Abstract
With the outbreak of Coronavirus (2019) (COVID-19), as of late March 2020, understanding how the cause of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) transmitted is one of the most important questions that researchers are seeking to answer; because this effort helps to reduce the spread of disease. The COVID-19 is highly transmissible and deadly. Despite "tracking the call" and carefully examining patient contact, it is not yet clear how the virus is transmitted from one sick person to another. Why it is so transmissible? Can viruses be transmitted through speech and exhalation aerosols? How far can these aerosols go? How long can an aerosol containing a virus stay in the air? Is the virus amount in these aerosols enough to lead to an infection? There is no consensus on aerosols' role in the transmission of SARS-CoV-2. Findings show that SARS-CoV-2 aerosol transmission is possible. Therefore, to effectively reduce SARS-CoV-2, precautionary control strategies for aerosol transfer should be considered. Our aim is to review the evidence of the aerosol transmission containing SARS-CoV-2.
Keywords: SARS-CoV-2, Airborne particles, Aerosol transmission, COVID-19
1. Introduction
The unprecedented pandemic of the COVID-19 poses a threat to public health worldwide. There is widespread agreement between members of the infectious disease community, about possible methods of transmitting the respiratory virus (Tellier et al., 2019; Morawska and Cao, 2020). A person becomes susceptible to infection through "direct" or "indirect" physical contact with the touch of a virus-infected hand. Direct contact indicates that the virus is transmitted through person-to-person contact (such as handshake) between an infected host and a susceptible person. While indirect contact means transmission through "fomite", which is caused by contact with an object such as a paper tissue or hand-rail that has been infected with a virus. On the other hand, airborne transmission can occur through two different modes and does not require physical contact between an infected person and a susceptible person (Lindsley et al., 2013; Bourouiba, 2020). During sneezing or coughing, a "droplet spray" of virus-laden airway fluid, usually more than 5 μm in diameter, directly affects a susceptible person. Alternatively, a susceptible individual can inhale microscopic particles and aerosol evaporated from the remaining solid components of the respiratory aerosol, which are droplets tiny enough (<5 μm) to stay airborne for hours. That is not clear which mechanisms play a major role in COVID-19 transmission. Much research on airborne diseases before the COVID-19 has focused on "exhalation" events such as coughing and sneezing (Lindsley et al., 2013; Bourouiba et al., 2014; Dhand and Li, 2020). There is now strong evidence; although many infected people who transmit the COVID-19 either at least symptomatic or are not at all symptomatic. In China, asymptomatic individuals were found to be positive for the SARS-CoV-2, and transmission of the virus from asymptomatic carriers has been identified (Chan et al., 2020; Zou et al., 2020; Hu et al., 2020; Rothe et al., 2020). Recently, epidemiologists have estimated that more than 85 % of infections in China (Wuhan), were "undocumented" prior to the travel restrictions, with "limited, mild or no symptoms" and never they were tested. Remarkably, the modeling shows that 79 % of cases are actually infected by undocumented peoples. In addition, inspecting of average delay timing between initial manifestations of symptoms and infection indicates that " … pre-symptomatic [virus] shedding in some cases may be typically documented" (Li et al., 2020; Yongjian et al., 2020). In fact, it seems that a large number of patients who are sufficiently sick and in need of hospital treatment may themselves have been infected by people who did not appear to be ill. By definition, asymptomatic people do not have a significant cough or sneeze (Dhand and Li, 2020; Lednicky et al., 2020; Kenarkoohi et al., 2020). Indirect and or direct contact modes and also the transfer of aerosol are the main possible transmission modes. Much of public health messages have rightly focused on the possibility of indirect and or direct transmission through contaminated hands, and focus on the importance of greeting without shaking hands with others and washing hands thoroughly. There are important reasons to believe that aerosols play a role in high COVID-19 transmissibility (Matson et al., 2020; Booth et al., 2005). In the 2003 epidemic, air sampling showed that the viable aerosolized virus was emitted into the air by hospitalized SARS patients (Booth et al., 2005; Tang et al., 2020). The SARS-CoV-1 virus is the closest known in humans to SARS-CoV-2 which is responsible for the COVID-19 pandemic. Recent experiments have shown that the SARS-CoV-2 aerosol remains viable in the air with a 1-h half-life. As a result, SARS-CoV-2 aerosol and fomite transmission are acceptable because the virus may remain to survive and infectious in the aerosols and on the surfaces for hours and up to days (Van Doremalen et al., 2020). In addition to coughing and sneezing, has been shown that both breathing and normal speech emit large amounts of aerosol particles (Duguid, 1946; Papineni and Rosenthal, 1997). Most people who are unfamiliar with aerosols particles are completely unaware of their existence, these exhaled particles are typically diameter about 1 μm and therefore invisible by the naked eye. These particles are large enough to carry the SARS-CoV-2 virus, and also they are within the correct range of size and can be easily inhaled deep into a susceptible person's respiratory tract (Yongjian et al., 2020; Tang et al., 2020; Heyder et al., 1986).
Recent studies on viral respiratory disease (such as influenza) have shown that a viable virus can be emitted from infected peoples by speaking even breathing, without sneezing or coughing (Dhand and Li, 2020; Yan et al., 2018). Normal and ordinary speech converts significant amounts of respiratory particles into airborne aerosols. Experimental research has shown that vocalization emits up more aerosols than breathing (Morawska et al., 2009), also, a recent study indicated the louder one speech, the more aerosols are produced (Asadi et al., 2019). COVID-19 is a severe respiratory infection, and recent studies clearly identified the presence of the SARS-CoV-2 in a tract of the respiratory system (Zhu et al., 2019). So particles derived from breath and speech may contain viruses. These particles may be due in part to the mechanism of "liquid film bursting" in alveoli in the pulmonary, and or through the vibration of the vocal cords during a speech (Johnson et al., 2011). The findings suggest that particles and aerosols in the air reach the brain and affect CNS health (Ehsanifar et al., 2021), with changes in the blood-brain barrier (BBB) or leakage and transmission along the olfactory nerve to the olfactory bulb (OB) and active Microglia are the main components (Ehsanifar et al., 2019a, 2019b, 2020, 2021a, 2021b).
2. How is COVID-19 transmitted via expiratory particles?
Exhaled aerosol particles (either outdoors or indoors) transport in a plume or puff that travels in the direction of the background air motion (Wei and Li, 2016) but in the classic Wells-Riley transmission model, it is assumed that the room air is well mixed (Wells, 1934; Xie et al., 2007). The close to each other people may not transmit the virus due to the countervailing background air movement, just as the movement of air transmits virus-containing aerosol particles from the infected person to a close healthy person, the virus can be transmitted to distant people through the movement of air (Cole et al., 2020; Chia et al., 2020). Increasing air speeds may lead to the transfer of more exhaled particles to reach more susceptible individuals, or increase turbulence in the air, thus diluting the concentration of particles and reducing the likelihood of infection. In addition, exhaled droplets and particles may be deposited so rapidly by gravity that they are removed from the air before inhalation (Wei and Li, 2016; Chia et al., 2020). However, given the large number of exhaled particles that are emitted during respiration and speaking, and given COVID-19 high transmissibility, a face-to-face talking with one asymptomatic infected person is an important hypothesis that may be sufficient to transmit COVID-19 even if both are careful not to touch.
3. The importance of airborne aerosols particles transmission
An overview of why understanding airborne transmission is important for SARS-CoV-2, based on epidemiological and prevalence data and Knowing how the virus is transmitted is essential to know how to prevent it from spreading (Morawska and Cao, 2020; Klompas et al., 2020).
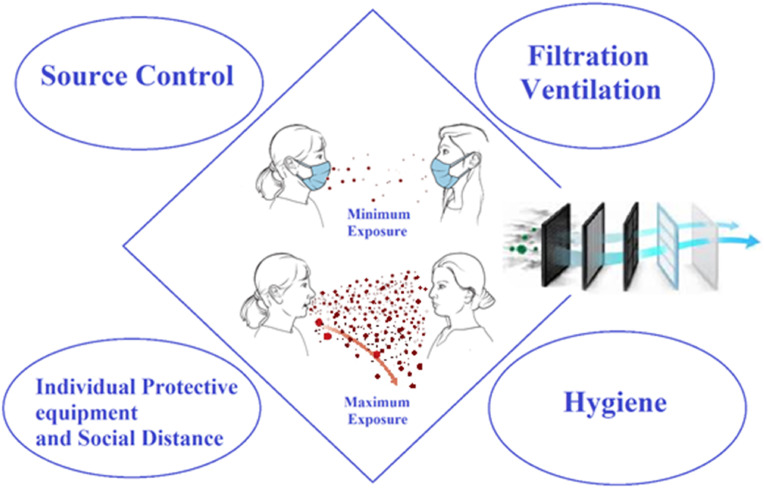
Information of transmission is required to inform the interventions, such as ventilation, mask use, physical distance, cleaning and disinfection. The four interventions general categories and their impact on transmission are shown in Fig. 1 (Tellier et al., 2019; Morawska and Cao, 2020; Van Doremalen et al., 2020).
Fig. 1.
Four basic categories of interventions to reduce the spread of SARS-CoV-2 that can result in a significant reduction in the risk of SARS-CoV-2 airborne transmission. Source control: Source control can significantly reduce, if not completely eliminate, the number of aerosols released into the air. Filtration/Ventilation: With effective filtration/ventilation, the number of viral aerosols can be greatly reduced. Individual protective equipment and social distance: These interventions can offer some protection by reducing a person's exposure. Hygiene: Research indicates that the highest viral loads or positive samples can be found on the floor. Cleaning the floor can reduce the possibility of resuspension.
The role of fomites in the transmission is unclear, but the SARS-CoV-2 is the respiratory virus that can be detected on the surfaces for 48 h or more. According to research, the virus is also present in a variety of body fluids. Perhaps the most worrying aspect of transmission evaluation is that 40–45 % of infected people are asymptomatic. It has now been well established that transmission of the SARS-CoV-2 by asymptomatic peoples is possible (Stadnytskyi et al., 2020; Zhang et al., 2020). This transmission makes it very challenging to track calls and fully evaluate transfer modes. The current understanding of how the virus prevalence is still evolving. While the SARS-CoV-2 genetically is similar to the other respiratory viruses but these similarities are not necessarily the same in terms of the transmission dynamics (Zhou et al., 2020; Wu et al., 2020; Wang et al., 2020). Recognition of SARS-CoV-2 is progressing rapidly. Also, experts' understanding and Knowledge of virus infection are increasing over time. For example, based on phylogenetic similarities, the course of infection was believed to be similar for SARS-CoV-2 and SARS (Stadnytskyi et al., 2020; Yao et al., 2020).
But after they found that the prevalence of SARS-CoV-2 in asymptomatic individuals was quite different, the agency modified the recommendation of the mask to include all people who go to public places. Initially thought was that SARS-CoV-2 transmitted through respiratory droplets that an infected person produces when speaking, sneezing, or coughing, droplets that can inoculate virus-infected mucous membranes in people up to 6 feet apart (Yao et al., 2020; Prather et al., 2020).
4. Airborne aerosols and respiratory viruses transmission
Respiratory viruses may be transmitted through indirect contact, direct contact person-to-person, large droplet spray, airborne aerosol, or a combination of all of these. Disease-carrying aerosols contain substances < 5 μm, while infectious disease physicians traditionally consider large droplet sprays to contain substances > 5 μm at close range (6.6 feet or < 2 m). It is generally believed that droplets and aerosols >5 μm settle within 6 feet away from the infected person who produces them, due to gravity (Duguid, 1946; Morawska et al., 2009; Asadi et al., 2019). Nevertheless, the use of 5 μm as a definite for these definitions is not supported by novel aerosol science, and the creation of a false dichotomy between what is considered an aerosol and what has considered a droplet undermines the notion of transmission (Klompas et al., 2020; Prather et al., 2020). The route of virus exposure is crucial to understanding transmission. Aerosols and droplets sprayed on the body and its mucous membranes, a kind of contact transmitter, while aerosols particles are inhaled by the respiratory system (Dhand and Li, 2020). This distinction now follows disease severity, infectious dose, and control strategies. At a close range, both inhalation and contact transmission pathways are possible, but in a longer range, when the droplets have settled rapidly transmission through the inhalation pathway is important. According to aerosol science, the aerosol size is not 5 μm, so an aerosol > 5 μm is inhalable and extends over 6 feet. Also, the aerosols may be formed through the resuspension of static aerosols or settled dust (Duguid, 1946; Prather et al., 2020). The virus is found in saliva or respiratory fluid, which varies in size from aerosols and droplets from 0.2 μm to 100 μm. In general, these aerosols particles and drops are produced through breathing, speaking, and talking with aerosols, coughing, and rapid drops accompanied by coughing (Dhand and Li, 2020; Heyder et al., 1986).
5. Production of aerosols and droplets by individuals and its distribution in air
Individuals produce droplets and aerosols in a wide range of concentrations and sizes. The smallest airborne aerosols particles are probably to be caused by the bursting of bronchial fluid film or laryngeal vibrations related to singing and speech. Larger droplets and aerosol particles are related to specific articulation movements of speaking as well as sneezing and coughing (Dhand and Li, 2020; Stadnytskyi et al., 2020). The majority of the aerosols particles observed in the human breath is < 10 μm. Breathing, speaking and singing generates ~100–1000 × more aerosol (<100 μm) than droplets (>100 μm) (Prather et al., 2020; Zhou et al., 2018). Aerosols exposure is far greater than droplets exposure, except for coughs, at intervals of less than 0.5 m. A large proportion of the SARS-CoV-2 transmission involves asymptomatic peoples who do not cough or sneeze, suggesting that aerosol particle exposure is an important pathway of transmission (Prather et al., 2020). The production of aerosol is very widely for different activities and people. For example, speaking louder than whispering or softer talking produces more aerosol material (Stadnytskyi et al., 2020). Activities such as singing which involves deeper breathing emit more aerosol particles and droplets from the lower respiratory tract. More research is needed on these changes in which respiratory aerosols are produced and the activities that most lead to aerosol production. About 20 % of individuals produce 80 % of the aerosols in the room, while about 10 % of the COVID-19 cause about 80 % of infections. To explain the diversity in the production of infectious aerosols, it is necessary to better understand the mechanisms of production and composition of fluids that lead to different sizes and numbers. Immediately after inhalation, due to the evaporation of water and liquids in them, droplets and aerosols begin to shrink. This rapid evaporation reduces the size of the aerosol particles to ~30–50 % of the initial size (Nicas et al., 2005). The virus in aerosols or droplets is subject to biological decay because of ultraviolet (UV) light exposure, desiccation and or other factors (Schuit et al., 2020). The droplets settle quickly, while the aerosols can be transported over long distances. These aerosols are transported by a stream of exhaled gas emitted by individuals and can then be carried quickly over large distances (Bourouiba, 2020; Van Doremalen et al., 2020). In the indoor space, by reducing the velocity of the exhalation cloud that carries them, background ventilation airflow scatters the particles remaining in the air. Eventually, these aerosols particles "deposit" or land on the surfaces. If these land on sensitive cells such as in the eyes, bronchi, distal lungs, they may cause infection (Bourouiba, 2020). That is not yet clear how many infectious droplets or aerosols are needed to cause an infection. All breathing, exhaling, talking, singing, coughing and sneezing, create persistence of droplets and airborne aerosol particles suspended in exhaled air multi-phase turbulent gas cloud. Exhaled clouds, with airborne particles and droplets of any size, can move to 8 m if sneezing (Bourouiba, 2020; Yao et al., 2020). All exhalations emit the high-momentum clouds with polluted droplets inside when breathing, talking, singing, coughing, and sneezing, but the arrival distance is different, and the size of the aerosol and droplets within them are different. Another pathway of transmission can be by regeneration dust or aerosol particles containing the virus from on the clothing or other surfaces and or on floors, as well as aerosolization of fomites. Half of the airborne aerosols particles in a room can be due to resuscitation by walking on the floors. Contaminated tissue rubbing produces particles thousands, and has been shown guinea pigs transmit viruses through their fur (Asadi et al., 2020).
6. The size of the infectious aerosol particles and droplets and how long they may remain infectious
SARS-CoV-2 infected humans can produce the infectious aerosols particles that may transmit the disease after adequate exposure. The spread of SARS-CoV-2 aerosols and disease transmission between humans is supported by the widespread contamination and positive results of RNA samples in air and on the surfaces detected in the COVID-19 patient's room at the hospital (Chia et al., 2020; Ong et al., 2020; Santarpia et al., 2020). Although positive RNA samples indicated the infectious virus possibility, except in sub-micron-sized particles, the results of cell culture were inconclusive (Santarpia et al., 2020). Recently, an important study was able to measure the infectious virus using the "gentler" collection method (Lednicky et al., 2020). Generally, these studies suggest that COVID-19 patients may produce aerosols with reproducible capable intact virions in the cell culture. The half-life of viruses in the aerosol is 1.1 h approximately. The Goldberg drum is used to hold airborne aerosols and particles and measure the stability of the infectious virus. With this method, founded that the SARS-CoV-2 half-life is a little up an hour (Van Doremalen et al., 2020). In other studies, infectious aerosols particles after 16 h found in the Goldberg drum that demonstrating virus stability (Tang et al., 2020). UV light greatly reduces the stability of the virus, and humidity and lower temperatures may increase the stability (Schuit et al., 2020). According to studies, the half-life of the virus decreases with increasing humidity. In another study, the virus half-life was reduced to 55 min, due to high humidity (Matson et al., 2020; Morris et al., 2020). Studies show that in warmer temperatures the virus infectivity disappears faster. The sunlight effect on the coronavirus was investigated using simulation of UV light with sunlight in spring, summer and autumn. These findings indicated that the SARS-CoV-2 half-life was reduced to less than 10 % or 6 min compared to that seen in dark environments (Schuit et al., 2020). Using simulated saliva in a laboratory experiment, the virus was found to be unstable at the high relative humidity in the tissue culture, while another study did the opposite (Smither et al., 2020). Since there is no data on actual respiratory secretions in human, SARS-CoV-2 in sputum and mucus transferred to surfaces, has a fewer half-life than the SARS-CoV-2 in the tissue culture medium (Matson et al., 2020), stability in respiratory tract outlet aerosols is likely to be lower compared to laboratory experiments using culture media. The size of the aerosol and droplets containing the influenza virus on exhalation can differ depending on the strain of the virus, and the size can affect the infection. Studies have shown that influenza transmission was mediated by the aerosols >1.5 m, even if 77 % of aerosols exhaled were ≤1.5 μm (Tang et al., 2020; Zhou et al., 2018). Smaller aerosols particles may play a significant role in some influenza virus strains than other strains, which indicating strain variations in the predominant mode of transmission. The place where the different sizes of aerosols particles deposit in the lungs are primarily determined by the size of these aerosols, with very fine 1 μm aerosols high probability to deposit deep in the lungs and cause more disease with the lower dose.
7. Conclusion
Aerosols are an important transport route for SARS-CoV-2, as aerosols particles can contain the infectious SARS-CoV-2, and remain suspended in the air for hours, which may be up to several meters transported from the source. The asymptomatic people mostly can often emit aerosols <10 μm in size and produce few droplets. Proving the aerosol particles transmission path is challenging and requires further research. Particularly, methods are needed that can sample large volumes and different sizes of air without harming the virus. For further understanding, a unified method is needed to quantify respiratory flow propagation across studies. Also, there is a need for risk assessment and a better understanding of transmission pathways to develop multi-scale models (different scales at models coupling) that take into account the physical processes related to transmission dynamics. To better understand the conditions that can increase the transmission risk, more study is needed to determine the range of human infectious dose, the difference in contamination rate with aerosol size, and the impact of the external factors on the disease severity. It is important to know how the infectious dose and inoculation pathway affect transmission. Awareness of the size distribution of viral aerosols is important in the exhaled breath, and knowledge of the mediated aerosols size range is also important for transmission. The limitations of the animal models must be considered when predicting human effects. Additionally, the impact of the repeated exposure, as well as the impact of the virus deposition site, must be determined. For disease severity, further research is needed on a combination of the underlying health issues such as sex, age, genetic and other factors. There is limited data about sex and age and is needed more human information to determine these factors' effect on the risk of transmission and the rate of infection. More aggressive mitigation strategies should be used in high-risk populations; treatment research should be analyzed in terms of age and gender, and other external items such as air pollutants on susceptible individuals should be considered. It would also be helpful to do more research in these cases:
Research on the characteristics of human-made SARS-CoV-2 infectious aerosols and variability in the infectious aerosols particle production rate from person to person. The infectious aerosols production during illness; and the relationship between aerosol particle dose and response via the aerosol pathway. Also, there is a need to design aerosol collectors that optimize the preservation of intact viruses. The persistence of man-made aerosols in different ambient and how to describe these environmental items in the high-risk settings (such as dental offices, long-term care centers, restaurants, bars and schools). The effect of temperature, sunlight and humidity on the virus stability in real respiratory secretions. Optimal face coverage characteristics, to support the source control. Further understanding of the effective face coverage strategies in the different environments for knowledge of clear public guidance.
Author statement
Mojtaba Ehsanifar: Conceptualization, Methodology, Writing-review & editing, Supervision.
Declaration of competing interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by Dr. Ehsanifar Research Lab., Tehran, Iran.
References
- Asadi S., et al. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S., et al. Influenza A virus is transmissible via aerosolized fomites. Nat. Commun. 2020;11(1):1–9. doi: 10.1038/s41467-020-17888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T.F., et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191(9):1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. Jama. 2020;323(18):1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Bourouiba L., Dehandschoewercker E., Bush J.W. Violent expiratory events: on coughing and sneezing. J. Fluid Mech. 2014;745:537–563. [Google Scholar]
- Chan J.F.-W., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11(1):1–7. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M., Ozgen C., Strobl E. 2020. Air Pollution Exposure and COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand R., Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;202(5):651–659. doi: 10.1164/rccm.202004-1263PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. Epidemiol. Infect. 1946;44(6):471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanifar M., et al. Exposure to nanoscale diesel exhaust particles: oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol. Environ. Saf. 2019;168:338–347. doi: 10.1016/j.ecoenv.2018.10.090. [DOI] [PubMed] [Google Scholar]
- Ehsanifar M., et al. Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol. Environ. Saf. 2019;176:34–41. doi: 10.1016/j.ecoenv.2019.03.090. [DOI] [PubMed] [Google Scholar]
- Ehsanifar Mojtaba, et al. Exposure To urban air pollution nanoparticles and CNS disease. On. J. Neur. & Br. Disord. 2021;5(5):520–526. doi: 10.32474/OJNBD.2021.05.000223. [DOI] [Google Scholar]
- Ehsanifar M.Y.Z., Karimian M., Behdarvandi M. Anxiety and depression following diesel exhaust nano-particles exposure in male and female mice. Journal of Neurophysiology and Neurological Disorders. 2020;6(101):1–8. [Google Scholar]
- Ehsanifar M., et al. Learning and memory disorders related to hippocampal inflammation following exposure to air pollution. Journal of Environmental Health Science and Engineering. 2021 doi: 10.1007/s40201-020-00600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanifar M., et al. Hippocampal inflammation and oxidative stress following exposure to diesel exhaust nanoparticles in male and female mice. Neurochem. Int. 2021;145:104989. doi: 10.1016/j.neuint.2021.104989. [DOI] [PubMed] [Google Scholar]
- Heyder J., et al. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986;17(5):811–825. [Google Scholar]
- Hu Z., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G., et al. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011;42(12):839–851. [Google Scholar]
- Kenarkoohi A., et al. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci. Total Environ. 2020;748:141324. doi: 10.1016/j.scitotenv.2020.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M., Baker M.A., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. Jama. 2020 doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- Lednicky J.A., et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley W.G., et al. A cough aerosol simulator for the study of disease transmission by human cough-generated aerosols. Aerosol. Sci. Technol. 2013;47(8):937–944. doi: 10.1080/02786826.2013.803019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson M.J., et al. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg. Infect. Dis. 2020;26(9):2276. doi: 10.3201/eid2609.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40(3):256–269. [Google Scholar]
- Morris S.B., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March–August 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69(40):1450. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2005;2(3):143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1997;10(2):105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368(6498):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- Rothe C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., et al. MedRxiv; 2020. Aerosol and Surface Transmission Potential of SARS-CoV-2. [Google Scholar]
- Schuit M., et al. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020;222(4):564–571. doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smither S.J., et al. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerg. Microb. Infect. 2020;9(1):1415–1417. doi: 10.1080/22221751.2020.1777906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnytskyi V., et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(22):11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020;144:106039. doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19(1):1–9. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Li Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Contr. 2016;44(9):S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells W.F. On air-borne infection. Study II. Droplets and droplet nuclei. Am. J. Hyg. 1934;20:611–618. [Google Scholar]
- Wu X., et al. medRxiv; 2020. Exposure to Air Pollution and COVID-19 Mortality in the United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., et al. How far droplets can move in indoor environments-revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17(3):211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Yan J., et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115(5):1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., et al. Vol. 731. Science of The Total Environment; 2020. On airborne transmission and control of SARS-Cov-2; p. 139178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongjian Z., et al. Science of the total environment; 2020. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China; p. 138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(26):14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., et al. Defining the sizes of airborne particles that mediate influenza transmission in ferrets. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115(10):E2386–E2392. doi: 10.1073/pnas.1716771115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]