Abstract
To evaluate the duration of topical brimonidine therapy before the onset of brimonidine-related allergic conjunctivitis and the clinical characteristics associated with the development of brimonidine allergy.
We retrospectively enrolled patients who presented brimonidine allergy from December 1, 2008 to November 30, 2020. The duration of brimonidine treatment, concomitant medications, benzalkonium chloride (BAK) exposure, change in IOP, and season of onset were evaluated.
292 patients were included, among which 147 were female and 145 were male. The mean age was 58.3 ± 13.6 years old. The mean (median) duration of brimonidine therapy was 266.6 (196) days, with a peak at 60–120 days. The duration was similar whether the patients received brimonidine monotreatment or in combination with other glaucoma drugs, with or without BAK. The IOP increased by 5.6% after brimonidine allergy (P < .001), which was even higher in the brimonidine monotherapy group (9.2%, P < .001). There was no significant IOP elevation in patients treated with multiple glaucoma medications.
Around half of brimonidine allergy occurred within 6 months, with a peak in 2 to 4 months. The duration did not differ in patients receiving brimonidine monotherapy or multiple glaucoma medications. The presence of BAK did not affect the duration either. When brimonidine allergy occurred, there was a loss of IOP control, especially in patients receiving brimonidine monotherapy. It is recommended to switch to other types of glaucoma medications for better IOP control.
Keywords: allergy, brimonidine, conjunctivitis, intraocular pressure
1. Introduction
Brimonidine is a safe and effective alpha 2 -adrenergic agonist which reduces 20–30% of intraocular pressure (IOP). It also gains popularity through its neuroprotective effect and holds the second largest market share in glaucoma medications.[1–3] Nonetheless, adverse effects occur when using topical brimonidine including ocular allergy, blurred vision, dry mouth, headache, sedation, drowsiness and hypotension.[4,5] Allergic follicular conjunctivitis is the most common presentation of ocular allergy, leading to itching, foreign-body sensation, tearing and conjunctival hyperemia (Fig. 1.) The resultant discomfort may stop patients from using the medication for glaucoma control.[6]
Figure 1.
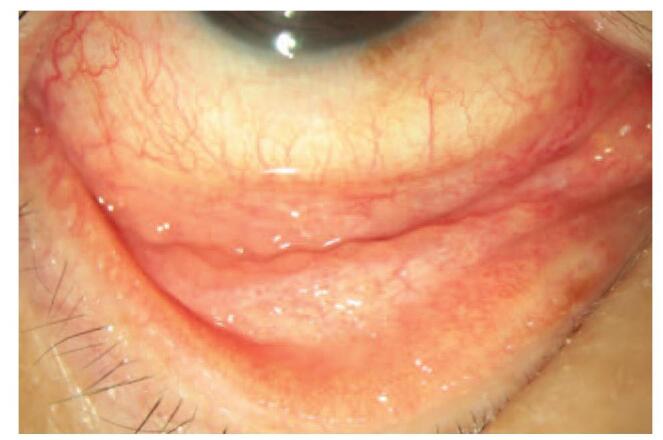
Allergic follicular conjunctivitis. A typical allergic follicular conjunctivitis. Multiple follicles on fornix, bulbar and palpebral conjunctiva, along with conjunctival hyperemia.
Allergic reactions associated with alpha-adrenergic agonist do not occur instantaneously after instillation of topical medication. Though not clearly understood, it is postulated that adrenergic agonists reduce the volume of conjunctival cells and by which widen intracellular spaces. Potential allergens may subsequently enter subepithelial tissue and result in follicular reaction.[7] The incidence of brimonidine allergy ranged from 4.7% to 25% in western countries. The mean interval from initiation of brimonidine to the onset of allergic follicular conjunctivitis was 6 to 9 months.[5,8–10] However, the interval differed widely among studies, ranging from 14 days to 12 months.[11,12]
The purpose of the present study was to evaluate the duration of brimonidine use before the onset of ocular allergy, seasons of onset, and IOP change after allergy. Furthermore, the effect of the combined use of glaucoma medications and the effect of benzalkonium chloride (BAK) exposure was also addressed.
2. Material and methods
This is a retrospective study conducted in Chang Gung Memorial Hospital (Taoyuan, Taiwan) from December 1, 2008 to November 30, 2020. The research protocol was approved by the institutional review board and adhered to the tenets of the Declaration of Helsinki (202100029B0). We included patients with brimonidine allergy, defined as bulbar or tarsal conjunctival follicular reactions which disappeared after discontinuation of topical brimonidine use. The topical medication containing brimonidine included brimonidine tartrate 0.15% preserved with purite (Alphagan P; Allergan Inc, California, U.S.), brimonidine 0.2%-timolol 0.5% (Combigan; Allergan Inc, California, U.S.), and Brimonidine 0.2%-brinzolamide 1% (Simbrinza; Alcon inc, Geneva, Switzerland). The duration of brimonidine use before allergy was defined as the interval between the first administration of brimonidine to the diagnosis of brimonidine allergy, during which topical brimonidine was continuously used.
Demographics including age, gender, type of glaucoma, IOP change, number of glaucoma medications, preservatives BAK, and the duration of brimonidine use before allergy were recorded. The IOP was measured with non-contact tonometer (NCT; Nidek Co., Ltd., Aichi, Japan). IOP at the onset of brimonidine allergy and before the onset were compared. Exclusion criteria were as following: unknown medication history, brimonidine use before visit in our hospital, and poor medication adherence. When measuring IOP change, we excluded patients who received ocular laser or surgery or patients with incomplete IOP documentation. When brimonidine allergy occur in bilateral eyes, one eye was randomly selected and included in the study.
Patients were subsequently divided into brimonidine monotreatment group and combination treatment group. They were also grouped based on BAK exposure or not. The duration of brimonidine use before allergy and IOP change were compared accordingly in each group.
Statistical analyses were performed using SPSS v26.0 for Windows (SPSS Inc, Chicago, IL, USA). The demographic data, such as age, gender, diagnosis and the number of concurrent medications were summarized with descriptive statistics. Continuous variables were expressed as the mean ± standard deviation or median with range in the parentheses. Categorical variables were expressed as count and percentage. The difference of the duration of brimonidine use before allergy were compared using Mann–Whitney U test, and the IOP change was compared using pair sample t-test. The P value < .05 indicated statistical significance.
3. Results
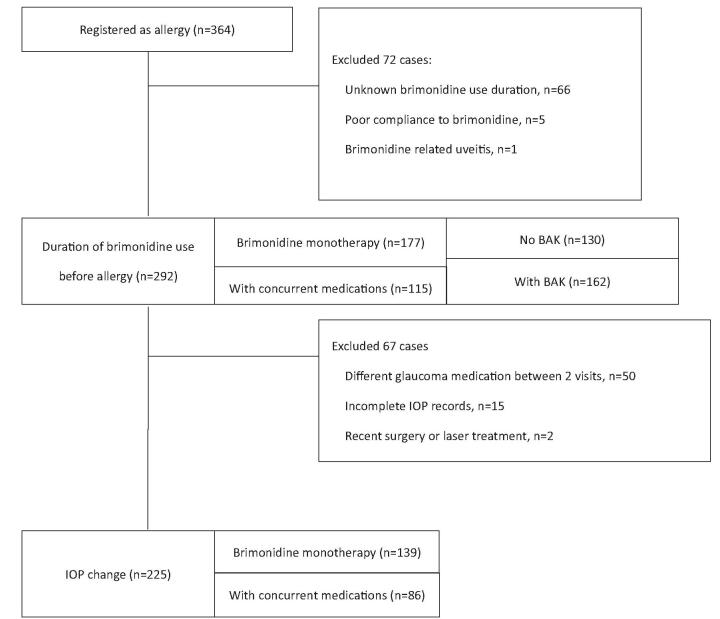
There were 364 patients who had documented brimonidine allergy on the medical chart. 72 patients were excluded from this study: 66 patients had unknown duration of brimonidine use, 5 patients were poorly adhered to the treatment, and 1 patient developed brimonidine-related uveitis (Fig. 2.) A total of 292 patients were included into the study. The mean age was 58.3 ± 13.6 years old, and 50.3% of the patients were female. 125 patients (42.8%) had normal tension glaucoma, 82 patients (28.1%) had primary open angle glaucoma, 50 patients (17.1%) had primary angle closure glaucoma, and 17 patients (5.8%) had ocular hypertension (Table 1.)
Figure 2.
Flowchart of the selection of the study patients.
Table 1.
Demographic and clinical data of patients.
| Parameter | Patients (n = 292) |
| Age (year) | 58.3 ± 13.6 (20 to 89)∗ |
| Gender | No (%) |
| Female | 147 (50.3) |
| Male | 145 (49.7) |
| Diagnosis | No (%) |
| NTG | 125 (42.8) |
| POAG | 82 (28.1) |
| PACG | 50 (17.1) |
| OHT | 17 (5.8) |
| Others | 18 (6.2) |
| No. of glaucoma medications | No (%) |
| 1 (Brimonidine monotherapy) | 177 (60.6) |
| 2† | 64 (21.9) |
| 3‡ | 20 (6.8) |
| 4§ | 31 (10.6) |
| BAK | |
| Without BAK | 162 (55.5) |
| With BAK | 130 (44.5) |
Data presented as mean ± standard deviation (minimum to maximum).
Brimonidine combined with any one of β-blockers, carbonic anhydrase inhibitors (CAI), or prostaglandin analogues (PGA).
Brimonidine combined with any two of β-blockers, CAI, or PGA.
Brimonidine combined with β-blockers, CAI and PGA.
(β-blockers included betaxolol, carteolol and timolol. CAI included dorzolamide and brinzolamide. PGA included latanoprost, travoprost, bimatoprost and tafluprost.)
BAK = benzalkonium chloride, NTG = normal tension glaucoma, OHT = ocular hypertension, PACG = primary angle closure glaucoma, POAG = primary open angle glaucoma.
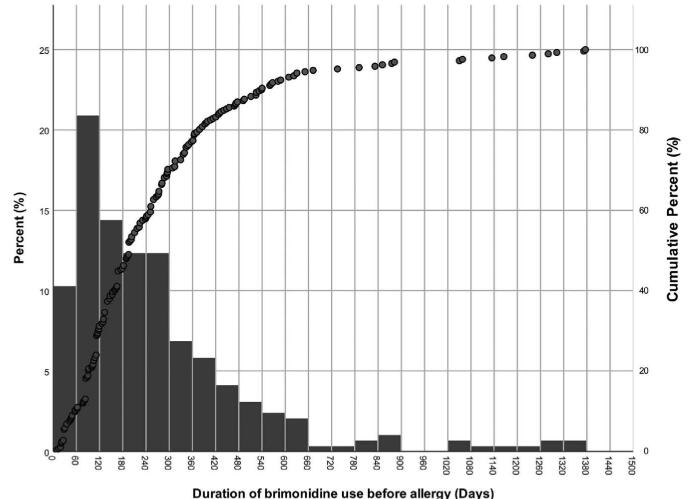
The mean duration of brimonidine use before allergy was 266.6 days, ranging from 9 to 1376 days, and the median was 196 days (Table 2.) The duration showed a right skewed distribution (Fig. 3.) In 21% of the patients, the allergy occurred within 120 days and peaked within 60–120 days. About 75% of the patients developed allergy within first year of brimonidine use, and there were less than 5% of the patients developed allergy after more than two years of brimonidine use.
Table 2.
Comparison of the duration before brimonidine allergy.
| All | Brimonidine monotherapy | With concurrent medications | Brimonidine with PGA | Brimonidine with β-blocker | Brimonidine without BAK | Brimonidine with BAK | |
| 292 | 177 | 115 | 34 | 27 | 162 | 130 | |
| Mean ± SD (days) | 266.6 ± 243.0 | 241.9 ± 215.7 | 304.5 ± 276.5 | 315.3 ± 285.5 | 301.6 ± 280.6 | 251 ± 230.4 | 285.3 ± 257.6 |
| Median (days) | 196 | 182 | 217 | 245 | 196 | 189.5 | 197.5 |
| Minimum (days) | 9 | 19 | 9 | 28 | 28 | 19 | 9 |
| Maximum (days) | 1376 | 1302 | 1376 | 1376 | 1372 | 1302 | 1376 |
| P value∗ | 0.095∗ | 0.084∗ | 0.285∗ | 0.318† |
SD = standard deviation, PGA = prostaglandin analogue.
Compare with the group of brimonidine monotherapy by Mann–Whitney test.
Compare with the group of brimonidine without BAK by Mann–Whitney test.
Figure 3.
Histogram and cumulative percentage of the duration before brimonidine allergy. This picture shows the relationship between the occurrence and use time of brimonidine allergy. The Y-axis on the left is for the histogram, which represents the proportion of occurrence in each 60 days, and the y-axis on the right is for the dot plot, which represents the cumulative percentage.
The mean (median) duration of brimonidine use before allergy in brimonidine monotreatment group was 241.9 ± 215.7(182) days, whereas the mean (median) duration was 304.5 ± 276.5 (217) days in combination treatment group (P = .095). Further comparison between brimonidine monotherapy and combination with prostaglandin analogue (PGA), or between brimonidine monotherapy and combination with β-blocker, neither showed statistical significance (P = .084 and .285, respectively.) (Table 2) Moreover, in patients who had received BAK or not, the duration was 285.3 ± 257.6 (162) days and 251 ± 230.4 (130) days, respectively (P = .318).
In the 225 patients who was continuously using the same medications before the onset of allergy, 139 had brimonidine only, and 86 had multiple glaucoma medications. The mean IOP before ocular allergy was 14.39 ± 3.64 mmHg, increased to 15.20 ± 4.04 mmHg after the onset of ocular allergy, which was 0.81mmHg (5.6%) higher (P < .001). In the brimonidine monotreatment group, the mean IOP was 1.25 mmHg (9.2%) higher after ocular allergy, from 13.62 mmHg to 14.87 mmHg (P < .001). There was no significant IOP change in patients with combination therapy (P = .786) (Table 3.)
Table 3.
Intraocular pressure changes during Brimonidine allergy.
| Number of eyes | Number of glaucoma medications | IOP before allergy | IOP during allergy | Difference (mmHg; %) | 95% CI | P | |
| All | 225 | 1–4 | 14.39 ± 3.64 | 15.20 ± 4.04 | +0.81; +5.6 | +0.43 to +1.19 | <.001 |
| Brimonidine monotherapy | 139 | 1 | 13.62 ± 3.11 | 14.87 ± 3.59 | +1.25; +9.2 | +0.83 to +1.66 | <.001 |
| Brimonidine with other medications∗ | 86 | 2–4 | 15.62 ± 4.08 | 15.73 ± 4.63 | +0.10; +0.6 | -0.61 to +0.80 | .786 |
| Brimonidine with PGA | 26 | 2 | 14.20 ± 4.10 | 13.45 ± 3.65 | -0.75; -5.2 | -1.91 to +0.41 | .194 |
| Brimonidine with β-blocker | 20 | 2 | 15.58 ± 4.49 | 15.64 ± 4.68 | +0.07; +0.4 | -1.41 to +1.53 | .933 |
Other medications included topical β-blockers, CAI and PGA. β-blockers included betaxolol, carteolol and timolol. CAI included dorzolamide and brinzolamide. PGA included latanoprost, travoprost, bimatoprost and tafluprost.
CAI = carbonic anhydrase inhibitors, CI = confidence interval, IOP = intraocular pressure, PGA = prostaglandin analogue.
Compared with brimonidine monotreatment patients, the mean IOP after the onset of allergy was similar in those who had combination treatment with PGA (before: 14.20 ± 4.10 mmHg, after: 13.45 ± 3.65 mmHg, -0.75 mmHg, P = .194), or with β-blocker (before: 15.58 ± 4.49 mmHg, after: 15.64 ± 4.68 mmHg, +0.07 mmHg, P = .933).
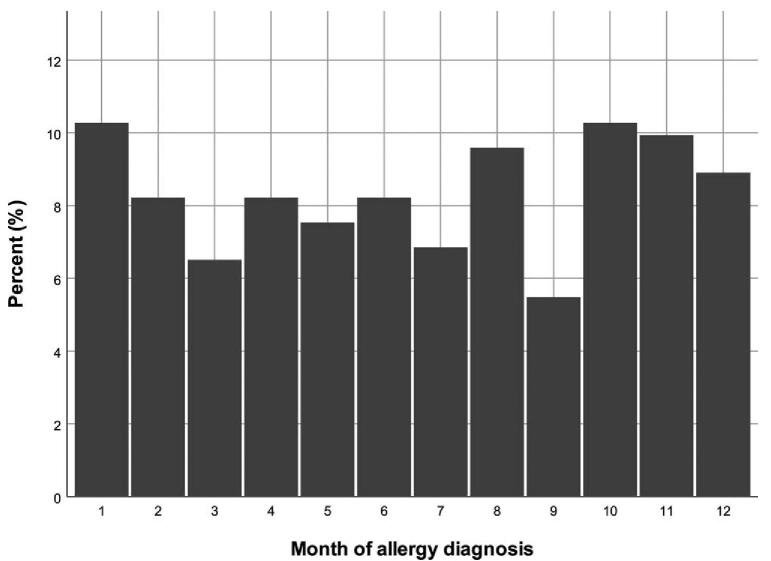
The number of patients who developed brimonidine allergy in each month of the year was illustrated in Table 4 and Figure 4. Although more patients developed brimonidine allergy from October to January, the difference in monthly distribution was not significant (P = .600.)
Table 4.
The month when brimonidine allergy was diagnosed.
| Number of patients | Percent (%) | |
| Jan | 30 | 10.3 |
| Feb | 24 | 8.2 |
| Mar | 19 | 6.5 |
| Apr | 24 | 8.2 |
| May | 22 | 7.5 |
| Jun | 24 | 8.2 |
| Jul | 20 | 6.8 |
| Aug | 28 | 9.6 |
| Sep | 16 | 5.5 |
| Oct | 30 | 10.3 |
| Nov | 29 | 9.9 |
| Dec | 26 | 8.9 |
| P = .600 |
Figure 4.
The histogram of brimonidine allergy diagnosed each month.
4. Discussion
In the present study, the mean and median duration of brimonidine use before allergy was 266 and 196 days respectively. Previous studies have shown that the duration of brimonidine use before the onset of allergy varies. Pierre Blondeau reported a mean duration of 204 days in a 25-months follow-up among 36 Canadian patients.[8] Williams et al reported that the mean duration of brimonidine use was 8.2 months before the development of allergic reactions.[9] Watts et al reported that the median duration of brimonidine therapy was 12 months in patients who developed allergic reaction.[12] To our understanding, the present study had the longest follow-up and had the largest number of patients among similar studies. Although the average duration of brimonidine use was longer in our study due to data skewness, the median duration of brimonidine allergy was around 6 months, which was similar to previous reports. Our result showed that brimonidine allergy peaked in 60–120 days, which was much shorter to mean or median duration. Therefore, clinicians must pay attention to the development of allergic reactions in the first few months after the prescription of brimonidine. The longest duration in the present study was 1376 days, suggesting that the allergic reaction may still occur in patients who have no previous adverse response for a long time, although less than 5% of patients developed brimonidine allergy after 2 years.
A prospective randomized trial reported that the combination use of brimonidine and timolol reduced the incidence of brimonidine allergy.[13,14] It was assumed that β-blocker resulted in vessel constriction and reduced conjunctival hyperemia, thus lowering the incidence of allergic reaction.[15] Some studies also claimed that the proinflammatory properties of PGA might promote the development of brimonidine allergy through vessel dilatation.[16,17] However, in the present study, the duration of brimonidine use before allergy did not differ between patients treated with brimonidine monotherapy, β-blocker combination therapy, or PGA combination therapy. In short, the combined use of glaucoma medication has no effect on the duration of brimonidine use before allergy.
In a previous study, BAK exposure resulted in a higher incidence of brimonidine allergy compared to BAK-free treatment.[18–20] In the present study, the monotreatment regimen of brimonidine was BAK free (0.15% brimonidine, Alphagan P; Allergan Inc, California, U.S.). Our result showed that the duration of brimonidine use before allergy did not differ between BAK exposed group or BAK free group. It is likely that BAK exposure affect the incidence but not the time of brimonidine allergy.
The IOP control of brimonidine appeared to be weakened when the allergic reaction developed. According to Watts P, the IOP increased from 20 mmHg to 28 mmHg when patients developed brimonidine allergy.[12] Although the mechanism was unclear, it was speculated that the follicular conjunctivitis increased conjunctival and episcleral blood flow, which might resultant in IOP elevation.[7,12,21] In the present study, the IOP increased by 1.25 mmHg (9%, P < .001) after the onset of allergy in the monotreatment group, indicating that the IOP control was weakened when brimonidine allergy occurred. Changing brimonidine to other glaucoma drugs not only relieve the allergic symptoms, but also better control the IOP. The IOP increase was not significant in the combination treatment group, possibly because the effect was blunted by other glaucoma medications. Besides, most of the patients enrolled in our study had normal tension glaucoma, and their baseline IOP never exceeded 21 mmHg. The IOP elevation after brimonidine allergy was less obvious.
There was no seasonal trend in the development of brimonidine allergy in the present study, and the result was similar to previous report.[8] Although statistically insignificant, more allergy occurred during October to January. This is the winter season in Taiwan when the incidence of allergic rhinitis and conjunctivitis was higher.[22,23] It was possible that potential allergen entered conjunctiva through the widened intercellular junction after prolonged brimonidine treatment and resulted in follicular conjunctivitis.
There were some limitations of the present study. First, the retrospective design may have introduced selection or information bias. Second, the incidence of brimonidine allergy was not available because only patients demonstrated brimonidine allergy were enrolled, and the total prescription number of brimonidine was not known.
5. Conclusion
The median duration of brimonidine use before the development of allergic response was 194 days, with a peak in 60–120 days. The duration did not differ between patients receiving brimonidine monotherapy or in combination with other glaucoma drugs. The presence of BAK did not affect the duration either. When brimonidine allergy occurred, there was a loss of IOP control, especially in patients receiving brimonidine monotreatment. It is recommended to switch to other type of glaucoma medication for better IOP control.
Author contributions
Conceptualization: Wei-Wen Su.
Data curation: Po-Han Yeh, Shian-Sen Shie, Yung-Sung Lee, Wei-Wen Su.
Formal analysis: Po-Han Yeh.
Investigation: Su-Chin Shen, Henry Shen-Lih Chen.
Methodology: Yung-Sung Lee.
Resources: Shian-Sen Shie.
Supervision: Wei-Chi Wu, Wei-Wen Su.
Writing – original draft: Po-Han Yeh, Wei-Wen Su.
Writing – review & editing: Po-Han Yeh, Yu-Chun Cheng, Wei-Wen Su.
Footnotes
Abbreviations: BAK = benzalkonium chloride, IOP = intraocular pressure, PGA= prostaglandin analogue.
How to cite this article: Yeh PH, Cheng YC, Shie SS, Lee YS, Shen SC, Chen HL, Wu WC, Su WW. Brimonidine related acute follicular conjunctivitis: Onset time and clinical presentations, a long-term follow-up. Medicine. 2021;100:29(e26724).
The authors have no funding to disclose.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- [1].Derick RJ, Robin AL, Walters TR, et al. Brimonidine tartrate: a one-month dose response study. Ophthalmology 1997;104:131–6. [DOI] [PubMed] [Google Scholar]
- [2].Lowry EA, Chansangpetch S, Lin SC. Use of Topical intraocular pressure-lowering medications in the us population: results from the NHANES study 1999 to 2014. J Glaucoma 2019;28:772–6. [DOI] [PubMed] [Google Scholar]
- [3].Chiu SL, Chu CL, Muo CH, Chen CL, Lan SJ. Trends in glaucoma medication expenditures under universal health coverage: a national population-based longitudinal survey in Taiwan. J Ophthalmol 2015;2015:243401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Novack GD, O’Donnell MJ, Molloy DW. New glaucoma medications in the geriatric population: efficacy and safety. tr Soc 2002;50:956–62. [DOI] [PubMed] [Google Scholar]
- [5].LeBlanc RP. Twelve-month results of an ongoing randomized trial comparing brimonidine tartrate 0.2% and timolol 0.5% given twice daily in patients with glaucoma or ocular hypertension. Brimonidine Study Group 2. Ophthalmology 1998;105:1960–7. [DOI] [PubMed] [Google Scholar]
- [6].Katz LJ. Brimonidine tartrate 0.2% twice daily vs timolol 0.5% twice daily: 1-year results in glaucoma patients. Am J Ophthalmol 1999;127:20–6. [DOI] [PubMed] [Google Scholar]
- [7].Butler P, Mannschreck M, Lin S, Hwang I, Alvarado J. Clinical experience with the long-term use of 1% apraclonidine. Incidence of allergic reactions. Arch Ophthalmol 1995;113:293–6. [DOI] [PubMed] [Google Scholar]
- [8].Blondeau P, Rousseau JA. Allergic reactions to brimonidine in patients treated for glaucoma. Can J Ophthalmol 2002;37:21–6. [DOI] [PubMed] [Google Scholar]
- [9].Williams GC, Orengo-Nania S, Gross RL. Incidence of brimonidine allergy in patients previously allergic to apraclonidine. J Glaucoma 2000;9:235–8. [DOI] [PubMed] [Google Scholar]
- [10].Schuman JS. Clinical experience with brimonidine 0.2% and timolol 0. 5% in glaucoma and ocular hypertension. Surv Ophthalmol 1996;41:S27–37. [DOI] [PubMed] [Google Scholar]
- [11].Manni G, Centofanti M, Sacchetti M, et al. Demographic and clinical factors associated with development of brimonidine tartrate 0.2%-induced ocular allergy. J Glaucoma 2004;13:163–7. [DOI] [PubMed] [Google Scholar]
- [12].Watts P, Hawksworth N. Delayed hypersensitivity to brimonidine tartrate 0.2% associated with high intraocular pressure. Eye (Lond) 2002;16:132–5. [DOI] [PubMed] [Google Scholar]
- [13].Craven ER, Walters TR, Williams R, Chou C, Cheetham JK, Schiffman R. Brimonidine and timolol fixed-combination therapy versus monotherapy: a 3-month randomized trial in patients with glaucoma or ocular hypertension. J Ocul Pharmacol Ther 2005;21:337–48. [DOI] [PubMed] [Google Scholar]
- [14].Sherwood MB, Craven ER, Chou C, et al. Twice-daily 0.2% brimonidine-0.5% timolol fixed-combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12-month randomized trial. Arch Ophthalmol 2006;124:1230–8. [DOI] [PubMed] [Google Scholar]
- [15].Motolko MA. Comparison of allergy rates in glaucoma patients receiving brimonidine 0.2% monotherapy versus fixed-combination brimonidine 0. 2%-timolol 0 5% therapy. Curr Med Res Opin 2008;24:2663–7. [DOI] [PubMed] [Google Scholar]
- [16].Gandolfi S, Simmons ST, Sturm R, Chen K, VanDenburgh AM. Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther 2001;18:110–21. [DOI] [PubMed] [Google Scholar]
- [17].Parrish RK, Palmberg P, Sheu WP. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 2003;135:688–703. [DOI] [PubMed] [Google Scholar]
- [18].Katz LJ. Twelve-month evaluation of brimonidine-purite versus brimonidine in patients with glaucoma or ocular hypertension. J Glaucoma 2002;11:119–26. [DOI] [PubMed] [Google Scholar]
- [19].Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol 2009;9:447–53. [DOI] [PubMed] [Google Scholar]
- [20].Goto Y, Ibaraki N, Miyake K. Human lens epithelial cell damage and stimulation of their secretion of chemical mediators by benzalkonium chloride rather than latanoprost and timolol. Arch Ophthalmol 2003;121:835–9. [DOI] [PubMed] [Google Scholar]
- [21].Shin DH, Glover BK, Cha SC, Kim YY, Kim C, Nguyen KD. Long-term brimonidine therapy in glaucoma patients with apraclonidine allergy. Am J Ophthalmol 1999;127:511–5. [DOI] [PubMed] [Google Scholar]
- [22].Chang CJ, Yang HH, Chang CA, Tsai HY. Relationship between air pollution and outpatient visits for nonspecific conjunctivitis. Invest Ophthalmol Vis Sci 2012;53:429–33. [DOI] [PubMed] [Google Scholar]
- [23].Kao CC, Huang JL, Ou LS, See LC. The prevalence, severity and seasonal variations of asthma, rhinitis and eczema in Taiwanese schoolchildren. Pediatr Allergy Immunol 2005;16:408–15. [DOI] [PubMed] [Google Scholar]