Abstract
Currently, the impact of chemotherapy (CT) on survival outcomes in elderly patients with nasopharyngeal carcinoma (NPC) receiving radiation therapy (RT) remains controversial. This retrospective study aims to investigate survival outcomes in a cohort of elderly NPC patients receiving RT alone or together with CT.
Clinical data on 529 NPC patients aged 65 years and older extracted from the Surveillance, Epidemiology, and End Results registry (2004–2015) was collected and retrospectively reviewed. In this cohort, 74 patients were treated with RT alone and 455 individuals received RT and CT. We used propensity score matching with a 1:3 ratio to identify correlations between patients based on 6 different variables. Kaplan–Meier analysis was used to evaluate overall (OS) and cancer-specific survival (CSS). The differences in OS and CSS between the 2 treatment groups were compared using the Log-rank test and Cox proportional hazards models.
The estimated 5-year OS and CSS rates for all patients were 49.5% and 59.3%, respectively. The combination of RT and CT provided longer OS than RT alone (53.7% vs 36.9%, P = .002), while no significant difference was observed in CSS (61.8% vs 51.7%, P = .074) between the 2 groups. Moreover, multivariate analysis demonstrated that the combination of CT and RT correlated favorably with OS and CSS. Subgroup analyses showed that the combination of RT and CT correlated better with both OS and CSS in patients with stage T3 or N2 or stage III.
Among NPC patients aged 65 years and older, treatment with RT and CT provided longer OS than RT alone. Furthermore, the combination of RT and CT showed a better correlation with OS and CSS in NPC patients with stage T3 or N2 or stage III.
Keywords: chemotherapy, nasopharyngeal carcinoma, radiation therapy, SEER, survival
1. Introduction
Nasopharyngeal carcinoma (NPC) is endemic in southeast Asia, northern Africa, and middle Europe, where the incidence is 15 to 50 cases per 100,000 people annually.[1] The latest data reported by the Global database of cancer epidemiology[2] indicated that 129,079 patients were newly diagnosed with NPC around the world in 2018. Of these patients, the rate of Chinese patients accounted for 47.7%. The proportion of NPC varies depending on age, ethnicity, and geographical origin in the endemic area[1] and non-endemic area.[3,4] NPC risk by age reaches the first peak at the ages 15 to 24 years followed by a second peak at ages 65 to 79 years.[5] In addition, due to life expectancy and the increasingly older population, the number of elderly patients with NPC is expected to rise in the future. However, the standard therapy for elderly patients was not recommended, since patients were either excluded from clinical trials or already recruited.[6–8] Hence, the role of chemotherapy (CT) on survival outcomes of elderly NPC patients remains unclear.
The first line of treatment for locoregionally advanced NPC recommended by the latest National Comprehensive Cancer Network guideline on 2020[9] is concurrent chemo-radiotherapy (CCRT) with induction chemotherapy (IC) or adjuvant chemotherapy (AC). Although these treatment regimens are recommended for newly diagnosed elderly NPC patients, there is insufficient evidence on the effectiveness of these strategies in elderly NPC patients. In 2 previous retrospective studies, conventional radiation therapy (RT) combined with CT correlated well with favorable survival and manageable complications in elderly NPC patients with good performance status.[10,11] However, less than 40% of patients included in these studies were treated with RT and CT. Furthermore, an investigational study conducted by Verma et al indicated that CCRT, compared to RT alone, provided better survival outcomes in NPC patients aged ≥70 years.[12] In contrast, another study performed by Mi et al showed that compared with intensity-modulate radiotherapy (IMRT) alone, combining CT with IMRT provided a similar survival and higher grade 3 toxicities.[13] Given this evidence, most oncologists usually recommend RT alone for treating elderly NPC patients.[14,15] It is unclear whether CT should be added to the treatment of elderly NPC patients. Thus, the present study aimed to assess survival outcomes of RT combined with CT compared to RT alone in elderly NPC patients (aged ≥65 years).
2. Materials and methods
2.1. Database and patient selection
All patients with histology-proven NPC were selected from the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute in the United States. The SEER 18 database[16] was obtained from the SEER program with SEER∗Stat software, version 8.3.6 (www.seer.cancer.gov/seerstat). We selected patients using the following criteria: age at diagnosis greater than 65 years old and NPC as the first malignancy diagnosed from 2004 to 2015. The American Joint Committee on Cancer 6th or 7th edition staging was used to define the disease stage of the patients. Individuals with stage UNK or the presence of distant metastases (M1) were excluded. The therapeutic scheme for NPC patients aged ≥65 years was either RT or RT combined with CT. The study was approved by the Medical Ethics Committee and the institutional review board of Zhejiang Cancer Hospital. But informed consent can not be obtained from these patients because the information about all patients was publicly available.
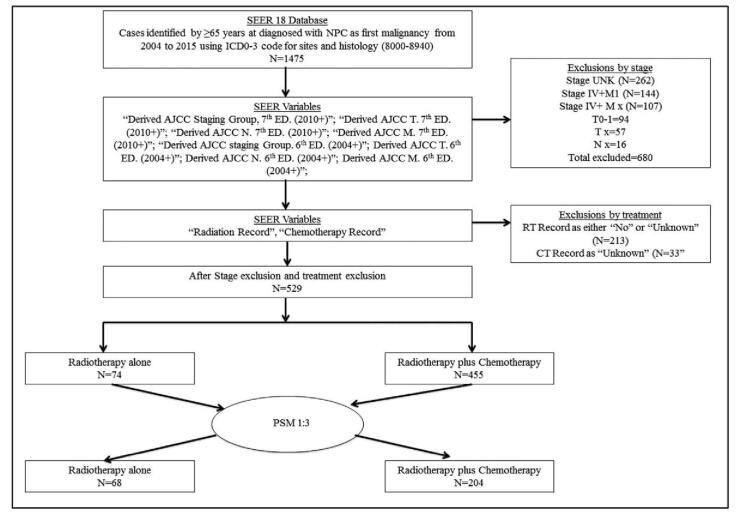
The flowchart of protocol for patient selection and analysis is shown in Figure 1. A total of 1475 patients with histology-confirmed NPC were registered on SEER database. A total of 529 NPC patients aged ≥65 years old receiving RT with or without CT were identified. A cohort of 272 NPC patients, identified by propensity score matching (PSM) with the ratio of 1:3, were enrolled in our study. All patients received RT with or without CT. The information collected prospectively included patient demographics, histology, staging, therapeutic strategy, overall survival (OS) and cancer-specific survival (CSS).
Figure 1.
Flowchart of protocol for patient selection and analysis. Selected patients were aged ≥65 years old and diagnosed with NPC from 2004 to 2015. They either received RT combined with CT or RT alone. AJCC = American Joint Committee on Cancer, ICD-03 = International Classification of Diseases for Oncology, 3rd Edition, NPC = nasopharyngeal carcinoma, PSM = propensity score matching, SEER = Surveillance, Epidemiology, and End Results.
2.2. Statistical analyses
The trends in treatment options, 5-year OS, and CSS were examined using SEER∗Stat software. All data were analyzed by IBM SPSS Statistical software, version 25.0. We used vital status and follow-up time from diagnosis date to calculate OS, as well as cancer-specific death classification to compute CSS. The Kaplan–Meier method was used to generate survival curves and log-rank tests were used to compare the survival distribution of patients based on different variables. We used a Cox regression model to conduct multivariate analyses to identify significant prognosticators. Hazard ratios (HRs) and 95% confidence intervals (CIs) for each prognosticator were calculated. If P value <.05, the differences between groups were considered statistically significant.
We adjusted potential biases related to treatment strategy using PSM analysis.[17,18] The propensity scores were calculated for each patient using logistic regression adjusted for age (65–69 year, ≥70 years), gender, histopathologic scoring, race, T stage, N stage, and clinical stage. We used all the above variables to conduct PSM with a 1:3 nearest neighbor matching algorithm at a caliper of 0.02.
3. Results
3.1. Patient characteristics
A total of 272 patients with an average age of 72 years old (range 65–88 years) were selected. In this cohort, the proportion of male patients was 58.8%, patients aged ≥70 years old amounted to 50.7%, and the percentage of individuals with stages III and IV NPC was 81.6%. Sixty-eight patients received RT alone and 204 patients were treated with a combination of RT and CT. Data analysis revealed no statistically significant differences in age, sex, histopathological score, race, T-stage, and clinical-stage between 2 treatment groups. Table 1 summarizes the cohort characteristics of pair-matched patients.
Table 1.
Basic characteristics of pair-matched patients aged ≥65 years old diagnosed with stages II–IVB NPC from 2004 to 2015 selected from the SEER database.
| Characteristics | Total (N = 272) | RT (N = 68) | RT + CT (N = 204) | P |
| Age | ||||
| <70 yrs | 89 | 23 | 66 | .941 |
| ≥70 yrs | 183 | 45 | 138 | |
| Gender | ||||
| Female | 112 | 27 | 85 | .887 |
| Male | 160 | 41 | 119 | |
| WHO history type | ||||
| I | 50 | 10 | 40 | .636 |
| II | 34 | 10 | 24 | |
| III | 188 | 48 | 140 | |
| T stage∗ | ||||
| T1 | 83 | 17 | 66 | .359 |
| T2 | 79 | 24 | 55 | |
| T3 | 78 | 17 | 61 | |
| T4 | 32 | 10 | 22 | |
| N stage∗ | ||||
| N0 | 85 | 29 | 56 | .021 |
| N1 | 123 | 21 | 102 | |
| N2 | 46 | 11 | 35 | |
| N3 | 18 | 7 | 11 | |
| Clinical stage∗ | ||||
| II | 117 | 24 | 93 | .179 |
| III | 105 | 27 | 78 | |
| IV | 50 | 17 | 33 |
AJCC = American Joint Committee on Cancer, CT = chemotherapy, NPC = nasopharyngeal carcinomaRT = radiotherapy, SEER = the Surveillance, Epidemiology, and End Results.
The 6th/7th AJCC/Union for International Cancer Control staging system.
3.2. Survival analyses
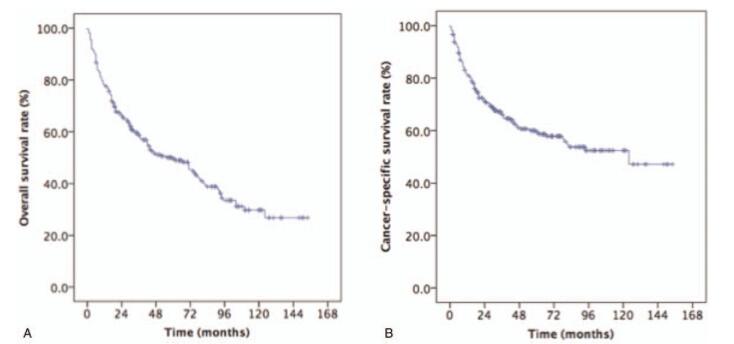
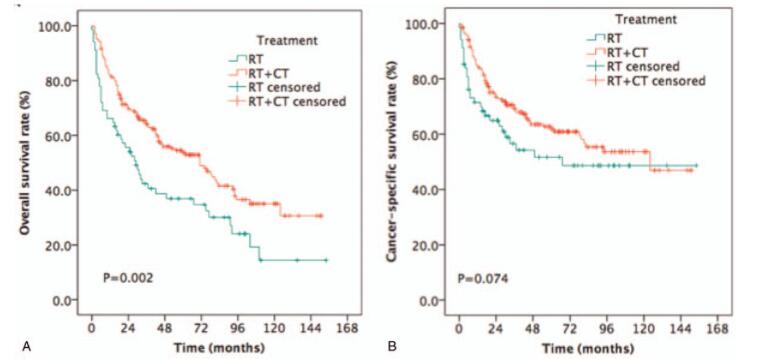
The median survival time was 34 months (range: 3–154 months). The estimated OS and CSS rates at 5 years were 49.5% and 59.3%, respectively (Fig. 2). RT/CT provided longer 5-year OS rates compared to RT alone (53.7% vs 36.9%, P = .002, Fig. 3A) for NPC patients aged ≥65 years old. Although RT/CT treatment also increased the estimated 5-year CSS rate, the difference between the 2 groups was not statistically significant (61.8% vs 51.7%, P = .074, Fig. 3B).
Figure 2.
Kaplan–Meier estimates of survival in newly diagnosed NPC patients aged ≥65 years old. (A) OS and (B) CSS. CSS = cancer-specific survival, NPC = nasopharyngeal carcinoma, OS = overall survival.
Figure 3.
Kaplan–Meier estimates of survival in newly diagnosed NPC patients aged ≥ 65 years old treated with RT or RT combined with CT. (A) OS and (B) CSS. CSS = cancer-specific survival, CT = chemotherapy, NPC = nasopharyngeal carcinoma, OS = overall survival, RT = radiotherapy.
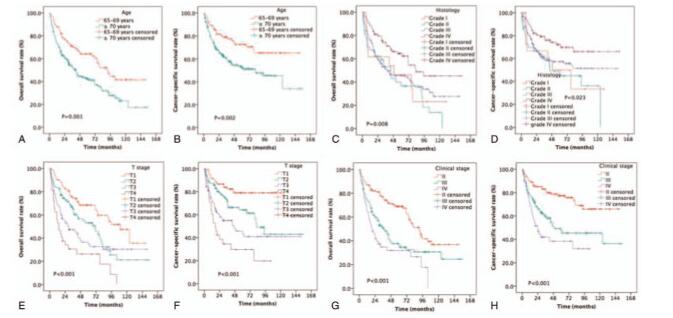
The estimated OS rates at 5 years were 64.2% and 42.1% for patients aged 65–70 years and ≥70 years old, respectively (P = .001, Fig. 4A). The estimated 5-year CSS rates were 72.7% and 52.4% for patients aged 65–69 years and ≥70 years old, respectively (P = .002, Fig. 4B). The estimated OS rates at 5 years were 46.2%, 36.5%, 45.5%, and 64.4% for NPC patients with grades I, II, III, and IV, respectively (P = .008, Fig. 4C). The estimated 5-year CSS rates were 50.0%, 45.0%, 57.3%, and 72.3% for patients with grades I, II, III, and IV, respectively (P = .023, Fig. 4D). The estimated 5-year OS rates were 68.4%, 53.1%, 34.6%, and 26.3% for patients with stages T1, T2, T3, and T4, respectively (P < .001, Fig. 4E). The estimated CSS rates at 5 years were 78.9%, 64.1%, 45.8%, and 29.7% for patients with stages T1, T2, T3, and T4, respectively (P < .001, Fig. 4F). The estimated OS rates at 5 years were 68.8%, 36.0%, and 31.8% for patients with stages II, III, and IV, respectively (P < .001, Fig. 4G). The estimated 5-year CSS rates were 77.4%, 49.3%, and 38.4% for patients with stages II, III, and IV, respectively (P < .001, Fig. 4H).
Figure 4.
Kaplan–Meier estimates of the survival in newly diagnosed NPC patients aged ≥65 years based on different variables. (A) OS and age; (B) CSS and age; (C) OS and histopathological score; (D) CSS and histopathological score; (E) OS and T stage; (F) CSS and T stage; (G) OS and clinical stage; and (H) CSS and clinical stage. CSS = cancer-specific survival, NPC = nasopharyngeal carcinoma, OS = overall survival.
3.3. Identification of prognosticators
Several potential prognosticators such as age, gender, histopathological type, race, T stage, N stage, clinical stage, and therapeutic regimen were examined using univariable Cox regression. Our results showed that age, histopathological score, T stage, and clinical stage were significant prognosticators for OS and CSS, while therapeutic strategy was only associated with OS (Table 2).
Table 2.
Univariable and multivariable analysis of both OS and CSS in selected newly diagnosed NPC patients with ≥65 years from the 18 SEER database.
| Univariate analysis | Multivariate analysis | |||||||
| 5-year OS | 5-year CSS | 5-year OS | 5-year CSS | |||||
| Factors | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Age | ||||||||
| 65–69 yrs | 1 | 1 | 1 | 1 | ||||
| ≥70 yrs | 0.548 (0.382–0.788) | .001 | 1.989 (1.268–3.119) | .003 | 0.523 (0.357–0.767) | .001 | 0.479 (0.298–0.772) | .002 |
| Sex | ||||||||
| Female | 1 | 1 | 1 | 1 | ||||
| Male | 1.099 (0.793–1.524) | .569 | 1.159 (0.782–1.719) | .462 | 0.796 (0.562–1.127) | .199 | 0.740 (0.485–1.131) | .164 |
| Histology | ||||||||
| Type I | 1 | 1 | 1 | 1 | ||||
| Type II | 0.721 (0.461–1.128) | .152 | 0.818 (0.490–1.365) | .442 | 0.841 (0.519–1.360) | .479 | 0.944 (0.539–1.655) | .842 |
| Type III | 0,583 (0.334–1.015) | .056 | 0.536 (0.269–1.068) | .076 | 0.534 (0.295–0.965) | .038 | 0.475 (0.229–0.984) | .045 |
| Race | ||||||||
| NHW | 1 | 1 | 1 | 1 | ||||
| NHB | 1.082 (0.591–1.979) | .798 | 1.224 (0.559–2.683) | .613 | 0.911 (0.488–1.701) | .769 | 1.045 (0.464–2.352) | .915 |
| NHAIAN | 1.163 (0.538–2.515) | .702 | 1.541 (0.597–3.926) | .372 | 0.884 (0.401–1.950) | .761 | 1.083 (0.410–2.860) | .872 |
| NHAPI | 0.57 (0.161–2.02) | .384 | 0.996 (0.257–3.853) | .995 | 0.550 (0.145–2.083) | .379 | 0.928 (0.219–3.927) | .919 |
| Hispanic | 0.882 (0.460–1.693) | .706 | 1.083 (0.471–2.487) | .852 | 0.773 (0.389–1.533) | .461 | 0.916 (0.399–2.280) | .954 |
| T stage∗ | ||||||||
| T1 | 1 | 1 | 1 | 1 | ||||
| T2 | 0.25 (0.149–0.42) | <.001 | 0.172 (0.09–0.328) | <.001 | 0.186 (0.103–0.339) | <.001 | 0.134 (0.065–0.277) | <.001 |
| T3 | 0.431 (0.265–0.702) | .001 | 0.367 (0.209–0.646) | .001 | 0.342 (0.203–0.577) | <.001 | 0.296 (0.161–0.542) | <.001 |
| T4 | 0.517 (0.358–0.931) | .024 | 0.595 (0.35–1.014) | .056 | 0.524 (0.314–0.874) | .013 | 0.532 (0.302–0.940) | .030 |
| N stage∗ | ||||||||
| N0 | 1 | 1 | 1 | 1 | ||||
| N1 | 0.935 (0.487–1.796) | .841 | 0.921 (0.445–1.905) | .825 | 0.527 (0.259–1.072) | .077 | 0.505 (0.229–1.114) | .091 |
| N2 | 0.667 (0.351–1.269) | .218 | 0.574 (0.279–1.184) | .133 | 0.750 (0.387–1.457) | .396 | 0.648 (0.306–1.374) | .258 |
| N3 | 0.866 (0.429–0.931) | .687 | 0.681 (0.303–1.528) | .351 | 0.795 (0.384–1.645) | .536 | 0.637 (0.274–1.478) | .294 |
| Clinical stage∗ | ||||||||
| II | 1 | 1 | ||||||
| III | 0.368 (0.239–0.566) | <.001 | 0.281 (0.167–0.472) | <.001 | ||||
| IV | 0.695 (0.461–1.046) | .081 | 0.664 (0.418–1.054) | .083 | ||||
| Therapy strategy | ||||||||
| RT | 1 | 1 | 1 | 1 | ||||
| RT + CT | 0.586 (0.416–0.825) | .002 | 0.683 (0.447–1.042) | .077 | 1.886 (1.312–2.712) | .001 | 1.624 (1.039–2.538) | .033 |
AJCC = American Joint Committee on Cancer, CI = confidence interval, CSS = cancer-specific survival, CT = chemotherapy, HR = hazards ratio, NHAIA = non-Hispanic American Indian/Alaska Native, NHAPI = non-Hispanic Asia or Pacific Islander, NHB = non-Hispanic Black, NHW = non-Hispanic White, NPC = nasopharyngeal carcinoma, OS = overall survival, RT = radiotherapy, SEER = the Surveillance, Epidemiology, and End Results.
The 6th/7th AJCC/Union for International Cancer Control staging system.
Moreover, multivariable Cox regression demonstrated that treatment with RT/CT was associated with longer OS (HR = 1.886, 95% CI: 1.312–2.712, P = .001) and CSS (HR = 1.624, 95% CI: 1.039–2.538, P = .033) compared to treatment with RT alone. Furthermore, the ≥70 years age group was associated with poorer OS and CSS compared to patients aged 65–69 years old (OS HR = 0.523, 95% CI: 0.357–0.767, P = .001; CSS HR = 0.479, 95% CI: 0.298–0.772, P = .002). Advanced T stage and World Health Organization (WHO) histology type III were also associated with poorer OS and CSS.
3.4. Subgroup analysis
To identify which newly diagnosed NPC patients aged ≥65 years old would benefit from the combination of RT and CT, we performed subgroup analysis. RT combined with CT provided longer OS (P = .041) and CSS (P = .048) in NPC patients with stage T3 (Table 3). Patients with stage N2 or a stage III also showed a significantly longer OS and CSS when treated with RT combined with CT. Interestingly, the male or ≥70 years or WHO histology type III patients had a more favorable OS (male P = .015; ≥ 70 years P = .007; type III P = .001) when receiving RT/CT than RT alone.
Table 3.
Subgroup analysis of both OS and CSS in newly diagnosed NPC patients aged ≥65 years selected from the 18 SEER database treated with RT or RT/CT.
| CSS | OS | |||||
| Characteristics | RT | RT + CT | P | RT | RT + CT | P |
| Age | ||||||
| 65–69 yrs | 66.1 | 74.8 | .386 | 50.5 | 69.4 | .051 |
| ≥70 yrs | 42.3 | 55.2 | .071 | 29.2 | 46.0 | .007 |
| Gender | ||||||
| Female | 53.2 | 68.5 | .066 | 46.7 | 58.1 | .056 |
| Male | 49.8 | 57.2 | .407 | 30.7 | 50.6 | .015 |
| Histology | ||||||
| Type I | 60.0 | 64.2 | .353 | 40.0 | 58.8 | .164 |
| Type II | 64.0 | 73.5 | .711 | 56.0 | 61.0 | .935 |
| Type III | 47.2 | 58.9 | .092 | 32.3 | 51.0 | .001 |
| T stage∗ | ||||||
| T1 | 87.8 | 77.4 | .566 | 52.3 | 72.6 | .004 |
| T2 | 54.0 | 68.2 | .398 | 44.5 | 57.0 | .541 |
| T3 | 29.4 | 47.4 | .041 | 20.6 | 38.8 | .048 |
| T4 | 22.9 | 31.6 | .358 | 20 | 28.3 | .512 |
| N stage∗ | ||||||
| N0 | 47.5 | 53.8 | .342 | 40.1 | 47.5 | .271 |
| N1 | 70.5 | 67.1 | .771 | 50.1 | 60.1 | .057 |
| N2 | 15.2 | 64.4 | .015 | 9.1 | 48.2 | .006 |
| N3 | 64.3 | 36.4 | .172 | 26.8 | 36.4 | .579 |
| Clinical stage∗ | ||||||
| II | 87.5 | 75.3 | .452 | 69.9 | 68.5 | .344 |
| III | 26.4 | 57.0 | .007 | 16.2 | 43.5 | .003 |
| IV | 42.0 | 36.9 | .972 | 25.7 | 34.5 | 0727 |
AJCC = American Joint Committee on Cancer, CSS = cancer-specific survival, CT = chemotherapy, NPC = nasopharyngeal carcinoma, OS = overall survival, RT = radiotherapy, SEER = Surveillance, Epidemiology, and End Results.
The 6th or 7th AJCC/Union for International Cancer Control staging system.
4. Discussion
In this study, we selected newly diagnosed NPC patients aged 65 years and above from the 18 SEER database and evaluated survival outcomes depending on their treatment regimen: RT and CT combined, or RT alone. Our findings indicated that the combination of RT and CT was associated with a significantly longer OS when compared to RT alone.
Univariate and multivariate analysis revealed that RT combined with CT was associated with a significant improvement in OS and CSS compared to RT alone. Moreover, the older age group (≥70 years) was associated with poorer OS and CSS than younger patients (65–69 years old). Subgroup analysis showed that RT combined with CT provided longer OS and CSS in patients with stage T3, stage N2, or stage III than RT alone. Interestingly, male or ≥70 years or grade III patients also showed a more favorable OS when treated with RT combined with CT than RT alone.
Several meta-analyses of randomized studies indicated that treatment with RT and CT improves 5-year survival by 4% to 6% and reduces the risk of mortality by 18%.[19] CCRT with or without AC has shown better OS than other treatments and has become the first therapy for locoregionally advanced NPC, despite the occurrence of acute adverse events.[20–22] Moreover, 2 previous meta-analyses revealed that adding IC before CCRT, compared with CCRT alone, decreases distant failure in locoregionally advanced NPC patients.[23,24] Wang et al performed another meta-analysis and showed that IC prior to CCRT significantly increased OS and progression-free survival (PFS).[25] To date, CCRT combined with IC or AC has been recommended as the standard therapy for locoregionally advanced NPC. However, it is unclear whether this strategy should also be used in elderly NPC patients.
Because of the increase in life expectancy and demographic changes in age, an increasing number of elderly patients have been diagnosed with NPC.[20,26] The older NPC patients, in contrast to younger individuals, have a significantly higher risk of death and cancer progression.[27] Therefore, it is very important for elderly NPC patients to receive the appropriate therapeutic regimen. In the era of conventional RT, the combination of RT and CT has been shown to provide survival advantages for elderly NPC patients.[28–31] Moreover, NPC patients aged >65 years old have often received insufficient cycles and doses of CT due to their poor performance status.[10] Verma et al indicated that CCRT provided more favorable survival outcomes in NPC patients aged ≥70 years old compared with RT alone,[12] while Mi et al showed that IMRT alone provided less grade 3 side events and comparable 3-year of OS (72.1% vs 72.5%, P = .735), PFS (65.9% vs 70.1%, P = .735), distant metastasis-free survival (76.4% vs 71.6%, P = .735), and loco-regional recurrence-free survival (90.8% vs 98.0%, P = .735) compared to IMRT combined with CT.[13]
Furthermore, factors including poor performance status, comorbidities, reduced organ function, and decreased social support affected the tolerance of CCRT in older patients. A higher number of grade 3 and 4 CT-related side events was correlated with cancer patients with comorbidities.[32] Goto et al indicated that factors such as comorbidities, stage, and administration, were associated with poor prognosis in the older population.[33] Several retrospective studies reported that therapy-related complications were not increased in the elderly patients,[34,35] while other studies reported that the proportion of adverse events improved with increasing age.[36,37] Furthermore, older patients experienced comorbidities and metabolic changes,[27] as well as a greater number of treatment-related complications.[32,38] Vercelli et al revealed that improving complications were closely related to poor prognosis in older patients.[36] To illustrate the role of performance status on survival, Müller von der Grün et al showed that the patients with ECOG performance status of 2 to 3 had a poor OS and PFS.[39] All these factors should be taken into consideration when assigning therapeutic regimens to elderly NPC patients.
To identify which newly diagnosed elderly NPC patients would benefit from the combination of RT and CT, we performed subgroup analysis. Treatment with RT and CT provided longer OS and CSS in patients with stage T3, stage N2 or histological grade III. Interestingly, male or grade III patients had a more favorable OS when receiving RT and CT than RT alone. According to these results, the appropriate therapeutic strategy was used for each group of elderly NPC patients.
Although SEER database provided publicly available data to investigate this clinical problem, several limitations were observed in this study. Firstly, treatment information regarding CT regimen, CT sequence, delays in CT, and CT-related toxicity was not registered in the database and therefore, a comprehensive assessment of the role of CT was not conducted. Secondly, RT dosing, RT planning, target volumes, RT-related toxicities were not evaluated in this study due to a lack of information about RT, and RT-related adverse events. Furthermore, SEER records did not include information about locoregional relapse, distant metastasis, so we were not able to assess loco-regional recurrence-free survival and distant metastasis-free survival.
Although there are some limitations in the present study, we have demonstrated that the combination of RT and CT offers some OS and CSS advantages in elderly NPC patients (aged ≥65 years) over RT alone. Future directions should include recording information on CT regimen and target treatment given to elderly NPC patients and longitudinal monitoring of the combined treatment effect.
5. Conclusion
The present study showed that the combination of RT and CT provided longer OS and CSS in newly diagnosed NPC patients (aged ≥65 years) selected from the 18 SEER database compared to RT alone. Moreover, the patients with stage T3 or N2 or stage III may also benefit from RT/CT combination treatment.
Author contributions
Acquisition of data: Yan Lu, Chuner Jiang, Fengqin Yan, Zhimin Ye, Yongfeng Piao.
Conception and design: Fangzheng Wang, Jianfeng Hua, Haitao Jiang, Zhenfu Fu, Yangming Jiang.
Conceptualization: Zhenfu Fu, Jianfeng Hua.
Data analysis and interpretation: Fangzheng Wang, Yangming Jiang.
Data curation: Chuner Jiang, Fengqin Yan, Yongfeng Piao, Zhimin Ye, Haitao Jiang, Yan Lu.
Drafting the article and revising it critically for important intellectual content: Yan Lun, Fangzheng Wang, Zhenfu Fu, Yangming Jiang.
Final approval of manuscript: All authors
Formal analysis: Yangming Jiang.
Methodology: Zhenfu Fu.
Writing – original draft: Fangzheng Wang, Yan Lu.
Footnotes
Abbreviations: AC = adjuvant chemotherapy, AJCC = American Joint Committee on Cancer, CCRT = concurrent chemoradiotherapy, CI = confidence interval, CSS = cancer-specific survival, CT = chemotherapy, HR = hazards ratio, IBM SPSS = International Business Machines Corporation statistical product and service solutions, IC = induction chemotherapy, IMRT = intensity-modulated radiotherapy, NHAIA = non-Hispanic American Indian/Alaska Native, NHAPI = non-Hispanic Asia or Pacific Islander, NHB = non-Hispanic Black, NHW = non-Hispanic White, NPC = nasopharyngeal carcinoma, OS = overall survival, PFS = progression-free survival, PSM = propensity score matching, RT = radiotherapy, SEER = the Surveillance, Epidemiology, and End Results.
How to cite this article: Lu Y, Hua J, Yan F, Jiang C, Piao Y, Ye Z, Fu Z, Jiang H, Wang F, Jiang Y. Combined radiotherapy and chemotherapy versus radiotherapy alone in elderly patients with nasopharyngeal carcinoma: a SEER population-based study. Medicine. 2021;100:29(e26629).
YL and CJ contributed equally to this work.
This study was supported by grants from the Medical and Health Science and Technology Program of Zhejiang Province (Nos. 2020KY084, 2019KY041, 2013KYB033, 2009B026, 2006A016, 2005B012, and 2004B014), National Natural Science Foundation of China (No. 81502647), Key Laboratory of Head and Neck Cancer Translational Research of Zhejiang Province (klab 2020).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Tang LL, Chen WQ, Xue WQ, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett 2016;374:22–30. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [3].Geara FB, Nasr E, Tucker SL, et al. Nasopharyngeal cancer in the Middle East: experience of the American University of Beirut Medical Center. Int J Radiat Oncol Biol Phys 2005;61:1408–15. [DOI] [PubMed] [Google Scholar]
- [4].Setton J, Han J, Kannarunimit D, et al. Long-term patterns of relapse and survival following definitive intensity-modulated radiotherapy for non-endemic nasopharyngeal carcinoma. Oral Oncol 2016;53:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bray F, Haugen M, Moger TA, Tretli S, Aalen OO, Grotmol T. Age-incidence curves of nasopharyngeal carcinoma worldwide: bimodality in low-risk populations and aetiologic implications. Cancer Epidemiol Biomarkers Prev 2008;17:2356–65. [DOI] [PubMed] [Google Scholar]
- [6].Wang C, Tang X, Wang J, Song J, Xu Y. Induction chemotherapy plus concurrent chemoradiotherapy vs concurrent chemoradiotherapy in elderly patients with advanced nasopharyngeal carcinoma. Otolaryngol Head Neck Surg 2017;157:233–8. [DOI] [PubMed] [Google Scholar]
- [7].Cao C, Hu Q, Chen X. Intensity-modulated radiotherapy for elderly patients with nasopharyngeal carcinoma. Head Neck 2018;40:590–5. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Y, Yi JL, Huang XD, et al. Inherently poor survival of elderly patients with nasopharyngeal carcinoma. Head Neck 2015;37:771–6. [DOI] [PubMed] [Google Scholar]
- [9]. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines Head and Neck Cancers. Version 1. 2020. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx#head-and-neck. Accessed February 12, 2020. [Google Scholar]
- [10].Zeng Q, Xiang YQ, Wu PH, Lv X, Qian CN, Guo X. A matched cohort study of standard chemo-radiotherapy versus radiotherapy alone in elderly nasopharyngeal carcinoma patients. PLoS One 2015;10:e0119593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu H, Chen QY, Guo L, et al. Feasibility and efficacy of chemoradiotherapy for elderly patients with loco-regionally advanced nasopharyngeal carcinoma: results from a matched cohort analysis. Radiat Oncol 2013;8:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Verma V, Surkar SM, Moreno AC, Lin C, Simone CB. Practice patterns and outcomes of chemoradiotherapy versus radiotherapy alone for older patients with nasopharyngeal carcinoma. Cancer Med 2018;7:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mi JL, Meng YL, Wu HL, et al. Comparison of intensity-modulated radiation therapy alone vs. intensity-modulated radiation therapy combine with chemotherapy in elderly nasopharyngeal carcinoma patients (aged >65 years). Strahlenther Oncol 2020;196:270–9. [DOI] [PubMed] [Google Scholar]
- [14].Sze HC, Ng WT, Chan OS, Shum TC, Chan LL, Lee AW. Radical radiotherapy for nasopharyngeal carcinoma in elderly patients: the importance of comorbidity assessment. Oral Oncol 2012;48:162–7. [DOI] [PubMed] [Google Scholar]
- [15].Guo QJ, Jiang WP, Lin SJ, et al. Radiation therapy for locoregionally advanced nasopharyngeal carcinoma in elderly patients. J Radiat Oncol 2012;13:323–32. [Google Scholar]
- [16]. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER∗Stat Database: Incidence—SEER 18 Regs Custom Data (with additional treatment fields), Nov 2016 Sub (2004-2015 varying)—Linked to County Attributes—Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. Available at: https://seer.cancer.gov/data. Accessed July 22, 2018. [Google Scholar]
- [17].Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making 2009;29:661–77. [DOI] [PubMed] [Google Scholar]
- [18].Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013;32:2837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Al-Sarraf M, Reddy MS. Nasopharyngeal carcinoma. Curr Treat Options Oncol 2002;3:21–32. [DOI] [PubMed] [Google Scholar]
- [20].Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310–7. [DOI] [PubMed] [Google Scholar]
- [21].Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2010;102:1188–98. [DOI] [PubMed] [Google Scholar]
- [22].Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 2006;64:47–56. [DOI] [PubMed] [Google Scholar]
- [23].OuYang PY, Xie C, Mao YP, et al. Significant efficacies of neoadjuvant chemotherapy and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, control trials. Ann Oncol 2013;24:2136–46. [DOI] [PubMed] [Google Scholar]
- [24].Chen YP, Guo R, Liu N, et al. Efficacy of the additional neoadjuvant chemotherapy to concurrent chemoradiotherapy for patients with locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis of randomized controlled trials. J Cancer 2015;6:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang MM, Tian HM, Li G, et al. Significant benefits of adding neoadjuvant chemotherapy before chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Oncotarget 2016;7:48375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leu YS, Chang YF, Lee JC, Lo AC, Chen YJ, Chen HW. Prognosis of nasopharyngeal carcinoma in the elderly is worse than in younger individuals—experience of a medical institute. Int J Gerontol 2004;8:81–4. [Google Scholar]
- [27].Yancik R, Havlik RJ, Wesley MN, et al. Cancer and comrbidity in older patients: a descriptive profile. Ann Epidemiol 1996;6:399–412. [DOI] [PubMed] [Google Scholar]
- [28].Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol 2002;20:2038–44. [DOI] [PubMed] [Google Scholar]
- [29].Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. JNCI J Natl Cancer Inst 2005;97:536–9. [DOI] [PubMed] [Google Scholar]
- [30].Zhang L, Zhao C, Peng PJ, et al. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol 2005;23:8461–8. [DOI] [PubMed] [Google Scholar]
- [31].Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 2003;21:631–7. [DOI] [PubMed] [Google Scholar]
- [32].Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol 2011;29:106–17. [DOI] [PubMed] [Google Scholar]
- [33].Goto Y, Kodaira T, Fuwa N, et al. Alternating chemotherapy in patients with nasopharyngeal cancer: prognostic factors and proposal for individualization of therapy. J Radiat Res 2013;54:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu MH, Chen HW, Su WH, Lai YL, Chang KH. Patterns of care and outcome in elderly patients with cervical cancer: a retrospective analysis. Int J Gerontol 2011;5:89–93. [Google Scholar]
- [35].Christman K, Muss Hb, Case LD, Stanley V. Chemotherapy of metastatic breast cancer in the elderly: the Piedmont Oncology Association experience. JAMA 1992;268:57–62. [PubMed] [Google Scholar]
- [36].Vercelli M, Capocaccia R, Quaglia A, Casella C, Puppo A, Coebergh JW. Relative survival in elderly European cancer patients: evidence for health care inequalities. The EUROCARE Working Group. Crit Rev Oncol Hematol 2000;35:161–79. [DOI] [PubMed] [Google Scholar]
- [37].Liu HC, Chen YC, Chen CH, Chen YJ. Esophagectomy in elderly patients with esophageal cancer. Int J Gerontol 2010;4:176–9. [Google Scholar]
- [38].Argiris A, Li Y, Murphy BA, Langer CJ, Forastiere AA. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol 2004;22:262–8. [DOI] [PubMed] [Google Scholar]
- [39].Müller von der Grün J, Martin D, Stöver T, Ghanaati S, Rödel C, Balermpas P. Chemoradiotherapy as definitive treatment for elderly patients with head and neck cancer. Biomed Res Int 2018;2018:3508795–13508795. [DOI] [PMC free article] [PubMed] [Google Scholar]