Instruments, Tokyo, Japan).
Cell counts
The efficiency rate of GnRH neurogeneration was calculated by manual GnRH cell count over the number of the total cells (DAPI-stained cells). For example, the comparison between Protocols 1 and 2 was made by counting cell bodies of GnRH positive neurons on the entire coverslip and the total cell number was assessed by DAPI staining and NIS elements software (Nikon), obtained from days 24 to 27 of the FGF8 treatment. For the GnRH cell count, we were not able to use computer-based programs including a high-content imaging platform, as GnRH neurons are not uniform in shape and size (Fig. 1C, 1D, 1F-1I, and 1K), and they aggregated and formed thick clusters (see Fig. 1D, 1I, and 1K). As total numbers increased, however, the approach to count the entire slide was inefficient. Accordingly, we decided to count a portion of the coverslip to extrapolate the total number. Using previously assessed GnRH positive cells in the entire slide, we placed a 20 × 20 grid (ie, a 6 × 6 mm section with 300 µm2) and counted the number of GnRH positive cells in randomly selected 5, 10, 20, and 30 grid sections. We found that counting 30 grid sections (~1% of the total area of the coverslip) accurately reflected the entire coverslip. This method was applied to all statistical analyses except for comparison between the 2 protocols in which we counted the entire slide and developmental changes in mCherry. Numbers of mCherry GnRH (mCh-GnRH) neurons were determined by counting all red cells in an 8-mm2 area per slide. For statistical analysis, we always counted at least 3 independent coverslips from at least 2 different nonconsecutive passages per treatment and derived the average ± SEM. For all stem cell cultures, we used passage 39 to 49 in H9 cells or mCherry labeled H9 cells and passage 56 to 66 in IMR90. Note that cell count of GnRH neurons in older cultures (ie, over FGF8 day 30) was not easy to perform, as they aggregate forming thick clusters and fair cell count was not possible; therefore, we did not count above this age (see Results section).
Generation of a stem cell line carrying the fluorescent marker mCherry-labeled GnRH gene
The transgenic mCh-GnRH cell line was generated by inserting a P2A-mCherry transgene downstream of the GnRH coding sequence, as schematically illustrated in Figshare Data Repository Figure 1 in (26) at the Human Stem Cell Core at the University of Wisconsin-Madison using a CRISPR/Cas9 targeting approach (29-31).
CRISPR/Cas9 targeting design and plasmid generation
Single guide RNA (sgRNA) design targeting the C-terminal locus of GnRH was performed using the crispr.mit.edu design tool. To utilize a Cas9D10A nickase, 2 sgRNAs targeting opposite DNA strands (sgRNA-1A: TACTTAAGTCATGTTAGTAA.TGG and sgRNA-1B: ACCCATTAAATACCTGTAAA.TGG) were cloned into an sgRNA expression plasmid from the laboratory of Su-Chun Zhang (Addgene #68463) (32).
Electroporation, selection, and growth
hESC (H9) were generally cultured and electroporated as described in Chen et al (32), although this protocol was modified for standard feeder-free maintenance conditions. Human ESCs were cultured in TeSR-E8 media (StemCell Technologies) on matrigel substrate (Corning). Twenty-four hours prior to electroporation, when cells were approximately 80% confluent, media was supplemented with rho kinase (ROCK) inhibitor (0.5 µM, Calbiochem, H-1152P). Cells were lifted and singularized with 0.5 mM EDTA in PBS (GIBCO) for 3 to 4 min, washed 2 times with Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12, and harvested in PBS. Cells were dispersed into single cells, and 1 × 107 cells were electroporated with appropriate combination of plasmids using the Gene Pulser Xcell System (Bio-Rad) at 250 V, 500 μF in a 0.4-cm cuvette (Phenix Research Products). The plasmid cocktail contained 15 µg of CAG-Cas9D10A plasmid (Kiran Musunuru, Addgene #44720), 6 µg each sgRNA plasmid (sgRNA-1A and sgRNA-1B), and 30 µg of a donor plasmid designed to insert a P2A-mCherry construct downstream of the GnRH coding sequence. This donor plasmid has approximately 1-kb homology arms consisting of the sequence upstream and downstream of the GnRH stop codon. Between the homology arms, the plasmid is designed to remove the stop codon and insert a P2A linker sequence and the mCherry gene (Clontech) along with a LoxP-flanked PGK-puromycin cassette to facilitate selection of cells with proper homologous repair (33). Following electroporation, cells were replated on matrigel in 0.5 µM ROCK inhibitor at a low density (original 1 × 107 cells replated to three 6-well plates). After approximately 5 days, cells were treated with puromycin (0.5 µg/mL, Invivogen, ant-pr-1) to select for cells incorporating the plasmid. Puromycin was resupplemented daily until hESC colonies grew to sufficient size (approximately 10 days). At this point, puromycin was removed and 0.5 µM ROCK inhibitor was added 24 h prior to clone picking.
Genotyping
Single-cell colonies were manually selected and mechanically disaggregated. Genomic DNA was isolated from a portion of these colonies using QuickExtract DNA Extraction Solution 1.0 (Epicentre). All genotyping and subsequent sequencing was performed via Q5 polymerase-based PCR (NEB). For genotyping, 3 pairs of primers were used to assess genomic integration of the transgenic construct. The transgene integration PCR (Geno 2F with 3’ close 2R) identified clones with correct targeting of the transgenic construct, the homozygous/heterozygous PCR (Geno 2F with 3’ close 2R) identified whether these clones exhibited monoallelic or biallelic transgenic integration, and the plasmid retention PCR (T3 with PGK up 2R) identified whether there was retention or nonspecific integration of the plasmid that may erroneously express the mCherry protein [see Figshare Data Repository Figure 1 in (26)]. Clone 1 was identified as a heterozygous transgenic line, and Clone 6 was identified as a homozygous transgenic line without nonspecific plasmid retention, so the transgenic integration was sequence validated for these clones. Purified PCR fragments, identified via agarose gel and purified using a Zymoclean Gel DNA Recovery Kit (Zymo Research), identified via agarose gel and purified using a Zymoclean Gel DNA Recovery Kit (Zymo Research), were submitted to Quintara Biosciences for Sanger sequencing to identify clones with the proper genetic modification and to ensure sequence fidelity of the reporter construct.
Off-target analysis
To identify whether the CRISPR-Cas9 system produced any nonspecific genome editing, we analyzed suspected off-target sites for genome modification in Clone 6.
Using the 5 highest-likelihood off-target sites predicted by the crispr.mit.edu algorithms for both of the sgRNAs that were used (sgRNA-1A and sgRNA-1B), we designed genotyping primers [see Figshare Data Repository Table 2 in (26)] to amplify these regions via Q5-polymerase PCR. PCR products were identified via agarose gel, purified using a Zymoclean Gel DNA Recovery Kit, and submitted to Quintara Biosciences for Sanger sequencing. No off-target CRISPR activity was observed.
CRE/LoxP recombination
To ensure proper GnRH-P2A-mCherry expression, we wanted to remove the floxed PGK-puromycin cassette. For this experiment, cells from Clone 1 and Clone 6 were lifted and singularized with 0.5 mM EDTA for 3 to 4 min, washed 2 times with Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12, and harvested in TeSR-E8 media with 0.5 µM ROCK inhibitor. Cells were pelleted by centrifugation (1000 rpm for 1.5 min). Remaining media was aspirated, leaving approximately 50 µL of media in the tube. A recombinant TAT-CRE recombinase (10 µL; Excellgen) was added to the cells, and the pellet was resuspended. The cells were allowed to sit in the media-CRE solution for 20 min at 37°C. After this time, cells were triturated to ensure single-cell dispersion and plated in 6-well plates for clonal selection in TeSR-E8 media with 0.5 µM ROCK inhibitor. Following growth of clones, individual colonies were selected as previously described and PCR verified to exhibit loss of the PGK-puromycin cassette. Final clones were sequenced to again reconfirm sequence fidelity of the GNRH-P2A-mCherry expression construct, and karyotyping was performed by WiCell (Madison, WI, USA). Clone 6, Cre subclone 1, was expanded and used for all subsequent fluorescent reporter experiments.
Physiological Characterization of Generated GnRH Neurons
Electrophysiological recording
Whole-cell patch recordings were performed on 10 mCh-GnRH neurons between 20 and 34 days after the start of FGF8 exposure. Coverslips were immersed in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-based artificial cerebrospinal fluid recording solution consisting of (mM) 128 NaCl, 5 KCl, 2 CaCl2, 2 MgSO4, 30 glucose, and 25 HEPES. Solutions were adjusted to 7.3 pH and 300 to 310 mOsm. Patch pipettes made of borosilicate glass were fire polished to resistances of 5 to 10 MΩ. Recording pipettes were filled with a K-gluconate based intracellular solution consisting of (mM) 140 K-gluconate, 10 NaCl, 10 HEPES, 5 EGTA, 0.5 CaCl2, 10 Na2 phosphocreatine, and 4 Mg ATPs. Intracellular recording solutions were corrected to 7.2 pH and 290 mOsm. mCh-GnRH cells were identified using an Olympus BX50WI microscope under 40× immersed-lens objective and visualized through a U-MWIY cube (EX 545-580; EM 600). Recordings were amplified and telegraphed in current clamp mode via MultiClamp 700B (Axon Instruments, San Jose, CA, USA) and digitized by Digidata1440A (Axon Instruments). Data were analyzed online and post hoc using pClamp 10 (Axon Instruments) software, Clampex and Clampfit 10.
Calcium imaging
[Ca2+]i levels were assessed by the method described previously (13,15,17) with some modifications. Culture medium (2 mL) was mixed with 18 µM fura-2 AM (Life Technologies) and 6 µL of a mixture of pluronic F-127 (BASF Corp., Parsippany, NY, USA) and dimethylsulfoxide (1:2) by vortexing for 15 sec. Cells on a coverslip were incubated in fura-2 for 20 to 40 min at 37°C under 5% CO2. The coverslip was then placed in a Dvorak-Stotler recording chamber. Fluorescence imaging of the dye-loaded cells was achieved with an inverted microscope. A culture was viewed through a ×20 objective lens, and a 750 × 750 µm recording field with mCh-GnRH cells were selected for data capture. Cultures were continuously perifused at a speed of 50 µL/min with Brain Phys medium without Phenol Red (05791, StemCell Tech., Cambridge, MA, USA), under 95% O2 and 5% CO2 at room temperature (22°-25°C).
[Ca2+]i was monitored as a function of the ratio of the 510-nm fura-2 emission excited by illuminations at 340 and 380 nm with a Lambda DG-4 light source and filter exchanger (Sutter Instruments, Novato, CA, USA). Fura-2 fluorescence was recorded at 10 sec intervals with a CCD camera (Hamamatsu, Hamamatsu City, Japan) and Nikon NIS imaging software (Nikon). The ratio of the fluorescence intensities (∆F/F0) from the 340- and 380-nm excitation wavelength-evoked images were used to calculate the free [Ca2+]i levels as described previously (13,15,17). All [Ca2+]i oscillation experiments were conducted between days 20 and 50 of FGF8 treatment.
Perifusion experiments and GnRH measurements
To examine the spontaneous GnRH release pattern and responses of GnRH neurons to various secretagogues, perifusion experiments were conducted using Sykes-Moore chambers as described previously (11,17). Briefly, GnRH neurons on 2 thermanox coverslips were placed face to face, separated by a rubber O-ring, forming a chamber with a volume of 200 µL. Cultured cells were perifused with a modified Krebs-Ringer phosphate buffer (11,17) with 0.1% glucose (pH = 7.4) under 95% O2 and 5% CO2 at 37°C. Perifusates were collected at 30 µL/min in 10-min fractions for 6 h using the ACUSYST (Endotronics, Minneapolis, MN, USA) perifusion system. Various known exocytotic stimuli, such as human kisspeptin-10 (hKP10, 1-100 nM), senktide (1-100 nM, neurokinin B agonist), and E2 (10 nM) for GnRH neurons, or vehicle for control, were applied for 20 min periods at approximately 2-h intervals. To test the viability of cells, cultures were challenged with 56 mM KCl at the end of experiments. All samples were stored at −80°C for later GnRH radioimmunoassay. GnRH concentrations in media samples were measured in 200 µL aliquots (single) with radioimmunoassay using antiserum R42 [see Figshare Data Repository Table 3 in (26)] as described previously (34). After the perifusion experiment, cells were fixed with 4% paraformaldehyde (pH = 7.6) and immunostained for GnRH and β-tubulin or the presence of mCh-GnRH neurons. This experiment was conducted between days 22 and 63 of FGF8 treatment.
Developmental Changes in the Transcriptome of Human GnRH Neurons
Sample preparations
To uncover the genes up- and downregulated during the stem cell differentiation of GnRH neurons, we prepared human stem cells (H9, IMR90, mCh-H9) treated with FGF8, FGF8 + kisspeptin or NDM alone and collected at days 0, 8, 15, and 25. This generated a total 12 groups with n = 3 in each group (n consists of 3 different passages). Total RNA was extracted from all 108 samples, standard messenger RNA (mRNA) libraries were prepared, and subjected to RNA sequencing (RNAseq) analysis.
RNAseq was conducted by the University of Wisconsin, Biotechnology Center, using an Illumina NovaSeq 6000 platform.
RNASeq preprocessing
The average size of RNAseq libraries (n = 108) was 20 329 051 reads with a SD of 2 941 894, and the read length was 151 base pairs. FastQC was used for quality control of the sequencing data (35). The read pairs were aligned to the human reference genome (GRCh37 Ensembl release 75) using STAR 2.5.3 (36) with the following parameters “--outFilterMultimapNmax 1 -- outFilterMismatchNoverLmax 0.1-- alignEndsType local.” STAR was also used to quantify read counts per gene based on GTF file (GRCh37 Ensembl release 75). Picard’s CollectInsertSizeMetrics, CollectRnaSeqMetrics, and EstimateLibraryComplexity were used for quality control of the aligned data. Based on GTF definition, nonprotein-coding genes were filtered out. These filtered genes were used for differential expression analysis. To perform quality control on the samples, the correlation between each sample using hierarchical clustering and principal component analysis plots were tested for confounding effects of metadata for the samples.
Differential gene expression analysis
Differential expression analysis for RNAseq data from H9 samples between points of the time-series experiment was performed using the R package DESeq2 version 1.26.0 (37). To determine the strongest individual gene effects in GnRH differentiation, differential expression analysis was performed for FGF8-treated samples between days 8 and 15, as well as between days 15 and 25. Counts per million (CPM) were calculated based on the number of uniquely mapped reads and 0.1 CPM cutoff threshold was used to filter the genes in less than 50% of the samples within the respective comparison groups, resulting in 14 260 genes for day 8 and day 15 that were included in the DESeq2 analysis and 14 785 genes for day 15 and day 25 included in the analysis. To correct for the hidden confounding variables, Surrogate Variable Analysis (SVAseq), version 3.34.0 (38), was used. The hidden variables estimated using SVAseq were regressed out in the differential expression analysis by incorporating surrogate variables into the DESeq2 model, and log2-fold changes (lfc) and P-values (corrected for multiple testing using Benjamini-Hochberg adjusted P-values) were estimated. Significant differentially expressed genes (DEGs) with adjusted P-value < 0.05 and absolute lfc > 1. To understand the uniqueness of transcriptomes associated with GnRH neurogenesis in vitro from those in other types of neurons derived from hiPSC, we compared the genes showing differential expression between day 25 and day 0 of FGF8-induced GnRH neurons to hiPSC-derived Ngn2-induced neuron (iNs) from the Talkowski Lab. For iNs, RNAseq was performed on both the hiPSCs and iN samples (day 21 post-Ngn2 induction), and differential gene expression analysis was performed between the hiPSCs and iN samples using the same methods as described in this study.
Time-series expression analysis for FGF8-treated H9 cell line samples from day 0 to day 25 was also performed using DESeq2. CPM-filtered and SVAseq corrected 15 798 genes were used in the time-course differential expression analysis. The significant time-series DEGs were selected through a likelihood ratio test. The DEGs with adjusted P-value < 0.05 were used for gene ontology (GO) term and KEGG pathway enrichment analyses. GO term enrichment analysis was performed using R package topGO, version 2.24 (39). KEGG pathway enrichment analysis was performed using the R package clusterProfiler, version 3.16.1 (40). The significantly enriched GO classes and pathways were selected (Benjamini-Hochberg adjusted P-value < 0.05).
Statistical Analysis
For all statistical analysis except for RNAseq analyses (as previously described), we used GraphPad-Prism (San Diego, CA, USA) statistical software. The efficiency rate between Protocols 1 and 2, kisspeptin, and PCR data were examined using 2-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc analysis. For time-lapse analysis of mCh-GnRH neurons and perifusion experiments (percentage values over baseline level, which was defined as the average of the 3 points before challenges) were subjected to 2-way ANOVA repeated measures with Bonferroni’s post hoc analysis. Pulsatility of GnRH release was determined with Pulsar algorithm as described previously (11). For analysis of calcium imaging data, identification of [Ca2+]i peaks and synchronization of [Ca2+]i peaks among individual GnRH neurons in a culture were conducted as previously described for primary GnRH neurons derived from the monkey placode (13,41). Subsequently, the interpulse interval (IPI) in single GnRH neurons and synchronization interval among individual GnRH neurons were calculated as described previously (13,41). For remaining data analysis, a Students t-test was applied. Significance was attained at P ≤ 0.05.
Results
Generation of GnRH Neurons: Exposure to FGF8 at 2 Different Developmental Stages
Immunohistochemical staining data indicated FGF8 treatment of hESC with both Protocols 1 and 2 resulted in GnRH neurogeneration (Fig. 1C, 1D, 1H, 1I and 1K). Similarly, the same FGF8 treatment of hiPSC with Protocols 1 and 2 resulted in GnRH neurogeneration (Fig. 1F and 1G). We confirmed GnRH staining with several antibodies to GnRH, including polyclonal antibodies GF6 (Fig. 1C-1G) and SW1 (Fig. 1I and 1K) and the monoclonal antibody LRH13 (Fig. 1H). A comparison of the efficiency rate between the 2 protocols by cell count further indicated that Protocol 2 yielded 5-fold more GnRH neurons than Protocol 1 (Fig. 1B). Note that the cell count was conducted in cultures younger than FGF8 treatment day 28, as GnRH neurons had a propensity to form thick multilayer clusters when they reached 4 to 6 weeks of FGF8 treatment, and an accurate cell count became considerably difficult. Importantly, with either hESC or hiPSC Protocol 2 (Fig. 1D and 1G) was far more efficient than Protocol 1 (Fig. 1C and 1F). Regardless of the origin (hESC or hiPSC), in both protocols there were small numbers of GnRH neurons without any FGF8 treatment, as a result of self-patterning (Fig. 1B and 1E). Collectively, we concluded that the FGF8 exposure of less differentiated NE cells is more efficient in generating GnRH neurons.
Small Molecules That Facilitate GnRH Neurogenesis
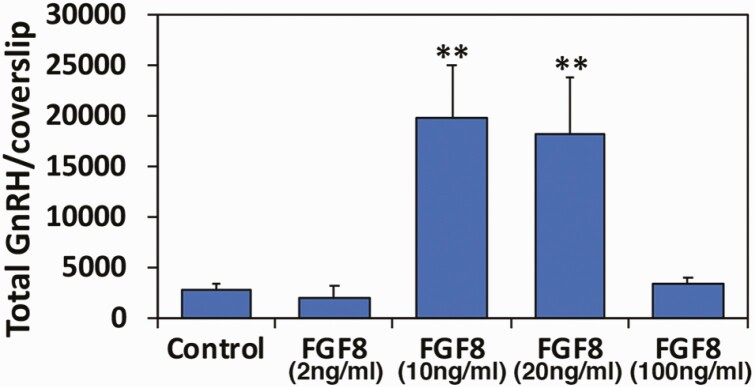
From this point we decided to use Protocol 2 exclusively. Comparing the effectiveness of FGF8 doses ranging from 2 to 100 ng/mL we found that 10 ng/mL was optimal for GnRH neurogeneration (Fig. 2). Increasing the dose to 20 ng/mL did not increase the number of GnRH neurons, and 100 ng/mL was ineffective over control (Fig. 2). Moreover, we examined the effects of FGF21, as it has been reported that FGF21 is also involved in IHH disease (42). The results suggested that FGF21 did not have any trophic effect resulting in GnRH neurogeneration (Fig. 3B).
Figure 2.
Determination of FGF8 doses for GnRH generation from hESC using Protocol 2. FGF8 at 10 ng/mL is sufficient. Total cell number of a single coverslip was ~200 000. ** P < 0.01 vs control (NDM alone). n = 3-6.
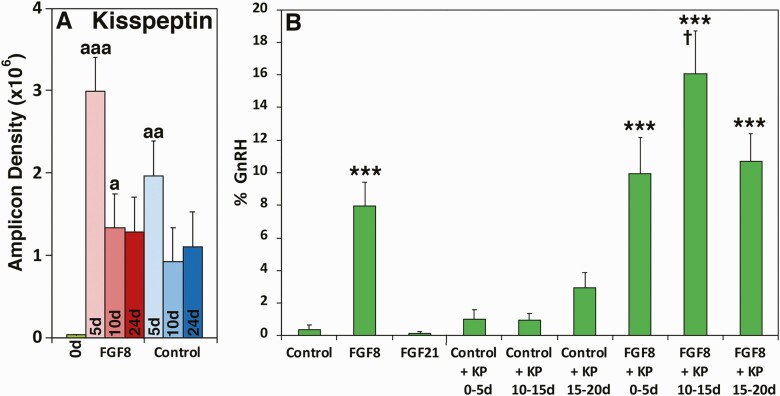
Figure 3.
(A) Changes in kisspeptin mRNA expressions during GnRH neurogeneration by FGF8 treatment. mRNA levels on days 0 (green), 5 (pale pink), 10 (dark pink), and 24 (red) in the presence of FGF8 and on days 5 (pale blue), 10 (blue), and 24 (dark blue) in the absence of FGF8 (NDM control) were assessed by RT-PCR (all, n = 4). aaa = P < 0.001, aa = P < 0.01, and a = P < 0.05 vs FGF8 day 0 (NE stage). Note that regardless of the presence or absence of FGF8, kisspeptin mRNA expressions on day 5 were significantly higher than day 0. (B) Effects of various molecules on GnRH neurogeneration from hESC. The mean ± SEM efficiency rate (n = 6, green). Exposure of NE cells to FGF8, but not to FGF 21, was effective in GnRH neurogeneration and addition of KP10 to FGF8 in NDM for a 5-day period, especially, days 10 to 15, significantly increased the efficiency rate of GnRH neurogeneration as well as the maximum efficiency rate. **P < 0.01, ***P < 0.001 vs control (NDM alone); † = P < 0.05 vs FGF8 alone.
Next we examined an additive effect of various molecules with FGF8 that potentially enhance GnRH neurogeneration. First, we examine the effects of kisspeptin, one of the most important upstream regulators for GnRH release (43), which was reported to increase the number of hypothalamic GnRH neurons in embryonic zebra fish (44). We also observed that kisspeptin mRNA levels in cultures were generally elevated during the differentiation of stem cells, and specifically on day 5 in the presence of FGF8, kisspeptin expression was significantly higher than other time periods measured (Fig. 3A). Results indicated that hKP10 treatment between days 10 and 15 in the presence of FGF8 increased the efficiency rate almost double over FGF8 alone, whereas hKP10 + FGF8 treatments between days 0 and 5 or days 15 and 20 were effective, but not as effective as hKP10 + FGF8 treatment between days 10 and 15 (Fig. 3B). Addition of hKP10 for the entire FGF8 treatment period did not show further improvement over FGF8 alone (data not shown). hKP10 treatment alone between days 15 and 20 had a trend to increase the efficiency rate over NDM control, although it was not statistically significant (Fig. 3B). Second, we examined the effects of RA and SHH, as they have been implicated in olfactory epithelial differentiation (45). Neither RA nor SHH were effective in increasing GnRH neurogeneration (see Figshare Data Repository Figure 2 in (26)).
Notch Inhibitors and Antimitotic Substances
It has been reported that after a fair amount of a specific type of neurons are generated, weeding mitotic cells or using notch inhibitors to synchronize neurogeneration helped the efficiency rate (21,22). As such, we tested the effects of 2 notch inhibitors, CE and DAPT, and the antimitotic compound, FdUR, on days 14 to 18 and days 18 to 22 of FGF8 treatment. The results indicated that DAPT at 2 and 10 µM doses for days 14 to 18 had a tendency to increase efficiency rates over those without the notch inhibitors, although the effect was statistically insignificant [see Figshare Data Repository Figure 3 in (26)]. CE at 0.05 and 0.1µM did not help the efficiency rate [see Figshare Data Repository Figure 3 in (26)]. Applications of both DAPT and CE at both doses examined sporadically reduced the total number of cells on coverslips, resulting in irregular results. FdUR at 10 and 20 µM doses on either days 14 to 18 or days 18 to 22 completely failed to improve the efficiency rate (data not shown). The effectiveness of the notch inhibitors needs further examination by changing different concentrations and/or the day of application.
Time Course of GnRH Neurogeneration
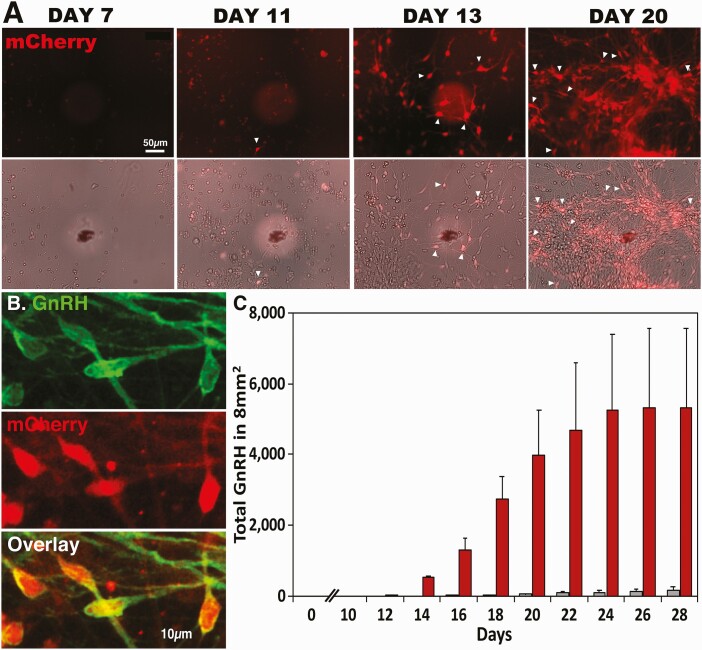
Upon GnRH expression in this cell line, the P2A peptide self-cleaves, resulting in separate GnRH and mCherry expression in the cell. Genotyping efforts revealed Clones 1 (heterozygous) and 6 (homozygous) as transgenic line without confounding plasmid retention, so we performed CRE recombination to remove the PGK-puromycin resistance cassette [see Figshare Data Repository Figure 1A and 1B in (26)]. Subsequent PCR confirmed successful Cre recombinase activity and excision of the resistance cassette [see Figshare Data Repository Figure 1C in (26)]. Clone 1, Cre subclone 1, and Clone 6, Cre subclone 1, were tested for transgenic sequence validation (and wild-type allele sequence validation for Clone 1 subclone 1), karyotyped, and screened for off-target Cas9 activity to verify this clone met our quality standards [see Figshare Data Repository Figure 1D in (26)]. Clone 6, Cre subclone 1, was expanded and used for all subsequent fluorescent reporter experiments. We refer to this cell line as “mCh-GnRH.” Differentiation of mCh-GnRH ESCs using Protocol 2 confirmed mCherry expression in GnRH peptide-containing neurons (Fig. 4B). Quantitative analysis indicated that 591 out of 600 mCherry positive cells on 6 coverslips (100 cells/coverslip) also expressed the GnRH peptide (98.5 ± 0.5%). Subsequent real-time live-cell image analysis (Fig. 4A) indicated that mCh-GnRH neurons started to appear on days 11 to 15 after FGF8 exposure, and the number of GnRH neurons continued to increase up to day 24 and reached a plateau on day 26 under Protocol 2 [Fig. 4C; also see Figshare Data Repository Table 4 in (26)]. The results from 2-way ANOVA indicated that there were significant interactions (P < 0.05), treatment effects (P < 0.0001), and time effects (P < 0.05). As expected from the results seen in Figure 1E, without any FGF8, a small number of GnRH neurons appeared between days 15 and 26, although they were not significant [Fig. 4C; also see Figshare Data Repository Table 4 in (26)].
Figure 4.
Fluorescent mCherry-labeled GnRH neurons derived from hESC allowed us to clarify the time course of GnRH neurogeneration. Using the CRISPR/Cas9 gene editing system, hESCs were modified such that mCherry is expressed when the GnRH gene turns on. (A) Live cell imaging (top row) and phase contrast imaging (second raw) were taken on days 7, 11, 13, and 20 of the FGF8 exposure at the same slide position, as indicated by a black marker with a white shadow circle. Note that because of the migratory nature of GnRH neurons, GnRH neurons seen one day are not visible in the same location in the later days. (B) An overlay microphotograph (bottom panel) indicates that mCherry expressing cells (red, middle panel) are also GnRH peptide positive (green, top panel). Note that while the peptide staining is seen in the cell body as well as in neurites, mRNA expression is limited to the cell body. (C) GnRH neurons counted in an 8-mm2 area showed that GnRH neurons are seen as early as days 11 and 12 of FGF8 exposure, and the number of GnRH expressing cells progressively increased between days 12 and 24, reaching the plateau. Red bars indicate cultures exposed to FGF8, whereas gray bars indicate cultures exposed to NDM alone. n = 4/group. For statistical analyses, see Supplementary Table 4 in (26).
Physiological Characterization of Generated GnRH Neurons
The generation of mCh-GnRH neurons allowed us to characterize electrophysiological properties, intracellular calcium [Ca2+]i signaling, and GnRH release pattern in human GnRH neurons.
Electrophysiological characteristics of GnRH neurons derived from human ES cells
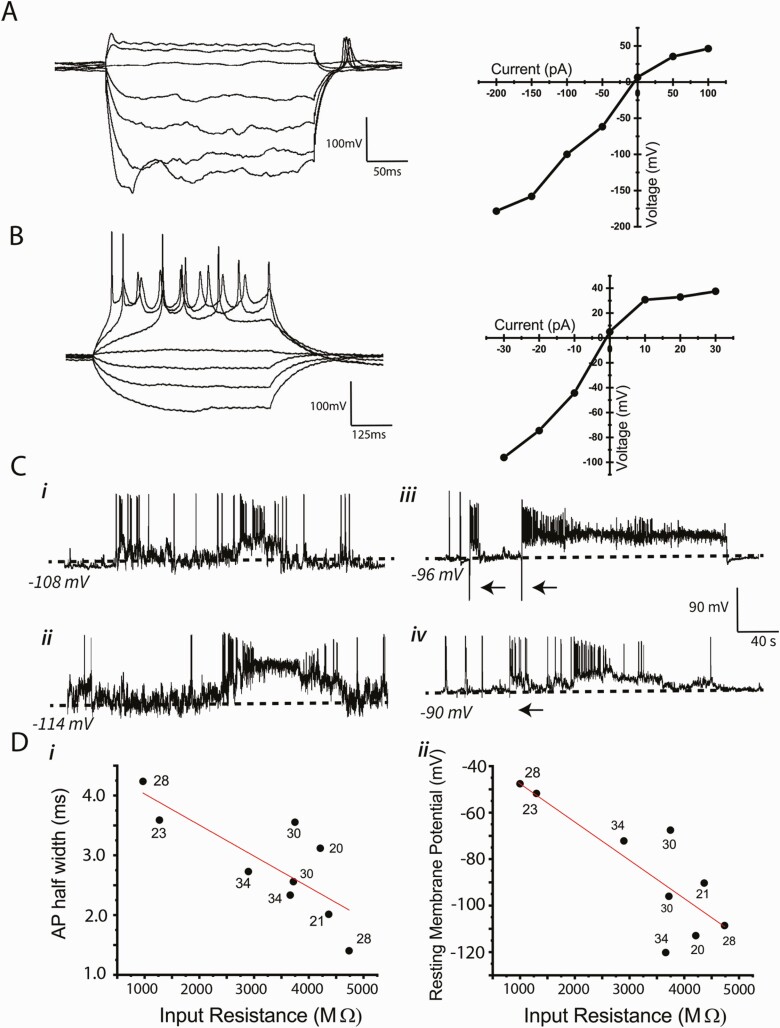
We recorded electrical activity from 10 mCh-GnRH neurons between days 20 and 34. Recordings from cells younger than day 20 were unsuccessful, due to weaker mCherry fluorescence under a conventional microscope used for electrophysiological recording and to the fragility of the cells. The summary of electrophysiological characteristics of the 10 GnRH neurons and examples from which we successfully obtained stable recordings are shown in Table 1 and Figure 5, respectively. Several findings are noteworthy. First, the differentiated human GnRH neurons had much more hyperpolarized resting membrane potentials and smaller action potential amplitudes compared with GnRH neurons reported for other mammalian species (Table 1) (46-49). Second, among the human differentiated GnRH cells between days 20 and 34, there were no significant age-related differences in resting membrane potential, input resistance, membrane capacitance, or action potential threshold, amplitude, or half-width (Fig. 5D). Third, depolarizing current pulses induced trains of action potentials in all cells except 1, and in that cell, which had a relatively depolarized resting membrane potential, hyperpolarizing current pulses induced “rebound” action potentials (Fig. 5A and 5B). Thus, action potentials could be induced by current injection in all cells. Fourth, all cells displayed spontaneous action potentials, whether occurring individually or in short bursts (Fig. 5C). Finally, there were negative correlations between action potential half width and input resistance (P = 0.012) (Fig. 5D, panel i) and between membrane potential and input resistance (P = 0.007) (Fig. 5D, panel ii).
Table 1.
Electrical properties of human GnRH neurons derived from embryonic stem cells: Comparison to GnRH neurons in mice and guinea pigs
| Human embryonic stem cell derived | Mouse green fluorescent protein-labeleda | Guinea pig Hypothalamusb | Mouse Olfactory placode derivedc | |
|---|---|---|---|---|
| (n = 10) | (n = 26) | (n = 8) | (n = 13) | |
| Resting potential (mV) | –88.1 ± 8.5 | –55.7 ± 3.7 | –55.0 ± 8.5 | –50.8 ± 3.6 |
| Input resistance (GΩ) | 3.3 ± 0.4 | 1.2 ± 0.5 | 0.5 ± 0.2 | 2.4 ± 2.0 |
| Membrane capacitance (pF) | 21.8 ± 3.8 | 25.9 ± 11.3 | 46.0 | 10 |
| AP half width (msec) | 2.9 ± 0.3 | 1.2 ± 0.3 | 2.5 ± 0.7 | 2 |
| AP Amplitude (mV) | 54.3 ± 2.7 | 79.5 ± 9.8 | NR | 80 |
| Threshold (mV) | –45.4 ± 1.5 | –32.2 ± 3.9 | NR | –37.5 |
Figure 5.
Electrophysiological characteristics of GnRH-expressing neurons derived from hESC. (A and B) Examples of voltage responses to 50 pA (A) or 10 pA (B) current steps in 2 different neurons. (right) I-V plots corresponding to traces to the left. In one of the cells (A) (28 days, Vm = −48 mV), no action potentials were elicited by depolarizing current steps, but rebound action potentials followed termination of hyperpolarization. In the other cell (B) (34 days, Vm = −72 mV), depolarizing steps elicited action potential trains but not rebound potentials. (C) Examples of bimodal resting membrane potentials (ie, i = 0 pA) in 4 cells. Traces i (28 days) and ii (22 days) show spontaneous transitions between hyperpolarized and depolarized levels, with activity consisting of single action potentials or brief bursts of action potentials. Traces iii (30 days) and iv (21 days) show transitions to sustained depolarized levels induced by brief 100 pA hyperpolarizing current pulses (arrows). Note that broken lines indicate the resting membrane potentials. (D) Relationship between electrophyiological characteristics of generated GnRH neurons. The age of the cell when recorded is labeled on each point. Although there were negative correlations between action potential half width and input resistance (i, R2 = 0.62, F1,7 = 11.45, P = 0.012) and between membrane potential and input resistance (ii, R2 = 0.67, F1,7 = 14.63, P = 0.007), both were developmental age independent.
One additional striking property that was observed in 6 of the 10 cells was a propensity to switch between 2 semistable “resting” membrane potentials (ie, i = 0 under current clamp). These cells were silent, or nearly so, at their hyperpolarized states, but they jumped spontaneously to a depolarized level marked by abundant bursts of spontaneous activity (Fig. 5C, panels i and ii), and then returned suddenly back to their hyperpolarized state. The depolarized states lasted for several seconds up to tens of seconds, and they could sometimes be induced by hyperpolarizing current pulses (Fig. 5C, panels iii and iv), but never by depolarizing pulses.
Taken together, these data indicate that by the time or shortly after the GnRH gene turns on, as judged by mCherry expression, GnRH neurons appear to have mature cell membrane characteristics and firing patterns.
Intracellular calcium signaling
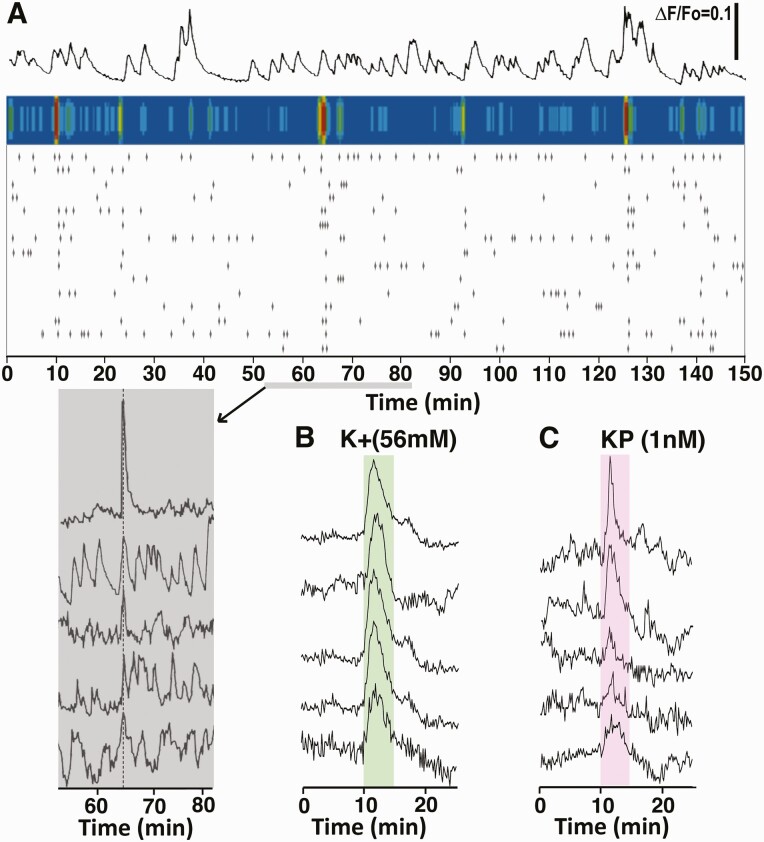
Previously, we have shown that primary GnRH neurons derived from monkey embryos exhibit periodical [Ca2+]i oscillations and they synchronize at an interval of approximately 53 min (13,41). As such, we examined whether similar [Ca2+]i oscillations are seen in stem cell–derived GnRH neurons. Systematic analysis with 33 cultures indicated that individual GnRH neurons exhibit periodical [Ca2+]i oscillations with an IPI of 18.3 ± 1.3 min, and they also synchronized with an IPI of 46.5 ± 3.0 min, although there were considerable variations among GnRH neurons (Fig. 6A). Neither the IPI of the periodical [Ca2+]i oscillations nor their synchronization changed over culture days after day 20 up to day 50 [see Figshare Data Repository Figure 4 in (26)]. Similarly, modifications with culture media, such as addition of Neural Supplement B or Brain Phys medium, did not change the IPIs of [Ca2+]i oscillations or their synchronization intervals. Moreover, stem cell–derived GnRH neurons readily responded to hKP10 and K+ challenges (Fig. 6B and 6C).
Figure 6.
An example of the pattern of [Ca2+]i oscillations in a GnRH neuron (top row) and the raster plot of significant [Ca2+]i peaks in 15 individual neurons (third row) in the field on a single coverslip (A) are shown. Similar to primary GnRH neurons, GnRH neurons derived from hESC exhibited periodical synchronizations of [Ca2+]i oscillations, shown by the colored heat map above the raster plot (second row). The shaded area (bottom row) shows a close up profile of synchronized pulses from 5 cells during the second synchronization. The [Ca2+]i response to 56 mM K+ (B) and kisspeptin (C) are also shown. The [Ca2+]i responses to high K+ and KP10 were uniform among the cells.
GnRH release
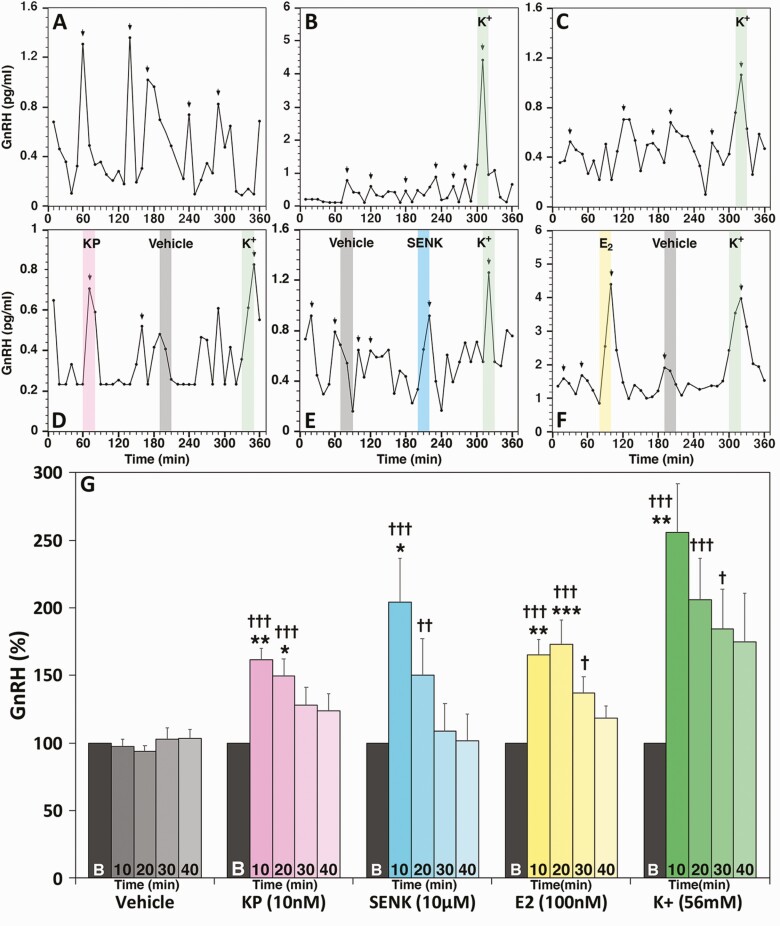
Release of the decapeptide hormone from hESC- (Fig. 7A and 7B) and hiPSC- (Fig. 7C) derived GnRH neurons are both pulsatile with IPI of 59.5 ± 3.3 (n = 17) and 62.5 ± 6.7 (n = 3) min, respectively. They also respond to known GnRH secretagogues, such as the kisspeptin agonist, hKP10 (Fig. 7D), the neurokinin B (NKB) agonist senktide (Fig. 7E), E2 (Fig. 7F), and high K+ (Fig. 7B-7F). Group data indicate that the hKP10- and senktide-stimulated GnRH release was significantly elevated for the first 20 min, while the effects of E2 and K+ lasted for 30 min (Fig. 7G). Vehicle challenges did not induce any changes (Fig. 7G).
Figure 7.
GnRH neurons derived from hESC (A and B) and hiPSC (C) released the GnRH decapeptide in a pulsatile manner. They also responded to 10 nM KP10 (D), 10 µM senktide (E), 100 nM E2 (F) and 56 mM K+ (B-F). Arrows indicate GnRH peaks identified by Pulsar algorithm. Results of statistical analyses for all secretagogues are shown in G. n = 9-20. * = P < 0.05, ** = P < 0.01, *** = P < 0.001 vs baseline values; † = P < 0.005, †† = P < 0.01, ††† = P < 0.001 vs vehicle at the corresponding time. Note that while examples of GnRH release were shown as actual concentrations (pg/mL), the data of statistical analysis were shown as percentage change from the baseline (an average of the 30 min prior to the secretagogue challenge).
Developmental Changes in the Transcriptome of Human GnRH Neurons
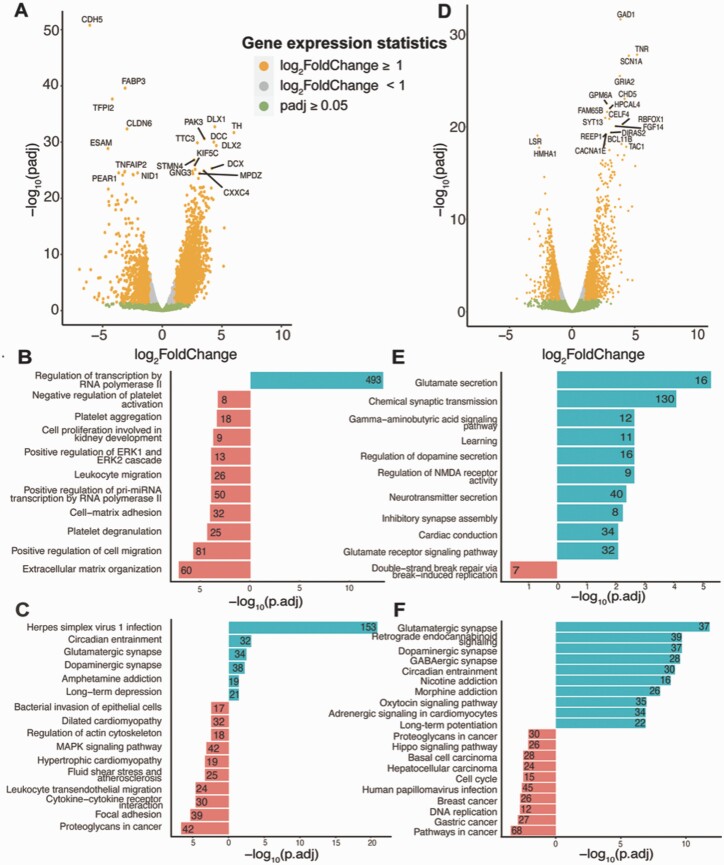
To interrogate developmental transcriptional changes in human stem cell–derived GnRH neurons, we performed gene expression analysis of the FGF8-treated human stem cell (H9)-derived GnRH neurons on days 0, 8, 15, and 25. To compare day 8 (n = 3) to day 15 (n = 3) samples, gene expression CPM filtered counts were normalized using DESeq2, which resulted in 3883 DEGs: 2850 upregulated DEGs and 1033 downregulated DEGs [Fig. 8A; also see Figshare Data Repository Table 5A in (26)]. These upregulated DEGs from day 8 to day 15 were enriched for GO terms associated with regulation of transcription by RNA polymerase II [Fig. 8B; also see Figshare Data Repository Table 5B in (26)] and KEGG pathways associated with herpes simplex virus 1 infection, circadian entertainment, glutamatergic synapse, dopaminergic synapse, and amphetamine addiction [Fig. 8C; also see Figshare Data Repository Table 5C in (26)]. Comparison of day 15 (n = 3) and day 25 (n = 3) samples found 14 785 genes expressed at measurable abundance (as stated in the Methods section), with 2590 DEGs (1489 upregulated and 1101 downregulated DEGs) [Fig. 8D; also see Figshare Data Repository Table 5D in (26)]. The GO terms associated with upregulated genes from days 15 to 25 were glutamate secretion, chemical synaptic transmission, gamma-aminobutyric acid signaling pathway, and neurotransmitter secretion [Fig. 8E; also see Figshare Data Repository Table 5E in (26)], whereas KEGG pathway terms included glutamatergic synapse, retrograde endocannabinoid signaling, dopaminergic synapse, GABAergic synapse, and oxytocin signaling pathway [Fig. 8F; also see Figshare Data Repository Table 5F in (26)]. Interestingly, the GnRH signaling pathway was also an associated with upregulated DEGs from days 15 to 25 and included 15 GnRH-relevant DEGs (adjusted P-value 0.027) [see Figshare Data Repository Table 5F in (26)]. These unbiased analyses strongly supported the experimental validation of GnRH neuron generation.
Figure 8.
Differential expression analysis of days 8, 15, and 25. (A) Volcano plot for DEGs from day 8 to day 15. The top 20 genes with adjusted P-value < 0.05 and highest log2 (fold change) are labeled. The number of upregulated genes is 2960, while the number of downregulated genes is 1075. (B) GO enrichment terms for DEGs from days 8 and 15 (up- and downregulated genes are as previously defined). The numbers at the end of the bars indicate the number of genes corresponding to the respective GO enrichment terms. (C) KEGG pathways for DEGs from day 8 to day 15. The numbers at the end of the bars indicate the number of genes corresponding to the respective KEGG pathway terms. (D) Volcano plot for DEGs between days 15 and 25. Top 20 genes with adjusted P-value < 0.05 and highest log2 (fold change) are labeled. The number of upregulated genes is 1475, while the number of downregulated genes is 1143. (E) GO enrichment terms for DEGs from days 15 and 25. (F) KEGG pathways for DEGs from day 15 to day 25.
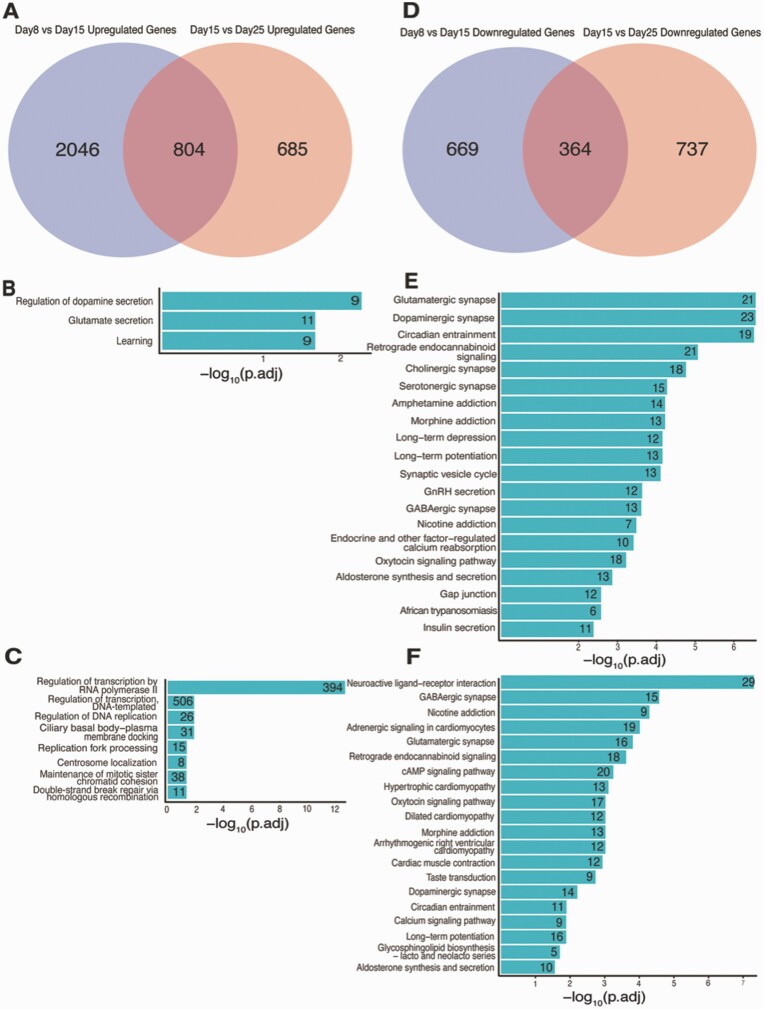
We next evaluated the changes in DEGs from days 8 to 25. Among the 2850 DEGs that were upregulated between days 8 and 15 comparison, 28% (n = 804) were also upregulated from days 15 to 25 [Fig. 9A; also see Figshare Data Repository Table 6A in (26)], while 35% (n = 364) of the 1033 downregulated DEGs from days 8 and 15 were consistently reduced from days 15 to 25 [Fig. 9D; also see Figshare Data Repository Table 6A in (26)]. Results were even more consistent when comparing later stages of development, as over half (53%) of 1489 upregulated DEGs from days 15 and 25 were also upregulated from days 8 to 15 [see Figshare Data Repository Table 6A in (26)], and 33% of 1101 downregulated DEGs were commonly downregulated across these comparisons [see Figshare Data Repository Table 6A in (26)]. This overlap of up- and downregulated DEGs is highly significant (hypergeometric test P-value = 1.6 × 10−213 and 1.6 × 10−174, respectively). For the 804 overlapping upregulated DEGs, the top associated GO terms were regulation of dopamine secretion, glutamate secretion, and learning [Fig. 9B; also see Figshare Data Repository Table 6B in (26)], and associated KEGG pathways included glutamatergic synapse, dopaminergic synapse, circadian entrainment, retrograde endocannabinoid signaling, GABAergic synapse, synaptic vesicle cycle, and GnRH secretion [Fig. 9E; also see Figshare Data Repository Table 6C in (26)]. When considering time-specific characteristics of gene regulation, we observed enrichment of pathways associated with regulation of transcription by RNA polymerase II, regulation of transcription, DNA-templated and regulation of DNA replication among DEGs uniquely upregulated from days 8 to 15 but not from days 15 to 25 [Fig. 9C; also see Figshare Data Repository Table 6D-6F in (26)], while neuroactive ligand-receptor interaction, GABAergic synapse, nicotine addiction, glutamatergic synapse and retrograde endocannabinoid signaling KEGG pathways were enriched for DEGs specifically upregulated from days 15 to 25 but not days 8 to 15 [Fig. 9F; also see Figshare Data Repository Table 6G in (26)].
Figure 9.
Differences in the DEGs between days 8 and 15 and between days 15 and 25. (A) Overlapping set of upregulated genes between days 8 and 15 and the set of upregulated genes between days 15 and 25. (B) GO enrichment terms for overlapping upregulated genes (n = 840). (C) GO enrichment terms for genes that are upregulated between days 8 and 15, but not between days 15 and 25 (n = 2120). (D) Venn diagram for the set of downregulated genes between days 8 and 15 and the set of upregulated genes between days 15 and 25. (E) KEGG pathways for overlapping upregulated genes (n = 840). (F) KEGG pathways for genes that are upregulated between days 15 and 25, but not between days 8 and 15 (n = 635).
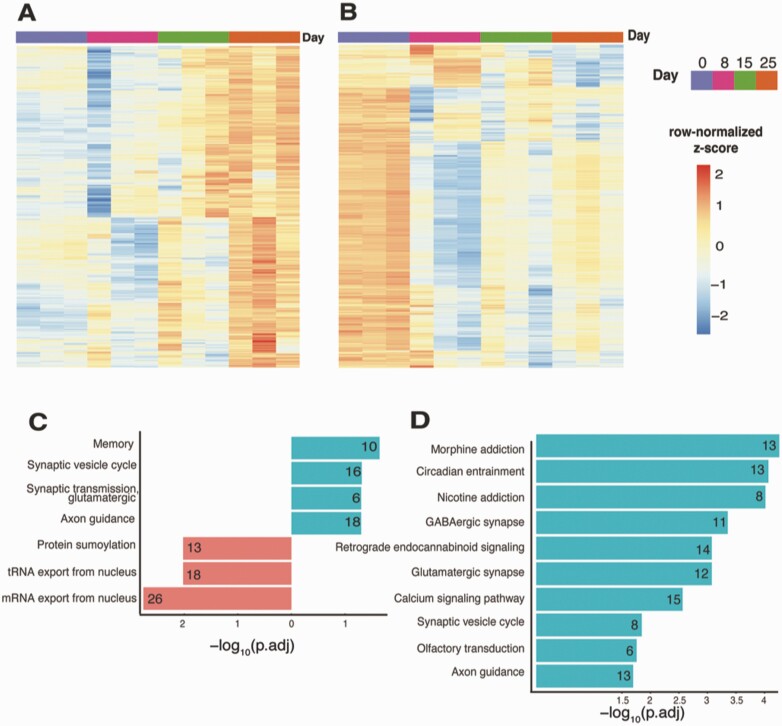
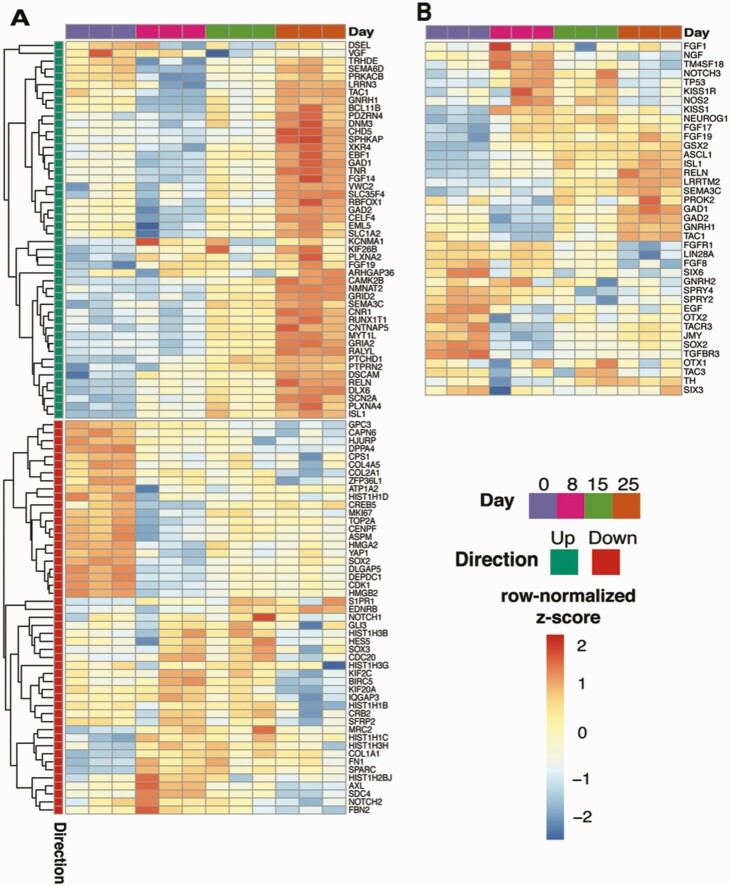
The time-course analysis of FGF8 treatment samples from days 0 to 25 identified 1355 genes that displayed increased expression levels with adjusted P-value < 0.05. From these 1355 genes, we further subdivided the genes using hierarchical clustering with 3 branches and focused on the cluster of 439 genes that peak in expression levels on day 25 [Fig. 10A; also see Figshare Data Repository Table 7A in (26)]. From GO enrichment analysis, these upregulated genes were associated with memory, synaptic vesicle cycle, glutamatergic synaptic transmission, and axon guidance [see Figshare Data Repository Table 7B in (26)], while the 1403 downregulated genes (Fig. 10B) were associated with protein sumoylation, transfer RNA export from the nucleus and mRNA export from the nucleus [Fig. 10C; also see Figshare Data Repository Table 7C in (26)]. The top KEGG pathway terms associated with these 439 upregulated genes were morphine addiction, circadian entrainment, nicotine addiction, GABAergic synapse, retrograde endocannabinoid signaling, and glutamatergic synapse [see Figshare Data Repository Table 7D in (26)], while the 1403 downregulated genes were not significantly associated with any statistically significant pathway terms (Fig. 10D). Furthermore, we confirmed the expression pattern of known genes involved in GnRH neurogeneration from stem cells with FGF8 treatment (50) (Fig. 11A). Importantly, despite the methodological differences, including the difference in the stem cell differentiation date/age and RNAseq with mix cell population vs single-cell RNAseq between the present study and Lund et al (50), almost all (44 of the top 50, hypergeometric test P-value = 5.3 × 10−63) of the upregulated genes from Lund et al displayed similar expression pattern changes this study, while the downregulated genes did not significantly overlap. Furthermore, we reached a similar conclusion from an additional analysis by testing if the transcriptome signals originate from non-GnRH cells in the culture. We compared the expression profiles for different neurotransmitter receptors, namely GABA, glutamate, and acetylcholine receptors, along with neuropeptides such as kisspeptin, neurokinin B, and dynorphin. We observed that 57 of such genes were found in the RNAseq data at the detection limits, 19 of which were differentially expressed between days 25 and 0. These DEGs showed both up- (n = 15) and downregulation (n = 4) [see Figshare Data Repository Figure 5 in (26)]. Finally, we compiled the developmental expression pattern of all genes based on publications from IHH patients and development of GnRH neurons for future studies and provide these data in Figure 11B (2,21,51-58).
Figure 10.
Genes with expression pattern during time-course treatment with FGF8 from day 0 to day 25. (A) Gene expression patterns for 439 upregulated genes from day 0 to day 25. (B) Gene expression patterns for 1403 downregulated genes from day 0 to day 25. (C) GO enrichment terms for genes with correlated expression pattern during time-course FGF8 treatment. (D) KEGG pathway terms for genes with correlated expression pattern during time-course FGF8 treatment.
Figure 11.
Gene expression changing during the GnRH development. (A) Gene expression heatmap for top 49 up- and 42 downregulated genes, aligned with 50 up and down genes (each) differentially expressed between days 20 and 27 in FGF8-treated mCh-expressing GnRH neurons reported by Lund et al (50). (B) Gene expression heatmap for the genes of our interests during the GnRH neuronal differentiation from stem cells. The color scale of heatmaps represents row-scaled z-scored DESeq2 normalized counts.
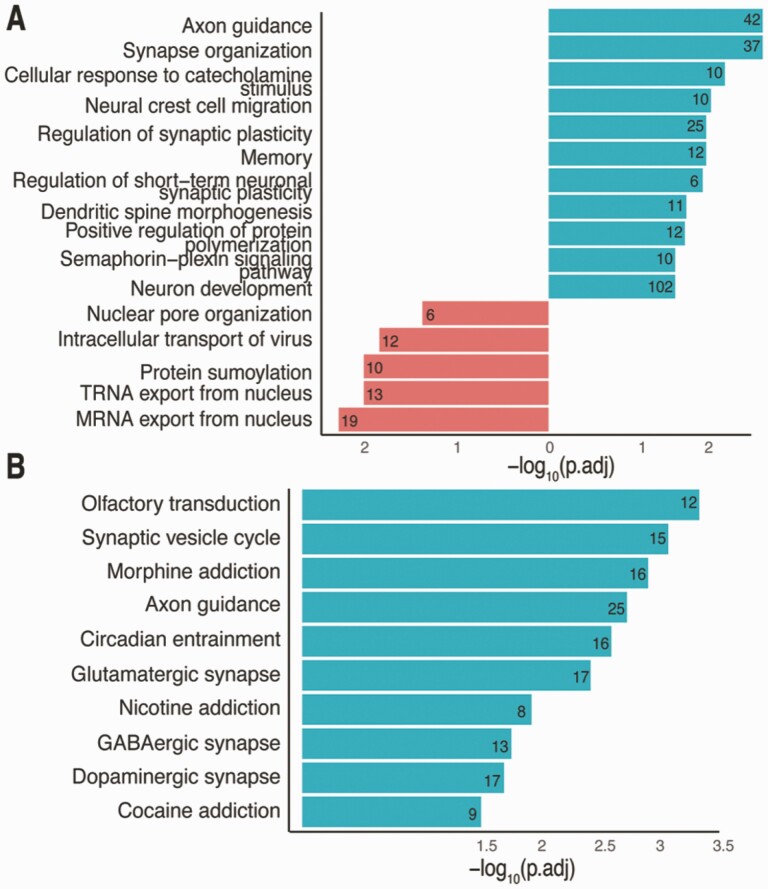
We evaluated DEGs (|lfc| > 1 and false discovery rate < 0.05) from iPSC to iNs in comparison with those from GnRH maturation, revealing 935 (overlap P-value = 5.4 × 10−73) and 755 (overlap P-value = 2.9 × 10−13) genes up- and downregulated, respectively, common to both neuron maturation procedures. These overlaps are highly significant and suggest that both procedures result in neuronal cell types, as shown by the functional and pathway enrichments (Fig. 12A and 12B). Interestingly, the GnRH signaling pathway that was significantly enriched in DEGs between days 15 and 25 in FGF8-induced GnRH neurons (adjusted P-value 0.027, 15 genes corresponding to this pathway) was not significant in genes overlapping between the GnRH and iN maturation protocol. This indicates that GnRH maturation protocol leads to a different type of neurons as compared to iNs.
Figure 12.
Comparison of FGF8 treated GnRH maturation protocol with iPSC-derived iNs. (A) Number of genes overlapping between up- and downregulated DEGs from the GnRH protocol from day 0 to day 25 and iPSC to iN development. (B) GO enrichment terms for overlapping up- and downregulated genes between the GnRH protocol and iPSC-iN development. (C) KEGG pathway terms for overlapping up-regulated genes between the GnRH protocol and iPSC-iN development.
Discussion
In the present study we were successful in establishing a reliable method for generation of GnRH neurons from both hESC and hiPSC. We further observed that stem cell–derived GnRH neurons exhibit physiological characteristics similar to those seen in olfactory placode–derived primary GnRH neurons. Importantly, although the treatment with FGF8 alone was effective in generating GnRH neurons, FGF8 plus kisspeptin yielded a higher efficiency rate, on average, 10% to 16% of GnRH decapeptide expressing neurons over the total cells (Figs. 1B and 3B). These numbers were far from a “pure population.” There might be, however, a potential limitation to reach close to 100%. As seen in the results of notch inhibitors and an antimitotic drug, elimination of non-GnRH cells dramatically reduced the total number of cells, resulting in a significant loss of GnRH neurons in the culture. We have also noticed that stem cell–derived GnRH neurons prefer to grow on the top of nonneuronal cells and form thick multilayered clusters (Figs. 1D and 4A), but not form ganglia. This characteristic is very similar to that in primary GnRH neurons. They always grow on the top of nonneuronal cells (eg, fibroblasts or epithelial cells) (41), which differ from other types of neurons, such as spinal motor neurons (59). Whether the efficiency rate improves by modifications of plating substrates other than matrigel, such as neural cell adhesion molecule (60), or by 3-dimensional cultures with proper scaffolds remains to be investigated.
When we started this project, there was no publication on this topic. Since then, 2 groups have reported the generation of human GnRH neurons from stem cells using FGF8 (27,28). The basic method of Lund et al (27) is similar to our Protocol 2, while our Protocol 1 has some similarities with Poliandri et al (28). In Protocol 2, we could count nearly 40% efficiency rate in parts of cultures, but in other parts of the same cultures the efficiency rate was less than 1% to 2%, yielding an overall average of 10%. Nonetheless, this study is unique, as we focused on the developmental window of GnRH neurogeneration using mCherry-labeled GnRH neurons and RNAseq, and physiological characterization of stem cell–derived GnRH neurons using elecrophysiological analysis, calcium imaging, and pulsatile GnRH release.
Mutations in FGFR1 and FGF21 result in IHH (52). FGF8 action is mediated largely through FGFR1 and some through FGFR3 in rodents (61,62). FGF21 action is also medicated though FGFR1, but it requires the coreceptor β-Klotho (63). In the present study we found that while the exposure of hESCs to FGF8 generated GnRH neurons, FGF21 was ineffective (Fig. 3). The results from this study, then, indicate that β-Klotho is unlikely required for GnRH neurogeneration. Whether additional members of FGF family, such as FGF17, are involved in the GnRH neurogeneration remain to be examined. Among small molecules tested, we found that addition of hKP10 supplementation during days 10 to 15, FGF8 exposure had a synergistic action with FGF8. Apparently, kisspeptin possesses a neuro-proliferative action. Exposure of embryonic, not adult, zebra fish to kisspeptin increases the number of hypothalamic GnRH neurons (44). In our study, kisspeptin alone was not as effective as FGF8. Neuro-proliferative action of kisspeptin during the GnRH neurogeneration appears to be an evolutionally well-conserved feature. Nevertheless, a question arises: why is kisspeptin supplement on days 10 to 15 FGF8 most effective? Regardless of FGF8 treatment, KISS1 expressions assessed by RT-PCR were significantly elevated on day 5 (Fig. 3A), and RNAseq analysis indicates that both KISS1 and KISS1R expressions appeared to be elevated between days 8 and 15 (Fig. 11B). Perhaps, hKP10 supplementation during days 0 to 5 is less effective, because kisspeptin in stem cell cultures is already high, whereas KISS1R between days 0 and 8 is low (Fig. 11B). Interestingly, transgenic male mice studies indicate that highly specific transsynaptic neural pathways from kisspeptin neurons to GnRH neurons are established by the mid-point of GnRH neurogeneration in vivo (64,65), suggesting kisspeptin can influence the ontogeny of GnRH neurons in vivo. Among other small molecules, failure of RA and SHH to increase GnRH neurogeneration needs further examination, as the doses and timing of drug applications require a delicate balance.
Generation of mCh-GnRH carrying hESC allowed us to find the exact time course necessary for stem cell transformation into GnRH neurons, to count GnRH producing cells more accurately, and to conduct experiments for physiological characterization of GnRH neurons. Live cell imaging suggests that expression of the GnRH gene was visible as early as day 11 FGF8 exposure, and the number progressively increased for an additional 2 weeks until it reached a plateau. Importantly, RNAseq analysis also indicates that GnRH neurogenesis start between days 8 and 15 FGF8 exposure, and then it accelerated between days 15 and 25 (Fig. 11B). Available reports in vivo show that GnRH neurons in human fetuses are not visible on E28 to E32, but they are seen in the olfactory placode between E42 and E56 (27,60). Thus, the in vitro maturation of GnRH neurons from hESCs seems to recapitulate in vivo maturation of human GnRH neurons.
Because of the fluorescent mCherry marker labeling, we were able to conduct extensive physiological characterization of hESC-derived GnRH neurons. In fact, to our knowledge, these are the first successful single-cell patch clamp recordings from human GnRH neurons reported. We found that human GnRH neurons had more hyperpolarized resting membrane potentials (approximately −90 mV) and smaller action potential amplitudes (approximately 50 mV) compared with GnRH neurons of other mammalian species (approximately −55 mV and 80 mV, respectively) (Table 1) (46-49). The majority of these cells did, however, exhibit spontaneous depolarizing plateaus that triggered trains of action potentials. All GnRH neurons older than day 20 FGF8 exposure that we were successful in recording exhibited similar electrophysiological characteristics and included spontaneous action potentials occurring individually and in bursts, indicating that day 20 FGF8 exposure may be sufficient to achieve GnRH neural maturation. Because in this study we did not examine GnRH neurons older than day 34, questions of whether highly hyperpolarized resting membrane potentials and whether the propensity of membrane potentials switching between 2 semistable “resting” potentials, change in older GnRH neurons (over day 35) remain to be investigated. The exceptionally hyperpolarized resting membrane potentials in human GnRH neurons need further examination. We can, however, add our preliminary observations that monkey primary GnRH neurons also display highly hyperpolarized resting membrane potentials, such as −100 mV (Abe and Terasawa, unpublished observation).
For [Ca2+]i imaging analysis, we applied the same method and criteria used for the primary GnRH cell cultures in nonhuman primates, as described previously (13,41). We found that similar to primary GnRH neurons, individual GnRH neurons exhibited periodical [Ca2+]i oscillations with the IPI of 18.3 ± 1.3 min and individual [Ca2+]i oscillations synchronized periodically at the IPI of 46.5 ± 3.0 min. However, in contrast to primary GnRH neurons that exhibit relatively uniform oscillatory [Ca2+]i patterns (13), the individual pattern of stem cell–derived GnRH neurons was highly variable (Fig. 6), synchronization peaks were not as conspicuous as seen in primary GnRH neurons, and the IPI was ~2-fold longer than that in primary GnRH neurons (8.2 ± 0.7 min). Importantly, however, the synchronization interval of stem cell–derived GnRH neurons was very similar to that in primary GnRH neurons (52.8 ± 3.0 min) (13), as well as the IPI of GnRH release in vitro (46.6 ± 4.0 min) (11) and in vivo (~60 min) (9,66,67). We hypothesize that GnRH neurons release the decapeptide hormone at the time of their synchronization.
While primary GnRH neurons derived from embryonic olfactory placode exhibit relatively uniform [Ca2+]i patterns (13,41), they are quite variable among individual hESC-derived GnRH neurons. We speculate that the highly variable [Ca2+]i patterns in hESC-derived GnRH neurons as compared to those in placode-derived GnRH neurons are attributable to the difference in the cell composition in the 2 culture systems. Primary GnRH neurons derived from embryonic monkey olfactory placode contain a lot of nonneural cells, but they do not contain other type of neurons or glia (7,41), whereas cultures of stem cell–derived GnRH neurons contain a substantial number of glia and other types of neurons including γ-amino butyric acid (GABA), neuropeptide Y, and tyrosine hydroxylase positive neurons (Fig. 13). As such, the presence of other types of neurons and glia adjacent to GnRH neurons is the more likely explanation, as they can influence GnRH neuronal activity synaptically or nonsynaptically. Species differences (ie, human GnRH neurons in this study vs nonhuman primate GnRH neurons in the primary culture) cannot be excluded. Nevertheless, despite highly variable [Ca2+]i patterns in individual GnRH neurons in this study, they exhibited a synchronization interval similar to that seen in primary GnRH neurons, suggesting that GnRH neurons themselves are the pacemaker for the pulsatile peptide release [see Terasawa (68) for further discussion].
Figure 13.
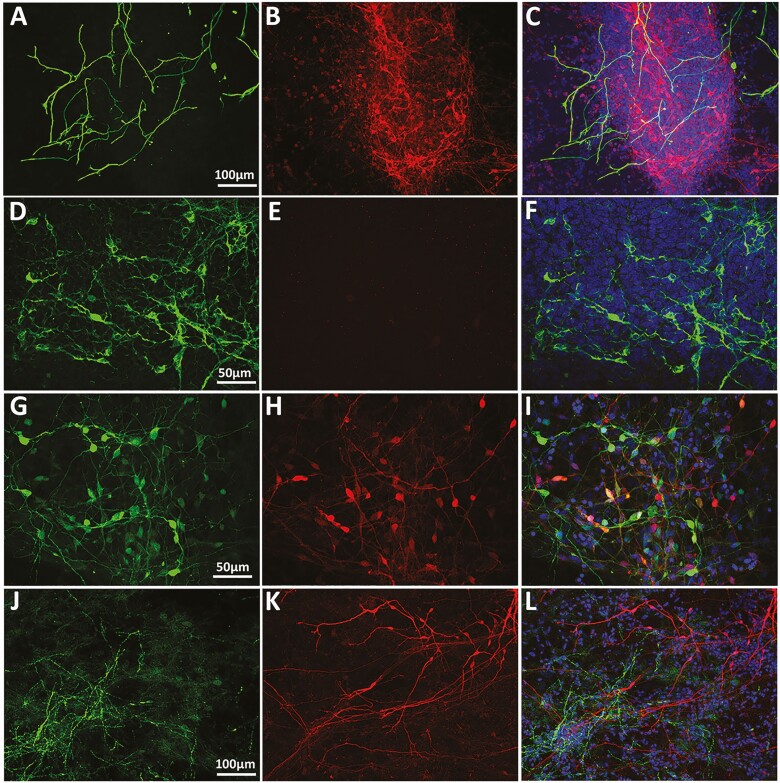
Left column: glial fibrillary acidic protein–positive cells (A) and neurons immunopositive to antibodies against tyrosine hydroxylase (D), GABA (G), and neuropeptide Y (J) are shown in Green. Middle column: red = mCherry-positive GnRH neurons (B, E, H, and K). Right column: overlay. Note that a subset of GABA neurons colocalize with GnRH neurons (I). Also, while glia, GABA, and neuropeptide Y neurons are closely associate with GnRH neurons, tyrosine hydroxylase–positive neurons are located in a completely different location within the slide.
In this study we have further shown that stem cell–derived GnRH neurons release the 10 amino acid GnRH peptide spontaneously or in response to known secretagogues, such as kisspeptin and NKB. Moreover, direct stimulatory action of E2 on GnRH neurons is consisted with previous observations in the median eminence vivo (69). Importantly, the IPIs of hESC and hiPSC are both ~60 min (59.5 ± 3.3 and 62.5 ± 6.7 min, respectively), which are very similar to the reported IPI in pulsatile LH release in humans (70,71), release of LH and GnRH and multiple unit activity associated with LH pulses in monkeys in vivo (9,66,67,72,73), and cultured placode–derived GnRH neurons (11). Additionally, we have previously shown that GnRH release occurs in response to kisspeptin, NKB, and E2 (17,74-78) in vivo. Stimulatory actions of kisspeptin and NKB on GnRH/LH release have also been reported in many other mammalian species, including in rhesus monkeys (78-81). Note that the secretagogue-induced GnRH release could be indirectly through other interneurons, as stem cell–derived cultures contain other neurons (Fig. 13).
In the present study, we designated the first day of FGF8 exposure as day 0, not counting the first 7 to 10 days of the CDM exposure (and 7-10 days of neural induction medium in Protocol 1), whereas works by Lund et al (27,50) designated the first day of the CDM exposure as day 0, and 10 days later epithelial cells are exposed FGF8 (day 10). As such, there is a 10-day difference in FGF8 exposure between our study and Lund et al (27,50). Additionally, while for the RNAseq analysis we used mixed cell groups throughout the stem cell differentiation by FGF8 exposure (ie, days 0, 8, 15, and 28), Lund et al (50) used mixed cell groups for their day 20 (day 10 of FGF8 exposure) and Td-Tomato-labeled GnRH neurons only for day 27 (day 17 of FGF8 exposure) in the RNAseq analysis. Despite these differences between the present study and Lund et al (50), the profile of the top 44 upregulated genes is essentially identical.
Prior to the GnRH neurogeneration the presence of genes Sox2, Ascl1, and Sox2 in the developing lateral olfactory epithelial and Fgf8 in the medical olfactory epithelial has been described (82,83). Indeed, the results from RNAseq (Fig. 11B) indicate that GSX2, ASCL1, and SOX2 were elevated on days 15 and 25. In contrast, GnRH was low on day 8, slightly elevated on day 15, and reached the highest level on day 25. These observations are consistent with developmental changes in the generation of olfactory placode followed by the birth of GnRH neurons (23,45,58). Importantly, the intensity of elevated GnRH-1 gene expression is parallel to the increased number of mCh-GnRH neurons.
Genetic analyses of IHH patients suggest that the mutations in ANOS1 (KAL1), PROKR2, PRK2, FGF17, FGF19, TAC3R, TAC3, SPRY4, and SEMA3A, 3C, and 3E are all involved in infertility or abnormal timing of puberty (2,51,52,54,56,84-87). The developmental profiles of the present study suggest that expressions of these genes were elevated on days 15 and 25 along with the GnRH gene. In contrast, both FGF8 and FGFR1 were elevated on day 8, but on day 15 they started to decline, reaching their lowest levels on day 25. Although this might indicate the timing of the importance of FGF8 and FGFR1 presence olfactory placode/GnRH neurogeneration, it is also possible that the presence of FGF8 in media might have changed the expression pattern. Additionally, a highly similar differential gene expression pattern between FGF8-derived GnRH neurons and iNs (Fig. 12B and 12C) suggests that during the differentiation and maturation the FGF8-derived GnRH neuron acquires panneuronal characteristics. On the other hand, the absence of GnRH signaling genes in the DEGs shared between 2 maturation protocols indicate that the timing and dose of FGF8 treatment protocol applied to hiPSC are unique to GnRH neurogeneration when compared to neurogeneration by Ngn2 treatment.
In the present study we observed that the rate-limiting enzyme for GABA synthesis from glutamate, GAD1 and GAD2, prominently increased along with GnRH gene and genes involved in IHH, and the importance of GABA and glutamate GAD1 and GAD2 in regulation of GnRH release and puberty has been well documented [see Terasawa and Fernandez (88)]. In fact, the prominent increase in GAD1 and GAD2 might be related to GnRH neuronal function, such as cell migration, as GABA is involved in the cell migration of GnRH neurons (89-91). Similarly, genes RELN and LRRTM, which significantly activated on days 15 and 25, are likely involved in synaptic formation (92,93) as well as neuronal cell migration (94) after GnRH neuron are generated.
The developmental profiles of the genes that involved in GnRH transcription, such as OTX1, OTX2, TP53, SIX3, and SIX6 (57,95-99) were weakly associated with changes in GnRH neurogeneration, although they are not as prominent as the genes associated with IHH. Interestingly, on day 25 the expression of SIX3 was slightly elevated, whereas SIX6 was suppressed (Fig. 11B). It has been reported that Six6 is activational, while Six3 appears to be a repressional transcription factor for the GnRH gene (57).
In summary, here we report a reliable method for generation of human GnRH neurons from hiPSC as well as ESC. Importantly, with our method we can harvest approximately 10 000 to 20 000 GnRH neurons per single slide. This is equivalent to having 5 to 10 primate brains, as human and macaque brains contain a total of ~2000 GnRH neurons (100). We also generated a hESC line, in which mCherry fluorescent marker is expressed when the GnRH gene turns on. Furthermore, we conducted extensive physiological characterization experiments in stem cell–derived GnRH neurons and found that they exhibit characteristics similar to GnRH neurons in vivo and primary GnRH neurons in vitro. Finally, we characterized developmental profiles of the GnRH transcriptome. Collectively, benefits of human GnRH neurons generated by the method described in this article are multifold: they will (1) help with research on human GnRH neurobiology, (2) be useful for establishing a disease model for IHH, (3) provide a potential tool for cell transplantation therapies in IHH patients, and (4) be useful for contraceptive drug screening.
Acknowledgments
The authors thank Drs. Su-chun Zhang, Department of Neuroscience and Waisman Center, University of Wisconsin-Madison, for his comments on this project; Dr. Stephanie Seminara, Harvard Reproductive Sciences Center, for her comments on the manuscript, and Dr. Nelly Pitteloud, Lausanne University Hospital, Switzerland, for her comments regarding FGF21.
Financial Support: This work was supported by Grant R21HD092009 (to E.T.) and R01HD096326 (to M.E.T.) from the Eunice Kennedy Shriver Institute of Child Health and Human Development and by UW-ICTR grant (UL1TR002373) from the NIH National Center for Advancing Translational Sciences. The work was made possible by support from the NIH Office of the Director for the Wisconsin National Primate Research Center (P51OD011106). Generation of the fluorescent reporter cell line was supported in part by a core grant to the Waisman Center from the NICHD (U54HD090256) and by a UW2020 Grant from the University of Wisconsin and the Wisconsin Alumni Research Foundation (to A.B. and Su-Chun Zhang).
Author Contributions: E.T., R.A.P., A.B., and K.L.K. designed experiments; K.L.K., A.J.P., A.G.F, and B.I.F. conducted experiments; J.S., R.Y., and S.E. conducted differential gene expression analysis; and E.T., K.L.K., A.J.P., and M.E.T. wrote the manuscript.
Additional Information
Disclosure Summary: The authors declare no conflicts of interest.
Data Availability
All data presented in this manuscript are available upon request.
References
- 1. Seminara SB, Hayes FJ, Crowley WF Jr. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19(5):521-539. [DOI] [PubMed] [Google Scholar]
- 2. Pitteloud N, Quinton R, Pearce S, et al. . Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117(2):457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bianco SD, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2009;5(10):569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan YM, de Guillebon A, Lang-Muritano M, et al. . GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2009;106(28):11703-11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouloux PM, Hu Y, MacColl G. Recent advances in the pathogenesis of Kallmann’s syndrome. Prog Brain Res. 2002;141:79-83. [DOI] [PubMed] [Google Scholar]
- 6. Lima Amato LG, Latronico AC, Gontijo Silveira LF. Molecular and genetic aspects of congenital isolated hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am. 2017;46(2):283-303. [DOI] [PubMed] [Google Scholar]
- 7. Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology. 1993;133(5):2379-2390. [DOI] [PubMed] [Google Scholar]
- 8. Fueshko S, Wray S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol. 1994;166(1):331-348. [DOI] [PubMed] [Google Scholar]
- 9. Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull. 1988;21(1):117-121. [DOI] [PubMed] [Google Scholar]
- 10. Kokoris GJ, Lam NY, Ferin M, Silverman AJ, Gibson MJ. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (HPG) male mice. Neuroendocrinology. 1988;48(1):45-52. [DOI] [PubMed] [Google Scholar]
- 11. Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999;140(3):1432-1441. [DOI] [PubMed] [Google Scholar]
- 12. Constantin S, Caraty A, Wray S, Duittoz AH. Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology. 2009;150(7):3221-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Terasawa E, Schanhofer WK, Keen KL, Luchansky L. Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci. 1999;19(14):5898-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24(28):6326-6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terasawa E, Keen KL, Grendell RL, Golos TG. Possible role of 5’-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol. 2005;19(11):2736-2747. [DOI] [PubMed] [Google Scholar]
- 16. Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146(10):4312-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23(3):349-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology. 2009;150(3):1400-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338(6211):161-164. [DOI] [PubMed] [Google Scholar]
- 20. Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86(20):8132-8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falardeau J, Chung WC, Beenken A, et al. . Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118(8):2822-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung WC, Moyle SS, Tsai PS. Fibroblast growth factor 8 signaling through fibroblast growth factor receptor 1 is required for the emergence of gonadotropin-releasing hormone neurons. Endocrinology. 2008;149(10):4997-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forni PE, Wray S. GnRH, anosmia and hypogonadotropic hypogonadism–where are we? Front Neuroendocrinol. 2015;36:165-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forni PE, Taylor-Burds C, Melvin VS, Williams T, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J Neurosci. 2011;31(18):6915-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006;16(2):132-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keen KL, Petersen AJ, Figueroa AG, et al. . GnRH neurogeneration from human stem cell. Figshare Data Repository. 2012. ProMED-mail website. https://figshare.com/articles/journal_contribution/GnRH_Neurogeneration_from_Human_Stem_Cell/14691129. Accessed June 1, 2021.
- 27. Lund C, Pulli K, Yellapragada V, et al. . Development of gonadotropin-releasing hormone-secreting neurons from human pluripotent stem cells. Stem Cell Reports. 2016;7(2):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poliandri A, Miller D, Howard S, et al. . Generation of kisspeptin-responsive GnRH neurons from human pluripotent stem cells. Mol Cell Endocrinol. 2017;447:12-22. [DOI] [PubMed] [Google Scholar]
- 29. Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109(39):E2579-E2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mali P, Yang L, Esvelt KM, et al. . RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Cao J, Xiong M, et al. . Engineering human stem cell lines with inducible gene knockout using CRISPR/Cas9. Cell Stem Cell. 2015;17(2):233-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim JH, Lee SR, Li LH, et al. . High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One. 2011;6(4):e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology. 1989;125(1):92-99. [DOI] [PubMed] [Google Scholar]
- 35. Andrews S. FastQC: A quality control tool for high throughput sequence data.2010. ProMED-mail website, version 0.10.1 released on May 3, 2012. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed May 12, 2020.
- 36. Dobin A, Davis CA, Schlesinger F, et al. . STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alexa A, Rahnenführer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. 2006;22(13):1600-1607. [DOI] [PubMed] [Google Scholar]
- 40. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richter TA, Keen KL, Terasawa E. Synchronization of Ca(2+) oscillations among primate LHRH neurons and nonneuronal cells in vitro. J Neurophysiol. 2002;88(3):1559-1567. [DOI] [PubMed] [Google Scholar]
- 42. Xu C, Messina A, Somm E, et al. . KLB, encoding β-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol Med. 2017;9(10):1379-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723-3736. [DOI] [PubMed] [Google Scholar]
- 44. Zhao Y, Lin MC, Mock A, Yang M, Wayne NL. Kisspeptins modulate the biology of multiple populations of gonadotropin-releasing hormone neurons during embryogenesis and adulthood in zebrafish (Danio rerio). PLoS One. 2014;9(8):e104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. LaMantia AS, Bhasin N, Rhodes K, Heemskerk J. Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron. 2000;28(2):411-425. [DOI] [PubMed] [Google Scholar]
- 46. Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19(6):2037-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17 beta and mu-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136(5):2341-2344. [DOI] [PubMed] [Google Scholar]
- 48. Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci U S A. 1995;92(9):3918-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci. 2010;30(40):13373-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lund C, Yellapragada V, Vuoristo S, et al. . Characterization of the human GnRH neuron developmental transcriptome using a GNRH1-TdTomato reporter line in human pluripotent stem cells. Dis Model Mech. 2020;13(3):dmm040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Franco B, Guioli S, Pragliola A, et al. . A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353(6344):529-536. [DOI] [PubMed] [Google Scholar]
- 52. Miraoui H, Dwyer AA, Sykiotis GP, et al. . Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am J Hum Genet. 2013;92(5):725-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hardelin JP, Dodé C. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev. 2008;2(4-5):181-193. [DOI] [PubMed] [Google Scholar]
- 54. Topaloglu AK, Reimann F, Guclu M, et al. . TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tommiska J, Sørensen K, Aksglaede L, et al. . LIN28B, LIN28A, KISS1, and KISS1R in idiopathic central precocious puberty. BMC Res Notes. 2011;4:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cariboni A, André V, Chauvet S, et al. . Dysfunctional SEMA3E signaling underlies gonadotropin-releasing hormone neuron deficiency in Kallmann syndrome. J Clin Invest. 2015;125(6):2413-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Larder R, Clark DD, Miller NL, Mellon PL. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. J Neurosci. 2011;31(2):426-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kramer PR, Wray S. Midline nasal tissue influences nestin expression in nasal-placode-derived luteinizing hormone-releasing hormone neurons during development. Dev Biol. 2000;227(2):343-357. [DOI] [PubMed] [Google Scholar]
- 59. Du ZW, Chen H, Liu H, et al. . Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun. 2015;6:6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwanzel-Fukuda M, Crossin KL, Pfaff DW, Bouloux PM, Hardelin JP, Petit C. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J Comp Neurol. 1996;366(3):547-557. [DOI] [PubMed] [Google Scholar]
- 61. Mott NN, Chung WC, Tsai PS, Pak TR. Differential fibroblast growth factor 8 (FGF8)-mediated autoregulation of its cognate receptors, Fgfr1 and Fgfr3, in neuronal cell lines. PLoS One. 2010;5(4):e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chung WC, Matthews TA, Tata BK, Tsai PS. Compound deficiencies in multiple fibroblast growth factor signalling components differentially impact the murine gonadotrophin-releasing hormone system. J Neuroendocrinol. 2010;22(8):944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ogawa Y, Kurosu H, Yamamoto M, et al. . BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104(18):7432-7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kumar D, Periasamy V, Freese M, Voigt A, Boehm U. In utero development of Kisspeptin/GnRH neural circuitry in male mice. Endocrinology. 2015;156(9):3084-3090. [DOI] [PubMed] [Google Scholar]
- 65. Kumar D, Candlish M, Periasamy V, Avcu N, Mayer C, Boehm U. Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology. 2015;156(1):32-38. [DOI] [PubMed] [Google Scholar]
- 66. Woller MJ, McDonald JK, Reboussin DM, Terasawa E. Neuropeptide Y is a neuromodulator of pulsatile luteinizing hormone-releasing hormone release in the gonadectomized rhesus monkey. Endocrinology. 1992;130(4):2333-2342. [DOI] [PubMed] [Google Scholar]
- 67. Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5(1):41-50. [DOI] [PubMed] [Google Scholar]
- 68. Terasawa E. Mechanism of pulsatile GnRH release in primates: Unresolved questions. Mol Cell Endocrinol. 2019;498:110578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kenealy BP, Kapoor A, Guerriero KA, et al. . Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33(49):19051-19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Veldhuis JD, Winters SJ. Nature of alpha subunit secretion in men: circadian rhythms, pulsatile release and secretory profiles. J Androl. 1989;10(3):248-258. [DOI] [PubMed] [Google Scholar]
- 71. Wennink JM, Delemarre-van de Waal HA, van Kessel H, Mulder GH, Foster JP, Schoemaker J. Luteinizing hormone secretion patterns in boys at the onset of puberty measured using a highly sensitive immunoradiometric assay. J Clin Endocrinol Metab. 1988;67(5):924-928. [DOI] [PubMed] [Google Scholar]
- 72. Williams CL, Thalabard JC, O’Byrne KT, et al. . Duration of phasic electrical activity of the hypothalamic gonadotropin-releasing hormone pulse generator and dynamics of luteinizing hormone pulses in the rhesus monkey. Proc Natl Acad Sci U S A. 1990;87(21):8580-8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. O’Byrne KT, Thalabard JC, Grosser PM, et al. . Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology. 1991;129(3):1207-1214. [DOI] [PubMed] [Google Scholar]
- 74. Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology. 2012;153(2):825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Kisspeptin and Neurokinin B signaling network underlies the pubertal increase in GnRH release in female rhesus monkeys. Endocrinology. 2017;158(10):3269-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Garcia JP, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Role of kisspeptin and neurokinin b signaling in male rhesus monkey puberty. Endocrinology. 2018;159(8):3048-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149(3):1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Roseweir AK, Kauffman AS, Smith JT, et al. . Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ruiz-Pino F, Navarro VM, Bentsen AH, et al. . Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology. 2012;153(10):4818-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Navarro VM. Interactions between kisspeptins and neurokinin B. Adv Exp Med Biol. 2013;784:325-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Clarkson J, Han SY, Piet R, et al. . Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216-E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kawauchi S, Shou J, Santos R, et al. . Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132(23):5211-5223. [DOI] [PubMed] [Google Scholar]
- 83. Tucker ES, Lehtinen MK, Maynard T, et al. . Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development. 2010;137(15):2471-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dodé C, Levilliers J, Dupont JM, et al. . Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33(4):463-465. [DOI] [PubMed] [Google Scholar]
- 85. Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308(5730):1923-1927. [DOI] [PubMed] [Google Scholar]
- 86. Hanchate NK, Giacobini P, Lhuillier P, et al. . SEMA3A, a gene involved in axonal pathfinding, is mutated in patients with Kallmann syndrome. Plos Genet. 2012;8(8):e1002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Känsäkoski J, Fagerholm R, Laitinen EM, et al. . Mutation screening of SEMA3A and SEMA7A in patients with congenital hypogonadotropic hypogonadism. Pediatr Res. 2014;75(5):641-644. [DOI] [PubMed] [Google Scholar]
- 88. Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22(1):111-151. [DOI] [PubMed] [Google Scholar]
- 89. Tobet SA, Chickering TW, King JC, et al. . Expression of gamma-aminobutyric acid and gonadotropin-releasing hormone during neuronal migration through the olfactory system. Endocrinology. 1996;137(12):5415-5420. [DOI] [PubMed] [Google Scholar]
- 90. Fueshko SM, Key S, Wray S. GABA inhibits migration of luteinizing hormone-releasing hormone neurons in embryonic olfactory explants. J Neurosci. 1998;18(7):2560-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Heger S, Seney M, Bless E, et al. . Overexpression of glutamic acid decarboxylase-67 (GAD-67) in gonadotropin-releasing hormone neurons disrupts migratory fate and female reproductive function in mice. Endocrinology. 2003;144(6):2566-2579. [DOI] [PubMed] [Google Scholar]
- 92. Wasser CR, Herz J. Reelin: neurodevelopmental architect and homeostatic regulator of excitatory synapses. J Biol Chem. 2017;292(4):1330-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Roppongi RT, Karimi B, Siddiqui TJ. Role of LRRTMs in synapse development and plasticity. Neurosci Res. 2017;116:18-28. [DOI] [PubMed] [Google Scholar]
- 94. Dairaghi L, Flannery E, Giacobini P, et al. . Reelin can modulate migration of olfactory ensheathing cells and gonadotropin releasing hormone neurons via the canonical pathway. Front Cell Neurosci. 2018;12:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kam KY, Jeong KH, Norwitz ER, Jorgensen EM, Kaiser UB. Oct-1 and nuclear factor Y bind to the SURG-1 element to direct basal and gonadotropin-releasing hormone (GnRH)-stimulated mouse GnRH receptor gene transcription. Mol Endocrinol. 2005;19(1):148-162. [DOI] [PubMed] [Google Scholar]
- 96. Layman WS, Hurd EA, Martin DM. Reproductive dysfunction and decreased GnRH neurogenesis in a mouse model of CHARGE syndrome. Hum Mol Genet. 2011;20(16):3138-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rave-Harel N, Miller NL, Givens ML, Mellon PL. The Groucho-related gene family regulates the gonadotropin-releasing hormone gene through interaction with the homeodomain proteins MSX1 and OCT1. J Biol Chem. 2005;280(35):30975-30983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Roth CL, Mastronardi C, Lomniczi A, et al. . Expression of a tumor-related gene network increases in the mammalian hypothalamus at the time of female puberty. Endocrinology. 2007;148(11):5147-5161. [DOI] [PubMed] [Google Scholar]
- 99. Xie H, Hoffmann HM, Meadows JD, et al. . Homeodomain proteins SIX3 and SIX6 regulate gonadotrope-specific genes during pituitary development. Mol Endocrinol. 2015;29(6):842-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Goldsmith PC, Lamberts R, Brezina LR. Gonadotropin-releasing horome neurons and pathways in the primate hypothalamus and forebrain. In: Norman RL, ed. Neuroendocrine Aspects of Reproduction. Academic Press; 1983:7-43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this manuscript are available upon request.