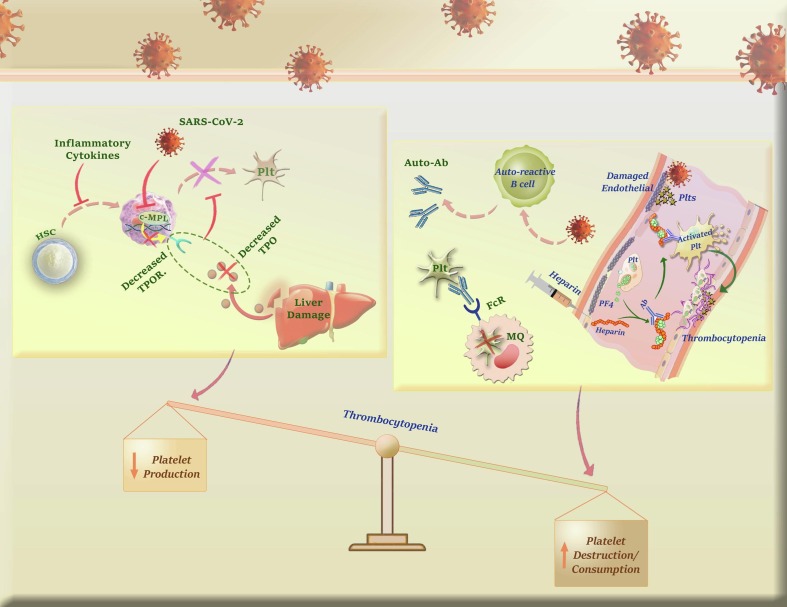
Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Thrombocytopenia, Platelet, Pathophysiology, Prognosis
Abbreviations: ACE2, Angiotensin-converting enzyme 2; MPA, Monocyte-platelet aggregate; AMI, Acute myocardial infarction; MPR, Mean platelet volume/Platelet count ratio; Ang-1, Angiopoietin 1; MPV, Mean platelet volume; APAS, Antiphospholipid antibody syndrome; NPA, Neutrophil–platelet aggregate; APLA, Anti-phospholipid antibodies; PDW, Platelet distribution width; BM, Bone marrow; PE, Pulmonary embolism; BSH, British Society of Hematology; P-LCR, Platelet-large cell ratio; CFU, Colony-forming unit; PLT, Platelet; CVC, Cenicriviroc; PRRs, Pattern recognition receptors; DIC, Disseminated intravascular coagulation; PT, prothrombin time; DRVVT, Dilute Russell's viper venom time; PTT, Partial thromboplastin time; DVT, Deep vein thrombosis; RP, Reticulated platelet; ECMO, Extracorporeal membrane oxygenation; S protein, Spike protein; FDA, Food and drug administration; SARS-CoV-2, Severe acute respiratory syndrome–related coronavirus; FV, Factor V; sICAM-1, Soluble intercellular adhesion molecule 1; HCV, Hepatitis C virus; sIL-6R, Soluble IL-6R; HIT, Heparin-induced thrombocytopenia; SIV, Simian immunodeficiency virus; HIV, Human immunodeficiency virus; SOCS1, Cytokine signaling 1; hrsACE2, Human recombinant soluble ACE2; SOFA, Sequential Organ Failure Assessment; HSCs, Hematopoietic stem cells; SP, Severe pneumonia; HPCs, Hematopoietic progenitor cells; sP-selectin, soluble form; HUS, Hemolytic-uremic syndrome; TCM, Traditional Chinese medicine; ICU, Intensive care unit; TCZ, Tocilizumab; IFN-α, Interferon alpha; TGF-β, Transforming growth factor beta; IL-6, Interleukin 6; TLR7, Toll-like receptor 7; IPF, Immature platelet fraction; TMA, Thrombotic microangiopathy; ITP, Immune thrombocytopenic purpura; TNF-α, Tumor necrosis factor alpha; IVIg, Intravenous immunoglobulin; TPO, Thrombopoietin; JYS, Jianpi Yiqi Shexue; TPOR, TPO receptor; LA, Lupus anticoagulants; TPO-RAs, TPO receptor agonists; mAbs, Monoclonal antibodies; TTP, Thrombotic thrombocytopenic purpura; MK, Megakaryocyte; VE, Vascular endothelial; MLP, Myeloproliferative leukemia protein; VEGF, Vascular endothelial growth factor
Abstract
Despite endorsed and exponential research to improve diagnostic and therapeutic strategies, efforts have not yet converted into a better prospect for patients infected with the novel coronavirus (2019nCoV), and still, the name of SARS-CoV-2 is coupled with numerous unanswered questions. One of these questions is concerning how this respiratory virus reduces the number of platelets (PLTs)? The results of laboratory examinations showed that about a quarter of COVID-19 cases experience thrombocytopenia, and more remarkably, about half of these patients succumb to the infection due to coagulopathy. These findings have positioned PLTs as a pillar in the management as well as stratifying COVID-19 patients; however, not all the physicians came into a consensus about the prognostic value of these cells. The current review aims to unravel the contributory role of PLTs s in COVID-19; and also to summarize the original data obtained from international research laboratories on the association between COVID-19 and PLT production, activation, and clearance. In addition, we provide a special focus on the prognostic value of PLTs and their related parameters in COVID-19. Questions on how SARS-CoV-2 induces thrombocytopenia are also responded to. The last section provides a general overview of the most recent PLT- or thrombocytopenia-related therapeutic approaches. In conclusion, since SARS-CoV-2 reduces the number of PLTs by eliciting different mechanisms, treatment of thrombocytopenia in COVID-19 patients is not as simple as it appears and serious cautions should be considered to deal with the problem through scrutiny awareness of the causal mechanisms.
1. Introduction
It has been about a year and a half that the COVID-19 disaster paralyzed the health care system. The first attempts to obviate the disease were more centered around how to treat the disease, but the failure in this field indicated that SARS-CoV-2 is more than a respiratory virus that can be easily treated with common anti-viral agents. These failures together with the delay in vaccine production caused us to give a closer look at COVID-19 pathogenesis so that a window would be opened through which we can conquer this virus. The results of the in-depth analyses revealed that SARS-CoV-2 has a high tropism for the cells which express ACE2; however, this was not all the story. It became evident that SARS-CoV-2 can directly or indirectly attack some blood cells such as platelets (PLT); for example, a disclosure indicated that about a quarter of COVID-19 patients have experienced thrombocytopenia (PLT less than 150 × 109/L), especially at the first week after admission to the hospital [1].
Of note, it should be mentioned that thrombocytopenia is not always an early event in COVID-19, as a considerable number of patients may experience it during disease progression several days after infection (late-phase thrombocytopenia). While a group of patients referred to hospital with thrombocytopenia immediately after infection, some other patients experience thrombocytopenia about fourteen days after the onset of disease symptoms or even after discharge from the hospital [2]. In a study conducted by Guan et al., it has been reported that 36.2% of patients with COVID-19 have a PLT count lower than 150,000/mm3 on admission [1]. The same results were also reported by Maquet et al. who indicated that from 253 COVID-19 cases who referred to the hospital, 24.9% were diagnosed with thrombocytopenia [3]. On the other hand, some studies have a consensus that COVID-19-related thrombocytopenia is a delayed event during infection [2]. Rampotas et al. indicated that thrombocytopenia in COVID-19 patients occurs in ICU and threatens the life of severe COVID-19 cases [4]. Another study declared that while the number of PLTs is normal at the time of admission, those patients who experienced thrombocytopenia after ICU admission have lower survival than those with a normal PLT count [5].
So far, the mechanisms that lead to the delayed thrombocytopenia have not been well described. Perhaps hospital-related sepsis can be the main reason why a COVID-19 patient at the ICU may develop thrombocytopenia. Juan et al. indicated that a sepsis-induced coagulopathy score of more than 4 in ICU admitted COVID-19 patients is an indicator of poor prognosis for patients and increases the hospital mortality rate up to 42% [6]. As a result of the bacterial sepsis during hospitalization, pathogen-associated molecular patterns (PAMPs) and host-derived damage-associated molecular patterns (DAMPs) are released, which in turn, recruit the innate arm of the immune system by producing the pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α. Simultaneously, viral infection can also activate B cells to produce pro-inflammatory cytokines and autoantibodies that interact with the PLTs’ glycoproteins. Once pro-inflammatory cytokines are produced, monocytes/macrophages and neutrophils are attracted to the site of infection to face pathogenes. But this is not all the story; the pro-inflammatory cytokines can alternatively stimulate the coagulation system and the exposure of the tissue factor on the surface of monocytes/macrophages or neutrophils can subsequently activate thrombin. As a result, the coagulation cascade is propagated, and eventually, a considerable number of PLTs are consumed. It should be also noted that since bacterial sepsis may hamper the expression of plasminogen activator inhibitor-1 (PAI-1), therefore, fibrinolysis is interrupted and more vigorously the number of PLTs will drop in COVID-19 patients admitted to ICU [7].
Along with sepsis, there are other reasons why a COVID-19 patient may experience thrombocytopenia during hospitalization. For instance, thrombotic thrombocytopenic purpura and atypical hemolytic uremia syndrome can induce severe thrombocytopenia at the pre-terminal stages of COVID-19 and put patients at more risk of mortality [8]. Also, it should not be forgotten that drug-induced thrombocytopenia might have a solid foot in this event. Heparin, antibiotics, and antiviral agents such as azithromycin and hydroxychloroquine are commonly administrated for COVID-19 patients [9], and there is no doubt that each of these drugs may be responsible for delayed thrombocytopenia. In this list, perhaps heparin is the most eminent drug that its name comes with thrombocytopenia. It has been claimed that within 5–10 days after the administration of heparin as a prophylaxis for COVID-19 patients, heparin-induced thrombocytopenia (HIT) might occur [10]. Nham et al. also indicated that ceftriaxone, levofloxacin, and lopinavir/ritonavir could induce reversible severe thrombocytopenia [11]. Apart from the drugs, hemodialysis and extracorporeal membrane oxygenation (ECMO) could also induce thrombocytopenia in COVID-19 patients [10].
Either induced early or delayed, thrombocytopenia can prolong patients’ hospitalization, increase their need for ventilation, and increase the 28-days mortality risk for patients [12]. In plain words, alteration in the PLT count may play a critical role in the progression of the disease: the lower is the PLT level, the poorer is the outcome and the higher is the risk of mortality [13]. Alongside thrombocytopenia, increased D-dimer levels further confirm that COVID-19 should be studied from the perspective of coagulopathy, as well [14], [15]. Given the striking effects of SARS-CoV-2 on the homeostasis system, this review allocates to provide a discussion about the prognostic value of PLTs and their related parameters in COVID-19 patients. We also provide brief information about the pathophysiology of thrombocytopenia and PLT- or thrombocytopenia-related treatment strategies in COVID-19.
2. Prognostic factors associated with platelets in COVID-19
2.1. Platelet count
As mentioned, PLT count is a valuable prognostic marker in COVID-19 patients since a considerable number of studies have reported the association between low PLT levels and the severity of the disease. In a recent meta-analysis, Bashash et al. have indicated that thrombocytopenia could remarkably increase the risk of disease progression and proposed that the daily monitoring of the platelet count can properly mirror the outcome of COVID-19 patients [16]. The results of another study delineated that from 1476 COVID-19 cases, 238 lost their lives during hospitalization who had experienced thrombocytopenia [13]. Jiang et al. also reported that the severity of thrombocytopenia was even much higher in non-survivors as compared to the critically ill survivors [17]. Inline, Lippi et al. have designed a meta-analysis on 1779 patients and re-concluded that the reduction in PLT count is associated with the mortality rate in patients [18]. Interestingly, Zhao et al. have introduced PLT count as a dynamic sensitive parameter to virus infection at the early stage of the disease, since platelets have a short life cycle and their storage at BM is critically low [19]. The authors suggested that among numerous inflammatory factors and molecules, PLTs are reliable cells for stratifying patients according to the medical care they would require.
The incidence of thrombocytopenia can also affect other laboratory findings. It has been reported that as compared to patients without thrombocytopenia, those with lower PLT count have lower oxygen levels as well as higher levels of blood urea, total bilirubin, lactate dehydrogenase, six-point ordinal scale score, and sequential organ failure assessment (SOFA) score [12]. Bao et al. have put a step a little higher and indicated that thrombocytopenia in COVID-19 victims is a mirror of bone marrow damage, intravascular coagulation defect, and vascular endothelial activation [8]. As a predicting tool for critically ill older patients, Zhu et al. made a flow chart and indicated that thrombocytopenia together with elevated partial thromboplastin time (PTT) could lead to a 28-day mortality [12]. Taken together, the most obvious conclusion is that the incidence of thrombocytopenia is a critical event in SARS-CoV-2 infection that not only may exacerbate the respiratory symptoms but also increase the risk of mortality.
2.2. Mean platelet volume (MPV)
Having established the association between PLT count and the outcome of the COVID-19 patients, very soon attention has been attracted to other PLT-related parameters. Guclu et al. showed that MPV may also have a predicting potential in COVID-19. Their results delineated that a one-unit increase in MPV level on the third day of hospitalization can increase the mortality rate by 1.76 times [20]. In agreement, He et al. observed that high MPV together with rapid elevation in platelet-large cell ratio (P-LCR) and platelet distribution width (PDW) within 2 weeks of disease diagnosis could increase the risk of death by 2–5 times [21]. Zhong et al. introduced a ratio called platelet mean volume/platelet count ratio (MPR) which is determined by dividing MPV by platelet count [22]. They used this ratio for predicting the outcome of 85 COVID-19 patients and suggested that those patients who had higher levels of mean platelet volume/platelet count ratio at the first, second, and third days after diagnosis developed severe pneumonia (SP); indicating that mean platelet volume/platelet count ratio is an independent risk stratifying factor for SP. In another study, Comer et al. proposed that the combination of PLT count, MPV, and neutrophil ratio could determine whether a patient would require an intensive care unit (ICU) [5]. Although a mounting number of studies are welcoming MPV as a risk stratifying factor, some other investigations failed to find any association between MPV and COVID-19 severity. In this vein, Aktaş et al. reported that there is no correlation between MPV value and other laboratory findings such as the number of WBC, CRP, and procalcitonin levels in both elderly and younger COVID-19 patients [23]. Ozenen et al. did the same comparison in children and they reached the same results [24]. Although the controversial results partially reduce the enthusiasm in monitoring MPV in COVID-19 patients, it should not be forgotten that perhaps the combination of MPV and other laboratory/clinical findings can make an algorithm for anticipating COVID-19 progression.
2.3. Immature platelet fraction (IPF)
Another interesting finding in COVID-19 patients __whether they have a normal or low number of PLT__ is the increase in the population of IPF: a group of young PLTs with the elevated ability to aggregate in response to agonists [25]. At the early stage of the disease, IPF might have a normal range; however, with the progress of the disease and in keeping with the decrease in PLT count, its level gradually elevates to mount the reference range. Increased level of IPF can be easily explained as a compensatory response to excessive PLT consumption. Moreover, the identification of such a group of highly-activated PLTs also answers a basic question concerning the development of clotting events in those intubated COVID-19 patients in ICU who has normal platelet counts [26]. Cohen et al. have compared the risk of clotting events in three groups of COVID-19, acute myocardial infarction (AMI), and stable patients with cardiovascular risk factors [27]. Their results showed that COVID-19 patients had the highest levels of IPF as compared to their counterparts and they were more prone to develop thrombotic events. Inline, Wu et al. have also found the elevated percentage of reticulated platelet (RP) in COVID-19 patients, and more interestingly, have found a relationship between PR% and the clinical outcome of the patients [28]. To sum up, these results suggest that increased turnover and hyperactivation of PLTs might have a respectable share in the induction of thrombotic events, especially in critically ill COVD-19 patients.
2.4. Increased P-selectin level
Another interesting finding in COVID-19 patients is the elevated levels of P-selectin (CD62P) not only on the surface of circulating PLTs but also in the serum of the patients in a soluble form (sP-selectin) [29]. Yatim et al. showed that the increase in sP-selectin levels at the time of diagnosis could increase the risk of disease severity, intubation, and death in patients [30]. In agreement, Goshua et al. indicated that those patients who were hospitalized at ICU showed elevated levels of sP-selectin [31]. The same results were achieved in a study by Vassiliou et al. who showed that the elevation in sP-selectin levels in ICU patients is an indicator of disease mortality [32].
2.5. Leukocyte-platelet aggregation
As mentioned, the identification of a group of hyperactivated PLTs in severe COVID-19 patients added another layer of complexity to the pathogenesis of the disease [33]. However, PLTs are not the only accuser as leukocytes can reinforce PLT aggregation through secretion of tissue factor (TF) __a coagulating factor that initiates the clotting process. In this vein, the monocyte-platelet aggregates (MPA) are detectable in severe cases of COVID-19 [34]. Joncour et al. also succeeded to find neutrophil–platelet aggregate (NPA) in critically ill COVID-19 patients, shedding more light on the roles of leukocytes in the stimulation of PLTs and propagation of the coagulation process that eventually lead to the formation of fibrin and D-dimers [35]. Indeed, the identification of PLT-leukocyte aggregates cast a flash of light on the COVID-19-related coagulopathy as the incidence of microthrombotic complication, as well as arterial/venous thrombosis in severe cases of the infection, may be explained, at least partly, under the umbrella of these aggregates [36].
2.6. Up- or down-regulation of coagulation factors
Endothelial cells were once considered an ally for PLTs to propagate the coagulation process in COVID-19 patients based on the findings indicating the high expression of von-Willebrand factor (vWF) on these cells [31]. In agreement, Zhou et al. indicated that the elevated expression of vWF together with the lower enzymatic activity of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) could increase the risk of death in COVID-19 patients. Subsequent observations have also found elevated levels of other endothelial-related biomarkers such as soluble E-selectin, angiopoietin 1 and 2 (Ang-1 and Ang-2, respectively), soluble intercellular adhesion molecule 1 (sICAM-1), vascular endothelial growth factor (VEGF), and soluble vascular endothelial (VE)-cadherin in ICU patients [37]. Of note, Zhou et al. showed that D-dimer level is approximately 9-fold higher in COVID-19 non-survivors as compared to survivors [14]. Inline, the mean value of D-dimers in severe patients was significantly higher than non-severe cases (X2 = 6.34, P = 0.01), highlighting that elevation of this parameter may effectively contribute to mirror the progression of disease toward an unfavorable clinical picture [16].
Another coagulating factor whose level might have a prognostic value is fibrinogen. The association between this protein and the risk of SARS has been well-established in previous studies [38]. A high level of factor V (FV) has also been accused to be involved in the severity of COVID-19. A recent study conducted by Harvard Medical School investigators showed that elevation in the serum level of FV could increase the risk of deep vein thrombosis (DVT) or pulmonary embolism (PE); however, it seems that in critically ill COVID-19 patients FV reduction is associated with the development of disseminated intravascular coagulation (DIC). Taken together, it seems that laboratory monitoring of coagulopathy parameters (i.e., platelet count, D-dimer, fibrinogen, and coagulation-based tests) undeniably assists clinicians to better stratify COVID-19 patients due to the risk of thrombosis [39]. To provide a well-conceptualized overview of the prognostic factors associated with platelets in COVID-19, we summarized all these data in Table 1 .
Table 1.
A summary of the prognostic factors associated with platelets in COVID-19.
| Patients No. | Age (Y) | Male (%) | Location | Outcome | Ref | |
|---|---|---|---|---|---|---|
| Platelets count | ||||||
| 1476 | M: 57 | 52.6 | China | Thrombocytopenia is associated with increased risk of in-hospital mortality, and compared with survivors, non-survivors were more likely to have thrombocytopenia and had lower nadir platelet counts.. | [13] | |
| Non-survivors: 29 Survivors: 503 |
M: 64 M: 48 |
48.346.1 | China | Early decrease in platelet count was associated with mortality in patients with COVID-19., as the platelet count among non-survivors decreased gradually within 1 week after admission. | [19] | |
| 7613 | NA | NA | NA | Thrombocytopenia might be a risk factor for COVID-19 progressing into a more severe state, as compared to non-severe patients, severe COVID-19 had a lower platelet count. | [17] | |
| 178 | M: 64 | 59.6 | China | Thrombocytopenia correlated with DIC rate and survival. Six out of 7 deaths had thrombocytopenia during hospitalization, and platelet count decreased subsequently until death. | [8] | |
| 167 | M: 66 | 67.07 | China | Thrombocytopenia was associated with the deterioration of respiratory function and baseline platelet count was associated with long-term mortality in critically ill COVID-19 patients. | [12] | |
| 3383 | NA | NA | China, Singapore | Decreased number of platelets more commonly associates with severe COVID-19; however, whether thrombocytopenia may result in diseases severity or the severity may decrease platelets is open to debate. | [16] | |
| 1779 | NA | NA | China, Singapore | Low platelet count is associated with increased risk of severe disease and mortality in patients with COVID-19, and thus should serve as clinical indicator of worsening illness during hospitalization. | [40] | |
| 215 | M: 64 | 55.81 | Turkey | Although thrombocytopenia was more likely occur in non-survivors, there was no correlation between platelet level & mortality. | [20] | |
| 251 | M: 8.92 | 55.5 | Turkey | Severe COVID‐19 patients had significantly more common thrombocytopenia than non-severe cases. | [24] | |
| Non-severe: 71 Severe: 44 |
M: 48 M: 64 |
54.92 69 |
Canada | Platelet counts were in the lower range in both severe and non-severe COVID-19 patients in comparison to the expected count in healthy volunteers; however, the platelet-lymphocyte ratio was lower in severe patients compared with nonsevere patients. | [41] | |
| 74 | M: 65.1 | 59 | Iran | Lower platelet count was detected among non-survivors compared to survivors. | [42] | |
| NA | NA | NA | NA | Thrombocytopenia is a significant finding in patients with severe type of COVID −19. Immune mediated platelet destruction might account for the delayed-phase thrombocytopenia in a group of patients. | [2] | |
| 575 | NA | NA | UK | Thrombocytopenia is common in an ICU setting due to endogenous and iatrogenic factors. Despite that, thrombocytopenia in patients with severe COVID-19 infections is surprisingly uncommon. | [4] | |
| Non-severe: 20 Severe: 34 |
M: 69 M: 59 |
65 62 |
Ireland | While platelet counts did not differ on the day of admission between patients subsequently designated to have had either a severe or non-severe disease course, significantly decreased platelet counts were observed among the severe COVID-19 patients at the time of transfer to the ICU relative to the non-severe group. | [5] | |
| 567 | M: 63 | 52.2 | Turkey | Decreased platelet count together with older age, presence of heart failure, clinical severity of the disease at presentation, ferritin level on admission, and increase in AST level during hospitalization may predict the mortality risk of these patients. | [43] | |
| 53 | M: 58.4 | 45.3 | Iran | There was an association between higher mortality rates and decreased platelet count. | [44] | |
| 500 | M: 44.24 | 50 | India | Platelet count may be a simple, economic, rapid and commonly available laboratory parameter that could straightforwardly discriminate between COVID patients with and without severe disease. | [45] | |
| 64 | M: 57.11 | 47.7 | Greece | Platelets were slightly lower in severe patients compared to the moderate ones, but it was not statistically significant. | [46] | |
| 516 | M: 67 | 66.9 | Italy | Using Cox regression analysis, platelet count was a predictor of mortality. | [47] | |
| 44 | M: 67.5 | 63.63 | Italy | In the univariate analysis for disease severity, thrombocytopenia together with male sex, respiratory frequency greater than 22, and cancer as comorbidity were significantly associated with higher odds for severe disease. | [48] | |
| 110 | M: 56.9 | 43.6 | Korea | Lymphocyte count and platelet count were significantly lower in the severe group than the non-severe group. | [49] | |
| 2054 | M: 59 | 52.6 | Brazil | In the multivariate Poisson regression model, various factors including low blood platelet count were independently associated with a higher risk of death. | [50] | |
| MPV | ||||||
| 215 | M: 64 | 55.81 | Turkey | Oxygen saturation at admission and the MPV difference between the first and third days of hospitalization were significant parameters for predicting mortality. | [20] | |
| 112 | M: 61 | 65.2 | China | Patients with high MPV (HR 3.73; P = 0.0034) were significantly associated with the worse survival. | [21] | |
| 85 | M: 43 | 51.8 | China | Compared with mild patients, patients with severe pneumonia showed a higher MPR level; proposing high MPR level as an independent risk factor for severe pneumonia in COVID-19. | [22] | |
| Non-severe: 20 Severe: 34 |
M: 69 M: 59 |
65 62 |
Ireland | Increased MPV and decreased platelet were associated with disease severity in COVID-19 upon hospitalization and intensive care unit admission. | [5] | |
| 37 | M: 54 | 56.8 | Turkey | They did not determine difference in MPV level, mortality and prognosis between COVID-19 patients. | [23] | |
| 251 | M: 8.92 | 55.5 | Turkey | MPV values are not associated with COVID‐19 disease severity; however, MPV can be used with other parameters such as WBC, CRP, procalcitonin, D‐dimer to predict hospitalization. | [24] | |
| 100 | M: 47.1 | 57 | India | High PDW shows significant association with mortality and cut-off values for PDW as 17% significantly associated with mortality. | [51] | |
| 640 | NA | NA | Turkey | MPV could be used as a simple and cost-effective tool to predict COVID-19 in subjects with diabetes in primary care. | [52] | |
| 81 | M: 60 | 49 | USA | There was a significant correlation between D-dimer levels and MVP; but a negative correlation between MPV and GFR in critically ill cohort. | [53] | |
| IPF | ||||||
| 47 |
M: 56 |
59.6 |
Israel | Patients with COVID-19 have increased IPF compared to stable patients with cardiovascular risk factors, suggesting that the enhanced platelet turnover may have a role in the development of thrombotic events in COVID-19 patients. | [27] | |
| 982 | M: 71 | 68 | Europa | IPF tended to increase with disease severity. | [54] | |
| 81 | M: 60 | 49 | USA | This study found increased IPF (greater than7) in COVID-19-infected subjects who had evidence of elevated D-dimers and AKI. | [53] | |
| Increased P-selectin level | ||||||
| Non-severe: 20 Severe: 34 |
M: 69 M: 59 |
65 62 |
Ireland | Circulating levels of soluble P-selectin increased in COVID-19 patients compared to the control cohorts and intriguingly, could differentiate between non-severe and severe COVID-19 cohorts. | [5] | |
| Non-severe: 71 Severe: 44 |
M: 48 M: 64 |
54.92 59 |
Canada | Platelet activation, assessed by surface expression of P-selectin and CD63, was evident in patients with severe COVID-19 and strongly correlated with levels of D-dimers. | [41] | |
| 68 | M: 62 | 60 | USA | Soluble P-selectin concentrations were significantly higher in ICU patients than non-ICU cases. | [31] | |
| Cohort 1: 60 Cohort 2: 50 |
M: 58 M: 56 |
76.7 78 |
Europa France |
Soluble P-selectin was a biomarker for poor outcome and could serve as a soluble marker associated with death. Transcriptional analysis identifed SELPLG RNA level as a biomarker for mechanical ventilation. | [30] | |
| Survivors: 28 Non-Survivors: 10 |
X: 62 X: 68 |
82.1 80 |
Greece | Soluble P-selectin was significantly elevated in ICU non-survivors compared to survivors, and also associated with a higher mortality probability in the Kaplan–Meier analysis. | [32] | |
| 46 | M: 65.2 | NA | Italy | A higher P-selectin plasma concentration was found in COVID-19 patients regardless of ICU admission. | [55] | |
| Healthy: 20 Non-severe: 37 Severe: 64 Non-Survivors: 18 |
M: 41.40 M: 44.52 M: 55.53 M: 63.77 |
40 62.16 60.9 50 |
Mexico | A significant difference was found in P-selectin in non-severe and healthy donors when compared to severe COVID-19 cases and deceased patients. Indee, a higher P-Selectin concentrations was found in the severe and death COVID-19 groups compared with patients in the non-Severe COVID-19 group and healthy donors. | [56] | |
| Leukocyte-platelet aggregation | ||||||
| Mild: 6 Severe: 35 |
M: 32 M: 57 |
33.3 46.8 |
Brazil | Increased platelet activation and platelet-monocyte aggregate formation are observed in severe COVID-19 patients, but not in patients presenting mild COVID-19 syndrome. | [34] | |
| 27 | M: 71 | 52 | France | Levels of NPA and MPA were significantly higher in severe COVID-19 patients relative to those with moderate disease. | [35] | |
| Up- or down-regulation of coagulation factors | ||||||
| 68 | X: 62 | 60 | USA | Mortality was significantly correlated with vWF antigen and soluble thrombomodulin among COVID-19 cases. Soluble thrombomodulin greater than 3.26 ng/mL was associated with lower lower likelihood of survival. | [31] | |
| Survivors: 28 Non-Survivors: 10 |
X: 62 X:68 |
82.1 80 |
Greece | ICU admission levels of Ang-2, sICAM-1, and vWF were higher in COVID-19 critically ill patients who will not survive. | [32] | |
| 183 | M: 54 | 53.55 | China | Abnormal coagulation results, especially markedly elevated D-dimer and FDP were common in non-survivors. | [57] | |
| 1621 |
X: 69 | 53.2 | China | The associations between coagulation factors, vWF and ADAMTS13, and COVID-19 severity are essentially causal; highlighting the importance of dynamically monitoring the plasma levels of these factors in COVID-19. | [37] | |
| 191 | M: 56 | 62 | China | D-dimer values were nearly 9-fold higher in patients who died than in those who survived. | [14] | |
| 41 | M: 49 | 73 | China | PT and D-dimer level on admission were higher in ICU patients than non-ICU patients. | [58] | |
| 449 | M: 65 | 59.68 | China | D-dimer, PT, and age were positively, and platelet count was negatively correlated with 28-day mortality. | [59] | |
| 138 | M: 56 | 54.3 | China | The level of D-dimer was significantly higher in non-survivors than in survivors. | [60] | |
| 1099 | M: 47 | 58.1 | China | D-dimer was higher in patients with severe COVID-19 than in those without. | [61] | |
| 192 | M: 69.4 | 58.3 | Italy | No association was found between lupus anticoagulant and mortality as well as mechanical ventilation. | [62] | |
| Healthy: 20 Non-severe: 37 Severe: 64 Non-Survivors: 18 |
M: 41.40 M: 44.52 M: 55.53 M: 63.77 |
40 62.16 60.9 50 |
Mexico | Concentrations of D-dimer and plasminogen activator inhibitor-1 were significantly elevated in severe COVID-19 patients. A significant difference was found in PAI-1 in non-severe and healthy donors when compared to severe and deceased COVID- 19 patients. vWF levels were also significantly different between severe patients and non-severe cases. | [56] | |
| 2277 | NA | NA | China | Screening abnormal coagulation parameters such as decreased platelet, prolonged PT, and elevated D-dimer were beneficial for predicting the severity and prognosis of COVID-19. | [15] | |
DIC: Disseminated intravascular coagulation; AST: Aspartate transaminase; MPV: Mean platelet volume; MPR: Platelet mean volume/platelet count ratio; WBC: White blood cells; CRP: C-reactive protein; PDW: Platelet distribution width; GFR: Glomerular filtration rates; IPF: Immature platelet fraction; AKI: Acute kidney injury; ICU: Intensive treatment unit; SELPLG: selectin P ligand; NPA: Neutrophil–platelet aggregate; MPA: Monocyte–platelet aggregate; vWF: von-Willebrand factor; Ang-2: Angiopoietin-2; sICAM-1: Soluble intercellular adhesion molecule-1; FDP: Fibrin and fibrinogen degradation product; ADAMTS13: A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; PT: Prothrombin time; PAI-1: plasminogen activator inhibitor 1; Y: Year; M: Median; X: Mean; NA: Not available.
3. Pathophysiology for thrombocytopenia in COVID-19
3.1. Decreased platelet production
3.1.1. Cytokine storm
When it comes to COVID-19, one of the first mechanisms that have been described for the disease pathogenesis was the cytokine storm __a process in which the excessive production of cytokines can prompt tissue damage by inducing positive feedback on immune cells [63]. A wealth of evidence declared that one of the most sensitive cells to increased levels of cytokines is the hematopoietic progenitor cell. For example, it has been indicated that transforming growth factor-beta (TGF-β) has deteriorating effects on megakaryocyte (MK)-colony-forming unit (CFU-meg), leading to suppression of megakaryocytopoiesis and subsequently thrombocytopenia [64], [65]. Liao et al. have also proposed another mechanism for SARS-CoV-2-induces thrombocytopenia [66]. The authors suggested that the propagation of systematic inflammation leading by Interleukin 6 (IL-6), IL-1, IL-2, IL-2R, IL-7, IL-10, and tumor necrosis factor-alpha (TNFα) could harm the BM microenvironment and eventually suppress hematopoiesis [67]. The excessive production of inflammatory cytokines in the BM microenvironment also leads to the release of ACE2 from the cells. Once ACE2 is separated, it loses its ability to control the renin-angiotensin system, an event that makes a vicious cycle of pro-inflammatory cytokine production [68].
3.1.2. Direct infection of HSCs
Some studies have come to a consensus that SARS-CoV-2 could directly target hematopoietic cells and bone marrow stromal cells. This idea has arisen from the genetic analysis which indicated that SARS-CoV-2 and human SARS-CoV share 82% nucleotide homology [69]. It has been suggested that through recruitment of CD13 or CD66a, SARS-CoV-2 internalizes into BM cells and megakaryocytes, propagates apoptotic signaling within the cells, and thereby induces thrombocytopenia [70]. ACE2 has also been proposed by Ropa et al. as a binding site for Spike (S) protein of SARS-CoV-2 to penetrate hematopoietic stem cells/hematopoietic progenitor cells (HSCs/HPCs). Overall, either via CD13, CD66a, or ACE2, evidence shows that SARS-CoV-2 may probably induce thrombocytopenia through attacking to HSCs/HPCs [71].
3.1.3. Decreased production of TPO
The ability of SARS-CoV-2 to bind to ACE2 has another advantage for the virus rather than reduction of HSCs/HPCs. In the perspective of megakaryocytopoiesis, the liver is a repertoire for TPO production. Like many other viruses such as simian immunodeficiency virus (SIV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV), SARS-CoV-2 can impair liver function, decrease TPO secretion, and thereby, attenuate the production of PLTs [68], [72], [73]. Deruelle et al. have suggested that the alteration in the BM microenvironment balance during SARS-CoV-2 infection could harm the ability of hepatocytes to produce TPO [74]. Interferon-alpha (IFN-α) is one of the accusers of this event, as this cytokine can hamper the expression of TPO in hepatocytes [75], [76], [77]. In contrast, Manne et al. claimed that SARS-CoV-2 infection does not affect TPO production but diminishes the expression of myeloproliferative leukemia protein (c-MPL) on megakaryocytes [29]. Whether decreasing the TPO levels or the expression of c-MPL, the results of these investigations apparently suggested that SARS-CoV-2 put an obstacle in the way of TPO to induce differentiation in the megakaryocytic lineage.
3.1.4. Damage to the pulmonary capillary beds
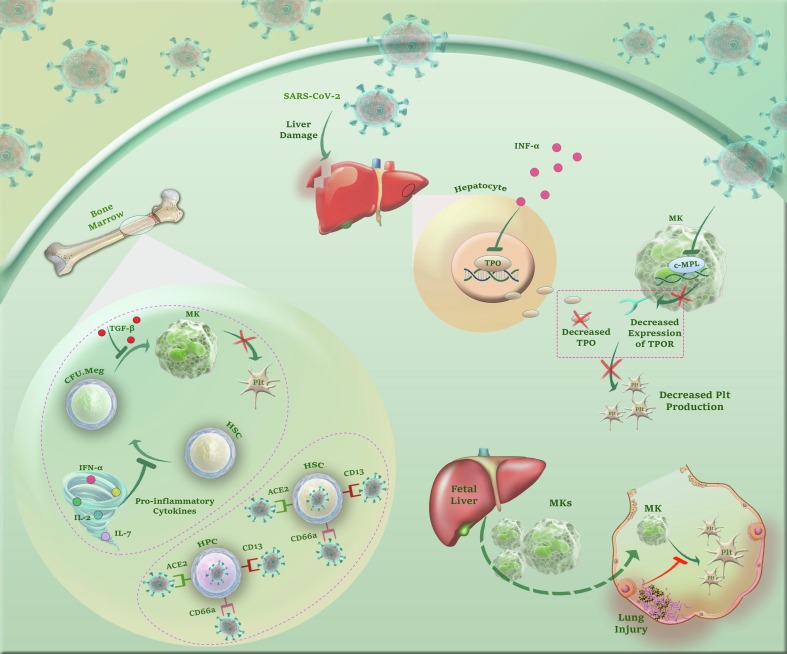
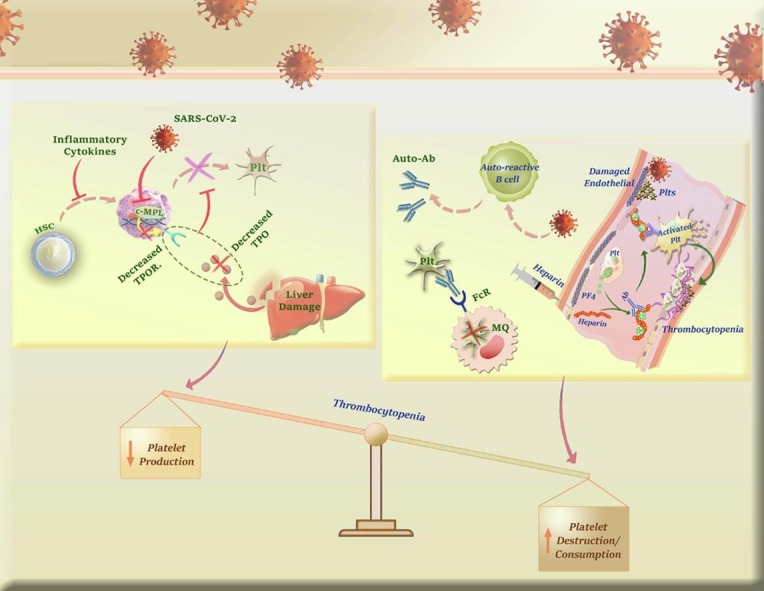
Aschoff has raised a theory over hundred years ago [78], suggesting that mature megakaryocytes left fetal liver capillary sinusoids [79] and the placenta [80], [81] to reside in pulmonary capillary beds of the lungs, where they gradually release circulating PLTs. This theory was later examined by several investigations and interestingly it became apparent that lung injury could impair PLT production [82]. Given this, it is reasonable to assume that SARS-CoV-2-induced damage to the pulmonary capillary beds may, at least partly, be in charge of thrombocytopenia in COVID-19 patients, especially those with severe lung injury. Inline, The results of the studies conducted by Xu et al. and Zhang et al. proposed that persistent hypertension, low oxygen levels, and diffused alveolar damage go hand in hand to disturb the integrity of pulmonary capillary beds, and eventually hinder PLT release [63], [83]. Fig. 1 summarizes plausible mechanisms by which SARS-CoV-2 decreases PLTs production.
Fig. 1.
A glance at the mechanisms by which SARS-CoV-2 reduces PLTs production. Either via CD13, CD66a or ACE2 SARS-CoV-2 may probably induce thrombocytopenia through attacking to HSCs/HPCs. Besides, the excessive secretion of inflammatory cytokines not only may suppress differentiation of HSCs to megakaryocytic progenitors but also hamper the maturation process of MKs. Apart from cytokine storm, SARS-CoV-2 can impair liver function, decrease TPO secretion, downregulate surface expression of TPO receptor on MKs via inhibition of c-MPL gene expression, and thereby, attenuate the production of PLTs. Finally, according to the assumptions proposing the lungs as the plausible reservoirs for MKs, it is reasonable to assume that SARS-CoV-2-induced damage to the pulmonary capillary beds may, at least partly, be in charge of thrombocytopenia in COVID-19 patients, especially those with severe lung injury.
3.2. Increased platelet destruction/clearance
3.2.1. Immune thrombocytopenic purpura (ITP)
Another mechanism proposed for COVID-19-induced thrombocytopenia is the induction of immune thrombocytopenic purpura (ITP) [63]. This idea has been raised from the piece of evidence suggesting the incidence of ITP after SARS-CoV-2 infection, especially in elderly and moderate-to-severe cases [84]. Bomhof et al. suggested that ITP might not happen during active infection but it may occur 10 days after the disease disappearance [85]. Notably, the clinical diagnosis of ITP is critical as it can increase the risk of bleeding as well as thromboembolic events in patients [86]. Revuz et al. also proposed ITP as an important reason for hemorrhagic symptoms in COVID-19 patients [87].
Toll-like receptor 7 (TLR7) is the main member of pattern recognition receptors (PRRs) on the innate immune cells that recognizes SARS-CoV-2; however, it should not be forgotten that it can inadvertently activate autoreactive B cells to produce autoantibodies [88]. Humbert et al. have recognized autoantibody against PLT glycoproteins in COVID-19 patients [88]. The autoantibody-coated PLTs are then demolished by the reticuloendothelial system and this is when thrombocytopenia would occur [83]. Apart from the improper response of the immune system, Lee et al. suggested that the incidence of thrombocytopenia due to ITP may be due to some genetic abnormalities [89]. The authors succeeded to recognize a heterozygous truncation variant in a suppressor of cytokine signaling 1 (SOCS1) in two individuals suffering from COVID-19, which leads to constitute activation of IFNs signaling. They claimed that the more IFNs are produced, the more immune response waves would be evolved and the more PLTs would be trapped by immune complexes. Taken together, ITP __whether initiated by inaccurate immune responses or due to genetic abnormalities__ is a possible mechanism which may explain COVID-19-induced thrombocytopenia.
3.2.2. Lupus anticoagulants (LA)
The presence of the anti-phospholipid antibodies (APLA), anticardiolipin IgA, and anti–β2-glycoprotein IgA and IgG in severe COVID-19 cases suggested that perhaps the incidence of thrombocytopenia is due to antiphospholipid antibody syndrome (APAS) [90]. The incidence of antiphospholipid antibody syndrome in COVID-19 has been reported in several studies. Helms et al. suggested the correlation between lupus circulating anticoagulants and the risk of patients’ hospitalization in ICU [91]. The results of Bowles et al. also proposed that COVID-19 patients with prolonged aPTT were mostly positive for anti-phospholipid antibodies [92]. The results of both lupus anticoagulant–sensitive aPTT and Russell’s viper-venom time (DRVVT) revealed that in most COVID-19 cases the titer of lupus anticoagulants was higher than the healthy individuals. Showers et al. also indicated that the serum levels of anticardiolipin antibodies gradually increase within 5 weeks in critically ill COVID-19 patients [93]. All of these findings suggest that COVID-19-induced thrombocytopenia may be due to the presence of anti-phospholipid antibodies, which target PLTs and increase their clearance from the reticuloendothelial system.
3.2.3. Direct infection of platelets
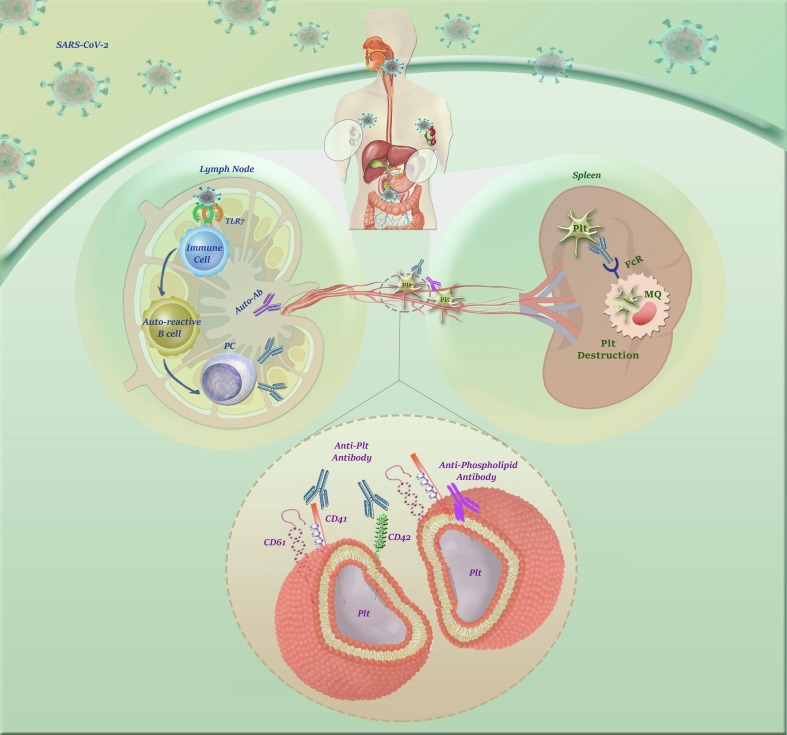
There is a consensus that PLTs are also some auxiliary members of the innate arm of the immune system that could attack the pathogens via their pattern recognition receptors such as C-type lectin receptors, TLR3, TLR7, and TLR9 [94]. This unique property can lead to the infection of these cells, as well. Given the similarity between SARS-CoV-2 and HCoV-229E, a virus that induces apoptosis in PLTs through interaction with CD13, Xu et al. have claimed this hypothesis that probably SARS-CoV-2 induces thrombocytopenia through direct infection of PLTs [63]. This suggestion was later examined by Zhang et al. who indicated that PLTs of COVID-19 cases have overexpressed αIIbβ3, P-selectin, and ACE2, which are all the binding sites for the Spike protein (S protein) of the virus [95]. The authors declared that the application of recombinant human ACE2 and anti-Spike monoclonal antibody could significantly reduce the incidence of thrombocytopenia, suggesting that perhaps SARS-CoV-2 use ACE2 to enter into host PLTs. Fig. 2 . provides a brief overview of the mechanisms by which SARS-CoV-2 may increase PLTs destruction.
Fig. 2.
A glance at the mechanisms by which SARS-CoV-2 increases PLTs destruction. The presence of the anti-phospholipid antibodies (APLA) in severe COVID-19 cases suggested that perhaps the incidence of thrombocytopenia is due to antiphospholipid antibody syndrome (APAS). Also, it has been claimed that toll-like receptor 7 (TLR7) on the innate immune cells can inadvertently activate autoreactive B cells to produce autoantibodies against PLT glycoproteins. The autoantibody-coated PLTs are then demolished by the reticuloendothelial system, an event which in turn leads to immune-mediated thrombocytopenia.
3.3. Increased platelet consumption
3.3.1. Lung injury; activation, aggregation, and consumption of platelets
Amid diverse discussed mechanisms that lead to thrombocytopenia in COVID-19 patients, the effect of lung injury could not be neglected. Once SARS-CoV-2 binds to ACE2 expressed on lung endothelial cells, it enforces endothelial cells to release vWF from Weibel-Palade bodies. The exposed vWF provokes GPIb-IX-V-mediated PLT activation so that the activated PLTs adhere to the injury site to release tissue repair growth factors and ameliorate SARS-CoV-2-induced tissue damage; notably, the more massive the tissue damage is, the more PLTs are consumed. Escher et al. suggested not only the activity of vWF significantly increased in COVID-19 patients, the level of FVIII __a coagulation factor that is stabilized by vWF__ was also elevated [96]. Inline, Helms et al. reported the same results concerning the elevated levels of vWF antigen, FVIII, D-dimer, and fibrinogen in 150 COVID-19 patients [91]. Blasi et al. and Martinellia et al. provided evidence of this disclosure from another perspective; the authors conducted two separate studies in which their results declared that the enzymatic activity of ADAMTS13 __an enzyme that cleaves vWF__ is diminished in COVID-19 patients [97]. Following these studies, Capecchi et al. reported that acute infection with SARS-CoV-2 may result in thrombotic thrombocytopenic purpura (TTP) through producing specific IgG against ADAMTS13 [98]. All these results clearly illustrate a schematic suggesting that lung tissue damage induced by SARS-CoV-2 can provoke thrombocytopenia mainly through increasing PLT consumption.
3.3.2. Increased coagulation activity
3.3.2.1. Thrombotic microangiopathy (TMA)
The first evidence of thrombotic microangiopathy (TMA) in COVID-19 was reported by Jhaveri et al. who succeeded to identify widespread microthrombi in the kidney of the patients [99]. The results of the first four autopsies from New Orleans also suggested thrombotic microangiopathy as a fatal mechanism in severe cases and proposed that damage to the small blood vessels of vital tissues, eventually followed by intravascular coagulopathy, will probably result in disease mortality [100]. The incidence of thrombotic microangiopathy sparkled a light on another mechanism that might be involved in SARS-CoV-2 pathogenesis; the activation of the complement system [101]. Gavriilaki et al. proposed that the excessive production of complement terminal products could interact with PLTs, and thereby, damage their cellular membranes [102]. By using a complement functional panel, Alizadeh et al. realized the hyperactivity of this cascade in a COVID-19 patient who experienced thrombocytopenia, anemia, and renal failure; as the activity of ADAMTS13 was normal, they diagnosed a thrombotic microangiopathy condition termed hemolytic-uremic syndrome (HUS) [103]. With keeping in mind that HUS might have an idiopathic etiology since no genetic abnormalities were detected in the patient, the authors claimed that there is a possibility that HUS was the secondary response to SARS-CoV-2 infection. With this piece of evidence, it is reasonable to assume that thrombotic microangiopathy together with the excessive activation of complement could decrease the number of PLTs, especially in the severe type of the disease.
3.3.2.2. DIC
It has been proposed that dysfunction in blood coagulation associates with poor prognosis and high mortality in COVID-19. Indeed, coagulopathy is a leading cause of mortality in severe cases [104], and DIC may occur in the majority of SARS-CoV-2-related deaths [105]. In a study conducted on 183 patients, 71.4% of non-survivors were diagnosed with overt DIC [105]. The excess of D-dimer levels of more than 2 mg/L can be an indicator of disease mortality with a sensitivity of 92% and a specificity of 83% [106]. With regard to these laboratory features and based on the definition of International Society on Thrombosis and Haemostasis (ISTH) form DIC, it could be indicated that SARS-CoV-2 can induce DIC in patients, and thereby, increase the risk of thrombosis. However, it has been demonstrated that COVID-19-associated coagulopathy, herein referred to as CAC, is different from the type of DIC that is described in bacterial sepsis, as in patients with COVID-19, DIC may be accompanied by elevated D-dimer and fibrinogen levels but not with abnormal prothrombin time (PT) [107]. The moderate increase in PT (about 3 s) has been reported only in critically severe cases of COVID-19 [105]. One of the main reasons why many reports emphasized the difference between CAC and classic DIC is the severity of thrombocytopenia. As compared to sepsis-associated DIC, at which more severe thrombocytopenia could be observed, CAC could either induce a mild reduction in PLT count or even do not exert any effect on PLTs [108]. It has been claimed that rather than thrombocytopenia, the incidence of CAC is mostly associated with an elevated risk of venous and arterial thrombosis; suggesting that COVID-19 coagulopathy might have more overlap with the hemophagocytic syndrome, antiphospholipid syndrome, and thrombotic microangiopathy than with classic DIC that is triggered due to the bacterial infections [7].
Whether the coagulopathy caused by SARS-CoV-2 is the same as DIC, or whether it causes mild or severe thrombocytopenia, the important point is that the formation of fibrin clots, as revealed by the elevated D-dimer levels, in small vessels can finally lead to PLT consumption and thereby the reduction in PLT count. SARS-CoV-2-induced tissue damage can also expose tissue factor on one hand, and on the other hand, suppress the activity of protein C, both of which lead to excessive PLT aggregation and consumption [7], [109]. Moreover, low-grade CAC together with localized pulmonary thrombotic microangiopathy lead to organ dysfunction in severe cases. Multi-organ failure, in turn, provokes pro-inflammatory responses which subsequently trigger the coagulation cascade in which more PLTs are consumed. In a study conducted by Bao et al., it has been indicated that CAC rate has a significant correlation with admission PLT count. Actually, severe cases of COVID-19 showed the trend of lower platelet count, higher level of D-Dimer, and a higher rate of DIC at 1 week after admission [8].
3.3.3. Heparin-induced thrombocytopenia (HIT)
An important drug that its administration may be associated with thrombocytopenia is heparin __which is usually prescribed to COVID-19 patients as prophylaxis for thrombosis [110]. When it comes to heparin and thrombocytopenia, the first mechanism is the induction of heparin-induced thrombocytopenia (HIT). Bhattacharjee et al. reported that within two weeks after administration of heparin the PLT level decreased to 50–70 × 09/L [84]. Another evidence for HIT has been observed in a 63-year-old man with COVID-19 who was admitted to the hospital with normal PLT count and functional liver; his PLT count significantly dropped 11–12 days after administration of heparin, and further analysis revealed the incidence of HIT due to the presence of anti-PF4/heparin antibody [111]. Taken together, although
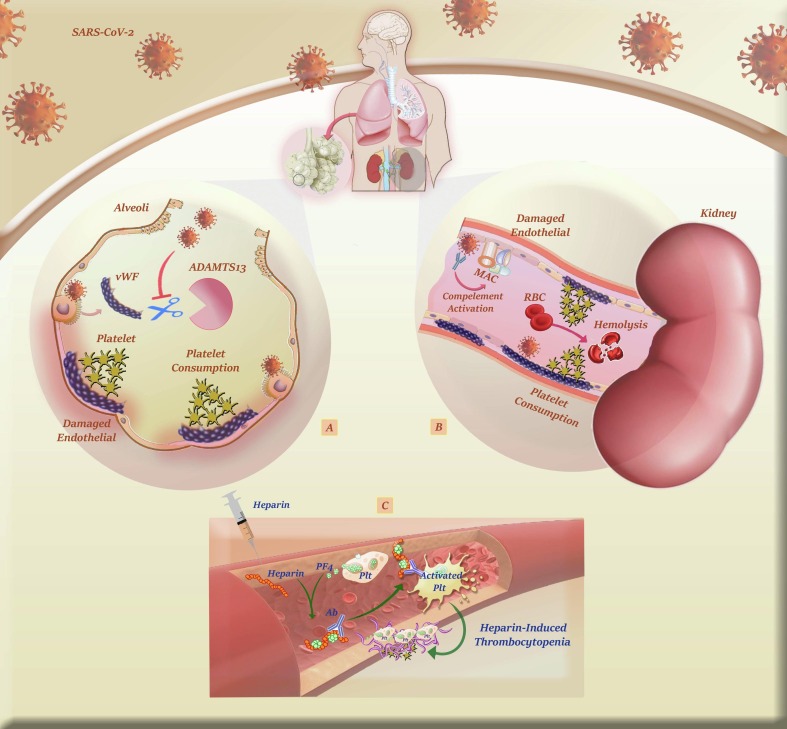
the incidence of HIT is low in COVID-19, it should not be forgotten that serious cautions should be taken, especially in hospitalized patients, to rapidly diagnose this phenomenon and stop the drug administration. Fig. 3 . provides a brief overview of the mechanisms by which SARS-CoV-2 may increase PLTs consumption.
Fig. 3.
A brief overview of the mechanisms by which SARS-CoV-2 increases PLTs consumption. A) Once SARS-CoV-2 binds to ACE2 expressed on lung endothelial cells, it enforces the cells to release vWF. Subsequently, this multimeric protein provokes GPIb-IX-V-mediated activation of PLTs so that they can adhere to the injury site to ameliorate SARS-CoV-2-induced tissue damage. B) Complement-mediated damage to kidney endothelial cells in COVID-19 patients can also result in increased consumption of PLTs together with the emergence of microangiopathic hemolytic anemia. C) Administration of heparin may be in charge of thrombocytopenia in COVID-19 cases, as well. Indeed, heparin-induced thrombocytopenia (HIT) is an immune complication of heparin therapy caused by antibodies to complexes of platelet factor 4 (PF4) and heparin. Pathogenic antibodies to PF4/heparin bind and activate cellular FcγRIIA on platelets to propagate a hypercoagulable state culminating in life-threatening thrombosis.
4. Thrombocytopenia-related treatment in COVID-19
4.1. Monoclonal antibodies (mAbs)
4.1.1. Inhibition of inflammatory cytokines
Numerous anti-inflammatory drugs have been tested for COVID-19 patients, but only tocilizumab (TCZ) enjoyed unprecedented success and got the food and drug administration (FDA) approval [112]. Tocilizumab can bind to both membraneous IL-6R (mIL-6R) and soluble IL-6R (sIL-6R) [113]. The results of a study conducted in Italy indicated that intravenous administration of tocilizumab not only improved the outcome of ICU patients but also exerted a sustained effect in patients [114]. Given the success of tocilizumab, more attempts were done to evaluate the therapeutic effect of other inhibitors of interleukin receptors in COVID-19 patients. Anakinra, an antagonist of IL-1R, was also efficient, safe, and successful to prevent multi-organ failure in COVID-19 patients [115]. Nemchand et al. suggested that this antagonist could block IL-1β secretion from the macrophages and thereby prevent the incidence of tissue damage in patients [116]. As Anakinra prevents tissue damage, it can barricade endothelial exposure, suppress propagation of the coagulation cascade, and subsequently, prevent the excessive aggregation of PLTs. Noteworthy, Cavalli et al. have attested that COVID-19 patients who received IL-1R inhibitor had a much better outcome and longer survival as compared to those who were treated with IL-6R inhibitor [117]. Despite efficacy, it seems that Anakinra has a long way to receive FDA approval for the treatment of critically ill COVID-19 patients.
4.1.2. Inhibition of the complement system
The aberrant activation of the complement cascade and the incidence of thrombotic microangiopathy have paved the way for the entrance of complement inhibitors into the therapeutic strategies of COVID-19 [118], [119]. Diurno et al. have tested the efficacy of eculizumab in five ICU hospitalized COVID-19 patients and reported that all cases successfully recovered [120]. Annane et al. also administrated eculizumab to severe COVID-19 cases and found that blockage of C5 is associated with a rapid reduction in lactate, urea, total and conjugated bilirubin levels, and improved hypoxia [121]. Moreover, the authors found that eculizumab could recover the PLT count and improve PT within 15 days. AMY-101 is a compstatin-based complement C3 inhibitor whose efficacy has been tested in COVID-19 patients, especially those with severe pneumonia [122]. In this vein, Mastellos et al. suggested that AMY-101 properly follows the footprints of eculizumab in hampering the hyper-inflammation condition [123].
4.1.3. Inhibition of chemokines and chemokine receptors
Given the importance of chemokines in the induction of thrombocytopenia, it is reasonable to assume that the administration of some chemokine inhibitors might be advantageous for recovering PLT count [124]. Cenicriviroc (CVC) is a small chemokine receptor antagonist whose therapeutic potentials have recently been tested in the pre-clinical models. It has been declared that cenicriviroc could halt the replicative capacity of the SARS-CoV-2 virus and stimulate anti-viral immune responses in COVID-19 patients [125]. Interestingly, Patterson et al. have also evaluated the efficacy of leronlimab, an antibody against CCR5, in COVID-19 patients and showed this agent could effectively reduce IL-6 level, restore the population of CD8+ and CD4+ T cells, and remove SARS-CoV2 plasma viremia [126]. Despite promising efficacy, leronlimab did not enter into the clinical trial due to some restrictions. However, it seems CCR5 blockage might probably bring advantages for COVID-19 patients. Indeed, it should be noted that among all the tested chemokine and chemokine receptor inhibitors, only anti-CXCL8 has reached phase II of clinical trials for the treatment of COVID-19 [127].
4.2. TPO receptor (TPOR) agonists
Since ITP is one of the main clinical complications in COVID-19, many physicians used TPO receptor agonists (TPO-RAs) as a strategy to overcome thrombocytopenia; however, one of the main concerns is the risk of thrombotic side effects [128], [129]. Given this, the British Society of Hematology (BSH) indicated that it is better to use steroids in COVID-19 patients rather than TPO receptor agonists [130]. In agreement, Pavord et al. indicated that administration of TPO receptor agonists could increase the risk of arterial or venous thrombosis within two weeks [10]. To obviate this challenge, Mah_evas et al. suggested TPO receptor agonists administration together with intravenous immunoglobulin (IVIg; 1–2 g/kg) or prednisone [131]. Their results of 2-month follow-up declared no sign of thrombosis in COVID-19 patients who received TPO receptor agonists according to this schedule. Inline, Lorenzo-Villalba et al. tested the therapeutic potential of TPO receptor agonists named eltrombopag together with IVIG in two COVID-19 patients; their results suggested that this combination could significantly improve the clinical outcome of the patients and prolong their survival [132].
4.3. Corticosteroids
Another approach that has been proposed for COVID-19–related ITP is the administration of corticosteroids, which simultaneously could conquer the systemic inflammatory responses, as well [133]. Dexamethasone was one of the first corticosteroids that has been administrated to hospitalized patients and showed successful results in diminishing the mortality rate [134]. Albani et al. indicated that corticosteroids can not reduce mortality; however, these drugs reduce ICU admission in COVID-19 patients [135]. Of note, some reports delineated that corticosteroids are not beneficial for COVID-19 patients unless they are administrated at an optimal timing, optimal dosage, and in the right schedule [136]. Hu et al. have evaluated the impact of corticosteroids in the treatment of ITP in a COVID-19 patient who did not respond to IVIG and platelet transfusion. In response to methylprednisolone (40 mg/day), not only the number of lymphocytes was significantly increased but also thrombocytopenia quickly went into remission [137]. More interesting, the number of PLTs remained stable and the symptoms of pneumonia improved gradually. The results of this study clearly suggest corticosteroids could counteract the deteriorating effects of the immune system on PLTs and treat thrombocytopenia in COVID-19 patients.
4.4. IVIG
Xie et al. administrated IVIG for critically ill COVID-19 patients and reported this treatment approach could reduce the need of the patients for mechanical ventilation and improve their laboratory examinations and outcomes in less than 48 h [138]. Based on this initial success, Herth et al. treated 12 COVID-19 patients with IVIG and delineated that the duration of hospitalization was significantly lower in this group as compared to those who did not receive IVIG [139]. Shao et al. also suggested that administration of high doses of IVIG to critically ill COVID-19 patients could hamper multi-organ failure and reduce 60-day mortality in severe cases [140]. The most interesting results have been published in a study by Martincic et al. who treated critically ill COVID-19 patients with ITP with dexamethasone (40 mg/day) and IVIG (1 g/Kg) [141]. When they analyzed the complete blood count of the patients, they realized the number of PLTs raised by almost 2,000/mm3 within 12 h after concomitant treatment. Moreover, this combination stopped bleeding and improved oxygenation.
4.5. Recombinant human ACE2
Human recombinant soluble ACE2 (hrsACE2) is one of the most critical therapeutic options that binds to the Spike protein of the virus, and thereby, prevents tissue injury [95]. When administrated intravenously to severe COVID-19 patients for 7 days, human recombinant soluble ACE2 could remarkably reduce the angiotensin II level, hamper IL-6 and IL-8-mediated inflammatory responses, and subsequently, prevent lung injury [142]. Interestingly, the results of both phase I (NCT00886353) and phase II (NCT01597635) clinical studies were positive about the use of human recombinant soluble ACE2 in the treatment of COVID-19 [143], [144]. In another study, the combination of human recombinant soluble ACE2 and remdesivir were tested in Vero E6 and kidney organoids [145]. This combination not only hampered the entrance of SARS-CoV-2 into the cells but also restricted the RNA replication of the virus. It seems that if the efficacy of human recombinant soluble ACE2 would be approved in COVID-19, this approach can robustly ameliorate the devastating effects of SARS-CoV-2 on critical organs and will indirectly improve thrombocytopenia.
4.6. Platelet transfusion
One of the therapeutic approaches that is considered for COVID-19 patients with thrombocytopenia and life-threatening hemorrhage is platelet transfusions, but serious caution should be measured as unnecessary transfusions may increase the risk of thrombotic events in patients with coagulopathy or ITP [10]. Wu et al. indicated that platelet transfusion together with 10 mg dexamethasone could remarkably increase PLT count by 30 × 109/L and ameliorate the bleeding in COVID-19 patients [146]. Lévesque et al. also treated a COVID-19 patient with ITP and bleeding event with IVIG (1 g/Kg), dexamethasone (40 mg/day), and several PLT and RBC transfusions [147]. The authors indicated that this strategy was successful in the cessation of bleeding and increasing the number of PLT in patients. However, when Martincic et al. has treated the COVID-19-induced ITP only with PLT transfusion, the patient lost his life within 24 hr [141]. Since the incidence of bleeding in COVID-19 patients is rare, PLT transfusion should be only considered for those with active bleeding. If a thrombocytopenic patient did not develop bleeding, it is recommended to manage the patient according to the ISTH guidelines for DIC.
4.7. Traditional Chinese medicine (TCM)
From the first episodes of the COVID-19 pandemic, tremendous attention has been attracted to traditional medicines and their impacts on the outcome of the patients. Amid them, traditional Chinese medicine (TCM) has enjoyed unprecedented success. An et al. indicated that traditional Chinese medicine could effectively increase the number of WBC, decreased the serum levels of IL-6 and procalcitonin, and more importantly elevated the number of PLTs in patients [148]. Inline,Chen et al. treated one group of patients with Jianpi Yiqi Shexue (JYS) granules and the other group with prednisone; although prednisolone more significantly increased the number of PLTs, Jianpi Yiqi Shexue could stop bleeding more rapidly in the patients [149]. Notably, they found that the combination of prednisolone and Jianpi Yiqi Shexue was more efficient in ITP as compared to either agent alone. Shen-Cao granule is another traditional Chinese medicine with the ability to increase the number of PLTs [150]. Hirano et al. also evaluated the efficacy of EK-49 and ascorbic acid in idiopathic ITP patients and suggested that while these agents could overcome thrombocytopenia, the conventional treatments such as prednisolone and azathioprine failed to recover low platelets [151]. Taken together, it seems that traditional Chinese medicine could be positioned as a pillar of thrombocytopenia treatment; however, it should not be forgotten that the safety and the tolerability of these agents have not been tested in COVID-19 patients yet. A list of clinical trials summarizing all these approaches was presented in Table 2 .
Table 2.
A list of clinical trials investigating the efficacies of therapeutic approaches in COVID-19.
| Drug | Mechanism | No. | Phase | Status | Aim and outcome | Identifier | |
|---|---|---|---|---|---|---|---|
| Monoclonal Abs | |||||||
| Tocilizumab | mAb against IL-6 | 243 | Phase 3 | Completed | This is a randomized, double blind, multi-center study to evaluate the effects of tocilizumab compared to placebo on the multi-organ dysfunction and outcomes of hospitalized patients with COVID-19. | NCT04356937 | |
| Sarilumab | mAb against IL-6 | 420 | Phase 3 | Completed | An adaptive Phase 3, randomized, double-blind, placebo-controlled study to assess efficacy and safety of Sarilumab in adult patients hospitalized with severe or critical COVID-19. | NCT04327388 | |
| Siltuximab(Sylvant) | IL-6 neutralization | 220 | NA | Completed | This observational study evaluated efficacy and safety of Siltuximab for treatment of SARS-CoV-2 infection complicated with serious respiratory complications. | NCT04322188 | |
| Canakinumab | IL-1R antagonist | 451 | Phase 3 | Completed | This is a multicenter, randomized, double-blind, placebo-controlled study to assess the efficacy and safety of Canakinumab-plus-SOC in patients with COVID-19-induced pneumonia and CRS. | NCT04362813 | |
| Anakinra | IL-1R antagonist | 80 | Phase 2 | Recruiting | Anakinra had showed survival benefits in MAS and sepsis and showed promising outcomes for the use in COVID-19. | NCT04643678 | |
| Anakinra | IL-1R antagonist | 170 | Phase 2 | Not yet recruiting | This study will determine the efficacy of IL-1R blockade in reducing the need for mechanical ventilation and/or 28-day mortality among patients with COVID-19 who have features of CSS and severe respiratory failure. | NCT04603742 | |
| BMS-986253 | Anti-IL-8 | 138 | Phase 2 | Recruiting | This is the first in-human study to evaluate whether neutralizing IL-8 with BMS-986253 can help improve the health condition of severe hospitalized COVID-19 patients. | NCT04347226 | |
| Infliximab | TNFα blocker | 17 | Phase 2 | Completed | This is a prospective, single center, phase 2 trial to assess the efficacy of TNFα inhibitor therapy in hospitalized adult patients with severe or critical COVID-19. | NCT04425538 | |
| AMY-101 | C3 Inhibitor | 144 | Phase 2 | Not yet recruiting | This study will assess the efficacy and safety, as well as PK and PD of AMY-101 in patients with severe COVID-19. | NCT04395456 | |
| Eculizumab | C5 Inhibitor | NA | NA | Available | Eculizumab will be used to modulate the activity of the distal complement preventing the formation of MAC. By this, mortality can be halted while the patient has time to recover from the virus with supportive medical care. | NCT04288713 | |
| Vilobelimab (IFX-1) | anti-C5a antibody | 390 | Phase 2/3 | Recruiting | Consists of i) Phase 2, open-label, randomized evaluating BSC + IFX-1 (Arm A) and BSC alone (Arm B); ii) Phase 3, double-blind, placebo-controlled, randomized comparing SOC + IFX-1 (Arm A) and SOC + placebo-to-match (Arm B). | NCT04333420 | |
| Cenicriviroc (CVC) | CCR2/CCR5 Inhibitor | 183 | Phase 2 | Recruiting | To evaluate the safety and efficacy of Cenicriviroc to reduce the severity of COVID-19. Also, to test if patients with pre-existing conditions, who have an increased risk of severe COVID-19 progression, benefit more. | NCT04500418 | |
| Maraviroc | CCR5 antagonists | 9 | Phase 1 | Completed | This study seeks to establish whether one week of treatment with Maraviroc, used at its approved dosage for HIV, is safe and tolerable in patients with SARS-CoV-2. | NCT04435522 | |
| Leronlimab (PRO 140) | CCR5 antagonist | 56 | Phase 2 | Active, not recruiting | The purpose of this study is to assess the safety and efficacy of Leronlimab administered as weekly subcutaneous injections in subjects experiencing prolonged symptoms (greater than12 weeks) of COVID-19. | NCT04678830 | |
| Reparixin | CXCR1/2 antagonist | 55 | Phase 2 | Terminated | To evaluate the efficacy and safety of Reparixin treatment as compared to the control arm in adult patients with severe COVID-19 pneumonia. | NCT04794803 | |
| Anti-platelet agents | |||||||
| Tirofiban | IIb/IIIa receptor inhibitor | 5 | Phase 2 | Completed | This study will evaluate the effects of compassionate-use treatment with IV tirofiban 25 mcg/kg, associated with acetylsalicylic acid IV, clopidogrel PO and fondaparinux 2.5 mg s/c, in COVID-19 patients. | NCT04368377 | |
| clopidogrel | P2Y12 antagonist | 750 | Phase 4 | Recruiting | This study will evaluate the efficacy of full-dose vs. standard prophylactic dose anticoagulation and of antiplatelet vs. no antiplatelet therapy for prevention of thrombosis in critically-ill COVID-19 patients. | NCT04409834 | |
| Ticagrelor | P2Y12 antagonist | 2000 | Phase 4 | Recruiting | This is a randomized, open label, adaptive platform trial to compare the effectiveness of antithrombotic strategies for prevention of adverse outcomes in COVID-19 cases. | NCT04505774 | |
| Prasugrel | P2Y12 antagonist | 128 | Phase 3 | Not yet recruiting | The prevention of thrombogenic platelet activity with a P2Y12 inhibitor is superior to fixed dose enoxaparin alone. This treatment is feasible in all patients, regardless of the treatment regimen, except for specific contraindications. | NCT04445623 | |
| Aspirin | Cyclooxygenase Inhibition | 128 | Phase 3 | Enrolling by invitation | The early use of aspirin in COVID-19 patients, which has the effects of inhibiting virus replication, anti-platelet aggregation, anti-inflammatory and anti-lung injury, is expected to be beneficial. | NCT04365309 | |
| Dipyridamole | Inhibition of cAMP-phosphodiesterase | 100 | Phase 2 | Recruiting | Dipyridamole, which has anti-platelet and anti-inflammatory effects, may be useful in COVID-19 cases. | NCT04424901 | |
| TPO receptor agonists | |||||||
| EPAG | TPOR agonist | 120 | Phase 2 | Recruiting | A prospective, multicenter, randomized, open-label study to investigate the efficacy and safety of Eltrombopag plus rhTPO versus Eltrombopag as treatment for corticosteroid-resistant or relapsed ITP during the COVID-19 pandemic. | NCT04516837 | |
| Corticosteroids | |||||||
| Corticosteroid | Anti-inflammation | 86 | NA | Completed | This is a prospective randomized controlled trails to explore the effectiveness and safety of glucocorticoids in the treatment of novel coronavirus pneumonia. | NCT04273321 | |
| Corticosteroid | Anti-inflammation | 450 | Phase 4 | Recruiting | Timing of corticosteroids administration is very important in COVID-19 for the recovery and mortality decrease. | NCT04530409 | |
| Corticosteroid | Anti-inflammation | 184 | Phase 3 | Recruiting | Early use of corticosteroids, low dose, in mild disease, can decrease progression to respiratory failure and death. | NCT04451174 | |
| Dexamethasone | Anti-inflammation | 284 | Phase 3 | Recruiting | To randomly evaluate the efficacy and safety of the use of dexamethasone, a parenteral corticosteroid approved in Argentina, in patients COVID-19-induced ARDS. | NCT04395105 | |
| MP | Anti-inflammation | 173 | NA | Completed | A trial to analyze the association of low dose prolonged infusion of MP for patients with severe acute respiratory syndrome with composite primary end-point (ICU referral, need for intubation, in-hospital death at day 28). | NCT04323592 | |
| IVIG | |||||||
| IVIG | Immunomodulation | 76 | Phase 3 | Completed | To assess the efficacy of IVIG (medication trade name: Bioven) in the high immunomodulatory dose in complex treatment of severe pneumonia caused by SARS-CoV-2. | NCT04500067 | |
| IVIG | Immunomodulation | 50 | Phase 1/2 | Completed | To assess clinical efficacy and safety of single dose of Intravenously administered IVIG developed from convalescent plasma of recovered COVID-19 individual in severe and critically ill patients. | NCT04521309 | |
| IVIG | Immunomodulation | 10 | Phase 4 | Not yet recruiting | To evaluate the effect of IVIG on the hospital length of stay as well as production of inflammatory and non-inflammatory cytokines, biomarkers for endothelial injury, and biomarkers for coagulation via Mass Spectrometry. | NCT04616001 | |
| IVIG | Immunomodulation | 100 | Phase 2 | Completed | To determine if high dose intravenous IVIG plus SMT can reduce the proportion of participants dying or requiring ICU admission on or before day 29 or who are dependent on high flow oxygen devices or mechanical ventilation. | NCT04432324 | |
| Recombinant human ACE2 | |||||||
| RhACE2 APN01 | Neutralizing SARS-CoV-2 | 200 | Phase 2 | Completed | To evaluate the effect of rhACE2 as a treatment for patients with COVID-19 to block viral entry and decrease viral replication. | NCT04335136 | |
| rhACE2 | Neutralizing SARS-CoV-2 | ---- | NA | Withdrawn | This is an open label, randomized, controlled, pilot clinical study in patients with COVID-19, to obtain preliminary biologic, physiologic, and clinical data in patients with COVID-19 treated with rhACE2 or control patients. | NCT04287686 | |
| ATR | Neutralizing SARS-CoV-2 | 1500 | Phase 4 | Recruiting | A pragmatic prospective, open-label, randomized controlled trial to examine the effectiveness of ARBs on improving the outcomes of people who tested positive for COVID-19. | NCT04394117 | |
| Anticoagulation therapy | |||||||
| Tinzaparin or Dalteparin | Anticoagulant | 166 | NA | Completed | To evaluate the efficacy of anticoagulation regime on the outcomes of critically ill patients via description of baseline characteristics and comorbidities before admission, and associate it with 28 days survival, survival outside ICU, thromboembolic event, and bleeding complications. | NCT04412304 | |
| Enoxaparin | Anticoagulant | 77 | Phase 2 | Terminated | A randomized open label trial to compare effectiveness of two dosing regimens currently used for prevention of clotting events in COVID-19 positive inpatients. | NCT0435927 | |
| Rivaroxaban | Anticoagulant | 400 | Phase 2 | Recruiting | Patients randomized into the rivaroxaban arm receive rivaroxaban OD until day 7 post randomization or hospital discharge, whichever occurs later, followed by a 28-day-phase of prophylactic anticoagulation. | NCT04416048 | |
| Heparin/ P2Y12 | Anticoagulant | 2000 | Phase 4 | Recruiting | A randomized, open label, adaptive platform trial to compare the effectiveness of antithrombotic strategies for prevention of adverse outcomes in COVID-19 positive inpatients | NCT04505774 | |
| Heparin sodium | Anticoagulant | 200 | Phase 4 | Recruiting | The combination of inhalation heparin combined with prophylactic doses of LMWH could reduce the progression to severe forms of the disease, and consequently the need for intensive care units and mechanical ventilation. | NCT04530578 | |
| Antithrombin | Anticoagulant | 48 | Phase 2 | Completed | A pilot clinical trial, single-center, exploratory, open, randomized, controlled, to study the efficacy and safety of human Antithrombin in patients with confirmed COVID-19 disease and criteria high risk to develop SARS. | NCT04745442 | |
| Antithrombin | Anticoagulant | 300 | NA | Recruiting | A multi-center, multinational, non-interventional, observational, retrospective, patient record study to assess changes in coagulation parameters in patients with severe COVID-19 receiving/not treatment with Antithrombin. | NCT04651400 | |
| TXA | Antifibrinolytic | 60 | Phase 2 | Not yet recruiting | A controlled trial of the drug TXA in inpatients recently admitted to the hospital with the diagnosis of COVID19. It is hypothesized that TXA will reduce the infectivity and virulence of the virus. | NCT04338126 | |
| TXA | Antifibrinolytic | 100 | Phase 2 | Not yet recruiting | A randomized, double-blind placebo controlled exploratory trial in order to determine whether TXA reduces infectivity and virulence of the SARS-CoV-2 virus. | NCT04550338 | |
| TXA | Antifibrinolytic | 100 | Phase 2 | Recruiting | A controlled trial of TXA in outpatients who were recently diagnosed with COVID-19. It is hypothesized that TXA will reduce the infectivity and virulence of the virus. | NCT04338074 | |
| Platelet transfusion | |||||||
| PRP | Increased platelets | 100 | NA | Recruiting | To evaluate the effect of PRP and cord blood in improving the symptoms of patients with COVID-19. | NCT04393415 | |
| aaPRP | Anti-inflammation | 30 | Phase 2 | Recruiting | To evaluate the potential of aaPRP and the outcomes for treating severe COVID-19 patients in ICU. | NCT04715360 | |
| Nebulized platelet lysate | Anti-inflammation; Immunomodulation | 1 | NA | Active, not recruiting | To evaluate and compare nebulized platelet lysate to placebo control of saline administered via handheld nebulizer 1X daily for eight weeks to determine its effect on lung function in patients with post-COVID-19 ARDS syndrome. | NCT04487691 | |
mAb: Monoclonal antibody; IL: Interleukin; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; SOC: Standard-of-care; CRS: Cytokine release syndrome; MAS: Macrophage activation syndrome; CSS: Cytokine storm syndrome; TNFα: Tumor necrosis factorα; PK: Pharmacokinetics; PD: Pharmacodynamics; BSC: Best supportive care; CVC: Cenicriviroc; CCR: C-C chemokine receptor; HIV: Human immunodeficiency virus; CXCR: CXC chemokine receptor; EPAG: Eltrombopag; TPOR: Thrombopoietin receptor; rhTPO: Recombinant human thrombopoietin; ITP: Immune thrombocytopenia; ARDS: Acute respiratory distress syndrome; MP: Methylprednisolone; ICU: Intensive treatment unit; IVIG: Intravenous immunoglobulin; SMT: Standard medical treatment; ACE2: Angiotensin-converting enzyme; rhACE2: Recombinant human angiotensin-converting enzyme 2; ATR: Angiotensin receptor blockers; ARBs: Angiotensin II receptor blockers; OD: Once daily; LMWH: Low-molecular-weight heparin; TXA: Tranexamic acid; PRP: Platelet-rich plasma; aaPRP: Autologous activated platelet-rich plasma; No.: Number of patients; NA: Not available.
5. Conclusion and future perspective
Numerous attempts were done to tackle thrombocytopenia in COVID-19 patients, but unfortunately, it seems that SARS-CoV-2 reduces the number of PLTs by using different mechanisms from patient to patient. For example, while the virus might induce thrombocytopenia by targeting the HSCs or megakaryocytes in a group of patients, it may increase the clearance of PLTs due to excessive response of the immune system in some other cases (Fig. 4 ). As the mechanism differs, the strategies to cure thrombocytopenia might be different, as well; in this vein, the interdiction of PLT transfusion for patients with ITP and heparin administration for those with HIT are good examples. These challenges proposed that the treatment of thrombocytopenia in COVID-19 patients is not as simple as it appears and serious cautions should be considered to deal with the problem. Thus far, several FDA-approved approaches such as tocilizumab, eculizumab, and ravulizumab succeeded to recover the PLT count; however, no drug or treatment has yet been found to efficiently increase platelets in COVID-19 patients. Perhaps, the key to propose an appropriate approach for treating thrombocytopenia is in the hand of further molecular and sequencing analysis of the virus to shed more light on the precise mechanisms through which SARS-CoV-2 disturbs the tune balance between pro- and anticoagulant pathways, diminishes the number of PLTs, and orchestrates catastrophic events to make the disease more complicated.
Fig. 4.
The brief story behind the inhibitory effects of SARS-CoV-2 on platelets. Upon infection with SARS-CoV-2, a group of events takes place to impose destructive effects on platelets. The unrestrained production of inflammatory cytokines coupled with decreased interaction of TPO/ TPO receptor can result in thrombocytopenia via hampering PLTs production. On the other hand, improper immune responses lead to autoantibody-mediated destruction of PLTs in the reticuloendothelial system. Besides, increased consumption of PLTs either due to SARS-CoV-2-induced tissue injury or heparin-induced thrombocytopenia may be responsible for the low number of PLTs in COVID-19 cases. Overall, all the above-mentioned events go hand in hand to disturb the tune balance between pro- and anticoagulant pathways, diminish the number of PLTs, and orchestrate catastrophic events to make the disease more complicated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors would like to express their gratitude to Shahid Beheshti University of Medical Sciences (Tehran, Iran) for supporting this study.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Guan W.-J., Ni Z.-y., Hu Y.u., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-l., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L.i., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. Clinical characteristics of coronavirus disease 2019 in China. New England J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashemieh M., Tabatabaee S., Radfar M., Fahim P. COVID-19 Associated Thrombocytopenia in Children: An Emerging Issue. Int. J. Pediatrics. 2021;9(6):13635–13642. [Google Scholar]
- 3.Maquet J., Lafaurie M., Sommet A., Moulis G. Thrombocytopenia is independently associated with poor outcome in patients hospitalized for COVID-19. Br. J. Haematol. 2020;190(5):e276–e279. doi: 10.1111/bjh.16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rampotas A., Pavord S. Platelet aggregates, a marker of severe COVID-19 disease. J. Clin. Pathol. 2020 doi: 10.1136/jclinpath-2020-206933. [DOI] [PubMed] [Google Scholar]
- 5.Comer S.P., Cullivan S., Szklanna P.B., Weiss L., Cullen S., Kelliher S., Smolenski A., Murphy C., Altaie H., Curran J., O’Reilly K., Cotter A.G., Marsh B., Gaine S., Mallon P., McCullagh B., Moran N., Ní Áinle F., Kevane B., Maguire P.B., Siegerink B. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19(2):e3001109. doi: 10.1371/journal.pbio.3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.J.E. Gómez-Mesa, S. Galindo-Coral, M.C. Montes, A.J.M. Martin, Thrombosis and Coagulopathy in COVID-19, Current problems in cardiology (2020) 100742. [DOI] [PMC free article] [PubMed]
- 7.Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M. The unique characteristics of COVID-19 coagulopathy. Crit. Care. 2020;24(1):1–8. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao C., Tao X., Cui W., Yi B., Pan T., Young K.H., Qian W. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Experim. Hematol. Oncol. 2020;9(1):1–8. doi: 10.1186/s40164-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bomhof G., Mutsaers P.G.N.J., Leebeek F.W.G., Boekhorst P.A.W., Hofland J., Croles F.N., Jansen A.J.G. COVID-19-associated immune thrombocytopenia. Br. J. Haematol. 2020;190(2) doi: 10.1111/bjh.v190.210.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavord S., Thachil J., Hunt B.J., Murphy M., Lowe G., Laffan M., Makris M., Newland A.C., Provan D., Grainger J.D., Hill Q.A. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Br. J. Haematol. 2020;189(6):1038–1043. doi: 10.1111/bjh.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nham E., Ko J.-H., Jeong B.-H., Huh K., Cho S.Y., Kang C.-I., Chung D.R., Peck K.R. Severe Thrombocytopenia in a Patient with COVID-19. Infection & chemotherapy. 2020;52(3):410. doi: 10.3947/ic.2020.52.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y., Zhang J., Li Y., Liu F., Zhou Q., Peng Z., Cox D. Association between thrombocytopenia and 180-day prognosis of COVID-19 patients in intensive care units: A two-center observational study. PLoS ONE. 2021;16(3):e0248671. doi: 10.1371/journal.pone.0248671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X., Yang Q., Wang Y., Wu Y., Xu J., Yu Y., Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020;18(6):1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang A., Leng Y., Zhang Y.i., Wu K., Ji Y., Lei S., Xia Z. Meta-analysis of coagulation parameters associated with disease severity and poor prognosis of COVID-19. Int. J. Infect. Dis. 2020;100:441–448. doi: 10.1016/j.ijid.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bashash D., Hosseini-Baharanchi F.S., Rezaie-Tavirani M., Safa M., Dilmaghani N.A., Faranoush M., Abolghasemi H. The prognostic value of thrombocytopenia in COVID-19 patients; a systematic review and meta-analysis. Arch. Acad. Emergency Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang S.Q., Huang Q.F., Xie W.M., Lv C., Quan X.Q. The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br. J. Haematol. 2020;190(1):e29–e33. doi: 10.1111/bjh.16817. [DOI] [PubMed] [Google Scholar]
- 18.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease, (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2019;506(2020):145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Xiaofang, Wang Kun, Zuo Peiyuan, Liu Yuwei, Zhang Meng, Xie Songpu, Zhang Hao, Chen Xinglin, Liu Chengyun. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients—indications for predictive, preventive, and personalized medical approach. EPMA J. 2020;11(2):139–145. doi: 10.1007/s13167-020-00208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Güçlü E., Kocayiğit H., Okan H.D., Erkorkmaz U., Yürümez Y., Yaylacı S., Koroglu M., Uzun C., Karabay O. Effect of COVID-19 on platelet count and its indices. Revista da Associação Médica Brasileira. 2020;66(8):1122–1127. doi: 10.1590/1806-9282.66.8.1122. [DOI] [PubMed] [Google Scholar]
- 21.He J., Wei Y., Chen J., Chen F., Gao W., Lu X. Dynamic trajectory of platelet-related indicators and survival of severe COVID-19 patients. Crit. Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-03339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong Qingyang, Peng Jie. Mean platelet volume/platelet count ratio predicts severe pneumonia of COVID-19. J. Clin. Lab. Anal. 2021;35(1) doi: 10.1002/jcla.v35.110.1002/jcla.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aktaş A., Sener K., Yılmaz N., Tunç M., Yolcu S. Is Mean Platelet Volume Useful for Predicting the Prognosis of COVID-19 Diagnosed Patients? Age. 2020;54(18,191):18,191. [Google Scholar]
- 24.Guner Ozenen G., Sahbudak Bal Z., Umit Z., Bilen N.M., Yildirim Arslan S., Yurtseven A., Saz E.U., Burcu B., Sertoz R., Kurugol Z. Demographic, clinical, and laboratory features of COVID-19 in children: The role of mean platelet volume in predicting hospitalization and severity. J. Med. Virol. 2021;93(5):3227–3237. doi: 10.1002/jmv.26902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hille Laura, Lenz Maximilian, Vlachos Andreas, Grüning Björn, Hein Lutz, Neumann Franz‐Josef, Nührenberg T.G., Trenk Dietmar. Ultrastructural, transcriptional, and functional differences between human reticulated and non-reticulated platelets. J. Thromb. Haemost. 2020;18(8):2034–2046. doi: 10.1111/jth.14895. [DOI] [PubMed] [Google Scholar]
- 26.Ranucci Marco, Ballotta Andrea, Di Dedda Umberto, Bayshnikova Ekaterina, Dei Poli Marco, Resta Marco, Falco Mara, Albano Giovanni, Menicanti Lorenzo. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen Amir, Harari Emanuel, Cipok Michal, Laish-Farkash Avishag, Bryk Gabriel, Yahud Ella, Sela Yaron, Lador Nili Karp, Mann Tal, Mayo Ami, Lev Eli I. Immature platelets in patients hospitalized with Covid-19. J. Thromb. Thrombol. 2021;51(3):608–616. doi: 10.1007/s11239-020-02290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Q. Wu, J. Ren, D. Hu, P. Jiang, G. Li, N. Anjum, G. Wang, G. Gu, J. Chen, X. Wu, An elevated percentage of reticulated platelet is associated with increased mortality in septic shock patients, Medicine 94(19) (2015). [DOI] [PMC free article] [PubMed]
- 29.B.K. Manne, F. Denorme, E.A. Middleton, I. Portier, J.W. Rowley, C. Stubben, A.C. Petrey, N.D. Tolley, L. Guo, M. Cody, Platelet gene expression and function in patients with COVID-19, Blood, The Journal of the American Society of Hematology 136(11) (2020) 1317-1329. [DOI] [PMC free article] [PubMed]
- 30.N. Yatim, J. Boussier, R. Chocron, J. Hadjadj, L. Barnabei, B. Charbit, T.-A. Szwebel, N. Carlier, F. Pène, C. Azoulay, Dysregulated primary hemostasis in critically ill COVID-19 patients, (2020).
- 31.Goshua George, Pine Alexander B, Meizlish Matthew L, Chang C-Hong, Zhang Hanming, Bahel Parveen, Baluha Audrey, Bar Noffar, Bona Robert D, Burns Adrienne J, Dela Cruz Charles S, Dumont Anne, Halene Stephanie, Hwa John, Koff Jonathan, Menninger Hope, Neparidze Natalia, Price Christina, Siner Jonathan M, Tormey Christopher, Rinder Henry M, Chun Hyung J, Lee Alfred I. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassiliou A.G., Keskinidou C., Jahaj E., Gallos P., Dimopoulou I., Kotanidou A., Orfanos S.E. ICU admission levels of endothelial biomarkers as predictors of mortality in critically ill COVID-19 patients. Cells. 2021;10(1):186. doi: 10.3390/cells10010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wool G.D., Miller J.L. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2021;88(1):15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.E.D. Hottz, I.G. Azevedo-Quintanilha, L. Palhinha, L. Teixeira, E.A. Barreto, C.R. Pão, C. Righy, S. Franco, T.M. Souza, P. Kurtz, Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19, Blood, The Journal of the American Society of Hematology 136(11) (2020) 1330-1341. [DOI] [PMC free article] [PubMed]
- 35.Le Joncour Alexandre, Biard Lucie, Vautier Mathieu, Bugaut Helene, Mekinian Arsene, Maalouf Georgina, Vieira Matheus, Marcelin Anne-Geneviève, Rosenzwajg Michelle, Klatzmann David, Corvol Jean-Christophe, Paccoud Olivier, Carillion Aude, Salem Joe-Elie, Cacoub Patrice, Boulaftali Yacine, Saadoun David. Neutrophil-Platelet and Monocyte-Platelet Aggregates in COVID-19 Patients. Thromb. Haemost. 2020;120(12):1733–1735. doi: 10.1055/s-0040-1718732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarbock Alexander, Singbartl Kai, Ley Klaus. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Investig. 2006;116(12):3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Y. Zhou, Z. Liu, H. Yang, J. Wang, M.J. Li, Coagulation factors and COVID-19 severity: Mendelian randomization analyses and supporting evidence, medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 38.Di Micco P., Russo V., Carannante N., Imparato M., Rodolfi S., Cardillo G., Lodigiani C. Clotting factors in COVID-19: epidemiological association and prognostic values in different clinical presentations in an Italian cohort. J. Clin. Med. 2020;9(5):1371. doi: 10.3390/jcm9051371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vajari M.K., Shirin M., Pourbagheri-Sigaroodi A., Akbari M.E., Abolghasemi H., Bashash D. COVID-19-related coagulopathy: a review of pathophysiology and pharmaceutical management. Cell Biol. Int. 2021 doi: 10.1002/cbin.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y.-P., Li G.-M., He J., Liu Y., Li M., Zhang R., Li Y.-L., Wu Y.-Z., Diao B. Combined use of the neutrophil-to-lymphocyte ratio and CRP to predict 7-day disease severity in 84 hospitalized patients with COVID-19 pneumonia: a retrospective cohort study. Annals Translational Med. 2020;8(10):635. doi: 10.21037/atm-20-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Y. Zaid, F. Puhm, I. Allaeys, A. Naya, M. Oudghiri, L. Khalki, Y. Limami, N. Zaid, K. Sadki, R.B. El Haj, Platelets can contain SARS-CoV-2 RNA and are hyperactivated in COVID-19, medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 42.Sayad Babak, Rahimi Zohreh. Blood coagulation parameters in patients with severe COVID-19 from Kermanshah Province. Islamic Republic of Iran, Eastern Mediterranean Health J. 2020;26(9):999–1004. doi: 10.26719/emhj.20.105. [DOI] [PubMed] [Google Scholar]
- 43.Turgutalp Kenan, Ozturk Savas, Arici Mustafa, Eren Necmi, Gorgulu Numan, Islam Mahmut, Uzun Sami, Sakaci Tamer, Aydin Zeki, Sengul Erkan, Demirelli Bulent, Ayar Yavuz, Altiparmak Mehmet Riza, Sipahi Savas, Mentes Ilay Berke, Ozler Tuba Elif, Oguz Ebru Gok, Huddam Bulent, Hur Ender, Kazancioglu Rumeyza, Gungor Ozkan, Tokgoz Bulent, Tonbul Halil Zeki, Yildiz Alaattin, Sezer Siren, Odabas Ali Riza, Ates Kenan. Determinants of mortality in a large group of hemodialysis patients hospitalized for COVID-19. BMC Nephrol. 2021;22(1) doi: 10.1186/s12882-021-02233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kashefizadeh A., Ohadi L., Golmohammadi M., Araghi F., Dadkhahfar S. Clinical features and short-term outcomes COVID-19 in Tehran, Iran: An analysis of mortality and hospital stay. Acta Bio Med. Atenei Parmensis. 2020;91(4) doi: 10.23750/abm.v91i4.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kashiv P., Kamendu A., Kumar J., Kumar N. Study of platelet count in covid-19 positive patients admitted in a tertiary care hospital of South Bihar. Int. J. Health Clin. Res. 2021;4(2):263–268. [Google Scholar]
- 46.M. Lagadinou, E. Salomou, N. Zareifopoulos, M. Marangos, C. Gogos, D. Velissaris, Prognosis of COVID-19: Changes in laboratory parameters, Age (yrs) 62(13.4) (2020) 47-16.42. [PubMed]
- 47.Fumagalli Carlo, Rozzini Renzo, Vannini Matteo, Coccia Flaminia, Cesaroni Giulia, Mazzeo Francesca, Cola Maria, Bartoloni Alessandro, Fontanari Paolo, Lavorini Federico, Marcucci Rossella, Morettini Alessandro, Nozzoli Carlo, Peris Adriano, Pieralli Filippo, Pini Riccardo, Poggesi Loredana, Ungar Andrea, Fumagalli Stefano, Marchionni Niccolò. Clinical risk score to predict in-hospital mortality in COVID-19 patients: a retrospective cohort study. BMJ Open. 2020;10(9):e040729. doi: 10.1136/bmjopen-2020-040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.M. Colaneri, P. Sacchi, V. Zuccaro, S. Biscarini, M. Sachs, S. Roda, T.C. Pieri, P. Valsecchi, A. Piralla, E. Seminari, Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020, Eurosurveillance 25(16) (2020) 2000460. [DOI] [PMC free article] [PubMed]
- 49.Jang J.G., Hur J., Choi E.Y., Hong K.S., Lee W., Ahn J.H. Prognostic factors for severe coronavirus disease 2019 in Daegu, Korea. J. Korean Med. Sci. 2020;35(23) doi: 10.3346/jkms.2020.35.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcolino M.S., Ziegelmann P.K., Souza-Silva M.V., Nascimento I., Oliveira L.M., Monteiro L.S., Sales T.L., Ruschel K.B., Martins K.P., Etges A.P.B. Clinical characteristics and outcomes of patients hospitalized with COVID-19 in Brazil: results from the Brazilian COVID-19 Registry. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gowda S.B., Gosavi S., Rao A.A., Shastry S., Raj S.C., Menon S., Suresh A., Sharma A. Prognosis of COVID-19: Red Cell Distribution Width, Platelet Distribution Width, and C-Reactive Protein. Cureus. 2021;13(2) doi: 10.7759/cureus.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozder A. A novel indicator predicts 2019 novel coronavirus infection in subjects with diabetes. Diabetes Res. Clin. Pract. 2020;166 doi: 10.1016/j.diabres.2020.108294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taha Muhanad, Sano Dahlia, Hanoudi Samer, Esber Zahia, Elahi Morvarid, Gabali Ali, Chopra Teena, Draghici Sorin, Samavati Lobelia. Platelets and renal failure in the SARS-CoV-2 syndrome. Platelets. 2021;32(1):130–137. doi: 10.1080/09537104.2020.1817361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.J. Linssen, A. Ermens, M. Berrevoets, M. Seghezzi, G. Previtali, H. Russcher, A. Verbon, J. Gillis, J. Riedl, E. de Jongh, A novel haemocytometric COVID-19 prognostic score developed and validated in an observational multicentre European hospital-based study, Elife 9 (2020) e63195. [DOI] [PMC free article] [PubMed]
- 55.Agrati Chiara, Bordoni Veronica, Sacchi Alessandra, Grassi Germana, Petrosillo Nicola, Nicastri Emanuele, Del nonno Franca, D'offizi Giampiero, Palmieri Fabrizio, Marchioni Luisa, Capobianchi Maria rosaria, Antinori Andrea, Ippolito Giuseppe, Bibas Michele. Elevated P-Selectin in severe Covid-19: considerations for therapeutic options. Mediterranean J. Hematol. Infect. Dis. 2021;13(1):e2021016. doi: 10.4084/mjhid.2021.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.S. Lopez-Castaneda, N. García-Larragoiti, A. Cano-Mendez, K. Blancas-Ayala, G. Damian-Vázquez, A.I. Perez-Medina, L.D. Chora-Hernández, C. Arean-Martínez, M.E. Viveros-Sandoval, Inflammatory and Prothrombotic Biomarkers Associated With the Severity of COVID-19 Infection, Clinical and Applied Thrombosis/Hemostasis 27 (2021) 1076029621999099. [DOI] [PMC free article] [PubMed]
- 57.Arachchillage Deepa R.J., Laffan Mike. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(5):1233–1234. doi: 10.1111/jth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang Ning, Bai Huan, Chen Xing, Gong Jiale, Li Dengju, Sun Ziyong. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.W. Alhazzani, M. Møller, Y. Arabi, M. Loeb, M. Gong, E. Fan, & Du, B.(2020). Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Intensive care medicine 1-34. [DOI] [PMC free article] [PubMed]
- 62.Gazzaruso Carmine, Mariani Giuseppe, Ravetto Carolina, Malinverni Laura, Tondelli Elena, Cerrone Maria, Sala Vittorio, Bevilacqua Luigi, Altavilla Teodoro, Coppola Adriana, Gallotti Pietro. Lupus anticoagulant and mortality in patients hospitalized for COVID-19. J. Thromb. Thrombol. 2021;52(1):85–91. doi: 10.1007/s11239-020-02335-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Panyang, Zhou Qi, Xu Jiancheng. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020;99(6):1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Mo, Ng Margaret H.L., Li Chi Kong, Chan Paul K.S., Liu Chang, Ye Jie Yu, Chong Beng H. Thrombopoietin levels increased in patients with severe acute respiratory syndrome. Thromb. Res. 2008;122(4):473–477. doi: 10.1016/j.thromres.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang M., Ng M.H., Li C.K. Thrombocytopenia in patients with severe acute respiratory syndrome. Hematology. 2005;10(2):101–105. doi: 10.1080/10245330400026170. [DOI] [PubMed] [Google Scholar]
- 66.Liao D., Zhou F., Luo L., Xu M., Wang H., Xia J., Gao Y., Cai L., Wang Z., Yin P. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671–e678. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virologica Sinica. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357(6377):420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.J. Ropa, S. Cooper, M.L. Capitano, W. Van’t Hof, H.E. Broxmeyer, Human hematopoietic stem, progenitor, and immune cells respond ex vivo to SARS-CoV-2 spike protein, Stem cell reviews and reports (2020) 1-13. [DOI] [PMC free article] [PubMed]
- 72.K.A.M. Pate, C.E. Lyons, J.L. Dorsey, S.E. Queen, R.J. Adams, C.N. Morrell, J.L. Mankowski, TGFβ mediated downregulation of thrombopoietin is associated with platelet decline in asymptomatic SIV infection, Journal of acquired immune deficiency syndromes (1999) 65(5) (2014) 510. [DOI] [PMC free article] [PubMed]
- 73.Afdhal N., McHutchison J., Brown R., Jacobson I., Manns M., Poordad F., Weksler B., Esteban R. Thrombocytopenia associated with chronic liver disease. J. Hepatol. 2008;48(6):1000–1007. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Deruelle E. Ben Hadj Salem O, Sep Hieng S, Pichereau C, Outin H, Jamme M, Immune thrombocytopenia in a patient with COVID-19. Int. J. Hematol. 2020;112:883–888. doi: 10.1007/s12185-020-02943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 76.Yamane A., Nakamura T., Suzuki H., Ito M., Ohnishi Y., Ikeda Y., Miyakawa Y. Interferon-α2b–induced thrombocytopenia is caused by inhibition of platelet production but not proliferation and endomitosis in human megakaryocytes, Blood. J. American Soc. Hematol. 2008;112(3):542–550. doi: 10.1182/blood-2007-12-125906. [DOI] [PubMed] [Google Scholar]
- 77.Wang Q., Fox N., Kaushansky K. Interferon-alpha directly inhibits thrombopoietin-induced megakaryocyte proliferation and differentiation. Zhonghua xue ye xue za zhi= Zhonghua Xueyexue Zazhi. 2001;22(6):296–299. [PubMed] [Google Scholar]
- 78.Aschoff L. Ueber capilläre Embolie von riesenkernhaltigen Zellen. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin. 1893;134(1):11–25. [Google Scholar]
- 79.J.A. Graeve, P. De Alarcon, Megakaryocytopoiesis in the human fetus, Archives of disease in childhood 64(4 Spec No) (1989) 481-484. [DOI] [PMC free article] [PubMed]
- 80.Woods M., Greaves M., Lawford P., Trowbridge E. Isolation of Megakaryocytes from Human Placentae. Platelets. 1994;5(2):109–112. doi: 10.3109/09537109409005521. [DOI] [PubMed] [Google Scholar]
- 81.Martin J., Slater D., Trowbridge E. Abnormal intrapulmonary platelet production: a possible cause of vascular and lung disease. The Lancet. 1983;321(8328):793–796. doi: 10.1016/s0140-6736(83)91851-2. [DOI] [PubMed] [Google Scholar]
- 82.Yang J., Yang M., Xu F., Li K., Lee S.K., Ng P.-C., Tam J.S., Yuen P.M., Fok T.-F. Effects of oxygen-induced lung damage on megakaryocytopoiesis and platelet homeostasis in a rat model. Pediatr. Res. 2003;54(3):344–352. doi: 10.1203/01.PDR.0000079186.86219.29. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y., Zeng X., Jiao Y., Li Z., Liu Q., Ye J., Yang M. Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thromb. Res. 2020;193:110–115. doi: 10.1016/j.thromres.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhattacharjee S., Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Comprehensive Clin. Med. 2020:1–11. doi: 10.1007/s42399-020-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bomhof G., Mutsaers P.G., Leebeek F.W., Te Boekhorst P.A., Hofland J., Croles F.N., Jansen A.G. COVID-19-associated immune thrombocytopenia. Br. J. Haematol. 2020;190(2):e61–e64. doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merli M., Ageno W., Sessa F., Salvini M., Caramazza D., Mora B., Rossi A., Rovelli C., Passamonti F., Grossi P. Recurrence of immune thrombocytopenia at the time of SARS-CoV-2 infection. Ann. Hematol. 2020;99(8):1951–1952. doi: 10.1007/s00277-020-04130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Revuz S., Vernier N., Saadi L., Campagne J., Poussing S., Maurier F. Immune thrombocytopenic purpura in patients with COVID-19. European J. Case Rep. Internal Med. 2020;7(7) doi: 10.12890/2020_001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Humbert S., Razanamahery J., Payet-Revest C., Bouiller K., Chirouze C. COVID-19 as a cause of immune thrombocytopenia. Medecine et maladies infectieuses. 2020;50(5):459. doi: 10.1016/j.medmal.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.P.Y. Lee, C.D. Platt, S. Weeks, R.F. Grace, G. Maher, K. Gauthier, S. Devana, S. Vitali, A.G. Randolph, D.R. McDonald, Immune dysregulation and multisystem inflammatory syndrome in children (MIS-C) in individuals with haploinsufficiency of SOCS1, Journal of Allergy and Clinical Immunology 146(5) (2020) 1194-1200. e1. [DOI] [PMC free article] [PubMed]
- 90.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Gandet F.F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bowles L., Platton S., Yartey N., Dave M., Lee K., Hart D.P., MacDonald V., Green L., Sivapalaratnam S., Pasi K.J. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N. Engl. J. Med. 2020;383(3):288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Showers C.R., Nuovo G.J., Lakhanpal A., Siegel C.H., Aizer J., Elreda L., Halevi A., Lai A.R., Erkan D., Magro C.M. A Covid-19 patient with complement-mediated coagulopathy and severe thrombosis. Pathobiology. 2021;88(1):14–22. doi: 10.1159/000512503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hottz E., Bozza F., Bozza P. Platelets in immune response to virus and immunopathology of viral infections. Front Med. 2018;5(121):2018. doi: 10.3389/fmed.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. Journal of hematology & oncology. 2020;13(1):1–22. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blasi A., von Meijenfeldt F.A., Adelmeijer J., Calvo A., Ibañez C., Perdomo J., Reverter J.C., Lisman T. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J. Thromb. Haemost. 2020;18(10):2646–2653. doi: 10.1111/jth.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.M. Capecchi, C. Mocellin, C. Abbruzzese, I. Mancini, D. Prati, F. Peyvandi, Dramatic presentation of acquired thombotic thrombocytopenic purpura associated with COVID-19, haematologica 105(10) (2020) e540-e540. [DOI] [PMC free article] [PubMed]
- 99.Jhaveri K.D., Meir L.R., Chang B.S.F., Parikh R., Wanchoo R., Barilla-LaBarca M.L., Bijol V., Hajizadeh N. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98(2):509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating COVID-19–related systemic thrombosis? Circulation. 2020;141(22):1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 101.Gavriilaki E., Sakellari I., Gavriilaki M., Anagnostopoulos A. Thrombocytopenia in COVID-19: pathophysiology matters. Ann. Hematol. 2020:1–2. doi: 10.1007/s00277-020-04183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gavriilaki E., Brodsky R.A. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br. J. Haematol. 2020;189(6):e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 103.Alizadeh F., O’Halloran A., Alghamdi A., Chen C., Trissal M., Traum A., DeCourcey D. Toddler With New Onset Diabetes and Atypical Hemolytic-Uremic Syndrome in the Setting of COVID-19. Pediatrics. 2021;147(2) doi: 10.1542/peds.2020-016774. [DOI] [PubMed] [Google Scholar]
- 104.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thrombosis Haemostasis : JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levi M., Iba T. COVID-19 coagulopathy: is it disseminated intravascular coagulation? Intern. Emerg. Med. 2020:1–4. doi: 10.1007/s11739-020-02601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhattacharjee S., Banerjee M., Pal R. COVID-19 Associated Hemophagocytic Lymphohistiocytosis and Coagulopathy: Targeting the Duumvirate. Indian Pediatr. 2020;57(9):827–833. doi: 10.1007/s13312-020-1962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.P. Lingamaneni, S. Gonakoti, K. Moturi, I. Vohra, M. Zia, Heparin-induced thrombocytopenia in COVID-19, Journal of investigative medicine high impact case reports 8 (2020) 2324709620944091. [DOI] [PMC free article] [PubMed]
- 112.Stahel P.F., Barnum S.R. Complement inhibition in coronavirus disease (COVID)-19: a neglected therapeutic option. Front. Immunol. 2020;11:1661. doi: 10.3389/fimmu.2020.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin. Drug Invest. 2020;40(6):511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Focà E., Andreoli L., Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmunity Rev. 2020:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Filocamo G., Mangioni D., Tagliabue P., Aliberti S., Costantino G., Minoia F., Bandera A. Use of anakinra in severe COVID-19: a case report. Int. J. Infect. Dis. 2020;96:607–609. doi: 10.1016/j.ijid.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nemchand P., Tahir H., Mediwake R., Lee J. Cytokine storm and use of anakinra in a patient with COVID-19. BMJ Case Reports CP. 2020;13(9) doi: 10.1136/bcr-2020-237525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Din C.T., Boffini N. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gavriilaki E., Brodsky R.A. Complementopathies and precision medicine. J. Clin. Investigat. 2020 doi: 10.1172/JCI136094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wong C., Lam C., Wu A., Ip W., Lee N., Chan I., Lit L., Hui D., Chan M., Chung S. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Diurno F., Numis F., Porta G., Cirillo F., Maddaluno S., Ragozzino A., De Negri P., Di Gennaro C., Pagano A., Allegorico E. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 121.Annane D., Heming N., Grimaldi-Bensouda L., Frémeaux-Bacchi V., Vigan M., Roux A.-L., Marchal A., Michelon H., Rottman M., Moine P. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study. EClin. Med. 2020;28 doi: 10.1016/j.eclinm.2020.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C., Huber-Lang M., Piemontese S., Assanelli A., Garlanda C. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mastellos D.C., da Silva B.G.P., Fonseca B.A., Fonseca N.P., Auxiliadora-Martins M., Mastaglio S., Ruggeri A., Sironi M., Radermacher P., Chrysanthopoulou A. Complement C3 vs C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020;220 doi: 10.1016/j.clim.2020.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deruelle E., Salem O.B.H., Hieng S.S., Pichereau C., Outin H., Jamme M. Immune thrombocytopenia in a patient with COVID-19. Int. J. Hematol. 2020;112(6):883–888. doi: 10.1007/s12185-020-02943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Okamoto M., Toyama M., Baba M. The chemokine receptor antagonist cenicriviroc inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;182 doi: 10.1016/j.antiviral.2020.104902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patterson B.K., Seethamraju H., Dhody K., Corley M.J., Kazempour K., Lalezari J., Pang A.P., Sugai C., Mahyari E., Francisco E.B. CCR5 inhibition in critical COVID-19 patients decreases inflammatory cytokines, increases CD8 T-cells, and decreases SARS-CoV2 RNA in plasma by day 14. Int. J. Infect. Dis. 2021;103:25–32. doi: 10.1016/j.ijid.2020.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park J.H., Lee H.K. Re-analysis of single cell transcriptome reveals that the NR3C1-CXCL8-neutrophil axis determines the severity of COVID-19. Front. Immunol. 2020;11:2145. doi: 10.3389/fimmu.2020.02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siegal D., Crowther M., Cuker A. Thrombopoietin receptor agonists in primary immune thrombocytopenia. Seminars Hematol. Elsevier. 2013:S18–S21. doi: 10.1053/j.seminhematol.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murt A., Eskazan A.E., Yılmaz U., Ozkan T., Ar M.C. COVID-19 presenting with immune thrombocytopenia: a case report and review of the literature. J. Med. Virol. 2021;93(1):43–45. doi: 10.1002/jmv.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sahu K.K., Siddiqui A.D., Rezaei N., Cerny J. Challenges for management of immune thrombocytopenia during COVID-19 pandemic. J. Med. Virol. 2020;92(11):2277–2282. doi: 10.1002/jmv.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahévas M., Moulis G., Andres E., Riviere E., Garzaro M., Crickx E., Guillotin V., Malphettes M., Galicier L., Noel N. Clinical characteristics, management and outcome of COVID-19-associated immune thrombocytopenia: a French multicentre series. Br. J. Haematol. 2020;190(4):e224–e229. doi: 10.1111/bjh.17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lorenzo-Villalba N., Zulfiqar A.-A., Auburtin M., Schuhmacher M.H., Meyer A., Maouche Y., Keller O., Andres E. Thrombocytopenia in the course of COVID-19 infection. European J. Case Rep. Internal Med. 2020;7(6) doi: 10.12890/2020_001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garcia D. Corticosteroids for ITP: A Comparison of Two Approaches. Hematologist. 2016;13(3) [Google Scholar]
- 134.R.C. Group, Dexamethasone in hospitalized patients with Covid-19, New England Journal of Medicine 384(8) (2021) 693-704. [DOI] [PMC free article] [PubMed]
- 135.Albani F., Fusina F., Granato E., Capotosto C., Ceracchi C., Gargaruti R., Santangelo G., Schiavone L., Taranto M.S., Tosati C. Corticosteroid treatment has no effect on hospital mortality in COVID-19 patients. Sci. Rep. 2021;11(1):1–6. doi: 10.1038/s41598-020-80654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yousefifard M., Ali K.M., Aghaei A., Zali A., Neishaboori A.M., Zarghi A., Safari S., Hashemi B., Forouzanfar M.M., Hosseini M. Corticosteroids on the Management of Coronavirus Disease 2019 (COVID-19): A Systemic Review and Meta-Analysis. Iranian J. Public Health. 2020;49(8):1411. doi: 10.18502/ijph.v49i8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu Z., Chen W., Liang W., Xu C., Sun W., Yi Y. Severe exacerbation of immune thrombocytopenia and COVID-19: the favorable response to corticosteroid-based therapy—a case report. Ann. Hematol. 2020:1–3. doi: 10.1007/s00277-020-04070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xie Y., Cao S., Dong H., Li Q., Chen E., Zhang W., Yang L., Fu S., Wang R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. infect. 2020;81(2):318. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herth F.J., Sakoulas G., Haddad F. Use of Intravenous Immunoglobulin (Prevagen or Octagam) for the Treatment of COVID-19: Retrospective Case Series. Respiration. 2020:1–9. doi: 10.1159/000511376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shao Z., Feng Y., Zhong L., Xie Q., Lei M., Liu Z., Wang C., Ji J., Liu H., Gu Z. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin. Transl. Immunol. 2020;9(10) doi: 10.1002/cti2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martincic Z., Skopec B., Rener K., Mavric M., Vovko T., Jereb M., Lukic M. Severe immune thrombocytopenia in a critically ill COVID-19 patient. Int. J. Infect. Dis. 2020;99:269–271. doi: 10.1016/j.ijid.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zoufaly A., Poglitsch M., Aberle J.H., Hoepler W., Seitz T., Traugott M., Grieb A., Pawelka E., Laferl H., Wenisch C. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respiratory Med. 2020;8(11):1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haschke M., Schuster M., Poglitsch M., Loibner H., Salzberg M., Bruggisser M., Penninger J., Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52(9):783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- 144.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21(1):1–9. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.V. Monteil, H. Kwon, P. Prado, A. Hagelkrüys, R.A. Wimmer, M. Stahl, A. Leopoldi, E. Garreta, C.H. Del Pozo, F. Prosper, Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell 181(4) (2020) 905-913. e7. [DOI] [PMC free article] [PubMed]
- 146.Wu X., Luo D., Liu Y., Zeng Y., Gong Y. Continuous thrombocytopenia after SARS-CoV-2 nucleic acid negative in a non-severe COVID-19 patient for several months. BMC Infect. Dis. 2020;20(1):1–5. doi: 10.1186/s12879-020-05495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lévesque V., Millaire É., Corsilli D., Rioux-Massé B., Carrier F.-M. Severe immune thrombocytopenic purpura in critical COVID-19. Int. J. Hematol. 2020;112(5):746–750. doi: 10.1007/s12185-020-02931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.An Y.-W., Yuan B., Wang J.-C., Wang C., Liu T.-T., Song S., Liu H.-Q. Clinical characteristics and impacts of traditional Chinese medicine treatment on the convalescents of COVID-19. Int. J. Med. Sci. 2021;18(3):646. doi: 10.7150/ijms.52664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen K., Zhang Y., Ma W., Wang C., Wang J., Zhang J., Chen X. New mechanisms of the TCM spleen-based treatment of immune thrombocytopenia purpura from the perspective of blood neurotransmitters. J. Traditional Chinese Med. Sci. 2017;4(2):106–112. [Google Scholar]
- 150.Yu C., Jiang Z., Hou A., Mu Y., Liu W., Tan S. Shen-Cao granules formulated based on traditional Chinese medicine alleviates bone marrow suppression caused by platinum-based anticancer reagents. Medicine. 2017;96(19) doi: 10.1097/MD.0000000000006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hirano A., Ueoka H. Successful treatment of idiopathic thrombocytopenic purpura by Chinese herbal medicine EK-49 and ascorbic acid in an elderly patient developing chronic subdural hematoma. Geriatrics Gerontol. Int. 2007;7(1):83–88. doi: 10.3143/geriatrics.38.224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.