Abstract
Background
JAM3 gene, located on human chromosome 11q25, encodes a member of the junctional adhesion molecule (JAM) family. Mutations of this gene are associated with hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts (HDBSCC).
Case report
Herein, we present a newborn male with a prenatal suspicion of bilateral cataracts but without fetal ultrasound findings of cortical malformations. He was postnatally diagnosed with a clinical picture of HDBSCC and Early-onset Developmental and Epileptic Encephalopathy (DEE), associated to a homozygous variant of JAM3 gene.
Conclusion
Identification of this variant in affected individuals has implications for perinatal and postnatal management and genetic counseling. To the best of our knowledge, this is the first case reported of a child with a JAM3 variant in Italy, from a different ethnic background than the other reported children until now (Saudi Arabian, Turkish, Afghani, and Moroccan origin). JAM3 screening could be requested in prenatal diagnosis of fetal congenital cataracts and included in Next-Generation DNA Sequencing panels.
Keyword: JAM3; Seizures; Cerebral hemorrhages; Cataracts; Neonate; Infant; COL4A1
Background
JAM3, located on human chromosome 11q25, is a gene that encodes a member of the junctional adhesion molecule (JAM) family [1–3].
Mutations of this gene are associated with hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts (HDBSCC, OMIM #613,730) [4, 5].
By genome wide linkage analysis followed by candidate gene sequencing of a Saudi Arabian family with HDBSCC, Mochida et al. identified a homozygous loss-of-function mutation in the JAM3 gene (OMIM*606,871). Affected individuals either died in early infancy or survived with profound developmental delay, spasticity, and seizures [4]. These findings showed how much JAM3 is important for maintaining the integrity of cerebral endothelial cells (avoiding hemorrhages) and for normal lens development in humans (avoiding cataract), confirming data seen in JAM3-deficient mice [6–8].
Three years later, in affected members of 3 unrelated families with HDBSCC (one Turkish, one Afghani, and one Moroccan), Akawi et al. identified 3 different homozygous loss-of-function mutations in JAM3 gene [5], with a similar phenotype to that reported by Mochida in 2010 [4].
Herein, we present a newborn male with a prenatal suspicion of bilateral cataracts but without fetal ultrasound findings of cortical malformations. He was diagnosed postnatally with a clinical picture of HDBSCC associated to a mutation of JAM3 gene: the 24-month follow-up was described.
Results
Clinical report
An Italian child was born to healthy but consanguineous parents at 37 weeks of gestational age (GA). Both parents were from central Italy. His 36-year-old mother had no symptomatic infections and was negative on TORCH infection screening during pregnancy. He was the first child and had no family history of neurological disorders or inherited cataracts. Bilateral crystalline hyperechogenities were previously detected at ultrasound assessment of 20 weeks GA. However, fetal magnetic resonance imaging (MRI) excluded a cataract and ischemic–hemorrhagic cerebral abnormalities at 20 weeks and 5 days GA.
The baby was delivered at term via a normal spontaneous delivery, with an Apgar score of 9 and 10 at 1 and 5 min after birth, respectively. The child’s auxological parameters at birth were all appropriate for gestational age according INeS Charts: weight 2750 g (25th centile), length 48 cm (25–50th centile); head circumference 34 cm (50th centile). No major malformations were detected, except for a bilateral cataract. At about 12 h of life, he was irritable and exhibited excessive startle, hypotonia, and apnea. Therefore, he was transferred from maternity room to our Neonatal Intensive Care Unit (NICU), where a cranial ultrasound showed periventricular leukomalacia.
Focal seizures started in second day of life, presenting with non-motor/autonomic signs (cyanosis and tachycardia) followed by bilateral myoclonic jerks and generalized hypertonia. Electroclinical and electrographic only episodes were observed. Seizures became more and more frequent until status epilepticus that was refractory to phenobarbital; the infant required mechanical ventilation. The status epilepticus was controlled with continuous infusion of midazolam. An Early-onset Developmental and Epileptic Encephalopathy (DEE) was documented. Multiple intraventricular hemorrhages (IVH) and intraparenchymal hemorrhages (IPH) were detected on brain magnetic resonance imaging (MRI): hemorrhages were observed, in particular, in left hemisphere (frontal and temporo-occipital regions), without midline shift. Other abnormalities included multiple periventricular calcifications, porencephalic cysts, and hypoplasia of cerebellar vermis (Fig. 1). The progressive post-hemorrhagic hydrocephalus (PHH) was managed with a ventriculo-subgaleal shunt (VSGS) on 12th day of life.
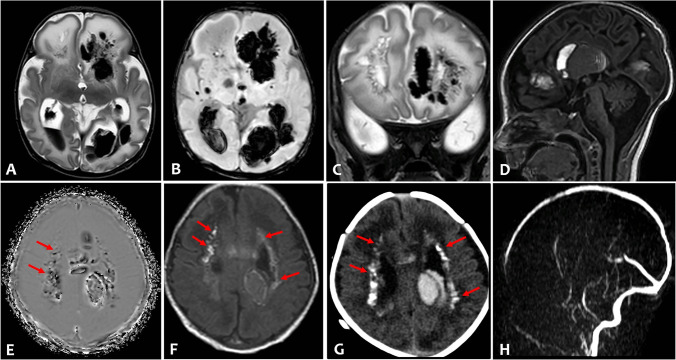
Fig. 1.
Axial T2-weighted (A) and susceptibility weighted imaging (B) of brain MRI showed severe intraventricular hemorrhage and bilateral multiple intraparenchymal hemorrhages, prevailing in the left hemisphere, and enlargement of the posterior horns of the lateral ventricles. Coronal T2-weighted (C) showed multiple cysts in the frontal periventricular white matter. Sagittal T1-weighted (D) revealed also a large intraventricular clot in the III ventricle and hypoplasia of cerebellar vermis. Multiple periventricular calcifications (arrows) were demonstrated on phase axial susceptibility weighted image (E), on T1-weighted (F) image, and on corresponding axial CT (G). There was no evidence of venous thrombosis and vascular malformation on MR venogram (H) and time of flight angiography (not shown)
An extensive metabolic examination for plasma and urinary amino acids and urinary organic acids, and screening for galactosemia were all normal. Creatine kinase (CK) concentration was normal. Initially a TORCH infection was suspected, but all tests for toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus were negative for the child and her mother. Therefore, we hypothesized that the patient had an underlying genetic disease that was associated with vascular vulnerability. Array-comparative genomic hybridization (array-CGH) was ordered and did not disclose any alterations.
A complete heart assessment revealed a myocardial hypertrophy. Subclinical arterial hypertension was noticed at 1 month of age, and the presence of renal hypertension was identified based on the elevation of the plasma renin activity; however, abdomen ultrasonography examination detected no internal organ defects, and no apparent stenosis was detected in either of the renal arteries. An oral calcium channel blocker (amlodipine 0.2 mg/kg/day) was transiently administered with a good control of blood pressure.
Ophthalmological examination showed congenital bilateral cataract, without other abnormalities. Vitrectomy was performed at 2 months of life.
A good seizure control was gradually obtained using levetiracetam and phenobarbital. The baby was then discharged from NICU with a strict follow-up program.
Genetic analysis
Considering suspected cortical malformations and cataract, the coding regions of COL4A1 and JAM3 genes were analyzed by performing a Next Generation Sequencing by Nextera (Illumina) assay for targeted resequencing. After bioinformatics analysis and variant filtering and prioritization, the patient was found to carry a novel homozygous variant in exon 6 of JAM3 gene: c.690 T > G (NM_032801.5) p.Cys230Trp (NP_116190.3). This homozygous variant was confirmed by Sanger sequencing. His father and his mother were both heterozygous carriers (Fig. 2). JAM3 mutations have been reported as associated to recessive forms of brain hemorrhages, subependymal calcifications and cataract (OMIM #606,871). This variant has not been reported until now in literature and is highly likely to be pathogenic according to American College of Medical Genetics (ACMG) criteria [9]. We also assessed the pathogenicity of this mutation using Poly-Phen2 software (http://genetics.bwh.harvard.edu/pph2/index.shtml) that identified it as “Probably Damaging”, suggesting that this novel variant has high pathogenicity. Identified variant was classified according to the HGVS nomenclature (www.hgvs.org/mutnomen).
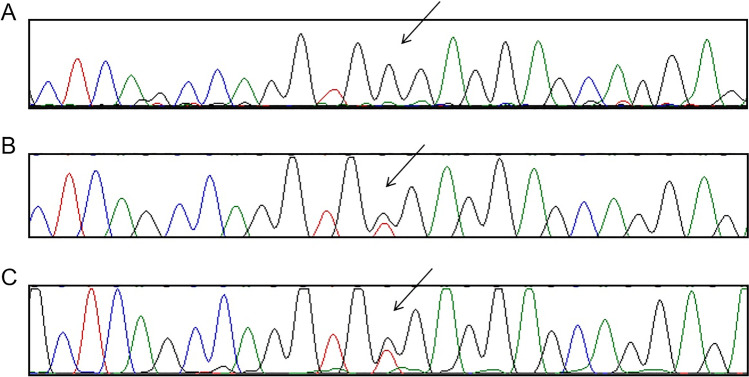
Fig. 2.
(A) Sanger sequencing confirmed the homozygous variation, c.690 T > G, in the proband. (B and C) Sanger sequencing shown that the parents were both carrying the heterozygous variation
Follow-up
During a 2-year follow-up (Fig. 3A–D), he showed a marked growth impairment; an Early-Onset Developmental and Epileptic Encephalopathy (DEE) was confirmed, associated with spastic tetraparesis. He received percutaneous endoscopic gastrostomy (PEG) for feeding.
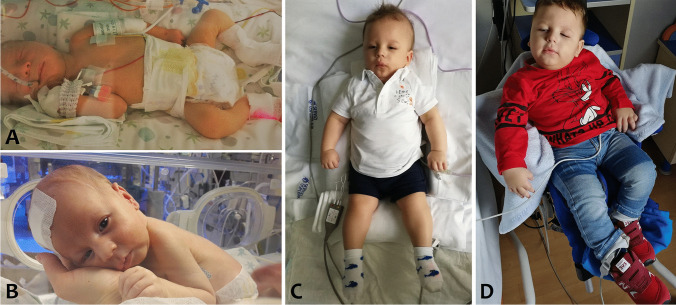
Fig. 3.
The patient during NICU stay (A–B), at 12 months (C) and at 24 months (D)
Serial head ultrasound and brain MRI showed a gradual worsening of ventricular dilatation (Fig. 4A,F; B,G): a ventriculoperitoneal shunt (VPS) was implanted at 4 months of life. Subsequent MRI (Fig. 4C, H; D,I) showed enlargement of porencephalic cystic and reduction of white-matter volume (destructive changes), and consensual gradual worsening of ventricular dilatation. At 2 years of life, brain MRI showed a plastic re-organization of porencephalic cysts and lateral ventricles with reduction of signs of cerebrospinal fluid’s decompensation (Fig. 4E,L).
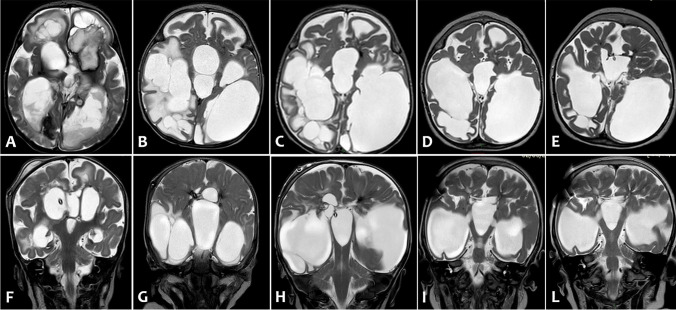
Fig. 4.
Axial (A–E) and coronal (F-L) T2-weighted images of serial brain MRI: (A,F) 1 month of life, (B–G) 3 months of life, (C–H) 4 months of life, (D–I) 15 months of life, (E–L) 2 years of life. Serial MRI scans showed porencephalic cystic evolution and reduced white-matter volume (indicative of destructive changes), and consensual gradual worsening of ventricular dilatation unless a ventriculoperitoneal shunt was implanted at 4 months of life. Last two MRI exams (D-I and E-L) showed a plastic re-organization of porencephalic cysts and lateral ventricles with reduction of signs of cerebrospinal fluid’s decompensation
The epileptic disorder was characterized by focal drug-resistant epilepsy, requiring to different antiepileptic drugs (AEDs) be added/substituted. Awake and sleep electroencephalogram (EEG) studies showed a severe alteration of brain electrical activity due to the organization of the background rhythm (a diffuse theta rhythm with posterior predominance) and due to the presence of intermittent bursts of high-voltage sharp waves and spikes. At time of writing, he receives topiramate, clonazepam, vigabatrin, lamotrigine with a sufficient seizure control.
Microphthalmia is evident; ocular motility is poorly evaluable, with horizontal and rotary nystagmus jerks. Spontaneous mobility is reduced with intermittent occurrences of abrupt and synchronized movements of the limbs. He shows a spastic quadriplegia with axial hypotonia. He has no head control and no functional use of the hands. A spinal X-ray performed at two years showed a dorsal-lumbar scoliosis. He wears ankle–foot orthosis, and he sits on a polyfunctional chair.
During the Italian COVID-19 s wave, he was found positive for SARS-CoV-2 by real-time PCR on nasopharyngeal swab. However, he is still alive and never developed fever or any signs of infection.
Discussion
Dreadful cerebrovascular diseases could be related to impaired integrity of blood vessels, as we describe in this clinical report of a term neonate with early-onset multiple intraventricular hemorrhages (IVH) and intraparenchymal hemorrhages (IPH).
Recent studies have tested the hypothesis that IVH may be secondary to variability in some risk genes [10]. Among these genes, COL4A1 gene (OMIM*120,130), located on human chromosome 13q34, encodes type IV collagen alpha-1 chain protein, which is expressed in all human tissues, especially in vessels and brain. To date, many pathogenic COL4A1 mutations have been identified. A wide spectrum of clinical manifestations, associated with COL4A1 mutations, may be caused by aberration of basement membranes of blood vessels. Neurological features are the most prominent but are rather nonspecific: antenatal intracranial hemorrhage, porencephaly, brain small-vessel disease with hemorrhage or vascular leukoencephalopathy have been reported [11–17]. Ophthalmological features associated with COL4A1 mutations are heterogeneous and include congenital cataracts [18].
Similarly, dysfunction of the tight junction components (occludin — OCLN, and junctional adhesion molecules — JAMs) has been associated with an overlap clinical presentation. There are three types of JAMs: JAM1 (expressed in endothelial and epithelial cells) and JAM2 and JAM3 (expressed only in vascular endothelial cells). JAM-1 is a ligand involved in trans-endothelial migration of leukocytes [19]. JAM2 plays a key role in blood–brain-barrier permeability and bi-allelic JAM2 variants have been related to early-onset recessive primary familial brain calcification (OMIM #618,824): Schottlaender et al. suggested that defective cell-to-cell adhesion and dysfunction of the movement of solutes through the paracellular spaces in the neurovascular unit could be a key mechanism in central nervous system calcifications [20]. JAM-3 functions similarly to JAM-2 and its mutations have been identified to cause hemorrhagic destruction of the brain [4, 5]. According to findings by Daniele et al. (2007), mouse JAM3 apical expression was also found on the embryonic retinal neuroepithelia, and its deficiency caused nuclear cataracts [21].
Conversely, OCLN is associated with autosomal recessive Pseudo-TORCH syndrome 1 (OMIM #251,290): affected individuals have congenital microcephaly, intracranial calcifications, simplified gyration and polymicrogyria, and severe developmental delay [22]. Patients with OCLN mutations did not have intracranial hemorrhages or congenital cataracts; only two siblings reported by Slee et al. (1999) also presented with congenital cataracts [23].
Herein, we reported a novel homozygous variant in the tight-junction gene JAM3 in an Italian child with severe hemorrhagic destruction of the brain and bilateral congenital cataracts as cardinal features. He was diagnosed postnatally with a clinical picture of HDBSCC. Echocardiography displayed no anatomical heart defects, but only myocardial hypertrophy. We did not observe in our patient thrombocytopenia, hepatomegaly, renal anomalies, or other organ involvement as previously reported in some patients [4, 5]. As reported by Akawi et al., the disorder is likely due to the total loss of the protein function as a result of the mutation [5].
COL4A1-related disease could have an overlap clinical presentation: according Tonduti et al., raised CK concentration, in addition to intracranial calcifications, is to be considered a useful pointer to a final diagnosis of COL4A1-related disease [24].
Our clinical report stresses the importance of screening also any JAM3 mutation when CK concentration is normal, such as in our patient. A normal CK concentration, in addition to intracranial calcifications and diffuse cerebral hemorrhages, should therefore lead to analyze JAM3 gene in the suspicion of HDBSCC.
To the best of our knowledge, this is the first case reported of a child with a JAM3 variant in Italy, from a different ethnic background than the other reported children until now (Saudi Arabian, Turkish, Afghani, and Moroccan origin).
Furthermore, congenital cataract is a genetically heterogeneous disease [25]. Next-generation DNA sequencing (NGS) technologies have been validated to determine the precise genetic cause of bilateral congenital cataracts in about 75% of individuals but JAM3 is not usually included in these panels [26, 27]. Our results contribute to expand the mutational spectrum of genes causing congenital cataract, in approaching the prenatal diagnosis.
Conclusion
In conclusion, we reported a phenotype resembling a TORCH infection caused by a novel variant in JAM3. We suggest COL4A1 and JAM3 testing in patients with post-hemorrhagic hydrocephalus, porencephalic cysts, and cataract as well as suspected TORCH infection without any proven etiological factors. Furthermore, JAM3 screening could be requested in prenatal diagnostic work-up of fetal congenital cataracts and included in NGS panels.
This is important, as identification of this variant in affected individuals has implications for perinatal and postnatal management and genetic counseling. In addition, making this diagnosis may help tailor appropriate screening tests for organs typically catastrophically involved.
Conflict of interest
The authors declare no potential conflict of interest with respect to the research, authorship and/or publication of this article.
Ethical approval
The authors declare that they have followed the protocols of their Institutional Review Board on the publication of the patient's data and the Helsinki Declaration; no patient is recognizable from data within this article. We obtained written informed consent from the parents beforehand in order to report these findings.
Acknowledgements
The authors thank the parents for their kindness and gratitude toward us.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arrate MP, Rodriguez JM, Tran TT, et al. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J Biol Chem. 2001;276:45826–45832. doi: 10.1074/jbc.M105972200. [DOI] [PubMed] [Google Scholar]
- 2.Liang TW, Chiu HH, Gurney A, et al. Vascular endothelial-junctional adhesion molecule (VE-JAM)/JAM 2 interacts with T, NK, and dendritic cells through JAM 3. J Immun. 2002;168:1618–1626. doi: 10.4049/jimmunol.168.4.1618. [DOI] [PubMed] [Google Scholar]
- 3.Santoso S, Sachs UJH, Kroll H, et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mochida GH, Ganesh VS, Felie JM, et al. A homozygous mutation in the tight-junction protein JAM3 causes hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Am J Hum Genet. 2010;87:882–889. doi: 10.1016/j.ajhg.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akawi NA, Canpolat FE, White SM, et al. Delineation of the clinical, molecular and cellular aspects of novel JAM3 mutations underlying the autosomal recessive hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Hum Mutat. 2013;34:498–505. doi: 10.1002/humu.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gliki G, Ebnet K, Aurrand-Lions M, et al. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 7.Imhof BA, Zimmerli C, Gliki G, et al. Pulmonary dysfunction and impaired granulocyte homeostasis result in poor survival of Jam-C-deficient mice. J Pathol. 2007;21:198–208. doi: 10.1002/path.2163. [DOI] [PubMed] [Google Scholar]
- 8.Daniele LL, Adams RH, Durante DE, et al. Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium. J Comp Neurol. 2007;505:166–176. doi: 10.1002/cne.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adén U, Lin A, Carlo W, et al. Candidate gene analysis: severe intraventricular hemorrhage in inborn preterm neonates. J Pediatr. 2013;163(5):1503–6.e1. doi: 10.1016/j.jpeds.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smigiel R, Cabala M, Jakubiak A, et al. Novel COL4A1 mutation in an infant with severe dysmorphic syndrome with schizencephaly, periventricular calcifications, and cataract resembling congenital infection. Birth Defects Res A Clin Mol Teratol. 2016;106(4):304–307. doi: 10.1002/bdra.23488. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenbelt KD, Pistorius LR, De Tollenaer SM, et al. Prenatal genetic confirmation of a COL4A1 mutation presenting with sonographic fetal intracranial hemorrhage. Ultrasound Obstet Gynecol. 2012;39(6):726–727. doi: 10.1002/uog.11070. [DOI] [PubMed] [Google Scholar]
- 13.Livingston J, Doherty D, Orcesi S, et al. COL4A1 mutations associated with a characteristic pattern of intracranial calcification. Neuropediatrics. 2011;42(6):227–233. doi: 10.1055/s-0031-1295493. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen RJ, Peeters-Scholte C, Van Vugt JJ, et al. Fetal origin of brain damage in 2 infants with a COL4A1 mutation: fetal and neonatal MRI. Neuropediatrics. 2011;42(1):1–3. doi: 10.1055/s-0031-1275343. [DOI] [PubMed] [Google Scholar]
- 15.Meuwissen ME, de Vries LS, Verbeek HA, et al. Sporadic COL4A1 mutations with extensive prenatal porencephaly resembling hydranencephaly. Neurology. 2011;76(9):844–846. doi: 10.1212/WNL.0b013e31820e7751. [DOI] [PubMed] [Google Scholar]
- 16.Bilguvar K, DiLuna ML, Bizzarro MJ, et al. COL4A1 mutation in preterm intraventricular hemorrhage. J Pediatr. 2009;155(5):743–745. doi: 10.1016/j.jpeds.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries LS, Koopman C, Groenendaal F, et al. COL4A1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann Neurol. 2009;65(1):12–18. doi: 10.1002/ana.21525. [DOI] [PubMed] [Google Scholar]
- 18.Coupry I, Sibon I, Mortemousque B, et al. Ophthalmological features associated with COL4A1 mutations. Arch Ophthalmol. 2010;128(4):483–489. doi: 10.1001/archophthalmol.2010.42. [DOI] [PubMed] [Google Scholar]
- 19.Ostermann G, Weber KS, Zernecke A, et al. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3(2):151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 20.Schottlaender LV, Abeti R, Jaunmuktane Z, et al. Bi-allelic JAM2 variants lead to early-onset recessive primary familial brain calcification. Am J Hum Genet. 2020;106(3):412–421. doi: 10.1016/j.ajhg.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniele LL, Adams RH, Durante DE, et al. Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium. J Comp Neurol. 2007;505(2):166–176. doi: 10.1002/cne.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Driscoll MC, Daly SB, Urquhart JE, et al. Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am J Hum Genet. 2010;87(3):354–364. doi: 10.1016/j.ajhg.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slee J, Lam G, Walpole I. Syndrome of microcephaly, microphthalmia, cataracts, and intracranial calcification. Am J Med Genet. 1999;84(4):330–333. doi: 10.1002/(SICI)1096-8628(19990604)84:4<330::AID-AJMG4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Tonduti D, Pichiecchio A, La Piana R, et al. COL4A1-related disease: raised creatine kinase and cerebral calcification as useful pointers. Neuropediatrics. 2012;43(5):283–288. doi: 10.1055/s-0032-1325116. [DOI] [PubMed] [Google Scholar]
- 25.Deng H, Yuan L. Molecular genetics of congenital nuclear cataract. Eur J Med Genet. 2014;57(2–3):113–122. doi: 10.1016/j.ejmg.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Gillespie RL, O'Sullivan J, Ashworth J et al (2014) Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmol 121(11):2124–37.e1–2 [DOI] [PubMed]
- 27.Astiazarán MC, García-Montaño LA, Sánchez-Moreno F, et al. Next generation sequencing-based molecular diagnosis in familial congenital cataract expands the mutational spectrum in known congenital cataract genes. Am J Med Genet A. 2018;176(12):2637–2645. doi: 10.1002/ajmg.a.40524. [DOI] [PubMed] [Google Scholar]