Abstract
The Capsule Endoscopy Crohn's Disease Activity Index (CECDAI) was recently reported as a new scoring system to evaluate the mucosal lesions of patients with Crohn's disease (CD). We investigated whether CECDAI is useful for assessing the necessity of early additional treatment in patients with CD in clinical remission.
Twenty-one patients with small intestinal CD in clinical remission underwent capsule endoscopy (CE). The CECDAI and Lewis score (LS) were used to evaluate the intestinal lesions. We analyzed the correlations between several biomarkers and CECDAI or LS and examined the changes in therapeutic regimens based on the CECDAI.
CE identified intestinal abnormalities in most CD patients in clinical remission: 81.0% and 85.7%, as assessed using CECDAI and LS, respectively. A significant positive correlation was observed between the CDAI and LS (P = .025), as well as between CDAI and CECDAI (P = .014) in these cases. Compared to LS, CECDAI scores were more evenly distributed. No significant correlations were observed between endoscopic scores and serum markers, including CRP, hemoglobin, and albumin levels. Additional treatment was performed significantly more often in patients with moderate-severe disease activity (CECDAI ≥5.8) (P = .012) than in those with normal (CECDAI <3.5) and mild (3.5≤CECDAI<5.8) disease activity. Resection of the small intestine did not affect the small bowel transit time or CE score.
CECDAI is useful in evaluating mucosal lesions in small bowel CD patients in clinical remission and helps in assessing the requirement for additional treatment for these patients, including those who undergo intestinal resection.
Keywords: capsule endoscopy, Crohn's diseases, inflammatory bowel disease
1. Introduction
Crohn's disease (CD) is a chronic inflammatory disease characterized by discontinuous patterns of full-thickness granulomatous inflammation and fistulas at any site in the gastrointestinal tract.[1] In recent years, remarkable progress has been made in developing therapeutic drugs and treatment strategies for CD, and options for treatment continue to increase.[2] Although the therapeutic goal of CD has been to achieve “clinical remission” so far, it has become apparent that achieving endoscopic “mucosal healing” or “deep remission” greatly contributes to the improvement of the patient's prognosis, such as lower surgical and hospitalization rates.[3,4] The evaluation of clinical disease activity may not be correlated with mucosal healing, and even the patients who are actually in clinical remission may have active mucosal lesions. Therefore, in order to evaluate “deep remission”, a method to objectively evaluate images is necessary, and endoscopy is one of the essential tools.
Recently, small intestine endoscopic examination, such as single-balloon enteroscopy and double-balloon enteroscopy, have been introduced to directly evaluate mucosal lesions of the small intestine.[5,6] However, it is not easy to perform small intestine endoscopy on patients in clinical remission, as it is difficult for the patients to endure large amounts of whole bowel irrigation before the endoscopic examination that itself is painful for the patients. On the other hand, the small intestinal capsule endoscopy is not accompanied by the pain associated with the examination, so the patient's acceptability is considered to be high. Also, studies which evaluate the small intestinal lesions of CD are gradually increasing.[7–9]
There are several CD scoring systems in endoscopy, such as Crohn's disease endoscopic index of severity (CDEIS),[10] Simple Endoscopic Score for Crohn's disease (SES-CD),[11] and Rutgeerts’ score,[12] but there is no established capsule endoscopic scoring system for mucosal lesions in CD patients.
Lewis score (LS) is often used as a capsule endoscopic scoring system for small bowel lesions,[13] but it is not specialized for CD lesions. The ability of LS to evaluate the mucosal lesions of CD has not been sufficiently studied. A new capsule endoscopic scoring system for CD called Capsule endoscopy Crohn's disease activity index scoring system (CECDAI) was proposed.[14] Recently, there have been some studies comparing CECDAI with LS regarding the usefulness of capsule endoscopy in CD.[15,16] However, it is still not yet clear whether this scoring system can quantitatively evaluate mucosal lesions in the small intestine during clinical remission and whether mucosal lesions after intestinal resection can be evaluated. In this study, we report on small intestinal capsule endoscopy for CD patients in clinical remission and investigate whether CECDAI can be used to evaluate mucosal lesions adequately and help in deciding whether additional treatment is required.
2. Material and methods
2.1. Patient population
From December 2012 to December 2016, a retrospective, single-center study was conducted with 21 patients. We included 21 patients diagnosed with the small intestine type of CD between the ages of 17 years and 71 years, whose clinical remission status was maintained for at least three months or more. Clinical remission was defined as a Crohn's disease activity index (CDAI) score of <150 points.
2.2. Capsule endoscopy
All examinations were performed with the PillCamTM SB2 or SB3 system (Medtronic Japan Co., Ltd.). Prior to the capsule endoscopic procedure, all patients, including seven who underwent small intestinal resection for stenosis and fistula, were assessed with a PillCamTM Patency Capsule (PC) (Medtronic Japan Co., Ltd.). The PC test was considered negative if the capsule was eliminated from the small bowel within 33 h from ingestion according to the manufacturer's instructions. The preparation for the capsule endoscopy included a 12 h overnight fast. After swallowing the capsule, 200 ml of polyethylene glycol was administered for one hour. The patients were allowed to drink clear liquids after two hours and to eat an ordinary diet after four hours. In the cases where the capsule did not reach the duodenum within two hours after swallowing, the patients were administrated 10 mg of metoclopramide.
2.3. Assessment of mucosal lesions
The results of CE were evaluated based on LS and CECDAI.[13,14] LS was automatically calculated using RAPIDTM Reader Software and CECDAI was evaluated using the laminated printout of the scoring system. The images were reviewed independently by two experts on CE. The final endoscopic activity scores were based on consensus between the two experts. The severity of small bowel lesions was classified according to the CECDAI scores as follows: normal (CECDAI <3.5), mild disease activity (3.5≤ CECDAI <5.8), and moderate-severe disease activity (CECDAI ≥5.8).[17,18] After evaluation of the severity of small bowel lesions by CE, additional medications including initiation or increase in dose of biologics and/or thiopurines were left to the investigators’ judgment.
2.4. Measurement of blood biomarkers
Hemoglobin (Hb), albumin (Alb), and C-reactive protein (CRP) levels were evaluated by a blood test performed between two weeks before and two weeks after the capsule endoscopy was performed. Blood samples from all the patients were collected and measured by the Department of Laboratory Medicine of Hamamatsu University School of Medicine. Data regarding these biological parameters were collected from the patients’ records.
2.5. Study design
We conducted a retrospective four-year observational study. The primary endpoint was the correlation of two capsule endoscopic scoring systems (LS, CECDAI) with laboratory values (Hb, Alb, CRP). The secondary endpoint was the changes in therapeutic regimens based on the CECDAI.
2.6. Statistics
Statistical analysis was performed using statistical software (SPSS for Windows, Version 16.0; SPSS Inc., Chicago, Illinois, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan). Continuous variables were presented as the mean ± standard deviation or the mean ± standard error of the mean, whereas groups were compared using the Student's t-test or the Mann–Whitney U test unless stated otherwise. Categorical variables are presented as percentages, and the analyses were performed using the Fisher's exact test. A P-value <.05 was considered statistically significant.
2.7. Ethical statement
Informed consent was obtained from all the patients after explaining the purpose of the study and the nature of the procedures involved. Similarly, the study protocol was reviewed and approved by the Institutional Review Board of Hamamatsu University School of Medicine (Registration number 15–221). Further, the investigation was conducted in accordance with the principles of Good Clinical Practice and in adherence to the Declaration of Helsinki at all times.
3. Results
3.1. Patients’ baseline demographics
Table 1 shows the demographic features of patients with CD. The baseline data of all the enrolled patients are shown in Table S1 Supplemental Digital Content. The average age of the 21 patients was 40.2 years (range 17–71 years), and the average disease duration was 7.1 years. The disease distribution in Montreal classification was 66.7% (n = 14), 19.0% (n = 4), 14.3% (n = 3) for B1, B2 and B3 respectively. Surgical intestinal resection had been performed in seven cases, namely, all cases of B2 and B3. The length of intestinal resection was 21.3 cm on average, the shortest being 17 cm, and the longest was 30 cm. All anastomosing methods were Kono-S anastomoses.[17] Regarding baseline medications, 95.2%, 38.1%, 38.1, and 42.9% of patients used 5-aminosalicylates, immunomodulators, elemental diet, and biologics, respectively. In all cases, capsule endoscopies of the small intestine were performed after passability by the PC was confirmed. The average scores of LS and CECDAI were 583.7 ± 37.9 and 3.8 ± 3.7, respectively. The average values of the blood test data, when the capsule endoscopy was performed, were CRP of 0.14 ± 0.16 mg/dL, Hb of 13.6 ± 1.4 g/dL, and Alb of 4.3 ± 0.4 g/dL.
Table 1.
Demographic features of patients with Crohn's disease (CD).
| Variable | n = 21 |
| Age (year) | 40.2 ± 16.1 (17–71) |
| Gender (male/female) | 14/7 |
| Duration of disease (year) | 7.86 ± 7.1 (0–22) |
| Age at diagnosis (year) | 32.3 ± 16.1 (14–65) |
| Montreal classification | |
| A1/A2/A3 | 1 (4.8%) / 14 (66.7%) / 6 (28.5%) |
| L1/L2/L3/L4 | 13 (61.9%) / 0 (0.0%) / 8 (38.1%) / 0 (0%) |
| B1/B2/B3 | 14 (66.7%) / 4 (19.0%) / 3 (14.3%) |
| Smoking | 11 (52.4%) |
| Surgery required | 7 (33.3%) |
| Length of resected small bowel (cm) | 21.3 ± 3.7 (17–30) |
| Perianal disease | 5 (23.8%) |
| Concomitant use | |
| 5-Aminosalicylates | 20 (95.2%) |
| Immunomodulators | 8 (38.1%) |
| Elemental diet | 8 (38.1%) |
| Biologics | 9 (42.9%) |
| CDAI (points) | 69.5 ± 37.9 (20–141) |
| LS (points) | 583.7 ± 1061.8 (0–3720) |
| CECDAI (points) | 3.8 ± 3.7 (0–13) |
| Small bowel transit time (min) | 235.7 ± 116.9 (42–554) |
| CRP (mg/dL) | 0.14 ± 0.16 |
| Hb (g/dL) | 13.6 ± 1.4 |
| Alb (g/dL) | 4.3 ± 0.4 |
A1 below16y, A2 between17 and 40y, A3 above 40y.
L1 ileal, L2 colonic, L3 ileocolonic, L4 isolated upper disease.
B1 non-stricturing, non-penetrating, B2 stricturing, B3 penetrating.
CDAI Crohn's disease activity index.
LS Lewis capsule endoscopy scoring index.
CECDAI Capsule endoscopy Crohn's disease activity index scoring system.
3.2. Correlations between CDAI and scoring systems of capsule endoscopy in the patients with CD in clinical remission
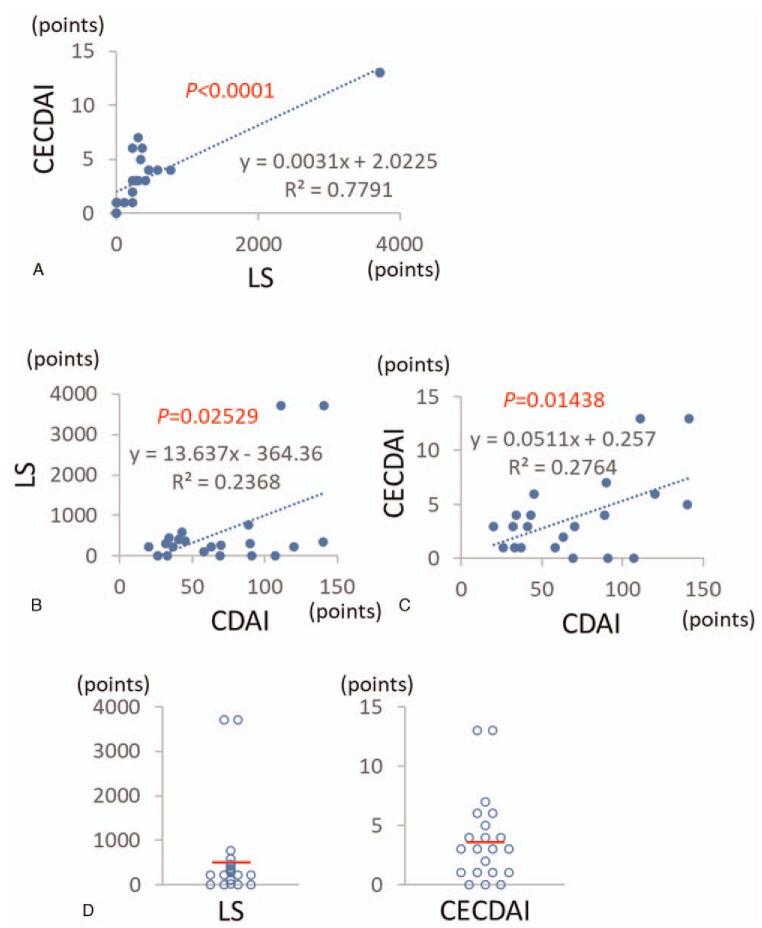
As shown in Figure 1A, LS and CECDAI showed a significant positive correlation. Next, we examined the relationship between CECDAI and CDAI in patients with CD in clinical remission. A statistically significant positive correlation (P = .025) was observed between LS and CDAI (Fig. 1B). A significant positive correlation (P = .014) was also observed between CECDAI and CDAI (Fig. 1C). From these results, it was observed that the capsule endoscopic score becomes higher proportionally to the clinical score in clinical remission cases.
Figure 1.
LS and CECDAI in the patients with CD in clinical remission. (A). Correlation between LS and CECDAI. (B). Correlation between CDAI and LS. (C). Correlation between CDAI and CECDAI (D). Distribution of the endoscopic scores of LS and CECDAI.
Figure 1D shows the distribution of LS and CECDAI in CD patients in clinical remission. There were four cases (19.0%) in LS and three cases (14, 3%) in CECDAI with an endoscopic score of 0 point, and there were 17 cases (81.9%) in LS and 18 cases (85.7%) in CECDAI with an endoscopic score of 1 or more. LS has a wide range of scores from 0 to 3720 points. Two cases with a score of 3720 points were prominent, and most of the other cases aggregated to 500 points or less, so the scores were distributed quite unevenly. On the other hand, CECDAI exists from score 0 to 13 points. Two cases with 13 points were higher than other scores, but scores were distributed relatively uniformly compared to LS. These results showed that the CECDAI is a more evenly distributed than LS in the capsule endoscopy data for CD patients in clinical remission.
3.3. Correlations between CE scoring system and serum biomarkers
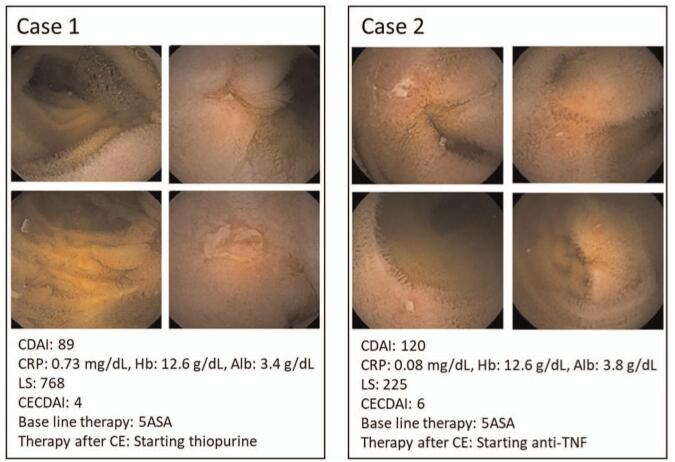
We investigated whether serum biomarkers including CRP, Hb, and Alb correlate with CE scoring systems in clinical remission cases. CRP (Fig. 2A), Hb (Fig. 2B), and Alb (Fig. 2C) showed no correlation with either LS or CECDAI. Figure 3 shows two cases in which CE was performed on CD patients in remission. Case 1 had a mild elevation of serum CRP and hypoalbuminemia, while Case 2 had no elevation of serum CRP and very mild hypoalbuminemia. As CE identified multiple small ulcers and erosions in the small intestine in both cases, thiopurine was added to the therapeutic regimen for Case 1 and anti-TNF was added for Case 2. These results indicated that the serum biomarkers do not always correlate with the CE scoring systems during the clinical remission phase in CD patients.
Figure 2.
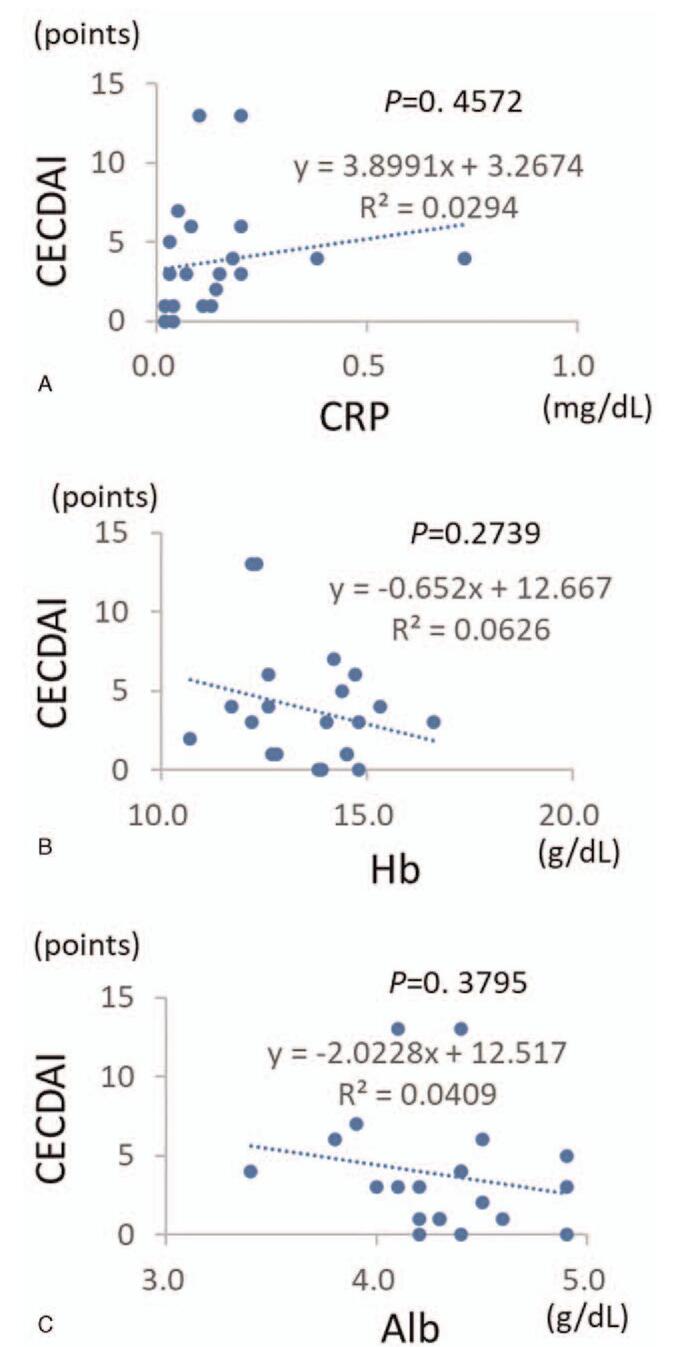
Correlations between the capsule endoscopy scores and the blood biomarkers. (A). Correlation between CECDAI and CRP. (B). Correlation between CECDAI and Hb. (C) Correlation between CECDAI and Alb.
Figure 3.
Images of capsule endoscopy in patients with CD in clinical remission. Multiple small ulcers and aphthoid lesions are seen in the small intestine.
3.4. Changes of therapeutic regimen of CD patients in clinical remission after CE
Next, we examined how the therapeutic regimen of CD patients was changed according to the CECDAI. There were six cases in which treatment was changed after the capsule endoscopy was performed, with the addition of thiopurine in one case and anti-TNF in four cases, and increasing existing anti-TNF in another (Fig. 4A). Previous reports indicate that a CECDAI score of less than 3.5 is normal, 3.5 to 5.8 indicate mild disease activity, and 5.8 and above indicate moderate-severe disease activity.[18,19] As shown in Figure 4B, treatment was changed after the capsule endoscopy in 8.3% cases of normal activity, 25.0% of mild disease activity, and 80.0% of moderate-severe disease activity. In other words, the proportion of patients whose treatment changed increased significantly (P = .012) with the increase in CECDAI score.
Figure 4.
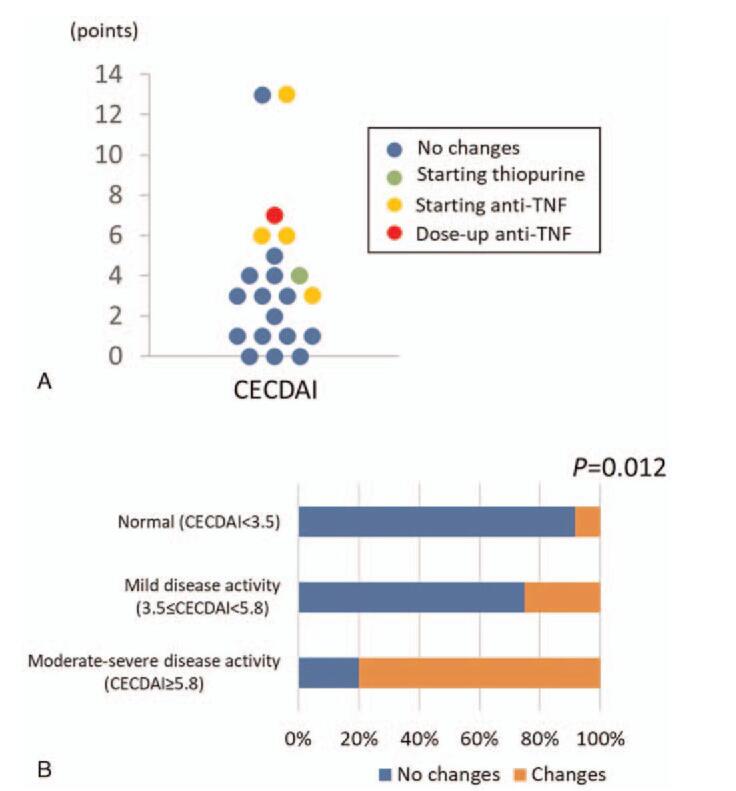
Changes in therapeutic regimen based on the CECDAI score. (A). Distribution of patients who required changes in therapeutic regimen. (B) Percentage of patients who required changes in therapeutic regimen based on the CECDAI score.
3.5. Effect of intestinal resection on small bowel transit time and scoring systems of CE
Finally, we examined whether the history of small bowel resection had an influence on small bowel transit time and scoring systems of capsule endoscopy. There was no significant difference in small bowel transit time between patients with and without a surgical history (Fig. 5A). Furthermore, there was no significant difference in endoscopic score, including both LS and CECDAI, between patients with and without a history of surgery (Fig. 5B). There was no significant correlation between LS and small bowel transit time and between CECDAI and small bowel transit time (Fig. 5C). Although the intestinal resection lengths of the surgical cases of this study were all shorter than 30 cm (Table 1), it was shown that the history of small bowel resection had no influence on small bowel transit time and the capsule endoscopy scores if intestinal resection length was shorter than 30 cm.
Figure 5.
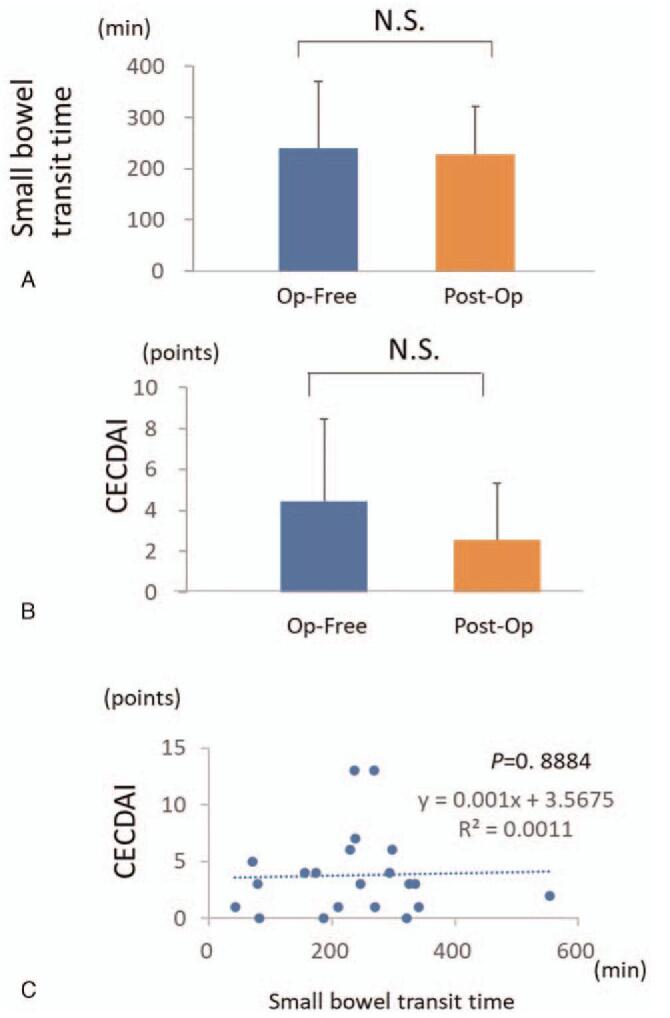
Effect of intestinal resection on small bowel transit time and CECDAI. (A). Effect of intestinal resection on small bowel transit time. (B). Effect of intestinal resection on CECDAI. (C). Correlation between small bowel transit time and CECDAI. Op-free: Surgical-free; Post-op: Post-surgical.
4. Discussion
Several studies have reported on the use of LS as a capsule endoscopic score for evaluating small bowel lesions in CD, and it is said to be very useful as a follow-up tool for patients diagnosed with CD; however, the range of the scores is very wide (0–4800).[19] LS is not a scoring system specialized for CD because it was originally created based on mucosal lesions of patients with nonsteroidal anti-inflammatory drug-induced enteropathy.[13] CECDAI, on the other hand, is a scoring system specialized for CD proposed by Gal et al[14] and the score range is 0 to 36, which is smaller than LS. In this study, both LS and CECDAI positively correlated with CDAI in clinical remission cases, and furthermore, it was shown that LS and CECDAI are significantly correlated with each other. Since scores are uniformly distributed in CECDAI compared to the uneven distribution of LS in the case of clinical remission, it seems that CECDAI will be able to make more objective comparative examinations.
We also examined whether the serum markers could predict endoscopic findings in clinical remission cases, but CRP, Hb, and Alb levels did not show correlations with endoscopic scores. Since it is difficult to identify endoscopic relapse before clinical relapse by measuring serum markers such as CRP and ESR,[23] fecal markers, such as calprotectin and lactoferrin, are used for predicting endoscopic recurrence.[20–22] However, whether fecal calprotectin can serve as a surrogate marker for mucosal healing remains to be clarified.[24] Currently, there is no significant correlation between serum or fecal biomarkers and endoscopic scores. Therefore, there seems to be no useful method other than the direct observation of mucosal condition by endoscopic examination to judge the status of healing.
As mentioned above, it has been reported that the clinical course of patients will be affected if mucosal healing is not achieved even in the clinical remission state.[22] Therefore, the mucosal evaluation by capsule endoscopy during clinical remission may have a significant influence on the patient's prognosis.[25,26] It has been reported that a higher score of LS is related to more frequent changes in treatment, and LS is known to be a useful tool for therapeutic management of CD.[27,28] However, there have been no reports on the impact of CECDAI on the treatment changes for patients with CD. Our study showed that the proportion of patients whose treatment changed remarkably increased as CECDAI increased (P = .012). Although this research does not prospectively evaluate differences in treatment changes between the group in which the capsule endoscope was performed and the group in which the capsule endoscope was not performed, it is well known that the achievement of mucosal healing is important for the prognosis of CD patients. Therefore, quantitative evaluation of small bowel lesions using CECDAI, during clinical remission period, is extremely critical for a better long-term prognosis of CD patients.
In CD patients who underwent ileocecal resection, more than 80% of endoscopic relapse is observed in the ileum on the oral side of the anastomotic site (neo-terminal ileum) within 12 months after operation,[12] but clinical relapse is seen from 3 to 5 years after surgery. Conversely, there seems to be a high probability of the presence of active lesions that need treatment on the intestinal mucosa at the time of onset of clinical relapse was observed. Kusaka et al examined cases in which capsule endoscopy was performed within three months of “curative” surgery, and endoscopic activity was observed in 84.0% of the cases.[26] However, it has not been examined whether the capsule endoscopic scoring system can be applied to cases after intestinal resection in the same manner as it is for non-resection cases. We clarified that the presence or absence of intestinal resection during clinical remission did not affect intestinal transit time and the capsule endoscopic scoring system including both of LS and CECDAI. In other words, it was revealed that capsule endoscopes can appropriately evaluate small intestinal mucosa in cases where the intestinal resection length is less than 30 cm.
The limitations of this study include its retrospective design and the small sample size. In addition, treatment methods for each patient, whose active mucosal lesions have been found in capsule endoscopy, are left to their respective attending physician, and no definite treatment design has been set up, and there has been no follow-up on changes in mucosal lesions by capsule endoscopy after the additional treatment. However, it seems that the index to consider additional treatment in clinical remission cases may concretely be proposed as a case of moderate to severe with CECDAI of 5.8 or more.
5. Conclusions
We found that CECDAI is a very useful scoring system to evaluate the extent of mucosal lesions when performing capsule endoscopy in remission cases of CD. Since CECDAI scores are more evenly distributed than LS values, it seems that the difference in the scores among patients, as well as the change in the score with time in each individual, can be more objectively evaluated. Even in surgical cases, CECDAI can be used to evaluate mucosal lesions properly if the intestinal resection length is relatively short. It is a useful scoring system for maintaining the quality of life of CD patients. However, to confirm these preliminary results, additional research using a multicenter prospective study is required.
Acknowledgments
Not applicable.
Author contributions
Conceptualization: Takahiro Miyazu, Natsuki Ishida, Ryosuke Takano, Satoshi Osawa, Takahisa Furuta, Ken Sugimoto.
Data curation: Takahiro Miyazu, Natsuki Ishida, Ryosuke Takano, Yasushi Hamaya, Moriya Iwaizumi, Satoshi Osawa, Takahisa Furuta, Ken Sugimoto.
Formal analysis: Takahiro Miyazu, Natsuki Ishida, Ryosuke Takano, Yasushi Hamaya, Satoshi Osawa, Ken Sugimoto.
Funding acquisition: Takahiro Miyazu, Natsuki Ishida, Yasushi Hamaya, Shinya Tani, Satoshi Osawa, Ken Sugimoto.
Investigation: Takahiro Miyazu, Natsuki Ishida, Satoshi Tamura, Mihoko Yamade, Shinya Tani, Ken Sugimoto.
Methodology: Natsuki Ishida, Satoshi Tamura, Mihoko Yamade, Shinya Tani, Ken Sugimoto.
Project administration: Natsuki Ishida, Satoshi Tamura, Mihoko Yamade, Takahisa Furuta, Ken Sugimoto.
Resources: Ryosuke Takano, Satoshi Tamura, Mihoko Yamade, Takahisa Furuta, Ken Sugimoto.
Software: Ryosuke Takano, Satoshi Tamura, Mihoko Yamade, Takahisa Furuta, Ken Sugimoto.
Supervision: Ryosuke Takano, Satoshi Tamura, Mihoko Yamade, Yasushi Hamaya, Moriya Iwaizumi, Satoshi Osawa, Takahisa Furuta, Ken Sugimoto.
Validation: Ryosuke Takano, Satoshi Tamura, Mihoko Yamade, Moriya Iwaizumi, Satoshi Osawa, Takahisa Furuta, Ken Sugimoto.
Visualization: Natsuki Ishida, Ryosuke Takano, Satoshi Tamura, Mihoko Yamade, Moriya Iwaizumi, Ken Sugimoto.
Writing – original draft: Takahiro Miyazu, Natsuki Ishida, Ryosuke Takano, Shinya Tani, Satoshi Osawa, Ken Sugimoto.
Writing – review & editing: Takahiro Miyazu, Natsuki Ishida, Ken Sugimoto.
Supplementary Material
Footnotes
Abbreviations: Alb = albumin; CD = Crohn's Disease; CDAI = Crohn's Disease Activity Index; CDEIS = Crohn's disease endoscopic index of severity; CE = capsule endoscopy; CECDAI = The Capsule Endoscopy Crohn's Disease Activity Index; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; Hb = hemoglobin; IBD = Inflammatory Bowel Disease; LS = Lewis score; PC = patency capsule; SES-CD = Simple Endoscopic Score for Crohn's disease.
How to cite this article: Miyazu T, Ishida N, Takano R, Tamura S, Yamade M, Hamaya Y, Tani S, Iwaizumi M, Osawa S, Furuta T, Sugimoto K. Usefulness of the capsule endoscopy Crohn's disease activity index in assessing the necessity of early additional treatment in patients with Crohn's disease in clinical remission. Medicine. 2021;100:29(e26550).
The authors have no funding to disclose.
The authors have no conflicts of interest to disclose.
Data Availability Statement: The data used to support the findings of this study are available from the corresponding author upon request.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–29. [DOI] [PubMed] [Google Scholar]
- [2].Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- [3].D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008;371:660–7. [DOI] [PubMed] [Google Scholar]
- [4].Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- [5].Frantz DJ, Dellon ES, Grimm IS, Morgan DR. Single-balloon enteroscopy: results from an initial experience at a U.S. tertiary-care center. Gastrointest Endosc 2010;72:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sunada K, Yamamoto H, Yano T, Sugano K. Advances in the diagnosis and treatment of small bowel lesions with Crohn's disease using double-balloon endoscopy. Therap Adv Gastroenterol 2009;2:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lewis BS. Expanding role of capsule endoscopy in inflammatory bowel disease. World J Gastroenterol 2008;14:4137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Niv E, Fishman S, Kachman H, Arnon R, Dotan I. Sequential capsule endoscopy of the small bowel for follow-up of patients with known Crohn's disease. J Crohns Colitis 2014;8:1616–23. [DOI] [PubMed] [Google Scholar]
- [9].Esaki M, Matsumoto T, Ohmiya N, et al. Capsule endoscopy findings for the diagnosis of Crohn's disease: a nationwide case-control study. J Gastroenterol 2019;54:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989;30:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- [12].Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- [13].Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008;27:146–54. [DOI] [PubMed] [Google Scholar]
- [14].Gal E, Geller A, Fraser G, Levi Z, Niv Y. Assessment and validation of the new capsule endoscopy Crohn's disease activity index (CECDAI). Dig Dis Sci 2008;53:1933–7. [DOI] [PubMed] [Google Scholar]
- [15].Yablecovitch D, Lahat A, Neuman S, et al. The Lewis score or the capsule endoscopy Crohn's disease activity index: which one is better for the assessment of small bowel inflammation in established Crohn's disease? Therap Adv Gastroenterol 2018;11:1756283X17747780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Omori T, Kambayashi H, Murasugi S, et al. Comparison of Lewis score and capsule endoscopy Crohn's disease activity index in patients with Crohn's disease. Dig Dis Sci 2020;65:1180–8. [DOI] [PubMed] [Google Scholar]
- [17].Kono T, Fichera A, Maeda K, et al. Kono-S anastomosis for surgical prophylaxis of anastomotic recurrence in Crohn's disease: an international multicenter study. J Gastrointest Surg 2016;20:783–90. [DOI] [PubMed] [Google Scholar]
- [18].Koulaouzidis A, Douglas S, Plevris JN. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn's Disease Activity Index. Dig Dis Sci 2012;57:987–93. [DOI] [PubMed] [Google Scholar]
- [19].Hall B, Holleran G, Chin JL, et al. A prospective 52 week mucosal healing assessment of small bowel Crohn's disease as detected by capsule endoscopy. J Crohns Colitis 2014;8:1601–9. [DOI] [PubMed] [Google Scholar]
- [20].Lehmann FS, Burri E, Beglinger C. The role and utility of faecal markers in inflammatory bowel disease. Therap Adv Gastroenterol 2015;8:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Höög CM, Bark LÅ, Broström O, Sjöqvist U. Capsule endoscopic findings correlate with fecal calprotectin and C-reactive protein in patients with suspected small-bowel Crohn's disease. Scand J Gastroenterol 2014;49:1084–90. [DOI] [PubMed] [Google Scholar]
- [22].Peyrin-Biroulet L, Ferrante M, Magro F, et al. Results from the 2nd scientific workshop of the ECCO. I: impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis 2011;5:477–83. [DOI] [PubMed] [Google Scholar]
- [23].Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis 2009;15:1851–8. [DOI] [PubMed] [Google Scholar]
- [24].Kopylov U, Nemeth A, Koulaouzidis A, et al. Small bowel capsule endoscopy in the management of established Crohn's disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis 2015;21:93–100. [DOI] [PubMed] [Google Scholar]
- [25].Aggarwal V, Day AS, Connor S, et al. Role of capsule endoscopy and fecal biomarkers in small-bowel Crohn's disease to assess remission and predict relapse. Gastrointest Endosc 2017;86:1070–8. [DOI] [PubMed] [Google Scholar]
- [26].Kusaka J, Shiga H, Kuroha M, et al. Residual lesions on capsule endoscopy is associated with postoperative clinical recurrence in patients with Crohn's disease. Dig Dis Sci 2018;63:768–74. [DOI] [PubMed] [Google Scholar]
- [27].Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn's disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis 2014;8:1610–5. [DOI] [PubMed] [Google Scholar]
- [28].Santos-Antunes J, Cardoso H, Lopes S, Marques M, Nunes AC, Macedo G. Capsule enteroscopy is useful for the therapeutic management of Crohn's disease. World J Gastroenterol 2015;21:12660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.