Abstract
Objective:
Previous investigations yielded inconsistent results for diagnostic and prognostic predictive values of MicroRNAs (miRNAs) for acute myocardial infarction (AMI).
Methods and results:
We systematically searched on PubMed and Web of Science for articles explored association of miRNAs and AMI published from January 1989 to March 2019. For diagnostic studies, a summary of sensitivity, specificity, positive likelihood ratios (PLR), negative likelihood ratios (NLR), and diagnostic odds ratio (DOR), which indicated the accuracy of microRNAs in the differentiation of AMI and no AMI, were calculated from the true positive (TP), true negative (TN), false positive (FP), and false negative (FN) of each study. In addition, the summary receive-operating characteristics (SROC) curve was constructed to summarize the TP and FP rates. For follow-up study, we computed hazard ratios (HRs) and 95% confidence intervals (CIs) for individual clinical outcomes. The meta-analysis showed a sensitivity [0.72 (95% CI: 0.61--0.81)] and specificity [0.88 (95% CI: 0.79--0.94)] of miR-1 for AMI. In addition, miR-133 showed a sensitivity [0.73 (95% CI: 0.55--0.85)] and specificity [0.88 (95% CI: 0.74--0.95)] for AMI. Moreover, the present study showed a sensitivity [0.83 (95% CI: 0.74--0.89)] and specificity [0.96 (95% CI: 0.82--0.99)] of miR-208 for AMI. A significant association was found between miR-208 and mortality after AMI (HR 1.09, 95% CI 1.01--1.18). It also indicated a sensitivity [0.84 (95% CI: 0.70--0.92)] and specificity [0.97 (95% CI: 0.87--0.99)] of miR-499 for AMI.
Conclusions:
Circulating miR-1, miR-133, miR-208, and miR-499 showed diagnostic values in AMI.
Keywords: acute myocardial infarction, meta-analysis, microRNAs
1. Introduction
Acute myocardial infarction (AMI) is one of the most common causes of morbidity and mortality worldwide, which causes over one-third of deaths in developed nations annually.[1–6] Owing to the lifestyle changes and effective therapeutic strategies [including percutaneous coronary intervention (PCI) and coronary artery bypass graft], the mortality from AMI reduced considerably in recent decades.[7,8] However, with the growing population of AMI patients, the survival rate for affected patients has remained almost unchanged.[9] A timely diagnosis and revascularization therapy within 3 hours after the onset of chest pain is recommended to repair the ischemic myocardium, which would decrease the mortality and ameliorate prognosis of AMI.[10] Thus, finding a biomarker is essential for early detection of AMI.
MicroRNAs (miRNAs) is a type of noncoding RNAs, composed of 19 to 25 nucleic acid and participates in signaling pathways associated with cell death, metabolism, stress response, cell proliferation, and differentiation.[11,12] MiRNA could be reliably detected in samples, such as plasma, serum, whole-blood, and peripheral blood mononuclear cell (PBMC), because circulating miRNAs are protected from degradation by being encapsulated in exosomes and microvesicles and by binding to transport proteins.[13,14] Thus, blood microRNA can be considered as biomarkers for diagnosis of various diseases. Recently, accumulating studies have demonstrated that miRNAs may be important biomarkers in the diagnosis and prognostic prediction of AMI.[15–17] However, different studies showed inconsistent results regarding miRNAs the diagnosis of MI. In order to enhance the strength of evidence, meta-analysis was conducted to summarize results of studies evaluating diagnostic and prognostic predictive values of miRNAs for MI.
2. Methods
The present study was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[18] We supplied a PRISMA 2009 checklist. Ethical approval was not applicable in the study.
2.1. Search strategy
We searched for articles published from January 1989 to March 2019 in PubMed and Web of Science databases. Search terms used were: (“microRNA” OR “miR”) AND (“myocardial infarction”). In addition, we removed duplicates. A total of 1099 articles were screened in the present study.
2.2. Inclusion criteria and exclusion criteria
The present study included all articles which explored association of microRNA and AMI. Moreover, these articles should be English language literatures.
We excluded articles if the study included patients suffering from other heart diseases, such as congenital heart disease, rheumatic heart disease, and so on. We dropped secondary processing of literature such as reviews and meta-analysis articles. In addition, case studies without group-level statistics were also excluded.
2.3. Data collection
Two individuals read titles and abstracts of articles. According to inclusion and exclusion criteria, we selected 74 articles to read full-texts. We recorded from these full-texts for following data: Author, publication years, country, participant demographics (sample size), specimen, AMI definition, detection methods, the time of circulating sampling follow-up periods. In each selected article with diagnostic study, we collected true positive (TP), true negative (TN), false positive (FP), and false negative (FN) directly or calculated them according to the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). For follow-up study, the hazard ratios (HRs) and 95% confidence intervals (95% Cis) for individual clinical outcomes were collected from these selected studies.
2.4. Meta-analysis for studies
We conducted a meta-analysis to summarize results, while at least 3 articles were displayed for the diagnostic and prognostic predictive values of each miRNA for AMI. All these statistical analyses were implemented with STATA 12.0 software and Meta-Disc Version 1.4. For diagnostic studies, a summary of sensitivity, specificity, positive likelihood ratios (PLR), negative likelihood ratios (NLR), and diagnostic odds ratio (DOR), which indicated the accuracy of microRNAs in the differentiation of AMI and no AMI, were calculated from the TP, FP, FN, and TN of each study. In addition, the summary receive-operating characteristics (SROC) curve was constructed to summarize the TP and FP rates.[19] For follow-up study, we computed HRs and CIs for individual clinical outcomes. Heterogeneity between studies was estimated with Q test, and the amount of variation derived from heterogeneity was assessed with computed I2. We performed fixed effects models to summarize effect size in absence of between study heterogeneity (Q test, P > .05). Inversely, we conducted random effects models to generate summary effect size with invariably high heterogeneity (Q test, P ≤ .05) of these studies.
3. Results
3.1. Search results
Supplementary Figure 1 showed the initial search results and step-by-step exclusion process on the basis of the exclusion criteria. Supplementary Table 1 showed study characteristics of the included 74 researches. Data were collected from 9 studies[6,20–27] for the diagnostic studies with miR-1 for AMI (AMI group: n = 732, control group: n = 480). Eight studies[5,6,16,22,28–31] were included for the diagnosis of AMI with miR-133 (AMI group: n = 1526, control group: n = 1759). Eleven studies[5,6,21,24,25,32–37] were included for miR-208 (AMI group: n = 2428, control group: n = 1863). In addition, 11 studies[5,6,21,24–26,32–34,38,39] were included for miR-499 (AMI group: n = 2490, control group: n = 1850).
Moreover, data were collected from 3 studies[5,40,41] for follow-up studies (AMI group: n = 1889, control group: n = 1570).
3.2. Meta-analysis results
3.2.1. Circulating miR-1 showed a diagnostic value for AMI
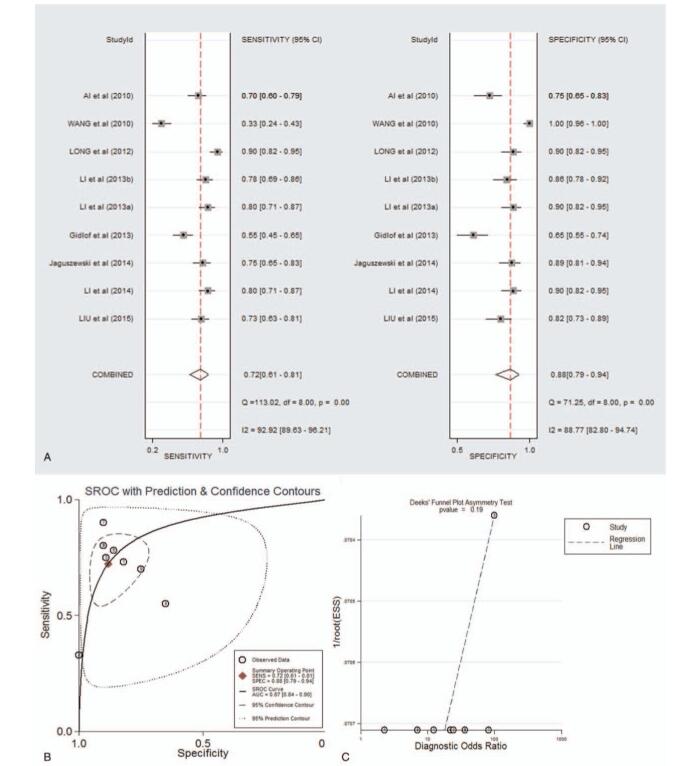
As shown in supplementary Table 2 and forest plot (Fig. 1A), the pooled parameters calculated were as follows: sensitivity, 0.72 (95% CI: 0.61--0.81); specificity, 0.88 (95% CI: 0.79--0.94); PLR, 6.1 (95% CI: 3.4--10.9); NLR, 0.31 (95% CI: 0.22--0.45); and DOR, 19 (95% CI: 10--40). The analysis showed a significant heterogeneity (sensitivity, I2 = 92.92%, P < .001; specificity, I2 = 88.77%, P < .001). Figure 1B showed the SROC curve, with an area under curve (AUC) of 0.87 (95% CI: 0.84--0.90). No statistically significant difference was found in a funnel plot for publication biases (P = .11, Fig. 1C).
Figure 1.
The sensitivity, specificity, DOR, SROC curve with AUC, and funnel graph of miR-1 in the diagnosis of AMI. (A) Sensitivity and specificity. (B) SROC curve with AUC. (C) Funnel graph. AMI = acute myocardial infarction; AUC = area under the curve; DOR = diagnostic odds ratio; SROC = summary receiver operator characteristic.
3.2.2. Circulating miR-133 showed a diagnostic value for AMI
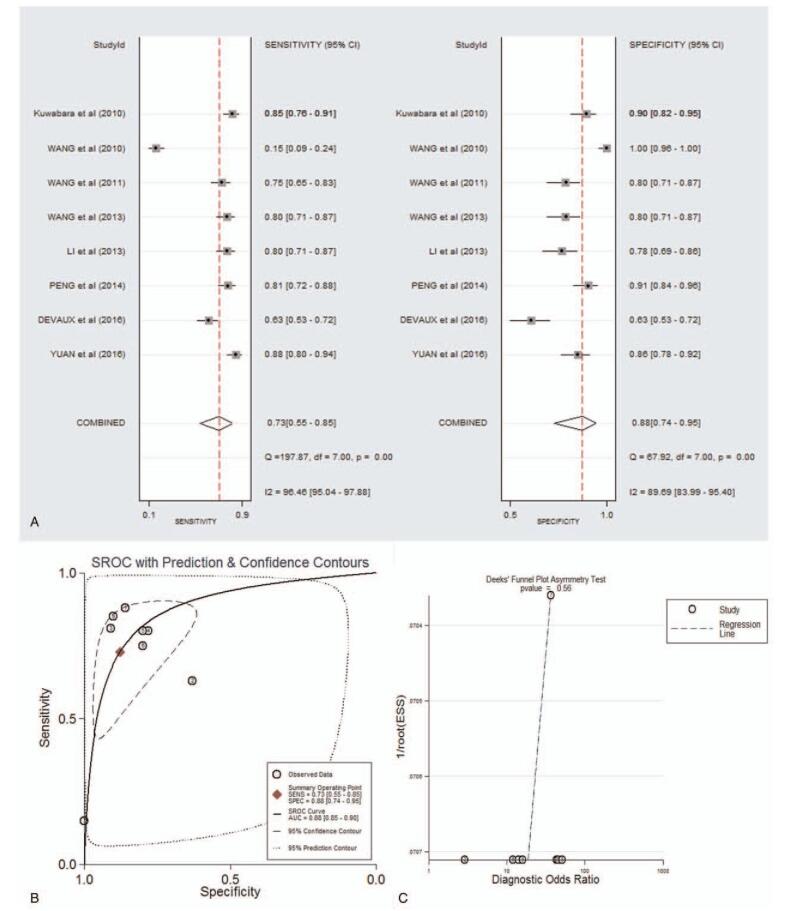
As shown in supplementary Table 2 and forest plot (Fig. 2.A), the pooled parameters were as follows: sensitivity, 0.73 (95% CI: 0.55–0.85); specificity, 0.88 (95% CI: 0.74–0.95); PLR, 6.0 (95% CI: 3.0–11.9); NLR, 0.31 (95% CI: 0.19–0.51); and DOR, 19 (95% CI: 9–40). The analysis showed a significant heterogeneity (sensitivity, I2 = 96.46%, P < .001; specificity, I2 = 89.69%, P < .001). Figure 2B showed the SROC curve, with an AUC of 0.88 (95% CI: 0.85–0.90). It showed no statistically significant difference in a funnel plot for publication biases (P = .56, Fig. 2C).
Figure 2.
The sensitivity, specificity, DOR, SROC curve with AUC, and funnel graph of miR-133 in the diagnosis of AMI. (A) Sensitivity and specificity. (B) SROC curve with AUC. (C) Funnel graph. AMI = acute myocardial infarction; AUC = area under the curve; DOR = diagnostic odds ratio; SROC = summary receiver operator characteristic.
3.2.3. Circulating miR-208 showed a diagnostic value and prognostic predictive value for AMI
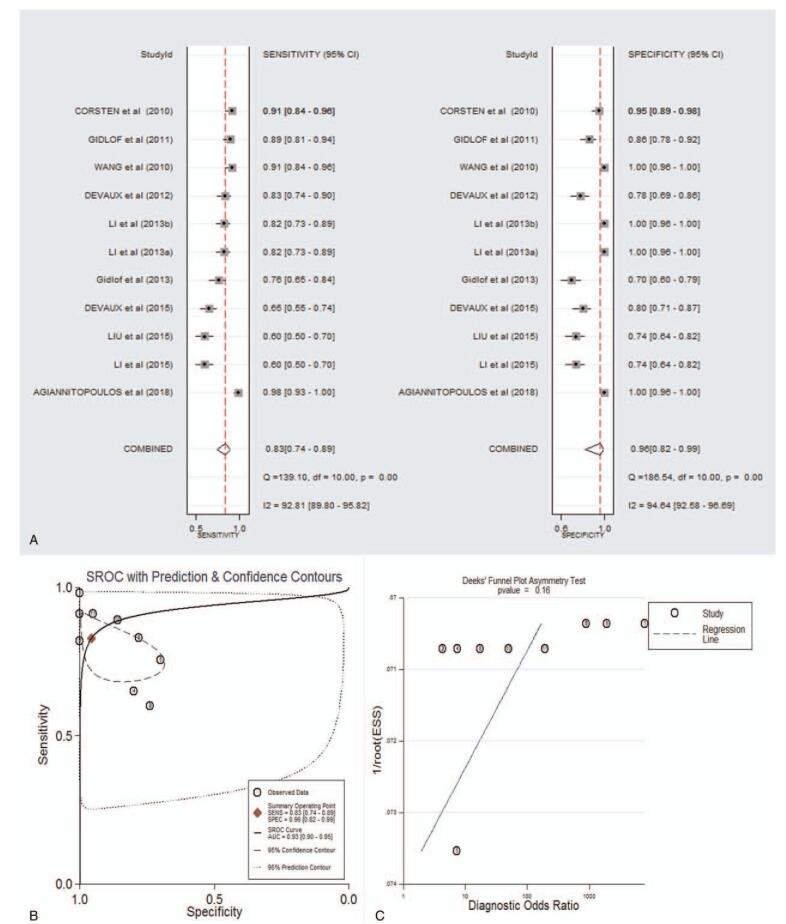
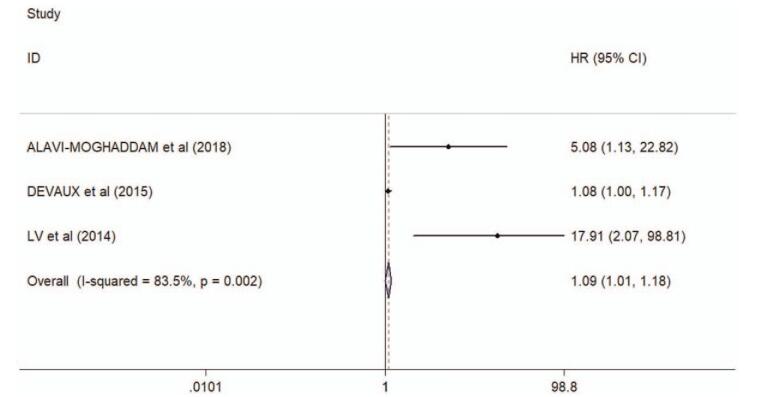
As shown in supplementary Table 2 and forest plot (Fig. 3A), the pooled parameters were as follows: sensitivity, 0.83 (95% CI: 0.74–0.89); specificity, 0.96 (95% CI: 0.82–0.99); PLR, 18.9 (95% CI: 4.0–88.3); NLR, 0.18 (95% CI: 0.11–0.30); and DOR, 105 (95% CI: 15–726). The analysis showed a significant heterogeneity (sensitivity, I2 = 92.81%, P < .001; specificity, I2 = 94.64%, P < .001). Figure 3B showed the SROC curve, with an AUC of 0.93 (95% CI: 0.90–0.95). It showed no statistically significant difference in a funnel plot for publication biases (P = .16, Fig. 3C). In addition, a significant association was found between miR-208 and mortality after AMI (HR 1.09, 95% CI 1.01--1.18, I2 = 83.5%, P = .002, Fig. 4).
Figure 3.
The sensitivity, specificity, DOR, SROC curve with AUC, and funnel graph of miR-208 in the diagnosis of AMI. (A) Sensitivity and specificity. (B) SROC curve with AUC. (C) Funnel graph. AMI = acute myocardial infarction; AUC = area under the curve; DOR = diagnostic odds ratio; SROC = summary receiver operator characteristic.
Figure 4.
Forest plots of the associations between miR-208 and mortality after AMI. AMI = acute myocardial infarction.
3.2.4. Circulating miR-499 showed a diagnostic value for AMI
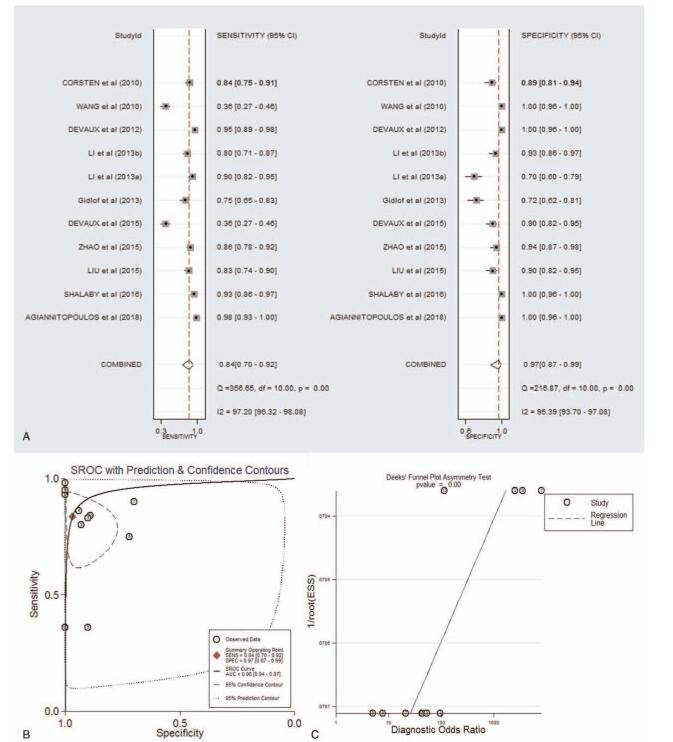
As shown in supplementary Table 2 and forest plot (Fig. 5A), the pooled parameters were as follows: sensitivity, 0.84 (95% CI: 0.70–0.92); specificity, 0.97 (95% CI: 0.87–0.99); PLR, 26.3 (95% CI: 6.1–113.9); NLR, 0.17 (95% CI: 0.09–0.33); and DOR, 154 (95% CI: 24–975). The analysis showed a significant heterogeneity (sensitivity, I2 = 97.20%, P < .001; specificity, I2 = 95.39%, P < .001). Figure 5B showed the SROC curve, with an AUC of 0.96 (95% CI: 0.94–0.97). It showed no statistically significant difference in a funnel plot for publication biases (P < .01, Fig. 5C).
Figure 5.
The sensitivity, specificity, DOR, SROC curve with AUC, and funnel graph of miR-499 in the diagnosis of AMI. (A) Sensitivity and Specificity. (B) SROC curve with AUC. (C) Funnel graph. AMI = acute myocardial infarction; AUC = area under the curve; DOR = diagnostic odds ratio; SROC = summary receiver operator characteristic.
4. Discussion
The study showed that miR-1, miR-133, miR-208, and miR-499 were the most common miRNAs explored for the diagnosis of AMI. In addition, the study indicated important values of the four miRNAs in the diagnosis of AMI. In addition, a significant association was found between miR-208 and mortality after AMI.
Recent studies demonstrated that miR-1, miR-133, miR-208, and miR-499 are identified as biomarkers for early AMI. Identifying new biomarkers for extremely early AMI diagnosis is important for making up the delayed plasma peak of cardiac troponin I (cTnI).[6] Wang et al[6] reported undetected level of miR-208a at 0 hours, but an increased level of more than 50-fold within 1 hour and a peak level at 3 hours after AMI in plasma of rat model, showing a peak before a detectable elevation of cTnI. After that, miR-208a declined to baseline within 24 hours, which enables the detection of minor cardiac events post-MI.[42] The study showed an increased level of miR-133a at 1 to 3 hours, a peak level at 3 to 12 hours, and a decreased level at 12 to 24 hours after AMI in rat plasma. In addition, Wang et al[6] found that miR-499 levels increased significantly in rat AMI models within 1 hour post AMI. Cheng et al[43] found that miR-1 showed an over 200-fold level at 6 hours post AMI in rat AMI models. These studies demonstrated that miRNAs are important biomarkers for early AMI diagnosis. Results of the present study that showed miR-1, miR-133, miR-208, and miR-499 had important values in the diagnosis of AMI were corresponding to a recent meta-analysis that showed predictive values of plasma miR-1, miR-208, and miR-499 in AMI.[26] In addition, the results were consistent with a meta-analysis for studies in Asian populations, which showed that miR-1, miR-133, and miR-499 may serve as promising diagnostic biomarkers in the early diagnosis of AMI.[44] The present systematically explored all studies involving associations between miRNAs and AMI and found that miR-1, miR-133, miR-208, and miR-499 are important biomarkers for early AMI diagnosis.
MiR-1 targets the synthesis of heat shock proteins (HSP)-60, HSP-70, and Bcl-2 and eventually promotes myoblast differentiation.[45] It is highly expressed in cardiomyocytes and skeletal muscle, and is released into the circulating after cardiac injury.[46] The meta-analysis showed a moderate sensitivity [0.72 (95% CI: 0.61--0.81)] and high specificity [0.88 (95% CI: 0.79--0.94)] of miR-1 for AMI, which was corresponding to a recent study which reported a markedly increased level of miR-1 in AMI patients, but only a mildly increased level of miR-1 in patients with other cardiovascular diseases.[6]
MiR-133 is expressed extensively in myocardial cells[6,47] and plays critical roles in myogenesis, cardiac development and hypertrophy.[48,49] The present study showed a moderate sensitivity [0.73 (95% CI: 0.55--0.85)] and high specificity [0.88 (95% CI: 0.74--0.95)] of miR-133 for AMI. In a cohort study, miR-133a was showed to distinguish ST-segment elevation acute myocardial infarction (STEMI), non-STEMI (NSTEMI), and unstable angina (UA).[50] In addition, in AMI patients, a study showed a positive association between the elevated plasma miR-133 and cTnI.[6] All these results supported that miR-133 is a powerful biomarker for the diagnosis of AMI.
MiR-208 was verified to be expressed in the heart through microarray analysis. In plasma of healthy individuals or non-AMI patients, miR-208 was undetectable, whereas miR-208 got to the peak level at 3 hours after AMI in plasma of rat model. The present study showed a high sensitivity [0.83 (95% CI: 0.74--0.89)] and specificity [0.96 (95% CI: 0.82--0.99)] of miR-208 for AMI. In addition, changes of plasma level of miR-208 were corresponding to plasma troponin-T, demonstrating that miR-208 was released from injured cardiomyocytes. Moreover, the levels of miR-208 rapidly decreased after PCI, suggesting that miR-208 might be important biomarkers for AMI prognosis.[51] The present study showed that a significant association was found between miR-208 and mortality after AMI. These results supported that circulating miR-208 showed a diagnostic value and prognostic predictive value for AMI.
MiRNA-499 is a newly discovered member of miRNAs encoded by myosin gene family, and is almost exclusively expressed in the heart. The present study showed a high sensitivity [0.84 (95% CI: 0.70–0.92)] and specificity [0.97 (95% CI: 0.87–0.99)] of miR-499 for AMI. Devaux et al[34] indicated that miR-499 was positive in 93% of patients, while cardiac troponin T (cTnT) was positive in 88% of AMI patients with chest pain < 3 hours, demonstrating that miR-499 is a sensitive biomarker for AMI diagnosis. In addition, Gidlof et al[21] suggested that miR-499 was correlated with the value of left ventricular ejection fraction in AMI patients, showing that circulating miR-499 could evaluate mortality risk.
Some recent studies indicated that microRNAs illustrated the potential advantages over traditional biomarkers. Circulating microRNAs have been recently reported to allow etiological diagnostic in the context heart failure.[52] Our study showed that miR-1, miR-133, miR-208, and miR-499 are the most common miRNAs explored for the diagnosis of AMI. Some recent studies also provided summary regarding the important role of miRNAs in the cardiovascular disease. A systematic review showed the prognostic role of platelet-derived microRNAs in the prediction of adverse cardiovascular events.[53] But the systematic review did not provide the quantitative summary on included studies. Regarding the prognostic effect of miR-133 on coronary atherosclerotic disease, De Rosa et al[54] found that miR-133 does not add significant prognostic information compared with traditional prognostic biomarkers. However, the number of studies exploring the role of miR133 in the prognosis of AMI was not sufficient to make a meta-analysis. Regarding miR-499-5p, in addition to their diagnostic value, Olivieri et al[55] found that circulating miR-499-5p levels are associated with 12-month cardiovascular mortality after NSTEMI in a sample of elderly/old subjects.[56] De Rosa et al[57] showed muscle-enriched miR-499 and miR-133 are released from the heart into the coronary circulation on MI. The present study, made a quantitative summary on the diagnostic values of miR-1, miR-133, miR-208, miR-499, and prognostic predictive value of miR-208 in AMI. False-positive results might be attributed to the influence by other molecules [such as small interfering RNA (siRNAs)]. False-negative results might be attributed to reduced amplification efficiency during polymerase chain reaction detection (PCR).
There are still some limitations in the present study. First, due to the limited numbers of included studies, we could not explore the prognostic predictive value of miR-1, miR-133, and miR-499 for AMI. Second, subgroups for the sample type, ethnicity, and many other factors could not be evaluated due to a limited number of articles.
5. Conclusion
The present meta-analysis suggested that circulating miR-1, miR-133, miR-208, and miR-499 showed diagnostic values in AMI. In addition, miR-208 showed a prognostic predictive value for AMI. Relative to miR-1 and miR-133, miR-208, and miR-499 were seemed as more reliable biomarkers in diagnosis of AMI.
Author contributions
Data curation: Gien-Kuo Lee, Yen-Ping Hsieh, Shang-Wei Hsu, Shou-Jen Lan.
Methodology: Yen-Ping Hsieh, Shang-Wei Hsu.
Writing – original draft: Yen-Ping Hsieh, Shou-Jen Lan.
Writing – review & editing: Shou-Jen Lan.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AMI = acute myocardial infarction, AUC = area under curve, CIs = confidence intervals, cTnT = cardiac troponin T, DOR = diagnostic odds ratio, FN = false negative, FP = false positive, HRs = hazard ratios, HSP = heat shock proteins, miRNAs = MicroRNAs, NLR = negative likelihood ratios, NPV = negative predictive value, PCI = percutaneous coronary intervention, PLR = positive likelihood ratios, PPV = positive predictive value, SROC = summary receive-operating characteristics, STEMI = ST-segment elevation acute myocardial infarction, TN = true negative, TP = true positive, UA = unstable angina.
How to cite this article: Lee GK, Hsieh YP, Hsu SW, Lan SJ. Exploring diagnostic and prognostic predictive values of microRNAs for acute myocardial infarction: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2021;100:29(e26627).
No potential conflict of interest was reported by the authors.
Supplemental digital content is available for this article.
References
- [1].Qian B, Katsaros D, Lu L, et al. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-beta1. Breast cancer research and treatment 2009;117:131–40. [DOI] [PubMed] [Google Scholar]
- [2].Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet (London, England) 2017;389:197–210. [DOI] [PubMed] [Google Scholar]
- [3].Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 2014;35:2950–9. [DOI] [PubMed] [Google Scholar]
- [4].Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. [DOI] [PubMed] [Google Scholar]
- [5].Devaux Y, Mueller M, Haaf P, et al. Diagnostic and prognostic value of circulating microRNAs in patients with acute chest pain. J Intern Med 2015;277:260–71. [DOI] [PubMed] [Google Scholar]
- [6].Wang GK, Zhu JQ, Zhang JT, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 2010;31:659–66. [DOI] [PubMed] [Google Scholar]
- [7].Cokkinos DV, Pantos C. Myocardial protection in man: from research concept to clinical practice. Heart Fail Rev 2007;12:345–62. [DOI] [PubMed] [Google Scholar]
- [8].Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 2014;35:2929. [DOI] [PubMed] [Google Scholar]
- [9].Kihara Y. After the triumph of cardiovascular medicine over acute myocardial infarction at the end of the 20th century. Can we predict the onset of acute coronary syndrome? Circ J 2011;75:2019–26. discussion 18. [DOI] [PubMed] [Google Scholar]
- [10].Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation 2015;132:241–50. [DOI] [PubMed] [Google Scholar]
- [11].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- [12].Plasterk RH. Micro RNAs in animal development. Cell 2006;124:877–81. [DOI] [PubMed] [Google Scholar]
- [13].Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008;105:10513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 2012;110:483–95. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Y, Liu YJ, Liu T, et al. Plasma microRNA-21 is a potential diagnostic biomarker of acute myocardial infarction. Eur Rev Med Pharmacol Sci 2016;20:323–9. [PubMed] [Google Scholar]
- [16].Yuan L, Liu X, Chen F, et al. Diagnostic and prognostic value of circulating MicroRNA-133a in patients with acute myocardial infarction. Clin Lab 2016;62:1233–41. [DOI] [PubMed] [Google Scholar]
- [17].Cortez-Dias N, Costa MC, Carrilho-Ferreira P, et al. Circulating miR-122-5p/miR-133b ratio is a specific early prognostic biomarker in acute myocardial infarction. Circ J 2016;80:2183–91. [DOI] [PubMed] [Google Scholar]
- [18]. Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Revista Española De Nutrición Humana Y Dietética. 2009. [PMC free article] [PubMed] [Google Scholar]
- [19].Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293–316. [DOI] [PubMed] [Google Scholar]
- [20].Ai J, Zhang R, Li Y, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun 2010;391:73–7. [DOI] [PubMed] [Google Scholar]
- [21].Gidlof O, Smith JG, Miyazu K, et al. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc Disord 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jaguszewski M, Osipova J, Ghadri JR, et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J 2014;35:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li C, Fang Z, Jiang T, et al. Serum microRNAs profile from genome-wide serves as a fingerprint for diagnosis of acute myocardial infarction and angina pectoris. BMC Med Genomics 2013;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li LM, Cai WB, Ye Q, et al. Comparison of plasma microRNA-1 and cardiac troponin T in early diagnosis of patients with acute myocardial infarction. World J Emerg Med 2014;5:182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li YQ, Zhang MF, Wen HY, et al. Comparing the diagnostic values of circulating microRNAs and cardiac troponin T in patients with acute myocardial infarction. Clinics (Sao Paulo, Brazil) 2013;68:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu X, Fan Z, Zhao T, et al. Plasma miR-1, miR-208, miR-499 as potential predictive biomarkers for acute myocardial infarction: an independent study of Han population. Exp Gerontol 2015;72:230–8. [DOI] [PubMed] [Google Scholar]
- [27].Long G, Wang F, Duan Q, et al. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int J Biol Sci 2012;8:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kuwabara Y, Ono K, Horie T, et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 2011;4:446–54. [DOI] [PubMed] [Google Scholar]
- [29].Peng L, Chun-guang Q, Bei-fang L, et al. Clinical impact of circulating miR-133, miR-1291 and miR-663b in plasma of patients with acute myocardial infarction. Diagn Pathol 2014;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang F, Long G, Zhao C, et al. Plasma microRNA-133a is a new marker for both acute myocardial infarction and underlying coronary artery stenosis. J Transl Med 2013;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang R, Li N, Zhang Y, et al. Circulating microRNAs are promising novel biomarkers of acute myocardial infarction. Intern Med (Tokyo, Japan) 2011;50:1789–95. [DOI] [PubMed] [Google Scholar]
- [32].Agiannitopoulos K, Pavlopoulou P, Tsamis K, et al. Expression of miR-208b and miR-499 in Greek patients with acute myocardial infarction. In Vivo (Athens, Greece) 2018;32:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Corsten MF, Dennert R, Jochems S, et al. Circulating microRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 2010;3:499–506. [DOI] [PubMed] [Google Scholar]
- [34].Devaux Y, Vausort M, Goretti E, et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem 2012;58:559–67. [DOI] [PubMed] [Google Scholar]
- [35].Gidlof O, Andersson P, van der Pals J, et al. Cardiospecific microRNA plasma levels correlate with troponin and cardiac function in patients with ST elevation myocardial infarction, are selectively dependent on renal elimination, and can be detected in urine samples. Cardiology 2011;118:217–26. [DOI] [PubMed] [Google Scholar]
- [36].Li C, Chen X, Huang J, et al. Clinical impact of circulating miR-26a, miR-191, and miR-208b in plasma of patients with acute myocardial infarction. Eur J Med Res 2015;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yan S, Liu K, Cao J, et al. Sudden drop” in blood pressure is associated with recanalization after thrombolysis. Medicine 2015;94:e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shalaby SM, El-Shal AS, Shoukry A, et al. Serum miRNA-499 and miRNA-210: a potential role in early diagnosis of acute coronary syndrome. IUBMB Life 2016;68:673–82. [DOI] [PubMed] [Google Scholar]
- [39].Zhao CH, Cheng GC, He RL, et al. Analysis and clinical significance of microRNA-499 expression levels in serum of patients with acute myocardial infarction. Genet Mol Res 2015;14:4027–34. [DOI] [PubMed] [Google Scholar]
- [40].2018;Alavi-Moghaddam M, Chehrazi M, Alipoor SD, et al. A preliminary study of microRNA-208b after acute myocardial infarction: impact on 6-month survival. 2018:2410451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lv P, Zhou M, He J, et al. Circulating miR-208b and miR-34a are associated with left ventricular remodeling after acute myocardial infarction. Int J Mol Sci 2014;15:5774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res 2017;120:381–99. [DOI] [PubMed] [Google Scholar]
- [43].Cheng Y, Tan N, Yang J, et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci 2010;119:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang Q, Ma J, Jiang Z, et al. Identification of microRNAs as diagnostic biomarkers for acute myocardial infarction in Asian populations: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang B, Lin H, Xiao J, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med 2007;13:486–91. [DOI] [PubMed] [Google Scholar]
- [46].Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol 2011;80:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chen JF, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 2006;38:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Care A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med 2007;13:613–8. [DOI] [PubMed] [Google Scholar]
- [49].Liu J, Hao DD, Zhang JS, Zhu YC. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. Biochem Biophys Res Commun 2011;413:342–7. [DOI] [PubMed] [Google Scholar]
- [50].Widera C, Gupta SK, Lorenzen JM, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol 2011;51:872–5. [DOI] [PubMed] [Google Scholar]
- [51].Sayed AS, Xia K, Yang TL, Peng J. Circulating microRNAs: a potential role in diagnosis and prognosis of acute myocardial infarction. Dis Markers 2013;35:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].De Rosa S, Eposito F, Carella C, et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur J Heart Fail 2018;20:1000–10. [DOI] [PubMed] [Google Scholar]
- [53].Pordzik J, Pisarz K, De Rosa S, et al. The potential role of platelet-related microRNAs in the development of cardiovascular events in high-risk populations, including diabetic patients: a review. Front Endocrinol (Lausanne) 2018;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].De Rosa R, De Rosa S, Leistner D, et al. Transcoronary concentration gradient of microRNA-133a and outcome in patients with coronary artery disease. Am J Cardiol 2017;120:15–24. [DOI] [PubMed] [Google Scholar]
- [55].Olivieri F, Antonicelli R, Spazzafumo L, et al. Admission levels of circulating miR-499-5p and risk of death in elderly patients after acute non-ST elevation myocardial infarction. Int J Cardiol 2014;172:e276–8. [DOI] [PubMed] [Google Scholar]
- [56].Olivieri F, Antonicelli R, Lorenzi M, et al. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int J Cardiol 2013;167:531–6. [DOI] [PubMed] [Google Scholar]
- [57].De Rosa S, Fichtlscherer S, Lehmann R, et al. Transcoronary concentration gradients of circulating microRNAs. Circulation 2011;124:1936–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.