Abstract
Several central and peripheral nervous system complications associated with the severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection have been recently described. An effective mass vaccination program is necessary to effectively reduce infection spread and, consequently, limit long-term sequelae, including those affecting the nervous system. Nevertheless, as more patients gain access to coronavirus disease 2019 (COVID-19) vaccines, it is important to report potential adverse events. Herein, we report a patient with previous history of post-infectious rhombencephalitis who developed an acute disseminated encephalomyelitis (ADEM) two weeks after being vaccinated for COVID-19.
Keywords: Neurological adverse events, Neurological complications, Vaccine, COVID-19, Encephalitis
1. Introduction
Acute disseminated encephalomyelitis (ADEM) is a demyelinating disorder of the central nervous system (CNS) that typically occurs in close temporal association with either an antecedent infection or, less frequently, following vaccination [1].
Herein, we report a patient with previous history of post-infectious rhombencephalitis who developed an ADEM-like, severe neuroinflammatory disorder of the CNS shortly after being vaccinated for SARS-CoV-2.
2. Case report
A 56-year-old female patient was referred for subacute onset over one week of unsteadiness of gait, predominantly on the left side, followed by clumsiness of left arm. The day before the onset of the symptoms, she experienced malaise and chills, without fever. Nasopharyngeal swab was negative for SARS-CoV-2 on reverse-transcriptase polymerase chain reaction (RT-PCR) assay. Two weeks prior to presentation, she received the first dose of the Pfizer-BioMTech COVID-19 vaccine (Comirnaty), without any acute allergic reactions.
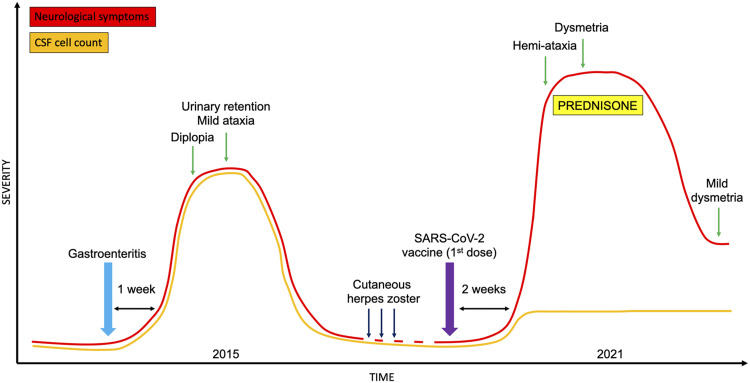
Her medical history was relevant for a previous clinical episode suggestive for post-infectious rhombencephalitis 5 years before this admission ( Fig. 1). At that time, the patient manifested diplopia, mild ataxia with left-ward deviation of gait, and urinary retention requiring catheterization one week after a febrile episode characterized by gastroenteritis and erythematous rash. Nuchal rigidity was not present. Brain magnetic resonance imaging (MRI), performed 6 days after onset, was unremarkable. Cerebrospinal fluid (CSF) showed pleocytosis (80 cells/mm3), while protein and glucose levels were within reference ranges. Electroencephalogram (EEG) was abnormal due to the presence of intermittent slowing in the delta range, predominantly over the fronto-temporal regions. A comprehensive microbiological and autoimmune screening was unrevealing. The patient spontaneously recovered and underwent regular follow-up, including a control brain MRI four months later, also unremarkable. Between 2016 and 2020 she was seen for recurrent episodes of cutaneous herpes zoster with resulting neuropathic pain, effectively treated with pregabalin. Neurological examination was normal during all these consultations, and the patient never complained of episodes of weakness, incoordination, sensory abnormalities, or visual loss.
Fig. 1.
Clinical evolution of the patient. Clinical features and their severity, treatment, and CSF cell count during the disease course. Abbreviations: CSF, cerebrospinal fluid; SARS-CoV-2, severe acute respiratory syndrome coronavirus.
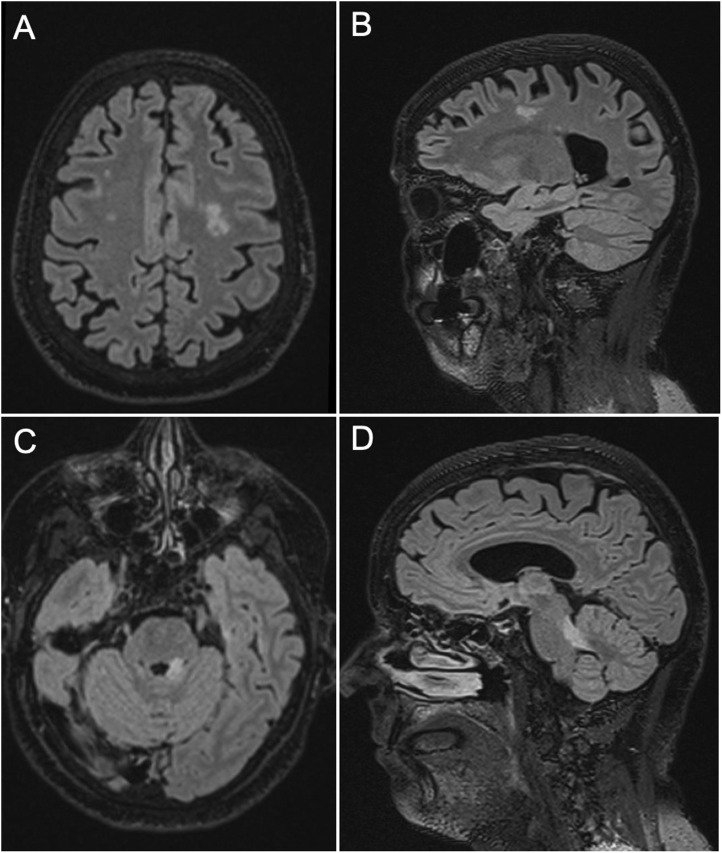
In the current admission, the patient was found to be alert and oriented. No cranial nerve deficits were documented, but horizontal gaze-evoked nystagmus was present on lateral gaze. Mild weakness on left upper limb was noticed (MRC 4/5), while sensory examination was unremarkable. Coordination testing revealed left sided dysmetria and the patient had a left hemi-ataxic gait (scale for the assessment and rating of ataxia [SARA] score: 7). Meningeal signs were negative. Brain MRI showed an area of hyperintensity on fluid attenuated inversion recovery (FLAIR) sequences involving the left cerebellar peduncle, with modest mass effect on the fourth ventricle, which was not present on the previous MRI examination ( Fig. 2). No contrast enhancement was observed and the lesion did not exhibit diffusion restriction. In addition, new supratentorial areas of hyperintensity on FLAIR sequences were observed, the largest in the left centrum semiovale (Fig. 2). No lesions in the corpus callosum were detected. Control EEG showed no abnormalities. CSF cell count, glucose, and protein levels were within reference ranges. Oligoclonal bands were searched in serum and CSF by isoelectric focusing, with negative results (type 1 pattern). Microbiological studies (including HSV, VZV, HHV6, EBV, CMV, TBE, Borrelia) and screening for antibodies targeting antigens associated with demyelinating disorders of the CNS (MOG, AQP4), as well as gangliosides (GM1, GM2, GM3, GM4, GD1a, GD1b, GD2, GD3, GT1a, GT1b, GQ1b) were all negative. In addition, an indirect immunofluorescence on primate brain slices along with a comprehensive screening for antibodies against extracellular and synaptic neuronal antigens (NMDAR, AMPAR, GABABR, LGI1, CASPR2, and DPPX) and intracellular antigens (Hu, Yo, Ri, Ma2, CV2/CRMP5, amphiphysin, GAD65) were performed in serum and CSF using commercial kits (Euroimmun, Lübeck, Germany), with negative results. A protective titer of neutralizing antibodies against SARS-CoV-2 Spike 1 was detected in serum (227 AU/ml, reference values: <0.8; Elecsys Roche Diagnostics, Switzerland), but we were not able to demonstrate the presence of anti-SARS-CoV2 antibodies in the CSF.
Fig. 2.
Brain magnetic resonance imaging (MRI) findings. FLAIR hyperintensity involving the frontal white matter, with the largest lesion on the left side (A: axial view, B: sagittal view) and the ipsilateral superior cerebellar peduncle (C: axial view, D: sagittal view). Abbreviations: FLAIR, Fluid attenuated inversion recovery.
A large cytokine panel (IL-6, IL-8, IL-1beta, TNF-alfa, sCD25, CXCL10, IFN-g, IL-10) was tested by multiplex ultrasensitive ELISA assay (BioTechne, USA). Serum analysis revealed increased concentration of IL-8 (59 pg/ml, reference range: 6.7–16.2), with similar concentration across the blood brain barrier (CSF/serum ratio of 0.98). Conversely, levels of IL-10 (1.7 pg/ml, reference range: 1.8–3.8) and TNF-alfa (5.4 pg/ml, reference range 7.8–12.2) were slightly reduced in serum, while IL-6 was normal (3.7 pg/ml; reference range 0.8 – 6.4). Of note a positive CSF/serum ratio was found for IL-10 (1.47), IFN-gamma (3.66) and most of all IL-6 (6.64). Complete blood count and markers of systemic autoimmunity (including ANA, ENA, ANCA, antiphospholipid antibodies, complement C3 and C4) were normal/negative. The patient was treated with corticosteroids (prednisone 75 mg q.d. with gradual tapering). At last follow-up, 50 days after symptoms onset and two weeks after completing corticosteroid treatment, the patient described a progressive improvement in gait stability, being able to walk without aid with only minor difficulties in tandem gait. Mild dysmetria and intention tremor of the left upper limb were still present (SARA score: 2). The second dose of the vaccine has been precautionary suspended and a control brain MRI (six months after onset) has been scheduled.
3. Discussion
We report a patient with previous history of post-infectious rhombencephalitis who developed a severe neuroinflammatory disorder following the first dose of mRNA-based vaccine to SARS-CoV-2.
The differential diagnosis spectrum in this case includes mainly inflammatory demyelinating disorders of the CNS (multiple sclerosis [MS], neuromyelitis spectrum disorders [NMOSD], and ADEM), Bickerstaff brainstem encephalitis, and autoimmune encephalitis. The absence of CSF-restricted oligoclonal bands, the lack of lesions in the corpus callosum, and the clinical evolution were not in favor of MS, although a longer follow-up is needed to rule out dissemination in time which is typical of MS. The patient did not experience working memory deficits, seizures, or psychiatric manifestations which are the core symptoms of autoimmune encephalitis and, in addition, a comprehensive neuronal antibody screening was negative. Similarly, the patient lacked ophthalmoplegia and consciousness disturbance, which are the cardinal manifestations of Bickerstaff encephalitis other than ataxia, and anti-GQ1b antibodies were negative. The close association with an infectious disease and vaccination pointed towards an ADEM-like disorder, even if not all features of the diagnostic criteria (initially proposed for the pediatric population [2]) were present, in particular no evidence of encephalopathy was detected in the second admission. Nevertheless, in a Danish nationwide study it was observed that only 35% of patients with a final diagnosis of ADEM fulfilled the above mentioned International Pediatric Multiple Sclerosis Study Group (IPMSSG) diagnostic criteria, suggesting that incomplete presentations exist [1]. ADEM is characterized by multifocal lesions involving asymmetrically the white matter which can be large and tumefactive, while CSF may be normal in as many as 60% of the cases [3]. In the differential diagnosis, it should be considered that lymphoma can also respond dramatically to corticosteroids, but the lesions are typically located in the basal ganglia or periventricular white matter and exhibit strong contrast enhancement [3]. In addition, no atypical lymphoid cells were detected in the CSF. Neuro-Behçet's syndrome may present with scattered, asymmetric, subcortical white matter lesions, without cortical involvement [3], but in this case the diagnosis was considered less likely due to the absence of orogenital ulcers or uveitis, therefore HLA testing was not performed. Finally, infectious diseases causing rhomboencephalitis (including Listeria monocytogenes) should also be considered, but in this case the lack of meningeal signs, pleocytosis and contrast-enhancing lesions render this diagnosis unlikely [4].
It is interesting to note that the patient showed an increased serum level of IL-8, the primary chemokine involved in neutrophil recruitment in acute inflammation and viral infection whose levels were demonstrated to be very high in COVID-19-associated Guillain-Barré syndrome and encephalopathy [5]. IL-8 was not significantly increased in CSF. Surprisingly, levels of IL-6, TNF-alfa and IL-10 were normal or even slightly reduced in serum, while in CSF both pro-inflammatory (IL-6, IFN-gamma) and anti-inflammatory cytokines (IL-10) were elevated with positive CSF/serum ratio, possibly indicating an unbalanced, but predominantly inflammatory process across the blood brain barrier.
Regarding the pathogenesis of this possible adverse event, immunological or genetic specificities of the patient may be relevant, as it was demonstrated for the role of HLA in other neurological autoimmune disorders [6]. Intriguingly, the patient here described had a previous history of recurrent herpes zoster, and it was shown that genetic variants in Toll-like receptor 3 (TLR3) - which are important in the recognition of foreign RNA - are associated with recurrence of zoster ophthalmicus [7], while TLR polymorphisms significantly affect vaccine response and, possibly, the risk of adverse events [8].
At the time of this report, 9442 reports of adverse reactions have been reported in the Vaccine Adverse Event Reporting System (VAERS) to the mRNA-based vaccines to SARS-CoV-2, including 6 cases of ADEM in the United States [9], but no clinical description was published yet regarding these cases. One case of ADEM was recently reported following inactivated SARS-CoV-2 vaccine in China [10].
4. Conclusion
Although the link between vaccination and the neurologic condition might have been just fortuitous, the possibility of a post-vaccine neuroinflammatory syndrome is very suggestive in this case. The potential implication is that particular caution may be needed in the vaccination of patients with previous history of post-infectious neurological disorders, but further prospective data is required before drawing definite conclusions. While waiting for large epidemiological data, it is important to keep in mind that a temporal association does not imply causation, and the experience so far is that SARS-CoV-2 vaccination is safe and should be recommended.
Ethical standards
The patient provided informed written consent for publication of this report. All procedures were performed in accordance with the institutional ethics committee (Comitato Etico Unico Regionale, CEUR) and the Declaration of Helsinki. The adverse event described herein was notified to the Italian Medicines Agency (AIFA).
Study funding
No targeted funding reported.
Data access, responsibility, and analysis
The Corresponding Author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors' contributions
Alberto Vogrig, Francesco Janes and Gian Luigi Gigli: Study concept and design. Alberto Vogrig, Francesco Janes, Gian Luigi Gigli, Francesco Curcio, Ilaria Del Negro, Serena D’Agostini, Martina Fabris and Mariarosaria Valente: Acquisition of data. Alberto Vogrig, Francesco Janes, Gian Luigi Gigli, Francesco Curcio, Ilaria Del Negro, Serena D’Agostini, Martina Fabris and Mariarosaria Valente: Analysis and interpretation of data. Alberto Vogrig: Drafting of the manuscript. Alberto Vogrig, Francesco Janes, Gian Luigi Gigli, Francesco Curcio, Ilaria Del Negro, Serena D’Agostini, Martina Fabris and Mariarosaria Valente: Critical revision of the manuscript for important intellectual content. Gian Luigi Gigli and Mariarosaria Valente: Study supervision. All authors read and approved the final manuscript.
Conflicts of interest
None reported.
References
- 1.Boesen M.S., Blinkenberg M., Koch‐Henriksen N., Thygesen L.C., Uldall P.V., Magyari M., Born A.P. Implications of the International Paediatric Multiple Sclerosis Study Group consensus criteria for paediatric acute disseminated encephalomyelitis: a nationwide validation study. Dev. Med. Child Neurol. 2018;60:1123–1131. doi: 10.1111/dmcn.13798. [DOI] [PubMed] [Google Scholar]
- 2.Krupp L.B., Tardieu M., Amato M.P., Banwell B., Chitnis T., Dale R.C., Ghezzi A., Hintzen R., Kornberg A., Pohl D., Rostasy K., Tenembaum S., Wassmer E., International Pediatric Multiple Sclerosis Study Group International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult. Scler. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 3.Osborn A.G., Hedlund G.L., Salzman K.L. Elsevier; 2017. Osborn’s Brain: Imaging, Pathology, and Anatomy. [Google Scholar]
- 4.Arslan F., Ertan G., Emecen A.N., Fillatre P., Mert A., Vahaboglu H. Clinical presentation and Cranial MRI findings of Listeria monocytogenes Encephalitis: a literature review of case series. Neurologist. 2018;23:198–203. doi: 10.1097/NRL.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 5.Gigli G.L., Vogrig A., Nilo A., Fabris M., Biasotto A., Curcio F., Miotti V., Tascini C., Valente M. HLA and immunological features of SARS-CoV-2-induced Guillain-Barré syndrome. Neurol. Sci. 2020;41:3391–3394. doi: 10.1007/s10072-020-04787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muñiz-Castrillo S., Vogrig A., Honnorat J. Associations between HLA and autoimmune neurological diseases with autoantibodies. Autoimmun. Highlights. 2020;11:2. doi: 10.1186/s13317-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang F., Glans H., Enoksson S.L., Kolios A.G.A., Loré K., Nilsson J. Recurrent Herpes Zoster Ophthalmicus in a patient with a novel toll-like receptor 3 variant linked to compromised activation capacity in fibroblasts. J. Infect. Dis. 2020;221:1295–1303. doi: 10.1093/infdis/jiz229. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrino P., Falvella F.S., Cheli S., Perrotta C., Clementi E., Radice S. The role of Toll-like receptor 4 polymorphisms in vaccine immune response. Pharm. J. 2016;16:96–101. doi: 10.1038/tpj.2015.21. [DOI] [PubMed] [Google Scholar]
- 9.Goss A.L., Samudralwar R.D., Das R.R., Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann. Neurol. 2021;89:856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao L., Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol. Belg. 2021 doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]