Abstract
Despite advances in surgery, chemotherapy, and radiation, there are limited treatment options for advanced head and neck squamous cell carcinoma (HNSCC) and survival remains very poor. Therefore, effective therapies are desperately needed. Recently, selective exploitation of DNA damage and replication stress responses has become a novel approach for cancer treatment. Wee1 kinase and Rad51 recombinase are two proteins involved in regulating replication stress and homologous recombination repair in cancer cells. In this study, we investigated the combined effect of Rad51 inhibitor (B02) and Wee1 inhibitor (AZD1775) in vitro and in vivo in various HNSCC cell lines. Clonogenic survival assays demonstrated that B02 synergized with AZD1775 in vitro in all HNSCC cell lines tested. The synergy between these drugs was associated with forced CDK1 activation and reduced Chk1 phosphorylation leading to induction of excessive DNA damage and replication stress, culminating in aberrant mitosis and apoptosis. Our results showed that elevated Rad51 mRNA expression correlated with worse survival in HNSCC patients with HPV-positive tumors. The combination of B02 and AZD1775 significantly inhibited tumor growth in vivo in mice bearing HPV-positive HNSCC tumors as compared to HPV-negative HNSCC. This differential sensitivity appears to be linked to HPV-positive tumors having more in vivo endogenous replication stress owing to transformation by E6 and E7 oncogenes. Furthermore, addition of B02 radiosensitized the HPV-negative HNSCC tumors in vitro and in vivo. In conclusion, our data implicate that a novel rational combination with Rad51 and Wee1 inhibitors holds promise as synthetic lethal therapy, particularly in high-risk HPV-positive HNSCC.
Keywords: HNSCC cells, mutant p53, TP53, Rad51, DNA damage, homologous recombination repair, CDK1, Chk1, replication stress, radiation, apoptosis, HPV-positive HNSCC, B02, AZD1775, Wee1
Introduction
Current treatment of head and neck squamous cell carcinoma (HNSCC) consists of multi-modality therapy with surgery, radiation, and chemotherapy (1,2). Despite significant improvements in these modalities, there are limited treatment choices for recurrent/metastatic and platinum refractory HNSCCs, and survival remains very poor. Therefore, novel therapeutic approaches are urgently needed. Wee1 is a tyrosine kinase that phosphorylates CDC2 at Tyr 15 and as such plays a pivotal role in the G2 DNA damage checkpoint (3-8). Recent clinical data show remarkable anti-tumor activity of the Wee1 kinase inhibitor AZD1775 (formerly known as MK-1775) in many cancer cells including HNSCC (9-12). This inhibitor acts to abrogate the G2 cell cycle checkpoint. HNSCC tumor cells harboring TP53 mutations are reliant on repairing DNA damage during arrest at this cell cycle checkpoint (6,13). Tumor cells that are unable to undergo cell cycle arrest at the G1 checkpoint are dependent on the S and G2 checkpoints for DNA repair and as such become more sensitive to G2 checkpoint abrogation (14-18). High levels of Rad51 expression have been observed in a variety of human malignancies including HNSCC, most likely contributing to increased cellular resistance of these malignancies to radiation and chemotherapy (19-21). During replication stress, Rad51 localizes with RPA32 to protect nascent DNA at stalled forks and mediates replication restart, thus allowing tumor cells to repair DNA damage through homologous recombination (HR) (22). We and others have shown that, in addition to its effects on the cell cycle, inhibition of Wee1 impairs Rad51-mediated homologous recombination repair through forced activation of CDK1, leading to senescence and/or apoptosis in HNSCC cells (3,6,23). As with any targeted therapy, response is often short-lived, and drug benefit could be achieved with novel therapeutic combinations. Taken together, these data suggest that the homologous recombination repair deficient state induced by Wee1 inhibition can be exploited to kill head and neck tumor cells that are resistant to conventional treatments. Therefore, we hypothesize that simultaneous targeting of Wee1 and Rad51 will result in greater cell killing in preclinical models of HNSCC. The Rad51 inhibitors, (RI-1 and B02) are currently under early preclinical testing in breast cancers and well tolerated in mice (24). The Rad51 inhibitor, B02 disrupts Rad51 binding to ssDNA during nucleofilament formation, thus affecting DNA repair (25). In the present study, we evaluate the efficacy of B02 alone and in combination with AZD1775 in vitro and in vivo in orthotopic mouse model of oral cancer.
Our data show that B02 displays differential sensitivity and synergizes with AZD1775 in vitro in various HNSCC cell lines. Mechanistically, these drugs interact synergistically to induce DNA damage, replication stress, and impaired Rad51-mediated HR through activation of CDK1 and decreased CHK1 phosphorylation, culminating in aberrant mitosis associated with apoptotic cell death. Interestingly, the combination of B02 with AZD1775 causes significant tumor growth inhibition in vivo in Human Papilloma Virus (HPV)-positive HNSCC tumor bearing mice as compared to HPV-negative HNSCC. Furthermore, addition of B02 radiosensitized the HPV-negative HNSCC tumors in vitro and in vivo. In summary, our data suggest that selective combined targeting of replication stress and Rad51-mediated HR repair may represent an effective therapeutic approach for killing high risk HPV-positive head and neck cancer.
Materials and Methods:
Cell lines and cell culture
HNSCC cell lines for this study were obtained from an established cell repository in the laboratory of Dr. Jeffrey N. Myers (University of Texas MD Anderson Cancer Center, Houston, TX). All cell lines were authenticated using short-tandem repeat analysis within six months of use for the current study. All HNSCC cell lines were cultured in appropriate media and maintained at 37°C in a humidified incubator containing 5% CO2, and periodically tested to ensure mycoplasma-free culture environment. All experiments were performed using cells from early passages. Identity, source, date of acquisition and culture conditions of all cell lines were described in Supplementary Materials and Methods section. The HNSCC cell line PCI-13 lacking endogenous p53 was obtained from the laboratory of Dr. Jennifer Grandis (University of Pittsburgh, Pittsburgh, PA) and engineered to stably express constructs containing either, wild-type (wt) p53 or high-risk EA (Evolutionary Action) score mutant p53 (G245D), and pBabe (null p53) as described previously (26). The RAD51 inhibitor B02 was purchased from Millipore-Sigma and prepared as 20 mmol/L stock solution in DMSO according to the manufacturer’s recommendations. The WEE1 inhibitor, AZD1775 was supplied by AstraZeneca through a collaborative agreement arranged by NCI-CTEP and was dissolved in DMSO at a stock concentration of 10 mmol/L. All drugs were stored at −20°C before use for all in vitro studies.
Generation of shRNA–expressing cells
The HMS-001 and UM-SCC-47 cells stably expressing shRNAs specific for Rad51, E7 or controls (scrambled shRNAs) lentiviral vectors respectively were generated using standard transfection protocol. See the Supplementary Materials and Methods section for details.
Clonogenic survival assay
To determine colony formation, HNSCC cells were seeded in 6-well plates (3 wells /treatment condition) at predetermined densities set for each cell line, and exposed to different fixed-ratio of B02 (0.625-25 μmol/L) and AZD1775 (0.0625-1.0 μmol/L) as a single agent or in combination. Controls (untreated) received 0.1% DMSO made in culture medium equivalent to the DMSO percentage in other treatment conditions. Cells were cultured for 10 to 14 days and clonogenic cell survival was determined as previously described (6). For radiosensitivity assays, cells were pretreated with different doses of B02, as indicated, then followed by exposure to either 2 Gray (Gy) radiation and the surviving colonies were determined.
Analysis of combined drug effects
The combination index (CI) and isobologram analyses assessing drug synergism was calculated via CalcuSyn Software (Biosoft) using the Chou–Talalay method (27). Details of the experiment were described in the Supplementary Materials and Methods section.
Western blot analysis
According to the IC50 values, minimally toxic and physiologically relevant drug doses were identified. Briefly, cells were treated in 10-cm plates with B02 (10 μmol/L) and AZD1775 (0.5 μmol/L) either alone or in combination for 16, 48 and 72 hours. Whole cell lysates were prepared, and western blot analyses were conducted as described previously (6). Antibodies used for Western blotting were described in Supplementary Materials and Methods section.
Cell cycle analysis and Annexin V-FITC/PI staining
HNSCC cells were seeded in 60-mm dishes, treated the next day with B02 (10 μmol/L), AZD1775 (0.5 μmol/L) either alone or in combination and then harvested at 24, 48, or 72 hours. The cell cycle analysis was performed as previously described (6). Apoptosis was detected using the Annexin V-FITC/PI staining kit obtained from BD Bioscience according to the manufacturer’s instructions.
Celigo Cell Count
Representative HNSCC cell lines (PCI-13-G245D, UM-SCC-47) were seeded in 96-well dishes, treated the next day with different doses of B02 (2.5-40 μmol/L), AZD1775 (0.0625-1.0 μmol/L) either alone or in combination for 24, 48, or 72 hours. Then, the cell growth was assayed overtime using the Celigo Cell Imaging Cytometer (Nexcelom) following treatment with these drugs according to manufacturers’ protocol.
Rad51 and Phospho-ɤH2AX focus formation
The UM-SCC-47 cells were plated onto glass coverslips and treated with drugs the following day as indicated. Detailed experimental procedures for the Rad51 and Phospho-ɤH2AX focus formation assay were described in Materials and Methods.
DNA fiber assay
DNA fiber analysis was performed as previously described (28). Briefly, PCI-13-G245D and UM-SCC-47 cells were treated with DMSO control, B02, AZD1775, or combination and then pulse-labeled with CldU for 15 minutes followed by IdU for 20 minutes. The experimental procedures were described in detail in the Supplementary Materials and Methods.
Orthotopic mouse model of oral tongue cancer and tumor growth delay
All animal experimentation was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas MD Anderson Cancer Center. Our orthotopic nude mouse tongue model has been previously described in the literature (6,23,26). The HPV-negative HNSCC (PCI-13-G245D) and HPV-positive HNSCC (UM-SCC-47) cells (50 X 103) suspended in 30 μL of DMEM only were injected into the lateral tongues of male athymic nude mice since oral cancer is mostly prevalent and represented in male patients. Mice were then randomized into different groups 8-11 days after injection and treatment was initiated when tumors were 4-6 mm3 in size. Mice were treated with B02 dissolved in cremophor/DMSO/PBS at ratio (1:1:3) and administrated intraperitoneally (i.p.) at a dose of 50 mg/kg three times a week (day 1, 3 and 5), and AZD1775 dissolved in 0.5% methylcellulose and DMSO at ratio (9:1) at a dose of 35 mg/kg given by oral gavage (p.o.) daily 5 times a week. Control mice received PBS or methylcellulose respectively. Treatment was continued for four weeks and tumor size and weight loss of the mice were monitored and recorded twice a week. Tongue tumors were measured with microcalipers, and tumor volume was calculated as (A)(B2)π/6, where A is the longest dimension of the tumor and B is the dimension of the tumor perpendicular to A. Mice were euthanized when they lost more than 20% of their preinjection body weight. Treatment with B02, radiation, or in combination was described in detail in the Supplementary Materials and Methods.
3D cell culture and in vivo TUNEL assay
A “3D cell culture” was established as described previously (29). Briefly, a total of 3000 HPV-positive HNSCC (MDA-HN-2C) cells, embedded in 150 μl of collagen gel (n = 3/group) were layered on top of the solidified collagen gel. The “3D tumors” were treated with a calculated corresponding maximum physiological (given to mice) doses of B02 (10 μmol/L) and AZD1775 (2.0 μmol/L) for 1 day or 5 days and effect of the drug on apoptosis was determined using the in vivo TUNEL assay as described previously (30).
TCGA HNSCC patient samples cohort analysis
Transcriptome data on 520 HNSCC samples (71 HPV-positive and 449 HPV-negative) from The Cancer Genome Atlas (TCGA) was obtained from NCI Genomic Data Commons (https://portal.gdc.cancer.gov/) (31). Respective mutation and clinical data were obtained from FireBrowse (http://firebrowse.org/). HPV status was determined in silico by detection of HPV transcripts, as previously described (31-33). To broadly understand HPV-driven expression of DNA damage repair (DDR) genes we performed unsupervised hierarchical clustering of HNSCC TCGA samples based on RNA expression of genes obtained from Gene Ontology (GO) searches for regulation of mitotic cell cycle, DNA duplex unwinding, DNA replication, DNA repair, DNA damage, checkpoint response, and replication fork. Genes were first vetted for differential expression in HPV-positive OPSCC samples (N = 52, HPV-16)) compared to HPV-negative tumors (N = 449) based on significance (Adj. P <0.05) and ≥1.5-fold change. This left 279 DDR genes that were used for clustering. Clustering of the DDR genes was also examined in panel of 73 HNSCC cell lines and 10 cervical squamous cell carcinoma lines with known HPV status. The genomic landscape of these HPV-positive cell lines was previously published (34).
Statistical analysis
A one-Way ANOVA test was carried out to analyze in vitro data. For mouse studies, a one-Way and 2-Way ANOVA tests were utilized to compare tumor volumes between control and treatment groups. Survival following drug treatment and radiation was analyzed by the Kaplan–Meier method and compared with log-rank test. All in vitro and in vivo data were expressed as ± standard error of the mean, ± SEM and P values <0.05 were considered significant. Analyses of in silico data were performed using JMP Pro 12.0.1 Software (SAS Institute Inc.). Differences in gene expression between groups were accessed by Wilcoxon test, and were considered significant when p-values were lower than 0.05. Sample categorization according to the expression of single genes, was determined by ROC analysis and Youden’s Index calculation, considering the 5-year overall survival as the variable of interest. Samples with RAD51 and WEE1 expression equal or below to the Youden’s Index were categorized as "Low RAD51, Low WEE1", while samples with expression above this index were categorized as "High RAD51, High WEE1" respectively. Associations between categorical variables and survival were determined by the log-rank test, considering a 5-year follow-up interval. For continuous variables, univariate Cox regression analysis was employed, again considering a 5-year follow-up interval, and the significance was established by the Wald and likelihood ratio tests.
Results
The Rad51 inhibitor, B02 displays differential sensitivity and synergizes with AZD1775 in vitro in HNSCC.
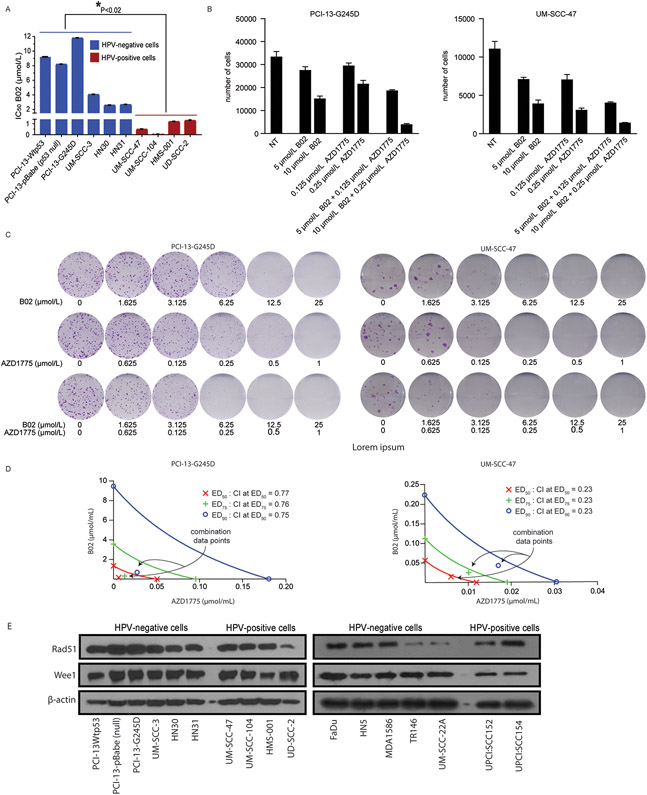
To determine the relative degree of sensitivity of HNSCC cells to B02 as single agent, HPV-negative HNSCC cells with natural TP53 statuses, isogenic HNSCC PCI13 cells with different TP53 mutational statuses, and HPV-positive HNSCC cells were treated with various concentrations of B02 alone (0-25 μmol/L) for 48 hours and subjected to clonogenic survival assays. Results shown in Figure 1A, demonstrated that the HPV-positive HNSCC cells were exquisitely sensitive to B02 as single agent in vitro (IC50; 0.02-1.65 μmol/L) compared to HPV-negative HNSCC (IC50; 3.0-12 μmol/L). Representative images of clonogenic survival assays reflecting the IC50 for B02 as a single agent are shown in Supplementary Figure S1. The cell growth with B02 and in combination with the Wee1 inhibitor, AZD1775 in two representative HNSCC cell lines (PCI13-G245D, UM-SCC-47) was assayed using the Celigo system which is capable of accurately counting adherent cells following drug treatment. After 48 hours treatment with B02, the PCI13-G245D and UM-SCC-47 showed a decline in cell number and less proliferation (Fig.1B, and Supplementary Fig. S2A-B). Furthermore, combination of B02 and AZD1775 resulted in greater cell killing compared to either drug alone in these cell lines (Fig. 1B, and Supplementary Fig. S2A-B). Similarly, clonogenic survival assays also demonstrated that combination with these drugs inhibited cell growth in PCI13-G245D and UMSCC-47 HNSCC cell lines respectively (Fig. 1C). We next examined whether B02 was synergistic when combined with AZD1775 treatment in the HNSCC cell lines tested, using the combination index (CI) method of Chou and Talalay (27). Conservative isobologram plots of effective doses (ED50; 50% inhibition), ED75 (75% inhibition), and ED90 (90% inhibition) obtained from Figure 1C, showed that the combination index (CI) at each inhibitory concentration is less than 1.0 (PCI-13-G245D; CI = 0.77, and UM-SCC-47; CI = 0.23), indicating strong synergism following combination treatment in these cells (Fig. 1D). Western blot analysis showed that the basal expression levels of Rad51 and Wee1 proteins were similar across all HNSCC cell lines tested (Fig. 1E).
Figure 1. The Rad51 inhibitor, B02 displays differential sensitivity and synergizes with AZD1775 in vitro in HNSCC.
A , IC50 values for B02 in selected HPV-negative and HPV-positive HNSCC cells treated with various concentrations of the drug for 48 hours and subjected to clonogenic survival assays as indicated. B, number of adherent cells (PCI-13-G245D and UM-SCC-47) measured with Celigo following treatment with B02 and in combination with AZD1775 for 72 hours. C, representative images of clonogenic survival assays in PCI13-G245D and UM-SCC-47 cell lines treated with combination of B02 and AZD1775. D, assessment of the degree of synergy between B02 and AZD1775 in these cells, using the Chou and Talalay method. B02 and AZD1775 were used at constant ratios (25:1 respectively). The CI values for combination of effective drug doses (ED) that result in growth inhibition of 50% (ED50; fraction affected; fa = 0.5), 75% (ED75; fa = 0.75), and 90% (ED90; fa = 0.90) were generated from the conservative isobolograms. The ED50 (red X), ED75 (green crosses) and ED90 (blue circles) graphed against fractional concentrations of B02 and AZD1775 on the y and x-axis, respectively are indicated. The diagonal line is the line of additivity. The conservative isobolograms show CI values less than 1.0 falling below diagonal line, indicate strong synergy. E, Western blot analyses of Rad51 and Wee1 expression at basal levels in representative HNSCC cell lines. The β-actin served as loading control. All treatments were performed in triplicate and each experiment was repeated at least three times. *P< 0.02, indicates significant difference between IC50 mean values for B02 in HPV-negative (blue) and HPV-positive (red) cells.
Rad51 knockdown induces DNA damage and replication stress responses and apoptosis. in HNSCC cells treated with Wee1 kinase inhibitor.
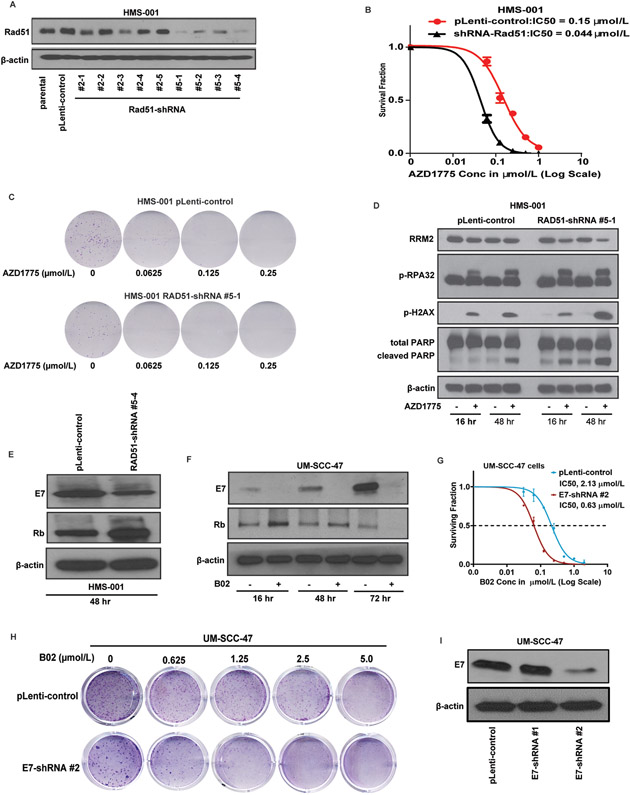
To confirm that sensitization of HNSCC cells to Wee1 inhibitor is specifically due to inhibition of Rad51, an HPV-positive HNSCC (HMS-001) cell line stably expressing shRNA-Rad51 was engineered and the sensitivity to the Wee1 inhibitor, AZD1775 was examined using clonogenic survival assays. Western blot analysis showed successful knockdown clones of Rad51 (Fig. 2A). The clonogenic survival of HMS-001-Rad51-shRNA cells (IC50 = 0.044 μmol/L) was found to be much lower than HMS-001-pLenti control cells (IC50 = 0.0.15 μmol/L) in response to AZD1775 treatment (Fig. 2B). Representative images of clonogenic survival assays reflecting the IC50 of AZD1775 for Rad51-shRNA clones are shown in Figure 2C. Furthermore, increased phosphorylation levels of markers of DNA damage (p-γH2AX (S134) and replication stress (pRPA32) responses was observed in AZD1775 treated Rad51-shRNA cells compared to their pLenti control cells respectively in a time-dependent manner (Fig. 2D). HMS-001 cells with Rad51-knockdown show remarkable induction of apoptosis as indicated by PARP1 cleavage. Decreased expression of the ribonucleotide reductase M2 (RRM2), a protein required for nucleotides supply during replication, was also seen in Rad51-shRNA expressing cells treated with AZD1775 (Fig. 2D).
Figure 2. Rad51 knockdown induces DNA damage and replication stress responses and apoptosis in HNSCC cells treated with Wee1 kinase inhibitor.
HPV-positive HNSCC (HMS-001) cells were stably transfected with pLenti-viral vector control or Rad51 shRNA plasmids and protein lysates were prepared as described in Methods. A, Western blot analyses showing successful knockdown clones of Rad51 (clones #5-1 and #5-4). Lysate from HMS-001 parental cells was used as positive control. B, clonogenic survival assay showing sensitivity of HMS-001 cells expressing Rad51-shRNA clone #5-1 to AZD1775 treatment. Data points represent the mean ± SEM of triplicate experiments. C, representative images of clonogenic survival assays for HMS-001 with pLenti-viral vector control and Rad51 shRNA clone#5-1 respectively. The HMS-001 cells with control vector and Rad51-shRNA #5-1 were then treated with/without AZD1775 for 16 and/or 48 hours and subjected to immunoblot analysis using antibodies as indicated. D, increased levels of phosphorylation of markers of DNA damage (p-γH2AX (S134), replication stress (pRPA32) responses, and decreased total levels of ribonucleotide reductase M2 (RRM2). PARP1 cleavage indicates induction of apoptosis in these cells. E, Rad51 knockdown inhibits E7 and enhances expression levels of Rb in HMS-001 cells. F, inhibition of E7 and enhanced Rb protein levels in UM-SCC-47 cells treated with 10 μmol/L B02 at various time point. G and H, clonogenic survival plot and representative clonogenic images showing increased sensitivity of UM-SCC-47 cells expressing E7-shRNA plasmid to treatment with B02 respectively. Knockdown of E7 was confirmed by immunoblotting (I). The β-actin served as loading control.
The HPV-positive HNSCC cell lines were exquisitley sensitive to B02 treatment compared to HPV-negative ones, suggesting that susceptibility to RAD51 inhibition is linked to expression of E6 and/or E7 oncogenes. Recent study has shown that E7 binds to the retinoblastoma tumor-suppressor gene (Rb) and inhibits its ability to repress the expressions of replication enzyme genes, thus pushing the cell cycle forward (35). Moreover, it has been demonstrated that repression of E6 and/or E7 oncogenes results in restoration of their respective tumor suppressors, p53 and pRb and induced apoptosis in HPV16-positive oropharyngeal cancer cell lines (36). Taken together, these studies implicate that that the HPV-positive tumors maybe dependent on E7 expression to tolerate replication stress and continue to survive in the presense of DNA damage. Therefore, we tested whether inhbition of Rad51 function could cause loss of oncogenic signaling through E7 in HPV-positive tumor cells. As shown in Figure 2E, knockdown of Rad51 was acompanied by decreased E7 and increased Rb protein levels in HMS-001 cells. These findings were further validated in UM-SCC-47 cells where treatment with B02 supressed E7 and enhanced Rb as early as 16 hours (Fig. 2F). Additionally, Knockdown of E7 further increased susceptabilty of HPV+HNSCC cells to B02 (IC50; 0.63 Vs. 2.13 μmol/L for E7-shRNA and pLenti-control respectively),suggesting a possible mechanism of the drug sensitivity through E7 effects on cell surviaval and proliferation (Fig. 2G-I).
Synergistic interaction of B02 and AZD1775 is mediated in part through forced activation of CDK1 and inhibition of Chk1 associated with impaired Rad51-mediated homologous recombination repair in HNSCC cells.
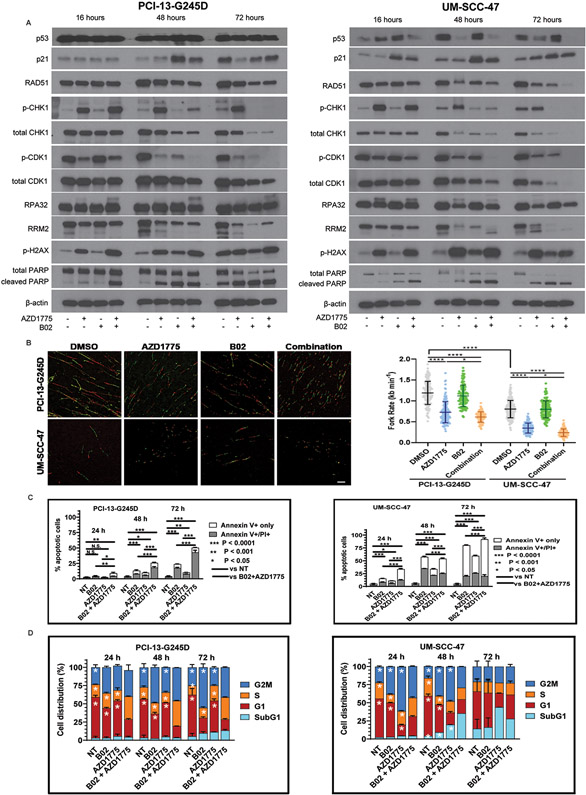
Our previous publications have demonstrated that Wee1 inhibition with AZD1775 can induce DNA damage and replication stress responses in HNSCC tumor cells through activation of CDK1 and Chk1 (23,37). Phosphorylation of Chk1 at S345 and S317 sites is required for Chk1 activation in cells. Therefore, we examined whether B02 and AZD1775 similarly affected phosphorylation and protein levels of CDK1 and Chk1 in PCI-13-G245D, UM-SCC-47, and UM-SCC-104 HNSCC cell lines by western blot analyses. The combination of B02 and AZD1775 markedly decreased the inhibitory phosphorylation of Tyr15 on CDK1 and total CDK1 protein levels in a time-dependent manner in these cells (Fig. 3A, and Supplementary Fig. S3A), suggesting a shift in cells stalled during S-phase. The Rad51 inhibitor, B02 sharply abrogated AZD1775-induced Chk1 phosphorylation at S345 and further decreased the total Chk1 protein levels in a time-dependent manner (Fig. 3A, and Supplementary Fig. S3A). Decreased Chk1 activation was associated with induction of the DNA damage marker, phospho-γH2AX following treatment with the drugs in these cells (Fig. 3A, and Supplementary Fig. S3A). The DNA damage was confirmed by immunofluorescence in the HPV+HNSCC (UM-SCC-47) cells using anti-phospho-γH2AX antibody. An increased nuclear phospho-Hγ2AX foci and/or immunostaining intensity were observed in these cells 24 hours after treatment with the combination compared to either drug alone (Supplementary Figure S4A-C). These findings raise the possibility that Rad51 and Wee1 inhibition cause lethal replicative stress in HNSCC. To test this possibility, the effects of B02 and AZD1775 on replication stress markers were examined in PCI-13-G245D, UM-SCC-47, and UM-SCC-104 HNSCC cells. Hyperphosphorylation of the replication stress marker RPA32 associated with reduced levels of the ribonucleotide reductase enzyme RRM2 was observed in these cells in a time-dependent manner consistent with induction of replication stress following treatment with drugs (Fig. 3A, and Supplementary Fig. S3A). Induction of replication stress (RS) was further confirmed in HNSCC cells with the DNA fiber assay. Consistent with our notion, B02 and AZD1775 treatment resulted in a significantly decreased median fork rate (higher replicative stress) in PCI-13-G245D cells (DMSO vs combination; 1.19 Vs 0.61 kb min−1, p<0.0001) and UM-SCC-47 cells (DMSO vs combination; 0.79 Vs 0.28 kb min−1, p<0.0001) respectively (Fig. 3B). Importantly, the UM-SCC-47 cells had higher basal RS than PCI-13-G245D cells (fork rate of 0.79 versus 1.19 kb min-1 respectively) (Fig. 3B). It has been reported that DNA damage causes an increase in p53 activity in HPV+ cell lines leading to p21 activation (38). Consistent with this finding, treatment with B02 increased p53 protein levels in the HPV-positive but not in the HPV-negative cells in a time-dependent manner (Fig. 3A, and Supplementary Fig. S3A). The p21 protein levels were also induced with B02 treatment in all cells tested in a time-dependent manner (Fig. 3A, and Supplementary Fig. S3A), consistent with recent report (39). This data suggest that B02 affects HNSCC cell growth during cell cycle transition through its effect on expression of cell cycle associated protein p21. Additionally, combination of B02 and AZD1775 completely blocked Rad51 protein expression, suggestive of impaired Rad51-mediated homologous recombination repair in HNSCC cells tested (Fig. 3A, and Supplementary Fig. S3A). The Rad51 nuclear foci is widely used as a surrogate marker of HR functionality (37,40). Gemcitabine has been shown to increase Rad51 foci formation and activate the HR repair in cancer cells (40). Therefore, we examined the status of homologous DNA repair by quantifying the Rad51 foci in HPV+HNDCC cells exposed to gemcitabine followed by treatment with B02 and AZD1775. The gemcitabine-increased focus formation was significantly inhibited upon addition of B02 and when combined with AZD1775 (Supplementary Fig. S5A-B). These results confirmed that the combination of the tested drugs inhibited the HR function in HNSCC cells. Moreover, similar to the results presented in Figure 2D, the treatment with B02 alone caused apoptosis which further increased upon addition of AZD1775 over time, as indicated by PARP1 cleavage (Fig. 3A, and Supplementary Fig. S3A). Apoptosis in these cells following treatment with drugs was further confirmed with AnnexinV/PI positive staining. In response to AZD1775 and B02 alone, percentage of early (Annexin V-positive, PI-negative) and late apoptosis (Annexin V-positive, PI-positive) in PCI13-G245D cells were less than 25% at all-time points tested. This percentage of apoptosis increased with the drug combination compared to single agent or untreated controls (Fig. 3B, and Supplementary Fig. S3B). Unlike the HPV-negative HNSCC (PCI-13-G245D) cells, treatment of HPV-positive HNSCC (UM-SCC-47 and UM-SCC-104) cells with B02 and/or AZD1775 resulted in earlier and higher levels of apoptotic death (Fig. 3B, and supplementary Fig. S3B), consistent with their complete PARP1 cleavage shown in Figure 3A. To better understand the longer-term effects of drug treatment, PCI-13-G245D, UM-SCC-47, and UM-SCC-104 HNSCC cells were treated with B02 and AZD1775, and examined at 24-72 hours for cell cycle kinetics. Treatment of PCI13-G245D cells with B02 and AZD1775 caused accumulation of cells in S phase (34.27 %) and G2/M phase (40.15 %) at 24 hours compared to untreated control (S: 17.84 %, G2/M: 25.97%), B02 (S: 23.39%; G2/M: 35.71%) or AZD1775 (S: 19.07 %, G2/M: 32.17%) alone respectively (Fig. 3C). In addition, drug combination decreased the cell number in the G1 phase and subsequently increased the sub-G1 population at 48 hours (Fig. 3C). Likewise, 24 hours of B02 and AZD1775 treatment induced slightly the S (26.99%, 20.21%) and G2/M (27.38%, 43.23%) cell cycle phase arrest in HPV-positive HNSCC (UM-SCC-47, UM-SCC-104) cells respectively, compared to untreated control (S: 20.24 %, G2/M: 23.09%), B02 (S: 11.42%; G2/M: 38.31%) or AZD1775 (S: 18.49 %, G2/M: 61.51%) alone respectively (Fig. 3C, and supplementary Fig. S3C). However, at 48 and 72 hours combination treatment, cell number in these phases gradually declined because proportion of cells went into apoptosis as revealed by increased subG1 fractions (UM-SCC-47, 35.40% and 27.90%; UM-SCC-104,10.06% and 18.48% respectively) (Fig. 3C, and Supplementary Fig. S3C). Collectively, these data suggest that the interactions between replicative stress and impaired homologous DNA recombination repair during cell cycle transition are likely involved in synergism observed with Rad51 and Wee1 inhibitors in HNSCC cells.
Figure 3. Synergistic interaction of B02 and AZD1775 is mediated in part through forced activation of CDK1 and inhibition of Chk1 associated with impaired Rad51-mediated homologous recombination repair in HNSCC cells.
PCI13-G245D and UM-SCC-47 cells were treated with B02, AZD1775, or in combination for 16, 48, 72 hours and protein lysates were subjected to western blot analysis with antibodies as indicated. A, levels of phosphorylation of Chk1 (S345), CDK1 (Y15), γH2AX (S139), RPA32, total p21, Rad51, Chk1, CDK1, RRM2. The presence of PARP-1 cleavage as marker of apoptosis is shown. The β-actin served as loading control. B, PCI13-G245D and UM-SCC-47 cells were treated with DMSO and drugs as indicated for 16 hours and then subject to DNA fiber analysis to quantify mean replication fork speed (rate). Lower speed indicates higher degree of replication stress. Scale bar, 10 μm. Data are mean±SD. C, Annexin/PI positive staining (percentage of dead cells) confirming induction of apoptosis in these cells following treatment with B02 and AZD1775. Unsynchronized PCI13-G245D and UM-SCC-47 cells were treated with B02, AZD1775 alone or in combination for various time points as indicated and subjected to cell cycle analysis. NT (no treatment) control. D, percentage of cell cycle distribution determined by flow cytometry was presented as bar graphs. An increase in S-phase and sub G1 fractions indicative of replicative stress and cumulative apoptosis respectively were observed over time. Data shown are representative of three independent experiments. *P< 0.0001; single treatment compared with combination treatment for cell cycle phases as indicated.
B02 synergizes with AZD1775 and radiation in vivo in an orthotopic mouse model of oral tongue cancer.
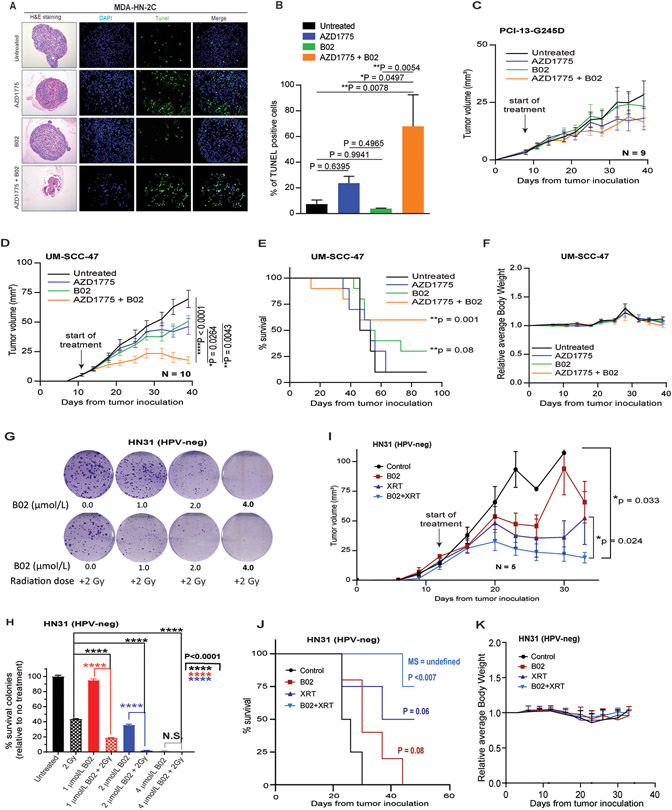
Recently, we have shown that the 3D cell culture systems are reliable models for monitoring cell growth and drug testing. This is due to their evident advantages in providing more physiologically relevant phenotypes similar to that in vivo (29,41). Therefore, HPV-positive HNSCC (MDA-HN-2C) cells established in our Laboratory (29) were grown in 3D collagen culture and treated with physiologically relevant doses of B02 and AZD1775 as indicated previously. The H&E staining of the 3D organoids is shown in Figure 4A. The cell death was monitored in the 3D culture organoids at day 5 following treatment with the drugs using the in vivo TUNEL assay. The TUNEL-positive staining was significantly increased in the 3D culture organoids treated with B02 and AZD1775, indicative of apoptosis (Fig. 4A-B), and consistent with 2D in vitro data.
Figure 4. B02 synergizes with AZD1775 and radiation in vivo in an orthotopic mouse model of oral tongue cancer.
A, hematoxylin and eosin (H&E) and TUNEL-positive staining determined at day 5 in the 3D culture organoids made from HPV-positive HNSCC (MDA-HN-2C) cells following treatment with equivalent physiological dose of B02 and AZD1775 for 5 days as described in Methods. B, quantification of the positive TUNEL staining shown in A. C and D, tumor growth curves in orthotopic mouse model oral tongues bearing HNSCC (PCI13-G245D and UM-SCC-47) cells following treatment with B02 (50 mg/kg), AZD1775 (35 mg/kg) either alone or in combination as described in methods. E and F, Kaplan-Meier analysis for survival and relative body weight loss during the treatment in UM-SCC-47 tumor bearing mice. Each treatment group contains 9-10 mice. G, representative clonogenic survival images of HPV-negative HNSCC (HN31) cells pre-treated with B02 two hours before radiation (XRT) as indicated. H, percentage of surviving colonies normalized to untreated control. Combinations of B02 plus XRT caused more tumor cell death than XRT or B02 alone. I, tumor growth curves in orthotopic mouse model oral tongues bearing HN31cells following treatment with B02, fractionated radiation dose (5 Gy), and in combination as described in Materials and Methods. J and K, Kaplan-Meier analysis for survival and relative body weight loss during the treatment with B02 and radiation in HN31 tumor bearing mice respectively. Each treatment group contains 5-6 mice. All in vivo data were expressed as ± standard error mean (±SEM) and P values < 0.05 were considered significant.
On the basis of results obtained from the 2D in vitro and the 3D organoids, the effect of B02, AZD1775 either alone or in combination was assessed in an orthotopic nude mouse model injected with HPV-negative mutant TP53 (PCI-13-G245D) and HPV-positive (UM-SCC-47) HNSCC cell lines in the tongue. Surprisingly, B02 (50 mg/kg, I.P., 3 times a week) or AZD1775 (35 mg/kg, P.O., 5 times a week) alone or in combination had little effects on tumor growth in mice bearing tumors with PCI-13-G245D cells (Fig. 4C, and Supplementary S6). These results suggest that the synergism seen with the combination in vitro in the HPV-negative HNSCC cells does not extrapolate to in vivo. The B02 or AZD1775 alone resulted in modest effects on tumor growth (p <0.101; p<0.059 respectively) compared to untreated controls (Fig. 4D, and Supplementary S6). Combination of these drugs significantly inhibited oral tumor growth, when compared with AZD1775 alone (P = 0.0264), B02 alone (P = 0.0043), or vehicle-treated group (P < 0.0001) in mice bearing UM-SCC-47 cells (Fig. 4D, and Supplementary S6). Furthermore, combined treatment with B02 and AZD1775 improved animal survival compared with animals in the untreated control and single treatment groups (Fig. 4E). None of the mice in the single or combination treatment arms showed more than 10% body weight loss or any signs of drug toxicity (Fig. 4F), suggesting that the combination of drugs is well tolerated during the study. Initial experiments to demonstrate B02 could sensitize HPV-positive HNSCC cell lines to radiation (XRT) were not interpretable because these cells were inherently too sensitive to 2 Gy radiation in vitro (data not shown). However, the radiosensitizing potential of B02 was readily apparent in vitro in the HPV-negative HN31 cells (Fig. 4G-H). Significant cell killing was achieved with 2 Gy XRT combined with B02 (1 or 2μmo/L) compared to 2 Gy XRT or either concentration of B02 alone (P<0.0001 for all comparisons). In the orthotopic tongue tumor model with HPV-negative HN31, XRT alone caused tumor growth delay (Fig. 4I) and modest increase in mouse survival (Fig. 4J). However, combination of B02 and XRT led to tumor growth inhibition (P = 0.024) and significant increase in survival time (P= 0.007) (Fig. 4I-J) compared to untreated mice. Furthermore, addition of B02 to radiation was well tolerated with no obvious weight loss observed in all mice tested (Fig. 4K).
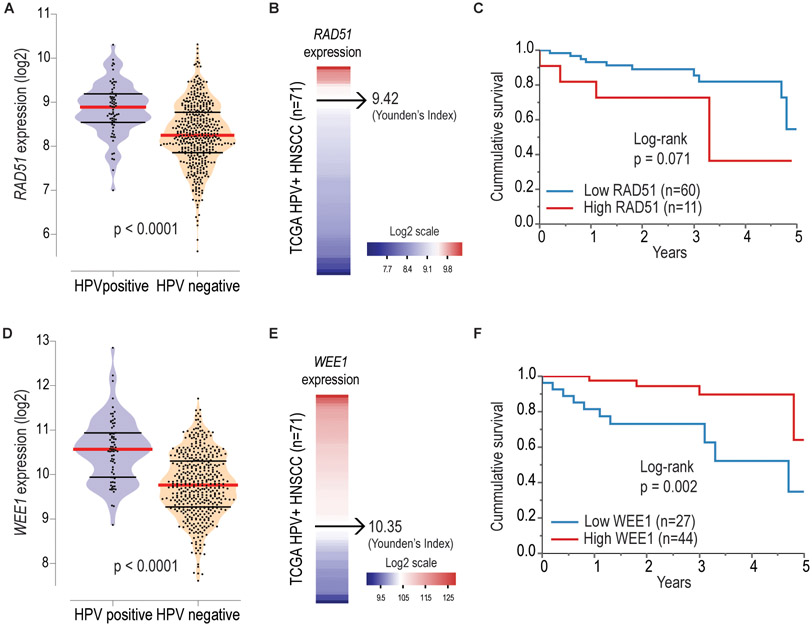
Association of higher RAD51 expression with poor overall survival in patients with HPV-positive HNSCC.
Recent report has shown that KRAS mutant lung cancer cells are highly dependent on RAD51 for survival and depletion of RAD51 resulted in enhanced DNA double strand breaks, defective colony formation, and cell death (42). Therefore, it is possible that HNSCC tumor cells can upregulate RAD51 expression to facilitate DNA damage repair and increase their survival. This cancer dependency could confer cell lethality when the Rad51 gene is severely inhibited. Therefore, we analyzed the Rad51 mRNA expression levels in the HNSCC TCGA patient cohort. The HPV-positive HNSCC patients (n=71) showed higher levels of RAD51 (8.84 ± 0.60 - mean/SD; p< 0.0001) than the HPV-negative HNSCC patients (n=449) (8.27 ± 0.71 - mean/SD) (Fig. 5A-B). Moreover, HPV-positive HNSCC patients with higher expression of RAD51 showed a tendency of lower survival (Figure 5C, p =0.071) (Fig. 5C). Similarly, HPV-positive samples showed increased levels of WEE1 (10.5±0.71- mean/SD; p< 0.0001) compared to HPV-negative samples (9.77±0.71) (Fig. 5D-E). Interestingly, patients with higher expression levels of WEE1 showed better survival compared to patients with lower levels of WEE1 (Figure 5F, p =0.002). Taken together, these data suggest that Rad51 represents a potential drug target in head and neck patients with HPV infection. In most cases, head and neck cancer patients from TCGA are not treated the same. This may influence the survival outcomes, and therefore uniformly treated cohorts are required to confirm these results.
Figure 5. Higher RAD51 expression is associated with poor overall survival in patients with HPV-positive HNSCC.
A, Violin plot represents RAD51 log2 transformed expression among 520 HNSCC according to HPV status. B, RAD51 gene expression range among HPV-positive HNSCC samples evaluated. C, Kaplan-Meier plot shows a trend towards worse survival rates among HPV-positive HNSCC with higher Rad51 expression. D, Violin plot represents WEE1 log2 transformed expression among 520 HNSCC according to HPV status. E, WEE1 gene expression range among HPV-positive HNSCC samples evaluated. F, Kaplan-Meier plot shows worse survival among HPV-positive HNSCC with lower WEE1 expression.
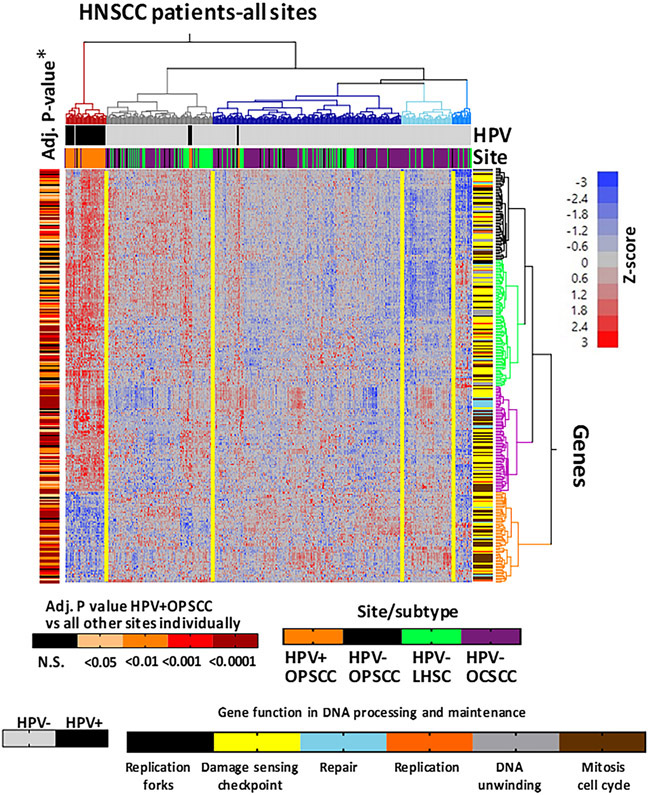
HPV-positive head and neck tumors differentially express hundreds of genes involved in DNA repair, processing and maintenance.
It is well established that HPV-positive tumors are dependent on the E6 and E7 oncogenes for survival, and these oncogenes drive expression of many DDR genes. Expression of such genes can potentially make these genomic subtypes of cancers vulnerable to drugs targeting replication stress and DNA damage repair. Therefore, we examined differential expression of DDR genes by performing unsupervised hierarchical clustering of HNSCC TCGA samples based on RNA expression of genes obtained from GO searches. Clustering of 279 DDR genes was presented in the heat map (Fig. 6) and in Supplementary Table S1. The analysis conclusively showed that more than 90% of HPV-positive tumors grouped together in their own cluster and overexpressed the same genes, indicating remarkable homogeneity. Similar results were obtained in a panel of 73 HNSCC cell lines and 10 cervical squamous cell carcinoma lines with known HPV status (Supplementary Fig. S7). The majority of HPV-positive cell lines clustered together, regardless of cancer type. Two HPV-positive cell lines that did not, contained a virus strain different from HPV-16 or HPV-18, are shown. Our analysis demonstrated an identifiable set of DNA replication and DDR genes upregulated in HPV-positive patient tumors
Figure 6. HPV-positive head and neck tumors differentially express hundreds of genes involved in DNA repair, processing and maintenance.
HNSCC TCGA samples were subjected to unsupervised hierarchical based on RNA expression of 279 DDR genes obtained from Gene Ontology searches. HPV-positive indicated by black boxes on top, with subsites beneath (orange = OPC; green = larynx; purple = oral cavity). Genes clustered vertically are annotated with colors specific for GO classifications (right) and Adjusted P value for differential expression for HPV-positive OPC is annotated vertically (left).
Discussion
It is well established that an intact DDR coupled with efficient homologous recombination is essential for detecting and repairing DNA breaks and maintaining genome stability in normal cells (43). Furthermore, if the DNA damage is extensive and cannot be repaired, mitotic slippage can also result in mitotic catastrophe and/or induction of apoptosis (43). Importantly, induction of replication stress can be exploited for tumor cell killing and therefore has been described as an Achilles’ heel of cancer (44). In recent years targeting Rad51, a protein involved in homologous recombination repair has become an area of intense investigation. In the present study, we demonstrated that HPV-positive HNSCC cell lines were much more sensitive to B02 treatment compared to HPV-negative ones in vitro, suggesting that susceptibility to RAD51 inhibition is linked to the biology of HPV oncogenic drivers E6 and/or E7. Park and colleagues have demonstrated that E7 expression increases the levels of Rad51 and delays radiation-induced DNA damage repair both in vitro and in vivo in HPV-positive HNSCC cells (45). Based on this study and our data, we envision that the HPV-positive tumors become more dependent on a regulatory feedback loop involving E7/Rb/E2F1/cyclin D1/Rad51 genes axis to tolerate the DNA damge and replication stress and continue to survive. Consequently, inhibition of Rad5 can disrupt this feedback loop dependency in these tumors. Our data provide evidence that Rad51 inhibition causes loss of oncogenic signaling through reduction in the E7 expression in HPV-positive tumor cells. However, it is not clear how this inhibitory effect occurs. Therefore, future experiments are required to examine if Rad51 inhibition directly blocks E6/E7 mRNA transcription and/or protein translation in these cells. Furthermore, in this study we present evidence that direct genetic ablation of E7 is sufficient and/or required for B02-mediated anti-tumor effects in HPV-positive cells. Expression of E6 and E7 oncogenes can induce chronic oncogene-induced replication stress and dysregulate DDR which make them more susceptible to drugs that target replication stress and homologous recombination repair. This possibility is supported by our data presented in Figure 6 and recent reports which demonstrate that expression of E6 and E7 is sufficient to induce reactive oxygen species (ROS) generation and induce DNA damage in cervical and head and neck cancer cells (46,47).
Mechanistically, we further provide evidence that B02 and AZD1775 interact synergistically to induce DNA damage, replication stress, and impaired Rad51-mediated HR through activation of CDK1 and decreased Chk1 phosphorylation, resulting in aberrant mitosis associated with apoptotic cell death. It is unclear how B02 inhibits Chk1 phosphorylation. Recent study has demonstrated that depletion of RAD51 with siRNA decreases Chk1 protein level in esophageal cancer cells, suggesting that RAD51 can act as an upstream regulator and positively controls the expression of Chk1 (48). Taken together, these data suggest that inhibition of Chk1 may be directly linked to Rad51 inhibition and it is unlikely due to off-target effects of the drug. It is possible that B02 inhibits Chk1 phosphorylation through direct inhibition of its upstream activator, the ATR kinase. Future experiment is required to confirm this possibility in HNSCC cells. Additionally, both B02 and AZD1775 led to decreased RRM2 in a time dependent manner, suggesting exhaustion of available deoxyribonucleoside triphosphate (dNTP) pools for replication. The drug combination resulted in greatest RRM2 inhibition. During DNA replication, if the amount of nucleotides or components of the replication machinery are not in adequate supply or properly assembled to complete replication, the cells become “stressed” and this is manifested by stalling of replication forks, creating synthetic lethality with DNA damage agents (49). Furthermore, Wee1 inhibition with AZD1775 has been shown to confer synthetic lethality in H3K36me3-deficient cancer cells by instigating dNTP starvation and replication stress (50). Consistent with previous report, a marked increase in the p21 protein levels was also observed in all cell lines tested with B02 treatment, suggesting growth arrest through regulation of the G1-S cell cycle transition (39). This mechanistic interaction between RAD51 and p21 will be further investigated in future experiments. In our study, it is not clear how B02 treatment induces p21 in TP53 mutant HNSCC cells. Preliminary data showed that B02 significantly increased the p21 mRNA levels in HPV-positive (UM-SCC-47) cells (unpublished observation). Future experiment is needed to test whether B02 induces p21 at the transcriptional level in the TP53 mutant cells.
In the HPV+HNSCC cells, the combination treatment caused a slight increase in number of cells entering the S-and G2/M phases and proportion of cells arrested in the sub-G1 phase indicating cell death. We do not know exactly in which cell cycle phase this death occurs. We think that the cells are going through replication stress and perhaps dying through apoptosis within the S-phase. Consistent with this, we previously demonstrated with live cell imaging that death through apoptosis could take place within S-phase during cell cycle in HNSCC following treatment with drugs that elicit replication stress (38). Future experiments will be required to explore this possibility. Interestingly, unlike the HPV-negative HNSCC, combination of B02 and AZD1775 causes significant tumor growth inhibition in vivo in HPV-positive HNSCC tumor bearing mice and significantly improved median survival. This may be related to HPV-positive tumors having more in vivo endogenous replication stress (ERS) owing to transformation by E6 and E7 oncogenes. In this case, if the tumors have more ERS, then two things happen. First, these tumors become more dependent on protein or genes that protect them from replication stress such as Rad51. Second, these tumors will be more sensitive to drugs that cause even more ERS such as the Wee1 inhibitor. Since, all the HNSCC cell lines tested have demonstrated comparable levels of Rad51 and Wee1 expression, the enhanced sensitivity to B02 monotherapy or in combination with AZD1775 can not be entirely explained by the expression of these proteins. Our data suggest that the biomarker for the drug combination response in HPV-positive cells is likely the high expression level of the DNA damage and replication stress gene signature, and Rad51 is one gene that is present in that signature. Because our data show that B02 can radiosensitize the HPV-negative head and neck tumors both in vitro and in vivo, we plan to compare current standard of care cisplatin plus radiation to B02 plus radiation particularly in the HPV-tumors with high levels of Rad51 expression. It will also be important to determine if integration of RAD51 inhibition into chemo-radiation algorithms can allow us to substitute a less toxic and potentially equally effective agent for HPV-positive HNSCC.
In summary, we provide evidence that HPV-positive HNSCC tumor cells are very sensitive to B02 as single agent and in combination with AZD1775 in vitro and in vivo. Our data implicate that the HPV-positive tumors may have adapted to become more dependent on their DDR genes for survival and to avoid replication stress. Taken together, our findings argue that a strategy aimed at simultaneous targeting of Rad51 and Wee1 function may represent an effective therapeutic strategy for killing high-risk head and neck cancer.
Supplementary Material
Financial Support:
This work was supported by the University of Texas MD Anderson Cancer Center-Oropharynx Cancer Program generously supported by Mr. and Mrs. Charles W. Stiefel awarded to A.A. Osman and J.N. Myers. This work used the services of MD Anderson’s Flow Cytometry and Cellular Imaging Core, and Bioinformatics Shared Resource which are supported by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (P30CA016672).
Abbreviations:
- HNSCC
head and neck squamous cell carcinoma
- HR
homologous recombination
- ERS
endogenous replication stress
- EA
Evolutionary Action
- STR
Short tandem repeat
- TCGA
The Cancer Genome Atlas
- HPV
human papillomavirus
- DDR
DNA damage repair
- CI
combination index
- RRM2
ribonucleotide reductase
- Rb
retinoblastoma protein, XRT, radiation, 3D, three-dimensional
- dNTP
deoxyribonucleoside triphosphate
- ED
effective drug dose
- GO
Gene Ontology
Footnotes
Conflicts of Interest Disclosure: Authors have no potential conflicts of interest to declare.
References
- 1.Adelstein DJ, Li Y, Adams GL, Wagner H Jr., Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21(1):92–98. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004; 350(19):1945–52. [DOI] [PubMed] [Google Scholar]
- 3.Aarts M, Sharpe R, Garcia-Murillas I, Gevensleben H, Hurd MS, Shumway SD, et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov 2012; 2(6):524–539. [DOI] [PubMed] [Google Scholar]
- 4.Beck H, Nahse V, Larsen MS, Groth P, Clancy T, Lees M, et al. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J Cell Biol 2010; 188(5):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck H, Nahse-Kumpf V, Larsen MS, O'Hanlon KA, Patzke S, Holmberg C, et al. Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol Cell Biol 2012; 32(20):4226–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osman AA, Monroe MM, Ortega Alves MV, Patel AA, Katsonis P, Fitzgerald AL, et al. Wee-1 kinase inhibition overcomes cisplatin resistance associated with high-risk TP53 mutations in head and neck cancer through mitotic arrest followed by senescence. Mol Cancer Ther 2015; 14(2):608–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portugal J, Mansilla S, Bataller M. Mechanisms of drug-induced mitotic catastrophe in cancer cells. Curr Pharm Des 2010; 16(1):69–78. [DOI] [PubMed] [Google Scholar]
- 8.Squire CJ, Dickson JM, Ivanovic I, Baker EN. Structure and inhibition of the human cell cycle checkpoint kinase, Wee1A kinase: an atypical tyrosine kinase with a key role in CDK1 regulation. Structure 2005; 13(4):541–550. [DOI] [PubMed] [Google Scholar]
- 9.Hirai H, Iwasawa Y, Okada M, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009; 8:2992–3009. [DOI] [PubMed] [Google Scholar]
- 10.Do K, Wilsker D, Ji J, Zlott J, Freshwater T, Kinders RJ, et al. Phase I Study of Single-Agent AZD1775 (MK-1775), a Wee1 Kinase Inhibitor, in Patients With Refractory Solid Tumors. J Clin Oncol 2015; 33(30):3409–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leijen S, van Geel RM, Sonke GS, de Jong D, Rosenberg EH, Marchetti S, et al. Phase II Study of WEE1 Inhibitor AZD1775 Plus Carboplatin in Patients With TP53-Mutated Ovarian Cancer Refractory or Resistant to First-Line Therapy Within 3 Months. J Clin Oncol 2016; 34(36):4354–4361. [DOI] [PubMed] [Google Scholar]
- 12.Mendez E, Rodriguez CP, Kao MC, Raju S, Diab A, Harbison RA, et al. A Phase I Clinical Trial of AZD1775 in Combination with Neoadjuvant Weekly Docetaxel and Cisplatin before Definitive Therapy in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res 2018; 24(12):2740–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moser R, Xu C, Kao M, Annis J, Lerma LA, Schaupp CM, et al. Functional kinomics identifies candidate therapeutic targets in head and neck cancer. Clin Cancer Res 2014; 20(16):4274–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leijen S, Beijnen JH, Schellens JH. Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents. Curr Clin Pharmacol 2010; 5(3):186–191. [DOI] [PubMed] [Google Scholar]
- 15.Powell SN, DeFrank JS, Connell P, Eogan M, Preffer F, Dombkowski D, et al. Differential sensitivity of p53(−) and p53(+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res 1995; 55(8):1643–1648. [PubMed] [Google Scholar]
- 16.Suganuma M, Kawabe T, Hori H, Funabiki T, Okamoto T. Sensitization of cancer cells to DNA damage-induced cell death by specific cell cycle G2 checkpoint abrogation. Cancer Res 1999; 59(23):5887–5891. [PubMed] [Google Scholar]
- 17.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O'Connor PM. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst 1996; 88(14):956–965. [DOI] [PubMed] [Google Scholar]
- 18.Yao SL, Akhtar AJ, McKenna KA, Bedi GC, Sidransky D, Mabry M, et al. Selective radiosensitization of p53-deficient cells by caffeine-mediated activation of p34cdc2 kinase. Nat Med 1996;2(10):1140–1143. [DOI] [PubMed] [Google Scholar]
- 19.Connell PP, Jayathilaka K, Haraf DJ, Weichselbaum RR, Vokes EE, Lingen MW. Pilot study examining tumor expression of RAD51 and clinical outcomes in human head cancers. Int J Oncol 2006; 28(5):1113–1119. [PubMed] [Google Scholar]
- 20.Klein HL. The consequences of Rad51 overexpression for normal and tumor cells. DNA Repair (Amst) 2008;7(5):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raderschall E, Stout K, Freier S, Suckow V, Schweiger S, Haaf T. Elevated levels of Rad51 recombination protein in tumor cells. Cancer Res 2002; 62(1):219–225. [PubMed] [Google Scholar]
- 22.Somyajit K, Saxena S, Babu S, Mishra A, Nagaraju G. Mammalian RAD51 paralogs protect nascent DNA at stalled forks and mediate replication restart. Nucleic Acids Res 2015; 43(20):9835–9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka N, Patel AA, Wang J, Frederick MJ, Kalu NN, Zhao M, et al. Wee-1 Kinase Inhibition Sensitizes High-Risk HPV+ HNSCC to Apoptosis Accompanied by Downregulation of MCl-1 and XIAP Antiapoptotic Proteins. Clin Cancer Res 2015; 21(21):4831–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang F, Mazin AV. A small molecule inhibitor of human RAD51 potentiates breast cancer cell killing by therapeutic agents in mouse xenografts. PLoS One 2014; 9(6):e100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang F, Mazina OM, Zentner IJ, Cocklin S, Mazin AV. Inhibition of homologous recombination in human cells by targeting RAD51 recombinase. J Med Chem 2012; 55(7):3011–3020. [DOI] [PubMed] [Google Scholar]
- 26.Osman AA, Neskey DM, Katsonis P, Patel AA, Ward AM, Hsu TK, et al. Evolutionary Action Score of TP53 Coding Variants Is Predictive of Platinum Response in Head and Neck Cancer Patients. Cancer Res 2015; 75(7):1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58(3):621–81. [DOI] [PubMed] [Google Scholar]
- 28.Fang Y, McGrail DJ, Sun C, Labrie M, Chen X, Zhang D, et al. Sequential Therapy with PARP and WEE1 Inhibitors Minimizes Toxicity while Maintaining Efficacy. Cancer Cell 2019; 35(6):851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka N, Osman AA, Takahashi Y, Lindemann A, Patel AA, Zhao M, et al. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral Oncol 2018; 87:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindemann A, Patel AA, Silver NL, Tang L, Liu Z, Wang L, et al. COTI-2, A Novel Thiosemicarbazone Derivative, Exhibits Antitumor Activity in HNSCC through p53-dependent and -independent Mechanisms. Clin Cancer Res 2019; 25(18):5650–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Center BITGDA. Analysis-ready standardized TCGA data from Broad GDAC Firehose 2016_01_28 run. Broad Institute of MIT and Harvard. Dataset. < 10.7908/C11G0KM9>. [DOI] [Google Scholar]
- 32.Chen Y, Yao H, Thompson EJ, Tannir NM, Weinstein JN, Su X. VirusSeq: software to identify viruses and their integration sites using next-generation sequencing of human cancer tissue. Bioinformatics 2013; 29(2):266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelber-Netto FO, Rao X, Guo T, Xi Y, Gao M, Shen L, et al. Variations in HPV function are associated with survival in squamous cell carcinoma. JCI Insight 2019; 4(1): e124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalu NN, Mazumdar T, Peng S, Shen L, Sambandam V, Rao X, et al. Genomic characterization of human papillomavirus-positive and -negative human squamous cell cancer cell lines. Oncotarget 2017; 8(49):86369–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res 2009; 15:6758–62. [DOI] [PubMed] [Google Scholar]
- 36.Rampias T, Sasaki C, Weinberger P, Psyrri A. 2009. E6 and E7 Gene Silencing and Transformed Phenotype of Human Papillomavirus 16–Positive Oropharyngeal Cancer Cells. J. Natl. Cancer Inst 2009; 101:412–423. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka N, Patel AA, Tang L, Silver NL, Lindemann A, Takahashi H, et al. Replication Stress Leading to Apoptosis within the S-phase Contributes to Synergism between Vorinostat and AZD1775 in HNSCC Harboring High-Risk TP53 Mutation. Clin Cancer Res 2017; 23(21):6541–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimple RJ, Smith MA, Blitzer GC , Torres AD, Martin JA, Yang RZ, Peet CR, Lorenz LD, Nickel KP, Klingelhutz AJ, Lambert PF, Harari PM. Enhanced Radiation Sensitivity in HPV-Positive Head and Neck Cancer. Cancer Res 2013; 73(15):4791–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Q, Cai D, Li M, Wu X. The homologous recombination protein RAD51 is a promising therapeutic target for cervical carcinoma. Oncol Rep 2017; 38(2):767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsels LA, Morgan MA, Tanska DM, Parsels JD, Palmer BD, Booth RJ, et al. Gemcitabine sensitization by checkpoint kinase 1 inhibition correlates with inhibition of a Rad51 DNA damage response in pancreatic cancer cells. Mol Cancer Ther 2009; 8: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka N, Zhao M, Tang L, Patel AA, Xi Q, Van HT, et al. Gain-of-function mutant p53 promotes the oncogenic potential of head and neck squamous cell carcinoma cells by targeting the transcription factors FOXO3a and FOXM1. Oncogene 2018; 37(10):1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J, Zhang Z, Zhao L, Li L, Zuo W, Han L. High expression of RAD51 promotes DNA damage repair and survival in KRAS-mutant lung cancer cells. BMB Rep 2019; 52(2):151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward A, Khanna KK, Wiegmans AP. Targeting homologous recombination, new preclinical and clinical therapeutic combinations inhibiting RAD51. Cancer Treat Rev 2015; 41(1):35–45. [DOI] [PubMed] [Google Scholar]
- 44.Kotsantis P, Petermann E, Boulton SJ. Mechanisms of Oncogene-Induced Replication Stress: Jigsaw Falling into Place. Cancer Discov 2018; 8(5):537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JW, Nickel KP, Torres AD, Lee D, Lambert PF, Kimple RJ. Human papillomavirus type 16 E7 oncoprotein causes a delay in repair of DNA damage. Radiother Oncol 2014; 113(3):337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marullo R, Werner E, Zhang H, Chen GZ, Shin DM, Doetsch PW. HPV16 E6 and E7 proteins induce a chronic oxidative stress response via NOX2 that causes genomic instability and increased susceptibility to DNA damage in head and neck cancer cells. Carcinogenesis 2015; 36(11):1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang N, Zhan T, Ke T, Huang X, Ke D, Wang Q, et al. Increased expression of RRM2 by human papillomavirus E7 oncoprotein promotes angiogenesis in cervical cancer. Br J Cancer 2014; 110(4):1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu X, Pan Q, Huang N, Wu J, Zhen N, Sun F, Li Z, Yang Q . RAD51 regulates CHK1 stability via autophagy to promote cell growth in esophageal squamous carcinoma cells. Tumor Biol 2016; 37:16151–16161. [DOI] [PubMed] [Google Scholar]
- 49.Dobbelstein M, Sorensen CS. Exploiting replicative stress to treat cancer. Nat Rev Drug Discov 2015; 14(6):405–23. [DOI] [PubMed] [Google Scholar]
- 50.Pfister SX, Markkanen E, Jiang Y, Sarkar S, Woodcock M, Orlando G, et al. Inhibiting WEE1 Selectively Kills Histone H3K36me3-Deficient Cancers by dNTP Starvation. Cancer Cell 2015; 28(5):557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.