Abstract
Acute heart failure (AHF) is a frequent medical condition that needs immediate evaluation and appropriate treatment. Patients with signs and symptoms of volume overload mostly require intravenous loop diuretics in the first hours of hospitalization. Some patients may develop diuretic resistance, resulting in insufficient and delayed decongestion, with increased mortality and morbidity. Urinary sodium measurement at baseline and/or during treatment has been proposed as a useful parameter to tailor diuretic therapy in these patients. This systematic review discusses the current sum of evidence regarding urinary sodium assessment to evaluate diuretic efficacy in AHF. We searched Medline, Embase, and Cochrane Clinical Trials Register for published studies that tested urinary sodium assessment in patients with AHF.
Keywords: Urinary sodium, Natriuresis, Acute heart failure, Volume overload, Diuretic
Introduction
Acute heart failure (AHF) is defined as rapid onset or worsening of symptoms and/or signs of heart failure (HF).1 In most cases, the main reasons for patients with AHF to seek medical care are increasing signs and symptoms of volume overload.2–5 Since sodium and water retention in the extracellular space are responsible for this mechanism,6 a cornerstone of the first-line treatment of AHF is represented by loop diuretics (LD), which promote sodium and water elimination in order to reduce extracellular fluid volume and achieve euvolaemia. Treating volume overload in the early hours of hospitalization is associated with lower in-hospital mortality.7 Furthermore, achieving effective decongestion in AHF is associated with improved survival and lower rate of re-hospitalizations.8–10
Tailoring of LD treatment for the single AHF patient is a dynamic process that involves clinical, haemodynamic, biochemical, and echocardiographic evaluation, starting in the first hours of hospitalization. The main goal is to achieve prompt decongestion by adequate diuretic dosing. However, the most commonly used parameters to assess diuretic response, such as fluid balance and weight changes, are often inaccurate, incomplete, and hard to standardize.11 Furthermore, monitoring these parameters requires time from the first diuretic dose, thereby delaying a potential early LD titration. However, evidence regarding optimal dosing and timing of diuretic treatment in AHF is still lacking.
In recent years, several studies have evaluated diuretic response using measuring urinary sodium content (UNa). A recent position paper by the Heart Failure Association (HFA) suggested to use spot UNa to follow-up diuretic response and adapt therapy.12
Sodium excretion can be measured either over a period of time with a urine collection or in a spot urine sample. The latter is clearly simpler and easier to obtain, cheaper, and available in most hospitals. Furthermore, it could also be used in the ambulatory setting.13 Compared with urine collection, a spot sample may be preferable in those situations where the clinical goal is to determine the immediate response to diuretic therapy in the first hours, but it only offers information regarding sodium concentration.
Objectives
This systematic review briefly summarizes the current treatment of AHF with volume overload and its common pitfalls, presents recent insights in UNa profiling in AHF, and highlights the current knowledge gaps.
Methods
Literature search
This systematic review followed the PRISMA guidelines.14 The population, intervention, comparison, and outcome (PICO) approach was used. We searched Medline (through PubMed), Embase, and Cochrane Clinical Trials Register (CENTRAL) for published studies that tested UNa assessment in patients with acute and/or decompensated HF. We used the following search terms: ‘heart failure’ AND (‘urinary sodium’ OR ‘urine sodium’ OR ‘natriuresis’ OR ‘diuretic resistance’).
Reference lists of all accessed full-text articles were searched for sources of potentially relevant information. The population of interest included patients of any age with acute and/or decompensated HF and UNa assessments in a hospital setting, regardless of UNa assessment protocol used. Given the lack of superior protocol clearly determining randomized controlled trial, all studies (observational, case-control, cohort prospective single-arm, post hoc analysis of randomized controlled trials) were considered. No time windows for UNa assessment and outcome measures or metrics of UNa assessment were pre-specified. The last search was run on 28 April 2020. We included all studies in English, published as full text. Abstracts and unpublished data were excluded.
Outcomes
Outcome measures considered were all-cause mortality, cardiovascular mortality, cardiac transplantation, (re-)hospitalization for AHF, mechanical circulatory or inotropic support, worsening HF, length of hospital stay, urine output, net fluid output, weight loss, natriuretic response, plasma NT-ProBNP levels, neurohormonal activation, and markers of tubular injury.
Eligibility criteria, study selection, and data extraction
Two independent reviewers (G.T. and A.G.) selected published studies of any study design, written in English and assessing pre-specified outcomes in AHF population, and screened all abstracts and titles to identify potentially eligible studies. All potentially eligible studies were then read in full to determine suitability for inclusion in the review. Decisions regarding the inclusion of studies required an agreement between both reviewers. Any discordances were discussed with a third author (J.D.). From each study, author(s), year of publication, study type, sample size, UNa assessment metrics, follow-up duration, and outcomes were extracted and collected into a centralized database.
Results
Search results
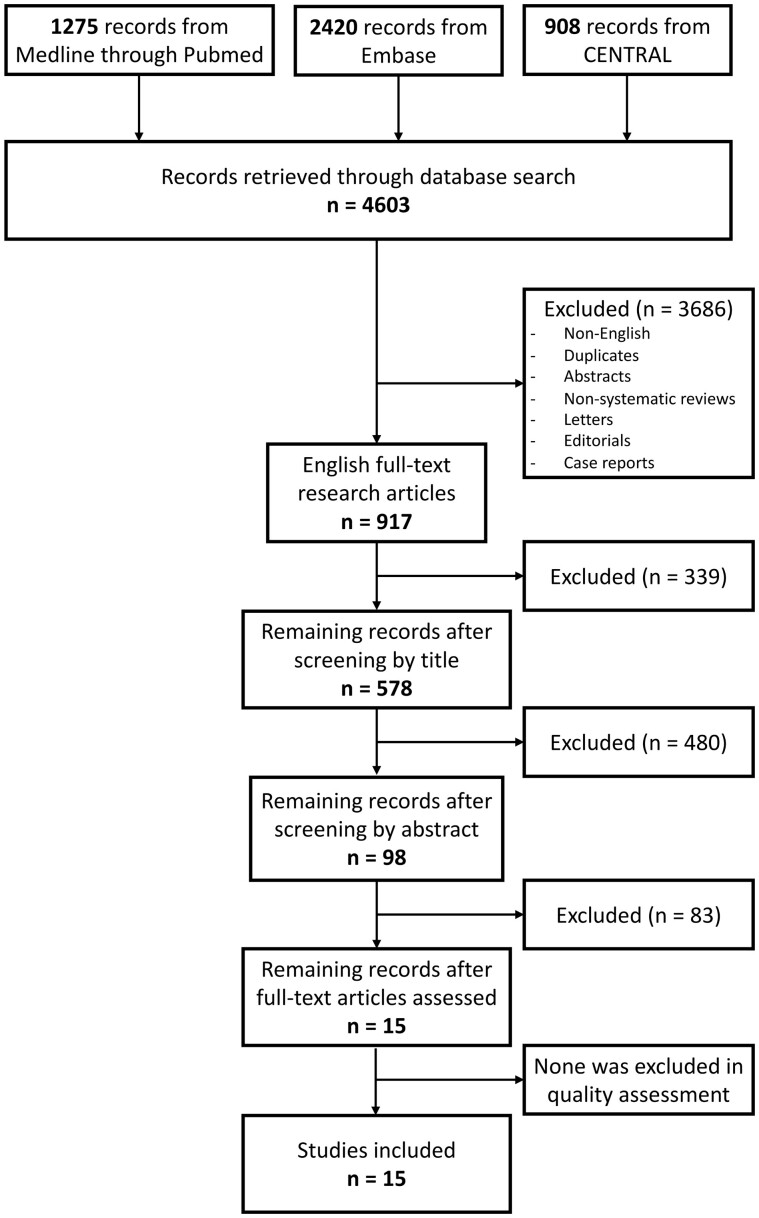
Figure 1 illustrates the study selection process. Out of a total of 4603 initial entries, 917 remained after eliminating duplicates, non-English papers, abstracts, non-systematic reviews, letters, editorials, and case reports. Of these, 578 were selected based on title relevance for further analysis of the abstracts, and 98 were selected for further analysis of the full-text article. Fifteen papers were considered eligible for inclusion. None was excluded in the quality assessment. No other published studies were retrieved after reviewing their reference lists. The systematic review finally included a total of 15 studies, which are presented in Table 1.
Figure 1.
Flow diagram of systematic review studies’ selection.
Table 1.
Studies to date that have investigated urinary sodium measurements as a predictor of clinical outcomes and diuretic response in AHF (integrated from Gupta et al.15)
| Author (year) | N | Study type | Sample | Follow-up | Threshold | Outcome |
|---|---|---|---|---|---|---|
| Singh et al. (2014)16 | 52 | Prospective, single-centre, observational | Spot sample at steady-state of continuous furosemide infusion | 5 months | UNa < 50 mmol | Diminished urine output, net fluid output and weight loss over 24 h. |
| UNa:UFurosemide ratio <2 mmol/mg | Increased risk of death, cardiac transplantation, or HF re-hospitalization. | |||||
| Verbrugge et al. (2014)17 | 50 | Prospective, single-centre, observational | Total sodium excretion during 72 h urine collection | 284 days | UNa 127 mmol/mg Bumetanide | Greater likelihood of all-cause mortality and AHF re-hospitalization. |
| Verbrugge et al. (2015)18 | 54 | Prospective, single-centre, observational | Total sodium excretion at 0–24 , 25–48, and 49–72 | 188 days | A stronger natriuretic response to diuretics is associated with a larger decrease in plasma NT-proBNP levels, less neurohumoral activation, and better clinical outcome (all-cause mortality and HF re-admission). | |
| Ferreira et al. (2016)19 | 100 | Single-centre, non-randomized, open-label case-control trial | Spot sample collected at Day 3 of LDs | 180 days |
UNa <60 mmol/L UNa/UK ratio <2 |
Higher rate of cardiovascular mortality and AHF re-hospitalization. |
| Testani et al. (2016)20 | 50 | Prospective, single-centre, observational | Spot sample 1–2 h after LD administration | — | UNa <60 mmol/L cumulative in 6 h (by equation) | Poor natriuretic response at 6 h can be predicted 1 or 2 h after diuretic administration with excellent accuracy using a spot urine sample. |
| Doering et al. (2017)21 | 187 | Post hoc analysis (STRATIFY and DECIDE trials) | Spot sample in the first 24 h (median 8.3 h after LD administration [IQR = 3.0, 12.9]) | 30 days | UNa <50 mmol/L | Greater likelihood of re-admission to the ED or hospital. |
| Luk et al. (2018)22 | 103 | Prospective, single-centre, observational | Spot sample collected at 1st urine void after LD administration [median 157 min (86–244)] | 90 days | UNa <60 mmol/L | Greater likelihood of reaching the composite endpoint (death at 90 days, mechanical circulatory/inotropic support); non-significant after adjustment for eGFR and home LD dose. |
| Honda et al. (2018)13 | 669 | Post hoc analysis (NaDEF registry) | Spot sample at admission | 560 days | UNa <74 mg/dL (32 mmol/L) | Greater likelihood of all-cause mortality and WHF. |
| Brinkley et al. (2018)23 | 176 | Prospective, single-centre, observational | Spot sample collected at 1st urine void after LD administration (within 3 h) | 30 days |
UNa <65 mmol/L Urine output <1200 mL |
Higher hospitalization rate (69% vs. 18%). |
| Collins et al. (2018)24 | 61 | Prospective, single-centre, observational | Spot sample collected 1 h after LD administration | Hospital stay | UNa <48 mmol/L | Increased risk of development of WHF. |
| Biegus et al. (2019)25 | 111 | Prospective, single-centre, observational | Spot sample collected at admission, 6, 24, and 48 h after LD administration | 1 year | Low spot UNa and lack to increase UNa in response to LDs are associated with poor diuretic response, markers of tubular injury and high risk of 1-year mortality. | |
| Hodson et al. (2019)26 | 316 | Post hoc analysis (ROSE-AHF trial) | Sodium excretion on the first 24 h urine collection | 6 months | Sodium excretion <2 g/day | Decreased 6-month survival. |
| Cunningham et al. (2019)27 | 298 | Post hoc analysis (ROSE-AHF trial) | Sodium excretion on the first 24 h urine collection | Hospital stay | UNa ≤60 mmol/L | Longer hospital Lower 72-h weight loss. |
| Galluzzo et al. (2020)28 | 80 | Post hoc analysis (DRAIN trial) | Spot sample collected 2 h after LD administration | Hospital stay | UNa ≤50 mmol/L | Lower daily urinary output, lower body weight loss, increased NT-ProBNP at 72 h. |
| Damman et al. (2020)29 | 175 | Prospective, single-centre, observational | Total sodium excretion in the first 6 h after LD administration | 257 days | UNa <89 mmol (lowest tertile) | Lower urinary output in the first 24 h. Higher mortality at follow-up. |
AHF, acute heart failure; ED, emergency department; eGFR, estimated glomerular filtration rate; HF, heart failure; LD, loop diuretic; WHF, worsening heart failure.
Comparison of urinary sodium metrics
The first important aspect of using UNa as an additional tool in AHF is the choice between different metrics to represent UNa quantitatively.
Total natriuresis is defined as the total amount of sodium excreted through the urine in a timed urine collection. Compared with the total amount of sodium intake, total natriuresis is an indicator of extracellular volume modifications. As a result, it indicates the reduction in extracellular volume that will eventually be achieved after administration of LD.30
UNa concentration is related to renal tubular sodium and water handling.30 Low values of UNa concentration in patients with HF, with the same sodium and water intake, reflect tubular sodium reabsorption mostly due to neurohormonal activation.31,32 In more severe HF, increased water reabsorption, due to increased antidiuretic hormone may also play a significant role.33 Interestingly, a recent study on stable chronic HF patients showed that patients who developed AHF had a chronically lower UNa concentration on a morning spot sample before diuretic administration and exhibited a further drop in UNa concentration during the week preceding hospitalization for AHF, compared with patients who did not develop AHF.34
Additionally, if UNa concentration is measured a few hours after diuretic administration, either in a spot sample or in a timed urine collection, it may be used as an indicator of diuretic response. This concept will be further developed in this review and has been advocated by the HFA to guide diuretic therapy, especially the first 24 h upon admission with AHF.12
Another evaluated metric of UNa is the fractional sodium excretion (FeNa), defined as the percentage of filtered sodium that is excreted in the urine. Theoretically, the most accurate reflection of renal tubular sodium handling, FeNa is calculated with a formula including urinary creatinine, plasma sodium, and plasma creatinine.35 According to older studies, baseline FeNa is reduced to <1% in patients with HF, and a baseline FeNa of <0.2% is associated with poor natriuretic response.36–38 A secondary analysis of the STRATIFY and DECIDE cohorts21 comparing three different definitions of diuretic resistance, showed that patients with AHF presenting a spot UNa <50 mmol/L within the first 12–24 h had a higher rate of 30-day hospital re-admission for AHF compared with those with spot UNa ≥50 mmol/L. On the contrary, patients fulfilling one of the other two definitions of diuretic resistance (FeNa <0.2% or urinary Na/K ratio <1.0) had no differences in re-hospitalization rate. More studies are needed to understand which UNa indicator would be best in the evaluation of AHF patients in terms of reproducibility, convenience, cost, plausibility, and provided information.
Urinary sodium as an indicator of diuretic response
The first observational study evaluating UNa as marker of diuretic response in AHF patients was undertaken by Singh et al.16 in 2009–10. Fifty-two consecutive patients with AHF receiving a continuous infusion of furosemide for at least 3 h and <24 h were enrolled, and a urine spot sample was obtained. Patients were followed for 5 days or until discharge, and adverse outcomes (i.e. death, re-hospitalizations, and cardiac transplant) were tracked as secondary endpoints for a median follow-up of 5 months. Investigators observed comparable correlations between UNa and FeNa with 24-h net urine output and 24-h weight loss. Furthermore, they demonstrated that insufficient UNa excretion on spot measurements was associated with relatively diminished net urine output and weight loss independent of measures of glomerular filtration. Interestingly, the absolute amount of spot UNa excretion during continuous intravenous infusion was far more predictive than FeNa. According to the authors, this was likely due to confounding effects of impaired UNa excretion secondary to underlying impairment of glomerular filtration.
When administering LDs in AHF, it is important to assess the response to therapy in the first hours, allowing for intervention if diuretic resistance is recognized. The most important study showing that an early measurement of UNa after an LD bolus predicts natriuretic response at 6 h was published 2016 by Testani et al.20 A physiology-derived equation to predict net sodium output using a spot urine sample obtained 1 or 2 h following LD administration was prospectively validated in 50 AHF patients using 6-h urine collections. Poor natriuretic response was defined as a cumulative sodium output of <50 mmol after 6 h, a threshold that would result in a positive sodium balance with twice-daily diuretic dosing. Following a median dose of 3 mg (2–4 mg) of intravenous bumetanide, 40% of the population had a poor natriuretic response at 6 h, with excellent correlation between measured and predicted sodium output. Overall, prediction of urine output was inferior to prediction of sodium output; however, prediction of urine output by the equation was similar to or better than results achieved using the clinically recorded partial fluid output. In summary, this study demonstrated that assessment of UNa in a spot urine sample 1–2 h following LD administration has an excellent correlation with total UNa output in a 6 h urine collection. Thus, it might allow an early evaluation of LD response, eventually detecting diuretic resistance and permitting more timely adjustments in therapy.12 This study was conducted in the inpatient setting, where subjects were enrolled up to 4 days after hospital admission. A similar protocol in ED was conducted by Collins et al.24 in 2016–17 in patients with AHF. Their goal was to determine the association of urine electrolyte patterns after the initial dose of intravenous LD with the development of in-hospital worsening heart failure (WHF). Urine electrolytes and urine output were collected at 1, 2, 4, and 6 h after diuretic administration. At 1 h after diuretic administration, patients who developed WHF were more likely to have significantly lower UNa and decreased UNa concentration than patients without WHF. Furthermore, all patients with WHF had a total UNa output of <35.4 mmol at 1 h, making this threshold 100% sensitive for predicting WHF. Although these results only represent a proof of concept, the authors agree that evaluating natriuresis within 1 h of LD administration could identify patients who may benefit from early treatment intensification.
Similar findings were shown by a recent subanalysis of a small trial on patients with decompensated HF and diluitional hyponatraemia.28 Patients were divided into two groups according to spot UNa excretion at 2 h from furosemide administration, with the threshold set at 50 mmol/L. Twenty-eight patients (35%) showed a low natriuretic response. As compared with the other patients, this group showed lower 24-h urinary output, lower body weight reduction after 48 h and increasing rather than reducing NT-proBNP at 72 h, indicating a peculiar AHF phenotype with protracted volume overload.
Another trial analysing patient-centred outcomes on a longer follow-up was conducted on 103 patients with AHF and volume overload.22 In contrast to the aforementioned studies, UNa was measured on the first urine sample produced after the first dose of LD and not at a predetermined time point. Clinical outcomes were compared dividing patients with UNa >60 mmol/L (n = 72) and UNa of <60 mmol/L (n = 31), with the primary endpoint of a composite of death at 90 days, mechanical circulatory support during admission, and requirement of inotropic support at discharge.
The median time between first intravenous LD dose administration and spot urine sample collection was 157 min (IQR 86–244) and did not differ between UNa categories. Patients with UNa <60 mmol/L were more than twice as likely to experience the primary endpoint, although it was non-significant after adjusting for renal function and baseline home LDs (P = 0.051). The small sample size and the arbitrary dichotomization of a continuous variable (UNa) are two major limitations accounted for by the authors. Furthermore, diuretic dose was not specified in the protocol, and not all patients were instructed to void prior to the first intravenous LD dose, so the UNa measurements were performed on a mixture of pre-diuretic residual urine and post-diuretic urine. However, the main message of both aforementioned studies was that UNa measurement is feasible even in an ED setting, and it can be an integrated marker of diuretic response in the first hours to stratify AHF patients.
Assessment of UNa in HF patients has been evaluated also in AHF patients receiving intravenous LD in special outpatient settings. Brinkley et al.23 prospectively followed 176 consecutive patients with advanced HF receiving intravenous furosemide for volume overload in an outpatient clinic. Spot UNa was measured in the first voided urine after diuretic infusion and compared with 3-h urine output and subsequent risk of 30-day hospitalization or ED visit. Spot UNa was significantly associated with urine output in a model adjusted for age, renal function, and blood urea nitrogen. Higher UNa was associated with lower risk of hospitalization or ED visit within 30 days in a model adjusted for haemoglobin and systolic blood pressure. Using a multivariable logistic regression model with optimal threshold from receiver operating characteristic curve analysis, patients with spot UNa <65 mmol/L and urine output <1200 mL after intravenous LD had a 69% rate of hospitalization in 30 days. Conversely, patients with spot UNa ≥65 mmol/L and urine output ≥1200 mL had only an 18% rate. Several limitations and sources of error (e.g. mixing of pre-diuretic residual urine with post-diuretic urine, non-steady-state furosemide kinetics, no predetermined timing of UNa measurement) in this study may prevent the standard use of the proposed cut-off of 65 mmol/L.39
Lastly, a post hoc analysis of the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF) trial, conducted in patients with AHF and concomitant renal dysfunction, showed a correlation between UNa levels and length of hospital stay (LOS). Patients with low UNa, defined as ≤60 mmol/L, had longer LOS (7 days vs. 5 days, P < 0.001) and lower 72-h weight loss (5.7 lb vs. 9.0 lb, P < 0.001) than patients with UNa >60 mmol/L. These associations persisted after controlling for baseline estimated glomerular filtration rate (eGFR) and outpatient furosemide dose. Interestingly, the associations were also observed when urine sodium concentration was measured from spot rather than cumulative sample at 24 h.27
In summary:
Spot UNa has a good correlation with natriuresis at 6 h (Testani et al.) and diuresis at 3 h (Brinkley et al.), 6 h (Testani et al.), and 24 h (Singh et al. and Galluzzo et al.).
Spot UNa after 1 h from intravenous LD dose is a predictor of risk for in-hospital WHF (Collins et al.).
UNa measured at the first void after diuretic administration may be used to stratify risk, predicting prolonged hospitalization (Cunningham et al.), 30-days re-hospitalizations (Brinkley et al.), and adverse clinical outcomes at 90-days follow-up (Luk et al.).
Urinary sodium as a prognostic marker
In the studies described so far, UNa was measured after diuretic administration. However, a retrospective analysis of the Japanese National cerebral and cardiovascular centre acute DEcompensated heart Failure (NaDEF) registry analysed the association between clinical outcomes and spot UNa collected on hospital admission.13 A total of 669 patients were stratified into tertiles based on UNa values. At baseline, tertiles did not differ in age, NYHA class, HF aetiology, or ejection fraction. However, patients with lower UNa were more likely to have a history of chronic kidney disease, to have experienced more HF hospitalizations and to receive oral diuretics at home. On admission, these patients had significantly higher plasma renin activity, aldosterone, cortisol, and dopamine levels. During hospitalization, patients with lower UNa had significantly less weight loss and higher diuretic requirement than those with higher UNa. During a median follow-up period of 560 days, lower UNa was significantly associated with the composite of all-cause death and WHF. In multivariable Cox-proportional hazards model, UNa remained an independent predictor of long-term adverse events.
Biegus et al.25 conducted a prospective, observational study on patients with AHF in which UNa was measured at baseline, 6, 24, and 48 h after initial treatment. In their population, spot UNa measured on admission did not correlate with outcome. However, serial assessments of UNa indicating either low levels or inability to increase UNa (vs. baseline value) in response to diuretic treatment at 6- and 48 h were associated with higher risk of all-cause mortality during 1-year follow-up, even after adjustment for prognosticators. Moreover, patients with a decrease or no change of UNa in the 6 and 48 h samples had a lower weight loss during hospitalization, and patients with a decrease or no change of UNa in the 48 h sample had a poorer diuretic response and a significant increase in the urinary levels of tubular injury biomarkers. These results support the hypothesis that AHF patients who have an increase in UNa within 48 h of diuretic treatment experienced more effective decongestion. In contrast, those with the opposite trend of UNa excretion achieve less effective decongestion, poorer diuretic efficacy, and an increase in tubular injury.
These findings are in agreement with another study,18 in which AHF patients with a stronger natriuretic response on a urine collection after three consecutive 24 h-intervals demonstrated more pronounced decreases in plasma NT-proBNP levels, while a weaker response was associated with higher peak plasma aldosterone levels and plasma renin activity. Moreover, natriuresis per LD dose predicted freedom from all-cause mortality or HF re-admissions, independently of baseline renal function.
Another key finding by Biegus et al.25 was the absence of difference in eGFR between patients with high and low UNa response. Thus, the authors highlighted an important aspect of AHF: renal capacity to maintain sodium excretion in response to LDs is disconnected from capacity to eliminate urea and other waste products of metabolism, as renal sodium handling takes place in urinary tubules not in glomerulus and mechanisms acting at the renal tubule are the main responsible for diuretic resistance.25
The fact that conventional markers of renal function might perform poorly as predictors of clinical outcome in AHF compared with UNa has also been confirmed by an older single-centre observational study.17 In this protocol, the incidence of worsening renal function during decongestive treatment differed substantially depending on the biomarker used to calculate eGFR. However, worsening renal function by any definition performed poorly as a predictor of clinical outcome. In contrast, the natriuretic response to diuretic therapy was strongly associated with all-cause mortality and AHF re-admissions on a median follow-up of 284 days.17
Two studies analysed the correlation between UNa levels and clinical outcomes at 6 months. The first was a single-centre, single-blind trial of 100 patients with AHF treated with standard therapy alone or with addition of spironolactone.19 In both groups, spot UNa levels >60 mmol/L and UNa/UK ratio >2 measured at Day 3 of hospitalization were associated with improved mid-term outcomes (composite of cardiovascular mortality and AHF re-hospitalizations at 180 days follow-up).
The second study was a retrospective analysis of the ROSE-AHF trial, in which authors examined natriuresis during the first 4 days of decongestive treatment in 316 patients with AHF.26 Overall, interpatient and day-by-day natriuretic response was highly variable. After 24 h, 28.5% of patients had a poor natriuretic response yielding positive sodium balance. This was independently associated with an increased risk of all-cause mortality at 6 months. Notably, fluid loss or changes in body weight were not associated with outcomes. An interesting finding was that a poor natriuretic response was even associated with worse outcomes in patients with a negative fluid balance. Based on their results, the authors suggest that assessment of natriuresis might be a better prognostic marker in AHF than net weight loss, urinary output, or fluid balance.
The impact of natriuretic response on long-term mortality was confirmed by a recent observational study including 175 patients in a tertiary cardiology centre in the Netherlands.29 In this cohort, total UNa output after 6 h was a strong predictor of all-cause mortality after a median follow-up of 257 days. When stratified for tertiles of UNa excretion at 6 h, this resulted in an HR 3.81 (95% CI 1.92–7.57, P < 0.001) for the lowest vs. the highest tertile, while the middle tertile did not show a significant difference with the highest tertile. After multivariable adjustment, this association remained significant. In addition, the authors observed that UNa excretion after 6 h was a strong predictor of total urinary volume after 24 h.
In summary:
In AHF, low UNa on admission (Honda et al.), or in the first days of hospitalization (Ferreira et al.), or a lack to increase UNa in response to intravenous LDs (Biegus et al., Hodson et al., Verbrugge et al., and Damman et al.) were associated with worse long-term outcomes.
Conventional markers of renal function (creatinine, cystatin C, and eGFR) are worse predictors of clinical outcome in AHF compared with UNa (Biegus et al. and Verbrugge et al.).
Poor natriuretic response is more strongly associated with survival in AHF than traditional parameters such as weight loss, urinary output, and fluid balance (Hodson et al.).
Conclusions
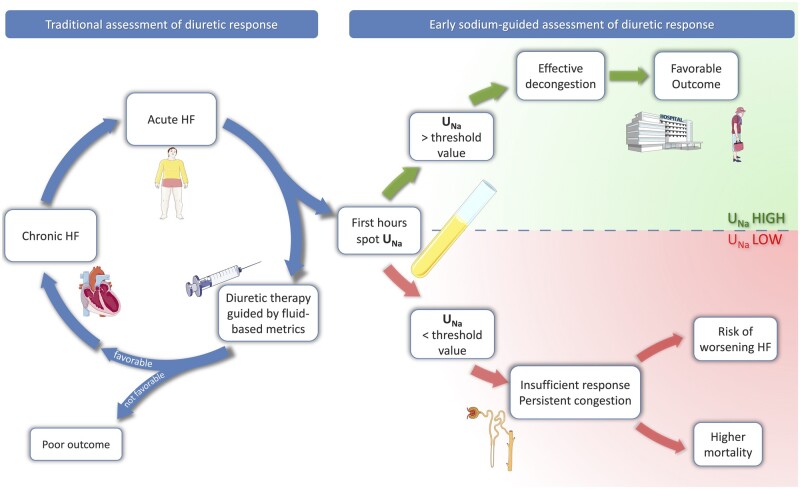
Measuring UNa in a spot urine sample and expressing its value as a concentration was the preferred option in the majority of the above-mentioned studies. However, the methodological differences between trials such as type and dosing regimens of LDs, timing, sources of error, and confounding factors, make it difficult to express a target UNa value at a precise timepoint. As already suggested in the recent HFA Position Paper, in the face of AHF with volume overload, a spot urine sodium content of <50–70 mmol/L after 2 h from intravenous LD administration generally identifies a patient with an insufficient diuretic response.12 This may permit a prompt stratification of AHF patient with higher risk of poor outcome, as supported by the evidence analysed in this review (Figure 2). Although this concept has yet to be formally validated, the early identification of a poor diuretic response possibly allows prompt intensification of LD dose, eventually using a strategy of combining diuretics with a different mode of action.40,41 Achieving a rapid and successful decongestion may reduce the time of organ damage due to AHF, allowing a quicker recovery and reducing hospital stay.
Figure 2.
Role of urinary sodium assessment in acute heart failure. HF, heart failure; UNa, urinary sodium, if needed: Figure modified from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License. http://smart.servier.com/).
The limitations of the single studies were elucidated in the text. Indeed, we know that insufficient natriuresis both in the first hours after presentation as well as during the hospitalization is related with diminished diuresis, increased risk to develop worsening HF, and poor outcome on the mid-term follow-up. However, current data are based on observational studies and only show association, as no randomized controlled trial has yet been completed comparing a systematic optimization of diuretic therapy driven by UNa profiling vs. the usual diuretic therapy guided by traditional fluid-based metrics. Such trials are needed in order to prove causality between influencing natriuresis and outcome, potentially opening a complete new and exciting scenario in targeting UNa in AHF therapy.
Funding
J.D. and W.M. are researchers for the Limburg Clinical Research Center (LCRC) UHasselt-ZOL-Jessa, supported by the foundation Limburg Sterk Merk (LSM), province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital.
Conflict of interest: none declared.
References
- 1. Ponikowski P, Voors AA,, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Meer P Van Der. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 2. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo-Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez-Fernandez S, Miani D, Filippatos G, Maggioni AP. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1242–1254. [DOI] [PubMed] [Google Scholar]
- 3. Gheorghiade M, Pang PS.. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557–573. [DOI] [PubMed] [Google Scholar]
- 4. Núñez J, Núñez E, Fonarow GC, Sanchis J, Bodí V, Bertomeu-González V, Miñana G, Merlos P, Bertomeu-Martínez V, Redón J, Chorro FJ, Llcer A. Differential prognostic effect of systolic blood pressure on mortality according to left-ventricular function in patients with acute heart failure. Eur J Heart Fail 2010;12:38–44. [DOI] [PubMed] [Google Scholar]
- 5. Martens P, Nijst P, Mullens W.. Current approach to decongestive therapy in acute heart failure. Curr Heart Fail Rep 2015;12:367–378. [DOI] [PubMed] [Google Scholar]
- 6. Mullens W, Verbrugge FH, Nijst P, Tang WHW.. Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur Heart J 2017;38:1872–1882. [DOI] [PubMed] [Google Scholar]
- 7. Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S.. Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017;69:3042–3051. [DOI] [PubMed] [Google Scholar]
- 8. Costanzo MR, Guglin ME, Saltzberg MT, Jessup M, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–683. [DOI] [PubMed] [Google Scholar]
- 9. Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 2012;5:54–62. [DOI] [PubMed] [Google Scholar]
- 10. Testani JM, Chen J,, McCauley BD, Kimmel SE, Shannon RP.. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010;122:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Testani JM, Brisco MA, Kociol RD, et al. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am J Med 2015;128:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mullens W, Damman K, Harjola V, Mebazaa A, Rocca HB, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:137–155. [DOI] [PubMed] [Google Scholar]
- 13. Honda S, Nagai T, Nishimura K, et al. Long-term prognostic significance of urinary sodium concentration in patients with acute heart failure. Int J Cardiol 2018;254:189–194. doi:10.1016/j.ijcard.2017.08.053 [DOI] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta R, Testani J, Collins S.. Diuretic resistance in heart failure. Curr Heart Fail Rep 2019;16:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh D, Shrestha K, Testani JM, et al. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail 2014;20:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verbrugge FH, Nijst P, Dupont M, Reynders C, Penders J, Tang WHW, Mullens W. Prognostic value of Glomerular filtration changes versus natriuretic response in decompensated heart failure with reduced ejection. J Card Fail 2014;20:817–824. [DOI] [PubMed] [Google Scholar]
- 18. Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, Dens J, Verhaert D, Vandervoort P, Tang WHW, Mullens W. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 2015;70:265–273. [DOI] [PubMed] [Google Scholar]
- 19. Ferreira JP, Girerd N, Medeiros PB, Santos M, Carvalho HC, Bettencourt P, Kénizou D, Butler J, Zannad F, Rossignol P. Spot urine sodium excretion as prognostic marker in acutely decompensated heart failure: the spironolactone effect. Clin Res Cardiol 2016;105:489–507. [DOI] [PubMed] [Google Scholar]
- 20. Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, Kula A, Chen M, Wilson FP, Darlington A, Bellumkonda L, Jacoby D, Tang WHW, Parikh CR. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Heart Fail 2016;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doering A, Jenkins CA, Storrow AB, et al. Markers of diuretic resistance in emergency department patients with acute heart failure. Int J Emerg Med 2017;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luk A, Groarke JD, Desai AS, Mahmood SS, Gopal DM, Joyce E, Shah SP, Lindenfeld J, Stevenson L, Lakdawala NK. First spot urine sodium after initial diuretic identifies patients at high risk for adverse outcome after heart failure hospitalization. Am Heart J 2018;203:95–100. [DOI] [PubMed] [Google Scholar]
- 23. Brinkley DM, Burpee LJ, Chaudhry SP, Smallwood JA, Lindenfeld JA, Lakdawala NK, Desai AS, Stevenson LW. Spot urine sodium as triage for effective diuretic infusion in an ambulatory heart failure unit. J Card Fail 2018;24:349–354. doi:10.1016/j.cardfail.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 24. Collins SP, Jenkins CA, Baughman A, et al. Early urine electrolyte patterns in patients with acute heart failure. ESC Heart Fail 2019;6:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biegus J, Zymliński R, Sokolski M, Todd J, Cotter G, Metra M, Jankowska EA, Banasiak W, Ponikowski P. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur J Heart Fail 2019;21:1–10. [DOI] [PubMed] [Google Scholar]
- 26. Hodson DZ, Griffin M, Mahoney D, Raghavendra P, Ahmad T, Turner J, Wilson FP, Tang WHW, Rao VS, Collins SP, Mullens W, Testani JM. Natriuretic response is highly variable and associated with 6-month survival: insights from the ROSE-AHF trial. JACC Heart Fail 2019;7:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cunningham JW, Sun J, Mc Causland FR, Ly S, Anstrom KJ, Lindenfeld J, Givertz MM, Stevenson LW, Lakdawala NK. Lower urine sodium predicts longer length of stay in acute heart failure patients: insights from the ROSE AHF trial. Clin Cardiol 2020;43:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galluzzo A, Frea S, Boretto P, Pidello S, Volpe A, Canavosio FG, Golzio PG, Bergerone S, De Ferrari GM. Spot urinary sodium in acute decompensation of advanced heart failure and dilutional hyponatremia: insights from DRAIN trial. Clin Res Cardiol 2020. doi:10.1007/s00392-020-01617-w [DOI] [PubMed] [Google Scholar]
- 29. Damman K, Ter Maaten JM, Coster JE, Krikken JA, Deursen VM, Krijnen HK, Hofman M, Nieuwland W, Veldhuisen DJ, Voors AA, Meer P. Clinical importance of urinary sodium excretion in acute heart failure. Eur J Heart Fail 2020. doi:10.1002/ejhf.1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verbrugge FH. Utility of urine biomarkers and electrolytes for the management of heart failure. Curr Heart Fail Rep 2019;16:240–249. [DOI] [PubMed] [Google Scholar]
- 31. Verbrugge FH. Editor’s Choice-Diuretic resistance in acute heart failure. Eur Heart J Acute Cardiovasc Care 2018;7:379–389. [DOI] [PubMed] [Google Scholar]
- 32. Tersalvi G, Dauw J, Martens P, Mullens W.. Impact of sacubitril-valsartan on markers of glomerular function. Curr Heart Fail Rep 2020;17:145–152. [DOI] [PubMed] [Google Scholar]
- 33. Kalra PR, Anker SD, Coats AJ.. Water and sodium regulation in chronic heart failure: the role of natriuretic peptides and vasopressin. Cardiovasc Res 2001;51:495–509. [DOI] [PubMed] [Google Scholar]
- 34. Martens P, Dupont M, Verbrugge FH, Damman K, Degryse N, Nijst P, Reynders C, Penders J, Tang WHW, Testani J, Mullens W. Urinary sodium profiling in chronic heart failure to detect development of acute decompensated heart failure. JACC Heart Fail 2019;7:404–414. [DOI] [PubMed] [Google Scholar]
- 35. Espinel CH. The FeNa test: use in the differential diagnosis of acute renal failure. JAMA 1976;236:579–581. [DOI] [PubMed] [Google Scholar]
- 36. Gabrielsen A, Bie P, Holstein-Rathlou NH, Christensen NJ, Warberg J, Dige-Petersen H, Frandsen E, Galatius S, Pump B, Sørensen VB, Kastrup J, Norsk P. Neuroendocrine and renal effects of intravascular volume expansion in compensated heart failure. Am J Physiol Regul Integr Comp Physiol 2001;281:R459–R467. [DOI] [PubMed] [Google Scholar]
- 37. Fifer MA, Molina CR, Quiroz AC, et al. Hemodynamic and renal effects of atrial natriuretic peptide in congestive heart failure. Am J Cardiol 1990;65:211–216. [DOI] [PubMed] [Google Scholar]
- 38. Dormans T P J, Gerlag P G G. Combination of high-dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure. European Heart Journal 1996;17:1867–1874. 10.1093/oxfordjournals.eurheartj.a014805 [DOI] [PubMed] [Google Scholar]
- 39. Martens P, Mullens W.. Spot urinary sodium in decompensated heart failure as a prognostic metric for successful ambulatory decongestion. J Card Fail 2018;24:355–356. [DOI] [PubMed] [Google Scholar]
- 40. Verbrugge FH, Grieten L, Mullens W.. New insights into combinational drug therapy to manage congestion in heart failure. Curr Heart Fail Rep 2014;11:1–9. [DOI] [PubMed] [Google Scholar]
- 41. Mullens W, Verbrugge FH, Nijst P, et al. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial: acetazolamide in decompensated heart failure with volume overload. Eur J Heart Fail 2018;20:1591–1600. [DOI] [PubMed] [Google Scholar]