Abstract
Background
Multiple sclerosis (MS) organizations have recommended that adults with MS obtain the COVID-19 vaccination. Vaccine hesitancy is a barrier to full COVID-19 inoculation in the general population. Whether vaccine hesitancy is also a barrier towards optimizing vaccination rates in the MS community is unknown. To investigate vaccine hesitancy and inform efforts to increase vaccine uptake in the MS population, we conducted a follow up survey of a national sample of adults with MS living in the United States who completed an initial survey early in the COVID-19 pandemic. The current study aimed to answer questions vital to understanding vaccine hesitancy, specifically: (1) What is the prevalence of COVID-19 vaccine hesitancy in early 2021? (2) What are the reasons for and factors associated with current hesitancy? (3) How has vaccine willingness and hesitancy changed from April/May 2020 to January/February 2021? and (4) Who has changed in their vaccine willingness?
Methods
Adults with MS living in the United States (N = 359) completed two online surveys (the first between 10 April 2020 and 06 May 2020; the second between 11 January 2021 and 08 February 2021) about their willingness and intent to obtain a COVID-19 vaccine. Participants also completed measures to assess factors potentially related to vaccine hesitancy, including demographics, MS variables, influenza vaccine history, vaccine concerns, and contextual factors, including perceived risk for SARS-CoV-2 infection, trust in COVID-19 information source, anxiety, and loneliness.
Results
Of the participants who completed the second survey in early 2021, 20.3% were vaccine hesitant, that is, either reporting that they were undecided (13.9%) or not intending to get vaccinated (6.4%). Vaccine hesitancy decreased between the two surveys, with nearly three-fourths (73.8%) of the second sample reporting that they planned to obtain the COVID-19 vaccine. Vaccine hesitancy was associated with having a lower level of education, being non-White, not having a recent flu vaccination, holding a lower perception of one's risk of getting COVID-19, and having lower trust in the Centers for Disease Control and Prevention. Participants who were vaccine hesitant reported concerns about the long-term effects of the vaccine, the vaccine approval process, and the potential impact of the vaccine given their own health conditions/history. Notably, 90% of the undecided group wanted additional information about the vaccine before deciding. Vaccine willingness changed over time, with many of those who were somewhat willing more willing to get the COVID-19 vaccine at survey 2. Individuals who were unwilling at survey 1 were highly likely to remain unwilling at survey 2.
Conclusion
Overall, COVID-19 vaccine hesitancy decreased during the pandemic, although one in five adults with MS were hesitant in early 2021. Of those who were undecided, most indicated that they wanted additional information about the vaccine before deciding whether to be vaccinated, suggesting additional educational efforts on the vaccine's safety, long-term effects, and potential health implications are still needed. Findings indicate that public health efforts may be best focused on those who are undecided, whose vaccine hesitancy may change over time and, possibly, with appropriate information or intervention.
Keywords: Coronavirus, COVID-19, Vaccine, Multiple sclerosis
1. Introduction
The Word Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend COVID-19 vaccination as the safest method to prevent the spread of SARS-CoV-2 and end the COVID-19 pandemic (Organization, 2020, Nguyen et al., 2021), In their COVID-19 Vaccine Guidance for People Living with Multiple Sclerosis (MS), the National Multiple Sclerosis Society (NMSS) recommended that people with MS get the COVID-19 vaccination as soon as it is available to them (NMSS, Society, 2021), Endorsed by the MS Coalition and other international MS organizations, the guidelines also recommended that adults who have progressive MS, are older, have a higher level of physical disability, and/or have other comorbid medical conditions (e.g., diabetes, obesity, heart disease, pregnancy), as well as Black and Hispanic individuals, be vaccinated as soon as possible given their higher risk for more severe disease and hospitalization. Vaccine hesitancy – the reluctance or unwillingness to be vaccinated – is a barrier to full COVID-19 inoculation in the general population (Dror et al., 2020, Lin et al., 2020). Whether vaccine hesitancy is also a barrier towards optimizing vaccination rates in the MS community is unknown.
During the early phase (April-May 2020) of the COVID-19 pandemic and prior to the vaccine becoming available, we conducted a national survey of adults with MS living in the United States (U.S.). Although two-thirds of the sample (66.0%) reported a definite willingness to receive the vaccine when available, 18.5% were only moderately willing and 15.4% were unwilling (Ehde et al., 2021). In the general U.S. population, COVID-19 vaccine hesitancy has fluctuated considerably since the start of the pandemic (Nguyen et al., 2021, Pew Research Center, 2020). In a nationally representative sample of randomly selected U.S. adults conducted in 2020, COVID-19 vaccine hesitancy ranged from a low of 27% in May, up to 49% in September, and down to 39% in November 2020 (Pew Research Center, 2020). An understanding of the prevalence of hesitancy and how hesitancy has changed over time in the MS population is needed to design and direct vaccination efforts in the MS community.
In addition to understanding how many people with MS are vaccine hesitant, it is important to know who is hesitant. In our 2020 survey, (Ehde et al., 2021) COVID-19 vaccine hesitancy was associated with having a lower level of education and a lower perception of one's risk for COVID-19 infection, as well as lower trust in COVID-19 information sources such as the CDC. People with MS may have additional concerns related to their MS and/or disease modifying therapy (DMT). For instance, specific reasons for vaccine hesitancy - such as concerns about vaccine safety, compatibility with personal health conditions, timing with DMTs, or religious beliefs – were not assessed in our earlier survey and have not been explored in a U.S. sample with MS. Social isolation, loneliness, and anxiety - which increased during the initial phase of the pandemic in the general population (Smith and Lim, 2020) and among people with MS (Alschuler et al., 2020) – are contextual factors that may also contribute to vaccine willingness. For example, COVID-19 vaccination might be viewed by some as a means towards decreasing social isolation and loneliness, whereas others may be anxious about or fearful of the vaccine and its effects.
To inform efforts to increase vaccine uptake in people with MS, we conducted a follow up survey of the sample of U.S. adults with MS who participated in our initial survey conducted early in the pandemic (Ehde et al., 2021, Alschuler et al., 2020). We aimed to answer four questions in this second survey, which was conducted between 11 January 2021 and 08 February 2021, shortly after vaccines became available in December 2020 in the U.S.: (1) What is the prevalence of COVID-19 vaccine hesitancy in 2021? (2) What are the reasons for and factors associated with current hesitancy, including demographics (including age, gender, education, geographic region), MS disease characteristics (course, disease severity) self-reported concerns about the vaccine (e.g., safety, side effects), influenza vaccination history, and contextual factors, including perceived risk for SARS-CoV-2 infection, trust in COVID-19 information source, anxiety, and loneliness? (3) How has vaccine hesitancy changed from April/May 2020 to January/February 2021? and (4) Who has changed in their vaccine willingness? Answers to these questions are critical to understanding vaccine hesitancy and to guiding public health efforts to increase vaccine uptake in the MS population.
2. Methods
2.1. Study design and participants
The original survey participants were recruited from a variety of public sources, including social media, research recruitment websites and registries (ParticipateinResearch.org and researchmatch.org), electronic newsletters disseminated via the University of Washington (UW), and other online sources, including social media, the study team's website and social media accounts. the National MS Society posted a link to the study on their COVID-19 research webpage and in their national emails announcing COVID-19 research opportunities to people with MS. All participants who completed this initial cross-sectional online survey early in the COVID-19 pandemic (between 10 April 2020 and 06 May 2020), self-reported an MS diagnosis, and provided contact information were emailed an invitation to participate in the second survey (between 11 January 2021 and 08 February 2021). Eligibility criteria were >18 years old, able to complete the survey in English, and were in the U.S. after 20 January 2020, the date of the first COVID-19 case in the U.S. Participants who did not respond to the initial contact were emailed a reminder to complete the survey once a week for three weeks and received one phone call reminder to complete the survey. Responses were reviewed as received, and participants with incomplete surveys were contacted by phone and email and asked to complete the survey. Participants’ responses from the first and second survey were linked by a unique record identification number. Respondents did not receive any incentives for survey completion. Additional details about the initial survey methods may be found elsewhere (Ehde et al., 2021, Alschuler et al., 2020). The University of Washington Human Subjects Division approved all study procedures.
2.2. Measures
Demographics (age, gender, race, ethnicity, marital status, education level), MS disease-related factors (disease duration, course, current DMT, and disease severity as measured by the Patient Determined Disease Steps [PDDS]), (Learmonth et al., 2013) were collected in the first survey (“survey 1”) and used in the present study (“survey 2”) to characterize the sample and use as correlates of vaccine hesitancy.
We assessed participants’ intentions to obtain a COVID-19 vaccination in two ways. To facilitate comparisons of our results to other studies on vaccine intentions in the general U.S. population, participants were asked to indicate their intent to get vaccinated when the vaccine was made available to them. Possible responses included “yes,” “no,” “undecided,” “I have already received one dose of the vaccine,” and “I have already received two doses of the vaccine.”1 Participants were considered vaccine hesitant if they indicated that they were undecided or not intending to get vaccinated. To allow us to compare our current findings with those of our initial survey, we used the same item from survey 1 to assess vaccine willingness, an item adapted from one used during influenza pandemics (Taylor et al., 2009). Responses were on a 5-point scale (1 = not at all willing, 2 = a little willing, 3 = moderately willing, 4 = very willing, and 5 = extremely willing).
Perceived risk for contracting COVID-19 was assessed using a 5-point scale (0 = no risk at all, 1 = a small risk, 2 = a moderate risk, 3 = a high risk, 4 = an extreme risk). Participants rated on a 5-point scale the places from which they receive their information about COVID-19 (e.g., healthcare providers, social media, where 0 = not at all to 4 = a lot) and on a 4-point scale the extent to which they trust the information sources (1 = do not trust at all to 4 = totally trust). Participants were asked whether they had obtained an influenza vaccination (shot or spray) in 2020. Self-reported concerns about the vaccine (described in detail in Table 2) were assessed as “not a concern,” a “minor concern,” or a “major concern.” Trust in information about COVID-19 from government officials and agencies (President Joseph Biden, President Donald Trump, and CDC), was assessed on a scale of 1 = do not trust at all to 4 = totally trust. How often participants accessed information in the past week from healthcare providers or the NMSS was assessed on a scale of 1 = not at all to 4 = a lot. As it may be expected that people who are lonely or anxious may be more eager to become vaccinated, anxiety was measured by the PROMIS Short Form v1.0-Anxiety 6a, (Cella et al., 2010) and loneliness by the PROMIS Loneliness Fixed Form (Hahn et al., 2010).
Table 2.
Frequency of vaccination concerns stratified by intent to receive vaccine
| Total | No | Undecided | Yesa | |
|---|---|---|---|---|
| Efficacy | ||||
| Not a concern | 136(38%) | 5(21.7%) | 19(38%) | 112(39.3%) |
| Minor concern | 130(36.3%) | 7(30.4%) | 15(30%) | 108(37.9%) |
| Major concern | 92(25.7%) | 11(47.8%) | 16(32%) | 65(22.8%) |
| Short-term side effects | ||||
| Not a concern | 158(44.1%) | 3(13.6%) | 15(30%) | 140(49%) |
| Minor concern | 163(45.5%) | 12(54.5%) | 23(46%) | 128(44.8%) |
| Major concern | 37(10.3%) | 7(31.8%) | 12(24%) | 18(6.3%) |
| Long-term side effects | ||||
| Not a concern | 69(19.2%) | 0(0%) | 4(8%) | 65(22.7%) |
| Minor concern | 181(50.4%) | 3(13%) | 11(22%) | 167(58.4%) |
| Major concern | 109(30.4%) | 20(87%) | 35(70%) | 54(18.9%) |
| Vaccine approval process | ||||
| Not a concern | 166(46.2%) | 3(13%) | 14(28%) | 149(52.1%) |
| Minor concern | 141(39.3%) | 5(21.7%) | 25(50%) | 111(38.8%) |
| Major concern | 52(14.5%) | 15(65.2%) | 11(22%) | 26(9.1%) |
| Being first to receive new vaccine | ||||
| Not a concern | 208(58.3%) | 7(30.4%) | 11(22.4%) | 190(66.7%) |
| Minor concern | 96(26.9%) | 4(17.4%) | 17(34.7%) | 75(26.3%) |
| Major concern | 53(14.8%) | 12(52.2%) | 21(42.9%) | 20(7%) |
| Health conditions/medical history | ||||
| Not a concern | 102(28.5%) | 3(13%) | 4(8.2%) | 95(33.2%) |
| Minor concern | 145(40.5%) | 4(17.4%) | 13(26.5%) | 128(44.8%) |
| Major concern | 111(31%) | 16(69.6%) | 32(65.3%) | 63(22%) |
| Religious beliefs | ||||
| Not a concern | 348(96.9%) | 18(78.3%) | 46(92%) | 284(99.3%) |
| Minor concern | 7(1.9%) | 3(13%) | 2(4%) | 2(0.7%) |
| Major concern | 4(1.1%) | 2(8.7%) | 2(4%) | 0(0%) |
| Want others more at risk to receive vaccine first | ||||
| Not a concern | 122(34.3%) | 9(39.1%) | 16(32%) | 97(34.3%) |
| Minor concern | 129(36.2%) | 9(39.1%) | 22(44%) | 98(34.6%) |
| Major concern | 105(29.5%) | 5(21.7%) | 12(24%) | 88(31.1%) |
| Cost of/access to vaccine | ||||
| Not a concern | 265(73.8%) | 18(78.3%) | 34(68%) | 213(74.5%) |
| Minor concern | 66(18.4%) | 3(13%) | 12(24%) | 51(17.8%) |
| Major concern | 28(7.8%) | 2(8.7%) | 4(8%) | 22(7.7%) |
| Dislike of needles | ||||
| Not a concern | 319(90.4%) | 18(81.8%) | 45(90%) | 256(91.1%) |
| Minor concern | 24(6.8%) | 1(4.5%) | 2(4%) | 21(7.5%) |
| Major concern | 10(2.8%) | 3(13.6%) | 3(6%) | 4(1.4%) |
| Want additional information first | ||||
| Not a concern | 218(60.9%) | 5(21.7%) | 5(10%) | 208(73%) |
| Minor concern | 90(25.1%) | 4(17.4%) | 23(46%) | 63(22.1%) |
| Major concern | 50(14%) | 14(60.9%) | 22(44%) | 14(4.9%) |
| COVID is not a big risk | ||||
| Not a concern | 255(72.9%) | 11(47.8%) | 27(55.1%) | 217(78.1%) |
| Minor concern | 38(10.9%) | 6(26.1%) | 15(30.6%) | 17(6.1%) |
| Major concern | 57(16.3%) | 6(26.1%) | 7(14.3%) | 44(15.8%) |
| More worried about vaccine than COVID | ||||
| Not a concern | 265(75.7%) | 4(17.4%) | 16(33.3%) | 245(87.8%) |
| Minor concern | 53(15.1%) | 4(17.4%) | 20(41.7%) | 29(10.4%) |
| Major concern | 32(9.1%) | 15(65.2%) | 12(25%) | 5(1.8%) |
Includes 21 individuals who already received at least one COVID-19 vaccination dose.
2.3. Analysis
Descriptive analyses (mean and standard deviations for continuous variables and counts and percentages for categorical variables) were used to characterize the study sample and vaccine willingness. Descriptive statistics were also used to depict characteristics between those who responded to the vaccine willingness question in the second survey and those who did not. A Sankey flow diagram characterized the change in vaccine willingness from survey 1 to survey 2. Descriptive statistics were also used to report the participants’ utilization of sources of information and their trust in the information sources with their vaccine willingness as well as their vaccine concerns and intent to vaccinate.
A hierarchical linear model was used to assess the association between demographic, MS disease, COVID-19, and contextual factors with the change in vaccine willingness. The association between vaccine willingness at survey 2 and demographic factors (age, gender, education, race, and location) were assessed in the first step; MS disease factors (PDDS and MS course) were assessed in the second step; COVID-19 factors (flu vaccination history and perceived risk of COVID-19) were assessed in the third step; and contextual factors (trust in CDC, anxiety, and loneliness) were assessed in the fourth step. Using the same steps, a hierarchical linear model with the change in vaccine willingness from survey 1 to survey 2 as the outcome was used to assess characteristics associated with a change in vaccine willingness.
A hierarchical ordinal logistic regression model using the same steps as the previous models, while additionally adjusting for vaccine willingness, was used to assess if the intent to vaccinate (yes, no, undecided) was associated with factors independent of vaccine willingness. In this model, those who had already received a vaccination were included as a “yes”.
3. Results
3.1. Sample chaacteristics
Of the 491 participants with MS who completed the first survey, 465 (94.7%) provided contact information and were invited to complete the second survey. Of those who were contacted, 12 (2.6%) declined and 1 (0.2%) had died. Reasons for declination included too much time or effort required (n = 5), not relevant (n = 2), other (n = 4), and no reason provided (n = 1). In addition, 78 participants (16.8%) did not respond. In survey 2, 359 (77.2%) completed the vaccine willingness questions and were included in reporting descriptive variables, and 312 (67.1%) completed both the vaccine questions and all predictors and were thus included in regression models. Those who responded to survey 2 tended to be slightly older (56.4 vs 53.7 years), had longer disease duration (17.3 vs 14.7 years), and were more likely to be men (18.7% vs 13.4%) and White (92.5% vs 85.0%; see Table 1 ).
Table 1.
Participant MS descriptive variables stratified by second survey response
| No Response | Response | Total | |
|---|---|---|---|
| Age (years) | 53.7(12.7) | 56.4(12.5) | 55.7(12.6) |
| Gender | |||
| Woman | 108(85%) | 287(79.9%) | 395(81.3%) |
| Man | 17(13.4%) | 67(18.7%) | 84(17.3%) |
| Non-binary | 0(0%) | 2(0.6%) | 2(0.4%) |
| Transgender | 1(0.8%) | 0(0%) | 1(0.2%) |
| Other/Prefer not to say/No answer | 1(0.8%) | 3(0.8%) | 4(0.8%) |
| Race | |||
| White | 108(85%) | 332(92.5%) | 440(90.5%) |
| More than one race | 9(7.1%) | 11(3.1%) | 20(4.1%) |
| Black/African American | 6(4.7%) | 6(1.7%) | 12(2.5%) |
| Prefer not to say | 3(2.4%) | 4(1.1%) | 7(1.4%) |
| Other | 1(0.8%) | 3(0.8%) | 4(0.8%) |
| American Indian/Alaska Native | 0(0%) | 2(0.6%) | 2(0.4%) |
| Asian | 0(0%) | 1(0.3%) | 1(0.2%) |
| Employment | |||
| Retired | 31(24.8%) | 116(32.4%) | 147(30.4%) |
| Employed full-time | 29(23.2%) | 111(31%) | 140(29%) |
| Unable to work | 29(23.2%) | 77(21.5%) | 106(21.9%) |
| Employed part-time | 12(9.6%) | 24(6.7%) | 36(7.5%) |
| Unemployed due to Covid-19 | 15(12%) | 16(4.5%) | 31(6.4%) |
| Unemployed unrelated to Covid-19 | 8(6.4%) | 11(3.1%) | 19(3.9%) |
| Student | 1(0.8%) | 3(0.8%) | 4(0.8%) |
| No response | 2(1.6%) | 1(0.3%) | 3(0.6%) |
| Education | |||
| 9th grade or less | 1(0.8%) | 0(0%) | 1(0.2%) |
| 10th-12th grade | 1(0.8%) | 0(0%) | 1(0.2%) |
| High school graduate or GED | 5(3.9%) | 18(5%) | 23(4.7%) |
| Vocational or technical school | 12(9.4%) | 16(4.5%) | 28(5.8%) |
| Some college | 28(22%) | 67(18.7%) | 95(19.5%) |
| College graduate | 50(39.4%) | 135(37.6%) | 185(38.1%) |
| Graduate or professional school | 30(23.6%) | 123(34.3%) | 153(31.5%) |
| Marital Status | |||
| Married | 59(47.2%) | 217(60.6%) | 276(57.1%) |
| Divorced | 24(19.2%) | 68(19%) | 92(19%) |
| Never married | 21(16.8%) | 44(12.3%) | 65(13.5%) |
| Widowed | 8(6.4%) | 12(3.4%) | 20(4.1%) |
| Domestic partner | 7(5.6%) | 13(3.6%) | 20(4.1%) |
| Legally separated | 5(4%) | 3(0.8%) | 8(1.7%) |
| Annulled | 1(0.8%) | 1(0.3%) | 2(0.4%) |
| No response | 2(1.6%) | 1(0.3%) | 3(0.6%) |
| MS disease duration (years) | 14.7(10.1) | 17.3(11.5) | 16.7(11.2) |
| MS disease course | |||
| Relapsing remitting | 83(65.9%) | 233(65.6%) | 316(65.7%) |
| Secondary progressive | 21(16.7%) | 59(16.6%) | 80(16.6%) |
| Primary progressive | 11(8.7%) | 37(10.4%) | 48(10%) |
| Unknown/No answer | 11(8.7%) | 21(5.9%) | 32(6.7%) |
| Clinically isolated syndrome | 0(0%) | 5(1.4%) | 5(1%) |
| Disease modifying therapy | |||
| Ocrelizumab | 2(28.6%) | 59(23.8%) | 61(23.9%) |
| Dimethyl fumarate | 0(0%) | 31(12.5%) | 31(12.2%) |
| Fingolimod | 0(0%) | 20(8.1%) | 20(7.8%) |
| Teriflunomide | 2(28.6%) | 19(7.7%) | 21(8.2%) |
| Alemtuzumab | 0(0%) | 7(2.8%) | 7(2.7%) |
| Siponimod | 0(0%) | 2(0.8%) | 2(0.8%) |
| Cladribine | 0(0%) | 1(0.4%) | 1(0.4%) |
| Diroximel fumarate | 0(0%) | 4(1.6%) | 4(1.6%) |
| Glatiramer acetate | 0(0%) | 49(19.8%) | 49(19.2%) |
| Interferons | 2(28.6%) | 20(8.1%) | 22(8.6%) |
| Natalizumab | 1(14.3%) | 19(7.7%) | 20(7.8%) |
| Other/Off-label | 0(0%) | 17(6.9%) | 17(6.7%) |
| No DMT | 120(94.5%) | 111(30.9%) | 231(47.5%) |
| Disability (PDDS) | |||
| 0 | 23(18.3%) | 71(19.8%) | 94(19.4%) |
| 1 | 20(15.9%) | 75(20.9%) | 95(19.6%) |
| 2 | 21(16.7%) | 42(11.7%) | 63(13%) |
| 3 | 16(12.7%) | 47(13.1%) | 63(13%) |
| 4 | 18(14.3%) | 44(12.3%) | 62(12.8%) |
| 5 | 9(7.1%) | 28(7.8%) | 37(7.6%) |
| 6 | 10(7.9%) | 31(8.7%) | 41(8.5%) |
| 7 | 9(7.1%) | 19(5.3%) | 28(5.8%) |
| 8 | 0(0%) | 1(0.3%) | 1(0.2%) |
| No answer | 1(0.8%) | 1(0.3%) | 2(0.4%) |
| Vaccine willingness at survey 1 | |||
| Not willing | 12(9.4%) | 17(4.7%) | 29(6.0%) |
| A little willing | 17(13.4%) | 29(8.1%) | 46(9.5%) |
| Moderately willing | 24(18.9%) | 66(18.4%) | 90(18.5%) |
| Very willing | 29(22.8%) | 90(25.1%) | 119(24.5%) |
| Extremely willing | 45(35.4%) | 157(43.7%) | 202(41.6%) |
The current sample was predominantly middle-aged (M = 56.4 years + 12.5 years), women (79.9%), White (92.5%), and college graduates or better (72%). Disease duration was 17.3 + 11.5 years, on average; 65.6% reported a relapsing-remitting disease course. Over two-thirds of the sample (69%) reported a DMT. At the time of the second survey, 12 (3.3%) participants reported that they had tested positive for COVID-19 at some point since the start of the pandemic. Most participants reported obtaining an influenza vaccination in 2020 (283, 79%). See Table 1 for additional descriptive statistics.
3.2. What is the prevalence of COVID-19 vaccine hesitancy in early 2021?
Of the participants who completed survey 2, 20.3% (73) were vaccine hesitant, that is, either reporting that they were undecided (50,13.9%) or not intending to get vaccinated (23, 6.4%). Nearly three-fourths of the sample (265, 73.8%) planned to get vaccinated. Only 21 participants (6.0%) had received at least one vaccine dose.
3.3. What are the reasons for and factors associated with current vaccine hesitancy?
Table 2 shows participants’ concerns about the COVID-19 vaccine. Respondents who were unwilling to get vaccinated endorsed major concerns about the long-term effects of the vaccine, the vaccine approval process, wanting more information about the vaccine, and the potential impact of the vaccine given their own health conditions/history. Participants who were undecided about the vaccine generally endorsed similar concerns as those who did not intend to get vaccinated. Notably, 90% (45) of those in the undecided group indicated that they wanted additional information about the vaccine before deciding. Religious beliefs, cost of the vaccine, and access to the vaccine were not significant concerns for either group.
Demographic factors (p = .002), COVID-19 factors (p < .001), and contextual factors (p < .001) were all associated with vaccine willingness (Table 3 ). In the full model, those with higher education (p = .007), who were White (β = 0.497, p = .028), had a history of flu vaccination (β = 0.804, p < .001), had a greater perceived risk of COVID-19 (β = 0.172, p = .008) greater trust in the CDC (β = 0.561, p < .001) were all significantly associated with a higher willingness to vaccinate.
Table 3.
Regression coefficients and p-values assessing the association between predictors and vaccine willingness at survey 2
| Univariate | Step 1 | Step 2 | Step 3 | Step 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| Overall | 0.002 | 0.009 | <0.001 | <0.001 | ||||||
| Step 1: p-value = .002 | ||||||||||
| Women | 0.013 | 0.942 | 0.07 | 0.678 | 0.046 | 0.789 | 0.124 | 0.446 | 0.055 | 0.705 |
| Age | 0.005 | 0.373 | 0.005 | 0.383 | 0.005 | 0.43 | 0.001 | 0.884 | 0.002 | 0.667 |
| Education | 0.001 | 0.001 | 0.002 | 0.008 | 0.007 | |||||
| High School or less | Ref | Ref | Ref | Ref | Ref | |||||
| Vocational/Some college | 0.003 | -0.008 | 0.051 | 0.189 | 0.228 | |||||
| College | 0.459 | 0.441 | 0.445 | 0.464 | 0.474 | |||||
| Graduate/Professional | 0.699 | 0.698 | 0.71 | 0.707 | 0.674 | |||||
| Race | 0.059 | 0.038 | 0.042 | 0.031 | 0.028 | |||||
| Other | -0.516 | -0.559 | -0.551 | -0.543 | -0.497 | |||||
| Urban | 0.239 | 0.134 | 0.546 | 0.12 | 0.591 | 0.143 | 0.493 | 0.158 | 0.399 | |
| Step 2: p-value = .683 | ||||||||||
| PDDS | -0.037 | 0.235 | -0.054 | 0.17 | -0.029 | 0.434 | -0.035 | 0.311 | ||
| MS Course | 0.875 | 0.716 | 0.653 | 0.832 | ||||||
| SIS/RRMS | Ref | Ref | Ref | Ref | ||||||
| PPMS | 0.109 | 0.182 | 0.085 | 0.043 | ||||||
| SPMS | 0.137 | 0.237 | 0.182 | 0.098 | ||||||
| Unknown | 0.01 | 0.168 | 0.295 | 0.209 | ||||||
| Step 3: p-value < .001 | ||||||||||
| Flu Vaccine | 1.088 | <0.001 | 0.982 | <0.001 | 0.804 | <0.001 | ||||
| Perceived risk of Covid-19 | 0.149 | 0.047 | 0.165 | 0.019 | 0.172 | 0.008 | ||||
| Step 4: p-value < .001 | ||||||||||
| Trust in CDC | 0.624 | <0.001 | 0.561 | <0.001 | ||||||
| Anxiety | 0.004 | 0.756 | 0.007 | 0.638 | ||||||
| Loneliness | -0.013 | 0.3 | -0.016 | 0.212 | ||||||
Note: Vaccine willingness was assessed on a 5-point scale: 1 = not at all willing, 2 = a little willing, 3 = moderately willing, and 5 = extremely willing.
Results for the ordinal logistic regression model can be found in Table 4 . COVID-19 factors were the only group of features to be significantly associated with an intent to vaccinate (p = .001). In the full model, history of flu vaccination (β = 2.075, p = .001) was the only factor to be associated with a greater odds of intending to get vaccinated after adjusting for vaccine willingness. No other factors were independently associated with the intent to vaccinate in the full model.
Table 4.
Ordinal logistic regression coefficients and p-values assessing the association between predictors and intent to vaccinate
| Univariate | Step 1 | Step 2 | Step 3 | Step 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| Overall | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Vaccine Willingness | 2.878 | <0.001 | 2.925 | <0.001 | 3.11 | <0.001 | 3.463 | <0.001 | 3.526 | <0.001 |
| Step 1: p-value = .804 | ||||||||||
| Women | -0.024 | 0.948 | 0.077 | 0.901 | -0.247 | 0.707 | -0.151 | 0.831 | 0.095 | 0.895 |
| Age | -0.001 | 0.964 | -0.009 | 0.656 | <0.001 | 0.994 | -0.019 | 0.409 | -0.033 | 0.21 |
| Education | 0.001 | 0.341 | 0.311 | 0.198 | 0.123 | |||||
| High school or less | Ref | Ref | Ref | Ref | Ref | |||||
| Vocational/Some college | 0.089 | -0.407 | -0.598 | -0.32 | -0.489 | |||||
| College | 0.728 | -0.421 | -0.979 | -1.009 | -1.062 | |||||
| Graduate/Professional | 1.625 | 0.657 | 0.223 | 0.527 | 0.749 | |||||
| Race | 0.089 | 0.432 | 0.219 | 0.202 | 0.149 | |||||
| Other | -0.865 | -0.626 | -1.002 | -1.177 | -1.429 | |||||
| Urban | 0.408 | 0.964 | -0.419 | 0.599 | -0.63 | 0.461 | -0.636 | 0.488 | -0.483 | 0.609 |
| Step 2: p-value = .165 | ||||||||||
| PDDS | -0.122 | 0.964 | -0.239 | 0.132 | -0.225 | 0.155 | -0.197 | 0.249 | ||
| MS Course | 0.705 | 0.126 | 0.153 | 0.09 | ||||||
| SIS/RRMS | ||||||||||
| PPMS | -0.232 | 0.121 | -0.24 | -0.484 | ||||||
| SPMS | 0.226 | 1.044 | 1.012 | 1.232 | ||||||
| Unknown | -0.466 | -1.658 | -1.597 | -1.801 | ||||||
| Step 3: p-value = .001 | ||||||||||
| Flu Vaccine | 1.89 | <0.001 | 2.08 | 0.001 | 2.075 | 0.001 | ||||
| Perceived risk of Covid-19 | 0.225 | 0.169 | -0.176 | 0.558 | -0.207 | 0.505 | ||||
| Step 4: p-value = .29 | ||||||||||
| Trust in CDC | 1.143 | <0.001 | 0.221 | 0.51 | ||||||
| Anxiety | 0.017 | 0.578 | 0.023 | 0.736 | ||||||
| Loneliness | -0.035 | 0.196 | -0.115 | 0.084 | ||||||
Note: Intent to vaccinate options included yes, undecided, or already vaccinated (1 or 2 doses). Those who had already received a vaccination were included as a “yes”.
3.4. How has vaccine willingness and hesitancy changed from April/May 2020 to January/February 2021?
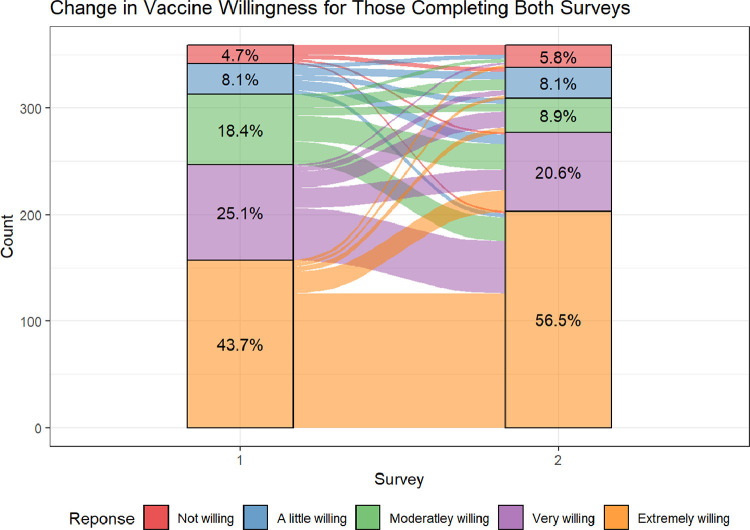
Of the participants who completed both surveys, more survey 2 participants were either extremely willing (56.5%) or very willing (20.6%) to get the vaccine than in survey 1. Approximately the same proportion were not at all (5.8%) or only a little willing (8.1%). Frequencies for each of the five response options are in Figure 1 .
Figure 1.
Change in Vaccine Willingness for Those Completing Both Survey.
3.5. Who has changed in their vaccine willingness?
A Sankey chart depicts the flow of vaccine willingness for participants who completed both survey 1 and survey 2 (Figure 1). As shown by the flows between the two time points, most cases of change in vaccine willingness were characterized by movement between adjacent categories (e.g., from very willing to either moderately willing or extremely willing). However, most of those who were not willing to be vaccinated at survey 1 remained unwilling at survey 2. More movement was seen between those who were moderately willing in either direction.
Contextual factors were associated with a change in vaccine willingness from survey 1 to survey 2 (p < .001). In the full model, being older (β = 0.012, p = .047), White (β = 0.58, p = .021) and having a greater trust in the CDC (β = 0.359, p < .001) were all positively associated with a higher change in vaccine willingness, while a history of flu vaccination (β = -0.439, p = .005) was associated with a lower change in vaccine willingness. See Table 5 for complete regression results.
Table 5.
Regression coefficients and p-values assessing the association between predictors and change in vaccine willingness
| Univariate | Step 1 | Step 2 | Step 3 | Step 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| Overall | 0.096 | 0.22 | 0.117 | <0.001 | ||||||
| Step 1: p-value = .096 | ||||||||||
| Women | 0.102 | 0.534 | 0.108 | 0.51 | 0.131 | 0.434 | 0.152 | 0.369 | 0.083 | 0.609 |
| Age | 0.009 | 0.088 | 0.008 | 0.134 | 0.005 | 0.364 | 0.007 | 0.208 | 0.012 | 0.047 |
| Education | 0.712 | 0.775 | 0.781 | 0.805 | 0.831 | |||||
| High School or less | Ref | Ref | Ref | Ref | Ref | |||||
| Vocational/Some college | 0.113 | 0.128 | 0.162 | 0.114 | 0.15 | |||||
| College | -0.089 | -0.046 | 0.006 | -0.006 | 0.019 | |||||
| Graduate/Professional | -0.017 | 0.051 | 0.123 | 0.128 | 0.121 | |||||
| Race | 0.019 | 0.023 | 0.022 | 0.017 | 0.021 | |||||
| Other | -0.61 | -0.598 | -0.601 | -0.628 | -0.58 | |||||
| Urban | -0.363 | 0.089 | -0.319 | 0.138 | -0.29 | 0.183 | -0.274 | 0.207 | -0.269 | 0.196 |
| Step 2: p-value = .706 | ||||||||||
| PDDS | 0.034 | 0.241 | 0.019 | 0.627 | 0.007 | 0.866 | -0.007 | 0.858 | ||
| MS Course | 0.324 | 0.59 | 0.674 | 0.752 | ||||||
| SIS/RRMS | Ref | Ref | Ref | Ref | ||||||
| PPMS | 0.156 | 0.082 | 0.085 | 0.097 | ||||||
| SPMS | 0.107 | -0.017 | 0.009 | -0.033 | ||||||
| Unknown | 0.474 | 0.372 | 0.338 | 0.256 | ||||||
| Step 3: p-value = .086 | ||||||||||
| Flu Vaccine | -0.308 | 0.048 | -0.322 | 0.044 | -0.439 | 0.005 | ||||
| Perceived risk of Covid-19 | 0.051 | 0.477 | 0.075 | 0.302 | 0.052 | 0.472 | ||||
| Step 4: p-value < .001 | ||||||||||
| Trust in CDC | 0.341 | <0.001 | 0.359 | <0.001 | ||||||
| Anxiety | 0.021 | 0.12 | 0.023 | 0.137 | ||||||
| Loneliness | 0.014 | 0.267 | 0.001 | 0.937 | ||||||
Note: Vaccine willingness was assessed on a 5-point scale: 1 = not at all willing, 2 = a little willing, 3 = moderately willing, and 5 = extremely willing.
4. Discussion
MS organizations strongly recommend that adults with MS get the COVID-19 vaccination. In this follow up survey of a sample of U.S. adults with MS, few of whom had been vaccinated or infected with COVID-19 at the time of participation, 20.3% were vaccine hesitant. Vaccine willingness increased between the two surveys, with 74% of the 2021 survey sample reporting that they were extremely or very willing to obtain the COVID-19 vaccine relative to 66% in the 2020 survey. Like the general population, (Robinson et al., 2021) vaccine hesitancy was associated with having a lower level of education, being non-White, not having a recent flu vaccination, holding a lower perception of one's risk of getting COVID-19, and having lower trust in the CDC. MS disease characteristics, including course and severity, were not associated with vaccine hesitancy. Among those who were vaccine hesitant, major concerns included the long-term effects of the vaccine, the vaccine approval process, and the potential impact of the vaccine given their own health conditions and history. They also reported wanting more information about the vaccine. Most of those who were unwilling to get vaccinated at the time of the first survey remained unwilling at the time of the second survey. Many of those who were unsure at survey 1 became at least moderately willing to be vaccinated at survey 2.
Our sample's vaccine hesitancy decreased between the first and second surveys, a finding consistent with those from a longitudinal survey conducted in the general U.S. population around the same times as our surveys (Pew Research Center, 2020). However, the proportion of MS survey participants who indicated that they had gotten (6.0%) or intended (73.8%) to get a COVID-19 vaccination was greater than the same U.S. sample, which found that 60% of Americans said that they would probably or definitely get the coronavirus vaccine. Thus, vaccine hesitancy appears to be lower among people with MS, relative to the general population. Nonetheless, 20.3% of our sample was vaccine hesitant, a finding consistent with the results of a survey conducted at a similar time point (late December 2020 to early January 2021) in a sample of MS patients from a single hospital system in Lisbon, Portugal (N = 256), in which 19.1% were vaccine hesitant (Serrazina et al., 2021).
These results have implications for which people with MS should be the focus of efforts to increase COVID-19 vaccine uptake. We found that vaccine willingness is not a dichotomy of willing and unwilling, and that willingness is not immutable, but rather, something that can change over time, especially among the important middle group that is characterized as undecided. Based on our findings, it appears that most undecided individuals’ opinions are changeable, with individuals in the willing and undecided groups generally becoming more willing (Figure 1). In contrast, individuals who were unwilling at survey 1 were highly likely to remain unwilling at survey 2. This suggests that public health efforts may be best focused on those who are undecided, who may change over time and, possibly, with appropriate information or intervention. Consistent with this, we found that ninety percent of those in the undecided group indicated that they wanted additional information about the vaccine before deciding. Those who were undecided also endorsed concerns about the long-term side effects of the vaccine, including the impact on their own health conditions, which was similar to what was found in the Portuguese MS sample, (Serrazina et al., 2021) suggesting potential types of information for providers to discuss with those who are undecided. Very few participants reported that religious beliefs – a factor cited for vaccine exemptions in the general U.S. population – was a concern for them.
Recognizing the potential for positively influencing vaccine willingness, the NMSS recently developed a COVID-19 Vaccine Conversation Tip Sheet for healthcare providers. It provides guidance on facilitating non-judgmental discussions about the vaccine (Society, 2021). It includes tips for eliciting and validating the hesitant patient's perspective, addressing vaccine misconceptions, and developing a plan for future steps or discussions. This Tip Sheet may be particularly helpful for conversations with patients who are undecided, as experts outside the MS field have recommended that healthcare providers first work to understand why their patients are undecided before attempting to persuade them to get the COVID-19 vaccine (Rosenbaum, 2021). It is possible that effective communications with and trust in providers may positively influence vaccination behaviors. Whether provider-patient communication strategies such as the ones suggested in the Covid-19 Tip Sheet decrease vaccine hesitancy and increase vaccination behaviors in people with MS is an important direction for future research. Although access to the vaccine was not a major concern for most of the undecided participants in our sample, interventions that make vaccination more convenient, such as providing vaccinations at convenient places (e.g., clinics, churches, job sites) or scheduling default vaccine appointments, have also been shown to increase vaccination rates in other populations (Brewer et al., 2017).
Several study limitations merit consideration. The survey 2 sample included 77% of the original survey 1 sample. Though vaccine hesitancy at survey 1 was higher among those who did not respond, the impact this had on the findings is unknown (Table 1). Although the survey enrolled participants from across the U.S., we did not randomly sample from all adults with MS living in the U.S. Participants were highly educated, on average, and disproportionately White. Given that COVID-19 vaccine hesitancy has been greater in people who identify as Black or Hispanic as well as those of lower education, (Khubchandani et al., 2021) our findings do not fully capture vaccine hesitancy in the overall MS population, which may limit the generalizability of the findings. Research examining the perspectives of people from other racial, ethnic, and socioeconomic groups, particularly as they have been disproportionately affected by the pandemic, is urgently needed. The sample also did not include many participants with significant disability on the PDSS. We did not ask participants about their political affiliations, although it has been associated with hesitancy in other surveys. All data were self-reported; we did not verify participants’ vaccination status or any other variables collected. Finally, this study's results, while informative, are reflective of two discreet time points during the COVID-19 pandemic; many factors, including vaccine availability, will change and may influence vaccine hesitancy and uptake.
In conclusion, this study suggests that vaccine hesitancy has decreased in the MS community during the COVID-19 pandemic, particularly among those who were moderately willing early in the pandemic. Given that most of those who are undecided wanted additional information about the COVID-19 vaccine before deciding, educational campaigns and other efforts are needed that focus on the vaccine's safety, long-term effects, and potential health implications specific to people with MS. Findings also indicate that MS providers and public health efforts may be best focused on those who are undecided, whose vaccine hesitancy may change over time and, possibly, with appropriate information or intervention.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
This study was partially supported by the Nancy and Buster Alvord Endowed Professorship in Multiple Sclerosis Research held by Dawn M. Ehde, PhD. Study data were collected and managed using REDCap electronic data capture tools hosted at the Institute of Translational Health Sciences (ITHS) which is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR002319. This same award also provides the research recruitment website, ParticipateinResearch.org, which was one of the sources for study participants. Recruitment for the study also included ResearchMatch. ResearchMatch is funded, in part, by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program and is funded by grants UL1TR000445, U24TR00157 9, and 5 U24 TR001579-02. The CTSA program is led by the NIH's National Center for Advancing Translational Sciences (NCATS). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Only two-dose vaccines were available at the time of survey 2.
References
- Organization WH. Coronavirus disease (COVID-19): Herd immunity, lockdowns, and COVID-19. https://www.who.int/news-room/q-a-detail/herd-immunity-lockdowns-and-covid-19. Published 2020. Updated 31 December 2020. Accessed 3 March 2021.
- Nguyen KH, Srivastav A., Razzaghi H., et al. COVID-19 Vaccination Intent, Perceptions, and Reasons for Not Vaccinating Among Groups Prioritized for Early Vaccination — United States, September and December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:217–222. doi: 10.15585/mmwr.mm7006e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror AA, Eisenbach N, Taiber S, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35(8):775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Tu P, Beitsch LM. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines (Basel) 2020;9(1) doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehde DM, Roberts MK, Herring TE, Alschuler KN. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult Scler Relat Disord. 2021;49 doi: 10.1016/j.msard.2021.102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance#section-0. Published 2021. Updated 01 March 2021. Accessed 4 March 2021.
- Smith BJ, Lim MH. How the COVID-19 pandemic is focusing attention on loneliness and social isolation. Public Health Res Pract. 2020;30(2) doi: 10.17061/phrp3022008. [DOI] [PubMed] [Google Scholar]
- Alschuler KN, Roberts MK, Herring TE, Ehde DM. Distress and risk perception in people living with multiple sclerosis during the early phase of the COVID-19 pandemic. Mult Scler Relat Disord. 2020;47 doi: 10.1016/j.msard.2020.102618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC neurology. 2013;13:37. doi: 10.1186/1471-2377-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, Raphael B, Barr M, Agho K, Stevens G, Jorm L. Public health measures during an anticipated influenza pandemic: Factors influencing willingness to comply. Risk Manag Healthc Policy. 2009;2:9–20. doi: 10.2147/RMHP.S4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J. Clin. Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EA, Devellis RF, Bode RK, et al. Measuring social health in the patient-reported outcomes measurement information system (PROMIS): item bank development and testing. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2010;19(7):1035–1044. doi: 10.1007/s11136-010-9654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39(15):2024–2034. doi: 10.1016/j.vaccine.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrazina F, Sobral Pinho A, Cabral G, Salavisa M, Correia AS. Willingness to be vaccinated against COVID-19: An exploratory online survey in a Portuguese cohort of multiple sclerosis patients. Mult Scler Relat Disord. 2021;51 doi: 10.1016/j.msard.2021.102880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center. Intent to get a COVID-19 vaccine rises to 60% as confidence in research and development process increases. December 2020.
- Society NMS. COVID-19 Vaccine Conversation Tip Sheet. https://nmsscdn.azureedge.net/NationalMSSociety/media/MSNationalFiles/Documents/HCP_flier_COVID19_TalkingPoints.pdf Published 2021. Accessed May 24 2021, 2021.
- Rosenbaum L. Escaping Catch-22 - Overcoming Covid Vaccine Hesitancy. N. Engl. J. Med. 2021;384(14):1367–1371. doi: 10.1056/NEJMms2101220. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing Vaccination: Putting Psychological Science Into Action. Psychol Sci Public Interest. 2017;18(3):149–207. doi: 10.1177/1529100618760521. [DOI] [PubMed] [Google Scholar]
- Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J. Community Health. 2021;46(2):270–277. doi: 10.1007/s10900-020-00958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]