Abstract
The hippocampus is particularly susceptible to neurodegeneration. Physical activity, specifically increasing cardiorespiratory fitness via aerobic exercise, shows promise as a potential method for mitigating hippocampal decline in humans. Numerous studies have now investigated associations between the structure and function of the hippocampus and engagement in physical activity. Still, there remains continued debate and confusion about the relationship between physical activity and the human hippocampus. In this review, we describe the current state of the physical activity and exercise literature as it pertains to the structure and function of the human hippocampus, focusing on four magnetic resonance imaging measures: volume, diffusion tensor imaging, resting state functional connectivity, and perfusion. We conclude that, despite significant heterogeneity in study methods, populations of interest, and scope, there are consistent positive findings, suggesting a promising role for physical activity in promoting hippocampal structure and function throughout the lifespan.
Keywords: exercise, cardiorespiratory fitness, perfusion, white matter, magnetic resonance imaging
INTRODUCTION
The hippocampus is essential in episodic and relational memory, and is highly susceptible to damage from a variety of conditions including hypoxia, encephalitis, epilepsy, and ischemia (Knierim, 2015). Adolescence and early adulthood are critical periods during which the hippocampus exhibits considerable reorganization and growth, which has important consequences for cognitive performance and academic achievement (Hueston et al., 2017). Furthermore, the hippocampus deteriorates earlier and more rapidly than other brain regions in populations experiencing neurodegeneration, including Alzheimer’s disease (AD; Frisoni et al., 2010), schizophrenia (Heckers & Konradi, 2002), depression (W. Liu et al., 2017), and multiple sclerosis (Sicotte et al., 2008), as well as in healthy older adults (Raz et al., 2005). These losses precede, and lead to, subsequent memory deficits and increased risk for neurologic diseases. Due to the importance of the hippocampus in memory formation and its sensitivity to disease processes, there is considerable scientific and public health interest in finding approaches for promoting the structural and functional integrity of the hippocampus throughout the human lifespan.
One widely accessible and cost-effective intervention for possibly enhancing the structure and function of the hippocampus is aerobic exercise. Aerobic exercise is a form of physical activity that requires oxygen, often challenges the oxygen capacity of the system, and engages large groups of muscles over extended periods of time. When aerobic exercise is habitual, it can lead to improvements in cardiorespiratory fitness, or the body’s ability to take in and deliver oxygen to working muscles (Manley, 1996). In this review, we use the term “physical activity”, broadly, to refer to all activities involving a period of movement, and the term “aerobic exercise” to refer to those studies that specifically focus on eliciting a cardiovascular response from the activity. Notably, aerobic exercise varies substantially in intensity level. Moderate-intensity activities (e.g., brisk walking) are often operationalized as requiring 3.0 to less than 6.0 metabolic equivalents (METs), whereas vigorous-intensity activities (e.g., running) require 6.0 or greater METs (2018 Physical Activity Guidelines Advisory Committee, 2018). Clinical trials that use aerobic exercise interventions frequently target moderate-to-vigorous intensity levels, particularly when recruiting from healthy young and midlife adult samples. However, even light-to-moderate intensity activity that demands 1.6 to less than 3.0 METs can elicit a substantial cardiovascular response in low fit or sedentary individuals (2018 Physical Activity Guidelines Advisory Committee, 2018). In general, clinical trials that utilize aerobic exercise interventions use the term “aerobic exercise” to refer to the behavioral intervention, regardless of the intensity, and we use the same terminology here when describing those results. In contrast, observational studies often use the more general term “physical activity” to appropriately capture a broad swath of activities assessed that are not necessarily aerobic in nature. Figure 1 depicts the various study designs and metrics of physical activity described in this review.
Figure 1.
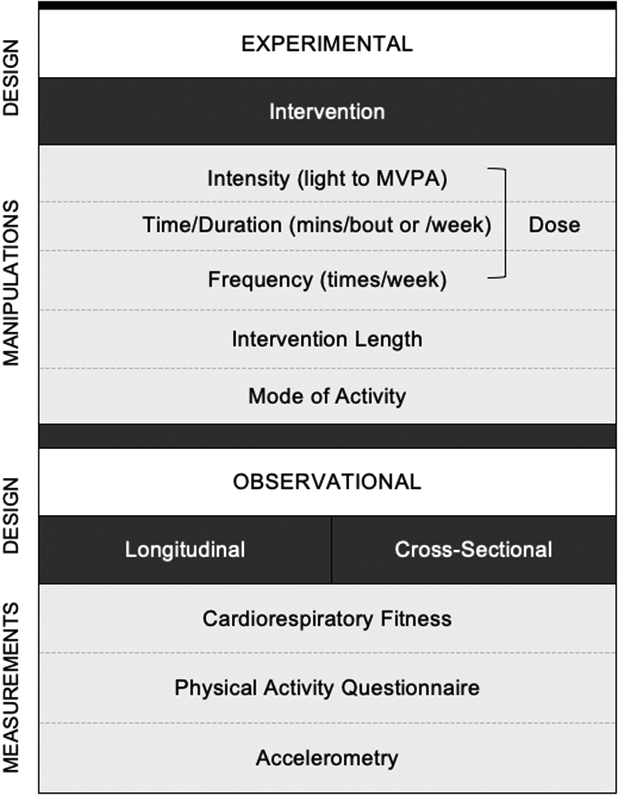
Conceptual illustration representing the diversity of study designs and physical activity related measurements employed in the studies included in this review.
Note. Exercise dose is defined as intensity (ranging from light to moderate-to-vigorous physical activity [MVPA]), duration (e.g. minutes per bout or week of exercise), and frequency (e.g. number of exercise bouts per week).
Rodent studies have demonstrated that aerobic exercise increases cell proliferation and survival, as well as angiogenesis, and alters gene expression for metabolic, growth factor, and signaling molecules and cascades in the hippocampus (Kerr et al., 2011; Mustroph et al., 2012; Rhodes et al., 2003; Tong et al., 2001; Van Praag et al., 1999). In humans, a major strength of neuroimaging is that it enables us to integrate multiple magnetic resonance imaging (MRI) measures to comprehensively characterize features of the structure and function of specific brain regions such as the hippocampus (Figure 2). Over the past 10 years, there has been a considerable number of human neuroimaging studies that target the hippocampus in the context of measurements of cardiorespiratory fitness or exposure to exercise. To facilitate interpretation, the frequency of the neuroimaging metrics and the measurements of physical activity and exercise in the cited literature are presented as a series of heat maps in Figure 3. Yet surprisingly, few reviews have integrated this literature. Instead, publications have either limited their reviews to specific measures of the hippocampus (i.e., volume) at the expense of functional or connectivity metrics, or have focused on whole-brain morphology, which could overlook hippocampus-specific effects (Erickson et al., 2014). For instance, Firth et al., (2018) reported that randomized clinical trials (RCTs) of aerobic exercise prevented age-related decline in left hippocampal volume and of bilateral hippocampal volume in older adults. However, this review did not include research in children, measures of hippocampal connectivity, white matter (WM), or function. Valkenborghs et al., (2019) systematically reviewed nine studies investigating the impact of exercise on brain structure and function in children and adolescents. They reported positive associations between exercise via RCTs and functional activation and connectivity in a number of regions, including the hippocampus and related functional networks. However, the authors also cited limitations due to a sparse number of studies and considerable heterogeneity across methods. Many other manuscripts have reviewed the effects of exercise on neuroimaging outcomes without specificity or focus on the hippocampus or tracts communicating with the hippocampus (Erickson et al., 2019; Sexton et al., 2016). Although such broad reviews are important, there is reason to focus the spotlight on the hippocampus given its dominant role in many disease states, its well-established cellular plasticity, and the clear links with exercise in rodents.
Figure 2.
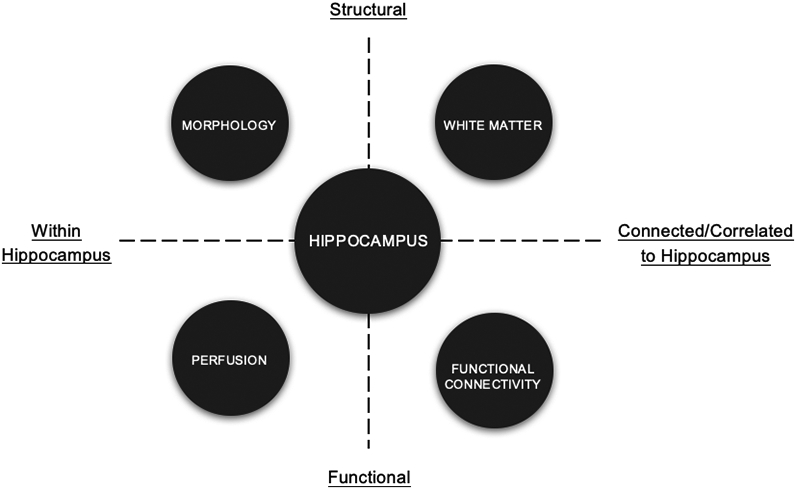
Conceptual illustration representing the neuroimaging measurements focused on in this review and how they can be viewed with respect to the hippocampus.
Note. This is a simplified conceptual scheme rather than a theoretical or mechanistic model.
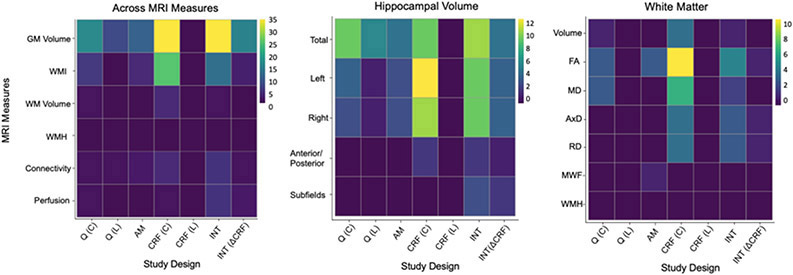
Figure 3.
Heat map illustration of the number of analyses outlined in this review, assessing hippocampal integrity (y-axis) using magnetic resonance imaging (MRI) measures of morphology (GM Volume), white matter integrity (WMI), white matter volume (WM Volume), white matter hyperintensities (WMH), functional connectivity, and perfusion to examine associations with physical activity measurements.
Note. Physical activity study designs (x-axis) include: (1) cross-sectional physical activity questionnaires (Q[C]); (2) longitudinal physical activity questionnaires (Q[L]); (3) accelerometry (AM); (4) cross-sectional cardiorespiratory fitness (CRF[C]); (5) longitudinal cardiorespiratory fitness (CRF[L]), including only observational studies and not randomized clinical trials; (6) interventions, including randomized clinical trials, training studies, and detraining studies, specifically measuring outcomes pre- and post-intervention (INT); and (7) interventions using the relative change in cardiorespiratory fitness as the main outcome (INT[ΔCRF]). The observations included in the heat maps are based on individual analyses within studies, as numerous studies conducted multiple analyses using various metrics of hippocampal integrity and physical activity. a) The number of analyses (n = 205) assessing any of the four magnetic resonance imaging measures reviewed in this study with relation to physical activity. b) The number of analyses (n = 124) assessing hippocampal morphology using total volume, left and right hippocampal seeds, anterior and posterior seeds, and subfields, examined in association with measures of physical activity. c) Number of analyses assessing (n = 58) white matter connections to the hippocampus using total volume, measures of white matter integrity, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AxD), radial diffusivity (RD), and myelin water fraction (MWF), and white matter hyperintensities (WMH), examined in association with measures of physical activity.
This review aims to address the following questions: (1) Do physical activity behaviors and related constructs, such as cardiorespiratory fitness, influence the volume, function, and integrity of the hippocampus across the lifespan?; (2) Do the effects of aerobic exercise interventions on hippocampal structure include both gray matter volume and WM connections?; (3) Does aerobic exercise influence hippocampal resting state connectivity or perfusion?; and (4) Based on this summary of the neuroimaging literature (see Table 1), are there broad conclusions and recommendations that could be generated about the effects of aerobic exercise on the hippocampus in humans? We will also address gaps in the literature and suggest future directions for the field.
Table 1.
Magnetic resonance imaging measures included in this review and their metrics.
| Magnetic Resonance Imaging Measures | Metrics |
|---|---|
| Volumetric | Using methods such as T1-weighted or T2-weighted imaging, we are able to characterize the morphology of the cortex and subcortical areas, including volume, shape, and thickness. |
| Diffusion Weighted Imaging and White Matter | White matter microstructural integrity may be characterized using a number of metrics including radial diffusivity (RD), mean diffusivity (MD), axial diffusivity (AxD), or the summary measure (FA). Other metrics of integrity include myelin water fraction. White matter volume and volume of white matter lesions can also be quantified using methods such as T1-weighted or T2-weighted imaging. |
| Functional Connectivity | Correlated functional activation in various brain regions, at rest and during task performance, allow for the detection of functionally connected brain regions. |
| Perfusion | Arterial spin labeling perfusion MRI provides a quantitative measure of blood flow and offers information about how the brain meets and regulates its metabolic demand. |
HIPPOCAMPAL MORPHOLOGY
Using MRI, the morphology of the hippocampus—including volume and shape—can be characterized. The findings from cross-sectional studies utilizing physical activity questionnaires are mixed (see Table 2), with some studies reporting that greater engagement in physical activity is associated with greater hippocampal volume (Gorham et al., 2019; Hashimoto et al., 2017; Killgore et al., 2013; McEwen et al., 2015; Nunley et al., 2017; Okonkwo et al., 2014; Tarkka et al., 2019), and other studies reporting null results (Bugg & Head, 2011; Head et al., 2012; Jeon et al., 2020; M. Ortega et al., 2015; Vemuri et al., 2012). For example, in a study of 61 healthy adults aged 18-45 years, more minutes per week exercising was associated with greater left and right hippocampal volume (Killgore et al., 2013). Many other studies have reported similar findings across healthy and patient populations, including schizophrenia and type 1 diabetes (Gorham et al., 2019; McEwen et al., 2015; Nunley et al., 2017; Okonkwo et al., 2014; Tarkka et al., 2019). Several studies of older adults (Bugg & Head, 2011; Head et al., 2012; Jeon et al., 2020; Vemuri et al., 2012) and adults with human immunodeficiency virus (HIV; M. Ortega et al., 2015) have failed to find similar associations, although Head and colleagues found decreased hippocampal volume among older adults with increased stress and lower physical activity levels (2012). These mixed findings might suggest that other unmeasured factors are either conflating or moderating the association.
Table 2.
A description of studies examining the association of physical activity or exercise with metrics of hippocampal health.
| Study (Year) |
Sample (Age) | Hippocampus (Defined As) |
Summary of Findings |
|---|---|---|---|
| MORPHOLOGY | |||
| Cross-sectional Physical Activity Questionnaire | |||
| Significant Findings | |||
| Gorham et al. (2019) | Children (9-11 years) | Total, left, and right | Greater number of sports was associated with larger total and left HV, but not right HV. |
| Hashimoto et al. (2017) | Older adults (58-94 years) | Total | Hippocampal atrophy was associated with leisure-time physical inactivity. |
| Killgore et al. (2013) | Healthy adults (18-45 years) | Left and right | More minutes per week exercising was associated with greater left and right HV. |
| McEwen et al. (2015) | Patients with first-episode schizophrenia (18-45 years) | Total, left, and right | Lower PA was associated with decreased total and left HV, but not right HV. |
| Nunley et al. (2017) | Middle-aged adults with and without type 1 diabetes (35-60 years) | Total | Higher PA was associated with larger HV among type 1 diabetes subjects but not control subjects. |
| Okonkwo et al. (2014) | Late-middle-aged adults (40-65 years) | Total | Increasing age was associated with decreased HV, but effect was attenuated by 43% for active participants compared to inactive subjects. |
| Tarkka et al. (2019) | Male monozygotic twins (mean age 34.5 years) | Cluster in left | More-active co-twins had greater HV than less-active co-twins. |
| Null Findings | |||
| Bugg et al. (2011) | Older adults (55-79 years) | Total | PA alone did not account for unique variance in HV. |
| Head et al. (2012) | Older adults (mean age 72.5 years) | Total | PA alone did not account for unique variance in HV. Compared to the high-exercise group, the low-exercise group had decreased HV with increasing stress. |
| Jeon et al. (2020) | Older adults with healthy cognition or MCI (55-90 years) | Total | Retrospective report of midlife PA was not associated with HV. |
| Ortega et al. (2015) | Adults with HIV (mean age 41.6 years) | Total | No HV differences between physically active and sedentary groups. |
| Vemuri et al. (2012) | Older adults with healthy cognition or MCI (70-90 years) | Total | PA was not associated with HV. |
| Longitudinal Physical Activity Questionnaire | |||
| Significant Findings | |||
| Barha et al., (2020) | Older adults (70-79 years) | Left and right | Maintaining time spent walking over 10 years predicted smaller left HV for women and larger left HV for men. Null results were found for right HV. |
| Best et al., (2017) | Older adults (70-79 years) | Total | Maintaining time spent walking over 10 years predicted less reduction in HV. |
| Erickson et al., (2010) | Older adults (≥65 years) | Total | Greater PA predicted greater HV 9 years later. |
| Maltais et al., (2019) | Older adults at risk of cognitive decline (mean age 75.3 years) | Total | Any level of PA was associated with a reduced decline in HV compared to inactivity over 3 years. |
| Smith et al., (2014) | Older adults (65-89 years) | Total | Those with low PA and one or both APOE ε4 alleles (High Risk/Low PA) had decreased HV compared to those all other groups over 18 months (i.e., Low Risk/Low PA, High Risk/High PA, Low Risk/High PA). |
| Tan et al., (2017) | Older adults (≥60 years) | Total | Greater PA was associated with greater HV over 10 years. |
| Null Findings | |||
| Reijs et al., (2017) | Older adults with cognitive complaints and MCI (≥55 years) | Total | Lower HV was associated with conversion to AD-type dementia over 2.4 years, but it was not modulated by PA. |
| Cross-sectional Accelerometer | |||
| Significant Findings | |||
| (Hamer et al., 2018) | Middle-aged adults (40-69 years) | Left and right | More overall PA was associated with greater left and right HV. |
| Klaren et al. (2015) | Adults with multiple sclerosis (18-64 years) | Total | More time spent in moderate-to-vigorous PA was associated with greater HV, but light PA behavior and sedentary behavior were not. |
| Makizako et al. (2015) | Older adults with MCI (≥65 years) | Total | More time spent in moderate PA was associated with greater HV, but light PA and total PA were not. |
| Raichlen et al. (2019) | Middle-aged to older adults (45-80 years) | Left and right | More time spent in moderate-to-vigorous PA was associated with greater left and right HV. |
| Varma et al. (2015) | Older adults (≥60 years) | Total | Greater amount, duration, and frequency of total daily walking activity and low-intensity daily walking activity were associated with larger HV among women but not men. |
| Verma et al. (2016) | Older adults (≥60 years) | Left and right shape | Greater number of steps/day was associated with outward shape differences along the subiculum of the left and right hippocampus in women but not men. Greater low-intensity walking activity was associated with outward shape differences along the subiculum of the left hippocampus in women but not men. Moderate-to-vigorous PA was not significantly correlated with hippocampal shape in men or women. |
| Null Findings | |||
| Alosco et al. (2015) | Older adults with heart failure (50-85 years) | Total | Daily step count was not associated with HV. |
| Spartano et al. (2019) | Mean age 53 years | Total | HV was not associated with time spent in moderate-to-vigorous PA, light PA, or total PA. |
| Cross-sectional Cardiorespiratory Fitness | |||
| Significant Findings | |||
| Aghjayan et al. (2020) | Middle-aged adults with overweight or obesity (18-55 years) | Left, right, and shape | Higher CRF was associated with greater left HV, with outward shape differences along the surface of the subiculum and CA1 regions. Null results were found for right HV. |
| Boots et al. (2015) | Middle-aged adults at risk for AD (40-65 years) | Total | Higher CRF was associated with greater HV. |
| Bugg et al. (2012) | Older adults with obesity (65-75 years) | Total | CRF accounted for unique variance in HV after controlling for age, gender, and waist circumference. |
| Chaddock et al. (2010) | Preadolescent children (9-10 years) | Left and right | Higher-fit children had greater left and right HV compared to lower-fit children. |
| Chaddock-Heyman et al. (2015) | Breast cancer survivors (41-73 years) | Total, left and right anterior, and left and right posterior | Higher CRF was associated with greater total HV. Lower-fit cancer survivors had smaller total, left and right anterior, and left and right posterior HV than higher-fit control subjects. Lower-fit cancer survivors had smaller left posterior HV compared to lower-fit control subjects. |
| Dougherty et al. (2017) | Older adults at risk for AD (50-75 years) | Total | Higher CRF was associated with greater HV for women but not men. |
| Erickson et al. (2009) | Older adults (59-81 years) | Left and right | Higher CRF was associated with greater left and right HV. |
| Esteban-Cornejo et al. (2017) | Children with overweight or obesity (8-11 years) | Cluster in left | Higher CRF was associated with greater left HV. |
| Fletcher et al. (2016) | Older adults (55-87 years) | Total | Higher CRF was associated with greater HV. |
| Herting et al. (2012) | Male adolescents (15-18 years) | Left and right | Higher CRF was associated with greater left and right HV. |
| Makizako et al. (2013) | Older adults with MCI (≥65 years) | Cluster in left | Higher exercise capacity was associated with greater left HV. |
| McAuley et al. (2011) | Older adults (60-80 years) | Total | Higher CRF was associated with greater HV. |
| Motl et al. (2015) | Adults with multiple sclerosis (18-64 years) | Total | Higher CRF was associated with greater HV. |
| Ortega et al. (2017) | Children (mean age 9.7 years) | Left and right shape | CRF was associated with the shape (expansions/contractions) of the right hippocampus, but not the left hippocampal. |
| Stillman et al. (2018) | Young adults (20-38 years) | Left and right anterior, and left and right posterior | Higher CRF was associated with greater left anterior HV. |
| Szabo et al. (2011) | Older adults (60-80 years) | Total | Higher CRF was associated with greater HV. |
| Null Findings | |||
| Cole et al. (2020) | Older adults (60-76 years) | Total, left, and right | CRF was not significantly associated with total, left, or right HV. |
| Engeroff et al. (2018) | Older adults (≥65 years) | Left and right | CRF was not significantly associated with left or right HV. |
| Honea et al. (2009) | Older adults with healthy cognition and early-stage AD (≥65 years) | Total | No significant relationship of CRF with HV in either group. |
| Raichlen et al. (2019) | Middle-aged to older adults (45-80 years) | Left and right | CRF was not associated with left or right HV. |
| Wood et al. (2016) | Master athletes and active adults (45-73 years) | Left and right | CRF was not associated with left or right HV regardless of group. |
| Randomized Clinical Trials | |||
| Significant Findings | |||
| Erickson et al., (2011) | Sedentary older adults (55-80 years) | Left and right anterior, and left and right posterior | The aerobic group had an increase in left and right HV compared to the control group, which had a decrease in left and right HV over the 12-month intervention. Aerobic exercise increased left and right anterior HV but not left or right posterior HV compared to the control group, which had a decrease in left and right anterior HV but not left and right posterior HV. Greater improvements in CRF were associated with greater increases in left and right anterior HV, and left and right posterior HV. |
| Jonasson et al., (2016) | Sedentary old adults (64-78 years) | Total | Increased CRF following the 6-month intervention was associated with increased HV. |
| Leavitt et al. (2014) | Female patients with memory impairment and multiple sclerosis (33-44 years) | Total | The aerobic group showed an increase in HV following the 3-month intervention but the non-aerobic group did not. |
| Lin et al., (2015) | Women with early psychosis (16-60 years) | Total, left, and right | Aerobic exercise over 12 weeks was associated with increased HV, mainly related to increases in the left hippocampus. Changes in CRF did not differ significantly across the groups. |
| Maass et al., (2015) | Sedentary older adults (60-77 years) | Head, body, and tail | Increased CRF was associated with increased hippocampal head volume over 12 weeks, although hippocampal head volume did not increase overall in the exercise group. Null results were found for the body and tail. |
| Morris et al., (2017) | Older adults with early-stage AD (≥55 years) | Total | Increased CRF was associated with increased HV over 26 weeks. |
| Niemann et al., (2014) | Older adults (62-79 years) | Total, left, and right | Total and left HV increased in the aerobic exercise group over the 12-month intervention. Null results were found for the right hippocampus. Changes in CRF significantly explained variance in total and left HV, but not right HV. |
| Pajonk et al., (2010) | Men with chronic schizophrenia and matched healthy subjects (20-51 years) | Total | Among both aerobic exercise groups, HV increased by 14% over the 12-week intervention. Greater changes in CRF were associated with larger increases in HV. |
| Rosano et al., (2017) | Sedentary older adults at risk for mobility disability (70-89 years) | Total, left, and right | The PA group had greater left, right, and total HV following a 24-month intervention, although the right hippocampus was attenuated after controlling for regional baseline volume. |
| ten Brinke et al., (2015) | Women with probable MCI (70-80 years) | Total, left, and right | Compared with the control group, the aerobic group significantly improved left, right, and total HV following a 6-month intervention. |
| Thomas et al., (2016) | Sedentary, young to middle-aged adults (mean age 33.7 years) | Anterior | The 6-week exercise intervention significantly increased anterior HV, but it returned to baseline after an additional 6 weeks following the completion of the study. |
| Null Findings | |||
| Bunketorp Käll et al., (2015) | Students in grades 5 and 6 | Left and right | Neither left nor right HV differed significantly between groups, despite greater increases in CRF among the boys in the intervention group compared to the boys in the control group. |
| Frederiksen et al., (2018) | Patients with mild to moderate AD (50-90 years) | Left, right, and subfields | There was no effect of a 16-week intervention on HV or subfield volume. There was no significant correlation between CRF and HV. |
| Krogh et al., (2014) | Adults with major depression (18-60 years) | Left, right, and total | There was no difference in left, right, or total HV between groups following a 3-month intervention. Changes in CRF were not associated with changes in HV. |
| Malchow et al., (2016) | Patients with schizophrenia and healthy controls (18-60 years) | Total, left, right, and subfields | No significant changes in HV or subfield volume were found in patients or controls following a 3-month intervention. |
| Scheewe et al., (2013) | Patients with schizophrenia and healthy controls (18-48 years) | Left and right | The 6-month intervention did not have a significant effect on HV in patients or controls. |
| Tarumi et al., (2019) | Patients with amnestic MCI (55-80 years) | Total | The 12-month intervention did not have a significant effect on HV, despite significant improvements in CRF in the exercise group compared to the control group. |
| WHITE MATTER | |||
| White Matter Integrity | |||
| Cross-sectional Physical Activity Questionnaire | |||
| Significant Findings | |||
| Rodriguez-Ayllon, Derks, et al., (2020) | Children (10 years) | Cingulum ROI: hippocampal cingulum and cingulate gyrus | PA was negatively correlated with MD among all WM tracts of interest. There was no association between total PA and FA when controlling for sociodemographic factors. |
| Gow et al., (2012) | Older Adults (mean age 69.5 years at baseline) | ROI: FA and MD composite factors, consisting of 12 ROIS, including the rostral cingulum bundles | Higher PA was significantly associated with higher composite FA, but not MD. |
| Null Findings | |||
| Maltais et al., (2020) | Older adults without dementia ≥ 70 years. | ROI included the hippocampal cingulum | Null relationship between PA and FA. Associations between lower PA and faster MD worsening did not include the hippocampal cingulum. |
| Cross-sectional Accelerometer | |||
| Significant Findings | |||
| Burzynska et al., (2014) | Low-fit older adults (60-78 years) | ROI included the anterior cingulum, temporal lobe WM, and medial temporal/para-hippocampal WM | Light PA was associated with temporal ROI FA. In addition, sedentary behavior was related to lower parahippocampal FA. |
| Bracht et al., (2016) | Adults (mean age 25.5 years) | ROI: fornix and bilateral parahippocampal cingulum | Positive correlation between activity level and MWF, but not FA, in the right parahippocampal cingulum. |
| Null Findings | |||
| Burzynska et al., (2017) | Low-active older adults (60–80 years) | ROIs included the fornix and anterior and posterior cingulum (as well as parahippocampal WM) | Neither light nor moderate-to-vigorous PA were correlated with change in FA in hippocampal ROIs. |
| Cross-sectional Cardiorespiratory Fitness (CRF) | |||
| Significant Findings | |||
| Chen et al., (2020) | Midlife to older adults (55–65 years) | ROIs included the hippocampal cingulum & cingulate gyrus | Higher CRF was associated with higher FA in multiple WM tracts, including the hippocampal cingulum. |
| Ding et al., (2018) | Older adults with healthy cognition (mean age 66 years) or amnestic MCI (mean age 65 years) | Whole-brain | Higher CRF was associated with higher FA, and lower MD and RD, in a number of WM tracts, including the cingulum. |
| Harasym et al., (2020) | Post-menopausal women (mean age 59 years) | ROIs included the hippocampal cingulum & cingulate gyrus | Higher CRF related to higher AD in the hippocampal cingulum (but no associations with FA, MD, or RD). |
| Marks et al., (2011) | Sedentary older adults (60–76 years) | ROI: bilateral anterior, middle, and posterior cingulum | Higher CRF was associated with higher FA in the left middle cingulum. |
| Oberlin et al., (2016) | Older adults (60–81 years) | Whole-brain | Higher CRF was associated with higher FA in a number of WM tracts, including the fornix and cingulum. |
| Null Findings | |||
| Burzynska et al., (2014) | Low-fit older adults (60-78 years) | ROIs included the anterior cingulum, temporal lobe WM, and medial temporal/para-hippocampal WM | Null associations between CRF and FA in hippocampal regions. After controlling for age and gender, and association between higher temporal FA and CRF was reduced to trend level. |
| Burzynska et al., (2017) | Low-active older adults (60–80 years) | ROIs included the fornix and anterior and posterior cingulum (and parahippocampal WM) | Null associations between CRF and FA in hippocampal regions. |
| Clark et al., (2019) | Sedentary older adults (57 to 86 years) | Whole-brain | Null associations between CRF and both FA and MD. |
| Perea et al., (2016) | Sedentary older adults with early stage AD (mean age 72.35 years) | Whole-brain and ROI including bilateral cingulum bundles. | No significant positive association between CRF and cingulum FA, MD, RD, or AD, although there was a trending relationship between higher CRF and lower RD in the right cingulum. |
| Teixeira et al., (2016) | Older adults with amnestic MCI (57–80) | Whole-brain | Significant associations between higher CRF and higher FA, lower MD, lower RD, and lower AD did not include WM connections to the hippocampus. |
| Tseng et al., (2013) | Sedentary older adults (66–82 years) and master athletes (61–80 years) | Whole-brain | Higher CRF was associated with higher FA in multiple WM pathways, but not in hippocampal projections. (Notably, relative to sedentary older adults, master athletes showed significantly lower MD (but not FA) in the hippocampal cingulum.) |
| Randomized Clinical Trials | |||
| Significant Findings | |||
| Voss et al., (2013) | Sedentary older adults (55-80 years) | Lobular ROIs: Frontal, Temporal, Parietal, and Occipital | Increased CRF over the course of the 12-month intervention was associated with increased temporal, frontal, and parietal FA (but not AD or RD) in the exercise group but not the control group. There were no significant main effects of group assignment. |
| Burzynska et al., (2017) | Low-active older adults (60–80 years) | ROIs included the fornix and anterior and posterior cingulum (as well as parahippocampal WM) | Significant group by time interaction, such that fornix FA increased in the dance group after 6-months of intervention, and decreased in the walking and control groups. RD and MD showed significantly less decrease in the dance group. |
| Null Findings | |||
| Clark et al., (2019) | Sedentary older adults (57–86 years) | Whole-brain | Lower FA and higher MD following 6-months of an aerobic exercise intervention did not include hippocampal WM connections. |
| Sexton et al., (2020) | Older adults (60–85 years) | Whole-brain | No significant effects of a 3-month aerobic exercise intervention on FA, AD, or RD. |
| Svatkova et al., (2015) | Patients with schizophrenia and healthy controls (18–48 years) | Whole-brain | The exercise group demonstrated significant increases in FA over the 6-month intervention in several WM pathways, but not including connections to the hippocampus. |
| White Matter Volume | |||
| Cross-sectional Physical Activity Questionnaire | |||
| Null Findings | |||
| Ho et al., (2011) | Older adults (mean age 77.9 years) | Whole-brain | Self-reported PA (kcal/weekly expenditure) was not significantly associated with volume in WM of hippocampal connections. |
| Cross-sectional Cardiorespiratory Fitness (CRF) | |||
| Significant Findings | |||
| Esteban-Cornejo et al., (2019) | Children (7–11 years) | Whole-brain | Higher CRF was associated with greater WM volume in regions including the cingulate gyrus, among children with overweight/obesity but not children with BMI in the healthy range. |
| Honea et al., (2009) | Older adults with healthy cognition or early-stage AD (≥65 years) | Whole-brain and ROIs targeting hippocampal and parahippocampal regions | In early-stage AD, higher CRF was associated with greater WM volume in the left hippocampal ROI. No significant association between CRF and WM volume in cognitively normal older adults. |
| Null Findings | |||
| Erickson et al., (2007) | Post-menopausal women (58–80 years) | Whole-brain | Significant positive associations between CRF and WM volume did not include hippocampal tracts. |
| Gordon et al., (2008) | Young adults (20–28 years) and older adults (65–81) | Whole-brain | Null association between CRF and WM volume. |
| Randomized Clinical Trials | |||
| Null Findings | |||
| Colcombe et al., (2006) | Sedentary older adults (60–79 years) | Whole-brain | Greater WM volume over 6-months of intervention in the exercise group did not include hippocampal tracts. |
| FUNCTIONAL CONNECTIVITY | |||
| Cross-sectional Accelerometer | |||
| Significant Findings | |||
| Prakash et al., (2011) | Midlife adults with multiple sclerosis (30–58 years) | Left and right hippocampal seeds | Higher PA was associated with higher rsFC of both the left and right hippocampus to the posteromedial cortex |
| Null Findings | |||
| Voss et al., (2016) | Older adults (60–80 years) | Network ROIs, including the DMN | No significant associations among rsFC networks and physical activity. |
| Cross-sectional Cardiorespiratory Fitness | |||
| Significant Findings | |||
| Flodin et al., (2017) | Sedentary older adults (64–78 years) | Whole-brain analyses and left and right hippocampal and parahippocampal seeds | Right medial temporal lobe positively correlated with rsFC to frontal, parietal, and occipital areas and negatively correlated to the right thalamus and right occipital cortex. |
| Ikuta & Loprinzi, (2019) | Midlife adults (mean age 42.5 years) | Left and right hippocampal and parahippocampal seeds | Higher CRF was associated with more positive parahippocampal, but not hippocampal interhemispheric rsFC. |
| Stillman et al., (2018) | Young adults (20–38 years) | Left and right anterior and posterior hippocampal seeds | Higher CRF was associated with more positive rsFC of the left and right anterior hippocampus to regions including the frontal pole, middle frontal gyrus, and parahippocampus. Negative correlation between CRF and rsFC of right anterior hippocampus and right superior frontal gyrus. |
| Voss et al., (2016) | Older adults (60–80 years) | Network ROIs, including the DMN, and exploratory analysis of correlations between whole-brain seeds and the DMN core | Positive associations between CRF and rsFC in multiple networks, with most robust results within the DMN. Positive associations between fitness level and rsFC between the DMN core and several seeds, one of which extended to the left and right hippocampus. |
| Randomized Clinical Trials | |||
| Significant Findings | |||
| Burdette et al., (2010) | Older adults (70–85 years) | Whole-brain | After 4-months of intervention, the exercise group showed greater rsFC within the hippocampus, and from the hippocampus to the anterior cingulate cortex, as compared to controls. |
| Flodin et al., (2017) | Sedentary older adults (64–78 years) | Whole-brain analyses and left and right hippocampal and parahippocampal seeds, as well as network ROIs including the DMN | Increase in CRF following 6-months of intervention was associated with increased rsFC between the right hippocampus and frontal and parietal regions, and decreased rsFC between the left hippocampus and right precentral gyrus. Increase in CRF was correlated with increased rsFC between the DMN and left prefrontal cortex. No significant group by time interactions on rsFC. |
| Leavitt et al., (2014) | Female patients with memory impairment and patients with multiple sclerosis (33-44 years) | Left hippocampal seed | Aerobic exercise group, but not the control group, showed an increase in rsFC from the left to the right hippocampus. |
| Voss et al., (2020) | Older adults (60–80) years | ROIs included posterior and anterior hippocampus and parahippocampal cortex seeds | Effects of acute bouts of moderate intensity exercise in hippocampal-cortical rsFC in the DMN did not survive correction for multiple comparisons, but did predict increases in rsFC in these regions following 12-weeks of aerobic exercise training. |
| Weng et al., (2017) | Young adults (mean age 23.2 years) and older adults (mean age 66.3 years) | Network ROIs, including the DMN and a hippocampal cortical network | Single session of moderate intensity exercise were associated with rsFC within the hippocampus, and from the hippocampal cortical network to the medial prefrontal cortex, temporal pole, and intracalcarine cortex. |
| PERFUSION | |||
| Cross-sectional Physical Activity Questionnaire | |||
| Significant Findings | |||
| Zlatar et al., (2014) | Sedentary older adults at genetic risk for AD (52-81 years) | Left and right | Greater sedentary time was associated with greater left hippocampal perfusion for APOE ε4 carriers but not noncarriers. Null results were found for the right hippocampus. |
| Cross-sectional Cardiorespiratory Fitness (CRF) | |||
| Significant Findings | |||
| Chaddock-Heyman et al., (2016) | Preadolescent children (7-9 years) | Total, anterior, and posterior | Higher CRF was associated with greater total and posterior hippocampal perfusion, but not anterior. |
| Randomized Clinical Trials | |||
| Significant Findings | |||
| Alfini et al., (2016) | Master athletes (≥50 years) | Left and right | Perfusion decreased in the left and right hippocampus after 10 days of no exercise training. |
| Burdette et al., (2010) | Older adults (≤85 years) | Total | The exercise training group had greater hippocampal perfusion than the control group following a 4-month intervention. |
| Maass et al., (2015) | Sedentary older adults (60-77 years) | Total | Increased hippocampal perfusion for the exercise group following a 3-month intervention, but not the control group. Increased CRF was associated with greater hippocampal perfusion. |
| Pereira et al., (2007) | Adults (21-45 years) | Dentate gyrus, CA1 subfield, subiculum, and entorhinal cortex | Perfusion in the dentate gyrus increased over a 3-month intervention. Increases in dentate gyrus perfusion were associated with increases in CRF. |
Notes. Within the WM section of the table, the “Hippocampus (Defined As) column” describes studies that: (1) first used an exploratory, whole-brain approach and then identified regions of significance (“exploratory”); and (2) defined regions-of-interest, including WM tracts connecting to the hippocampus, prior to running analyses (“ROI”). Findings defined as null specifically pertain to results targeting the hippocampus. WMH is not included in this table as few studies have examined measurements of physical activity and localized lesions and none in relation to hippocampal WM connections. CRF = cardiorespiratory fitness, HV = hippocampal volume, PA = physical activity, MCI = mild cognitive impairment, AD = Alzheimer’s disease, HIV = human immunodeficiency virus, WM = white matter, FA = fractional anisotropy, MD = mean diffusivity, RD = radial diffusivity, AxD = axial diffusivity, MWF = myelin water fraction, rsFC = resting state functional connectivity.
Are physical activity levels predictive of future changes in hippocampal volume? One longitudinal study of older adults found that greater amounts of walking at baseline, assessed via a validated physical activity questionnaire, predicted greater total hippocampal volume nine years later (Erickson et al., 2010). Many other longitudinal studies of healthy older adults utilizing validated questionnaires have demonstrated similar results (Barha et al., 2020; Best et al., 2017; Erickson et al., 2010; Maltais et al., 2019; Smith et al., 2014; Tan et al., 2017). In contrast, in a sample of older adults with subjective cognitive complaints and mild cognitive impairment (MCI), reduced total hippocampal volume was associated with conversion to AD over a 2.4-year follow-up and self-reported physical activity did not modulate this association (Reijs et al., 2017). These mixed findings suggest that more research is needed to examine the importance of physical activity with the trajectory of hippocampal volume changes among other populations. While questionnaires offer an easily accessible measure of physical activity, particularly with long-term follow-up, the reliability of self-report measures of physical activity may vary based on the type of instrument used, whether it has been validated, and the populations being tested. Thus, these self-report instruments may not provide accurate descriptions of physical activity behavior in all studies. Other measures, such as device-measured physical activity or cardiorespiratory fitness, may be better able to characterize associations with the hippocampus.
Studies using accelerometry to measure daily physical activity allow for a precise quantification of daily sedentary behaviors and walking activity, including light and moderate-to-vigorous physical activity. While several studies have failed to find an association between device-measured physical activity and hippocampal volume (Alosco et al., 2015; Spartano et al., 2019), the majority of studies have reported that greater physical activity was associated with larger hippocampal volume, with some evidence for regional specificity to the subiculum (see Table 2). The effects might also vary as a function of the intensity of the activity. That is, while some cross-sectional studies have reported that greater time spent engaging in moderate-to-vigorous intensity but not low-intensity physical activity was associated with greater hippocampal volume (Klaren et al., 2015; Makizako et al., 2015; Raichlen et al., 2019), others have found the amount of low-intensity but not moderate-to-vigorous intensity physical activity to be predictive of hippocampal volume (Varma et al., 2015, 2016). Despite this discrepancy related to the intensity of the activity, these cross-sectional results suggest that greater amounts of physical activity tend to be associated with greater hippocampal volume. Future investigations are necessary for determining whether the intensity of the physical activity is important for hippocampal volume as compared to other parameters of activity, such as the length, frequency, or type of activity.
Most studies that have examined the association between cardiorespiratory fitness and hippocampal volume have reported a significant positive association (see Table 2), but several have failed to find an association among older adults (Cole et al., 2020; Engeroff et al., 2018; Honea et al., 2009; Raichlen et al., 2019; Wood et al., 2016). Particularly, cross-sectional studies have reported that higher cardiorespiratory fitness levels are associated with greater hippocampal volume among healthy children and adults, with some evidence for regional specificity to the left anterior hippocampus (Boots et al., 2015; Chaddock et al., 2010; Dougherty et al., 2017; Erickson et al., 2009; Fletcher et al., 2016; Herting & Nagel, 2012; McAuley et al., 2011; F. B. Ortega et al., 2017; Stillman et al., 2018; Szabo et al., 2011). Similar cross-sectional results have been found among patients with conditions that often affect the volume of the hippocampus, including multiple sclerosis (Motl et al., 2015), MCI (Makizako et al., 2013), overweight or obesity (Aghjayan et al., 2020; Bugg et al., 2012; Esteban-Cornejo et al., 2017), and breast cancer survivors (Chaddock-Heyman et al., 2015). Interestingly, one study of 7-9-year-old children found that higher cardiorespiratory fitness levels were associated with expansion in some parts, and contraction in other parts, of the right hippocampus (F. B. Ortega et al., 2017). These findings suggest that cardiorespiratory fitness may be associated with total volume of the hippocampus because of more regionally-specific associations with particular subfields. This general pattern is consistent with the animal literature demonstrating that aerobic exercise increases cell proliferation and synaptic plasticity in a regionally specific manner (Van Praag et al., 1999). The reasons for why some studies fail to find this association remain a mystery but could be related to the population, the way in which cardiorespiratory fitness was measured, the range of fitness levels, or third variables that have been unaccounted for.
While cross-sectional studies provide insight into the association between cardiorespiratory fitness and hippocampal volume, studies that utilize RCT designs are better positioned to make causal inferences about engagement in exercise and changes to hippocampal morphology. Although changes to hippocampal volume have been studied within the context of aerobic exercise interventions, the results remain rather equivocal. A recent meta-analysis of 14 RCTs in adults found that aerobic exercise prevents a reduction in left hippocampal volume, but does not influence total or right hippocampal volume (Firth et al., 2018). An examination of the individual studies included in the meta-analysis indicates that aerobic exercise had a significant positive effect on total, regional, or lateral hippocampal volume depending on the population included in the study. This suggests that hippocampal volume is particularly sensitive to various factors including age, neurological health, and psychiatric health. However, a number of studies have failed to find significant effects in healthy adolescents and adults with a range of psychiatric and neurological conditions known to affect the hippocampus (Bunketorp Käll et al., 2015; Frederiksen et al., 2018; Krogh et al., 2014; Malchow et al., 2016; Scheewe et al., 2013; Tarumi et al., 2019). However, one of these studies reported an exercise adherence rate of only 36% (Krogh et al., 2014), raising methodological and theoretical questions about the inclusion of studies with both poor or good (>80%) adherence rates. A recent meta-analysis of exercise interventions found a significant reduction in control groups’ total hippocampal volume from pre- to post-intervention, but not left or right hippocampal volume, while an overall increase in the intervention group was not significant (Wilckens et al., 2021). When examining the characteristics of the samples and interventions, the overall effect was significant for adults 65 years and older, interventions 24 weeks or longer, and interventions providing up to 150 minutes of exercise per week (Wilckens et al., 2021). These findings suggest that the parameters of the intervention and sample characteristics may account for some of the heterogeneity in findings across the field.
Improvements in cardiorespiratory fitness may be a precondition for intervention effects on hippocampal volume. Several studies reported in Firth et al. (2018) found that exercise-induced increases in hippocampal volume were correlated with increases in cardiorespiratory fitness across healthy and patient populations (Erickson et al., 2011; Jonasson et al., 2016; Maass et al., 2015; Morris et al., 2017; Niemann et al., 2014; Pajonk et al., 2010). However, these findings were not unanimous. For example, one study found significant changes in hippocampal volume in women with early psychosis despite failing to find significant improvements in cardiorespiratory fitness among the intervention group (Lin et al., 2015). In children, no significant effect of a curriculum-based physical activity intervention was found on hippocampal volume despite significant increases in cardiorespiratory fitness among boys following the intervention (Bunketorp Käll et al., 2015). Thus, the extant literature suggests that (a) exercise interventions, at the very least, might be an effective method for preventing hippocampal atrophy and shrinkage – a finding that could have significant public health implications, (b) increases in cardiorespiratory fitness might be a critical precondition for finding effects of the intervention on the hippocampus, suggesting that studies of greater intensity and longer length might be more likely to alter hippocampal volume, and (c) effects might be moderated by age or the presence of disease states in which the hippocampus is affected. Notably, significant heterogeneity across intervention parameters, adherence, level of supervision, monitoring of intensity, and other factors make the conclusions about the impact of exercise interventions on hippocampal volume even muddier. Consistency and harmonization of methodology, a focus on greater adherence, and larger sample sizes are needed to clarify these results.
It remains poorly understood how long the effects of exercise on hippocampal morphology persist after the conclusion of the intervention. One study found in sedentary young to middle-aged adults that exercise-induced increases in hippocampal volume returned to baseline levels approximately six weeks following the completion of the six-week intervention (Thomas et al., 2016). This is likely not a surprising finding since maintaining positive consequences (i.e., hippocampal volume) of a behavioral change (i.e., exercise) often requires that the behavioral change itself also be maintained. As an analogy, dietary changes would likely influence glucose regulation but would not be maintained if someone returns to an unhealthy diet. This is an important consideration since most interventions fail to report the time between the conclusion of the intervention and when hippocampal morphology was assessed. Thus, it is likely that interventions that measure hippocampal volume several weeks or months after the completion of an intervention might be missing any positive benefits of the exercise intervention if their participants discontinue exercising. Unfortunately, because studies fail to report the average length of time between the end of the exercise intervention and when the assessment of hippocampal volume occurred, it is unknown whether this factor could explain some of the murkiness of the literature.
Taken together, these mixed results suggest that while aerobic exercise might have an effect on hippocampal volume, the effect could be small, transient, or there may be other factors moderating or better explaining it. For example, more robust and consistent results were found for older adults and patients with early AD, whereas results were heterogeneous for patients with schizophrenia, suggesting that one potential moderator may be the population studied. The parameters of the intervention, such as the mode, duration of exercise bout, frequency, intensity, and/or length of intervention are also likely moderating the impact of exercise on hippocampal morphology. Although there is strong support for a relationship between cardiorespiratory fitness and hippocampal volume, it remains equivocal whether increases in cardiorespiratory fitness via an aerobic exercise intervention are a necessary precondition for detecting changes in hippocampal volume. Furthermore, very few aerobic exercise interventions have targeted children, highlighting a need for future studies to elucidate the effect of exercise on hippocampal volume in youth.
HIPPOCAMPAL WHITE MATTER
The WM tracts most associated with the hippocampal formation include the fornix, which provides direct connection to the mammillary bodies of the hypothalamus, septal nuclei, and nucleus accumbens, as well as the cingulum, which interconnects the entorhinal cortex of the hippocampal formation to structures in the frontal, parietal, and temporal lobes. The health of WM pathways can be characterized using a number of techniques, including whole brain or regional volumetrics, identifying lesions, and assessing the microstructural integrity of WM fibers. Investigations using these measures have shown that WM structural health declines broadly with age and in the presence of pathology, such as AD (Bartzokis et al., 2001, 2004; Mielke et al., 2012; Villain et al., 2008; Westlye et al., 2009). As discussed in the systematic review conducted by Sexton and colleagues (2016), at present, few studies have investigated the potentially protective or enhancing effect of physical activity on WM structure, and the findings related to WM tracts connected to the hippocampus are mixed.
White Matter Microstructure
Diffusion tensor imaging (DTI) estimates the orientation, direction, and rate of water diffusion within brain tissue and provides detailed insight into the characteristics of WM fiber structure (Pfefferbaum & Sullivan, 2002). The most commonly used DTI parameter is fractional anisotropy (FA), a measure of the directional preference of water diffusion, which reflects various aspects of WM organization. Typically, higher FA values are positively related to axonal and myelin integrity, as well as fiber density. WM integrity, and specifically the integrity of the fornix and cingulum, has been consistently linked to aging and cognitive performance, including processing speed and memory function (Burzynska et al., 2017; Madden et al., 2008; Turken et al., 2008; Vernooij et al., 2009).
WM microstructure has received more attention than other indices of WM health in the exercise and cardiorespiratory fitness literature, although the findings are mixed and span widespread tracts that interconnect the bilateral frontal, temporal, parietal, and occipital gray matter, as well as global WM integrity (Chaddock-Heyman et al., 2014; Clark et al., 2019; K. Ding et al., 2018; Gow et al., 2012; Johnson et al., 2012; Maltais et al., 2020; Rodriguez-Ayllon, Esteban-Cornejo, et al., 2020; Teixeira et al., 2016; Tseng et al., 2013; Voss et al., 2013). In large part, studies focused on WM tract integrity have used an exploratory approach, rather than targeting specific pathways. Still, a considerable amount of evidence has shown associations between physical activity, and related measures such as cardiorespiratory fitness, and WM microstructure specific to hippocampal projections. In a study including two large, independent samples of 113 and 154 older adults (Oberlin et al., 2016), higher cardiorespiratory fitness was correlated with greater FA in the cingulum bundle and fornix. Furthermore, FA in the left cingulum partially mediated the relationship between cardiorespiratory fitness and spatial working memory, suggesting that WM integrity is a critical component in the relationship between cardiorespiratory fitness and elevated spatial memory performance. In healthy midlife to older adults, higher cardiorespiratory fitness was associated with higher FA in the right hippocampal segment of the cingulum and the left cerebral peduncle (F.-T. Chen et al., 2020). Similarly, in a sample of 15 older adults, Marks and colleagues (2011) found that cardiorespiratory fitness explained 28.5% of the variance in FA in the left cingulum. Another study that focused on both cardiorespiratory fitness and device-measured physical activity levels in low-fit older adults reported that greater engagement in light physical activity and higher cardiorespiratory fitness were associated with greater FA in the temporal lobe (Burzynska et al., 2014). However, the relationship with cardiorespiratory fitness was reduced to trend level upon correction for covariates.
Most studies of physical activity, cardiorespiratory fitness, and WM integrity have focused on FA. However, several other attributes of hippocampal WM microstructural health may be associated with physical activity. For instance, in children, WM fiber density (mean diffusivity) was associated with parent-reported physical activity across multiple WM tracts, including the hippocampal cingulum (Rodriguez-Ayllon, Derks, et al., 2020). A study of older adults with early AD did not find significant associations between cardiorespiratory fitness and FA in the cingulum bundles (Perea et al., 2016). However, the authors reported a trending association between higher cardiorespiratory fitness and lower myelin damage (radial diffusivity) in the right cingulum. Similarly, in post-menopausal women, Harasym and colleagues (2020) found a significant relationship between higher fitness and higher axonal integrity (axial diffusivity) in the hippocampal segments of the cingulum. Bracht and colleagues (2016) found that greater amounts of device-measured engagement in physical activity was associated with greater myelination (myelin water fraction), but not FA, in the right parahippocampal cingulum in healthy young adults. Their findings suggest that myelin health may underlie associations between physical activity and WM integrity. However, continued work combining multiple parameters of WM integrity will be necessary to shed light on which aspects of WM microstructure, particularly in the fornix and hippocampal cingulum, are sensitive to physical activity.
In contrast to the cross-sectional studies described above, there have been fewer published RCTs examining the impact of aerobic exercise training on WM integrity, and these results are mixed (Clark et al., 2019; Sexton et al., 2020). For instance, Burzynska and colleagues (2017) randomized 174 older adult participants into either a walking, dancing, walking/nutrition, or active control group. Only the dancing group showed an effect of training on FA, specifically in the fornix. In another study focusing on adults with schizophrenia and healthy controls, Svatkova and colleagues (2015) found that a six-month cycling intervention resulted in increased FA in multiple fiber tracts, although not pathways connected to the hippocampus. Further, Voss and colleagues (2013) conducted a year-long, aerobic exercise intervention in 70 older adults, consisting of an aerobic walking group and a stretching control group. Here, the authors did not find significant group-level effects of exercise training. However, greater post-intervention fitness levels were associated with increased frontal and temporal FA, as well as enhanced short-term memory. Even fewer exercise RCTs have focused on WM integrity in children. In a recent intervention, Chaddock-Heyman and colleagues (2014) randomized 143 children between the ages of 7 to 9 into a nine-month moderate-to-vigorous exercise training program or a waitlist control condition. The intervention group showed significantly greater increase in FA in the genu of the corpus callosum. In another intervention with 8 to 11-year-old children with overweight, the intervention group showed a greater increase in FA in the uncinate fasciculus after 8 months of exercise (Schaeffer et al., 2014). However, in both of these studies, the authors did not examine the effects of the intervention on hippocampal WM tracts, and thus, the influence of aerobic exercise on hippocampal WM in children requires further investigation.
This mixed pattern of findings raises a number of possible explanations. First, aerobic exercise interventions typically use a timeframe ranging between several months to a year. Although participating in continuous aerobic exercise increases cardiorespiratory fitness, it may be possible that more long-term maintenance of physical activity is necessary to influence WM projections to and from the hippocampus. Overall, the small number of exercise interventions, coupled with heterogeneity in study scope, demographics, length, and method of exercise, limit the generalizability of results and make interpretation difficult. Of further note, in comparison to other major WM pathways, fiber bundles communicating with the hippocampal formation are structurally narrow and winding (i.e., ‘crossing fibers’), posing a challenge for neuroimaging research focused on characterizing the structural health of these pathways (Maller et al., 2019; Nieuwenhuys et al., 1988). Thus, studies with high fidelity neuroimaging data that incorporate multiple measures of microstructural integrity may be particularly important in elucidating the associations between fitness and WM connections to and from the hippocampus.
White Matter Volume
While several investigations in older adults and children have found positive associations between cardiorespiratory fitness, self-reported physical activity, and exercise training using RCTs with WM volumes in various pathways (Colcombe et al., 2006; Erickson et al., 2007; Esteban-Cornejo et al., 2019; Ho et al., 2011), others have yielded null results (Gordon et al., 2008; Honea et al., 2009). Sexton and colleagues (2016) reported meta-analytic results, which revealed that higher levels of physical activity and cardiorespiratory fitness were significantly associated with higher global WM volume, albeit with small effect sizes. Their analyses did not establish support for associations between physical activity or fitness and localized WM volume in the temporal lobe (i.e., near the hippocampus). However, similar to studies of WM integrity, studies focused on WM volume and physical activity are highly variable in scope and methodology. Furthermore, there is a paucity of studies focusing on associations between exercise, fitness, and WM volume in tracts that communicate with the hippocampus. Accordingly, associations between exercise exposure, cardiorespiratory fitness and volume of the fornix and cingulum remain poorly understood.
One question raised by the mixed findings of studies targeting WM integrity and physical activity metrics is whether current techniques, such as DTI, are sensitive enough to detect differences in the WM changes that appear to be predicted by animal models (Lin Chen et al., 2020; Graham et al., 2019; Xiao et al., 2018). Techniques for WM imaging in humans have greatly improved over the past decade, which raises a potential challenge with interpreting results across the literature since earlier studies may obscure the sensitivity of findings from studies using more recent, higher resolution techniques. Nonetheless, there have been significant improvements in sensitivity across most imaging domains, which may be to the benefit of continued work examining the influence of physical activity on hippocampal health across neuroimaging metrics. Notably, as previously mentioned, Bracht and colleagues (2016) reported that myelination as a measure of WM integrity may be more sensitive than traditional DTI parameters, yet this approach has not been frequently used in the physical activity literature. Further, multishell methods of imaging have facilitated our ability to quantify microstructural integrity even in regions of crossing fibers and may enable increased sensitivity in human studies.
White Matter Hyperintensities
WM hyperintensities (WMH) are lesions that are considered to be clinically significant and are predictive of increased risk of cardiovascular events, stroke, dementia, and mortality (Debette & Markus, 2010). WM lesions specific to the fornix and cingulum bundles, in both animals and humans, have been linked to memory impairment and decline (Aggleton et al., 1995, 2010; Gaffan, 1994; Gaffan & Gaffan, 1991; Lockhart et al., 2012; Shamy et al., 2010). While several investigations have assessed the relationship between self-reported physical activity and WMH throughout the brain, many of these reports have been cross-sectional and yielded null findings (Ho et al., 2011; Rosano et al., 2010). In contrast, several studies reported that higher fitness and self-reported and device-measured physical activity was correlated with reduced overall lesion volume (Burzynska et al., 2014; Saczynski et al., 2008; Sen et al., 2012; Wirth et al., 2014). Similarly, one longitudinal investigation reported that greater amounts of self-reported physical activity at baseline was linked to lower lesion volume three years later (Gow et al., 2012). However, WMH are typically identified using global, rather than localized approaches, and at present, human studies of exercise or fitness have not specifically discussed WMH in hippocampal projections.
HIPPOCAMPAL FUNCTIONAL CONNECTIVITY
Whereas structural MRI studies reveal valuable information about the associations between aerobic exercise and hippocampal morphology, including white matter projections, other techniques such as resting state functional connectivity (rsFC) can provide further insight into the health of hippocampal circuitry (Gusnard & Raichle, 2001). rsFC measures temporal correlations in blood oxygen level dependent (BOLD) signal of spatially distributed brain regions at rest to identify “functional networks”, or networks of brain regions that tend to co-activate in various states. A number of such networks have received considerable attention in recent years, most prominently the default mode network (DMN). The DMN includes parts of the hippocampal formation and the medial frontal, posterior parietal, posterior cingulate, and retrosplenial cortices (Buckner et al., 2008). Disruptions in the connectivity of the DMN regions are found in aging (Andrews-Hanna et al., 2007), MCI (Lustig et al., 2003), and AD (Greicius et al., 2004), and correlate with impaired cognitive performance. Further, the regions of the DMN that are most sensitive to aging may also be sensitive to physical activity and cardiorespiratory fitness (Voss et al., 2016). Accordingly, there has recently been an increased interest in exercise as a method for enhancing the connectivity of the DMN and related functional brain networks and regions.
Cross-sectional reports demonstrate relationships between exercise engagement and/or cardiorespiratory fitness and rsFC between regions of the hippocampal formation and diffuse brain areas. Voss and colleagues (2016) found that higher cardiorespiratory fitness, but not accelerometry-measured physical activity, was associated with greater rsFC in a number of large-scale cortical networks in healthy older adults. These effects were strongest within the DMN, particularly in prefrontal and medial temporal regions, including the parahippocampal gyrus. In a study focused on connectivity among various seed locations, higher cardiorespiratory fitness associated with greater parahippocampal, but not hippocampal, interhemispheric rsFC in 284 healthy midlife adults (Ikuta & Loprinzi, 2019). Another recent investigation of 50 healthy young adults reported that higher cardiorespiratory fitness was associated with higher rsFC of the left anterior hippocampus to the frontal pole, middle frontal gyrus (MFG), and parahippocampus (Stillman et al., 2018). However, the authors also reported a surprising inverse relationship between cardiorespiratory fitness and rsFC in the right anterior hippocampus to the right superior frontal gyrus, reflecting possible hemispheric differences in the associations of cardiorespiratory fitness with hippocampal connectivity. Further, in an observational study, composite variables reflecting current physical activity and physical activity accumulated over a decade were associated with rsFC in the posterior cingulate cortex of the DMN, but not the hippocampus (Boraxbekk et al., 2016). Notably, the composite variables used to index physical activity reflect health characteristics commonly associated with physical activity, but do not clearly estimate fitness or engagement in exercise. Overall, these cross-sectional studies suggest that cardiorespiratory fitness and physical activity may be important for functional connectivity between medial temporal regions, including the hippocampus, and other brain structures that support memory function and executive functioning in adults.
Several RCTs have also investigated changes in rsFC between the hippocampus and multiple brain regions following exercise training, with mixed findings. One trial found that older adults participating in a four-month walking intervention showed increased overall connectivity between the hippocampus and the rest of the brain, particularly the anterior cingulate cortex (ACC), as compared to controls (Burdette et al., 2010). In contrast, Flodin and colleagues (2017) reported no significant differences in rsFC between older adults in an aerobic exercise and active control group after six months of intervention. However, post-hoc analyses revealed that improvements in cardiorespiratory fitness predicted higher rsFC of the right hippocampus to frontal and parietal areas, and lower rsFC of the left hippocampus to the precentral gyrus. Similar to findings reported by Stillman and colleagues (2018), these results indicate laterality differences in rsFC of the hippocampus in association with cardiorespiratory fitness, though in opposite directions. Although these hemispheric differences may be related to differential recruitment of resources, the discrepancies with regard to which hemisphere of the hippocampus showed increased rsFC makes interpretation difficult. It is possible that these differences relate to the age groups included in the studies, but research across the lifespan will be needed to clarify these questions.
Overall, evidence from observational and intervention research indicates that greater engagement in aerobic exercise and/or higher cardiorespiratory fitness may be beneficial to functional connectivity between the hippocampus and a diffuse array of brain regions critical for various cognitive functions. Yet studies focused on hippocampal rsFC are few in number, particularly aerobic exercise interventions. A handful of studies have investigated associations between acute bouts of aerobic exercise and rsFC, and they have reported that single sessions of light or moderate-intensity aerobic exercise predict rsFC in multiple networks. These networks include prefrontal, temporal, and parietal connections that overlap with the DMN (Voss et al., 2020) and the hippocampal-cortical network (Weng et al., 2017). Still, clarity regarding the similarities and differences in the effects of acute exercise on rsFC is warranted prior to drawing any conclusions. Moreover, cross-sectional studies of cardiorespiratory fitness and hippocampal connectivity have largely focused on young and midlife adults, whereas interventions have targeted older adults. Additional work in children and adolescents could provide valuable information about relationships between exercise, fitness, and hippocampal connectivity early in life.
Lastly, few studies have focused on hippocampal rsFC in patient populations in the context of physical activity and related metrics. One study of midlife adults with multiple sclerosis reported associations between rsFC and device-monitored physical activity levels from an accelerometer worn for 7 days during waking hours (Prakash et al., 2011). Higher levels of physical activity were associated with increased rsFC from the hippocampus to the posteromedial cortex, parahippocampal gyrus, superior frontal gyrus, and the medial frontal cortex. Similarly, Leavitt and colleagues (2014) reported preliminary results of an RCT, in which patients with multiple sclerosis in a three-month aerobic exercise group, but not controls, showed increased rsFC within the hippocampus. Together, these results suggest that patients with conditions involving altered hippocampal function might especially benefit from engaging in aerobic exercise.
HIPPOCAMPAL PERFUSION
Arterial spin labeling (ASL) perfusion MRI provides a quantitative measure of blood flow and offers information about how the brain meets and regulates its metabolic demand (Hales et al., 2014). Few cross-sectional studies have examined the relationship between hippocampal perfusion and physical activity or cardiorespiratory fitness. One study reported that greater device-measured sedentary time was associated with greater left hippocampal perfusion in sedentary older adults at genetic risk for AD (Zlatar et al., 2014). The authors speculated that the elevated perfusion might represent a compensatory mechanism for metabolic changes in preclinical AD. Among children, one study reported an association between higher cardiorespiratory fitness levels and greater hippocampal cerebral blood flow (CBF), with specific associations for the posterior hippocampus (Chaddock-Heyman et al., 2016). To date, no studies have examined the relationship between self-reported physical activity and hippocampal perfusion. Although there is promising evidence for differences in hippocampal perfusion as a function of cardiorespiratory fitness across the lifespan, future research is needed to explore these relationships further.
While few studies have examined the association between cardiorespiratory fitness and hippocampal CBF, a growing body of literature shows that aerobic exercise interventions increase hippocampal CBF (Burdette et al., 2010; Maass et al., 2015; Pereira et al., 2007). In particular, three to four months of an aerobic exercise intervention increased hippocampal CBF relative to non-exercising control groups in older adults (Burdette et al., 2010; Maass et al., 2015). Among midlife adults, three months of aerobic exercise increased CBF in the dentate gyrus subregion of the hippocampus (Pereira et al., 2007). When older adults with a history of long-term endurance training stopped all physical activity for 10 days, CBF within the left and right hippocampus decreased significantly, suggesting that exercise training was a critical component to hippocampal perfusion and that the benefits of exercise are transient or must be maintained (Alfini et al., 2016). While these effects have been examined in studies with small sample sizes (i.e., NPereira=11, NAlfini=12) and have not yet been studied in other patient populations, preliminary rodent studies have examined these effects in the context of Parkinson’s disease (Wang et al., 2015) and depression (Linmu Chen et al., 2017) and found similar results. Although the literature is scarce, these results suggest that hippocampal perfusion increases in response to exercise. Importantly, there have been no aerobic exercise interventions targeting children and hippocampal CBF, highlighting a need for future studies to elucidate the effect of exercise on hippocampal perfusion in youth.
There has been speculation for many years that the cognitive-enhancing effects of exercise might be occurring through increased CBF (Davenport et al., 2012). Animal literature demonstrates that exercise increases the production of new capillary beds, which provides increased nutrients and energy to the brain and might result in increased cerebral blood volume or flow (Swain et al., 2003). Evidence from RCTs of aerobic exercise interventions suggests that similar increases in hippocampal CBF can be detected in humans and might have an impact on disease or cognition. Increases in hippocampal perfusion have been associated with improvements in spatial object recall and recognition in older adults (Maass et al., 2015) and verbal recall among midlife adults (Pereira et al., 2007). Further work is needed to replicate these findings and extend them to other populations.
Of particular importance is whether increasing cardiorespiratory fitness through aerobic exercise interventions is a critical precondition for finding effects on hippocampal CBF. Several of the aforementioned interventions found that exercise-induced improvements in cardiorespiratory fitness were associated with increases in CBF in the hippocampus among older adults (Maass et al., 2015) and the dentate gyrus of midlife adults (Pereira et al., 2007). Although there is limited research, these results suggest that exercise interventions targeting improvements in cardiorespiratory fitness may increase hippocampal CBF. However, it remains to be tested whether changes in cardiorespiratory fitness are necessary to detect changes in hippocampal CBF following an intervention.
MECHANISMS
At present, the precise mechanisms by which engaging in physical activity influences the hippocampus remain poorly understood in humans. Nevertheless, exercise has been implicated in reducing multiple peripheral risk factors for neural decline, and thus has been described as uniquely positioned to promote brain health (Cotman et al., 2007). Rodent research has identified multiple cellular and molecular mechanisms that provide a foundation for explaining the associations among exercise, cardiorespiratory fitness, and the hippocampus in humans, specifically: (i) neurotrophins, (ii) stress hormones, (iii) inflammation, and (iv) insulin sensitivity. Although speculative, it is possible that one or more of these mechanisms explain the associations between exercise, fitness, and the hippocampal findings in humans.
Animal studies suggest that exercise training increases levels of growth factors and neurotrophins that influence cell proliferation and survival (Q. Ding et al., 2006; Van Praag et al., 2005), including brain-derived neurotrophic factor (BDNF; Rossi et al., 2006), vascular endothelial growth factor (VEGF; Fabel et al., 2003), and insulin-like growth factor 1 (IGF-1; Trejo, Carro, & Torres-Aleman, 2001). For example, BDNF is important in the growth and survival of neurons, axonal transport, long-term potentiation and memory formation (O’Bryant et al., 2009), and axonal growth (Ghiani et al., 2007). Importantly, exercise increases BDNF expression levels in the hippocampus (P. Liu & Nusslock, 2018; Neeper et al., 1995; Vaynman et al., 2004). Animal models have demonstrated greater BDNF synthesis during high-intensity exercise compared to low-intensity exercise and sedentary behaviors (de Almeida et al., 2013). Further, increases in hippocampal BDNF expression have been found after short (e.g., one week; Griffin et al., 2009) and long (e.g., four weeks; Y.-F. Liu et al., 2009) lengths of exercise. Human studies have reported that exercise-induced changes in hippocampal volume were correlated with changes to serum-derived BDNF among healthy older adults (Erickson et al., 2011). Aerobic exercise interventions have proven effective in increasing serum BDNF levels across several patient populations, including AD and schizophrenia (Choi et al., 2018; Green et al., 2011; Hock et al., 2000; H. Kim et al., 2014; Kuo et al., 2013). BDNF might also be an important mediator of cardiorespiratory fitness and exercise associations with white matter. Lower levels of plasma BDNF have been correlated with a steeper decline of WM volume in females (Driscoll et al., 2012). Single-nucleotide polymorphisms that influence the regulated secretion of BDNF have also been associated with lower WM volume and higher WMH burden (Huang et al., 2014). However, more research is needed to investigate whether exercise-related increases in BDNF, and other neurotrophic factors, influence the integrity of hippocampal WM projections. Nonetheless, alterations in neurotrophic factors may be one mechanism through which exercise contributes to overall increases in hippocampal health.
Changes to the hypothalamic-pituitary-adrenal (HPA) axis might be another mechanism by which engaging in exercise influences the hippocampus. Chronic stress has been associated with altered synaptic terminal structure in the rat hippocampus (Magariños et al., 1996) and suppressed hippocampal neurogenesis (Egeland et al., 2015; Gould & Tanapat, 1999). Likewise, decreases in dendritic spine density and severity of hippocampal atrophy have been associated with elevated release of corticosterone (Foy et al., 1987; Woolley et al., 1990). In contrast, voluntary exercise has been linked to reduced plasma cortisol and improved memory in young and old rodents (Estrada et al., 2019). Other work (D.-M. Kim & Leem, 2016) suggests that exercise reduces the impact of chronic stress on the rat hippocampus by enhancing the activity of Adenosine monophosphate-activated protein kinase (AMPK), which is important in the induction of BDNF expression and hippocampal neurogenesis (Yoon et al., 2008). Cross-sectional work focusing on older adult humans has indicated a convergent pattern of findings, such that self-reported physical activity moderated the association between lifetime stress and hippocampal volume and memory. Lower engagement in physical activity was associated with greater stress-related hippocampal decline when compared to higher physical activity engagement (Head et al., 2012). Further, research in young adult males has shown that varying intensities of exercise activate the HPA axis differently: moderate-to-vigorous intensity aerobic exercise increased cortisol and decreased adrenocorticotropic hormone concentrations but low-intensity activity had the opposite effect (Hill et al., 2008). However, further research is needed to determine the threshold necessary to induce a cortisol response. Summarily, engagement in exercise may mitigate the deleterious effects of chronic stress and its by-products on the hippocampus by modulating cortisol levels and the activity of AMPK and BDNF.
Chronic inflammation is a risk factor for a number of neurodegenerative diseases (Amor et al., 2010; Perry, 2004), and the hippocampus is quite susceptible to reduced decline as a result of chronic systemic and neuroinflammation (Barrientos et al., 2015). Engagement in habitual exercise may create an anti-inflammatory response by inducing long-term changes in circulating cytokines (Petersen & Pedersen, 2005). Studies in rats have shown compelling evidence of a long-term anti-inflammatory effect of aerobic exercise (Ryan & Nolan, 2016), and an associated reduction in hippocampal decline (Gomes da Silva et al., 2013; Lovatel et al., 2013). Although findings in humans are less consistent, there is also evidence of an immune-regulating influence of aerobic exercise in human adults (Sallam & Laher, 2015; Woods et al., 2011). Similarly, higher levels of cardiorespiratory fitness have been associated with lower circulating levels of pro-inflammatory cytokines, such as interleukin (IL) −6, and greater circulating levels of anti-inflammatory cytokines, such as IL-10 (Kullo et al., 2007; Wedell-Neergaard et al., 2018). However, research suggests that intense exercise may induce an excessive inflammatory response by down-regulating anti-inflammatory cytokine IL-10 and up-regulating the pro-inflammatory cytokines IL-1 and IL-1R1, whereas mild exercise may induce a mild inflammatory response by up-regulating the pro-inflammatory cytokine IL-1β and suppressing the pro-inflammatory cytokine TNF (Inoue et al., 2015). Accordingly, the potential anti-inflammatory effect of engaging in exercise may serve a protective role for the structural and functional health of the hippocampus depending on the intensity of the exercise.
Increased production of inflammatory cytokines, along with mitochondrial dysfunction, can also contribute to systemic insulin resistance, further accelerating neural decline (Pratchayasakul et al., 2015; Sripetchwandee et al., 2018). Healthy insulin signaling is essential for proper brain function. In the hippocampus, studies in rodents and healthy humans have suggested that the activation of insulin receptors results in insulin-induced enhancement of cognitive function (Biessels & Reagan, 2015). Here, too, preliminary studies in humans have found physical activity to improve metabolic function in both the brain and in the periphery by increasing insulin sensitivity and mitochondrial efficiency (Neufer et al., 2015). Such cellular and molecular changes then increase neural plasticity and promote the structural and functional integrity of the brain (Colcombe et al., 2004).
In all, research in animals and humans suggests multiple promising mechanistic explanations for associations between exercise, cardiorespiratory fitness, and the brain. It has also been suggested that exercise induces a cascade of systemic events that contribute to its impact on brain health and cognition, suggesting that the mechanisms outlined in this section may operate together, in sequence, or additively to promote hippocampal structure and function. Further work will be needed to elucidate nuances of how these mechanisms may operate and whether these explanations can account for broad influences on hippocampal structure and function, or if the effects are specific to certain metrics. For example, while hippocampal morphology and white matter connections may be mediated by BDNF expression, resting state connectivity and perfusion may be mediated by other mechanisms. An alternative perspective of mechanisms is to ask whether one measure of the hippocampus (e.g., volume) mediates its effects on other measures of the hippocampus (e.g., rsFC). Unfortunately, the majority of studies described in prior sections have utilized only a single neuroimaging technique in their analytical approach.
A major strength of neuroimaging is that it lends the ability to combine multiple MRI measures into a single analytical model to help better our understanding of the underlying mechanisms. In addition to larger datasets, studies that use multiple MRI measures allow us to assess how physical activity and exercise influence the structure and function of the hippocampus, as well as their interaction. A handful of studies have utilized a multi-modal analytical approach to better characterize the hierarchical nature of these metrics and attempt to resolve questions about whether one neuroimaging metric explains associations in another. Specifically, changes in hippocampal volume following an exercise intervention have been closely linked to changes in hippocampal perfusion, such that increased hippocampal blood flow largely accounted for increased volume (Maass et al., 2015). In contrast, Thomas and colleagues (2016) found that increases in hippocampal volume following an exercise intervention were better accounted for by an increase in myelinated fibers within hippocampal gray matter rather than increased vasculature. However, it cannot be ruled out whether other microstructural changes may also have contributed to increases in hippocampal volume. Furthermore, Stillman and colleagues (2018) found that associations between cardiorespiratory fitness and rsFC of the left anterior hippocampus were not explained by including left anterior hippocampal volume in the model, thereby indicating some independence of associations between rsFC and volumetric measures. Still, in light of the numerous metrics of hippocampal health that have been associated with cardiorespiratory fitness and exercise, the results of these multi-modal neuroimaging studies suggest that there may be mechanisms at multiple levels of hippocampal health.
CONCLUSION
This review highlights the considerable evidence that participation in aerobic exercise and increasing cardiorespiratory fitness are promising methods for promoting hippocampal health in humans. This conclusion comes from a summary of literature that includes much variability in experimental design, populations studied, measurements of physical activity and exercise, and neuroimaging measures of the hippocampus (see Figure 3). It is remarkable that, despite this level of heterogeneity in the science, there are consistent positive findings between physical activity, cardiorespiratory fitness, and hippocampal measurement. However, it is important to note that given the difficulty in publishing null-results, the synthesis of the studies included in this review might be a biased sample of all relevant studies. There are clearly significant gaps in knowledge (described in more detail below), but the bird’s eye view of the field indicates a positive association between physical activity and the hippocampus in humans. It is also clear from this review that there is a need for future RCTs of exercise to use high-quality study designs and high-fidelity neuroimaging techniques to make more definitive conclusions about the role of exercise on hippocampal outcomes. Lastly, there is a need for a meta-analysis to synthesize the data reported in this review using a statistically rigorous approach to address questions such as the clinical importance of the effect and the consistency of the effect.
Cognitive Implications
An important question that arises from this literature is: are these effects of exercise on the hippocampus just meaningless by-products of engaging in exercise or do they have relevance for memory formation or academic performance? Although some volumetric studies have found a relationship between increased hippocampal volume and improvements in memory following an exercise intervention (Erickson et al., 2011; Maass et al., 2015), other interventions examining volumetric changes have not reported such cognitive benefits (Jonasson et al., 2016; Thomas et al., 2016). Furthermore, increases in hippocampal perfusion have been associated with improvements in spatial object recall and recognition, and verbal recall (Maass et al., 2015; Pereira et al., 2007), and associations between cardiorespiratory fitness and the cingulum were associated with spatial working memory in older adults (Oberlin et al., 2016). Thus, it seems that often studies that find significant associations between metrics of physical activity or cardiorespiratory fitness and the hippocampus also report associations in relevant domains of cognition. Still, it is not known whether there is a dose-response relationship modulating this effect, and what time course may be necessary for the detection of potential cognitive benefits associated with fitness and exercise. Ultimately, as more studies elucidate the effects of exercise training on the hippocampus, they should also target the related cognitive impact of these changes. In addition, many studies utilized small sample sizes, which limits the ability to reliably interrogate correlations with cognitive performance. Even fewer studies employ hippocampal-dependent memory tasks that would presume to be more strongly associated with the hippocampal measures described in this review.
Gaps in the literature
Among the studies included in this review, there are a number of apparent gaps in the literature. Specifically: (i) we have very little information about the impact of exercise on the hippocampal formation in children and adolescents, (ii) we have little knowledge of the effects of exercise on the hippocampal formation in certain disease states in which the hippocampus is affected, (iii) we have a poor understanding of the persistence of effects because few studies conduct follow-up assessments after completion of an intervention, (iv) we know little about the effects of acute exercise on hippocampal function, and (v) we have little information about specific exercise intervention parameters that are necessary to achieve changes to hippocampal health.
The majority of studies described in this review were conducted in older adults. While this has significant implications for aging-related cognitive outcomes, healthy hippocampal development is also crucial earlier in the lifespan, as adolescence and early adulthood are critical periods during which the hippocampus exhibits considerable reorganization and growth. While several studies have found a relationship between cardiorespiratory fitness and hippocampal volume, perfusion, and WM microstructure in adolescents, the literature in this area is still sparse. Furthermore, few exercise interventions examining hippocampal volume, perfusion, white matter, and rsFC have targeted children, highlighting a critical area for future research. Although older adults are a population at more immediate risk for hippocampal degeneration, intervention early in life may set the stage for healthy behavioral habits that have consequences for brain development and maturation later in life. In addition, the literature focusing on particular disease states that affect the hippocampus is also small, indicating a need for future research to assess various patient populations and the impact on disease course. This is particularly relevant for conditions that involve hippocampal deterioration, such as dementia and MCI (Pennanen et al., 2004), schizophrenia (Harrison, 2004), and multiple sclerosis (Roosendaal et al., 2010).
Much remains to be established with regard to the characteristics that make an exercise training protocol sufficient to impart benefits to the hippocampus. For instance, although there is consistent support for a relationship between cardiorespiratory fitness and the hippocampus, there is less consensus about whether increasing cardiorespiratory fitness is a necessary precondition for detecting changes in hippocampal health in the context of aerobic exercise interventions. This is an important distinction and may help differentiate among forms of exercise that are most effective in targeting the hippocampus. Furthermore, few intervention studies examined how long the effects of exercise on hippocampal volume, perfusion, white matter, and rsFC persist after the conclusion of the intervention, hindering our ability to assess whether the effects are transient or more long-lasting. Studies with extended follow-up periods can help provide valuable information about the longevity of benefits. Further, RCTs are essential in allowing us to examine causality, but they also offer little insight into the process of change, which can be better captured by observational studies. In addition, experiments examining the impact of acute exercise may also help bridge our understanding of whether brief bouts of activity have any discernible impact on the hippocampus. Ultimately, the field will benefit from the inclusion of an array of well-designed study approaches that aim to address such nuances in the degree to which various forms of physical activity may be effective in promoting hippocampal health.
There was considerable variability in intervention parameters across studies, including the mode, duration of exercise bout, frequency, intensity, and length. Heterogeneity across adherence, level of supervision, and monitoring of intensity are among other factors that reduce our certainty in any conclusions about the impact of exercise interventions on hippocampal structure and function. Given the variability in intervention parameters, there might be significant pieces of information lost when grouping across studies of varying sensitivity and precision – particularly about the importance of specific parameters in detecting effects. Future studies examining which intervention factors are critical for influencing hippocampal health are needed. There is also a need for consistency and harmonization of methodology in the field of exercise research, a focus on greater adherence, and larger sample sizes in order to clarify these results.
In summary, there are many consistent findings between physical activity and multiple metrics of hippocampal health, specifically: gray matter volume, white matter structure, resting state functional connectivity, and perfusion. This review primarily focused on exercise training that was aerobic in nature; however, there are a number of other forms of mixed-aerobic and non-aerobic activities associated with brain aging and the hippocampus, such as Tai Chi Chuan and Baduanjin practice (Tao et al., 2016, 2017), yoga (Hariprasad et al., 2013), and resistance training (Cassilhas et al., 2012; Liu-Ambrose & Donaldson, 2009). Moreover, studies included in this review that utilized self-report estimates of physical activity may have captured leisure time activity that encompasses both aerobic and non-aerobic physical activity. Thus, future research should investigate how different forms of physical activity may influence the hippocampus and other brain regions. Although the current research is promising, much more work using longitudinal approaches, and rigorous and standardized intervention methods, across the lifespan will elucidate how exercise and fitness influence hippocampal health and which aspects of its structure and function are particularly good targets of intervention.
Acknowledgments
This research was supported by grants awarded from the National Institutes of Health to SLA (T32GM081760), to AL (5T32AG055381-03), and KIE (R01 DK095172). IEC was supported by a grant from the Alicia Koplowitz Foundation.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 2018 Physical Activity Guidelines Advisory Committee. (2018). 2018 Physical Activity Guidelines Advisory Committee Scientific Report. U.S. Department of Health and Human Services. [Google Scholar]
- Aggleton JP, Neave N, Nagle S, & Sahgal A (1995). A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: Evidence of a double dissociation between frontal and cingulum bundle contributions. Journal of Neuroscience, 15(11), 7270–7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, & Erichsen JT (2010). Hippocampal–anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions. European Journal of Neuroscience, 31(12), 2292–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghjayan SL, Jakicic JM, Rogers RJ, Esteban-Cornejo I, Peven JC, Stillman CM, Watt JC, & Erickson KI (2020). The fitness versus body fat hypothesis in relation to hippocampal structure. Psychophysiology, e13591. 10.1111/psyp.13591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfini AJ, Weiss LR, Leitner BP, Smith TJ, Hagberg JM, & Smith JC (2016). Hippocampal and Cerebral Blood Flow after Exercise Cessation in Master Athletes. Frontiers in Aging Neuroscience, 8, 184. 10.3389/fnagi.2016.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Brickman AM, Spitznagel MB, Sweet LH, Josephson R, Griffith EY, Narkhede A, Hughes J, & Gunstad J (2015). Daily Physical Activity Is Associated with Subcortical Brain Volume and Cognition in Heart Failure. Journal of the International Neuropsychological Society: JINS, 21(10), 851–860. 10.1017/S1355617715000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, & Valk PVD (2010). Inflammation in neurodegenerative diseases. Immunology, 129(2), 154–169. 10.1111/j.1365-2567.2009.03225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, & Buckner RL (2007). Disruption of large-scale brain systems in advanced aging. Neuron, 56(5), 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Best JR, Rosano C, Yaffe K, Catov JM, & Liu-Ambrose T (2020). Sex-Specific Relationship Between Long-Term Maintenance of Physical Activity and Cognition in the Health ABC Study: Potential Role of Hippocampal and Dorsolateral Prefrontal Cortex Volume. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(4), 764–770. 10.1093/gerona/glz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, & Maier SF (2015). Neuroinflammation in the normal aging hippocampus. Neuroscience, 309, 84–99. 10.1016/j.neuroscience.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, & Mintz J (2001). Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Archives of General Psychiatry, 58(5), 461–465. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, & Cummings JL (2004). Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiology of Aging, 25(7), 843–851. [DOI] [PubMed] [Google Scholar]
- Best JR, Rosano C, Aizenstein HJ, Tian Q, Boudreau RM, Ayonayon HN, Satterfield S, Simonsick EM, Studenski S, Yaffe K, Liu-Ambrose T, & Health, Aging and Body Composition Study. (2017). Long-term changes in time spent walking and subsequent cognitive and structural brain changes in older adults. Neurobiology of Aging, 57, 153–161. 10.1016/j.neurobiolaging.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, & Reagan LP (2015). Hippocampal insulin resistance and cognitive dysfunction. Nature Reviews Neuroscience, 16(11), 660–671. 10.1038/nrn4019 [DOI] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, LaRue A, Asthana S, Hermann BP, Sager MA, Johnson SC, & Okonkwo OC (2015). Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging and Behavior, 9(3), 639–649. 10.1007/s11682-014-9325-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraxbekk C-J, Salami A, Wåhlin A, & Nyberg L (2016). Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network—A multimodal approach. NeuroImage, 131, 133–141. 10.1016/j.neuroimage.2015.12.010 [DOI] [PubMed] [Google Scholar]
- Bracht T, Jones DK, Bells S, Walther S, Drakesmith M, & Linden D (2016). Myelination of the right parahippocampal cingulum is associated with physical activity in young healthy adults. Brain Structure and Function, 221(9), 4537–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: Anatomy, function, and relevance to disease. In The year in cognitive neuroscience 2008 (pp. 1–38). Blackwell Publishing. [DOI] [PubMed] [Google Scholar]
- Bugg JM, & Head D (2011). Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging, 32(3), 506–514. 10.1016/j.neurobiolaging.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Shah K, Villareal DT, & Head D (2012). Cognitive and neural correlates of aerobic fitness in obese older adults. Experimental Aging Research, 38(2), 131–145. 10.1080/0361073X.2012.659995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunketorp Käll L, Malmgren H, Olsson E, Lindén T, & Nilsson M (2015). Effects of a Curricular Physical Activity Intervention on Children’s School Performance, Wellness, and Brain Development. The Journal of School Health, 85(10), 704–713. 10.1111/josh.12303 [DOI] [PubMed] [Google Scholar]
- Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, & Rejeski WJ (2010). Using network science to evaluate exercise-associated brain changes in older adults. Frontiers in Aging Neuroscience, 2, 23. 10.3389/fnagi.2010.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Chaddock-Heyman L, Voss MW, Wong CN, Gothe NP, Olson EA, Knecht A, Lewis A, Monti JM, Cooke GE, Wojcicki TR, Fanning J, Chung HD, Awick E, McAuley E, & Kramer AF (2014). Physical Activity and Cardiorespiratory Fitness Are Beneficial for White Matter in Low-Fit Older Adults. PLoS ONE, 9(9), 1–10. 10.1371/journal.pone.0107413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Jiao Y, Knecht AM, Fanning J, Awick EA, Chen T, Gothe N, Voss MW, McAuley E, & Kramer AF (2017). White Matter Integrity Declined Over 6-Months, but Dance Intervention Improved Integrity of the Fornix of Older Adults. Frontiers in Aging Neuroscience, 9, 59. 10.3389/fnagi.2017.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassilhas RC, Lee KS, Venâncio DP, Oliveira MGM, Tufik S, & Mello MT (2012). Resistance exercise improves hippocampus-dependent memory. Brazilian Journal of Medical and Biological Research, 45(12), 1215–1220. 10.1590/S0100-879X2012007500138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, & Kramer AF (2010). A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research, 1358, 172–183. 10.1016/j.brainres.2010.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Chappell MA, Johnson CL, Kienzler C, Knecht A, Drollette ES, Raine LB, Scudder MR, Kao S-C, Hillman CH, & Kramer AF (2016). Aerobic fitness is associated with greater hippocampal cerebral blood flow in children. Developmental Cognitive Neuroscience, 20, 52–58. 10.1016/j.dcn.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Holtrop JL, Voss MW, Pontifex MB, Raine LB, Hillman CH, & Kramer AF (2014). Aerobic fitness is associated with greater white matter integrity in children. Frontiers in Human Neuroscience, 8. 10.3389/fnhum.2014.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Kienzler C, Drollette ES, Raine LB, Kao S-C, Bensken J, Weisshappel R, Castelli DM, Hillman CH, & Kramer AF (2018). Physical Activity Increases White Matter Microstructure in Children. Frontiers in Neuroscience, 12. 10.3389/fnins.2018.00950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Mackenzie MJ, Zuniga K, Cooke GE, Awick E, Roberts S, Erickson KI, McAuley E, & Kramer AF (2015). Higher cardiorespiratory fitness levels are associated with greater hippocampal volume in breast cancer survivors. Frontiers in Human Neuroscience, 9, 465. 10.3389/fnhum.2015.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F-T, Erickson KI, Huang H, & Chang Y-K (2020). The association between physical fitness parameters and white matter microstructure in older adults: A diffusion tensor imaging study. Psychophysiology, 57(5), e13539. 10.1111/psyp.13539 [DOI] [PubMed] [Google Scholar]
- Chen Lin, Chao F, Lu W, Zhang L, Huang C, Yang S, Qiu X, Yang H, Zhao Y, Wang S, Li C, & Tang Y (2020). Long-Term Running Exercise Delays Age-Related Changes in White Matter in Rats. Frontiers in Aging Neuroscience, 12. 10.3389/fnagi.2020.590530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Linmu, Zhou C, Tan C, Wang F, Gao Y, Huang C, Zhang Y, Jiang L, & Tang Y (2017). Stereological Study on the Positive Effect of Running Exercise on the Capillaries in the Hippocampus in a Depression Model. Frontiers in Neuroanatomy, 11, 93. 10.3389/fnana.2017.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, Aronson J, Zhang C, Miller SJ, Lesinski A, Chen JW, Kim DY, van Praag H, Spiegelman BM, Gage FH, & Tanzi RE (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science (New York, N.Y.), 361(6406). 10.1126/science.aan8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Guadagni V, Mazerolle EL, Hill M, Hogan DB, Pike GB, & Poulin MJ (2019). Effect of aerobic exercise on white matter microstructure in the aging brain. Behavioural Brain Research, 373, 112042. 10.1016/j.bbr.2019.112042 [DOI] [PubMed] [Google Scholar]
- Colcombe, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, & Elavsky S (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 3316–3321. 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, & Kramer AF (2006). Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(11), 1166–1170. [DOI] [PubMed] [Google Scholar]
- Cole RC, Hazeltine E, Weng TB, Wharff C, DuBose LE, Schmid P, Sigurdsson G, Magnotta VA, Pierce GL, & Voss MW (2020). Cardiorespiratory fitness and hippocampal volume predict faster episodic associative learning in older adults. Hippocampus, 30(2), 143–155. 10.1002/hipo.23151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, & Christie L-A (2007). Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences, 30(9), 464–472. 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Davenport MH, Hogan DB, Eskes GA, Longman RS, & Poulin MJ (2012). Cerebrovascular reserve: The link between fitness and cognitive function? Exercise and Sport Sciences Reviews, 40(3), 153–158. 10.1097/JES.0b013e3182553430 [DOI] [PubMed] [Google Scholar]
- de Almeida AA, Gomes da Silva S, Fernandes J, Peixinho-Pena LF, Scorza FA, Cavalheiro EA, & Arida RM (2013). Differential effects of exercise intensities in hippocampal BDNF, inflammatory cytokines and cell proliferation in rats during the postnatal brain development. Neuroscience Letters, 553, 1–6. 10.1016/j.neulet.2013.08.015 [DOI] [PubMed] [Google Scholar]
- Debette S, & Markus HS (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. Bmj, 341, c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Tarumi T, Zhu DC, Tseng BY, Thomas BP, Turner M, Repshas J, Kerwin DR, Womack KB, Lu H, Cullum CM, & Zhang R (2018). Cardiorespiratory Fitness and White Matter Neuronal Fiber Integrity in Mild Cognitive Impairment. Journal of Alzheimer’s Disease, 61(2), 729–739. 10.3233/JAD-170415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, & Gomez-Pinilla F (2006). Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience, 140(3), 823–833. [DOI] [PubMed] [Google Scholar]
- Dougherty RJ, Schultz SA, Boots EA, Ellingson LD, Meyer JD, Van Riper S, Stegner AJ, Edwards DF, Oh JM, Einerson J, Korcarz CE, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, Asthana S, Hermann BP, … Cook DB (2017). Relationships between cardiorespiratory fitness, hippocampal volume, and episodic memory in a population at risk for Alzheimer’s disease. Brain and Behavior, 7(3), e00625. 10.1002/brb3.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Martin B, Yang An, Maudsley S, Ferrucci L, Mattson MP, & Resnick SM (2012). Plasma BDNF Is Associated with Age-Related White Matter Atrophy but Not with Cognitive Function in Older, Non-Demented Adults. PLoS ONE, 7(4), 1–6. 10.1371/journal.pone.0035217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland M, Zunszain PA, & Pariante CM (2015). Molecular mechanisms in the regulation of adult neurogenesis during stress. Nature Reviews Neuroscience, 16(4), 189–200. 10.1038/nrn3855 [DOI] [PubMed] [Google Scholar]
- Engeroff T, Füzéki E, Vogt L, Fleckenstein J, Schwarz S, Matura S, Pilatus U, Deichmann R, Hellweg R, Pantel J, & Banzer W (2018). Is Objectively Assessed Sedentary Behavior, Physical Activity and Cardiorespiratory Fitness Linked to Brain Plasticity Outcomes in Old Age? Neuroscience, 388, 384–392. 10.1016/j.neuroscience.2018.07.050 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, & Kramer AF (2007). Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiology of Aging, 28(2), 179–185. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE, & FOR 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE*. (2019). Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Medicine and Science in Sports and Exercise, 51(6), 1242–1251. 10.1249/MSS.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, & Weinstein AM (2014). Physical activity, fitness, and gray matter volume. Neurobiology of Aging, 35 Suppl 2, S20–28. 10.1016/j.neurobiolaging.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, & Kramer AF (2009). Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus, 19(10), 1030–1039. 10.1002/hipo.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, & Kuller LH (2010). Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology, 75(16), 1415–1422. 10.1212/WNL.0b013e3181f88359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, & Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences, 108(7), 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Cornejo I, Cadenas-Sanchez C, Contreras-Rodriguez O, Verdejo-Roman J, Mora-Gonzalez J, Migueles JH, Henriksson P, Davis CL, Verdejo-Garcia A, Catena A, & Ortega FB (2017). A whole brain volumetric approach in overweight/obese children: Examining the association with different physical fitness components and academic performance. The ActiveBrains project. NeuroImage, 159, 346–354. 10.1016/j.neuroimage.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Esteban-Cornejo I, Rodriguez-Ayllon M, Verdejo-Roman J, Cadenas-Sanchez C, Mora-Gonzalez J, Chaddock-Heyman L, Raine LB, Stillman CM, Kramer AF, Erickson KI, Catena A, Ortega FB, & Hillman CH (2019). Physical Fitness, White Matter Volume and Academic Performance in Children: Findings From the ActiveBrains and FITKids2 Projects. Frontiers in Psychology, 10. 10.3389/fpsyg.2019.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada C, Cuenca L, Cano-Fernandez L, Gil-Martinez AL, Sanchez-Rodrigo C, González-Cuello AM, Fernandez-Villalba E, & Herrero MT (2019). Voluntary exercise reduces plasma cortisol levels and improves transitory memory impairment in young and aged Octodon degus. Behavioural Brain Research, 373, 112066. [DOI] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, & Palmer TD (2003). VEGF is necessary for exercise-induced adult hippocampal neurogenesis. European Journal of Neuroscience, 18(10), 2803–2812. [DOI] [PubMed] [Google Scholar]
- Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, & Ward PB (2018). Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage, 166, 230–238. 10.1016/j.neuroimage.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Fletcher MA, Low KA, Boyd R, Zimmerman B, Gordon BA, Tan CH, Schneider-Garces N, Sutton BP, Gratton G, & Fabiani M (2016). Comparing Aging and Fitness Effects on Brain Anatomy. Frontiers in Human Neuroscience, 10, 286. 10.3389/fnhum.2016.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodin P, Jonasson LS, Riklund K, Nyberg L, & Boraxbekk C-J (2017). Does aerobic exercise influence intrinsic brain activity? An aerobic exercise intervention among healthy old adults. Frontiers in Aging Neuroscience, 9, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, & Thompson RF (1987). Behavioral stress impairs long-term potentiation in rodent hippocampus. Behavioral and Neural Biology, 48(1), 138–149. [DOI] [PubMed] [Google Scholar]
- Frederiksen KS, Larsen CT, Hasselbalch SG, Christensen AN, Høgh P, Wermuth L, Andersen BB, Siebner HR, & Garde E (2018). A 16-Week Aerobic Exercise Intervention Does Not Affect Hippocampal Volume and Cortical Thickness in Mild to Moderate Alzheimer’s Disease. Frontiers in Aging Neuroscience, 10, 293. 10.3389/fnagi.2018.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, & Thompson PM (2010). The clinical use of structural MRI in Alzheimer disease. Nature Reviews Neurology, 6(2), 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D (1994). Scene-specific memory for objects: A model of episodic memory impairment in monkeys with fornix transection. Journal of Cognitive Neuroscience, 6(4), 305–320. [DOI] [PubMed] [Google Scholar]
- Gaffan D, & Gaffan EA (1991). Amnesia in man following transection of the fornix: A review. Brain, 114(6), 2611–2618. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Ying Z, de Vellis J, & Gomez-Pinilla F (2007). Exercise decreases myelin-associated glycoprotein expression in the spinal cord and positively modulates neuronal growth. Glia, 55(9), 966–975. 10.1002/glia.20521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes da Silva S, Simões PSR, Mortara RA, Scorza FA, Cavalheiro EA, da Graça Naffah-Mazzacoratti M, & Arida RM (2013). Exercise-induced hippocampal anti-inflammatory response in aged rats. Journal of Neuroinflammation, 10(1), 827. 10.1186/1742-2094-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, & Gratton G (2008). Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology, 45(5), 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham LS, Jernigan T, Hudziak J, & Barch DM (2019). Involvement in Sports, Hippocampal Volume, and Depressive Symptoms in Children. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 4(5), 484–492. 10.1016/j.bpsc.2019.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, & Tanapat P (1999). Stress and hippocampal neurogenesis. Biological Psychiatry, 46(11), 1472–1479. 10.1016/S0006-3223(99)00247-4 [DOI] [PubMed] [Google Scholar]
- Gow AJ, Bastin ME, Munoz Maniega S, Valdes Hernandez MC, Morris Z, Murray C, Royle NA, Starr JM, Deary IJ, & Wardlaw JM (2012). Neuroprotective lifestyles and the aging brain: Activity, atrophy, and white matter integrity. Neurology, 79(17), 1802–1808. 10.1212/WNL.0b013e3182703fd2 [DOI] [PubMed] [Google Scholar]
- Graham LC, Grabowska WA, Chun Y, Risacher SL, Philip VM, Saykin AJ, Sukoff Rizzo SJ, & Howell GR (2019). Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiology of Aging, 80, 154–172. 10.1016/j.neurobiolaging.2019.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Matheson SL, Shepherd A, Weickert CS, & Carr VJ (2011). Brain-derived neurotrophic factor levels in schizophrenia: A systematic review with meta-analysis. Molecular Psychiatry, 16(9), 960–972. 10.1038/mp.2010.88 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, & Menon V (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences, 101(13), 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, & Kelly AM (2009). Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus, 19(10), 973–980. 10.1002/hipo.20631 [DOI] [PubMed] [Google Scholar]
- Gusnard DA, & Raichle ME (2001). Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience, 2(10), 685. [DOI] [PubMed] [Google Scholar]
- Hales PW, Kawadler JM, Aylett SE, Kirkham FJ, & Clark CA (2014). Arterial spin labeling characterization of cerebral perfusion during normal maturation from late childhood into adulthood: Normal “reference range” values and their use in clinical studies. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 34(5), 776–784. 10.1038/jcbfm.2014.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Sharma N, & Batty GD (2018). Association of objectively measured physical activity with brain structure: UK Biobank study. Journal of Internal Medicine, 284(4), 439–443. 10.1111/joim.12772 [DOI] [PubMed] [Google Scholar]
- Harasym D, Turco CV, Nicolini C, Toepp SL, Jenkins EM, Gibala MJ, Noseworthy MD, & Nelson AJ (2020). Fitness Level Influences White Matter Microstructure in Postmenopausal Women. Frontiers in Aging Neuroscience. 10.3389/fnagi.2020.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariprasad VR, Varambally S, Shivakumar V, Kalmady SV, Venkatasubramanian G, & Gangadhar BN (2013). Yoga increases the volume of the hippocampus in elderly subjects. Indian Journal of Psychiatry, 55(Suppl 3), S394–S396. 10.4103/0019-5545.116309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ (2004). The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology, 174(1), 151–162. 10.1007/s00213-003-1761-y [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Araki Y, Takashima Y, Nogami K, Uchino A, Yuzuriha T, & Yao H (2017). Hippocampal atrophy and memory dysfunction associated with physical inactivity in community-dwelling elderly subjects: The Sefuri study. Brain and Behavior, 7(2), e00620. 10.1002/brb3.620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Singh T, & Bugg JM (2012). The moderating role of exercise on stress-related effects on the hippocampus and memory in later adulthood. Neuropsychology, 26(2), 133–143. 10.1037/a0027108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, & Konradi C (2002). Hippocampal neurons in schizophrenia. Journal of Neural Transmission (Vienna, Austria: 1996), 109(5–6), 891–905. 10.1007/s007020200073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, & Nagel BJ (2012). Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behavioural Brain Research, 233(2), 517–525. 10.1016/j.bbr.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EE, Zack E, Battaglini C, Viru M, Viru A, & Hackney AC (2008). Exercise and circulating cortisol levels: The intensity threshold effect. Journal of Endocrinological Investigation, 31(7), 587–591. 10.1007/BF03345606 [DOI] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Dinov ID, Stein JL, Rosano C, & Toga AW (2011). The effects of physical activity, education, and body mass index on the aging brain. Human Brain Mapping, 32(9), 1371–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, & Otten U (2000). Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Archives of Neurology, 57(6), 846–851. 10.1001/archneur.57.6.846 [DOI] [PubMed] [Google Scholar]
- Honea R, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, & Burns JM (2009). Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer’s disease. Alzheimer Disease and Associated Disorders, 23(3), 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-C, Liu M-E, Chou K-H, Yang AC, Hung C-C, Hong C-J, Tsai S-J, & Lin C-P (2014). Effect of BDNF Val66Met polymorphism on regional white matter hyperintensities and cognitive function in elderly males without dementia. Psychoneuroendocrinology, 39, 94–103. 10.1016/j.psyneuen.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Hueston CM, Cryan JF, & Nolan YM (2017). Stress and adolescent hippocampal neurogenesis: Diet and exercise as cognitive modulators. Translational Psychiatry, 7(4), e1081. 10.1038/tp.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, & Loprinzi PD (2019). Association of cardiorespiratory fitness on interhemispheric hippocampal and parahippocampal functional connectivity. European Journal of Neuroscience. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T, Rakwal R, & Soya H (2015). Long-Term Mild, rather than Intense, Exercise Enhances Adult Hippocampal Neurogenesis and Greatly Changes the Transcriptomic Profile of the Hippocampus. PLoS ONE, 10(6). 10.1371/journal.pone.0128720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SY, Byun MS, Yi D, Lee J-H, Ko K, Sohn BK, Lee J-Y, Ryu S-H, Lee DW, Shin SA, Kim YK, Kang KM, Sohn C-H, & Lee DY (2020). Midlife Lifestyle Activities Moderate APOE ε4 Effect on in vivo Alzheimer’s Disease Pathologies. Frontiers in Aging Neuroscience, 12, 42. 10.3389/fnagi.2020.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, & Gold BT (2012). Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage, 59(2), 1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson LS, Nyberg L, Kramer AF, Lundquist A, Riklund K, & Boraxbekk C-J (2016). Aerobic exercise intervention, cognitive performance, and brain structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Frontiers in Aging Neuroscience, 8, 336. 10.3389/fnagi.2016.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr AL, Cheng S-Y, & Jones TA (2011). Experience-dependent neural plasticity in the adult damaged brain. Journal of Communication Disorders, 44(5), 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Olson EA, & Weber M (2013). Physical Exercise Habits Correlate with Gray Matter Volume of the Hippocampus in Healthy Adult Humans. Scientific Reports, 3. 10.1038/srep03457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-M, & Leem Y-H (2016). Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience, 324, 271–285. 10.1016/j.neuroscience.2016.03.019 [DOI] [PubMed] [Google Scholar]
- Kim H, Song B, So B, Lee O, Song W, & Kim Y (2014). Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: A pilot study. Psychiatry Research, 220(3), 792–796. 10.1016/j.psychres.2014.09.020 [DOI] [PubMed] [Google Scholar]
- Klaren RE, Hubbard EA, Motl RW, Pilutti LA, Wetter NC, & Sutton BP (2015). Objectively Measured Physical Activity Is Associated with Brain Volumetric Measurements in Multiple Sclerosis. Behavioural Neurology, 2015, 482536. 10.1155/2015/482536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ (2015). The hippocampus. Current Biology: CB, 25(23), R1116–1121. 10.1016/j.cub.2015.10.049 [DOI] [PubMed] [Google Scholar]
- Krogh J, Rostrup E, Thomsen C, Elfving B, Videbech P, & Nordentoft M (2014). The effect of exercise on hippocampal volume and neurotrophines in patients with major depression–A randomized clinical trial. Journal of Affective Disorders, 165, 24–30. 10.1016/j.jad.2014.04.041 [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Khaleghi M, & Hensrud DD (2007). Markers of inflammation are inversely associated with VO2 max in asymptomatic men. Journal of Applied Physiology (Bethesda, Md.: 1985), 102(4), 1374–1379. 10.1152/japplphysiol.01028.2006 [DOI] [PubMed] [Google Scholar]
- Kuo F-C, Lee C-H, Hsieh C-H, Kuo P, Chen Y-C, & Hung Y-J (2013). Lifestyle modification and behavior therapy effectively reduce body weight and increase serum level of brain-derived neurotrophic factor in obese non-diabetic patients with schizophrenia. Psychiatry Research, 209(2), 150–154. 10.1016/j.psychres.2012.11.020 [DOI] [PubMed] [Google Scholar]
- Leavitt VM, Cirnigliaro C, Cohen A, Farag A, Brooks M, Wecht JM, Wylie GR, Chiaravalloti ND, DeLuca J, & Sumowski JF (2014). Aerobic exercise increases hippocampal volume and improves memory in multiple sclerosis: Preliminary findings. Neurocase, 20(6), 695–697. 10.1080/13554794.2013.841951 [DOI] [PubMed] [Google Scholar]
- Lin J, Chan SK, Lee EH, Chang WC, Tse M, Su WW, Sham P, Hui CL, Joe G, Chan CL, Khong PL, So KF, Honer WG, & Chen EY (2015). Aerobic exercise and yoga improve neurocognitive function in women with early psychosis. NPJ Schizophrenia, 1(0), 15047. 10.1038/npjschz.2015.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, & Nusslock R (2018). Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Frontiers in Neuroscience, 12. 10.3389/fnins.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, & Cui R (2017). The role of neural plasticity in depression: From hippocampus to prefrontal cortex. Neural Plasticity, 2017, 6871089. 10.1155/2017/6871089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-F, Chen H, Wu C-L, Kuo Y-M, Yu L, Huang A-M, Wu F-S, Chuang J-I, & Jen CJ (2009). Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: Roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. The Journal of Physiology, 587(Pt 13), 3221–3231. 10.1113/jphysiol.2009.173088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T, & Donaldson MG (2009). Exercise and cognition in older adults: Is there a role for resistance training programmes? British Journal of Sports Medicine, 43(1), 25–27. 10.1136/bjsm.2008.055616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SN, Mayda AB, Roach AE, Fletcher E, Carmichael O, Maillard P, Schwarz CG, Yonelinas AP, Ranganath C, & DeCarli C (2012). Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Frontiers in Human Neuroscience, 6, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatel GA, Elsner VR, Bertoldi K, Vanzella C, dos Santos Moysés F, Vizuete A, Spindler C, Cechinel LR, Netto CA, & Muotri AR (2013). Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiology of Learning and Memory, 101, 94–102. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, & Buckner RL (2003). Functional deactivations: Change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences, 100(24), 14504–14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lövden M, Lindenberger U, Bäckman L, Braun-Dullaeus R, Ahrens D, Heinze H-J, Müller NG, & Düzel E (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular Psychiatry, 20(5), 585–593. 10.1038/mp.2014.114 [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, & Huettel SA (2008). Cerebral white matter integrity mediates adult age differences in cognitive performance. Journal of Cognitive Neuroscience, 21(2), 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, & Fuchs E (1996). Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. Journal of Neuroscience, 16(10), 3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makizako H, Liu-Ambrose T, Shimada H, Doi T, Park H, Tsutsumimoto K, Uemura K, & Suzuki T (2015). Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(4), 480–486. 10.1093/gerona/glu136 [DOI] [PubMed] [Google Scholar]
- Makizako H, Shimada H, Doi T, Park H, Yoshida D, & Suzuki T (2013). Six-minute walking distance correlated with memory and brain volume in older adults with mild cognitive impairment: A voxel-based morphometry study. Dementia and Geriatric Cognitive Disorders Extra, 3(1), 223–232. 10.1159/000354189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow B, Keeser D, Keller K, Hasan A, Rauchmann B-S, Kimura H, Schneider-Axmann T, Dechent P, Gruber O, Ertl-Wagner B, Honer WG, Hillmer-Vogel U, Schmitt A, Wobrock T, Niklas A, & Falkai P (2016). Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophrenia Research, 173(3), 182–191. 10.1016/j.schres.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Maller JJ, Welton T, Middione M, Callaghan FM, Rosenfeld JV, & Grieve SM (2019). Revealing the Hippocampal Connectome through Super-Resolution 1150-Direction Diffusion MRI. Scientific Reports, 9(1), 2418. 10.1038/s41598-018-37905-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltais M, Boisvert-Vigneault K, Rolland Y, Vellas B, de Souto Barreto P, & MAPT/DSA Study Group. (2019). Longitudinal associations of physical activity levels with morphological and functional changes related with aging: The MAPT study. Experimental Gerontology, 128, 110758. 10.1016/j.exger.2019.110758 [DOI] [PubMed] [Google Scholar]
- Maltais M, Rolland Y, Boisvert-Vigneault K, Perus L, Mangin J-F, Grigis A, Chupin M, Bouyahia A, Gabelle A, Delrieux J, Vellas B, & de Souto Barreto P (2020). Prospective associations between physical activity levels and white matter integrity in older adults: Results from the MAPT study. Maturitas, 137, 24–29. 10.1016/j.maturitas.2020.04.012 [DOI] [PubMed] [Google Scholar]
- Manley AF (1996). Physical Activity And Health: A Report Of The Surgeon General. DIANE Publishing. [Google Scholar]
- Marks BL, Katz LM, Styner M, & Smith JK (2011). Aerobic fitness and obesity: Relationship to cerebral white matter integrity in the brain of active and sedentary older adults. Br J Sports Med, 45(15), 1208–1215. [DOI] [PubMed] [Google Scholar]
- McAuley E, Szabo AN, Mailey EL, Erickson KI, Voss M, White SM, Wójcicki TR, Gothe N, Olson EA, Mullen SP, & Kramer AF (2011). Non-Exercise Estimated Cardiorespiratory Fitness: Associations with Brain Structure, Cognition, and Memory Complaints in Older Adults. Mental Health and Physical Activity, 4(1), 5–11. 10.1016/j.mhpa.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen SC, Hardy A, Ellingson BM, Jarrahi B, Sandhu N, Subotnik KL, Ventura J, & Nuechterlein KH (2015). Prefrontal and Hippocampal Brain Volume Deficits: Role of Low Physical Activity on Brain Plasticity in First-Episode Schizophrenia Patients. Journal of the International Neuropsychological Society: JINS, 21(10), 868–879. 10.1017/S1355617715000983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Ceritoglu C, Brown T, Albert M, & Lyketsos CG (2012). Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimer’s & Dementia, 8(2), 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, Wilkins HM, Brooks WM, Billinger SA, Swerdlow RH, & Burns JM (2017). Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. Public Library of Science One, 12(2), e0170547. 10.1371/journal.pone.0170547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl RW, Pilutti LA, Hubbard EA, Wetter NC, Sosnoff JJ, & Sutton BP (2015). Cardiorespiratory fitness and its association with thalamic, hippocampal, and basal ganglia volumes in multiple sclerosis. NeuroImage. Clinical, 7, 661–666. 10.1016/j.nicl.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, & Rhodes JS (2012). Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience, 219, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Góauctemez-Pinilla F, Choi J, & Cotman C (1995). Exercise and brain neurotrophins. Nature, 373(6510), 109. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, … Laughlin MR (2015). Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metabolism, 22(1), 4–11. 10.1016/j.cmet.2015.05.011 [DOI] [PubMed] [Google Scholar]
- Niemann C, Godde B, & Voelcker-Rehage C (2014). Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Frontiers in Aging Neuroscience, 6, 170. 10.3389/fnagi.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, & Huijzen C. van. (1988). The Human Central Nervous System: A Synopsis and Atlas (3rd ed.). Springer-Verlag. 10.1007/978-3-662-10343-2 [DOI] [Google Scholar]
- Nunley KA, Leckie RL, Orchard TJ, Costacou T, Aizenstein HJ, Jennings JR, Erickson KI, & Rosano C (2017). Physical activity and hippocampal volume in middle-aged patients with type 1 diabetes. Neurology, 88(16), 1564–1570. 10.1212/WNL.0000000000003805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin LE, Verstynen TD, Burzynska AZ, Voss MW, Prakash RS, Chaddock-Heyman L, Wong C, Fanning J, Awick E, & Gothe N (2016). White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. Neuroimage, 131, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM, & Diaz-Arrastia R (2009). Brain-derived neurotrophic factor levels in Alzheimer’s disease. Journal of Alzheimer’s Disease, 17(2), 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik R, Gallagher CL, Dowling NM, Carlsson CM, Bendlin BB, LaRue A, Rowley HA, Christian BT, Asthana S, Hermann BP, Johnson SC, & Sager MA (2014). Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology, 83(19), 1753–1760. 10.1212/WNL.0000000000000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FB, Campos D, Cadenas-Sanchez C, Altmäe S, Martínez-Zaldívar C, Martín-Matillas M, Catena A, & Campoy C (2017). Physical fitness and shapes of subcortical brain structures in children. The British Journal of Nutrition, 1–10. 10.1017/S0007114516001239 [DOI] [PubMed] [Google Scholar]
- Ortega M, Baker LM, Vaida F, Paul R, Basco B, & Ances BM (2015). Physical Activity Affects Brain Integrity in HIV + Individuals. Journal of the International Neuropsychological Society : JINS, 21(10), 880–889. 10.1017/S1355617715000879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajonk F-G, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Müller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, & Falkai P (2010). Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry, 67(2), 133–143. 10.1001/archgenpsychiatry.2009.193 [DOI] [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala E-L, Vainio P, Vanninen R, Partanen K, & Soininen H (2004). Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiology of Aging, 25(3), 303–310. 10.1016/S0197-4580(03)00084-8 [DOI] [PubMed] [Google Scholar]
- Perea RD, Vidoni ED, Morris JK, Graves RS, Burns JM, & Honea RA (2016). Cardiorespiratory fitness and white matter integrity in Alzheimer’s disease. Brain Imaging and Behavior, 10(3), 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, & Small SA (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5638–5643. 10.1073/pnas.0611721104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH (2004). The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain, Behavior, and Immunity, 18(5), 407–413. 10.1016/j.bbi.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Petersen AMW, & Pedersen BK (2005). The anti-inflammatory effect of exercise. Journal of Applied Physiology, 98(4), 1154–1162. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, & Sullivan EV (2002). Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage, 15(3), 708–718. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Patterson B, Janssen A, Abduljalil A, & Boster A (2011). Physical activity associated with increased resting-state functional connectivity in multiple sclerosis. Journal of the International Neuropsychological Society, 17(6), 986–997. [DOI] [PubMed] [Google Scholar]
- Pratchayasakul W, Sa-nguanmoo P, Sivasinprasasn S, Pintana H, Tawinvisan R, Sripetchwandee J, Kumfu S, Chattipakorn N, & Chattipakorn SC (2015). Obesity accelerates cognitive decline by aggravating mitochondrial dysfunction, insulin resistance and synaptic dysfunction under estrogen-deprived conditions. Hormones and Behavior, 72, 68–77. 10.1016/j.yhbeh.2015.04.023 [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Klimentidis YC, Bharadwaj PK, & Alexander GE (2019). Differential associations of engagement in physical activity and estimated cardiorespiratory fitness with brain volume in middle-aged to older adults. Brain Imaging and Behavior. 10.1007/s11682-019-00148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, & Acker JD (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex (New York, N.Y.: 1991), 15(11), 1676–1689. 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Reijs BLR, Vos SJB, Soininen H, Lötjonen J, Koikkalainen J, Pikkarainen M, Hall A, Vanninen R, Liu Y, Herukka S-K, Freund-Levi Y, Frisoni GB, Frölich L, Nobili F, Rikkert MO, Spiru L, Tsolaki M, Wallin ÅK, Scheltens P, … Visser PJ (2017). Association Between Later Life Lifestyle Factors and Alzheimer’s Disease Biomarkers in Non-Demented Individuals: A Longitudinal Descriptive Cohort Study. Journal of Alzheimer’s Disease: JAD, 60(4), 1387–1395. 10.3233/JAD-170039 [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T Jr, & Gage FH (2003). Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behavioral Neuroscience, 117(5), 1006. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ayllon M, Derks IPM, van den Dries MA, Esteban-Cornejo I, Labrecque JA, Yang-Huang J, Raat H, Vernooij MW, White T, Ortega FB, Tiemeier H, & Muetzel RL (2020). Associations of physical activity and screen time with white matter microstructure in children from the general population. NeuroImage, 205, 116258. 10.1016/j.neuroimage.2019.116258 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ayllon M, Esteban-Cornejo I, Verdejo-Román J, Muetzel RL, Migueles JH, Mora-Gonzalez J, Solis-Urra P, Erickson KI, Hillman CH, Catena A, Tiemeier H, & Ortega FB (2020). Physical Activity, Sedentary Behavior, and White Matter Microstructure in Children with Overweight or Obesity: Medicine & Science in Sports & Exercise, 52(5), 1218–1226. 10.1249/MSS.0000000000002233 [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Hulst HE, Vrenken H, Feenstra HEM, Castelijns JA, Pouwels PJW, Barkhof F, & Geurts JJG (2010). Structural and Functional Hippocampal Changes in Multiple Sclerosis Patients with Intact Memory Function. Radiology, 255(2), 595–604. 10.1148/radiol.10091433 [DOI] [PubMed] [Google Scholar]
- Rosano C, Guralnik J, Pahor M, Glynn NW, Newman AB, Ibrahim TS, Erickson K, Cohen R, Shaaban CE, MacCloud RL, & Aizenstein HJ (2017). Hippocampal Response to a 24-Month Physical Activity Intervention in Sedentary Older Adults. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 25(3), 209–217. 10.1016/j.jagp.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Venkatraman VK, Guralnik J, Newman AB, Glynn NW, Launer L, Taylor CA, Williamson J, Studenski S, Pahor M, & Aizenstein H (2010). Psychomotor Speed and Functional Brain MRI 2 Years After Completing a Physical Activity Treatment. The Journals of Gerontology: Series A, 65A(6), 639–647. 10.1093/gerona/glq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, & Berardi N (2006). Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. European Journal of Neuroscience, 24(7), 1850–1856. [DOI] [PubMed] [Google Scholar]
- Ryan SM, & Nolan YM (2016). Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: Can exercise compensate? Neuroscience & Biobehavioral Reviews, 61, 121–131. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Jonsdottir MK, Sigurdsson S, Eiriksdottir G, Jonsson PV, Garcia ME, Kjartansson O, van Buchem MA, Gudnason V, & Launer LJ (2008). White matter lesions and cognitive performance: The role of cognitively complex leisure activity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(8), 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam N, & Laher I (2015, December 28). Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases [Review Article]. Oxidative Medicine and Cellular Longevity; Hindawi. 10.1155/2016/7239639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheewe TW, van Haren NEM, Sarkisyan G, Schnack HG, Brouwer RM, de Glint M, Hulshoff Pol HE, Backx FJG, Kahn RS, & Cahn W (2013). Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: A randomised controlled trial in patients with schizophrenia and healthy controls. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 23(7), 675–685. 10.1016/j.euroneuro.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Sen A, Gider P, Cavalieri M, Freudenberger P, Farzi A, Schallert M, Reichmann F, Watzinger N, Zweiker R, & Schmidt R (2012). Association of cardiorespiratory fitness and morphological brain changes in the elderly: Results of the Austrian Stroke Prevention Study. Neurodegenerative Diseases, 10(1–4), 135–137. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, & Johansen-Berg H (2016). A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage, 131, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Betts JF, Dennis A, Doherty A, Leeson P, Holloway C, Dall’Armellina E, Winkler AM, Demnitz N, Wassenaar T, Dawes H, & Johansen-Berg H (2020). The effects of an aerobic training intervention on cognition, grey matter volumes and white matter microstructure. Physiology & Behavior, 223, 112923. 10.1016/j.physbeh.2020.112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamy JL, Carpenter DM, Fong SG, Murray EA, Tang CY, Hof PR, & Rapp PR (2010). Alterations of white matter tracts following neurotoxic hippocampal lesions in macaque monkeys: A diffusion tensor imaging study. Hippocampus, 20(8), 906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, & Bookheimer SY (2008). Regional hippocampal atrophy in multiple sclerosis. Brain: A Journal of Neurology, 131(Pt 4), 1134–1141. 10.1093/brain/awn030 [DOI] [PubMed] [Google Scholar]
- Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Hazlett KE, Figueroa CM, Kandah CC, Kay CD, Matthews MA, & Rao SM (2014). Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer’s disease. Frontiers in Aging Neuroscience, 6, 61. 10.3389/fnagi.2014.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartano NL, Davis-Plourde KL, Himali JJ, Andersson C, Pase MP, Maillard P, DeCarli C, Murabito JM, Beiser AS, Vasan RS, & Seshadri S (2019). Association of Accelerometer-Measured Light-Intensity Physical Activity With Brain Volume: The Framingham Heart Study. JAMA Network Open, 2(4), e192745. 10.1001/jamanetworkopen.2019.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripetchwandee J, Chattipakorn N, & Chattipakorn SC (2018). Links Between Obesity-Induced Brain Insulin Resistance, Brain Mitochondrial Dysfunction, and Dementia. Frontiers in Endocrinology, 9. 10.3389/fendo.2018.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman CM, Uyar F, Huang H, Grove GA, Watt JC, Wollam ME, & Erickson KI (2018). Cardiorespiratory fitness is associated with enhanced hippocampal functional connectivity in healthy young adults. Hippocampus, 28(3), 239–247. 10.1002/hipo.22827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svatkova A, Mandl RCW, Scheewe TW, Cahn W, Kahn RS, & Hulshoff Pol HE (2015). Physical Exercise Keeps the Brain Connected: Biking Increases White Matter Integrity in Patients With Schizophrenia and Healthy Controls. Schizophrenia Bulletin, 41(4), 869–878. 10.1093/schbul/sbv033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, & Greenough WT (2003). Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience, 117(4), 1037–1046. 10.1016/S0306-4522(02)00664-4 [DOI] [PubMed] [Google Scholar]
- Szabo AN, McAuley E, Erickson KI, Voss M, Prakash RS, Mailey EL, Wójcicki TR, White SM, Gothe N, Olson EA, & Kramer AF (2011). Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology, 25(5), 545–553. 10.1037/a0022733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZS, Spartano NL, Beiser AS, DeCarli C, Auerbach SH, Vasan RS, & Seshadri S (2017). Physical Activity, Brain Volume, and Dementia Risk: The Framingham Study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(6), 789–795. 10.1093/gerona/glw130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Liu J, Egorova N, Chen X, Sun S, Xue X, Huang J, Zheng G, Wang Q, & Chen L (2016). Increased hippocampus–medial prefrontal cortex resting-state functional connectivity and memory function after tai chi chuan practice in elder adults. Frontiers in Aging Neuroscience, 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Liu J, Liu W, Huang J, Xue X, Chen X, Wu J, Zheng G, Chen B, Li M, Sun S, Jorgenson K, Lang C, Hu K, Chen S, Chen L, & Kong J (2017). Tai Chi Chuan and Baduanjin Increase Grey Matter Volume in Older Adults: A Brain Imaging Study. Journal of Alzheimer’s Disease, 60(2), 389–400. 10.3233/JAD-170477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkka IM, Hautasaari P, Pesonen H, Niskanen E, Rottensteiner M, Kaprio J, Savić AM, & Kujala UM (2019). Long-Term Physical Activity May Modify Brain Structure and Function: Studies in Young Healthy Twins. Journal of Physical Activity & Health, 16(8), 637–643. 10.1123/jpah.2018-0416 [DOI] [PubMed] [Google Scholar]
- Tarumi T, Rossetti H, Thomas BP, Harris T, Tseng BY, Turner M, Wang C, German Z, Martin-Cook K, Stowe AM, Womack KB, Mathews D, Kerwin DR, Hynan L, Diaz-Arrastia R, Lu H, Cullum CM, & Zhang R (2019). Exercise Training in Amnestic Mild Cognitive Impairment: A One-Year Randomized Controlled Trial. Journal of Alzheimer’s Disease: JAD, 71(2), 421–433. 10.3233/JAD-181175 [DOI] [PubMed] [Google Scholar]
- ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, & Liu-Ambrose T (2015). Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. British Journal of Sports Medicine, 49(4), 248–254. 10.1136/bjsports-2013-093184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CVL, Rezende TJ, Weiler M, Nogueira MH, Campos BM, Pegoraro LF, … & Balthazar ML (2016). Relation between aerobic fitness and brain structures in amnestic mild cognitive impairment elderly. Age, 38(3), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Dennis A, Rawlings NB, Stagg CJ, Matthews L, Morris M, Kolind SH, Foxley S, Jenkinson M, Nichols TE, Dawes H, Bandettini PA, & Johansen-Berg H (2016). Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. NeuroImage, 131, 162–170. 10.1016/j.neuroimage.2015.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, & Cotman CW (2001). Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiology of Disease, 8(6), 1046–1056. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, & Torres-Aleman I (2001). Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. Journal of Neuroscience, 21(5), 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Huang H, & Zhang R (2013). White matter integrity in physically fit older adults. Neuroimage, 82, 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken U, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, & Gabrieli JD (2008). Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage, 42(2), 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkenborghs SR, Noetel M, Hillman C, Nilsson M, Smith J, Ortega F, & Lubans DR (2019). The Impact of Physical Activity on Brain Structure and Function in Youth: A Systematic Review. Pediatrics, 144(4). 10.1542/peds.2018-4032 [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, & Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience, 2(3), 266. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Shubert T, Zhao C, & Gage FH (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience, 25(38), 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Chuang Y-F, Harris GC, Tan EJ, & Carlson MC (2015). Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus, 25(5), 605–615. 10.1002/hipo.22397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Tang X, & Carlson MC (2016). Hippocampal sub-regional shape and physical activity in older adults. Hippocampus, 26(8), 1051–1060. 10.1002/hipo.22586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, & Gomez-Pinilla F (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European Journal of Neuroscience, 20(10), 2580–2590. 10.1111/j.1460-9568.2004.03720.x [DOI] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, Kantarci K, Senjem ML, Gunter JL, Boeve BF, Petersen RC, & Jack CR (2012). Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Annals of Neurology, 72(5), 730–738. 10.1002/ana.23665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, & Breteler MM (2009). White matter microstructural integrity and cognitive function in a general elderly population. Archives of General Psychiatry, 66(5), 545–553. [DOI] [PubMed] [Google Scholar]
- Villain N, Desgranges B, Viader F, De La Sayette V, Mézenge F, Landeau B, Baron J-C, Eustache F, & Chételat G (2008). Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. Journal of Neuroscience, 28(24), 6174–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wójcicki TR, & White SM (2013). The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Human Brain Mapping, 34(11), 2972–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, Fanning J, Awick E, Gothe NP, & Olson EA (2016). Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage, 131, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Weng TB, Narayana-Kumanan K, Cole RC, Wharff C, Reist L, Dubose L, Sigurdsson G, Mills JA, Long JD, Magnotta VA, & Pierce GL (2020). Acute Exercise Effects Predict Training Change in Cognition and Connectivity: Medicine & Science in Sports & Exercise, 52(1), 131–140. 10.1249/MSS.0000000000002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Guo Y, Myers KG, Heintz R, & Holschneider DP (2015). Recruitment of the prefrontal cortex and cerebellum in Parkinsonian rats following skilled aerobic exercise. Neurobiology of Disease, 77, 71–87. 10.1016/j.nbd.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedell-Neergaard A-S, Krogh-Madsen R, Petersen GL, Hansen ÅM, Pedersen BK, Lund R, & Bruunsgaard H (2018). Cardiorespiratory fitness and the metabolic syndrome: Roles of inflammation and abdominal obesity. Public Library of Science One, 13(3), e0194991. 10.1371/journal.pone.0194991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng TB, Pierce GL, Darling WG, Falk D, Magnotta VA, & Voss MW (2017). The Acute Effects of Aerobic Exercise on the Functional Connectivity of Human Brain Networks. Brain Plasticity, 2(2), 171–190. 10.3233/BPL-160039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H. akon, Tamnes CK, Østby Y, & Fjell AM (2009). Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex, 20(9), 2055–2068. [DOI] [PubMed] [Google Scholar]
- Wilckens KA, Stillman CM, Waiwood AM, Kang C, Leckie RL, Peven JC, Foust JE, Fraundorf SH, & Erickson KI (2021). Exercise interventions preserve hippocampal volume: A meta-analysis. Hippocampus, 31(3), 335–347. 10.1002/hipo.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Haase CM, Villeneuve S, Vogel J, & Jagust WJ (2014). Neuroprotective pathways: Lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiology of Aging, 35(8), 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KN, Nikolov R, & Shoemaker JK (2016). Impact of Long-Term Endurance Training vs. Guideline-Based Physical Activity on Brain Structure in Healthy Aging. Frontiers in Aging Neuroscience, 8, 155. 10.3389/fnagi.2016.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JA, Wilund KR, Martin SA, & Kistler BM (2011). Exercise, Inflammation and Aging. Aging and Disease, 3(1), 130–140. [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, & McEwen BS (1990). Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. Journal of Neuroscience, 10(12), 4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Wang F, Luo Y, Chen L, Chao F, Tan C, Gao Y, Huang C, Zhang L, Liang X, Tang J, Qi Y, Jiang L, Zhang Y, Zhou C, & Tang Y (2018). Exercise protects myelinated fibers of white matter in a rat model of depression. Journal of Comparative Neurology, 526(3), 537–549. 10.1002/cne.24350 [DOI] [PubMed] [Google Scholar]
- Yoon H, Oh YT, Lee JY, Choi JH, Lee JH, Baik HH, Kim SS, Choe W, Yoon K-S, Ha J, & Kang I (2008). Activation of AMP-activated protein kinase by kainic acid mediates brain-derived neurotrophic factor expression through a NF-kappaB dependent mechanism in C6 glioma cells. Biochemical and Biophysical Research Communications, 371(3), 495–500. 10.1016/j.bbrc.2008.04.102 [DOI] [PubMed] [Google Scholar]
- Zlatar ZZ, Wierenga CE, Bangen KJ, Liu TT, & Jak AJ (2014). Increased hippocampal blood flow in sedentary older adults at genetic risk for Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD, 41(3), 809–817. 10.3233/JAD-132252 [DOI] [PMC free article] [PubMed] [Google Scholar]