Abstract
Colchicine is a unique, sophisticated anti-inflammatory agent that has been used for decades for the prevention of acute inflammatory flares in gout and familial Mediterranean fever. In recent years, clinical trials have demonstrated its potential in a range of cardiovascular (CV) conditions. Colchicine is avidly taken up by leucocytes, and its ability to bind to tubulin and interfere with microtubular function affects the expression of cytokines and interleukins, and the ability of neutrophils to marginate, ingress, aggregate, express superoxide, release neutrophil extracellular traps, and interact with platelets. In patients with acute and recurrent pericarditis, clinical trials in >1600 patients have consistently shown that colchicine halves the risk of recurrence [relative risk (RR) 0.50, 95% confidence interval (CI) 0.42–0.60]. In patients with acute and chronic coronary syndromes, multicentre randomized controlled trials in >11 000 patients followed for up to 5 years demonstrated that colchicine may reduce the risk of CV death, myocardial infarction, ischaemic stroke and ischaemia-driven revascularization by >30% (RR 0.63, 95% CI 0.49–0.81). The use of colchicine at doses of 0.5–1.0 mg daily in CV trials has proved safe. Early gastrointestinal intolerance limits its use in ∼10% of patients; however, ∼90% of patients tolerate it well over the long term. Despite isolated case reports, clinically relevant drug interactions with moderate to strong CYP3A4 inhibitors/competitors or P-glycoprotein inhibitors/competitors are rare if this dosage of colchicine is used in the absence of advanced renal or liver disease. The aim of this review is to summarize the contemporary data supporting the efficacy and safety of colchicine in patients with CV disease.
Keywords: Colchicine, Pericarditis, Acute coronary syndrome, Chronic coronary syndrome, Coronary artery disease, Atrial fibrillation, Heart failure, Inflammasome
Graphical Abstract
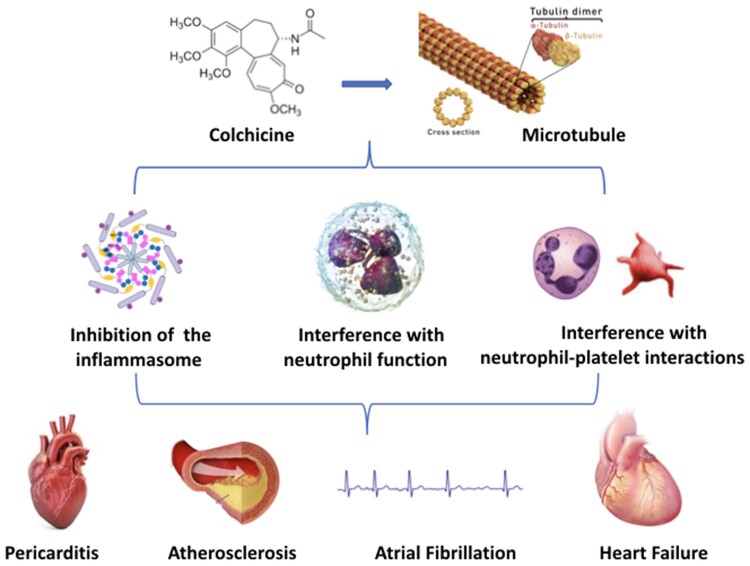
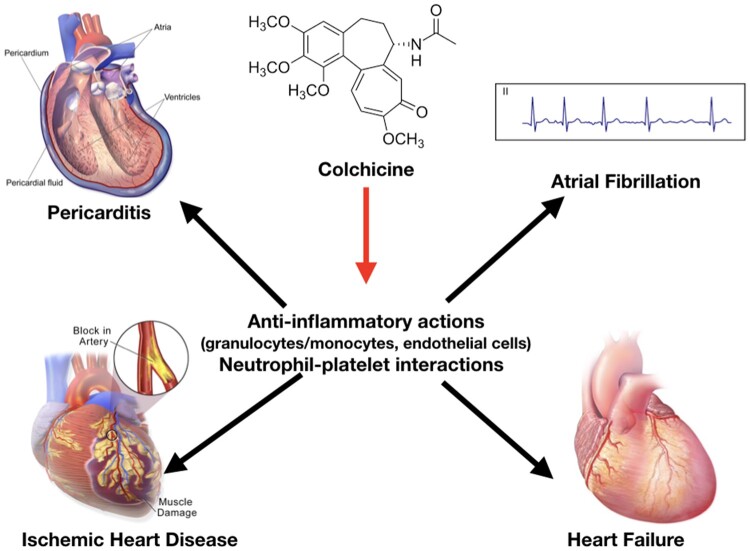
The central mechanism of the anti-inflammatory action of colchicine is the inhibition of microtubule function leading to the inhibition of granulocyte function, interference with selectin expression and neutrophil–platelet interactions, and non-specific inhibition of the assembly of the inflammasome in inflammatory cells. These actions could exert therapeutic effects in different cardiovascular diseases (e.g. pericarditis, acute and chronic coronary syndromes, atrial fibrillation, and heart failure).
Introduction
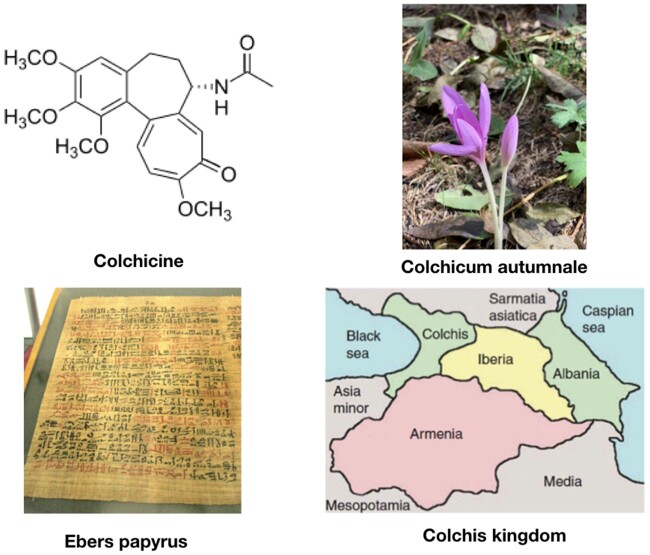
Colchicine is one of the oldest remedies still in use. It is derived from the bulb-like corms of the Colchicum autumnale plant, also known as autumn crocus. Its history as an herbal remedy for joint pain goes back to Egyptian times, and it was first mentioned in the medical literature in the Ebers Papyrus, an Egyptian medical manuscript written around 1500 BC (Figure 1).1 , 2 ,1w Colchicum extract was first described as a treatment for acute gout by Pedanius Dioscorides in De Materia Medica (first century AD). Use of colchicine continued over centuries and Colchicum corms were used by Avicenna, the famous Persian physician, and were recommended by Ambroise Paré in the 16th century. They were also mentioned in the London Pharmacopoeia in 1618.1 The active ingredient, colchicine, was isolated in the early 1800 s by the French chemists Pierre-Joseph Pelletier and Joseph Bienaimé Caventou, and remains in use today as a purified natural product.2w The name ‘colchicine’ is derived from the ancient and legendary kingdom of Colchis from where Jason recovered the Golden Fleece and where C. autumnale plants were widespread.1 , 2
Figure 1.
Colchicine is the active principle derived from Colchicum autumnale plants. The drug has been cited as medical remedy for the first time in the ancient Ebers papyrus (1500 BC). The name ‘colchicine’ is after the ancient and legendary kingdom of Colchis, where Colchicum autumnale plants were widespread.
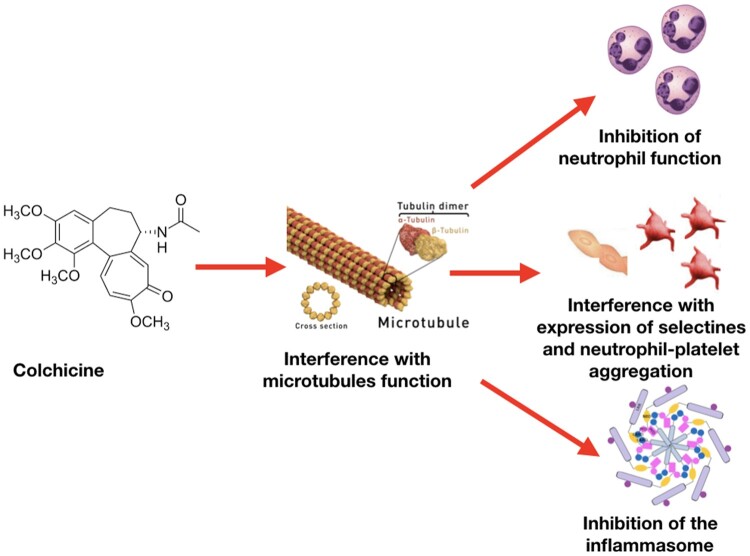
Despite its use over centuries, the exact mechanism of action of colchicine is still under investigation. In the 1950s and 1960s, the microtubule was identified as the primary cellular target. Microtubules are key constituents of the cellular cytoskeleton and are essential to several cellular functions, including maintenance of cell shape, intracellular trafficking, cytokine secretion, cell migration, and regulation of ion channels and cell division. Colchicine binds to tubulin heterodimers and alters the tubulin conformation, preventing any further growth of microtubules at low doses, but promoting their depolymerisation at high doses.3 Anti-inflammatory effects of colchicine are derived from a combination of actions (Figure 2). The effect of colchicine on tubulin affects the assembly of inflammasome and the expression of interleukin (IL)-1β, and other ILs, including IL-18 by macrophages; and impairs neutrophil chemotaxis, adhesion, mobilization, recruitment, production and release of superoxide, and the expression of neutrophil extracellular traps (NETs). Moreover, colchicine decreases neutrophil L-selectin expression, and modulates E-selectin expression on the cell surface of endothelial cells, thereby impairing neutrophil recruitment. In addition, colchicine may interfere with neutrophil-platelet interactions, which play a role in atherothrombosis.4–7 ,3w–5w
Figure 2.
Colchicine anti-inflammatory actions start with the interference with microtubule assembly and function and its capability to concentrate in inflammatory cells with limited expression of P-glycoprotein (e.g. granulocytes). Anti-inflammatory effects of colchicine are derived from a combination of different actions: (i) inhibition of granulocytes, (ii) interference with qualitative and quantitative expression of selectins on endothelial and inflammatory cells and platelet aggregation stimulated by inflammation, and (iii) non-specific inhibition of the inflammasome by interference with the assembly of its components when inflammation is stimulated.
The aim of this article is to critically review the usefulness of colchicine in the treatment of a range of cardiovascular (CV) conditions, focusing on the most relevant clinical studies. A literature review was performed including studies published up to January 2021. Bibliographic databases were searched (MEDLINE/PubMed, BioMed Central, the Cochrane Collaboration Database of Randomized Trials, Scopus, ClinicalTrials.gov, EMBASE, Google Scholar) using the search terms ‘colchicine’ AND ‘cardiovascular disease’ OR ‘coronary artery disease’ OR ‘pericarditis’ OR ‘atrial fibrillation’ OR ‘heart failure’. The research was restricted to English language. The authors independently screened titles and abstracts of all studies, while potentially eligible studies were appraised as full text. The most relevant papers are included in the reference list (Supplementary material online, Figure).
Pharmacology
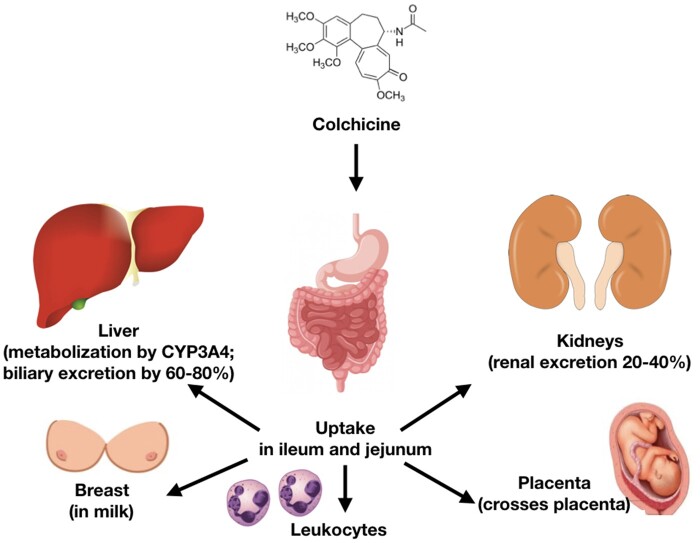
Colchicine is absorbed by the jejunum and ileum. Bioavailability is variable (mean 45%); however, peak serum concentrations are usually reached within 0.5–3 h of oral administration and decline over the next 2 h but subsequently rise due to enterohepatic recycling.4 Colchicine becomes highly concentrated in leucocytes, especially neutrophils, due to their limited expression of P-glycoprotein (P-gp). After single 1 mg oral dose, intracellular concentrations within neutrophils peak within 48 h in healthy subjects, which explains why its acute biological effects require 24–48 h to fully develop. Colchicine has an elimination half-life of 27–31 h. Thus, after discontinuation, its biological effects on leucocytes decline within 48 h.8 ,5w Between 10% and 30% of the drug is protein-bound. Colchicine is partially metabolized in the liver by de-acetylation with an elimination half-life of 12–30 min and is excreted by the kidneys (20–40%) and in the bile (60–80%). Decreased clearance through either of these two pathways may increase the risk of drug accumulation.6w Two major interactions of colchicine with specific proteins modulate its pharmacokinetics beyond tubulin: cytochrome P450 3A4 (CYP3A4) and P-gp. CYP3A4 metabolizes colchicine in the liver (Figure 3). P-gp is an ATP-dependent phospho-glycoprotein located in the cell membrane and responsible for the excretion of the drug in the intestine, liver, kidney, and blood–brain barrier.6w
Figure 3.
Colchicine uptake occurs in the ileum and jejunum. The drug is metabolized by the liver through cytochrome P450 3A4 (CYP3A4) and excreted into the bile and urine through P-glycoprotein. Colchicine can also cross the placenta and enters breast milk by P-glycoprotein.
Potential drug–drug interactions with colchicine
The risk of serious drug–drug interaction (DDIs) in patients taking colchicine relates to the prescribed dose, the presence of advanced renal or liver disease, and the nature of adjunctive medication. Table 1 provides a list of commonly used medications metabolized via CYP3A4 and P-gp grouped by potency,9 ,7w and lists the maximum doses of colchicine that have been reported as being safe in patients with and without advanced renal or liver disease.10
Table 1.
Safe use of colchicine with commonly used drugs that affect clearance of colchicine10
| CYP3A4 inhibitors | P-glycoprotein inhibitors | Safe colchicine use |
|---|---|---|
| Strong | ||
| Clarithromycin | Clarithromycin | Concomitant use of colchicine is generally avoided at any dose as an overlap of therapy for short periods may be rarely toxic even in patients with normal renal function.10 ,8w |
| Telithromycin | Itraconazole | |
| Ketoconazole | Ketoconazole | |
| Voriconazole | Voriconazole | |
| Fluconazole | Fluconazole | |
| Moderate | ||
| Cyclosporine | HIV medications (ritonavir) | Doses up to 0.5 mg daily are likely safe in patients with normal renal and liver function. |
| Ritonavir | In patients with renal or liver failure avoid if possible or reduce colchicine dose to alternate day.11 , 12 , 22 ,9w | |
| Mild | ||
| Erythromycin | Diltiazem | Doses up to 1.0 mg daily. |
| Ciprofloxacin | Verapamil | No dose adjustment required in patients with normal renal or liver function.11–13 ,9w |
| Cobicistat | Amiodarone | |
| Imatinib | Carvedilol | |
| Atorvastatin | Quinidine | |
| Grapefruit | Ranolazine | |
| Erythromycin | ||
| Simvastatin | ||
Concomitant use of colchicine must be carefully considered in all patients prescribed with several specific drugs, including clarithromycin and anti-fungal agents, even in the absence of severe renal or liver dysfunction.11 In patients without advanced renal or liver disease, long-term colchicine has been safely used at doses up to 1.0 mg daily in combination with other medication without dose adjustments.8w Colchicine has been used at doses up to 1.0 mg daily in the presence of mild renal and liver disease; however, because the risk of DDIs is enhanced with drugs that have effects on CYP3A4 and P-gp, it is prudent to limit the dose to 0.5 mg daily. If colchicine is required in patients with severe renal or liver disease, doses of 0.5 mg should be administered no more than on alternate days .12 ,9w
Concomitant use with statins
Despite isolated case reports of myotoxicity after concomitant use of colchicine and statin therapy,13 , 14 ,10w–14w a recent review of DDIs associated with statin use by the American Heart Association (AHA) did not raise concern about the co-administration of colchicine in patients without advanced renal disease.15 This advice is consistent with evidence from large placebo-controlled CV trials that included patients on moderate and high-dose statin therapy, which showed a low incidence of myotoxicity (<1%) that was no different compared to those taking placebo.15 , 16
The experience with DDIs associated with the use of colchicine in patients with CV disease therefore mirrors the experience of its use in familial Mediterranean fever (FMF) and gout, and confirms that serious DDIs are rare when therapy is administered at low doses, is not prescribed concomitantly with a few selective drugs, and is used cautiously in patients with advanced liver (e.g. Child-Pugh score C) or renal disease (estimated glomerular filtration rate <30 mL/min).
Safety and long-term tolerance
While a deliberate overdose of colchicine may be fatal in up to 10% of cases,15w judicious use of colchicine at doses of 0.5–1.0 mg daily has proven safe, as evidenced by a decade of observations in a wide range of patients with FMF, gout, pericarditis, and more recently, coronary disease.17–21 In a recent systematic review focused on adverse events in patients treated with colchicine in trials for CV indications, the occurrence of any adverse event was reported in 15.3% of patients treated with colchicine vs. 13.9% of patients treated with placebo [relative risk (RR) 1.26, 95% confidence interval (CI) 0.96–1.64, P = 0.09].16
Nonetheless, lower gastrointestinal side effects are common and result in early treatment discontinuation, limiting colchicine use in ∼10% of patients. These effects are dose-related, usually occur within days of starting therapy, may settle spontaneously during ongoing treatment, but invariably settle once colchicine is discontinued.
In contrast to early intolerance, over prolonged follow-up in the LoDoCo2 trial, late intolerance to colchicine was uncommon (3.4%) and equal to placebo.21 The incidence of self-reported myalgia was higher in patients on colchicine (21% vs. 18.5%; RR 1.16, 95% CI 1.02–1.32, P = 0.03), but this was not a common cause for treatment discontinuation. As indicated above, the incidence of myotoxicity associated with raised creatinine kinase was rare (<1%) and did not differ between those assigned to colchicine or placebo. Other adverse effects of colchicine use, including hepatotoxicity, neutropenia or agranulocytosis, rashes, infection, or death, have not featured in CV trials of colchicine; however, all trials to date were not sufficiently powered to detect differences in the incidence of these rare events.16
In a recent review of drug-induced agranulocytosis,22 over 120 drugs including colchicine were listed as potentially causal. However, in the current literature only one case report is described in a patient taking low-dose therapy with no history of renal or liver disease .16w
Prolonged use of colchicine has been associated with a transient rise in liver enzymes in ∼2% of patients, but as with statin therapy this did not result in treatment discontinuation or severe liver dysfunction. Other reported possible side-effects, including alopecia and neuropathy, have rarely been reported (<1% of users), and appear to resolve rapidly after colchicine withdrawal.14 , 16 Although colchicine crosses the placenta, continuous use at doses of 0.5–1.0 mg daily during pregnancy does not increase the risk of birth defects or pregnancy loss in FMF. Despite entering breast milk, colchicine is considered safe during breast feeding.17 , 18
Hence, when used at doses up to 1.0 mg daily in patients without advanced renal or liver disease, colchicine is safe. In the 90% of patients who do not develop early treatment intolerance, long-term use at this dosage proved to be safe and well tolerated.
Cardiovascular indications for colchicine
For over a century, treatment and prevention of acute gout was the most common clinically approved indication for short and long-term use of colchicine. Over 50 years ago, the safety and effectiveness of continuous life-long colchicine for the prevention of acute inflammatory flares in patients with FMF led to its regulatory approval for this purpose. Long-term colchicine has also been used off-label for the management of Behçet syndrome and pseudogout.17 Almost 35 years ago, colchicine was introduced in the field of cardiology for the treatment and prevention of recurrent pericarditis,17w and in the last 15 years, its utility and safety have been assessed for secondary prevention of coronary atherosclerosis,19 for the prevention of atrial fibrillation (AF) in specific settings (post-operative and after ablation), and for the prevention of heart failure (Figure 4).20 From a clinical perspective, the utility of low-dose colchicine in patients with CV disease is enhanced by its lack of effect on bleeding risk, blood pressure, QT interval, arrhythmias, and by the low risk of DDIs, when used concomitantly with commonly prescribed CV medications in patients without advanced renal or liver disease.
Figure 4.
Anti-inflammatory actions of colchicine have been studied for different cardiovascular indications: pericarditis (well-established and recommended by guidelines), acute and chronic coronary syndromes (emerging indication), prevention of post-operative atrial fibrillation and atrial fibrillation after ablation, and heart failure (still ongoing and to be well-defined).
Colchicine for the treatment of acute and recurrent pericarditis
The use of colchicine for the treatment of pericarditis was first proposed in 1987 by Bayes de Luna et al.17w The rationale for its use in this setting stems from the observation that colchicine was safe and highly effective in preventing acute flares of polyserositis in patients with FMF. Following a series of case reports and almost 20 years after Bayes de Luna’s letter, the first randomized, open-label trials of colchicine for the treatment of acute (COPE trial)23 and recurrent pericarditis (CORE trial)24 were published (Table 2). In both trials, colchicine was used on top of standard anti-inflammatory therapy. Participants were randomized to colchicine at a loading dose of 1–2 mg, followed by a maintenance dose of 0.5–1.0 mg daily (adjusted according to body weight) for 3–6 months. These trials demonstrated the effectiveness of colchicine and, aside from diarrhoea occurring in 8–10% of patients, colchicine therapy was safe and well tolerated.
The efficacy of colchicine therapy was then confirmed in subsequent double-blind randomized controlled trials in patients with acute and recurrent pericarditis, including the CORP,25 ICAP,26 and CORP-2 trials.27 In these trials, no loading dose was administered but daily dose was weight-adjusted (0.5 mg once daily for patients <70 kg or 0.5 mg twice daily). Therapy was continued for 3 months in patients with acute pericarditis, and for 6 months in patients with recurrent pericarditis.
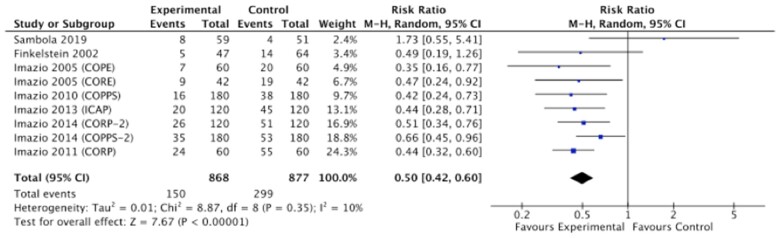
In contrast, one recent small open-label study reported neutral effects of colchicine in patients with acute idiopathic pericarditis who had not received corticosteroids.28 However, in this study, the use of colchicine was delayed, and the diagnostic criteria for pericarditis differed from previous trials and those outlined in the European Society of Cardiology guidelines.29 Nonetheless, as shown in Figure 5, when taken together, these trials convincingly demonstrated that colchicine halves the risk of recurrent pericarditis over 18 months.
Figure 5.
Risk of pericarditis in patients treated with or without colchicine in different settings (acute, recurrent pericarditis, and prevention of the post-pericardiotomy syndrome). CI, confidence interval; M-H, Mantel-Haenszel.
Colchicine for the prevention of postpericardiotomy syndrome
In 2002, Finkelstein et al.30 evaluated the effect of colchicine in patients undergoing cardiac surgery. Participants were randomized to either colchicine 1.5 mg daily or placebo for 1 month. The incidence of post-pericardiotomy syndrome was halved in patients taking colchicine (11% vs. 22%), with a trend towards statistical significance. A few years later, two large randomized controlled trials assessed the effect of colchicine 0.5–1.0 mg daily for 1 month in patients undergoing cardiac surgery.31 , 32 In the COPPS trial, colchicine reduced the incidence of post-pericardiotomy syndrome at 12 months compared with placebo (9% vs. 21%, P < 0.01)31; and in the COPPS-2 trial, colchicine also reduced the incidence of post-pericardiotomy syndrome (19% vs. 29%, P < 0.01), but did not reduce the occurrence of pericardial or pleural effusion.32 More recently, in a randomized trial by Meurin et al.,33 colchicine did not reduce effusion volume nor prevent late cardiac tamponade in a cohort of 197 patients with moderate to large-sized non-inflammatory persistent effusion 7–30 days after cardiac surgery in the absence of pericarditis.
A summary of the main studies in the setting of pericarditis is reported in Table 2. Overall, in patients with pericarditis, colchicine added on top of standard anti-inflammatory therapies halved the risk of recurrence and, in patients undergoing cardiac surgery, it halved the incidence of post-pericardiotomy syndrome (RR 0.50, 95% CI 0.42–0.60) (Figure 5).
Table 2.
Studies on colchicine for the treatment of pericardial diseases
| Study | Study design | Dosing | Clinical setting | Patients | Main results |
|---|---|---|---|---|---|
| COPE trial23 (2005) | Randomized trial (open-label) | Colchicine, 1 mg on first day, followed by 0.5 mg daily (if <70 kg) or 1 mg twice daily followed by 0.5 mg twice daily (if ≥70 kg), for 3 months | Acute pericarditis | 120 | Reduction of recurrent pericarditis (11% vs. 32%, P < 0.01, NNT 5) and symptoms persistence at 72 h (12% vs. 37%, P < 0.01) |
| CORE trial24 (2005) | Randomized trial (open-label) | Colchicine, 1 mg on first day, followed by 0.5 mg daily (if <70 kg) or 1 mg twice daily followed by 0.5 mg twice daily (if ≥70 kg), for 6 months | First recurrence of pericarditis | 84 | Reduction of recurrent pericarditis (24% vs. 51%, P = 0.02, NNT 4) and symptoms persistence at 72 h (10% vs. 31%, P = 0.03) |
| CORP trial25 (2011) | Double-blind RCT | Colchicine, 1 mg on first day followed by 0.5 mg daily (if <70 kg) or 1 mg twice daily followed by 0.5 mg twice daily (if ≥70 kg), for 6 months | First recurrence of pericarditis | 120 | Reduction of recurrent pericarditis (24% vs. 55%, P < 0.01) and symptoms persistence at 72 h (23% vs. 53%, P < 0.01) |
| ICAP trial26 (2013) | Double-blind RCT | Colchicine, 0.5 mg daily (if <70 kg) or 0.5 mg twice daily (if ≥70 kg), for 3 months | Acute pericarditis | 240 | Reduction of recurrent or incessant pericarditis (17% vs. 37%, P < 0.01, NNT 4) and symptoms persistence at 72 h (19% vs. 40%, P < 0.01) |
| CORP-2 trial27 (2014) | Double-blind RCT | Colchicine, 0.5 mg daily (if <70 kg) or 0.5 mg twice daily (if ≥70 kg), for 6 months | Recurrent pericarditis (second or subsequent recurrence) | 240 | Reduction of recurrent pericarditis (22% vs. 42%, P < 0.01, NNT 5) |
| Sambola et al.28 (2019) | Randomized trial (open label) | Colchicine, 0.5 mg twice daily (if <70 kg) or 1 mg twice daily (if ≥70 kg), for 3 months | Acute pericarditis | 110 | Failure to reduce recurrent pericarditis (13% vs. 8%, P = NS) |
| Finkelstein et al.30 (2002) | Randomized trial (open-label) | Colchicine, 1.5 mg daily from the third postoperative day, for 1 month | Post-pericardiotomy syndrome following cardiac surgery | 163 | Failure to reduce post-pericardiotomy syndrome (11% vs. 22%, P = 0.135) |
| COPPS trial31 (2010) | Double-blind RCT | Colchicine, 1 mg on the third postoperative day followed by 0.5 mg daily (if <70 kg) or 1 mg twice daily followed by 0.5 mg twice daily (if ≥70 kg), for 1 month | Post-pericardiotomy syndrome following cardiac surgery | 360 | Reduction of post-pericardiotomy syndrome (9% vs. 21%, P < 0.01) |
| COPPS-232 (2014) | Double-blind RCT | Colchicine from 48 to 72 h before surgery, 0.5 mg daily (if <70 kg) or 0.5 mg twice daily (if ≥70 kg), for 1 month | Post-pericardiotomy syndrome following cardiac surgery | 360 | Reduction of post-pericardiotomy syndrome (19% vs. 29%, P < 0.01) although it did not reduce occurrence of postoperative AF (34% vs. 42%, P = NS) or pericardial/pleural effusion (57% vs. 59%, P = NS) |
| Meurin et al.33 (2015) | Double-blind RCT | Colchicine, 1 mg daily for 2 weeks | Pericardial effusion following cardiac surgery | 197 | Failure to reduce effusion volume on a 0–4 scale (−1.1 ± 1.3 vs. −1.3 ± 1.3 grades) or late cardiac tamponade (7% vs. 6%, P = NS) |
AF, atrial fibrillation; NNT, number needed to treat; RCT, randomized controlled trial.
Colchicine for secondary prevention of chronic coronary disease
Investigations on the use of colchicine for secondary prevention in patients with coronary disease stemmed from the well-known role that inflammation plays in the chronic and acute phases of disease, and the observation that long-term use of colchicine was safe and effective for secondary prevention of acute inflammatory flares in patients with gout and FMF.34 , 35
The normal vascular endothelium is protective having antiplatelet, anticoagulant, vasodilator, and profibrinolytic actions.18w,19w The resting endothelium is also anti-inflammatory, as it acts to prevent leucocyte adhesion. Coronary risk factors including systemic arterial hypertension, hyperlipidaemia, and diabetes mellitus may promote endothelial dysfunction and trigger activation of endothelial cells, activating a proinflammatory process that leads to the early steps and progression of atherosclerosis.20w,21w
Accumulation of free cholesterol within the vessel wall predisposes to ongoing spontaneous self-assembly of free cholesterol into its crystalline forms, which can induce inflammatory injury by activating the innate immune response. Appreciating the role that cholesterol crystals play in the transformation of atheroma into the atherosclerotic plaque, and how this may result in acute plaque disruption, added plausibility to the potential value of colchicine.36 , 37 This insight was further enhanced by the CANTOS trial, which confirmed that IL-1β plays a central role in the atherosclerotic process.38
Unlike canakinumab used in CANTOS to specifically inhibit IL-1β, colchicine has much broader anti-inflammatory effects beyond inhibition of IL-1β. As indicated above, colchicine may accumulate within macrophages, inhibiting assembly of inflammasome and the expression of IL-1β. It may also dampen the production of several other proinflammatory cytokines, including IL-18. As colchicine accumulates in the endothelium, it reduces the expression of selectins that promote ingress of circulating leucocytes, and accumulation of colchicine in neutrophils affects their ability to marginate, aggregate and express cytokines, express NETs and interact with platelets, leading to a reduction of platelet aggregation.22w–24w
In 2007, Nidorf et al. demonstrated that in patients with stable coronary disease and elevated high-sensitivity C-reactive protein (hs-CRP) despite statin and antiplatelet therapy, colchicine 0.5 mg twice daily consistently decreased hs-CRP after 30 days of treatment, suggesting that colchicine had anti-inflammatory effects over statin and antiplatelet therapy.39
In 2013, Nidorf et al. conducted the first clinical trial of colchicine in patients with coronary disease. The low-dose colchicine (LoDoCo) pilot study was a randomized open-label trial conducted with a PROBE design in 532 patients with stable coronary artery disease, who were enrolled regardless of baseline hs-CRP.40 Patients receiving long-term colchicine 0.5 mg daily had a significant reduction of composite CV events [acute coronary syndrome (ACS), out-of-hospital cardiac arrest, non-cardioembolic ischaemic stroke] [5.3% vs. 16%; hazard ratio (HR) 0.33, 95% CI 0.18–0.59]. The benefit was mainly driven by a decreased occurrence of unstable angina.
Subsequently, two observational studies in patients treated for gout demonstrated that those prescribed with colchicine had a significantly reduced risk of CV events, including myocardial infarction, transient ischaemic attack and stroke (odds ratio 0.51, 95% CI 0.30–0.88), and all-cause mortality (HR 0.27, 95% CI 0.17–0.43).41 , 42
In 2020, the LoDoCo2 trial was published.21 In this double-blind placebo-controlled trial, a total of 5522 patients from Australia and the Netherlands were randomized to either colchicine 0.5 mg daily or placebo. The primary endpoint was a composite of CV death, spontaneous myocardial infarction, ischaemic stroke, or ischaemia-driven coronary revascularization. Over 90% of patients enrolled proved tolerant to colchicine and were randomized into the trial. At a median follow-up of 29 months, colchicine significantly reduced the primary endpoint compared with the placebo group (HR 0.69, 95% CI 0.57–0.83; P < 0.001) without significant side effects. As in the LoDoCo pilot study, the benefits of colchicine were seen soon after therapy was initiated and continued to accrue over the course of the trial. The treatment effect extended beyond the primary composite to include major adverse CV events and the individual outcomes of myocardial infarction and unplanned coronary revascularization.21
Colchicine for the prevention of restenosis following coronary angioplasty and surgical revascularization
Studies evaluating the use of colchicine for the prevention of coronary artery restenosis after percutaneous coronary intervention (PCI) have provided mixed results (Table 3). Two studies in the pre-stent era showed a neutral effect of colchicine in the prevention of restenosis following plain old balloon angioplasty.25w,26w However, in a later study on diabetic patients undergoing PCI with bare-metal stent, colchicine was associated with a lower rate of in-stent restenosis (16% vs. 33%, P < 0.01)43 and a similar trend was reported in a sub-analysis of the COLCOT trial.27w
Table 3.
Studies on colchicine for the prevention of chronic coronary syndromes
| Study | Study design | Dosing | Clinical setting | Patients | Main results |
|---|---|---|---|---|---|
| Nidorf et al.39 (2007) | Prospective study | Colchicine 0.5 mg twice daily for 1 month plus aspirin and high-dose atorvastatin | Stable coronary artery disease patients with elevated hs-CRP | 64 | Reduction of hs-CRP (from 4.58 ± 2.05 mg/L to 1.78 ± 1.38 mg/L, P < 0.01) |
| LoDoCo trial40 (2013) | Randomized trial (observer blinded) | Colchicine 0.5 mg daily for a median of 36 months plus statins and standard secondary prevention drugs | Stable coronary artery disease | 532 | Reduction of cardiovascular events (ACS, out-of-hospital cardiac arrest, non-cardioembolic ischaemic stroke): 5.3% vs. 16% (HR 0.33, 95% CI 0.18–0.59) |
| LoDoCo2 trial21 (2020) | Double-blind RCT | Colchicine 0.5 mg daily vs. placebo | Stable coronary artery disease | 5522 | Reduction of CV death, myocardial infarction, ischaemic stroke, or ischaemia-driven coronary revascularization: 6.8% vs. 9.6% (HR 0.69, 95% CI 0.57–0.83) |
| O’Keefe et al.25w (1992) | Double-blind RCT | Colchicine 0.6 mg twice daily for 6 months | Patients undergoing POBA | 197 | Failure to reduce restenosis (46% vs. 47%, P = NS) |
| Freed et al.26w (1995) | Open-label pilot trial | Colchicine 0.6 mg twice daily for 6 months | Patients undergoing POBA | 50 | Failure to inhibit restenosis (restenosis rate of 53%) |
| Deftereos et al.43 (2013) | Double-blind RCT | Colchicine 0.5 mg twice daily for 6 months | Diabetic patients undergoing PCI with bare-metal stent | 196 | Reduction of in-stent restenosis (16% vs. 33%, P < 0.01) |
| Giannopoulos et al.28w (2015) | Double-blind RCT | Colchicine, 0.5 mg twice daily (half dose if <60 kg), for 10 days | On-pump coronary artery bypass grafting | 59 | Reduction of peak high-sensitivity troponin T concentration within 48 h (616 pg/mL vs. 1613 pg/mL, P < 0.01) and CK-MB concentration (44.6 ng/mL vs. 93 ng/mL, P < 0.01) |
ACS, acute coronary syndrome; CI, confidence interval; CK-MB, creatine kinase-myocardial brain fraction; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; PCI, percutaneous coronary intervention; POBA, plain old balloon angioplasty; RCT, randomized controlled trial.
In a trial on patients undergoing on-pump coronary artery bypass grafting, colchicine reduced perioperative myocardial damage assessed with peak troponin T and creatine kinase-myocardial brain fraction (CK-MB) concentrations within 48 h.28w
Colchicine for secondary prevention following acute coronary syndromes
Early trials of colchicine in the setting of an ACS designed to assess the effect of colchicine on biomarkers of inflammation and myocardial injury have provided mixed results. In a pilot randomized trial including patients with ACS or stroke, colchicine failed to reduce 30-day hs-CRP (median 1.0 mg/L vs. 1.5 mg/L, P = 0.22).29w In the observational study by Akodad et al. on patients presenting with ST-elevation myocardial infarction (STEMI), colchicine also had a neutral effect on hs-CRP peak values during the index hospitalization.30w On the contrary, in the randomized trial by Deftereos et al. in patients with STEMI, short-term colchicine reduced CK-MB (3144 vs. 6184 ng/mL, P < 0.01) and infarct size on magnetic resonance imaging (18.3 vs. 23.2 mL/1.73 m2, P = 0.02).31w
A remodelling effect of colchicine on atherosclerotic plaques was shown in a study by Vaidya et al. in patients with a recent ACS. Patients receiving colchicine had a reduction of both hs-CRP and low attenuation plaque volume on coronary computed tomography angiography.32w
The only trial sufficiently powered to assess the clinical effects of colchicine following an ACS was the COLCOT trial.44 In this trial, patients with a recent (<1 month) myocardial infarction were randomized to colchicine 0.5 mg daily or placebo and followed up for 4 years. Patients assigned to colchicine had a lower incidence of the composite of CV death, cardiac arrest, myocardial infarction, stroke, or urgent hospitalizations for angina (5.5% vs. 7.1%; HR 0.77, 95% CI 0.61–0.96). The outcome was mainly driven by a reduction in the incidence of stroke and urgent revascularization for angina. A sub-study of COLCOT suggested that the treatment effect was more marked when colchicine was initiated within 3 days of the onset of myocardial infarction (HR 0.52, 95% CI 0.32–0.84); however, the major benefits were accrued well after hospital discharge.45 In contrast to the LoDoCo2 trial, colchicine use was associated with a low but increased incidence of hospitalization for (non-fatal) pneumonia (0.9% vs. 0.4%, P = 0.03).21 , 44
Two additional studies of colchicine in ACS were published in 2020: the COLCHICINE-PCI trial46 and the COPS trial.47 COLCHICINE-PCI investigated the effects of acute preprocedural oral administration of 1.8 mg of colchicine on PCI-related myocardial injury.46 Among 400 subjects undergoing PCI, preprocedural administration of colchicine attenuated the increase in IL-6 and hs-CRP after PCI when compared with placebo but had no effect on enzymatic measures of infarct size. The lack of treatment effect on myocardial injury was in contrast to that on infarct size in patients undergoing elective surgical revascularization. This difference in outcome likely reflects the clinical setting of each trial, as in COLCHICINE-PCI colchicine therapy was started late in the course of an evolving infarction, and did not control for the complexity of coronary stenting, which may have resulted in a greater risk of myocardial injury due to atheroembolism.
In the COPS trial, 795 patients with an ACS were randomized to either colchicine (0.5 mg twice daily for the first month, then 0.5 mg daily for 11 months) or placebo.47 The primary outcome was a composite of all-cause mortality, ACS, ischaemia-driven (unplanned) urgent revascularization, and non-cardioembolic ischaemic stroke. Although underpowered to assess the effect on clinical outcome, over the 12-month follow-up, there were 24 events in the colchicine group compared with 38 events in the placebo group (P = 0.09, log-rank). However, in contrast to the LoDoCo and COLCOT trials, there was a trend towards a higher rate of all-cause mortality (8 vs. 1; P = 0.017, log-rank), mostly due to a higher number of non-CV deaths in the colchicine group (5 vs. 0; P = 0.024, log-rank). As in the COLCOT trial, the incidence of other adverse effects including gastrointestinal effects did not differ between groups (colchicine 23.0% vs. placebo 24.3%).
Critique of the trials of colchicine in cardiovascular disease
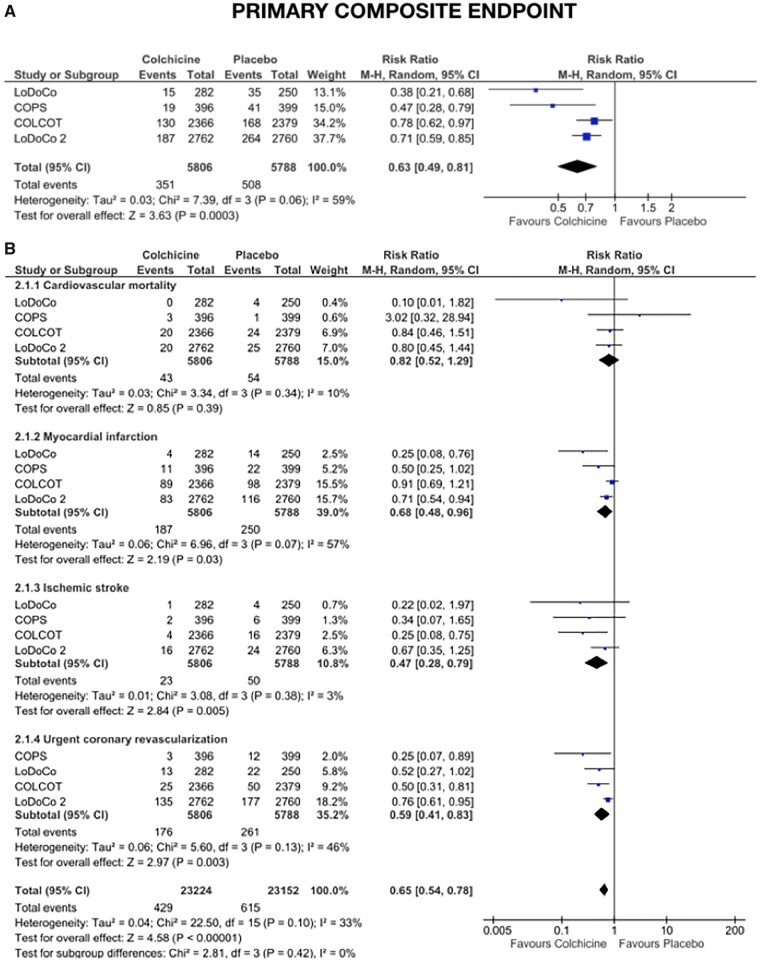
To date, four independent randomized controlled trials—LoDoCo,40 LoDoCo2,21 COLCOT,44 and COPS47—evaluating the effect of colchicine in a broad spectrum of >11 000 patients with acute and chronic coronary disease followed for up to 5 years, demonstrated that colchicine may reduce the risk of CV death, myocardial infarction, ischaemic stroke and ischaemia-driven revascularization by >30% (RR 0.63, 95% CI 0.49–0.81) (Figure 6). Although each has employed a simple pragmatic design, each has limitations and all have raised important questions.
Figure 6.
Forest plot of the primary clinical efficacy endpoint derived from main randomized controlled trials in acute and chronic coronary syndromes (A for the primary composite endpoint and B for the single components of the primary composite endpoint). CI, confidence interval; M-H, Mantel-Haenszel.
Specifically, none used clinical or biological markers of risk or inflammation for the selection of participants. Each recruited predominantly men. None reported cholesterol levels or blood pressure at enrolment. The COLCOT trial did not report the rate of dropout due to early intolerance to colchicine, only the composite outcome was found to be significantly reduced, and there was a higher incidence of (non-fatal) respiratory infections. The COPS trial had a truncated follow-up, the primary outcome included all-cause mortality rather than CV mortality and, as noted, a higher incidence of non-CV death was recorded in patients receiving colchicine. By design, LoDoCo2 excluded ∼10% of enrolled patients who proved intolerant to colchicine, which may in part explain why the effect size appeared greater than the other trials, and why it was able to demonstrate an effect on individual outcomes including myocardial infarction and unplanned revascularization. Nonetheless, a regional difference in the effect of colchicine was observed and, as in the COPS trial, a low but disproportionate number of participants randomized to colchicine were found to have died from non-CV causes (incidence, 0.7 vs. 0.5 events per 100 person-years; HR 1.51; 95% CI 0.99–2.31).47
Despite the lack of data on cholesterol levels and blood pressure at randomization, most patients in these trials were receiving moderate or high-dose statin therapy, and an equal proportion in each treatment group was taking lipid-lowering and anti-hypertensive therapy. Although overall data do not appear sufficient to establish if some patients may benefit more than others, subgroup analyses showed consistent effects of colchicine in a broad range of patients. The concern related to the imbalance in the number of non-CV deaths in LoDoCo2 and COPS has largely been addressed by meta-analyses that have demonstrated that colchicine does not increase the risk of all-cause mortality or non-CV death,33w–35w and the lack of association between colchicine and death from sepsis or cancer, or between the duration of therapy and non-CV death (in the COPS trial 3/5 patients died late of a non-CV cause after <30 day exposure to colchicine) makes it unlikely that a biological explanation will be found for these observations. Despite the regional variance in treatment effect noted in LoDoCo2, the effect of colchicine was directionally consistent between regions, and the consistent results of COLCOT and COPS clearly indicate that the effects of colchicine are not region-dependent.
Impressively the results of these trials have been broadly consistent. The LoDoCo and COLCOT trials confirmed that colchicine reduced the risk of the composite outcome of myocardial infarction, ischaemic stroke, unplanned revascularization and CV death, and when the primary outcome of the COPS trial is aligned with LoDoCo, LoDoCo2, and COLCOT (by excluding non-CV death) the effect on the composite CV outcome was also significant (Table 4). Furthermore, combined data from these trials confirm that colchicine reduces the risk of the individual outcomes of myocardial infarction, ischaemic stroke, and unplanned revascularization, and demonstrate a (non-significant) trend towards a reduction in CV death. Finally, these trials also indicate that, when used judiciously, colchicine 0.5 mg daily does not increase the risk of sepsis, cancer, neutropenia, myotoxicity, or bleeding.
Table 4.
Studies on colchicine for the prevention of acute coronary syndromes
| Study | Study design | Dosing | Clinical setting | Patients | Main results |
|---|---|---|---|---|---|
| Raju et al.29w (2012) | Double-blind RCT | Colchicine 1.0 mg daily for 1 month | ACS or stroke | 82 | Failure to reduce hs-CRP at 30 days (median 1.0 mg/l vs. 1.5 mg/l, P = 0.22) |
| Deftereos et al.31w (2015) | Double-blind RCT | Loading dose of 2 mg followed by 0.5 mg twice daily for 5 days | STEMI | 151 | Reduction of CK-MB plasma concentration (3144 ng/mL vs. 6184 ng/mL, P < 0.01) and infarct size by magnetic resonance imaging (18.3 mL/1.73 m2 vs. 23.2 mL/1.73 m2, P = 0.02) |
| Akodad et al.30w (2017) | Prospective study | Colchicine 1 mg once daily plus OMT for 1 month | STEMI | 44 | Failure to reduce CRP peak value during the index hospitalization (29.03 mg/L vs. 21.86 mg/L, P = 0.36), even after adjustment for the culprit artery (27 mg/L vs. 25 mg/L, P = 0.79) |
| Vaidya et al.32w (2018) | Prospective study | Colchicine 0.5 mg daily plus OMT for 12 months | Recent ACS (<1 month) | 80 | Reduction of LAPV (15.9 mm3 vs. 6.6 mm3, P = 0.008) and hs-CRP (1.10 mg/L vs. 0.38 mg/L, P < 0.01) |
| COLCOT trial44 (2019) | Double- blind RCT | Colchicine 0.5 mg daily for a median of 20 months | Recent myocardial infarction (<1 month) | 4745 | Reduction of CV events (composite of CV death, cardiac arrest, myocardial infarction, stroke, or urgent hospitalizations for angina): 5.5% vs. 7.1% (HR 0.77, 95% CI 0.61–0.96) |
| COLCHICINE-PCI46 (2020) | Double-blind RCT | Preprocedural oral administration of 1.8 mg of colchicine | 50% patients with an ACS | 400 | The primary outcome of PCI-related myocardial injury did not differ between colchicine (n = 206) and placebo (n = 194) groups (57.3% vs. 64.2%, P = 0.19) |
| COPS trial47 (2020) | Double-blind RCT | Colchicine 0.5 mg twice daily for the first month, then 0.5 mg daily | ACS | 795 |
The primary outcome of all-cause mortality, ACS, ischaemia-driven (unplanned) urgent revascularization, and non-cardioembolic ischaemic stroke did not differ between colchicine (n = 396) and placebo (n = 399): 24 vs. 38 events (P = 0.09) The composite of CV death, ACS, stroke and unplanned revascularization 0.54 (0.29–0.99) |
ACS, acute coronary syndrome; CK-MB, creatine kinase-myocardial brain fraction; CV, cardiovascular; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; LAPV, low attenuation plaque volume; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; STEMI, ST-elevation myocardial infarction.
Thus, collectively the current trials of colchicine in patients with CV disease suggest that colchicine slows the progression of atherosclerosis by limiting plaque growth, reducing the risk of plaque instability and the risk of in-stent restenosis,43 ,27w,33w–35w and indicate that when used judiciously, it is safe. As such, they lay the foundation to support repurposing colchicine for secondary prevention in patients with CV disease on top of statin and antiplatelet therapy.
In the next 3–5 years, the CLEAR SYNERGY study, the CONVINCE trial (NCT02898610), and the COLCARDIO trial (ACTRN12616000400460) will collectively recruit >9000 patients and will undoubtedly provide further insights into the efficacy, long-term safety and tolerability of colchicine 0.5 mg daily in various subsets of patients with CV disease.
Colchicine for the prevention of atrial fibrillation
Table 5 provides a summary of the main studies in the setting of AF.
Table 5.
Studies on colchicine for the prevention of atrial fibrillation
| Study | Study design | Dosing | Clinical setting | Patients | Main results |
|---|---|---|---|---|---|
| COPPS-POAF48 (2011) | Double-blind RCT | Colchicine, 1 mg on third postoperative day followed by 0.5 mg daily (if <70 kg) or 1 mg twice daily followed by 0.5 mg twice daily (if ≥70 kg), for 1 month | Atrial fibrillation following cardiac surgery | 336 | Reduction of postoperative atrial fibrillation (12% vs. 22%, P = 0.021) |
| Deftereos et al.38w (2012) | Double-blind RCT | Colchicine 0.5 mg twice daily for 3 months | Atrial fibrillation recurrence following pulmonary vein isolation | 161 | Reduction of postoperative atrial fibrillation (16% vs. 34%, P < 0.01), CRP at day 4 (−1.18 mg/L vs. −0.46 mg/L, P < 0.01) and IL-6 at day 4 (−0.50 pg/mL vs. −0.10 pg/mL, P < 0.01) |
| Deftereos et al.50 (2014) | Double-blind RCT | Colchicine 0.5 mg twice daily for 3 months | Atrial fibrillation recurrence following pulmonary vein isolation | 223 | Reduction of postoperative atrial fibrillation (31% vs. 49%, P = 0.01), improvement of the physical domain of quality of life scores at 12 months (63.6 ± 13.8 vs. 52.5 ± 18.1, P < 0.01) |
| END-AF trial36w (2016) | Double-blind RCT | Colchicine 0.5 mg twice daily plus 2 mg before surgery (half dose if <70 kg) for 8 days (mean) | Atrial fibrillation following cardiac surgery | 360 | Failure to reduce the occurrence of postoperative atrial fibrillation (14% vs. 20%, P = NS) |
| Zarpelon et al.37w (2016) | Double-blind RCT | Colchicine 1 mg twice daily before surgery, then 0.5 mg twice daily until discharge | Atrial fibrillation following cardiac surgery | 140 | Failure to reduce the occurrence of postoperative atrial fibrillation (7% vs. 13%, P = NS) |
CRP, C-reactive protein; IL-6, interleukin-6; RCT, randomized controlled trial.
Following bypass surgery
Atrial fibrillation is the most common complication after cardiac surgery and is a common cause of prolonged and recurrent hospitalization following surgery. Due to its efficacy in the prevention of the post-pericardiotomy syndrome, several small trials have investigated the use of colchicine for the prevention of postoperative AF. In a sub-study of the COPPS trial (the COPPS-POAF sub-study), colchicine 1 mg daily started on the third postoperative day, followed by 0.5–1 mg daily, reduced the incidence of postoperative AF at 30 days compared to placebo (12% vs. 22%, P = 0.021).48 Two more recent trials reported neutral results; however, both trials were limited by shorter periods of observation,36w,37w suggesting the need for additional studies to assess the effect of preoperative colchicine for the prevention of postoperative AF.
Following pulmonary vein ablation
Early AF recurrence following pulmonary vein isolation has been associated with local inflammation triggered by ablation. The use colchicine to prevent AF relapses after ablation has been assessed in two randomized trials by Deftereos et al.49 ,38w In the first trial conducted in 2012, colchicine 0.5 mg twice daily started on the day of ablation and continued for 3 months, reduced the incidence of postoperative AF (16% vs. 34%, P < 0.01), CRP at day 4 (−1.18 mg/L vs. −0.46 mg/L, P < 0.01) and IL-6 at day 4 (−0.50 pg/mL vs. −0.10 pg/mL, P < 0.01).38w In the second trial, which included a larger cohort of patients, colchicine reduced the incidence of postoperative AF (31% vs. 49%, P = 0.01) and improved the physical domain of the quality of life scores at 12 months (63.6 ± 13.8 vs. 52.5 ± 18.1, P < 0.01).49
Colchicine in patients with heart failure
The involvement of inflammatory pathways in the pro-fibrotic process associated with adverse ventricular remodelling following myocardial infarction has led to trials aimed at assessing whether inhibiting inflammation with colchicine can reduce the risk of progressive heart failure. To date, the evidence is limited to a single randomized controlled trial that assessed the efficacy and safety of 6-month colchicine (0.5–1 mg daily) on New York Heart Association (NYHA) class in 267 patients with stable heart failure with reduced left ventricular ejection fraction (<40%).50 After 6 months, colchicine use proved safe and reduced hs-CRP, but did not improve left ventricular dimensions or NYHA class.
Despite these negative results, two ongoing clinical trials are exploring the effect of colchicine on ventricular remodelling following myocardial infarction. The effects of a short course of colchicine during the acute phase of STEMI on ventricular remodelling will be specifically evaluated in the COVERT-MI trial (NCT03156816). The CLEAR SYNERGY study (NCT03048825) will evaluate whether colchicine (alone or associated with spironolactone) can reduce the 1-year risk of major adverse CV events or a composite of CV death and heart failure.
Conclusions
Colchicine is a sophisticated anti-inflammatory agent that has been used for centuries for the treatment and prevention of gout, and for over 50 years for the prevention of acute inflammatory flares in patients with FMF. Almost 35 years ago, the observed benefits of colchicine in the prevention and treatment of polyserositis in patients with FMF led to its introduction into CV medicine for the treatment and prevention of pericarditis. Subsequent randomized trials in over 1600 patients confirming its safety and efficacy have resulted in it being adopted in the European guidelines29 and registered in some countries (e.g. Italy, Austria) as first-line therapy for this indication. Over the last 15 years, clinical trials in >11 000 patients with coronary disease have shown that long-term low-dose colchicine can be safely used on top of lipid-lowering and antiplatelet therapy in the absence of advanced renal or liver disease to improve disease-free survival. Over the next 3–5 years, ongoing trials will add information about the benefits of colchicine in CV disease in a further 9000 patients.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: M.I. has been Advisory Board member for ACARPIA (colchicine), KINIKSA (rilonacept), and SOBI (anakinra), M.C. reported no disclosures.
Supplementary Material
Contributor Information
Massimo Imazio, Cardiology, Cardiothoracic Department, University Hospital “Santa Maria della Misericordia”, ASUFC, Piazzale Santa Maria della Misericordia 15, 33100 Udine, Italy.
Mark Nidorf, GenesisCare, 3/140 Mounts Bay Rd, Perth, Western Australia, Australia.
References
- 1. Nerlekar N, Beale A, Harper RW. Colchicine—a short history of an ancient drug. Med J Aust 2014;201:687–688. [DOI] [PubMed] [Google Scholar]
- 2. Bayes-Genis A, Adler Y, de Luna AB, Imazio M. Colchicine in pericarditis. Eur Heart J 2017;38:1706–1709. [DOI] [PubMed] [Google Scholar]
- 3. Leung YY, Yao Hui LL, Kraus VB. Colchicine—update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015;45:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther 2014;36:1465–1479. [DOI] [PubMed] [Google Scholar]
- 5. Vaidya K, Tucker B, Kurup R, Khandkar C, Pandzic E, Barraclough J, Machet J, Misra A, Kavurma M, Martinez G, Rye KA, Cochran BJ, Patel S. Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention. J Am Heart Assoc 2021;10:e018993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res 2018;371:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 2018;269:262–271. [DOI] [PubMed] [Google Scholar]
- 8. Chiabrando JG, Bonaventura A, Vecchié A, Wohlford GF, Mauro AG, Jordan JH, Grizzard JD, Montecucco F, Berrocal DH, Brucato A, Imazio M, Abbate A. Management of acute and recurrent pericarditis: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:76–92. [DOI] [PubMed] [Google Scholar]
- 9. Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH. Update on colchicine, 2017. Rheumatology (Oxford) 2018;57:i4–i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: old and new. Am J Med 2015;128:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum 2011;63:2226–2237. [DOI] [PubMed] [Google Scholar]
- 12. Ben‐Chetrit E. Colchicine dose reduction in patients with normal liver and kidney function: comment on the article by Terkeltaub et al. Arthritis Rheum 2011;63:3647–3648. [DOI] [PubMed] [Google Scholar]
- 13. Solak Y, Acikgoz SB, Yildirim M. Colchicine toxicity: an exaggerated reality? Am J Med 2015;128:e11. [DOI] [PubMed] [Google Scholar]
- 14. Stewart S, Yang KCK, Atkins K, Dalbeth N, Robinson PC. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2020;22:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiggins BS, Saseen JJ, Page RL, Reed BN, Sneed K, Kostis JB, Lanfear D, Virani S, Morris PB; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Hypertension; Council on Quality of Care and Outcomes Research; and Council on Functional Genomics and Translational Biology. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2016;134:e468–e495. [DOI] [PubMed] [Google Scholar]
- 16. Andreis A, Imazio M, Avondo S, Casula M, Paneva E, Piroli F, De Ferrari GM. Adverse events of colchicine for cardiovascular diseases: a comprehensive meta-analysis of 14 188 patients from 21 randomized controlled trials. J Cardiovasc Med (Hagerstown) 2021;doi:10.2459/JCM.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 17. Ozen S, Demirkaya E, Erer B, Livneh A, Ben-Chetrit E, Giancane G, Ozdogan H, Abu I, Gattorno M, Hawkins PN, Yuce S, Kallinich T, Bilginer Y, Kastner D, Carmona L. EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis 2016;75:644–651. [DOI] [PubMed] [Google Scholar]
- 18. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, Marder W, Guyatt G, Branch DW, Buyon J, Christopher-Stine L, Crow-Hercher R, Cush J, Druzin M, Kavanaugh A, Laskin CA, Plante L, Salmon J, Simard J, Somers EC, Steen V, Tedeschi SK, Vinet E, White CW, Yazdany J, Barbhaiya M, Bettendorf B, Eudy A, Jayatilleke A, Shah AA, Sullivan N, Tarter LL, Birru Talabi M, Turgunbaev M, Turner A, D'Anci KE. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020;72:529–556. [DOI] [PubMed] [Google Scholar]
- 19. Imazio M, Andreis A, Brucato A, Adler Y, De Ferrari GM. Colchicine for acute and chronic coronary syndromes. Heart 2020;106:1555–1560. [DOI] [PubMed] [Google Scholar]
- 20. Andreis A, Imazio M, De Ferrari GM. Colchicine for the treatment of cardiovascular diseases: old drug, new targets. J Cardiovasc Med (Hagerstown) 2021;22:1–8. [DOI] [PubMed] [Google Scholar]
- 21. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA, Tijssen JGP, Cornel JH, Thompson PL; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383:1838–1847. [DOI] [PubMed] [Google Scholar]
- 22. Andres E, Villalba NL, Zulfiqar A-A, Serraj K, Mourot-Cotte R, Gottenberg JC. State of art of idiosyncratic drug-induced neutropenia or agranulocytosis, with a focus on biotherapies. J Clin Med 2019;8:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, Moratti M, Gaschino G, Giammaria M, Ghisio A, Belli R, Trinchero R. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005;112:2012–2016. [DOI] [PubMed] [Google Scholar]
- 24. Imazio M, Bobbio M, Cecchi E, Demarie D, Pomari F, Moratti M, Ghisio A, Belli R, Trinchero R. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med 2005;165:1987–1991. [DOI] [PubMed] [Google Scholar]
- 25. Imazio M, Brucato A, Cemin R, Ferrua S, Belli R, Maestroni S, Trinchero R, Spodick DH, Adler Y; CORP (COlchicine for Recurrent Pericarditis) Investigators. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med 2011;155:409–414. [DOI] [PubMed] [Google Scholar]
- 26. Imazio M, Brucato A, Cemin R, Ferrua S, Maggiolini S, Beqaraj F, Demarie D, Forno D, Ferro S, Maestroni S, Belli R, Trinchero R, Spodick DH, Adler Y; ICAP Investigators. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013;369:1522–1528. [DOI] [PubMed] [Google Scholar]
- 27. Imazio M, Belli R, Brucato A, Cemin R, Ferrua S, Beqaraj F, Demarie D, Ferro S, Forno D, Maestroni S, Cumetti D, Varbella F, Trinchero R, Spodick DH, Adler Y. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet 2014;383:2232–2237. [DOI] [PubMed] [Google Scholar]
- 28. Sambola A, Roca Luque I, Mercé J, Alguersuari J, Francisco-Pascual J, García-Dorado D, Sagristà-Sauleda J. Colchicine administered in the first episode of acute idiopathic pericarditis: a randomized multicenter open-label study. Rev Esp Cardiol (Engl Ed) 2019;72:709–716. [DOI] [PubMed] [Google Scholar]
- 29. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W; ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finkelstein Y, Shemesh J, Mahlab K, Abramov D, Bar-El Y, Sagie A, Sharoni E, Sahar G, Smolinsky AK, Schechter T, Vidne BA, Adler Y. Colchicine for the prevention of postpericardiotomy syndrome. Herz 2002;27:791–794. [DOI] [PubMed] [Google Scholar]
- 31. Imazio M, Trinchero R, Brucato A, Rovere ME, Gandino A, Cemin R, Ferrua S, Maestroni S, Zingarelli E, Barosi A, Simon C, Sansone F, Patrini D, Vitali E, Ferrazzi P, Spodick DH, Adler Y; COPPS Investigators. COlchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS): a multicentre, randomized, double-blind, placebo-controlled trial. Eur Heart J 2010;31:2749–2754. [DOI] [PubMed] [Google Scholar]
- 32. Imazio M, Brucato A, Ferrazzi P, Pullara A, Adler Y, Barosi A, Caforio AL, Cemin R, Chirillo F, Comoglio C, Cugola D, Cumetti D, Dyrda O, Ferrua S, Finkelstein Y, Flocco R, Gandino A, Hoit B, Innocente F, Maestroni S, Musumeci F, Oh J, Pergolini A, Polizzi V, Ristić A, Simon C, Spodick DH, Tarzia V, Trimboli S, Valenti A, Belli R, Gaita F; COPPS-2 Investigators. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 Randomized Clinical Trial. JAMA 2014;312:1016–1023. [DOI] [PubMed] [Google Scholar]
- 33. Meurin P, Lelay-Kubas S, Pierre B, Pereira H, Pavy B, Iliou MC, Bussière JL, Weber H, Beugin JP, Farrokhi T, Bellemain-Appaix A, Briota L, Tabet JY; French Society of Cardiology. Colchicine for postoperative pericardial effusion: a multicentre, double-blind, randomised controlled trial. Heart 2015;101:1711–1716. [DOI] [PubMed] [Google Scholar]
- 34. Nidorf SM. Embracing colchicine as a new cornerstone therapy for coronary disease. Trends Cardiovasc Med 2020. Nov 3; doi: 10.1016/j.tcm.2020.10.012 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Libby P. Inflammation in atherosclerosis-no longer a theory. Clin Chem 2021;67:131–142. [DOI] [PubMed] [Google Scholar]
- 36. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nidorf SM, Fiolet A, Abela GS. Viewing atherosclerosis through a crystal lens: how the evolving structure of cholesterol crystals in atherosclerotic plaque alters its stability. J Clin Lipidol 2020;14:619–630. [DOI] [PubMed] [Google Scholar]
- 38. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1113. [DOI] [PubMed] [Google Scholar]
- 39. Nidorf M, Thompson PL. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am J Cardiol 2007;99:805–807. [DOI] [PubMed] [Google Scholar]
- 40. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–410. [DOI] [PubMed] [Google Scholar]
- 41. Crittenden DB, Lehmann RA, Schneck L, Keenan RT, Shah B, Greenberg JD, Cronstein BN, Sedlis SP, Pillinger MH. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol 2012;39:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solomon DH, Liu C-C, Kuo I-H, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis 2016;75:1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deftereos S, Giannopoulos G, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, Driva M, Hahalis G, Pyrgakis V, Alexopoulos D, Manolis AS, Stefanadis C, Cleman MW. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J Am Coll Cardiol 2013;61:1679–1685. [DOI] [PubMed] [Google Scholar]
- 44. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie MA, Dubé MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L'Allier PL, Guertin MC, Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 45. Bouabdallaoui N, Tardif J-C, Waters DD, Pinto FJ, Maggioni AP, Diaz R, Berry C, Koenig W, Lopez-Sendon J, Gamra H, Kiwan GS, Blondeau L, Orfanos A, Ibrahim R, Grégoire JC, Dubé M-P, Samuel M, Morel O, Lim P, Bertrand OF, Kouz S, Guertin M-C, L’Allier PL, Roubille F. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J 2020;41:4092–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shah B, Pillinger M, Zhong H, Cronstein B, Xia Y, Lorin JD, Smilowitz NR, Feit F, Ratnapala N, Keller NM, Katz SD. Effects of acute colchicine administration prior to percutaneous coronary intervention: COLCHICINE-PCI randomized trial. Circ Cardiovasc Interv 2020;13:e008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, Sriamareswaran R, Htun NM, Wilson W, Stub D, van Gaal W, Howes L, Collins N, Yong A, Bhindi R, Whitbourn R, Lee A, Hengel C, Asrress K, Freeman M, Amerena J, Wilson A, Layland J. Colchicine in patients with acute coronary syndrome: the Australian COPS Randomized Clinical Trial. Circulation 2020;142:1890–1900. [DOI] [PubMed] [Google Scholar]
- 48. Imazio M, Brucato A, Ferrazzi P, Rovere ME, Gandino A, Cemin R, Ferrua S, Belli R, Maestroni S, Simon C, Zingarelli E, Barosi A, Sansone F, Patrini D, Vitali E, Trinchero R, Spodick DH, Adler Y; COPPS Investigators. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation 2011;124:2290–2295. [DOI] [PubMed] [Google Scholar]
- 49. Deftereos S, Giannopoulos G, Efremidis M, Kossyvakis C, Katsivas A, Panagopoulou V, Papadimitriou C, Karageorgiou S, Doudoumis K, Raisakis K, Kaoukis A, Alexopoulos D, Manolis AS, Stefanadis C, Cleman MW. Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Heart Rhythm 2014;11:620–628. [DOI] [PubMed] [Google Scholar]
- 50. Deftereos S, Giannopoulos G, Panagopoulou V, Bouras G, Raisakis K, Kossyvakis C, Karageorgiou S, Papadimitriou C, Vastaki M, Kaoukis A, Angelidis C, Pagoni S, Pyrgakis V, Alexopoulos D, Manolis AS, Stefanadis C, Cleman MW. Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. JACC Heart Fail 2014;2:131–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.