Abstract
Nerve guidance conduits with multifunctional features could offer microenvironments for improved nerve regeneration and functional recovery. However, the challenge remains to optimize multiple cues in nerve conduit systems due to the interplay of these factors during fabrication. Here, a modular assembly for the fabrication of nerve conduits was utilized to address the goal of incorporating multifunctional guidance cues for nerve regeneration. Silk-based hollow conduits with suitable size and mechanical properties, along with silk nanofiber fillers with tunable hierarchical anisotropic architectures and microporous structures, were developed and assembled into conduits. These conduits supported improved nerve regeneration in terms of cell proliferation (Schwann and PC12 cells) and growth factor secretion (BDNF, brain-derived neurotrophic factor) in vitro, and the in vivo repair and functional recovery of rat sciatic nerve defects. Nerve regeneration using these new conduit designs was comparable to autografts, providing a path towards future clinical impact.
Keywords: hierarchical architecture, nerve conduit, module assembly, silk nanofiber, peripheral nerve regeneration
Graphical Abstract
Silk-based nerve conduits containing silk nanofiber fillers with tunable hierarchical anisotropic architectures were fabricated through a modular assembly process. The conduits promoted the proliferation of schwann cells and the axonal outgrowth of PC12 cells in vitro and also accelerated the repair and functional recovery of rat sciatic nerve defects in vivo, comparable to autografts.
1. Introduction
Peripheral nerve injury (PNI) usually leads to serious disabilities.[1] Autografts as the gold standard for PNI are limited by donor sources, the need for secondary operations, size mismatches and the potential morbidity of donor sites.[2–4] Several nerve conduits composed of synthetic and natural biopolymers have been used to treat large PNIs and are considered as suitable alternative to autografts in clinical use.[2,5,6] However, these clinical conduits fail to achieve functional recovery comparable to autografts.[7] Tissue engineering-based strategies with the seeded cells have been utilized to improve axon growth and functional recovery of PNI.[8] The short survival time of the seeded cells along with the short half-life of growth factors reduced the effectiveness of the designs towards nerve regeneration.[9] These factors, along with the cost for the growth factors and cell culture challenges further hindered clinical translation.[10] Thus, more accessible and effective strategies are needed.
Multiple physical cues, such as structural hierarchy, mechanical properties, topographic anisotropy and electrical/magnetic stimulation can regulate cell proliferation, migration and differentiation, upregulate expression of growth factors and cytokines, and also induce neurite extension and functional recovery.[11–13] Unlike cell and growth factor-based strategies, these physical cues provide relatively stable control of the process and should facilitate clinical translation.[9] Electrospinning, 3D printing, microfluidic extrusion, molding, photolithography and crosslinking have all been used to fabricate nerve conduits with specific physical features.[14–16] Multiple physical cues were also introduced into same nerve guidance graft systems to achieve improved outcomes and functional recovery.[17–19] Although recent studies using some of these approaches have revealed significant improvements in axonal regeneration and peripheral nerve repair,[3,13,20] challenges remain to optimize these physical factors.
Biomaterials that can be tuned with multiple physical cues to optimize peripheral nerve responses can be effective.[21,22] Silk is a useful option for this goal due to its biocompatibility, tunable mechanical properties, degradability, loading capacity for electrical and magnetic agents, availability in terms of supply, and options for device fabrication.[23–26] Silk aqueous solutions can be used to fabricate these devices,[27] and can support nerve cell adhesion and growth, including neural stem cells, schwann cells and olfactory ensheathing cells.[28,29] Silk-based nerve grafts with anisotropic topographies and microluminal-nanoluminal hierarchical structures have been developed to improve peripheral nerve regeneration.[30,31] Cells, growth factors, and conductive and magnetic cargos have been loaded into silk-based nerve conduits for improved outcomes.[3,30,32] While these studies suggest that silk is a suitable material for the regeneration of peripheral nerve functions, optimized physical cues through improved fabrication methods remains to be addressed.
Silk nanofibers were recently assembled in aqueous solution, providing building units to fabricate neuro-conductive niches for peripheral nerve repair.[27] Through tuning the arrangement of the silk nanofibers under electrical fields, anisotropic hierarchical microstructures and gradients of physical cues were introduced to enhance the proliferation and migration of cells.[33,34] Different bioactive cargos, such as inhibitors, growth factors, and graphene nanoparticles were loaded into the materials for tissue regeneration,[24,29,35] with options to optimize the specific material features.[27] However, no silk nanofiber-based conduits with optimized physical cues have been developed to date to stimulate peripheral nerve regeneration.
In the present study, silk nanofibers with different concentrations were treated under electric fields to form aligned micro-nano hierarchical structures with optimized features and then used as nerve conduit fillers. These aligned hydrogels were introduced to flexible silk conduits to study nerve fiber growth and axonal extension. Both in vitro and in vivo results indicated that the fillers with both aligned nanofibers and anisotropic lamella chambers with optimal sizes supported the best functional recovery after PNI.
2. Results and Discussion
2.1. Fabrication of silk conduits with hierarchical silk nanofiber fillers
Conduits containing fillers with hierarchical anisotropic architectures stimulated axon extensions and functional recovery for the regeneratio of long nerve defects.[36–40] However, these technologies are usually time-consuming, complex and challenging to control. Here, a modular assembly strategy was used to fabricate nerve conduits with hierarchical anisotropic architectures, providing a flexible and controllable option (Scheme 1). Silk conduits and anisotropic silk nanofiber fillers were prepared through mold casting and electric fields. The structures and properties of the conduits and fillers were optimized before modular packaging.
Scheme 1.

Schematic fabrication of silk conduits with hierarchical silk nanofiber fillers. Module I and Module II showed the preparation process of the hollow silk conduits and the anisotropic silk nanofiber hydrogels. The silk conduits were prepared through coating the knitted silk tubes in the concentration process at 60°C while the aligned hydrogels were fabricated through treating silk nanofiber solutions under the electrical fields. Then silk conduits were inserted into the anisotropic hydrogels to assemble the conduits. After the freeze drying process, the nerve guidance conduit was prepared and used in the in vitro and in vivo studies.
Silk conduits have been prepared via multiple methods such as electrospinning, mold casting, membrane rolling, dipping and horseradish peroxidase (HRP) crosslinking.[3,31,41] The tubes prepared by mold casting, dipping and electrospinning usually have inferior mechanical properties. Membrane rolling and crosslinking methods improve these features, however, further improvement is necessary to optimize utility for nerve repair.[42,43] We have previously developed silk fiber-reinforced conduits for artificial blood vessels with good mechanical strength.[44] These silk fiber-reinforced conduits had good flexibility and controllable diameters, suggesting possible applications in peripheral nerve grafts. Therefore, silk fibers were twined on a metal rod mold (1.5 mm) and then immersed in silk solutions to prepare the conduits. Following treatment at 60°C, the silk fiber-reinforced tubes formed on the mold (Scheme 1). FTIR spectroscopy indicated a high beta-sheet content (42.8%±2.02) of the silk tubes due to the temperature-induced structural transformation (Figure 1A). The conduits had a thickness of about 150 μm with elongation at break of 21.5±0.02 % and a tensile stress of 6.7±0.64 MPa, suggesting suitability as nerve grafts (Figure 1B). Suture retention strength was about 3.8±0.9 N in the wet state, which met requirements.[44]
Figure 1.
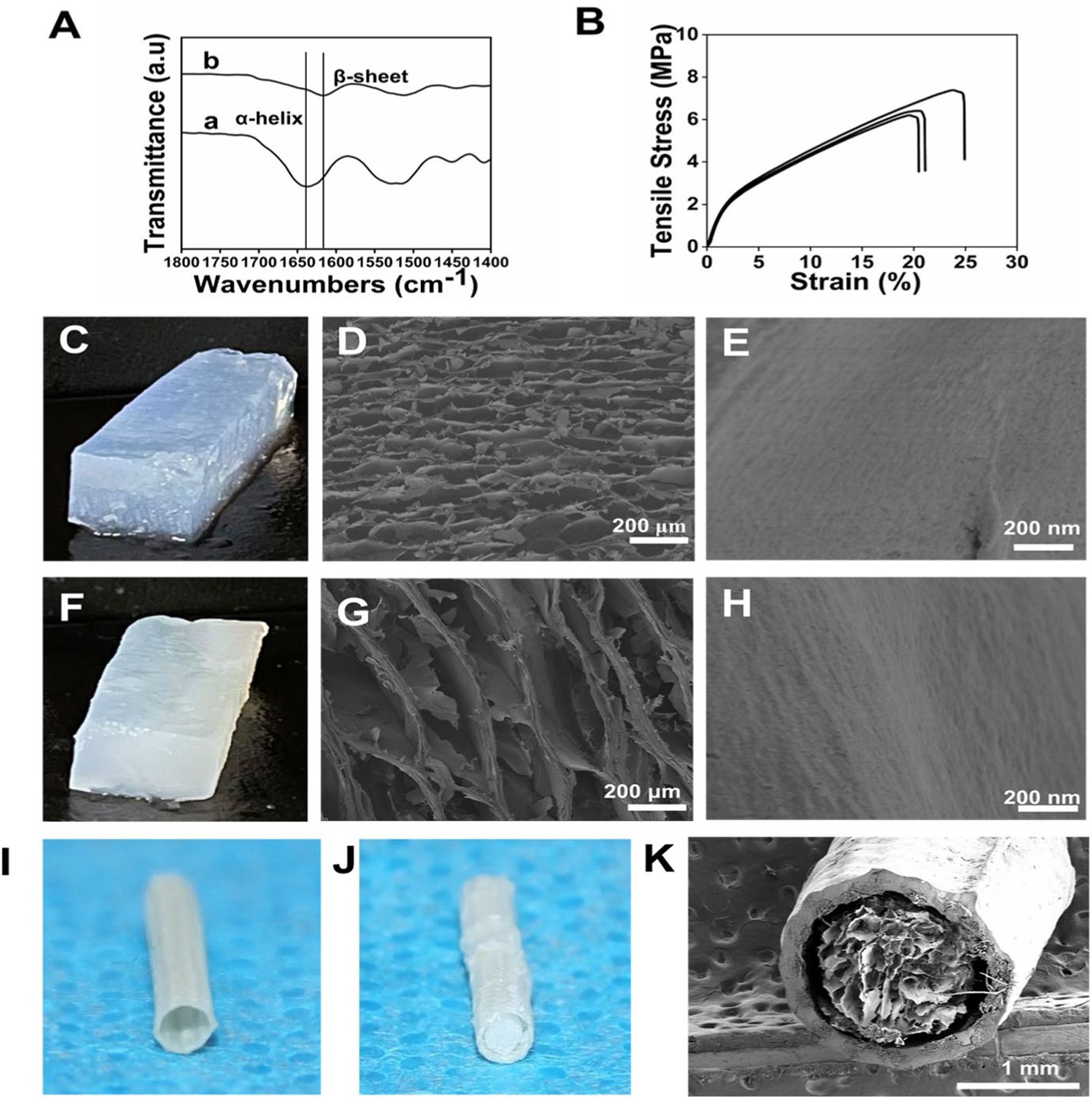
Fabrication of silk conduits with hierarchical silk nanofiber fillers: (A) FTIR analysis before (a) and after (b) treatment at 60°C; (B) Mechanical properties of hollow silk conduits; (C) Photographs of the aligned hydrogels derived from 1% silk nanofiber solutions; (D) SEM images of fillers derived from 1% silk nanofiber solutions; (E) High magnification images of the lamellae of fillers; (F) Photographs of the aligned hydrogels derived from 2% silk nanofiber solutions; (G) SEM images of fillers derived from 2% silk nanofiber solutions; (H) High magnification images of the lamellae of fillers; (I) Photograph of the hollow silk conduits; (J) Photograph of the silk conduits filled with the aligned hydrogels; (K) SEM images of silk conduits filled with silk nanofiber fillers.
Hollow conduits often have inferior regenerative capacity for large neural gaps.[45] Laminar fillers are an option to create neuro-conductive niches for better functional recovery.[46] The hierarchical structures of fillers, such as ECM-similar nanofibers, aligned topography and microarchitectures can influence axonal outgrowth and Schwann cell migration.[47] Tuning multiple physical cues is an effective strategy for the design of bioactive nerve conduits.[48–50] Electrospinning and unidirectional freeze drying have been applied to introduce filler alignment.[51,52] However, controlling luminal pore size without compromising anisotropy remains difficult for both electrospinning and unidirectional freeze drying.[49] Silk nanofibers composed of beta-sheet rich structures have negative charges with a zeta potential of −31.4±2.50 mv, endowing motility in electrical fields. These silk nanofibers migrated in an electric field to form hydrogels with hierarchical anisotropic structures with aligned lamellae (Figure 1 C, F).[27] The laminar spacing was tuned by changing the concentration of the silk nanofibers. Previous studies revealed that aligned pores with diameters of 50 μm facilitated axonal outgrowth and Schwann cell migration.[53,54] Silk nanofiber solutions with concentrations of 1% and 2% were used to fill the silk conduits, since the diameters of the lamellae inside the hydrogels were 50–80 μm and 100–200 μm, respectively.[33] Compared to previous porous silk-based fillers,[20] the introduction of optimized hierarchical aligned structures would facilitate better cell migration and nerve regeneration.
A stiffness of below 1 KPa provides suitable mechanical cues to induce neural differentiation and stimulate the migration of Schwann cells.[29,55] When the compressive force was orthogonal to the lamellae, the modulus of the aligned hydrogels from 1% and 2% silk nanofiber solutions were 0.63±0.14 and 0.89±0.16 KPa, respectively.[29] Therefore, the mechanical properties of these aligned hydrogels further strengthened the fillers in the peripheral nerve conduits. To fill the conduits, the hollow conduits were inserted into the aligned hydrogels along the direction of the lamellae. After freeze-drying, the guidance conduits containing hierarchically aligned sponge fillers were prepared (Figure 1K). SEM images revealed aligned lamellae structures with sizes of 50–80 μm and 100–200 μm, respectively (Figure 1D, G). High magnification images of the lamellae of the fillers from 1% and 2% silk nanofibers revealed anisotropic topography (Figure 1E, H).
2.2. In vitro biological response to the anisotropic silk nanofiber fillers
Previous studies revealed that anisotropic topographies played a critical role in inducing neural differentiation and stimulating the migration of Schwann cells,[37,45] which are important supportive cells for peripheral nerve regeneration. The Schwann cells were distributed homogeneously in the scaffolds (Figure 2A). The cells exhibited orientation along the parallel lamellae on the aligned fillers (ASFN-1 and ASFN-2) while homogeneously spread on isotropic porous silk nanofiber fillers (RSFN-1, RSFN-2) (Figure 2A). DNA content revealed sustained and improved proliferation on the aligned fillers, thus cytocompatibility. Although both anisotropic and isotropic fillers were composed of the same silk nanofibers, higher cell numbers were achieved on the anisotropic fillers, suggesting guidance by the aligned physical cues (Figure 2B). Previous studies suggested that aligned porous structures of 50 μm provided improved regulation of nerve cells.[53,54] Therefore, compared to ASFN-2 fillers, the cells on the ASFN-1 fillers proliferated better, possibly due to the size of the aligned porous structures.
Figure 2.

In vitro biological response to the anisotropic silk nanofiber fillers: (A) CLSM images of Schwann cells cultured on the different fillers for 1, 3, and 7 days; (B) Cell proliferation of different samples cultured for 1, 3, and 7 days; (C) Distribution of orientation angles of Schwann cell nuclei when cultured on the silk nanofiber fillers for 7 days; (D) ELISA of BDNF secreted by the cells on the silk nanofiber fillers (n = 3). RSFN-2, isotropic porous silk nanofiber fillers with silk concentration of 2 wt%, RSFN-1, isotropic porous silk nanofiber fillers with silk concentration of 1 wt%, ASFN-2, aligned porous silk nanofiber fillers with silk concentration of 2 wt%, ASFN-1, aligned porous silk nanofiber fillers with silk concentration of 1 wt%. Data presented as mean ± SD, n=3, the error bars indicate the SD, p-values are calculated using two-way ANOVA with Turkey’s multiple comparison tests (b) and one-way ANOVA with Sidak’s multiple comparison tests (c, d), *p < 0.05, **p < 0.01, and ***p < 0.001. NS means no statistical difference (P > 0.05).
Compared to RSFN-2 and RSFN-1 fillers, the orientation angle of Schwann cells on the ASFN-2 fillers and ASFN-1 fillers decreased (Figure 2C). Schwann cells support neurite extension via secreted growth factors. The expression of typical proteins such as brain-derived neurotrophic factor (BDNF) was investigated to evaluate the influence of the aligned physical cues on this factor. As hypothesized, higher expression of BDNF was found for the cells cultured on the aligned fillers, with the highest expression in the ASFN-1 group (Figure 2D). The axons of PC12 cells extended and aligned along the anisotropic lamellae on the ASFN-1 and ASFN-2 fillers, while homogeneous extensions of axons were observed for the cells cultured on the isotropic porous silk nanofiber fillers (Figure S2). Significantly longer axon extension was achieved for the ASFN-1 and ASFN-2 study groups, confirming the role of the aligned structures on axonal outgrowth (Figure S2A–2B). Previous silk fillers stimulated cell proliferation but showed inferior secretion of cytokines and axonal outgrowth.[32] Better proliferation, extension and secretion behaviors of the cells on the anisotropic fillers further confirmed that the aligned structures regulated the biological responses of nerve cells to facilitate neurite outgrowth.
2.3. Peripheral nerve regeneration in vivo
The neuro-inductive capacity of the silk nanofiber fillers suggested promising applications for peripheral nerve repair. Thus, the silk conduits containing the aligned porous silk nanofiber fillers were implanted into rat sciatic nerve defects (length 10 mm, diameter 1.5 mm) to assess nerve regeneration (Figure S3A–F).[1, 32] Compared to some previous nerve defect model,[56,57] the length of nerve defects here is bigger to avoid the unwanted self-healing behavior. Autografts were also implanted as positive controls. A total of 48 Sprague-Dawley (SD) rats were divided into 6 groups randomly, with 8 rats in each group. The samples were collected and analyzed at 4 and 12 weeks postoperatively.
All rats survived without adverse reactions during the in vivo experimental process. After 12 weeks, nerve-like tissues regenerated inside the conduits and bridged the two stumps without destruction of the lumens. The functional recovery of the sciatic nerve defects was investigated after 12 weeks of surgery. The sciatic function index (SFI) was used to evaluate motor function recovery of the damaged nerves.[46] Walking footprint photographs and SFI values of various groups are shown in Figure 3A. Gradual improvement of functional recovery was achieved following the introduction of the porous fillers. Autograft transplantation is the gold standard and supported the best nerve recovery.[50] The SFI for the autografts was −55.3, which decreased to −82 for the rats treated with hollow silk conduits. For the rats treated with ASFN-1, the SFI was −66.3 without a significant difference with the autograft group (Figure 3B). Electrophysiology was also used to assess functional recovery. The best motor nerve conduction velocity was obtained for the ASFN-1 group, which also compared well to the autografts (Figure 3C). The results confirmed that the conduits with the hierarchical anisotropic architectures provided the best neural inductive niche, superior to hollow conduits and to conduits with porous fillers without anisotropic structures.
Figure 3.

Evaluation of motor function restoration after 12 weeks: (A) Typical footprint photographs; (B) Quantitative analysis of SFI values; (C) Motor nerve conduction velocity (m/s). HC, hollow silk conduits, RSFN-2-C, the composite conduits of RSFN-2 with silk conduit, RSFN-1-C, the composite conduits of RSFN-1 with silk conduit, ASFN-2-C, the composite conduits of ASFN-2 with silk conduit, ASFN-1-C, the composite conduits of ASFN-1 with silk conduit. Data presented as mean ± SD, n=3, the error bars indicate the SD, p-values are calculated using one-way ANOVA with Sidak’s multiple comparison tests, *p < 0.05, **p < 0.01, and ***p < 0.001. NS means no statistical difference (P > 0.05).
Histological results were obtained by staining longitudinal sections of samples with hematoxylin-eosin (HE) after 12 weeks post-implantation. The regenerated nerves had extended into all the conduits and the transected stumps were also collected after 12 weeks. Compared to the silk nanofiber-filled conduits, significantly fewer tissues were found in the hollow conduits, indicating the stimulating role of the silk nanofiber fillers (Figure 4A). The extended tissues increased gradually in the RSFN-1, RSFN-2, ASFN-2, ASFN-1, respectively, due to the pores and aligned structures. Similar to previous studies,[53,54,58] the porous structures facilitated the ingrowth of nerves (RSFN-1 and RSFN-2) and the introduction of aligned structures further stimulated neurite extension (ASFN-2 and ASFN-1). The best tissue regeneration was achieved in the ASFN-1 groups due to the optimal neural inductive niche as also shown in vitro. The regeneration of nerve tissues in the ASFN-1 group was similar to that of the autograft group, further confirming the role of physical cues in directing nerve regeneration.
Figure 4.

HE staining of regenerated nerves: (A) Longitudinal HE staining of the graft segments at 12 weeks postoperatively; (B) transverse-sectioned nerve images from the middle section after 4 and 12 weeks postoperatively. Black arrows indicate red blood cells/vessels while green arrows point to regenerated nerve fibers. HC, hollow silk conduits, RSFN-2-C, the composite conduits of RSFN-2 with silk conduit, RSFN-1-C, the composite conduits of RSFN-1 with silk conduit, ASFN-2-C, the composite conduits of ASFN-2 with silk conduit, ASFN-1-C, the composite conduits of ASFN-1 with silk conduit.
HE stained images of cross sections obtained from the middle positions of the conduits revealed the quality of the nerves (Figure 4B). At 12 weeks postoperatively, few inflammatory cells were found in all groups, indicating the biocompatibility of the silk-based materials. Although a significantly higher density of blood vessels appeared in the hollow conduits, RSFN-2 and RSFN-1 groups, only a few nerve fibers appeared and were sparse in the middle area, while other regions were occupied by connective tissue (Figure 4B). The results suggested inferior nerve regeneration in these groups. Significantly higher ratios of the regenerated nerve fibers were found in the groups with aligned silk nanofiber fillers, where the highest amount of bundles of myelinated nerve fibers was achieved in the ASFN-1 group. Interestingly, compared to the hollow conduit group and RSFN group, the regenerated tissues in the ASFN-1 group were similar with the autograft group, where higher ratios of nerve bundles were accompanied with fewer blood vessels. Previous studies suggested the positive influence of angiogenesis on nerve regeneration.[59,60] The conduits collected after 4 weeks post-implantation were also stained by HE to study the angiogenesis at early regeneration stages. In contrast to 12 weeks, better vascularization appeared in the ASFN-1 group, suggesting that the ASFN-1 conduits provided a better microenvironment to tune angiogenesis and nerve regeneration in vivo, achieving nerve tissue extension comparable to autologous nerve transplantation. Although further study is necessary to reveal the relationships of angiogenesis and nerve regeneration in the system, similar angiogenesis behavior was found in wound healing where early angiogenesis facilitated ingrowth of granulation tissue, while excessive angiogenesis at later healing stage resulted in scar formation.[24,61] Our present results suggest the possibility of improving peripheral nerve repair through tuning angiogenesis in the regenerative process.
Soluble protein 100 and Neurofilament 200 (S-100 and NF-200) are typical markers of Schwann cells and neurons in which the amount of S-100 represents the functions of Schwann cells and the maturation of the nerves, while NF-200 is an indicator of neuronal size and conduction velocity.[62] Similar to the HE staining results, gradually higher positive expression of S-100 and NF-200 proteins appeared in the hollow conduits, RSFN-1, RSFN-2, ASFN-2 and ASFN-1 groups, respectively. The density and distribution of both S-100 and NF-200 proteins in the ASFN-1 groups was similar to that in the autograft group (Figure 5A–C). The results further indicated the best functional recovery of peripheral nerves in the ASFN-1 group. Statistical analysis of the S-100 and NF-200 positive areas confirmed this, without a significant difference with the autograft group (Figure 5B–5C), revealing the neural inductive capacity of the hierarchical anisotropic architectures in the ASFN-1 study group.
Figure 5.

Immunohistochemical staining of regenerated nerves at 12 weeks postoperatively: (A) S-100 and NF-200 staining of regenerated nerve sections; (B) Percentage of S-100 positive area; (C) Percentage of NF-200 positive area. HC, hollow silk conduits, RSFN-2-C, the composite conduits of RSFN-2 with silk conduit, RSFN-1-C, the composite conduits of RSFN-1 with silk conduit, ASFN-2-C, the composite conduits of ASFN-2 with silk conduit, ASFN-1-C, the composite conduits of ASFN-1 with silk conduit. Data presented as mean ± SD, n=3, the error bars indicate the SD, p-values are calculated using one-way ANOVA with Sidak’s multiple comparison tests, *p < 0.05, **p < 0.01, and ***p < 0.001. NS means no statistical difference (P > 0.05).
Peripheral nerve transection usually results in gastrocnemius muscle atrophy including muscle fiber shrinkage, collagen fiber proliferation and a decline in muscle weight.[63] Masson’s trichrome staining was used to visualize muscle fibers (red) and collagen fibers (blue) of the gastrocnemius muscle after 12 weeks post-implantation (Figure 6A). A significant degeneration of gastrocnemius muscle as well as an increase of collagen fibers were found in the hollow conduits, RSFN-1 and RSFN-2 groups, suggesting deficits in the regeneration of the sciatic nerve. Muscle atrophy was reduced in the ASFN-1 and ASFN-2 groups, since anisotropic SFN fillers stimulated nerve regeneration. The morphology of muscle tissues in the ASFN-1 group remained similar to that in the autograft group, suggesting the best functional recovery of the transected nerves with this study group (Figure 6B). The weight decline of the gastrocnemius muscle in the different groups exhibited the same tendency as observed with the Masson’s staining results. Aside from the autograft group, the ASFN-1 group maintained the highest muscle weight (Figure 6C).
Figure 6.

Microstructural evaluation of the gastrocnemius muscles on the injured side and cross sections of the regenerated nerves 12 weeks postoperatively: (A) Masson’s trichrome staining of the gastrocnemius muscles; (B) Average percentage of collagen fiber area on the injury side; (C) Wet weight of gastrocnemius muscles on the injured side; (D) TEM of sections located at the middle section of the defects; (E) Diameter of the myelinated axons; (F) Thickness of myelin sheath. HC, hollow silk conduits, RSFN-2-C, the composite conduits of RSFN-2 with silk conduit, RSFN-1-C, the composite conduits of RSFN-1 with silk conduit, ASFN-2-C, the composite conduits of ASFN-2 with silk conduit, ASFN-1-C, the composite conduits of ASFN-1 with silk conduit. Data presented as mean ± SD, n=3, the error bars indicate the SD, p-values are calculated using one-way ANOVA with Sidak’s multiple comparison tests, *p < 0.05, **p < 0.01, and ***p < 0.001. NS means no statistical difference (P > 0.05).
The structure of the regenerated nerve fibers, including the thickness of the myelin sheath and axon diameters are important indicators of regenerated nerve quality.[64] The best nerve regeneration was in the ASFN-1 group and these were investigated with TEM to examine nerve microstructures. The hollow conduit and autograft groups were studied as negative and positive controls, respectively (Figure 6D–F). Both axon diameter and thickness of the myelin sheath in the ASFN-1 group were significantly larger than that in the hollow conduits and RSFN-1 groups. The TEM results also suggested that the microstructure of the regenerated nerve fibers in the ASFN-1 group were comparable to the autograft group.
3. Conclusion
Optimizing multiple physical and biological cues is feasible to develop peripheral nerve conduits with comparable regenerative capacity to autografts. However, neural inductive biomaterials with multiple cues are challenging to prepare due to the interplay of these different features during the device fabrication process. The modular assembly strategy used here provides an option to achieve this goal with significant control. Silk nanofiber fillers with hierarchical anisotropic structures were developed and then assembled with the silk-based conduits, thus a combination of two key fabrication steps. Functional nerve restoration comparable to autografts demonstrated the value of this modular assembly strategy. Considering the versatility of silk nanofibers as carriers of various cargos,[65,66] additional physical and biological cues can be introduced into the conduits towards even more versatile conduits in the future, to potentially provide suitable replacement options to autografts.
4. Experimental Section
Preparation of silk Solutions:
Silk solution was prepared through a traditional LiBr solvent system.[27] Silk fibers were degummed in Na2CO3 solution (0.02 M) at 100°C for 20 min. After rinsing and drying treatments, the degummed fibers were dissolved in LiBr solution (9.3 M) at 60°C for 4h and then dialyzed against distilled water for 72h. After centrifugation twice at 9,000 rpm for 20 min, aqueous silk solutions with a concentration of about 6 wt% were obtained and stored at 4°C for further use.
Fabrication of Hollow Silk Conduits:
The silk conduits were produced via a mesh-reinforced tube fabrication method.[44] The silk yarn mesh tubes were braided around a diameter of 1.5 mm stainless steel mold with a 2/2 pattern at a take-up rate of 1.2 m min−1 on a high-speed rope braiding machine (JC 2–24, Zhangjiagang Textile Machinery Co., Ltd, Jiaxing, People’s Republic of China). After degumming with Na2CO3 solution, the mesh tubes were nested inside silk solution vertically to form the mesh-reinforced tubes directly after the evaporation of silk solution at 60°C, and silk fiber-reinforced tubes formed on the mold automatically.
Fabrication of Aligned Silk Nanofiber Hydrogels:
The 6 wt% silk solution was concentrated to 20 wt% at 60°C, and then diluted to 1 wt% and 2 wt% at 60°C until silk nanofiber formation.[23] The silk nanofibers (1 wt% and 2 wt%) were treated at 50V DC for 30 min and termed ASFN-1 and ASFN-2. The 1 wt% and 2 wt% silk nanofibers without electric field were prepared and termed RSFN-1 and RSFN-2.
Module Assembly of Silk Conduits:
The hollow silk conduits were inserted into the silk nanofiber hydrogels along the direction of the aligned lamellae to embed the hydrogel inside the conduit directly. The composite silk conduits were then prepared after lyophilization under −80°C for 48 h. According to the silk concentration of fillers, the composite conduits were termed ASFN-1-C and ASFN-2-C. As controls, homogeneous silk nanofiber hydrogels without electric field treatment were also embed inside the conduits and termed RSFN-1-C and RSFN-2-C.
Characterization of Silk Conduits:
Fourier transform infrared spectroscopy (FTIR, Nicolet FTIR 5700, Thermos Scientific, FL, USA) was used to evaluate the secondary conformations, the beta-sheet contents were measured using PeakFit software. The mechanical properties of the hollow conduits were evaluated with a tensile testing machine (Instron 5967, Instron, Norwood, MA). The tensile properties of the wet conduits (length 3 cm) were measured at a crosshead speed of 20 mm min−1 with a 500 N load cell. The suture retention strength was also evaluated based on the reported method[44]. One end of a wet conduit was clamped and 8–0 polyester suture (BV-130–5 needle, Ethicon, Somerville, U.K.) was passed through the conduit, 2 mm from the edge. The suture was pulled at a rate of 20 mm min−1.
Zeta Potential of Silk Nanofibers:
Zeta potential was obtained with a Zetasizer (Nano ZS, Malvern, Worcestershire, U.K.) at 25°C.
Scanning Electron Microscopy (SEM) of the Silk Nanofiber Fillers:
Scanning electron microscopy (SEM, S-4800, Hitachi, Tokyo, Japan) was performed to measure the microstructure of the silk nanofiber fillers structures. The samples were coated with gold and measured at 3 kV.
Morphology of Schwann Cell Cultured in the Silk Nanofiber Fillers:
Schwann cells were purchased from Shanghai Institutes for Biological Science (Shanghai, People’s Republic of China), and cultured with DMEM containing 10% FBS and 1% penicillin-streptomycin. Before cell culture, different silk nanofiber fillers (ASFN-1, ASFN-2, RSFN-1, RSFN-2) were sterilized with 60Co γ-irradiation at 25 kGy. Schwann cells (5.0 × 104 cells per well) were seeded in the fillers and cultured for 1, 3 and 7 days.
The collected samples were stained with FITC-phalloidin and DAPI. CLSM (Confocal laser scanning microscope, Olympus FV10 inverted microscope, Nagano, Japan) was used to examine cell morphology. At least 50 cells were measured using ImageJ software to calculate the orientation angle of cell nucleus. The proliferation of Schwann cells was evaluated by DNA content using the Omega’s Tissue DNA kit following the manufacturer protocols. The extracted DNA content was measured at an excitation wavelength of 480 nm and emission wavelength of 530 nm using a BioTeK spectrofluorometer (BioTeK Synergy 4, Winooski, VT, USA) and calculated based on a standard curve. Schwann cells were seeded in the different fillers at 5.0 × 104 cells per well and cultured for 2 days. The supernatant of each group was collected for BDNF (Brain-derived neurotrophic factor) measurement. The concentrations of BDNF in the supernatant were examined using ELISA (Rat BDNF PicoKine ELISA Kit, EK0308, Boster, Wuhan, People’s Republic of China). The absorbance at 450 nm was examined using a BioTeK spectrofluorometer (BioTeK Synergy 4, Winooski, VT, USA). The amount of BDNF was calculated based on a standard curve and the sample measurements were at least N=3.
The Morphology of PC12 Cultured in the Silk Nanofiber Fillers:
PC12 cells purchased from Shanghai Institutes for Biological Science (Shanghai, People’s Republic of China) were cultured with RPMI 1640 medium containing 10% FBS and 1% penicillin-streptomycin. PC12 cells were seeded in the different fillers at 3.0×104 cells per well and cultured for 1 and 5 days. The samples were stained with FITC-phalloidin and DAPI and measured using CLSM (Confocal laser scanning microscope, Olympus FV10 inverted microscope, Nagano, Japan). More than 20 cells were measured to calculate the spread length of PC12 using ImageJ software.
Surgical Procedures:
All animal experiments were approved by the Animal Care and Experiment Committee of the Soochow University. Briefly, 48 male Sprague Dawley rats, weighing 200–220 g, were randomized to six groups (8 rats per group): HC Group (Hollow silk conduits), RSFN-2-C group, RSFN-1-C, ASFN-1-C, ASFN-2-C, and autograft group. After anaesthetization with 1 % sodium pentobarbital solution, the right sciatic nerve was carefully exposed to produce a 10 mm nerve defect. The proximal and distal ends of the injured nerve were bridged with the different nerve conduits and sutured with 8–0 suture. For the autograft group, the 10 mm transected nerve segment was sutured in place with 8–0 suture. After implantation of the conduits, the muscle and skin were sutured in layers with 4–0 sutures. All rats were raised in a constant temperature 25°C and 60% humidity room after surgery.
Behavior Analysis:
After 12 weeks post-implantation, walking track analysis was performed to evaluate the limb functional recovery.[62] The SFI values were obtained based on the following formula: SFI=(−38.3×(EPL-NPL)÷NPL)+(109.5×(ETS-NTS)÷NTS)+(13.3×(EIT-NIT)÷NIT)−8.8. TS, PL and IT were the distance between the first toe and the fifth toe, from the third toe to the heal, and from the second toe to the fourth toe, respectively. These indexes were measured and calculated based on experimental legs (E) and normal legs (N). The SFI value close to −100 indicates total loss of function while that near 0 represents normal nerve function.
Electrophysiology:
The electrophysiological properties of the regenerated sciatic nerves was measured after 12 weeks post-surgery. After anesthetization with 1% sodium pentobarbital solution an electrical stimulator was placed at distal and proximal nerve graft, and stimulation current was adjusted at 3.0 mA. The motor nerve conduction velocity was evaluated using Electromyography (Keypoint, Medtronic, Minneapolis, USA).
Histological Analyses and Immunohistochemical Staining:
At 4 and 12 weeks after the conduit implantation, regenerated sciatic nerves were collected, fixed with 4% paraformaldehyde, embedded in paraffin and sectioned in 8 μm thick slices for histological analysis and immunohistochemical staining. According to procedures reported previously,[60] the slices were stained with H&E and different primary antibodies (Mouse anti NF-200 antibody, 1:200, GB13141 and Rabbit anti S-100 antibody, 1:500, GB11397), respectively. The images of the stained slices were recorded with an inverted microscope (Axio Vert A1, Carl Zeiss, Oberkochen, Germany). ImageJ software was used to evaluate the expression of the antigens. Masson’s trichrome stained sections were used to evaluate collagen synthesis and were prepared according to previous studies.[48] At week 12 after surgery, the bilateral gastrocnemius of rats in each group were excised and weighed to calculate wet weight recovery ratio (injured side/normal side). Transmission Electron Microscopy (TEM, Hitachi, HT7700, Ibaraki, Japan) was also used to analyze the regenerated nerves after 12 weeks post-implantation. Ultrathin sections (thickness: 50.0 nm) were cut from the middle portion of the regenerated nerves and stained with lead citrate and uranyl acetate. The diameter of myelinated nerve fibers and the thickness of myelinated sheath were measured with ImageJ software.
Statistics:
All data were statistically analyzed by using SPSS v.16.0 software (IBM SPSS Statistics, US). One-way AVOVA was used to compare differences between groups. Comparisons between two groups were evaluated by t-test. Values are presented as means ± standard deviations. *P < 0.05, ** P < 0.01, and *** P < 0.001 were considered to be significant.
Supplementary Material
Acknowledgements
Q. Lu, F. Zhang and W. Cheng have the same contribution to the work. The authors thank the National Key R&D Program of China (2016YFE0204400), and the NIH (P41EB027062, R01NS094218).
Footnotes
Conflict of Interest
All authors have no financial/commercial Conflict of Interest.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Weinan Cheng, Department of Orthopedics, The First Affiliated Hospital of Xiamen University, Xiamen 361000, P. R. China.
David L. Kaplan, Department of Biomedical Engineering, Tufts University, Medford, Massachusetts 02155, United States
References
- [1].Quan Q, Meng H, Chang B, Hong L, Li R, Liu G, Cheng X, Tang H, Liu P, Sun Y, Peng J, Zhao Q, Wang Y, Lu S, Biomaterials 2019, 207, 49. [DOI] [PubMed] [Google Scholar]
- [2].Arslantunali D, Dursun T, Yucel D, Hasirci N, Hasirci V, Med. Devices-Evid. Res 2014, 7, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Giannaccini M, Calatayud MP, Poggetti A, Corbianco S, Novelli M, Paoli M, Battistini P, Castagna M, Dente L, Parchi P, Lisanti M, Cavallini G, Junquera C, Goya GF, Raffa V, Adv. Healthcare Mater 2017, 6, 1601429. [DOI] [PubMed] [Google Scholar]
- [4].Li X, Zhang Q, Luo Z, Yan S, You R, Biointerphases 2019, 14, 061001. [DOI] [PubMed] [Google Scholar]
- [5].Wang GW, Yang H, Wu WF, Zhang P, Wang JY, Biomaterials 2017, 131, 145. [DOI] [PubMed] [Google Scholar]
- [6].Zhang W, Zhang L, Liu J, Zhang L, Zhang J, Tang P, Biomaterials 2017, 142, 90. [DOI] [PubMed] [Google Scholar]
- [7].Bu Y, Wang X, Li L, Hu X, Tan D, Li Z, Lai M, Qiu X, Sun F, Wang H, Yang F, Wu D, Guo J, Adv. Healthcare Mater 2020, 9, 2000268. [DOI] [PubMed] [Google Scholar]
- [8].Burnstine-Townley A, Eshel Y, Amdursky N, Adv. Funct. Mater 2019, 30, 1901369. [Google Scholar]
- [9].Chen X, Ge X, Qian Y, Tang H, Song J, Qu X, Yue B, Yuan WE, Adv. Funct. Mater 2020, 30, 2004537. [Google Scholar]
- [10].Li R, Li DH, Zhang HY, Wang J, Li XK, Xiao J, Acta. Pharmacol. Sin 2020, 41, 1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang Z, Jorgensen ML, Wang Z, Amagat J, Wang Y, Li Q, Dong M, Chen M, Biomaterials 2020, 253, 120108. [DOI] [PubMed] [Google Scholar]
- [12].Eftekhari BS, Eskandari M, Janmey PA, Samadikuchaksaraei A, Gholipourmalekabadi M, Adv. Funct. Mater 2020, 30, 1907792. [Google Scholar]
- [13].Kim JI, Hwang TI, Lee JC, Park CH, Kim CS, Adv. Funct. Mater 2019, 30, 1907330. [Google Scholar]
- [14].Broguiere N, Luchtefeld I, Trachsel L, Mazunin D, Rizzo R, Bode JW, Lutolf MP, Zenobi-Wong M, Adv. Mater 2020, 32, 1908299. [DOI] [PubMed] [Google Scholar]
- [15].Carvalho CR, Costa JB, da Silva Morais A, Lopez-Cebral R, Silva-Correia J, Reis RL, Oliveira JM, Adv. Healthcare Mater 2018, 7, 1800186. [DOI] [PubMed] [Google Scholar]
- [16].Joung D, Lavoie NS, Guo SZ, Park SH, Parr AM, McAlpine MC, Adv. Funct. Mater 2020, 30, 1906237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kasper M, Deister C, Beck F, Schmidt CE, Adv. Healthcare Mater 2020, 9, 2000174. [DOI] [PubMed] [Google Scholar]
- [18].Kato-Negishi M, Onoe H, Ito A, Takeuchi S, Adv. Healthcare Mater 2017, 6, 1700143. [DOI] [PubMed] [Google Scholar]
- [19].Kim BJ, Park M, Park JH, Joo S, Kim MH, Kang K, Choi IS, Adv. Healthcare Mater 2018, 7, 1800289. [DOI] [PubMed] [Google Scholar]
- [20].Rao F, Yuan Z, Li M, Yu F, Fang X, Jiang B, Wen Y, Zhang P, Artif. Cells Nanomed. Biotechnol 2019, 47, 491. [DOI] [PubMed] [Google Scholar]
- [21].Pawelec KM, Yoon C, Giger RJ, Sakamoto J, Biomaterials 2019, 216, 119263. [DOI] [PubMed] [Google Scholar]
- [22].Jiang H, Qian Y, Fan C, Ouyang Y, Front. Bioeng. Biotechnol 2020, 8, 582646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bai S, Han H, Huang X, Xu W, Kaplan DL, Zhu H, Lu Q, Acta Biomater. 2015, 20, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ding Z, Zhou M, Zhou Z, Zhang W, Jiang X, Lu X, Zuo B, Lu Q, Kaplan DL, ACS Biomater. Sci. Eng 2019, 5, 4077. [DOI] [PubMed] [Google Scholar]
- [25].Ding Z, Ma J, He W, Ge Z, Lu Q, Kaplan DL, J. Mater. Chem. B 2017, 5, 4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Han F, Liu S, Liu X, Pei Y, Bai S, Zhao H, Lu Q, Ma F, Kaplan DL, Zhu H, Acta Biomater. 2014, 10, 921. [DOI] [PubMed] [Google Scholar]
- [27].Lu Q, Bai S, Ding Z, Guo H, Shao Z, Zhu H, Kaplan DL, Adv. Mater. Interfaces 2016, 3, 1500687. [Google Scholar]
- [28].Li S, Hang Y, Ding Z, Lu Q, Lu G, Chen H, Kaplan DL, ACS Biomater. Sci. Eng 2020, 6, 2847. [DOI] [PubMed] [Google Scholar]
- [29].Wang L, Song D, Zhang X, Ding Z, Kong X, Lu Q, Kaplan DL, ACS Biomater. Sci. Eng 2018, 5, 613. [DOI] [PubMed] [Google Scholar]
- [30].Zhao Y, Liang Y, Ding S, Zhang K, Mao H, Yang Y, Biomaterials 2020, 255, 120164. [DOI] [PubMed] [Google Scholar]
- [31].Chang W, Shah MB, Zhou G, Walsh K, Rudraiah S, Kumbar SG, Yu X, Biomed. Mater 2020, 15, 035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Magaz A, Faroni A, Gough JE, Reid AJ, Li X, Blaker JJ, Adv. Healthcare Mater 2018, 7, 1800308. [DOI] [PubMed] [Google Scholar]
- [33].Wang L, Lu G, Lu Q, Kaplan DL, ACS Biomater. Sci. Eng 2018, 4, 933. [DOI] [PubMed] [Google Scholar]
- [34].Xu G, Ding Z, Lu Q, Zhang X, Zhou X, Xiao L, Lu G, Kaplan DL, Protein Cell 2020, 11, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cheng W, Ding Z, Zheng X, Lu Q, Kong X, Zhou X, Lu G, Kaplan DL, Biomater. Sci 2020, 8, 2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hu X, Wang X, Xu Y, Li L, Liu J, He Y, Zou Y, Yu L, Qiu X, Guo J, Adv. Healthcare Mater 2020, 9, 1901570. [DOI] [PubMed] [Google Scholar]
- [37].Li A, Hokugo A, Yalom A, Berns EJ, Stephanopoulos N, McClendon MT, Segovia LA, Spigelman I, Stupp SI, Jarrahy R, Biomaterials 2014, 35, 8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dixon AR, Jariwala SH, Bilis Z, Loverde JR, Pasquina PF, Alvarez LM, Biomaterials 2018, 186, 44. [DOI] [PubMed] [Google Scholar]
- [39].Wang J, Xiong H, Zhu T, Liu Y, Pan H, Fan C, Zhao X, Lu WW, ACS Nano 2020, 14, 12579. [DOI] [PubMed] [Google Scholar]
- [40].Yang L, Jurczak KM, Ge L, Rijn P, Adv. Healthcare Mater 2020, 9, 2000117. [DOI] [PubMed] [Google Scholar]
- [41].Rose JC, Laporte LD, Adv. Healthare Mater 2018, 7, 1701067. [Google Scholar]
- [42].Schilling C, Mack T, Lickfett S, Sieste S, Ruggeri FS, Sneideris T, Dutta A, Bereau T, Naraghi R, Sinske D, Knowles TPJ, Synatschke CV, Weil T, Knöll B, Adv. Funct. Mater 2019, 29, 1809112. [Google Scholar]
- [43].Shahriari D, Loke G, Tafel I, Park S, Chiang PH, Fink Y, Anikeeva P, Adv. Mater 2019, 31, 1902021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu S, Dong C, Lu G, Lu Q, Li Z, Kaplan DL, Zhu H, Acta Biomater. 2013, 9, 8991. [DOI] [PubMed] [Google Scholar]
- [45].Singh A, Asikainen A, Teotia AK, Shiekh PA, Huotilainen E, Qayoom I, Partanen J, Seppala J, Kumar A, ACS Appl. Mater. Interfaces 2018, 10, 43327. [DOI] [PubMed] [Google Scholar]
- [46].Sun B, Zhou Z, Wu T, Chen W, Li D, Zheng H, El-Hamshary H, Al-Deyab SS, Mo X, Yu Y, ACS Appl. Mater. Interfaces 2017, 9, 26684. [DOI] [PubMed] [Google Scholar]
- [47].Marcus M, Baranes K, Park M, Choi IS, Kang K, Shefi O, Adv. Healthcare Mater 2017, 6, 1700267. [DOI] [PubMed] [Google Scholar]
- [48].Li X, Yang W, Xie H, Wang J, Zhang L, Wang Z, Wang L, ACS Appl. Mater. Interfaces 2020, 12, 36860. [DOI] [PubMed] [Google Scholar]
- [49].Lackington WA, Ryan AJ, O’Brien FJ, ACS Biomater. Sci. Eng 2017, 3, 1221. [DOI] [PubMed] [Google Scholar]
- [50].Wang S, Kempen DHR, De Ruiter GCW, Cai L, Spinner RJ, Windebank AJ, Yaszemski MJ, Lu L, Adv. Funct. Mater 2015, 25, 2715. [Google Scholar]
- [51].Dewle A, Pathak N, Rakshasmare P, Srivastava A, ACS Biomater. Sci. Eng 2020, 6, 779. [DOI] [PubMed] [Google Scholar]
- [52].Puhl DL, Funnell JL, Nelson DW, Gottipati MK, Gilber RJ, Bioengineering 2020, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wieringa PA, de Pinho ARG, Micera S, van Wezel RJA, Moroni L, Adv. Healthcare Mater 2018, 7, 1701164. [DOI] [PubMed] [Google Scholar]
- [54].Hu X, Huang J, Ye Z, Xia L, Li M, Lv B, Shen X, Luo Z, Tissue Eng. Part A 2009, 15, 3297. [DOI] [PubMed] [Google Scholar]
- [55].Fuhrmann T, Anandakumaran PN, Shoichet MS, Adv. Healthcare Mater 2017, 6, 1601130. [DOI] [PubMed] [Google Scholar]
- [56].Zhang SJ, Wu WL, Yang KY, Chen YZ, Liu HC, Neural Regen. Res 2017, 12, 1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang C, Zhang P, Wang Y, Yu K, Kou Y, Jiang B, Artif. Cells Blood Substit. Immobil. Biotechnol 2010, 38, 103. [DOI] [PubMed] [Google Scholar]
- [58].Lutzweiler G, Barthes J, Koenig G, Kerdjoudj H, Mayingi J, Boulmedais F, Schaaf P, Drenckhan W, Vrana NE, ACS Appl. Mater. Interfaces 2019, 11, 19819. [DOI] [PubMed] [Google Scholar]
- [59].Grasman JM, Ferreira JA, Kaplan DL, Adv. Funct. Mater 2018, 28, 1803822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhu L, Wang K, Ma T, Huang L, Xia B, Zhu S, Yang Y, Liu Z, Quan X, Luo K, Kong D, Huang J, Luo Z, Adv. Healthcare Mater 2017, 6, 1600860. [DOI] [PubMed] [Google Scholar]
- [61].Han H, Ning N, Liu S, Lu Q, Fan Z, Lu H, Lu G, Kaplan DL, Adv. Funct. Mater 2016, 26, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hou Y, Wang X, Zhang Z, Luo J, Cai Z, Wang Y, Li Y, Adv. Healthcare Mater 2019, 8, 1900913. [DOI] [PubMed] [Google Scholar]
- [63].Xie H, Yang W, Chen J, Zhang J, Lu X, Zhao X, Huang K, Li H, Chang P, Wang Z, Wang L, Adv. Healthcare Mater 2015, 4, 2195. [DOI] [PubMed] [Google Scholar]
- [64].Zou JL, Liu S, Sun JH, Yang WH, Xu YW, Rao ZL, Jiang B, Zhu QT, Liu XL, Wu JL, Chang C, Mao HQ, Ling EA, Quan DP, Zeng YS, Adv. Funct. Mater 2018, 28, 1705739. [Google Scholar]
- [65].Zhang X, Zhang Z, Xiao L, Ding Z, He J, Lu G, Lu Q, Kaplan DL, Biomacromolecules 2020, 21, 1022. [DOI] [PubMed] [Google Scholar]
- [66].Zheng X, Ding Z, Cheng W, Lu Q, Kong X, Zhou X, Lu G, Kaplan DL, Adv. Healthcare Mater 2020, 9, 2000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


