Abstract
Background:
Alcohol Use Disorder (AUD) is a complex psychiatric disease characterized by high alcohol intake as well as by hyperkatifeia and hyperalgesia during withdrawal. A role for Sigma-1 Receptors (Sig-1Rs) in the rewarding and reinforcing effects of alcohol has started to emerge in recent years, as rat studies have indicated that Sig-1R hyperactivity may result in excessive alcohol drinking. Sig-1R studies in mice are very scarce, and its potential role in alcohol-induced hyperalgesia is also unknown.
Methods:
In this study, we investigated the role of Sig-1R in alcohol drinking and associated hyperalgesia in male mice, using an intermittent access 2-bottle choice model of heavy drinking.
Results:
The Sig-1R antagonist BD-1063 was found to dose-dependently reduce both alcohol intake and preference, without affecting either water or sucrose intake, suggesting that the effects are specific for alcohol. Notably, the ability of BD-1063 in suppressing ethanol intake correlated with the individual baseline levels of alcohol drinking, suggesting that the treatment was more efficacious in heavy drinking subjects. In addition, BD-1063 was able to reverse alcohol-induced hyperalgesia during withdrawal, as assessed using an automatic Hargreaves test, without affecting thermal sensitivity in alcohol-naïve animals or locomotor activity in either group.
Conclusions:
These data show anti-alcohol effects of Sig-1R antagonism in heavy drinking mice as well as its efficacy in reducing alcohol-induced hyperalgesia, thereby laying the foundation for the development of novel treatments for AUD and associated pain states.
Keywords: Addiction, Drinking, Dependence, Alcoholism, Pain, Hyperkatifeia, Allostasis
Introduction
It is estimated that 88,000 deaths per year can be attributed to alcohol and over 14 million American adults (5.9% of the population) were diagnosed with severe problematic drinking, which is medically diagnosed as alcohol use disorder (AUD) (2018 NSDUH, Stahre et al., 2014). AUD is conceptualized as a repeated cycle of binge drinking and associated euphoria, emergence of a negative emotional state followed by preoccupation and anticipation or craving (Koob and Le Moal, 2005, Koob and Volkow, 2016). One of the hypothesized mechanisms of compulsive drinking is through the development of negative reinforcement by which drinking would transiently relieve the hyperkatifeia (i.e. negative emotional symptoms) present during withdrawal; this causes the hedonic set point to gradually shift to an allostatic hedonic state (Koob, 2020, Koob and Le Moal, 2001). In addition to negative affective states, sensory dimensions of pain (hyperalgesia, i.e. low pain threshold) have also been proposed to be part of the abstinence syndrome that contributes to continued alcohol use (Egli et al., 2012). Indeed, patients with a history of chronic alcohol use report more severe pain, which disrupts daily activities, and these same individuals report drinking more frequently to manage pain compared to non-problem drinkers (Brennan et al., 2005). Furthermore, neural circuits activated by cycles of alcohol intoxication and withdrawal overlap with those that are hyperactive during chronic pain states (Egli et al., 2012, Robins et al., 2019).
The Sigma-1 receptor (Sig-1R) has been proposed as a promising target for the treatment of AUD. Originally misclassified as an opioid receptor, Sig-1R is now recognized as a molecular chaperone that exists predominantly on the mitochrondrion-endoplasmic reticulum interface and serves as a calcium sensor (Alonso et al., 2000, Hayashi and Su, 2001, Hayashi and Su, 2003, Martin et al., 1976, Pasternak, 2017). Upon activation, it dissociates from its binding partner binding immunoglobulin protein (BiP) and moves toward the cellular periphery, where it modulates a variety of effectors ranging from voltage gated ion channels, G-protein-coupled receptors, kinases and neurotransmitter transporters (Aydar et al., 2002, Balasuriya et al., 2012, Hong et al., 2017, Kinoshita et al., 2012, Kourrich et al., 2013, Navarro et al., 2010). We, as well as others, have found that Sig-1R antagonists reduce alcohol self-administration (Sabino et al., 2009a), motivation to drink (Sabino et al., 2009a), alcohol-induced conditioned place preference (Bhutada et al., 2012), and reinstatement of both conditioned place preference (Bhutada et al., 2012) and operant alcohol seeking behavior (Martin-Fardon et al., 2012) (see (Quadir et al., 2019) for a review). While these studies have been conducted mainly in rats, it remains unclear whether Sig-1R also mediates heavy alcohol drinking in mice.
Sig-1R antagonists have been shown to alleviate neuropathic, inflammatory, and visceral pain (Merlos et al., 2017a). For example, S1RA was shown to dose dependently inhibit both phases of formalin induced nociception, capsaicin-induced mechanical and thermal hyperalgesia, as well as partial sciatic nerve injury (SNI)-induced mechanical and thermal hyperalgesia (Romero et al., 2012). In another study, mice with SNI were allowed to operantly self-administer S1RA, which abolished SNI-induced anhedonia and mechanical allodynia (Bura et al., 2013). The Sig-1R antagonist BD-1047 has also been shown to be effective in treating chronic constriction injury (CCI)-induced mechanical allodynia (Choi et al., 2013, Moon et al., 2015, Moon et al., 2013, Roh et al., 2008, Son and Kwon, 2010). Additionally, S1RA inhibits mechanical allodynia induced by both carrageenan and complete Freund’s adjuvant, two commonly used models of inflammatory pain (Gris et al., 2014, Tejada et al., 2014). Sig-1Rs have also been investigated in the treatment of visceral pain, induced via intracolonic administration of capsaicin (Gonzalez-Cano et al., 2013). Indeed, Sig-1R antagonists BD-1063, NE-100 and S1RA were able to reduce mechanical allodynia and associated abdomen licking, stretching and retracting behaviors (Gonzalez-Cano et al., 2013). Similarly, Sig-1R knockout mice do not develop mechanical allodynia in models of SNI-induced neuropathy, paclitaxel induced neuropathy, or intracolonic capsaicin (Castany et al., 2018, de la Puente et al., 2009, Gonzalez-Cano et al., 2013, Nieto et al., 2012, Sanchez-Fernandez et al., 2014). Together these studies confer a strong role for Sig-1R in mediating inflammatory, neuropathic and visceral pain. However, whether Sig-1R contributes to hyperalgesia induced by heavy alcohol drinking is unknown.
The aim of the present study was to examine the effect of the selective Sig-1R antagonist BD-1063 on both alcohol drinking and associated hyperalgesia using a mouse model of heavy drinking.
Materials and Methods
Subjects
Male C57BL/6J mice (7 weeks old upon arrival, N=50) were purchased from Jackson laboratory (Bar Harbor, ME, USA). Mice were single-housed with Teklad Diet 2918 and water ad libitum in a humidity- and temperature-controlled AAALAC-approved vivarium on a 12h reverse light/dark cycle (lights off at 10:00 am). Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Principles of Laboratory Animal Care, and were approved by the Institutional Animal Care and Use Committee (IACUC) of Boston University.
Drugs
Ethanol (20% v/v) was prepared from 190-proof ethanol diluted in tap water. Sucrose (1.15 % w/v; Sigma Aldrich, St. Louis, MO) was also dissolved in tap water. BD-1063 x 2 HBr salt (1-[2-(3,4-Dichlorophenyl)ethyl]-4-methylpiperazine dihydrobromide) was synthesized according to the previously reported procedure (de Costa et al., 1993). BD-1063 was solubilized in sterile, isotonic saline and administered intraperitoneally (i.p., 10 ml/kg). Drug dose was based on the salt weight (BD-1063 x 2 HBr), such that the highest dose used, 30 mg/kg, corresponds to 18.75 mg/kg of free base (BD-1063). Doses were chosen based on previous studies from our laboratory and others (Blasio et al., 2015, Brammer et al., 2006, Cottone et al., 2012, Hiranita et al., 2010, Moore et al., 2017, Nguyen et al., 2005, Nguyen et al., 2014, Nieto et al., 2014).
Intermittent Access 2-Bottle Choice (IA2BC) to Ethanol
Upon arrival, mice (n=10) were acclimated for 1 week to the presence of two 50-ml conical tubes (Fisher Scientific, Pittsburgh, PA) equipped with rubber stoppers and 2.5” long straight metal-ball bearing sipper tubes (Ancare, Bellmore, NY) filled with tap water. Mice were then subject to an intermittent access to ethanol paradigm for several weeks (Fig. 1). In this model of heavy alcohol drinking, one water bottle is replaced with a water bottle containing 20% (v/v) ethanol (EtOH) on alternating days; 2h into the dark cycle, pre-weighed bottles were provided and then removed and weighed again 24h later, as done previously (Hwa et al., 2011, Quadir et al., 2020a, Quadir et al., 2020c). It is important to note this is a chronic alcohol consumption protocol, where mice receive 24h access to alcohol every other day. In these studies, Sig-1R antagonist experiments began after several weeks of drinking (see Fig 1 for exact timeline). To account for spillage, two additional sets of bottles were pre-weighed placed on cages lacking mice. Water mice, control for the pain and locomotor activity experiments, received an identical treatment, except that the bottles were both filled with tap water.
Figure 1:
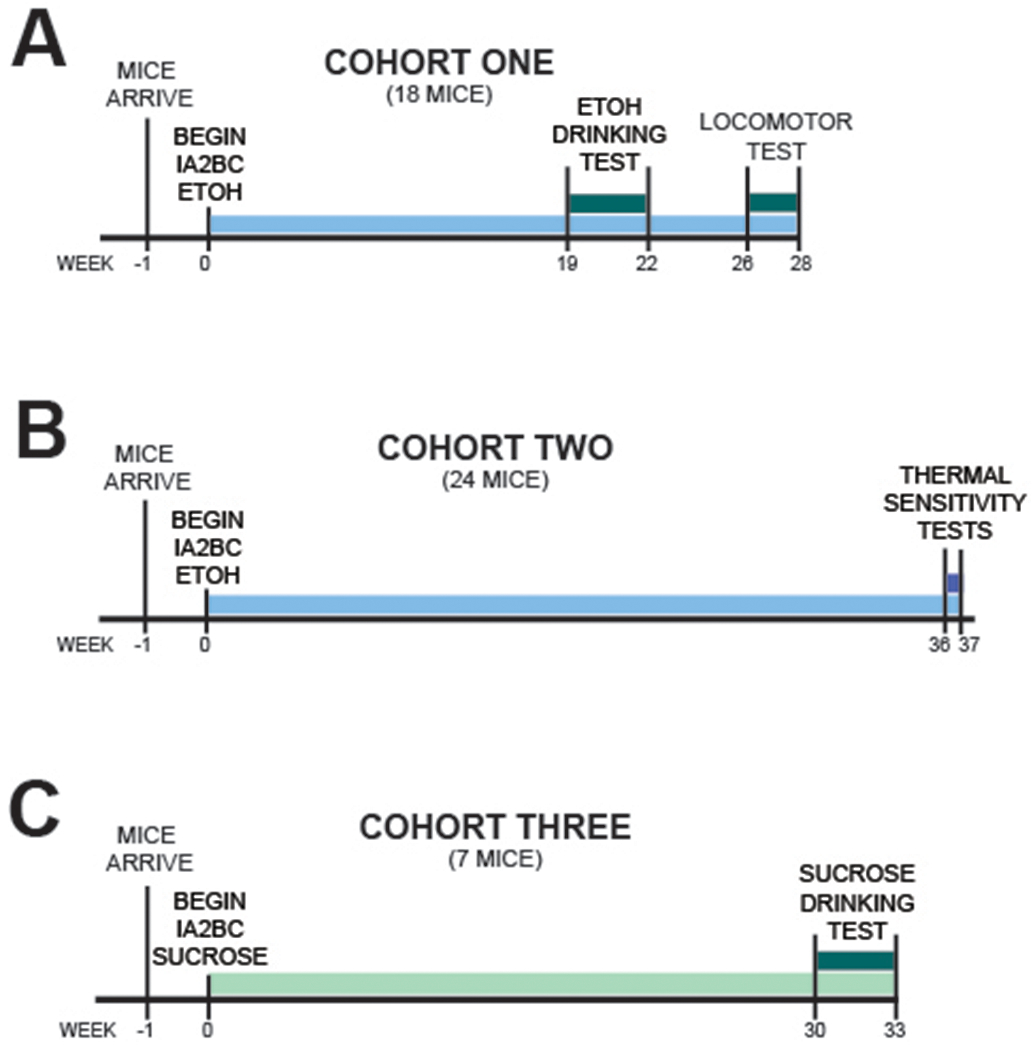
Experimental Timeline.
On test days, 30 min before bottles on time, food was removed and BD-1063 was administered (0, 10, 30 mg/kg, i.p.) in a balanced Latin square, within-subject design. 30 min after injection, pre-weighed bottles and food were provided to the animals and ethanol, water, and food intake were recorded at 2h, 6h, and 24h.
Intermittent Access 2-Bottle Choice (IA2BC) to Sucrose
A separate cohort of animals (n=7) underwent a procedure identical to the one above, except that 1.15% (w/v) sucrose was provided instead of ethanol. Drug treatments were conducted as described for the ethanol drinking experiment.
Thermal Sensitivity Testing (Hargreaves Test)
Sensitivity to thermal stimuli was assessed in a separate set of mice (n=23, 11-12 per group, IA2BC and water controls), using a Plantar Test Analgesia Meter equipped with a heat-flux infrared radiometer (IITC, Woodland Hills, CA) with glass preheated to 32 oC and artificial intensity set to 30, similarly to our previous work (Quadir et al., 2020a). the two test days occurred 24h after the last alcohol drinking session. Mice were first habituated to the preheated glass for 1hr , after which they were administered BD-1063 (0, 30 mg/kg, i.p.). 30 min later, they were tested for thermal sensitivity: an infrared beam was shined onto alternating paws (3-5 times per paw) and latency to withdraw was recorded; a 20 sec cutoff was used to avoid tissue damage. Latencies were first averaged for each paw, then averaged per animal (Cheah et al., 2016, Quadir et al., 2020c, Saika et al., 2015). On the days that followed the tests, mice were placed back on the regular intermittent drinking paradigm and allowed access to ethanol for two drinking sessions before being tested again for thermal sensitivity. BD-1063 was administered using a balanced, Latin square within-subject design, where doses were counterbalanced across test days.
Locomotor Activity
In order to confirm that any behavioral effects seen were not confounded by potential stimulatory or sedative effects of BD-1063, effects of BD-1063 on locomotor activity was examined using an Opto-M3 activity system (Columbus Instruments, Columbus, OH) as reported previously (Dore et al., 2013, Iemolo et al., 2016, Moore et al., 2020). The same animals used in the ethanol drinking test were used for locomotor activity, after 4 further weeks of undisturbed drinking (see Fig. 1). On the day prior to locomotor testing, mice were habituated to the room and apparatus for 3h under red light. On test day, mice were habituated to the locomotor apparatus for 1h, and then were administered BD-1063 (0, 30 mg/kg) in a mixed design; two treatment free alcohol sessions were allowed between test days. After 30 min pretreatment time, beam breaks were recorded for 120 min. All locomotor testing occurred in the mice home cage.
Statistics
Intake data were analyzed with repeated measure two-way ANOVAs, with Dose and Time as within-subject factors. Thermal sensitivity data analyzed using a mixed design two-way ANOVA, with Dose as a within-subject factor and ethanol as a between-subjects factor. A three way ANOVA was used to analyze locomotor activity, with Dose and time as within subjects factors and ethanol as a between subjects factor. Post-hoc comparisons were performed using student’s Newman-Keuls test. The threshold for significance was set to p ≤0.05.
Results
Effect of BD-1063 on Ethanol Intake
We found a highly significant effect of BD-1063 on ethanol intake [Dose: F(2,18)= 18.36, p≤0.001; Time: F(1,9)= 0.73, n.s. Time x Dose: F(2,18)= 0.46, n.s.]; post-hoc analysis showed that the 30 mg/kg dose significantly reduced alcohol intake by 43% and 46% at the 2h and 6h time point, respectively (Fig. 2A). Notably, the efficacy of the highest dose of BD-1063 (30 mg/kg) in suppressing alcohol intake significantly correlated with the individual baseline levels of drinking at both the 2h (r(8) = 0.65, p ≤0.05) and the 6h time point (r(8) = 0.70, p ≤0.05), as shown in Fig. 2B, suggesting that BD-1063 exerted a higher relative suppression of ethanol intake in heavy drinking mice, as compared to low drinking mice. BD-1063 also significantly affected preference for ethanol [Time x Dose: F(2,18)=1.31,n.s.; Time: F(1,9)=2.07,n.s.; Dose: F(2,18)=4.86, p≤0.05]; post-hoc analysis showed that the 30 mg/kg dose reduced preference by 32% and 27% at the 2h and 6h time points respectively (Fig. 1D). We found no effect of BD-1063 on water intake [Dose: F(2,18)= 0.05, n.s.; Time: F(1,9)= 3.74, n.s.; Time x Dose: F(2,18)= 0.07, n.s.] (Fig. 2C), total fluid intake [Time x Dose: F(2,18)=0.33, n.s.; Time: F(1,9)=2.98, n.s.; Dose: F(2,18)=2.18, n.s.] (data not shown), or food intake [Time x Dose: F(2,18)=2.28, n.s.; Time: F(1,9)=7.72, p ≤0.05; Dose: F(2,18)=0.02, n.s.] (Fig. 2E). The effect of BD-1063 on ethanol intake and preference did not extend to the 24h time point [Intake: F(2,18)=2.86, n.s.; Preference: F(2,18)=0.65, n.s.] (data not shown).
Figure 2:
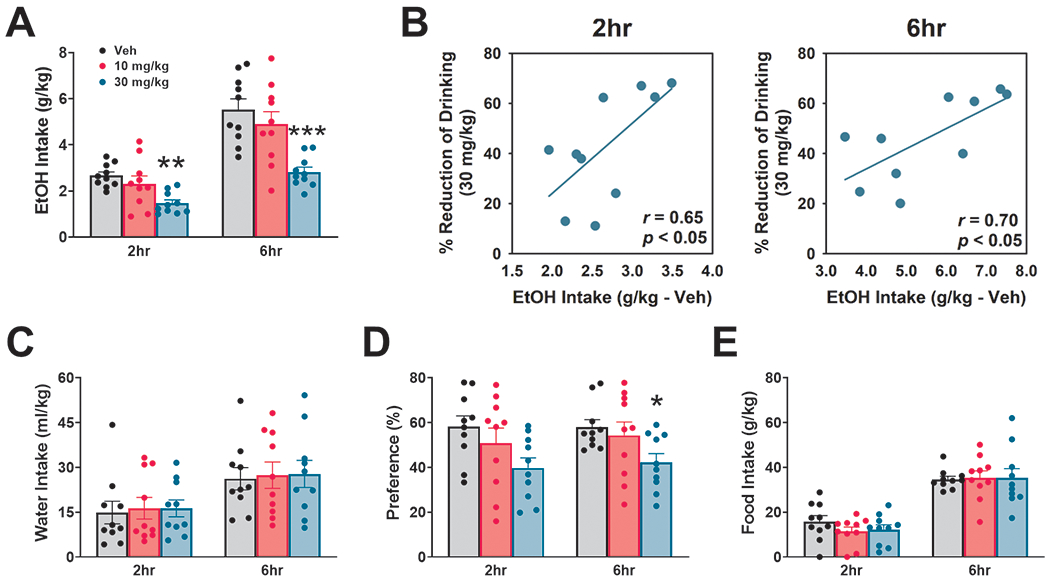
Effect of BD-1063 on ethanol (EtOH) intake (A), water intake (C), ethanol preference (D), and food intake (E). Data are normalized by body weight and represent Mean ± SEM. * p≤ 0.05, ** p≤ 0.01, *** p≤ 0.001 vs. Veh (Newman Keul’s test). (B) Correlation between the % reduction of ethanol intake by the 30 mg/kg dose of BD-1063 and the 2hr (left) and 6hr (right) ethanol intake under vehicle (Veh) conditions.
Effect of BD-1063 on Sucrose Intake
BD-1063 was found to affect sucrose intake, despite not reliably in one direction across time [Dose: F(2,12)=0.45, n.s.; Time: F(1,6)= 20.83, p ≤0.01; Time x Dose: F(2,12)=4.05, p ≤0.05]; neither individual one-way ANOVA at each time point, nor post-hoc analysis revealed any significant differences among groups, as shown in Fig. 3A. We found no effect of BD-1063 on water intake [Dose: F(2,12)=1.51,n.s.;Time: F(1,6)= 40.61, p ≤0.001; Time x Dose: F(2,12)=0.74, n.s.], as shown in Fig. 3B. BD-1063 had no effects on either sucrose or water intake at 24h[Sucrose: F(2,12)=2.25, n.s.; Water: F(2,12)=0.38, n.s.] (data not shown).
Figure 3:
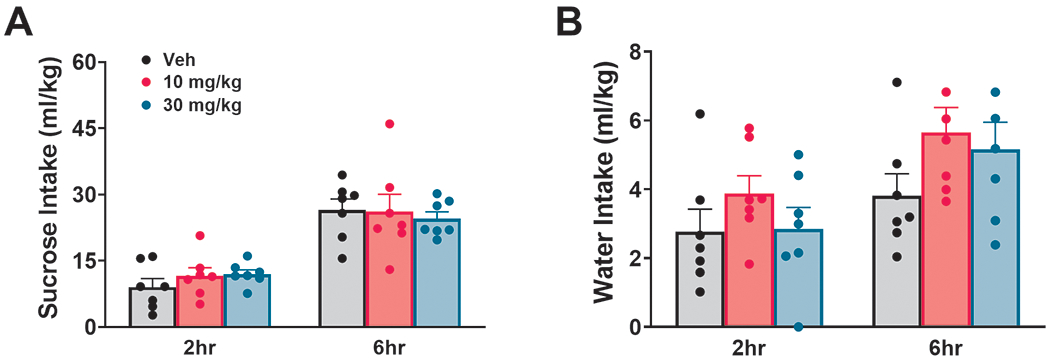
Effect of BD-1063 on sucrose intake (A) and water intake (B). Data are normalized by body weight and represent Mean ± SEM.
Effect of BD-1063 on Thermal Pain Sensitivity
Mice with a history of alcohol drinking showed higher thermal pain sensitivity in the Hargreaves test during withdrawal, as compared to controls, as measured by a 20% reduction in latency to paw withdrawal, as shown in Fig. 4A. Pretreatment with BD-1063 significantly affected thermal sensitivity [Dose: F(1,20)=4.51, p ≤ 0.05; Ethanol: F(1,20)= 2.90, n.s.; Dose x Ethanol: F(1,20)=3.88, p = 0.06]. While BD-1063 had no effect on paw withdrawal latency in alcohol-naïve, control mice, it was instead able to completely normalize it in ethanol-withdrawn mice, which resulted in a latency that was statistically indistinguishable from the water-exposed controls. Interestingly, no correlations were found between either the individual baseline levels of drinking and the threshold for thermal sensitivity under vehicle (r(9) = 0.27, n.s.) (Fig. 4B), or between the individual baseline levels of drinking and the efficacy of BD-1063 to reverse the threshold reduction in ethanol-exposed mice (r(9) = −0.32, n.s.) (Fig. 4C).
Figure 4:
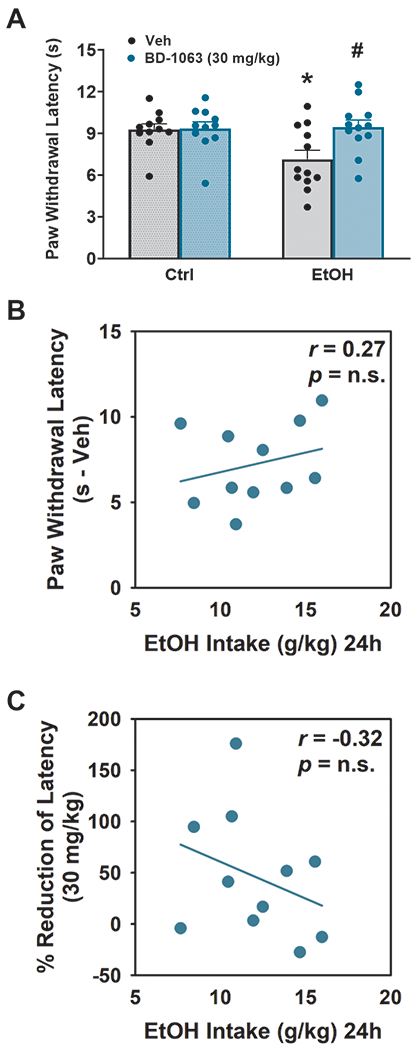
Effect of BD-1063 on thermal sensitivity (paw withdrawal threshold) (A). Data represent Mean ± SEM. * p≤ 0.05 vs. Veh; # p≤ 0.05 vs. Ctrl (Newman Keul’s test). . (B) Lack of correlation between the Paw withdrawal latency under vehicle (Veh) conditions and the 24h ethanol intake under vehicle (Veh) conditions. (C) Lack of correlation between the % reduction of decreased paw withdrawal latency by the 30 mg/kg dose of BD-1063 and the 24h ethanol intake under vehicle (Veh) conditions.
Effect of BD-1063 on Locomotor Activity
We found no effect of BD-1063 on locomotor activity across Time in either group [Dose: F(1,14)= 4.18, n.s. (p =0.06) ; Time: F(11,154)= 6.76, p ≤0.001; Ethanol: F(1,14)= 0.02, n.s.; Dose x Ethanol: F(1,14)= 0.19, n.s.; Dose x Ethanol x Time: F(11,154)= 1.69, n.s. (p =0.08)], as shown in Fig. 5A, or on total beam breaks [Dose: F(1,14)=4.18,n.s.; Ethanol: F(1,14)= 0.02, n.s.; Dose x Ethanol: F(1,14)= 0.19, n.s.], as shown in Fig. 5B.
Figure 5:
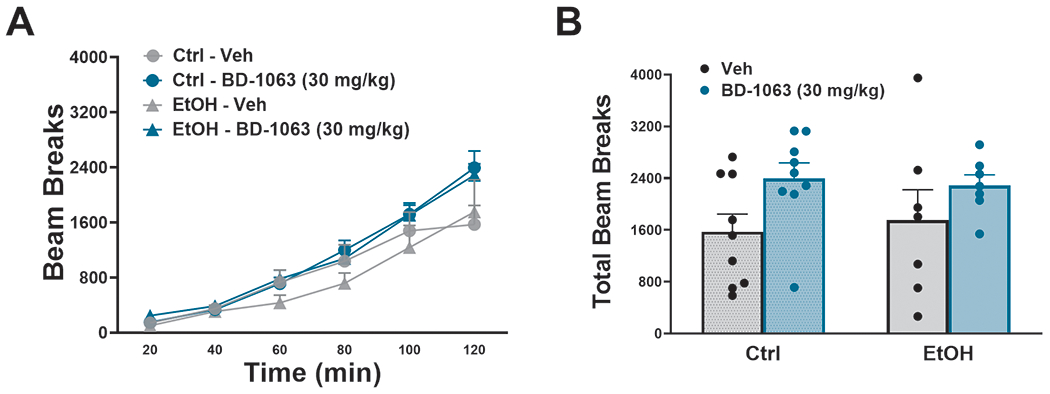
Effect of BD-1063 on locomotor activity across time (A) and during the entire observation period (B). Data represent Mean ± SEM.
Discussion
The current study investigated the role of Sig-1R in excessive alcohol drinking and withdrawal-induced hyperalgesia in mice using an intermittent, two bottle choice access to ethanol paradigm. We found that the Sig-1R antagonist BD-1063 decreased both alcohol intake and preference without affecting concurrent water, total fluid, or food intake. In addition, this effect was selective for alcohol, as BD-1063 had no effect on sucrose intake. BD-1063 also reduced thermal hyperalgesia brought about by chronic alcohol drinking, without affecting pain sensitivity in control animals. There was a trend (p=0.06) to a stimulatory effect of BD-1063 (regardless of history of ethanol drinking) in locomotor activity; however, this weak, non-significant increase cannot explain the effects seen in the drinking or pain tests, as BD-1063 had no effect on either sucrose drinking or on thermal sensitivity in ethanol naïve mice.
Sig-1R antagonists have been investigated as a potential therapeutic for substance use disorders since the early 2000s, when it was found that the Sig-1R is critical for the actions of both cocaine- and alcohol in a conditioned place preference test (Maurice et al., 2003, Romieu et al., 2000, Romieu et al., 2002). Since then, our and other laboratories have shown that antagonism of Sig-1R is able to block a variety of alcohol addiction-related behaviors (reviewed in (Quadir et al., 2019)). In the context of home-cage access to ethanol, Sig-1R antagonists decrease ethanol drinking in rats under a continuous 24h access model (Blasio et al., 2015, Sabino et al., 2009b); in the context of operant self-administration, Sig-1R agonists and antagonists were shown to exert a bidirectional modulation of alcohol intake in a fixed ratio 1 as well as a progressive ratio schedule of reinforcement (Sabino et al., 2011, Sabino et al., 2009a). Here, we show the ability of the Sig-1R antagonist BD-1063 to dose-dependently reduce high levels of ethanol intake in a different species, mice, and in a chronic, intermittent access to ethanol drinking paradigm. This model, initially proposed by Wise in 1973 and adapted into both rats and mice (Carnicella et al., 2014, Hwa et al., 2011, Melendez, 2011, Sabino et al., 2013, Wise, 1973), is known to induce high levels of alcohol intake and strong withdrawal behavioral phenotypes, such as heightened pain sensitivity, aggression, and cognitive deficits (George et al., 2012, Hwa et al., 2015, Quadir et al., 2020a), as well as extensive molecular and biochemical phenotypes (reviewed in (Carnicella et al., 2014)). Notably, the ability of BD-1063 in suppressing ethanol intake correlated with the individual baseline levels of alcohol drinking, suggesting that this specific pharmacological treatment is more efficacious the more an animal drinks. In addition, BD-1063 reduced preference for the ethanol solution at the 6h time point, and did not affect concurrent water intake or food intake, which speaks against a general malaise or performance suppressing effect of the drug.
In addition, the effects of BD-1063 were selective for alcohol, as BD-1063 treatment did not alter sucrose intake. One interesting note is that across the various drinking models, discrepant effects of Sig-1R antagonist have been shown in the context of sucrose intake; indeed, one study using continuous (24/7) access to alcohol found that Sig-1R antagonists increase sucrose intake (Sabino et al., 2009b), while another study showed no effect in operant behavior (Tapia et al., 2019). A reason for the discrepancy may be related to the different ligands employed; indeed, while this study used BD-1063, the previous ones used NE-100 and PD144418.
While several studies have shown increased sensitivity to noxious thermal stimuli during alcohol withdrawal, many of these involved rats and/or were performed using experimenter-administered alcohol (Avegno et al., 2018, Fu et al., 2015, Roltsch Hellard et al., 2017). The present study shows instead the emergence of a hyperalgesic phenotype in mice that have been voluntarily drinking alcohol through an intermittent access paradigm, a phenotype that we have recently shown in this model (Quadir et al., 2020b). Indeed, the Sig-1R antagonist BD-1063 was able to completely reverse the alcohol-induced thermal hyperalgesia observed during withdrawal, suggesting a role of Sig-1R activation in alcohol-induced pain states. Interestingly, BD-1063 did not affect thermal sensitivity in alcohol-naïve mice, consistently with previous studies showing no inherent effects of Sig-1R ligands on nociceptive pain (e.g. pain states not induced by an external factor) (Chien and Pasternak, 1995, Entrena et al., 2009, Kim et al., 2008). This is also in line with studies finding a role of Sig-1R in sensitized (i.e. induced by nerve injury or inflammatory agent) but not baseline conditions (Castany et al., 2018, de la Puente et al., 2009, Gonzalez-Cano et al., 2013, Nieto et al., 2012, Sanchez-Fernandez et al., 2014). Interestingly, the baseline levels of drinking did not correlate with either the chronic alcohol-induced reduction in thermal sensitivity under vehicle conditions or with the ability of BD-1063 to reverse the threshold reduction; this finding is consistent with what we reported previously (Quadir et al., 2020a) and suggests that there may be a threshold of ethanol intake that elicits hyperalgesia in mice, above which the degree of the resulting pain state does not change. As assessed by measuring general motor activity, BD-1063 was found not to have any sedative effects, indicating the observed anti-hyperalgesic effects cannot be explained simply by a reduction in motor activity which would also increase the latency to paw withdrawal. Of note is that BD-1063 showed a trend to instead increasing motor activity which, however, did not reach significance.
Anti-hyperalgesic effects of Sig-R antagonists have been extensively studied in the context of neuropathic pain, and they are thought to involve both central and peripheral sites (Merlos et al., 2017a, Merlos et al., 2017b, Sanchez-Fernandez et al., 2017). Although Sig-R ligands are unable to bind opioid receptors directly, Sig-R receptor inhibition has been shown to enhance analgesia induced by opioid drugs in nociceptive pain at both central and peripheral sites (Mei and Pasternak, 2002, Prezzavento et al., 2017, Sanchez-Fernandez et al., 2013) and to increase the anti-hyperalgesic effects of endogenous opioid peptides produced by immune cells that accumulate at inflamed sites (Tejada et al., 2017). This modulation of morphine-induced analgesia is due to Sig-Rs physically interacting with opioid receptors to restrain their functioning (Sanchez-Fernandez et al., 2017), such that Sig-R antagonism would inhibit pain hypersensitivity by “releasing the brake” (i.e. disinhibiting) and thereby enabling opioids (whether endogenous or exogenous) to better exert their antinociceptive effects. Since we have recently shown an interesting cross-talk between the opioid and Sig-R system in regards to the modulation of heavy alcohol drinking (Valenza et al., 2020), it is conceivable that a similar mechanism involving the opioid signaling may apply to the anti-alcohol effects of Sig-1R antagonism. In particular, we speculate that Sig-1 antagonism may potentiate the effects of endogenous opioids, released following alcohol drinking, at mu and delta opioid receptors and, therefore, make alcohol more reinforcing.
Alternatively, Sig-1R antagonism may decrease alcohol intake by relieving the alcohol-induced hyperalgesia present during withdrawal, thereby blocking the negatively reinforced vicious cycle. Within the brain, there are various areas that overlap in chronic alcohol use and chronic pain that may be contributing to these effects (Egli et al., 2012, Robins et al., 2019). These areas, which include, among others, the prefrontal cortex, the anterior cingulate cortex, and the nucleus accumbens, all contain high densities of both Sig-1R and mu opioid receptors (Alonso et al., 2000, Baldo, 2016, Carcole et al., 2019, Cheng et al., 2008, Gianoulakis, 2001, Richard and Fields, 2016). Future studies will directly probe the role of Sig-1R in these areas in mediating the effects observed here. Although we did not examine the effect of BD-1063 on ethanol pharmacokinetics, previous studies have found no effect of Sig-1R antagonism on blood alcohol levels (Sabino et al., 2009b); we, therefore, expect the observed effects to be pharmacodynamic and centrally-mediated. One limitation of this study is that only males were examined; therefore, future work will need to ascertain the role of the Sig-1R system in alcohol drinking and alcohol-induced pain states also in females. BD-1063 has preferential, nanomolar affinity for Sig-1R, being 30-fold selective for Sig-1R versus Sig-2R sites (Brammer et al., 2006, Matsumoto and Mack, 2001). Still, it is possible that at the systemic doses administered here, that BD-1063 binds both SigR subtypes. BD-1063 was chosen in this study because of its already established efficacy in models of addiction, but future studies will be needed to ascertain whether other Sig-1R antagonists, such as NE-100, S1RA, and PD144418 share similar effects.
In conclusion, our data provide novel insights into neurobiological mechanisms underlying excessive alcohol drinking and alcohol-induced hyperalgesia, and suggest Sig-1R as a potential medication target for AUD.
Acknowledgments
We thank Kristin Doucette and Aya Zeabi for their technical help. This publication was made possible thanks to grant numbers AA024439 (VS), AA025038 (VS), and AA026051 (PC) from the National Institute on Alcohol and Alcoholism (NIAAA), and the Boston University’s Undergraduate Research Opportunities Program (UROP). The work of the Drug Design and Synthesis Section, MTMDB, NIDA, and NIAAA was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA). The authors declare no conflict of interest.
References cited
- (2018 NSDUH) 2018 National Survey on Drug Use and Health (NSDUH), in Series 2018 National Survey on Drug Use and Health (NSDUH), www.samhsa.gov, pp Table 5.4A—Alcohol Use Disorder in Past Year among Persons Aged 12 or Older, by Age Group and Demographic Characteristics: Numbers in Thousands, 2017 and 2018.
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T (2000) Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience 97:155–170. [DOI] [PubMed] [Google Scholar]
- Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, Edwards S, Middleton JW, Gilpin NW (2018) Central Amygdala Circuits Mediate Hyperalgesia in Alcohol-Dependent Rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 38:7761–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB (2002) The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34:399–410. [DOI] [PubMed] [Google Scholar]
- Balasuriya D, Stewart AP, Crottes D, Borgese F, Soriani O, Edwardson JM (2012) The sigma-1 receptor binds to the Nav1.5 voltage-gated Na+ channel with 4-fold symmetry. The Journal of biological chemistry 287:37021–37029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA (2016) Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis. Trends in neurosciences 39:366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutada PS, Mundhada YR, Ghodki YR, Chaware P, Dixit PV, Jain KS, Umathe SN (2012) Influence of sigma-1 receptor modulators on ethanol-induced conditioned place preference in the extinction-reinstatement model. Behavioural pharmacology 23:25–33. [DOI] [PubMed] [Google Scholar]
- Blasio A, Valenza M, Iyer MR, Rice KC, Steardo L, Hayashi T, Cottone P, Sabino V (2015) Sigma-1 receptor mediates acquisition of alcohol drinking and seeking behavior in alcohol-preferring rats. Behavioural brain research 287:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammer MK, Gilmore DL, Matsumoto RR (2006) Interactions between 3,4-methylenedioxymethamphetamine and sigma1 receptors. European journal of pharmacology 553:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH (2005) Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction 100:777–786. [DOI] [PubMed] [Google Scholar]
- Bruna J, Videla S, Argyriou AA, Velasco R, Villoria J, Santos C, Nadal C, Cavaletti G, Alberti P, Briani C, Kalofonos HP, Cortinovis D, Sust M, Vaque A, Klein T, Plata-Salaman C (2018) Efficacy of a Novel Sigma-1 Receptor Antagonist for Oxaliplatin-Induced Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Phase IIa Clinical Trial. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 15:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bura AS, Guegan T, Zamanillo D, Vela JM, Maldonado R (2013) Operant self-administration of a sigma ligand improves nociceptive and emotional manifestations of neuropathic pain. Eur J Pain 17:832–843. [DOI] [PubMed] [Google Scholar]
- Carcole M, Zamanillo D, Merlos M, Fernandez-Pastor B, Cabanero D, Maldonado R (2019) Blockade of the Sigma-1 Receptor Relieves Cognitive and Emotional Impairments Associated to Chronic Osteoarthritis Pain. Frontiers in pharmacology 10:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S (2014) Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castany S, Gris G, Vela JM, Verdu E, Boadas-Vaello P (2018) Critical role of sigma-1 receptors in central neuropathic pain-related behaviours after mild spinal cord injury in mice. Scientific reports 8:3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M, Andrews MR, Chew DJ, Moloney EB, Verhaagen J, Fassler R, Fawcett JW (2016) Expression of an Activated Integrin Promotes Long-Distance Sensory Axon Regeneration in the Spinal Cord. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:7283–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZX, Lan DM, Wu PY, Zhu YH, Dong Y, Ma L, Zheng P (2008) Neurosteroid dehydroepiandrosterone sulphate inhibits persistent sodium currents in rat medial prefrontal cortex via activation of sigma-1 receptors. Experimental neurology 210:128–136. [DOI] [PubMed] [Google Scholar]
- Chien CC, Pasternak GW (1995) Sigma antagonists potentiate opioid analgesia in rats. Neuroscience letters 190:137–139. [DOI] [PubMed] [Google Scholar]
- Choi SR, Roh DH, Yoon SY, Kang SY, Moon JY, Kwon SG, Choi HS, Han HJ, Beitz AJ, Oh SB, Lee JH (2013) Spinal sigma-1 receptors activate NADPH oxidase 2 leading to the induction of pain hypersensitivity in mice and mechanical allodynia in neuropathic rats. Pharmacological research 74:56–67. [DOI] [PubMed] [Google Scholar]
- Cognition-Clinical-Trials (2018) Clinical Trial of CT1812 in Mild to Moderate Alzheimer’s Disease, in Series Clinical Trial of CT1812 in Mild to Moderate Alzheimer’s Disease, https://ClinicalTrials.gov/show/NCT02907567. [Google Scholar]
- Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J, Iyer MR, Steardo L, Rice KC, Hayashi T, Sabino V (2012) Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37:2593–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Costa BR, He XS, Linders JT, Dominguez C, Gu ZQ, Williams W, Bowen WD (1993) Synthesis and evaluation of conformationally restricted N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamines at sigma receptors. 2. Piperazines, bicyclic amines, bridged bicyclic amines, and miscellaneous compounds. Journal of medicinal chemistry 36:2311–2320. [DOI] [PubMed] [Google Scholar]
- de la Puente B, Nadal X, Portillo-Salido E, Sanchez-Arroyos R, Ovalle S, Palacios G, Muro A, Romero L, Entrena JM, Baeyens JM, Lopez-Garcia JA, Maldonado R, Zamanillo D, Vela JM (2009) Sigma-1 receptors regulate activity-induced spinal sensitization and neuropathic pain after peripheral nerve injury. Pain 145:294–303. [DOI] [PubMed] [Google Scholar]
- Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V (2013) CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38:2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S (2012) Alcohol dependence as a chronic pain disorder. Neuroscience and biobehavioral reviews 36:2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entrena JM, Cobos EJ, Nieto FR, Cendan CM, Gris G, Del Pozo E, Zamanillo D, Baeyens JM (2009) Sigma-1 receptors are essential for capsaicin-induced mechanical hypersensitivity: studies with selective sigma-1 ligands and sigma-1 knockout mice. Pain 143:252–261. [DOI] [PubMed] [Google Scholar]
- Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J (2015) Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. International journal of physiology, pathophysiology and pharmacology 7:136–144. [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF (2012) Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings of the National Academy of Sciences of the United States of America 109:18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C (2001) Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. Journal of psychiatry & neuroscience : JPN 26:304–318. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cano R, Merlos M, Baeyens JM, Cendan CM (2013) Sigma1 receptors are involved in the visceral pain induced by intracolonic administration of capsaicin in mice. Anesthesiology 118:691–700. [DOI] [PubMed] [Google Scholar]
- Gris G, Merlos M, Vela JM, Zamanillo D, Portillo-Salido E (2014) S1RA, a selective sigma-1 receptor antagonist, inhibits inflammatory pain in the carrageenan and complete Freund’s adjuvant models in mice. Behavioural pharmacology 25:226–235. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP (2001) Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proceedings of the National Academy of Sciences of the United States of America 98:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP (2003) Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108–15 cells. The Journal of pharmacology and experimental therapeutics 306:726–733. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL (2010) Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. The Journal of pharmacology and experimental therapeutics 332:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong WC, Yano H, Hiranita T, Chin FT, McCurdy CR, Su TP, Amara SG, Katz JL (2017) The sigma-1 receptor modulates dopamine transporter conformation and cocaine binding and may thereby potentiate cocaine self-administration in rats. The Journal of biological chemistry 292:11250–11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011) Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism, clinical and experimental research 35:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Nathanson AJ, Shimamoto A, Tayeh JK, Wilens AR, Holly EN, Newman EL, DeBold JF, Miczek KA (2015) Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology 232:2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Seiglie M, Blasio A, Cottone P, Sabino V (2016) Pituitary adenylate cyclase-activating polypeptide (PACAP) in the central nucleus of the amygdala induces anxiety via melanocortin receptors. Psychopharmacology 233:3269–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, Kim KW, Beitz AJ, Lee JH (2008) Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. British journal of pharmacology 154:1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Matsuoka Y, Suzuki T, Mirrielees J, Yang J (2012) Sigma-1 receptor alters the kinetics of Kv1.3 voltage gated potassium channels but not the sensitivity to receptor ligands. Brain research 1452:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2020) Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biological psychiatry 87:44–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 24:97–129. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2005) Neurobiology of Addiction, Academic Press. [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A (2013) Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell 152:236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Strong EM, Weiss F (2012) Effect of sigma(1) receptor antagonism on ethanol and natural reward seeking. Neuroreport 23:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE (1976) The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. The Journal of pharmacology and experimental therapeutics 197:517–532. [PubMed] [Google Scholar]
- Matsumoto RR, Mack AL (2001) (+/−)-SM 21 attenuates the convulsive and locomotor stimulatory effects of cocaine in mice. European journal of pharmacology 417:R1–2. [DOI] [PubMed] [Google Scholar]
- Maurice T, Casalino M, Lacroix M, Romieu P (2003) Involvement of the sigma 1 receptor in the motivational effects of ethanol in mice. Pharmacology, biochemistry, and behavior 74:869–876. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW (2002) Sigma1 receptor modulation of opioid analgesia in the mouse. The Journal of pharmacology and experimental therapeutics 300:1070–1074. [DOI] [PubMed] [Google Scholar]
- Melendez RI (2011) Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcoholism, clinical and experimental research 35:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos M, Burgueno J, Portillo-Salido E, Plata-Salaman CR, Vela JM (2017a) Pharmacological Modulation of the Sigma 1 Receptor and the Treatment of Pain. Advances in experimental medicine and biology 964:85–107. [DOI] [PubMed] [Google Scholar]
- Merlos M, Romero L, Zamanillo D, Plata-Salaman C, Vela JM (2017b) Sigma-1 Receptor and Pain. Handbook of experimental pharmacology 244:131–161. [DOI] [PubMed] [Google Scholar]
- Minerva-Clinical-Trials (2020) Study to Evaluate Efficacy and Safety of Roluperidone (MIN-101) in Adult Patients With Negative Symptoms of Schizophrenia, in Series Study to Evaluate Efficacy and Safety of Roluperidone (MIN-101) in Adult Patients With Negative Symptoms of Schizophrenia, https://ClinicalTrials.gov/show/NCT03397134. [Google Scholar]
- Moon JY, Choi SR, Roh DH, Yoon SY, Kwon SG, Choi HS, Kang SY, Han HJ, Kim HW, Beitz AJ, Oh SB, Lee JH (2015) Spinal sigma-1 receptor activation increases the production of D-serine in astrocytes which contributes to the development of mechanical allodynia in a mouse model of neuropathic pain. Pharmacological research 100:353–364. [DOI] [PubMed] [Google Scholar]
- Moon JY, Roh DH, Yoon SY, Kang SY, Choi SR, Kwon SG, Choi HS, Han HJ, Beitz AJ, Lee JH (2013) Sigma-1 receptor-mediated increase in spinal p38 MAPK phosphorylation leads to the induction of mechanical allodynia in mice and neuropathic rats. Experimental neurology 247:383–391. [DOI] [PubMed] [Google Scholar]
- Moore CF, Leonard MZ, Micovic NM, Miczek KA, Sabino V, Cottone P (2020) Reward sensitivity deficits in a rat model of compulsive eating behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 45:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CF, Schlain GS, Mancino S, Sabino V, Cottone P (2017) A behavioral and pharmacological characterization of palatable diet alternation in mice. Pharmacology, biochemistry, and behavior 163:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Wade E, Sayed S, Ahle G, Kiraly DD, Welch A, Collins KA, Soleimani L, Iosifescu DV, Charney DS (2017) Dextromethorphan/quinidine pharmacotherapy in patients with treatment resistant depression: A proof of concept clinical trial. Journal of affective disorders 218:277–283. [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, Cortes A, Casado V, Canela EI, Ortiz J, Fuxe K, Lluis C, Ferre S, Franco R (2010) Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proceedings of the National Academy of Sciences of the United States of America 107:18676–18681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR (2005) Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology 49:638–645. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Robson MJ, Healy JR, Scandinaro AL, Matsumoto RR (2014) Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PloS one 9:e89985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FR, Cendan CM, Canizares FJ, Cubero MA, Vela JM, Fernandez-Segura E, Baeyens JM (2014) Genetic inactivation and pharmacological blockade of sigma-1 receptors prevent paclitaxel-induced sensory-nerve mitochondrial abnormalities and neuropathic pain in mice. Molecular pain 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FR, Cendan CM, Sanchez-Fernandez C, Cobos EJ, Entrena JM, Tejada MA, Zamanillo D, Vela JM, Baeyens JM (2012) Role of sigma-1 receptors in paclitaxel-induced neuropathic pain in mice. The journal of pain : official journal of the American Pain Society 13:1107–1121. [DOI] [PubMed] [Google Scholar]
- Pasternak GW (2017) Allosteric Modulation of Opioid G-Protein Coupled Receptors by Sigma1 Receptors. Handbook of experimental pharmacology 244:163–175. [DOI] [PubMed] [Google Scholar]
- Prezzavento O, Arena E, Sanchez-Fernandez C, Turnaturi R, Parenti C, Marrazzo A, Catalano R, Amata E, Pasquinucci L, Cobos EJ (2017) (+)-and (−)-Phenazocine enantiomers: Evaluation of their dual opioid agonist/sigma1 antagonist properties and antinociceptive effects. European journal of medicinal chemistry 125:603–610. [DOI] [PubMed] [Google Scholar]
- Quadir SG, Cottone P, Sabino V (2019) Role of Sigma Receptors in Alcohol Addiction. Frontiers in pharmacology 10:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir SG, Rohl CD, Zeabi A, Moore CF, Cottone P, Sabino V (2020a) Effect of Different Standard Rodent Diets on Ethanol Intake and Associated Allodynia in Male Mice. Alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir SG, Tanino SM, Rohl CD, Sahn JJ, Yao EJ, Cruz LDR, Cottone P, Martin SF, Sabino V (2020b) The Sigma-2 receptor / transmembrane protein 97 (sigma2R/TMEM97) modulator JVW-1034 reduces heavy alcohol drinking and associated pain states in male mice. Neuropharmacology:108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir SG, Tanino SM, Rohl CD, Sahn JJ, Yao EJ, Cruz LDR, Cottone P, Martin SF, Sabino V (2020c) The Sigma-2 receptor / transmembrane protein 97 (sigma2R/TMEM97) modulator JVW-1034 reduces heavy alcohol drinking and associated pain states in male mice. Neuropharmacology 184:108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Fields HL (2016) Mu-opioid receptor activation in the medial shell of nucleus accumbens promotes alcohol consumption, self-administration and cue-induced reinstatement. Neuropharmacology 108:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins MT, Heinricher MM, Ryabinin AE (2019) From Pleasure to Pain, and Back Again: The Intricate Relationship Between Alcohol and Nociception. Alcohol Alcohol 54:625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Na HS, Lee JH (2008) Intrathecal injection of the sigma(1) receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology 109:879–889. [DOI] [PubMed] [Google Scholar]
- Roltsch Hellard EA, Impastato RA, Gilpin NW (2017) Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addiction biology 22:692–701. [DOI] [PubMed] [Google Scholar]
- Romero L, Zamanillo D, Nadal X, Sanchez-Arroyos R, Rivera-Arconada I, Dordal A, Montero A, Muro A, Bura A, Segales C, Laloya M, Hernandez E, Portillo-Salido E, Escriche M, Codony X, Encina G, Burgueno J, Merlos M, Baeyens JM, Giraldo J, Lopez-Garcia JA, Maldonado R, Plata-Salaman CR, Vela JM (2012) Pharmacological properties of S1RA, a new sigma-1 receptor antagonist that inhibits neuropathic pain and activity-induced spinal sensitization. British journal of pharmacology 166:2289–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T (2000) Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. Neuroreport 11:2885–2888. [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T (2002) Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 26:444–455. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP (2011) Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36:1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L Jr., Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP (2009a) The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34:1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Steardo L, Koob GF, Zorrilla EP (2009b) Selective reduction of alcohol drinking in Sardinian alcohol-preferring rats by a sigma-1 receptor antagonist. Psychopharmacology 205:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Kwak J, Rice KC, Cottone P (2013) Pharmacological characterization of the 20% alcohol intermittent access model in Sardinian alcohol-preferring rats: a model of binge-like drinking. Alcoholism, clinical and experimental research 37:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika F, Kiguchi N, Kobayashi Y, Kishioka S (2015) Peripheral alpha4beta2 nicotinic acetylcholine receptor signalling attenuates tactile allodynia and thermal hyperalgesia after nerve injury in mice. Acta Physiol (Oxf) 213:462–471. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez C, Entrena JM, Baeyens JM, Cobos EJ (2017) Sigma-1 Receptor Antagonists: A New Class of Neuromodulatory Analgesics. Advances in experimental medicine and biology 964:109–132. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez C, Montilla-Garcia A, Gonzalez-Cano R, Nieto FR, Romero L, Artacho-Cordon A, Montes R, Fernandez-Pastor B, Merlos M, Baeyens JM, Entrena JM, Cobos EJ (2014) Modulation of peripheral mu-opioid analgesia by sigma1 receptors. The Journal of pharmacology and experimental therapeutics 348:32–45. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez C, Nieto FR, Gonzalez-Cano R, Artacho-Cordon A, Romero L, Montilla-Garcia A, Zamanillo D, Baeyens JM, Entrena JM, Cobos EJ (2013) Potentiation of morphine-induced mechanical antinociception by sigma(1) receptor inhibition: role of peripheral sigma(1) receptors. Neuropharmacology 70:348–358. [DOI] [PubMed] [Google Scholar]
- Son JS, Kwon YB (2010) Sigma-1 Receptor Antagonist BD1047 Reduces Allodynia and Spinal ERK Phosphorylation Following Chronic Compression of Dorsal Root Ganglion in Rats. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology 14:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X (2014) Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Preventing chronic disease 11:E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford-Clinical-Trials PET/MRI in the Diagnosis of Chronic Pain, in Series PET/MRI in the Diagnosis of Chronic Pain, https://ClinicalTrials.gov/show/NCT03556137.
- Tapia MA, Lee JR, Gereau GB, Moore JM, Weise VN, Mason KL, Cessac ME, Bodeen JL, Miller DK, Will MJ (2019) Sigma-1 receptor antagonist PD144418 suppresses food reinforced operant responding in rats. Behavioural brain research 362:71–76. [DOI] [PubMed] [Google Scholar]
- Tejada MA, Montilla-Garcia A, Cronin SJ, Cikes D, Sanchez-Fernandez C, Gonzalez-Cano R, Ruiz-Cantero MC, Penninger JM, Vela JM, Baeyens JM, Cobos EJ (2017) Sigma-1 receptors control immune-driven peripheral opioid analgesia during inflammation in mice. Proceedings of the National Academy of Sciences of the United States of America 114:8396–8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada MA, Montilla-Garcia A, Sanchez-Fernandez C, Entrena JM, Perazzoli G, Baeyens JM, Cobos EJ (2014) Sigma-1 receptor inhibition reverses acute inflammatory hyperalgesia in mice: role of peripheral sigma-1 receptors. Psychopharmacology 231:3855–3869. [DOI] [PubMed] [Google Scholar]
- UPenn-Clinical-Trials (2019) 18F ISO-1 PET/CT in Breast Cancer, in Series 18F ISO-1 PET/CT in Breast Cancer, https://ClinicalTrials.gov/show/NCT02284919. [Google Scholar]
- Urfer R, Moebius HJ, Skoloudik D, Santamarina E, Sato W, Mita S, Muir KW (2014) Phase II trial of the Sigma-1 receptor agonist cutamesine (SA4503) for recovery enhancement after acute ischemic stroke. Stroke 45:3304–3310. [DOI] [PubMed] [Google Scholar]
- Valenza M, Blasio A, DiLeo A, Cottone P, Sabino V (2020) Sigma receptor-induced heavy drinking in rats: Modulation by the opioid receptor system. Pharmacology, biochemistry, and behavior 192:172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29:203–210. [DOI] [PubMed] [Google Scholar]


