Abstract
An updated description of the genus Atheniella, combining macro- and micromorphological characters that elaborate on the original generic characterisation, is presented. Atheniella is characterised by a brightly coloured pileus, all tissues inamyloid and pileipellis covered with simple to branched excrescences. Previously, nine Atheniella species were known globally, of which three species were accepted in China. Three newly-recognised species classified in the genus are here formally described from Yunnan Province: Atheniella flavidasp. nov., A. rutilasp. nov. and A. taoyaosp. nov. The new species are characterised by a yellow, orange, pink or red pileus, fusiform cheilocystidia and pleurocystidia, non-smooth pileipellis, stipitipellis smooth or with cylindrical ornamentation, caulocystidia fusiform or subglobose, if present and all tissues inamyloid. Morphological descriptions, photographs, line drawings and comparisons with closely-related taxa are presented for the new species. A phylogenetic analysis of sequence data for the rDNA internal transcribed spacer region and nuclear large ribosomal subunit (ITS + nLSU) supported that Atheniella is resolved as monophyletic and also supported the taxonomic recognition of the new species. A key to the 12 species of Atheniella is also provided.
Keywords: new taxon, polygenes, taxonomy, white basidiospores
Introduction
The genus Atheniella Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry is a small mycenoid genus, formerly treated as Mycena (Pers.) Roussel sect. Adonideae (Fr.) Quél., that was elevated to genus rank by Redhead et al. (2012). Atheniella is characterised macroscopically by its habit resembling that of Mycena owing to the small basidiomata, white lamellae, hollow stipe and is saprophytic on rotten wood or plant debris (Kühner 1938; Smith 1947; Redhead et al. 2012; Aronsen and Læssøe 2016). Redhead et al. (2012) noted that the brightly coloured pileus (e.g. yellow, orange, pink or red) and all tissues unreactive in Melzer’s Reagent are diagnostic characters that distinguish Atheniella from Mycena. Given the change in taxonomic rank, formalisation of new combinations in Atheniella was required for species formerly classified in Mycena sect. Adonideae (Grgurinovic 2003; Redhead et al. 2012; Aravindakshan and Manimohan 2015; Gminder and Böhning 2016; Lehmann and Lüderitz 2018, 2019). In previous publications, nine taxa were recognised in Atheniella, comprising three new species and six new combinations, of which Atheniella adonis (Bull.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry is the type species (Grgurinovic 2003; Redhead et al. 2012; Aravindakshan and Manimohan 2015; Gminder and Böhning 2016; Lehmann and Lüderitz 2018, 2019). An infrageneric classification for Atheniella has not been proposed since the genus was established (Redhead et al. 2012).
Previous taxonomic studies of Atheniella are incomplete because of insufficient species representation and a lack of phylogenetic evidence and only four taxa of Atheniella have been included in phylogenetic studies (Moncalvo et al. 2002; Matheney et al. 2006). Based on a phylogenetic reconstruction for more than 800 euagaric taxa derived from a nuclear ribosomal large subunit RNA gene (nLSU) sequence dataset, Moncalvo et al. (2002) established the Mycenaceae (Clade 47) to include 10 genera, including Mycena. However, Mycena aurantiidisca (Murrill) Murrill (≡ Atheniella aurantiidisca (Murrill) Redhead, Moncalvo, Vilgalys, Desjardin & B.A.Perry) and Mycena adonis (Bull.) Gray (≡ Atheniella adonis) were separated from the Mycenaceae to form an independent lineage termed the “adonis” clade (Clade 26). Matheney et al. (2006) agreed with Moncalvo et al. (2002) in the establishment of the Mycenaceae and that the “adonis” group should be excluded from Mycena, but differed in the phylogenetic placement of Atheniella spp. Mycena amabillissima (Peck) Sacc. (≡ Atheniella amabillissima (Peck) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry) and M. aurantiidisca (≡ Atheniella aurantiidisca) was placed in the “hydropoid” subclade of the Marasmioid clade (IV) (Matheney et al. 2006).
Species of Atheniella are widespread in temperate regions, but also distributed in the tropical zone (Smith 1935a, b, c, 1937, 1939; Maas Geesteranus 1980, 1990, 1992a, b; Redhead 1984; Perry 2002; Grgurinovic 2003; Robich 2003; Uehling et al. 2012; Aravindakshan and Manimohan 2015; Osono 2015; Aronsen and Læssøe 2016). Previous studies of Atheniella Kühner ex Singer during the last century focused on species distributed in Europe and North America (Murrill 1916; Tyler 1991; Emmett 1992; Gyosheva and Ganeva 2004; Friedrich 2006; Miersch and Karasch 2011; Miersch 2013; Pérez-De-Gregorio 2015; Norvell 2016; Aime et al. 2018). In contrast, few investigations of Atheniella taxa in Australia and Asia have been conducted. However, progress in clarifying the relationship between Mycena and Atheniella has been achieved in recent years (Miyamoto et al. 1998, 2000; Grgurinovic 2003; Aravindakshan and Manimohan 2015; Na 2019; Na and Bau 2018, 2019a, b).
Three Atheniella species, namely A. adonis, A. aurantiidisca and A. flavoalba (Fr.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry, were previously recognised in China (Bau and Liu 2011; Li et al. 2015; Na 2019). During our ongoing research on Mycena s.l., three new mycenoid species belonging to Atheniella were discovered in Yunnan Province, southwest China and are formally described here as A. flavida Q. Na & Y.P. Ge, A. rutila Q. Na & Y.P. Ge and A. taoyao Q. Na & Y.P. Ge. In addition, the generic morphological description of Atheniella is updated and a key for identification of the 12 species of Atheniella currently known is provided.
Materials and methods
Morphological examination
Macroscopic descriptions were prepared, based on freshly-collected specimens, whereas micromorphological descriptions relied on dried material. In the descriptions, colour abbreviations follow Kornerup and Wanscher (1978). Microscopic observations were conducted on dried specimens mounted in 5% potassium hydroxide (KOH) and stained with Congo red when necessary. Melzer’s Reagent was used to test whether spores and tissues were amyloid (Horak 2005). Twenty mature basidiospores from each basidiocarp were measured in lateral view and one or two basidiocarps were examined per specimen. The basidiospore dimensions were recorded; the notation [a/b/c] used at the beginning of each basidiospore description indicates that a basidiospores from b basidiocarps of c specimens were measured. Measured dimensions (length × width) are presented as (d) e–f–g (h) × (i) j–k–l (m), where d is the minimum length, e–g represents the range of at least 90% of values, f is the average length and h is the maximum length; width (i–m) is expressed in the same manner. In addition, Q is the length: width ratio of a spore and Q ± SD is the average Q of all basidiospores ± the sample standard deviation. Authority abbreviations follow those used in Index Fungorum (https://www.indexfungorum.org). Voucher specimens have been deposited in the Fungarium of the Fujian Academy of Agricultural Sciences (FFAAS), China.
DNA extraction, PCR amplification and DNA sequencing
Genomic DNA was extracted from tiny pieces of lamellae using the NuClean Plant Genomic DNA Kit (Kangwei Century Biotechnology Co., Beijing, China). The internal transcribed spacer (ITS) region and the nuclear large subunit (nLSU) of rDNA were amplified with the primer pairs ITS1/ITS4 and LROR/LR7, respectively (White et al. 1990; Hopple and Vilgalys 1999). The PCR thermocycling protocol (for both ITS and nLSU) was 94 °C for 4 min, followed by 34 cycles of 94 °C for 45 sec, 52 °C for 45 sec and 72 °C for 1 min and final extension for 10 min at 72 °C. The new sequences were submitted to GenBank (Table 1). The nBLAST tools (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were used to compare the sequence identity with sequences in the NCBI databases. The GenBank accession numbers for the ITS and nLSU sequences are as follows: Atheniella flavida (MW969653–MW969654; MW969665), A. rutila (MW969658–MW969659; MW969668) and A. taoyao (MW969655–MW969657; MW969666–MW969667).
Table 1.
Sequenced specimens used in phylogenetic analysis. New species are marked in bold.
| No. | Taxa | Voucher | Locality | ITS Sequences ID | nLSU Sequences ID | Reference |
|---|---|---|---|---|---|---|
| 1 | Atheniella adonis (Bull.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | H6036863 | FINLAND | MW540691 | – | Unpublished |
| 2 | A. adonis (Bull.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | 1058 | CANADA | KJ705189 | – | Unpublished |
| 3 | A. adonis (Bull.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | DAOM174885 | – | – | AF261361 | Moncalvo et al. (2002) |
| 4 | A. amabillissima (Peck) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | AFTOL–ID 1686 | USA | DQ490644 | DQ457691 | Matheney et al. (2006) |
| 5 | A. amabillissima (Peck) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | TUR183733 | FINLAND | MW540719 | – | Unpublished |
| 6 | A. amabillissima (Peck) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | BD–2020a | FINLAND | MW540733 | – | Unpublished |
| 7 | A. aurantiidisca (Murrill) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | UBC: F15202 | CANADA | DQ384585 | – | Unpublished |
| 8 | A. aurantiidisca (Murrill) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | AFTOL–ID 1685 | USA | DQ490646 | DQ470811 | Matheney et al. (2006) |
| 9 | A. aurantiidisca (Murrill) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | UBC: F33062 | CANADA | MF908459 | – | Unpublished |
| 10 | A. aurantiidisca (Murrill) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | HMJAU 43811 | CHINA | MT497546 | – | Unpublished |
| 11 | A. aurantiidisca (Murrill) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | MF06837 | USA | MT636967 | – | Unpublished |
| 12 | A. aurantiidisca (Murrill) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | DAOM216791 | – | – | AF261360 | Moncalvo et al. (2002) |
| 13 | A. flavoalba (Fr.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | 604 | ITALY | JF908464 | – | Osmundson et al. (2013) |
| 14 | A. flavoalba (Fr.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | CBS 359.50 | FRANCE | MH856659 | MH868175 | Vu et al. (2019) |
| 15 | A. flavoalba (Fr.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | CBS 258.53 | FRANCE | MH857185 | MH868723 | Vu et al. (2019) |
| 16 | A. flavoalba (Fr.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | H6032608 | FINLAND | MW540661 | – | Unpublished |
| 17 | A. flavoalba (Fr.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry | H6036822 | FINLAND | MW540676 | – | Unpublished |
| 18 | A. flavida Q. Na & Y.P. Ge | FFAAS0350 | CHINA, Type | MW969653 | MW969665 | This study |
| 19 | A. flavida Q. Na & Y.P. Ge | FFAAS0355 | CHINA | MW969654 | – | This study |
| 20 | A. rutila Q. Na & Y.P. Ge | FFAAS0354 | CHINA, Type | MW969658 | MW969668 | This study |
| 21 | A. rutila Q. Na & Y.P. Ge | FFAAS0356 | CHINA | MW969659 | – | This study |
| 22 | A. taoyao Q. Na & Y.P. Ge | FFAAS0351 | CHINA | MW969655 | – | This study |
| 23 | A. taoyao Q. Na & Y.P. Ge | FFAAS0352 | CHINA, Type | MW969656 | MW969666 | This study |
| 24 | A. taoyao Q. Na & Y.P. Ge | FFAAS0353 | CHINA | MW969657 | MW969667 | This study |
| 25 | Hemimycena albicolor (A.H. Sm.) Elborne | MICH 11456 | USA | MK169368 | – | Unpublished |
| 26 | H. gracilis (Quél.) Singer | AFTOL–ID 1732 | USA | DQ490623 | DQ457671 | Matheney et al. (2006) |
| 27 | H. lactea (Pers.) Singer | F33274 | CANADA | MH718253 | – | Unpublished |
| 28 | H. lactea (Pers.) Singer | MQ18R237–QFB30753 | CANADA | MN992168 | – | Unpublished |
| 29 | H. mairei (E.–J. Gilbert) Singer | CBS 263.47 | FRANCE | MH856248 | DQ457671 | Vu et al. (2019) |
| 30 | H. mairei (E.–J. Gilbert) Singer | CBS 265.47 | FRANCE | MH856249 | MH867780 | Vu et al. (2019) |
| 31 | H. ochrogaleata (J. Favre) M.M. Moser | 409d | ITALY | JF908431 | – | Osmundson et al. (2013) |
| 32 | H. tortuosa (P.D. Orton) Redhead | PDD:95759 | NEW ZEALAND | HQ533011 | – | Unpublished |
| 33 | H. tortuosa (P.D. Orton) Redhead | FRDBI 18076639 | UK | MW487985 | – | Unpublished |
| 34 | Hydropus scabripes (Murrill) Singer | GG355_86 | NETHERLANDS | GU234149 | – | Geml et al. (2009) |
| 35 | Mycena abramsii (Murrill) Murrill | 231a | VENICE | JF908400 | – | Osmundson et al. (2013) |
| 36 | M. abramsii (Murrill) Murrill | HMJAU 43282 | CHINA | MH396626 | – | Na and Bau (2019) |
| 37 | M. abramsii (Murrill) Murrill | HMJAU 43468 | CHINA | MH396627 | – | Na and Bau (2019) |
| 38 | M. adscendens Maas Geest. | Aronsen120803 | NORWAY | KT900140 | – | Larsson and Aronsen (2015) |
| 39 | M. adscendens Maas Geest. | Orstadius329–05 | NORWAY | KT900141 | – | Larsson and Aronsen (2015) |
| 40 | M. adscendens Maas Geest. | Aronsen061119 | NORWAY | KT900142 | – | Larsson and Aronsen (2015) |
| 41 | M. adscendens Maas Geest. | Aronsen120826 | NORWAY | KT900143 | – | Larsson and Aronsen (2015) |
| 42 | M. alnetorum J. Favre (=M. abramsii (Murrill) Murrill) | CM14–RG2 | USA | KU295552 | – | Unpublished |
| 43 | M. amicta (Fr.) Quél. | 4745–HRL 1312 | CANADA | KJ705188 | – | Unpublished |
| 44 | M. amicta (Fr.) Quél. | CBS 352.50 | FRANCE | MH856655 | MH868170 | Vu et al. (2019) |
| 45 | M. amicta (Fr.) Quél. | CBS 254.53 | FRANCE | MH857183 | – | Vu et al. (2019) |
| 46 | M. amicta (Fr.) Quél. | H6036851 | FINLAND | MW540687 | – | Unpublished |
| 47 | M. arcangeliana Bres. | 252b | ITALY | JF908401 | – | Osmundson et al. (2013) |
| 48 | M. arcangeliana Bres. | 252f | ITALY | JF908402 | – | Osmundson et al. (2013) |
| 49 | M. cinerella (P. Karst.) P. Karst. | Aronsen051014 | SWEDEN | KT900146 | – | Larsson and Aronsen (2015) |
| 50 | M. cinerella (P. Karst.) P. Karst. | 173 | RUSSIA | MF926553 | – | Malysheva et al. (2017) |
| 51 | M. citrinomarginata Gillet | 317h | ITALY | JF908416 | – | Osmundson et al. (2013) |
| 52 | M. citrinomarginata Gillet | AD4TN | TUNISIA | KU973883 | – | Unpublished |
| 53 | M. clavicularis (Fr.) Gillet | 615i | ITALY | JF908466 | – | Osmundson et al. (2013) |
| 54 | M. clavicularis (Fr.) Gillet | 615b | ITALY | JF908467 | – | Osmundson et al. (2013) |
| 55 | M. diosma Krieglst. & Schwöbel | KA13–1230 | KOREA | KR673698 | – | Kim et al. (2015) |
| 56 | M. diosma Krieglst. & Schwöbel | 320f | ITALY | JF908417 | – | Osmundson et al. (2013) |
| 57 | M. entolomoides T. Bau | HMJAU 43048 | CHINA | MG654736 | – | Na and Bau (2018) |
| 58 | M. entolomoides T. Bau | HMJAU 43052 | CHINA | MG654737 | – | Na and Bau (2018) |
| 59 | M. entolomoides T. Bau | HMJAU 43126 | CHINA | MG654738 | – | Na and Bau (2018) |
| 60 | M. filopes (Bull.) P. Kumm. | 3782 | FRANCE | KJ705175 | – | Unpublished |
| 61 | M. filopes (Bull.) P. Kumm. | KA12–1699 | KOREA | KR673631 | – | Kim et al. (2015) |
| 62 | M. filopes (Bull.) P. Kumm. | 287f | ITALY | JF908410 | – | Osmundson et al. (2013) |
| 63 | M. floridula (Fr.) Quél. (=Atheniella adonis) | 259 | ITALY | JF908405 | – | Osmundson et al. (2013) |
| 64 | M. floridula (Fr.) Quél. (=Atheniella adonis) | 259a | ITALY | JF908406 | – | Osmundson et al. (2013) |
| 65 | M. floridula (Fr.) Quél. (=Atheniella adonis) | CBS 360.50 | FRANCE | MH856660 | MH868176 | Vu et al. (2019) |
| 66 | M. floridula (Fr.) Quél. (=Atheniella adonis) | HMJAU 43193 | CHINA | MK309770 | – | Unpublished |
| 67 | M. floridula (Fr.) Quél. (=Atheniella adonis) | HMJAU 43213 | CHINA | MK309771 | – | Unpublished |
| 68 | M. floridula (Fr.) Quél. (=Atheniella adonis) | HMJAU 43613 | CHINA | MK309772 | – | Unpublished |
| 69 | M. galopus (Pers.) P. Kumm. | BIOUG19840–F07 | CANADA | MF908430 | – | Dewaard (2017) |
| 70 | M. leaiana (Berk.) Sacc. | 1028 | ITALY | JF908376 | – | Osmundson et al. (2013) |
| 71 | M. leaiana (Berk.) Sacc. | CNH03 (TENN) | USA | MF686520 | – | Unpublished |
| 72 | M. meliigena (Berk. & Cooke) Sacc. | 39 | ITALY | JF908423 | – | Osmundson et al. (2013) |
| 73 | M. meliigena (Berk. & Cooke) Sacc. | 39d | ITALY | JF908429 | – | Osmundson et al. (2013) |
| 74 | M. metata (Fr.) P. Kumm. | 313b | ITALY | JF908412 | – | Osmundson et al. (2013) |
| 75 | M. olivaceomarginata (Massee) Massee | GG436–86 | NETHERLANDS | GU234119 | – | Geml et al. (2012) |
| 76 | M. olivaceomarginata (Massee) Massee | CBS 228.47 | FRANCE | MH856228 | MH867756 | Vu et al. (2019) |
| 77 | M. olivaceomarginata (Massee) Massee | CBS 229.47 | FRANCE | MH856229 | MH867757 | Vu et al. (2019) |
| 78 | M. olivaceomarginata (Massee) Massee | HK47–15 | NORWAY | MT153141 | – | Thoen et al. (2020) |
| 79 | M. pearsoniana Dennis ex Singer | FCME25817 | USA | JN182198 | – | Harder et al. (2012) |
| 80 | M. pearsoniana Dennis ex Singer | TENN61544 | USA | JN182199 | – | Harder et al. (2012) |
| 81 | M. pearsoniana Dennis ex Singer | TENN61384 | USA | JN182200 | – | Harder et al. (2012) |
| 82 | M. pelianthina (Fr.) Quél. | CBH164 | DENMARK | FN394548 | – | Unpublished |
| 83 | M. pelianthina (Fr.) Quél. | 108b | ITALY | JF908379 | – | Osmundson et al. (2013) |
| 84 | M. pelianthina (Fr.) Quél. | 108f | ITALY | JF908380 | – | Osmundson et al. (2013) |
| 85 | M. plumbea P. Karst. | JN198391 | CHINA | JN198391 | – | Wu et al. (2013) |
| 86 | M. plumbea P. Karst. | 420526MF0010 | CHINA | MG719769 | – | Wang et al. (2017) |
| 87 | M. polygramma (Bull.) Gray | 439b | ITALY | JF908433 | – | Osmundson et al. (2013) |
| 88 | M. polygramma (Bull.) Gray | 439f | ITALY | JF908434 | – | Osmundson et al. (2013) |
| 89 | M. pura (Pers.) P. Kumm. | TENN65043 | USA | JN182202 | – | Harder et al. (2012) |
| 90 | M. pura f. alba (Gillet) Kühner | CBH410 | USA | FN394595 | – | Unpublished |
| 91 | M. purpureofusca (Peck) Sacc. | F19748 | CANADA | HQ604766 | – | Unpublished |
| 92 | M. purpureofusca (Peck) Sacc. | G. Alfredsen | NORWAY | JQ358809 | – | Unpublished |
| 93 | M. rosea Gramberg | 938a | ITALY | JF908488 | – | Osmundson et al. (2013) |
| 94 | M. rosea Gramberg | Champ–21 | SPAIN | KX449424 | – | Perez-Izquierdo et al. (2017) |
| 95 | M. rubromarginata (Fr.) P. Kumm. | 407q | ITALY | JF908430 | – | Osmundson et al. (2013) |
| 96 | M. rubromarginata (Fr.) P. Kumm. | TL–12780 | DENMARK | KX513845 | KX513849 | Perry (2016) |
| 97 | M. seminau A.L.C. Chew & Desjardin | ACL136 | MALAYSIA | KF537250 | KJ206952 | Chew et al. (2015) |
| 98 | M. seminau A.L.C. Chew & Desjardin | ACL308 | MALAYSIA | KF537252 | KJ206964 | Chew et al. (2015) |
| 99 | M. seynii Quél. | 71l | ITALY | JF908469 | – | Osmundson et al. (2013) |
| 100 | M. seynii Quél. | 71h | ITALY | JF908470 | – | Osmundson et al. (2013) |
| 101 | M. silvae–nigrae Maas Geest. & Schwöbel | 515 | ITALY | JF908452 | – | Osmundson et al. (2013) |
| 102 | M. silvae–nigrae Maas Geest. & Schwöbel | CC 13–12 | USA | KF359604 | – | Baird et al. (2014) |
| 103 | M. stylobates (Pers.) P. Kumm. | 455 | ITALY | JF908439 | – | Osmundson et al. (2013) |
| 104 | M. supina (Fr.) P. Kumm. | 128a | ITALY | JF908388 | – | Osmundson et al. (2013) |
| 105 | M. tenax A.H. Sm. | p187i | USA | EU669224 | – | Unpublished |
| 106 | M. tenax A.H. Sm. | OSC 113746 | USA | EU846251 | – | Unpublished |
| 107 | M. vulgaris (Pers.) P. Kumm. | 447h | ITALY | JF908435 | – | Osmundson et al. (2013) |
| 108 | M. vulgaris (Pers.) P. Kumm. | 3781 | CANADA | KJ705177 | – | Unpublished |
| 109 | M. zephirus (Fr.) P. Kumm. | KA13–1265 | KOREA | KR673722 | – | Kim et al. (2015) |
Sequence alignment and phylogenetic analysis
A dataset comprising concatenated sequences for the ITS and nLSU regions from 45 accessions of three genera (Atheniella, Hemimycena Singer and Mycena) was compiled. A total of 112 sequences downloaded from GenBank and 11 sequences newly generated in this study were aligned and adjusted manually using BioEdit 7.0.4.1 and Clustal X (Thompson et al. 1997; Hall 1999). Gaps in the alignments were treated as missing data. The alignment was deposited with TreeBase (submission ID, 28111; study accession URL: http://purl.org/phylo/treebase/phylows/study/TB2:S28111). Hydropus scabripes (Murrill) Singer was chosen as the outgroup. The aligned dataset consisted of 836 ITS and 879 nLSU nucleotide sites (including gaps). The best-fit evolutionary model was determined using Modeltest 2.3 for each of the ITS and nLSU data partitions for Bayesian Inference (BI), which was implemented with MrBayes 3.2.6 (Ronquist and Huelsenbeck 2003; Nylander 2004). Markov Chain Monte Carlo (MCMC) chains sampling every 100th generation until the topological convergence diagnostic value was less than 0.01 (Ronquist and Huelsenbeck 2003). Maximum Likelihood (ML) analysis was performed using raxmlGUI 1.5b1 and topological support was assessed using the rapid bootstrapping algorithm with 1000 replicates (Stamatakis et al. 2004). Topology support values, greater than 75% bootstrap support (ML) and 0.95 Bayesian posterior probabilities (BPP), are shown for relevant branch nodes.
Results
Phylogenetic analysis
The concatenated dataset comprised 45 taxa and 1715 sites. The GTR + G evolutionary model was selected for both ITS and nLSU regions. The optimal evolutionary model for the 5.8S and nLSU partitions was lset nst = 6, rates = invgamma and prset statefreqpr = dirichlet (1,1,1,1). The BI and ML phylogenetic reconstructions were consistent in topology and, thus, only the BI tree is presented (Fig. 1).
Figure 1.
Maximum Likelihood and Bayesian tree concatenated ITS + nLSU dataset (ML ≥ 75%, BPP ≥ 0.90 are indicated). The tree is rooted with Hydropus scabripes. The new species are marked by red dot.
The phylogenetic tree contained four major clades. Both Atheniella and Mycena were resolved as monophyletic. The six species of Hemimycena were resolved into two clades. Each of the four clades corresponded with high statistical support (ML bootstrap [BS] ≥ 84%, BI posterior probability [BPP] = 1).
The Atheniella Clade formed a sister group to the Hemimycena 1, Hemimycena 2 and Mycena clades with high statistical support (BS = 84%, BPP = 1.00). Samples of the three new species were placed in the Atheniella Clade and formed monophyletic lineages, each with high statistical support (A. flavida, BS = 100%, BPP = 1.00; A. rutila, BS = 100%, BPP = 1.00; A. taoyao, BS = 100%, BPP = 1.00; Fig. 1). The phylogenetic tree resolved Atheniella flavida as forming a monophyletic lineage, which was sister to the majority of accessions included within the Atheniella Clade, consisting of A. adonis, A. amabillissima, A. flavoalba, Mycena floridula and the other two new species. Recognition of Atheniella taoyao and A. rutila was well supported, with these two species respectively indicated to be sister to accessions of A. amabillissima and to accessions of A. flavoalba and Mycena floridula (Fr.) Quél. Atheniella flavoalba was polyphyletic with accessions placed in two distinct lineages together with accessions of Mycena floridula. Accessions of Atheniella adonis were distributed amongst three lineages and were difficult to distinguish genetically from accessions of A. flavoalba in two lineages.
Taxonomy
Atheniella
Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry
96E7183A-B6A5-52F3-9A03-673A3A15CFD1
550101
Diagnosis.
Basidiomata small, mycenoid. Pileus conical, campanulate, to hemispherical, often with a small papilla when young, flattening or concave at centre with age; brightly coloured, white, creamy, yellow, orange, pinkish, reddish, sometimes yellow or deep brown at centre when old, the margin frequently fading to white, creamy, yellowish-white or yellow in the mature period; delicately pubescent, pruninose, glabrescent with age, translucent-striate, barely or shallowly sulcate, margin flattened and waved. Context thin and fragile, white. Lamellae ascending, adnate, adnexed, decurrent with tooth, faces concolorous with the sides. Stipe cylindrical, hollow, fragile, pruinose, almost smooth when old, base with coarse fibrils; white, yellow, orange, pink, sometimes base tinged deeper yellow with age. Odour and taste inconspicuous. Basidiospores globose, subglobose, ellipsoid, narrowly ellipsoid to cylindrical, smooth, thin-walled, hyaline, guttulate, inamyloid, white in prints. Basidia clavate, hyaline, thin-walled, 2- or 4-spored. Cheilocystidia fusiform, clavate, subutriform, long-stalked, hyaline, thin-walled. Pleurocystidia similar to cheilocystidia. Pileipellis hyphae covered with simple to branched excrescences, hyaline. Hyphae of the stipitipellis smooth or with simple cylindrical excrescences, hyaline; caulocystidia cylindrical, lageniform, subglobose, if present, hyaline, thin-walled. All tissues non-reactive in iodine. Clamps present or absent.
Habit and habitat.
Saprophytic on grass, moss, rotten wood or plant debris (leaves, pine needles and twigs).
Type species.
Atheniella adonis (Bull.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry
Etymology.
Intentionally spelled to achieve phonetic harmony and uniqueness, the epithet alludes to the mythical goddess Athena (the combination of beautiful colouration, spear-like stature and shield-like pileus) and her ancient Mycenaean origin. Gender: feminine.
Key to species of Atheniella
| 1 | Growing on twigs of Filipendula ulmaria | A. ulmariae |
| – | Growing on lawn or broadleaf-conifer mixed forest | 2 |
| 2 | Pileus yellowish-white, yellow to orange | 3 |
| – | Pileus pink or red | 7 |
| 3 | Cheilocystidia fusiform, thick-walled in the middle portion | A. delectabilis |
| – | Cheilocystidia fusiform, uniformly thin-walled | 4 |
| 4 | Clamps absent in all tissues | A. flavida |
| – | Clamps present in all tissues | 5 |
| 5 | Basidiospores broadly ellipsoid | A. leptophylla |
| – | Basidiospores narrowly ellipsoid | 6 |
| 6 | Caulocystidia up to 60 μm | A. flavoalba |
| – | Caulocystidia less than 20 μm | A. aurantiidisca |
| 7 | Lamellae decurrent | A. taoyao |
| – | Lamellae adnate to adnexed | 8 |
| 8 | Pileipellis with gelatinous hyphae | Mycena rohitha (≡ A. rohitha) |
| – | Pileipellis without gelatinous hyphae | 9 |
| 9 | Cheilocystidia with several large irregular excrescences or otherwise nodulose | Mycena wubabulna (≡ A. wubabulna) |
| – | Cheilocystidia entirely smooth | 10 |
| 10 | Stipe tinged coral-red and base yellowish with age | A. amabillissima |
| – | Stipe constantly white with age | 11 |
| 11 | Stipitipellis smooth; caulocystidia clavate to fusiform | A. adonis |
| – | Stipitipellis with simple cylindrical excrescences; caulocystidia not seen | A. rutila |
Atheniella flavida
Q. Na & Y.P. Ge sp. nov.
D60D42A0-3BEC-524A-B9F9-CB06F7CF1BD1
839378
Figure 2.
Basidiomata of Atheniella species a–cAtheniella adonis (Bull.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry d–fAtheniella aurantiidisca (Murrill) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry g–iAtheniella flavida Q. Na & Y.P. Ge j–lAtheniella flavoalba (Fr.) Redhead, Moncalvo, Vilgalys, Desjardin & B.A. Perry m–pAtheniella rutila Q. Na & Y.P. Ge q–sAtheniella taoyao Q. Na & Y.P. Ge. Scale bars: 10 mm (a–f, j–l, n–p), 5 mm (g–i, q–s). Photographs a,b, d–h, j–o, q, r by Qin Na; c, i, p, s by Yupeng Ge.
Figure 3.
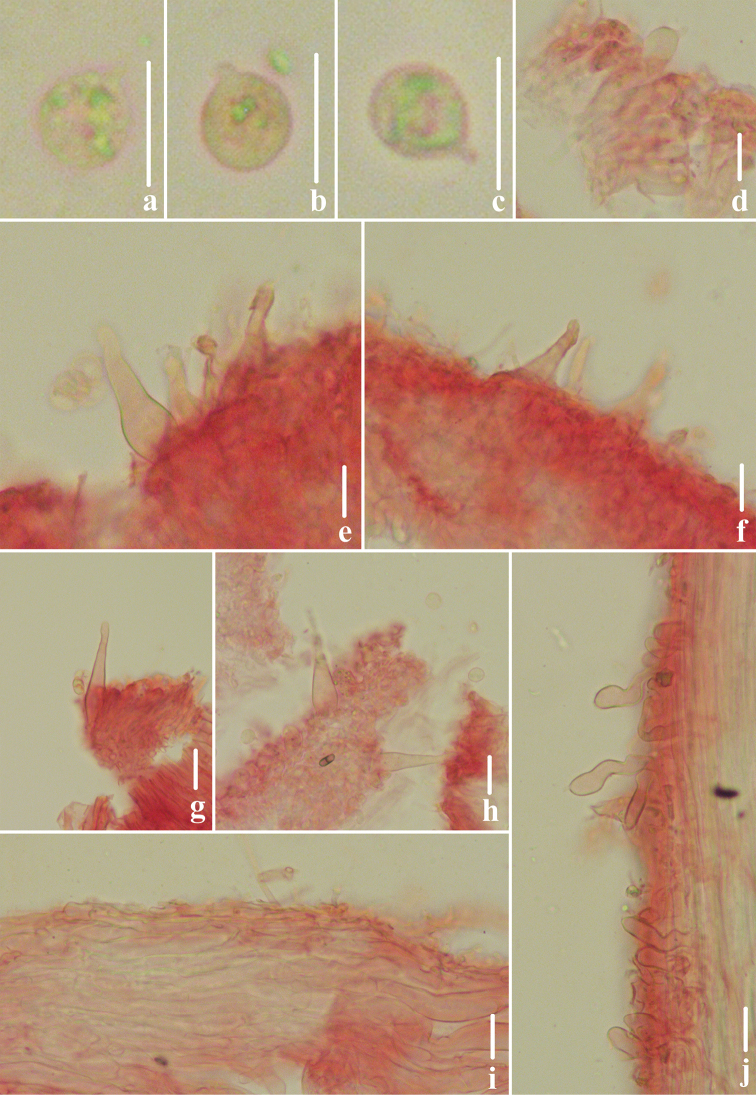
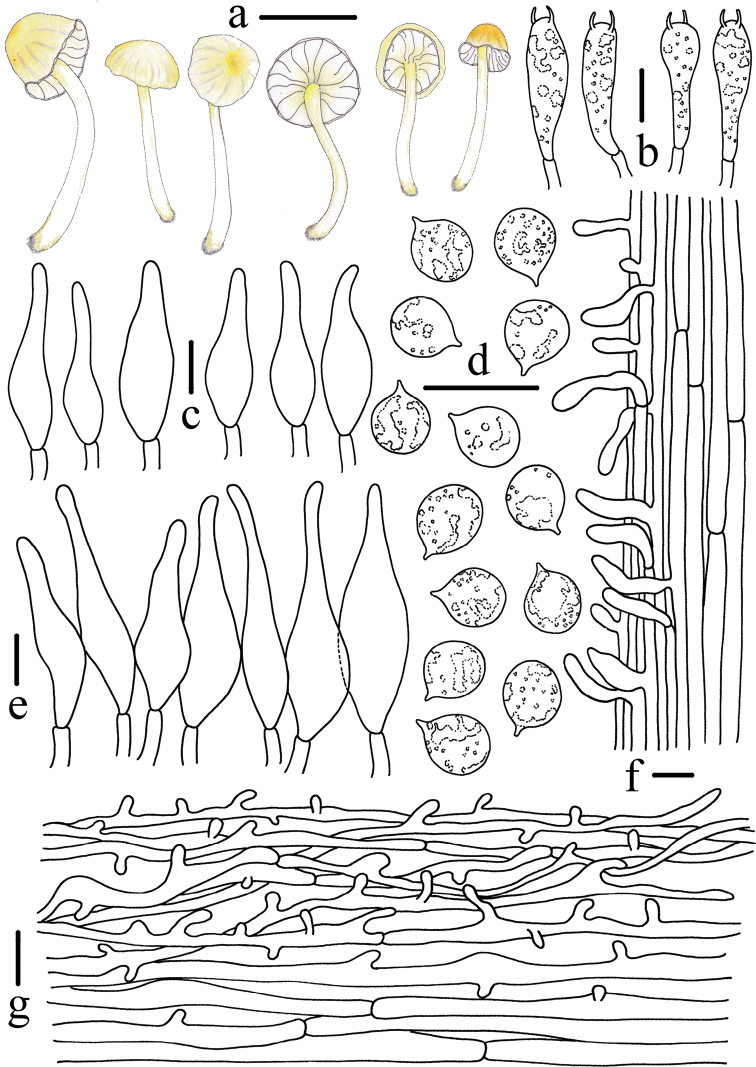
Microscopic features of Atheniella flavida (FFAAS0350, holotype) a–c basidiospores d basidia e, f cheilocystidia g, h pleurocystidia i pileipellis j stipitipellis and caulocystidia. Scale bars: 10 μm (a–j).
Figure 4.
Morphological features of Atheniella flavida (FFAAS0350, holotype) a basidiomata b basidia c pleurocystidia d basidiospores e cheilocystidia f stipitipellis and caulocystidia g pileipellis. Scale bars: 10 mm (a); 10 μm (b–g). Drawings by Qin Na and Yupeng Ge.
Diagnosis.
Pileus colour changing from orange-yellow to yellow, slightly concave at centre with age, pruninose. Lamellae narrowly adnate. Stipe densely pruinose. Basidiospores globose to subglobose, inamyloid. Cheilocystidia and pleurocystidia fusiform, thin-walled. Pileipellis with mass of excrescences. Caulocystidia cylindrical or lageniform. All tissues non-reactive in iodine. Clamps absent.
Holotype.
China. Yunnan Province, Yuxi City, Xinping County, Mopanshan National Forest Park, 25 Jul 2020, Qin Na, Yupeng Ge and Zewei Liu, FFAAS0350 (Collection No. MY0182).
Etymology.
Refers to the yellow basidiomata.
Description.
Pileus 2.6–4.8 mm in diam., conic when young, becoming almost hemispherical and slightly concave at centre with age, orange-yellow (4A8) when young, fading to cream-yellow (3A4–3A6) at maturity, margin light yellow (3A3), sulcate, translucent-striate, delicately pubescent, pruninose, glabrescent with age, margin waved. Context very thin and fragile, pure white. Lamellae narrowly adnate, ascending, cream-white (3A2) to light yellow (3A3), faces concolorous with the sides, decurrent with a short tooth. Stipe slender, 5.5–12 × 0.5–0.8 mm, cylindrical, hollow, fragile, bright yellow (4A6), densely pruinose on the entire surface, almost smooth when old, base with sparse white fibrils. Odour and taste inconspicuous.
Basidiospores [60/3/2] (6.5) 6.7–7.2–7.8 (8.3) × (5.7) 5.9–6.5–7.1 (7.8) μm [Q = 1.03–1.22, Q = 1.11 ± 0.043] [holotype [40/2/1] (6.6) 6.7–7.2–7.6 (7.9) × (5.8) 5.9–6.4–6.9 (7.4) μm, Q = 1.04–1.20, Q = 1.10 ± 0.041], globose to subglobose, hyaline, guttulate, thin-walled, inamyloid. Basidia 20–29 × 5–8 μm, hyaline, clavate, 2-spored. Cheilocystidia abundant, 36–51 × 8–11 μm, fusiform, long-stalked, hyaline, thin-walled. Pleurocystidia similar to cheilocystidia, 28–43 × 6–10 μm. Pileipellis hyphae 2–6 μm wide, cutis; covered with mass of excrescences, 3.3–8.2 × 1.2–3.4 μm, hyaline. Hyphae of the stipitipellis 2–8 μm wide, hyaline, smooth; caulocystidia cylindrical or lageniform, 14–37 × 5–11 μm, hyaline, thin-walled. All tissues non-reactive in iodine. Clamps not seen in all tissues.
Habit and habitat.
Solitary to scattered on rotten wood in evergreen broad-leaf forest, Cephalotaxus, Cunninghamia, Keteleeria, Podocarpus, Pseudotaxus, Pseudotsuga, Sequoia, Taxus, Torreya and Tsuga.
Other specimens examined.
China. Yunnan Province, Yi Autonomous Prefecture, Chuxiong City, Zixishan, 27 Jul 2020, Qin Na, Yupeng Ge and Zewei Liu, FFAAS0355 (Collection No. MY0234).
Remarks.
Atheniella flavida is considered to be a distinct species in Atheniella on account of the pileus colour changing from orange-yellow to yellow, globose to subglobose basidiospores and caulocystidia comparatively small (Maas Geesteranus 1980, 1990, 1992a, 1992b; Perry 2002; Robich 2003; Aronsen and Læssøe 2016). Four species with a yellow or orange pileus are recorded: A. aurantiidisca, A. delectabilis (Peck) Lüderitz & H. Lehmann, A. flavoalba and A. leptophylla (Peck) Gminder & Böhningare (Smith 1935b; Maas Geesteranus 1980; Robich 2003; Aronsen and Læssøe 2016). Atheniella flavoalba, which is the most widely distributed species in the Northern Hemisphere, often seen in northeast China (Fig. 2a–c), shows the most morphological similarities to A. flavida; however, the former differs in forming cylindrical spores (6.5–9 × 3–4.5 μm) and the caulocystidia are fusiform and clavate to globose (Perry 2002; Robich 2003; Aronsen and Læssøe 2016; Na 2019). In contrast to A. flavida, A. aurantiidisca, which had been found in Yunnan Province and Tibet Autonomous Region of China (Fig. 2d–f) and A. leptophylla are easily mistaken for the new species (Robich 2003; Aronsen and Læssøe 2016; Na 2019). However, the pileus of A. aurantiidisca and A. leptophylla is constantly distinctly orange and caulocystidia of the two species are larger (up to 50 μm long) (Robich 2003; Aronsen and Læssøe 2016). Atheniella delectabilis, which was formerly named Hemimycena delectabilis (Peck) Singer on account of the white to yellowish-white pileus, decurrent lamellae and inamyloid basidiospores, is easily mistaken for A. flavida by the light yellowish pileus and the similar shape and size of cheilocystidia and caulocystidia. However, A. delectabilis is distinguishable from A. flavida by its decurrent lamellae and cylindrical spores (7–9 × 3–4 μm) (Smith 1935b; Malysheva and Morozova 2009). In addition, A. delectabilis produces cheilocystidia that are partially thick-walled (Smith 1935b).
Atheniella rutila
Q. Na & Y.P. Ge sp. nov.
7B1AE07F-7AD5-5D1E-B577-2AEE315C0C95
839379
Figure 5.
Microscopic features of Atheniella rutila (FFAAS0354, holotype) a–c basidiospores d basidia e, f cheilocystidia g, h pleurocystidia i pileipellis j stipitipellis. Scale bars: 10 μm (a–j).
Figure 6.
Morphological features of Atheniella rutila (FFAAS0354, holotype) a basidiomata b pleurocystidia c basidia d basidiospores e cheilocystidia f stipitipellis g pileipellis. Scale bars: 5 mm (a); 10 μm (b–g). Drawings by Qin Na and Yupeng Ge.
Diagnosis.
Pileus campanulate to hemispherical, concave with age, slightly pruinose. Lamellae adnate to adnexed, white. Stipe base with dense white fibrils. Basidiospores cylindrical, inamyloid. Pleurocystidia similar to cheilocystidia, fusiform, with a long neck. Pileipellis covered with numerous excrescences. Hyphae of the stipitipellis with simple cylindrical excrescences. Caulocystidia not seen. All tissues non-reactive in iodine. Clamps absent.
Holotype.
China. Yunnan Province, Lincang City, Wulaoshan National Forest Park, 31 Jul 2020, Qin Na, Yupeng Ge and Zewei Liu, FFAAS0354 (Collection No. MY0210).
Etymology.
Refers to the bright red-tinted pileus.
Description.
Pileus 2.0–10.2 mm in diam., campanulate to hemispherical, applanate or slightly concave at centre when old, deep salmon (10A7) to bright red (10A8), shallowly sulcate, translucent-striate, delicately pubescent, glabrescent when old. Context white, thin, very fragile. Lamellae broadly adnate to adnexed, ascending, white, concolorous with the sides, basally interveined with age. Stipe 5.0–15.8 × 1.0–2.0 mm, cylindrical, hollow, fragile, transparent, pruninose, glabrescent when old, base slightly swollen, covered with dense white fibrils. Odour and taste indistinctive.
Basidiospores [60/3/2] (7.2) 7.7–8.6–9.8 (10.1) × (3.6) 4.1–4.6–5.3 (5.5) μm [Q = 1.71–2.05, Q = 1.85 ± 0.079] [holotype [40/2/1] (7.2) 7.5–8.5–9.7 (10.0) × (3.6) 4.1–4.6–5.2 (5.5) μm, Q = 1.72–1.99, Q = 1.86 ± 0.086], narrowly ellipsoid to cylindrical, hyaline in water and 5% KOH, inamyloid, smooth. Basidia 19–28 × 5–8 μm, 2-spored, clavate, hyaline. Cheilocystidia 32–45 × 8–11 μm, abundant, fusiform, with a long neck, thin-walled and hyaline. Pleurocystidia similar to cheilocystidia, 27–42 × 7–12 μm. Pileipellis hyphae 2–5 μm wide, covered with numerous excrescences, 3.2–6.9 × 0.8–1.7 μm, hyaline. Hyphae of the stipitipellis 2–7 μm wide, non-dextrinoid, hyaline, with simple cylindrical excrescences, 4.6–14.3 × 2.9–5.2 μm. All tissues non-reactive in iodine. Clamps absent in all tissues.
Habit and habitat.
Scattered on rotten wood in evergreen broadleaf and Pinus mixed forest.
Other specimens examined.
Yunnan Province, Puer City, Xiaoheijiang National Forest Park, 1 Aug 2020, Qin Na, Yupeng Ge and Zewei Liu, FFAAS0356 (Collection No. MY0235).
Remarks.
Atheniella rutila is considered to be a distinct species in Atheniella on account of the bright red pileus, white stipe, narrowly ellipsoid to cylindrical and inamyloid spores and characters of the cystidia, pileipellis and stipitipellis (Maas Geesteranus 1980, 1990, 1992a, b; Perry 2002; Grgurinovic 2003; Robich 2003; Aravindakshan and Manimohan 2015; Aronsen and Læssøe 2016). Atheniella amabillissima is difficult to distinguish from A. rutila owing to the reddish basidiomata, but the pileus of A. amabillissima fades to white with age or has a dirty yellowish disc, and the spores are smaller (7–9 × 3–4 μm) (Smith 1935b). Atheniella adonis shows certain morphological similarities to A. rutila in possessing tiny and pinkish-red basidiomata, white lamellae and cylindrical basidiospores. However, A. adonis differs in producing a pileus with a white margin, longer stipe and clavate to fusiform caulocystidia (Perry 2002; Robich 2003; Aronsen and Læssøe 2016). In comparison with Atheniella rutila, Mycena rohitha (≡ A. rohitha) and M. wubabulna (≡ A. wubabulna) have gelatinous pileus hyphae and narrower basidiospores (Grgurinovic 2003; Aravindakshan and Manimohan 2015).
Atheniella taoyao
Q. Na & Y.P. Ge sp. nov.
A96D1736-AF4F-5D87-9846-2408998FE214
839380
Figure 7.
Microscopic features of Atheniella taoyao (FFAAS0352, holotype) a–c basidiospores d basidia e cheilocystidia f, g pleurocystidia h pileipellis i stipitipellis. Scale bars: 10 μm (a– i).
Figure 8.
Morphological features of Atheniella taoyao (FFAAS0352, holotype) a basidiomata b basidia c pleurocystidia d basidiospores e cheilocystidia f stipitipellis and caulocystidia g pileipellis. Scale bars: 5 mm (a); 10 μm (b–g). Drawingsby Qin Na and Yupeng Ge.
Diagnosis.
Pileus pinkish to light reddish. Lamellae decurrent. Stipe pruninose, base slightly swollen. Basidiospores narrowly ellipsoid to cylindrical, inamyloid. Cheilocystidia and pleurocystidia fusiform. Pileipellis hyphae covered with excrescences. Stipitipellis smooth, caulocystidia of two types, fusiform or subglobose. All tissues non-reactive in iodine. Clamps absent.
Holotype.
China. Yunnan Province, Yuxi City, Xinping County, Mopanshan National Forest Park, 25 Jul 2020, Qin Na, Yupeng Ge and Zewei Liu, FFAAS0352 (Collection No. MY0184).
Etymology.
Refers to the pinkish to reddish basidiomata. Tao Yao is a poem in the “The Book of Songs” that praises a young woman, whose beauty is compared to a flowering peach tree and who will be married and assume a new role in life.
Description.
Pileus 1.4–5.8 mm in diam., campanulate or hemispherical, obtusely umbonate in the centre, flattening with age, translucent-striate, light pink-salmon (8A3), light coral red (8B7), fading light pink (8A2) or white to the margin, delicately pubescent, glabrescent with age, with a flat margin. Context pure white, thin, fragile. Lamellae decurrent dentate, ascending, sparse, pure white, edges concolorous with the sides. Stipe 46–58 × 0.5–1.0 mm, central, terete, almost equal, hollow, fragile, transparent, pruninose, glabrescent with age, base slightly swollen, with tiny, white, fine hairs. Odourless, taste mild.
Basidiospores [80/4/3] (7.4) 7.7–8.3–9.1 (9.4) × (3.9) 4.1–4.5–5.0 (5.5) μm [Q = 1.73–2.08, Q = 1.85 ± 0.076] [holotype [40/2/1] (7.4) 7.7–8.2–9.0 (9.2) × (4.0) 4.1–4.4–5.0 (5.4) μm, Q = 1.75–1.99, Q = 1.84 ± 0.079], narrowly ellipsoid to cylindrical, hyaline, guttulate, thin-walled, inamyloid. Basidia 20–31 × 5–7 μm, hyaline, clavate, 2-spored. Cheilocystidia 23–42 × 5–10 μm, fusiform, long-stalked, hyaline. Pleurocystidia similar to cheilocystidia, 20–40 × 5–9 μm. Pileipellis hyphae 1–5 μm wide, cutis; covered with numbers of cylindrical or fusiform excrescences, 3.5–10.4 × 1.4–4.3 μm, hyaline. Hyphae of the stipitipellis 3–10 μm wide, hyaline, smooth; caulocystidia fusiform, 16.5–24.9 × 5.3–11.5 μm or subglobose, 11.8–16.5 × 9.1–12.9 μm. All tissues non-reactive in iodine. Clamps not seen.
Habit and habitat.
Scattered to gregarious on living wood in evergreen broadleaf forest, for example, Cephalotaxus, Cunninghamia.
Other specimens examined.
Yunnan Province, Yuxi City, Xinping County, Mopanshan National Forest Park, 25 Jul 2020, Qin Na, Yupeng Ge and Zewei Liu, FFAAS0351 (Collection No. MY0183); Yunnan Province, Yuxi City, Xinping County, Shimenxia, 26 Jul 2020, Qin Na, Yupeng Ge and Zewei Liu, FFAAS0353 (Collection No. MY0185).
Remarks.
Atheniella taoyao is unique in Atheniella because of the light pink-salmon pileus, decurrent lamellae and the two types of caulocystidia. Atheniella adonis most closely resembles A. taoyao, but the former differs in having adnate to adnexed lamellae, stipe with pink at the apex and larger caulocystidia (15–50 × 3.5–13.5 μm) (Aronsen and Læssøe 2016). Atheniella amabillissima is closely allied to A. taoyao, but differs in the larger basidiomata (pileus 3–20 mm in diam.), pileus fading to white or yellow with age, stipe tinted with coral red and yellow with age and the cheilocystidia are up to 65 μm in length (Smith 1935b). Aravindakshan and Manimohan (2015) described the species Mycena rohitha Aravind. & Manim. (≡ Atheniella rohitha) collected from India. This taxon differs from Atheniella taoyao in its orange stipe and gelatinous pileus hyphae (Aravindakshan and Manimohan 2015). Mycena wubabulna, a species described by Grgurinovic (2003) that should be transferred to Atheniella, is readily identified by its yellowish stipe base and cylindrical basidiospores (7.5–10.6 × 3.1–4.7 μm; Q = 2.3).
Discussion
The present phylogenetic analysis showed that Atheniella formed a distinct clade independent of Hemimycena and Mycena with high BPP and BS support and, thus, supported segregation of the genus from the Mycenaceae (Moncalvo et al. 2002; Matheney et al. 2006). This finding also supported the view of Redhead et al. (2012) that Atheniella, formerly treated as Mycena sect. Adonideae, should be elevated to generic rank. Atheniella is more closely related to Mycena than to Hemimycena, based on genetic distance, in accordance with the greater similarity of Atheniella to Mycena spp. in morphological characters. The presence of pileocystidia and the morphological differences of the cheilocystidia, caulocystidia and stipitipellis can be used to distinguish Atheniella species from Hemimycena and Mycena.
Atheniella was originally established by Redhead et al. (2012) to accommodate four species: A. adonis, A. amabillissima, A. aurantiidisca and A. flavoalba. In recent years, the number of recognised species of Atheniella has increased to nine, but the description of the genus was incomplete and not detailed (Redhead et al. 2012; Gminder and Böhning 2016; Lehmann and Lüderitz 2019). With description of the new species in the present study, the generic description for Atheniella requires updating. Amongst Atheniella species, the bright colour of the pileus may be uniform or be tinted at the centre, but fades to white at the margin, the lamellae are adnate to decurrent and the stipe colour sometimes changes to yellow or pink towards the base. With regards to micromorphological characters, Atheniella produces globose to cylindrical spores, caulocystidia are present or absent and the stipitipellis is smooth or has projections.
Atheniella is closely allied to Hemimycena, Mycena sect. Aciculae Kühner ex Singer and Mycena sect. Oregonenses Maas Geest., based on morphology (Maas Geesteranus 1980, 1992a, b). Species of Hemimycena lack bright yellow, pink to red basidiomata, produce larger spores and pileocystidia are often seen (Antonín and Noordeloos 2004; Malysheva and Morozova 2009). M. acicula (Schaeff.) P. Kumm., which is the sole species classified in Mycena sect. Aciculae, shares an orange-coloured pileus, non-amyloid spores and ornamentation of the pileipellis, but the stipitipellis is covered with numerous excrescences and is embedded in gelatinous material (Maas Geesteranus 1990; Robich 2003; Aronsen and Læssøe 2016). A longer stipe (up to 60 mm) with yellow fibrils at the base, cheilocystidia fusiform or lageniform with a rounded apex and caulocystidia with yellow contents are morphological characters that distinguish Mycena sect. Oregonenses from Atheniella (Maas Geesteranus 1990).
Morphological and molecular evidence support classification of the three newly-recognised species as members of Atheniella. The three species share white lamellae, a pruninose stipe base without a basal disc, inamyloid basidiopores, fusiform and thin-walled cheilocystidia, pileipellis covered with excrescences and are unreactive in Melzer’s Reagent. In addition, the three species grow on rotten wood or other plant debris. A. flavida is mainly distinguished from A. taoyao and A. rutila by its distinctly yellowish-orange to yellow pileus and globose spores. The pinkish or reddish basidiomata support the inclusion of A. taoyao and A. rutila in Atheniella. Compared with A. rutila, A. taoyao is readily discriminated, based on the light pink basidiomata, narrow ellipsoid basidiospores and subglobose or fusiform caulocystidia. A. amabillissima shows the most morphological similarities to A. taoyao and A. rutila; however, A. amabillissima has a pileus which fades to white with age, smaller spores and longer cheilocystidia (Smith 1935b).
It is noteworthy that the taxonomic status of Mycena floridula remains unresolved (Josserand 1930; Kühner 1938; Aronsen and Læssøe 2016). This species was formerly classified in Mycena sect. Adonideae as a form of M. flavoalba with a pink pileus (Josserand 1930; Kühner 1938; Aronsen and Læssøe 2016). More recently, it was proposed that the name M. floridula was a synonym of A. adonis (Redhead et al. 2012). The phylogenetic reconstructions in our study and accessions of M. floridula indicated that M. floridula was closely related to A. flavoalba, with little genetic distinction between the two taxa. Therefore, we tentatively accept M. floridula as a pink form of A. flavoalba, but emphasise that a detailed appraisal of the morphological and molecular variation of A. flavoalba is required.
Supplementary Material
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 3190012), the Natural Science Foundation of Shandong Province (No. ZR2020QC001, No. ZR2019PC028), the Major Scientific and Technological Innovation Projects of Key R & D Programs in Shandong Province (No. 2019JZZY010717) and the Innovation Team of Shandong Agricultural Industry Technology System (2016 No.18). We sincerely thank Drs Jun–qing Yan (Jiangxi Agriculture University, Nanchang) for their kind help during fieldwork.
Citation
Ge Y, Liu Z, Zeng H, Cheng X, Na Q (2021) Updated description of Atheniella (Mycenaceae, Agaricales), including three new species with brightly coloured pilei from Yunnan Province, southwest China. MycoKeys 81: 139–164. https://doi.org/10.3897/mycokeys.139.67773
References
- Aime MC, Bell CD, Wilson AW. (2018) Deconstructing the evolutionary complexity between rust fungi (Pucciniales) and their plant hosts. Studies in Mycology 89: 143–152. 10.1016/j.simyco.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonín V, Noordeloos ME. (2004) A monograph of the genera Hemimycena, Delicatula, Fayodia, Gamundia, Myxomphalia, Resinomycena, Rickenella, and Xeromphalina (Tribus Mycenae sensu Singer, Mycena excluded) in Europe. IHW-Verlag.
- Aravindakshan DM, Manimohan P. (2015) Mycenas of Kerala. SporePrint Books, Calicut, India. doi: 10.13140/RG.2.1.2116.4003 [DOI]
- Aronsen A, Larsson E. (2016) Studier i släktet Mycena-2. Svensk Mykologisk 37(3): 26–31. [Google Scholar]
- Aronsen A, Læssøe T. (2016) The Genus Mycena s.l. Fungi of Northern Europe (Vol. 5). Narayana Press, Gylling, Denmark.
- Bau T, Liu Y. (2011) Four new records of Mycena from China. Mycosystema 30(4): 653–657. doi: 10.13346/j.mycosystema.2011.04.024 [DOI] [Google Scholar]
- Emmett EE. (1992) British Mycena species–1. Mycologist 6(2): 72–76. 10.1016/S0269-915X(09)80453-9 [DOI] [Google Scholar]
- Friedrich S. (2006) Threatened and protected macromycetes in the Wkrzanska Forest. Acta Mycologica 41(2): 229–240. 10.5586/am.2006.025 [DOI] [Google Scholar]
- Gminder A, Böhning T. (2016) Index Fungorum 302: 1–1.
- Grgurinovic CA. (2003) The genus Mycena in south–eastern Australia. Fungal Diversity Press, Canberra.
- Gyosheva M, Ganeva A. (2004) New and rare macromycetes and bryophytes from montane peat habitats in Bulgaria. Mycologia Balcanica 1: 9–13. [Google Scholar]
- Hall TA. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. 10.1021/bk-1999-0734.ch008 [DOI] [Google Scholar]
- Hopple JS, Vilgalys R. (1999) Phylogenetic relationships in the mushroom genus Coprinus and dark-spored allies based on sequence data from the nuclear gene coding for the large ribosomal subunit RNA: divergent domains, outgroups, and monophyly. Molecular Phylogenetics & Evolution 13(1): 1–19. 10.1006/mpev.1999.0634 [DOI] [PubMed] [Google Scholar]
- Horak E. (2005) Röhrlinge und Blätterpilze in Europa: Bestimmungsschlüssel für Polyporales (pp), Boletales, Agaricales, Russulales. Elsevier, Spektrum Akad Verlag.
- Josserand M. (1930) Note sur deux mycènes: M. flavo-alba (Fr.) Q. et M. floridula (Fr.) Q. Bullc. Soc. myc. Fr. 46(1): 38–42. [Google Scholar]
- Kornerup A, Wanscher JHK. (1978) The Methuen Handbook of Colour. Eyre Methuen, London.
- Kühner R. (1938) Le genre Mycena (Fries). Encyclopédie Mycologique X. P. Lechevalier.
- Lehmann V, Lüderitz O. (2018) Index Fungorum 416: 1–1.
- Lehmann V, Lüderitz O. (2019) Index Fungorum 416: 1–1.
- Li Y, Li TH, Yang ZL, Bau T, Dai YC. (2015) Atlas of Chinese Macrofungal Resources. Central Chinese Farmer Press, Zhengzhou, China.
- Maas Geesteranus RA. (1980) Studies in Mycenas–15. Persoonia 11: 93–120. [Google Scholar]
- Maas Geesteranus RA. (1990) Conspectus of the Mycenas of the Northern Hemisphere–14, Sections Adonidae, Aciculae, and Oregonenses. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen (Ser C), Amsterdam, North–Holland 93(2): 163–186. [Google Scholar]
- Maas Geesteranus RA. (1992a) Mycenas of the Northern Hemisphere I. Studies in Mycenas and other papers. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen, Amsterdam, North–Holland.
- Maas Geesteranus RA. (1992b) Mycenas of the Northern Hemisphere II. Studies in Mycenas and other papers. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen, Amsterdam, North–Holland.
- Malysheva EF, Morozova OV. (2009) Notes on Hemimycena from European Russia. Czech Mycology 61(1): 27–71. 10.33585/cmy.61103 [DOI] [Google Scholar]
- Matheney PB, Curtis JM, Hofstetter V, Aime M, Moncalvo J, Ge Z, Yang Z, Slot J, Ammirati J, Baroni T, Bougher N, Hughes K, Lodge D, Kerrigan R, Seidl M, Aanen D, DeNitis M, Daniele G, Desjardin D, Kropp B, Norvell L, Parker A, Vellinga E, Vilgalys R, Hibbett D. (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98(6): 982–995. 10.1080/15572536.2006.11832627 [DOI] [PubMed] [Google Scholar]
- Miersch J, Karasch P. (2011) Mycena oregonensis new for Bavaria and Mycena leptophylla, two apricot-coloured mycenas. Mycologia Bavaric 12: 19–26. [Google Scholar]
- Miersch J. (2013) Records of orange-capped mycena Mycena aurantiidisca in Bavaria and Saxony-Anhalt-new for Germany. Mycologia Bavaric 14: 13–21. [Google Scholar]
- Miyamoto T, Igarashi T, Takahashi K. (1998) Notes on three species of Mycena new to Japan from Picea forests of Hokkaido. Mycoscience 3(39): 337–342. 10.1007/BF02464018 [DOI] [Google Scholar]
- Miyamoto T, Igarashi T, Takahashi K. (2000) Lignin-degrading ability of litter-decomposing basidiomycetes from Picea forests of Hokkaido. Mycoscience 41(2): 105–110. 10.1007/BF02464317 [DOI] [Google Scholar]
- Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduine SJW, Larsson E, Baroni TJ, Thorn RG, Jacobsson S, Clémençon H, MillerJr OK. (2002) One hundred and seventeen clades of euagarics. Molecular Phylogenetics and Evolution 23: 357–400. 10.1016/S1055-7903(02)00027-1 [DOI] [PubMed] [Google Scholar]
- Murrill WA. (1916) Pleurotus, Omphalia, Mycena and Collybia published in North American Flora. Mycologia 8(4): 218–221. 10.1080/00275514.1916.12018883 [DOI] [Google Scholar]
- Na Q. (2019) Taxonomy and Molecular Phylogeny of Mycena in China. Doctoral Dissertation of Jilin Agriculture University, Jilin, China. 10.27163/d.cnki.gjlnu.2019.000016 [DOI]
- Na Q, Bau T. (2018) New species of Mycena (Mycenaceae, Agaricales) with colored lamellae and three new species records from China. Phytotaxa 361(3): 266–278. 10.11646/phytotaxa.361.3.2 [DOI] [Google Scholar]
- Na Q, Bau T. (2019a) Mycena section Sacchariferae: three new species with basal discs from China. Mycological Progress 18: 483–493. 10.1007/s11557-018-1456-8 [DOI] [Google Scholar]
- Na Q, Bau T. (2019b) Recognition of Mycena sect. Amparoina sect. nov. (Mycenaceae, Agaricales), including four new species and revision of the limits of sect. Sacchariferae. Mycokeys 52: 103–124. 10.3897/mycokeys.52.34647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell LL. (2016) Mushrooms of the Redwood Coast–a comprehensive guide to the fungi of coastal northern California. Mycotaxon 131(3): 723–731. 10.5248/131.723 [DOI] [Google Scholar]
- Nylander J. (2004) MrModeltest v.2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala.
- Osono T. (2015) Effects of litter type, origin of isolate, and temperature on decomposition of leaf litter by macrofungi. Journal of Forest Research 20(1): 77–84. 10.1007/s10310-014-0462-1 [DOI] [Google Scholar]
- Pérez-De-Gregorio MÀ. (2015) Mycena leptophylla from the Iberian Peninsula. Micologia e Vegetazione Mediterrane 29(2): 127–131. [Google Scholar]
- Perry BA. (2002) A taxonomic investigation of Mycena in California. Doctoral dissertation, San Francisco State University, California, USA.
- Redhead SA. (1984) Mycological observations, 4–12: on Kuehneromyces, Stropharia, Marasmius, Mycena, Geopetalum, Omphalopsis, Phaeomarasmius, Naucoria and Prunulus. Sydowia 246–270.
- Redhead SA, Moncalvo JM, Vilgalys R, Desjardin DE, Perry BA. (2012) Index Fungorum 14: 1–1.
- Robich G. (2003) Mycena d’Europa. Associazione Micologica Bresadola, Trento, Italy.
- Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Smith AH. (1935a) Studies in the Genus Mycena–I. American Journal of Botany 22(10): 858–877. 10.2307/2435962 [DOI] [Google Scholar]
- Smith AH. (1935b) Studies in the Genus Mycena–II. Mycologia 27(6): 586–604. 10.1080/00275514.1935.12017103 [DOI] [Google Scholar]
- Smith AH. (1935c) Studies in the Genus Mycena–III. Mycologia 28(5): 410–430. 10.2307/3754114 [DOI] [Google Scholar]
- Smith AH. (1937) Notes on agarics from the western United States. Bulletin of the Torrey Botanical Club 64(7): 477–487. 10.2307/2481096 [DOI] [Google Scholar]
- Smith AH. (1939) Studies in the Genus Mycena–V. Mycologia 31(3): 267–285. 10.1080/00275514.1939.12017341 [DOI] [Google Scholar]
- Smith AH. (1947) North American species of Mycena. University Michigan Press, Ann Arbor, Michigan.
- Stamatakis A, Ludwig T, Meier H. (2004) RAxML–III: a fast program for maximum likelyhood–based inference of large phylogenetic trees. Bioinformatics 21(4): 456–463. 10.1093/bioinformatics/bti191 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F. (1997) The Clustal–X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 63: 215–228. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyier G. (1991) Ecology of the genus Mycena in beech (Fagus sylvatica), oak (Quercus robur) and hornbeam (Carpinus betulus) forest of Sweden. Nordic Journal of Botany 11(1): 111–121. 10.1111/j.1756-1051.1991.tb01807.x [DOI] [Google Scholar]
- Uehling JK, Henkel TW, Vilgalys R, Smith ME. (2012) Membranomyces species are common ectomycorrhizal symbionts in Northern Hemisphere forests. Mycorrhiza 22(7): 577–581. 10.1007/s00572-012-0457-8 [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR protocols: a guide to methods and applications.Academic, San Diego, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.