Abstract
To investigate the correlation between the serum albumin level and the prognosis of patients with Bell's palsy.
We retrospectively analyzed the clinical records of 311 inpatients with Bell's palsy (BP) in our hospital between September 2018 and October 2019. The patients were divided into 2 groups: the recovered group (with the House-Brackmann grade ≤ 2) and the unrecovered group (with the House-Brackmann grade > 2), according to the follow-up results within 3 months after discharge. Blood test indicators (white blood cell count, neutrophil-to-lymphocyte ratio, red cell distribution width, serum albumin level, globulin level) and basic clinical data (age, sex, course of the disease, inpatient days, comorbidity of hypertension, diabetes, and hepatitis B) of the 2 groups were compared to explore whether they were correlated with the prognosis of patients with Bell's palsy.
The serum albumin level of patients with BP in the unrecovered group was significantly lower than that of the recovered group (medians [interquartile range], 40.75 [38.40, 43.85] vs 44 [42.10, 46.20], P < .001). Multivariate binary logistic regression revealed that serum albumin (odds ratio 0.772, 95% confidence interval 0.711–0.839, P < .001) was a protective factor for BP prognosis.
Serum albumin is a protective factor for the prognosis of BP. Although more prospective clinical controlled trials are needed, our study provides valuable and crucial prognostic information for physicians.
Keywords: Bell's palsy, facial paralysis, prognosis, serum albumin
1. Introduction
Bell's palsy (BP, also named idiopathic facial paralysis), a single neuropathy of the cerebral nerves, is the most common cause of peripheral facial paralysis.[1] BP usually appears as unilateral facial motor dysfunction. The clinical manifestations of severe cases are as follows: forehead wrinkles disappear, eyes cannot be closed, the nose cannot be scrunched, teeth cannot be exposed when smiling, and air leakage occurs when blowing. These symptoms can seriously affect daily life and social activities and induce psychological problems easily.[2] Epidemiological investigation shows that about 11.5 to 53.3 patients per 100,000 population suffer from such pain.[1]
Many studies have explored factors related to Bell's palsy prognosis, including age, the severity of initial facial paralysis, diabetes, hypertension, dyslipidemia, and body mass index.[3,4] Biomarkers including C-reactive protein to albumin ratio,[5] white blood cell count,[5,6] neutrophil to lymphocyte ratio[6,7] were also reported to be associated with the recovery of BP.
Serum albumin is the most abundant protein in the plasma, which has various essential biological functions. Serum albumin is well known as a nutritional marker, and it is also an inflammatory marker.[8–12] Additionally, serum albumin is already noted as a critical biomarker in many autoimmune diseases, such as rheumatoid arthritis and myasthenia gravis disease,[10,13] so its effect on the immune state of the body cannot be ignored. The clinical significance of serum albumin in terms of prediction for the prognosis of certain diseases has been reported, such as ulcerative colitis,[14] cirrhosis,[15] and cardiovascular diseases.[16]
In this study, we used retrospective analysis to investigate the indicative function of the serum albumin level for BP prognosis. It was clarified that the serum albumin level in BP patients in the unrecovered group was significantly lower than that in the recovered group, suggesting that serum albumin can be used as a clinical protective factor for the prognosis of BP. To our knowledge, this is the first time to explore the relationship between serum albumin and the prognosis of BP.
2. Methods
2.1. Study population
This study retrospectively analyzed the records of patients between 18 and 60 years old who were diagnosed with BP and admitted to our hospital between September 2018 and October 2019. We excluded the patients with peripheral facial palsy due to acute otitis media, trauma, congenital causes, neoplasm, Ramsay Hunt syndrome, and those who were undergoing a pregnant period. The House-Brackmann Facial Nerve Grading System (H-B)[17] was used to determine the severity of facial motor dysfunction at admission and during the follow-up period after discharge. The patients’ facial motor function was rated as H-B grade 4 to 6 at the first visit to our hospital. The patients we included all received acupuncture therapy and oral prednisone.
Baseline demographic and clinical characteristics, including age, sex, course of diseases, comorbidity of diabetes, hypertension and hepatitis B, and H-B grade, were recorded at admission and during follow-up.
The Institutional Review Board approved this study of our hospital. The informed consent of patients was waived because this is a retrospective study.
2.2. Biomarker analysis
All patients’ blood samples were collected at the time of admission, and biomarkers, including serum albumin (ALB), globulin (GLOB), white blood cell, and red blood cell distribution width (RDW), were recorded. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the absolute neutrophil count's value by the value of the absolute lymphocyte count. The albumin-to-globulin ratio was also calculated. Complete blood count measurements were analyzed using the LH780 Coulter blood cell counter (Beckman Coulter Inc, Brea, CA). Serum albumin and globulin levels were analyzed using Architect C16000 automated analyzer (Abbott Diagnostics, Abbott Park, IL).
2.3. Follow-up and assessment of clinical outcome
We followed up with all the included patients in this study. Facial motor function rated by H-B was recorded every month until clinical recovery (H-B grade ≤ 2) or at least 3 months after discharge (when they came back to the hospital for further treatment or via the video call). Till the end of the third month after discharge, patients with H-B grade ≤ 2 were classified into the recovered group. Accordingly, those with H-B grade > 2 were classified into the unrecovered group. We used a multivariate binary logistic regression model to analyze the relationship between each variable and the recovery of Bell's palsy.
2.4. Statistical analysis
All statistical analyses were performed using SPSS statistical software (version 25.0, IBM Corp., Armonk, NY), with P < .05 defined as statistically significant. The normal distribution of the data was assessed with the Shapiro–Wilk test. Among numerical variables, those with a normal distribution were expressed as means ± standard deviation, and those without normal distribution were shown as medians (interquartile range). A number and percentage indicated categorical variables. Two independent samples t test was used to compare 2 groups of numerical variables with normal distributions. The Mann–Whitney U test was used to compare 2 groups of numerical variables without normal distributions. The chi-square test was used to evaluate the difference in categorical variables between the recovered and unrecovered patients. A multivariate binary logistic regression was used to explore potential factors related to BP prognosis. Receiving operator characteristics curve (ROC) analysis test was used to detect the most significantly associated factor with BP prognosis and determine a cut-off value.
3. Results
3.1. Baseline characteristics
We collected the baseline information of 311 patients with a mean age of 39.7 ± 11.8 years (range, 18–60 years), including 169 males (54.3%) and 142 females (45.7%). Out of the 311 patients, 32 (10.3%) had diabetes, 48 (15.4%) had hypertension, and 30 (9.6%) had hepatitis B. As for the severity of the facial motor dysfunction at admission, 88 (28.3%) were diagnosed as grade 4, 111 (35.7%) as grade 5, 112 (36.0%) as grade 6 (Table 1).
Table 1.
Baseline demographic and clinical characteristics of patients (n = 311).
| Item | Value |
| Age (yrs, mean ± SD) | 39.7 ± 11.8 |
| Sex (M/F) | 169 (54.3%)/142 (45.7%) |
| Hypertension (with/without) | 48 (15.4%)/263 (84.6%) |
| Diabetes (with/without) | 32 (10.3%)/279 (89.7%) |
| Hepatitis B (with/without) | 30 (9.6%)/281 (90.4%) |
| Severity of palsy (H-B grade) | |
| IV | 88 (28.3%) |
| V | 111 (35.7%) |
| VI | 112 (36.0%) |
F, female; H-B, House-Brackmann Facial Nerve Grading System; M, male; SD, standard deviation.
3.2. Clinical outcome (facial motor function)
In the third month after discharge, 195 patients with H-B grade ≤ 2 were categorized into the recovered group, and 116 patients with H-B grade > 2 were classified into the unrecovered group.
Tables 2 and 3 display a univariate analysis to determine whether the variables were connected to the recovery of patients with BP within 3 months after discharge. Results showed the course of the disease, inpatient days, RDW, ALB, and GLOB significantly impacted the recovery. However, no significant difference was identified between the 2 groups about sex, age, white blood cell, NLR, albumin-to-globulin ratio, and status of being sick with hypertension, diabetes, and hepatitis B. Interestingly, ALB and GLOB were significantly higher in the recovered group than in the unrecovered group (ALB: 44 vs 40.75, P < .001; GLOB: 27.60 vs 26.50, P = .036). By contrast, RDW was significantly higher in the unrecovered group than in the recovered group (13.10 vs 12.90, P = .018). The course of the disease and inpatient days in the unrecovered group were found longer than that in the recovered group (18 vs 8, P = .008 and 15.5 vs 13, P = .001, respectively).
Table 2.
Demographic and clinical characteristics of patients in the recovered group and unrecovered group.
| Variable | Category | Recovered (n) | Unrecovered (n) | χ2 | P |
| Sex | Male | 111 | 58 | 1.284 | .257 |
| Female | 84 | 58 | |||
| Hypertension | With | 29 | 19 | 0.127 | .722 |
| Without | 166 | 97 | |||
| Diabetes | With | 23 | 9 | 1.284 | .257 |
| Without | 172 | 107 | |||
| Hepatitis B | With | 15 | 15 | 2.290 | .130 |
| Without | 180 | 101 |
A chi-square test was used to compare gender, hypertension, diabetes, and hepatitis B between recovered and unrecovered patients in the third month after discharge. P < .05 is considered statistically significant.
Table 3.
Demographic, clinical characteristics, and biochemical indicators of patients in the recovered group and unrecovered group.
| Variable | Recovered | Unrecovered | Z | P |
| Age (yrs) | 38 (28, 49) | 42 (33, 49.75) | 1.867 | .062 |
| Course (d) | 8 (4, 34) | 18 (6, 41) | 2.646 | .008∗ |
| Days | 13 (9, 19) | 15.5 (11, 20) | 3.194 | .001∗ |
| WBC (109/L) | 8.31 (6, 10.51) | 7.70 (5.33, 10.28) | 1.343 | .179 |
| NLR (%) | 4.67 (2.25, 7.13) | 4 (2.04, 7.19) | 0.432 | .666 |
| RDW (%) | 12.90 (12.50, 13.30) | 13.10 (12.53, 13.70) | 2.367 | .018∗ |
| ALB (g/L) | 44 (42.10, 46.20) | 40.75 (38.40, 43.85) | 6.866 | <.001∗ |
| GLOB (g/L) | 27.60 (24.30, 30.60) | 26.50 (24.10, 28.98) | 2.097 | .036∗ |
| A/G | 1.58 (1.44, 1.81) | 1.55 (1.42, 1.69) | 1.955 | .051 |
Mann–Whitney U test was used to compare age, course, days, WBC, NLR, RDW, ALB, GLOB, A/G between the patients recovered and unrecovered. P < .05 is considered statistically significant.
Significant difference. A/G, serum albumin-to-globulin ratio; ALB, serum albumin; Course, course of the disease (d); Days, days of inpatient; GLOB, globulin; M, median; NLR, neutrophil-to-lymphocyte ratio (absolute neutrophil count/absolute lymphocyte count); RDW, red blood cell distribution width; WBC, white blood cell.
3.3. Correlation between ALB and clinical outcome
To further explore the potential factors related to Bell's palsy prognosis, we established a multivariate binary logistic regression model (Table 4). In the multivariate binary logistic regression analysis, ALB significantly affected the recovery from BP (odds ratio 0.772, 95% confidence interval 0.711–0.839, P < .001). The serum albumin level was positively correlated with the prognosis of patients with Bell's palsy. It was also evident that the inpatient day was negatively correlated with the recovery of Bell's palsy (odds ratio 1.061, 95% confidence interval 1.014–1.110, P = .011).
Table 4.
Multivariate binary logistic regression for the prognosis of Bell's palsy.
| 95%CI | ||||||
| Variable | β | Wald | P | OR | Lower | Upper |
| Age | 0.004 | 0.103 | .748 | 1.004 | 0.981 | 1.027 |
| GLOB (g/L) | −0.054 | 2.659 | .103 | 0.948 | 0.889 | 1.011 |
| ALB (g/L) | −0.258 | 37.862 | <.001∗ | 0.772 | 0.711 | 0.839 |
| RDW (%) | 0.092 | 0.709 | .400 | 1.096 | 0.885 | 1.358 |
| Course | 0.008 | 2.424 | .119 | 1.008 | 0.998 | 1.018 |
| Days of inpatient | 0.059 | 6.53 | .011∗ | 1.061 | 1.014 | 1.110 |
The multivariate binary logistic regression model was used to further analyze the factors related to the prognosis of Bell's palsy. ALB, albumin; CI, confidence interval; GLOB, globulin; OR, odds ratio; RDW, red blood cell distribution width. P < .05 is considered statistically significant.
Significant difference.
Figure 1 presents the ROC analysis of the factors, including ALB, GLOB, RDW, course, age, and inpatient days. ROC analysis of prognostic factors of BP indicated that ALB had a greater AUC value (0.73) compared to GLOB (0.57) (P < .001 vs P = .036) and other factors. Accordingly, ALB might be the most significant factor associated with the prognosis of BP. The cut-off value of ALB for the prognosis of BP was 41.5 g/L with a sensitivity of 81.03% and specificity of 58.62%, and the cut-off value of GLOB for the prognosis was 29.2 g/L with a sensitivity of 39.49% and specificity of 81.03%.
Figure 1.
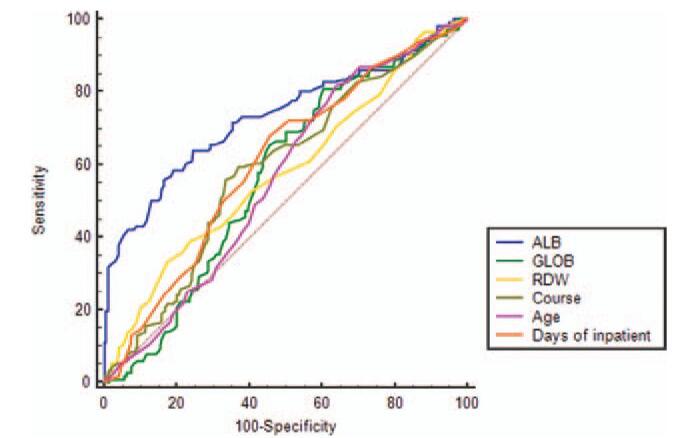
ROC curves for ALB, GLOB, RDW, age, course, and days of inpatient. The area under the ROC curve for ALB was broader than the other 5, indicating that ALB was superior to the other 5 in terms of capability to predict the prognosis. Sensitivity/specificity to predict the prognosis by ALB at 41.5 g/L was 81.03%/58.62%, by GLOB at 29.2 g/L was 39.49%/81.03%, by RDW at 13.5% was 82.56%/33.62%, by course at 15 d was 66.67%/56.03%, by age at 31 yrs was 35.90%/81.90%, and by days of inpatient at 13 d was 54.36%/68.10%. ALB (AUC: 0.73, P < .001), GLOB (AUC: 0.57, P = .036). ALB, albumin; GLOB, globulin; RDW, red blood cell distribution width; ROC, receiving operator characteristics curve.
4. Discussion
The exact pathogenesis of BP remains uncertain, inflammation caused by viral infection has always been considered one of the causes of BP,[18] and some suggested that facial nerve ischemia may play an important role.[19] However, there are few reports on the mechanism of secondary edema after facial nerve injury. Past evidence showed that edema was most evident within 3 weeks after onset, and in patients with severe nerve damage, the swelling continued even after 10 weeks.[20] Inflammatory cell infiltration was observed in histopathological specimens of patients in the acute phase,[21] suggesting that edema is possibly secondary to inflammation in the acute phase. In the later phase, the relationship between inflammation and edema is not so obvious, and another reason for edema may be that the nerve has been denatured or ischemia.[20] The degree of nerve damage is directly related to the prognosis of BP.
Our study used a simple blood test to find that serum ALB level is associated with Bell's palsy prognosis. The albumin level was higher in the recovered group than in the unrecovered group. Multivariate binary logistic regression revealed that serum albumin (odds ratio 0.772, 95% confidence interval 0.711–0.839, P < .001) is a protective factor for the prognosis of BP. In the ROC analysis, the cut-off value of ALB for the prognosis of BP was found as 41.5 g/L (normal range at 40–55 g/L), which could remind clinicians to pay more attention to patients with albumin levels lower than normal.
Edema and oxidative stress are associated with the development of BP, indicating that ALB may play a role in recovering BP.[22] Albumin is primarily synthesized by the liver and released into the blood circulation. It takes around 30 minutes to synthesize and secrete albumin.[23,24] Physiologically, the degradation rate of albumin is comparable to synthesis. Thus it is usually maintained at a certain concentration in the blood.[23] Albumin is thought to be a negative acute-phase protein.[5]In an inflammatory state, vascular endothelial permeability increases. Various components in blood vessels, including albumin, leak out into the tissue space, resulting in decreased albumin concentration in plasma.[25] In patients with liver dysfunction or malnutrition, albumin synthesis is reduced. The body preferentially synthesizes acute reactive protein under an emergency state, which further reduces albumin synthesis. Therefore, a low albumin level may indicate persistent inflammation and malnutrition.
Albumin plays a predominant role in the maintenance of plasma colloidal osmotic pressure.[26] Different serum albumin levels may lead to varying degrees of edema, and thus to a large extent, determine the degree of nerve damage. A low level of albumin is detrimental for the facial nerve to release from edema. The edematous nerve is compressed in the narrow facial nerve canal, forming a vicious circle of ischemia-edema-ischemia, ultimately leading to nerve degeneration and even necrosis. Recent studies have also shown that Bell's palsy's pathogenesis is related to oxidative stress, which refers to an oxidant–antioxidant imbalance due to a decline in total antioxidant capacity.[22] Serum albumin has many biochemical properties; one of them is the antioxidant function. It plays an essential role in the antioxidant system with other substances.[26,27]
Globulin was also found related to the prognosis of BP in the univariate analysis. Autoimmunity has always been discussed as one of the pathogenesis of BP.[28] Low serum globulin level presents low immunity and thereby leads to poor recovery of patients. However, only 8 of 311 patients have a globulin level lower than normal (20–40 g/L). Insufficient sample size may explain why there was no significant result in multivariate binary logistic regression. Whether globulin impacts the recovery of BP patients needs to be further investigated in the future.
In previous studies, NLR and RDW were potential prognostic markers for BP.[6,29] NLR has been reported as an indicator of inflammation in patients with idiopathic peripheral facial palsy and related to the severity of paralysis, the worse facial motor function in the acute phase, and the higher NLR.[18] However, in our study, NLR showed no significant relation with the prognosis of BP. RDW is considered a novel biomarker of inflammation,[30] and BP patients with higher RDW were found to have worse recovery.[29] Although a similar result was presented in the univariate analysis in our study, it was found that RDW was not statistically significant after being adjusted by multivariate binary logistic regression analysis. One possible explanation is its relatively weak influence compared to ALB. Besides, different treatment options before admission and differences from onset to the first visit to our hospital may lead to this result.
However, our study remained a clinical phenomenon found in clinical practice, which requires randomized controlled trials to validate our findings, that is, whether rising the serum albumin level via variable therapies can improve the prognosis of Bell's palsy. Based on our study, more investigation can further elucidate the relationship between the serum albumin level and BP. First of all, this is a retrospective study. The use of drugs or therapy methods before coming to our hospital was not clear. As a result, whether the recovery is related to drug usage can be verified. Secondly, typical inflammatory biomarkers such as the C-reactive protein or erythrocyte sedimentation rate can be considered. Thirdly, the serum albumin level can be tracked in the long run to establish the connection during the follow-up period.
5. Conclusion
Our study preliminarily discovered that the serum albumin level in the recovered group was higher than in the unrecovered group, indicating that serum albumin is a potentially protective factor for BP prognosis. Therefore, serum albumin can be used as a novel marker to predict BP patients’ prognosis, which provides a guide for clinicians in treating patients with BP.
Author contributions
Conceptualization: Wenfang Shang, Haiyu Hu, Mengxia Shen, Lihua Xuan.
Data curation: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Zelin Yu, Lihua Xuan.
Formal analysis: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Lihua Xuan.
Investigation: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Zelin Yu, Lihua Xuan.
Methodology: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Zelin Yu, Lihua Xuan.
Project administration: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Lihua Xuan.
Resources: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Zelin Yu, Lihua Xuan.
Software: Wenfang Shang, Haiyu Hu, Mengxia Shen.
Supervision: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Zelin Yu, Lihua Xuan.
Validation: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Zelin Yu, Lihua Xuan.
Visualization: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Zelin Yu, Lihua Xuan.
Writing – original draft: Wenfang Shang, Mengxia Shen
Writing – review & editing: Wenfang Shang, Haiyu Hu, Mengxia Shen, Jiangxia Wu, Zelin Yu, Lihua Xuan.
Footnotes
Abbreviations: ALB = albumin, BP = Bell's palsy, GLOB = globulin, H-B = House-Brackmann Facial Nerve Grading System, NLR = neutrophil-to-lymphocyte ratio, RDW = red blood cell distribution width, ROC = receiving operator characteristics curve.
How to cite this article: Shang W, Hu H, Shen M, Wu J, Yu Z, Xuan L. Investigating the correlation between serum albumin level and the prognosis of Bell's palsy. Medicine. 2021;100:29(e26726).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Baugh RF, Basura GJ, Ishii LE, et al. Clinical practice guideline: Bell's palsy. Otolaryngol – Head Neck Surg: official journal of American Academy of Otolaryngology-Head and Neck Surgery 2013;149: Suppl: S1–27. [DOI] [PubMed] [Google Scholar]
- [2].Mun SJ, Park KT, Kim Y, Park JH, Kim YH. Characteristics of the perception for unilateral facial nerve palsy. Eur Arch Oto-rhino-laryngol 2015;272:3253–9. [DOI] [PubMed] [Google Scholar]
- [3].Zhao H, Zhang X, Tang YD, Zhu J, Wang XH, Li ST. Bell's palsy: clinical analysis of 372 cases and review of related literature. Eur Neurol 2017;77:168–72. [DOI] [PubMed] [Google Scholar]
- [4].Choi SA, Shim HS, Jung JY, et al. Association between recovery from Bell's palsy and body mass index. Clin Otolaryngol: official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 2017;42:687–92. [DOI] [PubMed] [Google Scholar]
- [5].Cayir S, Hizli O, Kayabasi S. Is C-reactive protein to albumin ratio an indicator of poor prognosis in Bell's palsy? Eur Arch Oto-rhino-laryngol 2020;277:115–9. [DOI] [PubMed] [Google Scholar]
- [6].Bucak A, Ulu S, Oruc S, et al. Neutrophil-to-lymphocyte ratio as a novel-potential marker for predicting prognosis of Bell palsy. Laryngoscope 2014;124:1678–81. [DOI] [PubMed] [Google Scholar]
- [7].Atan D, İkincioğullari A, Köseoğlu S, et al. New predictive parameters of Bell's palsy: neutrophil to lymphocyte ratio and platelet to lymphocyte ratio. Balkan Med J 2015;32:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Heidari B. C-reactive protein and other markers of inflammation in hemodialysis patients. Caspian J Intern Med 2013;4:611–6. [PMC free article] [PubMed] [Google Scholar]
- [9].Firouzjahi A, Monadi M, Karimpoor F, et al. Serum C-reactive protein level and distribution in chronic obstructive pulmonary disease versus healthy controls: a case–control study from Iran. Inflammation 2013;36:1122–8. [DOI] [PubMed] [Google Scholar]
- [10].Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release: Off J Control Release Soc 2008;132:171–83. [DOI] [PubMed] [Google Scholar]
- [11].Rezvani AR, Storer BE, Storb RF, et al. Decreased serum albumin as a biomarker for severe acute graft-versus-host disease after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011;17:1594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cehreli R, Yavuzsen T, Ates H, Akman T, Ellidokuz H, Oztop I. Can inflammatory and nutritional serum markers predict chemotherapy outcomes and survival in advanced stage nonsmall cell lung cancer patients? BioMed Res Int 2019;2019:1648072.doi:10.1155/2019/1648072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Weng Y-Y, Yang D-H, Qian M-Z, et al. Low serum albumin concentrations are associated with disease severity in patients with myasthenia gravis. Medicine 2016;95:e5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khan N, Patel D, Shah Y, Trivedi C, Yang YX. Albumin as a prognostic marker for ulcerative colitis. World J Gastroenterol 2017;23:8008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Carvalho JR, Verdelho Machado M. New insights about albumin and liver disease. Ann Hepatol 2018;17:547–60. [DOI] [PubMed] [Google Scholar]
- [16].Suzuki S, Hashizume N, Kanzaki Y, Maruyama T, Kozuka A, Yahikozawa K. Prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. PLoS One 2019;14:e0219044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Engstrom M, Jonsson L, Grindlund M, Stålberg E. House-Brackmann and Yanagihara grading scores in relation to electroneurographic results in the time course of Bell's palsy. Acta Oto-laryngol 1998;118:783–9. [DOI] [PubMed] [Google Scholar]
- [18].Kilickaya MM, Tuz M, Yariktas M, Yasan H, Aynali G, Bagci O. The importance of the neutrophil–lymphocyte ratio in patients with idiopathic peripheral facial palsy. Int J Otolaryngol 2015;2015:981950.doi:10.1155/2015/981950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang J, Dai C, Zhao H, Huang X. Establishment of animal model for ischemic facial palsy in rat. J Clin Otorhinolaryngol 1999;13:464–5. [PubMed] [Google Scholar]
- [20].Yanagihara N, Honda N, Hato N, Murakami S. Edematous swelling of the facial nerve in Bell's palsy. Acta Oto-laryngol 2000;120:667–71. [DOI] [PubMed] [Google Scholar]
- [21].Liston SL, Kleid MS. Histopathology of Bell's palsy. Laryngoscope 1989;99:23–6. [DOI] [PubMed] [Google Scholar]
- [22].Terzi S, Dursun E, Yilmaz A, et al. Oxidative stress and antioxidant status in patients with Bell's palsy. J Med Biochem 2017;36:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Caironi P, Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus 2009;7:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hafkenscheid JC, Yap SH, van Tongeren JH. Measurement of the rate of synthesis of albumin with 14C-carbonate: a simplified method. Zeitschrift fur klinische Chemie und klinische Biochemie 1973;11:147–51. [DOI] [PubMed] [Google Scholar]
- [25].Dahn MS, Jacobs LA, Smith S, Lange MP, Mitchell RA, Kirkpatrick JR. The significance of hypoalbuminemia following injury and infection. Am Surg 1985;51:340–3. [PubMed] [Google Scholar]
- [26].Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology 2005;41:1211–9. [DOI] [PubMed] [Google Scholar]
- [27].Irshad M, Chaudhuri PS. Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol 2002;40:1233–9. [PubMed] [Google Scholar]
- [28].Greco A, Gallo A, Fusconi M, Marinelli C, Macri GF, de Vincentiis M. Bell's palsy and autoimmunity. Autoimmun Rev 2012;12:323–8. [DOI] [PubMed] [Google Scholar]
- [29].Horibe Y, Tanigawa T, Shibata R, et al. Efficacy of the red blood cell distribution width for predicting the prognosis of Bell palsy: a pilot study. Eur Arch Oto-Rhino-Laryngol 2017;274:2303–6. [DOI] [PubMed] [Google Scholar]
- [30].Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008;117:163–8. [DOI] [PubMed] [Google Scholar]


