Abstract
NK cells play a crucial role in the clearance of human viruses but their activity is significantly impaired in chronic hepatitis B infected patients (CHB). Cooperation with dendritic cells (DCs) is pivotal for obtaining optimal NK cell antiviral function, thus we investigated whether HBV might impact the ability of DCs to sustain NK cell functions. Human DCs were poor stimulators of IFN-γ production by NK cells when exposed to HBV, while maintained capability to trigger NK cell cytotoxicity. HBV prevented DC maturation but did not affect their expression of HLA class I, thus allowing DCs to evade NK cell lysis. Tolerogenic features of DCs exposed to HBV were further supported by their increased expression of IL-10 and immunosuppressive enzyme indoleamine-2, 3-dioxygenase that contributed to the impairment of DC-mediated NK cell IFN-γ production and proliferation respectively. HBV could also inhibit the expression of inducible immunoproteasome (iP) subunits on DCs. In fact, NK cells could induce iP subunit expression on DCs but they failed in the presence of HBV. Remarkably, circulating BDCA1+DCs isolated from CHB patients were functionally compromised, hence altering, in turn, NK cell responses. The abnormal NK/DC interplay caused by HBV may significantly impair the efficacy of antiviral immune response in CHB patients.
Keywords: Dendritic cells, Natural Killer cells, Hepatitis B virus, Interleukin-12, Interferon-γ, Immunoproteasome
Introduction
Hepatitis B virus (HBV) is a major cause of inflammatory liver disease of variable severity that affects millions of people worldwide. Despite the availability of vaccine, more than 257 million people are still infected with HBV. (1)
The pathophysiology of HBV infection is complex and closely linked to host immune response. The ineffective and weak immune response toward HBV is thought to be the fundamental underlying cause for evolution of HBV infection until the establishment of chronic disease. (2, 3)
Progression of persistent infection, together with cell death and regeneration of infected hepatocytes, succeed one another leading to chronic inflammation and fibrosis often associated with the risk of developing liver cirrhosis and hepatocellular carcinoma. (4)
More importantly, HBV infection is often associated with other co-morbidities, including several recurrent bacterial and viral infections, thus suggesting that HBV might interfere with the development of an effective immune response directed not only against HBV but also toward other microorganisms or tumors. (5–7) Indeed, HBV is not directly cytopathic and has to evolve mechanisms to escape from the control of the host’s immune system. (8) Innate immunity controls viral infections immediately after contact with the pathogens in order to limit the viral spread and shape an efficient development of adaptive immune response. (9,10) One of the main players in the innate response during viral infection are Natural Killer (NK) cells, which exert both direct cytotoxicity of infected cells and non-cytolytic activity through the release of a large amount of IFN-γ, a relevant cytokine able to control the pathogenic processes by limiting viral production and spread. (11)
However, several studies indicate that NK cells from both peripheral blood (PB) and hepatic compartment of patients (pts) with chronic hepatitis B (CHB), along the progression of the infection, display functional defects that limit their antiviral efficacy. Among them, the marked reduction in IFN-γ release represents a major flaw. (12–14) Conversely, NK cell cytotoxic activity is maintained in CHB pts, suggesting that NK cells might also be implicated in liver damage associated with the chronic infection. (15) Dendritic cells (DCs) sustain NK cell activity during viral infection and several studies have demonstrated that a relevant functional cross-talk occurs between these two cell populations soon after their interaction. (16–18) Among the functional events that take place upon NK/DC interaction a critical role has been assigned to IL-12 and IL-15, which act as potent inducers of NK cell effector functions. IL-12 secretion by DCs efficiently promotes the production of IFN-γ by NK cells, while IL-15 is mainly involved in the stimulation of NK cell proliferation and survival. (19,20) Activated NK cells, in turn, are able to eliminate immature DCs, expressing lower levels of HLA class I, that would not be able to optimally prime a T cell response, rather leading to immune tolerance. Thus, NK cells are thought to play an important regulatory role by selectively editing DCs during the course of immune response. (16,21,22) DC dysfunctions during HBV infection have already been reported, mainly in terms of defective maturation state and a skewed balance toward IL-10 production at the expense of IL-12 release. (23–25) These recent findings raise the possibility that the antiviral functions of NK cells could also be negatively affected. To gain more insight into the effect of HBV on different aspects of NK/DC cross-talk, in this study (i) we have characterized the ability of human DCs exposed to HBV to stimulate proliferation, cytokine release and cytotoxicity of NK cells and (ii) we have investigated whether NK cells can edit autologous DCs and their immunogenic properties.
Materials and Methods
Samples collection
Peripheral blood mononuclear cells (PBMCs) samples and sera were collected at the University Hospital “G. Martino” (University of Messina) either from healthy donors (HD) admitted to the blood transfusion center or from CHB pts from the Liver Unit. The institutional ethics committee approved the study and all participants gave written informed consent according to the Declaration of Helsinki. CHB pts had been treated with anti-HBV antiviral therapy and followed-up by testing for liver biochemistry and HBV DNA every 3–6 months once antiviral therapy was started. HBV DNA became undetectable from month 6 after therapy initiation. Sera utilized in this study were collected on the day of therapy initiation and after 18–24 months (Table S1). PBMCs were collected from HD and pts with (i) with HBeAg-negative chronic hepatitis and high serum HBV DNA levels (> 80.000.000 IU/mL) and (ii) under nucleos(t)ide analogs (NUC)-therapy with complete control of the infection (HBV DNA negative/antibody to hepatitis B surface antigen positive), and with (iii) HBeAg-negative chronic infection (inactive carrier status with serum HBV DNA levels <2.000 IU/mL). Pts characteristics are summarized in Table S2. All pts enrolled in this study were negative for antibodies against hepatitis C, hepatitis D and human immunodeficiency viruses. Sera and PBMCs were stored at −80° C until use. IL-10 concentration was assessed on both CHB and HD sera by ELISA assay.
Serum HBV DNA and HBsAg Quantifications.
Serum HBV DNA was quantified using the COBAS AmpliPrep-COBAS TaqMan HBV test (Roche Diagnostics, Monza, Italy) with a lower limit of detection of 20 IU/mL. HBsAg was quantified on the same sera with LumipulseGHBsAg-Quant assay according to the manufacturer’s instructions [Fujirebio Italia, Pomezia (Roma), Italy], with the lowest limit of detection of HBsAg at 0.005 IU/mL.
Cell Purification and Isolation
Monocytes isolated from HD-PBMCs were sorted as CD14+ by using FACSAria cell sorter (BD Bioscience, NJ, USA) and cultured in the presence of rhIL-4 (20 ng/mL, Miltenyi Biotec, Germany) and rhGM-CSF (25 ng/mL, Sargramostim, Leukine©) in RPMI 1640 medium supplemented with 10% fetal bovine serum and with penicillin (100 U/ml) and streptomycin (100 μg/ml). (26) Following a 6 day of incubation at 37°C, non-adherent cells displayed the CD14-/CD11c+/CD83- phenotype, which is characteristic of immature DCs. Alternatively, BDCA1+DCs were directly isolated from HD or CHB pts at different stage of infection, as Lin-(CD19, CD3, CD14)/HLADR+/CD11c+/BDCA1+.
NK cells were isolated from both HD and CHB pts by negative magnetic separation (NK Cell Isolation Kit/ Miltenyi Biotec, Germany) or sorted as CD3-/CD56+ by FACSAria cell sorter. Cell populations sorted displayed a higher than 95% purity.
mAbs and multicolor flow cytometry
The mAbs used in this study are listed in Supplemental information Table S3. mAbs specific for Ag-presentation machinery subunits (27,28), listed in Table S4, were purified from mouse ascites by affinity chromatography on Protein G. The purity and activity of mAb preparations were monitored by SDS-PAGE and by binding assays with cells with the appropriate phenotype. Sample acquisition was performed on FACSCantoII or FACSymphony (BD Biosciences, NJ, USA) flow cytometers. Data were acquired by FACS Diva (BD Biosciences, NJ, USA) and analysed by FlowJoVX (Tree Star Inc, OR, USA) software.
RT-PCR
mRNA was isolated from immature DCs exposed for 24h or not to HBV (DCs or HBV-DCs). mRNA was isolated by using RNeasy MicroKit (QIAGEN, Germany). cDNA was synthesized by using Quantitect Reverse Transcription Reagents (QIAGEN, Germany) and IDO gene expression (Cat. Num. Hs00984148_m1, ThermoFisher Scientific, MA, USA) was assayed by qPCR (Quant Studio DX real-time PCR system ThermoFisher Scientific, MA, USA). mRNA content was normalized to β-actin expression. Mean relative gene expression was determined by using DDCT method.
Statistical Analysis
A paired Student’s t-test or One-Way Anova test was used to evaluate statistical significance. P-values lower than 0.05 were considered statistically significant. Statistics were calculated using GraphPad Prism 4 software.
Supplemental Information
Supplemental information includes figures, tables and detailed experimental procedures.
Results
DCs exposed to HBV are impaired in maturation and in inducing IFN-γ production by NK cells
The major functional defect of NK cells observed in HBV patients is an impairment in INF-γ production. (12–14) Because DCs represent early activators of NK cell response (16), we analysed whether HBV could affect the ability of monocyte-derived DCs to stimulate INF-γ secretion by NK cells. We first analysed the stimulatory capability of DCs treated with purified HBV virions and we observed a low IFN-γ production by NK cells, thus suggesting that HBV exerts a weak activating effect on DCs (Figure 1A). Then, we evaluated whether HBV virions can exert an inhibitory effect on DCs also when they are stimulated by a strong maturation stimulus such as Poly I:C, a potent TLR-3 ligand mimicking viral infection. DCs were stimulated by Poly I:C, in the presence or absence of HBV particles for 24h and the production of IFN-γ by NK cells was evaluated upon DC stimulation. Our results showed that Poly I:C-stimulated DCs, in the presence of HBV, display reduced capabilities to stimulate IFN-γ production by NK cells (Figure 1A, S1). IFN-γ production by NK cells is largely sustained by IL-12 produced by DCs. (19,20) We therefore analyzed the production of IL-12 by DCs exposed to HBV either before or following Poly I:C stimulation. HBV fails to induce IL-12 production by DCs and, remarkably, the abundant release of this cytokine occurring following TLR3-ligand stimulation was drastically reduced in the presence of HBV particles (Figure 1A).
Figure. 1. IL-12/IL-10 skew in HBV-exposed DCs results in impaired DC maturation and production of IFN-γ by NK cells.
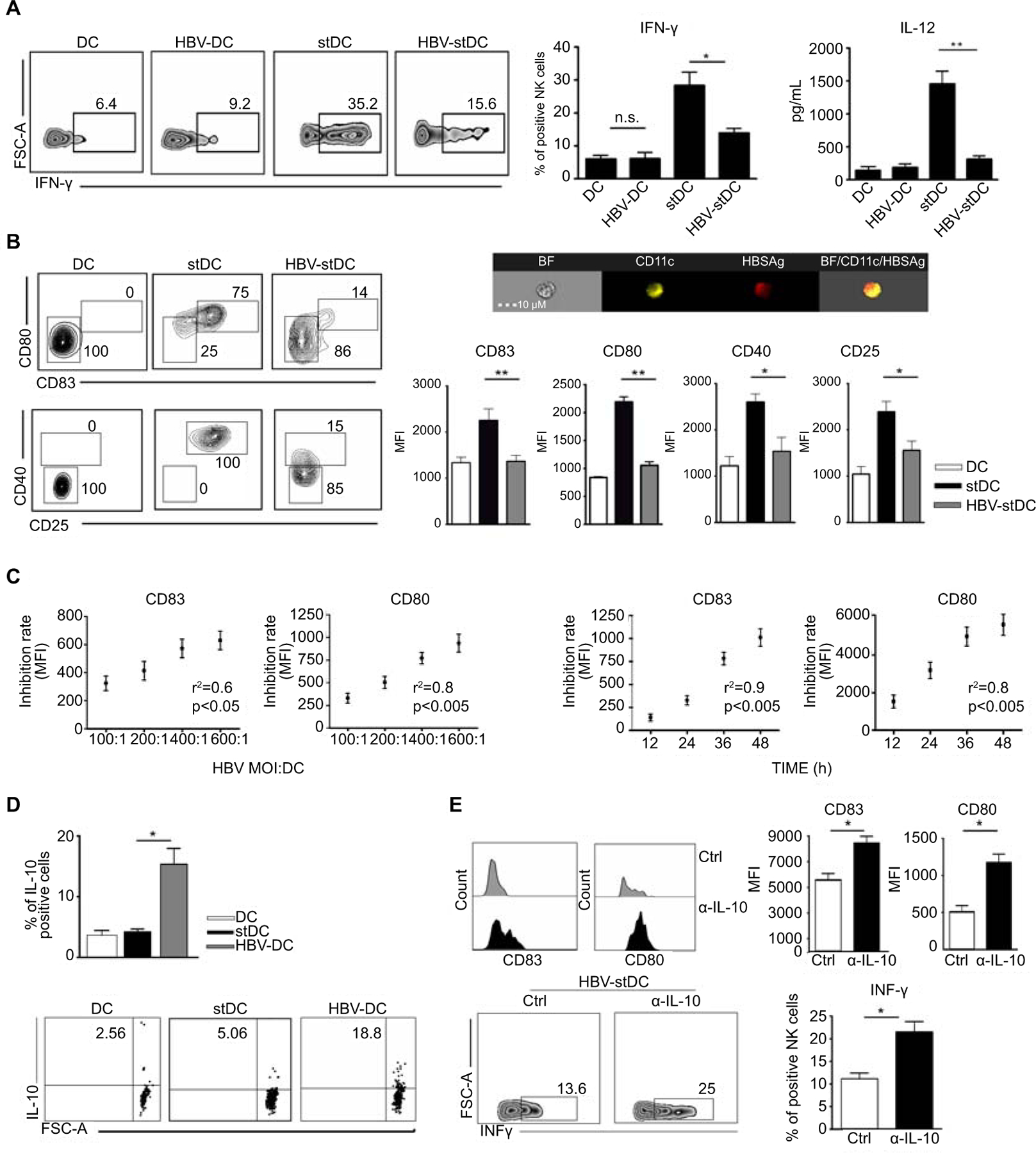
(A) Representative dot plots and relative statistical analysis show the percentage of IFN-γ positive NK cells assessed by intracellular staining, following co-culture with immature DCs exposed or not to HBV virions (HBV-DCs or DCs) or Poly I:C-stimulated DCs exposed or not to HBV virions (HBV-stDCs or stDCs). Bars diagrams represent mean values ± SEM of IFN-γ+ cells (n=6); Associated IL-12 concentration was detected in culture supernatants of DCs in the same culture conditions. Bars diagrams represent mean values ± SEM of cytokine concentration (n=3).
(B) Representative dot plots and Mean Fluorescence Intensity (MFI) values of costimulatory/activation molecules analyzed on DCs, stDCs, HBV-stDCs. Bars diagrams represent mean values ± SEM from seven independent experiments; Representative multispectral imaging flow cytometry shows DC internalization of HBV virions assessed upon 24h of viral exposure.
(C) Correlations between either HBV virion dose or time of exposure and inhibition rate of DC expression of the indicated molecules. Graphs represent difference of MFI values between stDCs and HBV-stDCs.
(D) IL-10 production was assessed on DCs, stDCs and HBV-DCs upon 24h of culture by intracellular staining. Bars represent mean values ± SEM obtained in three independent experiments.
(E) HBV-stDCs maturation and the induced IFN-γ production by NK cells were assessed in the presence of anti-IL-10 blocking mAb. Bars represent mean values ± SEM obtained in at least three independent experiments.
*p <0.05, **p <0.005
It has been previously reported that DCs from CHB patients are not able to acquire a proper activated phenotype. (24,25) We confirmed that HBV can be internalized by DCs, as assessed by multispectral imaging flow cytometry after 24h of exposure to HBV (Figure 1B, S2) and can at the same time inhibit DC maturation upon stimulation by Poly I:C (Figure 1B). DC impairment in the expression of the main costimulatory molecules and activation markers upon exposure to HBV virions was time- and dose-dependent (Figure 1C). One potential mechanism underlying HBV immune evasion relies on increased level of IL-10 (24,25). In our setting, HBV could induce IL-10 production by DCs (Figure 1D) that exerted both autocrine and paracrine effect on DCs and NK cells respectively, since IL-10 blocking restored both DC maturation and, in turn, IFN-γ production by NK cells (Figure 1E, S3).
HBV-exposed DCs can express high levels of HLA class I molecules thus eluding NK cell killing
NK cells play an important regulatory role by editing autologous immature, potentially tolerogenic, DCs that would not be able to optimally prime T-cells during the adaptive immune responses. DC editing process by NK cells is mainly regulated by the level of HLA class I molecule expression on DCs. It is well established that mature, activated, DCs upregulate the expression of not only co-stimulatory molecules but also of HLA molecules. NK cells are then able to discriminate between mature and immature DCs by killing the latter because of their lower HLA class I expression level on DC membrane. (16,22,30)
Therefore, beside the classical maturation markers, we also analysed HLA class I expression on DCs upon exposure to HBV. Remarkably, HBV was able to upregulate HLA class I expression on DCs, despite it prevented DC maturation in terms of T cell costimulatory molecules (Figure 2A).
Figure 2. HBV-exposed DCs are protected from NK cell killing by the expression of high level of HLA-I molecules.

(A) HLA class I expression was assessed on DCs (dotted line), stDCs (black line), HBV-DCs (grey line) by flow cytometry. One representative experiment out of three is shown. Bars represent mean values ± SEM HLA-I+ cells obtained in three independent experiments.
(B) NK cell cytolytic ability was assessed in the presence of anti-HLA class I blocking mAb or isotype matched irrelevant antibody (IgM ctrl). Cytotoxicity was assessed as percentage of CD107a+ NK cells following culture with autologous DCs, stDCs, HBV-stDCs. Bars represent mean values ± SEM of CD107a+ cells obtained in at least three independent experiments
(C) Expression of CCR6 and CCR7 was assessed by flow cytometry on DCs, stDCs or HBV-stDCs. One experiment out of four performed is shown.
Functional experiments, performed to evaluate in this context DC killing by NK cells, revealed that HBV-DCs were protected from autologous NK cell killing (Figure 2B) because of their increased HLA class I expression. Thus, HBV-DCs are not able to acquire T cell stimulating activity, but preserve the capability of upregulating the expression of HLA class I molecules, thus sheltering themselves from NK cell attack. These results suggest that HBV might allow DCs to survive and re-circulate in an immature and potentially tolerogenic state.
In agreement with this hypothesis, despite their immature state, when stimulated by Poly I:C, HBV-DCs down-regulate CCR6 expression and up-regulate CCR7 expression, thus acquiring also the potential to gain access to lymph nodes, where they could potentially exert a tolerogenic role (Figure 2C).
NK cells fail to induce the expression of antigen presentation machinery components in HBV-exposed DCs
HBV-exposed monocyte-derived DCs have been reported to induce a lower T-cell response. It has been proposed that their antigen-presenting capacity may be functionally defective during HBV infection. (25) A main mechanism involved in the Ag-presenting capability of DCs is ensured by the correct assemblement of their Ag-presentation machinery. The proteasome, a large protein complex responsible for degradation of intracellular proteins, is expressed in all non-immune cells and consists of a set of constitutive subunits. Immunoproteasomes (iP), an alternative derived form of proteasome, composed by the coordinate induction of IFN-γ-inducible subunits, such as LMP2, LMP7 and LMP10, is selectively expressed in activated DCs and can enhance their ability to generate antigenic peptides. (31,32)
When DCs were stimulated by IFN-γ in the presence of HBV, we observed a defective expression of iP subunits LMP-2 and LMP-7, while the expression of constitutive proteasome components does not seem to be altered (Figure 3A).
Figure 3. NK cells can induce immunoproteasome subunits expression in DCs, but fail in HBV-DCs.

(A) The expression of immunoproteasome (iP) subunits was assessed by flow cytometry in DCs or HBV-DCs stimulated with rh-IFN-γ. mAbs specific for the indicated subunits were employed. Bars represent mean values ± SEM of MFI obtained in four experiments.
(B) Expression analysis of LMP-2 and LPM-7 iP subunits was assessed in stDCs or HBV-stDCs upon co-culture with NK cells. IFN-γ stimulated DCs were employed as positive control. Bars represent mean values ± SEM of MFI obtained in five experiments.
*p <0.05, **p <0.005.
Considering that one of the main outcomes of NK/DC cross-talk is the IFN-γ release by NK cells we investigated whether NK cells may directly have a role in the expression of iP repertoire in DCs treated or not with HBV virions.
Interestingly, we found that NK cells were able to effectively induce the expression of both LMP2 and LMP7 iP subunits in control DCs but they failed in DC exposed to HBV (Figure 3B). The failure of NK cells to induce iP subunit expression in HBV-DCs is consistent with the lower IFN-γ production during their cross-talk with DCs.
HBV inhibits DC-induced NK cell proliferation by triggering IDO expression in DCs
DCs sustain NK cell activity also by inducing their proliferation, which is associated with better control of viral infection. (16) Therefore, we assessed whether HBV could influence DC-induced NK cell proliferation.
HBV impaired the ability of DCs to promote proliferation of resting NK cells, since the frequency of Ki67+ NK cells upon stimulation with DCs was significantly lower in the presence of HBV (Figure 4A).
Figure. 4. HBV inhibits DC-induced NK cell proliferation by upregulating IDO expression in DCs.

(A) NK cell proliferation was assessed as percentage of Ki67+ NK cells upon 5 days of co-culture with stDCs or HBV-stDCs. Bars represent mean values ± SEM of Ki67+ cells. (n=4).
(B) Expression of mbIL-15 and IL-15Rα was assessed on DCs, HBV-DCs and stDCs. Bars represent mean values ± SEM of positive cells. (n=3).
(C) Flow cytometry analysis of IDO expression and (D) RT-PCR analysis of IDO mRNA assessed in DCs or HBV-DCs. Bars represent mean values ± SEM of IDO+ cells. (n=4).
(E) NK cell proliferation was assessed as percentage of Ki67+ NK cells upon 5 days of co-culture with HBV-stDCs in the presence or absence of IDO inhibitor 1MT or stDCs as control. Bars represent mean values ± SEM of Ki67+ cells obtained in four experiments.
*p <0.05, **p <0.005
In this regard, previous studies reported a reduced NK cell frequency in HBV infected patients. (33,34) The defective ability of DCs exposed to HBV to stimulate NK cell proliferation might play a role in this reduction. In an attempt to find a possible explanation for the inhibition of NK cell proliferation following DC stimulation, we evaluated the main mechanisms involved in this process.
IL-15 is a relevant cytokine involved in DC-induced NK cell proliferation that exerts its biological effects in a membrane-bound form (mbIL-15), requiring the expression of IL-15Rα for its trans-presentation to NK cells. It is noteworthy that DCs have been shown to upregulate both mbIL-15 and IL-15Rα following TLR stimulation. (20) We analyzed the expression of these molecules on HBV-DCs and found that HBV did not affect the expression of both mbIL-15 and its receptor on DCs, which therefore maintain their capability for IL-15 trans-presentation to NK cells (Figure 4B).
Considering that HBV could skew DCs toward a potentially tolerogenic phenotype, we analysed also the expression of Indoleamine 2,3 dioxygenase (IDO), a tolerogenic enzyme involved in suppression of both T and NK cell proliferation. (35,36) We observed that exposure to HBV significantly increased both IDO protein and mRNA expression in DCs (Figure 4C, D). To confirm the involvement of IDO in the inhibition of DC-induced NK cell proliferation, we performed NK/DC co-culture experiments in the presence of the specific IDO inhibitor, 1-Methyl Tryptophan (1MT). We found that NK cell proliferation was significantly restored in the presence of 1MT, indicating that HBV impairs the capability of DCs to stimulate NK cell expansion by upregulating IDO expression (Figure 4E).
HBV-exposed DCs are competent in stimulating NK cell cytotoxicity
NK/DC cross-talk can enhance NK cell cytolytic ability in order to remove infected or neoplastic cells. (16) Thus, we investigated whether HBV can also alter DC capability to support NK cell cytotoxic activity.
To this purpose, resting NK cells were cultured with DCs, previously stimulated by Poly I:C, either in the presence or absence of HBV, and then tested for their killing ability against tumor cells, such as the classical NK cell target K562 eritro-leukemia cell line and the liver cancer cell line HepG2.
Although HBV-exposed DCs were poor stimulators of IFN-γ production by NK cells, they maintain the ability to enhance NK cell cytotoxicity against both K562 and HepG2 cells, assessed by either target-based killing assay or degranulation assay (Figure 5A, B).
Figure 5. HBV does not affect the ability of DCs to potentiate NK cell cytotoxic.

(A) NK cell cytolytic ability was assessed by both target-based killing assay and (B) CD107a degranulation assay. NK cells were previously co-cultured with stDCs or HBV-stDCs for 12h. Then, HepG2 or K562 target cell lines were added to the cultures for additional 6h. Analysis of killed target cells, identified as PKH-26+/7AAD+ cells, or frequency of CD107a+ NK cells were assessed by flow cytometry. Ctrl: NK cells and target cells. One representative experiment for each assay is shown. Bars represent mean values ± SEM of a triplicate.
Sera of CHB patients can impair DC functions, which are restored upon antiviral therapy
HBV particles are known to be present in CHB pts sera and the amount of HBV DNA is used to establish viral load. Therefore, we tested whether sera collected from these pts might be sufficient to alter DC activity.
Sera of CHB pts induced significant dysfunctions in DCs in terms of both expression of costimulatory molecules/activation markers and capability to induce IFN-γ production by NK cells (Figure 6A-B). Moreover, by analysing in detail the features of CHB pts sera, we observed that inhibition of DC maturation associated with viral load but not with HBsAg sera levels (Figure 6C).
Figure 6. DC functions are impaired in the presence of CHB sera but restored upon antiviral therapy.

(A) Expression of the indicate molecules was assessed on DCs and stDC in the presence of either CHB or HD sera, as control. One representative experiment out of six is shown. Bars represent mean values ± SEM of MFI of the indicated molecules.
(B) Representative dot plots and statistical analysis show IFN-γ+ NK cells following co-culture with stDCs, previously cultured in the presence of serum derived from CHB pts or HD. Bars represent mean values ± SEM of IFN-γ+ cells (n=5).
(C) Correlation between MFI values of CD80 and either viral load or HBsAg level contained in the four different CHB sera employed for DC culture. Correlation was expressed as correlation coefficient r2.
(D) IL-12 concentration was analysed in culture supernatants of stDCs cultured in the presence of sera collected from four CHB pts either before or after antiviral-therapy. Bars represent mean values ± SEM of IL-12 production by stDCs in four independent experiments.
**p <0.005, ***p<0.0005
Antiviral therapy with nucleoside analogs is effective in reducing viral load (37) and in CHB pts who undergo antiviral treatment IFN-γ production by NK cells is restored. (38) Therefore, we investigated whether DCs cultured with sera collected from pts undergoing antiviral therapy similarly restored IL-12 production. Remarkably, in all four pts analysed, the amount of IL-12 produced by DCs was significantly increased when DCs were stimulated in the presence of sera collected following antiviral therapy (Figure 6D). These results indicated that effective anti-viral therapy can result in the recovery of DC functions.
Circulating BDCA1+DC exposed to both HBV and CHB sera are poor stimulators of NK cell antiviral function
Besides monocyte-derived DCs, BDCA1+DCs represent another main human DC subset circulating in peripheral blood (39). Similarly to monocyte-derived counterpart, the ability to activate NK cells, enhance their cytolytic activity and inducing IFN-γ release, has been previously reported also for BDCA1+DCs. (40,41) On the other hand, this subset of myeloid circulating DCs have only recently be explored in the context of HBV infection. (42) We therefore investigated whether HBV might affect the cross-talk with NK cells also in BDCA1+ DCs.
In the presence of both purified HBV and serum from CHB pts, blood BDCA+DCs displayed significant impairment in the expression of the main maturation markers and when cultured with NK cells, they failed in inducing IFN-γ production (Figure 7A-B). The strong inhibition observed in CHB serum condition might suggest that, in addition to virus, other soluble factors could exert an inhibitory effect on DCs, such as the immunosuppressive cytokine IL-10. (43) In agreement with the above-mentioned results, sera of CHB pts contained relevant amount of IL-10 that associated with DC inhibition (Figure 7C). Moreover, as their monocyte-derived DC counterpart, HBV-BDCA1+DCs showed an increased expression of IDO, at both mRNA and protein level, suggesting a skewing toward a more tolerogenic profile (Figure 7D). These results indicate that circulating BDCA1+DCs could be functionally impaired in CHB patients because of HBV presence and might in turn negatively cross-regulate NK cell antiviral function.
Figure 7. Circulating BDCA1+DCs are functionally impaired in the presence of HBV, resulting in defective interactions with NK cells.

(A) Expression of activation markers was assessed on stBDCA1+DCs cultured in the presence of HBV particles or CHB sera. Ctrl: stBDCA1+DCs in HD sera. Bars represent mean values ± SEM of MFI of the indicated molecules. (n=5)
(B) Representative dot plots show IFN-γ production by NK cells cultured with stBDCA1+DCs in the presence of either HBV particles or CHB serum. Ctrl: Cultures performed in HD serum. One representative experiment is shown. Bars represent mean values ± SEM of IFN-γ+ cells obtained in five different experiments.
(C) IL-10 concentration was assessed by ELISA assay in CHB or HD sera, as control. IL-10 content correlation with the expression of CD80 on stBDCA1+DCs cultured in CHB sera. Bars represent the mean values ± SEM of IL-10 concentration.
(D) Flow cytometry and RT-PCR analysis of IDO expression was assessed on BDCA1+DCs exposed or not to HBV (n=4). Bars diagrams represent mean values ± SEM of the percentage of cells expressing respectively either IDO enzyme or the associated mRNA.
*p <0.05, **p <0.005 ***p <0.0005.
Circulating BDCA1+DCs and NK cells from CHB patients display an altered cross-talk
In order to investigate whether the impairment of NK-DC cross-talk in the presence of HBV, observed in vitro, may also occur in CHB pts, we isolated and analysed circulating BDCA1+DCs and NK cells directly from pts. In order to obtain a clearer picture of NK-DC interplay during CHB, we collected peripheral blood from pts in different virological conditions: (i) with HBeAg-negative chronic hepatitis and high serum HBV DNA levels (> 80.000.000 IU/mL) and (ii) under nucleos(t)ide analogs (NUC)-therapy with complete control of the infection (HBV DNA negative/antibody to hepatitis B surface antigen positive), and with (iii) HBeAg-negative chronic infection (inactive carrier status with serum HBV DNA levels <2.000 IU/mL) (Table S2). We then analyzed CHB BDCA1+DCs and NK cell separately, by crossing them with NK cells and BDCA1+DCs healthy counterpart respectively, with the aim of understanding the relative contribution of each cell type in sustaining antiviral response. Figure 8B depicts a shift in BDCA1+DC capability to stimulate NK cell activity between HBeAg-negative chronic hepatitis and the other virological conditions. In particular, BDCA1+DCs isolated from pts with HBeAg negative chronic hepatitis failed in inducing optimal amount of IFN-γ by NK cells, and this capability was partially recovered in HBeAg-negative chronic hepatitis pts under NUC treatment and virtually unaltered in BDCA1+DCs isolated from pts with HBeAg-negative chronic infection compared to HD control. NK cell functionality differed among the distinct virological conditions of chronic HBV infection. Even if stimulated by fully competent healthy DCs, NK cells isolated from patients with HBeAg-negative chronic hepatitis displayed an impaired IFN-γ production that, instead, appeared relatively constant and adequate in inactive carriers and in patients under NUC-therapy (Figure 8A). Altogether, these data revealed that the reduction of IFN-γ produced by NK cells can depend on an impaired cross-talk with DCs; however, in pts with HBeAg-negative chronic hepatitis, NK cells can be already impaired, most likely because of an altered microenvironment associated to the high levels of viral particles and antigens present in these pts.
Figure 8. BDCA1+DCs directly isolated from HBeAg-negative chronic hepatitis pts display subverted functions and impaired NK cell activating properties.

(A) BDCA1+DCs and NK cells were isolated from pts with (i) HBeAg-negative chronic hepatitis and high serum HBV DNA levels (> 80.000.000 IU/mL) or (ii) under NUC-therapy and with (iii) HBeAg-negative chronic infection (inactive carrier status with serum HBV DNA levels <2.000 IU/mL). Representative dot plots show IFN-γ production by HD-NK cells cultured with stBDCA1+DCs derived from pts at the indicated virological conditions (upper dot plots) or by NK cells derived from the same pts at the different virological conditions cultured with HD stBDCA1+DCs (lower dot plots). Results shown are representative of n=2 HBeAg-negative chronic hepatitis, n=4 HBeAg-negative chronic hepatitis under NUC-therapy, n=3 HBeAg-negative chronic infection (inactive carrier). As control, co-culture of NK cells and DCs isolated from HDs are depicted (upper central dot plot) (n=3).
(B) HBV DNA was assessed in BDCA1+DCs isolated from 4 CHB pts (HBsAg-positive) and 2 HBsAg-negative individuals by using nested-PCR and specific primers for Core, Pol, S, and X HBV genomic regions. M, DNA molecular-weight marker; amplification product size is indicated along left or right margin. Negative control: DNA-free reaction buffer from 1sr round PCR; Negative control 2: DNA-free reaction buffer from 2nd round PCR; Positive control: HBV DNA derived from the recombinant plasmid pFC80, which contains head-to-tail tetramer of full-length HBV DNA (ayw) inserted in the EcoRI site of pBR322.
(C) Flow cytometry and RT-PCR analysis of IDO expression (upper panel) and PD-L1 expression (lower panel) was assessed in BDCA1+DCs isolated from pts with HBeAg-negative chronic hepatitis. Bars represent mean values ± SEM of the percentage of cells expressing respectively either IDO enzyme or the associated mRNA, or the frequency of PD-L1+ cells. (n=3)
(D) NK cell proliferation was assessed by flow cytometry as percentage of Ki67+ NK cells upon 5 days of co-culture with stBDCA1+DCs directly isolated from pts with HBeAg-negative chronic hepatitis. Proliferation assay was carried out in the presence or absence of IDO inhibitor 1MT. One representative experiment out of three is shown. Bars represents mean value ± SEM of Ki67+ cells obtained in triplicate experiments.
In line with the significant NK/DC cross-talk impairment observed in pts with HBeAg-negative chronic hepatitis, BDCA1+DCs freshly isolated from this pts showed the presence of HBV genomic DNA sequences, as Core, Pol, S and X viral genomic regions (Figure 8B), which were not detectable in T lymphocytes, isolated from the same pts, used as control (Figure S4). Moreover, circulating BDCA1+DCs isolated from pts with HBeAg-negative chronic hepatitis displayed tolerogenic features as demonstrated by high level of IDO and expression of the inhibitory immune checkpoint ligand PD-L1 (Figure 8C). In addition to induce low level of IFN-γ production, DCs isolated from these patients were poor stimulators of NK cell proliferation that, similarly to in vitro results, was rescued in the presence of IDO inhibitor (Figure 8D). In conclusion, results obtained investigating the cross-talk between DCs and NK cells directly isolated from chronically HBV infected pts with different viremia levels are in line with data obtained upon in vitro exposure to HBV and confirmed the abnormal NK/DC interplay occurring during HBV infection.
Discussion
NK cell functions are finely regulated by the interaction with other immune cells, especially DCs. NK cells in CHB infection might be altered per se (12–15) or, as our study suggests, may be defective because of an altered cross-talk with DCs.
Our data, obtained either upon in vitro cell exposure to the virus or directly isolating cells from CHB pts, reveal that DCs, in the presence of HBV, are not able to properly activate NK cells. In particular, when exposed to HBV, DCs are poor stimulators of both IFN-γ release and proliferation of NK cells, events both relevant for the optimal control of viral spread.
NK cell functional defects are usually combined and an impaired cytotoxicity is usually associated with a deficiency in cytokine production. Recent findings, however, have indicated that NK cells in CHB are characterized by a functional dichotomy, featuring a conserved or enhanced cytotoxicity and a concomitant reduced production of IFN-γ and tumor necrosis factor-α. (13,14)
In line with this observation, in our setting, DCs maintain the capability to enhance NK cell cytotoxicity. The mechanism underlying the conserved capability of HBV-DCs to stimulate NK cell cytotoxicity represents a point that calls for further investigation. In this regard, in Chronic Hepatitis C Virus infection (HCV), an altered IFN-α signaling has been reported. This defect in cytokine production is caused by an increased IFN-α-stimulated STAT1 phosphorylation polarizing NK cells toward cytotoxicity and a concomitant reduced IFN-α-induced STAT4 phosphorylation yielding to reduced IFN-γ mRNA levels in NK cells. As a result, HCV-induced IFN-α leads to chronic liver inflammation via cytotoxic mechanisms but without achieving viral clearance because of insufficient IFN-γ production. (44) In HBV infection, this functional dichotomy has not been completely clarified and DCs, as well as the signals they provided to NK cells, could be crucial to explain this phenomenon. Moreover, although it is largely believed that HBV-related CHB is associated with an inadequate response of liver-infiltrating Cytotoxic T Lymphocytes (CTL), a loco-regional skewing towards cytotoxic activity by NK cells, which are abundant in the liver where they represent up to 31% of the hepatic lymphocytes, might significantly contribute to the progression of liver injury (45)
On the other hands, it is well known that NK cells, by editing immature DCs ensure the survival of the most immunogenic DCs (16,22) HBV-DCs, despite their immature features in terms of costimulatory molecule expression and cytokine release, would escape from immune-editing mediated by NK cell because of the upregulation of HLA class I, fundamental to activate CTL but, at the same time, also to restrain their selection by NK cells.
Along the same line, antigen machinery components, crucial for the antigen presentation capability of DCs are also altered by HBV. In particular, we here showed that LMP2 and LMP7 subunits of iP are not expressed in HBV-exposed DCs. A fully functional iP in DCs is of paramount importance in the processing of antigens by the endogenous pathway. LMP2 loss has been shown to have a marked effect on CTL repertoire. Indeed, although LMP7 and LMP10 are able to assemble functional iP, the lack of LMP2 results in a functionally defective iP. (32,44,46) We have here showed that NK cells have the potential to induce iP subunits in DCs but the presence of HBV makes them refractory to NK cell stimulation, most likely because of the insufficient IFN-γ production during their interactions. As a result, DCs in CHB pts might recirculate displaying non-immunogenic features accompanied by an incomplete or defective assembly of iP. These abnormalities would be highly detrimental for their antigen presentation, thus possibly imprinting a tolerogenic response and/or inducing a defective anti-viral adaptive response. In line with that, circulating BDCA1+DCs derived from CHB pts express high level of the tolerogenic enzyme IDO, which is involved in the suppression of proliferation of different lymphocyte subsets, including NK cells, as we have here shown. Altogether, these latter findings reveal how HBV, affecting the cross-talk between DCs and NK cells, might cause a broad immune impairment leading to an inefficient response of both the innate and adaptive arms, therefore unable to efficiently control viral spread.
It is clear that the outcome of HBV infection is the result of complex interactions between HBV and the host immune system. Pts suffering from chronic infection have a weak or at least functionally impaired immune response leading to a state of immune tolerance. This facilitates HBV persistence and, potentially, also an insufficient control of other concomitant infections that may occur in CHB pts. (5–7)
Despite current antiviral therapies are able to exert a potent suppression of HBV replication in liver, a viral rebound often occurs upon cessation of treatment, thus resulting in a failure to completely cure HBV infection. This clinical scenario is most likely caused by the inability of the immune system to recognize cells harboring HBV and to eliminate cells actively producing the virus. Specific immunotherapies can offer the possibility to eradicate or at least to maintain low levels of HBV replication under the control of a functional host antiviral response. Different therapeutic strategies have been lately developed, targeting both innate and adaptive immunity. The latter have been designed to enhance HBV-specific T cell responses, while the former aim at enhancing innate responses exploiting the antiviral efficacy of cytokines or PRR agonists. (47)
Our current data support the potential of DC-based immunotherapy approach for the treatment of CHB, since the recovery of DC functions might restore NK cell antiviral activity as well as their cross-talk, with a consequent optimal activation also of antigen-specific CTL. Moreover, the possibility to combine a DC-based immunotherapy, aimed to obtain an effective immune response, with antiviral agents targeting HBV replication could render current treatments more effective and therapeutically advantageous for pts affected by CHB.
In conclusion, our data provide information about the complex virus-host immune interactions highlighting the relevant role of DCs in the impairment of NK cells observed in CHB pts. The altered NK/DC cross-talk caused by HBV might represent another possible mechanism leading to persistent infections and should be taken into account for designing novel immune-therapeutic strategies to manage chronic HBV infection and counteract the associated infectious co-morbidities.
Supplementary Material
Acknowledgments
Financial Support
This work was in part supported by grants awarded by Associazione Italiana Ricerca sul Cancro (AIRC) IG11650; by Programma Operativo Fondo Europeo di Sviluppo Regionale (FESR) 2007–2013 - linea d’intervento 4.1.2.A; by Italian Ministry of Health, Ricerca Finalizzata 2018; by Italian Ministry of Education, University and Research (MIUR), PRIN 2017 (to G.F.); by Gilead Sciences Fellowship Program FP-2015–365 (to T.P.); by NIH grants RO1DE028172, RO3CA239193 and by DOD grant W81XWH-16–1-0500 (to S.F.).
List of Abbreviations
- DC
Dendritic cells
- NK
Natural Killer
- CHB
Chronic Hepatitis B
- Poly I:C
Polyinosinic:polycytidylic acid
- iP
Immunoproteasome
- IDO
Indoleamine-2,3-dioxygenase
Footnotes
Conflict of Interest: The authors declare no conflict of interest that pertains to this work.
References
- 1.Global Hepatitis Report, 2017. ISBN 978–92-4–156545-5_World Health Organization 2017 [Google Scholar]
- 2.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection J. Hepatol 2017;67(2):370–398 [DOI] [PubMed] [Google Scholar]
- 3.Liang TJ. Hepatitis B: the virus and disease. Hepatology 2009; 49:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med 2007;204 (3):667–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen MH, Lim JK, Burak Ozbay A, Fraysse J, Liou I, Meyer N et al. Advancing Age and Comorbidity in a US Insured Population‐Based Cohort of Patients With Chronic Hepatitis B. Hepatology 2019; 69(3):959–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu A, Le A, Zhang J, Wong C, Wong C, Henry L et al. Increasing co-morbidities in chronic hepatitis B patients: experience in primary care and referral practices during 2000–2015. Clin Transl Gastroenterol 2018; 9(3): 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei XL, Qiu MZ, Jin Y, Huang YX, Wang RY, Chen WW et al. Hepatitis B virus infection is associated with gastric cancer in China: an endemic area of both diseases. Br J Cancer 2015;112 (7):1283–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg W. Mechanisms of immune escape in viral hepatitis. Gut 1999; 44(5): 759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol 2003;4:175–81 [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol. Rev 2009;227:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY et al. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol 2002; 169(8):4279–87. [DOI] [PubMed] [Google Scholar]
- 12.Lunemann S, Malone DF, Hengst J, Port K, Grabowski J, Deterding K et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis 2014;209 (9):1362–73 [DOI] [PubMed] [Google Scholar]
- 13.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 2009;137:1151–1160. [DOI] [PubMed] [Google Scholar]
- 14.Maini MK, Peppa D. NK cells: a double-edged sword in chronic hepatitis B virus infection. Front Immunol 2013;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Zhang S, Zou Z, Shi J, Zhao J, Fan R, et al. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatology 2011; 53(1):73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferlazzo G, Tsang M, Moretta L, Melioli G, Steinman R, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med 2002; 195:343–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal Activating Interaction between Natural Killer Cells and Dendritic Cells. J Exp Med 2002; 195(3):327–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol 2003;4(2):175–81 [DOI] [PubMed] [Google Scholar]
- 19.Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 2004;104(10):3267–75 [DOI] [PubMed] [Google Scholar]
- 20.Ferlazzo G, Pack M, Thomas D, et al. : Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. PNAS. USA 2004; 101(47):16606–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Arico M, Moretta L, et al. NK- dependent DC maturation is mediated by TNF-alpha and IFN-gamma released upon engagement of the NKp30 triggering receptor. Blood 2005;106(2):566–71 [DOI] [PubMed] [Google Scholar]
- 22.Della Chiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol 2003;33 (6):1657–66. [DOI] [PubMed] [Google Scholar]
- 23.van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology 2004;40:738–46. [DOI] [PubMed] [Google Scholar]
- 24.Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology 2009;126 (2):280–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckebaum S, Cicinnati VR, Zhang X, Ferencik S, Frilling A,Grosse-Wilde H, et al. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology 2003;109(4): 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994;179(4):1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens 2003;62:385–393, [DOI] [PubMed] [Google Scholar]
- 28.Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X et al. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens 2005;66:185–194. [DOI] [PubMed] [Google Scholar]
- 29.Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G et al. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology 2006;130 (3):823–37 [DOI] [PubMed] [Google Scholar]
- 30.Ferlazzo G, Semino C, Melioli G.HLA class I molecule expression is up-regulated during maturation of dendritic cells, protecting them from natural killer cell-mediated lysis. Immunol Lett 2001;76(1):37–41. [DOI] [PubMed] [Google Scholar]
- 31.Aki M, Shimbara N, Takashina M, Akiyama K, Kagawa S, Tamura T et al. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J Biochem 1994; 115(2):257–69. [DOI] [PubMed] [Google Scholar]
- 32.Hensley SE, Zanker D, Dolan BP, David A, Hickman HD, Embry AC et al. Unexpected role for the immunoproteasome subunit LMP2 in antiviral humoral and innate immune responses. J Immunol 2010;184 (8):4115–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Duan X, Wan Z, Zang H, You S, Yang R, et al. Lower number and decreased function of natural killer cells in hepatitis B virus related acute-on-chronic liver failure. Clin Res Hepatol Gastroenterol 2016;40(5):605–613. [DOI] [PubMed] [Google Scholar]
- 34.Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thélu MA, Sturm N et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol 2009;51(3):458–67. [DOI] [PubMed] [Google Scholar]
- 35.Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, Simon H et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 2002;196 (4):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 2012; 72:1407–15 [DOI] [PubMed] [Google Scholar]
- 37.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354(10):1001–10 [DOI] [PubMed] [Google Scholar]
- 38.Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis. B. J. Hepatol 2011; 54, 209–218 [DOI] [PubMed] [Google Scholar]
- 39.Breton G, Zheng S, Valieris R, Tojal da Silva I, Satija R, Nussenzweig MC. Human dendritic cells (DCs) are derived from distinct circulating precursors that are precommitted to become CD1c+ or CD141+ DCs 2016; 213(13):2861–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol 2005;174(2):727–34. [DOI] [PubMed] [Google Scholar]
- 41.Della Chiesa M, Romagnani C, Thiel A, Moretta L, Moretta A. Multidirectional interactions are bridging human NK cells with plasmacytoid and monocyte-derived dendritic cells during innate immune responses. Blood 2006;108(12):3851–8. [DOI] [PubMed] [Google Scholar]
- 42.Ouaguia L, Leroy V, Dufeu-Duchesne T, Durantel D, Decaens T, Hubert M et al. Circulating and Hepatic BDCA1+, BDCA2+, and BDCA3+ Dendritic Cells Are Differentially Subverted in Patients With Chronic HBV Infection. Front Immunol 2019; 10: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falasca K, Ucciferri C, Dalessandro M, Zingariello P, Mancino P, Petrarca C, et al. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann Clin Lab Sci 2006;36:144–0 [PubMed] [Google Scholar]
- 44.Miyagi T, Takehara T, Nishio K, Shimizu S, Kohga K, Li W et al. Altered interferon-alpha-signaling in natural killer cells from patients with chronic hepatitis C virus infection. J Hepatol 2010; 53(3):424–30 [DOI] [PubMed] [Google Scholar]
- 45.Robek MD, Garcia ML, Boyd BS, Chisari FV. Role of immunoproteasome catalytic subunits in the immune response to hepatitis B virus. J Virol 2007;81(2):483–91. Epub 2006 Nov [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Norbury CC, Cho Y, Yewdell JW, Bennink JR. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8 (+) T cells at the levels of T cell repertoire and presentation of viral antigens. J Exp Med 2001;193 (11):1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertoletti A, Le Bert N. Immunotherapy for Chronic Hepatitis B Virus Infection. Gut Liver 2018;12 (5):497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


