Abstract
Background/purpose
Superspreading events (SSEs) are pivotal in the spread of SARS-CoV-2. This study aimed to investigate an SSE of COVID-19 in a hospital and explore the transmission dynamics and heterogeneity of SSE.
Methods
We performed contact tracing for all close contacts in a cluster. We did nasopharyngeal or throat swabbing for SARS-CoV-2 by real-time RT-PCR. Environmental survey was performed. The epidemiological and clinical characteristics of the SSE were studied.
Results
Patient 1 with congestive heart failure and cellulitis, who had onset of COVID-19 two weeks after hospitalization, was the index case. Patient 1 led to 8 confirmed cases, including four health care workers (HCW). Persons tested positive for SARS-CoV-2 were HCW (n = 4), patient 1's family (n = 2), an accompanying person of an un-infected in-patient (n = 1), and an in-patient admitted before the SSE (n = 1). The attack rate among the HCW was 3.2 % (4/127). Environmental survey confirmed contamination at the bed rails, mattresses, and sink in the room patient 1 stayed, suggesting fomite transmission. The index case's sputum remained positive on illness day 35. Except one asymptomatic patient, at least three patients acquired the infection from the index case at the pre-symptomatic period. The effective reproduction number (Rt) was 0.9 (8/9).
Conclusion
The host factor (heart failure, longer viral shedding), transmissibility of SARS-CoV-2 (Rt, pre-symptomatic transmission), and possible multiple modes of transmission altogether contributed to the SSE. Rapid response and advance deployment of multi-level protection in hospitals could mitigate COVID-19 transmission to one generation, thereby reducing its impact on the healthcare system.
Keywords: SARS-CoV-2, COVID-19, Superspreading event, Outbreak, Hospital
Introduction
Coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China in late December 2019. On March 11, 2020, the WHO declared COVID-19 a global pandemic. The virus causing COVID-19, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can spread efficiently in humans, with a basic reproductive number of 2.2–2.5, as determined in Wuhan, suggesting high transmissibility.1 , 2 Large outbreaks of SARS-CoV-2 infection in the community setting have been reported.3, 4, 5 However, previous experience of a hospital outbreak of MERS-CoV infection in Korea, where one single patient infected many others in an overcrowded hospital setting, raised the concern about the existence of potential super-spreaders of SARS-CoV-2, that were first reported during SARS epidemics.6, 7, 8, 9
From February 26 to March 10, 2020, an outbreak of SARS-CoV-2 infection occurred in a large hospital in Taiwan from an index case with congestive heart failure, which eventually led to 8 confirmed cases of COVID-19. The index case spread the virus to 8 other persons, including 4 health care workers (HCW), during her stay at the emergency room and ward before she was diagnosed and transferred to a negative pressure isolation room for subsequent treatment. The study aimed to report the hospital's response to a cluster of COVID-19 associated with a SARS-CoV-2 superspreading event (SSE) and the dynamics and heterogeneity of the transmission.
Methods
Identification of index case
Chang Gung Memorial Hospital is a tertiary hospital located in northern Taiwan with 3700 beds for in-patient care. On February 14, 2020, one patient with history of coronary artery disease and heart failure was admitted to a general ward (ward 5C) after seeking initial care in the emergency room. She expressed no contact with suspected cases or visitors from foreign countries. In addition to the pre-existing heart failure, she was admitted to the ward for medical treatment of cellulitis. On February 19, mild fever (37.6 °C) developed but quickly resolved on the following day. February 19 was designated as the day of illness onset for the index case (patient 1). The patient experienced fever again and cough on February 23. Next day, she underwent throat swabbing for rapid influenza diagnostic test (RIDT), which was performed by a nurse practitioner (patient 5). Chest radiograph showed right upper lung pneumonia on the same day. An infectious disease (ID) physician was consulted for the pneumonia, and a joint meeting held by the physicians of different specialties decided to screen for COVID-19. After SARS-CoV-2 infection was confirmed in this patient on February 27, contact investigation and environmental survey were initiated immediately.
Definition of close contacts
The contact tracing included medical staff, accompanying persons, visitors, and patients in the emergency room and ward 5C. According to the guidelines of Taiwan CDC, close contact is defined by contacting the index case within 2 m without using appropriate personal protective equipment (PPE) and without a minimum requirement of exposure time in hospital settings.10 For HCW to perform aerosol generating procedures (AGP) such as throat swabbing or intubation, PPE is considered appropriate only when N95 respirators are used. HCW are categorized as close contacts if they wear only surgical masks when performing AGP. Patients, accompanying persons, and visitors were considered as close contacts when they stay within 2 m aside the index case. We identified close contacts through review of security video footage, medical chart records, nurse records, and shift schedules. We also interviewed hospital staff, visitors, patients, and accompanying persons to gather essential information. The effective reproduction number (R t) is defined as the mean number of secondary cases generated by a primary case in a period.11
Environmental survey
We sampled the surfaces of the environment and equipment by the Dacron swab (Copan Diagnostics, Corona, CA). The swabs were put onto 0.8 mL of viral transport medium for subsequent tests. Follow-up survey was performed according to the results of the initial investigation. For swabbing the ground surface of the room, 900 cm squares were delineated and sampled. All samples were processed immediately after the collection.
Collection of respiratory specimens and RT-PCR
Respiratory specimens of the nasopharyngeal or throat swabs were collected where appropriate. All samples were maintained in a transport medium (UTM-RT, Copan Diagnostics, Murrieta, CA) for further analysis. We used reverse transcription polymerase chain reaction (RT-PCR) to detect SARS-CoV-2. RNA was extracted from the specimens using LabTurbo system (Taigen, Taiwan) according to the manufacturer's instructions, except that the specimen was pretreated with Proteinase K prior to RNA extraction. RT-PCR was performed according to a proposed protocol.12 In brief, the amplification targeted the following genes: RNA-dependent RNA polymerase gene (RdRp), envelope (E) gene, and nucleocapsid (N) gene. We used the E gene and N gene assays as the first-line screening targets, followed by confirmatory testing with the RdRp gene assay.
Results
Contact tracing and investigation
As of March 10, 2020, we performed nasopharyngeal swabbing for HCW (n = 127), in-patients (n = 55), and visitors who accompanied their family in the hospital (n = 27). Those patients who needed in-patient care were transferred to negative pressure isolation rooms or single rooms and quarantined for 14 days after the last day of exposure. HCW considered as close contacts were quarantined at home or a designated dormitory area for 14 days. All in-patients and HCW tested negative were re-assessed and re-tested again before the end of quarantine period. The investigation confirmed eight cases, including four HCW (Table 1 and Fig. 1 ). The detailed investigation information was available in the Supplementary Methods.
Table 1.
Clinical characteristics and laboratory testing results of the hospital COVID-19 cluster.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Description | Index case | Cleaner | Nurse | Nurse | Nurse practitioner | The index patient's family | An un-infected in-patient's family who stayed in the ward 5C | An in-patient admitted to 5C before the cluster was identified | The index patient's family |
| Age (year) | 57 | 56 | 29 | 27 | 42 | 26 | 58 | 56 | 24 |
| Sex | Female | Female | Female | Female | Female | Female | Female | Female | Male |
| Underlying conditions | Heart failure, coronary artery disease | Hypertension | Pregnancy | No | No | No | Hypertension | Liver cirrhosis, hepatocellular carcinoma | No |
| Presenting symptoms | Fever and cough | Cough | Cough and rhinorrhea | Cough and rhinorrhea | Muscle ache and mild fever (37.5 °C) | No symptoms | Fever, cough, and muscle ache | Cough | Cough |
| Chest radiograph | Pneumonia | Normal | NA | Normal | Normal | Normal | Pneumonia | Pneumonia | NA |
| HRCT | GGO | GGO | NA | Normal | GGO | NA | GGO | GGO | NA |
| LOS | 43 | 14 | 23 | 21 | 31 | NA | 39 | 28 | NA |
| Outcome | Mortality | Survival | Survival | Survival | Survival | Survival | Survival | Survival | Survival |
| White blood cell count (109/L) | 2.1 | 9.1 | 5.3 | 6.4 | 3.6 | NA | 7.1 | 3.4 | NA |
| Lymphocyte (109/L) | 0.2 | 2.4 | 1.3 | 2.0 | 1.2 | NA | 1.2 | 0.7 | NA |
| Platelet count (109/L) | 158 | 325 | 249 | 273 | 174 | NA | 198 | 186 | NA |
| D-dimer (ng/mL) | 1902 | 191 | 574 | <170 | <170 | NA | 429 | 1539 | NA |
| C-reactive protein (mg/L) | 73.6 | 2.5 | 2.6 | 1.5 | 3.4 | NA | 66 | 10.4 | NA |
| Lactate dehydrogenase (U/L) | 690 | 177 | 189 | 212 | 227 | NA | 327 | 265 | NA |
| Creatine (mg/dL) | 1.4 | 0.6 | 0.4 | 0.7 | 0.6 | NA | 0.6 | 0.6 | NA |
| AST (U/L) | 45 | 27 | 21 | 21 | 38 | NA | 32 | 41 | NA |
| ALT (U/L) | 16 | 25 | 18 | 13 | 23 | NA | 29 | 31 | NA |
| Bilirubin (mg/dL) | 1.3 | 1.2 | 0.2 | 0.3 | 0.7 | NA | 0.5 | 0.3 | NA |
| Ct valuea | |||||||||
| E gene | 23.9 | 26.6 | 28.8 | 26.4 | 29.9 | 24.1 | 27.5 | 26.6 | 24.95 |
| RdRp-1 | 29.8 | 26.9 | 25.6 | 24.0 | 37.6 | 25.2 | 29.8 | 28.1 | 27.03 |
| RdRp-2 | 24.2 | 28.0 | 25.6 | 23.9 | 30.8 | 25.4 | 26.7 | 27.3 | 26.02 |
| N gene | 28.5 | 26.6 | 28.8 | 26.4 | 32.3 | 28.1 | 31.7 | 32.0 | 29.73 |
Ct = cycles of thresholds; GGO = ground-glass opacities; HRCT = High-resolution computed tomography; LOS = length of stay; NA = not available.
RT-PCR of the respiratory specimens collected at the time of diagnosis.
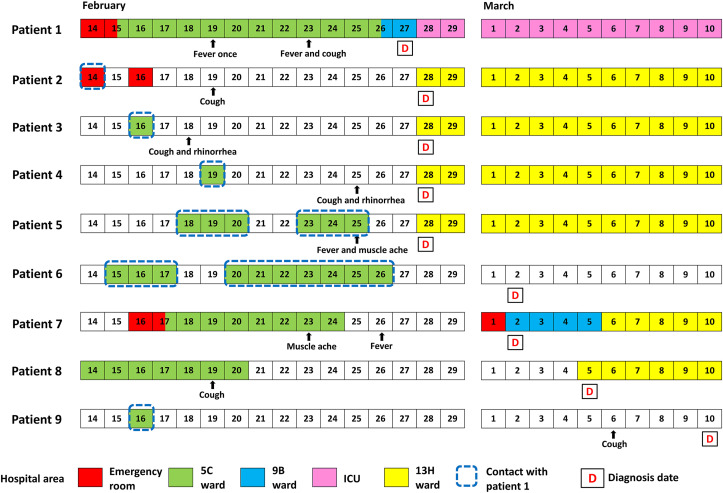
Figure 1.
Transmission dynamics of patients at the superspreading event associated with COVID-19 in a hospital. The designated wards for isolation of COVID-19 cases were 9B, 13H and ICU. The cluster involved four hospital staff (patients 2–5), two families (patients 6 and 9) of the index case (patient 1), one accompanying person for an un-infected in-patient (patient 7), and one in-patient who stayed in the same ward (patient 8). Patients 2, 3, and 8 may acquire infection earlier than symptom onset of the index case. Patient 2 was a cleaner and performed cleansing work for the bed next to the bed of Patient 1 at the emergency room on February 14. Patient 3 was a nurse who cared patient 1 on February 16, and she also cared an un-infected in-patient (patient 7's family) from February 21 to February 23. Patient 4 was a nurse who cared patient 1 on February 19. Patient 5 was a nurse practitioner who wore a surgical mask to swab patient 1 for a rapid influenza diagnostic test on February 24. She joined the team to care patient 1 for several days before the cluster. Patient 6 was patient 1's daughter who accompanied patient 1 during her admission in the ward 5C. She was asymptomatic throughout the entire course. Patient 7 accompanied her un-infected husband during his admission to ward 5C for treatment before the cluster. Patient 9 was the index case's son who stayed in the ward 5C for only one day on February 16. Patient 8 was admitted to the ward 5C from February 14 to February 20 for radiofrequency ablation of liver tumor. Patient 9 was tested negative twice before the end of quarantine on March 3. He developed cough on March 6, and a third testing was positive for SARS-CoV-2 on March 10. Except the asymptomatic patient 6, the serial intervals (in parentheses) of the infected were: patient 2 (0), patient 3 (−1), patient 4 (6), patient 5 (6), patient 7 (4), patient 8 (0), and patient 9 (16).
Environmental contamination
On February 28, there were 20 samples collected from the room that patient 1 stayed in the ward 5C. SARS-CoV-2 RNA was found in four samples. The sample area of the floor was 30 × 30cm. There were four samples checked for the floor in the room. Two samples were done for the floor area near the index patient and two were done for the surrounding floor areas. The contaminated sites at the room patient 1 stayed were the bed rails and mattresses of neighboring beds (one bed for another un-infected patient and another for the accompanying person) and the sink (Fig. 2 ). It is possible that these sites were contaminated by the index case when she approached toilet or closet near the door of the room. On February 29, 104 surface samples were collected from the exposure sites of the emergency room. All were tested negative for SARS-CoV-2.
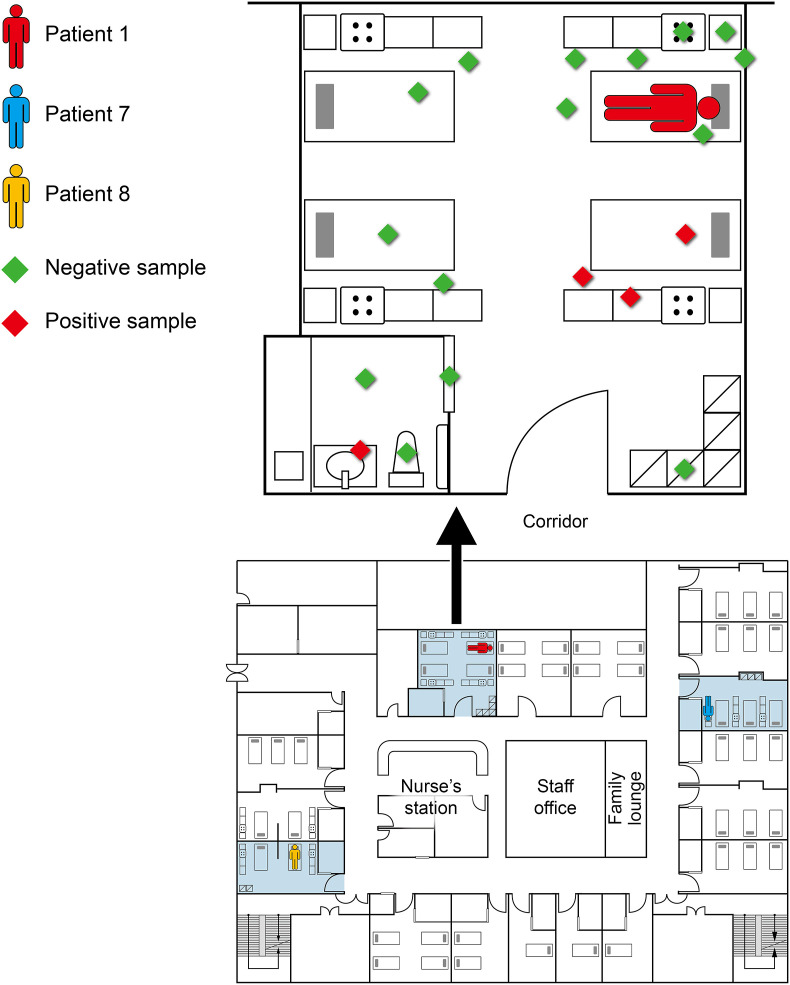
Figure 2.
Floor plan of the ward 5C, the locations of the patients 1, 7, and 8 during their stay in the ward, and results of environmental sampling in the event. Environmental sampling was performed at the room patient 1 stayed. Sites sampled were: 1) Sites next to the environment of patient 1: the table, the chair, the bed, the bedrails, wall, and floor; 2) Sites near other patients who stayed at the same room: the beds, the floor, and the lockers; and 3) Bathroom: the door handle, the sink, floor, and the toilet. The contaminated sites were the bed rails and mattresses of neighboring beds (one bed for another un-infected patient and another for the accompanying person) and the sink. The red diamonds indicate the contaminated sites, and the green diamonds the sites sampled with no contamination.
Complete cleaning of the emergency room and the ward 5C was performed immediately after the identification of patient 1. Follow-up survey of the environment was conducted on March 1. Samples were collected from previously contaminated surfaces of the room. All 25 samples were negative for SARS-CoV-2.
Infection control measures
After Taiwan CDC started the border control in January 2020, the hospital implemented patient traffic control policy, based on risk stratification of the patients and visitors entering the hospital. Universal mask wearing, reinforcement of hand hygiene, and restriction of visitors were also implemented in early February. Ward 5C was closed after cleaning and disinfection. We re-enforced the infection control measures implemented in the hospital to all HCW. Hospital-wide areas including wards and ICUs were allocated and assigned to 20 ID physicians of the hospital. Each ID physician worked with infection control nurses when there were suspected cases of COVID-19 in the responsible region. This workflow and assigned areas were integrated to the computerized antimicrobial stewardship program, which has been deployed since 2004.13 When ID physicians reviewed antibiotic prescriptions, they would review relevant clinical information including vital signs, laboratory data, and chest images simultaneously. Throat or nasopharyngeal swabbing would then be performed for suspected cases in a single room or an isolation room with negative pressure, if deemed necessary. We reduced the workload for HCW by reducing the bed number of the hospital. Beds in emergency room and wards were separated at least 2 m apart. There were no further new cases linked to the cluster after March 10, 2020.
Index case and the event
The index case was admitted due to heart failure and cellulitis. As shown in Table 1, laboratory results reflected leukopenia (2.1 × 109/L) and lymphopenia (0.2 × 109/L). She developed fever on February 19 (illness day 0) and cough on day 4. She was intubated due to hypoxemic respiratory failure on illness day 9. Extracorporeal membrane oxygenation (ECMO) was applied from day 21 to day 35 due to pulseless ventricular tachycardia. Viral load detected by RT-PCR from throat peaked on day 9 (threshold cycle (Ct) value: 21.3) and remained detectable until day 19. The lower respiratory tract (sputum) was initially tested negative for SARS-CoV-2 on day 9, but the viral load of sputum rose on day 12 (Ct value: 16.7). The sputum was still positive on day 35. Eventually the patient died of sepsis, heart failure, ventricular fibrillation, and refractory shock on day 39. The index case did not receive remdesivir treatment since the patient was intubated with mechanical ventilation. Steroids were not used because the evidence for dexamethasone use was limited at that time.
Overall, we performed nasopharyngeal swabbing for HCW (n = 127), the in-patients admitted in the ward 5C (n = 57) and accompanying persons for their family admitted in the ward 5C (n = 27), as of March 5, 2020 in the investigation. Persons tested positive for SARS-CoV-2 were summarized in Fig. 1. In this outbreak, the secondary attack rate for HCW was 3.2 % (4/127). The R t was calculated 8/9 (0.9) as there were 9 infectors (primary cases) and 8 infectees (secondary cases). The pre-symptomatic transmission was observed among the infected in at least patients 2, 3, and 8, and possibly in patient 9, with a wide distribution of the serial intervals (range, −1 to 16) (Fig. 1). All but the index case had mild symptoms only; one case (patient 6) was totally asymptomatic. Half of them had no underlying diseases. The index case and patient 6 had a relatively higher viral shedding (lower Ct values) at the time of diagnosis (Table 1), indicating it is the longer duration of shedding, rather than the higher viral load at diagnosis, that characterized the super-spreader in this superspreading event. Furthermore, we were able to isolate SARS-CoV-2 from five patients in the cluster who showed higher initial viral load. Whole genome sequencing revealed that all of the 5 isolates belong to the same clade, with only four nucleotide changes in two, while the remaining three showing identical viral genome.14
Discussion
SSEs are among the distinguishing features in disease transmission caused by human coronaviruses, which were readily described in SARS-CoV and MERS-CoV infections.6, 7, 8, 9 Recent studies of transmission modeling also proved that SSE could increase the overall disease transmission heterogenicity of COVID-19.15 , 16 The drivers of SSEs vary, generally including pathogen, host, environmental, behavior, and response factors. Although several nosocomial clusters caused by SARS-CoV-2 have been reported in the pandemic, there is no comprehensive investigational report of an SSE yet.17, 18, 19 Herein we described an SSE associated with an index case, who was infected with SARS-CoV-2 and transmitted the virus before symptom onset in a large hospital. Surface samples taken in the room showed environmental contamination by the patient, suggesting probable fomite transmission, in addition to the transmission via respiratory droplets. Hospital response included screening for the hospital staff listed as close contacts, restriction of the number of visitors, reinforcement of adherence to infection control measures to all HCW, and environmental cleansing and disinfection. Early contact tracing and quarantine for the close contacts successfully limited the spread of SARS-CoV-2 in our hospital to one generation of transmission. In contrast, previous SSEs from SARS-CoV or MERS-CoV usually involved multiple generations of spread.6, 7, 8, 9 The secondary attack rate for HCW was 3.2 %, which is significantly lower than that reported from a SARS SSE.7 The R t was less than 1, due to the advance deployment of control measures such as universal mask-wearing and reinforcement of hand hygiene before the event. The study highlights the importance of early detection, contact tracing and quarantine, and the timely implementation of infection control measures to confine an SSE in a hospital.
Although there is no consistent definition of SSE, reports suggested that most infected cases were transmitted by 10–20 % primary cases in an outbreak associated with an SSE.5, 6, 7, 8, 9 Xu et al. has estimated that the threshold of observing SSEs is set as 3.78, meaning the occurrence of an SSE if 4 or more secondary cases were infected by one primary case.15 We presumed that the clustered cases were secondary to the index case and that transmissions occurred within the ward. In this study, the investigation of the transmission chain was based on the timing of events, albeit it is possible that all transmissions within this SSE were neither nosocomial nor related to the putative index case.
The index case presented to the hospital with heart failure and later transmitted the virus to at least 3 persons before symptom start (patients 2, 3, 8 and possibly patient 9), which accounts for approximately half of the infected in the cluster. Silent spreading of SARS-CoV-2, or pre-symptomatic transmission, has been reported during the pandemic in February 2020.20 , 21 As the estimated serial interval of COVID-19 is shorter or close to its median incubation period, a significant proportion of secondary transmission may occur prior to disease onset. This phenomenon could be due to the high level of virus shedding in the upper respiratory tract during the pre-symptomatic period of the patients with COVID-19.22, 23, 24 Likewise, a contact tracing study by Taiwan CDC found that the transmissibility of SARS-CoV-2 was high before and immediately after symptom onset.10 Consequently, symptom-driven surveillance alone would miss asymptomatic cases and was not sufficient to control the spreading of COVID-19 in healthcare settings.25 The containment of the spreading of SARS-CoV-2 in the hospital heavily relied on rapid identification of the SSE and response, based on our understanding on the transmission dynamics associated with the virus.26
COVID-19 can cause cardiac complications, including myocardial injury, heart failure, and arrhythmia.27 , 28 In particular, patients with heart failure are at increased risk for severe disease caused by SARS-CoV-2.29 The respiratory symptoms could become more severe when patients had chronic cardiovascular diseases, which might be associated with an increased level of angiotensin-converting enzyme 2 expression.30 The index patient of our cluster had coronary artery disease and myocardial infarction 1 year before admission, which may make the patient more vulnerable to myocardial injury and cytokine storm of COVID-19. In accordance with our observation, a high prevalence of cardiovascular disease associated with COVID-19 was reported; up to 22 % of critically ill patients experienced myocardial injury from COVID-19.31 , 32 Patients with concomitant chronic cardiovascular disease and COVID-19 usually showed a worse in-hospital outcome.30 , 33 , 34 Respiratory symptoms of COVID-19 could be confused with symptoms attributed to heart failure, which might lead to more droplets, thereby causing a superspreading event. Our study suggests that particular attention should be paid to patients with cardiovascular disease during the pandemic of COVID-19.
We noticed that the index case was asked to wear surgical mask after admission according to our policy but frequently took it off because of respiratory distress caused by heart failure. In Asian countries, facemask wearing is a common practice among people who get sick and visit the hospital.35 Taiwan CDC has enforced compulsory facemask policies for the people taking public transit or visiting the hospital since COVID-19 pandemic occurred. Although there is no evidence to support that wearing a facemask could reduce the risk of transmission of COVID-19, facemasks are commonly used as part of droplet precautions when caring for patients with respiratory symptoms.36 It was confirmed that facemask wearing can reduce the virions of seasonal human coronavirus in aerosols, with a trend toward reduced detection of the virus in respiratory droplets.37 Therefore, use of facemask may reduce the viral shedding and prevent transmission of COVID-19 by the infected people, whether they are symptomatic or not.38 As SARS-CoV-2 can be transmitted asymptomatically or pre-symptomatically, hospital transmission can be reduced if all patients, visitors, and HCW wear facemasks in the hospital during the pandemic.37
Environmental contamination by droplets or fomite was confirmed in the room where the patient has stayed. Nursing cares, with or without AGP, carried opportunities for droplet transmission or fomite transmission by close contact.39 , 40 Although patient 5 (a nurse practitioner) has cared patient 1 for only a short period of time, she might be infected through droplets or aerosols emission generated by the swabbing procedure for RIDT on February 24. Furthermore, two other nurses (patients 3 and 4) have cared the patient for only one shift. It remains intriguing that other nurses in the ward 5C, who were on regular shifts caring the patient for days, were not infected. Moreover, we did not identify direct exposure between the index case and patients 7 and 8. Based on the available information and evidence, we concluded that patients 7 and 8 were infected by fomite transmission. Since SARS-CoV-2 remains infectious in aerosols for hours and on surfaces for days, our findings indicate that pre-symptomatic transmission of SARS-CoV-2 through fomite or aerosols is of concern in crowded hospital settings.38 , 39
Our study has some limitation. Responding to the outbreak, environmental cleansing was performed immediately after the identification of the index case such that public areas in the ward 5C were not sampled. However, the sampling of the surfaces in the public areas including family lounge, nurse's station, and staff office, was negative for the virus after cleaning. The viral source of the index case was not identified. We acknowledged that delayed recognition of the index case is one reason for a super-spreading event to occur. The incubation period of COVID is known to be two to 14 days, though symptoms typically appear within five days after exposure. In the investigation, the index case revealed significant fever and respiratory symptoms 9 days after admission. COVID infection was confirmed early when full-blown covid symptoms appeared. Therefore, prevention of transmission relies on quickly recognizing and identifying the infected cases and associated SSEs in the hospital settings. Although the environmental survey was limited to the room in which the index case stayed, our results support that fomite transmission and asymptomatic transmission are both important modes of transmission for the SSE in our hospital.
Conclusions
This is a detailed report and analysis of an SSE of COVID-19 in a hospital setting. This hospital cluster was characterized by non-specific symptoms and heart failure of the index patient, pre-symptomatic transmission, mild symptoms in the infected healthcare workers, evidence of environmental contamination, and timely response of the hospital to this outbreak. Rapid response and advance deployment of multi-level protection did mitigate the COVID-19 outbreak to one generation of transmission, significantly reducing its impact on the healthcare system. The study showed that the SSE is the combined result of multiple contributing factors including host factors, transmissibility of the virus, environment, and multiple modes of transmission. The study advanced our understanding on the superspreading event of SARS-CoV-2 and the way to stop its spread in the healthcare setting. Early recognition of the index case and prompt multidisciplinary interventions are key to mitigating COVID-19 transmission and preventing an SSE to occur.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board (202000853B0), Chang Gung Memorial Hospital.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by Ministry of Science and Technology, Taiwan (grant MOST109-2327-B-182-002). We thank the help from Health Bureau and North Regional Control Center, Centers for Disease Control, Taiwan in this investigation. We thank all the patients and their families in this study, and we express our condolences to families of the patient who died from COVID-19. We also thank our colleagues in the Department of Thoracic Medicine for caring the patients of this cluster, and the entire staff of the Infection Control Working Group during the outbreak situation who were able to work on this investigation in parallel with their clinical duties; the names of the staff are: Li-Yueh Huang, Yueh-Pi Chiu, Kuei-Chu Hou, Mei-Lien Chen, Yu-Chuan Huang, Li-Mei Tsai, Yu-Hua Su, Hsiu-Ping Wu, Shu-Ling Liu, Hsiao-Ni Wang, Li-Fang Chang, Shu-Hui Shen, Yun-Chi Hung, En-Chi Liu, Yi-Chuan Chen, Chiu-Lan Yeh, Hsiao-Chi Chang, Yu-Ching Chen, Ya-Ting Wu, Ching-Yu Wang, Yi-Rong Lu, Mao-Cheng Ge, Jeng-How Yang, and Yen-Mu Wu.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2021.07.006.
Contributor Information
The Infection Control Working Group:
Li-Yueh Huang, Yueh-Pi Chiu, Kuei-Chu Hou, Mei-Lien Chen, Yu-Chuan Huang, Li-Mei Tsai, Yu-Hua Su, Hsiu-Ping Wu, Shu-Ling Liu, Hsiao-Ni Wang, Li-Fang Chang, Shu-Hui Shen, Yun-Chi Hung, En-Chi Liu, Yi-Chuan Chen, Chiu-Lan Yeh, Hsiao-Chi Chang, Yu-Ching Chen, Ya-Ting Wu, Ching-Yu Wang, Yi-Rong Lu, Mao-Cheng Ge, Jeng-How Yang, and Yen-Mu Wu
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382 doi: 10.1056/NEJMoa2001316. 1199‒207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30260-9. 689‒97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pung R., Chiew C.J., Young B.E., Chin S., Chen M.I.C., Clapham H.E., et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30528-6. 1039‒46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böhmer M.M., Buchholz U., Corman V.M., Hoch M., Katz K., Marosevic D.V., et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20 doi: 10.1016/S1473-3099(20)30314-5. 920‒8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamner L., Dubbel P., Capron I., Ross A., Jordan A., Lee J., et al. High SARS-CoV-2 attack rate following exposure at a choir practice - skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6919e6. 606‒10. [DOI] [PubMed] [Google Scholar]
- 6.Cho S.Y., Kang J.M., Ha Y.E., Park G.E., Lee Ji Y., Ko J.H., et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388:994–1001. doi: 10.1016/S0140-6736(16)30623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Z., Ning F., Zhou W., He X., Lin C., Chin D.P., et al. Superspreading SARS events, beijing, 2003. Emerg Infect Dis. 2004;10 doi: 10.3201/eid1002.030732. 256‒60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi S., Jung E., Choi B.Y., Hur Y.J., Ki M. High reproduction number of Middle East respiratory syndrome coronavirus in nosocomial outbreaks: mathematical modelling in Saudi Arabia and South Korea. J Hosp Infect. 2018;99 doi: 10.1016/j.jhin.2017.09.017. 162‒8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assiri A., McGeer A., Perl T.M., Price C.S., Rabeeah A.A.A., Cummings D.A.T., et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369 doi: 10.1056/NEJMoa1306742. 407‒16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H., et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inglesby T.V. Public health measures and the reproduction number of SARS-CoV-2. J Am Med Assoc. 2020;323:2186–2187. doi: 10.1001/jama.2020.7878. [DOI] [PubMed] [Google Scholar]
- 12.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan Y.Y., Lin T.Y., Huang C.T., Deng S.T., Wu T.L., Leu H.S., et al. Implementation and outcomes of a hospital-wide computerized antimicrobial stewardship program in a large medical center in Taiwan. Int J Antimicrob Agents. 2011;38 doi: 10.1016/j.ijantimicag.2011.08.011. 486‒92. [DOI] [PubMed] [Google Scholar]
- 14.Gong Y.N., Tsao K.C., Hsiao M.J., Huang C.G., Huang P.N., Huang P.W., et al. SARS-CoV-2 genomic surveillance in Taiwan revealed novel ORF8-deletion mutant and clade associated with infections in Middle East. Emerg Microb Infect. 2020;9 doi: 10.1080/22221751.2020.1782271. 1457‒66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X.K., Liu X.F., Wu Y., Ali S.T., Du Z., Bosetti P., et al. Reconstruction of transmission pairs for novel coronavirus disease 2019 (COVID-19) in mainland China: estimation of super-spreading events, serial interval, and hazard of infection. Clin Infect Dis. 2020;71(12):3163–3167. doi: 10.1093/cid/ciaa790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Li Y., Wang L., Li M., Zhou X. Evaluating transmission heterogeneity and super-spreading event of COVID-19 in a metropolis of China. Int J Environ Res Publ Health. 2020;17:3705. doi: 10.3390/ijerph17103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickman H.M., Rampling T., Shaw K., Martinez-Garcia G., Hail L., Coen P., et al. Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2021;72(4):690–693. doi: 10.1093/cid/ciaa816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y.K., Peng S., Li L.Q., Wang Q., Ping W., Zhang N., et al. Clinical and transmission characteristics of COVID-19 - a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. 2020;40:295–300. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan M., Khan H., Khan S., Nawaz M. Epidemiological and clinical characteristics of coronavirus disease (COVID-19) cases at a screening clinic during the early outbreak period: a single-centre study. J Med Microbiol. 2020;69 doi: 10.1099/jmm.0.001231. 1114‒23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020;323 doi: 10.1001/jama.2020.2565. 1406‒7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93 doi: 10.1016/j.ijid.2020.02.060. 284‒6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles' Heel of current strategies to control COVID-19. N Engl J Med. 2020;382 doi: 10.1056/NEJMe2009758. 2158‒60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang L., Zhang X., Zhang X., Wei Z., Zhang L., Xu J., et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect. 2020;80:e1–e13. doi: 10.1016/j.jinf.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26 doi: 10.1038/s41591-020-0869-5. 672‒5. [DOI] [PubMed] [Google Scholar]
- 25.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382 doi: 10.1056/NEJMoa2008457. 2081‒90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frieden T.R., Lee C.T. Identifying and interrupting superspreading events-implications for control of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2606.200495. 1059‒66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loungani R.S., Rehorn M.R., Newby L.K., Katz J.N., Klem I., Mentz R.J., et al. A care pathway for the cardiovascular complications of COVID-19: insights from an institutional response. Am Heart J. 2020;225:3–9. doi: 10.1016/j.ahj.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasoni D., Italia L., Adamo M., Inciardi R.M., Lombardi C.M., Solomon S.D., et al. COVID 19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22 doi: 10.1002/ejhf.1871. 957‒66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFilippis E.M., Reza N., Donald E., Givertz M.M., Lindenfeld J., Jessup M. Considerations for heart failure care during the coronavirus disease 2019 (COVID-19) pandemic. JACC Heart Fail. 2020;8 doi: 10.1016/j.jchf.2020.05.006. 681‒91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17 doi: 10.1038/s41569-020-0360-5. 259‒60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141 doi: 10.1161/CIRCULATIONAHA.120.046941. 1648‒55. [DOI] [PubMed] [Google Scholar]
- 32.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S., et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6924e2. 759‒65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116 doi: 10.1093/cvr/cvaa106. 1666‒87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liuxingbingxue Zazhi. 2020;41 145‒51. [Google Scholar]
- 35.Feng S., Shen C., Xia N., Song W., Fan M., Cowling B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30134-X. 434‒6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacIntyre C.R., Chughtai A.A. Facemasks for the prevention of infection in healthcare and community settings. BMJ. 2015;350:h694. doi: 10.1136/bmj.h694. [DOI] [PubMed] [Google Scholar]
- 37.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.H., McDevitt J.J., Hau B.J.P., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368 doi: 10.1126/science.abc6197. 1422‒4. [DOI] [PubMed] [Google Scholar]
- 39.Doremalen N van, Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2004973. 1564‒7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Guo M., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582 doi: 10.1038/s41586-020-2271-3. 557‒60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.