Abstract
Background:
Campylobacter fetus subsp. fetus is the causal agent of sporadic abortion and infertility in bovines that produces economic losses in livestock.
Aims:
This study evaluates the capability of C. fetus subsp. fetus to invade and survive in bovine endometrial epithelial cells and attempts to describe a pathogenic mechanism of this microorganism.
Methods:
Primary culture of bovine endometrial epithelial cells was challenged with C. fetus subsp. fetus. Intracellular bacteria, represented by the number of genomic copies (g.c.) were quantified at 0, 2, 4, 10, and 24 hours post-infection (h.p.i.), by quantitative polymerase chain reaction (qPCR). The presence of intracellular bacteria was evaluated by immunofluorescence and immunohistochemistry.
Results:
The results showed that only viable C. fetus subsp. fetus could invade endometrial cells. The g.c. number in assays with viable C. fetus subsp. fetus reached an average value of 656 g.c., remained constant until 4 h.p.i., then decreased to 100 g.c, at 24 h.p.i. In assays with non-viable microorganisms, the average value of g.c. was less than 1 g.c. and never changed. The intracellular presence of this bacteria was confirmed at 2 h.p.i. by immunofluorescence and immunohistochemistry.
Conclusion:
The results suggest that only C. fetus subsp. fetus viable can invade bovine endometrial epithelial cells but will not replicate in them, indicating that the endometrial cells do not represent a replication niche for this pathogen. Nonetheless, this invasion capability suggests that this type of cell could be employed by the pathogen to spread to other tissues.
Key Words: Bovine endometrial cells, Campylobacter fetus, Intracellular survival
Introduction
Campylobacter fetus subsp. fetus is frequently isolated from the intestinal tract of asymptomatic cattle, goats, and sheep. In animals, C. fetus subsp. fetus exhibits a tropism for placental and reproductive tract tissues and is one of the major causes of both sporadic and epidemic septic abortions (Viejo et al., 2001 ▶; Iraola et al., 2012 ▶). The diseases produced by C. fetus subsp. fetus generates considerable economic losses, representing a significant problem in animal production (Michi et al., 2016 ▶; Iraola et al., 2017 ▶). Campylobacter fetus is a microaerophilic Gram-negative spiral-shaped bacterium that resides in the epithelial crypts of the bovine prepuce, and is transmitted to the cow during service or artificial insemination with contaminated semen (Mshelia et al., 2010 ▶; Gard, 2016 ▶; Michi et al., 2016 ▶). Campylobacter fetus subsp. fetus can attach in an irreversible way to bull spermatozoa and affect sperm quality (Cagnoli et al., 2020 ▶).
Even though C. fetus is an animal health problem, its pathogenicity mechanisms are unknown. Considering the pathogenesis of Campylobacter sp. infections, this microorganism must have mechanisms involved in the colonization or invasion of tissues. Virulence factors such as adhesions, secretion systems, and anti-phagocytic layers have been identified in the genome of C. fetus (Kienesberger et al., 2014 ▶; Kaakoush et al., 2015 ▶). Nonetheless, it is still necessary to describe the pathogenesis mechanisms associated with cell infection in animal tissues.
During an infection of a heifer’s reproductive system, the endometrial epithelial cells are the first kind of cells that might interact with C. fetus. Some authors had demonstrated that C. fetus is able to adhere and invade many human epithelial cell lines like Hep-2 (Konkel and Joens, 1989 ▶), INT407 (Graham, 2003 ▶), Caco-2 (Baker and Graham, 2010 ▶), and HT-29/B6 (Bücker et al., 2017 ▶). Endometrial epithelial cells could play an important role in C. fetus infections because they act as a physical and immunological barrier in heifer reproductive organs during this process (Agostinis et al., 2019 ▶). In this study,
the capability of C. fetus subsp. fetus to invade and survive in bovine endometrial epithelial cells was evaluated using quantitative polymerase chain reaction (qPCR) and microscopy, to establish basic aspects of BGV pathogenesis.
Materials and Methods
Strains and bacteria culture conditions
Campylobacter fetus subsp. fetus ATCC 27374 (Oliveira et al., 2016 ▶) was grown under a microaerophilic atmosphere (85% N2, 10% CO2, and 5% O2, CampyGen, Oxoid) on selective Campylobacter agar supplemented with 5% sheep blood at 37°C for 96 h. To evaluate if the bacterial invasion was dependent on cell viability, C. fetus subsp. fetus was inactivated by heat treatment at 56°C for 90 min (C. fetus subsp. fetus heat-inactivated). Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028 was used as a positive control in invasion assays, considering its capability to invade different types of epithelial cells (Hall and Jones, 1977 ▶; Brumell et al., 1999 ▶, Paukszto et al., 2020 ▶). Salmonella typhimurium was grown on Luria-Bertani (LB) agar and subcultivated in a hyperosmolar LB broth for 24 h at 37°C.
Primary bovine endometrial epithelial cell culture
Approximately 5 cm2 of uterus tissue was obtained from three slaughtered cows from a slaughterhouse. The tissue was washed three times with Hank’s solution supplemented with gentamicin (1.6 mg/ml) and transported to the laboratory in the same solution on ice (~ 6°C). The tissue was cut into pieces (approximately 3 mm2) and washed with phosphate-buffered saline (PBS) pH = 7.2 (1.9 mM NaH2PO4, 8.1 mM Na2HPO4, 154 mM NaCl). Two grams of tissue fragments were partially disaggregated with 40 ml of digestion solution, Dulbecco’s modified eagle medium (DMEM) supplemented with 1.13 mg/ml of collagenase type I, and 1 UI/ml DNAse I) at 37°C for 1.5 h on stirring. After this time, the cell suspension was centrifuged at 1500 × g for 5 min, and 30 ml of supernatant was recovered and centrifuged at 3500 × g for 10 min. The cell pellet was washed three times with 5 ml of DMEM/PBS (2:1), filtered (40 µm diameter/pore) and transferred into 25 cm2 cell culture flasks (Nunc, Thermo Fisher Scientific, USA) with 5 ml of DMEM-primary culture medium [50 mM hydroxyethyl piperazineethanesulfonic acid (HEPES), 5% vol/vol fetal bovine serum, 1% v/v antibiotic/antifungal solution (penicillin G 10,000 U, streptomycin 5,000 µg, and amphotericin B 12.5 µg per ml)]. Cells were incubated for 24 h at 37°C. On the next day, the medium was removed, the cells were washed with DMEM/PBS (2:1) three times and 5 ml of DMEM-primary culture medium was finally added. For fibroblast depuration, one-minute trypsinization was performed daily for three consecutive days (Kisselbach et al., 2009 ▶).
Evaluation of C. fetus intracellular survival
Gentamicin protection assays were performed following a standard protocol (Elsinghorst, 1994 ▶). Campylobacter fetus subsp. fetus was grown on Campylobacter selective agar supplemented with 5% sheep blood at 37°C for 72 h under a microaerophilic atmosphere. Bacteria biomass was collected in PBS and diluted in minimum essential medium (MEM) to adjust a multiplicity of infection (MOI) 100:1. Endometrial cell monolayers (10,000 cells/well) were infected and centrifuged at 800 × g for 5 min to maximized cell-bacteria contact. Plates were incubated at 37°C for 2 h. Then, monolayers were washed twice with PBS and incubated with DMEM medium supplemented with 5% fetal bovine serum and 100 µg/ml gentamicin. At 0, 2, 4, 10, and 24 hours post-infection (h.p.i.), three wells were washed with PBS and intracellular bacteria were recuperated by the addition of 1 ml of 1% Triton X-100. The cell lysates were collected in microtubes and kept frozen until the intracellular microorganism quantification assays were done.
Gentamicin sensitivity evaluation in C. fetus subsp. fetus
Before evaluating bacterial intracellular survival by gentamicin protection assays, sensitivity to this antibiotic was determined using the disc diffusion method. Briefly, C. fetus subsp. fetus was grown in blood agar for 96 h at 37°C under a microaerophilic atmosphere. Bacteria were suspended at 2 × 106 colony-forming unit/ml (CFU/ml), and 100 µl were plated on Mueller Hinton agar. Sterilized paper filter discs were set on an agar plate, and gentamicin was added to each disc in the following concentrations: 0, 25, 50, 100, 200, and 300 µg/ml. The plates were incubated under a microaerophilic atmosphere at 37°C for 72 h. The inhibition zones surrounding the discs were measured and compared with inhibition zones produced by ciprofloxacin (100 µg/ml) to determine antimicrobial sensitivity (Luangtongkum et al., 2007 ▶).
DNA extraction from infected cells
Cell lysates recuperated from intracellular survival assays were centrifuged at 12,000 × g for 20 min at 4°C. The pellet was recovered and suspended in 500 µL of lysis solution (10 mM Tris-HCl, pH = 8, 25 mM ethylenediaminetetraacetic acid (EDTA), pH = 8, and 2% sodium lauryl sulfate). Then 50 µg of proteinase K was added and incubated at 56°C for an h. Later, 300 µL of phenol/chloroform (1:1) was added, the tube was homogenized and centrifuged at 10,000 × g for 2 min. The supernatant was recovered, and 50 µL of 3 M sodium acetate pH = 6.0 and 1 ml of 100% ethanol were added. Tubes were incubated at -20°C for 2 h and then centrifuged at 13,300 × g for 20 min at 4°C. The pellet was washed with 70% ethanol and suspended with 20 µL of TE (10 mM Tris, pH = 8, 1 mM EDTA, pH = 8). The obtained DNA was quantified by spectrophotometry at 260 nm (Bio-Rad® SmartSpec Plus Spectrophotometer, USA).
Intracellular microorganism quantification by qPCR
Specific primers designed for DNA sequences of 16s rRNA and invA genes were used to quantify C. fetus subsp. fetus and S. typhimurium, respectively. Primer sequences for C. fetus were 5´-GAG ATC ACC AGG AAT ACC C-3´ and 5´-CAC CTG TCT CAA CTT TCT AGC-3´ and for S. typhimurium were 5´-GTG AAA TTA TCG CCA CGT TCG GGC AA-3´ and 5´-TCA TCG CAC CGT CAA AGG AAC C-3´. For the qPCR assays, a commercial kit (Power SYBR Green Applied Biosystems) was used, following the manufacturer’s protocol. Cycle conditions for C. fetus subsp. fetus amplification were as follow: initial denaturation of 95°C for 60 s, followed by 40 cycles of denaturation at 94°C for 60 s, annealing at 50°C for 60 s, and extension of 72°C for 60 s. For S. typhimurium, qPCR conditions included an initial denaturation of 95°C for 60 s, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension of 72°C for 30 s. Standard curves (1 ng, 100 pg, 10 pg, 1pg, and 0.1 pg of chromosomal DNA from both microorganisms) were used for quantification.
Immunofluorescence and immunohistochemistry
Bovine endometrial epithelial cells were infected as previously described, washed with PBS and fixed with 2% of paraformaldehyde for 20 min at room temperature. For the detection of intracellular bacteria, cells were permeabilized with 0.1% of Triton X-100 for 10 min. For the immunofluorescence analysis, cells were incubated for 30 min at 37°C with 50 µg/ml of phalloidin-fluorescein isothiocyanate (FITC) diluted with 1% of dimethyl sulfoxide (DMSO), 1 mg/ml propidium iodide, Alexa Fluor 488 conjugate rabbit antibody anti-cytokeratin 18, rabbit anti-C. fetus antibodies and Alexa Fluor 594 conjugated goat antibody anti-rabbit (IgG). The samples were observed using an epifluorescence microscope (E800, Nikon, Japan). For the immunohistochemistry analysis, cells were incubated for 30 min at 37°C, with rabbit anti-C. fetus antibodies and horseradish peroxidase-conjugated goat antibodies anti-rabbit IgG. It was used as a staining solution with 0.05% of 3-amino-9-ethylcarbazole, 0.015% of H2O2 in 0.05 M of sodium acetate pH = 5.5. Second staining was applied to provide contrast (Diffquick Staining System, Millipore). The samples were observed using an optic microscope (Velab, Mex). To evaluate the intracellular presence of C. fetus subsp. fetus in both assays, an experimental control without permeabilization was included.
Statistical analysis
Six assays with three replicates for each treatment were performed. Each treatment was subjected to the Shapiro-Wilk test, and the difference in intracellular bacterial number in each treatment was analyzed through Student’s t-test.
Results
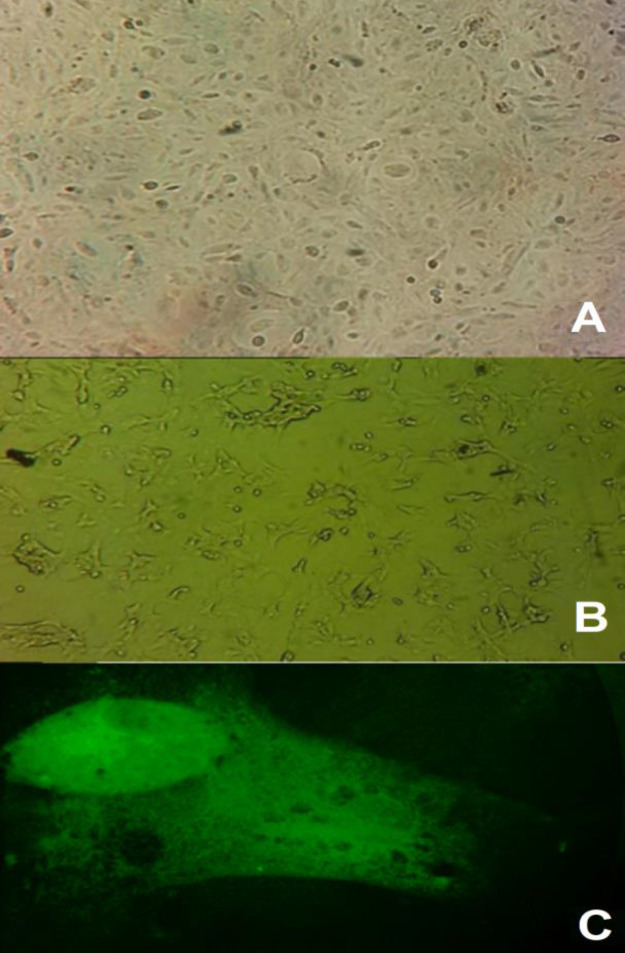
Primary bovine endometrial epithelial cell culture was established until the second week of incubation. The cells in the monolayer showed a polygonal epithelial-like morphology (Fig. 1A). The cells remained viable until the seventh passage, while later sub-cultures showed abnormal morphology (rounded cells), and their proliferation rates decreased to 50% (Fig. 1B). For cell-type confirmation, cytokeratin 18 was visualized by immunofluorescence. The results showed the presence of this protein in more than 90% of the cells (Fig. 1C).
Fig. 1.
Bovine endometrial epithelial cells cultures. (A) Normal appearance of epithelial-like cells after 7th passaging (×20), (B) Appearance of epithelial cells of bovine endometrium at 10th passaging (×20), and (C) Immunofluorescence assay of bovine endometrial cells stained with Alexa 488 (green) showed the presence of Cytokeratin 18 (×40)
Before intracellular survival evaluation assays, the sensibility of C. fetus subsp. fetus to gentamicin was determined. The results showed that C. fetus subsp. fetus was not able to grow around paper filter discs with 25 µg/ml of gentamicin. However, it was able to grow in DMEM without gentamicin (data not shown). The inhibition area observed with 25 µg/ml was similar to the one obtained with ciprofloxacin (~27 mm diameter). With higher concentrations of gentamicin, the inhibition was superior.
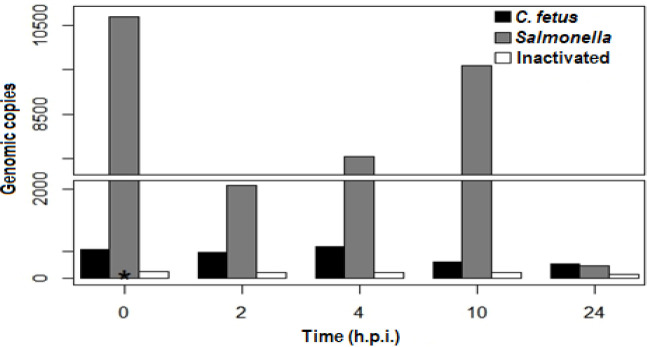
The results of intracellular survival assays with C. fetus subsp. fetus viable (non-heat-inactivated) showed that in the first 4 h.p.i., the number of genomic copies (g.c.) remained almost the same. At 0 h.p.i., there was an average (~) of 633.85 g.c. per 10,000 cells in the well (g.c./cell-well), at 2 h.p.i. were ~640.2 g.c./cell-well, and at 4 h.p.i. were ~696 g.c./cel-well. Later, the number of g.c. decreased significantly. At 10 h.p.i., there were ~350.3 g.c./cell-well and at 24 h.p.i. there were ~282.0 g.c./cel-well (Fig. 2). At this point, it is important to mention that the cellular monolayer remained intact until the last time (data not shown). Infection with C. fetus subsp. fetus non-viable (heat-inactivated) showed only ~1 g.c. in all post-infection times (Fig. 2). This data suggests that C. fetus subsp. fetus can invade bovine endometrial epithelial cells but cannot grow inside them. It also indicates that invasion was only induced by viable microorganisms. In contrast, the number of g.c. obtained from cells infected with S. typhimurium slightly decreased in the first 2 h.p.i. At 0 h.p.i., there were ~3280.2 g.c./cell-well, which decreased to ~2690.0 g.c./cell-well at 2 h.p.i. Later, at 4, 10, and 24 h.p.i. the g.c. increased to ~7550, ~9590.7, and ~28400.2 g.c./cell-well, respectively. These results indicate that S. typhimurium can invade and replicate inside endometrial epithelial cells.
Fig. 2.
Intracellular survival assays. The bovine endometrial epithelial cells were infected with C. fetus ATCC 27374, S. typhimurium, and C. fetus subsp. fetus non-viable (heat-inactivated). Intracellular bacteria were quantified by qPCR at 0, 2, 4, 10, and 24 h.p.i
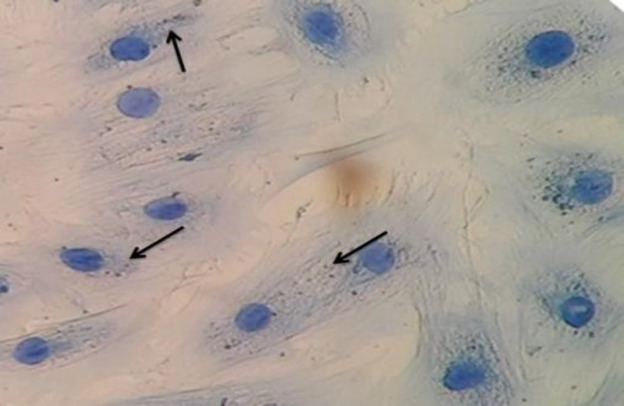
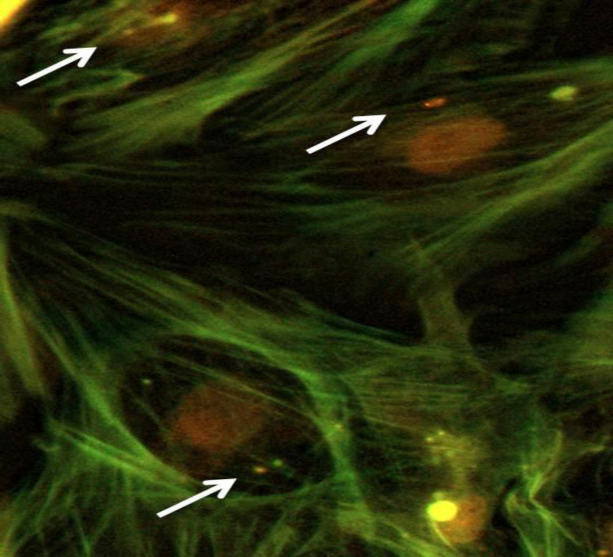
The presence of C. fetus subsp. fetus inside of epithelial cells was confirmed by immunohistochemistry (Fig. 3) and immunofluorescence (Fig. 4). The results of both assays at 2 h.p.i. revealed intracellular bacteria in permeabilized cells but not in the non-permeabilized cells. Considering that C. fetus subsp. fetus did not proliferate, its presence was not evaluated at a later time post-infection. These results demonstrate that C. fetus subsp. fetus can invade cells, confirming the results obtained in qPCR.
Fig. 3.
Immunohistochemistry of intracellular C. fetus ATCC 27374 in bovine endometrial epithelial cells (×40). The microorganisms observed inside the cell are in brown and the cells are in blue color. The black arrows show the intracellular bacteria
Fig. 4.
Immunofluorescence of intracellular C. fetus in bovine endometrial epithelial cells. The cytoskeleton was stained with phalloidin-FITC (green), nucleus with propidium iodide, and intracellular microorganisms with Alexa 594 (red). The white arrow shows C. fetus inside the cells
Discussion
Campylobacter fetus subsp. fetus produces sporadic infections in the bovine genital tract. However, until now, the virulence mechanisms have not been well described. In this work, the ability of C. fetus subsp. fetus to invade bovine endometrial epithelial cells was demonstrated. In previous studies, invasion of Campylobacter spp. in epithelial cells was proved in which only immortalized cellular lines were used (Konkel and Joens, 1989 ▶; Graham, 2003 ▶; Watson and Galán, 2008 ▶; Baker and Graham, 2010 ▶; Bücker et al., 2017 ▶). In this study, primary cell cultures were used to avoid possible alteration in the phenomena (Alge et al., 2006 ▶). Our results showed the ability of C. fetus subsp. fetus to invade bovine endometrial epithelial cells, but not to survive inside them. This could suggest that these cells do not represent a replicative niche. However, some aspects are necessary to resolve, like the role of a hormonal environment (Wang et al., 2010 ▶).
The comparison between the results of the viable and nonviable (heat-inactivated) C. fetus subsp. fetus, showed that the ability to invade endometrial cells is an active bacterial process rather than a cellular one since this bacterium cannot invade the epithelial cells. In several intracellular microorganisms, this process involves the expression of many genes to achieve the intracellular niche, or in the case of Campylobacter jejuni, translocation through the epithelial cells (Dersch, 2003 ▶; Baker and Graham, 2010 ▶).
In the present work, the intracellular presence of C. fetus subsp. fetus was demonstrated by qPCR. This technique was previously used to quantify fastidious microorganisms (Blakes et al., 2015 ▶; Ricchi et al., 2017 ▶). The data obtained by qPCR was confirmed by immunofluorescence and immunohistochemistry, suggesting that this pathogen possesses mechanisms that enable the invasion of the cells. In other studies, qPCR has been used for evaluating intracellular proliferation, especially for viable but nonculturable microorganisms which are difficult to grow in the laboratory (González-Escalona et al., 2006 ▶; Wattanaphansak et al., 2010 ▶; Blakes et al., 2015 ▶). In this regard, research performed with T84 cells revealed that the intracellular survival capability of C. jejuni could not be established since culture is complicated after the infection. In this study, a qPCR was used to evaluate bacterial proliferation (Watson and Galán, 2008 ▶).
In intracellular survival assays, the genomic copy number analysis showed that the number of intracellular bacteria remains constant until 4 h.p.i., and then decreases to 24 h. These suggest that C. fetus subsp. fetus cannot evade bactericidal mechanisms of the cell and that bacterial and DNA degradation starts at 4 h.p.i. However, the absence of proliferation inside the cells could be due to another mechanism, such as the lack of metabolism needed for survival inside the cells. Although this is a hypothesis, more analysis is required to prove it. These results agree with the ones obtained previously with INT407 cell infections (Graham, 2003 ▶). In these assays, viable microorganisms were obtained only until 4 h.p.i. It was also observed that C. fetus subsp. fetus was less invasive than S. typhimurium (used as a positive control of infection). This microorganism was able to replicate inside this cell type. This result can be normal, considering that the type III secretion system gives bacteria the ability to invade and multiply in many types of cells (Yoon et al., 2011 ▶; Boumart et al., 2014 ▶).
The type of cell used in this study showed the typical morphology of endometrial epithelial cells, described as cuboid shape cells that form a cobblestone-like structure in monolayers (Eritja et al., 2010 ▶). In this work, the cells maintained their morphology and viability until the seventh passage. Other authors indicated that cell viability can be preserved until 40 passes by using growth promoters (Feng et al., 2012 ▶). However, a free hormone cell culture media was used in our experiment to avoid interference with bactericidal mechanisms of endometrial cells. For cell-type confirmation, cytokeratin 18 protein was detected by immunofluorescence. This protein has been previously described in other studies, where primary endometrial epithelial cells were established as models for hormonal effect analyses (Eritja et al., 2010 ▶; Feng et al., 2012 ▶; Haeger et al., 2015 ▶). In this work, more than 90% of the cells showed the presence of cytokeratin 18. These results confirm that this primary cell culture agrees with bovine endometrial epithelial cells and can be used to evaluate intracellular survival. On the other hand, the absence of fibroblast-like cells demonstrated that the trypsinization process was useful to eliminate this cell-type as previously described (Kisselbach et al., 2009 ▶).
Other studies have shown the translocation capability of C. jejuni where the cell monolayer always keeps its integrity (Baker and Graham, 2010 ▶; Day et al., 2010 ▶). In this work, the monolayer of endometrial cells remained without perceptible changes; thus, C. fetus subsp. fetus might have the same translocation ability as C. jejuni since the bovine endometrial epithelial cells are not the proliferative niche for this microorganism. This mechanism of translocation could represent to C. fetus the possibility to access other cell types, such as stromal cells or macrophages, where the pathogen might proliferate (Louwen et al., 2012 ▶).
Future investigations in C. fetus subsp. fetus pathogenicity mechanisms may shed light on the translocation ability of this pathogen and help identify the replication niche, understand the pathogenesis of campylobacteriosis and develop new strategies for prevention and treatment. In conclusion, C. fetus subsp. fetus can invade bovine endometrial epithelial cells but cannot survive inside them. This suggests the existence of other proliferative niches for this pathogen and establishes the possibility that endometrial cells are only used by this pathogen to reach submucosa tissue and induce uterine inflammations associated with abortions.
Acknowledgment
This research was supported by Universidad Autónoma Metropolitana, Unidad Xochimilco; Project “Molecular pathogenesis of bacterial infections.”
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agostinis C, Mangogna A, Bossi F, Ricci G, Kishore U. Uterine immunity and microbiota: A shifting paradigm. Front Immunol. 2019;10:1–11. doi: 10.3389/fimmu.2019.02387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alge CS, Hauck SM, Priglinger SG, Kampik A, Ueffing M. Differential protein profiling of primary versus immortalized human RPE cells identifies expression patterns associated with cytoskeletal remodeling and cell survival. J. Proteome Res. 2006;5:862–878. doi: 10.1021/pr050420t. [DOI] [PubMed] [Google Scholar]
- Baker NT, Graham LL. Campylobacter fetus translocation across Caco-2 cell monolayers. Microb. Pathog. 2010;49:260–272. doi: 10.1016/j.micpath.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Blakes A, Day NPJ, Atwal S, Giengkam S, Blacksell SD, Paris DH. Improved quantification, propagation, purification, and storage of the obligate intracellular human pathogen Orientia tsutsugamushi. PLoS Negl. Trop. Dis. 2015;9:1–20. doi: 10.1371/journal.pntd.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumart Z, Velge P, Wiedemann A. Multiple invasion mechanisms and different intracellular behaviors: a new vision of Salmonella–host cell interaction. FEMS Microbiol. Lett. 2014;361:1–7. doi: 10.1111/1574-6968.12614. [DOI] [PubMed] [Google Scholar]
- Brumell JH, Steele-Mortimer O, Finlay BB. Bacterial invasion: force feeding by Salmonella. Curr. Biol. 1999;9:277–280. doi: 10.1016/s0960-9822(99)80178-x. [DOI] [PubMed] [Google Scholar]
- Bücker R, Krug SM, Fromm A, Nielsen HL, Fromm M, Nielsen H. Campylobacter fetus impairs barrier function in HT-29/B6 cells through focal tight junction alterations and leaks. Ann. N. Y. Acad. Sci. 2017;1405:189–201. doi: 10.1111/nyas.13406. [DOI] [PubMed] [Google Scholar]
- Cagnoli CI, Chiapparrone ML, Cacciato CS, Rodríguez MG, Aller JF, Catena MDC. Effects of Campylobacter fetus on bull sperm quality. Microb. Pathog. 2020;149:1–5. doi: 10.1016/j.micpath.2020.104486. [DOI] [PubMed] [Google Scholar]
- Day AS, Nahidi L, Kaakoush NO, Mitchell HM, Zhang L, Leach ST. Host Attachment, invasion, and stimulation of proinflammatory cytokines by Campylobacter concisus and other non-Campylobacter jejuniCampylobacter species. J. Infect. Dis. 2010;202:1855–1865. doi: 10.1086/657316. [DOI] [PubMed] [Google Scholar]
- Dersch, P. Molecular and cellular mechanisms of bacterial entry into host cells. In: Herwald, H (Ed.), Host response mechanisms in infectious diseases. 1st Edn. Switzerland: Karger; 2003. pp. 183–209. [DOI] [PubMed] [Google Scholar]
- Elsinghorst EA. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- Eritja N, Llobet D, Domingo M, Santacana M, Yeramian A, Matias-Guiu X. A novel three-dimensional culture system of polarized epithelial cells to study endometrial carcinogenesis. Am. J. Pathol. 2010;176:2722–2731. doi: 10.2353/ajpath.2010.090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Cao Y, Liu Z, Fu Y, Liu B, Li D. Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signaling molecules expression in bovine endometrial epithelial cells. Vet. Immunol. Immunopathol. . 2012;151:20–27. doi: 10.1016/j.vetimm.2012.09.039. [DOI] [PubMed] [Google Scholar]
- Gard J. Bovine genital campylobacteriosis-A review. Int. J. Vet. Sci. Res. 2016;2:29–31. [Google Scholar]
- Graham LL. Campylobacter fetus adheres to and enters INT 407 cells. Can. J. Microbiol. 2003;48:995–1007. doi: 10.1139/w02-096. [DOI] [PubMed] [Google Scholar]
- González-Escalona N, Fey A, Höfle MG, Espejo RT, Guzmán CA. Quantitative reverse transcription polymerase chain reaction analysis of Vibrio cholerae cells entering the viable but non-culturable state and starvation in response to cold shock. Environ. Microbiol. 2006;8:658–666. doi: 10.1111/j.1462-2920.2005.00943.x. [DOI] [PubMed] [Google Scholar]
- Haeger JD, Hambruch N, Dantzer V, Hoelker M, Schellander K, Klisch K. Changes in endometrial ezrin and cytokeratin 18 expression during bovine implantation and in caruncular endometrial spheroids in vitro. Placenta. 2015;36:821–831. doi: 10.1016/j.placenta.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Hall GA, Jones PW. A study of the pathogenesis of experimental Salmonella dublin abortion in cattle. J. Comp. Pathol. 1977;87:53–65. doi: 10.1016/0021-9975(77)90079-2. [DOI] [PubMed] [Google Scholar]
- Iraola G, Forster SC, Kumar N, Lehours P, Bekal S, García-Peña FJ. Distinct Campylobacter fetus lineages adapted as livestock pathogens and human pathobionts in the intestinal microbiota. Nat. Commun. 2017;8:1367–1370. doi: 10.1038/s41467-017-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraola G, Hernández M, Calleros L, Paolicchi F, Silveyra S, Velilla A, Carretto L, Rodríguez E, Pérez R. Application of a multiplex PCR assay for Campylobacterfetus detection and subspecies differentiation in uncultured samples of aborted bovine fetuses. J. Vet. Sci. 2012;13:371–376. doi: 10.4142/jvs.2012.13.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienesberger S, Sprenger H, Wolfgruber S, Halwachs B, Thallinger GG. Comparative genome analysis of Campylobacter fetus subspecies revealed horizontally acquired genetic elements important for virulence and niche specificity. PLOS One. 2014;9:1–13. doi: 10.1371/journal.pone.0085491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselbach L, Merges M, Bossie A, Boyd A. CD90 expression on human primary cells and elimination of contaminating fibroblasts from cell cultures. Cytotechnology. 2009;59:31–44. doi: 10.1007/s10616-009-9190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel ME, Joens LA. Adhesion to and invasion of HEp-2 cells by Campylobacter spp. Infect. Immun. 1989;57:2984–2990. doi: 10.1128/iai.57.10.2984-2990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwen R, Nieuwenhuis EES, van Marrewijk L, Horst-Kreft D, de Ruiter L. Campylobacter jejuni translocation across intestinal epithelial cells is facilitated by ganglioside-like lipooligosaccharide structures. Infect. Immun. 2012;80:3307–3318. doi: 10.1128/IAI.06270-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luangtongkum T, Morishita TY, El-Tayeb AB, Ison AJ, Zhang Q. Comparison of antimicrobial susceptibility testing of Campylobacter spp by the agar dilution and the agar disk diffusion methods. J. Clin. Microbiol. 2007;45:590–594. doi: 10.1128/JCM.00986-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michi AN, Favetto PH, Kastelic J, Cobo ER. A review of sexually transmitted bovine trichomoniasis and campylobacteriosis affecting cattle reproductive health. Theriogenology. 2016;85:781–791. doi: 10.1016/j.theriogenology.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Mshelia GD, Amin JD, Woldehiwet Z, Murray RD, Egwu GO. Epidemiology of bovine venereal campylobacteriosis: geographic distribution and recent advances in molecular diagnostic techniques. Reprod. Domest. Anim. 2010;45:221–230. doi: 10.1111/j.1439-0531.2009.01546.x. [DOI] [PubMed] [Google Scholar]
- Oliveira LM, Resende DM, Dorneles EM, Horácio EC, Alves FL, Gonçalves LO, Tavares GS, Stynen AP, Lage AP, Ruiz JC. Complete genome sequence of type strain Campylobacter fetus subsp fetus ATCC 27374 Genome Announc. enome Announc. 2016:e01344–16. doi: 10.1128/genomeA.01344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukszto L, Mikolajczyk A, Jastrzebski JP, Majewska M, Dobrzyn K, Kiezun M, Smolinska N, Kaminski T. Transcriptome, spliceosome and editome expression patterns of the porcine endometrium in response to a single subclinical dose of Salmonella Enteritidis lipopolysaccharide. Int. J. Mol. Sci. 2020;2:1–25. doi: 10.3390/ijms21124217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricchi M, Bertasio C, Boniotti MB, Vicari N, Russo S, Tilola M. Comparison among the quantification of bacterial pathogens by qPCR, dPCR, and cultural methods. Front Microbiol. 2017;8:1–15. doi: 10.3389/fmicb.2017.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo G, Gomez B, De Miguel D, Del Valle A, Otero L, De La Iglesia P. Campylobacter fetus subspecies fetus bacteremia associated with chorioamnionitis and intact fetal membranes. Scand. J. Infect. Dis. 2001;33:126–127. doi: 10.1080/003655401750065517. [DOI] [PubMed] [Google Scholar]
- Wang HB, Lü SH, Lin QX, Feng LX, Li DX, Duan CM. Reconstruction of endometrium in vitro via rabbit uterine endometrial cells expanded by sex steroid. Fertil. Steril. 2010;93:2385–2395. doi: 10.1016/j.fertnstert.2009.01.091. [DOI] [PubMed] [Google Scholar]
- Watson RO, Galán JE. Campylobacterjejuni survives within epithelial cells by avoiding delivery to lysosomes. PLOS Pathog. 2008;4:1–15. doi: 10.1371/journal.ppat.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanaphansak S, Gebhart CJ, Anderson JM, Singer RS. Development of a polymerase chain reaction assay for quantification of Lawsonia intracellularis. J. Vet. Diagn. Invest. 2010;22:598–602. doi: 10.1177/104063871002200416. [DOI] [PubMed] [Google Scholar]
- Yoon H, Gros P, Heffron F. Quantitative PCR-based competitive index for high-throughput screening of Salmonella virulence factors. Infect. Immun. 2011;79:360–368. doi: 10.1128/IAI.00873-10. [DOI] [PMC free article] [PubMed] [Google Scholar]