Abstract
Cutaneous metastasis (CM) occurs infrequently and usually presents during the later stages of cancer, and has a poor prognosis. Although there are insufficient current data, cancer treatment changes could have a positive impact on the outcome. This retrospective study aimed to review the pattern and prognosis of CM in patients with solid malignancy in a tertiary cancer center in Thailand.
We reviewed the medical records of cancer patients diagnosed with CM between October 2009 and August 2015 at Chulabhorn Hospital, a tertiary cancer center in Thailand. Patients with primary skin cancer and hematological malignancies were excluded. We collected and analyzed data, including the time of cancer diagnosis and CM, type of cancer, clinical characteristics, and survival outcome.
Of 11,418 patients, there were 33 (0.3%) were diagnosed with CM. Breast cancer was the most common primary cancer (12 cases, 36%). Skin nodules were commonly detected on the anterior chest wall. Also, 79% of CM patients had concomitant visceral metastasis. The median overall survival of those with CM was 9.21 months (95% confidence interval 4.75–83.38 months) regardless of presentation either at onset or disease recurrence (P = .083). However, the change of management was affected in 78% diagnosed with a later stage of CM. No statistical difference in survival was observed between breast cancer and non-breast cancer patients (8.79 vs 9.21 months, P = .613).
Despite CM being a sign of poor prognosis, it may still be an indicator for changing cancer patients’ treatment. Hence, early CM diagnosis and prompt novel therapy may positively affect outcomes for cancer patients.
Keywords: breast cancer, cancer center, cutaneous metastasis, prognosis, solid malignancy
1. Introduction
Cutaneous metastasis (CM) occurs infrequently and usually during the late stages of cancer and has a poor prognosis. The incidence of CM is 0.5% to 9%,[1–6] with different spectra of clinical manifestations, type of primary cancer, and natural history of the disease, and treatment is limited to only a few options. The changes in cancer care in the past few decades could have a positive effect on patient outcomes.[7] Novel treatment strategies, including targeted therapy, have been explored in some specific cancer types, such as breast and lung cancers. However, there are limited data on patients with CM in the current cancer diagnosis and treatment. Thus, this retrospective study aimed to review the pattern and prognosis of CM in patients with solid malignancy in a tertiary cancer center in Thailand, which may differ from the data from other countries.
2. Methods
2.1. Study population
We comprehensively reviewed the medical records of cancer patients diagnosed with CM from solid malignancies between October 2009 and August 2015 in Chulabhorn Hospital, a tertiary care cancer center in Bangkok, Thailand. CM was classified using the International Classification of Disease (ICD)-10, code C79.2 (secondary malignant neoplasm of skin). CM was confirmed by histopathology or clinical and radiographic examination. We excluded patients with CM caused by transplantation, iatrogenic implantation, or direct extension of the primary tumor, and those with primary skin cancer, melanoma, and hematological malignancies. Data collection included a diagnosis of primary cancer, the interval between diagnosis of primary cancer and CM, clinical characteristics of CM, and presence or absence of visceral metastasis. The cut-off for survival was August 2016. All patient personal data were kept confidential in compliance with our institute's data protection guidelines. The study was reviewed and approved by the Human Research and Ethics Committee, Chulabhorn Research Institute (Certificate No. 009/2558).
2.2. Statistical analysis
Categorical data were expressed as frequency (percentage). Continuous data were presented as mean and standard deviation if normally distributed, or median and interquartile range if they showed skewed deviation. Kaplan–Meier estimates were used for survival analysis. All analyses were performed using STATA/SE version 16 software (StataCorp LLC, College Station, Texas, USA). A P value ≤ .05 was considered statistically significant.
3. Results
3.1. Clinical characteristics of cancer patients with CM
Among 11,418 patients with solid malignancies, 33 (0.3%) were diagnosed with CM. There were 16 males and 17 females. The age at the time of CM diagnosis ranged from 34 to 72 years, with a median age of 55. CM was confirmed by histopathology in 16 (48%) cases. The characteristics of patients are summarized in Table 1.
Table 1.
Clinical characteristics of cancer patients with CM.
| Characteristics | Total (n = 33) |
| Male sex | 16 (48) |
| Median age (range), yr | 55 (34–72) |
| Type of primary cancer | |
| Breast | 12 (36) |
| Head and neck | 4 (12) |
| Pancreas | 3 (9) |
| Bile duct | 2 (6) |
| Large intestine | 2 (6) |
| Esophagus | 2 (6) |
| Hepatocellular carcinoma | 2 (6) |
| Lung | 2 (6) |
| Thyroid | 1 (3) |
| Kidney | 1 (3) |
| Ovary | 1 (3) |
| Uterine cervix | 1 (3) |
| Site of CM | |
| Anterior chest wall | 16 (48) |
| Abdominal wall | 9 (27) |
| Neck area | 6 (18) |
| Other | 2 (6) |
| Histological subtype of primary cancer | |
| Adenocarcinoma | 23 (70) |
| Squamous cell carcinoma | 6 (18) |
| Other | 4 (12) |
| CM as first presentation | 10 (30) |
| Presence of visceral organ metastasis | 26 (79) |
Results are presented as no. (%), unless otherwise stated. CM = cutaneous metastasis.
Breast cancer was the most common primary cancer (12 cases), followed by head and neck cancer (four cases) and pancreatic cancer (three cases). The types of primary cancer differed between men and women. Breast cancer was the most common primary cancer among women (12 cases), whereas head and neck cancer was the most common primary cancer among men (four cases).
Skin nodules were the most frequently observed lesion pattern of CM (20 cases, 61%), followed by the mass lesion (10 cases, 30%). However, some patients had more than 1 type of skin lesions, such as ulcerated nodules concomitant with masses or erythematous to purplish patches. The predominant sites included anterior chest wall (16 cases, 48%), abdominal wall (9 cases, 27%) and neck (6 cases, 18%). A specific form of cutaneous metastasis called Sister Mary Joseph nodule was noted in 3 patients with pancreatic cancer (2 cases) and ovarian cancer (1 case). Additionally, the main histological subtypes were adenocarcinoma (Fig. 1A), with account for 70% of CM (23 cases), with squamous cell carcinoma subtype (Fig. 1B) accounting for 18% (6 cases). All cases with skin biopsy had a similar cell type to primary cancer, straightforwardly.
Figure 1.
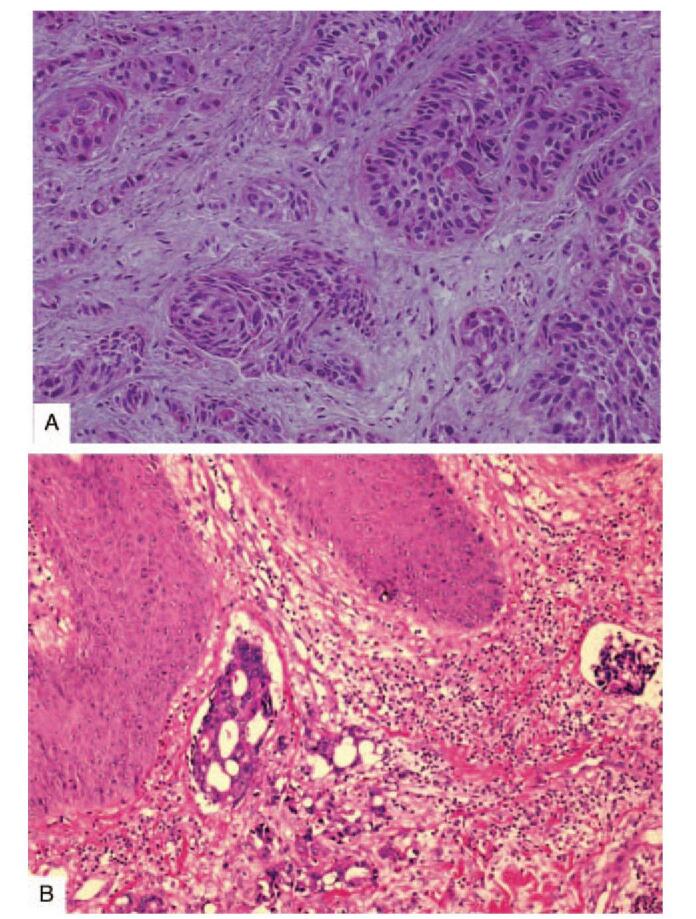
Metastatic carcinoma to skin (A) Squamous cell carcinoma metastasis to the abdominal wall in a patient with esophageal squamous cell carcinoma (hematoxylin and eosin, 40 ×). (B) Adenocarcinoma metastasis to the abdominal wall in a patient with sigmoid colon adenocarcinoma (hematoxylin and eosin, 40 ×).
Ten cases (30%), including breast (five cases), pancreatic (two cases), bile duct (two cases), and hypopharyngeal (one case) cancers, presented with CM as the first clinical manifestation. Meanwhile, the other 23 cases (70%) were observed at the time of cancer recurrence. The median duration between diagnosis of primary malignancy and CM was 9.2 months (range 2.1–69.6 months).
Concomitant visceral metastasis was detected with CM in most patients at diagnosis (26 cases, 79%). Lung, bone, and liver were the most common sites of metastasis (Table 2).
Table 2.
Visceral metastases among 26 cases of cutaneous metastasis.
| No. of cases | |
| Lung | 15 |
| Bone | 12 |
| Liver | 9 |
| Adrenal gland | 5 |
| Peritoneum | 5 |
| Pleura | 4 |
| Brain | 4 |
| Diaphragm | 1 |
| Spleen | 1 |
Some cases had more than one metastatic site
3.2. Treatment received after the diagnosis of CM
The treatments after the diagnosis of CM were determined according to the current guidelines and patients’ status. Among 10 cancer cases with CM as the first presentation, 9 patients received systemic chemotherapy, while 1 patient died before start treatment. The diagnosis of CM affected the change of management in 18 out of 23 patients (78%) with the late stage of the disease. To be described: there were 15 cases with the restart or change of systemic treatment followed by local radiation (6 cases), surgical resection (1 case), as shown in supplemental Table 1.
3.3. Survival outcome in patients with CM
The median overall survival among cancer patients after diagnosis with CM was 9.21 months (95% confidence interval 4.75–83.38 months) (Fig. 2). However, the survival was longer in those with CM as the first clinical manifestation compared to the later stage of CM disease, despite no statistical significance (not reached vs. 7.87 months, P = .083) (Fig. 3).
Figure 2.
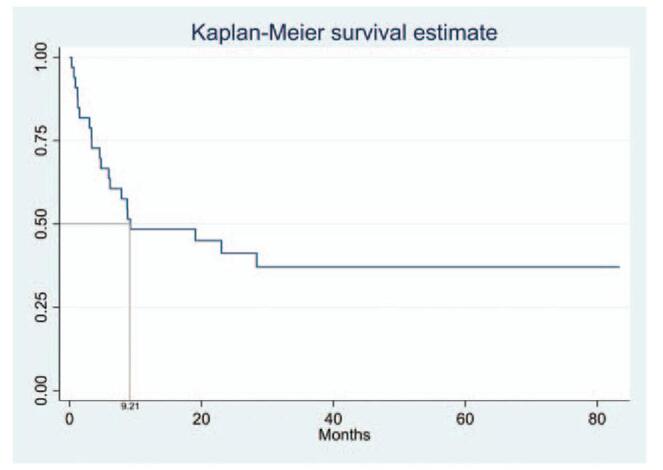
Survival analysis after the development of cutaneous metastasis in all cancer patients, with median overall survival of 9.21 months (95% confidence interval, 4.75–83.38 months).
Figure 3.
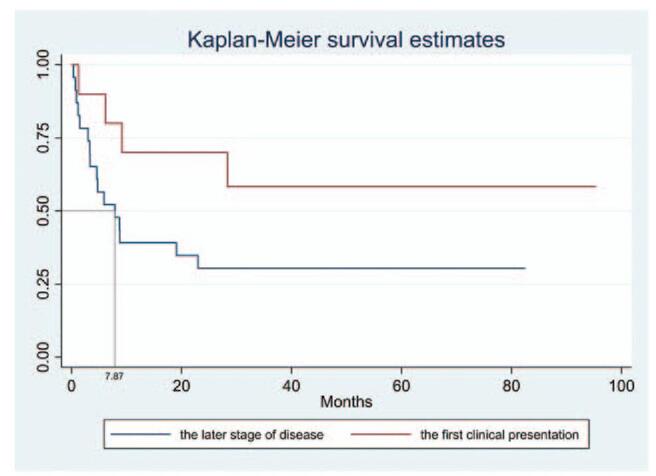
Survival analysis in cancer patients with cutaneous metastasis as the first presentation or late in the disease. Median overall survival did not differ significantly (Not reached vs 7.87 months, P = .083).
3.4. Survival outcome among breast cancer patients
Among 12 breast cancer patients, there were six with human epidermal growth factor receptor (HER) 2-positive disease, three with triple-negative breast cancer, two with estrogen-receptor-positive/HER2-negative breast cancer, and one with unknown estrogen receptor/HER2 status. There was no statistical difference in survival between breast cancer and non-breast cancer patients (8.79 vs 9.21 months, P = .613) (Fig. 4).
Figure 4.
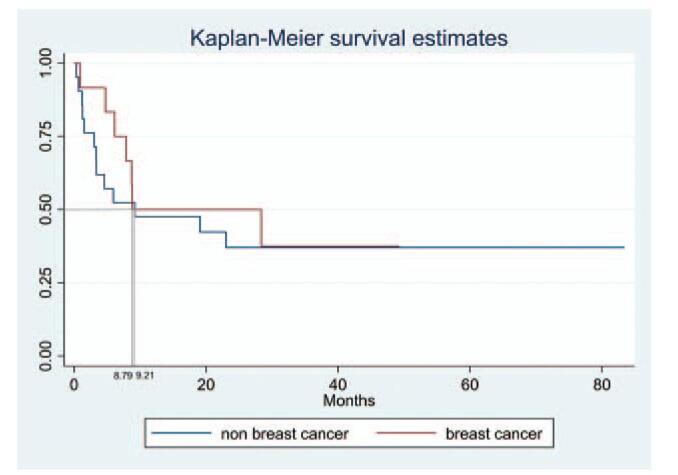
Survival analysis after the development of cutaneous metastasis in breast and non-breast cancer patients. Median overall survival did not differ significantly (8.79 vs 9.21 months, P = .613).
4. Discussion
Our study revealed CM among cancer patients who received treatment between 2009 and 2015 at a tertiary cancer center in Thailand. To our knowledge, our study was the first study on CM in the Thai population, which different compared to data from previous studies in other countries.
The prevalence of CM in our study was low (0.3%), compared with previous reports in Caucasian populations (0.5%–9.0%).[1–6] Nevertheless, this prevalence was similar to that in other Asian countries, including Taiwan (1.02%)[8] and India (0.5%).[9] It may have been affected by differences in frequency of primary tumors, sex, age, and ethnicity.
Breast cancer is the most common primary cancer with CM, while the anterior chest wall is the most frequently found CM site.[7] The rate of CM from breast cancer varied from 18.6% to 26.5% in some studies and was higher than in other cancers.[8,10–15] This can be explained by breast cancer ‘s superficial location and its direct contiguity with the overlying skin.[16] Furthermore, the site of CM tends to occur close to the region of primary cancer.[2] For instance, CM among breast and lung cancer patients usually involves the chest wall.[4] In contrast, malignancy of the gastrointestinal tract is likely to involve the abdominal area. However, the regional distribution of CM may not always be predictable and sometimes related to metastatic spread mechanism.[16] The mechanism for CM varies and includes direct invasion and hematogenous and lymphatic spreading. Nonetheless, CM can occur anywhere on the skin.
The present and previous studies have shown that CM is more common from adenocarcinoma than other histological types of cancer.[2,4,8,10–15] This may also be associated with breast cancer prevalence, as the most common cancer type with CM. Another possibility is the “seed and soil hypothesis,” stating that the skin may provide a favorable environment for certain cancer cells.
In our study, two cases of bile duct cancer had CM (one on the back and the other on the chest wall), concomitant with other visceral metastases. Bile duct cancer or cholangiocarcinoma are rare worldwide but are the most common cancers in Thailand. Lui et al[17] retrospectively reviewed 30 cholangiocarcinoma patients with CM between 1978 and 2014. They demonstrated that 50% of patients had CM at the drainage site, with complications from the percutaneous biliary drainage, and the scalp was the most frequent site of CM.
In our study, patients with CM had a poor prognosis with an average of 9.21 months. Gül et al[15] and Gan et al[12] reported the presence of other organ metastases in 20% and 48% of CM patients, respectively. Unlike other studies, most of our patients (79%) were detected with advanced-stage cancer with other visceral metastases, similar to the Taiwanese study.[18]
However, the survival of patients with CM as the first clinical manifestation was longer than those with CM as recurrence of disease, despite no significance. Nonetheless, a longer follow-up analysis needed to be confirmed.
CM may not always indicate a grave prognosis and depends on each type of cancer. Hu et al[18] demonstrated that median survival following CM diagnosis in patients with breast cancer with CM only, breast cancer with visceral metastases, and non-breast cancer was 57.43, 25.22, and 6.04 months, respectively. Additionally, Lookingbill et al[4] revealed that the survival of patients with breast or endometrial cancer was longer than for other types of cancer. However, the survival between breast and non-breast cancer patients was not different in our study. While different molecular subtypes of breast cancer affected the disease prognosis, but not mentioned in previous studies.[4,18] Most of our breast cancer cases were HER-2 positive and triple-negative breast cancer - markers of poor prognosis. Whereas, further studies with a large number were recommended to ratify these preliminary results.
Importantly, CM could be a clinical indicator for disease recurrence. We observed three cases (two breasts and one colon cancer) with CM as the only cancer recurrence sign. Despite the different cutaneous metastases presentation, the firm, rapidly growing erythematous nodule(s), or eruption of multiple skin nodules were frequently observed.[19] Moreover, these presentations may be asymptomatic or associated with pain and tenderness.[20] However, the CM could also be presented as macules, infiltrated plaques, tumors with telangiectasia, and even in bullous or pigmented tumors mimicking other benign or malignant dermatologic disorders. While there remained no single diagnosis criteria for CM.[20,21] The known history of cancer, location of the lesion(s) close to primary cancer, and some specific CM forms, such as carcinoma erysipeloides, En cuirasse, Sister Mary Joseph nodule, maybe the clues for the diagnosis of CM.[19] The differentiation between the primary skin carcinoma and the metastatic carcinoma was also crucial but somewhat hard to define clinically in many cases due to different clinical presentations and some common features, especially in squamous cell carcinoma. In the meantime, the high index of suspicion in lesions with the uncertain diagnosis was critical following a malignant potential, either the primary skin carcinoma or the CM. Thus, clinicians should be aware of these signs during follow-up.
Newly detected CM may lead to restarting or changing chemotherapy or radiotherapy lines and even changing the treatment plan from curative to palliative. A skin biopsy should be performed for suspicious lesions, particularly rapidly growing nodules of undetermined nature, persistent indurated erythema, and nonhealing ulcers.[3,15] Although CM usually have a histological pattern similar to its primary tumor, there may also be more undifferentiated cells. A careful histopathological examination to often reveal important clues or the optimum immunohistochemistry staining should be done for diagnosis confirmation.[20] The early detection of CM could prolong survival, especially in patients with no other widespread metastases.[22] As well, a multidisciplinary approach should help determine optimal care towards improving patients’ quality of life.
4.1. Limitations
There were some limitations to our study. First, this was a retrospective study in one cancer center, which an inadequate number of subjects to determine the real incidence in our country. Second, CM could not be confirmed by pathological diagnosis in 52% of the patients. After a comprehensive review, this group of patients showed strong evidence of recurrence or disease progression of other visceral metastases, so the clinician urgently decided to change treatment or retreat these patients. Nonetheless, pathological examination of CM might delay treatment.
Nonetheless, a prospective multicenter study with the complete documented data, including the histopathologic results with the optimal immunohistochemistry panel in larger population should be recommended to elucidate more accurate information in CM patients.
5. Conclusions
Despite CM being a sign of poor prognosis, it may still be an indicator for changing cancer patients’ treatment. Hence, early CM diagnosis and prompt novel therapy may positively affect outcomes in cancer patients.
Acknowledgments
The authors would like to thank the following people who kindly contributed in many aspects to the success of this project: Thaniya Sricharunrat MD, Kamonwan Soonklang PhD, and Ms. Sunattee Kessung. We thank Cathel Kerr, BSc, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Author contributions
Conceptualization: Phurichaya Teyateeti, Teerapat Ungtrakul
Data curation: Phurichaya Teyateeti
Formal analysis: Phurichaya Teyateeti, Teerapat Ungtrakul
Funding acquisition: Teerapat Ungtrakul
Investigation: Phurichaya Teyateeti, Teerapat Ungtrakul
Methodology: Teerapat Ungtrakul
Project administration: Teerapat Ungtrakul
Resources: Phurichaya Teyateeti, Teerapat Ungtrakul
Software: Phurichaya Teyateeti, Teerapat Ungtrakul
Validation: Phurichaya Teyateeti, Teerapat Ungtrakul
Supervision: Teerapat Ungtrakul
Writing – original draft: Phurichaya Teyateeti
Writing – review & editing: Teerapat Ungtrakul
Supplementary Material
Footnotes
Abbreviations: CM = cutaneous metastasis, HER = human epidermal growth factor receptor.
How to cite this article: Teyateeti P, Ungtrakul T. Retrospective review of cutaneous metastasis among 11,418 patients with solid malignancy: a tertiary cancer center experience. Medicine. 2021;100:29(e26737).
Funding sources The study was granted by Chulabhorn Royal Academy.
The study was reviewed and approved by the Human Research and Ethics Committee, Chulabhorn Research Institute (Certificate No. 009/2558).
Consent for publication was not applicable.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74–85. [DOI] [PubMed] [Google Scholar]
- [2].Reingold IM. Cutaneous metastases from internal carcinoma. Cancer 1966;19:162–8. [DOI] [PubMed] [Google Scholar]
- [3].Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol 1990;22:19–26. [DOI] [PubMed] [Google Scholar]
- [4].Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol 1993;29(2 Pt 1):228–36. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol 1995;33(2 Pt 1):161–82. quiz 183-166. [DOI] [PubMed] [Google Scholar]
- [6].Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J 2003;96:164–7. [DOI] [PubMed] [Google Scholar]
- [7].Strickley JD, Jenson AB, Jung JY. Cutaneous metastasis. Hematol Oncol Clin North Am 2019;33:173–97. [DOI] [PubMed] [Google Scholar]
- [8].Hu SC, Chen GS, Wu CS, Chai CY, Chen WT, Lan CC. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol 2009;60:379–87. [DOI] [PubMed] [Google Scholar]
- [9].Nibhoria S, Tiwana KK, Kaur M, Kumar S. A clinicopathological and immunohistochemical correlation in cutaneous metastases from internal malignancies: a five-year study. J Skin Cancer 2014;2014:793937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Handa U, Kundu R, Dimri K. Cutaneous metastasis: a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol 2017;61:47–54. [DOI] [PubMed] [Google Scholar]
- [11].Queiros CS, Filipe PL, Soares de Almeida L. Cutaneous metastases from solid neoplasms in the 21st century: a retrospective study from a Portuguese tertiary care center. J Eur Acad Dermatol Venereol 2020;34:1218–24. [DOI] [PubMed] [Google Scholar]
- [12].Gan EY, Chio MT, Tan WP. A retrospective review of cutaneous metastases at the National Skin Centre Singapore. Australas J Dermatol 2015;56:01–6. [DOI] [PubMed] [Google Scholar]
- [13].Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol 2017;27:609–14. [DOI] [PubMed] [Google Scholar]
- [14].Chatterjee D, Kaur R, Madakshira MG, Bhattacharjee R, De D, Saikia UN. Cutaneous metastasis from solid organ cancer: a histopathological and immunohistochemical study of 74 cases from a tertiary care centre of North India. Australas J Dermatol 2020;61:87–90. [DOI] [PubMed] [Google Scholar]
- [15].Gul U, Kilic A, Gonul M, Külcü Cakmak S, Erinçkan C. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol 2007;87:160–2. [DOI] [PubMed] [Google Scholar]
- [16].Alcaraz I, Cerroni L, Rutten A, Kutzner H, Requena L. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol 2012;34:347–93. [DOI] [PubMed] [Google Scholar]
- [17].Liu M, Liu BL, Liu B, et al. Cutaneous metastasis of cholangiocarcinoma. World J Gastroenterol 2015;21:3066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hu SC, Chen GS, Lu YW, Wu CS, Lan CC. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol 2008;22:735–40. [DOI] [PubMed] [Google Scholar]
- [19].Ko CJ, McNiff JM. Bolognia JL, Schaffer JV, Cerroni L. Cutaneous Metastases. Dermatology. 4th ed.China: Elsevier Saunders; 2018;2160-2167. [Google Scholar]
- [20].Wong CY, Helm MA, Kalb RE, Helm TN, Zeitouni NC. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci 2013;5:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nashan D, Müller ML, Braun-Falco M, Reichenberger S, Szeimies RM, Bruckner-Tuderman L. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol 2009;135:01–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marcoval J, Moreno A, Peyri J. Cutaneous infiltration by cancer. J Am Acad Dermatol 2007;57:577–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


