Abstract
Background:
Alcohol excites neurons of the ventral tegmental area (VTA) and the release of dopamine from these neurons is a key event in ethanol reward and reinforcement. Many mechanisms for ethanol actions on neurons of the VTA have been proposed, but antagonists generally do not eliminate ethanol-induced excitation of VTA neurons. We have previously demonstrated that the ion channel KCNK13 plays an important role in ethanol excitation of mouse VTA neurons. Here, we elaborate on that finding and further assess the importance of KCNK13 in rats.
Methods:
Rats (Sprague-Dawley and Fisher 344) were used in these studies. In addition to single unit electrophysiology in brain slices, we used quantitative PCR and immunohistochemistry to discern the effects of ethanol and the brain slice preparation method on the expression levels of Kcnk13 and KCNK13.
Results:
Interestingly, immunohistochemistry demonstrated that the levels of KCNK13 were significantly reduced during procedures normally used to prepare brain slices for electrophysiology, such that there is a reduction of about 75% in KCNK13 protein at the time that electrophysiological recordings would normally be made. Extracellular recordings demonstrated that ethanol excitation of VTA neurons was reduced after knockdown of Kcnk13 using a small interfering RNA (siRNA) delivered via the recording micropipette. Real-time PCR demonstrated that expression of Kcnk13 was altered in a time-dependent manner after alcohol withdrawal.
Conclusions:
KCNK13 plays an important role in ethanol stimulation of rat VTA neurons, and KCNK13 is dynamically regulated by cell damage, by ethanol exposure, and during withdrawal. KCNK13 is a novel alcohol-sensitive protein and further investigation of this channel may offer new avenues for the development of agents useful in altering the rewarding effect of alcohol.
Keywords: THIK-1, dopamine, Kcnk13, alcohol withdrawal, two-pore potassium channel
Introduction
Dopaminergic (DA) neurons of the ventral tegmental area (VTA) are important mediators of alcohol reward and reinforcement (Koob and Volkow, 2010). The mechanism of activation of VTA neurons by alcohol has been explored, with a number of possible neurotransmitters and ion channels being implicated in ethanol-induced excitation (Morikawa and Morrisett, 2010). Evidence that acutely dissociated VTA neurons are activated by ethanol suggests that a key factor for alcohol-induced excitation of these neurons is a molecular target located on the VTA neurons themselves (Brodie et al., 1999). Similarly, ethanol excitation of VTA neurons still occurs when GABAA, GABAB, NMDA, AMPA, metabotropic glutamate, muscarinic and nicotinic cholinergic receptors are blocked (Nimitvilai et al., 2016), again suggesting that the primary target of alcohol is neither pre-synaptic terminals nor receptors of these neurotransmitters. One possibility for the target of alcohol action is an ion channel on the membrane of the VTA neuron. Although evidence indicates that several ion channels (Okamoto et al., 2006, Rivera-Meza et al., 2014, Herman et al., 2015) are affected by ethanol in the VTA, blocking those channels fails to prevent alcohol effects in VTA neurons (McDaid et al., 2008, Koyama et al., 2007, Appel et al., 2003).
Leak potassium channels participate in maintaining the resting membrane potential and control of neuronal excitability. These potassium channels are constitutively open and maintain the negative resting membrane potential (Goldstein et al., 2001). There are at least 15 different two-pore potassium channels having a variety of pharmacological and physiological profiles. Some two-pore potassium channels are activated by volatile anesthetics and others are inhibited (Enyedi and Czirjak, 2010). The Tandem-pore Halothane-Inhibited potassium (THIK) channels, KCNK12 and KCNK13, differ from other leak channels in that they are inhibited by isoflurane, halothane, gadolinium, and by high extracellular calcium concentration (Enyedi and Czirjak, 2010). There are only a few reports assessing the function of THIK channels in the central nervous system. We demonstrated that KCNK13 channels in mouse VTA neurons are important for ethanol excitation (You et al., 2019). It is important to examine rat VTA neurons as well as mouse, as there has been speculation regarding differences between rat and mouse in some studies of alcohol in VTA neurons (Okamoto et al., 2006). Ethanol inhibition of KCNK13 in the VTA is behaviorally important, as downregulation of this protein altered alcohol drinking (You et al., 2019). Here, we provide evidence that KCNK13 is important for ethanol excitation of rat VTA neurons, that Kcnk13 gene expression is regulated by alcohol, and that the expression of the channel protein is downregulated as a result of the typical method for brain slice preparation. Our results indicate that KCNK13 may be an important target of ethanol action on VTA neurons.
Materials and Methods
Animals
Male rats (Fisher 344) were purchased from Envigo (Indianapolis, IN). Rats used in the acute studies were 90–120 grams on arrival; male Sprague-Dawley rats treated with Lieber-DeCarli diet were 250 gm on arrival. Sprague-Dawley rats were used for the alcohol withdrawal studies in this report because of their characterization in the Lieber-DeCarli diet model in our laboratory and that of the Center for Alcohol Research in Epigenetics (You et al., 2018a, You et al., 2018b). We do not compare Fisher 344 and Sprague-Dawley data directly.
For some of the studies included as supplemental data, male C57BL/6J mice (C57) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were 4–5 weeks old on arrival and used for siRNA electrophysiology experiments within two weeks of arrival.
All rats and mice were treated in compliance with the NIH Guide for the Care and Use of Laboratory Animals. All experimental methods in this study were approved by the Animal Care Committee of the University of Illinois at Chicago.
Lieber-DeCarli Diet:
For the results shown in Figure 6 below, Sprague-Dawley rats (270–290 gm) were given access to ethanol in a Lieber-DeCarli diet protocol. Chronic ethanol administration of oral Lieber-DeCarli ethanol-containing diet feeding was performed as described previously (27). Male adult Sprague–Dawley rats were single cage housed, randomly assignment to three groups were used: control diet fed (C) and 24h (n=4) or 72h (n=16) withdrawal from ethanol diet (W).
Rats were given Lieber-DeCarli diet (Bio-Serv, Inc., Frenchtown, NJ, USA) as their only source of both food and fluid. Diet was provided daily between 5:00 and 6:00 p.m, right before the beginning of the dark cycle of the housing facility. Control and ethanol diet rats were pair-fed. For the first three days, all rats received control diet. Then the pair-fed control group (C) continued to receive the control liquid diet for the entire length of treatment. Rats in the ethanol-fed group (W) were gradually introduced to ethanol over a 7-day period (concentrations increased daily from day 1 to day 7; ethanol concentrations were 1.8%, 3.2%, 4.5%, 5.4%, 6.3%, 7.2%, 8.1% v/v, respectively) and then maintained on 9% (v/v) ethanol-containing Lieber-DeCarli liquid diet for 15 days. The rats in the withdrawal group (W) were then withdrawn from ethanol for either 24 hours before sacrifice or 72 hours before sacrifice. Tissue taken from these areas containing the VTA were then used for mRNA expression.
Electrophysiology
The brain slice preparation technique was similar to that described previously (Brodie et al., 1990, Nimitvilai et al., 2016). The rats were anesthetized with isoflurane and immediately subjected to decapitation. After removing the parietal and occipital plates of the skull, chilled cutting solution was poured over the cortex to begin chill the brain; the brain was then rapidly removed from the cranium. The brain was placed cortex down on a chilled platform and ice-cold cutting solution was poured over the whole brain, which was then roughly dissected to a smaller block to remove the cerebral cortices and tissue posterior to the rostral pons and anterior to the optic chiasm. This block of tissue was mounted to the chuck of the vibratome (Campden 7000smz, Lafayette Instruments, Lafayette, IN) and submerged into chilled (4° C) cutting solution in the vibratome. Coronal sections (400 μm) of the VTA were cut in chilled cutting solution and then immediately placed in the recording chamber in which artificial cerebrospinal fluid (aCSF) was flowing continuously (2 ml/min; 35° C). Standard aCSF was used in these experiments (in mM: NaCl 126, KCl 2.5, NaH2PO4 1.24, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, glucose 11). The composition of the cutting solution was (in mM): KCl 2.5, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, glucose 11, and sucrose 220. All solutions were saturated with 95% O2/ 5% CO2 (pH=7.4).
Recording electrodes were positioned in the VTA under a dissecting microscope using a precision micromanipulator (Siskiyou Corporation, Grants Pass, OR, USA). Neurons within the lateral VTA had electrophysiological criteria for DA neurons (Lacey et al., 1989, Mueller and Brodie, 1989, Brodie et al., 1988). Not all VTA neurons in this study were tested but every neuron tested with baclofen (0.1–1 μM) was inhibited; sensitivity to baclofen is characteristic of DA neurons but not GABAergic neurons in the VTA (Margolis et al., 2012).
Except for siRNA, drugs were infused into the aCSF using calibrated pumps from stock solutions. Most of the salts and ethanol (95%; 190 proof) were purchased from Sigma (St. Louis, MO). Isoflurane was purchased from Henry Schein Animal Health (Dublin, OH).
Extracellular recording electrodes (1.5 mm diameter glass tubing with filament, resistance 2–4 MΩ (Sutter Instruments, Novato, CA); tip diameter approximately 0.5 – 0.7 μm) were filled with 0.9% NaCl. A high-gain extracellular amplifier (x-Cell, FHC, Inc., Bowdoin, ME) and an IBM-PC-based data acquisition system (ADInstruments, Inc.) were used to collect firing rate data. Drug effects were calculated as change in firing rate compared to pre-drug baseline using one-minute averages. The change in firing rate is expressed as a percentage of the initial firing rate to control for changes in firing rate that could occur over time.
For siRNA delivery, siRNA targeting Kcnk13 and Kcnk12 was purchased from Dharmacon (ON-TARGETplus siRNA). As the sequence of Kcnk12 is related to but different from that of Kcnk13, siKcnk12 was used as control siRNA. It should be noted that in experiments in which siRNA was included in the recording micropipette, the amplitude of the action potential signal was used as an index of distance from the electrode tip to the neuron being recorded, and the amplitude of the action potential signal was maximized (2–4 mV) in order to insure that the siRNA was delivered as efficiently as possible to the neuron.
Immunofluorescent staining of brain sections
Rats were randomly assigned to one of three groups. One set of rats were sacrificed using isoflurane followed by perfusion with chilled saline and 4% paraformaldehyde (PFA); subsequently, VTA brain slices (250 μm) were made. The second set of rats were sacrificed using isoflurane; brain slices (250 μm) were made from the brains of these rats as for electrophysiology, and then immersed into 4% PFA for fixation. The third set treated the same as the second set, except that the brain slices were incubated in aCSF for 1 hours before fixation with PFA. Brain tissue was processed for immunohistochemistry as described previously (Kharazia et al., 2003). Primary antibodies for TH and KCNK13 detection (Fig 2) were mouse anti-TH (EMD Millipore, Burlington, MA, catalog number MAB318) and rabbit anti-KCNK13 (Novus, Littleton, CO, catalog number NBP2–41132). Images of KCNK13 expression in VTA (KCNK13 and TH) were obtained using a Zeiss LSM 710 confocal microscope (Carl Zeiss, Thornwood, NY). Intensity of immunofluorescence was quantified using the ImageJ software (National Institutes of Health).
Quantitative real-time PCR (qPCR)
For validation of siRNA knockdown of gene expression, rats were sacrificed using isoflurane; brain slices containing VTA were made same as for electrophysiology. Slices then were incubated in aCSF containing siRNA (targeting Kcnk13, Kcnk12 or non-targeting control siRNA, Dharmacon ON-TARGETplus siRNA) for 2 hours before shock freezing for measurement of mRNA expression. For chronic ethanol studies, rats were sacrificed either 24 or 72 hours after alcohol withdrawal from a 15 day Lieber-DeCarli diet regimen (You et al., 2018b). The VTA was dissected on ice using RNase-free conditions. RNA was isolated from VTA tissue using the RNeasy mini kit (Qiagen, Valencia, CA) and subjected to first strand cDNA synthesis using reverse transcriptase (Thermo Fisher, Waltham, MA). Quantitative real-time PCR was used to determine the mRNA level of Kcnk13 or Kcnk12. Relative expression was calculated using the delta Cq method. The expression of Gapdh was used as a reference for the expression levels of Kcnk13 and Kcnk12.
Statistical Analysis
Origin (Originlab, Northampton, MA) or GraphPad Prism version 6.05 (GraphPad Software, Inc., La Jolla, CA) were used for statistical analysis. Mean data are shown as mean ± S.E.M. Differences in ethanol response (Figure 1B) and mRNA expression (Figures 3, 4, 6) were assessed with unpaired t tests (between subjects). One-way ANOVA was used to assess significance of levels of immunoreactivity (Figure 2). Two-way ANOVAs were used to compare concentration-responses between groups (Figures 1A, 1C, 5, S1). As comparisons were made between immunofluorescence under different conditions, a one-way ANOVA was used to establish statistical significance for differences between the groups. Bonferroni post-hoc tests were used for multiple means comparison testing for ANOVAs as appropriate.
Results
Ethanol excitation in the VTA is reduced by high extracellular calcium or gadolinium, and is mimicked by isoflurane.
While there are no specific antagonists for most two-pore potassium channels, several non-selective agents can interfere with these ion channels, including external gadolinium and volatile anesthetics like halothane and isoflurane (Enyedi and Czirjak, 2010, Goldstein et al., 2001), and high external calcium ion (Ca++) (Goldstein et al., 2001, Rajan et al., 2000). Some of these conditions, including high external calcium ion concentration, reduce ethanol excitation in mouse VTA neurons (You et al., 2019), pointing to the possibility that a specific leak potassium channel might underlie ethanol-induced excitation in the VTA of rats as well. In initial experiments with rat VTA neurons, we increased the extracellular Ca++ concentration from 2.4 mM to a total of 10 mM. We began with normal aCSF and increased the calcium concentration for each neuronal response recorded; we did not randomize the regular calcium/high calcium administration. We cannot rule out an order-dependence of the calcium effect, although we have never observed an order-dependent effect of ethanol administration. The high calcium condition blocked ethanol-induced excitation (Figure 1A). Neurons in this experiment initially were spontaneously active at a rate of 1.51 ± 0.3 Hz; in high extracellular Ca++ medium, ethanol excitation reverted to a concentration-dependent inhibition (two-way repeated measures ANOVA, for the effect of calcium F1,3=11.43, P<0.05, n=4 cells from 3 rats); this is similar to the action of high external calcium concentration on ethanol responses in mice (You et al., 2019).
Figure 1.
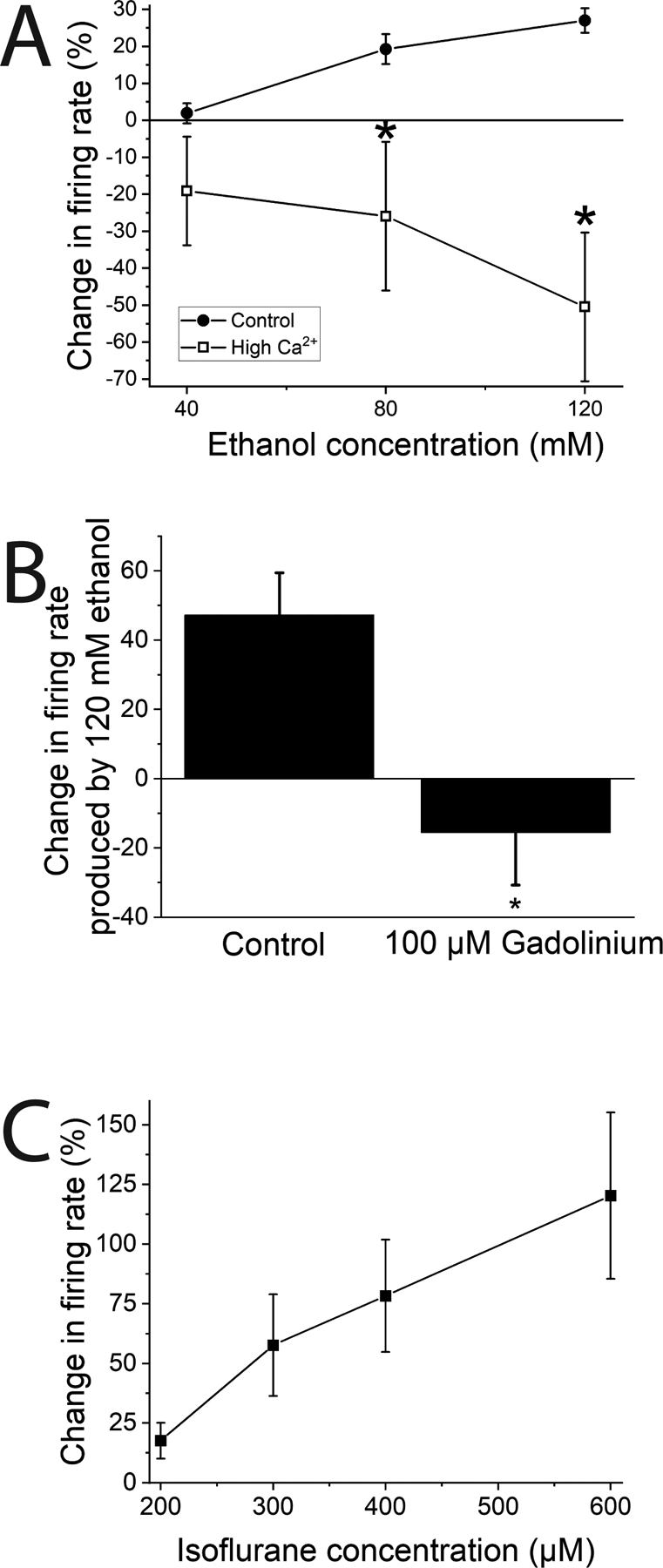
Pharmacological antagonism of KCNK13 and ethanol excitation of rat VTA neurons.
A. Ethanol excitation is reduced by high extracellular calcium concentration. Increased external calcium concentration (final total external calcium: 7.5–12.5 mM) blocked ethanol the excitatory effect of ethanol on VTA neurons. (two-way ANOVA, F1,3 = 11.43; p<0.05; means comparison with Bonferroni test, * = P < 0.05; n=4 cells from 3 rats).
B. Gadolinium reduces ethanol excitation of rat VTA neurons. The excitatory effect of ethanol (120 mM) was reduced by 100 μM gadolinium. (paired-sample t-test, DF=7, t-statistic = 2.80, *=P<0.05; n=8 cells from 4 rats).
C. Isoflurane excites VTA neurons. Isoflurane (200–600 μM) increased firing rate of VTA neurons in a concentration-dependent manner. (one way repeated measures ANOVA, F3,6 = 8.27; p<0.02; n=8 cells from 4 rats)
Gadolinium ion inhibits KCNK13 channels (Rajan et al., 2001). We tested the excitatory effect of 120 mM ethanol in the presence of gadolinium (100 μM). The initial firing rate was 1.58 ± 0.3 Hz. Ethanol was tested, and then the same concentrations were tested in the presence of gadolinium (100 μM). In the presence of gadolinium there was a reduction of the excitatory effect of 120 mM ethanol; in the absence of gadolinium, 120 mM ethanol increased the firing rate by 47.2 ± 12.2%, and in the presence of 100 μM gadolinium, 120 mM ethanol decreased the firing rate by 15.6% (paired t-test, DF=7, t-statistic 2.804, P<0.05, n=8 cells from 4 rats) (Figure 1B).
Since volatile anesthetics can affect tandem-pore potassium channels, we also assessed how isoflurane affected spontaneous neuronal firing rate of rat VTA neurons. Initial firing rate of neurons in this experiment was 1.58 ± 0.21 Hz. Isoflurane (200 – 600 μM) mimicked the excitatory effect of ethanol in a concentration-dependent manner (one-way repeated measures ANOVA, F5,10=44.47, P<0.001 n=8 cells from 4 rats) (Figure 1C). Some leak potassium channels are activated by volatile anesthetics (which should lead to hyperpolarization and inhibition) but both THIK channels (KCNK12 and KCNK13) are blocked by isoflurane (which should lead to depolarization and excitation), so we focused subsequent experiments on KCNK12 and KCNK13. It should be noted that all rats were anesthetized briefly with isoflurane prior to sacrifice, but as the excitatory effects of isoflurane were reversible with washout, and all slices were incubated in the recording chamber in drug-free aCSF for one hour before recording, the acute effect of the isoflurane anesthesia is likely to be minimal in these experiments.
KCNK13 immunoreactivity is downregulated by brain slice preparation procedure
For VTA neurons to reach the same level of ethanol excitation, higher concentrations of ethanol are needed in vitro (Brodie et al., 1990) compared to blood ethanol concentrations of ethanol achieved in vivo (Gessa et al., 1985). It has been shown that leak potassium channels are downregulated following axotomy (Ungless et al., 2002, Tulleuda et al., 2011, Acosta et al., 2014), and as there is neuronal damage that is a natural consequence of the in vitro brain slicing procedure, it is possible that a leak channel like KCNK13 is downregulated prior to electrophysiological recordings of VTA neurons under standard brain slicing methods. To examine this possibility, immunohistochemistry was performed in brain slices under three different conditions: with fixation in vivo perfusion, as would normally be performed for immunohistochemistry, and with fixation at two time points after making brain slices suitable for electrophysiology: Immediately after cutting the slice, and after one hour incubation in aCSF, which is the earliest time at which electrophysiological recordings would normally be performed. Brain slices were assessed for KCNK13 immunoreactivity as well as TH immunoreactivity. TH immunoreactivity was assessed as a control for general tissue deterioration and loss of dopamine neurons. The results of this experiment are shown in Figure 2. Figure 2A illustrates typical immunoreactivity of brain slices as observed by confocal microscope. There was a significant decrease in TH immunoreactivity after one-hour incubation in aCSF compared to the other two conditions (one-way ANOVA, F2,51 = 6.72, P<.005, n=18) (Figure 2B). In contrast, there was a decrease in KCNK13 immunoreactivity in both conditions in which fixation occurred in vitro, and the brain slice incubated for one hour in aCSF prior to fixation had significantly less KCNK13 immunoreactivity than the other two conditions (one-way ANOVA, F2,51 = 37.19, P<.001, n=18) (Figure 2C). When controlling for possible tissue deterioration by expressing KCNK13 immunoreactivity as a percentage of TH immunoreactivity, there was a significant time-dependent decrease in KCNK13 reactivity (one-way ANOVA, F2,51 = 15.04, P<.001, n=18) (Figure 2D). In fact, the level of KCNK13 immunoreactivity at the time that electrophysiological recordings would be made was less than 25% of the level in the tissue subjected to fixation in vivo.
Figure 2.
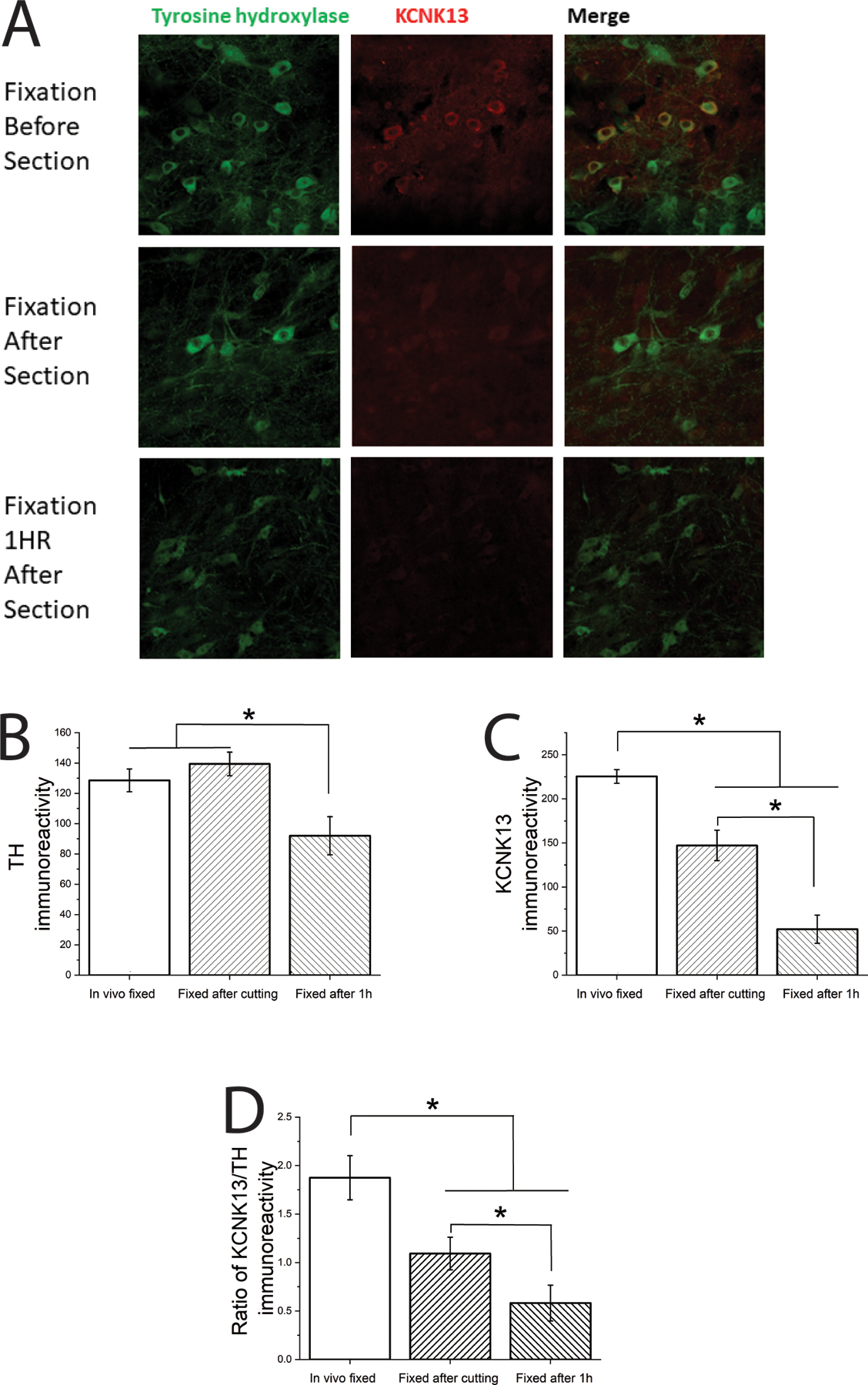
Brain slice preparation and incubation in aCSF reduces KCNK13 immunoreactivity
A. Immunohistochemistry: KCNK13 and TH in VTA slices VTA slices (250 μm thick) were processed three different ways: After in vivo perfusion with paraformaldehyde (Fixation before section), in vitro fixation immediately after brain slices were prepared as for electrophysiology (Fixation after section), and in vitro fixation after brain slices were prepared as for electrophysiology and incubated for 1 hour as we would before electrophysiological recording (Fixation 1 HR after section)
B. TH immunoreactivity was significantly reduced after 1 hour incubation in aCSF compared to the other two conditions (one-way ANOVA, F2,51 = 6.72, P<0.005, means comparison with Bonferroni test *=P<0.05, n=18 (6 per group));
C. KCNK13 immunoreactivity was significantly reduced in a time-dependent manner over the three conditions (one-way ANOVA, F2,51 = 37.19, P<0.001, means comparison with Bonferroni test *=P<0.05, n=18 (6 per group));
D. When calculated as a ratio of TH to KCNK13 immunoreactivity, there was a significant time-dependent reduction in KCNK13 (one-way ANOVA, F2,51 = 15.04, P<0.001, means comparison with Bonferroni test *=P<0.05, n=18 (6 per group)), suggesting that the reduction in KCNK13 was not a function of a general reduction in immunoreactivity over time.
siKcnk13 exposure downregulates Kcnk13 mRNA
As there is no selective antagonist of KCNK13, we tested whether siRNA could downregulate Kcnk13 mRNA in brain slices containing the VTA. We incubated VTA slices with siRNA that targeted Kcnk13 for two hours. The results of these experiments are shown in Figure 3. We observed that incubation with siKcnk13 produced a decrease in expression of Kcnk13 mRNA of 30.8%, to 0.721 ± 0.07 compared to 1.04 ± 0.11 in slices incubated with a scrambled control siRNA (two-sample t-test, t=2.41, P < 0.05, n=6 samples from 6 rats per group).
Figure 3.
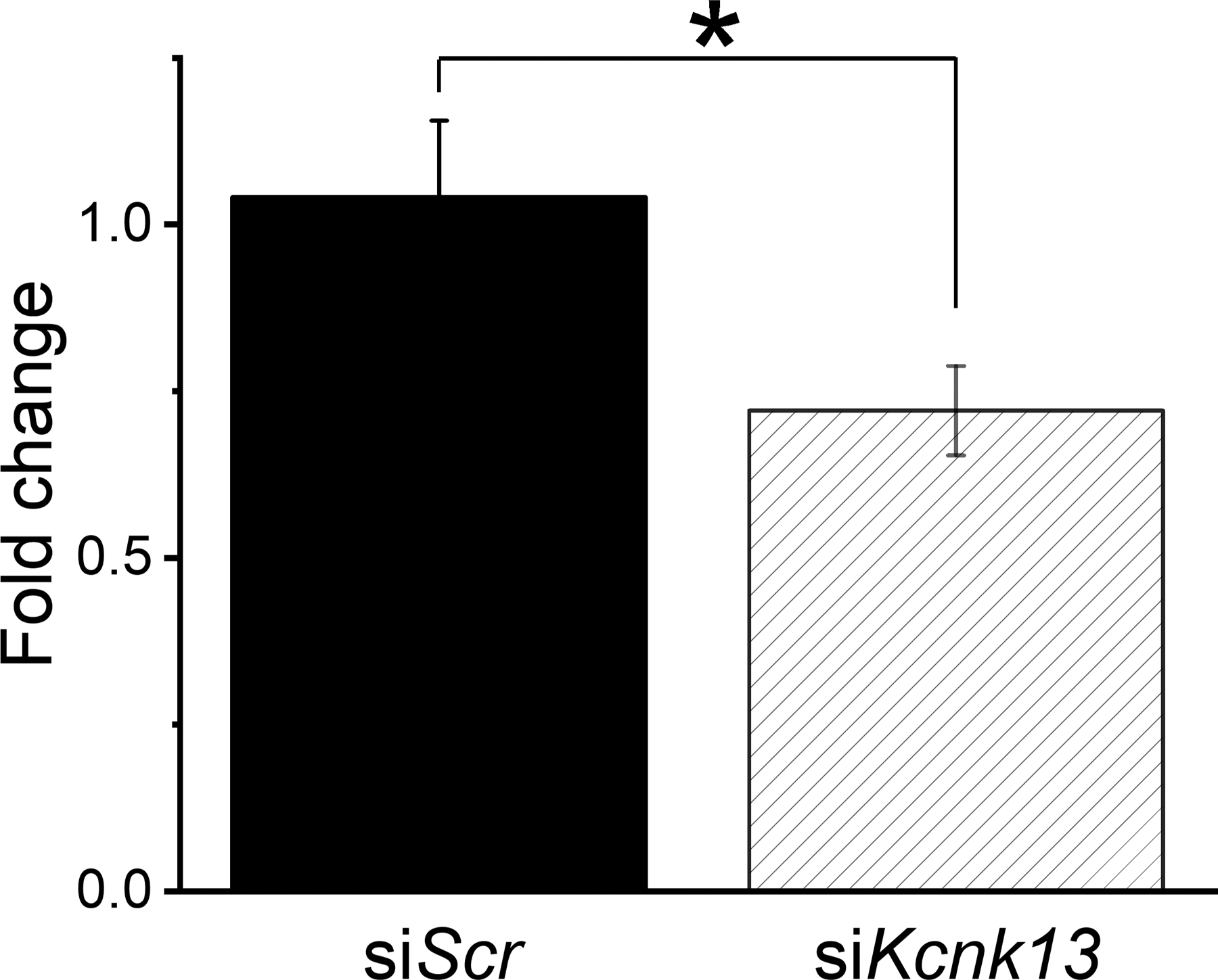
Incubation for two hours with siRNA targeting Kcnk13 reduces Kcnk13 mRNA
Incubation of coronal brain slices of the VTA (400 μm thick) with siRNA targeting Kcnk13 reduced the levels of Kcnk13 mRNA. The siKcnk13 reduced the levels of Kcnk13 by 30.8%, from 1.04 ± 0.11 to 0.721 ± 0.07 (two-sample t-test, t=2.41, * = P<0.05, n=6 per group).
As stated above in the Introduction, KCNK13 and KCNK12 are closely related potassium channels. In a separate experiment, we compared the effects of siKcnk13 and siKcnk12 on the expression of Kcnk12 and Kcnk13. We incubated VTA slices with siRNA that targeted either Kcnk12 or Kcnk13 for two hours. The results of qPCR on these slices is shown in Figure 4. Expression of Kcnk13 was reduced by siKcnk13-exposure (n=12 from 12 rats) but not by siKcnk12-exposure (n=13 from 13 rats) compared to the siControl group (n=14 from 14 rats) (one way ANOVA, F2,36 = 7.77, P<0.002) (Figure 4A). Kcnk12 expression was reduced by siKcnk12 (n=12 from 12 rats) compared the siKcnk13-exposed group (n=13 from 13 rats) but not compared to the siControl-exposed group (n=12 from 12 rats) (one way ANOVA, F2,34 = 3.28, P<0.05) (Figure 4B). These experiments demonstrate that downregulation by siRNA was specific to the mRNA for each gene.
Figure 4.
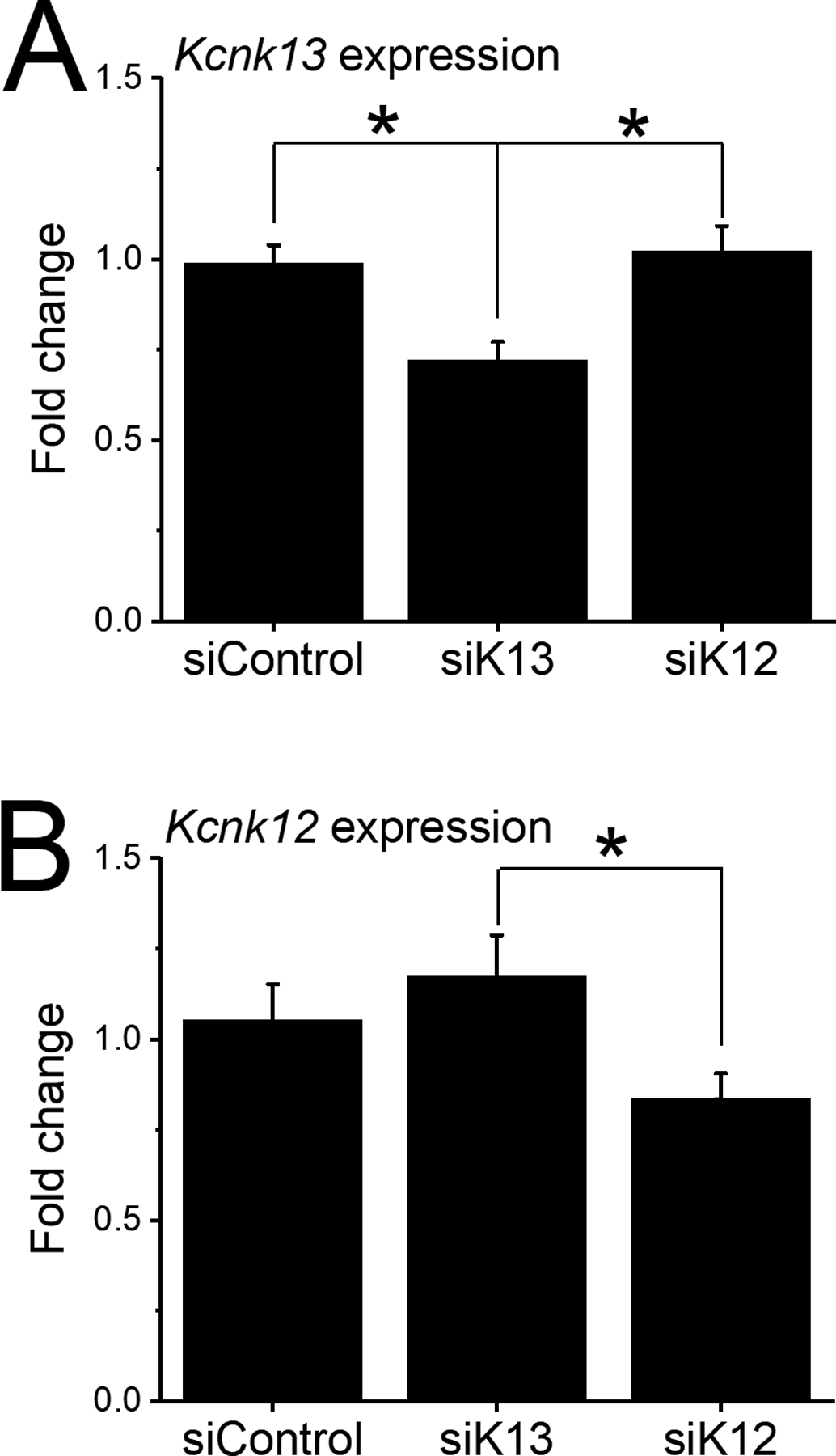
Selectivity of two-hour incubation with siRNA targeting Kcnk12 and Kcnk13.
Comparison was made in the effect of incubation of coronal brain slices of the VTA (400 μm thick) with siRNA targeting Kcnk12, Kcnk13 or scrambled siRNA (siScr).
A. siKcnk13 or siScr incubation: Incubation with siKcnk13 significantly reduced the level of Kcnk13 (n=12) compared to siScr (n=14) and to Kcnk12 (n=13) Kcnk13 levels (one-way ANOVA, F2,36=7.77, P<0.01; means comparison with Bonferroni test, * = P<0.05 for significance).
B. siKcnk12 or siScr incubation: Incubation with siKcnk12 significantly reduced the level of Kcnk12 (n=12) compared to Kcnk13 (n=13) but not to siScr; there was no significant difference between Kcnk13 and Scr groups (one-way ANOVA, F2,34=3.28, * = P<0.05; means comparison with Bonferroni test, P<0.05 for significance).
siKcnk13 decreases ethanol excitation of rat VTA neurons
We used exposure to siKcnk13 to specifically affect KCNK13 channels to determine their contribution to ethanol-induced excitation. siKcnk13 (or siKcnk12 as a control) was included in the electrode filling solution, and ethanol responses were assessed at the beginning of the recording (as control condition since at this point the effects of the siRNA would be minimal) and two hours after the initiation of the recording to study the effect of Kcnk13 downregulation. We used siKcnk12 as a control instead of a scramble control as Kcnk12 is closely related to Kcnk13 and is a more rigorous control than a scramble. The initial firing rate of the neurons exposed to siKcnk13 was 1.51 ± 0.27 Hz, and that of those exposed to siKcnk12 was 1.52 ± 0.21 Hz. Inclusion of siKcnk13 produced no significant change in baseline firing rate (at 2 h, the firing rate was 1.60 ± 0.24; paired t-test t-statistic = 0.715, DF=7, P>0.05) a significant time-dependent reduction of the excitatory effect of ethanol (Two-way RM ANOVA, F1,7=6.58 P<0.05, n=8 cells from 7 rats) (Figure 5A), whereas the inclusion of siKcnk12 has no significant effect on ethanol excitation of VTA neurons (Two-way RM ANOVA, F1,6=0.002 P>0.05, n=7 cells from 5 rats) (Figure 5B). Using this same method, similar results were obtained with mouse VTA neurons (Supplementary Data), consistent with our previously reported reduction of ethanol excitation of VTA neurons treated in vivo with shRNA targeting Kcnk13 (You et al., 2019).
Figure 5.
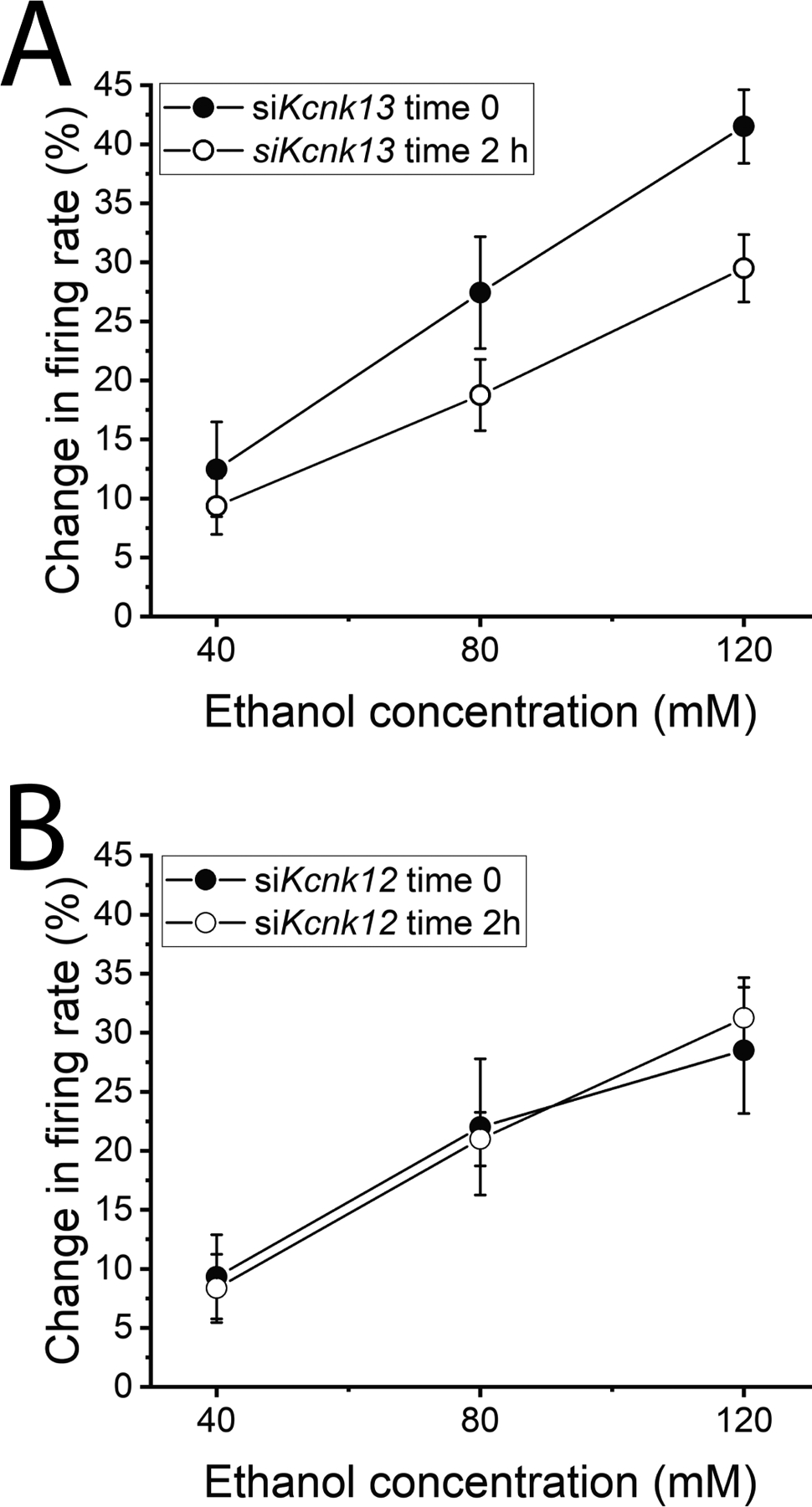
Electrophysiology: siKcnk13, not siKcnk12, reduced ethanol excitation of rat VTA neurons
A. siKcnk13 was included in the recording pipette. Change in firing rate of VTA neurons produced by ethanol early in the recording and at least 2 hours after initiation of the recording. The response to ethanol was significantly reduced by the inclusion of siKcnk13in the recording pipette. (Two-way RM ANOVA, F1,7=6.58 P<0.05, n=8)
B. siKcnk12 included in the recording pipette. Change in firing rate of VTA neurons produced by ethanol early in the recording and at least 2 hours after initiation of the recording. The response to ethanol was not significantly reduced by the inclusion of siKcnk12 in the recording pipette. (Two-way RM ANOVA, F1,6=0.002 P>0.05, n=7)
Expression of Kcnk13 is altered by in vivo ethanol exposure
In order to assess whether Kcnk13 expression was altered in response to ethanol exposure, we performed a number of qPCR experiments. We examined the expression levels of Kcnk13 mRNA during withdrawal from chronic ethanol treatment. These results are illustrated in Figure 6. During withdrawal from Lieber-DeCarli diet, there is a time-dependent change in Kcnk13 expression. At 24 h withdrawal from Lieber-DeCarli diet, there was an increase in expression of Kcnk13 mRNA; control expression level of Kcnk13 was 1.02 ± 0.11, and 24 after withdrawal from ethanol diet, the expression level was 1.61 ± 0.22 (two-sample t-test, DF=7, t-statistic = −2.54, P<0.05; n=5 samples from 5 rats/group). Interestingly, when measured 72 h after withdrawal, Kcnk13 expression was significantly decreased; control expression level of Kcnk13 was 1.04 ± 0.07, and 72 after withdrawal from ethanol diet, the expression level was 0.79 ± 0.0.04 (two-sample t-test, DF=30, t-statistic = 2.95, P<0.01; n=16 samples from 16 rats per group).
Figure 6.
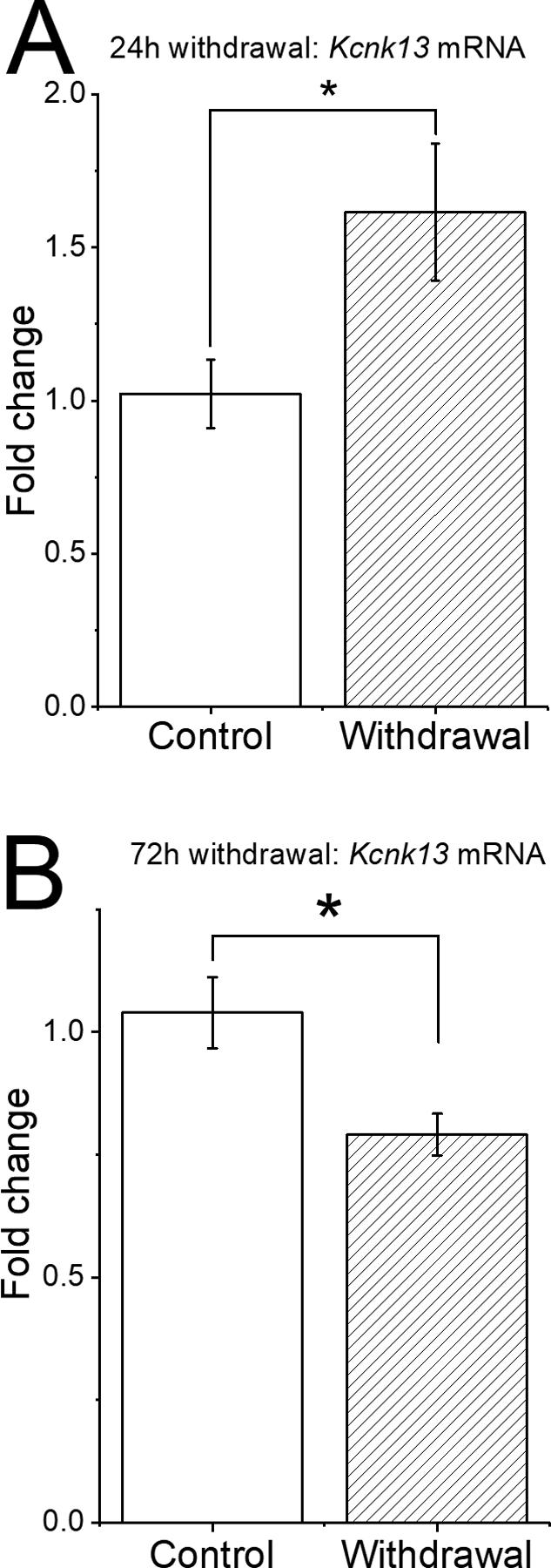
Withdrawal from Lieber-DeCarli diet produces time-dependent changes in Kcnk13 expression in the VTA of Sprague-Dawley rats.
A. After 24-hour withdrawal from a 15-day course of alcohol intake via Lieber-DeCarli diet, there was a significant increase in Kcnk13 expression compared to the VTA of rats given control diet. * = P< 0.05.
B. After 72-hour withdrawal from a 15-day course of alcohol intake via Lieber-DeCarli diet, there was a significant decrease of Kcnk13 expression compared to the VTA of rats given control diet. * = P< 0.01.
Discussion
Using pharmacological and molecular methods, we report that KCNK13 channels play a key role in ethanol excitation of VTA neurons of rats. In regard to some controversy and speculation about the similarity of mechanism of alcohol-induced excitation of VTA neurons of rats versus mice (for example, (Okamoto et al., 2006)), it is important to establish that KCNK13 is also important for ethanol responses in rat VTA neurons. The response of KCNK13 of VTA neurons to ethanol is likely to be behaviorally relevant, as Kcnk13 knockdown in the VTA increased ethanol consumption in mice (You et al., 2019). Among the cellular elements involved in alcohol effects in the VTA (You et al., 2018a), in this report and in our previously published work (You et al., 2019), we show that KCNK13 channels are coded by ethanol-sensitive genes (as shown by the responsiveness of Kcnk13 to ethanol treatment) and appear to be important for ethanol-induced excitation of VTA neurons.
Leak potassium channels have been shown to be downregulated following cellular trauma such as axotomy. Leak potassium current has been reported to be reduced after axotomy in Aplysia (Ungless et al., 2002). Axotomy induces downregulation of the specific leak potassium channels TREK2 (in rat nociceptors) (Acosta et al., 2014) and TRASK (Tulleuda et al., 2011). The decrease in KCNK13 immunoreactivity that we observed (Figure 2 and 3) is consistent with the observations made for these other leak potassium channels, as axotomy occurs during brain slice preparation. Concentrations of ethanol needed to activate DA-like VTA neurons in rodent brain slices (20–120 mM, although lower concentrations under some conditions can increase activity (Avegno et al., 2016, Mrejeru et al., 2015)) are higher than those achieved during ethanol intake by humans (e.g., 0.08 g/dl alcohol concentration is about 17mM). Similarly, lower ethanol concentrations evoke dopamine release as measured with in vivo microdialysis (Vena and Gonzales, 2015) or in vivo voltammetry (Signs et al., 1987, Jones et al., 2006). The results shown in Figure 2 indicates that there is a relatively rapid and profound decrease in KCNK13 immunoreactivity subsequent to standard brain slice preparation procedures. In fact, the level of KCNK13 that would be present at the beginning of a brain slice recording could be decreased by 75% compared to the level present in the intact animal. As a 22.2% decrease in Kcnk13 mRNA and a 15.1% decrease in KCNK13 immunoreactivity produced a 50% decrease in ethanol excitation in the mouse VTA (You et al., 2019), a 75% decrease in a major target of alcohol such as KCNK13 may explain the lower ethanol efficacy in brain slice experiments. Although it may be surprising that additional downregulation with siRNA (Figure 5) could affect the response to ethanol subsequent to a decrease of 75% from the native levels, this observation indicates the importance of KCNK13 in the response of VTA neurons to ethanol. We also noted that siRNA reduction of Kcnk13 over two hours did not alter the baseline firing rate; an increase in baseline firing might be expected with the downregulation of a leak potassium channel like KCNK13. There may be numerous reasons for the lack of effect on baseline firing, including compensation by upregulation of other leak potassium channels during the KCNK13 downregulation. We speculate that the importance of leak potassium channels in regulating the firing rate of these neurons could induce this compensation in the event of downregulation of KCNK13. Studies of the mechanisms of the downregulation of KCNK13 would be useful in elucidating the regulation of this ion channel by axotomy or other trauma.
Isoflurane produced excitation similar to ethanol (Figure 1C). KCNK13 channels are inhibited by anesthetics like halothane and isoflurane, so this observation is consistent with the presence of these channels on VTA neurons. Isoflurane is non-specific in that it also activates some two-pore channels, like the TREK channel (Pavel et al., 2020). However, in the presence of isoflurane, it is likely that the effects of ethanol on KCNK13 would be occluded; studies of the VTA in intact, anesthetized preparations may not be suitable for studying this action of ethanol, and other actions of ethanol may appear to predominate (e.g., effects on GABA versus dopamine neurons of the VTA (Gallegos et al., 1999)). It is also possible that the exposure to isoflurane immediately prior to sacrifice could alter KCNK13 expression, but the brief duration of exposure before sacrifice, and the time between sacrifice and the first recording (at least one hour) should minimize the consequences of that isoflurane exposure. Likewise, exposure to ethanol in the recording chamber at time 0 could alter expression of KCNK13 within the two-hour time, and that could compete with the siKcnk13 reduction in function. Additional careful experiments will be needed to rule out this possibility, but it is clear that siKcnk13 reduces both Kcnk13 mRNA and ethanol excitation of VTA neurons.
In addition to the involvement in the electrophysiological response to ethanol, Kcnk13 expression is altered by in vivo ethanol exposure. Acute ethanol administration increased the expression of Kcnk13 mRNA after four hours in mice rather than one hour (You et al., 2019). This increase in Kcnk13 mRNA expression was correlated with a significant increase in the effect of ethanol to excite VTA neurons (You et al., 2019). The increase in mRNA expression points to the dynamic regulation of Kcnk13 levels, and the cellular importance of this ion channel in VTA neurons. In the present study, we demonstrated a change in Kcnk13 mRNA correlated with ethanol withdrawal. At 24 hours after removal of alcohol-containing Lieber-DeCarli diet, there was a significant increase in Kcnk13 mRNA, similar to levels we observed after acute ethanol treatment in mice. At 72 hours of withdrawal, there was a significant decrease in Kcnk13 levels. As a decrease in Kcnk13 mRNA was correlated with an increase in alcohol intake in mice (You et al., 2019), it is tempting to speculate that decreased expression of Kcnk13 in the VTA during protracted withdrawal may be one factor in alcohol seeking behavior. Studies designed to elucidate the mechanisms of the dynamic regulation of KCNK13 could yield useful information about novel means to reduce alcohol seeking during protracted withdrawal.
Numerous studies have implicated other neurotransmitters and ion channels that participate in ethanol-induced excitation. Ethanol affects M-current (Koyama et al., 2007), h-current (Okamoto et al., 2006, Brodie and Appel, 1998) and barium-sensitive potassium conductances (McDaid et al., 2008, Herman et al., 2015). KCNK13-mediated excitation appears to be important for excitation of VTA neurons. A variety of targets of alcohol action could alter membrane properties (such as membrane resistance) and influence excitation mediated by KCNK13 blockade, including ion channels (Okamoto et al., 2006, McDaid et al., 2008, Herman et al., 2015), and neurotransmitter receptors (Larsson et al., 2002, Ericson et al., 2008, Steffensen et al., 2009, Theile et al., 2011, Steffensen et al., 2000). Blocking these receptors and ion channels does not block ethanol-induced excitation of VTA neurons (please see (Nimitvilai et al., 2016)), whereas treatments that block KCNK13 (e.g., gadolinium (Figure 1A) or high extracellular Ca++ (Figure 1B; (You et al., 2019))), can block ethanol-induced excitation of VTA neurons, suggesting that KCNK13 is a key target of ethanol in the VTA. KCNK13 deserves in-depth studies as it has a crucial role in ethanol stimulation of VTA neurons.
Supplementary Material
Acknowledgments:
Thanks to Amynah Pradhan and Alycia Tipton for the generous contribution of tissue samples for the 72-hour withdrawal study. Thanks to Dr. Subhash C. Pandey for the use of his laboratory for the molecular studies. The work reported here was supported by PHS Grant R01AA05846 (MSB) and P50AA022538 (MSB). None of the authors have any conflicts of interest to declare.
Footnotes
Supplementary Data:
Additional information (Figure S1) is available in the Supplementary Materials section.
Financial Disclosures:
All authors reported no related financial interests or potential conflicts of interest.
References
- Acosta C, Djouhri L, Watkins R, Berry C, Bromage K, Lawson SN (2014) TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J Neurosci 34:1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SB, Liu Z, McElvain MA, Brodie MS (2003) Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J. Pharmacol. Exp. Ther 306:437–446. [DOI] [PubMed] [Google Scholar]
- Avegno EM, Salling MC, Borgkvist A, Mrejeru A, Whitebirch AC, Margolis EB, Sulzer D, Harrison NL (2016) Voluntary adolescent drinking enhances excitation by low levels of alcohol in a subset of dopaminergic neurons in the ventral tegmental area. Neuropharmacology 110:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Appel SB (1998) The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin. Exp. Res 22:236–244. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB (1999) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin. Exper. Res 23:1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV (1988) Ethanol increases the firing of dopamine neurons of the ventral tegmental area in vitro. Alcohol Clin. Exper. Res 12:323. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain. Res 508:65–69. [DOI] [PubMed] [Google Scholar]
- Enyedi P, Czirjak G (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90:559–605. [DOI] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Chau P, Soderpalm B (2008) Nicotinic acetylcholine receptors in the anterior, but not posterior, ventral tegmental area mediate ethanol-induced elevation of accumbal dopamine levels. J Pharmacol Exp Ther 326:76–82. [DOI] [PubMed] [Google Scholar]
- Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC (1999) Adaptive responses of gamma-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J. Pharmacol. Exp. Ther 291:1045–1053. [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G (1985) Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain. Res 348:201–203. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N (2001) Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci 2:175–184. [DOI] [PubMed] [Google Scholar]
- Herman MA, Sidhu H, Stouffer DG, Kreifeldt M, Le D, Cates-Gatto C, Munoz MB, Roberts AJ, Parsons LH, Roberto M, Wickman K, Slesinger PA, Contet C (2015) GIRK3 gates activation of the mesolimbic dopaminergic pathway by ethanol. Proc Natl Acad Sci U S A 112:7091–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA (2006) Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse 60:251–255. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Jacobs KM, Prince DA (2003) Light microscopic study of GluR1 and calbindin expression in interneurons of neocortical microgyral malformations. Neuroscience 120:207–218. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Brodie MS, Appel SB (2007) Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J. Neurophysiol 97:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA (1989) Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J. Neurosci 9:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Soderpalm B, Engel JA (2002) Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol 28:157–167. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL (2006) The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J. Physiol 577:907–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, Fields HL (2012) Identification of rat ventral tegmental area GABAergic neurons. PLoS One 7:e42365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS (2008) Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J. Neurophysiol 100:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA (2010) Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol 91:235–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrejeru A, Marti-Prats L, Avegno EM, Harrison NL, Sulzer D (2015) A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience 290:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AL, Brodie MS (1989) Intracellular recording from putative dopamine-containing neurons in the ventral tegmental area of Tsai in a brain slice preparation. J. Neurosci. Methods 28:15–22. [DOI] [PubMed] [Google Scholar]
- Nimitvilai S, You C, Arora DS, McElvain MA, Vandegrift BJ, Brodie MS, Woodward JJ (2016) Differential Effects of Toluene and Ethanol on Dopaminergic Neurons of the Ventral Tegmental Area. Front Neurosci 10:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H (2006) Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J. Neurophysiol 95:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel MA, Petersen EN, Wang H, Lerner RA, Hansen SB (2020) Studies on the mechanism of general anesthesia. Proc Natl Acad Sci U S A 117:13757–13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, Karschin A, Derst C (2001) THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem 276:7302–7311. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Muller R, Daut J, Karschin A, Derst C (2000) TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem 275:16650–16657. [DOI] [PubMed] [Google Scholar]
- Rivera-Meza M, Quintanilla ME, Bustamante D, Delgado R, Buscaglia M, Herrera-Marschitz M (2014) Overexpression of hyperpolarization-activated cyclic nucleotide-gated channels into the ventral tegmental area increases the rewarding effects of ethanol in UChB drinking rats. Alcohol Clin Exp Res 38:911–920. [DOI] [PubMed] [Google Scholar]
- Signs SA, Yamamoto BK, Schechter MD (1987) In vivo electrochemical determination of extracellular dopamine in the caudate of freely-moving rats after a low dose of ethanol. Neuropharmacology 26:1653–1656. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Nie Z, Criado JR, Siggins GR (2000) Ethanol inhibition of N-methyl-D-aspartate responses involves presynaptic gamma-aminobutyric acid(B) receptors. J. Pharmacol. Exp. Ther 294:637–647. [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, Criado JR (2009) Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol. Biochem. Behav 92:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA (2011) GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience 172:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulleuda A, Cokic B, Callejo G, Saiani B, Serra J, Gasull X (2011) TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Gasull X, Walters ET (2002) Long-term alteration of S-type potassium current and passive membrane properties in aplysia sensory neurons following axotomy. J Neurophysiol 87:2408–2420. [DOI] [PubMed] [Google Scholar]
- Vena AA, Gonzales RA (2015) Temporal profiles dissociate regional extracellular ethanol versus dopamine concentrations. ACS Chem Neurosci 6:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Savarese A, Vandegrift BJ, He D, Pandey SC, Lasek AW, Brodie MS (2019) Ethanol acts on KCNK13 potassium channels in the ventral tegmental area to increase firing rate and modulate binge-like drinking. Neuropharmacology 144:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Vandegrift B, Brodie MS (2018a) Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 235:1711–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Vandegrift BJ, Zhang H, Lasek AW, Pandey SC, Brodie MS (2018b) Histone Deacetylase Inhibitor Suberanilohydroxamic Acid Treatment Reverses Hyposensitivity to gamma-Aminobutyric Acid in the Ventral Tegmental Area During Ethanol Withdrawal. Alcohol Clin Exp Res 42:2160–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


