ABSTRACT
A 63-year-old man with nonalcoholic steatohepatitis cirrhosis who underwent orthotopic liver transplant presented 1 year later with obstructive jaundice because of a biliary stricture. This anastomotic stricture was initially believed to be ischemic, but further investigation revealed malignant biliary obstruction because of encasement of the bile duct by a mass arising from liver segment VII, later determined to be post-transplant lymphoproliferative disorder with widespread metastasis. After reduction of immunosuppression and systemic chemotherapy, he experienced complete remission. This case illustrates the need to consider post-transplantation lymphoproliferative disorder–related biliary stricture in any postorthotopic liver transplantation transplant patient presenting with obstructive jaundice.
INTRODUCTION
Obstructive jaundice because of biliary stricture is a common complication after liver transplantation. Vascular injury or surgical anastomotic failure is a common cause of post-transplant biliary obstruction, but biliary obstruction can also be caused by post-transplant lymphoproliferative disorder (PTLD), an immunosuppression-mediated lymphoproliferative disorder usually associated with Epstein-Barr virus (EBV). Reduction of immunosuppression and treatment with cytotoxic chemotherapy may lead to complete resolution of this potentially life-threatening condition. It is important to consider this rare lymphoid malignancy in patients who present with post-transplant biliary obstruction in the presence of EBV and systemic symptoms such as fever and night sweats.
CASE REPORT
A 63-year-old man with nonalcoholic steatohepatitis cirrhosis underwent orthotopic liver transplant (OLT). Laboratory evaluation suggests pretransplant EBV exposure occurred in both donor and recipient. Five months after transplant, mixed hepatocellular-cholestatic jaundice prompted a liver ultrasound which showed choledocholithiasis, and he underwent endoscopic retrograde cholangiopancreatography (ERCP) and biliary sphincterotomy (Figures 1 and 2). Liver biopsy also revealed grade II acute cellular rejection. Although he was treated with antirejection immunosuppression protocol, laboratory testing revealed persistent moderate liver injury. Approximately 6 months after transplant, he developed intermittent fevers, weight loss, and sweating episodes. Laboratory testing revealed cytomegalovirus and EBV viremia. In response, valganciclovir was initiated, and mycophenolate and tacrolimus dosing were decreased.
Figure 1.

Endoscopic retrograde cholangiopancreatography performed at the time of presentation. The post-transplant anatomy showed a candy cane bend of recipient duct with small cystic duct stump. A tight stricture at the surgical anastomosis contained a single localized stenosis 8 mm in length. Multiple unsuccessful attempts were made to traverse this stricture with a wire. The upper third of the main bile duct was diffusely dilated. The largest diameter was 12 mm.
Figure 2.
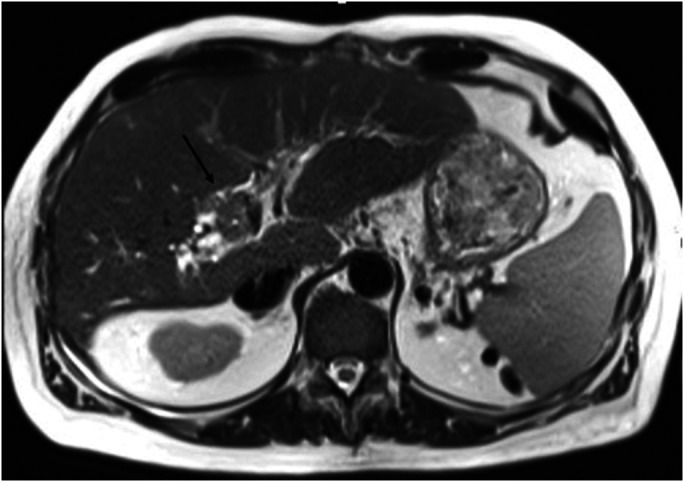
This demonstrates an isointense mass on T2-weighted imaging, which involves hepatic segment 8 and extends toward the porta hepatis with resultant stricture of the post-transplant biliary anastomosis and subsequent intrahepatic biliary dilatation.
One year after transplantation, an acute elevation in total bilirubin prompted a computed tomography (CT) scan which revealed increased diameter of the common bile duct (Figure 3). Repeat ERCP revealed an 8-mm stricture at the choledochocholedochostomy which could not be traversed. Brushings obtained during this procedure were nondiagnostic. He then received external-to-internal biliary drainage through percutaneous transhepatic cholangiography (PTC). This anastomotic stricture was initially believed to be ischemic, but magnetic resonance cholangiopancreatography revealed a 3.1 × 1.9 × 2.6-cm soft-tissue mass arising from segment VIII of the liver, extending into the porta hepatis and encasing 2.5 cm of the common bile duct at the anastomosis. Notably, this finding was not observed on the CT scan 2 weeks earlier. Ultrasound-guided biopsy showed EBV-positive, CD20-negative, diffuse large B-cell lymphoma (Figure 4). Positron emission tomography scan showed stage IV disease with hypermetabolic activity throughout the hepatic parenchyma with diffuse lymph node and bony metastasis (Figure 5). Tacrolimus was discontinued, and he underwent 6 cycles of chemotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Repeat positron emission tomography/CT completed 1 month after chemotherapy showed complete treatment response without evidence of active malignancy. The percutaneous drain was removed after cholangiogram demonstrated patent flow through the common bile duct.
Figure 3.
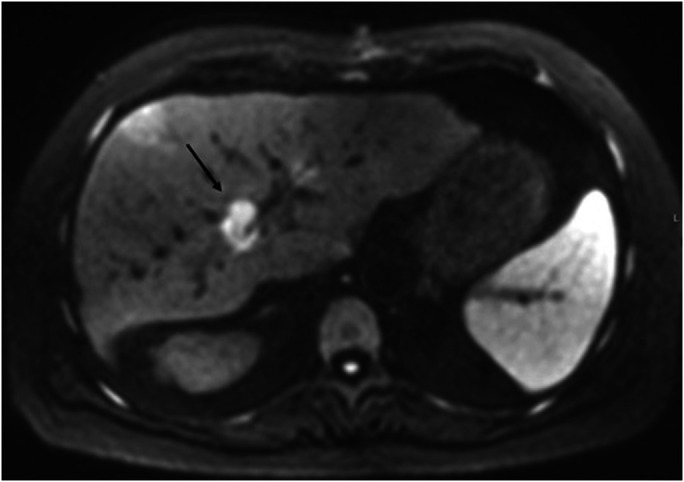
On diffusion-weighted imaging, the mass demonstrates marked diffusion restriction, with corresponding low signal on apparent diffusion coefficient.
Figure 4.
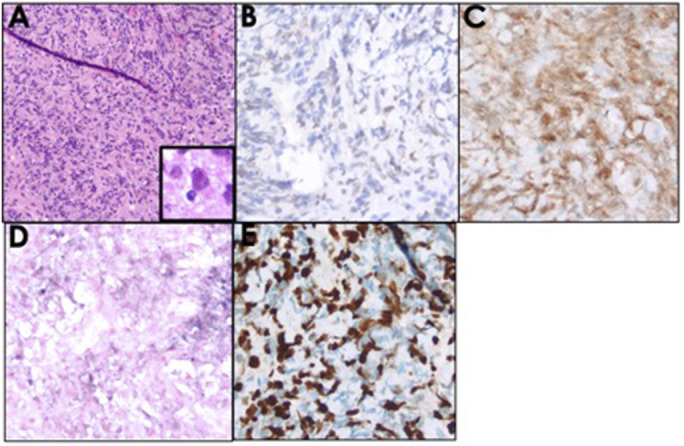
The liver core biopsy showing (A) a focal infiltrate of inflammatory cells mixed with occasional large atypical lymphoid cells having prominent nucleoli (A with inset, hematoxylin and eosin stain). Although negative for CD20 (not shown), by immunohistochemistry, the large atypical cells were positive for (B) Pax5 and (C) Oct2, consistent with B-cell lineage. The atypical B cells were positive for (D) EBER RNA by in situ hybridization and had (E) a high Ki-67 proliferation index (∼90%).
Figure 5.
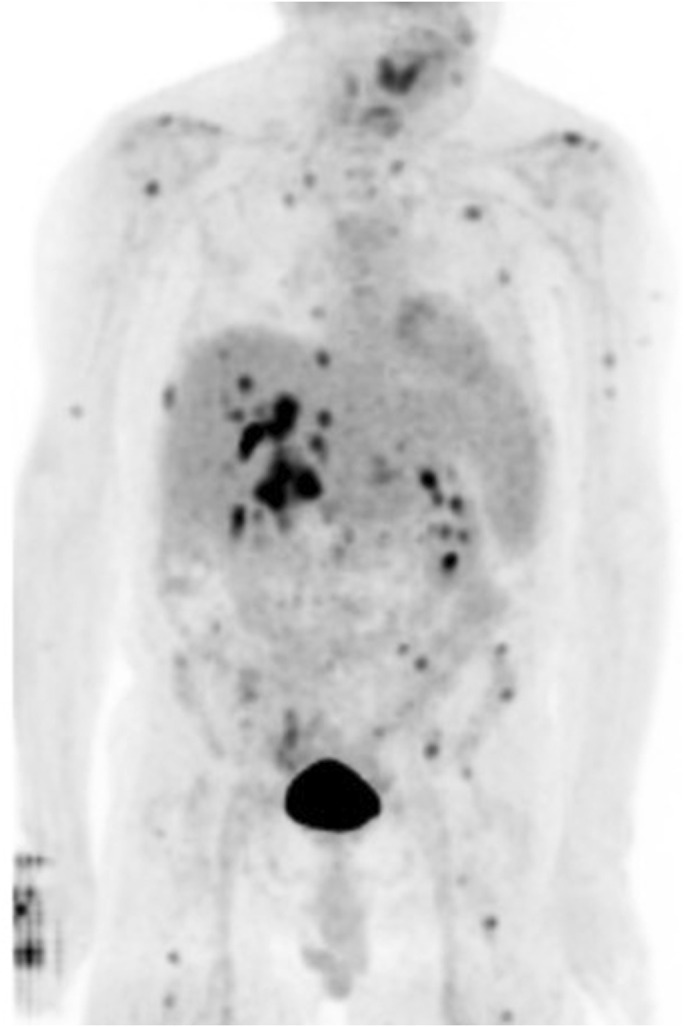
18F-fluorodeoxyglucose (FDG)-positron emission tomography demonstrates intense FDG uptake within the mass, with multifocal hypermetabolic metastatic disease involving several lymph nodes groups as well as multiple osseous structures. Numerous hypermetabolic osseous foci consistent with metastatic disease as seen in the base of the skull, left scapula, bilateral ribs, lumbar spine, left iliac bone, left sacrum, and bilateral proximal femurs as described above.
DISCUSSION
Biliary complications after OLT are reported in up to 40% of cases and usually involve biliary strictures.1 Biliary strictures are classified as anastomotic or nonanastomotic and are usually secondary to preservation injury, hepatic artery thrombosis and/or stenosis, chronic rejection, cytomegalovirus infection, blood group type incompatibility, and recurrence of an underlying biliary disease, such as primary sclerosing cholangitis.1 Most strictures within the first 3 months are anastomotic because of either anastomotic ischemia or failure of suturing technique.2 Post-OLT biliary stricture resulting from PTLD is quite rare.
The presentation of post-transplant biliary stricture is generally the result of biliary outflow obstruction. Clinical findings include jaundice, right upper quadrant pain, and pruritis, and laboratory findings may reflect cholestatic and hepatic injury. Biliary dilation is not a consistent finding in post-transplant biliary stricture, and ultrasound may therefore fail to provide a diagnosis.3 It has been suggested that biliary fibrosis after transplant causes the donor bile ducts to become less pliable, with a diminished capacity to dilate.4 Therefore, MRI with magnetic resonance cholangiopancreatography has become the diagnostic imaging modality of choice. Direct cholangiography, either through ERCP or PTC, is generally reserved for definitive management. ERC is generally preferred to PTC because it is effective, widely available, and has relatively fewer complications PTC.4 Surgical reconstruction is primarily reserved for salvage therapy when ERC and PTC have failed.
PTLD includes a heterogenous group of lymphoproliferative disorders that occur after solid organ or allogenic hematopoietic stem cell transplantation, generally in the setting of EBV. PTLD may occur in up to 5.5% of adults after liver transplant.5 Major risk factors for PTLD after solid organ transplant include multiorgan and intestinal transplantation, degree of immunosuppression (especially of T cells), higher risk within the first year after transplant, and EBV seronegativity of the transplant recipient.6 This is believed to be due to the fact that the recipient has neither virus-specific antibodies to neutralize infectious virions released by infected B cells nor virus-specific T cells to control the outgrowth of subsequently infected recipient B cells.6 This patient was EBV-seropositive before transplant.
The clinical presentation of PTLD is heterogenous and may range from incidental asymptomatic findings to fulminant organ failure and tumor lysis syndrome.5 Other typical symptoms include fever, lymphadenopathy, weight loss, or splenomegaly. Disease may be localized to 1 site or to the allograft, or it may present as diffuse disease with multiorgan failure. Bone marrow and central nervous system involvement may also occur.6 The gold standard for the diagnosis of PTLD is histopathological examination.5,7 Therapies for PTLD differ from the management of lymphoproliferative disorders in immunocompetent patients. The cornerstone of the initial therapy of PTLD is reduction of immunosuppression, which leads to regression of PTLD in 20%–80% of cases.5 Potential additional therapies include surgical extirpation of localized disease, local radiation therapy, rituximab monotherapy, immunochemotherapy, chemotherapy, stem cell transplantation, and cellular immunotherapy.5 This case demonstrates the difficulties of prevention and diagnosis of PTLD-related biliary stricture in a highly complex patient. It illustrates the need to consider PLTD-related biliary stricture in any post-OLT transplant patient presenting with obstructive jaundice.
DISCLOSURES
Author contributions: D. Karb wrote the manuscript and is the article guarantor. S. Satyavada, R. Shah, and M. Ismail edited the manuscript. E. Alencherry and R. Beck provided the images. SM Cohen revised the manuscript for intellectual content.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Sagarika Satyavada, Email: Sagarika.Satyavada@UHhospitals.org.
Raj Shah, Email: Raj.shah@uhhospitals.org.
Mayada Ismail, Email: Mayada.Ismail@UHhospitals.org.
Erin Alencherry, Email: Erin.Alencherry@UHhospitals.org.
Rose Beck, Email: Rose.Beck@UHhospitals.org.
Stanley Martin Cohen, Email: Stanley.Cohen@UHhospitals.org.
REFERENCES
- 1.Baron PW, Heneghan MA, Suhocki PV, et al. Biliary stricture secondary to donor B-Cell lymphoma after orthopic liver transplantation. Liver Transplant. 2001;7(1):62–7. [DOI] [PubMed] [Google Scholar]
- 2.Morard I, Mentha G, Rubbia L, et al. Post transplantation lymphoproliferative disorder (PTLD) presenting as biliary duct obstruction. Open Transplant J. 2008;2(1):62–5. [Google Scholar]
- 3.St. Peter S, Rodriquez-Davalos MI, Rodriguez-Luna HM, Harrison EM, Moss AA, Mulligan DC. Significance of proximal biliary dilatation in patients with anastomotic strictures after liver transplantation. Dig Dis Sci. 2004;49(7–8):1207–11. [DOI] [PubMed] [Google Scholar]
- 4.Villa NA, Harrison ME. Management of biliary strictures after liver transplantation. Gastroenterol Hepatol (N Y). 2015;11(5):316–28. [PMC free article] [PubMed] [Google Scholar]
- 5.Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378(6):549–62. [DOI] [PubMed] [Google Scholar]
- 6.Kamdar KY, Rooney CM, Heslop HE. Posttransplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant. 2011;16(3):274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swerdlow SH. (ed.). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]


