Abstract
Objectives
Repetitive transcranial magnetic stimulation (TMS) is a promising treatment for suicidality, but it is underlying neural mechanisms remain poorly understood. Our prior findings indicated that frontostriatal functional connectivity correlates with the severity of suicidal thoughts and behaviors. In this secondary analysis of data from an open label trial, we evaluated whether changes in frontostriatal functional connectivity would accompany suicidality reductions following TMS. We also explored the relationship between frontostriatal connectivity change and underlying white matter (WM) organization.
Materials and Methods
We conducted seed‐based functional connectivity analysis on participants (N = 25) with comorbid post‐traumatic stress disorder and depression who received eight weeks of 5 Hz TMS to left dorsolateral prefrontal cortex. We measured clinical symptoms with the Inventory of Depressive Symptomatology‐Self Report (IDS‐SR) and the PTSD Checklist for DSM‐5 (PCL‐5). We derived suicidality from IDS‐SR item 18. Magnetic resonance imaging data were collected before TMS, and at treatment end point. These data were entered into analyses of covariance, evaluating the effect of suicidality change across treatment on striatal and thalamic functional connectivity. Changes in other PTSD and depression symptoms were included as covariates and results were corrected for multiple comparisons. Diffusion connectometry in a participant subsample (N = 17) explored the relationship between frontal WM integrity at treatment baseline and subsequent functional connectivity changes correlated with differences in suicidality.
Results
Suicidal ideation decreased in 65% of participants. Reductions in suicidality and functional connectivity between the dorsal striatum and frontopolar cortex were correlated (p‐False Discover Rate‐corrected < 0.001), after covariance for clinical symptom change. All other results were nonsignificant. Our connectometry results indicated that the integrity of frontostriatal WM may circumscribe functional connectivity response to TMS for suicide.
Conclusions
Targeted reduction of fronto‐striatal connectivity with TMS may be a promising treatment for suicidality. Future research can build on this multimodal approach to advance individualized stimulation approaches in high‐risk patients.
Keywords: Diffusion magnetic resonance imaging, fMRI, suicidal ideation, suicide, transcranial magnetic stimulation
INTRODUCTION
Despite advances in mental health treatment, suicide is a leading cause of death in the United States (1) and worldwide, an estimated 800,000 people die by suicide every year (2). Almost 50% of individuals that die by suicide have a history of psychiatric illness (3). Clearly, novel, effective treatments for suicide are needed to meet the challenge of this public health crisis.
Repetitive transcranial magnetic stimulation (TMS) is an effective treatment for pharmacoresistant major depression (4, 5, 6) and anxiety disorders (7, 8). Most TMS protocols used to treat depression and anxiety stimulate the dorsolateral prefrontal cortex (DLPFC) (9). DLPFC plays a key role in domain‐general cognitive control of thought and behavior (10). It is hypothesized that upregulating DLPFC function via stimulation enhances control over cognitive and affective symptoms of psychiatric illness, including suicidal thoughts and behaviors.
The history of TMS as a suicide reduction intervention is modest, yet promising. Despite variance between TMS protocols, multiple studies have reported suicidality decreases in patients treated for depression (11, 12, 13, 14, 15, 16). One randomized controlled trial (RCT) reported a 44% reduction in “being bothered by thoughts of suicide,” after completing a course of high‐dose left DLPFC TMS (12). Similarly, a study pooling data from multiple sham‐controlled RCTs found that bilateral TMS was associated with reductions in suicidal ideation (17). In some cases, these reductions were independent of decreases in overall depression (13, 14, 17), or occurred regardless of the adequacy of depression symptom response (13, 14, 17). These observations suggest that TMS may possibly reduce suicidality through independent mechanisms.
Optimizing TMS as an antisuicidal intervention will require targeting the precise neural circuits underlying observed suicidality reductions. Unfortunately, optimization efforts are limited by the lack of essential circuit‐level data. Moreover, the extent to which suicidality reductions represent specific or nonspecific responses to TMS is under debate. RCTs specifically evaluating TMS for suicidality have not consistently demonstrated statistically significant separation between active and sham TMS (12, 13, 14). Though neural data alone cannot resolve this controversy, it may disambiguate circuits implicated in specific, from nonspecific, treatment responses. This information is critical for optimization of TMS as a suicide treatment.
Circuits underlying decision‐making networks, in particular the valence and cognitive control networks, are promising potential targets for optimizing TMS for suicidality. The valence network contributes to decision‐making through its involvement in reinforcement learning (18). Functional magnetic resonance imaging (fMRI) studies consistently demonstrate that normative valence activation during decision‐making is disrupted in adults with depression and history of suicide attempts (19, 20, 21), youths with behavioral disorders and ideation (22), and is sometimes correlated with impaired decision‐making (19, 20), an established correlate of suicidal thoughts and behaviors (23, 24, 25, 26, 27). The relationship between suicide and cognitive control is well documented, though not well defined at the process level (28). Nonetheless, aberrant fMRI activation of control regions is reported consistently in studies of decision‐making and suicide, though the directionality of reported effects is variable (reviewed in Reference 29).
Moreover, the relationship between decision‐making circuit function and suicide is robust enough to be observed in resting‐state functional connectivity (RSFC) (30). In a prior investigation, we used functional connectivity to identify seed‐to‐seed correlations between regions‐of‐interest (ROIs) that varied systematically with self‐reported suicidality in participants diagnosed with posttraumatic stress disorder (PTSD) (N = 50). Significant findings of this study included hyperconnectivity between striatal and anterior frontal cortex ROIs associated with the valence network, as well as between anterior cingulate and the pars orbitalis cognitive control ROIs.
If hyperconnectivity between these regions contributes to suicide and related problems with decision‐making, using neuromodulation to disrupt pathological signaling may produce antisuicidal effects. As a preliminary test of this hypothesis, we conducted a secondary functional connectivity analysis of resting‐state MRI in participants (N = 25) from an open label trial of 5 Hz TMS of left DLPFC for comorbid PTSD and depression (7). The present study's goal was to identify brain areas where changes in the functional connectivity of the striatum or thalamus following completion of the eight‐week TMS course varied systematically with post‐TMS differences in suicidality. We focused our analysis on the striatum and thalamus because of their established relationship with decision‐making, suicidality, and functional connectivity in our prior study (30). As both the valence and cognitive control networks are indirectly connected via the cortico‐striatothalamic cortical (CSTC) anatomical loops converging in the striatum and thalamus, we reasoned that modulation of either network would be reflected in striatothalamic functional connectivity changes.
Here, we employed seed‐to‐voxel analyses to evaluate changes in whole‐brain RSFC of the striatum and thalamus after completion of the TMS course. We hypothesized that changes in functional connectivity and suicidality would be correlated, such that connectivity would decrease in participants experiencing reductions in suicidal thoughts and behaviors. This expectation was informed by patterns of hyperconnectivity observed in our prior work (30), and evidence that left DLPFC TMS stimulation evokes striatal response (31). We also explored structural relationships at baseline (pre‐TMS) that potentially influence subsequent responsiveness to TMS in a subset of participants (n = 17) using diffusion MRI and deterministic fiber connectometry. Diffusion connectometry (32) is a novel approach that identifies white matter (WM) segments where local diffusion is statistically associated with variables of interest, here functional connectivity correlates of suicidality change.
MATERIALS AND METHODS
Participants and TMS Methods
Data for secondary functional connectivity analyses were obtained from a subset of participants with PTSD + Major Depressive Disorder (MDD) that took part in a trial of left DLPFC 5 Hz TMS and completed pre‐ and post‐TMS MRI scans (n = 25; age = 52.4 ± 10, female = 12). For inclusion in the parent study, participants were required to meet DSM‐IV criteria for both PTSD and MDD, with overall illness severity ratings of at least “moderately ill” on the Clinical Global Impressions‐Severity Scale for each diagnosis. Active suicidal intent or suicidal plan were exclusionary. All participants received up to eight weeks of unblinded 5 Hz TMS to the left DLPFC, targeted using the Beam/F3 method; treatment was delivered at 120% of motor threshold, 3000–4000 pulses for up to 40 sessions. Though this is a lower than the typical US Food and Drug Administration–cleared 10 Hz “dose” for MDD, naturalistic observations from our clinic indicated that lower frequency stimulation was better tolerated and achieved equivalent efficacy in rTMS for MDD. These observations were corroborated in a related case series (n = 10) examining acceptability and safety of 5 Hz TMS, as well as changes in PTSD and MDD symptoms (33). The trial found TMS was associated with statistically significant and clinically meaningful reductions in depressive and PTSD symptoms (all p < .001). For complete details of enrollment criteria, TMS methods, and results, see Carpenter et al. (7). We note that fMRI data collected at baseline only (not post‐TMS) were included in a previous, larger secondary analysis (N = 50) examining network relationships and suicidality (30). However, the analytic approach (seed‐to‐voxel, see p. 11) and brain connectivity and suicidality scores (post‐pre TMS difference) differ in the present study. All procedures were conducted at the Providence VA Medical Center, Brown University, or Butler Hospital in Providence, Rhode Island under the supervision of the Institutional Review Boards at each study site. Written informed consent was obtained for all participants in accordance with the Declaration of Helsinki.
Clinical Measures
Study participants completed the self‐reported PTSD Symptom Checklist for DSM‐5 (PCL‐5 (34)) and Inventory of Depressive Symptoms Self‐Report (IDS‐SR (35)) before and after their course of TMS. Pre‐ and post‐TMS suicidality scores were derived from the IDS‐SR “thoughts of suicide” item (item #18). Possible responses to item #18 include: (0) “no thoughts of death or suicide,” (1) “feeling that life is empty or wondering if it is worth living,” (2) “thinking of suicide or death several times a week for several minutes,” or (3) “thinking of suicide or death in detail multiple times each day, having a specific suicide plan, or having made an attempt.” We derived an index of lifetime suicidality from intake interviews and verified that previous psychiatric hospitalizations were related to suicidality by chart review. See Table 1 for clinical scores, demographic, and medical information.
Table 1.
Demographics and Clinical Measures by Change in Suicidal Severity.
| All | Decreased IDS‐SR item #18 | Increased IDS‐SR item #18 | No change IDS‐SR item #18 | |
|---|---|---|---|---|
| n | 25 | 11 | 3 | 11 |
| Age, mean (SD) | 52.4 (10.0) | 59.2 (6) | 47.0 (19.6) | 46.7 (10.0) |
| Females, n (%) | 12 (48) | 5 (45) | 2 (67) | 5 (45) |
| IDS‐SR | ||||
| Baseline, mean (SD) | 45.4 (12.4) | 45.9 (16.6) | 46.9 (15.9) | 43.6 (9.7) |
| End point, mean (SD) | 27.2 (17.9) | 20.6 (18.7) | 31.9 (15.5) | 30.0 (18.3) |
| % change, mean (SD) | 42.6 (31.3) | 58.8 (21.5) | 27.8 (33.3) | 33.2 (35.5) |
| PCL | ||||
| Baseline, mean (SD) | 49 (12.6) | 47.2 (13.2) | 47.6 (17.5) | 48.6 (12.9) |
| End point, mean (SD) | 28.5 (19.0) | 20.9 (20.0) | 31.1 (15.1) | 32.5 (19.2) |
| % change, mean (SD) | 41.8 (38.0) | 58.0 (33.4) | 29.3 (37.1) | 30.2 (42.2) |
| Medications* | ||||
| Antidepressants, n (%) | 18 (72) | 6 (55) | 2 (67) | 10 (91) |
| Benzodiazepines, n (%) | 10 (40) | 5 (45) | 1 (33) | 4 (36) |
| Anticonvulsants, n (%) | 5 (20) | 3 (27) | 0 (0) | 2 (18) |
| Antipsychotics, n (%) | 10 (40) | 5 (45) | 0 (0) | 5 (45) |
| Stimulants, n (%) | 7 (28) | 3 (27) | 1 (33) | 3 (27) |
Descriptive data for participants grouped according to change in IDS‐SR item #18 score following a course of 5 Hz TMS treatment for comorbid PTSD and depression.
*Note that medications were stable for at least six weeks prior to stimulation.
MRI Data Collection
Structural, functional, and diffusion neuroimaging data were acquired within one week of beginning and ending TMS. All imaging data were collected at the Brown University MRI Research Facility on either a Siemens (Erlangen, Germany) 3T TimTrio or 3T Prisma scanner using a 32‐channel head receiver coil. At each timepoint, we collected a high‐resolution T1‐weighted anatomical image (voxel size = 1.0 mm3, repetition time [TR] = 1900 msec, echo time [TE] = 2.98 msec, field‐of‐view [FOV] = 256 mm2) and one 8‐min run of “resting state” gradient‐echo echo‐planar imaging (EPI) T2*‐weighted functional MRI (voxel = 3.0 mm3, TR = 2500 ms, TE = 28 ms, flip angle = 90°, FOV = 64 × 64, 42 axial slices, 192 volumes). During resting‐state scans, participants were instructed to keep their eyes open and remain as still as possible. A 12‐min, 64 direction, diffusion‐weighted EPI scan (voxel size = 1.8 mm3, TR = 10,200 msec, TE = 103.0 msec, slices = 76, b = 1000 sec/mm2, b0 = 12) was collected during the pre‐TMS session from participants (n = 17). Diffusion data were collected for all participants; however, we restricted our analysis to participants scanned with the Prisma because small modifications of the diffusion sequence were made at the time of the model upgrade.
Functional Connectivity Preprocessing and Analysis
We used SPM12 (University College London; https://www.fil.ion.ucl.ac.uk/spm/) and the CONN toolbox (www.nitrc.org/projects/conn) for functional MRI preprocessing and analysis. Basic preprocessing included slice‐time correction, motion estimation and realignment, segmentation, normalization to Montreal Neurological Institute (MNI)‐152 atlas space, and spatial smoothing with a 6 mm full‐width half‐max Gaussian kernel. We implemented additional functional connectivity‐specific preprocessing steps to reduce further influence deleterious effects of motion and non‐neuronal signals on functional connectivity estimates (36). Per the ART Toolbox as implemented by CONN (37), we flagged high motion (translational > 0.5 mm, rotational > 0.02 radians) and global signal variance (>3sd) volumes from preprocessed data. We also used Anatomical CompCor (38) within CONN to deconstruct signal time courses (WM and cerebrospinal fluid [CSF]). Flagged volumes, five principal components each for WM and CSF, six motion parameters and their first derivatives, and the linear trend were regressed from subject‐level data to limit contributions of potential confound variables. CONN's regression‐based motion correction strategies and quality diagnostic tools available are aligned with those of recent alternatives such as XCP (39) and fMRIPrep (40). The resulting residuals were band‐pass filtered (high‐pass = 0.008, low‐pass = 0.1) after nuisance regression (41).
Seed ROIs and Subject‐Level Functional Connectivity Analyses
We measured whole‐brain seed‐to‐voxel functional connectivity in four a priori seeds in each hemisphere. Seed masks included the executive‐ and limbic‐projecting subregions of the striatum from the diffusion‐based parcellation of Tziortzi et al. (42), approximating commonly used dorsal and ventral divisions defined by cyto‐ or receptor‐architecture. Diffusion‐based frontal and temporal projecting zones of the thalamus from Behrens et al. (43) that overlap with portions of medial dorsal and the anterior complex of the thalamus. For each subject and seed, we extracted the average blood oxygen level‐dependent time course and computed its cross‐correlation with time courses from individual voxels, producing a whole‐brain map of bivariate Pearson's correlations. These Pearson's maps were then converted into Fisher‐transformed Z scores to improve conformation to assumptions of normality.
Second Level RSFC Analyses
Our second‐level analyses evaluated whether changes in striatal or thalamic functional connectivity following a course of TMS were associated with changes in suicidal severity. We operationalized these changes as the difference in IDS‐SR item #18 scores from baseline‐to‐endpoint. To identify brain regions where functional connectivity and suicidality differences were correlated, we entered subject‐level RSFC seed maps into an analysis of covariance (using age, percent change in overall PCL and IDS‐SR scores as covariates). Models evaluated the between‐subjects effect of change in suicidality on within‐subject functional connectivity differences across treatment. We adopted a dual‐thresholding procedure to correct for multiple comparisons (44). Significant voxels were identified using a voxel‐height threshold (p‐uncorrected < 0.001) but were only considered significant if they were part of a cluster of voxels exceeding an expected size threshold (p‐False Discover Rate‐corrected [FDR] < 0.05) based on random field theory estimate. Post hoc sensitivity analyses were conducted to assess potential effects of sex or scanner. Other sensitivity tests indicated that removing age as a covariate did not influence our findings. The association between change in suicidality and functional connectivity was weaker and nonsignificant when overall PTSD and depression symptoms we not regressed from models.
Diffusion MRI Preprocessing and WM Reconstruction
FSL (45) was used for diffusion preprocessing and visual quality assurance. Preprocessing included: 1) affine registration to the first nondiffusion‐weighted (b0 image) (46), 2) eddy current correction (47), and 3) reorientation of diffusion vectors (48). Diffusion image reconstruction was carried out with DSI Studio (http://dsi-studio.labsolver.org). Subjects' preprocessed diffusion data were submitted to Q‐space diffeomorphic reconstruction (QSDR) (49). QSDR is an extension of the deterministic generalized q‐sampling imaging (GQI) reconstruction algorithm (50). Because the GQI and QSDR model free reconstruction algorithms operate at the subvoxel level, they are less vulnerable to the limitations of tensor‐based methods, for example, partial volume effects, poor resolution of orientation in voxels with crossing fibers. In QSDR, spin distribution functions (SDFs) (50) are computed from voxels' diffusion signals and mapped directly into stereotaxic space (MNI Atlas). The resulting whole‐brain SDF map describes the density of oriented spins at every voxel.
Local Connectometry Analysis
DSI Studio also was used for connectometry analysis. Connectometry is executed at the level of the local connectome. Local connectomes describe relative similarity and density of oriented spins from adjacent voxels (32). For every subject and voxel, oriented spin densities were extracted and entered into the n‐by‐m local connectome matrix Y, where n is equal to the number of subjects, and m is the number of oriented spins. Connectometry analysis employs permutation‐based testing procedure to localize associations between local connectomes to group or other explanatory variables to shared WM bundles (for complete details, see Yeh et al. (32)). Our group analysis identified fiber segments where WM integrity at study baseline was associated with subsequent functional connectivity change after TMS. To this end, we treated subject‐level coefficients from significant functional connectivity clusters as explanatory variables in a linear regression testing their association with the nonpermuted matrix Y. For hypothesis testing, null distributions were obtained by random resampling of the matrix (5000 row permutations). We then regressed explanatory variables against permutations to obtain the null distribution. We applied a dual thresholding procedure to reduce false positives. First, we applied the threshold t > 1.0 to the unpermuted matrix to identify potential local connectomes associated with functional connectivity. We then subjected these fibers to two iterations of topology‐informed pruning to remove likely false connections (51). The second minimum length threshold of 20 voxels was used to filter out the remaining short, fragmented and likely false positive tracks. Length histograms were constructed for retained tracks and we derived the false discovery rate (FDR) for nonpermuted tracks from the null distribution of track lengths.
RESULTS
Clinical Measures
Table 1 describes demographics and descriptive statistics of the participant sample. Participants completed 36 ± 6 TMS sessions (median = 37 sessions, mode = 40 sessions, range 13–40 sessions). Suicidal ideation of any severity (i.e., IDSSR item 18 score of at least 1) was reported by 68% of participants at study baseline, with 10 participants reporting a score of one, five participants reporting a score of two, and two participants reporting a score of three. After TMS, suicidality decreased by a minimum of one point in n = 11 (65%) of participants who endorsed suicidality pretreatment, t 15 = 8.2, p < 0.00001. Suicidality increased in n = 3 (12%) participants; of note, including a one‐point increase in one participant that did not endorse suicidality at baseline (i.e., item 18 = 0). Across participants, mean change in overall PCL‐5 and IDS‐SR scores was 41.8% and 42.6%, respectively, in this subset of the original study cohort. In the parent sample, average decrease in PCL‐5 was 35.5% and IDS‐SR was 37.6% (see Carpenter et al. (7) for complete details).
Functional Connectivity
Our seed‐to‐voxel results indicated that frontostriatal connectivity decreased in participants whose suicide item scores were reduced after TMS; connectivity increased in those participants whose item scores increased. Using the left frontal projecting striatum seed, we observed a significant cluster in the right frontal pole (MNI: +28 +62 +04, k = 192, p‐FDR < 0.0001), as well as a second cluster in the left frontal pole that was marginally significant after multiple comparisons correction (MNI: −32 +54 –04, k = 55, p‐FDR = 0.07) (Fig. 1). Functional connectivity decreases between the right frontal projecting striatum seed and right frontal pole also were significantly correlated with changes in suicidality (MNI: +30 +54 –10, k = 66, p‐FDR < 0.05). Results for all other seeds were nonsignificant. Post hoc sensitivity tests indicated that removing age as a covariate did not influence our findings. The association between change in suicidality and functional connectivity was weaker when we did not regress overall PTSD and depression symptoms from the model.
Figure 1.
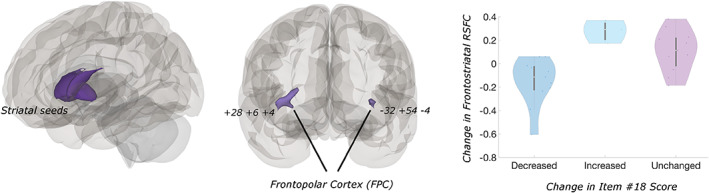
Decreases in frontostriatal functional connectivity are correlated with reductions in suicidality following TMS. Left: Location of striatal seeds. Seed corresponded to the “prefrontal” projecting subregion of striatum per Tziortzi et al. (45). Center: Frontopolar cortex clusters where changes in RSFC to striatum and suicidality are correlated (p‐FDR < 0.001). Right: Though changes in suicide self‐rating were analyzed as a continuous measure, violin plots depict distributions of RSFC change by group for illustrative purposes. White circles denote medians, whiskers illustrate interquartile rang. [Color figure can be viewed at wileyonlinelibrary.com]
Diffusion Connectometry
Our connectometry results indicated that pretreatment frontostriatal WM integrity was associated with the above changes in functional connectivity (i.e., changes that occurred with TMS). Changes in left frontopolar functional connectivity to the right striatum were associated with a broadly distributed set of local frontal WM pathways, including the left u fibers (76%), left corticothalamic pathway (12%), left cortico striatal pathway (7.8%), left inferior fronto occipital fasciculus (3.7%) (p‐FDR < 0.01) (Fig. 2). Baseline anatomical connectivity was not associated with subsequent change in functional connectivity for the right frontopolar clusters (all p‐FDR > 0.05).
Figure 2.
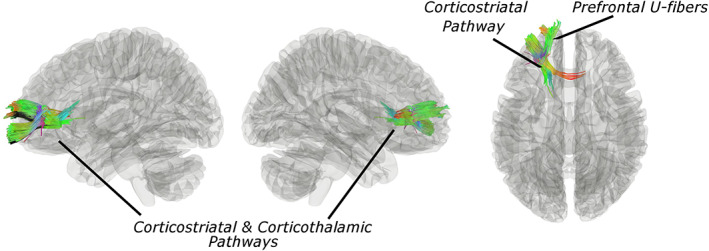
Frontostriatal WM integrity is associated with functional connectivity response to TMS for suicidality. Pathway segments where increased quantitative anisotropy at study baseline was associated with RSFC correlates in suicidal ideation (IDS‐SR item #18) after TMS (nonparametric p‐FDR < 0.05). Colors denote fiber orientations. [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
To our knowledge, this is the first multimodal neuroimaging investigation of neural mechanisms underlying suicidality reduction following an acute course of TMS therapy. We observed a significant association between reductions in suicidality and decreased frontostriatal functional connectivity. Importantly, this relationship was independent of improvement in other clinical symptoms. The independence of this effect implies that neuromodulation directed toward a distinct frontostriatal circuit may specifically reduce pathological signaling related to suicide.
The potential importance of this frontopolar striatal circuit in reducing suicidality aligns with the broader neuroimaging literature surrounding suicide, valance, and value‐based decision‐making (52, 53, 54, 55), and the association of valence disruption with emotional distortions in psychiatric illness (56). The cortical portions of this circuit include frontopolar and adjacent orbitofrontal cortex; prior studies have linked aberrant fMRI activation in these regions to impaired decision‐making and suicide (20, 57, 58). Our earlier work revealed evidence of frontostriatal hyperconnectivity in this circuit, even in the absence of a task (30). This earlier study also found of evidence of suicidality‐related hyperconnectivity in control networks. We restricted the scope of the present analysis to striatal and thalamic functional connectivity, reasoning that if control connectivity were modulated, it would likely occur involve CSTC subcortical structures, given the lack or weakness of direct anatomical connections between the DLPFC stimulation site and these regions. Our results did not support this expectation. Thus, though this awaits sham‐controlled replication, our findings suggest that administering left DLPFC TMS may be an effective means of reducing pathological valence hyperconnectivity, but not control network connectivity, in individuals struggling suicide. Future work should explore whether it is possible to remediate hyperconnectivity in control circuits using other stimulation targets.
Though our frontostriatal results are promising, their alignment with findings from a recent clinical trial (NCT01832805) of accelerated intermittent theta‐burst stimulation for depression, recommends they must be interpreted cautiously (16). This trial reported correlated reductions in suicidality and frontopolar perfusion (measured by arterial spin labeling) in participants receiving sham, but not active stimulation (16). The similarity of the circuit identified in this study to that of Baeken et al. is striking, especially given the differences in diagnostic criteria, magnetic pulse pattern (i.e., 5 Hz vs. theta‐burst protocols), and neuroimaging modality across studies. While our collective results may reflect the frontopolar cortex's involvement in a common process contributing to the resolution of suicidality, we would be remiss if we did not acknowledge that it may be nonspecific, rather than attributable to TMS. Additional research is needed to disambiguate nonspecific, from treatment effects of TMS. This caveat notwithstanding, this circuit's consistent implication in reducing suicidality nonetheless argues that optimizing frontostriatal engagement may improve treatment response, be it verum or nonspecific.
We do wish to be cautious about interpreting null effects. However, null findings for the remaining seeds are not wholly unexpected. Though all seeds are sites of CSTC loop convergence and have shown suicidality‐related hyperconnectivity, only modulation of the striatum after DLPFC stimulation has been demonstrated directly (31). Our observation of significant results for the frontal, but not temporal, striatal subregions correspond with this previous demonstration.
Last, our exploratory connectometry results further highlight the significance of frontostriatal circuits in suicidality. Our findings are broadly consistent with observations of reduced frontal WM integrity in those with history of suicidal thoughts or behaviors, including general decreases in frontal fractional anisotropy (59) and specific decreases in frontothalamic (60), frontostriatal (61), and inferior capsule (62) circuits. The convergence of these previous findings upon frontal striatothalamic circuits is noteworthy given demonstrations of acute modulation of striatal and thalamic fMRI activity following left DLPFC TMS (31), and evidence that DLPFC‐striatal functional connectivity at baseline predicts symptom response to left DLPFC TMS for depression (63). As part of the CSTC loop system (64, 65), these anatomical pathways are likely essential for efficient propagation of TMS‐evoked signals to the subcortex. Indeed, evidence links anisotropy in these circuits to the magnitude of TMS‐evoked responses in striatum and thalamus following frontal polar cortex stimulation (66). Though preliminary, our findings identify a structural pathway whose pretreatment integrity impacts engagement of functional targets. Thus, these data reveal a potential avenue for optimizing TMS delivery and for its development as an emerging treatment for suicidality. For example, future work could explore using metrics of baseline circuit integrity to calibrate optimal stimulation frequencies for target engagement.
Limitations
Because we lack sham‐controlled data, we cannot disambiguate specific from nonspecific brain correlates, a notable shortcoming given recent findings (16). Though this limits scientific interpretation, providing entirely inactive treatments to symptomatic and potentially suicidal patients does raise important ethical caveats. Potential medication effects may influence our results given our naturalistic sample. We are underpowered to address this statistically, but future work should evaluate potential effects of medications on response to TMS for suicidality, especially given research demonstrating impacts on motor cortex excitability (e.g., benzodiazepines as reviewed in Reference 67).
We also acknowledge the limitations of our single‐item measure of suicidality, derived from the IDS‐SR. This measure conflates severity and frequency and cannot parse severe ideation from behaviors. While not ideal, derived scores were necessary as change in suicidality was not a primary outcome of the parent study. An alternative option would have been to use the PHQ9 to derive this measure, as it also was collected in the parent study. The PHQ9 has been shown to predict changes in cumulative suicide risk at one year (68), though it generates more false positives than gold‐standard measures like the Columbia Suicide Severity Rating Scale when used for risk screening (69). We speculate that false positives may stem from the conflation of suicide and other forms of self‐harm in the phrasing of the PHQ9 suicide item. Thus, despite its limitations, we chose to derive our measure from the IDS‐SR because it asks about suicide, specifically. There are many validated measures designed to quantify suicidal thoughts and behaviors (reviewed in Reference 70) which should be incorporated into future studies of TMS and suicide. Though we treated overall depression and anxiety scores as model covariates, we acknowledge that this strategy cannot completely control for the influence of these factors (71, 72). We also note that we adopted a more liberal approach to multiple comparisons correction which we applied FDR‐correction at the seed, rather than the analysis level. Our study also is subject to the limitations inherent to all small sample studies, and we acknowledge that though promising, our findings should be regarded as preliminary.
CONCLUSIONS
While preliminary, our study adds to a growing literature demonstrating that TMS reduces suicidal thoughts and behaviors. Using a multimodal approach, we localized potential mechanisms of suicidality reduction to a specific frontostriatal circuit. This particular circuit's implication aligns with prior work, though additional research is needed to distinguish specific effects. Collectively, findings argue that the precise targeting of frontopolar circuits via diffusion‐guided individualized targeting (73, 74, 75) may enhance neuromodulation delivery precision to our highest‐risk patients. Combing these methodologies with innovations in 5 Hz TMS delivery, such as pulse energy optimization (76), may further improve its efficacy as an antisuicidal treatment.
Authorship Statement
Jennifer Barredo designed and conducted the secondary data analyses reported in this manuscript. Linda L. Carpenter and Noah S. Philip designed and conducted the original parent study, including patient recruitment, data collection, and data analysis. Jennifer Barredo prepared the manuscript draft with intellectual input from Yosef Berlow, Hannah R. Swearingen, Benjamin D. Greenberg, Linda L. Carpenter, and Noah S. Philip. All authors have approved the final manuscript.
Acknowledgements
The efforts of Jennifer Barredo, Hannah R. Swearingen, Noah S. Philip, and Benjamin D. Greenberg were supported in part by the VA RR&D Center for Neurorestoration and Neurotechnology. The Department of Veterans Affairs also supported Jennifer Barredo (IK2 CX001824) and Noah S. Philip (I01 RX002450). Noah S. Philip, Jennifer Barredo, Benjamin D. Greenberg, and Linda L. Carpenter received support from the COBRE Center for Neuromodulation (NIGMS P20 GM130452). Jennifer Barredo received support from a Brain Behavior Research Foundation Young Investigator Award. Yosef Berlow received NIH funding (R25 MH101076). The clinical trial was partially supported by an investigator‐initiated research grant to Linda L. Carpenter from Neuronetics to Butler Hospital. In the past three years, Noah S. Philip has been an unpaid scientific advisory board member for Neuronetics in 2017. Linda L. Carpenter reports additional grant/research support from NeoSync, and Janssen, and she has been a consultant for Magstim LTD, AffectNeuro, Inc. The other authors declare that they have no competing interests. The funders had no role in the conduct of the study, manuscript preparation, or the decision to submit for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of U.S. Department of Veterans' Affairs or other funders. This work was presented in part at the 2019 annual meeting of the American College of Neuropsychopharmacology (ACNP). We thank all our participants.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: The efforts of Jennifer Barredo, Hannah R. Swearingen, Noah S. Philip, and Benjamin D. Greenberg were supported in part by the VA RR&D Center for Neurorestoration and Neurotechnology. The Department of Veterans Affairs also supported Jennifer Barredo (IK2 CX001824) and Noah S. Philip (I01 RX002450). Noah S. Philip, Jennifer Barredo, Benjamin D. Greenberg, and Linda L. Carpenter received support from the COBRE Center for Neuromodulation (NIGMS P20 GM130452). Yosef Berlow received NIH funding (R25 MH101076). The clinical trial was partially supported by an investigator‐initiated research grant from Neuronetics to Butler Hospital.
Conflict of Interest: Dr. Philip has been an unpaid scientific advisory board member for Neuronetics since 2017. Dr. Carpenter reports grant/research support from Neuronetics, NeoSync, and Janssen, and has consulted for Magstim LTD, AffectNeuro, Inc. The other authors have no competing interests. The funders had no role in the study's conduct, manuscript preparation, or the decision to submit for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of U.S. Department of Veterans' Affairs or other funders.
REFERENCES
- 1.CDC . Web‐Based Injury Statistics Query Reporting System (WISQARS). https://www.cdc.gov/injury/wisqars/index.html [DOI] [PMC free article] [PubMed]
- 2.WHO . WHO Global Health Estimates. https://www.who.int/mental_health/prevention/suicide/suicideprevent/en/
- 3.Stone DM, Simon TR, Fowler KA et al. Vital signs: trends in state suicide rates — United States, 1999–2016 and circumstances contributing to suicide — 27 states, 2015. MMWR Morb Mortal Wkly Rep 2018;67:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George MS, Lisanby SH, Avery D et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham‐controlled randomized trial. Arch Gen Psychiatry 2010;67:507–516. [DOI] [PubMed] [Google Scholar]
- 5.O'Reardon JP, Solvason HB, Janicak PG et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007;62:1208–1216. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter LL, Janicak PG, Aaronson ST et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety 2012;29:587–596. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter LL, Conelea C, Tyrka AR et al. 5 Hz repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. J Affect Disord 2018;235:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip NS, Barredo J, Aiken E et al. Theta‐burst transcranial magnetic stimulation for posttraumatic stress disorder. Am J Psychiatry 2019;176:939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philip NS, Barredo J, Aiken E, Carpenter LL. Neuroimaging mechanisms of therapeutic transcranial magnetic stimulation for major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc 2011;17:759–765. [DOI] [PubMed] [Google Scholar]
- 11.Hadley D, Anderson BS, Borckardt JJ et al. Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment‐resistant depression in a clinical setting. J ECT 2011;27:18–25. [DOI] [PubMed] [Google Scholar]
- 12.George MS, Raman R, Benedek DM et al. A two‐site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatients. Brain Stimul 2014;7:421–431. [DOI] [PubMed] [Google Scholar]
- 13.Desmyter S, Duprat R, Baeken C, Bijttebier S, van Heeringen K. The acute effects of accelerated repetitive transcranial magnetic stimulation on suicide risk in unipolar depression: preliminary results. Psychiatr Danub 2014;26:48–52. [PubMed] [Google Scholar]
- 14.Desmyter S, Duprat R, Baeken C, Van Autreve S, Audenaert K, van Heeringen K. Accelerated intermittent theta burst stimulation for suicide risk in therapy‐resistant depressed patients: a randomized, sham‐controlled trial. Front Hum Neurosci 2016;10:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelnaim MA, Langguth B, Deppe M et al. Anti‐suicidal efficacy of repetitive transcranial magnetic stimulation in depressive patients: a retrospective analysis of a large sample. Front Psych 2019;10:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeken C, Wu GR, van Heeringen K. Placebo aiTBS attenuates suicidal ideation and frontopolar cortical perfusion in major depression. Transl Psychiatry 2019;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissman CR, Blumberger DM, Brown PE et al. Bilateral repetitive transcranial magnetic stimulation decreases suicidal ideation in depression. J Clin Psychiatry 2018;79:17m1152. [DOI] [PubMed] [Google Scholar]
- 18.Maia T, Frank M. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci 2011;14:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dombrovski AY, Szanto K, Clark L, Reynolds CF, Siegle GJ. Reward signals, attempted suicide, and impulsivity in late‐life depression. JAMA Psychiatry 2013;70:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jollant F, Lawrence NS, Olie E et al. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision‐making and suicidal behavior. Neuroimage 2010;51:1275–1281. [DOI] [PubMed] [Google Scholar]
- 21.Olie E, Ding Y, Le Bars E et al. Processing of decision‐making and social threat in patients with history of suicidal attempt: a neuroimaging replication study. Psychiatry Res 2015;234:369–377. [DOI] [PubMed] [Google Scholar]
- 22.Dir AL, Allebach CL, Hummer TA et al. Atypical cortical activation during risky decision making in disruptive behavior disordered youths with histories of suicidal ideation. Biol Psychiatry Cogn Neurosci Neuroimaging 2020;5:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dombrovski AY, Clark L, Siegle GJ et al. Reward/punishment reversal learning in older suicide attempters. Am J Psychiatry 2010;167:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szanto K, Bruine de Bruin W, Parker AM, Hallquist MN, Vanyukov PM, Dombrovski AY. Decision‐making competence and attempted suicide. J Clin Psychiatry 2015;76:e1590–e1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanyukov PM, Szanto K, Hallquist MN et al. Paralimbic and lateral prefrontal encoding of reward value during intertemporal choice in attempted suicide. Psychol Med 2016;46:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek K, Kwon J, Chae JH et al. Heightened aversion to risk and loss in depressed patients with a suicide attempt history. Sci Rep 2017;7:11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadlaczky G, Hokby S, Mkrtchian A et al. Decision‐making in suicidal behavior: the protective role of loss aversion. Front Psych 2018;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bredemeier K, Miller IW. Executive function and suicidality: a systematic qualitative review. Clin Psychol Rev 2015;40:170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmaal L, van Harmelen AL, Chatzi V et al. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry 2020;25:408–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barredo J, Aiken E, van 't Wout‐Frank M, Greenberg BD, Carpenter LL, Philip NS. Neuroimaging correlates of suicidality in decision‐making circuits in posttraumatic stress disorder. Front Psychiatry 2019;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanlon CA, Canterberry M, Taylor JJ et al. Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: a pilot study. PLoS One 2013;8:e67917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh FC, Badre D, Verstynen T. Connectometry: a statistical approach harnessing the analytical potential of the local connectome. Neuroimage 2016;125:162–171. [DOI] [PubMed] [Google Scholar]
- 33.Philip NS, Ridout SJ, Albright SE, Sanchez G, Carpenter LL. 5‐Hz transcranial magnetic stimulation for comorbid posttraumatic stress disorder and major depression. J Trauma Stress 2016;29:93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weathers F, Litz B, Keane T, Palmieri P, Marx B, Schnurr P. The PTSD Checklist for DSM‐5 (PCL‐5). https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- 35.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Med 1996;26:477–486. [DOI] [PubMed] [Google Scholar]
- 36.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield‐Gabrieli S, Nieto‐Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 38.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007;37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciric R, Rosen A, Erus G et al. Mitigating head motion artifact in functional connectivity MRI. Nat Protoc 2018;13:2801–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteban O, Markiewicz CJ, Blair RW et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 2019;16:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satterthwaite TD, Elliott MA, Gerraty RT et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. Neuroimage 2013;64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tziortzi AC, Haber SN, Searle GE et al. Connectivity‐based functional analysis of dopamine release in the striatum using diffusion‐weighted MRI and positron emission tomography. Cereb Cortex 2014;24:1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behrens T, Johansen‐Berg H, Woolrich MW et al. Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750–757. [DOI] [PubMed] [Google Scholar]
- 44.Woo CW, Krishnan A, Wager TD. Cluster‐extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 2014;91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 46.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 47.Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion‐weighted MRI. Magn Reson Med 2004;51:103–114. [DOI] [PubMed] [Google Scholar]
- 48.Leemans A, Jones DK. The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 2009;61:1336–1349. [DOI] [PubMed] [Google Scholar]
- 49.Yeh FC, Tseng WY. NTU‐90: a high angular resolution brain atlas constructed by Q‐space diffeomorphic reconstruction. Neuroimage 2011;58:91–99. [DOI] [PubMed] [Google Scholar]
- 50.Yeh FC, Wedeen VJ, Tseng WY. Generalized q‐sampling imaging. IEEE Trans Med Imaging 2010;29:1626–1635. [DOI] [PubMed] [Google Scholar]
- 51.Yeh FC, Panesar S, Barrios J et al. Automatic removal of false connections in diffusion MRI tractography using topology‐informed pruning (TIP). Neurotherapeutics 2019;16:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richard‐Devantoy S, Berlim MT, Jollant F. A meta‐analysis of neuropsychological markers of vulnerability to suicidal behavior in mood disorders. Psychol Med 2014;44:1663–1673. [DOI] [PubMed] [Google Scholar]
- 53.van Heeringen K, Bijttebier S, Desmyter S, Vervaet M, Baeken C. Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate‐based meta‐analysis of structural and functional MRI studies. Front Hum Neurosci 2014;8:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry 1999;156:181–189. [DOI] [PubMed] [Google Scholar]
- 55.Jollant F, Lawrence NL, Olie E, Guillaume S, Courtet P. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry 2011;12:319–339. [DOI] [PubMed] [Google Scholar]
- 56.Heller AS, Johnstone T, Shackman AJ et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto‐striatal brain activation. Proc Natl Acad Sci U S A 2009;106:22445–22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jollant F, Guillaume S, Jaussent I et al. Psychiatric diagnoses and personality traits associated with disadvantageous decision‐making. Eur Psychiatry 2007;22:455–461. [DOI] [PubMed] [Google Scholar]
- 58.Dombrovski AY, Szanto K, Siegle GJ et al. Lethal forethought: delayed reward discounting differentiates high‐ and low‐lethality suicide attempts in old age. Biol Psychiatry 2011;70:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olvet DM, Peruzzo D, Thapa‐Chhetry B et al. A diffusion tensor imaging study of suicide attempters. J Psychiatr Res 2014;51:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia Z, Wang Y, Huang X et al. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci 2014;39:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myung W, Han CE, Fava M et al. Reduced frontal‐subcortical white matter connectivity in association with suicidal ideation in major depressive disorder. Transl Psychiatry 2016;6:e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia Z, Huang X, Wu Q et al. High‐field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry 2010;167:1381–1390. [DOI] [PubMed] [Google Scholar]
- 63.Avissar M, Powell F, Ilieva I et al. Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimul 2017;10:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 65.Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci 2016;18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kearney‐Ramos TE, Lench DH, Hoffman M, Correia B, Dowdle LT, Hanlon CA. Gray and white matter integrity influence TMS signal propagation: a multimodal evaluation in cocaine‐dependent individuals. Sci Rep 2018;8:3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ziemann U, Reis J, Schwenkreis P et al. TMS and drugs revisited 2014. Clin Neurophysiol 2015;126:1847–1868. [DOI] [PubMed] [Google Scholar]
- 68.Simon GE, Rutter CM, Peterson D et al. Does response on the PHQ‐9 depression questionnaire predict subsequent suicide attempt or suicide death? Psychiatr Serv 2013;64:1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Na PJ, Yaramala SR, Kim JA et al. The PHQ‐9 item 9 based screening for suicide risk: a validation study of the Patient Health Questionnaire (PHQ)‐9 item 9 with the Columbia Suicide Severity Rating Scale (C‐SSRS). J Affect Disord 2018;232:34–40. [DOI] [PubMed] [Google Scholar]
- 70.Runeson B, Odeberg J, Pettersson A, Edbom T, Jildevik Adamsson I, Waern M. Instruments for the assessment of suicide risk: a systematic review evaluating the certainty of the evidence. PLoS One 2017;12:e0180292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol 2001;110:40–48. [DOI] [PubMed] [Google Scholar]
- 72.Westfall J, Yarkoni T. Statistically controlling for confounding constructs is harder than you think. PLoS One 2016;11:e0152719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liebrand LC, Caan MWA, Schuurman PR et al. Individual white matter bundle trajectories are associated with deep brain stimulation response in obsessive‐compulsive disorder. Brain Stimul 2019;12:353–360. [DOI] [PubMed] [Google Scholar]
- 74.Coenen VA, Schlaepfer TE, Bewernick B et al. Frontal white matter architecture predicts efficacy of deep brain stimulation in major depression. Transl Psychiatry 2019;9:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riva‐Posse P, Choi KS, Holtzheimer PE et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment‐resistant depression. Mol Psychiatry 2018;23:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halawa I, Reichert K, Anil S, Sommer M, Paulus W. P260 increasing pulse energy of 5Hz rTMS improves its efficacy in inducing excitatory aftereffects. Clin Neurophysiol 2020;131:e162–e163. [Google Scholar]


