Abstract
Aim
COVID-19 has spread globally with heavy impact on most countries and our therapeutic strategies in COVID-19 patients with diabetes are still limited. Recently, some new information was added to this field. We performed this updated meta-analysis to reveal the underlying effect of metformin on COVID-19 patients with diabetes.
Methods
We searched the PubMed, Embase and CNKI (China National Knowledge Infrastructure) databases for all articles. The odds ratio (OR) corresponding to the 95% confidence interval (95% CI) was used to assess the effect of metformin on COVID-19 patients with diabetes. The statistical heterogeneity among studies was assessed with the Q-test and I2 statistics.
Results
We collected 17 studies including 20,719 COVID-19 patients with diabetes. Our results found that metformin was associated with significantly decreased mortality and severity in COVID-19 patients with diabetes (OR = 0.64, 95% CI = 0.51–0.79 for mortality, and OR = 0.81, 95% CI = 0.66–0.99 for severity).
Conclusions
Our meta-analysis indicated that following metformin treatment might benefit the patients with T2DM, both the mortality and severity. However, patients with severe COVID-19 should be monitored closely for the development of lactic acidosis, acidosis, and decreased kidney function.
Keywords: Metformin, COVID-19, Diabetes, Meta-analysis
1. Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread throughout the world, afflicting more than 174.4 million people, resulting in more than 3.7 million deaths globally as of June 9, 2021, and with a mortality rate of about 2.1%. The epidemic of diabetes mellitus and its complications poses a major global health threat. The International Diabetes Federation (IDF) estimated that 1 in 11 adults had diabetes mellitus, the estimate is projected to rise to 642 million by 2040 globally [1]. The presence of diabetes mellitus, the individual degree of hyperglycaemia and the presence of typical complications of diabetes mellitus seem to be independently associated with COVID-19 severity and increased mortality [1], [2]. Especially the hyperglycaemia might modulate immune and inflammatory responses, thus predisposing patients to severe COVID-19 and possible lethal outcomes [2], [3].
Glucose-lowering medications might have effects on COVID-19 pathogenesis, and these effects could have implications for the management of patients with diabetes mellitus and COVID-19 [3]. Dipeptidyl peptidase 4 (DPP4) and the renin–angiotensin–aldosterone system (RAAS) are linked genetically and are associated with the risk of SARS-CoV-2 infection and possibly severity of COVID-19[4]. Glucagon-like peptide 1 (GLP1) analogues are not recommended in severe COVID-19 patients, because they will take time to become effective [5]. Sodium–glucose cotransporter 2 (SGLT2) inhibitors might cause adverse effects such as osmotic diuresis and dehydration in patients with COVID-19 and so cannot be recommended [6].
Metformin is a widely used oral glucose-lowering drug and is recommended as a first-line drug in recent treatment guidelines of the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) [7]. The clinical science of the potential relationships between metformin and COVID-19 patients with diabetes mellitus has been widely studied. However, knowledge in this field is emerging rapidly, with numerous publications appearing frequently. In the present study, we carried out this meta-analysis to detect the overall effects of metformin on COVID-19 patients with diabetes. This study was reported in accordance with the PRISMA statement for reporting systematic reviews and meta-analysis [8].
2. Methods
2.1. Publication search and inclusion criteria
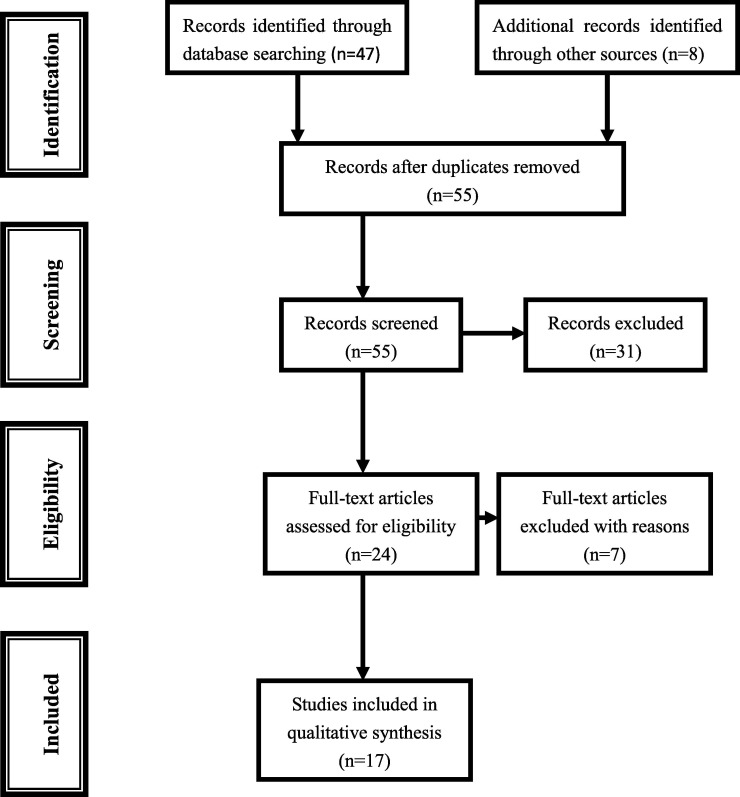
We searched the PubMed, Embase and CNKI (China National Knowledge Infrastructure) databases for all articles within a range of published years from 2019 to 2021 on the effect of metformin on COVID-19 patients with diabetes (last search was June 6, 2021). The following terms were used in this search: ‘metformin’, ‘diabetes’ and ‘COVID-19’. In order to identify the relevant publications, the references cited in the research papers were also scanned. Combining searches resulted in 47 abstracts. In addition, eight studies were identified through review articles and meta-analysis, for a total of 55 studies were screened after duplicated records were removed. After screening the titles and abstracts, 24 were retrieved for more detailed evaluation (Fig. 1 ). We used the Newcastle-Ottawa Scale (NOS) for assessing the quality of cohort studies and case-control studies based on three categories and eight items.
Fig. 1.
Flowchart for identification of studies.
We evaluated the eligible studies if all the following conditions were met: (1) evaluation on the effect of metformin on COVID-19 patients with diabetes; (2) inclusion of sufficient data or the data can be acquired from the manuscript or supplementary materials to calculate ORs and 95% CIs; and (3) the study was published in English.
2.2. Data extraction
Two authors (Kui Zhang and Wenxing Yang) independently reviewed and extracted the data needed. Disagreements were resolved through discussion among the authors to achieve a consensus. The following information was recorded for each study: first author, year of publication, region, outcome, number of metformin users, and number of patients (all of the data are shown in table 1 ).
Table 1.
Characteristics of literatures included in the meta-analysis.
| Reference | Year | Region | Outcome | No. of metformin users | No. of patients | Adjustment for covariates |
|---|---|---|---|---|---|---|
| Jiang N[20] | 2021 | China | Mortality ARDS |
100 | 328 | age, gender, weight, FBG, severity of COVID-19, Charlson comorbidity index, CHD, metformin therapy prior to hospitalization, DDI, creatinine and site |
| Li W[22] | 2021 | China | Mortality | 37 | 131 | age, BMI, glucose, triglyceride, CRP, D-dimer, and steroid use |
| Bramante CT[18] | 2021 | USA | Mortality | 2333 | 6256 | age, sex, comorbidities, alcohol abuse, HIV, asthma, inflammatory bowel disease, dementia, Charlson comorbidity index, and medications, and state |
| Lally MA[21] | 2021 | USA | Mortality | 127 | 775 | age, body mass index, hemoglobin A1c, estimated glomerular filtration rate, long stay (>90 days), and underlying psychoses |
| Cheng X[35] | 2020 | China | Mortality ARDS DIC Heart failure Acute kidney injury Acute heart injury |
678 | 1213 | age, gender, comorbidities, blood glucose, C-reactive protein, estimated glomerular filtration rate, alanine aminotransferase, and creatinine |
| Ghany R[19] | 2021 | USA | Mortality ARDS |
392 | 1139 | age, gender, Charlson score, diabetes, hypertension and ejection fraction |
| Luo P[40] | 2020 | China | Mortality | 104 | 283 | age, gender, underlying diseases, clinical severity |
| Oh TK[23] | 2021 | Korea | Mortality | 480 | 2047 | age, sex, underlying disability, Charlson Comorbidity Index |
| Li J[41] | 2020 | China | Mortality ventilation |
37 | 131 | age, body weight, BMI, oxygen desaturation, glucose, triglyceride, CRP, and D-dimers |
| Lalau J-D[36] | 2020 | France | Mortality IMV |
1496 | 2449 | sex, age, BMI, arterial hypertension, history of disease, active cancer, treated obstructive sleep apnoea, use of any of anti-diabetes drugs |
| Crouse A[42] | 2020 | USA | Mortality | 76 | 239 | age, race, sex, obesity and hypertension |
| Pérez-Belmonte LM[38] | 2020 | Spain | Mortality ICU admission mechanical ventilation in-hospital death |
825 | 1488 | age; gender; history of smoking, hypertension; dyslipidemia; chronic kidney disease; cerebrovascular disease; chronic obstructive pulmonary disease; atrial fibrillation; coronary artery disease; heart failure; obesity; dementia; Barthel Index score; and Charlson Comorbidity Index score |
| Wargny M[24] | 2021 | France | Mortality | 1553 | 2794 | sex, age, BMI, the patient’s history, routine medication, symptoms on admission, biological features |
| Chen Y[34] | 2020 | China | Mortality Poor prognosis |
43 | 120 | age, albumin, creatinine, glucose, CRP, and usage of a specific medication |
| Goodall JW[39] | 2020 | UK | Mortality | 210 | 981 | age, sex, comorbidities and medication usage |
| Kim MK[37] | 2020 | Korea | Mortality severe disease |
113 | 235 | age, sex, and the presence of underlying diseases |
| Gao Y[44] | 2020 | China | Life threatening complications | 56 | 110 | age, gender, blood glucose and LDH levels |
IMV, tracheal intubation for mechanical ventilation; severe disease, the necessity for the use of a high-flow nasal cannula, mechanical ventilation, CRRT, or ECMO, or admission to an ICU; CRP, C-reactive protein; BMI, body mass index.
2.3. Statistical analysis
The odds ratio (OR) corresponding to the 95% confidence interval (95% CI) was used to assess the effect of metformin on COVID-19 patients with diabetes. The statistical heterogeneity among studies was assessed with the Q-test and I2 statistics [9]. If there was no obvious heterogeneity, the fixed-effects model (the Mantel-Haenszel method) was used to estimate the summary OR [10]; otherwise, the random-effects model (the DerSimonian and Laird method) was used [11]. Finally, random effects models were used to calculate the overall OR estimates and 95% CIs to assess the effect of metformin on mortality and severity in COVID-19 patients with diabetes. To explore sources of heterogeneity across studies, we did logistic meta-regression analyses. We examined the following study characteristics: publication year, region, number of metformin users, and number of patients. Publication bias was evaluated with funnel plot and Begg’s rank correlation method [12]. The statistical analyses were performed by STATA 12.0 software (Stata Corp., College Station, TX).
3. Results
3.1. Characteristics of studies
Out of a total of 55 titles and abstracts, 24 were retrieved for more detail evaluation. Of the seven excluded studies, two papers were reviews, three papers lacked enough data, and two papers were excluded with duplicated data [13], [14] and the updated data were included. Finally, 17 studies met the inclusion criteria for this study, including 20,719 COVID-19 patients with diabetes. The details including first author, year of publication, region, outcome, number of metformin users, and number of patients in selected studies were listed in Table 1.
3.2. Quantitative synthesis
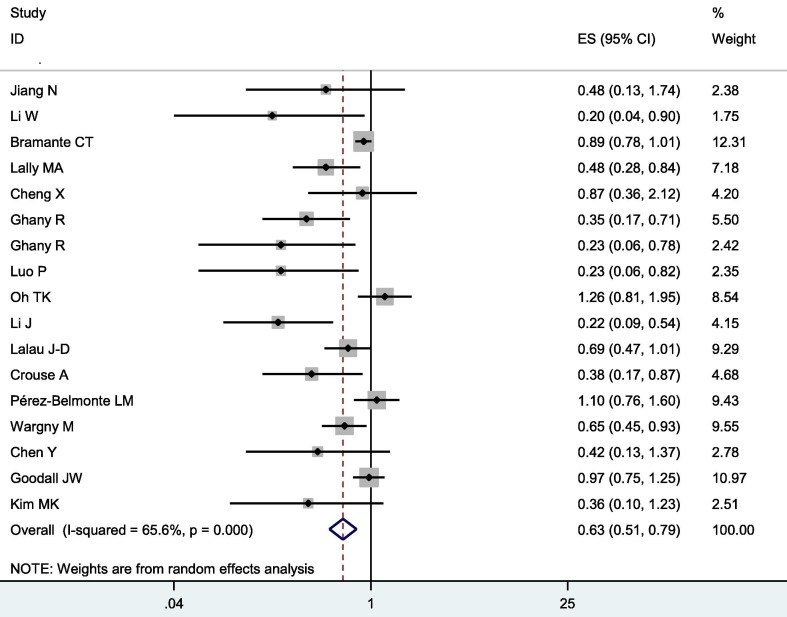
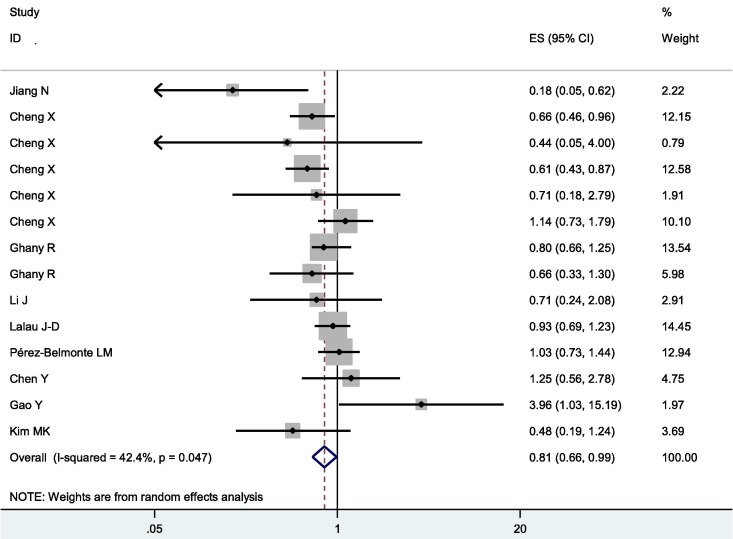
Overall, metformin was associated with significantly decreased mortality and severity in COVID-19 patients with diabetes (OR = 0.64, 95% CI = 0.51–0.79 for mortality, and OR = 0.81, 95% CI = 0.66–0.99 for severity, shown in Fig. 2 and Fig. 3 )
Fig. 2.
Forest plot of mortality following metformin treatment in COVID-19 patients with diabetes.
Fig. 3.
Forest plot of severity following metformin treatment in COVID-19 patients with diabetes.
3.3. Evaluation of heterogeneity
There was heterogeneity among studies in comparisons (P heterogeneity < 0.001, I2 = 65.6 %, Tau2 = 0.0996 for mortality, and P heterogeneity = 0.047, I2 = 42.2 %, Tau2 = 0.0493 for severity). Logistic meta-regression analyses found no possible factors that may substantially influence the initial heterogeneity.
3.4. Sensitivity analysis
The influence of a single study on the overall meta-analysis estimate was investigated by omitting one study at a time, and the omission of any study made no significant difference, indicating that our results were statistically reliable.
3.5. Publication bias
The Begg’s test was performed to evaluate the publication bias of selected literatures. No evidence of publication bias in our study was observed (P = 0.064 for mortality and P = 0.827 for severity).
4. Discussion
COVID-19 has spread globally with heavy impact on most countries and our therapeutic strategies in COVID-19 patients with diabetes are still limited. Diabetes was associated with poorer outcomes in COVID-19 patients [3], [15]. Previous meta-analysis indicated that metformin consumption was associated with lower mortality in COVID-19 patients among diabetic populations [16], [17]. However, whether to continue or withdraw metformin therapy in COVID-19 patients with diabetes remains contentious. Knowledge in this field is emerging rapidly, with numerous publications appearing frequently. Recently, some new information was added to this field [18], [19], [20], [21], [22], [23], [24], so we performed this updated meta-analysis to reveal the underlying effect of metformin on COVID-19 patients with diabetes.
Metformin can promote lifespan [25] and facilitates health [26], [27] through mitohormesis [28] and lysosomal pathway [25] to coordinate mTORC1 and AMPK [29] via host-microbe- metformin -nutrient interactions [30], [31]. Metformin has been reported to have anti-inflammation properties and reduced oxidative damage [32]. Metformin’s ability to reduce neutrophil counts and to reduce neutrophil extracellular traps have also been proposed as potential mechanisms for its beneficial use in patients with diabetes and COVID-19[33]. In previous clinical researches, some studies found that metformin use was not significantly associated with lower mortality in COVID-19 patients with diabetes [18], [20], [23], [34], [35], [36], [37], [38], [39]. However, others found that patients using metformin after admission were significantly more likely to survive than those who did not use [19], [21], [22], [24], [40], [41], [42]. Significantly, Oh TK et al found that metformin therapy might have potential benefits for the prevention of COVID-19 in Korean population [23]. Bramante CT et al revealed that metformin was associated with decreased mortality in women with obesity or type 2 diabetes who were admitted to hospital for COVID-19, but not in men [18], and they contributed the results to different cytokine responses to COVID-19 between genders. Most strikingly, research revealed that metformin use prior to the diagnosis of COVID-19 have more potential benefits in subjects with diabetes [42]. Our results with accumulated data indicated that metformin was associated with significantly decreased mortality in COVID-19 patients with diabetes.
Acute respiratory distress syndrome (ARDS) is one of the most common complications in patients with COVID-19. It is of great significance to prevent the incidence of ARDS for improving the outcome of patients [43]. The effect of metformin on the incidence of ARDS was controversial [19], [20]. Significantly, metformin use was significantly associated with reduced heart failure and inflammation [35]. However, some researches did not find any significant results of metformin use on clinical severity of the disease [37], and adverse outcomes [38]. Another study found that antidiabetic therapy with metformin was associated with a higher risk of disease progression in COVID-19 patients with diabetes during hospitalization [44], and the reason was that blood glucose and lactate dehydrogenase (LDH) levels of the metformin group were higher than those of the non-metformin group at admission [44]. Our results with accumulated data indicated that metformin was associated with significantly decreased severity in COVID-19 patients with diabetes.
A few limitations of our study should be considered. Although we did not observe significant publication bias, publication bias is possible in any meta-analysis. Moreover, there was heterogeneity among studies in overall comparisons. Although we performed logistic meta-regression analyses and stratified analysis to explore sources of heterogeneity across studies, we still found no possible factors that may substantially influence the initial heterogeneity, and the heterogeneity may potentially affect the results.
In conclusion, our meta-analysis indicated that following metformin treatment in COVID-19 patients with diabetes might decrease the mortality and severity. However, metformin use was significantly associated with a higher incidence of acidosis, particularly in cases with severe COVID19 [35]. Thus, patients with severe COVID-19 should be monitored closely for the development of lactic acidosis, acidosis, and decreased kidney function.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu L., She Z.-G., Cheng X.u., Qin J.-J., Zhang X.-J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim S., Bae J.H., Kwon H.-S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia I., Peiró C., Lorenzo Ó., Sánchez-Ferrer C.F., Eckel J., Romacho T. DPP4 and ACE2 in diabetes and COVID-19: therapeutic targets for cardiovascular complications? Front Pharmacol. 2020;11:1161. doi: 10.3389/fphar.2020.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nauck M.A., Meier J.J. Management of endocrine disease: are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181:R211–R234. doi: 10.1530/EJE-19-0566. [DOI] [PubMed] [Google Scholar]
- 6.Hahn K., Ejaz A.A., Kanbay M., Lanaspa M.A., Johnson R.J. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711–712. doi: 10.1038/nrneph.2016.159. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 10.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 11.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 13.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bramante C.T., Ingraham N.E., Murray T.A., Marmor S., Hovertsen S., Gronski J., et al. Observational Study of Metformin and Risk of Mortality in Patients Hospitalized with Covid-19. medRxiv. 2020 doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erener S. Diabetes, infection risk and COVID-19. Mol Metab. 2020;39:101044. doi: 10.1016/j.molmet.2020.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukito A.A., Pranata R., Henrina J., Lim M.A., Lawrensia S., Suastika K. The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2177–2183. doi: 10.1016/j.dsx.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarus G., Suhardi I.P., Wiyarta E., Rasyidah R.A., Barliana J.D. Is there a need to reconsider the use of metformin in COVID-19 patients with type 2 diabetes mellitus? Int J Diabetes Dev Ctries. 2021:1–6. doi: 10.1007/s13410-021-00924-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramante C.T., Ingraham N.E., Murray T.A., Marmor S., Hovertsen S., Gronski J., et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2(1):e34–e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghany R., Palacio A., Dawkins E., Chen G., McCarter D., Forbes E., et al. Metformin is associated with lower hospitalizations, mortality and severe coronavirus infection among elderly medicare minority patients in 8 states in USA. Diabetes Metab Syndr. 2021;15(2):513–518. doi: 10.1016/j.dsx.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang N., Chen Z., Liu L.i., Yin X., Yang H., Tan X., et al. Association of metformin with mortality or ARDS in patients with COVID-19 and type 2 diabetes: a retrospective cohort study. Diabetes Res Clin Pract. 2021;173:108619. doi: 10.1016/j.diabres.2020.108619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lally M.A., Tsoukas P., Halladay C.W., O'Neill E., Gravenstein S., Rudolph J.L. Metformin is associated with decreased 30-day mortality among nursing home residents infected with SARS-CoV2. J Am Med Dir Assoc. 2021;22(1):193–198. doi: 10.1016/j.jamda.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Li J., Wei Q., McCowen K., Xiong W., Liu J., et al. Inpatient use of metformin and acarbose is associated with reduced mortality of COVID-19 patients with type 2 diabetes mellitus. Res Sq. 2021 doi: 10.1002/edm2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh T.K., Song I.-A. Metformin use and risk of COVID-19 among patients with type II diabetes mellitus: an NHIS-COVID-19 database cohort study. Acta Diabetol. 2021;58(6):771–778. doi: 10.1007/s00592-020-01666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wargny M., Potier L., Gourdy P., Pichelin M., Amadou C., Benhamou P.-Y., et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64(4):778–794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J., Ou Y., Li Y., Hu S., Shao L.W., Liu Y. Metformin extends C. elegans lifespan through lysosomal pathway. Elife. 2017;6 doi: 10.7554/eLife.31268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizos C.V., Elisaf M.S. Metformin and cancer. Eur J Pharmacol. 2013;705(1-3):96–108. doi: 10.1016/j.ejphar.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Deng J., Sheng W., Zuo Z. Metformin attenuates Alzheimer's disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav. 2012;101(4):564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Haes W., Frooninckx L., Van Assche R., Smolders A., Depuydt G., Billen J., et al. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A. 2014;111(24):E2501–E2509. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C.-S., Li M., Ma T., Zong Y., Cui J., Feng J.-W., et al. Metformin activates AMPK through the lysosomal pathway. Cell Metab. 2016;24(4):521–522. doi: 10.1016/j.cmet.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Mannerås-Holm L., et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 31.Pryor R., Norvaisas P., Marinos G., Best L., Thingholm L.B., Quintaneiro L.M., et al. Host-microbe-drug-nutrient screen identifies bacterial effectors of metformin therapy. Cell. 2019;178(6):1299–1312.e29. doi: 10.1016/j.cell.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuiveling M., Vazirpanah N., Radstake T.R.D.J., Zimmermann M., Broen J.C.A. Metformin, a new era for an old drug in the treatment of immune mediated disease? Curr Drug Targets. 2018;19(8):945–959. doi: 10.2174/1389450118666170613081730. [DOI] [PubMed] [Google Scholar]
- 33.Dalan R. Metformin, neutrophils and COVID-19 infection. Diabetes Res Clin Pract. 2020;164:108230. doi: 10.1016/j.diabres.2020.108230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 35.Cheng X.u., Liu Y.-M., Li H., Zhang X., Lei F., Qin J.-J., et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;32(4):537–547.e3. doi: 10.1016/j.cmet.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalau J.-D., Al-Salameh A., Hadjadj S., Goronflot T., Wiernsperger N., Pichelin M., et al. Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19. Diabetes Metab. 2020;47 doi: 10.1016/j.diabet.2020.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M.K., Jeon J.-H., Kim S.-W., Moon J.S., Cho N.H., Han E., et al. The clinical characteristics and outcomes of patients with moderate-to-severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J. 2020;44(4):602. doi: 10.4093/dmj.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez-Belmonte L.M., Torres-Peña J.D., López-Carmona M.D., Ayala-Gutiérrez M.M., Fuentes-Jiménez F., Huerta L.J., et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. 2020;18(1) doi: 10.1186/s12916-020-01832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodall J.W., Reed T.A.N., Ardissino M., Bassett P., Whittington A.M., Cohen D.L., et al. Risk factors for severe disease in patients admitted with COVID-19 to a hospital in London, England: a retrospective cohort study. Epidemiol Infect. 2020;148 doi: 10.1017/S0950268820002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo P., Qiu L., Liu Y., Liu X.-L., Zheng J.-L., Xue H.-Y., et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J., Wei Q.i., Li W.X., McCowen K.C., Xiong W., Liu J., et al. Metformin use in diabetes prior to hospitalization: effects on mortality in Covid-19. Endocr Pract. 2020;26(10):1166–1172. doi: 10.4158/EP-2020-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crouse A, Grimes T, Li P, Might M, Ovalle F, Shalev A. metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 43.Zangrillo A., Beretta L., Scandroglio A.M., Monti G., Fominskiy E., Colombo S., et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020 doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y., Liu T., Zhong W., Liu R., Zhou H., Huang W., et al. Risk of metformin in patients with type 2 diabetes with COVID-19: a preliminary retrospective report. Clin Transl Sci. 2020;13(6):1055–1059. doi: 10.1111/cts.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]