Objectives:
To evaluate the effect of eye spray phospholipid concentration on symptoms and tear film stability.
Methods:
High-concentration (Tears Again, Optima Pharma GmbH, Hallbergmoos, Germany) and low-concentration (Ocuvers, Innomedis AG, Germany) phospholipid eye sprays were sprayed onto the closed eyelids of 30 subjects (33.2±1.8 years; 20 women) in a multicentered, prospective, crossover study. Ocular comfort (visual analog scale) and noninvasive tear film stability (NIBUT) of each eye were evaluated before application (along with the Ocular Surface Disease Index), 10 min after application, and 30 min after application.
Results:
Comfort (high concentration: 68.5±16.4 vs. low concentration: 70.7±14.5 phospholipid) and NIBUT (high concentration: 11.5±4.6 sec vs. low concentration: 11.2±6.0 sec phospholipid) were not different (P>0.3) between sprays before application, but comfort (by 12 points, P=0.001) and NIBUT (by 5 sec, P=0.016) were significantly better with a high-concentration phospholipid spray at both 10 min and 30 min time points than those with the low-concentration phospholipid spray.
Conclusions:
The liposomal eye spray with higher concentration of phospholipids significantly improved ocular comfort and tear film stability in contrast to the eye spray with lower concentration of phospholipids, hence practitioners need to choose an appropriate eye spray to maximize the patient benefit.
Key Words: Phospholipids, Tear film stability, Comfort, Tear film
Dry eye is a common disease, described as aqueous-deficient and/or evaporative dry eye,1 the latter being the most common type (78%).2,3 The lipid layer of the tears plays an important role in inhibiting tear film evaporation and in spreading the tears across the ocular surface.4 The lipid layer is a complex structure with an inner polar layer, interfacing with the aqueous phase and reducing surface tension, and a thicker, outer, nonpolar layer.5 The lipid layer stabilizes the tear film providing a surface tension decrease and a 90% to 95% aqueous evaporation reduction.6,7
Phospholipid liposomal spray is suggested to improve the polar properties of the lipid layer (such as its surface tension and solubility), enhancing lipid spread over the tear film.8–14 The spray is applied to the closed eyelids and supplements phospholipid liposomes to the lid margins, where the phospholipids mix with the present lipid reservoir from the meibomian glands. Improvements in symptomatology, visual acuity, lipid layer thickness, tear film stability, eyelid margin inflammation, tear production, and lid parallel conjunctival folds have been documented with the use of the original liposomal spray in patients with dry eye.8–15 However, as other sprays have become available with different concentrations of phospholipids, contradictory studies have been published on their relative effectiveness.14,16,17 These studies were based on a contralateral eye design, limiting the accurate reporting of symptomology.18 It is well established that normal eyes do not function as independent units, but rather there is cross-communication between right and left eyes.18 Hence this study aimed to determine the relative effectiveness of two liposomal eye sprays with different concentration of phospholipids on dry eye symptoms and tear film stability in a multicentered, prospective, crossover trial.
METHODS
Based on laboratory analyses of phospholipid concentrations of different sprays, one high-concentration and one low-concentration phospholipid eye spray was selected (Table 1).
Table 1:
Laboratory Analyses of Two Different Liposomal Eye Sprays (Spectral Service GmbH, Cologne, Germany; Analyses Certificate DIA76896-1, 02. and DIA74607-3; 2018)
| Product | Phospholipids | Weight Percent | Concentration |
| Ocuvers Spray hyaluron | Phosphatidylcholine | 0.04 | 0.4 mg/mL |
| Unidentifiable phospholipids | 0.08 | 0.8 mg/mL | |
| In total | 0.12 | 1.2 mg/mL | |
| Tears Again Sensitive | Phosphatidylcholine | 0.95 | 9.5 mg/mL |
| 2-Lysophosphatidylcholine | 0.03 | 0.3 mg/mL | |
| In total | 0.98 | 9.8 mg/mL |
Participants gave written informed consent before participating in the study. Ethical approval was given by the Research Ethics Committee of Aston University. All procedures were conducted in accordance with the Declaration of Helsinki (1983). Potential participants were excluded if they were contact lens wearers or had worn lenses within the last year, were younger than 18 years, had any ocular surface defect or pathology, were pregnant, or had used any ocular medications or drops on the study visit days.
The high-concentration (Tears Again Sensitive, Optima Pharmazeutische GmbH, Hallbergmoos, Germany) and low-concentration phospholipid (Ocuvers, Innomedis AG, Germany) formulations were sprayed from a distance of 10 cm onto each closed eyelids of 30 participants in a multicentered, prospective, double-blind, crossover study. Participants were randomized as to which spray they received bilaterally first, and there was a gap of 24 to 120 hr between trials.
Before application, each participant's symptoms were evaluated using the Ocular Surface Disease Index (OSDI) questionnaire.19 Total OSDI scores were calculated as recommended by Schiffman et al.20 Ocular comfort (“How is the current comfort of your eye?”) of each eye was reported on a visual analog scale (100 = perfect) and noninvasive tear breakup time (NIBUT) was determined using a Tearscope Plus (Keeler Ltd, Windsor, United Kingdom) with a fine grid insert21 before and again 10 min and 30 min after application of the sprays. Noninvasive tear breakup time was the time measured, in seconds, between the full opening of the eyelids after a complete blink and the first break in the tear film. Three consecutive readings were evaluated, and the median noted. Both the observer and the subject were masked from the previous results throughout the study. As both sprays are CE marked, have been commercially available for many years and have been evaluated in different independent studies,8,10,13–17,22 the safety of the spray was not part of the study design.
Statistical Analyses
The distribution of the data was tested using a Shapiro–Wilk test. As all the data were significantly different from a normal distribution, differences between treatments at each time point between arms were analyzed by Wilcoxon signed-rank tests and between consecutive measurements by Friedman tests (SPSS Statistics for Windows, version 20, IBM Corp., Armonk, NY). Thirty participants were recruited to allow a 90% power comparison based on previously reported noninvasive breakup time repeatability [21] to detect a difference of 2 sec (effect size=0.62; α=0.05; 1-β=0.90; G*Power v3.1.9.4: Franz Faul, University Kiel, Germany).
RESULTS
The 30 participants (aged 33.2±1.8 years; 20 women) had a mean OSDI score of 13.2±14.3 (SD). Comfort scores (Friedman test; P=0.003) and NIBUT (P=0.017) significantly increased after application of the high-concentration phospholipid spray, but (Friedman test; P=0.003) not when using the low-concentration phospholipid spray (P=0.424 and P=0.839, respectively).
When comparing both arms (high-concentration and low-concentration phospholipid sprays), comfort scores were not statistically different before treatment (Wilcoxon test; P=0.794) but were significantly better for the high-concentration phospholipid spray at the 10 min (P=0.006) and 30 min (P=0.001) time points (Fig. 1 and Table 2). Noninvasive tear breakup time was also not statistically different between arms before treatment (P=0.934) but was significantly better for the high-concentration phospholipid spray at the 10 min (P=0.038) and 30 min (P=0.027) time points (Fig. 2 and Table 2). No complications were reported from the use of either spray.
FIG. 1.
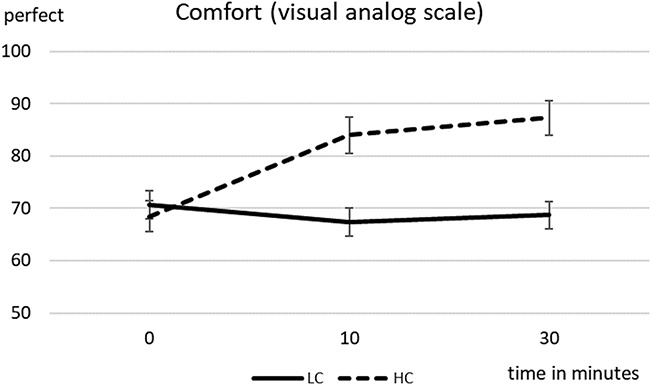
Mean comfort scores (on a 100 point, visual analog scale) and standard error over the observation time of 0 to 30 min (high-concentration (HC) and low-concentration (LC) phospholipid spray).
Table 2:
Comfort and Noninvasive Breakup Time (NIBUT) Mean and SD
| Phospholipid Concentration | Ocular Comfort | NIBUT | ||||
| Low | High | Significance | Low | High | Significance | |
| Time point | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||
| Baseline | 70.7±14.5 | 68.5±16.4 | 0.794 | 11.3±6.0 | 11.5±4.6 | 0.934 |
| 10 min | 67.4±14.8 | 84.0±19.1 | 0.006 | 11.8±5.3 | 16.7±8.8 | 0.038 |
| 30 min | 68.7±14.5 | 87.3±17.3 | 0.001 | 11.9±5.6 | 16.7±7.8 | 0.027 |
FIG. 2.
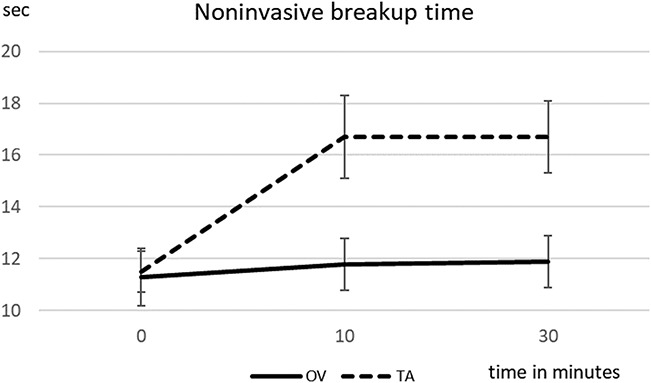
Mean noninvasive breakup time [sec] and standard error over the observation time of 0 to 30 min (high-concentration [HC] and low-concentration [LC] phospholipid spray).
DISCUSSION
This study aimed to determine the relative effectiveness of two liposomal eye sprays with different concentration of phospholipids on dry eye symptoms and tear film stability. Based on laboratory analyses, the concentration of the phospholipids of the high-concentration phospholipid spray was 8 times higher than that of the low-concentration phospholipid, but otherwise the sprays were similar in the delivery device; hence, the impact of lipid concentration on comfort and tear stability could be assessed. Use of the high-concentration phospholipid spray resulted in a significant effect on symptoms (reduction) and tear film stability (increase), whereas the lower-concentration phospholipid spray did not have any measurable effect, in the same participants. The findings of this study are in accordance with findings of many research groups in contact lens wearers and nonlens wearers.8,10,13–15,17,23 Previous studies have demonstrated that the high-concentration phospholipid spray improves tear film parameters from less than 10 min to 90 min after application.8,14 The converse findings of a single comparative contralateral trial, where a low-concentration phospholipid spray outperformed the high-concentration competitor, are hard to explain.16 The main limitation of this study was the single use of each spray and not examining a population with a wider range of dry eye symptoms. However, a marked improvement in both dryness symptoms and tear film stability was found with the concentration of the phospholipid, which would be expected to increase with more frequent use of the spray and in a population with drier eyes. In case of eyes with aqueous deficiency, phospholipids in conjunction with artificial tears might be beneficial and deserves future investigation.
In dry eye, increased evaporation of the tear film results in hyperosmolarity of the tear film, initiating a cascade of inflammatory processes resulting in epithelium damage, mucin deficiency, and reduced wettability of the cornea.24,25 This has been identified as the core mechanism of dry eye.24,26 Any improvement of the lipid layer suppresses the vicious cycle of dry eye disease, thus improving the epithelium and consequently the mucin layer and wettability of the cornea.7 This might be an important factor to be considered in the long-term treatment of patients with the evaporative form of dry eye.
This study showed that a high-concentration phospholipid spray improved tear film stability and also enhanced ocular comfort. Phospholipids are recognized to be important components within the tear film. They are vital in surface monolayer formation and for surfactant properties. Ninety-two percent of the meibum consists of neutral lipids, and the remaining 8% of polar lipids.27 The polar lipids consist of 70% phospholipids; the most predominant of which is phosphatidylcholine. Deficiency of these components prevents formation of a stable, continuous lipid layer, which, in turn, causes an increased tear evaporation rate.27,28 The liposomal spray Tears Again is a tear film supplement containing phosphatidylcholine derived from highly purified soy lecithin. The major phospholipid, phosphatidylcholine, is delivered in a stable form of liposomes to the closed eyelid. From there, they migrate, with blinking, across the eyelid margins to combine with the tear film.14 Improvement of tear film stability after application of a spray with a higher concentration of phosphatidylcholine confirms the positive effect this phospholipid has on the tear film.
CONCLUSION
The liposomal eye spray with a high concentration of phospholipids significantly improved ocular comfort and tear film stability in contrast to the eye spray with a lower concentration of phospholipids, which had no effect; hence, practitioners need to choose an appropriate phospholipid eye spray to maximize the benefit to patients with dry eyes due to a deficient lipid layer.
Footnotes
The authors have no conflicts of interest to disclose.
This investigator-initiated study was part funded by Optima Pharmazeutische GmbH, Hallbergmoos, Germany.
Contributor Information
Sonia Trave-Huarte, Email: travehus@aston.ac.uk.
James S. Wolffsohn, Email: j.s.w.wolffsohn@aston.ac.uk.
REFERENCES
- 1.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf 2017;15:539–574. [DOI] [PubMed] [Google Scholar]
- 2.Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea 2012;31:472–478. [DOI] [PubMed] [Google Scholar]
- 3.Heiligenhaus A, Koch JM, Kruse FE, et al. Diagnosis and and differentiation of dry eye disorders. Ophthalmologe 1995;92:6–11. [PubMed] [Google Scholar]
- 4.Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res 1961;1:39–45. [DOI] [PubMed] [Google Scholar]
- 5.Bron AJ, Tiffany JM, Gouveia SM, et al. Functional aspects of the tear film lipid layer. Exp Eye Res 2004;78:347–360. [DOI] [PubMed] [Google Scholar]
- 6.Lozato PA, Pisella PJ, Baudouin C. The lipid layer of the lacrimal tear film: Physiology and pathology. J Fr Ophtalmol 2001;24:643–658. [PubMed] [Google Scholar]
- 7.Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf 2017;15:438–510. [DOI] [PubMed] [Google Scholar]
- 8.Craig JP, Purslow C, Murphy PJ, et al. Effect of a liposomal spray on the pre-ocular tear film. Cont Lens Anterior Eye 2010;33:83–87. [DOI] [PubMed] [Google Scholar]
- 9.Khaireddin R, Schmidt KG. Vergleichende Untersuchung zur Therapie des evaporativen trockenen Auges. Klin Monbl Augenheilkd 2010;227:128–134. [DOI] [PubMed] [Google Scholar]
- 10.Dausch D, Lee S, Dausch S, et al. Comparative study of treatment of the dry eye syndrome due to disturbances of the tear film lipid layer with lipid-containing tear substitutes. Klin Monatsbl Augenheilkd 2006;223:974–983. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Dausch S, Maierhofer G, et al. A new therapy concept with a liposome eye spray for the treatment of the dry eye. Klin Monatsbl Augenheilkd 2004;221:825–836. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff G, Khaireddin R. Lipidsubstitution bei kontaktlinsenassoziiertem Trockenen Auge. Aktuelle Kontaktologie 2011;7:24–28. [Google Scholar]
- 13.Nosch DS, Joos RE, Job M. Prospective randomized study to evaluate the efficacy and tolerability of Ectoin containing Eye Spray (EES09) and comparison to the liposomal Eye Spray Tears Again (TA) in the treatment of dry eye disease. Cont Lens Anterior Eye 2020. doi: 10.1016/j.clae.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Pult H, Gill F, Riede-Pult BH. Effect of three different liposomal eye sprays on ocular comfort and tear film. Contact Lens Anterior Eye 2012;89:E1035–E1041. [DOI] [PubMed] [Google Scholar]
- 15.Wang MT, Ganesalingam K, Loh CS, et al. Compatibility of phospholipid liposomal spray with silicone hydrogel contact lens wear. Cont Lens Anterior Eye 2017;40:53–58. [DOI] [PubMed] [Google Scholar]
- 16.Hueck A, Wehrmann R. Comparison of the clinical efficacy of four different liposomal sprays for the treatment of dry eye. Open J Ophthalmol 2017;7:103–116. [Google Scholar]
- 17.Müller-Treiber A. Effect of the OmniTears lidspray and comparison of the effects of the OmniTears and LipoNit lidsprays. In: EAOO Warsaw 2014. Warsaw, Polan, 2014. [Google Scholar]
- 18.Novack GD, Asbell P, Barabino S, et al. TFOS DEWS II clinical trial design report. Ocul Surf 2017;15:629–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel M, Sickenberger W, Pult H. The effectiveness of questionnaires in the determination of contact lens induced dry eye. Ophthalmic Physiol Opt 2009;29:479–486. [DOI] [PubMed] [Google Scholar]
- 20.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease Index. Arch Ophthalmol 2000;118:615–621. [DOI] [PubMed] [Google Scholar]
- 21.Guillon JP. Use of the Tearscope Plus and attachments in the routine examination of the marginal dry eye contact lens patient. Adv Exp Med Biol 1998;438:859–867. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Tong L. Lipid-containing lubricants for dry eye: A systematic review. optom vis sci 2012. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Khaireddin R, Hueber A. Eyelid hygiene for contact lens wearers with blepharitis. Comparative investigation of treatment with baby shampoo versus phospholipid solution. Ophthalmologe 2013;110:146–153. [DOI] [PubMed] [Google Scholar]
- 24.2007 Report of the international dry eye workshop (DEWS). Ocul Surf 2007;5:65–204. [DOI] [PubMed] [Google Scholar]
- 25.Craig JP, Nelson JD, Azar DT, et al. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf 2017;15:802–812. [DOI] [PubMed] [Google Scholar]
- 26.Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf 2017;15:802–812. [DOI] [PubMed] [Google Scholar]
- 27.McCulley JP, Shine WE. Eyelid disorders: The meibomian gland, blepharitis, and contact lenses. Eye Contact Lens 2003;29:S93–S95. discussion S115-8. [DOI] [PubMed] [Google Scholar]
- 28.Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci 1997;74:8–13. [DOI] [PubMed] [Google Scholar]


