Abstract
Objective
BCG can improve the response to vaccines directed against viral infections, and also, BCG vaccination reduces all-cause mortality, most likely by protecting against unrelated infections. However, the effect of BCG vaccination on dendritic cell (DC) subsets is not well characterized.
Methods
We investigated the impact of BCG vaccination on the frequencies of DC subsets and type I and III interferons (IFNs) using whole blood and plasma samples in a group of elderly individuals (age 60-80 years) at one-month post-vaccination as part of our clinical study to examine the effect of BCG on COVID-19.
Results
Our results demonstrate that BCG vaccination induced enhanced frequencies of plasmacytoid DC (pDC) and myeloid DC (mDC). BCG vaccination also induced diminished plasma levels of type I IFNs, IFNα and IFNβ but increased levels of type III IFNs, IL-28A and IL-29.
Conclusions
Thus, BCG vaccination was associated with enhanced DC subsets and IL-28A/IL-29 in elderly individuals, suggesting its ability to induce non-specific innate immune responses.
Keywords: BCG, dendritic cells, type I and III interferons, COVID-19
Introduction
Bacillus Calmette–Guérin (BCG) is a live-attenuated vaccine primarily established to protect against childhood meningitis and disseminated tuberculosis (TB) (Foster et al., 2021). Several epidemiological findings suggest that BCG may increase the capacity of the immune system to fight against pathogens other than TB (Leentjens et al., 2015) and such non-specific responses augment both T cell-mediated adaptive and innate immune memory in a process called trained immunity; this could have important implications for improving vaccination strategies. (Netea and van Crevel, 2014). Bearing in mind the high morbidity and mortality due to COVID-19 in the elderly population, a possible protective effect through BCG in this group would be of clinical significance (O'Neill and Netea, 2020). However, the effect of BCG vaccination in protecting against heterologous infections in elderly individuals is still unclear as not many biological studies have been conducted so far to support this hypothesis.
Dendritic cells (DC) are the most effective antigen-presenting cells (APC), playing essential roles in bridging the innate and adaptive immune responses (Banchereau and Steinman, 1998). DCs are more efficient than other APCs, B cells and monocytes, in inducing T-cell proliferation (Inaba et al., 1989, Ludewig et al., 1999). Furthermore, DCs also play a significant role in establishing immunologic memory (Ludewig et al., 1999). Type I interferons (IFN-α/β) have a wide range of antiviral activities, which stimulate an antiviral response across various cell types (Mantlo et al., 2020). Like type 1 interferons, type III IFNs termed IFN lambda-1(IL-29), IFN lambda-2 (IL-28A), and IFN lambda-3 (IL-28B) also play important roles in antiviral immune activities (Zhou et al., 2018). Studies have also shown that type I and type III IFNs are able to inhibit SARS–CoV-2 replication (Felgenhauer et al., 2020), but the effect of BCG vaccination in inducing type I and III IFNs is not well studied.
Hence, we examined the induction of DC subsets in response to BCG vaccination in elderly individuals at baseline and one month post-vaccination along with baseline frequencies in unvaccinated individuals. We also examined the circulating levels of type I and type III IFNs following vaccination. We demonstrate that BCG vaccination induces significantly enhanced frequencies of DC subsets and altered type I and type III IFNs, suggesting that BCG can potentially boost immune responses in a non -specific or off-target manner in these elderly individuals.
Materials and Methods
Ethics statement
The study was approved by the Ethics Committees of NIRT (NIRT-INo:2020010). Informed written consent was obtained from all participants. The study is part of the clinical study entitled, Study to evaluate the effectiveness of the BCG vaccine in reducing morbidity and mortality in elderly individuals in COVID-19 hotspots in India (NCT04475302).
Study Population
Elderly individuals between 60 - 80 years of age residing in hotspots for SARS-Cov2 infection were included in the study between July 2020 and September 2020 in Chennai, India, after obtaining informed written consent from the study participants. The elderly population positive for SARS-Cov2 infection by either antibody (serology) or PCR test, HIV-infected or individuals with malignancy or on immunosuppressive drugs or transplant recipients and those on dialysis or anti-psychiatric medications or hypersensitivity to vaccinations, were not included in the study. Also, those who had been diagnosed with tuberculosis (TB) in the previous 6-months or were currently on anti-TB treatment were not included in the study.
Fifty four participants received a single dose of BCG vaccine (Freeze-dried) manufactured by Serum Institute of India, Pune. The adult dose of BCG vaccine was 0.1 mL injected intradermally over the distal insertion of the deltoid muscle onto the left humerus (approximately one-third down the left upper arm). In case of a previous vaccination scar or ulcer/injury, or tattoo on the left upper arm, vaccination was given in the right upper arm. Thirty two elderly individuals from the same hotspot area were not vaccinated and were considered as controls. Blood was drawn from the vaccinated participants at baseline (before vaccination) and one month following vaccination. Blood was drawn from the controls only at baseline.
Ex vivo analysis
All antibodies used in the study were from BD Biosciences (San Jose, CA), BD Pharmingen (San Diego, CA), eBioscience (San Diego, CA), or R&D Systems (Minneapolis, MN). Whole blood was used for ex vivo phenotyping, and it was performed on all \86 individuals. Briefly, to 250μl aliquots of whole blood, a cocktail of monoclonal antibodies specific for various immune cell types was added. Plasmacytoid DCs were classified as (Lin– HLA-DR+ CD123+) and myeloid DCs (Lin– HLA-DR+ CD11c+). Monocyte phenotyping was performed using antibodies directed against CD45-PerCP, CD14-Pacific Blue, HLA-DR-PE-Cy7 (clone L243; BD), and CD16-APC- Cy7. Eight-color flow cytometry was performed on a FACS Canto II flow cytometer with FACSDIVA software, version 6 (Becton Dickinson). The gating was set by forward and side scatter, and 100 000 gated events were acquired. Data were collected and analyzed using FLOW JO software (TreeStar, Ashland, OR).
ELISA
Circulating levels of IFNα and IFNβ were measured using Luminex Human Magnetic multiplex assay kit (R&D Systems). IL-28A, IL-28B, and IL-29 were measured using the DuoSet ELISA kit (R&D Systems). The lowest detection limits were as follows: IFNα, 3.9 pg/mL; IFNβ, 3.25 pg/ml; IL-28A, 62.5 pg/mL; IL-28B, 62.5 pg/mL and IL-29, 62.5 pg/mL. The lowest standard value was assigned to the samples that were below the threshold of detection.
Statistical analysis
Geometric means (GM) were used for measurements of central tendency. Wilcoxon signed-rank test was used to compare frequencies of immune subsets and type I and III interferons in the BCG vaccinated group at month 0 (M0) and month 1 (M1). Statistically significant differences between unvaccinated and BCG vaccinated M1 groups were analyzed using the Mann-Whitney test. Analyses were performed using Graph-Pad PRISM Version 9.0. Correlation matrix analysis was done using statistical software JMP 14.0 (SAS, Cary, NC, USA).
Results
Study population
The demographics of the study population are shown in Table I . From July 2020 through September 2020, 86 individuals were enrolled in the study, 54 in the vaccinated arm and 32 in the unvaccinated arm. All the vaccinated individuals were followed up at month 1 post-vaccination with no loss to follow-up. The median age was 65 (Range: 60-78) years in BCG vaccinated group and 63 years (Range: 60-80) in the unvaccinated group. There were 34 males and 20 females in the BCG vaccinated and 15 males and 17 females in the unvaccinated group. In the enrolled population, 26% of BCG vaccinated and 15% of unvaccinated individuals had diabetes mellitus, while 15% and 9% had cardiovascular disease, respectively. In our cohort, 4%-6% were current smokers, and 6% were alcoholics. Other baseline characteristics were similar between the two arms.
Table I.
Demographics of the study population.
| Vaccinated | Non-Vaccinated | |
|---|---|---|
| Subjects Enrolled | n=54 | n=32 |
| Age (Median) | 65 (60 -78) | 63 (60 -80) |
| Gender (M/F) | 34/20 | 15/17 |
| Height (Median) | 160 cm | 155 cm |
| Weight (Median) | 62 Kg | 63 Kg |
| Pulse rate (Median) | 86 | 88 |
| Systolic Blood Pressure (Median) | 132 | 140 |
| Diastolic Blood Pressure (Median) | 81 | 80 |
| SPOS% (Median) | 98 | 98 |
| Diabetes Mellitus no. (%) | 14 (26 %) | 5 (15 %) |
| Smoking, no. (%) | 2 (4 %) | 2 (6 %) |
| Alcoholism, no. (%) | 3 (6 %) | 2 (6 %) |
| Cardiovascular Disease, no. (%) | 8 (15 %) | 3 (9 %) |
| Respiratory Diseases, no. (%) | 5 (9 %) | 2 (6%) |
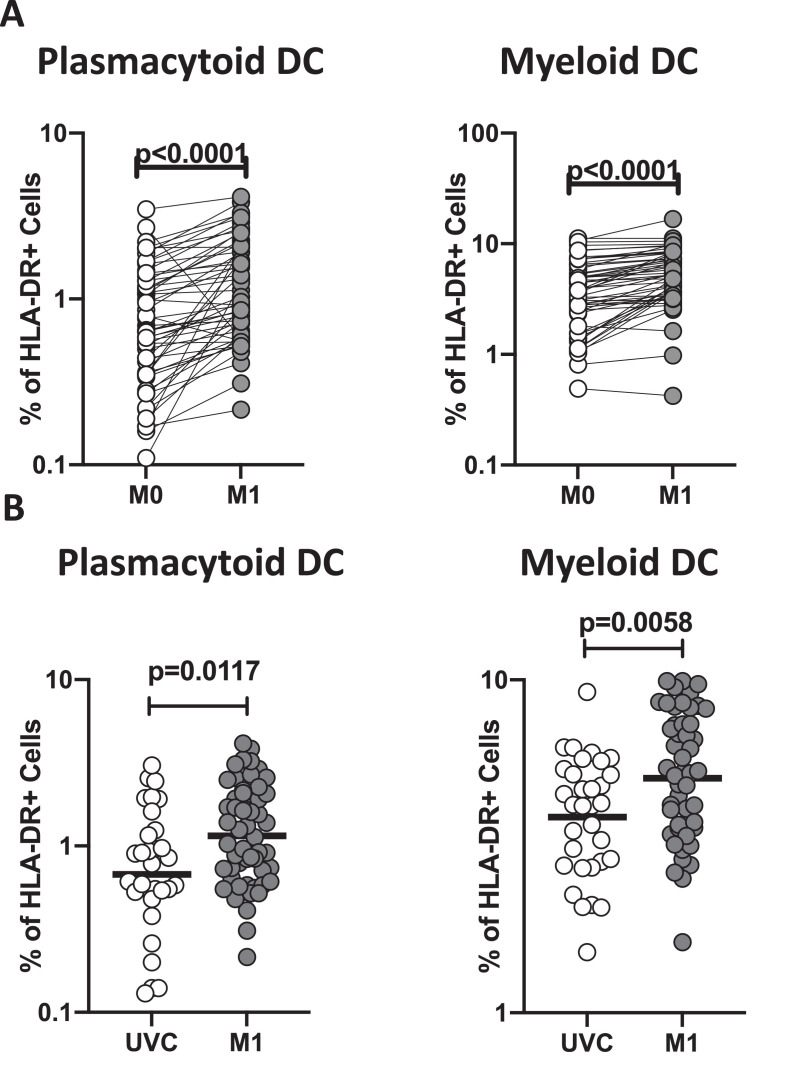
BCG vaccination induces enhanced frequencies of myeloid and plasmacytoid DCs
To assess the ex vivo phenotype of DC subsets following BCG vaccination, we compared the subsets at baseline or before BCG vaccination (M0) and at month 1 (M1) post-vaccination. As shown in Fig 1 A, the frequencies of myeloid and plasmacytoid DCs had significantly increased at M1 compared to M0 in BCG-vaccinated individuals. Next, we compared the frequencies of DC subsets in post-vaccinated individuals to unvaccinated controls. As shown in Fig 1B, BCG vaccinated individuals exhibited increased frequencies of both myeloid and plasmacytoid DCs compared to unvaccinated controls. A representative flow cytometry plot showing the gating strategy for DC subsets is shown in Sup. Fig. Thus, BCG vaccination induces enhanced frequencies of DC subsets in elderly individuals.
Figure 1.
BCG vaccination is associated with heightened frequencies of dendritic cell subsets
(A) Frequencies of dendritic cell (DC) subsets (plasmacytoid DC and myeloid DC) in BCG pre-vaccinated [M0] (n = 54) and month 1 following vaccination [M1] (n = 54). Data are shown in line diagrams, with each line representing a single individual. P values were calculated using the Wilcoxon matched pair tests with Holms correction for multiple comparisons. (B) Frequencies of dendritic cell (DC) subsets (plasmacytoid DC and myeloid DC) in BCG unvaccinated (UVC) (n = 32) and post vaccinated [M1] (n = 54) individuals. The data are represented as scatter plots, with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons.
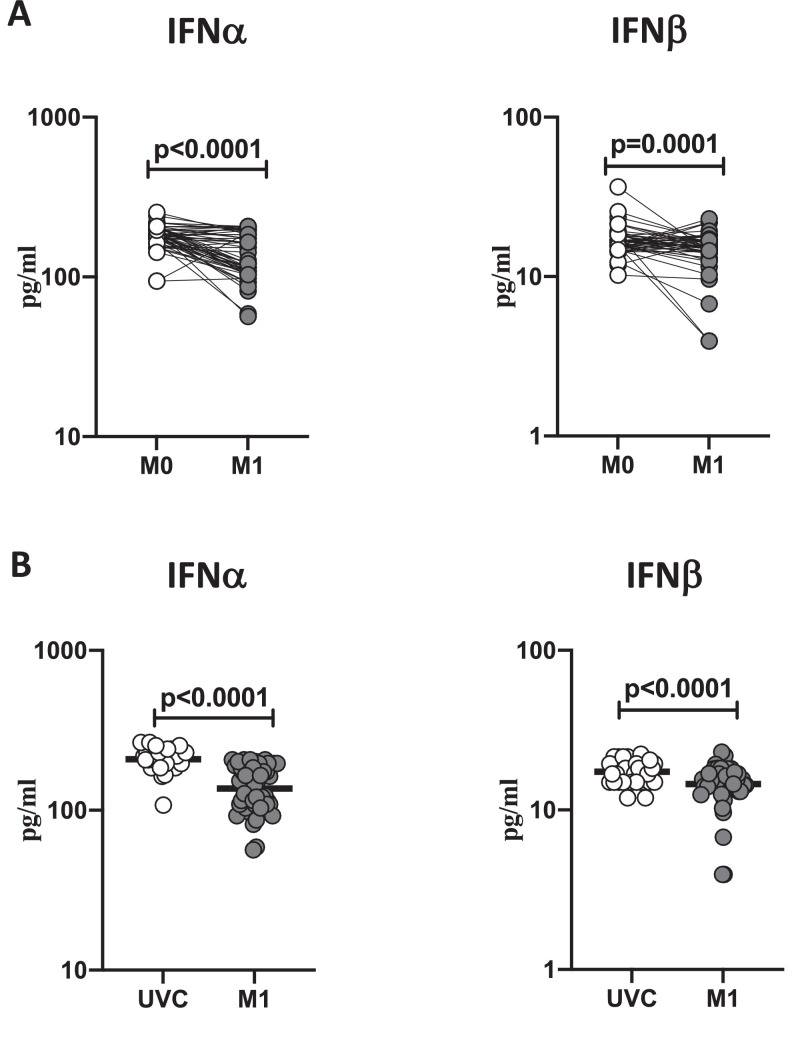
BCG vaccination induces diminished plasma levels of type I interferons
To study the plasma levels of type I IFNs following BCG vaccination, we compared the plasma levels of IFNα and IFNβ at baseline or before BCG vaccination (M0) and at month 1 (M1) post-vaccination. As shown in Figure 2 A, IFNα (p<0.0001) and IFNβ (p=0.0001) showed significantly diminished levels at M1 compared to M0. Next, we compared the plasma levels of type I IFNs in post-vaccinated individuals to unvaccinated controls. As shown in Fig 2B, BCG vaccinated individuals exhibited decreased circulating levels of IFNα (p<0.0001) and IFNβ (p<0.0001). Thus, BCG vaccination induces diminished systemic levels of type I IFNs in elderly individuals.
Figure 2.
BCG vaccination is associated with decreased circulating levels of type I interferons
(A) The plasma levels of type I IFNs like IFNα and IFNβ were measured in BCG pre-vaccinated [M0] (n = 54) and month 1 following vaccination [M1] (n = 54). Data are shown as line diagrams, with each line representing a single individual. p values were calculated using the Wilcoxon matched pair tests with Holms correction for multiple comparisons. (B) The plasma levels of type I IFNs like IFNα and IFNβ in BCG unvaccinated (UVC) (n = 32) and post vaccinated [M1] (n = 54) individuals. The data are represented as scatter plots, with each circle representing a single individual. p values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons
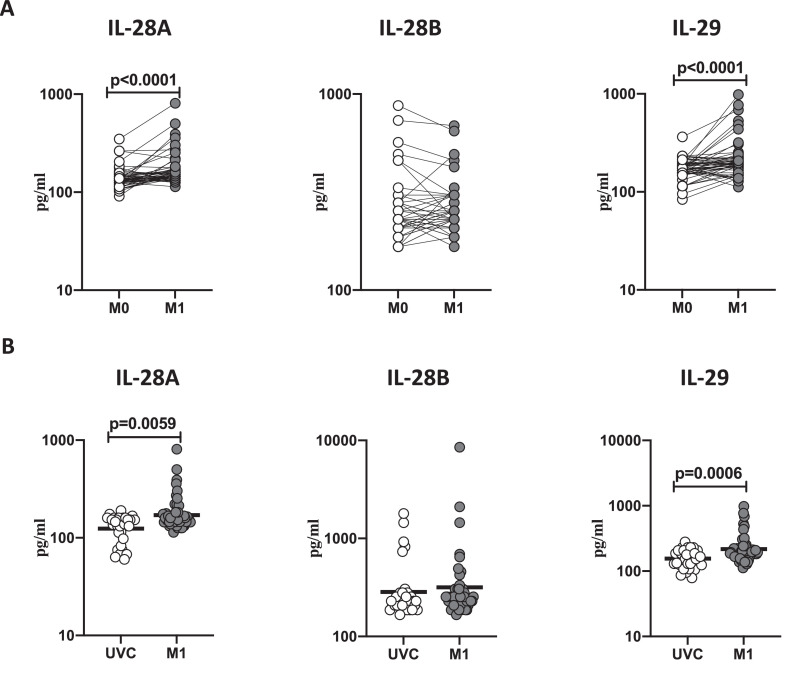
BCG vaccination induces enhanced plasma levels of type III interferons
To study the plasma levels of type III IFNs following BCG vaccination, we compared the plasma levels of IL-28A, IL-28B, and IL-29 at baseline or before BCG vaccination (M0) and at month 1 (M1) post-vaccination. As shown in Figure 3 A, IL-28A (p<0.0001) and IL-29 (p<0.0001) showed significantly increased levels at M1 compared to M0. Next, we compared the plasma levels of type III IFNs in post-vaccinated individuals to unvaccinated controls. As shown in Fig 3B, BCG vaccinated individuals exhibited significantly increased circulating levels of IL-28A (p=0.0059) and IL-29 (p=0.0006). Thus, BCG vaccination induces increased systemic levels of type III interferons in elderly individuals.
Figure 3.
BCG vaccination is associated with increased circulating levels of type III interferons
(A) The plasma levels of type III IFNs like IL-28A, IL-28B, and IL-29 were measured in BCG pre-vaccinated [M0] (n = 54) and month 1 following vaccination [M1] (n = 54). Data are shown as line diagrams, with each line representing a single individual; p values were calculated using the Wilcoxon matched pair tests with Holms correction for multiple comparisons. (B) The plasma levels of type III IFNs like IL-28A, IL-28B, and IL-29 in BCG unvaccinated (UVC) (n = 32) and post vaccinated [M1] (n = 54) individuals. The data are represented as scatter plots, with each circle representing a single individual. p values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons
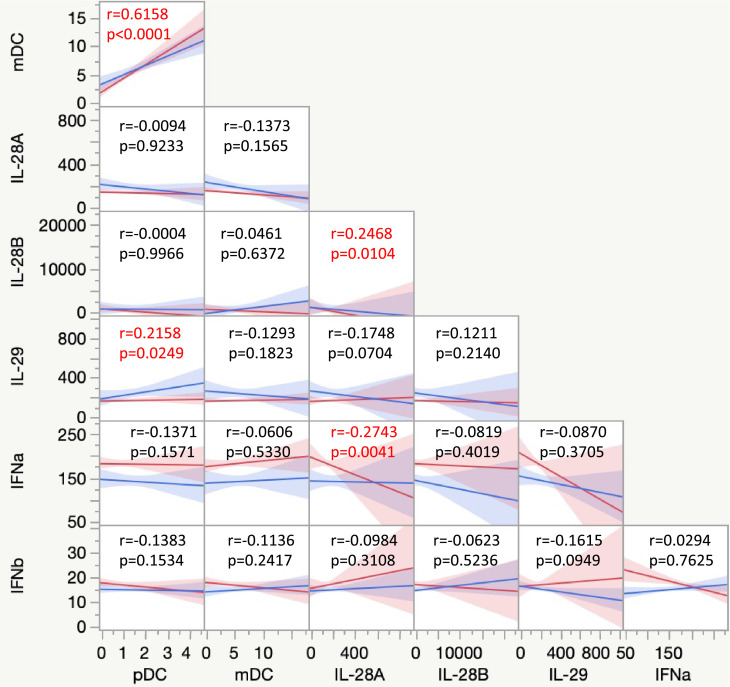
Associations between DC subsets and type I and III interferons
We wanted to identify correlations between frequencies of DC subsets (pDC and mDC) and type I (IFNα and IFNβ) and III IFNs (IL-28A, IL-28 B, and IL-29) in BCG vaccinated individuals. As shown in Fig. 4 , a multiparametric matrix correlation plot showed no significant correlation between the DC subsets and type I IFNs. However, a strong positive correlation between plasma levels of IL-29 with the frequencies of pDC was observed. No significant correlation was seen between IL-28A and IL-28B and DC subsets. Our results overall indicate a partial association between the DC subsets and type I and III IFNs.
Figure 4.
Relationship between DC subsets and type I and III interferons
Multiparametric matrix correlation plot of DC subsets and type I and III interferons in all individuals with BCG pre-vaccinated and month 1 following vaccination. Spearman's correlation coefficients are visualized. The blue line represents the x-axis parameter, and the red line represents the y-axis parameter.
Discussion
Naturally, the elderly population is at a greater risk for infection against new infectious episodes. We have chosen to investigate the elderly population residing in COVID-19 hotspots as it is known that this population is at a high risk of developing infections. Various clinical trials have shown that BCG vaccination limits the number of infections of all causes, especially respiratory tract infections, arguing for a protective effect (Giamarellos-Bourboulis et al., 2020, Madsen et al., 2020). Few other studies have determined the protective effect of BCG vaccination against SARS-CoV2 in elderly individuals (Ten Doesschate et al., 2020). Various findings over the years have reported that BCG vaccines can induce a dominant non-specific immune response, but it is still unclear if the BCG vaccine can offer meaningful protection against diseases like COVID-19. (Aspatwar et al., 2021). Actually, numerous clinical trials are ongoing to estimate the capacity of the BCG vaccine to modulate immunity against COVID-19. The main goal of these clinical trials is to determine if BCG vaccination lessens the incidence and severity of the SARS-CoV-2 infection. These studies will eventually help us to understand whether and to what extent BCG offers protection against SARS-CoV-2 (Aspatwar et al., 2021). In addition, studies have also reported that BCG vaccination in the elderly population resulted in decreased risk of pneumonia in tuberculin-positive individuals, indicating that administering BCG is one of the effective strategies for the prevention of pneumonia (Ohrui et al., 2005). In this current study, we wanted to evaluate the impact of BCG on DC subsets, type I and III interferons in elderly individuals residing in COVID-19 hotspots. As part of the study protocol, we studied the dendritic cells, Type 1 and III immune responses generated by BCG vaccination in a group of elderly individuals.
DCs encompass a diverse population of cells that play an important role in initiating, directing, and regulating adaptive immune responses (Soto et al., 2020). Activating the adaptive immune response requires the presentation of antigens to T cells by DC and macrophages (Hilligan and Ronchese, 2020). Delays in the activation of DCs often results in a delay in the induction of the adaptive immune response to pathogens. Thus, methods to either increase the frequencies of DCs or activate DCs would improve the protective efficacy of vaccines (Fucikova et al., 2019). Our data showing elevated frequencies of both mDC and pDC thus clearly illustrate a critical effect of BCG vaccination in possibly enhancing the innate immune response to specific and non-specific pathogens in elderly individuals. Moreover, while mDC is mainly involved in the process of antigen–presentation (Macri et al., 2018), pDC is the primary source of Type 1 interferons in the host (Leylek and Idoyaga, 2019). Thus, it is potentially likely that increased frequencies of pDC might heighten the propensity of pDC to mount Type I IFN responses against encountered pathogens.
Interferons constitute the first line of defense against microbial infections, particularly against viruses (Sa Ribero et al., 2020). Type I interferons and DCs share an overlying history; studies have been well reported that expression of type 1 IFNs by DC and their interface are one of the essential components of the innate and adaptive immune responses (Fitzgerald-Bocarsly and Feng, 2007). It is also known that cross-talk between pDC and mDC via type I IFNs has been involved in some pathological situations (Fitzgerald-Bocarsly and Feng, 2007). Even in our study, we observed a significant increase in the frequencies of DC subsets, whereas there was a significant decrease in the circulating level of IFNα and IFNβ one month post BCG vaccination, indicating that this inverse relationship may be involved in the containment of infection.
Type III IFNs typically act on epithelial cells in response to viral infection (Sommereyns et al., 2008). Plasmacytoid dendritic cells are the foremost producers of Type III IFNs (Yin et al., 2012, Zhang et al., 2013). Published data suggest that the impact of Type III IFNs on host immunity extends beyond its effect at the mucosal level to effects on systemic immune responses, specifically the innate and adaptive arms of the immune response (Zanoni et al., 2017). Thus, Type III IFNs are known to play a crucial role in adaptive immune responses to viral and bacterial infection, alter anti-tumor responses, and affect immunity (Lasfar et al., 2016, Lazear et al., 2015, Wack et al., 2015). Some studies have reported that Type III IFNs are the prominent IFNs produced after viral infection (Andreakos et al., 2017). In our study, we observed elevated circulating levels of IL-28A and IL-29 after one month of BCG vaccination, which in turn was correlated with the elevated levels of dendritic cells. However, their role in SAR-CoV-2 infections has not, to our knowledge, been explored.
In summary, our study highlights the effect of BCG vaccination in modulating the frequencies of DC subsets. Study limitations are that samples were collected only during the baseline visit and not at follow-up in control individuals and that all the measured data are reported only in percentages, not absolute numbers. Our study also reveals an effect of BCG in inducing a positive correlation with type III IFNs and DC subsets. The possible cellular mechanism is that type I or III IFNs may contribute to DC maturation, which is promoted by BCG vaccination. Although our study did not examine the mechanical changes in the immune system, our data reveal a vital role for BCG vaccination in boosting immune responses in the elderly population.
Acknowledgments
Acknowledgments
We thank the staff of the Department of Clinical Research, NIRT. We thank the data entry operators Mr. Jaiganesh and Mr. Vigneshwaran and all the ICER department staff members and Greater Chennai Corporation for the timely help.
Financial support
This work was supported by the Indian Council of Medical Research (ICMR). The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Author Contributions
Designed the study (SB, CP); conducted experiments (NPK, RA, AN, NS, RMR, VV); acquired data (NPK, RA, AN); analyzed data (NPK, RA); contributed reagents and also revised subsequent drafts of the manuscript (SB, CP); responsible for the enrolment of the participants and also contributed to acquisition and interpretation of clinical data (CP, BPK, BJ, DK, ST); wrote the manuscript (SB, NPK, CP). All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data and materials availability
All the reported data are available within the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.07.041.
Appendix. Supplementary materials
References
- Andreakos E, Salagianni M, Galani IE, Koltsida O. Interferon-lambdas: Front-Line Guardians of Immunity and Homeostasis in the Respiratory Tract. Front Immunol. 2017;8:1232. doi: 10.3389/fimmu.2017.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspatwar A, Gong W, Wang S, Wu X, Parkkila S. Tuberculosis vaccine BCG: the magical effect of the old vaccine in the fight against the COVID-19 pandemic. Int Rev Immunol. 2021:1–14. doi: 10.1080/08830185.2021.1922685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Felgenhauer U, Schoen A, Gad HH, Hartmann R, Schaubmar AR, Failing K, et al. Inhibition of SARS-CoV-2 by type I and type III interferons. J Biol Chem. 2020;295(41):13958–13964. doi: 10.1074/jbc.AC120.013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie. 2007;89(6-7):843–855. doi: 10.1016/j.biochi.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M, Hill PC, Setiabudiawan TP, Koeken V, Alisjahbana B, van Crevel R. BCG-induced protection against Mycobacterium tuberculosis infection: Evidence, mechanisms, and implications for next-generation vaccines. Immunol Rev. 2021 doi: 10.1111/imr.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucikova J, Palova-Jelinkova L, Bartunkova J, Spisek R. Induction of Tolerance and Immunity by Dendritic Cells: Mechanisms and Clinical Applications. Front Immunol. 2019;10:2393. doi: 10.3389/fimmu.2019.02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Dominguez-Andres J, et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell. 2020;183(2) doi: 10.1016/j.cell.2020.08.051. 315-23 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilligan KL, Ronchese F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol. 2020;17(6):587–599. doi: 10.1038/s41423-020-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Romani N, Steinman RM. An antigen-independent contact mechanism as an early step in T cell proliferative responses to dendritic cells. J Exp Med. 1989;170(2):527–542. doi: 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasfar A, Zloza A, de la Torre A, Cohen-Solal KA. IFN-lambda: A New Inducer of Local Immunity against Cancer and Infections. Front Immunol. 2016;7:598. doi: 10.3389/fimmu.2016.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Nice TJ, Diamond MS. Interferon-lambda: Immune Functions at Barrier Surfaces and Beyond. Immunity. 2015;43(1):15–28. doi: 10.1016/j.immuni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leentjens J, Kox M, Stokman R, Gerretsen J, Diavatopoulos DA, van Crevel R, et al. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J Infect Dis. 2015;212(12):1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- Leylek R, Idoyaga J. The versatile plasmacytoid dendritic cell: Function, heterogeneity, and plasticity. Int Rev Cell Mol Biol. 2019;349:177–211. doi: 10.1016/bs.ircmb.2019.10.002. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Oehen S, Barchiesi F, Schwendener RA, Hengartner H, Zinkernagel RM. Protective antiviral cytotoxic T cell memory is most efficiently maintained by restimulation via dendritic cells. J Immunol. 1999;163(4):1839–1844. [PubMed] [Google Scholar]
- Macri C, Pang ES, Patton T, O'Keeffe M. Dendritic cell subsets. Semin Cell Dev Biol. 2018;84:11–21. doi: 10.1016/j.semcdb.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Madsen AMR, Schaltz-Buchholzer F, Benfield T, Bjerregaard-Andersen M, Dalgaard LS, Dam C, et al. Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic: A structured summary of a study protocol for a randomised controlled trial in Denmark. Trials. 2020;21(1):799. doi: 10.1186/s13063-020-04714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, van Crevel R. BCG-induced protection: effects on innate immune memory. Semin Immunol. 2014;26(6):512–517. doi: 10.1016/j.smim.2014.09.006. [DOI] [PubMed] [Google Scholar]
- O'Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20(6):335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrui T, Nakayama K, Fukushima T, Chiba H, Sasaki H. [Prevention of elderly pneumonia by pneumococcal, influenza and BCG vaccinations] Nihon Ronen Igakkai Zasshi. 2005;42(1):34–36. doi: 10.3143/geriatrics.42.34. [DOI] [PubMed] [Google Scholar]
- Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16(7) doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4(3) doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto JA, Galvez NMS, Andrade CA, Pacheco GA, Bohmwald K, Berrios RV, et al. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front Immunol. 2020;11:1513. doi: 10.3389/fimmu.2020.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Doesschate T, Moorlag S, van der Vaart TW, Taks E, Debisarun P, Ten Oever J, et al. Correction to: Two Randomized Controlled Trials of Bacillus Calmette-Guerin Vaccination to reduce absenteeism among health care workers and hospital admission by elderly persons during the COVID-19 pandemic: A structured summary of the study protocols for two randomised controlled trials. Trials. 2020;21(1):555. doi: 10.1186/s13063-020-04521-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wack A, Terczynska-Dyla E, Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat Immunol. 2015;16(8):802–809. doi: 10.1038/ni.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Dai J, Deng J, Sheikh F, Natalia M, Shih T, et al. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol. 2012;189(6):2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Granucci F, Broggi A. Interferon (IFN)-lambda Takes the Helm: Immunomodulatory Roles of Type III IFNs. Front Immunol. 2017;8:1661. doi: 10.3389/fimmu.2017.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kodys K, Li K, Szabo G. Human type 2 myeloid dendritic cells produce interferon-lambda and amplify interferon-alpha in response to hepatitis C virus infection. Gastroenterology. 2013;144(2) doi: 10.1053/j.gastro.2012.10.034. 414-25 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JH, Wang YN, Chang QY, Ma P, Hu Y, Cao X. Type III Interferons in Viral Infection and Antiviral Immunity. Cell Physiol Biochem. 2018;51(1):173–185. doi: 10.1159/000495172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.