Abstract
Moderate physical exercise is associated with an irrefutable reduction in cardiac morbidity and mortality. The current guidelines recommend at least 150 min of moderate exercise or 75 min of vigorous exercise per week. Endurance athletes perform exercise at a level that is 10- to 20-fold greater than these recommendations. These athletes reveal several structural and functional cardiac adaptations including increased cardiac size, enhanced ventricular filling, and augmentation of stroke volume even at the highest heart rates. The long-term effects of endurance exercise on the heart are unknown. Endurance exercise is associated with a transient increase in serum concentrations of biomarkers of cardiac damage and ventricular dysfunction which improves within 72 h. Over the past decade, there have been emerging studies reporting attenuated mortality benefit amongst individuals who perform the highest volume of exercise. Studies in lifelong male athletes aged above 40 years old show a higher prevalence of high coronary artery calcium scores (>300 Agatston units), a higher coronary plaque burden, and myocardial fibrosis compatible with subclinical myocardial infarction compared with relatively sedentary healthy controls, raising speculation that lifelong intense exercise imposes chronic coronary stress on the heart. This review article will provide a critical analysis of the existing data.
Keywords: Athlete’s heart, Master athlete, Coronary artery disease, Coronary artery calcification, Myocardial fibrosis
Graphical Abstract
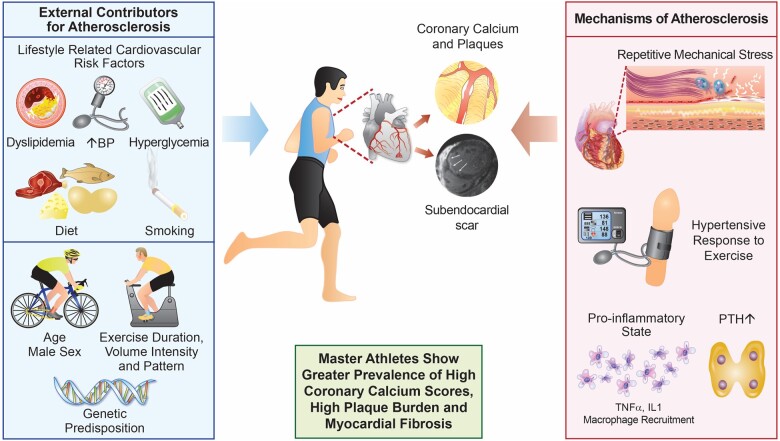
Life-long middle-aged male 1 endurance athletes demonstrate increased prevalence of high coronary calcium scores. Contributing factors include genetic predisposition, ageing and the prevalence or recognised atherosclerotic risk factors. Additionally, exercise is associated with mechanical flexing of the arteries, coronary inflammation and a hypertensive response to exercise. Some athletes may develop ischaemic scar and increased risk of ventricular arrhythmias, however longitudinal studies are necessary to investigate this further. BP, blood pressure; IL1, interleukin-1; PTH, parathyroid hormone; TNFα, tumour necrosis factor alpha.
 Listen to the audio abstract of this contribution.
Listen to the audio abstract of this contribution.
The benefits of exercise
The benefits of regular physical exercise on the human body are multiple and indisputable. The improvements in cardiovascular risk profile associated with exercise are partly secondary to its positive impact on atherosclerotic risk factors such as blood pressure, lipid profile, body mass index, and insulin resistance.1 Although intensive exercise acutely has a pro-inflammatory effect that potentially increases the risk of coronary inflammation, atherosclerotic plaque destabilization, and rupture, regular exercise generally exerts an anti-inflammatory effect on the vascular endothelium and triggers myokine release from skeletal muscle. Exercise promotes nitric oxide production from the vascular endothelium, thus improving vasodilatory capacity, vascular homeostasis, and deactivation of scavenging oxidative species.2 , 3 Exercise also stimulates angiogenesis, which increases tissue oxygen transport and inhibits cell adhesion molecules that otherwise mediate the inflammatory atherosclerotic process.4 According to animal studies, the protective effect of exercise on the endothelium is dose-dependent and varies depending on exercise protocol and vessels.2 There is currently no evidence suggesting any one exercise type has a preferential benefit on endothelial function over another. As with most beneficial effects of exercise, optimal endothelial function is achieved through regular moderate-intensity physical exercise. Extreme strenuous and exhaustive exercise contrarily increase oxidative stress and can result in a systemic inflammatory response, although this is usually short-lived and resolves within a few hours.4 , 5
Current physical activity guidelines recommend a minimum of 30 min of moderate physical activity per day 5 days per week or 25 min of vigorous activity per day, 3 days per week6; however, individuals engaging in endurance sports exercise at levels far beyond these recommendations. In the past decade, there have been several reports of a reverse J-shaped dose–response relationship between lifetime exercise exposure and cardiovascular morbidity, suggesting there is a threshold beyond which some of the aforementioned benefits of exercise are lost.7 , 8 These studies have led to an ongoing debate regarding the potential deleterious effect of a cumulative dose of high-intensity endurance training on the heart.
Ageing, exercise, and the cardiovascular system
The cardiovascular system undergoes a plethora of age-related structural and functional changes that impact on cardiac reserve. At a cellular level, there is a gradual decline in the number of myocytes and a compensatory increase in myocyte size, resulting in mild left ventricular (LV) hypertrophy.9 , 10 Functionally, there is an overall reduction in cardiac energetics, specifically, prolongation of relaxation time causing increased preload, an increase in vascular and myocardial stiffness, and loss of catecholamine sensitivity causing an increased afterload.11 These changes result in a reduced LV end-systolic volume and reduced ejection fraction reserve.10 The long-term physiological effects of exercise training attenuate some of these age-related changes through its anti-inflammatory and anti-oxidative effects. Exercise slows the rate of arterial stiffening and prevents the development of vascular disease,12 offsets age-related reductions in LV compliance and distensibility and attenuates the rate of fall in maximum oxygen consumption.13 , 14 However, exercise does not appear to slow the age-related reduction in maximum heart rate from reduced beta-adrenergic responsivity and intrinsic slowing of the sinoatrial node.10
Ageing and skeletal muscle
Sarcopenia, the loss of muscle mass with ageing, results from the replacement of type II ‘fast-twitch’ muscle fibres and motor units with fat and connective tissue, causing loss of contractile and metabolic myocyte function.15 Driving factors for sarcopenia include chronic low-grade inflammation, oxidative stress, impaired regenerative capacity, and impaired protein synthesis—despite feeding and exercise.16 Onset is usually in the 5th decade but only usually becomes evident in the 7th decade and explains why athletic performance deteriorates with age, in particular, explosive forms of exercise. Due to the more gradual decline in ‘slow-twitch’ fibres, individuals can continue to compete in endurance sports at elite level into the 5th and 6th decades. A retrospective analysis of 43 551 participants in ultra-marathons worldwide demonstrated the mean age of the top 10 ranked athletes was 43.4-years and 42.1-years in females and males, respectively. There was no deterioration in peak running times in athletes until they reached the 6th decade.17
Cardiovascular adaptation to exercise
Endurance athletes impose huge demands on the cardiovascular system and manifest the most profound physiological cardiovascular adaptations to exercise. The constellation of adaptations often referred to as the ‘athlete’s heart’ are well-defined in young athletes. These include profound sinus bradycardia, increased QRS voltages, cardiac chamber dilatation and increased LV wall thickness, enhanced diastolic ventricular filling, and high peak oxygen consumption.18 In addition, athletes demonstrate an increase in pulmonary artery diameter; however, pulmonary vascular resistance remains unchanged in most athletes.19 The magnitude of such adaptation varies according to age, sex, ethnicity, sporting discipline, and intensity of exercise. In general, adult male endurance athletes with a body surface area ≥2 m2 reveal the largest cardiac dimensions.
Cardiac adaptation in the master athlete
Cardiovascular adaptation to exercise in master athletes is less well-defined and more complex due to the interplay between training effects, ageing, and the cumulative impact of lifestyle-related cardiovascular risk factors. Despite this complex interaction, master athletes adapt similarly to their younger counterparts by increasing cardiac dimensions and mass.20 , 21 Merghani et al.22 demonstrated an up to 15% greater end-diastolic volume and 9.9% greater maximal wall thickness in master endurance athletes compared with non-athletic controls. Compared with young athletes, master athletes demonstrate increased LV wall thickness and left atrial size, but less extensive chamber dilatation. From a functional perspective, LV ejection fraction is unchanged; however, stroke volume is increased as are indices of diastolic function.22
Sudden cardiac death in master athletes
Sudden death during sport effects ∼1 in 50 000 individuals. Over 90% of all exercise-related sudden cardiac deaths (SCDs) occurs in the middle-aged and older population.23 Coronary atherosclerosis is the most common cause of SCD in master athletes accounting for more than 80% of deaths24 with a high male preponderance in both competitive and recreational sports.25 , 26 The acute effects of exercise increase the predilection for plaque rupture and resultant myocardial infarction by up to 10-fold.25 , 27 The risk of acute myocardial infarction and SCD is inversely related to the amount of habitual exercise performed.27 Individuals exercising up to five times per week have a 50-fold lower risk of myocardial infarction and a 7-fold lower risk of SCD compared with individuals who exercise infrequently.28 Commonly proposed mechanisms for myocardial infarction during exercise include a combination of endothelial erosion resulting in plaque rupture, neuro-hormonal activation, and hypercoagulability.26 However, plaque rupture is not the only mechanism by which atherosclerosis exacerbates sudden death during extreme exercise. In a case series of SCD during marathons in the USA, there was no evidence of thrombus or plaque rupture.29 Alternative mechanistic possibilities include demand ischaemia from obstructive coronary artery stenosis and coronary vasospasm from increased sympathetic activity.30 Other causes of exercise-related SCD in master athletes include the cardiomyopathies, myocarditis, valvular heart disease, and aortic rupture.24
Can exercise damage an otherwise healthy heart?
Endurance sporting events are associated with a transient rise in serum biomarkers of myocardial damage including troponin and brain natriuretic peptide (BNP); temporary right, and to a lesser extent, LV systolic dysfunction, and impaired myocardial relaxation. The significance of transient rises in biomarkers is unclear. The transient rise and normalization of serum cardiac troponin concentrations within 48 h suggest that the troponin originates from the cytosol rather than the sarcomere and enters the circulation due to increased myocyte membrane permeability. Episodes of acutely raised serum troponin may represent myocyte necrosis from subtle myocardial inflammation or microinfarction, such that repeated events could culminate in adverse cardiac remodelling and arrhythmias.31 , 32 Abnormal levels of BNP following an endurance event have been associated with right ventricular dysfunction and possibly represent prolonged and repeated wall stress due to a raised cardiac output. A small proportion of ostensibly healthy master athletes reveal atrial fibrillation, coronary artery calcification, and myocardial fibrosis,33 which has increased speculation about the potentially deleterious impact of chronic endurance exercise on cardiovascular health.
Middle-aged endurance athletes are at a five-fold increased risk of atrial fibrillation compared with sedentary counterparts. Proposed mechanisms include shortening of the atrial action potential due to increased vagal tone, and direct effects on the atrial myocardium, including mechanical stretch, inflammation, and fibrosis. Extrinsic contributing factors include male sex, tall stature, and the volume and intensity of exercise.34
Coronary artery disease in master athletes
The coronary artery calcium (CAC) score, as measured by computed tomography (CT), is a surrogate for atherosclerotic volume and a strong predictor of future adverse cardiac events in the general population.35 , 36 The CAC score is also used for risk stratification and intensifying therapy for recognized atherosclerotic risk factors. Although exercise is thought to be protective for coronary artery disease, there is mounting evidence that male master athletes have higher CAC scores and a greater prevalence of coronary atherosclerosis on coronary CT angiography (CCTA), compared with age and atherosclerotic risk-matched controls.22 , 37 Möhlenkamp and colleagues37 investigated CAC in 108 ostensibly healthy males aged 50–72 years who had run ≥5 marathons and compared them with 864 age-matched controls. The prevalence of a CAC score ≥100 Agatston units (AU) was similar in both groups (36%). After matching for Framingham score, CAC score was higher in marathon runners than controls (36% vs. 12%). The Measuring Athlete's Risk of Cardiovascular Events (MARC) study demonstrated amongst 318 middle-aged male endurance athletes (mean age 54.7-years), who could exercise to high workloads (318 ± 48 Watts), and of whom 94% had a low ESC 10-year risk score, that 16% had a CAC score ≥100 AU.38
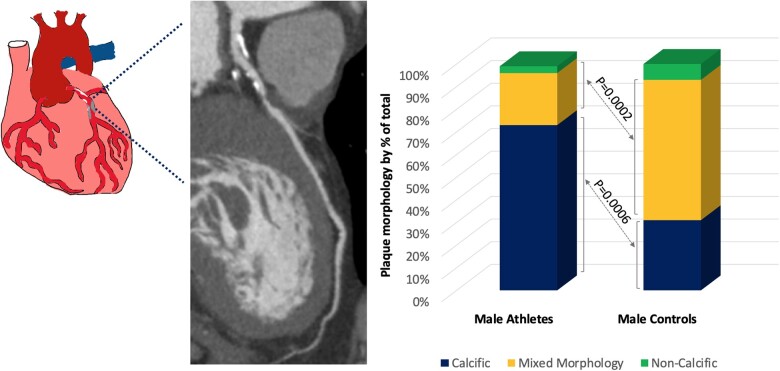
Our group recruited 152 master endurance athletes with a low Framingham risk score and 92 age and Framingham risk-matched controls. Athletes had a mean age of 51 years, a mean Framingham risk score of 4.33% and had been competing in endurance sports for a mean of 31 years. All participants underwent comprehensive testing including CCTA and CAC score. There was no difference in the prevalence of a CAC score of zero or a CAC score >70th percentile between the two groups; however, almost one in five male athletes had a CAC score ≥100 AU and 11.3% demonstrated a CAC score >300 AU compared with none of the male controls. Male athletes had a significantly greater proportion of calcific plaques, which are considered stable and less prone to rupture, compared with controls (72% vs. 31%; P = 0.0002). Controls had a significantly higher prevalence of mixed morphology plaque, which is more vulnerable to rupture resulting in acute coronary thrombosis22 (Figure 1).
Figure 1.
(A) Coronary computed tomography angiography demonstrating mixed morphology plaque in the proximal left anterior descending artery in a male master athlete. (B) Breakdown of coronary plaques according to morphology in male athletes and controls. Athletes have a higher prevalence of calcified plaques compared with controls.22
There are currently no studies amongst athletes examining markers of plaque vulnerability such as peri-coronary adipose tissue or CT markers such as spotty calcification, napkin ring sign, and positive remodelling. Such data could provide valuable insight into characterizing and quantifying the risk of coronary lesions and their relationship to other potentially related structural changes such as myocardial fibrosis, in athletes. One study of consecutive patients referred for CCTA demonstrated a lower prevalence of non-calcified plaque and high-risk markers amongst those regularly endurance training.39 A further study of patients with angina undergoing percutaneous coronary intervention used intravascular ultrasound, optimal coherence tomography, and cardiopulmonary exercise testing to demonstrate a smaller lipid volume, greater fibrous volume, and thicker fibrous cap in those with greater cardiorespiratory fitness (CRF).40
Association between intense exercise and coronary atherosclerosis
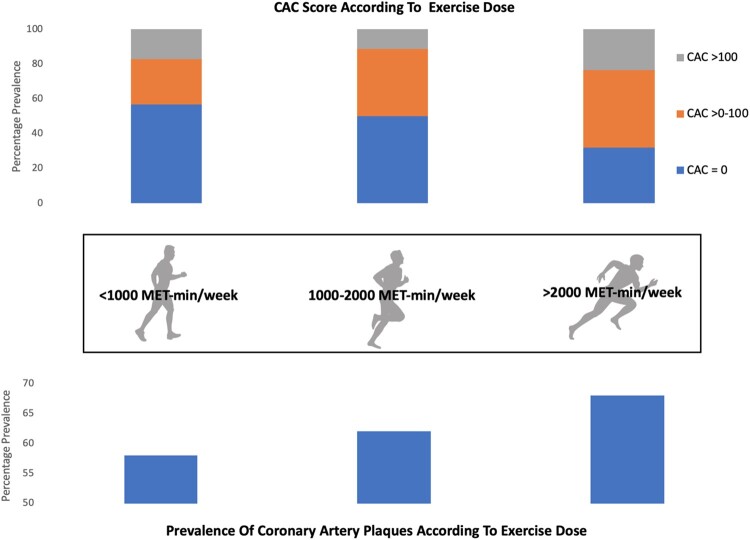
The MARC study investigators used their data to evaluate the relationship between coronary atherosclerosis and exercise dose (MET-min/week) in 284 men. Athletes were categorized as exercising for <1000, 1000–2000, or >2000 MET-min/week. Those athletes with the highest exercise dose had significantly greater CAC and more atherosclerotic plaques. There was no relationship between exercise dose and plaque morphology for mixed or non-calcified plaques; however, athletes who exercised the most had the highest prevalence of calcified plaques41 (Figure 2 and Supplementary material online, Table S1).
Figure 2.
Data from the Measuring Athlete's Risk of Cardiovascular Events study demonstrating the relationship between exercise dose and coronary artery calcium (CAC) (A) and prevalence of calcified plaques (B).41
Mechanisms for coronary atherosclerosis and coronary artery calcium in master athletes
There are several proposed mechanisms underlying the development of atherosclerotic coronary artery disease in male master athletes. These include prolonged and repetitive mechanical stress from flexing of the epicardial coronary arteries during vigorous cardiac contraction, an excessively high blood pressure during exercise,22 , 37 , 42 increased parathyroid hormone levels secondary to exercise, and the acute pro-inflammatory state associated with repeated prolonged bouts of intense exercise.43 Some athletes with increased CAC have a genetic predisposition or established risk factors for atherosclerosis.
The greater prevalence of calcified rather than mixed morphology plaques in male master athletes is also unexplained; however, different endothelial repair mechanisms may be in place compared with individuals with a high burden of atherosclerotic risk factors. It is possible that chronic endurance exercise accelerates calcification and stabilizes plaques akin to the protective effect of high-dose statins.44 , 45 Interval scanning of 1255 disease-free patients in the PARADIGM study revealed that statins resulted in slower progression of atherosclerosis, modestly lower overall plaque volume, lower fibrous plaque volume, and an increase in dense calcium volume—a marker of plaque stability.45 The mechanisms for these effects are poorly understood; the anti-inflammatory properties of statins, as with exercise, being one possibility.
Current studies suggest that the presence of CAC does not confer the same level of risk amongst individuals engaging in large volumes of intensive exercise as in the general population. Radford et al.46 evaluated the association between CRF, CAC score, and cardiovascular events over an 8.4-year follow-up period in a cohort of 8425 men. The study showed that a greater CRF reduced cardiovascular disease events and a higher calcium score increased them. When considered together, a greater continuous CRF attenuated the risk of cardiovascular disease events when adjusted for CAC score. For each MET increase in CRF across all CAC scores adjusted for risk factors, the event rate was reduced by 14%. DeFina and colleagues47 demonstrated that the most active individuals (≥3000 MET-min/week) had an 11% greater relative risk of a CAC score ≥100 AU compared with less active controls. Despite having more calcium, athletes exercising >3000 MET-min/week had no increase in mortality compared with individuals performing 1500–3000 and <1500 MET-min/week.
There are a number of potential explanations for the failure to demonstrate an increase in adverse outcomes in athletes with CAC. Lifelong endurance athletes develop an array of protective cardiovascular adaptations that result in significant coronary flow reserve. These include ischaemic pre-conditioning, coronary collateralization, and optimal vasodilatory capacity due to enhanced nitrous oxide production. In the general population, the CAC score is a reflection of overall atherosclerotic burden, therefore, we speculate that if exercise increases the extent and density of endothelial calcification, the overall atherosclerotic burden will be overestimated in athletes. Should this be the case, the use of the CAC score as a surrogate marker for quantifying coronary atherosclerotic volume, and therefore predicting cardiovascular morbidity and mortality risk, may not be as valid in athletes as in the general population. An alternative hypothesis is that CAC detected with CT occurs within the medial layer of the artery wall as part of the healing process following smooth muscle apoptosis, as opposed to the intimal layer—which is the site of rupture-prone atherosclerosis. There are currently no in vivo methods to differentiate these entities.
Sex differences in coronary artery calcification in master athletes
Knowledge of the pattern of coronary atherosclerosis specifically in the female master athlete is scant. A study of 26 lifelong female marathon runners, having run at least one marathon per year for 10–25 years, showed a lower calcium burden and less plaques compared with controls; however, the athletes also had a lower atherosclerotic risk factor profile. Athletes with plaques tended to be older and were amongst those who had been running marathons for the most years.48 Our own experience of 46 female athletes showed that there was no difference in CAC score or prevalence of plaque morphology compared with 38 age- and Framingham risk-matched controls.22 These, albeit small studies, suggest that female athletes may benefit from the protective effect of oestrogens pre-menopause; however, the impact of the menopause on the prevalence of CAC in female athletes is unknown.
Endurance exercise and sub-clinical infarction
Focal fibrosis affecting the right ventricular insertion points has been observed in >40% of male master athletes and >30% of female master athletes and considered a benign consequence of sustained repetitive right ventricular pressure and volume overload, with similar findings in pulmonary hypertension.49 Major myocardial fibrosis affecting the subendocardial layer, mid-wall, or subepicardial region has been reported in 11–17% of master athletes and is confined to males. The aetiology and mechanism for myocardial fibrosis are unclear; however, a subendocardial pattern is suggestive of ischaemic injury and a subepicardial pattern is compatible with a post-inflammatory response. In our experience of 152 master endurance athletes and 92 controls, 11% of master athletes revealed myocardial fibrosis compared with no controls. Of these, one-third revealed fibrosis compatible with previous myocardial infarction, the remaining two-thirds revealed a non-ischaemic scar pattern with a mid-wall or a subepicardial distribution, most often characteristic of acute or healed myocarditis,22 although a cardiomyopathic process acquired through lifelong intensive exercise also merits consideration. Of the athletes with scar compatible with myocardial infarction, only half had a coronary stenosis in the relevant coronary artery and in each case, the stenosis was ≤50%. Möhlenkamp et al.37 showed that a higher CAC percentile, in addition to number of marathons completed, was predictive for the presence of late gadolinium enhancement (LGE). These results suggest that subendocardial fibrosis in master athletes may represent sub-clinical myocardial infarction from demand ischaemia, micro-emboli, and coronary spasm. The prevalence of coronary events at 6-year follow-up was 57% amongst those with LGE vs. 8% in those without50 suggesting the presence of LGE in addition to coronary calcification increases the risk of adverse outcomes.
Myocardial fibrosis is an important finding due to its association with ventricular arrhythmias and increased mortality in the general population.51 Studies to date have not demonstrated a greater prevalence of ventricular arrhythmias in master athletes compared with controls52 nor any direct association between myocardial fibrosis, ventricular arrhythmias, and adverse events in master athletes.37 Our relatively limited experience of arrhythmias in master athletes with an ischaemic pattern of fibrosis revealed a higher prevalence of non-sustained ventricular tachycardia than those without fibrosis (43% vs. 7%) with all three athletes with ventricular tachycardia revealing a ≥50% stenosis in the left anterior descending artery.22 Further large-scale longitudinal studies are required to investigate the precise impact of myocardial scar in athletes.
Conclusion
Regular physical exercise is imperative for the maintenance of optimal health and longevity and should be globally encouraged. There is emerging evidence that a proportion of athletes show high CAC scores, a higher plaque burden and myocardial fibrosis compared with age- and Framingham-matched controls. The mechanism and significance of these findings are unclear. Current limited data find no association between a high CAC score and all-cause mortality in master athletes.
Future directions
Large prospective longitudinal studies in male master athletes are required to investigate the cause and significance of CAC, coronary plaques, and myocardial fibrosis in athletes. The potential major role of inflammation in these findings merits further exploration. Further characterization of the female veteran athlete’s heart, understanding the impact of the menopause, and explaining the physiological differences between females and males will greatly expand our current understanding of the impact of exercise on the older athlete’s heart.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Funded fully by a British Heart foundation Clinical research fellowship.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Gemma Parry-Williams, Cardiology Clinical and Academic Group, St. George’s University of London, Cranmer Terrace, London SW17 0RE, UK.
Sabiha Gati, National Heart and Lung Institute, Imperial College London & Royal Brompton and Harefield Hospitals NHS Foundation Trust, London SW3 6LY, UK.
Sanjay Sharma, Cardiology Clinical and Academic Group, St. George’s University of London, Cranmer Terrace, London SW17 0RE, UK.
References
- 1. Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee D-C, Earnest CP, Church TS, O’Keefe JH, Milani RV, Blair SN. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res 2015;117:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc 2006;38:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pagan LU, Gomes MJ, Okoshi MP. Endothelial function and physical exercise. Arq Bras Cardiol 2018;111:540–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Francescomarino SD, Sciartilli A, Valerio VD, Baldassarre AD, Gallina S. The effect of physical exercise on endothelial function. Sports Med (Auckland, NZ ) 2009;39:797–812. [DOI] [PubMed] [Google Scholar]
- 5. Suzuki K, Tominaga T, Ruhee RT, Ma S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants 2020;9:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelliccia A, Sharma S, Gati S, Bäck M, BöRjesson M, Caselli S, Collet J-P, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Stuart AG, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2005;26:1422–1445.15923204 [Google Scholar]
- 7. Lee D-C, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol 2014;64:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schnohr P, O’Keefe JH, Marott JL, Lange P, Jensen GB. Dose of jogging and long-term mortality: the Copenhagen City Heart Study. J Am Coll Cardiol 2015;65:411–419. [DOI] [PubMed] [Google Scholar]
- 9. Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res 1991;68:1560–1568. [DOI] [PubMed] [Google Scholar]
- 10. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 2003;107:346–354. [DOI] [PubMed] [Google Scholar]
- 11. Hollingsworth KG, Blamire AM, Keavney BD, Macgowan GA. Left ventricular torsion, energetics, and diastolic function in normal human aging. Am J Physiol Heart Circ Physiol 2012;302:H885–H892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM, Levine BD. The effect of lifelong exercise frequency on arterial stiffness. J. Physiol 2018;596:2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 2004;110:1799–1805. [DOI] [PubMed] [Google Scholar]
- 14. Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: relation to training status. Am J Physiol Heart Circ Physiol 2000;278:H829–H834. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 2012;3:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harridge SDR, Lazarus NR. Physical activity, aging, and physiological function. Physiology 2017;32:152–161. [DOI] [PubMed] [Google Scholar]
- 17. Zingg M, Rust CA, Lepers R, Rosemann T, Knechtle B. Master runners dominate 24-h ultramarathons worldwide-a retrospective data analysis from 1998 to. Extreme Physiol Med 2013;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baggish AL, Wood MJ. Athlete’s heart and cardiovascular care of the athlete: scientific and clinical update. Circulation 2011;123:2723–2735. [DOI] [PubMed] [Google Scholar]
- 19. Domenech-Ximenos B, Garza MS, la Prat-González S, Sepúlveda-Martínez Á, Crispi F, Perea RJ, Garcia-Alvarez A, Sitges M. Exercise-induced cardio-pulmonary remodelling in endurance athletes: not only the heart adapts. Eur J Prev Cardiol 2020;27:651–659. [DOI] [PubMed] [Google Scholar]
- 20. Bohm P, Kindermann W, Meyer T, Scharhag J. Response by Bohm et al to letter regarding article, “right and left ventricular function and mass in male elite master athletes: a controlled contrast-enhanced cardiovascular magnetic resonance study”. Circulation 2016;134:e364–e365. [DOI] [PubMed] [Google Scholar]
- 21. Grimsmo J, Grundvold I, Maehlum S, Arnesen H. Echocardiographic evaluation of aged male cross-country skiers. Scand J M Sci Sports 2011;21:412–419. [DOI] [PubMed] [Google Scholar]
- 22. Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, David S, Yeo T-J, Narain R, Malhotra A, Papadakis M, Wilson MG, Tome M, AlFakih K, Moon JC, Sharma S. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation 2017;136:126–137. [DOI] [PubMed] [Google Scholar]
- 23. Marijon E, Tafflet M, Celermajer DS, Dumas F, Perier M-C, Mustafic H, Toussaint J-F, Desnos M, Rieu M, Benameur N, Heuzey J-YL, Empana J-P, Jouven X. Sports-related sudden death in the general population. Circulation 2011;124:672–681. [DOI] [PubMed] [Google Scholar]
- 24. Marijon E, Uy-Evanado A, Reinier K, Teodorescu C, Narayanan K, Jouven X, Gunson K, Jui J, Chugh SS. Sudden cardiac arrest during sports activity in middle age; clinical perspective. Circulation 2015;131:1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med 1984;311:874–877. [DOI] [PubMed] [Google Scholar]
- 26. Chugh SS, Weiss JB. Sudden cardiac death in the older athlete. J Am Coll Cardiol 2015;65:493–502. [DOI] [PubMed] [Google Scholar]
- 27. Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion—protection against triggering by regular exertion. N Engl J Med 1993;329:1677–1683. [DOI] [PubMed] [Google Scholar]
- 28. Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 29. Kim JH, Malhotra R, Chiampas G, Hemecourt P d’ Troyanos C, Cianca J, Smith RN, Wang TJ, Roberts WO, Thompson PD, Baggish AL. Cardiac arrest during long-distance running races. N Engl J Med 2012;366:130–140. [DOI] [PubMed] [Google Scholar]
- 30. Kuroki N, Abe D, Suzuki K, Mikami M, Hamabe Y, Aonuma K, Sato A. Exercise-related resuscitated out-of-hospital cardiac arrest due to presumed myocardial ischemia: result from coronary angiography and intravascular ultrasound. Resuscitation 2018;133:40–46. [DOI] [PubMed] [Google Scholar]
- 31. Mousavi N, Czarnecki A, Kumar K, Fallah-Rad N, Lytwyn M, Han S-Y, Francis A, Walker JR, Kirkpatrick IDC, Neilan TG, Sharma S, Jassal DS. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol 2009;103:1467–1472. [DOI] [PubMed] [Google Scholar]
- 32. Gerche AL, Connelly KA, Mooney DJ, MacIsaac AI, Prior DL. Biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise. Heart 2008;94:860–866. [DOI] [PubMed] [Google Scholar]
- 33. Eijsvogels TMH, Fernandez AB, Thompson PD. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol Rev 2016;96:99–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guasch E, Mont L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Nat Rev Cardiol 2017;14:88–101. [DOI] [PubMed] [Google Scholar]
- 35. Bamberg F, Sommer WH, Hoffmann V, Achenbach S, Nikolaou K, Conen D, Reiser MF, Hoffmann U, Becker CR. Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J Am Coll Cardiol 2011;57:2426–2436. [DOI] [PubMed] [Google Scholar]
- 36. Möhlenkamp S, Lehmann N, Moebus S, Schmermund A, Dragano N, Stang A, Siegrist J, Mann K, Jöckel K-H, Erbel R. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol 2011;57:1455–1464. [DOI] [PubMed] [Google Scholar]
- 37. Möhlenkamp S, Lehmann N, Breuckmann F, Bröcker-Preuss M, Nassenstein K, Halle M, Budde T, Mann K, Barkhausen J, Heusch G, Jöckel K-H, Erbel R, Investigators MS, Investigators HNRS, on behalf of the Marathon Study Investigators and the Heinz Nixdorf Recall Study Investigators. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J 2008;29:1903–1910. [DOI] [PubMed] [Google Scholar]
- 38. Braber TL, Mosterd A, Prakken NH, Rienks R, Nathoe HM, Mali WP, Doevendans PA, Backx FJ, Bots ML, Grobbee DE, Velthuis BK. Occult coronary artery disease in middle-aged sportsmen with a low cardiovascular risk score: the Measuring Athlete’s Risk of Cardiovascular Events (MARC) study. Eur J Prev Cardiol 2016;23:1677–1684. [DOI] [PubMed] [Google Scholar]
- 39. Feuchtner G, Langer C, Barbieri F, Beyer C, Dichtl W, Bonaros N, Cartes-Zumelzu F, Klauser A, Schachner T, Friedrich G, Plank F, Senoner T. Relationship of exercise to coronary artery disease extent, severity and plaque type: a coronary computed tomography angiography study. J Cardiovasc Comput Tomogr 2019;13:34–40. [DOI] [PubMed] [Google Scholar]
- 40. Yoshikawa D, Ishii H, Kurebayashi N, Sato B, Hayakawa S, Ando H, Hayashi M, Isobe S, Okumura T, Hirashiki A, Takeshita K, Amano T, Uetani T, Yamada S, Murohara T. Association of cardiorespiratory fitness with characteristics of coronary plaque: assessment using integrated backscatter intravascular ultrasound and optical coherence tomography. Int J Cardiol 2013;162:123–128. [DOI] [PubMed] [Google Scholar]
- 41. Aengevaeren VL, Mosterd A, Braber TL, Prakken NHJ, Doevendans PA, Grobbee DE, Thompson PD, Eijsvogels TMH, Velthuis BK. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation 2017;136:138–148. [DOI] [PubMed] [Google Scholar]
- 42. Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res 2005;67:187–197. [DOI] [PubMed] [Google Scholar]
- 43. Franck G, Even G, Gautier A, Salinas M, Loste A, Procopio E, Gaston A-T, Morvan M, Dupont S, Deschildre C, Berissi S, Laschet J, Nataf P, Nicoletti A, Michel J-B, Caligiuri G. Haemodynamic stress-induced breaches of the arterial intima trigger inflammation and drive atherogenesis. Eur Heart J 2018;4:1. [DOI] [PubMed] [Google Scholar]
- 44. Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273–1282. [DOI] [PubMed] [Google Scholar]
- 45. Lee S-E, Chang H-J, Sung JM, Park H-B, Heo R, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Lee BK, Chun EJ, Cademartiri F, Maffei E, Marques H, Leipsic JA, Shin S, Choi JH, Chinnaiyan K, Raff G, Virmani R, Samady H, Stone PH, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging 2018;11:1475–1484. [DOI] [PubMed] [Google Scholar]
- 46. Radford NB, DeFina LF, Leonard D, Barlow CE, Willis BL, Gibbons LW, Gilchrist SC, Khera A, Levine BD. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle-age men. Circulation 2018;137:1888–1895. [DOI] [PubMed] [Google Scholar]
- 47. DeFina LF, Radford NB, Barlow CE, Willis BL, Leonard D, Haskell WL, Farrell SW, Pavlovic A, Abel K, Berry JD, Khera A, Levine BD. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol 2019;4:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roberts WO, Schwartz RS, Kraus SM, Schwartz JG, Peichel G, Garberich RF, Lesser JR, Oesterle SN, Wickstrom KK, Knickelbine T, Harris KM. Long-term marathon running is associated with low coronary plaque formation in women. Med Sci Sports Exerc 2017;49:641–645. [DOI] [PubMed] [Google Scholar]
- 49. Małek ŁA, Bucciarelli-Ducci C. Myocardial fibrosis in athletes—current perspective. Clin Cardiol 2020;43:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Möhlenkamp S, Leineweber K, Lehmann N, Braun S, Roggenbuck U, Perrey M, Broecker-Preuss M, Budde T, Halle M, Mann K, Jöckel K-H, Erbel R, Heusch G. Coronary atherosclerosis burden, but not transient troponin elevation, predicts long-term outcome in recreational marathon runners. Basic Res Cardiol 2014;109:391. [DOI] [PubMed] [Google Scholar]
- 51. Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 2006;113:2733–2743. [DOI] [PubMed] [Google Scholar]
- 52. Zorzi A, Mastella G, Cipriani A, Berton G, Monte AD, Gusella B, Nese A, Portolan L, Sciacca F, Tikvina S, Tollot S, Trovato D, Iliceto S, Schiavon M, Corrado D. Burden of ventricular arrhythmias at 12-lead 24-hour ambulatory ECG monitoring in middle-aged endurance athletes versus sedentary controls. Eur J Prev Cardiol 2018;25:2003–2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.