Abstract
Polychlorinated biphenyls (PCBs) are pervasive environmental contaminants implicated as risk factors for neurodevelopmental disorders (NDDs). Immune dysregulation is another NDD risk factor, and developmental PCB exposures are associated with early life immune dysregulation. Studies of the immunomodulatory effects of PCBs have focused on the higher-chlorinated congeners found in legacy commercial mixtures. Comparatively little is known about the immune effects of contemporary, lower-chlorinated PCBs. This is a critical data gap given recent reports that lower-chlorinated congeners comprise >70% of the total PCB burden in serum of pregnant women enrolled in the MARBLES study who are at increased risk for having a child with an NDD. To examine the influence of PCBs, sex, and genotype on cytokine levels, mice were exposed throughout gestation and lactation to a PCB mixture in the maternal diet, which was based on the 12 most abundant PCBs in sera from MARBLES subjects. Using multiplex array, cytokines were quantified in the serum and hippocampus of weanling mice expressing either a human gain-of-function mutation in ryanodine receptor 1 (T4826I mice), a human CGG premutation repeat expansion in the fragile X mental retardation gene 1 (CGG mice), or both mutations (DM mice). Congenic wildtype (WT) mice were used as controls. There were dose-dependent effects of PCB exposure on cytokine concentrations in the serum but not hippocampus. Differential effects of genotype were observed in the serum and hippocampus. Hippocampal cytokines were consistently elevated in T4826I mice and also in WT animals for some cytokines compared to CGG and DM mice, while serum cytokines were usually elevated in the mutant genotypes compared to the WT group. Males had elevated levels of 19 cytokines in the serum and 4 in the hippocampus compared to females, but there were also interactions between sex and genotype for 7 hippocampal cytokines. Only the chemokine CCL5 in the serum showed an interaction between PCB dose, genotype, and sex. Collectively, these findings indicate differential influences of PCB exposure and genotype on cytokine levels in serum and hippocampal tissue of weanling mice. These results suggest that developmental PCB exposure has chronic effects on baseline serum, but not hippocampal, cytokine levels in juvenile mice.
Keywords: CGG repeat expansion mutation, Chemokines, fmr1, Neurodevelopmental disorders, Neuroimmune interactions, Ryanodine receptor
Graphical Abstract
Highlights
-
•
Mice were developmentally exposed to a human-relevant mixture of PCBs.
-
•
PCBs altered cytokine levels in serum but not hippocampus from juvenile mice.
-
•
Genotype had a large influence on hippocampal cytokine concentrations.
-
•
Sex differences: males had increased levels of cytokines, especially in serum.
-
•
Interactions between factors were observed for a minority of cytokines.
1. Introduction
A number of environmental toxicants have been identified as risk factors for neurodevelopmental disorders (NDDs), including autism spectrum disorder (ASD) (Bölte et al., 2019; Kalkbrenner et al., 2014; Carter and Blizard, 2016; Ye et al., 2017; Rossignol et al., 2014). Polychlorinated biphenyls (PCBs) are of particular concern because these compounds modulate signaling pathways and neurodevelopmental processes implicated in the etiology of ASD and other NDDs (Stamou et al., 2013; Pessah et al., 2019; Klocke and Lein, 2020). PCBs are ubiquitous persistent organic pollutants that were widely used in many industrial applications, including as plasticizers in paints and rubber products and as insulating fluids in electrical equipment, due to their high chemical and thermal stability (Faroon and Ruiz, 2016). There are 209 PCB congeners with different chemical structures and properties based on the number and position of chlorine atoms around the biphenyl rings. PCBs were originally synthesized and sold as commercial mixtures, such as Aroclor 1248 and 1254, that vary in specific congener profile but contain more stable, higher-chlorinated PCB congeners (Kodavanti et al., 2001; Klocke et al., 2020). While levels of these “legacy” PCBs have generally decreased in human biological samples since their global production ban in 2001, they are still pervasive in human tissues, including in pregnant and nursing women (Rawn et al., 2017; Mannetje et al., 2013; Focant et al., 2013). A study of human post-mortem brain tissue found higher concentrations of PCB 95 in samples from patients with genetic NDDs associated with ASD as compared to neurotypical controls (Mitchell et al., 2012). Contemporary human exposures occur predominantly through inhalation and diet (Ravenscroft and Schell, 2018; Basra et al., 2018; Ampleman et al., 2015; Malisch and Kotz, 2014; Cimenci et al., 2013; Marin et al., 2011; Voorspoels et al., 2008; Baars et al., 2004), and PCBs readily cross the placenta and transfer into breast milk (Needham et al., 2011).
Epidemiological studies indicate that prenatal PCB exposure impacts early-life immune function (Dietert, 2014). Within a Norwegian mother and child cohort study, maternal dietary exposure to PCBs has been associated with congener- and sex-specific changes in gene expression of immune-related processes in cord blood samples from newborns (Hochstenbach et al., 2012), and with more frequent respiratory tract infections and reduced antibody response to a measles vaccine in children (Stølevik et al., 2013). Increased susceptibility to respiratory tract infections following prenatal PCB exposure has also been observed in Swedish (Glynn et al., 2008) and Inuit (Dallaire et al., 2004) infants. In Dutch children, prenatal PCB exposure was associated with lower antibody response to measles vaccine, while current PCB body burden was associated with a higher prevalence of chicken pox and recurrent middle-ear infections (Weisglas-Kuperus et al., 2000). Reduced antibody responses to vaccines, suggestive of depressed adaptive immune responses, were associated with PCB exposure in children from the Faroe Islands (Heilmann et al., 2006; Heilmann et al., 2010). Finally, secretion of the cytokine tumor necrosis factor-alpha (TNF-α) was negatively correlated with plasma lipid concentrations of PCBs in stimulated cord blood mononuclear cells from newborns in a remote Canadian coastal region (Bilrha et al., 2003). Together these studies identify the immune system as a likely target of developmental PCB exposure.
Rodent models of PCB exposure demonstrate altered cytokines in maternal serum (Xu et al., 2019) as well as fetal brain tissue and serum following gestational exposure to legacy PCB congeners or mixtures. Increased concentrations of inflammatory cytokines TNF-α, interleukin-1β (IL-1β), and interferon-gamma (IFN-γ) were observed in fetal serum following developmental exposure of rats to the coplanar (dioxin-like) congener PCB 126 (Ahmed et al., 2018). Following gestational exposure to a mixture of Aroclors 1242, 1248, and 1254 at 20 μg/kg in the maternal diet, sex-specific differences in hypothalamic cytokine expression were observed in postnatal day (P) 1 rats (Bell et al., 2018). Further, developmental exposure to an environmentally relevant mixture of Aroclors (Kostyniak et al., 2005) at 18 mg/kg through maternal diet was shown to increase serum IL-6 in male, but not female, rat offspring at P14 (Miller et al., 2010).
Collectively, this evidence indicates early and persistent PCB-induced cytokine dysregulation in multiple tissues as well as sex differences in response to PCB exposure. Even small fluctuations in cytokines may impact neurodevelopment, as these signaling molecules have essential roles in the development and function of the nervous system (Guidolin et al., 2018; Borsini et al., 2015; Yirmiya and Goshen, 2011) and are expressed in the brain in an age- and region-specific manner (Garay et al., 2013; Pousset, 1994). Furthermore, altered immune function is frequently observed in children with ASD (Meltzer and Van De Water, 2017), including cytokine dysregulation (Goines and Ashwood, 2013; Masi et al., 2015), and may be contributing factors in the pathogenesis of these disorders, which exhibit a strong sex bias (Hughes et al., 2018; Gładysz et al., 2018).
Previous studies of the immunomodulatory effects of PCBs have focused on the higher-chlorinated congeners found in legacy commercial mixtures. The immune effects of developmental exposure to contemporary, lower-chlorinated PCBs have not been previously characterized. This is a critical data gap given recent reports that the lower-chlorinated congeners PCB 28 and PCB 11 comprise >70% of the total PCB burden in serum of pregnant women at increased risk for having a child with an NDD (Sethi et al., 2019). To address this data gap, we used a mouse model of developmental PCB exposure to assess the immune effects of a mixture of PCBs based on the 12 most abundant PCB congeners identified in the serum of pregnant women (Sethi et al., 2019) enrolled in the MARBLES (Markers of Autism Risk in Babies - Learning Early Signs) study, who have had one child diagnosed with ASD and thus are at higher risk for their next child being diagnosed with an NDD (Hertz-Picciotto et al., 2018). This MARBLES PCB mix contains legacy higher-chlorinated congeners, such as PCBs 138, 153, and 180, as well as contemporary lower-chlorinated congeners, in the same relative proportion found in the MARBLES subjects. The contemporary congener PCB 11 was not found in the commercial Aroclor mixtures (Kodavanti et al., 2001); rather, it is an inadvertent byproduct of current pigment production processes (Hu and Hornbuckle, 2010; Guo et al., 2014; Shang et al., 2014) that has been widely documented in air (Hu et al., 2008; Choi et al., 2008; Heo et al., 2014) and other environmental samples (Bartlett et al., 2019), serum (Sethi et al., 2017) and commercial milk samples (Chen et al., 2017) from dairy cows, as well as serum samples from children and their mothers (Marek et al., 2013; Koh et al., 2015).
NDD risk and severity are widely posited to be determined by gene × environment interactions (Stamou et al., 2013; Schaafsma et al., 2017), therefore, we also investigated how genotype influences the cytokine response to developmental PCB exposure. Aberrant calcium homeostasis has been implicated in the pathophysiology of ASD (Krey and Dolmetsch, 2007; Palmieri et al., 2010) and other NDDs (Martins-de-Souza et al., 2009; Mizoguchi and Monji, 2017), and PCBs are known to alter calcium homeostasis in neurons (Pessah et al., 2019). Therefore, we focused on NDD-linked genotypes that dysregulate calcium signaling in neurons. Specifically, we leveraged mouse models engineered to express a human gain-of-function mutation in the RYR1 gene (T4826I-RYR1; T4826I mice) (Yuen et al., 2012) or a human CGG repeat expansion in the premutation range in the 5′ non-coding region of the fragile X mental retardation gene 1 (FMR1; CGG mice) (Berman et al., 2014). Ryanodine receptors (RyRs) are intracellular calcium channels involved in many critical functions in immune and neural cells including T cell activation (Wolf et al., 2015) and synaptic plasticity (Abu-Omar et al., 2018). Mutations in RYR1 and FMR1 are both linked to dysregulated calcium signaling and confer heightened sensitivity to environmental stressors in multiple cell types from mice (Barrientos et al., 2012; Cao et al., 2012; Cao et al., 2013) and affect immune function in humans and mice (Vukcevic et al., 2013; Careaga et al., 2014; Marek et al., 2012). To assess the effect of not only PCB dose, but also gene dose on immune response, we additionally studied a double mutant (DM) mouse (Keil et al., 2019) that expressed both of these prevalent but clinically subtle mutations in the human population (Robinson et al., 2006; Kim et al., 2013; Hagerman et al., 2011; Hagerman and Hagerman, 2013). Expression of these mutations, singly or in combination, has previously been shown to interfere with endpoints of relevance to NDDs in juvenile mice, including social behavior and alter dendritic arborization in hippocampal and cortical neurons (Keil et al., 2019).
In summary, cytokines influence social behavior (Eisenberger et al., 2017) and neuronal development (Sarder et al., 1996; Neumann et al., 2002; Chisholm et al., 2012), therefore, altered cytokine levels as a consequence of genetic factors and/or toxicant exposure may contribute to the effects of genetic and environmental factors on neurodevelopment (Dietert and Dietert, 2008). Understanding how genetic and environmental risk factors for NDDs affect cytokine and chemokine levels will provide insight regarding the complex relationships between etiological factors contributing to these disorders. Thus, in the current study, we tested the hypothesis that sex, genotype, and PCB dose interact to alter cytokine and chemokine profiles in serum and hippocampal tissue from mice developmentally exposed to a mixture of PCBs modeled after PCB congener profiles in the serum of pregnant women at risk for having a child with an NDD.
2. Material and methods
2.1. Animals
All procedures involving animals were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of California Davis Animal Care and Use Committee. All mice were housed in clear plastic shoebox cages with corn cob bedding and maintained on a 12 h light and dark cycle at 22 ± 2 °C with feed (Diet 5058, LabDiet, Saint Louis, MO) and water available ad libitum. Male and female mice of four different genotypes were investigated (Keil et al., 2019): (a) homozygous mice, which express a human gain-of-function mutation in RYR1 (T4826I-RYR1), referred to herein as T4826I mice; (b) female homozygous and male hemizygous FMR1 premutation mice that express the human X-linked CGG repeat expansion in the FMR1 gene in the range of 170–200 repeats, referred to as CGG mice; (c) mice expressing both the RYR1 and FMR1 mutations, referred to as double mutation (DM) mice; and (d) wildtype (WT) mice on a genetic background matching the mutant genotypes (75% C57BL/6J and 25% SVJ129), as determined by SNP analysis (Keil et al., 2019). To generate the WT control mice, C57BL/6J and SVJ129 WT mice were purchased from Jackson Labs (JAX, Sacramento, CA) and crossed to generate congenic WT mice. Genotyping of all animals was performed as previously described (Keil et al., 2019). The animals reported here are a subset of animals evaluated as part of a larger overall study designed to evaluate neurodevelopmental outcomes (Keil-Steitz and Lein, under review) in addition to effects on the gut microbiome and intestinal physiology (Rude et al., 2019) and cytokine levels (data reported here). For the cytokine analyses, 192 animals from 113 dams were included in the hippocampal tissue analysis and 176 animals from 101 dams were included in the serum analysis, with 92 animals from 72 dams overlapping between the two sets. Further details about the animals used in this study are provided in Supplementary Table 1.
2.2. Developmental PCB exposure
The MARBLES PCB mix (Sethi et al., 2019) used for dosing dams was composed of congeners (% of total mass) 28 (48.2%), 11 (24.3%), 118 (4.9%), 101 (4.5%), 52 (4.5%), 153 (3.1%), 180 (2.8%), 149 (2%), 138 (1.7%), 84 (1.5%), 135 (1.3%), and 95 (1.2%). Purified PCB congeners were synthesized by Dr. Hans-Joachim Lehmler (The University of Iowa, Iowa City, IA) and confirmed by gas chromatography to be >99% pure (Sethi et al., 2019). PCB congeners were suspended in organic peanut oil (Spectrum Organic Products, LLC, Melville, NY) and homogenously added to organic peanut butter (Trader Joe's, Monrovia, CA). Dams were dosed with peanut butter stocks at 0, 0.1, 1, or 6 mg/kg/day. Vehicle (0 mg/kg/day) was peanut oil added to peanut butter. The dose of 6 mg/kg/d, the highest dose tested in this study, was chosen because this dose of Aroclor 1254 in the maternal diet has been shown to result in levels of PCBs in the weanling rodent brain that are similar to those documented in human brain tissue (Yang et al., 2009).
Humans are exposed to the higher-chlorinated PCBs predominantly via the diet (Hu and Hornbuckle, 2010), while human exposure to the lower-chlorinated PCBs occurs via several routes, including direct contact with PCB-containing consumer products (Hu and Hornbuckle, 2010; Anezaki and Nakano, 2014); inhalation of PCBs in the air (Ampleman et al., 2015); and dietary ingestion, for example, of commercial milk products (Ampleman et al., 2015; Chen et al., 2017). Therefore, as the MARBLES mix included both higher- and lower-chlorinated congeners, we exposed dams to PCBs via the diet. All mice were habituated to the peanut butter mix for 3–5 days prior to the start of dosing by placing each mouse individually into a clean cage without bedding and presenting them with 0.1 g of the vehicle peanut butter mixture on a small disposable weigh boat. Mice that did not consume the peanut butter within 15–20 min by the fifth day were not advanced further in the study (this represented <2% of animals). Dosing began two weeks prior to mating to achieve steady state levels of PCBs in the dam prior to conception. Dosing continued throughout gestation and lactation up to P21. This timeframe corresponds to human brain development throughout gestation, e.g., rodent brain development during the first three weeks after birth corresponds to human brain development during the third trimester (Semple et al., 2013; Rice and Barone, 2000). Each dam was weighed prior to being transferred to a clean cage for dosing, and the amount of PCB mixture was adjusted for body weight to ensure the appropriate dose was consumed. Dams were not returned to their home cage until they had consumed all the peanut butter, which typically occurred within 15–20 min.
Dams were placed with stud males (two females per male) after two weeks of dosing. There was no determination of where females were in the ovarian cycle prior to mating. Within each cohort across the larger study, if no discernable plug was detected and/or a significant increase in body weight was not observed by the time other dams in the cohort had litters, the dam was marked in the database as not becoming pregnant and was removed from the study. For dams that gave birth, litters were culled to 8 pups at P2. Pups were weaned at P21, which terminated their PCB exposure. Weanlings were group housed with same genotype, same dose, same sex littermates until euthanized for sample collection. A subset of animals tested for social behavior as part of the larger overall study were singly housed for 2–3 days prior to euthanasia for sample collection. The behavioral status of the animals used for cytokine analysis is noted in Supplementary Table 1; only 39 animals (20% of hippocampal samples) used in this study underwent behavioral testing prior to collection of samples for cytokine analysis. The age at which samples were collected from pups ranged from P27-P32; however, the majority of samples (approximately 87% of serum samples and 85% of hippocampal samples) were collected at P28–30, with the mean age being P29 for all groups (Supplementary Fig. 1). The ages were balanced across groups and were within a tight range.
Immediately after euthanasia, blood was collected by cardiac puncture, allowed to clot and then centrifuged at 7000 rpm for 10 min. Serum was collected and frozen at −80 °C until further analysis. Brains were harvested immediately after blood was collected and microdissected on ice to obtain the hippocampus, which was snap-frozen in liquid nitrogen then stored at −80 °C until further analysis. Matched brain and serum from the same animal were not obtained from all animals; however, there were 92 animals from 72 dams from which both types of samples were collected.
2.3. Tissue processing
Proteins were isolated from hippocampal tissues using the Bio-Plex® cell lysis kit (Bio-Rad, Hercules, CA). Lysing solution was prepared using Bio-Plex cell lysis buffer and factors 1 and 2 from the kit and cOmplete™ cocktail protease inhibitors (Millipore Sigma, Burlington, MA). All samples and solutions were kept at 4 °C throughout processing. Hippocampal tissues were kept frozen until rinsing with Bio-Plex cell wash buffer from the kit, followed by homogenization in 200 μl of lysing solution using a probe sonicator (7–10 pulses of 1 s per sample at setting 5 on a VirSonic ultrasonic cell disruptor 100, VirTis, Gardiner, NY). Homogenized samples were frozen on dry ice for at least 10 min, then thawed, sonicated for 3 min, and centrifuged at 4500 rcf for 4 min at 4 °C. Supernatant was removed, and protein concentration was determined with the Pierce BCA assay (Thermo Fisher), according to manufacturer's instructions using lysing solution as the diluent for standards and blank. Samples were run in duplicate and absorption at 562 nm was read on a BioTek Synergy H1 hybrid microplate reader (Winooski, VT).
2.4. Cytokine and chemokine measurement
Cytokines and chemokines were measured in a multiplex analysis using Bio-Plex Pro™ Mouse 23-plex kits (Bio-Rad). The kit included the following analytes: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17A, IFN-γ, TNF-α, G-CSF (granulocyte colony-stimulating factor), GM-CSF (granulocyte-macrophage colony-stimulating factor), CXCL1 (C-X-C motif ligand 1)/KC (keratinocyte chemoattractant), CCL2 (C-C motif ligand 2)/MCP-1 (monocyte chemoattractant protein 1), CCL3/MIP-1α (macrophage inflammatory protein-1 alpha), CCL4/MIP-1β, CCL5/RANTES (regulated upon activation, normal T cells, expressed and secreted), and CCL11/Eotaxin. The working concentration range and limit of detection for each cytokine kit is noted in Supplementary Table 2. Samples were stored at −80 °C prior to analysis and prepared according to manufacturer's recommendations. Serum samples were centrifuged then diluted 1:4 in Bio-Plex sample diluent, and standards were prepared using standard diluent provided with kits. Hippocampal samples were centrifuged and first diluted to 2.5 μg/μl using lysing solution to normalize protein content. Samples were then combined 1:1 with Bio-Plex sample diluent to a final concentration of 1.25 μg/μl. Hippocampal blank and standards were prepared using the same matrix as samples (1:1 lysing solution:sample diluent). Assays were performed per manufacturer's instructions, and wash steps were carried out using a Bio-Plex Pro Wash Station (Bio-Rad). Samples were run in duplicate and evenly divided between plates so that one of each experimental group was represented on each plate. Plates were read on a Bio-Plex 200 System (Bio-Rad), and standard curves were optimized automatically in the Bio-Plex Manager software (version 6.1.1, Bio-Rad, RRID:SCR_014330). Cytokine concentrations were extrapolated from the standard curves. Overall, cytokine concentrations in samples were skewed to the lower end of detection. If concentrations fell below the lowest standard, values that could be extrapolated were used but concentrations that could not be extrapolated were assigned a value of half the limit of detection [LOD/2] for that plate. For hippocampal samples, standard curves for some cytokines were imported from other plates to normalize variability between plates due to low cytokine concentrations. All values for G-CSF in hippocampal samples fell below the lowest standard so this analyte was not included in the final results of hippocampal cytokines.
2.5. Statistical analysis
Generalized linear models were used to evaluate the effect of genotype, dose and sex on mean differences in each analyte (cytokine). For all analytes, a full model consisting of all main effects (genotype, sex, dose) with all 2- and 3-way interactions was fit first. Then, non-significant interactions were dropped to yield a final model fit consisting of all main effects and any significant interactions. To account for variation between plates, plate number was included as a main effect (p-values listed in Supplementary Table 2). The litter was the unit of analysis with pups considered repeated measures on the litter (Table 1). Generalized estimating equations were used to estimate model parameters, and a robust covariance estimate was used to account for correlation among littermates. Serum cytokine and chemokine concentrations were natural log (ln)-transformed to meet model assumptions of normality and homogeneity of variances. Significant tests for main effects or interactions were followed with post hoc analyses of all pairwise comparisons using Tukey's Honestly Significant Difference procedure. All statistical tests were two-sided and evaluated at a significance level of 0.05. Data analyses were performed using the Statistical Analysis System software (SAS, RRID:SCR_008567). GraphPad Prism 8 software (RRID:SCR_002798) was used for data visualization.
Table 1.
Summary of litter and animal numbers for each treatment group included in cytokine analysis. The numbers of dams/litters and (animals) used in this study are shown for serum (top) and hippocampal (bottom) samples. As an additional note, some M and F within genotypes and dose groups are from the same litter (not shown in this table).
| Serum: litter and (animal) numbers | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 mg/kg/d |
0.1 mg/kg/d |
1 mg/kg/d |
6 mg/kg/d |
|||||
| M | F | M | F | M | F | M | F | |
| WT | 4 (5) | 5 (5) | 5 (5) | 4 (6) | 4 (6) | 5 (5) | 4 (5) | 5 (6) |
| CGG | 5 (5) | 5 (6) | 4 (5) | 4 (6) | 5 (5) | 5 (8) | 5 (7) | 5 (7) |
| DM | 5 (5) | 4 (5) | 5 (5) | 5 (6) | 4 (6) | 4 (5) | 5 (5) | 4 (5) |
| T4826I | 5 (5) | 5 (5) | 5 (6) | 5 (5) | 5 (5) | 5 (6) | 5 (5) | 5 (5) |
| Hippocampal tissue: litter and (animal) numbers | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 mg/kg/d |
0.1 mg/kg/d |
1 mg/kg/d |
6 mg/kg/d |
|||||
| M | F | M | F | M | F | M | F | |
| WT | 5 (6) | 6 (6) | 5 (7) | 4 (6) | 4 (5) | 6 (6) | 6 (6) | 5 (6) |
| CGG | 6 (6) | 5 (6) | 5 (5) | 5 (6) | 6 (7) | 5 (6) | 5 (7) | 5 (6) |
| DM | 6 (6) | 4 (6) | 4 (5) | 5 (6) | 4 (6) | 4 (6) | 6 (6) | 5 (6) |
| T4826I | 6 (6) | 6 (6) | 5 (6) | 5 (6) | 6 (6) | 6 (6) | 5 (6) | 5 (6) |
All other analyses not related to the cytokines (e.g., dam mass, pup mass, anogenital distance) were conducted in Prism using 2-way ANOVA with post hoc Tukey's test to determine significant differences between groups.
3. Results
3.1. Effects of developmental PCB exposure on reproductive success and estrogen-sensitive developmental endpoints
Across all groups the pregnancy rate was 88% (Supplementary Table 3). The overall average among all groups for days from mating to litter date of birth was 26.6 ± 2 days, and there were no significant differences in the days from start of first mating to litter date of birth among any of the groups (Supplementary Fig. 2A). Dam mass at weaning was not altered by PCB exposure, but there was a significant main effect of genotype (Supplementary Fig. 2B).
To examine potential estrogenic effects of the PCB exposure, we evaluated anogenital distance, a parameter that can be altered by estrogenic compounds, in male and female mice at P7 (Supplementary Fig. 3). As expected, males had a greater anogenital distance than females across all genotypes (Supplementary Fig. 3A–D); however, there were effects of PCB dose and genotype on this parameter. In WT males, PCB exposure decreased anogenital distance at all doses (Supplementary Fig. 3A, E). There was a main effect of dose within the T4826I genotype, with the 0.1 mg/kg dose having decreased anogenital distance compared to vehicle or 1 mg/kg PCB dose groups (Supplementary Fig. 3B). Among the vehicle control groups, DM male mice had decreased anogenital distance compared to WT and T4826I male mice (Supplementary Fig. 3E). Main effects of genotype and PCB dose were also observed in female mice, with CGG females having greater anogenital distance than DM females and the PCB dose 0.1 mg/kg decreasing anogenital distance compared to the 1 mg/kg dose group (Supplementary Fig. 3F). These data indicate that PCBs may have estrogenic activity in WT male mice at P7 and in males the DM genotype phenocopies this effect.
Similarly, body weights of juvenile males were greater than females within each genotype (Supplementary Fig. 4A–D). An interaction between sex and dose was observed for the WT group, with WT males in the 1 mg/kg dose group exhibiting decreased body mass compared to WT males in the 0.1 mg/kg PCB dose group. Within the T4826I and CGG groups, there was a main effect of PCB dose with the 1 mg/kg dose decreasing body mass relative to the control in the CGG group, but increasing body mass relative to the vehicle and 0.1 mg/kg dose group in the T4826I mice. Within males, an interaction between dose and genotype was observed, and among the vehicle control groups, CGG and T4826I males had decreased body mass compared to WT males (Supplementary Fig. 4E). Within females, the 2-way interaction between dose and genotype was almost significant (p = 0.051), and there was a main effect of genotype, with CGG females having decreased body mass relative to all other genotypes and WT females showing increased body mass relative to T4826I females (Supplementary Fig. 4F). These results demonstrate that there are sex, genotype, and PCB dose effects and interactions for the body mass of juvenile animals.
3.2. There are significant effects of PCB dose, genotype and/or sex on a majority of cytokines in serum and hippocampal tissue
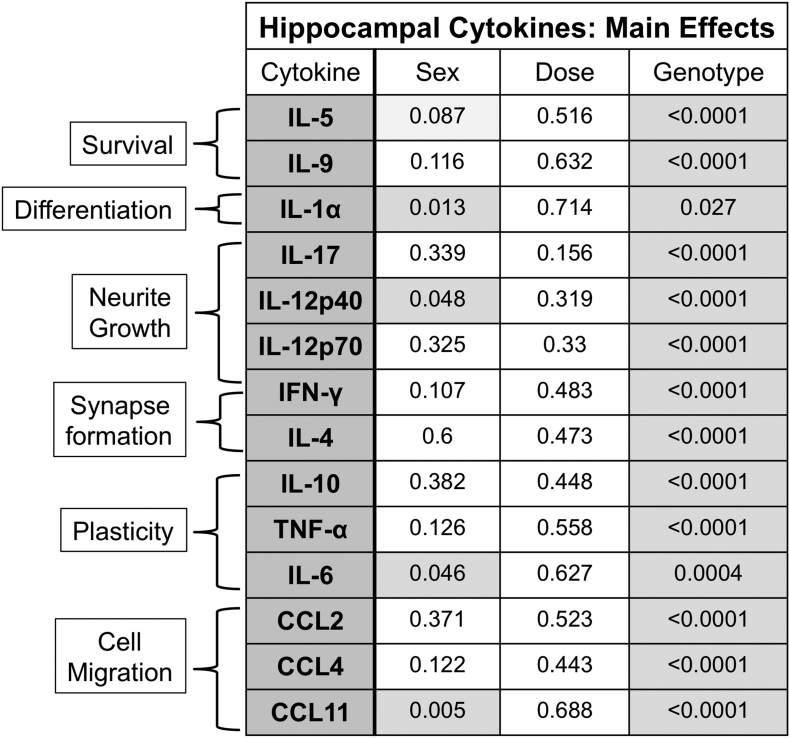
Circulating peripheral cytokines can provide important insight into biological processes and disease pathogenesis (Aziz, 2015). Cytokines also regulate many aspects of neurodevelopment (Guidolin et al., 2018; Yirmiya and Goshen, 2011) that may impact hippocampal neuronal connectivity and function (Wei et al., 2012; Petitto et al., 1999; Williamson and Bilbo, 2013). The hippocampus is critical for learning and memory (Albani et al., 2014), and recent evidence also indicates a role for this brain region in social behaviors (Montagrin et al., 2018; Phillips et al., 2019). Hippocampal alterations, such as changes in functional connectivity, have been noted in patients with ASD (Cooper et al., 2017; Raymond et al., 1995) and in preclinical models of NDDs (Bergdolt and Dunaevsky, 2019). Therefore, the effects of PCB dose, genotype, and sex, as well as the two-way and three-way interactions among these factors were examined for influences on baseline concentrations of 23 cytokines from serum and hippocampal tissue of juvenile mice developmentally exposed to the MARBLES PCB mixture. Significant interactions were only observed for 1 cytokine in the serum and for 7 cytokines in the hippocampus and will be described after discussing main effects.
Almost all of the serum cytokines, which are broadly categorized according to major immune function, displayed a main effect of PCB dose, sex, and/or genotype (Fig. 1). Cytokines that showed a significant main effect of all three factors include IL-1α, IFN-γ, IL-12p40, and CXCL1 (Fig. 1). In contrast, only main effects of genotype and sex were significant for the majority (14 out of 22) of hippocampal cytokines, which are broadly grouped according to neurodevelopmental function (Fig. 2). Genotype influences dominated in the hippocampus as indicted by the observation that 21 out of 22 hippocampal cytokines had a main effect or interaction involving genotype. Collectively, these observations highlight the differential influences of these factors on cytokine levels in serum vs. hippocampal tissue.
Fig. 1.
Summary of main effects on serum cytokines and chemokines in juvenile mice. Peripheral cytokines for which the final statistical models were reduced to main effects, categorized by primary immune function. Listed p-values are based on the Chi-Square distributions for each effect in the model with shaded boxes representing significant main effects (p < 0.05). PCB effects were observed for many cytokines, especially those associated with TH cells. Genotype effects were more limited and involved TH1 cytokines as well as innate inflammatory cytokines and chemokines. Differences due to sex were observed in numerous cytokines from many functional categories, but particularly for innate inflammatory cytokines and chemokines as well as cytokines functioning as growth and differentiation factors.
Fig. 2.
Summary of main effects on hippocampal cytokines and chemokines in juvenile mice. Hippocampal cytokines for which the final statistical models were reduced to main effects, categorized by function during neurodevelopment, although many of these cytokines can play multiple pleiotropic roles during different phases of brain development. Listed p-values are based on the Chi-Square distributions for each effect in the model with shaded boxes representing significant main effects (p < 0.05). Genotype was highly influential on hippocampal cytokine concentrations with sex showing a more modest influence. However, we did not find evidence that developmental PCB exposure altered hippocampal cytokine levels in juvenile mice.
3.3. Cytokine levels are increased in the serum, but not hippocampus, of juvenile mice following developmental PCB exposure
In the serum, PCB dose effects (Fig. 3) were found for a number of T cell cytokines and innate inflammatory cytokines and chemokines. Most differences were observed between the vehicle and 6 mg/kg groups (IL-1α, GM-CSF, IL-10, IL-4, IL-9, IL-13, IL-17, IFN-γ, IL-12p70, CCL3) and the 0.1 mg/kg and 6 mg/kg groups (IL-1α, TNF-α, GM-CSF, IL-10, IL-4, IL-9, IL-17, IFN-γ, IL-12p40, CXCL1), with the 6 mg/kg PCB exposure increasing cytokine concentrations (Fig. 3). The 1 mg/kg PCB dose increased serum IL-13 and CCL3 relative to vehicle animals. In contrast, no main effect of PCB dose or interactions involving PCB dose were observed for any hippocampal cytokines or chemokines (Fig. 2).
Fig. 3.
Dose-dependent effects of developmental PCB exposure on peripheral cytokines and chemokines in juvenile mice. PCB dose had a significant main effect on the level of innate inflammatory cytokines IL-1α (A), TNF-α (B), and GM-CSF (C); regulatory cytokine IL-10 (D); TH2 cytokines IL-4 (E), IL-9 (F), and IL-13 (G); TH17 cytokine IL-17 (H); TH1 cytokines IFN-γ (I), IL-12p40 (J), and IL-12p70 (K); and inflammatory chemokines CXCL1/KC (L) and CCL3/MIP-1α (M) in serum. In A–M, data are collapsed across genotypes and sexes, and each data point represents 1 animal (0 mg/kg: 41 animals from n = 26 litters; 0.1 mg/kg: 44 animals from n = 26 litters; 1 mg/kg: 46 animals from n = 24 litters; 6 mg/kg: 45 animals from n = 25 litters). Whiskers on box plots depict minimum and maximum values and p-values are indicated between groups as determined by the differences of least squares means with post hoc Tukey-Kramer adjustment for multiple comparisons.
3.4. Genotype has differential effects on serum and hippocampal cytokines
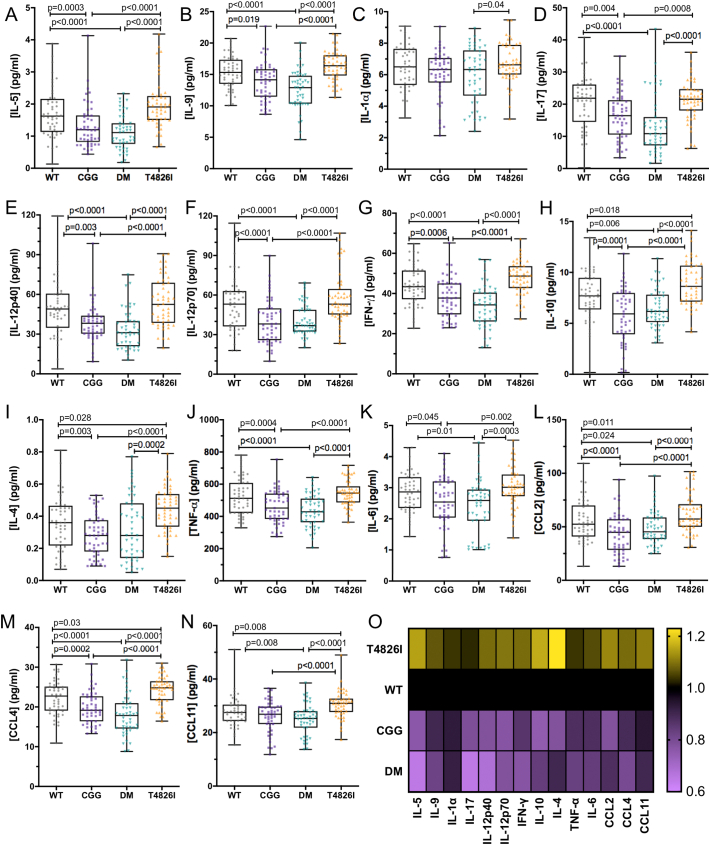
Genotype strongly influenced a large number of hippocampal cytokines, with CGG and DM animals showing decreased concentrations of IL-5, IL-9, IL-17, IL-12, IFN-γ, TNF-α, and IL-6 compared to T4826I and WT animals (Fig. 4). T4826I mice had elevated levels of IL-10, IL-4, CCL2, CCL4, and CCL11 compared to every other genotype (Fig. 4). Genotype affected relatively fewer cytokines in the serum (Fig. 5) than in the hippocampus, and these were limited to TH1 subsets and innate inflammatory cytokines and chemokines. Of the 6 cytokines altered by genotype in the serum, 4 of these (IL-1α, IFN-γ, IL-12p40, and CCL11) were also altered by genotype in the hippocampus, although the cytokine patterns and significant comparisons were different in each tissue. For example, DM animals had higher serum IL-1α than WT animals but lower hippocampal IL-1α than the T4826I group. Unlike in the hippocampus, all of the mutant genotypes generally had increased serum cytokine concentrations relative to WT (Fig. 5G), with the notable exception of CCL11, which was decreased in DM mice relative to WT and CGG mice. Most peripheral cytokine differences were observed between WT mice and the mutant genotypes. However, within the TH1 cytokines, CGG mice had the lowest concentrations of IFN-γ but the highest IL-12p40 levels, driving differences between the CGG group and the T4826I and DM groups. These patterns suggest differential influences of the genetic mutations between serum and hippocampal cytokine levels.
Fig. 4.
Genotype strongly influences cytokine and chemokine concentrations in hippocampal tissue from juvenile mice. Genotype had a significant main effect on: IL-5 (A), IL-9 (B), IL-1α (C), IL-17 (D), IL-12p40 (E), IL-12p70 (F), IFN-γ (G), IL-10 (H), IL-4 (I), TNF-α (J), IL-6 (K), CCL2/MCP-1 (L), CCL4/MIP-1β (M), and CCL11/Eotaxin (N) concentrations in the hippocampus. Heat map (O) depicting fold-changes in cytokine concentrations in different genotypes relative to WT (black, set to 1), with lower concentrations shown in purple (CGG and DM) and higher concentrations shown in yellow (T4826I). In A–N, data are collapsed across PCB dose groups and sexes, and each data point represents 1 animal (WT: 48 animals from n = 31 litters; CGG: 49 animals from n = 28 litters; DM: 47 animals from n = 26 litters; T4826I: 48 animals from n = 28 litters). Whiskers on box plots depict minimum and maximum values and p-values are indicated between groups as determined by the differences of least squares means with post hoc Tukey-Kramer adjustment for multiple comparisons.
Fig. 5.
Genotype influences peripheral cytokine and chemokine levels in juvenile mice. Genotype had a significant main effect on the concentration of innate inflammatory cytokines IL-1α (A) and G-CSF (B); TH1 cytokines IFN-γ (C) and IL-12p40 (D); and inflammatory chemokines CXCL1/KC (E) and CCL11/Eotaxin (F) in serum. Heat map (G) depicting fold-changes in cytokine concentrations in different genotypes relative to WT (black, set at 1.0), with lower concentrations shown in purple and higher concentrations shown in yellow (*p < 0.05 versus WT mice and #p < 0.05 versus CGG mice). In A–F, data are collapsed across PCB dose groups and sexes, and each data point represents 1 animal (WT: 43 animals from n = 28 litters; CGG: 49 animals from n = 26 litters; DM: 42 animals from n = 25 litters; T4826I: 42 animals from n = 23 litters). Whiskers on box plots depict minimum and maximum values and p-values are indicated between groups as determined by the differences of least squares means with post hoc Tukey-Kramer adjustment for multiple comparisons.
3.5. Juvenile males have increased peripheral and hippocampal cytokine levels compared to females
Sex influenced the concentrations of 19 cytokines in the serum (Fig. 6), with male mice showing increased levels of many cytokines from numerous functional classes, including innate inflammatory cytokines and chemokines, T cell cytokines, and cytokines that stimulate growth and differentiation. Cytokines with p < 0.001 include IL-1β, IL-10, IL-17, GM-CSF, IL-4, TNF-α, IFN-γ, CCL4, and CXCL1 (Fig. 6). Sex effects on hippocampal cytokines were limited to IL-1α, IL-6, IL-12p40, and CCL11 (Fig. 7), with male mice showing increased concentrations relative to female mice, as was observed in the serum. Notably, all 4 of the cytokines affected by sex in the hippocampus also showed a main effect of sex in the serum.
Fig. 6.
Male juvenile mice have increased peripheral cytokine and chemokine concentrations relative to age-matched females. Sex had a significant main effect on the concentration of serum cytokines from many functional categories, including early inflammatory cytokines IL-1α (A), IL-1β (B), IL-6 (C), and TNF-α (D); growth and differentiation factors IL-2 (E), IL-3 (F), G-CSF (G), and GM-CSF (H); regulatory cytokine IL-10 (I); TH2 factors IL-4 (J), IL-13 (K), and CCL11/Eotaxin (L); inflammatory chemokines CXCL1/KC (M), CCL2/MCP-1 (N), CCL3/MIP-1α (O), and CCL4/MIP-1β (P); TH1 cytokines IFN-γ (Q) and IL-12p40 (R); and TH17 cytokine IL-17 (S). In A–S, data are collapsed across genotypes and PCB dose groups, and each data point represents 1 animal (85 M and 91 F from n = 75 independent litters each). Whiskers on box plots depict minimum and maximum values for cytokines with p < 0.05 as determined by Chi-Square analysis.
Fig. 7.
Hippocampal cytokine levels are increased in juvenile male mice compared to age-matched females. Sex had a significant main effect on: IL-1α (A), IL-6 (B), IL-12p40 (C), and CCL11/Eotaxin (D) concentrations in hippocampal tissue. In A–D, data are collapsed across genotypes and PCB dose groups, and each data point represents 1 animal (96 animals from n = 84 (M) and n = 81 (F) independent litters). Whiskers on box plots depict minimum and maximum values for cytokines with p < 0.05 as determined by Chi-Square analysis.
3.6. A 3-way interaction was observed for CCL5 in the serum, and interactions between genotype and sex were found for seven hippocampal cytokines
The only cytokine showing a significant 3-way interaction between genotype, sex, and PCB dose was CCL5 in the serum (Fig. 8 with significant pairwise comparisons listed in Supplementary Table 4). Groups driving the majority of these differences include: WT males in the 0 and 1 mg/kg PCB dose groups, DM females in the 0 and 6 mg/kg dose groups, and DM males in the 0.1 and 1 mg/kg dose groups (Fig. 8B and Supplementary Table 4). While no evidence of PCB dose effects was found within a particular sex/genotype combination, there were notable differences between sexes and genotypes at baseline and for a given PCB dose. For example, in both the vehicle and 1 mg/kg dose groups, CGG, T4826I, and DM male and female mice all had significantly higher levels of serum CCL5 than WT males, similar to the pattern observed for other serum cytokines. At the 6 mg/kg dose, DM female mice had elevated concentrations of CCL5 compared to WT and CGG males and females. Within the T4826I genotype, females had higher CCL5 levels than males at 6 mg/kg, which was different from the pattern observed for other cytokines. These results indicate a sex effect of PCB exposure on CCL5 production for some dose groups and genotypes.
Fig. 8.
Serum concentrations of the chemokine CCL5/RANTES show a genotype*sex*dose interaction. A full statistical model was fit for the chemokine CCL5/RANTES in the serum of juvenile mice (A) with significant genotype*sex*dose pairwise comparisons (as determined by Tukey-Kramer post hoc test) listed in Supplementary Table 4. Whiskers on box plots depict minimum and maximum values, and each data point represents 1 animal (5–8 animals from n = 4–5 independent litters per group as detailed in Table 1). Listed p-values for interactions and main effects are based on the limiting Chi-Square distributions for each effect in the model. Heat map (B) of average CCL5/RANTES ln-transformed concentrations, with lower concentrations shown in purple and higher concentrations shown in yellow. Numbers indicate the count of significant (p < 0.05) pairwise comparisons against that group (Supplementary Table 4).
Serum concentrations of the chemokine CCL5/RANTES show a genotype*sex*dose interaction. A full statistical model was fit for the chemokine CCL5/RANTES in the serum of juvenile mice (A) with significant genotype*sex*dose pairwise comparisons (as determined by Tukey-Kramer post hoc test) listed in Supplementary Table 4. Whiskers on box plots depict minimum and maximum values, and each data point represents 1 animal (5–8 animals from n = 4–5 independent litters per group as detailed in Table 1). Listed p-values for interactions and main effects are based on the limiting Chi-Square distributions for each effect in the model. Heat map (B) of average CCL5/RANTES ln-transformed concentrations, with lower concentrations shown in purple and higher concentrations shown in yellow. Numbers indicate the count of significant (p < 0.05) pairwise comparisons against that group (Supplementary Table 4).
Several hippocampal cytokines had an interaction between genotype and sex, including CCL5, IL-2, CXCL1, IL-1β, IL-3, CCL3, and GM-CSF (Fig. 9 with significant pairwise comparisons listed in Supplementary Table 5). Within the DM group, sex differences were observed for CCL5, IL-2, and IL-1β, with female mice having lower levels than males. WT female mice had lower levels of CCL3 and GM-CSF than their male counterparts. T4826I mice of both sexes had elevated levels of CCL3 and GM-CSF compared to CGG and DM males and females, and other differences between the T4826I and CGG/DM groups were found for IL-2, IL-1β, CXCL1, and IL-3, as was observed for other cytokines with a main effect of genotype in the hippocampus.
Fig. 9.
Interactions between multiple factors influence hippocampal cytokines from juvenile mice. A significant genotype*sex interaction was found for hippocampal concentrations of CCL5/RANTES (A), IL-2 (B), CXCL1 (C), IL-1β (D), IL-3 (E), CCL3/MIP-1α (F), and GM-CSF (G), with significant multiple comparisons (as determined by Tukey-Kramer post hoc test) listed in Supplementary Table 5. In A–G, data are collapsed across PCB dose groups. Whiskers on box plots depict minimum and maximum values, and each data point represents 1 animal (WT: 24 animals from n = 20 litters (M), 24 animals from n = 21 litters (F); CGG: 25 animals from n = 22 litters (M), 24 animals from n = 20 litters (F); DM: 23 animals from n = 20 litters (M), 24 animals from n = 18 litters (F); T4826I: 24 animals from n = 22 litters (M), 24 animals from n = 22 litters (F)). Significant (p < 0.05) and trending (p < 0.1) p-values are listed for interactions and main effects as based on the Chi-Square distributions for each effect in the model.
3.7. The influence of PCB dose, genotype, and sex on the correlation between serum and hippocampal cytokine levels
It is possible that one or more of the factors examined in this study alter the relationship between brain and serum cytokines. To explore this possibility, we used a linear regression model to ask if there was any correlation between serum and hippocampal cytokines and if this was altered by PCB dose, genotype, or sex in the subset of animals from which both types of samples were collected (Supplementary Table 6). For IL-1α, there was an interaction with PCB dose, with the slopes of the lines for PCB doses 1 and 6 mg/kg being significantly greater than control. IL-10 and CCL2 showed a relationship between serum and hippocampal cytokines and both had an interaction with genotype. IL-9 also had an interaction with genotype, while IL-1β, GM-CSF, and CCL3 showed interactions with sex.
4. Discussion
This study examined the effects of PCB dose, genotype, and sex on basal levels of 23 cytokines and chemokines in serum and hippocampal tissue from juvenile mice exposed throughout gestation and lactation to a mixture of PCBs modeled after the congener profile found in the serum of mothers at risk for having a child with an NDD (Sethi et al., 2019; Hertz-Picciotto et al., 2018). We hypothesized interactions between these factors would influence cytokine production and that the mutant genotypes, particularly the DM animals, would be more susceptible to the effects of developmental PCB exposure. Contrary to our prediction, this was not observed for most cytokines, which were reduced to main effects models, and only serum CCL5 showed a significant interaction involving PCB dose (Fig. 10). In the serum, sex influenced the greatest number of cytokines, followed by PCB dose, and then genotype. In contrast, genotype was highly influential on hippocampal cytokine profile with sex having a more moderate effect and several cytokines showing an interaction between sex and genotype. Surprisingly, we did not find evidence that developmental PCB exposure affected cytokine levels in hippocampal tissue, which may reflect differential susceptibilities and/or recovery rates of cytokine-producing cells in the hippocampus as compared to the periphery at this age.
Fig. 10.
Summary of final models for cytokines from juvenile mice developmentally exposed to PCBs. Venn diagrams represent the main effects of sex, genotype, and PCB dose in the serum and sex and genotype in the hippocampus, summarizing cytokines and chemokines with significant p-values (p < 0.05) in each category. Consistent group trends within main effect categories are indicated by arrows. Cytokines for which the final statistical models included significant interactions are listed below the Venn diagrams.
A main effect of PCB exposure was observed for serum cytokines and chemokines, with dose-dependent effects noted for 13 cytokines, suggestive of developmental immunotoxicity (Dietert, 2014). The MARBLES PCB mix contains both lower-chlorinated congeners such as PCBs 11, 28, 52 (sum is 77% of mix) as well as higher-chlorinated, legacy congeners such as PCBs 138, 153, 180 (sum is 7.6% of mix), and there is previous evidence of immunomodulatory effects from exposure to both congener subclasses in various immune cells. Studies of human PBMCs demonstrate congener-specific gene expression patterns following ex vivo exposure to PCBs 138 and 153 (Ghosh et al., 2011), with sex affecting PBMC response to PCBs 52, 138, and 180 (Wens et al., 2011). Congener-specific gene regulation was also observed in human male PMBCs exposed to PCBs 28, 138, 153, and 180, with PCB 180 increasing expression of IL-6 and IL-1β after 24 h exposure (Leijs et al., 2019). In a comparative study, PCB 52 induced a higher production of reactive oxygen species from human neutrophil granules than PCBs 153 and 180 (Berntsen et al., 2016). Additionally, PCB 118, but not PCB 153, increased the percentage of CD4+ T cells that produce interleukin-4 (IL-4), suggestive of enhanced TH 2 differentiation (Gaspar-Ramírez et al., 2012). Exposure to PCBs 138, 153, and 180 reduced the phagocytic activity of human monocytes and neutrophils (Levin et al., 2005), and exposure of human mast cells to PCB 153 activated NF-κB signaling and induced expression of inflammatory cytokines IL-6, IL-1β, and TNF-α (Kwon et al., 2002). A study using adult male C57BL/6 mice treated with PCBs by oral gavage for 24 h found increased plasma IL-6, TNF-α, and CCL2 from PCB 118, increased plasma IL-6 from PCB 153, and no changes from PCB 126 (Choi et al., 2012). Collectively, this evidence indicates cell- and congener-specific responses to PCBs and suggests that both lower- and higher-chlorinated congeners contribute to PCB effects on serum cytokines observed in mice exposed developmentally to the MARBLES PCB mix.
Based on the observed serum cytokine profile, the peripheral immune cell populations dysregulated by developmental exposure to the MARBLES PCB mix likely include T cells and macrophages/monocytes (Tayal and Kalra, 2008). Prenatal PCB/dioxin exposure has been associated with changes in populations of T cells, monocytes, and granulocytes in blood from Dutch infants (Weisglas-Kuperus et al., 1995), and a follow-up study observed increases in overall T cell numbers and various T cell subsets in childhood (Weisglas-Kuperus et al., 2000). Further, Swedish infants with the highest prenatal exposure to PCBs 28, 52, and 101 had increased mean counts of lymphocytes and monocytes (Glynn et al., 2008), and children from a PCB-contaminated area in Slovakia demonstrated altered lymphocyte profiles (Horváthová et al., 2011). It is important to note that circulating cytokines may also be produced by nonimmune cells such as epithelial, endothelial, and adipose cells, all of which have shown relevant effects from PCB exposure, including increased transcription of inflammatory cytokines following exposure to legacy congeners in human endothelial cells (Liu et al., 2015) and adipose cells (Kim et al., 2012) and increased production of reactive oxygen species and cytotoxicity in human prostate epithelial cells exposed to a hydroxylated metabolite of PCB 11 (Zhu et al., 2013). Developmental exposure to the MARBLES PCB mix in mice also increased Il6, Il1β, Il12, and Il22 transcript levels in the gastrointestinal tract of juvenile male and female WT and DM offspring (Rude et al., 2019). These coincide with IL-12 increases in the serum of MARBLES PCB-exposed animals (in all 4 genotypes), although IL-22 was not measured in our samples. Overall, these findings suggest that PCBs increase baseline inflammatory markers in an age-, cell-, and congener-dependent manner, which may impair or alter immune responses to inflammatory challenges (Bell et al., 2018; Badry et al., 2020).
In contrast to observations of PCB effects on serum cytokines, no changes were found in cytokine levels in hippocampal tissue following developmental exposure to the MARBLES PCB mix. The lack of PCB effect on hippocampal cytokines was not because PCBs did not distribute to the developing brain. Analysis of PCB levels in brain tissue of littermates of the mice used in this study revealed dose-dependent PCB levels in the hippocampus of juvenile mice exposed to the MARBLES mix through the maternal diet that were not influenced by sex or genotype (Sethi et al., under review). Other studies have reported evidence of PCB distribution to brain tissue in offspring following developmental exposure (Kania-Korwel et al., 2017), with no effect of sex on brain levels of PCBs (Miller et al., 2010). There is also evidence of congener-specific tissue distribution following dietary exposure to PCBs (Kania-Korwel et al., 2017; Sipka et al., 2008), which may contribute to the observed differences in PCB effects on cytokine levels in serum versus the hippocampus. Previously, elevated levels of mcp1/ccl2 mRNA were found in the brains of adult male mice 24 and 48 h after oral administration of PCBs 77 and 104, but not after PCB 153, even though PCB 153 accumulated in the brain to a greater extent than the other two congeners (Sipka et al., 2008). Another study using a mouse model of developmental PCB exposure to compare disposition of PCBs 95 and 136 and their hydroxylated metabolites demonstrated generally higher levels of parent compounds in the brain than in the blood but higher levels of the sum of hydroxylated metabolites in whole blood of pups at P21 (Kania-Korwel et al., 2017), which might further contribute to the differential effects of PCB exposure on cytokine levels in the hippocampus and periphery. Furthermore, there is evidence to suggest long-term effects of PCB exposure on peripheral cytokine levels in humans, as victims of an accidental PCB poisoning through ingestion of contaminated rice bran oil showed higher serum levels of IL-17, IL-1β, TNF-α, and IL-23 than controls >40 years after the incident (Kuwatsuka et al., 2014). This suggests the possibility that developmental PCB exposure could create chronic changes in serum cytokines of offspring.
In the brain, cytokines are likely produced locally by glial cells (Lee et al., 2002; Hanisch, 2002; Choi et al., 2014; Dong and Benveniste, 2001), which may not be as susceptible to chronic effects of developmental PCB exposures. In addition, there are resident immune cell populations capable of cytokine production in the developing brain, such as mast cells in the hippocampus of P0 rats (Joshi et al., 2019), with unique functions as compared to their peripheral counterparts (Arcuri et al., 2019) that may also alter their response to PCBs. However, our results do not rule out the possibility of changes in highly localized cytokine concentrations in hippocampal tissue or in other brain regions due to PCBs. IL-6 was increased in the hypothalamus of adult female offspring (P145-147 rats) following developmental exposure through diet to a PCB mixture based on blood profiles of Inuit mothers in Arctic Canada that included PCB congeners 28, 52, 101, 118, 138, 153, and 180 in common with the MARBLES mix (Hayley et al., 2011). Other studies have found changes in brain cytokine transcripts following exposure to legacy mixtures of PCBs. This includes a study using adult male rats dosed intraperitoneally (2 mg/kg) with Aroclor 1254 that found increased Tnfα and Il6 transcript levels in brain tissue following 30 days of exposure (Sumathi et al., 2016). In a preclinical model of gestational PCB exposure through diet, a mixture of Aroclors increased mRNA expression of Tnfα in male hypothalamus and exerted sex-dependent effects on LPS challenge but had no main effect on serum cytokines in P1 rats (Bell et al., 2018). Whether these transcriptional changes are predictive of changes at the protein level has yet to be determined. Overall, these results are contrary to our findings but may reflect differences in age (Kania-Korwel et al., 2017), species, brain region, and/or the PCB exposure paradigm (congener profile or dose).
Genotype had a significant effect on basal cytokine levels in hippocampal tissue that was not further altered by PCB exposure. The T4826I genotype was associated with increased hippocampal cytokine levels relative to the CGG and DM genotypes, and in some cases, relative to WT as well. It is quite interesting that the DM animals were more similar to CGG animals in terms of hippocampal cytokine concentrations, particularly because they were more similar to T4826I animals with respect to serum cytokines, especially those of the TH1 category (IFN-γ, IL-12p40). This suggests differential effects of these genetic mutations on peripheral immune cells (Careaga et al., 2014; Bracci et al., 2007; Guse and Wolf, 2016) versus cytokine-producing cells in the hippocampus (Hodges et al., 2017; Reyes and Parpura, 2009; Klegeris et al., 2007). Furthermore, the lack of PCB effect on hippocampal cytokines, particularly in WT animals, is even more surprising given the large influence of the RYR1 mutation on hippocampal cytokine levels. Calcium released from internal stores via RyR activity is critical for cell signaling and function in neurons (Abu-Omar et al., 2018; Brini et al., 2014; Mellentin et al., 2007), glia (Reyes and Parpura, 2009; Klegeris et al., 2007), and immune (Wolf et al., 2015; Bracci et al., 2007; Trebak and Kinet, 2019) cells, including IL-2 production by T cells (Dadsetan et al., 2008). While the PCB mixture used in this study has been shown to increase RyR1 activity by 4-fold in WT, RyR1-enriched microsomal preparations from rabbit skeletal muscle (Sethi et al., 2019), this may not be enough to cause the calcium dysregulation needed to alter cytokine production as compared to homozygous mutations in RYR1. In skeletal muscle preparations from adult homozygous T4826I mice, the RyR1 channel open probability is increased 15-fold and resting cytoplasmic calcium levels are chronically elevated compared with WT (Barrientos et al., 2012). The FMR1 premutation also increases resting intracellular calcium concentrations and alters patterns of spontaneous calcium oscillations in hippocampal neurons (Cao et al., 2012) and cortical astrocytes (Cao et al., 2013) from mice, suggesting that calcium signaling may be too impaired in the mutant genotypes to be further altered by PCBs. It is also possible that PCBs may be acting primarily through pathways such as nuclear factor-kappa B (NF-κB) to modulate peripheral immune function (Kwon et al., 2002; Santoro et al., 2015; Wu et al., 2017).
Although we observed more variable effects of genotype on serum cytokine levels, other studies of RYR1 gain-of-function mutations and FMR1 premutation on immune function are consistent with our findings in hippocampal tissue. A different gain-of-function mutation in RYR1 (HET RYR1Y522S) resulted in a more active immune system in 8–10 week-old mice, including increased antibody levels and more efficient antigen presentation by dendritic cells (Vukcevic et al., 2013). Similarly, peripheral blood mononuclear cells (PBMCs) from patients with the malignant hyperthermia-linked gain-of-function V2168M RYR1 mutation have increased IL-1β production when stimulated with RyR agonists (Girard et al., 2001). In human female FMR1 premutation carriers, increased CGG repeat length is associated with decreased cytokine production at both baseline and following stimulation of monocytes and leukocytes (Careaga et al., 2014). This negative association is also observed in stimulated splenocytes from both sexes of 6-month-old CGG knock-in mice (Careaga et al., 2014). However, in PBMCs from human male FMR1 premutation carriers, increased secretion of the immunosuppressive cytokine IL-10 (but not inflammatory IL-6 or IL-8) is correlated with number of CGG repeats (Marek et al., 2012). Finally, adult male Fmr1 knock-out mice show reduced baseline expression of Il 6 and Tnf α in hippocampal tissue but transcript levels of Il 1β, Ifn γ, Il 10, and Mcp 1 are not different (Hodges et al., 2017). Collectively, these data indicate that CGG repeat expansions in FMR1 depress immune function and gain-of-function mutations in RYR1 increase immune function in both males and females of multiple species, although the exact nature of these changes vary between studies, likely owing to a wide range of ages and outcomes examined. This may also explain the lack of an additive effect of both mutations on cytokine concentrations in the DM group, as the FMR1 and RYR1 mutations had opposite effects on IFN-γ and IL-12p40 levels in the serum and for 13 of the 14 cytokines with genotype differences in the hippocampus.
It is well established that there are inherent sex differences in the rate of mouse brain maturation (McCarthy et al., 2018), and the neuroimmune system has been implicated in the regulation of this process (Nelson and Lenz, 2017; Lenz et al., 2018). In addition, the female brain remains responsive to the programming effects of steroid hormones after birth (McCarthy et al., 2018), which is significant as birth represents a shift in PCB exposure from gestational to lactational. How PCBs influence the sexual differentiation of the brain and whether these effects occur during specific developmental windows remains to be determined. Overall, differences in sexual differentiation of the brain could contribute to the sex and PCB effects on cytokine levels and other endpoints observed here. Sexual dimorphisms in immunity have also been widely observed, with both hormones and genetic factors associated with sex chromosomes contributing to these differences (Jaillon et al., 2019). Evidence indicates that estrogens and androgens influence the differentiation, lifespan, and effector functions of immune cells, often with opposing roles during immune system development and activation (Jørgensen, 2015; Kovats, 2015; Trigunaite et al., 2015). PCBs have also been shown to act as endocrine disruptors with some exhibiting estrogenic activity (Jansen et al., 1993). In our study, differences due to sex were observed in the anogenital distance of P7 animals and in body weights and cytokine levels of juvenile animals. However, in contrast to the findings for the vast majority of cytokines, interactions with PCB dose were observed for both P7 anogenital distance and juvenile body mass. These results raise the possibility that PCB-induced changes in estrogen signaling may contribute to the changes in body mass or the profile of cytokines analyzed here, a possibility to be examined in future studies.
Juvenile male mice had increased levels of 19 cytokines in the serum that overlapped with 4 cytokines (IL-1α, IL-6, IL-12p40, and CCL11) in the hippocampus. Our findings of elevated basal levels of cytokines in juvenile male mice compared to females are consistent with previous descriptions of human males producing stronger inflammatory responses than females prior to puberty (Klein and Flanagan, 2016), although most studies have examined sex differences in the context of an immune challenge. In whole blood samples from healthy prepubescent children, males have increased production of IL-1 and IL-6 relative to females following stimulation with a mitogen for monocytes (Casimir et al., 2010). In a study examining the effects of neonatal (P4) E. coli infection on subsequent lipopolysaccharide (LPS) challenge in juvenile (P24) rats, sex differences were observed in cytokine expression from prefrontal cortex, but not from hippocampus or spleen at 4 h post-challenge, and baseline sex differences in uninfected control animals were not noted in any tissue (Osborne et al., 2017). As immune challenge leads to a massive induction of cytokine production, it is possible that baseline differences are comparatively too subtle to detect. However, a recent report details sex and strain differences seen prior to an immune challenge in serum and brain tissue from P12-15 rats (Bruce et al., 2019), including increased cortical IL-1β, IL-17, IL-4, and IL-10, increased hippocampal IFN-γ and IL-1β, and increased serum MCP-1 in males. In that study (Bruce et al., 2019), more sex differences were observed in brain cytokines than serum, but this could be due to age (Garay et al., 2013) and species differences as compared with our mouse model. The elevated immune activity in males as compared to females early in life is hypothesized to contribute to male bias and sex differences observed in ASD (Kopec et al., 2018; McCarthy, 2019).
A question to be explored in future studies is the functional consequences of these baseline cytokines changes. Human epidemiological studies suggest functional immune changes in infancy and childhood as a result of prenatal PCB exposure, such as reduced antibody responses to vaccines and increased susceptibility to respiratory tract infections (Stølevik et al., 2013; Glynn et al., 2008; Dallaire et al., 2004; Weisglas-Kuperus et al., 2000), although cytokine differences were not examined in these studies. Altered baseline cytokines may shift important regulatory balances through influences on immune cells such as macrophages and T helper cell populations, thus altering the response to an immune challenge. There is also evidence that cytokines can alter blood brain barrier permeability (Almutairi et al., 2016), disruptions of which have been implicated in a number of neurological conditions (Moretti et al., 2015). It is further possible that PCB exposure, genotype, or sex may alter the relationship between serum and hippocampal cytokines. The results of our exploratory regression analysis suggest that PCB exposure may change the correlation of IL-1α between the brain and periphery, although these relationships and their significance will also need to be evaluated further in future studies.
The hippocampus is an important brain region for learning and memory (Albani et al., 2014), and hippocampal-dependent contextual learning is thought to begin around P24 (juvenile stage) in rodents (Schiffino et al., 2011). Cytokines impact the development of rodent hippocampal neurons at multiple stages, with effects on cell survival (Araujo and Cotman, 1993), differentiation (Mehler et al., 1993; Green et al., 2012), neurite outgrowth and morphology (Sarder et al., 1996; Neumann et al., 2002; Shen et al., 2010), and synapse formation and plasticity (Vikman et al., 2001; Vereyken et al., 2007; Maggio and Vlachos, 2018). Cytokines also influence behavior (Wei et al., 2012; Petitto et al., 1999; Nelson and Lenz, 2017; Kelley et al., 2003), and cytokine dysregulation in both the brain and periphery is noted frequently in ASD (Saghazadeh et al., 2019; Gładysz et al., 2018). Cytokines may be involved in the pathogenesis of NDDs, and their usefulness as biomarkers in ASD is being explored (Ashwood et al., 2011; Masi et al., 2017; Xu et al., 2015; Jones et al., 2017; Krakowiak et al., 2017). It seems unlikely that PCB-mediated developmental neurotoxicity is occurring through cytokine dysregulation in the juvenile hippocampus; however, PCB-induced cytokine alterations at other ages or in different brain regions cannot be ruled out. In addition, the relevance of PCB-induced peripheral cytokine alterations on brain development, behavior, and immune responses will need to be further explored.
5. Conclusions
Serum cytokines and chemokines in juvenile mice were influenced by sex, PCB dose, and genotype, while hippocampal cytokines and chemokines were influenced by genotype and sex but not developmental PCB exposure. Contrary to our expectations, the mutant genotypes were not more affected by developmental PCB exposure than the WT animals in terms of cytokine changes for the majority of those examined in this study. The differential influences of PCB dose, sex, and genotype imply different mechanisms regulating cytokine production between the brain and periphery and also demonstrate that the influences of these factors on cytokines in the serum and brain are not necessarily correlated. The biological significance and functional consequences of these changes will need to be explored in future studies. These results suggest that developmental PCB exposure has lasting effects on baseline peripheral, but not hippocampal, cytokine levels in juvenile mice.
The following are the supplementary data related to this article.
Supplementary material containing Supplementary Figs. 1–4 (1 - age distribution of animals used in this study; 2 - number of days from first meeting to parturition and dam mass at weaning; 3 - anogenital distance in P7 male mice; 4 - body mass in juvenile mice) and Supplementary Tables 4–6 (4 - significant pairwise comparisons for CCL5/RANTES; 5 - significant pairwise comparisons for hipocampal cytokines and chemokines; 6 - significant interactions that may influence the relationship between serum and hippocampal cytokine concentrations).
Additional details for animals included in serum and hippocampal cytokine analysis.
Cytokine multiplex assay details.
Summary of dam pregnancy outcomes.
List of abbreviations
- CGG
mice which express the human X-linked CGG premutation repeat expansion in the FMR1 gene
- DM
double mutation mice which express the FMR1 and RYR1 mutations
- MARBLES
Markers of Autism Risk in Babies - Learning Early Signs
- NDDs
neurodevelopmental disorders
- P
postnatal day
- PBMCs
peripheral blood mononuclear cells
- RyR
ryanodine receptor
- T4826I
mice which express a human gain-of-function mutation in RYR1
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by funding from the National Institute of Environmental Health Sciences [R01 ES014901 to PJL, T32 ES007059 to SS, P01 ES011269 to JV and PJL, and R00 ES029537 to KPKS], the National Institute of Child Health and Human Development [U54 HD079125 to JV and PJL], and the United States Environmental Protection Agency [RD83543201 to JV and PJL]. The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article of publication.
CRediT authorship contribution statement
Lauren Matelski: Conceptualization, Investigation, Validation, Data Curation, Writing - Original Draft, Visualization, Project administration. Kimberly P. Keil Stietz: Conceptualization, Methodology, Investigation, Resources. Sunjay Sethi: Conceptualization, Methodology, Investigation, Resources. Sandra L. Taylor: Software, Formal analysis, Data Curation. Judy Van de Water: Conceptualization, Supervision, Funding acquisition. Pamela J. Lein: Conceptualization, Supervision, Funding acquisition. All: Writing - Review and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Statistical support for this project was provided by the UC Davis MIND Institute's Intellectual and Developmental Disabilities Research Center (IDDRC) Biostatistics, Bioinformatics, and Research Design (BBRD) core (U54 HD079125). The authors wish to thank Lori Haapanen (UC Davis IDDRC Biological Analysis Core (U54 HD079125)) for advice and help with the Luminex data collection and analysis. We also wish to acknowledge Dr. Isaac N. Pessah (UC Davis) for providing the T4826I-RYR1 and double mutant strains of mice and Dr. Xueshu Li and Dr. Hans-Joachim Lehmler (University of Iowa) for synthesis of PCB congeners used in this study (P01 ES013661 to HJL). The graphical abstract for this article was created with BioRender.com.
References
- Abu-Omar N., Das J., Szeto V., Feng Z.-P. Neuronal ryanodine receptors in development and aging. Mol. Neurobiol. 2018;55:1183–1192. doi: 10.1007/s12035-016-0375-4. [DOI] [PubMed] [Google Scholar]
- Ahmed R.G., El-Gareib A.W., Shaker H.M. Gestational 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) exposure disrupts fetoplacental unit: fetal thyroid-cytokines dysfunction. Life Sci. 2018;192:213–220. doi: 10.1016/j.lfs.2017.11.033. [DOI] [PubMed] [Google Scholar]
- Albani S.H., McHail D.G., Dumas T.C. Developmental studies of the hippocampus and hippocampal-dependent behaviors: insights from interdisciplinary studies and tips for new investigators. Neurosci. Biobehav. Rev. 2014;43:183–190. doi: 10.1016/j.neubiorev.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutairi M.M.A., Gong C., Xu Y.G., Chang Y., Shi H. Factors controlling permeability of the blood-brain barrier. Cell. Mol. Life Sci. 2016;73:57–77. doi: 10.1007/s00018-015-2050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampleman M.D., Martinez A., DeWall J., Rawn D.F.K., Hornbuckle K.C., Thorne P.S. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ. Sci. Technol. 2015;49:1156–1164. doi: 10.1021/es5048039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anezaki K., Nakano T. Concentration levels and congener profiles of polychlorinated biphenyls, pentachlorobenzene, and hexachlorobenzene in commercial pigments. Environ. Sci. Pollut. Res. 2014;21:998–1009. doi: 10.1007/s11356-013-1977-2. [DOI] [PubMed] [Google Scholar]
- Araujo D.M., Cotman C.W. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. http://www.ncbi.nlm.nih.gov/pubmed/8422590 [DOI] [PubMed] [Google Scholar]
- Arcuri C., Mecca C., Giambanco I., Donato R. Parenchymal and non-parenchymal immune cells in the brain: a critical role in regulating CNS functions. Int. J. Dev. Neurosci. 2019;77:26–38. doi: 10.1016/j.ijdevneu.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N. Measurement of circulating cytokines and immune-activation markers by multiplex technology in the clinical setting: what are we really measuring? For. Immunopathol. Dis. Therap. 2015;6:19–22. doi: 10.1615/ForumImmunDisTher.2015014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars A.J., Bakker M.I., Baumann R.A., Boon P.E., Freijer J.I., Hoogenboom L.A.P., Hoogerbrugge R., van Klaveren J.D., Liem A.K.D., Traag W.A., de Vries J. Dioxins, dioxin-like PCBs and non-dioxin-like PCBs in foodstuffs: occurrence and dietary intake in The Netherlands. Toxicol. Lett. 2004;151:51–61. doi: 10.1016/j.toxlet.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Badry A., Jaspers V.L.B., Waugh C.A. Environmental pollutants modulate RNA and DNA virus-activated miRNA-155 expression and innate immune system responses: insights into new immunomodulative mechanisms. J. Immunotoxicol. 2020;17:86–93. doi: 10.1080/1547691X.2020.1740838. [DOI] [PubMed] [Google Scholar]
- Barrientos G.C., Feng W., Truong K., Matthaei K.I., Yang T., Allen P.D., Lopez J.R., Pessah I.N. Gene dose influences cellular and calcium channel dysregulation in heterozygous and homozygous T4826I-RYR1 malignant hyperthermia-susceptible muscle. J. Biol. Chem. 2012;287:2863–2876. doi: 10.1074/jbc.M111.307926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett P.W., Isaksson E., Hermanson M.H. ‘New’ unintentionally produced PCBs in the Arctic. Emerg. Contam. 2019;5:9–14. doi: 10.1016/j.emcon.2018.12.004. [DOI] [Google Scholar]
- Basra K., Scammell M.K., Benson E.B., Heiger-Bernays W. Ambient air exposure to PCBs: regulation and monitoring at five contaminated sites in EPA regions 1, 2, 4, and 5. New Solut. 2018;28:262–282. doi: 10.1177/1048291118763620. [DOI] [PubMed] [Google Scholar]
- Bell M.R., Dryden A., Will R., Gore A.C. Sex differences in effects of gestational polychlorinated biphenyl exposure on hypothalamic neuroimmune and neuromodulator systems in neonatal rats. Toxicol. Appl. Pharmacol. 2018;353:55–66. doi: 10.1016/j.taap.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdolt L., Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog. Neurobiol. 2019;175:1–19. doi: 10.1016/j.pneurobio.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R.F., Buijsen R.A., Usdin K., Pintado E., Kooy F., Pretto D., Pessah I.N., Nelson D.L., Zalewski Z., Charlet-Bergeurand N., Willemsen R., Hukema R.K. Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J. Neurodev. Disord. 2014;6:25. doi: 10.1186/1866-1955-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F., Berntsen H.F., Walaas S.I., Bogen I.L. Low-chlorinated non-dioxin-like polychlorinated biphenyls present in blood and breast milk induce higher levels of reactive oxygen species in neutrophil granulocytes than high-chlorinated congeners. Basic Clin. Pharmacol. Toxicol. 2016;119:588–597. doi: 10.1111/bcpt.12620. [DOI] [PubMed] [Google Scholar]
- Bilrha H., Roy R., Moreau B., Belles-Isles M., Dewailly E., Ayotte P. In vitro activation of cord blood mononuclear cells and cytokine production in a remote coastal population exposed to organochlorines and methyl mercury. Environ. Health Perspect. 2003;111:1952–1957. doi: 10.1289/ehp.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölte S., Girdler S., Marschik P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019;76:1275–1297. doi: 10.1007/s00018-018-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini A., Zunszain P.A., Thuret S., Pariante C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38:145–157. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Bracci L., Vukcevic M., Spagnoli G., Ducreux S., Zorzato F., Treves S. Ca2+ signaling through ryanodine receptor 1 enhances maturation and activation of human dendritic cells. J. Cell Sci. 2007;120:2232–2240. doi: 10.1242/jcs.007203. [DOI] [PubMed] [Google Scholar]
- Brini M., Calì T., Ottolini D., Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell. Mol. Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M., Streifel K.M., Boosalis C.A., Heuer L., González E.A., Li S., Harvey D.J., Lein P.J., Van de Water J. Acute peripheral immune activation alters cytokine expression and glial activation in the early postnatal rat brain. J. Neuroinflammation. 2019;16:200. doi: 10.1186/s12974-019-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Hulsizer S., Tassone F., Tang H., Hagerman R.J., Rogawski M.A., Hagerman P.J., Pessah I.N. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum. Mol. Genet. 2012;21:2923–2935. doi: 10.1093/hmg/dds118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Hulsizer S., Cui Y., Pretto D.L., Kim K.H., Hagerman P.J., Tassone F., Pessah I.N. Enhanced asynchronous Ca(2+) oscillations associated with impaired glutamate transport in cortical astrocytes expressing Fmr1 gene premutation expansion. J. Biol. Chem. 2013;288:13831–13841. doi: 10.1074/jbc.M112.441055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M., Rose D., Tassone F., Berman R.F., Hagerman R., Ashwood P. Immune dysregulation as a cause of autoinflammation in fragile X premutation carriers: link between FMRI CGG repeat number and decreased cytokine responses. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0094475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C.J., Blizard R.A. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochem. Int. 2016;101:83–109. doi: 10.1016/j.neuint.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Casimir G.J., Heldenbergh F., Hanssens L., Mulier S., Heinrichs C., Lefevre N., Désir J., Corazza F., Duchateau J. Gender differences and inflammation: an in vitro model of blood cells stimulation in prepubescent children. J. Inflamm. (Lond). 2010;7:28. doi: 10.1186/1476-9255-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Lin Y., Dang K., Puschner B. Quantification of polychlorinated biphenyls and polybrominated diphenyl ethers in commercial cows’ milk from California by gas chromatography–triple quadruple mass spectrometry. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm S.P., Cervi A.L., Nagpal S., Lomax A.E. Interleukin-17A increases neurite outgrowth from adult postganglionic sympathetic neurons. J. Neurosci. 2012;32:1146–1155. doi: 10.1523/JNEUROSCI.5343-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.D., Baek S.Y., Chang Y.S., Wania F., Ikonomou M.G., Yoon Y.J., Park B.K., Hong S. Passive air sampling of polychlorinated biphenyls and organochlorine pesticides at the Korean arctic and antarctic research stations: implications for long-range transport and local pollution. Environ. Sci. Technol. 2008;42:7125–7131. doi: 10.1021/es801004p. [DOI] [PubMed] [Google Scholar]
- Choi J.J., Choi Y.J., Chen L., Zhang B., Eum S.Y., Abreu M.T., Toborek M. Lipopolysaccharide potentiates polychlorinated biphenyl-induced disruption of the blood-brain barrier via TLR4/IRF-3 signaling. Toxicology. 2012;302:212–220. doi: 10.1016/j.tox.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.S., Lee H.J., Lim I., Satoh J.I., Kim S.U. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimenci O., Vandevijvere S., Goscinny S., Van Den Bergh M.-A., Hanot V., Vinkx C., Bolle F., Van Loco J. Dietary exposure of the Belgian adult population to non-dioxin-like PCBs. Food Chem. Toxicol. 2013;59:670–679. doi: 10.1016/j.fct.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Cooper R.A., Richter F.R., Bays P.M., Plaisted-Grant K.C., Baron-Cohen S., Simons J.S. Reduced hippocampal functional connectivity during episodic memory retrieval in autism. Cereb. Cortex. 2017;27:888–902. doi: 10.1093/cercor/bhw417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadsetan S., Zakharova L., Molinski T.F., Fomina A.F. Store-operated Ca2+ influx causes Ca2+ release from the intracellular Ca2+ channels that is required for T cell activation. J. Biol. Chem. 2008;283:12512–12519. doi: 10.1074/jbc.M709330200. [DOI] [PubMed] [Google Scholar]
- Dallaire F., Dewailly E., Muckle G., Vézina C., Jacobson S.W., Jacobson J.L., Ayotte P. Acute infections and environmental exposure to organochlorines in Inuit infants from Nunavik. Environ. Health Perspect. 2004;112:1359–1365. doi: 10.1289/ehp.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert R.R. Developmental immunotoxicity, perinatal programming, and noncommunicable diseases: focus on human studies. Adv. Med. 2014;2014 doi: 10.1155/2014/867805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert R.R., Dietert J.M. Potential for early-life immune insult including developmental immunotoxicity in autism and autism spectrum disorders: focus on critical windows of immune vulnerability. J. Toxicol. Environ. Heal. - Part B Crit. Rev. 2008;11:660–680. doi: 10.1080/10937400802370923. [DOI] [PubMed] [Google Scholar]
- Dong Y., Benveniste E.N. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Moieni M., Inagaki T.K., Muscatell K.A., Irwin M.R. In sickness and in health: the co-regulation of inflammation and social behavior. Neuropsychopharmacology. 2017;42:242–253. doi: 10.1038/npp.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroon O., Ruiz P. Polychlorinated biphenyls: new evidence from the last decade. Toxicol. Ind. Health. 2016;32:1825–1847. doi: 10.1177/0748233715587849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focant J.-F., Fréry N., Bidondo M.-L., Eppe G., Scholl G., Saoudi A., Oleko A., Vandentorren S. Levels of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans and polychlorinated biphenyls in human milk from different regions of France. Sci. Total Environ. 2013;452–453:155–162. doi: 10.1016/j.scitotenv.2013.02.057. [DOI] [PubMed] [Google Scholar]
- Garay P.A., Hsiao E.Y., Patterson P.H., McAllister A.K. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Ramírez O., Pérez-Vázquez F.J., Pruneda-Álvarez L.G., Orta-García S.T., González-Amaro R., Pérez-Maldonado I.N. Effect of polychlorinated biphenyls 118 and 153 on Th1/Th2 cells differentiation. Immunopharmacol. Immunotoxicol. 2012;34:627–632. doi: 10.3109/08923973.2011.648265. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Zang S., Mitra P.S., Ghimbovschi S., Hoffman E.P., Dutta S.K. Global gene expression and ingenuity biological functions analysis on PCBs 153 and 138 induced human PBMC in vitro reveals differential mode(s) of action in developing toxicities. Environ. Int. 2011;37:838–857. doi: 10.1016/j.envint.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard T., Cavagna D., Padovan E., Spagnoli G., Urwyler A., Zorzato F., Treves S. B-lymphocytes from malignant hyperthermia-susceptible patients have an increased sensitivity to skeletal muscle ryanodine receptor activators. J. Biol. Chem. 2001;276:48077–48082. doi: 10.1074/jbc.M107134200. [DOI] [PubMed] [Google Scholar]
- Gładysz D., Krzywdzińska A., Hozyasz K.K. Immune abnormalities in autism spectrum disorder-could they hold promise for causative treatment? Mol. Neurobiol. 2018;55:6387–6435. doi: 10.1007/s12035-017-0822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A., Thuvander A., Aune M., Johannisson A., Darnerud P.O., Ronquist G., Cnattingius S. Immune cell counts and risks of respiratory infections among infants exposed pre- and postnatally to organochlorine compounds: a prospective study. Environ. Health. 2008;7:62. doi: 10.1186/1476-069X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P.E., Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol. Teratol. 2013;36:67–81. doi: 10.1016/j.ntt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H.F., Treacy E., Keohane A.K., Sullivan A.M., O’Keeffe G.W., Nolan Y.M. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol. Cell. Neurosci. 2012;49:311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Guidolin D., Fede C., Tortorella C. Nerve cells developmental processes and the dynamic role of cytokine signaling. Int. J. Dev. Neurosci. 2018:1–15. doi: 10.1016/j.ijdevneu.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Guo J., Capozzi S.L., Kraeutler T.M., Rodenburg L.A. Global distribution and local impacts of inadvertently generated polychlorinated biphenyls in pigments. Environ. Sci. Technol. 2014;48:8573–8580. doi: 10.1021/es502291b. [DOI] [PubMed] [Google Scholar]
- Guse A.H., Wolf I.M.A. Ca2+ microdomains, NAADP and type 1 ryanodine receptor in cell activation. Biochim. Biophys. Acta - Mol. Cell Res. 2016;1863:1379–1384. doi: 10.1016/j.bbamcr.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Hagerman R., Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12:786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R., Au J., Hagerman P. FMR1 premutation and full mutation molecular mechanisms related to autism. J. Neurodev. Disord. 2011;3:211–224. doi: 10.1007/s11689-011-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U.K. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hayley S., Mangano E., Crowe G., Li N., Bowers W.J. An in vivo animal study assessing long-term changes in hypothalamic cytokines following perinatal exposure to a chemical mixture based on Arctic maternal body burden. Environ. Health. 2011;10:65. doi: 10.1186/1476-069X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C., Grandjean P., Weihe P., Nielsen F., Budtz-Jørgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3:1352–1359. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C., Budtz-Jørgensen E., Nielsen F., Heinzow B., Weihe P., Grandjean P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ. Health Perspect. 2010;118:1434–1438. doi: 10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J., Kim D., Lee G. Congener profiles and source-wise phase partitioning analysis of PCDDs/Fs and PCBs in Gyeonggi-do ambient air, South Korea. Int. J. Environ. Res. Public Health. 2014;11:11065–11080. doi: 10.3390/ijerph111111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I., Schmidt R.J., Walker C.K., Bennett D.H., Oliver M., Shedd-Wise K.M., LaSalle J.M., Giulivi C., Puschner B., Thomas J., Roa D.L., Pessah I.N., Van de Water J., Tancredi D.J., Ozonoff S. A prospective study of environmental exposures and early biomarkers in autism spectrum disorder: design, protocols, and preliminary data from the MARBLES study. Environ. Health Perspect. 2018;126 doi: 10.1289/EHP535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach K., van Leeuwen D.M., Gmuender H., Gottschalk R.W., Stølevik S.B., Nygaard U.C., Løvik M., Granum B., Namork E., Meltzer H.M., Kleinjans J.C., van Delft J.H.M., van Loveren H. Toxicogenomic profiles in relation to maternal immunotoxic exposure and immune functionality in newborns. Toxicol. Sci. 2012;129:315–324. doi: 10.1093/toxsci/kfs214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges S.L., Nolan S.O., Taube J.H., Lugo J.N. Adult Fmr1 knockout mice present with deficiencies in hippocampal interleukin-6 and tumor necrosis factor-α expression. Neuroreport. 2017;28:1246–1249. doi: 10.1097/WNR.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváthová M., Jahnová E., Palkovičová L., Trnovec T., Hertz-Picciotto I. Dynamics of lymphocyte subsets in children living in an area polluted by polychlorinated biphenyls. J. Immunotoxicol. 2011;8:333–345. doi: 10.3109/1547691X.2011.615767. [DOI] [PubMed] [Google Scholar]
- Hu D., Hornbuckle K.C. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010;44:2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Martinez A., Hornbuckle K.C. Discovery of non-aroclor PCB (3,3′-dichlorobiphenyl) in Chicago air. Environ. Sci. Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H.K., Mills Ko E., Rose D., Ashwood P. Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Front. Cell. Neurosci. 2018;12 doi: 10.3389/fncel.2018.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon S., Berthenet K., Garlanda C. Sexual dimorphism in innate immunity. Clin. Rev. Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- Jansen H.T., Cooke P.S., Porcelli J., Liu T.C., Hansen L.G. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod. Toxicol. 1993;7:237–248. doi: 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- Jones K.L., Croen L.A., Yoshida C.K., Heuer L., Hansen R., Zerbo O., Delorenze G.N., Kharrazi M., Yolken R., Ashwood P., Van De Water J. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol. Psychiatry. 2017;22:273–279. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen T.N. Sex disparities in the immune response. Cell. Immunol. 2015;294:61–62. doi: 10.1016/j.cellimm.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Joshi A., Page C.E., Damante M., Dye C.N., Haim A., Leuner B., Lenz K.M. Sex differences in the effects of early life stress exposure on mast cells in the developing rat brain. Horm. Behav. 2019;113:76–84. doi: 10.1016/j.yhbeh.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner A.E., Schmidt R.J., Penlesky A.C. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Curr. Probl. Pediatr. Adolesc. Health Care. 2014;44:277–318. doi: 10.1016/j.cppeds.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Lukasiewicz T., Barnhart C.D., Stamou M., Chung H., Kelly K.M., Bandiera S., Lein P.J., Lehmler H.-J. Editor’s highlight: congener-specific disposition of chiral polychlorinated biphenyls in lactating mice and their offspring: implications for PCB developmental neurotoxicity. Toxicol. Sci. 2017;158:101–115. doi: 10.1093/toxsci/kfx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil K.P., Sethi S., Wilson M.D., Silverman J.L., Pessah I.N., Lein P.J. Genetic mutations in Ca2+ signaling alter dendrite morphology and social approach in juvenile mice, Genes. Brain Behav. 2019;18:16–20. doi: 10.1111/gbb.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K.W., Bluthé R.-M., Dantzer R., Zhou J.-H., Shen W.-H., Johnson R.W., Broussard S.R. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003;17(Suppl. 1):S112–S118. doi: 10.1016/s0889-1591(02)00077-6. (http://www.ncbi.nlm.nih.gov/pubmed/12615196) [DOI] [PubMed] [Google Scholar]
- Kim M.J., Pelloux V., Guyot E., Tordjman J., Bui L.-C., Chevallier A., Forest C., Benelli C., Clément K., Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ. Health Perspect. 2012;120:508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Jarvik G.P., Browning B.L., Rajagopalan R., Gordon A.S., Rieder M.J., Robertson P.D., Nickerson D.A., Fisher N.A., Hopkins P.M. Exome sequencing reveals novel rare variants in the ryanodine receptor and calcium channel genes in malignant hyperthermia families. Anesthesiology. 2013;119:1054–1065. doi: 10.1097/ALN.0b013e3182a8a998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A., Choi H.B., McLarnon J.G., McGeer P.L. Functional ryanodine receptors are expressed by human microglia and THP-1 cells: their possible involvement in modulation of neurotoxicity. J. Neurosci. Res. 2007;85:2207–2215. doi: 10.1002/jnr.21361. [DOI] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Klocke C., Lein P.J. Evidence implicating non-dioxin-like congeners as the key mediators of polychlorinated biphenyl (PCB) developmental neurotoxicity. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke C., Sethi S., Lein P.J. The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): similarities and differences. Environ. Sci. Pollut. Res. Int. 2020;9:8885–8896. doi: 10.1007/s11356-019-06723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti P.R., Kannan N., Yamashita N., Derr-Yellin E.C., Ward T.R., Burgin D.E., Tilson H.A., Birnbaum L.S. Differential effects of two lots of aroclor 1254: congener-specific analysis and neurochemical end points. Environ. Health Perspect. 2001;109:1153–1161. doi: 10.1289/ehp.011091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W.X., Hornbuckle K.C., Thorne P.S. Human serum from urban and rural adolescents and their mothers shows exposure to polychlorinated biphenyls not found in commercial mixtures. Environ. Sci. Technol. 2015;49:8105–8112. doi: 10.1021/acs.est.5b01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec A.M., Fiorentino M.R., Bilbo S.D. Gut-immune-brain dysfunction in autism: importance of sex. Brain Res. 2018;1693:214–217. doi: 10.1016/j.brainres.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyniak P.J., Hansen L.G., Widholm J.J., Fitzpatrick R.D., Olson J.R., Helferich J.L., Kim K.H., Sable H.J.K., Seegal R.F., Pessah I.N., Schantz S.L. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015;294:63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P., Goines P.E., Tancredi D.J., Ashwood P., Hansen R.L., Hertz-Picciotto I., Van de Water J. Neonatal cytokine profiles associated with autism spectrum disorder. Biol. Psychiatry. 2017;81:442–451. doi: 10.1016/j.biopsych.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey J.F., Dolmetsch R.E. Molecular mechanisms of autism: a possible role for Ca2+ signaling. Curr. Opin. Neurobiol. 2007;17:112–119. doi: 10.1016/j.conb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kuwatsuka Y., Shimizu K., Akiyama Y., Koike Y., Ogawa F., Furue M., Utani A. Yusho patients show increased serum IL-17, IL-23, IL-1β, and TNFα levels more than 40 years after accidental polychlorinated biphenyl poisoning. J. Immunotoxicol. 2014;11:246–249. doi: 10.3109/1547691X.2013.835890. [DOI] [PubMed] [Google Scholar]
- Kwon O., Lee E., Moon T.C., Jung H., Lin C.X., Nam K.-S., Baek S.H., Min H.-K., Chang H.W. Expression of cyclooxygenase-2 and pro-inflammatory cytokines induced by 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153) in human mast cells requires NF-kappa B activation. Biol. Pharm. Bull. 2002;25:1165–1168. doi: 10.1248/bpb.25.1165. http://www.ncbi.nlm.nih.gov/pubmed/12230110 [DOI] [PubMed] [Google Scholar]
- Lee Y.B., Nagai A., Kim S.U. Cytokines, chemokines, and cytokine receptors in human microglia. J. Neurosci. Res. 2002;69:94–103. doi: 10.1002/jnr.10253. [DOI] [PubMed] [Google Scholar]
- Leijs M.M., Gan L., De Boever P., Esser A., Amann P.M., Ziegler P., Fietkau K., Schettgen T., Kraus T., Merk H.F., Baron J.M. Altered gene expression in dioxin-like and non-dioxin-like PCB exposed peripheral blood mononuclear cells. Int. J. Environ. Res. Public Health. 2019;16 doi: 10.3390/ijerph16122090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K.M., Pickett L.A., Wright C.L., Davis K.T., Joshi A., McCarthy M.M. Mast cells in the developing brain determine adult sexual behavior. J. Neurosci. 2018;38:8044–8059. doi: 10.1523/JNEUROSCI.1176-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Morsey B., Mori C., Nambiar P.R., De Guise S. Non-coplanar PCB-mediated modulation of human leukocyte phagocytosis: a new mechanism for immunotoxicity. J. Toxicol. Environ. Health. A. 2005;68:1977–1993. doi: 10.1080/15287390500227126. [DOI] [PubMed] [Google Scholar]
- Liu D., Perkins J.T., Petriello M.C., Hennig B. Exposure to coplanar PCBs induces endothelial cell inflammation through epigenetic regulation of NF-κB subunit p65. Toxicol. Appl. Pharmacol. 2015;289:457–465. doi: 10.1016/j.taap.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N., Vlachos A. Tumor necrosis factor (TNF) modulates synaptic plasticity in a concentration-dependent manner through intracellular calcium stores. J. Mol. Med. 2018;96:1039–1047. doi: 10.1007/s00109-018-1674-1. [DOI] [PubMed] [Google Scholar]
- Malisch R., Kotz A. Dioxins and PCBs in feed and food—review from European perspective. Sci. Total Environ. 2014;491–492:2–10. doi: 10.1016/j.scitotenv.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Mannetje A., Coakley J., Bridgen P., Brooks C., Harrad S., Smith A.H., Pearce N., Douwes J. Current concentrations, temporal trends and determinants of persistent organic pollutants in breast milk of New Zealand women. Sci. Total Environ. 2013;458–460:399–407. doi: 10.1016/j.scitotenv.2013.04.055. [DOI] [PubMed] [Google Scholar]
- Marek D., Papin S., Ellefsen K., Niederhauser J., Isidor N., Ransijn A., Poupon L., Spertini F., Pantaleo G., Bergmann S., Beckmann J.S., Jacquemont S., Tanackovic G. Carriers of the fragile X mental retardation 1 (FMR1) premutation allele present with increased levels of cytokine IL-10. J. Neuroinflammation. 2012;9:1–8. doi: 10.1186/1742-2094-9-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R.F., Thorne P.S., Wang K., Dewall J., Hornbuckle K.C. PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ. Sci. Technol. 2013;47:3353–3361. doi: 10.1021/es304455k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin S., Villalba P., Diaz-Ferrero J., Font G., Yusà V. Congener profile, occurrence and estimated dietary intake of dioxins and dioxin-like PCBs in foods marketed in the Region of Valencia (Spain) Chemosphere. 2011;82:1253–1261. doi: 10.1016/j.chemosphere.2010.12.033. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D., Gattaz W.F., Schmitt A., Rewerts C., Maccarrone G., Dias-Neto E., Turck C.W. Prefrontal cortex shotgun proteome analysis reveals altered calcium homeostasis and immune system imbalance in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2009;259:151–163. doi: 10.1007/s00406-008-0847-2. [DOI] [PubMed] [Google Scholar]
- Masi A., Quintana D.S., Glozier N., Lloyd A.R., Hickie I.B., Guastella A.J. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol. Psychiatry. 2015;20:440–446. doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- Masi A., Glozier N., Dale R., Guastella A.J. The immune system, cytokines, and biomarkers in autism spectrum disorder. Neurosci. Bull. 2017;33:194–204. doi: 10.1007/s12264-017-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.M. Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology. 2019;44:38–44. doi: 10.1038/s41386-018-0138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.M., Herold K., Stockman S.L. Fast, furious and enduring: sensitive versus critical periods in sexual differentiation of the brain. Physiol. Behav. 2018;187:13–19. doi: 10.1016/j.physbeh.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler M.F., Rozental R., Dougherty M., Spray D.C., Kessler J.A. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 1993;362:62–65. doi: 10.1038/362062a0. [DOI] [PubMed] [Google Scholar]
- Mellentin C., Jahnsen H., Abraham W.C. Priming of long-term potentiation mediated by ryanodine receptor activation in rat hippocampal slices. Neuropharmacology. 2007;52:118–125. doi: 10.1016/j.neuropharm.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Meltzer A., Van De Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology. 2017;42:284–298. doi: 10.1038/npp.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V.M., Kahnke T., Neu N., Sanchez-Morrissey S.R., Brosch K., Kelsey K., Seegal R.F. Developmental PCB exposure induces hypothyroxinemia and sex-specific effects on cerebellum glial protein levels in rats. Int. J. Dev. Neurosci. 2010;28:553–560. doi: 10.1016/j.ijdevneu.2010.07.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.M., Woods R., Chi L.-H., Schmidt R.J., Pessah I.N., Kostyniak P.J., LaSalle J.M. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ. Mol. Mutagen. 2012;53:589–598. doi: 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi Y., Monji A. Microglial intracellular Ca2+ signaling in synaptic development and its alterations in neurodevelopmental disorders. Front. Cell. Neurosci. 2017;11:1–9. doi: 10.3389/fncel.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagrin A., Saiote C., Schiller D. The social hippocampus. Hippocampus. 2018;28:672–679. doi: 10.1002/hipo.22797. [DOI] [PubMed] [Google Scholar]
- Moretti R., Pansiot J., Bettati D., Strazielle N., Ghersi-Egea J.-F., Damante G., Fleiss B., Titomanlio L., Gressens P. Blood-brain barrier dysfunction in disorders of the developing brain. Front. Neurosci. 2015;9:40. doi: 10.3389/fnins.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L.L., Grandjean P., Heinzow B., Jørgensen P.J., Nielsen F., Sjödin A., Patterson D.G., Turner W.E., Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol. 2011;45:1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L.H., Lenz K.M. The immune system as a novel regulator of sex differences in brain and behavioral development. J. Neurosci. Res. 2017;95:447–461. doi: 10.1002/jnr.23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H., Schweigreiter R., Yamashita T., Rosenkranz K., Wekerle H., Barde Y.-A. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J. Neurosci. 2002;22:854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne B.F., Caulfield J.I., Solomotis S.A., Schwarz J.M. Neonatal infection produces significant changes in immune function with no associated learning deficits in juvenile rats. Dev. Neurobiol. 2017;77:1221–1236. doi: 10.1002/dneu.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri L., Papaleo V., Porcelli V., Scarcia P., Gaita L., Sacco R., Hager J., Rousseau F., Curatolo P., Manzi B., Militerni R., Bravaccio C., Trillo S., Schneider C., Melmed R., Elia M., Lenti C., Saccani M., Pascucci T., Puglisi-Allegra S., Reichelt K.L., Persico A.M. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol. Psychiatry. 2010;15:38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- Pessah I.N., Lein P.J., Seegal R.F., Sagiv S.K. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol. 2019 doi: 10.1007/s00401-019-01978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto J.M., McNamara R.K., Gendreau P.L., Huang Z., Jackson A.J. Impaired learning and memory and altered hippocampal neurodevelopment resulting from interleukin-2 gene deletion. J. Neurosci. Res. 1999;56:441–446. doi: 10.1002/(SICI)1097-4547(19990515)56:4<441::AID-JNR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Robinson H.A., Pozzo-Miller L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife. 2019;8:1–32. doi: 10.7554/eLife.44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pousset F. Developmental expression of cytokine genes in the cortex and hippocampus of the rat central nervous system. Dev. Brain Res. 1994;81:143–146. doi: 10.1016/0165-3806(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Ravenscroft J., Schell L.M. Patterns of PCB exposure among Akwesasne adolescents: the role of dietary and inhalation pathways. Environ. Int. 2018;121:963–972. doi: 10.1016/j.envint.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Rawn D.F.K., Sadler A.R., Casey V.A., Breton F., Sun W., Arbuckle T.E., Fraser W.D. Dioxins/furans and PCBs in Canadian human milk: 2008–2011. Sci. Total Environ. 2017;595:269–278. doi: 10.1016/j.scitotenv.2017.03.157. [DOI] [PubMed] [Google Scholar]
- Raymond G.V., Bauman M.L., Kemper T.L. Hippocampus in autism: a Golgi analysis. Acta Neuropathol. 1995;91:117–119. doi: 10.1007/s004010050401. [DOI] [PubMed] [Google Scholar]
- Reyes R.C., Parpura V. The trinity of Ca2+ sources for the exocytotic glutamate release from astrocytes. Neurochem. Int. 2009;55:2–8. doi: 10.1016/j.neuint.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R., Carpenter D., Shaw M.-A., Halsall J., Hopkins P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum. Mutat. 2006;27:977–989. doi: 10.1002/humu.20356. [DOI] [PubMed] [Google Scholar]
- Rossignol D.A., Genuis S.J., Frye R.E. Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psychiatry. 2014;4 doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude K.M., Pusceddu M.M., Keogh C.E., Sladek J.A., Rabasa G., Miller E.N., Sethi S., Keil K.P., Pessah I.N., Lein P.J., Gareau M.G. Developmental exposure to polychlorinated biphenyls (PCBs) in the maternal diet causes host-microbe defects in weanling offspring mice. Environ. Pollut. 2019;253:708–721. doi: 10.1016/j.envpol.2019.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh A., Ataeinia B., Keynejad K., Abdolalizadeh A., Hirbod-Mobarakeh A., Rezaei N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: effects of age, gender, and latitude. J. Psychiatr. Res. 2019;115:90–102. doi: 10.1016/j.jpsychires.2019.05.019. [DOI] [PubMed] [Google Scholar]
- Santoro A., Ferrante M.C., Di Guida F., Pirozzi C., Lama A., Simeoli R., Clausi M.T., Monnolo A., Mollica M.P., Mattace Raso G., Meli R. Polychlorinated biphenyls (PCB 101, 153, and 180) impair murine macrophage responsiveness to lipopolysaccharide: involvement of NF-κB pathway. Toxicol. Sci. 2015;147:255–269. doi: 10.1093/toxsci/kfv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarder M., Abe K., Saito H., Nishiyama N. Comparative effect of IL-2 and IL-6 on morphology of cultured hippocampal neurons from fetal rat brain. Brain Res. 1996;715:9–16. doi: 10.1016/0006-8993(95)01291-5. [DOI] [PubMed] [Google Scholar]
- Schaafsma S.M., Gagnidze K., Reyes A., Norstedt N., Månsson K., Francis K., Pfaff D.W. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc. Natl. Acad. Sci. U. S. A. 2017;114:1383–1388. doi: 10.1073/pnas.1619312114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino F.L., Murawski N.J., Rosen J.B., Stanton M.E. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol. Learn. Mem. 2011;95:190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B.D., Blomgren K., Gimlin K., Ferriero D.M., Noble-Haeusslein L.J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Chen X., Kass P.H., Puschner B. Polychlorinated biphenyl and polybrominated diphenyl ether profiles in serum from cattle, sheep, and goats across California. Chemosphere. 2017;181:63–73. doi: 10.1016/j.chemosphere.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Morgan R.K., Feng W., Lin Y., Li X., Luna C., Koch M., Bansal R., Duffel M.W., Puschner B., Zoeller R.T., Lehmler H., Pessah I.N., Lein P.J. Comparative analyses of the 12 most abundant PCB congeners detected in human maternal serum for activity at the thyroid hormone receptor and ryanodine receptor. Environ. Sci. Technol. 2019;53:3948–3958. doi: 10.1021/acs.est.9b00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H., Li Y., Wang T., Wang P., Zhang H., Zhang Q., Jiang G. The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,3′-dichlorobiphenyl (PCB 11) Chemosphere. 2014;98:44–50. doi: 10.1016/j.chemosphere.2013.09.075. [DOI] [PubMed] [Google Scholar]
- Shen Y., Liu S., Zhan M., Luo J., Zhu L.-J. Interleukin-2 enhances dendritic development and spinogenesis in cultured hippocampal neurons. Anat. Rec. (Hoboken) 2010;293:1017–1023. doi: 10.1002/ar.21118. [DOI] [PubMed] [Google Scholar]
- Sipka S., Eum S.-Y., Son K.W., Xu S., Gavalas V.G., Hennig B., Toborek M. Oral administration of PCBs induces proinflammatory and prometastatic responses. Environ. Toxicol. Pharmacol. 2008;25:251–259. doi: 10.1016/j.etap.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamou M., Streifel K.M., Goines P.E., Lein P.J. Neuronal connectivity as a convergent target of gene × environment interactions that confer risk for autism spectrum disorders. Neurotoxicol. Teratol. 2013;36:3–16. doi: 10.1016/j.ntt.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stølevik S.B., Nygaard U.C., Namork E., Haugen M., Meltzer H.M., Alexander J., Knutsen H.K., Aaberge I., Vainio K., van Loveren H., Løvik M., Granum B. Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food Chem. Toxicol. 2013;51:165–172. doi: 10.1016/j.fct.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Sumathi T., Asha D., Nagarajan G., Sreenivas A., Nivedha R. L-Theanine alleviates the neuropathological changes induced by PCB (Aroclor 1254) via inhibiting upregulation of inflammatory cytokines and oxidative stress in rat brain. Environ. Toxicol. Pharmacol. 2016;42:99–117. doi: 10.1016/j.etap.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Tayal V., Kalra B.S. Cytokines and anti-cytokines as therapeutics—an update. Eur. J. Pharmacol. 2008;579:1–12. doi: 10.1016/j.ejphar.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Trebak M., Kinet J.P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019;19:154–169. doi: 10.1038/s41577-018-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigunaite A., Dimo J., Jørgensen T.N. Suppressive effects of androgens on the immune system. Cell. Immunol. 2015;294:87–94. doi: 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Vereyken E.J.F., Bajova H., Chow S., de Graan P.N.E., Gruol D.L. Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. Eur. J. Neurosci. 2007;25:3605–3616. doi: 10.1111/j.1460-9568.2007.05615.x. [DOI] [PubMed] [Google Scholar]
- Vikman K.S., Owe-Larsson B., Brask J., Kristensson K.S., Hill R.H. Interferon-gamma-induced changes in synaptic activity and AMPA receptor clustering in hippocampal cultures. Brain Res. 2001;896:18–29. doi: 10.1016/s0006-8993(00)03238-8. http://www.ncbi.nlm.nih.gov/pubmed/11277968 [DOI] [PubMed] [Google Scholar]
- Voorspoels S., Covaci A., Neels H. Dietary PCB intake in Belgium. Environ. Toxicol. Pharmacol. 2008;25:179–182. doi: 10.1016/j.etap.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Vukcevic M., Zorzato F., Keck S., Tsakiris D.A., Keiser J., Maizels R.M., Treves S. Gain of function in the immune system caused by a ryanodine receptor 1 mutation. J. Cell Sci. 2013;126:3485–3492. doi: 10.1242/jcs.130310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Chadman K.K., McCloskey D.P., Sheikh A.M., Malik M., Brown W.T., Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim. Biophys. Acta - Mol. Basis Dis. 2012;1822:831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N., Sas T.C.J., Koopman-Esseboom C., van der Zwan C.W., De Ridder M.A.J., Beishuizen A., Hooijkaas H., Sauer P.J.J. Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatr. Res. 1995;38:404–410. doi: 10.1203/00006450-199509000-00022. [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N., Patandin S., Berbers G.A.M., Sas T.C.J., Mulder P.G.H., Sauer P.J.J., Hooijkaas H. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ. Health Perspect. 2000;108:1203–1207. doi: 10.1289/ehp.001081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wens B., De Boever P., Maes M., Hollanders K., Schoeters G. Transcriptomics identifies differences between ultrapure non-dioxin-like polychlorinated biphenyls (PCBs) and dioxin-like PCB126 in cultured peripheral blood mononuclear cells. Toxicology. 2011;287:113–123. doi: 10.1016/j.tox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Williamson L.L., Bilbo S.D. Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain Behav. Immun. 2013;30:186–194. doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Wolf I.M.A., Diercks B., Gattkowski E., Czarniak F., Kempski J., Werner R., Schetelig D., Mittrücker H., Schumacher V., von Osten M., Lodygin D., Flügel A., Fliegert R., Guse A.H. Frontrunners of T cell activation: initial, localized Ca2+ signals mediated by NAADP and the type 1 ryanodine receptor. Sci. Signal. 2015;8:ra102. doi: 10.1126/scisignal.aab0863. [DOI] [PubMed] [Google Scholar]
- Wu H., Yu W., Meng F., Mi J., Peng J., Liu J., Zhang X., Hai C., Wang X. Polychlorinated biphenyls-153 induces metabolic dysfunction through activation of ROS/NF-κB signaling via downregulation of HNF1b. Redox Biol. 2017;12:300–310. doi: 10.1016/j.redox.2017.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Li X., Zhong Y. Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediat. Inflamm. 2015;2015:1–10. doi: 10.1155/2015/531518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Guo X., Li N., Pan Q., Ma Y.Z. Effects of quercetin on Aroclor 1254-induced expression of CYP 450 and cytokines in pregnant rats. J. Immunotoxicol. 2019;16:140–148. doi: 10.1080/1547691X.2019.1604585. [DOI] [PubMed] [Google Scholar]
- Yang D., Kim K.H., Phimister A., Bachstetter A.D., Ward T.R., Stackman R.W., Mervis R.F., Wisniewski A.B., Klein S.L., Kodavanti P.R.S., Anderson K.A., Wayman G., Pessah I.N., Lein P.J. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ. Health Perspect. 2009;117:426–435. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B.S., Leung A.O.W., Wong M.H. The association of environmental toxicants and autism spectrum disorders in children. Environ. Pollut. 2017;227:234–242. doi: 10.1016/j.envpol.2017.04.039. [DOI] [PubMed] [Google Scholar]
- Yirmiya R., Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yuen B., Boncompagni S., Feng W., Yang T., Lopez J.R., Matthaei K.I., Goth S.R., Protasi F., Franzini-Armstrong C., Allen P.D., Pessah I.N. Mice expressing T4826I-RYR1 are viable but exhibit sex- and genotype-dependent susceptibility to malignant hyperthermia and muscle damage. FASEB J. 2012;26:1311–1322. doi: 10.1096/fj.11-197582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Mapuskar K.A., Marek R.F., Xu W., Lehmler H.J., Robertson L.W., Hornbuckle K.C., Spitz D.R., Aykin-Burns N. A new player in environmentally induced oxidative stress: polychlorinated biphenyl congener, 3,3′-dichlorobiphenyl (PCB11) Toxicol. Sci. 2013;136:39–50. doi: 10.1093/toxsci/kft186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material containing Supplementary Figs. 1–4 (1 - age distribution of animals used in this study; 2 - number of days from first meeting to parturition and dam mass at weaning; 3 - anogenital distance in P7 male mice; 4 - body mass in juvenile mice) and Supplementary Tables 4–6 (4 - significant pairwise comparisons for CCL5/RANTES; 5 - significant pairwise comparisons for hipocampal cytokines and chemokines; 6 - significant interactions that may influence the relationship between serum and hippocampal cytokine concentrations).
Additional details for animals included in serum and hippocampal cytokine analysis.
Cytokine multiplex assay details.
Summary of dam pregnancy outcomes.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.