Abstract
Purpose
Postural Tachycardia Syndrome (POTS) – a syndrome characterized by orthostatic symptoms and a heart rate increase of at least 30 beats per minute in the absence of hypotension upon standing – is often accompanied by increased sympathetic activity and low blood volume. A common non-pharmacologic recommendation for POTS is a high-sodium diet with the goal of bolstering circulating blood volume. The objective of this study is to assess the effects of 6 days of high sodium on endothelial function in POTS.
Methods
14 patients with POTS and 13 age-matched healthy controls, all females, were studied following six days of low sodium (10 mEq/day) and six days of high-sodium (300 mEq/day) diets in a crossover manner. We measured endothelial function following reactive hyperemia in the brachial artery using Flow Mediated Dilation (FMD), Leg Blood Flow (LBF) using strain gauge plethysmography in the calf, and reactive hyperemic index (RHI) in the microcirculation of the hand using Pulsatile Arterial Tonometry.
Results
On the low-sodium diet, FMD% did not differ between POTS and Controls although peak brachial artery diameter was lower for POTS. RHI was higher for POTS than Controls, but there were no differences in post-ischemic LBF increase. On the high-sodium diet, there were no differences in FMD%, LBF increase or RHI between groups.
Conclusion
In summary, a high-sodium diet for six days did not induce endothelial dysfunction. This non-pharmacologic treatment used for patients with POTS does not negatively affect endothelial function when used for a sub-acute duration.
Keywords: Endothelial function, nitric oxide, autonomic nervous system, postural tachycardia syndrome, dietary salt
Introduction
Postural Tachycardia Syndrome (POTS) is characterized by an increase in heart rate of at least 30 beats per minute when upright in the absence of hypotension [12, 15, 40, 44] and orthostatic symptoms for more than 6 months. The orthostatic tachycardia is often accompanied by symptoms of sympathetic activation such as tremor and palpitations, and in some patients the exaggerated heart rate response is coupled with high muscle sympathetic nerve activity [28, 44].
Endothelial dysfunction, an impairment of vasodilatory responses in blood vessels, can increase long-term cardiovascular risk [3, 7]. Prior studies found bidirectional inverse relationships between sympathetic activation and nitric oxide (NO), which plays a key role in vasodilation [18]. Sander et al. and Young et al. found that inhibition of NO leads to hypertension and sympathoexcitation, respectively [42, 50]. Conversely, Hijmering et al. found that sympathetic activation reduces NO-mediated vasodilation [23].
In addition to sympathetic activation, many patients with POTS have been found to have a deficit in blood volume, and these patients are encouraged to follow a high-sodium (HS) diet in an attempt to boost circulating blood volume [14, 39, 40, 44]. In healthy normotensives, a HS diet decreases NO production [10] but does not always cause measurable decreases in endothelial function and effects on endothelial function may be increased in males as compared to females [10, 11, 22, 29, 33]. It is unknown whether a HS diet affects endothelial function in patients with POTS, a syndrome predominantly seen in females [43]. We have previously shown that patients with POTS respond similarly to the suppression of NO synthesis under autonomic blockade on an uncontrolled diet as controls. We concluded that there is no functional impairment of the endothelial response to endogenous NO in POTS.
A recent meta-analysis of salt-supplementation in patients with orthostatic intolerance only included one study of patients with POTS – a study looking at whether 24-hour sodium urine levels could be used to identify whether adolescents with POTS respond to oral sodium supplementation [31, 51]. No studies examined the long-term cardiovascular risk of high sodium diets [31]. This is the first study designed to measure the effect of a six-day high-sodium diet on endothelial function in patients with POTS. The goal of the present study is to assess the impact of dietary salt on endothelial function in different vascular beds (conduit arteries, resistance vessels and microcirculation) and different anatomic sites (upper vs. lower limbs) in healthy females and a cohort of patients with POTS. We test the hypothesis that six days of a high-sodium diet will result in a decrease of endothelial function in POTS.
Methods
The Vanderbilt Institutional Review Board approved this study (Vanderbilt IRB 091322) and it was registered on ClinicalTrials.gov (NCT1550315). Each participant gave their written informed consent prior to starting study activities.
General Study Description:
Premenopausal females with POTS (between the ages of 18 and 50 years) with age- and sex-matched healthy control participants were enrolled in this study. Only patients with a POTS diagnosis confirmed by the Vanderbilt Autonomic Dysfunction Center were enrolled. Due to the lower prevalence of POTS among males we chose just to enroll females in this study, eliminating the need to control for sex-related differences in our analyses. Healthy controls were screened and were required to have normal hematologic, renal and liver function lab work, a normal EKG, supine blood pressure < 145/95, and no history of cardiac, liver, blood or lung diseases. They were also pre-menopausal, non-smokers and did not have any illnesses or take medications which might impact autonomic function (for example, adrenocortical disease, diabetes, etc.).
A diet length of 5 to 7 days have been used in six previous studies of the effect of dietary sodium on endothelial function, which formed the basis for choosing a six-day diet period in this study [4, 9–11, 22, 29, 33]. After enrollment, participants received either six days of low-sodium (LS; 10 mEq/day) or high-sodium (HS; 300 mEq/day) diet in a randomized crossover design. Study periods were generally separated by one month and were scheduled so that study days occurred in the same phase of the participants’ menstrual cycle. The LS diet was ~10% of recommended sodium intake (2300 mg or ~100 mEq) and the HS diet was ~300% of the recommended sodium intake. Both study diets were alcohol-, caffeine- and methylxanthine-free and provided approximately 80 mEq/day of potassium (K+). Meals were prepared by the Vanderbilt CRC metabolic kitchen. Fluid intake was ad libitum throughout the study period. Patients with POTS were admitted to the Vanderbilt Clinical Research Center (CRC) prior to diet initiation, whereas controls were provided with the study diet on an outpatient basis for three days before being admitted on the fourth day of study diet. Diet success was assessed with 24-hour urinary sodium starting in the AM on the sixth day of the diet and ending the following morning.
Medications that would affect blood volume or autonomic function were discontinued for at least 5 half-lives prior to the study visit, except fludrocortisone, which was discontinued for at least 5 days prior to the study. Participants were allowed to continue selective serotonin reuptake inhibitors and birth control (not containing drosperinone) because these do not interfere with measurements of endothelial function [13, 20, 45]. Two participants with POTS were on citalopram (an SSRI), one participant with POTS was on oral birth control (orthotri-cyclen), and two participants had an IUD (one POTS and one HC). Testing of endothelial function was performed on the sixth day of each diet intervention.
Assessment of Endothelial Function:
Endothelial function was assessed using three different methods to measure NO-mediated dilatory responses to ischemia. Our primary outcome was brachial artery flow-mediated dilation (FMD), which assesses conduit artery responses to reactive hyperemia. Reactive hyperemia, the influx of blood into vasculature following ischemia, increases shear stress, which triggers the release of NO and facilitates endothelial-dependent vasodilation to decrease shear stress back to baseline levels [35].
Secondary outcomes were leg blood flow (LBF), which measures resistance vessel responses to reactive hyperemia in the calf and reactive hyperemia index (RHI), which is an indicator of microvascular endothelial function in response to ischemia [3, 19].
Flow Mediated Dilation (FMD):
Baseline and peak brachial arterial diameters, measured before and during ischemic reactive hyperemia, were used to calculate the absolute change (delta FMD; ΔFMD; peak-baseline brachial artery diameter) and percent change {percent FMD; FMD%; [(peak-baseline)/baseline] × 100%}in brachial arterial diameter [32]. Time to peak FMD (in seconds) was also recorded.
An ultrasound probe was placed over the brachial artery 3–5 cm above the elbow and baseline arterial diameter was obtained during a 60 second baseline period (iU22; Phillips, Bothell, WA). After this, a brachial blood pressure cuff was inflated to 50 mmHg above the subject’s baseline systolic blood pressure distal to the probe for 5 min to induce forearm ischemia. Following release of the cuff, brachial arterial diameter was continuously measured for 180 seconds to capture the peak dilatory response to ischemia. Images were analyzed as previously described. Briefly, an automated brachial wall detection software was used to determine baseline and peak brachial arterial diameter to limit the effect of investigator bias (Brachial analyzer 5.0; Medical Imaging Applications LLC, Iowa City, IA) [32]. In order to maximize the resolution of the brachial artery images for analysis, we did not simultaneously measure flow throughout the study. This improved the quality of recordings but prevented us from normalizing FMD% by shear stress.
Leg Blood Flow (LBF):
The percent change in muscle blood flow following ischemia is a measurement of endothelial function in the resistance vessels [3]. Traditionally the forearm has been the site of this measurement [3, 6]. In this study, we adapted this method to measure changes in blood flow to the calf. Use of the calf rather than the forearm was based on prior work by Stewart and colleagues, who found that some patients with POTS have reduced blood flow to the legs [47].
Mercury in-silastic strain gauges were wrapped around the widest circumference of the calf and inflatable cuffs were placed above and below the strain gauge to prevent venous outflow from the region of interest and to limit arterial flow to the distal limb [6]. The distal cuff (around the ankle) was inflated to 200 mmHg and the proximal cuff (two centimeters above the flexion of the knee) was inflated to ischemic levels for 5 minutes. This was followed by intermittent rapid inflation (50 mm Hg) and deflation in a 16 second cycle, for the last two minutes of the measurement. Rapid cuff inflation was achieved using a commercially available air source (Hokanson EC4, DE; Hokanson Inc, Indianapolis; IN). This method allows measurement of baseline and peak post-ischemic LBF (ml/100ml/min) and percent change in LBF {LBF%; [(peak-baseline)/baseline] × 100%}.
Reactive Hyperemia Index (RHI)
Peripheral microvascular endothelial function responses to ischemia in the hand (a partially NO-mediated response) can be assessed using peripheral artery tonometry (PAT) with an Endo-PAT device (Endo-PAT 2000, Itamar-Medical, Caesarea; Israel) [2, 5, 37]. In this method, a probe is placed on a finger of each hand to measure pulse volume by PAT and ischemia is applied to one of the upper arms using brachial cuff inflation to supra-systolic pressures. The RHI (a ratio of pulse volume before and after ischemia in the affected limb that is normalized by measurements in the contralateral arm) indicates peripheral microvascular endothelial function and is reported in arbitrary units (AU) [3, 5]. The device also calculated the augmentation index (a ratio of the second to first measured systolic pulse pressures) as an indicator of arterial stiffness, measured in AU [3].
Statistical Analysis
Our main outcome was the difference in FMD% between diets in the POTS group. Secondary outcomes were differences in LBF% and RHI between diets in the POTS group, differences in endothelial function (FMD%, LBF% and RHI) between diets for controls and differences between POTS and controls on each diet. Because the data were not normally distributed, we used Wilcoxon signed-rank tests for the primary outcome (differences in FMD% between HS and LS diets). We also used the Wilcoxon signed-rank test for our secondary outcomes of differences in LBF% and RHI between diets and differences in endothelial function between diets for controls. Differences between groups (POTS vs. controls) were assessed using Mann-Whitney rank-sum tests within each diet. Data were expressed as mean (SD) unless otherwise noted. Data were stored in a secure Research Data Capture (REDCap) database housed at Vanderbilt University [21]. Analyses were completed using Stata/IC 14.2 and figures were generated with GraphPad Prism 8.2.1.
Missing Data
Those who had missing data were included in calculations of group means and Mann-Whitney rank-sum comparisons of group means. However, for Wilcoxon signed-rank (matched-pairs) analyses comparing diets, the following numbers of full pairs were used: 11 POTS and 10 controls for FMD, 12 POTS and 13 controls for LBF. RHI was completed on both diets for all participants, although the AI was missing for one participant. Missing data was due to suboptimal quality of data recordings.
Results
Subject Characteristics
We enrolled a total of 27 female participants: 14 patients with POTS and 13 controls. There were no differences in age [35 (SD 8) vs 31 (SD 6)] or baseline body mass index [22.8 (SD 2.9) vs 23.4 (SD 2.6)] between POTS and controls. All participants were pre-menopausal.
Comparison between Diets: POTS
Weight, supine blood pressure and supine NE levels did not differ between the LS and HS diets. Supine HR was lower on the HS diet (73 bpm (SD 11) vs 66 (SD 9), p 0.01; Table 1). As expected, 24-hour urine sodium was increased on the HS diet (16 mmol/24hr (SD 7) vs 235 (SD 42), p 0.001; Table 1). HS diet had no measurable negative effect on endothelial function of females with POTS in any of the vascular beds assessed. FMD% (conduit artery function) was similar between LS and HS [11.8% (SD 4.8) vs. 12.8% (SD 3.8); p=0.3; Table 2]. Baseline brachial artery diameter [2.9 mm (SD 0.2) LS vs. 3.0 mm (SD 0.3) HS; p=0.3] and peak brachial artery diameter [3.2 mm (SD 0.3) LS vs. 3.3 mm (SD 0.3) HS, p=0.2] were not affected by the HS diet (Table 2). Similarly, average ΔFMD did not differ between diets [0.3 mm (SD 0.1) LS vs. 0.4 mm (SD 0.1) HS; p=0.3; Table 2]. HS did not increase the time to reach peak dilation [47 sec (SD 17) LS vs. 49 sec (SD 15) HS; p=0.9; Table 2].
Table 1.
Effect of Low and High-sodium Diet on Weight, Blood Pressure and Labs
| Measurements | POTS | Healthy Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low Sodium | High Sodium | P-value between Diets1 | Low Sodium | High Sodium | P-value between Diets1 | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Weight Day 6 (kg) | 63 | 8 | 63 | 8 | 0.8 | 61 | 7 | 62 | 8 | 0.07 |
| Supine Heart Rate, bpm | 73 | 11 | 66 | 9 | 0.01 | 66 | 10 | 64 | 12 | 0.15 |
| Supine Systolic Blood Pressure, mmHg | 104 | 8 | 107 | 10 | 0.5 | 107 | 7 | 108 | 10 | 0.6 |
| Supine Norepinephrine, pg/mL | 247* | 94 | 195 | 123 | 0.08 | 166 | 72 | 118 | 40 | 0.02 |
| 24-hour Urine Sodium, mmol/24 hr | 16 | 7 | 235 | 42 | < 0.01 | 18 | 8 | 263 | 61 | <0.01 |
p value between low and high-sodium diets calculated with Wilcoxon Sign-rank
p-value ≤ 0.05 between POTS and HC on LS diet, calculated with Mann-Whitney Rank-sum
Table 2.
Differences in Endothelial Function Between Diets and Groups
| Measurements | POTS | Healthy Controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Sodium | High Sodium | P-value between Diets1 | Low Sodium | High Sodium | P-value between Diets1 | |||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |||
| Baseline Brachial Artery Diameter, mm | 13 | 2.9 | 0.2 | 12 | 3.0 | 0.3 | 0.3 | 10 | 3.1 | 0.3 | 13 | 3.1 | 0.3 | 0.9 |
| Peak Brachial Artery Diameter, mm | 13 | 3.2* | 0.3 | 12 | 3.3 | 0.3 | 0.2 | 10 | 3.5 | 0.3 | 13 | 3.4 | 0.3 | 0.2 |
| Absolute Change Flow Mediated Dilation, mm | 13 | 0.3 | 0.1 | 12 | 0.4 | 0.1 | 0.3 | 10 | 0.4 | 0.1 | 13 | 0.3 | 0.1 | 0.1 |
| Percent Flow Mediated Dilation | 13 | 11.8 | 4.8 | 12 | 12.8 | 3.8 | 0.3 | 10 | 12.8 | 4.5 | 13 | 10.6 | 4.7 | 0.1 |
| Time to Peak Brachial Artery Diameter, sec | 13 | 47 | 17 | 12 | 49 | 15 | 0.9 | 10 | 47 | 21 | 13 | 39 | 15 | 0.4 |
| Baseline Calf Blood Flow, ml/100ml/min | 14 | 2.6 | 1.3 | 12 | 3.2 | 2.0 | 0.5 | 13 | 3 | 1.3 | 13 | 3.2 | 1.5 | 0.6 |
| Peak Calf Blood Flow, ml/100ml/min | 14 | 15.9 | 8.7 | 12 | 13.9 | 7.1 | 0.4 | 13 | 16.3 | 12.2 | 13 | 16.3 | 7.6 | 0.6 |
| Percent Change Calf Blood Flow | 14 | 536 | 261 | 12 | 375 | 154 | 0.1 | 13 | 408 | 257 | 13 | 445 | 215 | 0.5 |
| Reactive Hyperemia Index, A.U. | 14 | 2.5* | 0.6 | 14 | 2.3 | 0.5 | 0.3 | 13 | 1.9 | 0.4 | 13 | 1.8 | 0.4 | 1.0 |
| Augmentation Index, A.U. | 14 | 7.1 | 15.3 | 14 | 7.8 | 14.8 | 0.7 | 12 | −1.0 | 11.9 | 13 | 2.3 | 11.4 | 0.6 |
p value between low and high-sodium diets calculated with Wilcoxon Sign-rank
p-value ≤ 0.05 between POTS and HC on LS diet, calculated with Mann-Whitney Rank-sum
LBF% (resistance vessel function) did not differ between LS [536% (SD 260)] and HS diets [375% (SD 154), p=0.1; Table 2]. Baseline [2.6 ml/100ml/min (SD 1.3) vs. 3.2 ml/100ml/min (SD 2.0); p=0.5] and peak leg blood flow [15.9 ml/100ml/min (SD 8.7) vs. 13.9 ml/100ml/min (SD 7.1); p=0.4] did not vary between LS and HS diets (Table 2). Similarly, RHI [microvascular function, 2.5 AU (SD 0.6) vs. 2.3 AU (SD 0.5); p=0.3] and arterial stiffness [7.1 AU (SD 15.3) vs. 7.8 AU (SD 14.8); p=0.7] were not different between LS and HS diet (Table 2).
Comparison between Diets: Controls
Weight, supine HR and supine SBP did not differ between diets. However, supine NE was decreased on the HS diet (166 (SD 72) vs 118 (SD 40), p 0.02; Table 1). 24-hour urine sodium excretion was increased on the HS diet (p 0.002; Table 1). FMD% was similar between LS [12.8 % (SD 4.5)] and HS [10.6 % (SD 4.7); p=0.1] for controls (Table 2). Baseline brachial artery diameter [3.1 mm (SD 0.3) vs. 3.1 mm (SD 0.3); p=0.9] and peak brachial artery diameter [3.5 mm (SD 0.3) vs. 3.4 mm (SD 0.3); p=0.2] were not different between LS and HS diet (Table 2). Similarly, average ΔFMD did not differ between LS [0.4 mm (SD 0.1)] and HS diets [0.3 mm (SD 0.1); p=0.1; Table 2]. HS did not lengthen the time to reach peak dilation [47 sec (SD 21) LS vs. 39 sec (SD 15) HS; p=0.4; Table 2].
LBF% did not differ between LS [408 % (SD 257)] and HS diets [445 % (SD 215): p=0.5; Table 2]. Baseline [3.0 ml/100ml/min (SD 1.3) vs. 3.2 ml/100ml/min (SD 1.5); p=0.6] and peak leg blood flow ([16.3 ml/100ml/min (SD 12.2) vs. 16.3 ml/100ml/min (SD 7.6); p=0.6] did not vary between LS and HS diets (Table 2). RHI [1.9 AU (SD 0.4) LS vs. 1.8 AU (SD 0.4); p=1.0] and arterial stiffness [−1.0 AU (SD 11.9) vs. 2.3 AU (SD 11.4); p=0.6] were not different between LS and HS diet (Table 2).
Comparison between Groups: Low Sodium
On the LS diet, supine NE was significantly higher for POTS (247 (SD 94) vs 166 (SD 72) HC, p 0.03; Table 1). Supine HR and SBP did not differ between groups. POTS and controls had similar FMD% (p=0.6; Figure 1A) and average ΔFMD (p=0.2) in response to ischemia (Table 2). However, baseline brachial artery diameter tended to be lower for POTS (p=0.07) and peak brachial artery diameter was significantly lower post ischemia than seen in controls (p=0.05; Table 2). The time to peak FMD did not differ between POTS and controls.
Figure 1. Effect of Low- and High-Sodium Diets on Endothelial Function.
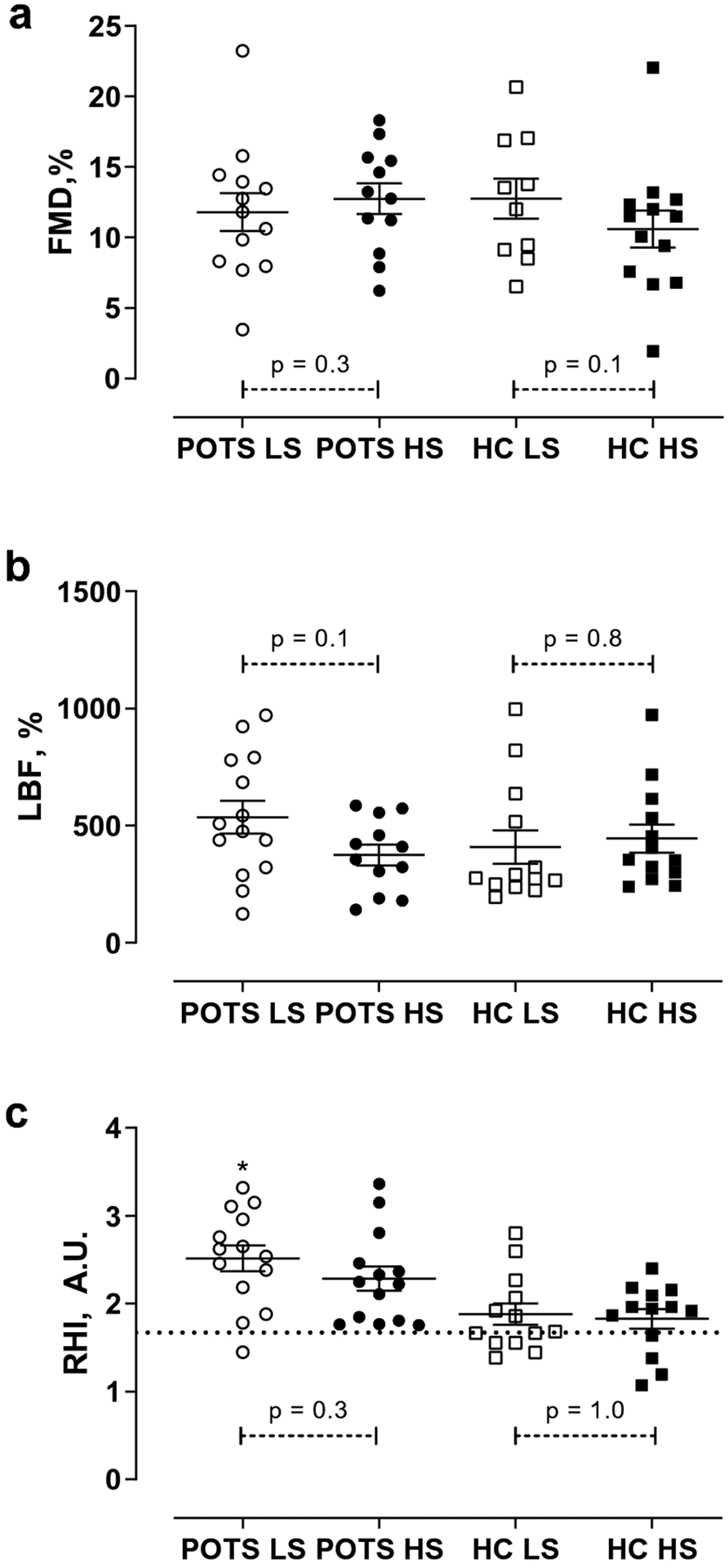
Percent change in Flow Mediated Dilation (FMD, %; Panel a), Leg Blood Flow (% LBF / min; Panel b) and Reactive Hyperemia Index (RHI; Panel c) on Low Sodium (LS) and High Sodium (HS) diets for POTS and controls (HC). Values are expressed as means with error bars indicating the SEM. Differences between diets were assessed using Mann Whitney Rank-Sum and differences between groups were assessed using Wilcoxon Sign-rank.* P < 0.01 for difference between POTS and controls on the LS diet.
Mean percent change in LBF did not differ between POTS and controls (p=0.2; Figure 1B, Table 2). Both baseline LBF and peak LBF were similar between groups (p=0.4 and 0.7 respectively; Table 2). RHI was higher for POTS than controls on the LS diet (p<0.01; Figure 1C, Table 2) while arterial stiffness did not vary between groups (p=0.2; Table 2).
Comparison between Groups: High Sodium
On the HS diet, there were no differences in supine SBP, HR or norepinephrine between groups. Patients with POTS and controls had similar FMD% (p=0.2; Figure 1A, Table 2) post ischemia. Baseline brachial artery diameter and peak brachial artery diameter and average ΔFMD did not differ between groups (p=0.4, 0.3 and 0.2 respectively; Table 2). The time to peak FMD tended to be longer for POTS than controls [49 sec (SD 15) vs. 39 sec (SD 15), p 0.09; Table 2] but did not reach statistical significance.
LBF% was not different between POTS and controls (p=0.7; Figure 1B, Table 2). Baseline and peak LBF were similar between the two groups on the HS diet (p=0.6 and 0.5 respectively, Table 2). RHI tended to be higher for POTS than controls on the HS diet but did not reach statistical significance (p=0.06; Figure 1C, Table 2). Arterial stiffness did not vary between groups (p=0.5; Table 2).
DISCUSSION
The main finding of the present study is that 6 days of high-sodium did not negatively affect endothelial function in POTS as compared to measurements on a low-sodium diet.
Conduit Artery Vessel Function
We did not find differences in FMD% between LS and HS diets for POTS. Prior studies of healthy and hypertensive cohorts have demonstrated that a HS diet decreases FMD% in comparison to measurements on a LS diet [29, 33]. However, Lennon-Edwards et al. found that the decrease in FMD% was markedly higher for men [29]. We did find a trend toward a prolonged time-to-peak FMD in POTS on the HS diet, but reassuringly we did not find differences in brachial arterial diameters or FMD% between diets.
Although Liao et al found higher FMD% in adolescent POTS than their healthy counterparts without controlling for diet [30], we did not find differences in FMD% between groups. On the LS diet, we did find that POTS had a lower peak brachial artery diameter following reactive hyperemia compared to controls. However, because POTS also tended to have a lower baseline brachial artery diameter, the lower peak brachial artery diameter did not lead to a measurable difference in FMD% between groups. Mitchell et al. found that coronary risk factors were related to the amount of shear stress achieved during FMD measurements, which they hypothesized may be the actual driving factor for lower observed FMD% in some populations [35]. It is possible that the patients with POTS did not achieve the same levels of shear stress on the LS diet, thus leading to a smaller peak brachial artery diameter.
Resistance Artery Vessel Function
A HS diet did not worsen LBF% in POTS either. Because prior studies have demonstrated that some patients with POTS have diminished blood flow to the legs accompanied by reduced endothelial function, we measured resistance artery function in the leg vessels as opposed to the traditional forearm site [46, 47]. We did not find differences in baseline LBF between groups prior to ischemia, perhaps indicating that we did not enroll any “low flow” POTS in this study [46, 47]. Consistent with past findings that forearm blood flow does not differ between HS and LS diets for hypertensives or normotensives, this study showed that a six-day HS diet does not have a negative impact on LBF in POTS or controls [10, 22].
Microvascular Function
Pulsatile arterial tonometry has been hailed as a simple and non-invasive method to measure endothelial function [2, 5, 36]. Additionally, it has been shown that the measurement can be reliably repeated [34]. An RHI cut-off value of 1.67 AU has commonly been used to indicate endothelial dysfunction [25, 36, 49].The mean RHI was above this cut-off in both groups on each study diet (Figure 1C). Interestingly only one POTS patient had an RHI less than 1.67 AU (LS visit), while seven controls had an RHI less than 1.67 AU (six at the LS visit and four at the HS visit). However, this isolated finding among otherwise healthy individuals in our study (with no indication of endothelial dysfunction detected with FMD% or LBF%) makes us hesitate to conclude there is endothelial dysfunction present in this healthy population. There is conflicting data on the use of RHI as a replacement for FMD to determine endothelial function. In some studies, there is a good correlation between Endopat and FMD while in others, there is no such correlation (such as Allan and colleagues’ study of healthy controls and adults with PAD) [1, 27]. Additionally, one study of young adults (ages 18 to 30) found that 47% had an RHI less than 1.67, calling into question the validity of that cut-off as an indicator of endothelial dysfunction in younger participants [38].
To complicate things further, we are measuring RHI during salt loading, which can produce changes in sympathetic activity and could result in alterations in microcirculation and therefore RHI [48]. Although RHI did not differ significantly within groups between LS and HS visits we did note differences between POTS and controls. The reason behind why POTS had higher RHI values than controls on the low salt diet (and tended to have higher values on the HS diet) is not clear to us. Microcirculatory responses to hyperemia are only partially driven by nitric oxide or the endothelium, so differences between POTS and controls could be driven by different responses to salt unloading or differences in the dominance of non-endothelial dependent mechanisms between the groups [37, 41]. Although it has been shown that a single HS meal does not have an acute effect on RHI in healthy adults, this study adds to our knowledge of the impact of sub-acute HS diets on RHI by demonstrating the RHI did not differ between diets for either POTS or controls [8].
Effect on Blood Pressure and Heart Rate
A Cochrane review of salt-reduction interventions has found that a low-sodium diet consistently increases NE levels (as compared to a normal salt diet) although blood pressure lowering effects are minimal in normotensives [16]. We found that NE levels were significantly higher for POTS than controls on the LS diet but differences between groups were not measured on the HS diet.
We did not find that the diet had any measurable effect on supine systolic blood pressure. Perhaps this is because our participants were normotensives, a group which often sees minimal reductions in blood pressure on a LS diet [16]. Another explanation for the lack of a measurable effect on blood pressure is the duration of the study. A 2017 study looking at the effect of salt reduction in a typical American diet found that the blood pressure lowering effects accumulated over the four-week study period [26]. A 2020 meta-analysis of sodium reduction did not find a relationship between the duration of diet and blood pressure changes, although they did caution that studies with short-term diets may underestimate the true impact of low-sodium diet on blood pressure [24]. Perhaps the short duration of the study diet may explain why our participants did not experience significant blood pressure reduction on the LS diet.
A prior meta-analysis found that dietary salt reduction causes modest increases in heart rate [17]. In our study, we did find that HR was significantly higher on the LS diet for POTS but did not find a change between diets for the HC. These findings seem to indicate that POTS are more sensitive to the negative effects of sodium restriction (increased NE levels and HR).
Limitations
Patients with POTS are a heterogenous group, which may explain why we were unable to replicate the endothelial dysfunction that Stewart et al. have found in their “low flow’ POTS [47]. Although we did not measure MSNA in this study, NE was higher for POTS than HC on the LS diet, which may indicate that POTS experience increased sympathetic activity compared to controls when sodium is restricted. Given prior literature showing that sodium has a greater effect on FMD% in males, sex may have contributed to the absence of differences in FMD% following the study diets [29]. Because simultaneous measurement of blood flow would affect the resolution of diameter imaging during FMD, we did not measure brachial artery blood flow during hyperemia. This limits our understanding of the effects of diet on blood flow and shear stress. An additional limitation is that the study was completed after only six days of the study diets. We have not measured endothelial function before and after a long-term HS diet or compared endothelial function between normal and HS diets. It is possible that prolonged HS diets might have different effects than measured in this six-day study. In clinical settings, we typically recommend that patients with POTS consume ~ 200 mEq of sodium daily [14, 44]. In this study, the HS diet was more extreme than that recommended to patients, and further research should be done to assess endothelial function with a more attainable daily sodium intake.
Conclusion
In conclusion, we found that a HS diet did not negatively impact endothelial function in patients with POTS. Although the effects of a HS diet still need to be studied in the long-term, this study may help to minimize concerns among clinicians or patients with POTS that a HS diet could contribute to long-term cardiac risk due to sodium-related endothelial dysfunction.
Funding
This work was supported by National Institutes of Health (NIH) grants R01 HL102387, PO1 HL56693, and UL1 TR000445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest The authors declare that they have no conflict of interest.
Ethics Approval This study was performed under the ethical standards of the Declaration of Helsinki. It was approved by the Vanderbilt Institutional Review Board (IRB 091322).
Availability of Data and Material The data that support the findings of this study are available from the corresponding author, [AG], upon reasonable request
Code Availability Analysis was completed by Emily C. Smith with Stata 14/IC 14.2 and code is available from the corresponding author [AG] upon reasonable request.
Registration ClinicalTrials.gov NCT01550315; March 9, 2012
References
- 1.Allan RB, Vun SV, Spark JI (2016) A Comparison of Measures of Endothelial Function in Patients with Peripheral Arterial Disease and Age and Gender Matched Controls. Int J Vasc Med 2016:2969740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axtell AL, Gomari FA, Cooke JP (2010) Assessing endothelial vasodilator function with the Endo-PAT 2000. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barac A, Campia U, Panza JA (2007) Methods for evaluating endothelial function in humans. Hypertension 49:748–760 [DOI] [PubMed] [Google Scholar]
- 4.Barić L, Drenjančević I, Matić A, Stupin M, Kolar L, Mihaljević Z, Lenasi H, Šerić V, Stupin A (2019) Seven-Day Salt Loading Impairs Microvascular Endothelium-Dependent Vasodilation without Changes in Blood Pressure, Body Composition and Fluid Status in Healthy Young Humans. Kidney Blood Press Res 44:835–847 [DOI] [PubMed] [Google Scholar]
- 5.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A (2004) Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 44:2137–2141 [DOI] [PubMed] [Google Scholar]
- 6.Casey DP, Curry TB, Joyner MJ (2008) Measuring muscle blood flow: a key link between systemic and regional metabolism. Curr Opin Clin Nutr Metab Care 11:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ, Hypertension WGoEaEFotESo (2005) Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens 23:7–17 [DOI] [PubMed] [Google Scholar]
- 8.Dickinson KM, Clifton PM, Keogh JB (2011) Endothelial function is impaired after a high-salt meal in healthy subjects. Am J Clin Nutr 93:500–505 [DOI] [PubMed] [Google Scholar]
- 9.Dickinson KM, Keogh JB, Clifton PM (2009) Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 89:485–490 [DOI] [PubMed] [Google Scholar]
- 10.Dishy V, Sofowora GG, Imamura H, Nishimi Y, Xie HG, Wood AJ, Stein CM (2003) Nitric oxide production decreases after salt loading but is not related to blood pressure changes or nitric oxide-mediated vascular responses. J Hypertens 21:153–157 [DOI] [PubMed] [Google Scholar]
- 11.Eisenach JH, Gullixson LR, Kost SL, Joyner MJ, Turner ST, Nicholson WT (2012) Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. J Appl Physiol (1985) 112:1049–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21:69–72 [DOI] [PubMed] [Google Scholar]
- 13.Friedman J, Cremer M, Jelani QU, Huang X, Jian J, Shah S, Katz SD (2011) Oral contraceptive use, iron stores and vascular endothelial function in healthy women. Contraception 84:285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garland EM, Celedonio JE, Raj SR (2015) Postural Tachycardia Syndrome: Beyond Orthostatic Intolerance. Curr Neurol Neurosci Rep 15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garland EM, Raj SR, Black BK, Harris PA, Robertson D (2007) The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 69:790–798 [DOI] [PubMed] [Google Scholar]
- 16.Graudal NA, Hubeck-Graudal T, Jurgens G (2017) Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev 4:CD004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graudal NA, Hubeck-Graudal T, Jürgens G (2016) Reduced Dietary Sodium Intake Increases Heart Rate. A Meta-Analysis of 63 Randomized Controlled Trials Including 72 Study Populations. Front Physiol 7:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadi HA, Carr CS, Al Suwaidi J (2005) Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1:183–198 [PMC free article] [PubMed] [Google Scholar]
- 19.Hamburg NM, Benjamin EJ (2009) Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med 19:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hantsoo L, Czarkowski KA, Child J, Howes C, Epperson CN (2014) Selective serotonin reuptake inhibitors and endothelial function in women. J Womens Health (Larchmt) 23:613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Matsuura H, Chayama K, Oshima T (2001) Sodium chloride loading does not alter endothelium-dependent vasodilation of forearm vasculature in either salt-sensitive or salt-resistant patients with essential hypertension. Hypertens Res 24:711–716 [DOI] [PubMed] [Google Scholar]
- 23.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ (2002) Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39:683–688 [DOI] [PubMed] [Google Scholar]
- 24.Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell NRC, Li Q, Lackland DT, Leung AA, Anderson CAM, MacGregor GA, He FJ (2020) Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ 368:m315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igari K, Kudo T, Toyofuku T, Inoue Y (2016) The Relationship between Endothelial Dysfunction and Endothelial Cell Markers in Peripheral Arterial Disease. PLoS One 11:e0166840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juraschek SP, Woodward M, Sacks FM, Carey VJ, Miller ER, Appel LJ (2017) Time Course of Change in Blood Pressure From Sodium Reduction and the DASH Diet. Hypertension 70:923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE (2003) Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 146:168–174 [DOI] [PubMed] [Google Scholar]
- 28.Lambert E, Eikelis N, Esler M, Dawood T, Schlaich M, Bayles R, Socratous F, Agrotis A, Jennings G, Lambert G, Vaddadi G (2008) Altered sympathetic nervous reactivity and norepinephrine transporter expression in patients with postural tachycardia syndrome. Circ Arrhythm Electrophysiol 1:103–109 [DOI] [PubMed] [Google Scholar]
- 29.Lennon-Edwards S, Ramick MG, Matthews EL, Brian MS, Farquhar WB, Edwards DG (2014) Salt loading has a more deleterious effect on flow-mediated dilation in salt-resistant men than women. Nutr Metab Cardiovasc Dis 24:990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Y, Chen S, Liu X, Zhang Q, Ai Y, Wang Y, Jin H, Tang C, Du J (2010) Flow-mediated vasodilation and endothelium function in children with postural orthostatic tachycardia syndrome. Am J Cardiol 106:378–382 [DOI] [PubMed] [Google Scholar]
- 31.Loughlin EA, Judge CS, Gorey SE, Costello MM, Murphy RP, Waters RF, Hughes DS, O’Donnell MJ, Canavan MD (2020) Lack of Evidence to Support Increased Salt For Orthostatic Intolerance Syndromes: A Systematic Review and Meta-Analysis. Am J Med [DOI] [PubMed] [Google Scholar]
- 32.Marinos A, Celedonio JE, Ramirez CE, Gottlieb J, Gamboa A, Hui N, Yu C, Stein CM, Biaggioni I, Shibao CA (2015) Time-Course Analysis of Flow Mediated Dilation for the Evaluation of Endothelial Function After a High-Fat Meal in African Americans. J Am Heart Assoc 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews EL, Brian MS, Ramick MG, Lennon-Edwards S, Edwards DG, Farquhar WB (2015) High dietary sodium reduces brachial artery flow-mediated dilation in humans with salt-sensitive and salt-resistant blood pressure. J Appl Physiol (1985) 118:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCrea CE, Skulas-Ray AC, Chow M, West SG (2012) Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med 17:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Keyes MJ, Levy D, Vasan RS, Benjamin EJ (2004) Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44:134–139 [DOI] [PubMed] [Google Scholar]
- 36.Moerland M, Kales AJ, Schrier L, van Dongen MG, Bradnock D, Burggraaf J (2012) Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. Int J Vasc Med 2012:904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P (2006) Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985) 101:545–548 [DOI] [PubMed] [Google Scholar]
- 38.Nilsson P, Östling G, Kennbäck C, Persson M (2016) Endothelial Function in Young Healthy Subjects. Journal of Hypertension 34 [Google Scholar]
- 39.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D (2005) Reninaldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 111:1574–1582 [DOI] [PubMed] [Google Scholar]
- 40.Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, Thibodeau-Jarry N, Sheldon RS (2020) Canadian Cardiovascular Society Position Statement on Postural Orthostatic Tachycardia Syndrome (POTS) and Related Disorders of Chronic Orthostatic Intolerance. Can J Cardiol 36:357–372 [DOI] [PubMed] [Google Scholar]
- 41.Rosenberry R, Nelson MD (2020) Reactive hyperemia: a review of methods, mechanisms, and considerations. Am J Physiol Regul Integr Comp Physiol 318:R605–R618 [DOI] [PubMed] [Google Scholar]
- 42.Sander M, Hansen PG, Victor RG (1995) Sympathetically mediated hypertension caused by chronic inhibition of nitric oxide. Hypertension 26:691–695 [DOI] [PubMed] [Google Scholar]
- 43.Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Diedrich A, Raj V, Sheldon RS, Biaggioni I, Robertson D, Raj SR (2019) The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med 286:438–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheldon RS, Grubb BP, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K (2015) 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 12:e41–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simoncini T, Fu XD, Caruso A, Garibaldi S, Baldacci C, Giretti MS, Mannella P, Flamini MI, Sanchez AM, Genazzani AR (2007) Drospirenone increases endothelial nitric oxide synthesis via a combined action on progesterone and mineralocorticoid receptors. Hum Reprod 22:2325–2334 [DOI] [PubMed] [Google Scholar]
- 46.Stewart JM, Medow MS, Minson CT, Taneja I (2007) Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293:H2161–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart JM, Medow MS, Montgomery LD (2003) Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 285:H2749–2756 [DOI] [PubMed] [Google Scholar]
- 48.Strazzullo P, Barbato A, Vuotto P, Galletti F (2001) Relationships between salt sensitivity of blood pressure and sympathetic nervous system activity: a short review of evidence. Clin Exp Hypertens 23:25–33 [DOI] [PubMed] [Google Scholar]
- 49.Syvänen K, Korhonen P, Partanen A, Aarnio P (2011) Endothelial function in a cardiovascular risk population with borderline ankle–brachial index. Vascular Health and Risk Management [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young CN, Fisher JP, Gallagher KM, Whaley-Connell A, Chaudhary K, Victor RG, Thomas GD, Fadel PJ (2009) Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J Physiol 587:4977–4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q, Liao Y, Tang C, Du J, Jin H (2012) Twenty-four-hour urinary sodium excretion and postural orthostatic tachycardia syndrome. J Pediatr 161:281–284 [DOI] [PubMed] [Google Scholar]


