Abstract
Many botanicals used for women’s health contain estrogenic (iso)flavonoids. The literature suggests that estrogen receptor beta (ERβ) activity can counterbalance ERα-mediated proliferation, thus, providing a better safety profile. A structure-activity relationship study of (iso)flavonoids was conducted to identify ERβ-preferential structures, overall estrogenic activity, and ER subtype estrogenic activity of botanicals containing these (iso)flavonoids. Results showed that flavonoids with prenylation on C8 position increased estrogenic activity. C8 Prenylated flavonoids with C2-C3 unsaturation resulted in increased ERβ potency and selectivity (e.g., 8-prenylapigenin (8-PA), EC50 (ERβ): 0.0035 ± 0.00040 μM), whereas 4′-methoxy or C3 hydroxy groups reduced activity (e.g., icaritin, EC50 (ERβ): 1.7 ± 0.70 μM). However, non-prenylated and C2-C3 unsaturated isoflavonoids showed increased ERβ estrogenic activity (e.g., genistein, EC50 (ERβ): 0.0022 ± 0.0004 μM). Licorice (Glycyrrhiza inflata, (EC50 (ERα): 1.1 ± 0.20; (ERβ): 0.60 ± 0.20 μg/mL), containing 8-PA, and red clover (EC50 (ERα): 1.8 ± 0.20; (ERβ): 0.45 ± 0.10 μg/mL), with genistein, showed ERβ-preferential activity as opposed to hops (EC50 (ERα): 0.030 ± 0.010; (ERβ): 0.50 ± 0.050 μg/mL) and Epimedium sagittatum (EC50 (ERα): 3.2 ± 0.20; (ERβ): 2.5 ± 0.090 μg/mL), containing 8-prenylnaringenin and icaritin, respectively. Botanicals with ERβ-preferential flavonoids could plausibly contribute to ERβ-protective benefits in menopausal women.
Keywords: Estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), (iso)flavonoids, phytoestrogens, prenylation
Graphical Abstract
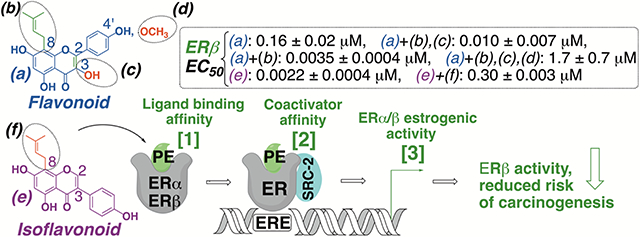
Introduction
Botanicals have been used for a long time as spices in food and beverages as well as ethnomedically because of the perceived health benefits they provide.1 Additionally, the release of the Women’s Health Initiative study (WHI) reports showed an increased risk of breast cancer and toxicities associated with conventional hormone therapy of conjugated equine estrogens and medroxyprogesterone.2 Since then, botanicals have increasingly become an alternate choice in women’s health, including usage for the alleviation of menopausal symptoms.1 This tendency is accentuated by the fact that botanicals are generally perceived to be a safer option than conventional drugs.1 Some of the most frequently used botanicals for women’s health are (1) red clover aboveground parts (Trifolium pratense L.), (2) hops strobuli (Humulus lupulus L.), (3) licorice roots/rhizomes (Glycyrrhiza sp.), and (4) horny goat weed leaves (Epimedium species).1 Red clover has been reportedly used to treat respiratory ailments in traditional medicine, and today it is contained in many botanical dietary supplements for the relief of menopausal complaints.3 Hops have been traditionally utilized in beer brewing and as a sedative and nowadays for the relief of menopausal symptoms.1 Licorice roots have been widely used as a sweetening agent in food and beverages as well as medicinally for inflammation, respiratory problems, and for the management of menopausal symptoms.1 Three licorice species, which include Glycyrrhiza inflata, to be referred to as “inflata licorice”, are used medicinally and are covered in international pharmacopeias as a group rather than in single-species monographs.4 Horny goat weed is the common name used for 50 different known species of Epimedium, which include E. sagittatum, E. grandiflorum, and E. koreanum to cite a few widely studied Epimedium species.5 These Epimedium species are well known in Traditional Chinese Medicine for natural hormone therapy, including the treatment of menstrual irregularities and osteoporosis.5 For the present studies, Epimedium sagittatum has been used for the pharmacological analysis.
All four of the presented botanicals contain phytoestrogenic (iso)flavonoids as major constituents and/or as biological significant phytoestrogens.1 These compounds can function similar to endogenous estrogens in humans.1 Spent hops contains the most potent ERα agonistic phytoestrogen known to date, 8-prenylnaringenin (8-PN), whereas red clover is known to contain the ERβ agonist genistein (GEN) and its precursor biochanin A.1 Alcoholic extracts from inflata licorice showed preferential activity for ERβ over ERα and contain the highest concentration of the ERβ-preferential flavone, 8-prenylapigenin (8-PA), in comparison to the remaining two approved medicinal species of licorice.4 Lastly, E. sagittatum contains glycosides of the major bioactive flavonol, icaritin (ICT), which is metabolized to desmethylicaritin (DMI) in vivo.6 Both ICT and DMI are reported to be estrogenic.5
When these botanicals are consumed, the bioavailable phytoestrogenic flavonoids can interact with the two known estrogen receptors (ER), ERα and ERβ.7 ERα expression is predominant in female organs such as mammary gland, uterus, ovary (thecal cells), and adipose tissue, while ERβ is mainly present in the breast (predominantly epithelial cells), ovary (granulosa cells), brain, as well as colon.7, 8 ERα activity drives cellular proliferation in estrogen responsive tissues such as breast and uterus, and studies point to potential increased risk of hormone–dependent cancers when selectively stimulated.7 For example, 8-PN, which is equipotent in both ER subtypes, has been shown to increase proliferative activity in breast and uterine tissues in murine models.4, 9, 10 In contrast, ERβ activity is reported to be antiproliferative, suppressing ERα-driven cellular proliferation, potentially reducing the risk of carcinogenesis.7 Recent research shows an increased interest in ERβ-selective ligands due to their potential antiproliferative properties and additional health benefits.7, 8
The present structure activity relationship (SAR) study analyzed respective phytoestrogenic (iso)flavonoids that are present in select botanicals and structurally similar ubiquitous (iso)flavonoids: 8-PN, 8-PA, naringenin (NRG), apigenin (API), icaritin (ICT), desmethylicaritin (DMI), kaempferol (KFL), GEN, dehydrogenistein (DGN), 8-prenylgenistein (8-PG) (Scheme 1). 8-PN, 8-PA, DMI, ICT, and 8-PG exhibit site-specific prenylation patterns, and all the tested (iso)flavonoids have other structural differences (Scheme 1). The primary aim of this study was to determine how structural variations to the conserved (iso)flavonoid pharmacophore modulate ER-subtype selectivity — specifically ERβ-preferential activity — and overall ER activity. The overarching hypothesis was that site-specific prenylation, (un)saturation, hydroxylation, and methylation can modulate ER-subtype affinity and activity of (iso)flavonoids.
Scheme 1.
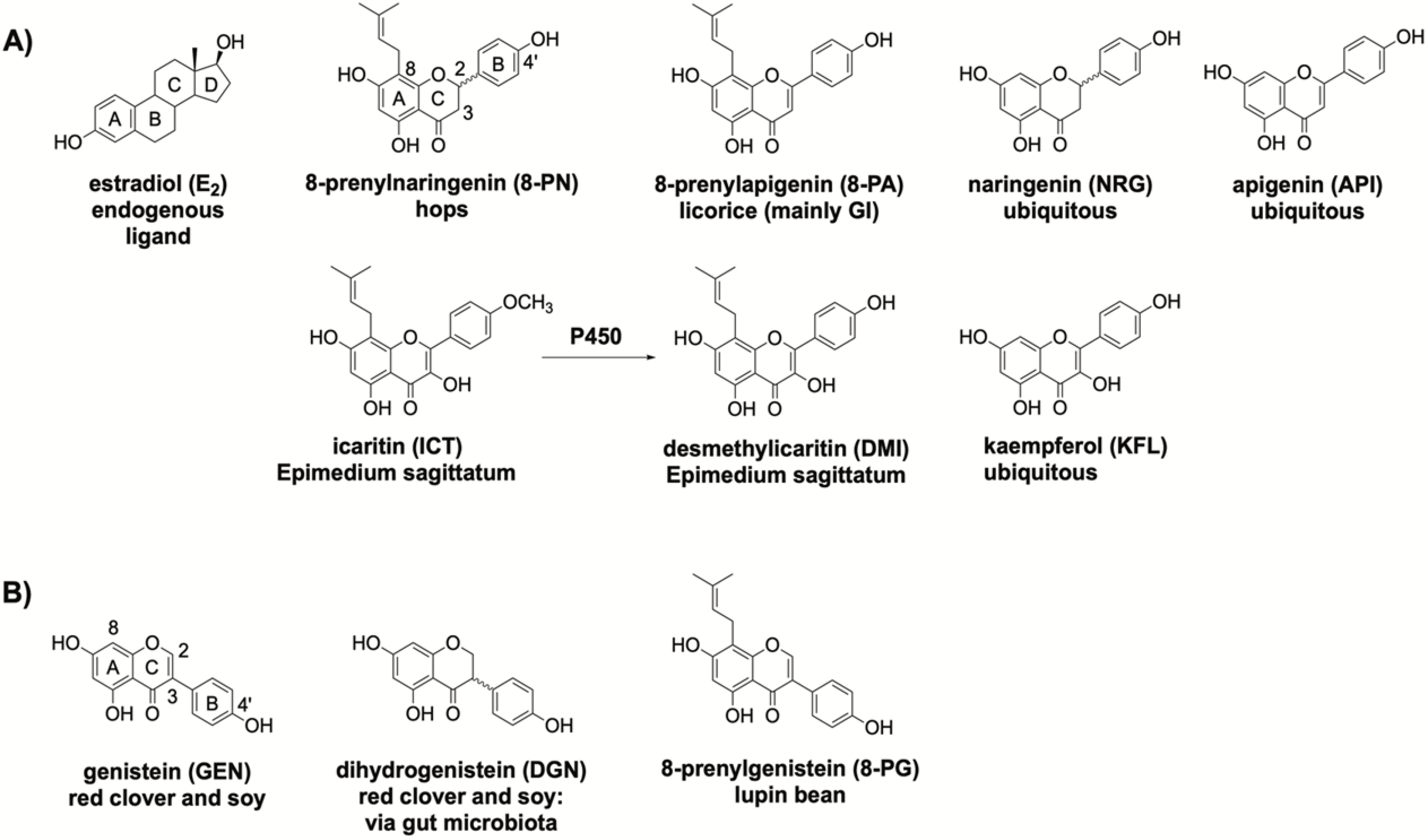
(A) Estradiol, endogenous ligand; identified bioactive flavonoids present in hops, licorice (mainly Glycyrrhiza inflata (GI)), Epimedium species (e.g., E. sagittatum) sold as horny goat weed, and present in ubiquitous plant sources as indicated. (B) Identified bioactive isoflavonoids present in red clover/soy and lupin bean, as well as DGN, a biotransformed metabolite of GEN.
Additionally, the estrogenic activity of selected extracts containing the key bioactive (iso)flavonoids — hops (8-PN), red clover (GEN), inflata licorice (8-PA), and E. sagittatum (ICT) — were also assessed. A related subaim was to determine whether the estrogenic activity of the studied extracts correlates with the observed estrogenic behavior of their respective identified bioactive (iso)flavonoids. This approach was intended to assess the extent of the influence of these bioactive compounds on the overall estrogenicity of their respective botanical extracts. Botanicals containing potent ERβ-preferential (iso)flavonoids may be able to provide the proposed ERβ-related protective benefits by counterbalancing ERα proliferative activity in vivo.
Materials and Methods
Chemicals and Reagents
General Experimental Procedures:
All chemicals were purchased from Thermo Fisher Scientific (Hanover Park, IL, USA) or Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was acquired from Gemini Bio-Products (West Sacramento, CA, USA).
Phytoconstituents:
8-Prenylnaringenin (8-PN), naringenin (NRG), apigenin (API), genistein (GEN), kaempferol (KFL) and icaritin (ICT) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Desmethylicaritin (DMI) was obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA); 8-Prenylapigenin (8-PA) and 8-Prenylgenistein (8-PG) were obtained from Ryan Scientific Inc. (Mount Pleasant, SC, USA). Dehydrogenistein (DGN) was obtained from TRC Canada (Toronto Research Chemicals Inc., Toronto, Canada). The purity of the phytoconstituents was determined as described previously using either the relative 100% or the absolute qHNMR method (supporting information for compound purity, S1).11 All commercial compounds (API, ICT, KFL, DMI, GEN, DGN, 8-PG) with a purity below 95% w/w or containing structurally related impurities were re-purified by semi-preparative HPLC-UV prior to performing the biological assays (supporting information, S2). Therefore, the final purity of all tested (iso)flavonoids was higher than 95%, determined by quantitative 1H NMR (qHNMR).
Botanical Extract Preparation
Leaves from Epimedium sagittatum, (Sieb. & Zucc.) Maxim., Berberidaceae, sold as horny goat weed were purchased from Starwest Botanicals (part # 209365–51, lot# 60901). The powder was analyzed by means of microscopic analyses, chemical profiling, and DNA barcoding (supporting information, S3).
The chosen licorice species for this study was Glycyrrhiza inflata Batalin (Fabaceae), collected in China (Kuqa County, Xinjiang Province, People’s Republic of China) and generously provided by Dr. Liang Zhao at Lanzhou Institute of Chemical Physics, CAS. G. inflata was identified using macroscopic/microscopic analyses, chemical profiling, and DNA barcoding as previously described.12 While the DNA barcoding data for the three Glycyrrhiza species are similar and require careful analysis of the nucleotide sequences at the four standardized DNA loci (rbcL, matK, and psbA-trnH, and ITS), the most discriminant DNA loci was found to be psbA-trnH and the matK marker within the G. inflata genotype.12 This method unequivocally distinguished the G. inflata material (BC 711) from the other two Glycyrrhiza species.12 The powdered roots of G. inflata (licorice) were extracted by hydro-alcoholic maceration and 8-PA content was determined by LC-MS to be 0.168 ± 0.014 % w/w as previously described (supporting information, S4).4
Red clover (Trifolium pratense L., Fabaceae) standardized extract was prepared by autohydrolysis using proprietary methods and manufactured by PureWorld Botanicals (acquired by Naturex, Inc., South Hackensack, NJ).13 Biochanin A and GEN content quantified by QM-qHNMR were 15.4 ± 0.6 and 0.64 ± 0.04 % w/w, respectively (supporting information, S4).14
Hops (Humulus lupulus L., Cannabaceae) extract was prepared with ethanol at Hopsteiner (New York, NY, USA) from spent hops.15 The contents (% w/w) of four markers, xanthohumol (XH), isoxanthohumol (IX), 6-prenylnaringenin (6-PN), and 8-PN, in freshly prepared extract samples were determined by HPLC-MS/MS to be 0.28 ± 0.03 for 8-PN (supporting information, S4), 1.22 ± 0.09 for 6-PN, 1.11 ± 0.07 for IX, 33.2 ± 2.8 for XH. Desmethylxanthohumol (DMX) content was determined in freshly prepared samples to be 0.63 ± 0.06 % w/w. It is known to isomerize to 6-PN/8-PN (5.7:1) in solution with time.16
For the enzymatic hydrolysis of E. sagittatum, an auto-hydrolyzed extract (70.0 mg) (supporting information, S5 and S7) was dissolved with 171.60 mg snailase in Milli-Q water (2 mL) and stirred at 40°C. After four days, enzymatic activity was stopped by adding methanol (2 mL). Aglycones were extracted with organic solvents (40 mL, hexanes, chloroform, and ethyl acetate). A total of 18.2 mg of snailase-hydrolyzed extract, enriched in icaritin, was obtained.17 The ICT concentration was determined to be 3.40 % w/w by HPLC-UV (supporting information, S4–S10).
Cell Culture conditions
The ERα, endometrial carcinoma cells (Ishikawa) were provided by Dr. R. B. Hochberg (Yale University, New Haven, CT). The MDA-MB-231/β41 breast carcinoma cell line, stably transfected with ERβ was a gift from Dr. Debra Tonetti (University of Illinois at Chicago, Chicago, IL). Both cell lines were maintained as previously described4 and authenticated by short tandem repeat profile analysis. The cells were in accordance with the Health Protection Agency Culture Collection in the UK (Ishikawa) and with ATCC (MDA-MB-231).
ERα and ERβ Competitive Binding assay
The protocol was used and validated for this analysis as previously described with minor modifications (supporting information S11).18–20 Briefly, the reaction mixture consisting of 5 μL of compound in DMSO, 5 μL of purified full length human recombinant ERα or ERβ (Invitrogen Carlsbad, CA) diluted in ER binding buffer (3 nM), and 5 μL of “Hot Mix” (400 nM) was prepared fresh with 95 Ci/mmol [3H] estradiol (E2) diluted in 1:1 ethanol:ER binding buffer (NEN Life Science Products, Boston, MA, USA). [3H]-E2 concentration of 20 nM was used as tracer in the reaction mixture in accordance with previously described methods.18 Then, ER binding buffer (85 μL) was added and the mixture was incubated at room temperature for 2 h. The test compounds did not exhibit solubility issues at the tested concentrations. Hydroxyapatite (BioRad, Herculus, CA) slurry (HAPS, 100 μL) was added to the reaction mixture while kept on ice for 15 min and vortexed every 5 min. A wash step was repeated three times by adding 900 μL of appropriate wash buffer for the respective receptor subtype to the reaction mixture, then vortexing, centrifuging at 10,000 g at 4 °C for 1 min and decanting. The HAPS pellet with receptor-ligand complex was re-suspended in 200 μL of ethanol (200 proof) and added to scintillation tubes. Reaction tubes were further rinsed with 200 μL of ethanol and rinse was added to scintillation tubes. Cytoscint [(4 ml; ICN (Costa Mesa, CA)] was added to the tube, and a Beckman LS 6500 liquid scintillation counter (Schaumburg, IL) was used to count radioactivity. The % inhibition of [3H] E2 was calculated using equation (1):
| (1) |
The % binding of sample was calculated by comparison with that of estradiol (50 nM). The data obtained were the mean of three analytical replicates in triplicates. % Relative Binding Affinity (RBA) values were calculated for each compound using equation (2) to quantitate binding activity relative to 17β-E2.10
| (2) |
RBA β/α values [(%RBA β of compound (A)] / [%RBA α of compound (A)] were used to express the measure of preferential interaction of each specific (iso)flavonoid for each ER subtype (α or β).21
Computational docking model
In silico docking analysis was performed to investigate the interactions of the (iso)flavonoids with ERα and ERβ. Binding sites of ERα and ERβ bound to genistein were obtained from Protein Data Bank (PDB ID: 1×7r and 1×7j, respectively) and uploaded to Molecular Operating Environment (MOE; Chemical Computing Group, version 2016.0208). All unnecessary water molecules were removed, and the structure was prepared using the quick prep option in MOE. Compounds were docked with triangle matcher placement with London dG scoring and induced fit refinement with GBVI/WSA dG scoring. The images are shown with the docked compound in the molecular surface showing hydrophobicity and lipophilicity of the binding site.
Time Resolved Fluorescence Energy Transfer (TR-FRET) assay in ERα and ERβ
The following agonist mode TR-FRET assay protocol was adapted from established procedures.22, 23 For ERα-ligand recruitment assessment, (iso)flavonoids at varied concentrations were incubated with 4 nM ERα−417 (amino acids 304–554; C381,530S; site-specifically labeled at C417 with biotin-maleimide), 1 nM streptavidin terbium chelate (LanthaScreen® Tb-Streptavidin; ThermoFisher Scientific catalog number: PV3965), and 100 nM steroid receptor coactivator 2 (SRC-2, residues 627–829; labeled nonspecifically with 5-iodoacetamidofluorescein) in 80 μL of TR FRET buffer. For ERβ-ligand recruitment assessment, (iso)flavonoids at varied concentrations were incubated with 7 nM ERβ-LBD (labeled with GST), 5 nM terbium-anti-GST antibody and 250 nM steroid receptor coactivator 2 (residues 627–829; labeled nonspecifically with 5-iodoacetamidofluorescein) in 80 μL of TR FRET buffer. TR FRET buffer contained 20 mM Tris-HCl, 10% glycerol, 50 mM NaCl, 0.02% Nonidet® P 40 substitute at pH 7.5 with a DMSO concentration of 3%. After a 1 hour incubation at RT for each respective ER subtype-ligand recruitment measure, TR-FRET readings were taken on a Biotek Neo2 reader using the software Gen5 v. 3.02 using excitation band of 360/40 nm with a 100 μs delay and a 500 μs collection time with emission readings at 495/5 nm and 520/25 nm. Experiments were performed in triplicate using Corning black, polystyrene, flat bottom, nonbinding surface area, 96-well half area assay plates. Graphpad Prism v. 7.02 was used to generate best fit curves of the data (ratio of emission at 520 nm/emission at 495 nm) to sigmoidal, 4PL, where X is log (concentration of inhibitor).
Functional estrogenic activity assays
The protocol for the Estrogen-Responsive Alkaline Phosphatase assay in ERα endometrial cancer cells (Ishikawa) used by Pisha and Pezzuto24 and ERβ-ERE-luciferase induction assay in ERβ positive breast cancer cells (MDA-MB-231/β41 cells) were validated and used as previously described (supporting information S12).4
Statistics
Graph Pad Prism® 7.0 (GraphPad software, San Diego, CA) Macintosh version was used for curve fitting (sigmoidal dose-response curve model with variable slope) and data analysis.
Results
Competitive binding assays of (iso)flavonoids in ERα and ERβ
Among the flavanones and flavones, 8-PN had the highest affinity (% RBA) for ERα, followed by 8-PA. 8-PN showed greater affinity for ERα over ERβ, while 8-PA demonstrated the strongest binding affinity for ERβ with preferential binding for ERβ over ERα (Table 1, Figure 1). API and NRG had significantly weaker affinity for ERα and ERβ than their prenylated congeners. The decreasing order of affinity for flavanones/flavones in ERα was 8-PN > 8-PA>> NRG ≈ API, while for ERβ the order was 8-PA >> 8-PN >> API > NRG (Table 1). The flavonols (DMI, KFL, ICT) showed significantly less overall ER affinity than the corresponding flavone, 8-PA (Table 1, Figure 1). The low affinity for ERα and ERβ by DMI and KFL was similar to the non-prenylated flavonoids, API and NRG (Table 1, Figure 1). However, all four flavonoids, API, NRG, DMI, and KFL, demonstrated low, but preferential ERβ affinity (Table 1, Figure 1). The 4′ methoxylated ICT had negligible binding affinity to ERα and ERβ (Table 1, Figure 1). All three isoflavonoids (GEN, DGN, 8-PG) had significantly weaker affinity for ERα than for ERβ. GEN had the strongest affinity for ERβ among the isoflavonoids (Table 1). The overall decreasing affinity ranking order in ERα was GEN >> DGN ≈ 8-PG and in ERβ GEN > DGN > 8-PG, demonstrating a pronounced decrease in affinity with isoflavonoid prenylation as in 8-PG (Table 1). 8-PN showed preferential affinity for ERα at RBA β/α of 0.3 (Table 1). In contrast, 8-PA and GEN had ERβ-preferential affinity with RBA β/α of 2.7 and 3.5, respectively (Table 1). The 8-PN and GEN RBA β/α values were consistent with previous publications.10, 18 Among all the (iso)flavonoids tested, 8-PA showed the strongest ERβ binding affinity, while GEN showed the best overall ERβ-preferential affinity (Table 1).
Table 1.
ERα and ERβ (iso)flavonoid ER subtype and coactivator affinitya
| Compounds | ERα | ERβ | RBA β/α | ||
|---|---|---|---|---|---|
| % RBAb | % RREc | % RBAb | % RREc | ||
| 17β-Estradiol | 100 | 100 | 100 | 100 | --- |
| Flavanones, Flavones | |||||
| 8-PN | 30 | 94.7 | 9 | 51.2 | 0.3 |
| 8-PA | 18 | 43.2 | 48 | 47.2 | 2.7 |
| NRG | <0.002 | 12.6 | 0.05 | 23.0 | --- |
| API | <0.002 | 15.4 | 0.6 | 32.8 | --- |
| Flavonols | |||||
| DMI | <0.002 | 9.1 | 0.5 | 16.6 | --- |
| ICT | <0.002 | 10.2 | <0.002 | 11.9 | --- |
| KFL | <0.002 | 31.1 | 0.2 | 18.8 | --- |
| Isoflavonoids | |||||
| GEN | 5.6 | 62.7 | 19.4 | 50.2 | 3.5 |
| DGN | <0.002 | 25.1 | 14 | 38.7 | --- |
| 8-PG | <0.002 | 33.5 | 0.9 | 17.2 | --- |
Values are expressed as the mean of at least three independent analytical determinations in triplicate. Method details for the ERα and ERβ competitive binding assay and for the (TR-FRET) assay are described in the Experimental Section.
% relative binding affinity (RBA)= (IC50 E2/ IC50 (iso)flavonoid))*100,
% relative recruitment efficacy (RRE) = (max FRET efficacy (iso)flavonoid) / max FRET efficacy E2)*100, E2 arbitrarily set at 100%.
Figure 1.
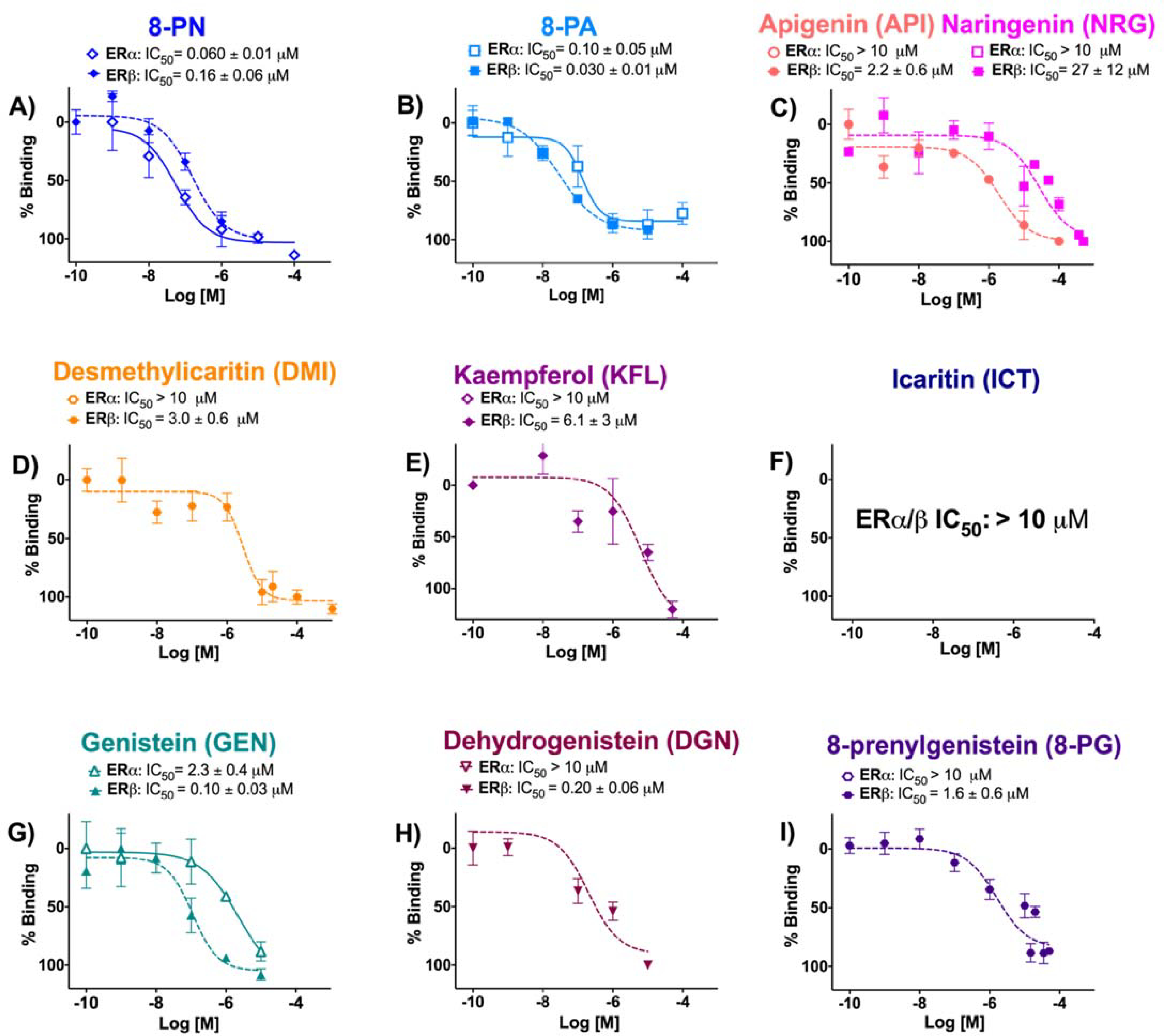
Competitive radioligand binding of flavanones, flavones (A - C), flavanols (D - F) and isoflavonoids (G - I) as dose-response evaluations to assess affinity for ERα and ERβ. Data represent average ± SEM (μM) from three independent determinations plus IC50 values.
In silico computational models
The present findings are further explained by our in silico docking results (Figure 2A & B), in which the C8-prenyl group on 8-PN interacts with lipophilic regions in the ERα binding pocket (Figure 2A). This interaction can provide stronger 8-PN binding to ERα, which in part helps explain the more potent IC50 values determined for 8-PN in comparison to its non-prenylated counterpart, NRG (Table 1, Figure 1A,1C). In contrast, the prenyl group of the isoflavonoid, 8-PG, interacts with hydrophilic regions in the ERβ binding pocket (Figure 2B), leading to an unfavorable binding interaction, as opposed to the non-prenylated counterpart, GEN, that exhibits strong potency in this ER subtype (Table 1, Figure 1G,1I).
Figure 2.
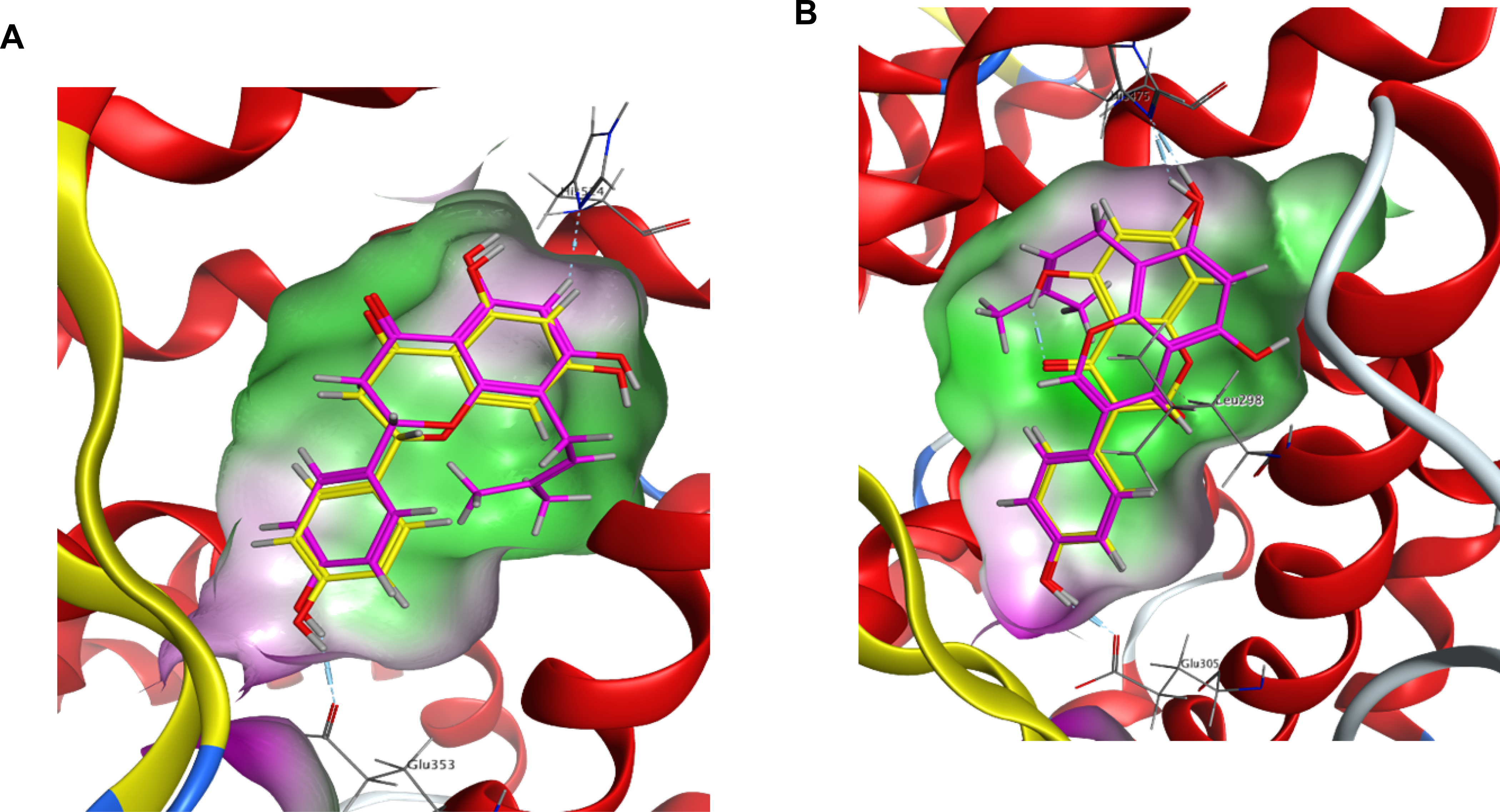
Computational modelling of ERα and ERβ binding pocket. The green regions of the binding pocket are lipophilic and contain non-polar residues, which interact favorably with a prenyl group; the purple regions are hydrophilic with polar amino acid residue that interact unfavorably with a prenyl group. (A) 8-PN (magenta) and NRG (yellow) are docked in the binding pocket of ERα. The prenyl group of 8-PN interacts with the lipophilic region of the ERα pocket creating favorably binding conditions. (B) GEN (yellow) and 8-PG (magenta) are docked in the ERβ binding pocket. GEN binds well in the ERβ pocket, while 8-PG places its prenyl group in a hydrophilic region creating unfavorable binding conditions.
(Iso)flavonoids-ER complex coactivator recruitment in ERα and ERβ
The % Relative Recruitment Efficacy (RRE)25 reflects a measure of the efficacy of the transcription factor, steroid receptor coactivator-2 (SRC-2), recruitment by each ER-ligand complex. This assay comparatively assesses the affinity of each respective (iso)flavonoid-ER complex for the SRC-2, relative to the affinity of the E2-ER complex reference. In ERα, 8-PN had the strongest % RRE, followed by 8-PA. The order of % RRE readings in ERα for flavanones and flavones were: 8-PN > 8-PA >> API ≈ NRG (Table 1). In ERβ, 8-PN and 8-PA showed comparable activity, while API showed more activity than NRG (Table 1, Figure 3). Both 8-PN and 8-PA had greater activity than API and NRG, giving an ERβ % RRE order of 8-PN ≈ 8-PA > API > NRG (Table 1, Figure 3). As observed in the ER-binding assay, the flavonols, DMI, ICT, and KFL, exhibited weak overall results, but KFL showed comparatively the highest activity in both ER subtypes. However, in comparison to 8-PA the flavonols had significantly lower overall % RRE values (Table 1). Lastly, GEN exhibited the highest % RRE in ERα and ERβ among the isoflavonoids, followed by 8-PG and DGN resulting in a ranking order of ERα: GEN > 8-PG > DGN and ERβ: GEN > DGN >> 8-PG (Table 1).
Figure 3.
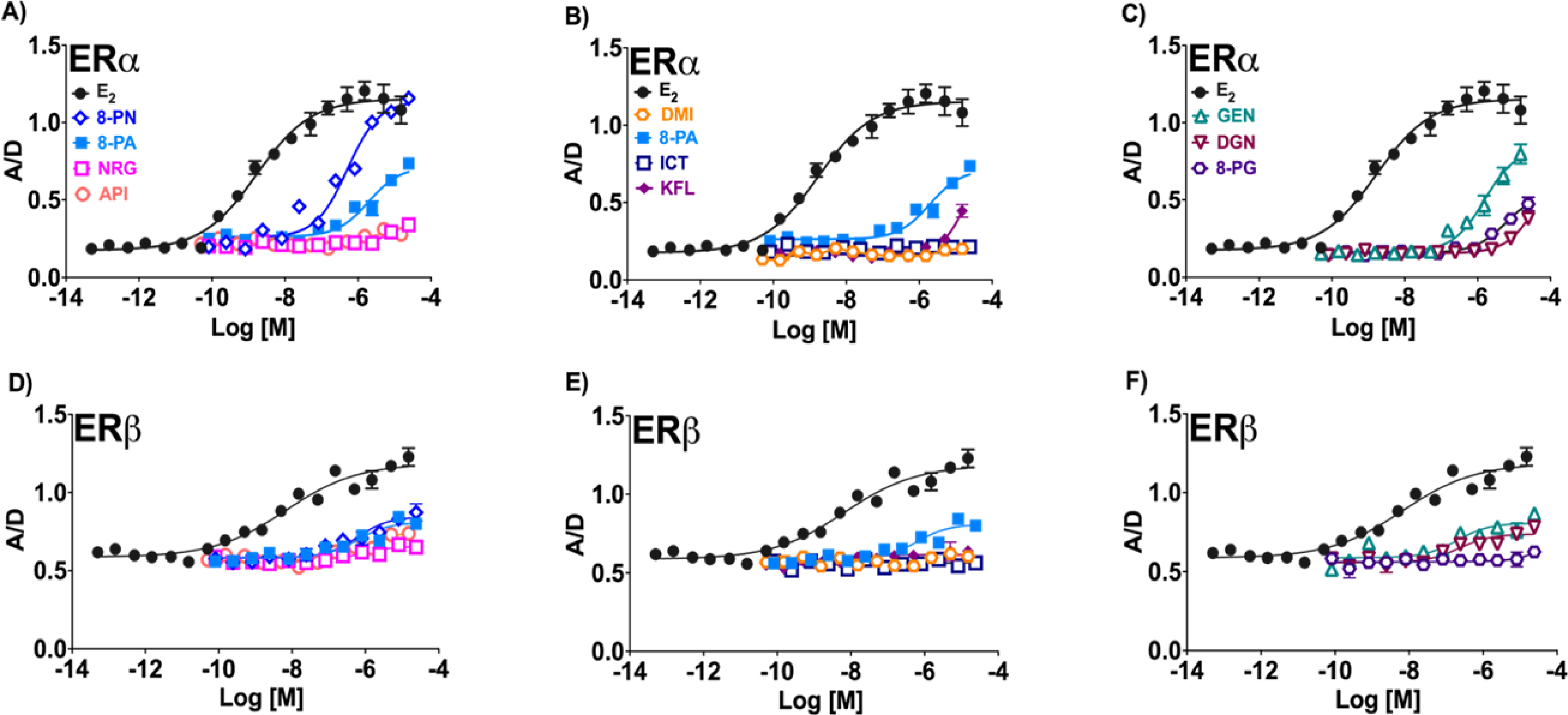
TR-FRET assessing coactivator transcription factor (SRC-2) recruitment by flavanones and flavones (A and D), flavonols (B and E), as well as isoflavonoids (C and F) for ERα and ERβ. The measure of respective ligand-ER recruitment efficacy is represented by A/D: the normalized acceptor molecule emission signals (A) /normalized donor molecule emission signal (D). Data represent average ± SEM from two or three independent determinations.
Comparative functional estrogenic activity of (iso)flavonoids
The functional activity of the (iso)flavonoids and select botanical extracts enriched in these compounds was determined in alkaline phosphatase activity assay in ERα+ endometrial carcinoma-(Ishikawa) cells and ERβ-luciferase assay in ERβ+ MDA-MB-231/β41 breast cancer cells with validated methods (supporting information S12). Prenylation at C8 on the A-ring of the flavonoids showed higher ERα and ERβ functional activity as observed in 8-PN, 8-PA, and DMI in comparison to their non-prenylated congeners, NRG, API, and KFL, with the exception of the 4′-O-methylated, ICT (Scheme 1A, Table 2, Figure 4A - D). However, prenylation at the same site on isoflavonoids, as in 8-PG, led to significantly lower functional activity compared to GEN and DGN (Scheme 1B, Table 2, Figure 5).
Table 2.
ERα- and ERβ-dependent Estrogenic Effects of Extracts and (Iso)Flavonoids.a
| Extractsb | ERα | ERβ | ||
|---|---|---|---|---|
| EC50 | Maximum efficacy | EC50 | Maximum efficacy | |
| Hops | 0.030 ± 0.010 | 72 ± 4.0 | 0.50 ± 0.050 | 107 ± 8.0 |
| Red clover | 1.8 ± 0.20 | 94 ± 12 | 0.45 ± 0.10 | 139 ± 7.0 |
| Inflata licorice | 1.1 ± 0.204 | 57 ± 6.04 | 0.60 ± 0.204 | 80 ± 104 |
| Epimedium sagittatum | 3.2 ± 0.20 | 53 ± 5.0 | 2.5 ± 0.090 | 53 ± 0.60 |
| Compoundsc | EC50 | Maximum efficacy | EC50 | Maximum efficacy |
| 17β-Estradiol | 0.030 ± 0.00d,4 | 100 ± 104 | 0.030 ± 0.00d,4 | 100 ± 4.04 |
| Flavanones, Flavones | ||||
| 8-PN | 0.0050 ± 0.00104 | 108 ± 184 | 0.0050 ± 0.000504 | 87 ± 9.04 |
| 8-PA | 0.050 ± 0.00604 | 93 ± 7.04 | 0.0035 ± 0.000404 | 104 ± 6.04 |
| NRG | 3.7 ± 0.50 | 69 ± 6.0 | 0.10 ± 0.040 | 99 ± 4.0 |
| API | 3.2 ± 0.030 | 45 ± 3.0 | 0.16 ± 0.020 | 118 ± 5.0 |
| Flavonols | ||||
| DMI | 0.20 ± 0.020 | 67 ± 12 | 0.010 ± 0.0070 | 90 ± 11 |
| ICT | 1.6 ± 0.40 | 53 ± 8.0 | 1.7 ± 0.70 | 69 ± 4.0 |
| KFL | 5.1 ± 4.0 | 36 ± 5.0 | 0.20 ± 0.050 | 75 ± 4.0 |
| Isoflavonoids | ||||
| GEN | 0.40 ± 0.050 | 117 ± 25 | 0.0022 ± 0.0004 | 131 ± 3.0 |
| DGN | 0.80 ± 0.10 | 84 ± 4.0 | 0.020 ± 0.010 | 114 ± 12 |
| 8-PG | 0.50 ± 0.20 | 49 ± 10 | 0.30 ± 0.0030 | 74 ± 7.0 |
Values are expressed as the mean ± SEM of at least three independent determinations in triplicate. ERα activity was determined in the estrogen-responsive alkaline phosphatase assay using ERα positive Ishikawa cells. ERβ-activity was determined using the ERE-luciferase induction assay in ERβ positive MDA-MB-231/β41 cells. Method details are described in the Experimental Section.
Values are expressed in μg/mL for extracts.
Values are expressed in μM for compounds.
nM
Figure 4.
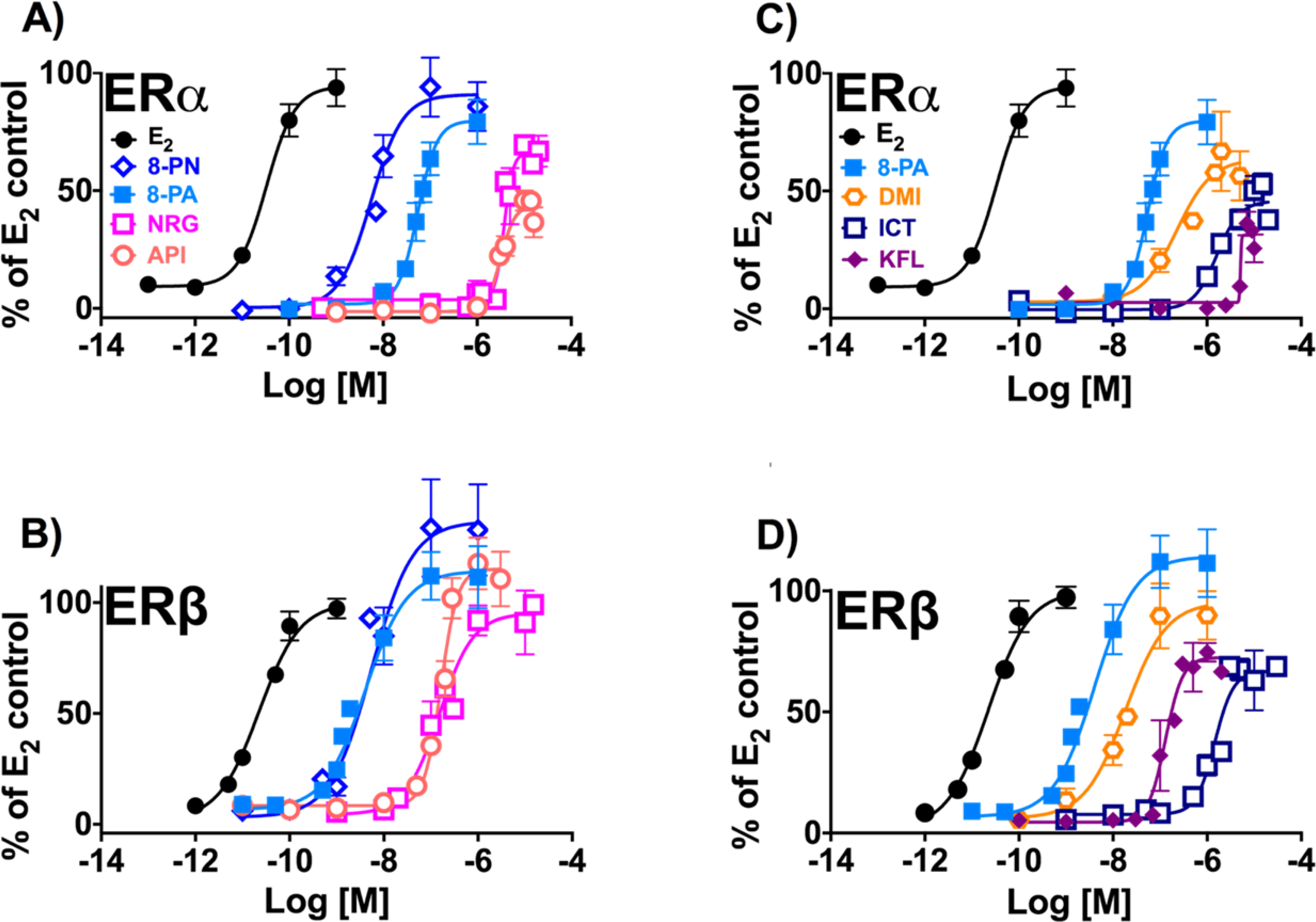
Induction of estrogenic effects in vitro by the flavanones/flavones (A and B) and flavonols (C and D): (A, C) ERα, estrogen-dependent induction of alkaline phosphatase activity in Ishikawa cells. ERβ, estrogen-dependent induction of ERE-luciferase activity in MDA-MB-231/β41 cells. Data represent average ± SEM from three independent determinations.
Figure 5.
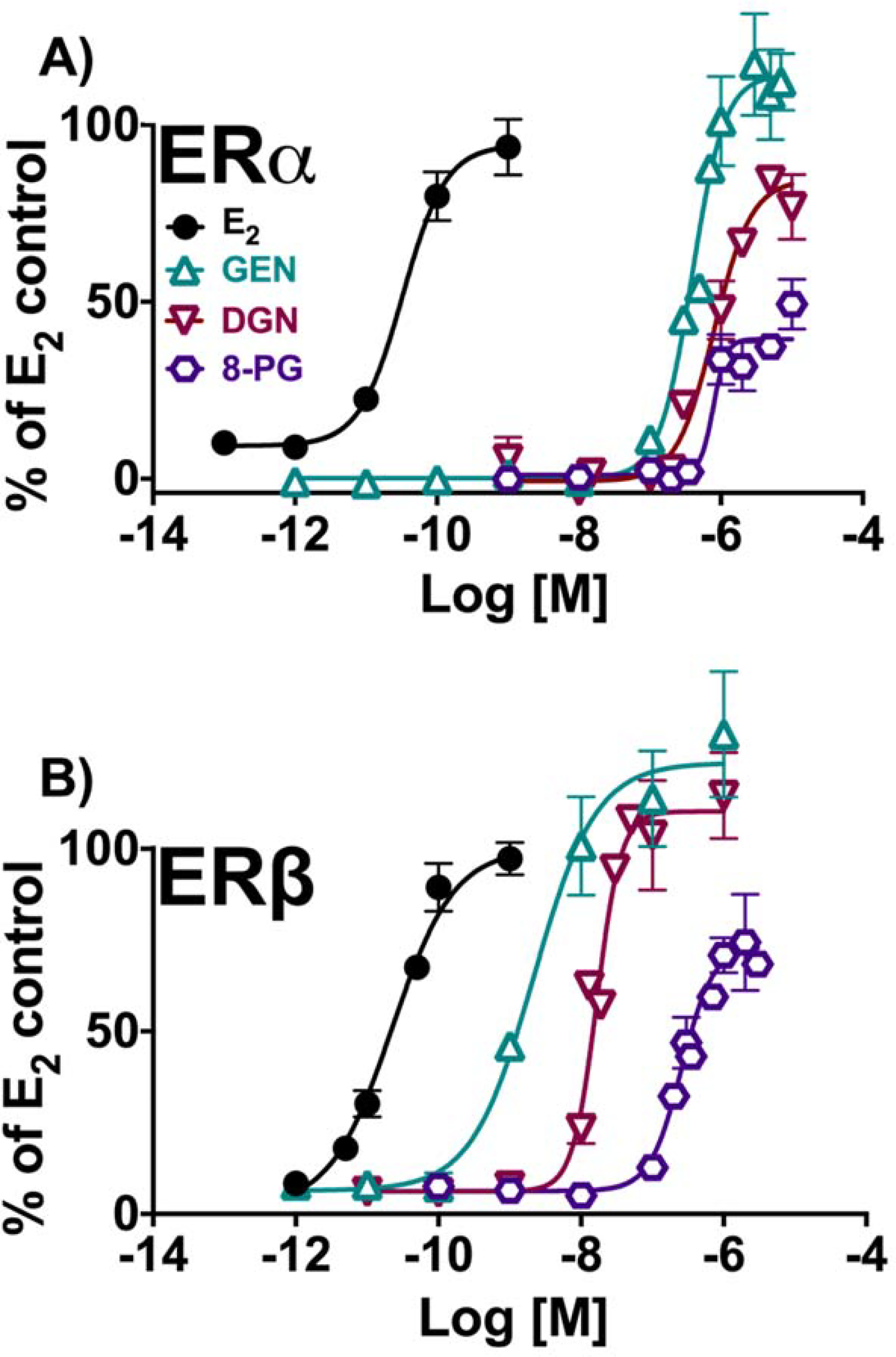
Induction of estrogenic effects in vitro by isoflavonoids: GEN, DGN, 8-PG. (A) Estrogen-dependent induction of alkaline phosphatase activity in Ishikawa cells. (B) Estrogen-dependent induction of ERE-luciferase activity in MDA-MB-231/β41 cells. Data represent average ± SEM from three independent determinations.
a). Flavanones and flavones.
The prenylated flavanone, 8-PN which has a saturated C2-bond in the C-ring showed the highest ERα potency, followed by the prenylated flavone, 8-PA, with C2-C3 unsaturation at the same position. The order of potency observed in the ERα assay is as follows: 8-PN > 8-PA >> NRG ≈ API (Table 2, Figure 4A). However, 8-PA demonstrated considerably higher ERβ potency and was the most ERβ-preferential over ERα among the flavonoids in this assay, while 8-PN was equipotent in both subtypes. The resulting ERβ potency order was 8-PA ≈ 8-PN >> NRG ≈ API (Table 2, Figure 4B). While NRG and API were the least estrogenic flavanone and flavone, respectively, both show some preferential ERβ potency (Table 2, Figure 4B).
b). Flavonols.
Similar to results from the ER-binding and FRET assay, C-ring hydroxylation at C3, as seen in DMI, ICT, and KFL, reduced the overall estrogenic activity of these compounds compared to 8-PA (Table 2, Figure 4C–D). Additional 4′C-O-methylation, as observed in ICT, further reduced the estrogenic potency compared to the non-methylated congener, DMI (Table 2, Figure 4C–D). In ERα the order of estrogenic activity was 8-PA >> DMI > ICT > KFL (Table 2, Figure 4C). However, in ERβ, O-methylation at the 4′ position seemed to obscure the potentiating effect of C8 prenylation of ICT, making the non-prenylated KFL more active than ICT for this ER subtype: 8-PA > DMI > KFL >> ICT (Table 2, Figure 4D). In comparison to the tested flavonols, 8-PA also showed the most ERβ-preferential activity.
c). Isoflavonoids.
In contrast to what was observed with flavonoids, prenylation of isoflavonoids resulted in a significant decrease of overall estrogenic efficacy and potency as observed in 8-PG (Scheme 1B, Table 2, Figure 5A & B). Saturation at the C2-C3 position in ring C also led to an overall reduction in estrogenic functional activity as observed for DGN in comparison to GEN. The isoflavonoids were significantly less potent ERα ligands than ERβ ligands with an order of ERα potency with GEN ≈ DGN ≈ 8-PG, while showing a more defined decreasing order of efficacy: GEN > DGN > 8-PG (Table 2, Figure 5A). In ERβ, GEN had the strongest potency as expected followed by DGN, with 8-PG showing the lowest potency and efficacy relative to the former compounds: GEN >> DGN >> 8-PG (Table 2, Figure 5B).
Functional estrogenic activity of extracts
The botanical extracts, each containing one of the studied flavonoids/flavonols (8-PN, 8-PA, ICT) and isoflavonoids (GEN) as bioactive markers, were analyzed for their ERα and ERβ potency. The tested hops extract was generally equipotent in both ERα-(Ishikawa) and ERβ (MDA-MB-231/β41) cell-based assays. In addition, the hops extract displayed the highest potency in the ERα assay compared to the other tested botanical extracts (Table 2, Figure 6). Hops, red clover, and inflata licorice extracts did show similar overall activity in ERβ. Red clover and inflata licorice extracts both demonstrated some preferential potency for ERβ over ERα. While red clover behaved as a full agonist in ERα and ERβ, inflata licorice mainly demonstrated partial agonistic activity in ERα. However, inflata licorice extract had greater functional efficacy in ERβ compared to ERα (Table 2, Figure 6). E. sagittatum showed the least overall estrogenic activity, behaving as a partial agonist in ERα and ERβ. The order of functional activity of botanical extracts for ERα was: hops > inflata licorice > red clover >> E. sagittatum (Table 2, Figure 6). In the ERβ assay the rank order was red clover ≈ hops ≈ inflata licorice >> E. sagittatum (Table 2, Figure 6).
Figure 6.
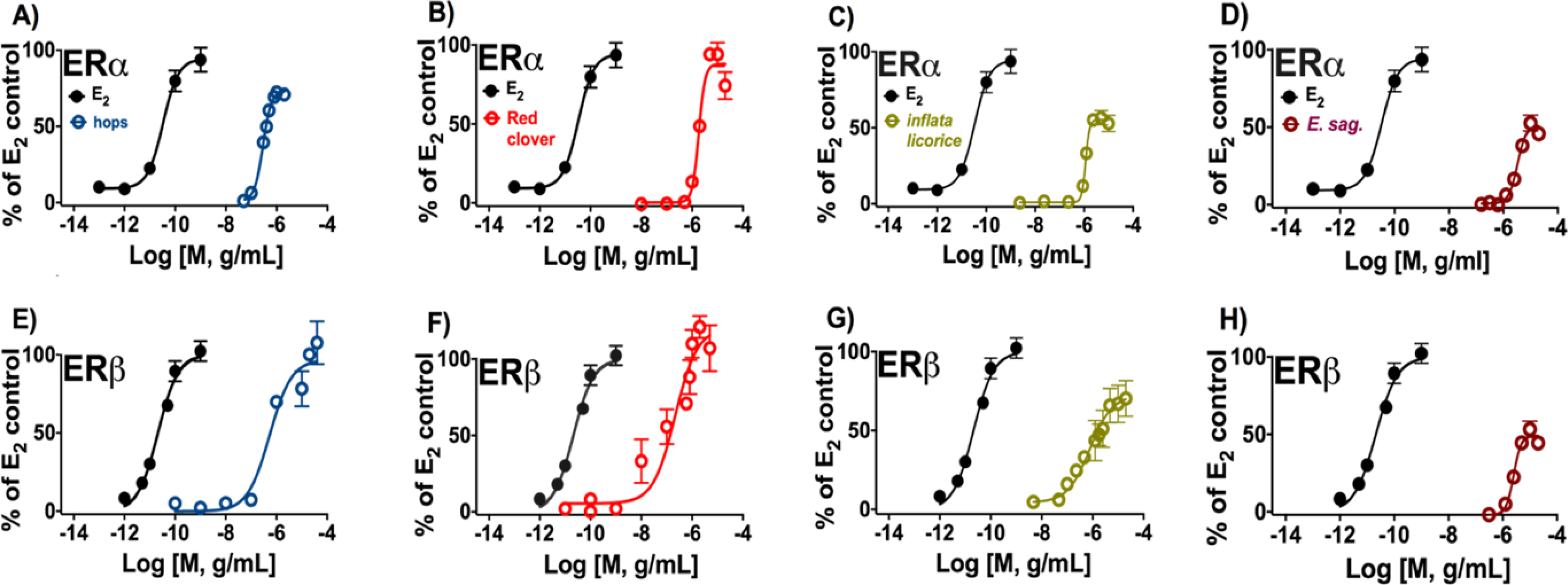
Induction of estrogenic effects in vitro by botanical extracts enriched in target (iso)flavonoids: (A, E) hops, (B, F) red clover, (C, G) inflata licorice, and (D, H) E. sagittatum. The panels A to D represent the ERα-dependent induction of alkaline phosphatase activity in Ishikawa cells. The panels E to H represent the ERβ-dependent induction of ERE-luciferase activity in MDA-MB-231/β41 cells. Data represent average ± SEM from three independent determinations.
Discussion
ERα and ERβ are co-expressed in various estrogen-sensitive tissues (e.g., mammary gland), but the relative ratio of these ER subtypes varies depending on the tissue.26, 27 For example, the uterus is reported to have a higher ERα to ERβ ratio, while ERβ is reported to be more prominently expressed than ERα in normal mammary tissue.26, 28 Therefore, the relative ratio of ERβ and ERα in a given tissue can influence the level of ERβ-driven protection from ERα-mediated estrogen carcinogenesis.26 Literature suggests a potential protective role of ERβ from carcinogenesis in normal cells. This is indicated by loss of ERβ expression in early breast carcinogenesis such as ductal carcinomas, as well as its inverse relationship in expression with Ki-67, a biomarker of cell proliferation.29 Literature also shows that in the presence of E2, ERβ activity increased in vitro expression of p21 WAF1/cip1, a tumor suppressor cellular protein that inhibits cell cycle progression, and subsequently inhibited the expression of the pro-proliferative proteins such as c-Myc and cyclin D1.30 In cells, p21WAF1/cip1 is reported to bind and inhibit the activity of the proliferating cell nuclear antigen (PCNA), responsible for DNA replication during cell proliferation.31
The initial step in induction of estrogenic activity involve the binding of the compound to the ER. Both of the ER subtypes contain polar binding residues in the ligand binding site and these interact with the A- and D-ring of the endogenous ligand E2 (Scheme 2).7 These specific regions contain amino acid residues crucial for ligand binding including arginine and glutamic acid (A-ring pocket) and histidine (D-ring pocket), which interact with select hydroxyl moieties on ligands, promoting receptor binding (Scheme 2, B.1 - B.3).7 One of the basic requirements for binding of a ligand to the ER is the presence of two optimally positioned hydroxyl groups (or bio-isosteres in some cases), approximately 11Å apart, separated by a hydrophobic core.7 This helps to form crucial hydrogen bonds with the respective amino acids for adequate ligand-binding affinity for both ER subtypes as in the case of E2 (Scheme 2).7 However, binding affinity is ligand-dependent.25 A majority of (iso)flavonoids used in our study generally harbor these structural characteristics and consequently displayed estrogenic activity (Tables 1 & 2, Scheme 1). Certain features in chemical structures such as planarity generally tend to favor ERβ selectivity as observed in the ERβ agonist GEN, although this feature has some limitations.7, 32 The ERβ active site is narrower than its counterpart, ERα.7 As a result, planar (iso)flavonoids, such as GEN, are able to fit into the active site with closer proximity to non-polar residues, thereby resulting in stronger hydrophobic interactions with amino acids such as methionine (Met 336).7 This is one likely explanation for the increased preferential binding activity for ERβ over ERα of planar (iso)flavonoids (Table 1, Figure 1). However, some non-planar compounds such as 8β-vinyl estradiol can still display pronounced ERβ selectivity due to ligand rigidity and optimally positioned substituents.7
Scheme 2.
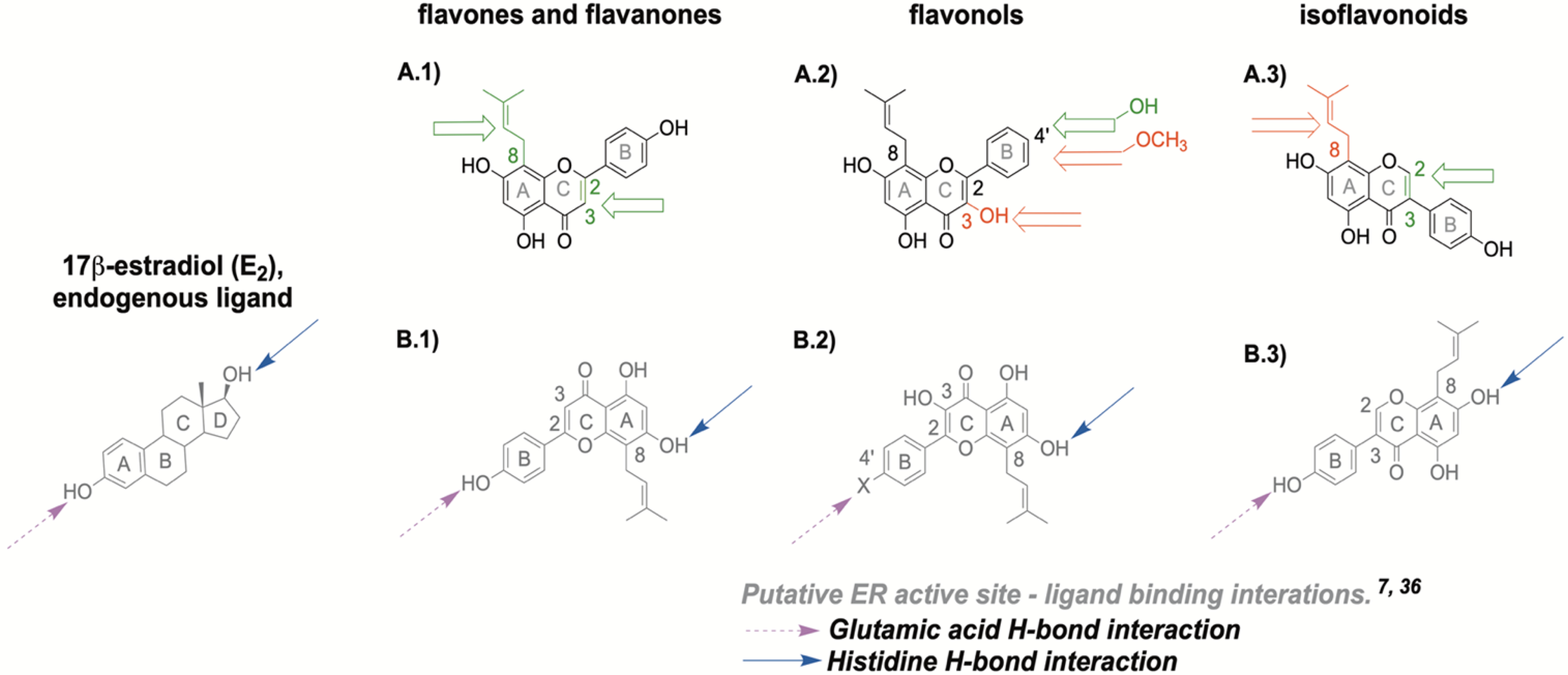
Tested flavonoid structures with putative ER binding interactions: (A.1) flavanone and flavone structure with A-ring prenylation at 8 position (C8) and (un)saturation at C2-C3. (A.2) flavonol structure with C3 hydroxyl group and B-4’ position for hydroxyl or methoxy group (A.3) isoflavonoid structure with C8 prenylation and (un)saturation at C2-C3. The open green arrow represents an increased ERβ activity when the considered substitution is present on the core flavonoid structure, whereas the open red arrow indicated a decreased activity. (B.1) ER binding interactions of flavanones and flavones, (B.2) of flavonols, (B.3) of isoflavonoids in relation to E2. Blue full-tailed arrows represent moieties with hydrogen bond (H-bond) interactions with histidine residue in the ER binding site; magenta dash-tailed arrows represent moieties with H-bond interactions with glutamic acid residues in ER binding site.
After the ligand has been bound to the ER, coregulators (e.g., SRC-2) are recruited to the ligand-ER complex.25 When complexed with the ER, the ligand structure also determines the resulting conformation of the bound ER, which in turn uniquely determines the respective strength of coactivator recruitment activity.25 Consequently, two different ligands with similar binding affinities can reflect different recruitment abilities,25 resulting in their respective functional biological activities. Literature suggests that binding affinity of a compound and subsequent coactivator recruitment are closely associated to the resulting biological response in the cell; however, there are some exceptions to this correlation.25, 33 Literature also shows that the SRC-2 coactivator has a moderately stronger affinity for ERα over ERβ.34 Collectively, this can explain the stronger TR-FRET assay readouts observed in our ERα data compared to ERβ (Table 1, Figure 3). In addition to SRC-2, there are two other isoforms, SRC-1 and SRC-3, which have even stronger reported affinity for ERα over ERβ.34
In general, the current SAR results from the binding assays mirror observations from the TR-FRET and functional estrogenic results in both ER subtypes (Table 1 & 2, Figure 1 - 5). Flavanone and flavone prenylation at C8 showed increased ligand binding, elevated SRC-2 recruitment to the ligand-ER complex, and higher functional activity compared to the non-prenylated congeners. In contrast, prenylation on the same site in isoflavonoids decreased these estrogenic activities (Table 1 & 2). The computational studies provide additional explanation for the observed estrogenic effects related to (iso)flavonoid prenylation. A hydrophobic groove is formed in the binding pocket of ER when bound by prenylated flavonoids similar to those tested in this study.32 This hydrophobic groove interacts with the prenyl group on the A-ring of flavonoids like 8-PN, as shown in the computational docking analysis (Figure 2A).32 The groove also likely envelops the prenyl group present on the C8 position of the A-ring of similar flavonoids such as 8-PA, stabilizing the compound in the pocket (Figure 2).32 This may explain the greater affinity of the 8-prenylated flavonoids for the ER and subsequent SRC-2 recruitment, as well as higher overall functional activity compared to their non-prenylated counterparts, NRG and API (Table 1 & 2, Figure 1 - 4).32 These outcomes are consistent with previous publications.10 However, prenylation on C-6, such as in 6-PN found in hops, leads to significantly lower functional estrogenic activity compared to what was observed with 8-PN demonstrating the importance of the correct positioning of the prenyl-group.15
The presence of a C3 hydroxyl group in flavonols resulted in lower overall estrogenic activity, (Table 1 & 2, Figure 1,3, & 4, Scheme 1A). However, the prenylated DMI had the highest overall estrogenic functional activity compared to the B-ring 4′-O-methylated ICT and non-prenylated KFL (Table 2, Figure 4C & 4D). Additionally, the functional potency of the unprenylated KFL with a 4′-OH group was significantly stronger than the prenylated and 4′-O-methylated ICT in ERβ (Scheme 1A, Table 2, Figure 4D). These results highlight the important role of the B-ring 4′-OH group in these (iso)flavonoid-receptor interactions as observed with KFL and ICT (Table 2, Figure 4D). This B-ring 4′-OH group mirrors the activity of the C3-hydroxyl group on the endogenous ligand, E2, because both moieties form hydrogen bonds with the same notable amino acid residues such as glutamic acid in the ER active site during ligand binding (Scheme 2).7, 35 Interestingly, non-prenylated KFL showed the highest overall SRC-2 recruitment activity compared to the prenylated congeners DMI and ICT especially in ERα in the coactivator affinity studies (Table 1, Figure 3). Additional comparative studies of coactivator recruitment, including ER conformations, by KFL and other similar structures may be beneficial to further explain these observations.
The isoflavonoid, 8-PG, and the flavone, 8-PA, are constitutional isomers. However, the B-ring in 8-PG is attached to the C-3-position instead of the 2-position, as it is the case with 8-PA (Scheme 1A and B). As a result, the B-ring in 8-PG will be positioned differently from that of flavonoids such as 8-PA in the ligand binding site (Figure 2B). This can lead to differences in binding affinity and activity (Figure 1 - 5). Interestingly, for isoflavonoids, the functional effects of prenylation are mainly observed in differences in the efficacy in ERα assays, while in ERβ, these effects are reflected in both potency and efficacy (Table 2, Figure 5). The reduction in estrogenic efficacy and potency that was observed for 8-PG as compared to non-prenylated GEN correlated with results from past studies and was further clarified in computational docking results (Figure 2B).36 API and GEN, which are also constitutional isomers of each other (Scheme 1), also bind differentially to the ER subtypes resulting in varying estrogenic activity (Table 2, Figure 4 & 5). While both compounds showed preferential ERβ activity, the isoflavone, GEN, was significantly more potent in ERβ (Figure 3 & 5). Similarly, in the ERβ assay, 8-PA also showed much stronger potency than the non-prenylated congener API, and comparable ERβ potency and ERβ-preferential activity to GEN (Table 2, Figure 4 & 5).4
The literature reports that ERα tends to be more transcriptionally dominant than ERβ37 and, therefore, the potential for ERα-dependent proliferation leading to increased risk of mammary carcinogenesis could be concerning.7 The present results show that 8-PA and GEN have stronger binding affinity to ERβ than to ERα and higher ERβ-preferential functional activity over ERα (Table 1 & 2, Figure 1, 4 & 5). Therefore, these compounds can be of clinical benefit in vivo, since sustained leverage towards ERβ cellular activity by these ERβ-preferential (iso)flavonoids could contribute to counter-balancing ERα pro-proliferative gene expression and maintain balanced overall activity in mammary tissue. Further studies are warranted to substantiate this observation.
Our results also indicate that the estrogenic activity of the tested extracts mimicked the potent/abundant bioactive (iso)flavonoids they contain. Prior studies have suggested 8-PN to be mainly responsible for the estrogenic activity observed in hops.15, 19 Similar to 8-PN, the studied hop extract was equipotent in both ER subtypes in functional assays (Table 2, Figure 5). The investigated red clover extract contains the potent isoflavonoid, GEN and its precursor biochanin A, present at 0.64% w/w and 15.2% w/w, respectively.14 Literature suggests that these two compounds contribute considerably to the observed estrogenic activity of red clover extracts.14, 20 Although some ERβ-preferential binding activity has been reported for biochanin A in competitive ER ligand binding assays,20 much of its reported in cell ERβ-preferential estrogenic activity is likely due to its metabolism to GEN.1 Similarly, in the current results, the tested red clover extract showed some ERβ-preferential activity over ERα (Table 2, Figure 6). Likewise, 8-PA, present in an inflata licorice extract at 0.17 % w/w, was also an ERβ-preferential flavonoid (Table 2, Figure 4).4 In addition, the flavanone, liquiritigenin (LigF), present at 0.12 % w/w in this inflata licorice extract has also been demonstrated to have ERβ preferential estrogenic activity.38, 39 However, 8-PA exhibited greater overall estrogenicity and a stronger ERβ-potency than LigF with an ERα EC50 for 8-PA of 0.050 μM (Table 2) and for LigF of 3.4 μM, as well as an ERβ EC50 for 8-PA of 0.0035 μM (Table 2) and for LigF of 0.037 μM.4, 40 The inflata licorice extract, like 8-PA and LigF, displayed overall preferential activity for ERβ over ERα (Table 2, Figure 6). Due to 8-PA’s higher estrogenic activity, 8-PA most likely had a greater contribution to the estrogenic activity of inflata licorice than LigF.
The considerable decrease in E2 serum levels occurring during perimenopause may lead to undesired symptoms.41 In clinical literature findings, isoflavones (e.g. genistein) from soy botanicals have been reported to alleviate menopausal symptoms without evidence of mammary tissue hyperproliferation, although these studies have known limitations.42 Women that use soy, red clover, and inflata licorice to alleviate menopausal symptoms may benefit from the ERβ-protective properties of their respective bioactive phytoestrogens, GEN and 8-PA. The botanical extract obtained from E. sagittatum had the lowest ERα and ERβ activity of all the extracts studied (Table 2, Figure 6). Epimedium species, including E. sagittatum, contain different ICT oligo-glycosides, also known as icariins.5 To mimic the hydrolysis of the glycosides in the intestine in vivo, the extract from E. sagittatum was hydrolyzed prior to biological analysis yielding an extract enriched in aglycones, including the weakly estrogenic ICT (Figure 4, S4 – S10). While DMI was only detected in traces in the hydrolyzed extract, ICT is metabolized by P450 enzymes to the estrogenic metabolite, DMI, (Scheme 1, Figure 4C & 4D) in vivo.43 The estrogenic results with E. sagittatum may mostly correlate with the effects observed with its weakly estrogenic major flavonol, ICT, and to a certain degree with its metabolite DMI. The present findings suggest that the estrogenic effects of the tested extracts could correlate with the order of activity of their corresponding bioactive (iso)flavonoids: in ERα: 8-PN/hops > 8-PA/inflata licorice ≈ GEN / red clover > DMI / E. sagittatum > ICT (Table 2); in ERβ: GEN/red clover ≥ 8-PA/inflata licorice ≈ 8-PN/spent hops >> DMI/E. sagittatum >> ICT/E. sagittatum (Table 2).
Because prenylated flavonoids were described to form cyclization products under some conditions,44 the stability of 8-PA (10 μM), as a prototype of a prenylated (iso)flavonoid, was analyzed in the alkaline phosphatase assay (estrogenic activity) in Ishikawa cells. After 24, 48, and 96 h of 8-PA incubation with Ishikawa cells, LC-MS/MS analysis of the media revealed that no 8-PA cyclization products were observed (supporting information, S13). This suggests that the bioactivity of 8-PA is due to the parent compound, 8-PA. As the other analyzed prenylated (iso)flavonoids have similar chemical properties, no cyclization products of these (iso)flavonoids are expected under the bioassay conditions. Therefore, the bioactivity is likely due to the parent (iso)flavonoids.
Like all botanicals, the four study botanicals contain many other constituents, such as various (iso)flavonoids, e.g., biochanin A and formononetin, in red clover,13 and other flavonoids and chalcones, such as XH, in hops.45 In addition to the sweet tasting triterpenoid, glycyrrhizic acid, a variety of flavonoids are characteristic compounds for licorice.1 Various flavonoids and lignins are also contained in E. sagittatum among other compounds.5 However, the focus of the current investigation was to analyze the SAR in respect to the ER-subtype activity of key (iso)flavonoids that have been described as major and/or biologically significant phytoestrogens in these four botanicals. These (iso)flavonoids were compared to ubiquitous (iso)flavonoids in food and plants, e.g. naringenin (Scheme 1). The primary aim of this SAR study was to analyze the influence of C8-prenylation, C3-hydroxy, C4’-methoxy groups, and C2-C3 (un)saturation on several endpoints: ERα/β-binding, (iso)flavonoid-ER complex coactivator (SRC-2) recruitment, and the overall ER-subtype activity of these (iso)flavonoids.
The analyzed phytoestrogens can activate both ER targets (ERα and ERβ), therefore, inducing various cellular pathways (e.g., ERα: relief of hot flashes, proliferation; ERβ: influence on memory, antiproliferation) leading to an overall “polypharmacological” effect.15, 46 Other constituents in the extract can also influence the specific phytoestrogen’s activity. In line with this, the concentration of the selected bioactive (iso)flavonoid in the extract, timing of supplementation, in vivo pharmacokinetics, estrogenic potency, and how their effect is influenced by the activity of other phytoconstituents in the extract, could influence how much a selected bioactive flavonoid modulates the activity of the botanical extract.1, 15 For example, existing reports show that hops neither increased nor decreased uterine tissue weight, while its bioactive flavonoid 8-PN, in dietary concentrations, increased uterine weight,1 likely indicating ERα activity.9
8-PA and GEN showed ERβ-preferential activity in vitro. Both compounds can contribute to the properties of inflata licorice and red clover, respectively, to regulate ERα pathways, and more notably provide ERβ activity with its associated benefits when consumed to alleviate menopausal symptoms. While the ERα activities of these extracts might offer favorable health benefits including assistance in management of postmenopausal symptoms, their ERβ-preferential activity can potentially provide a reduced risk of adverse effects and help maintain overall wellness. In vivo studies on estrogenic effects of GEN and red clover preparations have been reported;47 however, in vivo estrogenic studies evaluating the effects of 8-PA and inflata licorice are warranted based on the newly presented in vitro data. Finally, from the described SAR observations, potential estrogenic activity and related health benefits of similar (iso)flavonoids and of botanicals containing these types of phytoestrogens may be predicted.
Supplementary Material
Acknowledgments
We are thankful to R.B. Hochberg for providing the Ishikawa cells and Debra Tonetti for providing the ERβ stably transfected MDA-MB-231/β41. Shao-Nong Chen thanks Dr. Liang Zhao, LICP, CAS, for a generous gift of G. inflata plant material.
Funding
The present study was funded by grant # T32 AT007533 (predoctoral trainee: O.C.M.) awarded by the Office of the Director, National Institutes of Health (OD) and the National Center for Complementary & Integrative Health (NCCIH), and grant P50 AT000155 by NCCIH and ODS in support of the UIC Center for Botanical Dietary Supplements Research. Furthermore, this work was partially supported by grant U41 AT008706 (CENAPT). R.T.H. was supported by a NIH pre-doctoral fellowship (F31 AT010090). A. Hajirahimkhan was supported by a fellowship from the American Cancer Society (131667-PF-18–049-01-NEC).
Abbreviations used
- API
Apigenin
- BDS
Botanical Dietary Supplement
- DMI
Desmethylicaritin
- DGN
Dehydrogenistein
- DPN
2,3 bis-diarylpropionitrile
- ER
estrogen receptor
- GEN
Genistein
- HAPS
Hydroxyapatite Slurry
- ICT
Icaritin
- KFL
Kaempferol
- NRG
Naringenin
- PPT
Propylpyrazole triol
- 8-PA
8-prenylapigenin
- 8-PN
8-prenylnaringenin
- 8-PG
8-prenylgenistein
- RBA
Relative Binding Affinity
- RRE
Relative Recruitment Efficacy
- SAR
Structure Activity Relationship
- SRC
steroid receptor coactivator
- TR-FRET
Time Resolved Fluorescence Energy Transfer
Footnotes
Notes
The authors declare no competing financial interests.
Associated content: Supporting information
Supporting information (SI) is available free of charge on the ACS Publication website
This SI contains the qHNMR spectra and purity information of the analyzed (iso) flavonoids (S1); semi-preparative HPLC profiles for the re-purification of select (iso)flavonoids (S2); DNA barcoding sequences obtained for the botanical sold as horny goat weed (S3); percent composition (% w/w) of (iso)flavonoid per extract (S4); method for auto-hydrolysis of E. sagittatum (S5); calibration curve for the quantitation of icaritin in E. sagittatum preparations (S6); HPLC-UV profile of E. sagittatum extracts (S7); HPLC-UV profile crude methanol E. sagittatum extract with/without spiked icariin (S8); HPLC-UV profile of enzymatic extract with/without spiked icaritin standard (S9); LC-MS chromatogram of enzymatic extract confirming the identity of icaritin (S10); competitive Binding Assay method validation with estradiol in ERα and ERβ (S11); estrogenic functional assay method validation (S12); stability analysis of 8-PA under bioassay conditions (S13); related references (S14). All the NMR data and calculation spreadsheets related to the purity determination of the different (iso)flavonoids are made freely available via the Harvard Dataverse at https://doi.org/10.7910/DVN/HNMYE5.
References
- 1.Dietz BM; Hajirahimkhan A; Dunlap TL; Bolton JL, Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev 2016, 68 (4), 1026–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chlebowski RT; Hendrix SL; Langer RD; Stefanick ML; Gass M; Lane D; Rodabough RJ; Gilligan MA; Cyr MG; Thomson CA, Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA 2003, 289 (24), 3243–3253. [DOI] [PubMed] [Google Scholar]
- 3.Booth NL; Piersen CE; Banuvar S; Geller SE; Shulman LP; Farnsworth NR, Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause: a literature review. Menopause 2006, 13 (2), 251–264. [DOI] [PubMed] [Google Scholar]
- 4.Hajirahimkhan A; Mbachu O; Simmler C; Ellis S; Dong H; Nikolić D; Lankin DC; van Breemen RB; Chen SN; Pauli GF; Dietz BM; Bolton JL, Estrogen Receptor (ER) Subtype Selectivity Identifies 8-Prenylapigenin as an ERβ Agonist from Glycyrrhiza inflata and Highlights the Importance of Chemical and Biological Authentication. J. Nat. Prod 2018, 81, 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma H; He X; Yang Y; Li M; Hao D; Jia Z, The genus Epimedium: an ethnopharmacological and phytochemical review. J. Ethnopharmacol 2011, 134 (3), 519–541. [DOI] [PubMed] [Google Scholar]
- 6.Huang SSC; Komatsu K; Ren SSJ; Lien LL; Lien EJ, Phytohormones and antioxidants in Chinese herbs and natural food. Int. J. Oriental Med 2001, 26 (2), 66–86. [Google Scholar]
- 7.Nilsson S; Koehler KF; Gustafsson JÅ, Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug. Discov 2011, 10 (10), 778. [DOI] [PubMed] [Google Scholar]
- 8.Paterni I; Granchi C; Katzenellenbogen JA; Minutolo F, Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diel P; Thomae RB; Caldarelli A; Zierau O; Kolba S; Schmidt S; Schwab P; Metz P; Vollmer G, Regulation of gene expression by 8-prenylnaringenin in uterus and liver of Wistar rats. Planta Med. 2004, 70 (01), 39–44. [DOI] [PubMed] [Google Scholar]
- 10.Helle J; Kräker K; Bader MI; Keiler AM; Zierau O; Vollmer G; Welsh J; Kretzschmar G, Assessment of the proliferative capacity of the flavanones 8-prenylnaringenin, 6-(1.1-dimethylallyl) naringenin and naringenin in MCF-7 cells and the rat mammary gland. Mol. Cell Endocrinol 2014, 392 (1), 125–135. [DOI] [PubMed] [Google Scholar]
- 11.Pauli GF; Chen SN; Simmler C; Lankin DC; Gö T; Jaki BU; Friesen JB; McAlpine JB; Napolitano JG, Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay: miniperspective. J. Med. Chem 2014, 57 (22), 9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmler C; Anderson JR; Gauthier L; Lankin DC; McAlpine JB; Chen SN; Pauli GF, Metabolite profiling and classification of DNA-authenticated licorice botanicals. J. Nat. Prod 2015, 78 (8), 2007–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth NL; Overk CR; Yao P; Burdette JE; Nikolic D; Chen SN; Bolton JL; van Breemen RB; Pauli GF; Farnsworth NR, The chemical and biologic profile of a red clover (Trifolium pratense L.) phase II clinical extract. J. Altern. Complement. Med 2006, 12 (2), 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phansalkar RS; Simmler C; Bisson J; Chen SN; Lankin DC; McAlpine JB; Niemitz M; Pauli GF, Evolution of quantitative measures in NMR: quantum mechanical qHNMR advances chemical standardization of a red clover (Trifolium pratense) extract. J. Nat. Prod 2017, 80 (3), 634–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz BM; Chen SN; Alvarenga RFR; Dong H; Nikolić D; Biendl M; van Breemen RB; Bolton JL; Pauli GF, DESIGNER extracts as tools to balance estrogenic and chemopreventive activities of botanicals for women’s health. J. Nat. Prod 2017, 80 (8), 2284–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SN; Lankin DC; Chadwick LR; Jaki BU; Pauli GF, Dynamic residual complexity of natural products by qHNMR: solution stability of desmethylxanthohumol. Planta Med. 2009, 75 (7), 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin X; Zhang Z; Sun E; Li S; Jia X, Statistically designed enzymatic hydrolysis of an icariin/β-cyclodextrin inclusion complex optimized for production of icaritin. Acta. Pharm. Sin. B 2012, 2 (1), 83–89. [Google Scholar]
- 18.Hwang CS; Kwak HS; Lim HJ; Lee SH; Kang YS; Choe TB; Hur HG; Han KO, Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem 2006, 101 (4–5), 246–253. [DOI] [PubMed] [Google Scholar]
- 19.Milligan S; Kalita J; Pocock V; Van De Kauter V; Stevens J; Deinzer M; Rong H; De Keukeleire D, The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J. Clin. Endocr. Metab 2000, 85 (12), 4912–4915. [DOI] [PubMed] [Google Scholar]
- 20.Overk CR; Yao P; Chadwick LR; Nikolić D; Sun Y; Cuendet MA; Deng Y; Hedayat A; Pauli GF; Farnsworth NR, Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense). J. Agric. Food Chem 2005, 53 (16), 6246–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y; Gong P; Madak-Erdogan Z; Martin T; Jeyakumar M; Carlson K; Khan I; Smillie TJ; Chittiboyina AG; Rotte SC, Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J 2013, 27 (11), 4406–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speltz TE; Fanning SW; Mayne CG; Fowler C; Tajkhorshid E; Greene GL; Moore TW, Branching Out: γ-Methylated Hydrocarbon Stapled Peptides for the Estrogen Receptor/Coactivator Interaction. Angew. Chem. Int. Ed. Engl 2016, 55 (13), 4252–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunther JR; Du Y; Rhoden E; Lewis I; Revennaugh B; Moore TW; Kim SH; Dingledine R; Fu H; Katzenellenbogen JA, A set of time-resolved fluorescence resonance energy transfer assays for the discovery of inhibitors of estrogen receptor-coactivator binding. J. Biomol. Screen 2009, 14 (2), 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisha E; Pezzuto JM, Cell-based assay for the determination of estrogenic and anti-estrogenic activities. Methods Cell Sci. 1997, 19, 37–43. [Google Scholar]
- 25.Jeyakumar M; Carlson KE; Gunther JR; Katzenellenbogen JA, Exploration of dimensions of estrogen potency parsing ligand binding and coactivator binding affinities. J. Biol. Chem 2011, 286 (15), 12971–12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafsson JA, Estrogen receptor beta—a new dimension in estrogen mechanism of action. J. Endocrinol 1999, 163 (3), 379–383. [DOI] [PubMed] [Google Scholar]
- 27.Rietjens IM; Sotoca AM; Vervoort J; Louisse J, Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res 2013, 57 (1), 100–113. [DOI] [PubMed] [Google Scholar]
- 28.Park BW; Kim KS; Heo MK; Ko SS; Lee KS; Hong SW; Yang WI; Kim JH; Kim GE, Expression of estrogen receptor β in normal mammary and tumor tissues: is it protective in breast carcinogenesis? Breast Cancer Res. Treat 2003, 80 (1), 79–85. [DOI] [PubMed] [Google Scholar]
- 29.Roger P; Sahla ME; Mäkelä S; Gustafsson JÅ; Baldet P; Rochefort H, Decreased expression of estrogen receptor β protein in proliferative preinvasive mammary tumors. Cancer Res. 2001, 61 (6), 2537–2541. [PubMed] [Google Scholar]
- 30.Paruthiyil S; Parmar H; Kerekatte V; Cunha GR; Firestone GL; Leitman DC, Estrogen receptor β inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 2004, 64 (1), 423–428. [DOI] [PubMed] [Google Scholar]
- 31.Warbrick E; Lane DP; Glover DM; Cox LS, A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr. Biol 1995, 5 (3), 275–282. [DOI] [PubMed] [Google Scholar]
- 32.van de Schans MG; Ritschel T; Bovee TF; Sanders MG; de Waard P; Gruppen H; Vincken JP, Involvement of a hydrophobic pocket and helix 11 in determining the modes of action of prenylated flavonoids and isoflavonoids in the human estrogen receptor. ChemBioChem 2015, 16 (18), 2668–2677. [DOI] [PubMed] [Google Scholar]
- 33.Strunck E; Stemmann N; Hopert AC; Wünsche W; Frank K; Vollmer G, Relative binding affinity does not predict biological response to xenoestrogens in rat endometrial adenocarcinoma cells. J. Steroid Biochem 2000, 74 (3), 73–81. [DOI] [PubMed] [Google Scholar]
- 34.Wong CW; Komm B; Cheskis BJ, Structure−function evaluation of ERα and β interplay with SRC family coactivators. ER selective ligands. Biochemistry 2001, 40 (23), 6756–6765. [DOI] [PubMed] [Google Scholar]
- 35.Wang ZQ; Weber N; Lou YJ; Proksch P, Prenylflavonoids as nonsteroidal phytoestrogens and related structure–activity relationships. ChemMedChem 2006, 1 (4), 482–488. [DOI] [PubMed] [Google Scholar]
- 36.Kretzschmar G; Zierau O; Wober J; Tischer S; Metz P; Vollmer G, Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J. Steroid Biochem 2010, 118 (1–2), 1–6. [DOI] [PubMed] [Google Scholar]
- 37.Li X; Huang J; Yi P; Bambara RA; Hilf R; Muyan M, Single-chain estrogen receptors (ERs) reveal that the ERα/β heterodimer emulates functions of the ERα dimer in genomic estrogen signaling pathways. Mol. Cell. Biol 2004, 24 (17), 7681–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajirahimkhan A; Simmler C; Yuan Y; Anderson JR; Chen SN; Nikolić D; Dietz BM; Pauli GF; van Breemen RB; Bolton JL, Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS One 2013, 8 (7), e67947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunlap TL; Wang S; Simmler C; Chen SN; Pauli GF; Dietz BM; Bolton JL, Differential effects of glycyrrhiza species on genotoxic estrogen metabolism: licochalcone A downregulates P450 1B1, whereas isoliquiritigenin stimulates it. Chem. Res. Toxicol 2015, 28 (8), 1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mersereau JE; Levy N; Staub RE; Baggett S; Zogric T; Chow S; Ricke WA; Tagliaferri M; Cohen I; Bjeldanes LF, Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Mol. Cell Endocrinol 2008, 283 (1–2), 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JS; Ettinger B; Stanczyk FZ; Vittinghoff E; Hanes V; Cauley JA; Chandler W; Settlage J; Beattie MS; Folkerd E, Comparison of methods to measure low serum estradiol levels in postmenopausal women. J. Clin. Endocr. Metab 2006, 91 (10), 3791–3797. [DOI] [PubMed] [Google Scholar]
- 42.Cheng G; Wilczek B; Warner M; Gustafsson JÅ; Landgren B-M, Isoflavone treatment for acute menopausal symptoms. Menopause 2007, 14 (3), 468–473. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B; Chen X; Zhang R; Zheng F; Du S; Zhang X, Metabolite profiling, pharmacokinetics, and in vitro glucuronidation of icaritin in rats by ultra-performance liquid chromatography coupled with mass spectrometry. J. Anal. Methods Chem 2017, 2017, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao Z; Liu J; Jiang Y; Gong L; Yang B, Synthesis of prenylated flavonols and their potents as estrogen receptor modulator. Sci. Rep 2017, 7 (1), 12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chadwick LR; Nikolic D; Burdette JE; Overk CR; Bolton JL; van Breemen RB; Fröhlich R; Fong HH; Farnsworth NR; Pauli GF, Estrogens and Congeners from Spent Hops (Humulus lupulus). J. Nat. Prod 2004, 67 (12), 2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H; Wang J; Zhou W; Wang Y; Yang L, Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J. Ethnopharmacol 2013, 146 (3), 773–793. [DOI] [PubMed] [Google Scholar]
- 47.Overk CR; Guo J; Chadwick LR; Lantvit DD; Minassi A; Appendino G; Chen SN; Lankin DC; Farnsworth NR; Pauli GF; van Breemen RB; Bolton JL, In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin. Chem. Biol. Interact 2008, 176, 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


