Abstract
Background
In patients exposed to high-dose methotrexate (HDMTX; ≥1g/m2) with a history of elevated methotrexate (MTX) concentrations during previous doses, it is unclear whether prescribing high-dose leucovorin (HDLV) rescue limits future high levels or reduces the likelihood of acute kidney injury (AKI).
Methods
This retrospective, single-center study longitudinally followed adult lymphoma patients treated with HDMTX between 1/1/2011 and 10/31/2017 from diagnosis until 30 days after the last HDMTX dose. Endpoints included elevated MTX concentrations at 48 h (≥1.0 μmol/L) and incident AKI after each HDMTX dose.
Results
The 321 included patients had a median (IQR) age of 65 (57, 72) years, 190 (59%) were male, and 293 (91%) were Caucasian. There were 1558 HDMTX doses [median (IQR) 3 (2, 6) doses per patient] prescribed with 265 (83%) patients receiving more than one MTX dose. Those receiving HDLV rescue were more likely to have an elevated MTX concentration after that dose (OR = 2.69, 95% CI: 1.75–4.11, p < 0.001). Receiving HDLV rescue was associated with a greater likelihood of AKI after MTX (OR = 2.18, 95% CI: 1.38–3.43, p < 0.001). Hospital LOS was longer in those prescribed empiric HDLV rescue after MTX than those prescribed standard leucovorin with an estimated difference of 1.1 days, (95% CI: 0.5–1.7, p < 0.001).
Conclusion
Sequential HDMTX doses are associated with a significant incidence of elevated MTX levels and AKI during lymphoma management. HDLV rescue prescribed during subsequent MTX doses in patients with a previously elevated level was not associated with improved safety outcomes. The optimal supportive care strategy following HDMTX administration requires further investigation.
Keywords: Methotrexate, Leucovorin rescue, Nephrotoxicity, Therapeutic drug monitoring, Lymphoma
Introduction
Methotrexate (MTX) is a renally eliminated antimetabolite with longstanding use as a treatment for many hematologic and oncologic malignancies [1–5]. High-dose methotrexate (HDMTX; ≥ 1 g/m2) penetrates the blood-brain barrier making it an integral component of chemotherapy regimens for patients with lymphoma who have central nervous system (CNS) involvement or those at high risk for CNS relapse [6, 7]. MTX doses of 8 g/m2 for treatment of CNS lymphoma result in greater cytotoxic levels than intrathecal drug delivery of a 12-mg dose [8]. Systemic MTX at doses of at least 3.5 g/m2 has effectively prevented CNS recurrence in high-risk patients treated for systemic diffuse large B-cell lymphoma [9–13].
MTX competitively inhibits the enzyme dihydrofolate reductase, which blocks the conversion of dihydrofolate to its active form, depleting levels of DNA precursor tetrahydrofolates. This folate depletion ultimately leads to attenuated DNA synthesis as a result of cofactor loss necessary for purine and pyrimidine synthesis [14]. MTX-associated toxicities are a consequence of this decreased synthesis in a variety of cells and organs [14, 15]. The severity of these toxicities is directly proportional to the duration of elevated extracellular MTX concentrations [11, 15–17]. Leucovorin administration at 24 h after HDMTX therapy, termed leucovorin rescue, is necessary to improve the therapeutic index of MTX while reducing morbidity and mortality [18].
MTX-associated acute kidney injury (AKI) is a well-recognized complication that occurs in 5–30% of patients [19–21]. Doses of leucovorin are increased in patients with an elevated 48-h MTX concentration or a marked rise in serum creatinine to protect nonmalignant cells exposed to MTX in an effort to reduce life-threatening, systemic adverse events [4, 15, 22, 23]. High-dose leucovorin (HDLV) rescue mitigates myelosuppression in patients with high serum MTX concentrations or evident AKI [23–25]. Additionally, research demonstrated that sufficiently high leucovorin rescue doses could also prevent neurotoxicity, even after MTX doses up to 88 g/m2 [26, 27]. However, it is unknown whether proactively increasing the leucovorin dose in patients who previously experienced elevated 48-h serum MTX concentrations or MTX-related AKI can reduce AKI during subsequent MTX doses.
At our institution, standard leucovorin dosing (25 mg intravenous every 6 h) is delivered 24 h after the start of the HDMTX infusion and is continued until MTX levels are less than 0.1 μmol/L. If a patient experiences either a 50% increase in serum creatinine from baseline or a 48-h serum MTX concentration ≥1.0 μmol/L, HDLV rescue, defined as any dose greater than standard, is prescribed until MTX clearance and empirically with all subsequent MTX doses. The objective of this study was to determine if the use of empiric HDLV rescue impacts the incidence of future elevations in 48-h serum MTX concentrations. Secondary outcomes included the impact of empiric HDLV rescue on incidence of AKI and hospital length of stay (LOS).
Materials and methods
Study design and population
This retrospective, single-center study was conducted at the Mayo Clinic Rochester Methodist Hospital in Rochester, Minnesota. Consecutive adult patients hospitalized to receive HDMTX between January 2011 and October 2017 were reviewed for inclusion. Patients were included in the study if they were aged 18 years or older, diagnosed with lymphoma, and prescribed HDMTX as an infusion of 4 h or less. All patients had their consent for review of their medical records for research purposes verified through Minnesota Research Authorization prior to data abstraction. This study was approved by the Mayo Clinic Institutional Review Board.
Electronic medical records were reviewed from date of lymphoma diagnosis until 30 days after the last dose of HDMTX was administered. Baseline demographics were abstracted after malignancy diagnosis and prior to any chemotherapy administration. Laboratory information was collected at the time of diagnosis, prior to each HDMTX dose, and daily after HDMTX administration until hospital discharge. Body surface area (BSA) was calculated using the DuBois formula [28].
HDMTX was defined as any MTX dose ≥1 g/m2 [9, 15]. Per institutional protocol (Fig. 1), patients received leucovorin rescue of 25 mg intravenously every 6 h beginning 24 h after the start of the initial HDMTX infusion [10, 29]. If, at any time, the serum creatinine increased to greater than 50% of the pre-MTX value, the institutional protocol guided prescribers to increase leucovorin to 200 mg per meter squared (mg/m2) every 6 h and continue until the MTX level was < 0.1 μmol/L. Additionally, the leucovorin dose was adjusted in response to the serum MTX concentration drawn 48 h after the start of the MTX infusion according to the values in Table 1. Subsequent MTX doses would follow a similar process as the initial dosing algorithm in Fig. 1, except for the potential of an empirically prescribed HDLV rescue dose. A serum MTX concentration was considered elevated if it exceeded 1 μmol/L at 48 h. AKI was defined and staged with the Kidney Disease Improving Global Outcomes (KDIGO) criteria [30]. Any leucovorin dose greater than the standard dose empirically prescribed 24 h after HDMTX for doses beyond the initial MTX dose was defined as HDLV rescue.
Fig. 1.
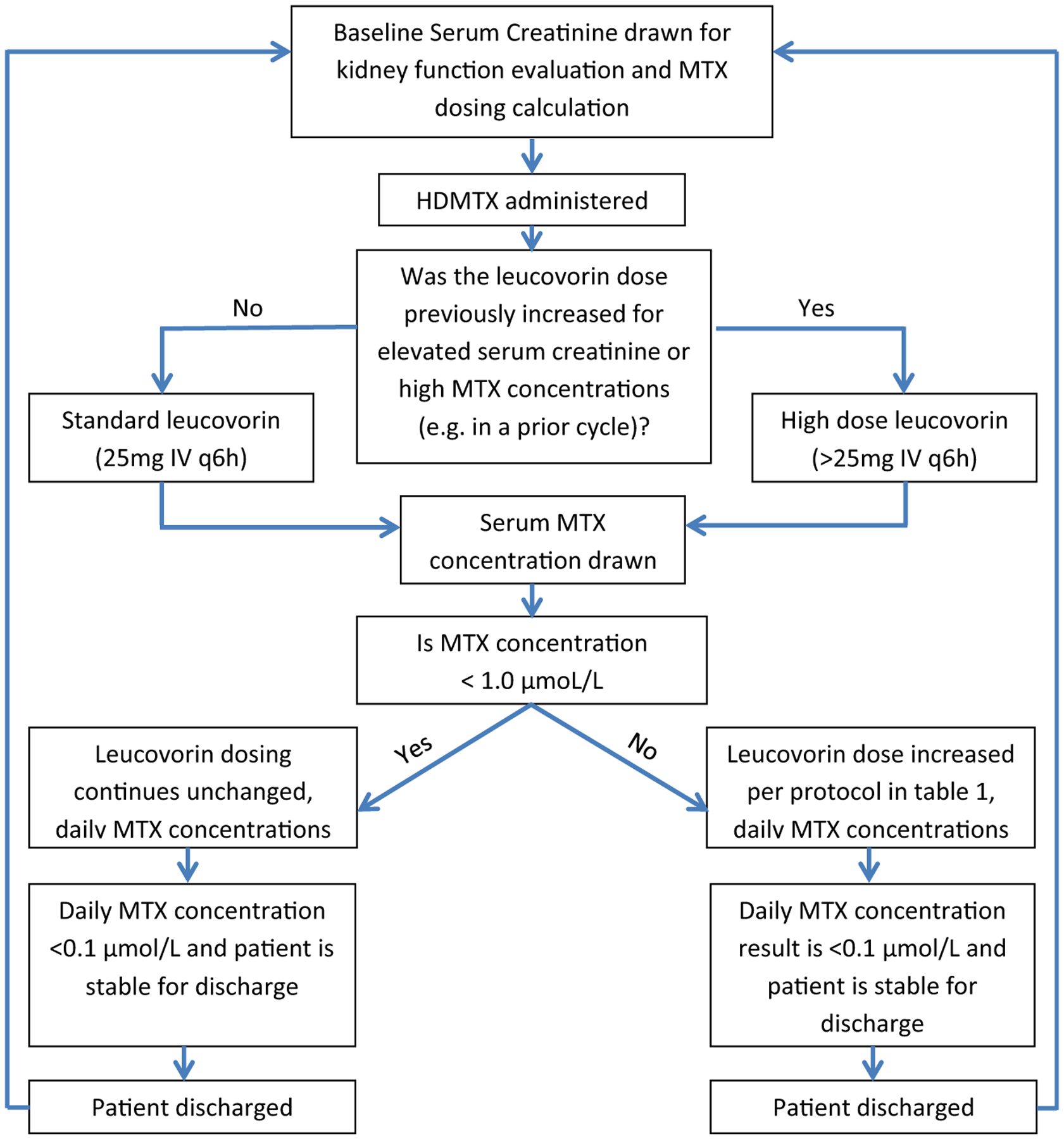
Institutional protocol for evaluating patients and prescribing HDMTX therapy
Table 1.
Leucovorin dose adjustment according to serum MTX concentration drawn 48 h after the start of the methotrexate infusion
| Methotrexate level* (μmol/L) | Leucovorin dose |
|---|---|
| 0.1–0.99 | 25 mg IV or PO every 6 h |
| 1–4.99 | 50 mg/m2 IV every 6 h |
| 5–9.99 | 100 mg/m2 IV every 6 h |
| 10–19.9 | 200 mg/m2 IV every 6 h |
| 20–50 | 500 mg/m2 IV every 6 h |
If, at any time, serum creatinine increases to greater than 50% of the pre-MTX value, leucovorin increases to 200 mg/m2 every 6 h and continues until MTX < 0.1 μmol/L
Statistical analysis
Baseline patient characteristics were summarized with counts and percentages for categorical variables, and with medians and interquartile ranges (IQR) for continuous variables. The study sought to characterize factors associated with elevated MTX concentrations, AKI, and hospital LOS. We provide two sets of analyses including one for the initial cycle and one for all cycles. Patients were followed until either MTX treatment was discontinued, the patient died, or the end of the study timeframe. Logistic regression was used to assess the association between baseline characteristics and the incidence of an elevated MTX concentration after the initial MTX dose. When evaluating all MTX doses prescribed to a patient, repeated measures logistic regression was used to estimate the association between patient characteristics and an elevated MTX concentration. Univariate and multivariable repeated measures logistic regression models were also used to estimate the association between leucovorin dose and each outcome of interest. Variables that were significant on univariate analysis (and without substantial missingness) were controlled for in the multivariable models. The cumulative incidence of elevated MTX and AKI were estimated after each MTX dose using the Kaplan-Meier method. Repeated measures linear regression was used to assess the association between HDLV and hospital LOS. All statistical tests were two-sided and p < 0.05 was considered to be statistically significant. The analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.4.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2015).
Results
Patient demographics and treatment information
The 321 patients who met inclusion criteria were prescribed a total of 1558 HDMTX doses [median (IQR) 3 [2, 6] doses per patient]. Fifty-six patients (17%) received only one MTX dose and were excluded from the analyses regarding empiric HLDR. The median (IQR) age was 65 (57, 72) years, 190 (59%) were male, and 293 (91%) were Caucasian. A majority (92%) of patients had an estimated creatinine clearance of more than 60mL/min prior to receipt of their initial MTX dose. Additional baseline demographics and clinical characteristics are provided in Table 2.
Table 2.
Summary and association of baseline demographics and laboratory values with an elevated methotrexate level at 48 h after the first HDMTX dose
| Characteristic | Initial cycle (N = 321) | Odds ratio† (95% CI) | p-value |
|---|---|---|---|
| Age (year), median (IQR) | 64.9 (56.7, 72.2) | 1.02 (1.00, 1.04) | 0.053 |
| Male, no. (%) | 190 (59.2%) | 2.16 (1.25, 3.72) | 0.006 |
| Race, no. (%) | |||
| Caucasian | 293 (91.3%) | 5.11 (1.19, 22.0) | 0.029 |
| Other/unknown | 28 (8.7%) | Reference | |
| Body surface area, median (IQR) | 2.0 (1.8, 2.1) | 1.18 (1.08, 1.30)‡ | <0.001 |
| Lymphoma diagnosis subtype, no. (%) | |||
| Diffuse large B-cell lymphoma (DLBCL) | 132 (41.1%) | Reference | |
| Primary DLBCL of the CNS | 106 (33.0%) | 2.41 (1.31, 4.45) | 0.005 |
| EBV positive DLBCL of elderly | 10 (31.2%) | 1.20 (0.24, 6.06) | 0.82 |
| Other | 73 (22.7%) | 2.41 (1.23, 4.72) | 0.010 |
| Bone marrow involvement, no. (%) | 46 (14.3%) | 2.10 (1.09, 4.06) | 0.027 |
| Percent involvement, median (IQR) | 30 (10, 70) | ||
| Serum creatinine, mg/dL, median (IQR) | 0.8 (0.7, 0.9) | 1.20 (1.08, 1.33)‡ | <0.001 |
| Estimated creatinine clearance, no. (%) | |||
| < 30 mL/min | 0 (0%) | ||
| 31–60 mL/min | 26 (8.2%) | 1.85 (0.80, 4.26) | 0.15 |
| > 60 mL/min | 291 (91.8%) | Reference | |
| Hemoglobin, g/dL, median (range) | 11.5 (7–16.2) | 0.83 (0.72, 0.95) | 0.008 |
| WBC count, × 109/L, median (IQR) | 7.1 (4.8, 10) | 0.97 (0.92, 1.03) | 0.36 |
| ANC count, × 109/L, median (IQR) | 5.2 (3.2, 8) | 1.01 (0.94, 1.09) | 0.70 |
| ALC count, × 109/L, median (IQR) | 0.8 (0.5, 1.3) | 0.98 (0.95, 1.02)‡ | 0.29 |
| Aspartate aminotransferase, median (IQR) | 21 (17, 28) | 1.05 (0.97, 1.14) | 0.22 |
| Alanine aminotransferase, median (IQR) | 27 (19, 46) | 1.10 (0.96, 1.06) | 0.68 |
| Alkaline phosphatase, median (IQR) | 72 (57, 92) | 0.97 (0.91, 1.04) | 0.42 |
| Total bilirubin, median (IQR) | 0.4 (0.3, 0.6) | 1.08 (1.01, 1.16)‡ | 0.025 |
| LDH, median (IQR) | 201 (167, 264) | 1.01 (0.99, 1.02) | 0.095 |
| Albumin, mg/dL, median (IQR) | 3.8 (3.2, 4.1) | 0.93 (0.88, 0.98)‡ | 0.012 |
CI confidence interval; IQR interquartile range
Odds ratios for continuous variables are per 1 unit increase unless otherwise specified
Per 0.1 unit increase
Per 10 unit increase
Only available in 203
Only available in 182
Chemotherapy regimen distribution included 43 patients (13%) prescribed HDMTX 8 g/m2 monotherapy, 161 patients (50%) prescribed HDMTX 8 g/m2 with Rituximab and Temozolomide (MRT), 96 patients (30%) prescribed HDMTX 3.5 g/m2 in combination with Rituximab, cyclophosphamide, doxorubicin, and prednisone (MR-CHOP), and 21 patients (7%) prescribed other HDMTX-containing combination chemotherapy regimens. The median (IQR) MTX dose was 6.3 g (4.3, 7.5) for 84 patients (26%) receiving MTX for prophylaxis against CNS relapse and 12.8 g (7.5, 16.8) for the 237 patients (74%) receiving MTX as treatment for active disease in the CNS.
Elevated 48-h serum methotrexate concentrations
First MTX dose
A total of 83 patients (26%) had an elevated MTX concentration at 48 h after their initial MTX dose. Factors associated with an elevated MTX level after the initial MTX dose were male sex, Caucasian race, higher BSA, primary DLBCL of the CNS, positive bone marrow involvement, a higher baseline serum creatinine, higher total bilirubin, lower hemoglobin, and a lower albumin.
Subsequent MTX doses
There were 265 patients who received more than one MTX dose and were included in the analysis to assess the potential protective effect of leucovorin dose increases during subsequent MTX doses. Prescribing standard leucovorin (based on the prior MTX concentration not being elevated) resulted in a 12% rate of elevated MTX concentrations during subsequent MTX (83/709 doses). Prescribing HDLV rescue (based on the prior elevated MTX concentration) resulted in a 34% rate of elevated MTX concentrations during the subsequent MTX dose (160/465 doses). The cumulative incidence of elevated MTX concentrations after three MTX doses was 44% (95% CI: 38–50) and 61% (95% CI: 53–68) after six MTX doses. The incidence of elevated MTX concentrations by number of MTX doses is shown in Fig. 2. Table 3 displays the association between the patient factors and the outcome of an elevated MTX concentration at 48 h after administration.
Fig. 2.
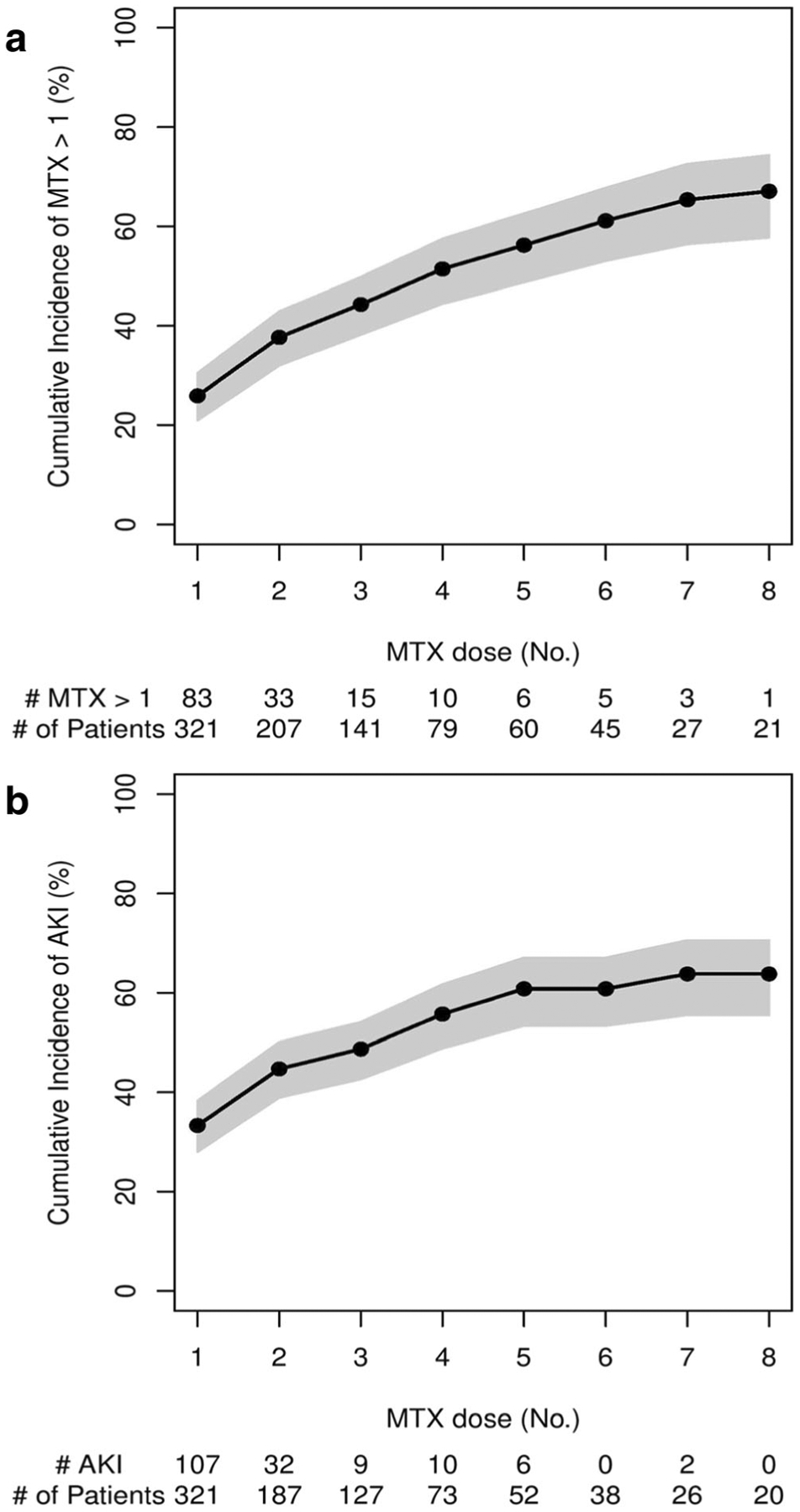
Cumulative incidence of elevated 48-h serum methotrexate concentrations and acute kidney injury after HDMTX administration
Table 3.
Multivariable association of patient characteristics and laboratory values with an elevated methotrexate level at 48 h or AKI after any subsequent MTX dose
| Odds ratio† (95% CI) | p-value | |
|---|---|---|
| Elevated 48-h serum Methotrexate concentration | ||
| Body surface area | 1.02 (0.92, 1.12)‡ | 0.72 |
| Lymphoma diagnosis subtype | ||
| DLBCL | Reference | |
| Primary DLBCL of the CNS | 1.57 (0.82, 2.99) | 0.17 |
| EBV positive DLBCL of elderly | 0.14 (0.01, 1.38) | 0.092 |
| Other | 1.07 (0.54, 2.14) | 0.84 |
| Serum creatinine, mg/dL (N = 1170) | 1.06 (0.97, 1.15)‡ | 0.21 |
| Hemoglobin, g/dL (N = 1173) | 0.74 (0.66, 0.83) | <0.001 |
| Intended MTX dose (8 g/m2 vs. other) | 0.88 (0.44, 1.78) | 0.734 |
| Delivered MTX dose, g | 1.11 (1.04, 1.18) | 0.001 |
| Empirically prescribed high-dose leucovorin rescue | 2.87 (1.87, 4.39) | <0.001 |
| Acute kidney injury | ||
| Male | 3.26 (1.80, 5.93) | <0.001 |
| Race | ||
| Caucasian | 3.58 (1.43, 8.89) | 0.007 |
| Other/unknown | Reference | |
| Body surface area | 0.95 (0.86, 1.04)‡ | 0.27 |
| Serum creatinine, mg/dL (N = 1185) | 1.02 (0.94, 1.12)‡ | 0.58 |
| Hemoglobin, g/dL (N = 1190) | 0.82 (0.72, 0.93) | 0.003 |
| ANC count, × 109/L (N = 1187) | 1.05 (1.00, 1.11) | 0.041 |
| Intended MTX dose (8 g/m2 vs. other) | 0.79 (0.39, 1.62) | 0.52 |
| Delivered MTX dose, g | 1.06 (0.99, 1.12) | 0.097 |
| Empirically prescribed high-dose leucovorin rescue | 1.92 (1.21, 3.034) | 0.006 |
ANC absolute neutrophil cell; CI confidence interval; DLBCL diffuse large B-cell lymphoma
Odds ratios for continuous variables are per 1 unit increase unless otherwise specified
Per 0.1 unit increase
As outcomes from the prior MTX dose impact the next dose, we analyzed all of the potential outcomes from the prior dose for an association with elevated MTX concentrations after administering a subsequent MTX dose (Table 4). After adjustment of relevant confounders, HDLV rescue with a prior MTX dose remained significantly associated with an elevated MTX concentration following the next MTX dose. Notably, those who had AKI with their prior MTX dose did not have significantly increased odds of having an elevated MTX concentration following the next MTX dose.
Table 4.
Multivariable association of patient outcome from prior MTX dose with an elevated methotrexate level at 48 h or AKI after the next MTX dose
| Group based on prior MTX dose | Univariate | Multivariable* | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Elevated 48-h serum methotrexate concentration† | ||||
| Neither HDL or AKI | Reference | Reference | ||
| HDL only | 3.78 (2.46, 5.82) | <0.001 | 2.73 (1.72, 4.33) | <0.001 |
| AKI only | 2.19 (1.10, 4.36) | 0.025 | 1.83 (0.91, 3.67) | 0.088 |
| Both HDL and AKI | 6.29 (3.73, 10.61) | <0.001 | 4.36 (2.51, 7.57) | <0.001 |
| Acute kidney injury‡ | ||||
| Neither HDL or AKI | Reference | Reference | ||
| HDL only | 1.78 (1.07, 2.99) | 0.028 | 1.57 (0.94, 2.63) | 0.084 |
| AKI only | 3.23 (1.75, 5.97) | <0.001 | 2.70 (1.45, 5.02) | 0.002 |
| Both HDL and AKI | 5.35 (3.20, 8.97) | <0.001 | 3.97 (2.29, 6.90) | <0.001 |
Adjusted for BSA, diagnosis group, SCr, hemoglobin, dose (8 vs. not), and delivered MTX dose
HDL only vs. both HDL and AKI: p-value = 0.056
HDL only vs. both HDL and AKI: p-value<0.001; AKI only vs. both HDL and AKI: p-value = 0.28
Acute kidney injury
First MTX dose
There were 107 patients (33%) who experienced AKI after their initial MTX dose. Among these AKI episodes, 81 cases (76%) were stage 1, 19 (18%) were stage 2, and 7 (6%) were stage 3. Seventy-eight (73%) patients who experienced AKI after the initial MTX dose went on to receive at least one subsequent MTX dose.
Subsequent MTX doses
The incidence of AKI by number of MTX doses received is displayed in Fig. 2. The cumulative incidence of AKI after three MTX doses was 49% (95% CI: 43–54) and was 61% (95% CI: 53–67) after six MTX doses. In those with a history of AKI, prescription of HDLV rescue with subsequent MTX doses was not protective against subsequent AKI.
As outcomes from the prior MTX dose impact the next dose, we analyzed the potential combinations of outcomes from the prior dose for an association with AKI after a subsequent MTX dose is prescribed (Table 4). After adjustment of relevant confounders, multivariable analysis demonstrated a persistently greater risk for AKI in the patients who experienced AKI during the prior dose. Importantly, those who received HDLV rescue due to previously elevated MTX concentration with their prior MTX dose did not have a significantly increased risk for having AKI following their next MTX dose. Patients who experienced AKI were more likely to have an MTX dose reduction for their next dose than patients without an AKI, even if patients experienced an elevated MTX concentration with that prior MTX dose.
Hospital length of stay
Overall median hospital LOS was 4.1 (3.8, 5.8) days. Hospital LOS was longer in those prescribed empiric HDLV rescue based on previous MTX doses (median LOS with empiric HDLV rescue: 5 (4.1, 6.1) days; with standard leucovorin doses: 4 (3.2, 4.3) days; estimated difference = 1.1 days, 95% CI: 0.5–1.7, p < 0.001).
Discussion
Our study demonstrated that elevated 48-h serum MTX concentrations and AKI occurred commonly in patients receiving HDMTX with a 26% and 33% incidence, respectively, after the initial MTX dose. In patients with a history of elevated MTX levels or MTX-associated AKI, empirical increases in leucovorin rescue during subsequent MTX doses did not appear protective of the kidney. In fact, the risk of a repeat episode of an elevated MTX concentration was higher in patients who were prescribed HDLV rescue. Notably, AKI with a previous MTX dose was not associated with an elevated MTX concentration following the next MTX dose; however, previous AKI was significantly associated with AKI following their next MTX dose. These results question the utility of empiric HDLV rescue as supportive care strategy for patients receiving HDMTX and highlight a need to improve renal assessment for HDMTX dosing.
Toxicity due to prolonged MTX exposure is an established concern without an optimal solution [31]. MTX-associated nephrotoxicity is an important contributor to dose accumulation and systemic toxicities such as myelosuppression [22, 24, 32]. MTX time-dependent and concentration-dependent relationships differ by target tissue and a specific concentration threshold specific to each organ must be exceeded before toxicity will occur [15, 33]. Renal cells, unlike stem cells in the bone marrow, are generally quiescent cells in the G0 phase with a low turnover; therefore, MTX should exhibit limited effects on these cells [34]. However, MTX is renally eliminated and the concentrations found in the kidney may be high enough to incite passive diffusion and cell death [35].
Abelson et al. [24] administered leucovorin 150mg every 3 h when renal impairment was identified and concluded that early intervention with HDLV rescue is paramount to prevent myelosuppression. Subsequently, it was suggested that the HDLV rescue doses be escalated in proportion to the prescribed MTX dose to achieve CSF penetration and prevent neurotoxicity [26]. It is unclear why we did not observe a beneficial effect of HDLV on the kidneys in high-risk patients. The chosen doses for leucovorin rescue in our study were based on the outcomes of previous MTX and leucovorin dose increases. Our HDLV rescue doses may not have been sufficient to prevent renal cell injury. However, increased medication cost corresponding to larger leucovorin doses and inventory depletion when prescribing mega-dose HDLV utilization during times of drug shortages must be considered [15, 36, 37]. Unfortunately, all patients experiencing AKI or elevated MTX levels in our institution received empiric HDLV rescue during subsequent MTX doses; therefore, it is unknown whether or not the incidence and severity of AKI would have been even higher if the standard leucovorin was prescribed instead of the employed HDLV rescue strategy. Future investigations could include different HDLV rescue doses across treatment groups to determine if any kidney-related benefit exists.
The variable incidence of MTX-associated nephrotoxicity depends on the patient population, MTX-based chemotherapy regimen chosen, MTX dosage prescribed, and AKI definition utilized [15, 19, 24, 32]. Our longitudinal evaluation of serial MTX doses resulted in a high AKI incidence despite empiric HDLV rescue, and patients who experienced AKI during the prior dose were at significantly increased risk for AKI following their next MTX dose. Repeated AKI and impaired glomerular filtration rate (GFR) place lymphoma patients at a heightened risk for progression to CKD and increased mortality [38–42].
Equally important to employing HDLV rescue to prevent systemic toxicity in the setting of prolonged MTX exposure is the attention that must be paid to optimizing and individualizing the MTX dose to improve drug safety without jeopardizing clinical effectiveness [24, 43]. One possibility is to improve MTX dose selection by increasing the accuracy in GFR assessment. The shortcomings of serum creatinine and estimated creatinine clearance as a surrogate for GFR are well-described elsewhere and can lead to GFR overestimation, particularly in patients with malignancy [44, 45]. Whether other renal biomarkers as a replacement for, or addition to, serum creatinine can improve GFR-estimation and MTX dose selection remains unknown and should be investigated [46].
After dose administration, prediction of toxicity through earlier AKI detection or serum MTX measurement would permit timely implementation of rescue measures. Novel biomarkers that indicate tubular epithelial cell stress or damage before overt AKI is detectable with creatinine deserve exploration for utility throughout nephrotoxic chemotherapy [47–49]. Treatment-associated AKI, which further potentiates delayed MTX elimination, is often not detected until several days after the damage occurs leaving a window of missed opportunity for early intervention, such reducing MTX concentrations by prescribing glucarpidase [24, 42, 43].
Limitations of this study include the retrospective, non-randomized design and dependence on the accuracy of electronic medical records. While patients were managed initially at our institution, it is possible that those who referred from a distant facility returned home for continued treatment and incurred an event that was not captured. We attempted to enroll consecutive patients over a substantial timeframe to establish an appropriately representative sample for analysis. Additionally, repeated measures logistic regression permitted the evaluation of patients longitudinally to assess the impact of sequential HDMTX doses. As stated previously, our investigation utilized individual patients receiving multiple doses and did not contain two comparator groups; therefore, it is unknown whether or not the incidence and severity of AKI would have been even higher if the standard leucovorin was prescribed instead of HDLV rescue. Given the unpredictability of MTX toxicity and the need to provide multiple doses over the course of lymphoma management, we feel this represents an accurate, real-world, clinical scenario. Lastly, it should be noted that the selection of the HDLV rescue dose was left up to provider discretion and likely based off of previous MTX administrations, since defined guidelines in this patient population were not previously available.
Conclusion
Patients receiving sequential HDMTX doses experienced a significant incidence of elevated MTX levels and AKI during lymphoma management. HDLV rescue prescribed during subsequent MTX doses did not limit future high levels, reduce the likelihood AKI, nor did it shorten hospital LOS. However, the benefits of empiric HDLV rescue on other systemic toxicities and on the ability to maintain the recommended administration schedule remain unknown and require further investigation.
Funding
This project was supported in part by CTSA Grant Number TL1 TR002380 from the National Center for Advancing Translational Science (NCATS) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI143882 (PI - E.F.B.).
Footnotes
Availability of data and material N/A.
Code availability N/A.
Publisher's Disclaimer: Disclaimer The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Ethics approval This retrospective chart review was approved by the Mayo Clinic Institutional Review Board and performed in accordance with the ethical standards of the 1964 Declaration of Helsinki with adherence to all relevant regulations of the US health insurance portability and accountability act (HIPAA), IRB No. 17–008432, Approved 11 October 2017.
Competing interests E.F.B. provides consultation for FAST Biomedical, unrelated to this work. All other authors declare no potential personal, financial, or ethical conflicts of interest regarding the contents of this manuscript.
References
- 1.Freeman AI, Weinberg V, Brecher ML, Jones B, Glicksman AS, Sinks LF, Weil M, Pleuss H, Hananian J, Burgert EO Jr, Gilchrist GS, Necheles T, Harris M, Kung F, Patterson RB, Maurer H, Leventhal B, Chevalier L, Forman E, Holland JF (1983) Comparison of intermediate-dose methotrexate with cranial irradiation for the post-induction treatment of acute lymphocytic leukemia in children. N Engl J Med 308:477–484 [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg SJ, Anderson JR, Gottlieb AJ et al. (1987) A randomized trial of high-dose methotrexate versus standard-dose methotrexate following cyclophosphamide, doxorubicin (adriamycin), vincristine, and prednisone with or without bleomycin in the therapy of diffuse large cell lymphoma: preliminary report of Cancer and Leukemia Group B Study 7851. NCI Monogr:77–80 [PubMed] [Google Scholar]
- 3.Delepine N, Delepine G, Cornille H, Brion F, Arnaud P, Desbois JC (1995) Dose escalation with pharmacokinetics monitoring in methotrexate chemotherapy of osteosarcoma. Anticancer Res 15: 489–494 [PubMed] [Google Scholar]
- 4.Isacoff WH, Townsend CM, Eiber FR, Forster T, Morton DL, Block JB (1976) High dose methotrexate therapy of solid tumors: observations relating to clinical toxicity. Med Pediatr Oncol 2:319–325 [DOI] [PubMed] [Google Scholar]
- 5.Holmboe L, Andersen AM, Morkrid L, Slordal L, Hall KS (2012) High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol 73: 106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubenstein JL, Gupta NK, Mannis GN, Lamarre AK, Treseler P (2013) How I treat CNS lymphomas. Blood 122:2318–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansara R (2018) Central nervous system prophylaxis strategies in diffuse large B cell lymphoma. Curr Treat Options in Oncol 19:52. [DOI] [PubMed] [Google Scholar]
- 8.Glantz MJ, Cole BF, Recht L, Akerley W, Mills P, Saris S, Hochberg F, Calabresi P, Egorin MJ (1998) High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol 16:1561–1567 [DOI] [PubMed] [Google Scholar]
- 9.Skarin AT, Zuckerman KS, Pitman SW, Rosenthal DS, Moloney W, Frei E 3rd, Canellos GP (1977) High-dose methotrexate with folinic acid in the treatment of advanced non-Hodgkin lymphoma including CNS involvement. Blood 50:1039–1047 [PubMed] [Google Scholar]
- 10.Batchelor T, Carson K, O’Neill A, Grossman SA, Alavi J, New P, Hochberg F, Priet R (2003) Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96–07. J Clin Oncol 21:1044–1049 [DOI] [PubMed] [Google Scholar]
- 11.Ferreri AJ, Reni M, Foppoli M et al. (2009) High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 374:1512–1520 [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, Cheson BD, Kaplan LD (2013) Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 31: 3061–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramson JS, Hellmann M, Barnes JA, Hammerman P, Toomey C, Takvorian T, Muzikansky A, Hochberg EP (2010) Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 116:4283–4290 [DOI] [PubMed] [Google Scholar]
- 14.Bertino JR (1963) The mechanism of action of the folate antagonists in man. Cancer Res 23:1286–1306 [PubMed] [Google Scholar]
- 15.Bleyer WA (1977) Methotrexate: clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev 4:87–101 [DOI] [PubMed] [Google Scholar]
- 16.Pinedo HM, Chabner BA (1977) Role of drug concentration, duration of exposure, and endogenous metabolites in determining methotrexate cytotoxicity. Cancer Treat Rep 61:709–715 [PubMed] [Google Scholar]
- 17.Joerger M, Huitema AD, Illerhaus G, Ferreri AJ (2012) Rational administration schedule for high-dose methotrexate in patients with primary central nervous system lymphoma. Leuk Lymphoma 53: 1867–1875 [DOI] [PubMed] [Google Scholar]
- 18.Levitt M, Mosher MB, DeConti RC, Farber LR, Skeel RT, Marsh JC, Mitchell MS, Papac RJ, Thomas ED, Bertino JR (1973) Improved therapeutic index of methotrexate with “leucovorin rescue”. Cancer Res 33:1729–1734 [PubMed] [Google Scholar]
- 19.Zhu JJ, Gerstner ER, Engler DA, Mrugala MM, Nugent W, Nierenberg K, Hochberg FH, Betensky RA, Batchelor TT (2009) High-dose methotrexate for elderly patients with primary CNS lymphoma. Neuro-Oncology 11:211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May J, Carson KR, Butler S, Liu W, Bartlett NL, Wagner-Johnston ND (2014) High incidence of methotrexate associated renal toxicity in patients with lymphoma: a retrospective analysis. Leuk Lymphoma 55:1345–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green MR, Chamberlain MC (2009) Renal dysfunction during and after high-dose methotrexate. Cancer Chemother Pharmacol 63: 599–604 [DOI] [PubMed] [Google Scholar]
- 22.Frei E 3rd, Jaffe NJ, Pitman S (1975) Letter: Limitations of methotrexate and citrovorum-factor treatments. N Engl J Med 292:107–108 [DOI] [PubMed] [Google Scholar]
- 23.Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA (1977) Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med 297:630–634 [DOI] [PubMed] [Google Scholar]
- 24.Abelson HT, Fosburg MT, Beardsley GP, Goorin AM, Gorka C, Link M, Link D (1983) Methotrexate-induced renal impairment: clinical studies and rescue from systemic toxicity with high-dose leucovorin and thymidine. J Clin Oncol 1:208–216 [DOI] [PubMed] [Google Scholar]
- 25.Stoller RG, Kaplan HG, Cummings FJ, Calabresi P (1979) A clinical and pharmacological study of high-dose methotrexate with minimal leucovorin rescue. Cancer Res 39:908–912 [PubMed] [Google Scholar]
- 26.Cohen IJ (2004) Defining the appropriate dosage of folinic acid after high-dose methotrexate for childhood acute lymphatic leukemia that will prevent neurotoxicity without rescuing malignant cells in the central nervous system. J Pediatr Hematol Oncol 26:156–163 [DOI] [PubMed] [Google Scholar]
- 27.Cohen IJ (2017) Neurotoxicity after high-dose methotrexate (MTX) is adequately explained by insufficient folinic acid rescue. Cancer Chemother Pharmacol 79:1057–1065 [DOI] [PubMed] [Google Scholar]
- 28.Du Bois D, Du Bois EF (1916) Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med XVII:863–871 [PubMed] [Google Scholar]
- 29.Glass J, Gruber ML, Cher L, Hochberg FH (1994) Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg 81:188–195 [DOI] [PubMed] [Google Scholar]
- 30.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL et al. (2012) Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2(2):1–138 [Google Scholar]
- 31.Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21:1471–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalia S, Price S, Forsyth P, Sokol L, Jaglal M (2015) What is the optimal dose of high-dose methotrexate in the initial treatment of primary central nervous system lymphoma? Leuk Lymphoma 56: 500–502 [DOI] [PubMed] [Google Scholar]
- 33.Chabner BA, Young RC (1973) Threshold methotrexate concentration for in vivo inhibition of DNA synthesis in normal and tumorous target tissues. J Clin Invest 52:1804–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomasova D, Anders HJ (2015) Cell cycle control in the kidney. Nephrol Dial Transplant 30:1622–1630 [DOI] [PubMed] [Google Scholar]
- 35.Pannu AK (2019) Methotrexate overdose in clinical practice. Curr Drug Metab 20:714–719 [DOI] [PubMed] [Google Scholar]
- 36.Shank BR, Seung AH, Kinsman K, Newman MJ, Donehower RC, Burton B (2017) Effects of the leucovorin shortage: pilot study investigating cost, efficacy, and toxicity comparison of low fixed-dose versus body surface area-adjusted leucovorin dosing in patients with resectable colon or metastatic colorectal cancer. J Oncol Pharm Pract 23:163–172 [DOI] [PubMed] [Google Scholar]
- 37.Cohen IJ (2013) Challenging the clinical relevance of folinic acid over rescue after high dose methotrexate (HDMTX). Med Hypotheses 81:942–947 [DOI] [PubMed] [Google Scholar]
- 38.Safadi S, Hommos MS, Enders FT, Lieske JC, Kashani KB (2020) Risk factors for acute kidney injury in hospitalized non-critically ill patients: a population-based study. Mayo Clin Proc 95:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng YC, Nie S, Li L, Li Y, Liu D, Xiong M, Wang L, Ge S, Xu G, on behalf of the EACH study investigators (2019) Epidemiology and outcomes of acute kidney injury in hospitalized cancer patients in China. Int J Cancer 144:2644–2650 [DOI] [PubMed] [Google Scholar]
- 40.Na SY, Sung JY, Chang JH, Kim S, Lee HH, Park YH, Chung W, Oh KH, Jung JY (2011) Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol 33:121–130 [DOI] [PubMed] [Google Scholar]
- 41.Ubukata M, Hara M, Nishizawa Y, Fujii T, Nitta K, Ohta A (2018) Prevalence and mortality of chronic kidney disease in lymphoma patients: a large retrospective cohort study. Medicine 97:e9615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demiralp B, Koenig L, Kala J, Feng C, Hamlett EG, Steele-Adjognon M, Ward S (2019) Length of stay, mortality, and readmissions among Medicare cancer patients treated with glucarpidase and conventional care: a retrospective study. Clinicoecon Outcomes Res 11:129–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nirenberg A, Mosende C, Mehta BM, Gisolfi AL, Rosen G (1977) High-dose methotrexate with citrovorum factor rescue: predictive value of serum methotrexate concentrations and corrective measures to avert toxicity. Cancer Treat Rep 61:779–783 [PubMed] [Google Scholar]
- 44.Levey AS, Inker LA (2017) Assessment of glomerular filtration rate in health and disease: a state of the art review. Clin Pharmacol Ther 102:405–419 [DOI] [PubMed] [Google Scholar]
- 45.Lameire N, Van Biesen W, Vanholder R (2008) Acute renal problems in the critically ill cancer patient. Curr Opin Crit Care 14:635–646 [DOI] [PubMed] [Google Scholar]
- 46.Barreto JN, McClanahan AL, Rule AD, Thompson CA, Frazee E (2018) Incorporating cystatin C to predict methotrexate elimination in patients with CNS lymphoma and suspicious renal function. Case Rep Hematol 2018:7169897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kashani K, Al-Khafaji A, Ardiles T et al. (2013) Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, Feldkamp T, Uettwiller-Geiger DL, McCarthy P, Shi J, Walker MG, Kellum JA, Sapphire Investigators (2014) Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 29:2054–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bihorac A, Chawla LS, Shaw AD, al-Khafaji A, Davison DL, DeMuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, Jortani S, Kleerup E, Koyner JL, Krell K, LeTourneau J, Lissauer M, Miner J, Nguyen HB, Ortega LM, Self WH, Sellman R, Shi J, Straseski J, Szalados JE, Wilber ST, Walker MG, Wilson J, Wunderink R, Zimmerman J, Kellum JA (2014) Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 189: 932–939 [DOI] [PubMed] [Google Scholar]


