Abstract
Background:
Polypharmacy and potentially inappropriate medications (PIM) are prevalent in older adults with cancer, but their associations with physical function are understudied. This study examined the associations of polypharmacy and PIM with physical function in older adults with cancer, and the optimal cut-off value for number of medications associated with physical functional impairment was obtained.
Methods:
This cross-sectional analysis used baseline data from a randomized study enrolling patients ≥70 with advanced cancer starting a new systemic cancer treatment. We categorized PIM using 2015 Beers criteria. Three validated physical function measures were assessed: Activity of Daily Living (ADL), Instrumental Activity of Daily Living (IADL), and Older Americans Resources and Services Physical Health (OARS PH). Optimal cut-off value for number of medications was determined by Youden’s index. We then performed separate multivariate logistic regressions to examine associations of polypharmacy and PIM with physical function measures.
Results:
Among 439 patients (mean age 76.9), Youden’s index identified ≥8 medications as the optimal cut-off value for polypharmacy; 43% were on ≥8 medications and 62% were on ≥ 1 PIM. On multivariate analysis, being on ≥8 medications was associated with impairments in ADL (OR 1.64 [95% CI 1.01-2.58]) and OARS PH (1.73 [1.01-2.98]). PIM was associated with impairments in IADL (1.72 [1.09-2.73]) and OARS PH (1.97 [1.15-3.37]). A cut-off of 5 medications was not associated with any of the physical functional measures.
Conclusion:
Physical function, an important component of outcomes for older adults with cancer, is associated with polypharmacy (defined as ≥8 medications) and with PIM. Future studies should evaluate the association of polypharmacy with functional outcomes in this population in a longitudinal fashion.
Introduction
More than half of new cancer cases occur in adults aged 65 years or older.1 In the United States, the older adult population is rapidly growing, resulting in an urgent need for aging-sensitive cancer care. Older adults with cancer are at high risk for polypharmacy (the concurrent use of multiple medications).2 Increasing comorbidities with age,3,4 “prescribing cascades” (the initiation of a medication to treat the adverse effects of another medication),5 and care fragmentation across multiple specialists6 all contribute to polypharmacy. In addition, the use of “complementary medicines” such as vitamins and supplements is common in this population, with a prevalence of up to 80%.7 Polypharmacy in conjunction with chemotherapeutic and supportive care medications can increase the potential for clinically significant drug-drug interactions and adverse outcomes.8 Polypharmacy is also associated with an increased risk of taking potentially inappropriate medications (PIM; medications with a risk higher than benefit in older adults)3,9. Previous studies have shown a high prevalence of PIM in older adults, and particularly in older adults with cancer3.
Older adults with cancer frequently have one or more geriatric impairments, which may affect treatment tolerance and worsen outcomes.10 The presence of these impairments can be determined using geriatric assessment (GA), which is a multidisciplinary systematic process using validated tools to assess domains such as comorbidity, functional status and physical performance, cognition, and psychological status.11 Physical functional impairment is associated with multiple adverse outcomes including decreased quality of life, increased chemotherapy toxicity, and increased mortality, 12 and maintenance of optimal physical functioning is important for these patients. Studying polypharmacy and PIM is particularly important in older adults with advanced cancer as it can highlight some distinctive issues that may not apply to other populations of older adults. First, older patients with advanced cancer generally have a poor prognosis; their disease is not curable, and they often prioritize physical functioning and functional independence over extending the duration of life when deciding on cancer treatment.13 Moreover, these patients do not have “time to benefit” from many medications, such as statins and antidiabetic medications, which may cause more harm than benefit.
In the general older adult population, polypharmacy and PIM are associated with physical function impairment, falls,14 and longitudinal functional decline.15 However, there are few data evaluating polypharmacy and/or PIM with physical function in older adults with cancer. Moreover, there are inconsistent cut-off values for the number of medications used to define polypharmacy in the literature. Though ≥5 medications is the most commonly used definition of polypharmacy in the literature 3,16, other cut-off values to define polypharmacy have been used, including three, six, nine, or ten medications17. A better understanding of the relationships between polypharmacy/PIM and physical function in this population could inform the development of interventions to optimize medication use in this population. For example, previous research has demonstrated that pharmacists play a significant role in assessment of medication appropriateness, with respect to quality and safety of prescribing.18 However, data about the feasibility of pharmacist-led medication review or other interventions are still lacking in older adults with advanced cancer.
The aim of this cross-sectional study was to examine the baseline association of polypharmacy and PIM with physical function in older adults with advanced cancer prior to receiving a new line of chemotherapy, and to determine a cut-off value for the number of medications most strongly associated with impairment on several validated physical functional measures. This study builds on work studying optimal cut-offs for polypharmacy in older adults newly diagnosed with cancer;19 unlike prior studies, we include only older adults with advanced cancer starting chemotherapy, who may be at the most risk of adverse outcomes from polypharmacy. We hypothesized that polypharmacy and PIM were associated with baseline impairment in physical function in older adults with advanced cancer starting chemotherapy.
Methods
2.1. Study Design
This is a cross-sectional analysis of baseline data from a nationwide multicenter study assessing whether providing GA information plus GA-guided recommendations to community oncologists reduces chemotherapy toxicity in older adults starting a new systemic treatment regimen for advanced cancer (URCC 13059, PI: Mohile; ClinicalTrials.gov identifier: NCT02054741). This study was conducted within the URCC NCI Community Oncology Research Program (NCORP) and approved by the Institutional Review Board at all participating sites. Enrollment of participants started in May 2014. A polypharmacy log was completed at baseline in the primary study by a clinical research associate at each study site in collaboration with the patient. In this secondary analysis, we included patients who had complete data in their polypharmacy logs at the time of analysis, including indication, frequency, doses, and route of administration (n=439). Those with incomplete data were excluded from this analysis (n=78).
2.2. Participants
Eligible patients were aged 70 years or older, diagnosed with an incurable stage III/IV solid tumor or lymphoma, impaired in at least one geriatric domain on GA, and planning to start a new systemic cancer treatment regimen within four weeks. Eligible treatment regimens included cytotoxic chemotherapy drug and therapies (such as some monoclonal antibody therapies or tyrosine kinase inhibitors) that have a similar prevalence of toxicity.
2.3. Medication Review
Prior to the initiation of the new cancer treatment regimen, a polypharmacy log was completed including all regular medications (both prescription and over the counter [OTC] medications, including “complementary” medications) received by the participants within two weeks of study enrollment. Antineoplastic therapies and supportive care medications specifically initiated in conjunction with cancer treatment were collected in a separate log and were not included in the medication count for this analysis.
PIM were captured using the 2015 American Geriatrics Society Beers criteria20 which consists of four parts: 1) medications that may be potentially harmful for people aged 65 and older; 2) potential drug-disease interactions; 3) 13 combinations known to cause harmful drug-drug interactions; and 4) potentially problematic medications to avoid or adjusted depending on kidney function. All parts of the Beers criteria were applied by a medical oncologist using baseline study forms (polypharmacy log, comorbidity assessment, and lab forms) in addition to the clinic notes of the participants.
2.4. Dependent Variables
Key dependent variables were three validated patient-reported instruments to measure physical function in older adults. All three dependent variables were categorized as binary variables (impaired vs non-impaired). For each instrument, difficulty performing any task assessed by the instrument was graded as an impairment for that instrument. The Katz Activities of Daily Living (ADL) assesses difficulty with bathing, dressing, eating, getting in or out of the bed, continence, and walking; ADL impairment means inability to perform one or more activities.21 The Instrumental activity of Daily Living (IADL) scale assesses activities related to independent living such as difficulty using a telephone and shopping; IADL impairment was present if patients reported needing help or being unable to perform one or more activities.22 The Older Americans Resources and Services Physical Health (OARS PH) survey assesses difficulty with 10 items including but not limited to physical activity and walking for long distances. OARS PH impairment was present if patients selected one or more responses for “my health limits me a lot”23.
2.5. Covariates
Our covariates consisted of sociodemographic, clinical, and GA variables. Socio-demographic variables included age (continuous), gender, race (white, black, and other), education (less than high school, high school graduate, and some college or more), and income (≤$50,000 and >$50,000 or declined to answer). Clinical variables included cancer type (gastrointestinal, lung, and other), cancer stage (stage III and IV), comorbidities (yes or no for the presence of ≥1 comorbidity that affected participant a “great deal,” or ≥3 that affected the participant “somewhat” based on the modified OARS comorbidity scale)24, and physician-reported Karnofsky Performance Score (KPS) (40-60, 70-80, and 90-100). GA variables included binary variables for impairment (yes/no) for OARS medical social support (impairment defined as any question answered with “some of the time,” “a little of the time,” or “none of the time)25, psychological status (impairment defined as ≥ 5 points on geriatric depression scale [GDS] or ≥ 19 points generalized anxiety disorder [GAD-7] scale)26,27 and nutritional status (impairment defined as ≤11 score on the Mini Nutrition Assessment [MNA])28.
Cognitive impairment was not included in the GA covariates, as in the 2015 Beers Criteria, cognitive impairment is one of the conditions used to assess drug-disease interactions to determine PIM. For example, benzodiazepines are considered PIM when received by individuals with cognitive impairment. To avoid the potential for collinearity in multivariate analyses, cognitive impairment was not included as a separate variable in our models.
2.6. Statistical Approach
For each of the three outcome measures, we used the receiver operating characteristic (ROC) curve analyses to calculate the area under the curve (AUC) and to determine the best cut-off value for number of concomitant medications (ordinal predictor) identifying association with physical functional impairments (binary outcome). On the ROC curve, each point corresponds to a specific cut-off value of the predictor, and increasing sensitivity decreases specificity (and vice versa). ROC curves facilitate an efficient identification of patients who may be at risk of physical functional impairment through utilization of Youden’s index.29 Youden’s index, calculated as the sum of the sensitivity and specificity minus one, captures the performance of a specific cut-of value in classifying binary outcomes. The maximum value of Youden index represents the maximum combination of sensitivity plus specificity on ROC curve, and it is frequently used to determine the optimal cutoff value for continuous covariates.
Next, we constructed separate multivariate logistic regression models to examine the associations of polypharmacy and PIM with ADL, IADL, and OARS PH. For each dependent variable, we tested the association with three independent variables: 1) Polypharmacy-A (using ≥5 concomitant medications, the most commonly used cut-off value in the literature); 2) Polypharmacy-B (using the cut-off value determined by the Youden’s Index), and 3) PIM (one or more medications according to the 2015 Beers criteria).
All models included age, gender, race, and cancer type as covariates, as these variables have been shown to correlate with physical function outcomes in older adults in prior studies30–33. To select other covariates for the models, we used backward stepwise procedures. An interaction term between age and gender was added to the models based on prior studies; other interaction terms were considered, but were not included based on literature review and clinical expertise.
The significance criteria (p-value) for entry and elimination of variables from the model (p=0.157) was chosen as it is close to the critical level for which the stepwise procedure is asymptotically equivalent to selection of model covariates using Akaike Information Criterion (AIC).34. Two-sided P-values of <0.05 were considered statistically significant. All analyses were conducted with SAS 9.4 and Stata 13.0.
Results
3.1. Baseline Characteristics of the Study Sample
Among 439 patients included in this analysis, the mean age was 76.9 (SD 5.4) years, 55% were males, 86% were white, and 82% had at least high school education. The most common cancer type was gastrointestinal cancer (34%) followed by lung cancer (29%). In this cohort, 30% had impairment in ADL, 55% in IADL, and 78% in OARS PH.
3.2. Prevalence of Polypharmacy and PIM
The mean number of medications reported was 7.1 (range 1-23), and 16% of these medications were “complementary” medications. Seventy-one percent of patients received ≥5 concurrent medications, 43% received ≥8 concurrent medications, and 24% received ≥10 concurrent medications (Figure 1).
Figure 1:
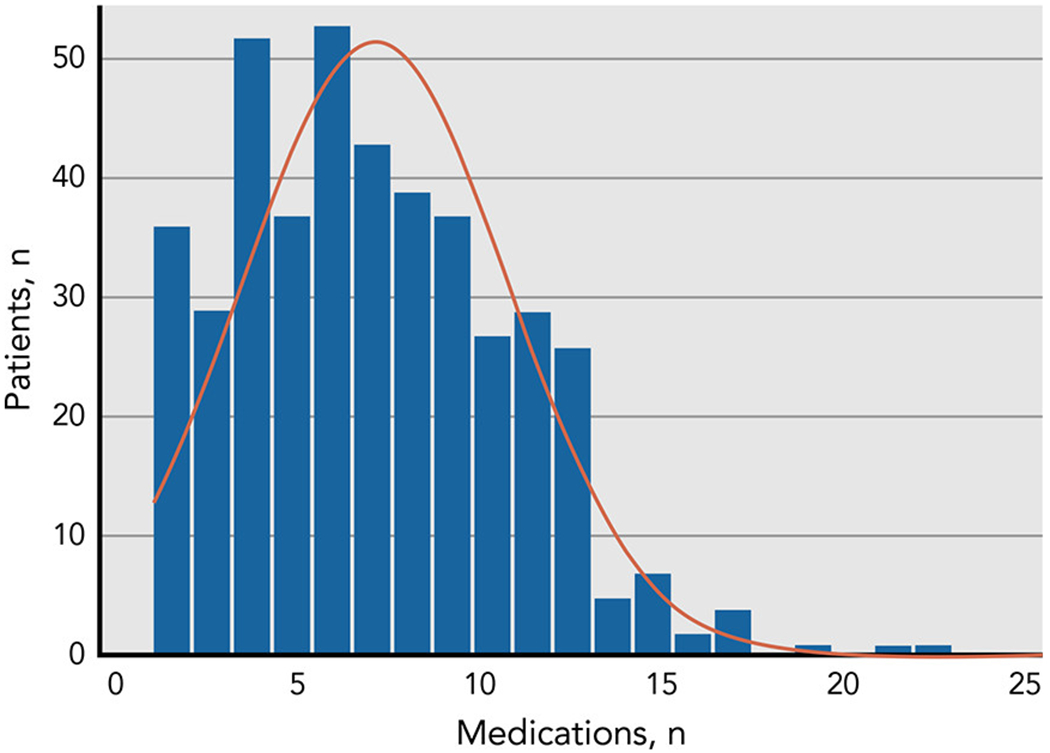
A histogram showing the number patients receiving different number of medications in the study sample
A total of 480 medications (16%) were considered inappropriate based on the 2015 Beers criteria. The mean number of PIM per patient was 1.2 (range: 0-8), and 62% of patients (n=273) received at least one PIM. Of these patients, 96%, (n=265) would have been captured using only the first part of the Beers Criteria (i.e., the list of medications not recommended for any adult older than 65, regardless of comorbidity or interactions). Common classes of PIM were proton pump inhibitors (36%), benzodiazepines (24%), non-steroidal anti-inflammatory drugs (18%), and first-generation antihistamines (15%).
The ROC curves for ADL, IADL, and OARS PH demonstrated AUC of 0.59, 0.58, and 0.59 respectively (Figure 2). The maximum value for the Youden’s Index was reached at n=8 medications for all of the examined measures, corresponding to the split of < 8 vs. ≥8 medications. When OTC medications were excluded from the medication counts, the cut-offs obtained from Youden’s Index were 6, 5, and 8 for ADL, IADL, and OARS PH respectively.
Figure 2:
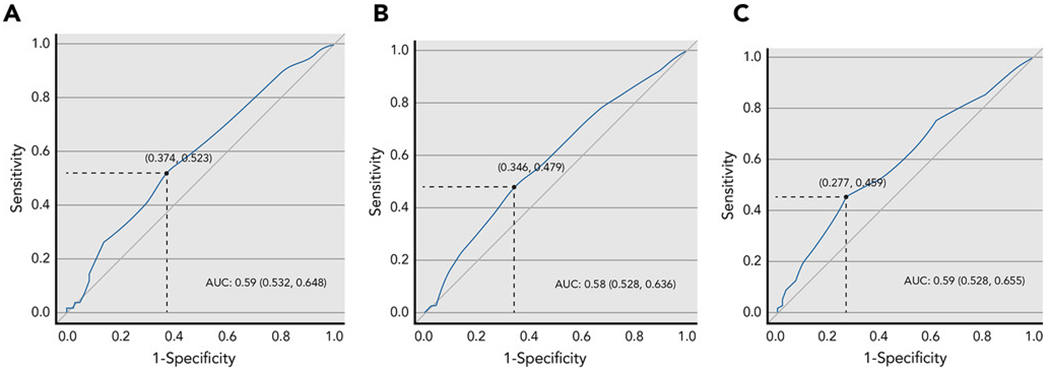
Receiver operating characteristic (ROC) curves for the examined physical function outcomes in relation to the number of medications. The AUC for ADL, IADL, and OARS PH were 0.59, 0.58, and 0.59 respectively. The maximum value for the Youden’s Index was reached at n=8 medications for all of the examined measures.
Table 1 shows the characteristics of the examined population according to the number of medications received (< 8 vs. ≥8). Patients who received ≥ 8 concurrent medications were more likely to be female (51% vs. 40%, P=0.02) and impaired on OARS comorbidity scale (82% vs. 56%, P<0.01).
Table 1:
Baseline characteristics of included patients in relation to the number of medications received
| Variables | N=439 (100%) | <8 medications (n=250) |
≥ 8 medications (n=189) |
P value | |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 76.9 (5.4) | 77.2 (5.5) | 76.6 (5.3) | 0.30 | |
| Gender, % | Male Female |
244 (55.4) 195 (44.6) |
150 (60.2) 99 (39.8) |
93 (48.9) 97 (51.1) |
0.02 |
| Race, %a | White Black Others |
375 (86.0) 37 (8.5) 24 (5.5) |
213 (86.2) 21 (8.5) 13 (5.3) |
162 (85.7) 16 (8.5) 11 (5.8) |
0.97 |
| Education, %b | Some college or more High school graduate <High school |
213 (48.7) 145 (33.2) 79 (18.1) |
122 (49.2) 81 (32.7) 45 (18.1) |
91 (48.1) 64 (33.9) 34 (18.0) |
0.96 |
| Income, %a | >$50,000 ≤$50,000 |
200 (45.9) 236 (54) |
120 (48.4) 128 (51.6) |
80 (42.6) 108 (57.4) |
0.24 |
| Cancer type, %c | Gastro-intestinal Lung Others |
148 (34.4) 125 (29.1) 158 (36.6) |
85 (34.4) 65 (26.3) 97 (39.3) |
63 (34.2) 60 (32.6) 61 (33.2) |
0.28 |
| Cancer stage, %c | Stage III Stage IV Others d |
54 (12.4) 366 (85) 11 (2.6) |
30 (12.2) 210 (85.4) 6 (2.4) |
24 (13.0) 156 (84.3) 5 (2.7) |
0.95 |
|
Comorbidities, %
|
Impaired e Non impaired |
297 (67.7) 142 (32.3) |
141 (56.4) 109 (43.6) |
156 (82.5) 33 (17.5) |
<0.001 |
| Physician reported KPS, %f | 40-60 70-80 90-100 |
64 (14.7) 242 (55.8) 128 (29.5) |
33 (13.4) 132 (53.4) 82 (33.2) |
31 (16.6) 110 (58.8) 46 (24.6) |
0.14 |
| Medical Social Support, % | Impaired g Non impaired |
127 (28.9) 312 (71.1) |
69 (27.6) 181 (72.4) |
58 (30.7) 131 (69.3) |
0.48 |
| Psychological status, % | Impaired h Non impaired |
289 (65.8) 150 (34.2) |
169 (67.6) 81 (32.4) |
120 (63.5) 69 (36.5) |
0.37 |
| Mini-nutritional assessment, % | Impaired i Non impaired |
279 (63.6%) 160 (36.4%) |
151 (60.4) 99 (39.6) |
128 (67.7) 61 (32.3) |
0.11 |
3 patients has missing data
2 patients have missing data
8 patients have missing data
others refers to cancer types which have different staging system such as small cell lung cancer.
impaired means presence of ≥1 comorbidity that affected participant a “great deal,” or ≥3 that affected the participant “somewhat” based on the modified OARS comorbidity scale
5 patients have missing data
impaired means answering any of the questions on OARS Medical Social Support as some of the time, a little of time, or none of the time
impaired means ≥ 5 points on Geriatric depression scale or ≥ 10 points on Generalized Anxiety Scale-7
impaired means ≤11 points on the Mini Nutrition Assessment
Abbreviations: KPS: Karnofsky Performance Score; SD: standard deviation
3.3. Association of Polypharmacy and PIM with Physical Function
In multivariate logistic regression models, the use of ≥8 medications was associated with impairment in ADL (odds ratio [OR] 1.64, 95% confidence interval [CI] 1.01-2.58) and OARS PH (1.73 [1.01-2.98]), but not with IADL (1.26 [0.81-1.97]. Taking ≥5 concurrent medications (i.e., using the most common definition for polypharmacy in the literature) was not associated with impairment of any of the physical function measures. PIM was associated with impairment in IADL (OR 1.72 [1.09-2.73]) and OARS PH (1.97, [1.15-3.37]) but not with ADL (1.42 [0.87-2.32]) (Table 2). Sensitivity analysis, using only the 265 patients receiving PIMs captured by the first part of the Beers Criteria, did not alter these results.
Table 2:
Multivariate analysis evaluating the association of polypharmacy and potentially inappropriate medications with physical function outcomes
| ADL impairment (30%) | IADL impairment (55%) | OARS PH impairment (78%) | ||
|---|---|---|---|---|
| Variables | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| PP | continuous | 1.05 (0.99-1.12) | 1.01 (0.95-1.08) | 1.03 (0.95-1.11) |
| PP-A | < 5 medications ≥ 5 medications |
Reference 1.44 (0.83-2.49) |
Reference 1.095 (0.68-1.77) |
Reference 1.23 (0.71-2.12) |
| PP-B (Youden’s Index) | < 8 medications ≥ 8 medications |
Reference 1.61 (1.01-2.58)* |
Reference 1.26 (0.81-1.97) |
Reference 1.73 (1.01-2.98)* |
| PIM | No Yes |
Reference 1.42 (0.87-2.32) |
Reference 1.72 (1.09-2.73)* |
Reference 1.97 (1.15-3.37)* |
Abbreviations: AOR: adjusted odds ratio; ADL: Activity of Daily Living; CI: confidence interval; IADL: Instrumental Activity of Daily Living; OARS PH: Older Americans Resources and Services Physical Health; PP: Polypharmacy; PIM: potentially inappropriate medications
ADL final model: included covariates were age, gender, race, cancer type, income, and KPS
IADL final model: included covariates were age, gender, race, cancer type, income, KPS, education level, and comorbidities
OARS PH final model: included covariates were age, gender, race, cancer type, income, KPS, and comorbidities
P value< 0.05
Discussion
In this cross-sectional analysis of baseline data from a large cohort of older adults with advanced cancer enrolled onto a clinical trial, we identified a high prevalence of polypharmacy (71% taking ≥5 medications, 43% taking ≥8 medications, and 24% taking ≥10 medications) and PIM (62% taking medications in the 2015 Beers Criteria). We found both polypharmacy (≥8 medications) and PIM were independently associated with physical function impairments measured using validated tools. To the authors’ knowledge, this is the first study demonstrating that PIM is associated with physical function impairment in older adults with cancer. Given the cross-sectional nature of the study, however, conclusions about causality cannot be drawn.
The definition of polypharmacy varies in the literature, with ≥5 medications as the most commonly used definition.35 Previous data suggests a prevalence of polypharmacy as high as 92% in older adults with cancer using this definition.4 Unlike many studies to date, 36,37 we included non-prescription medications (i.e., over-the-counter [OTC]) in addition to prescription medications when defining polypharmacy. Many OTC medications including pain medications (e.g., NSAIDs) and sleep aids (e.g., diphenhydramine) are prevalent and considered to be PIMs; they may lead to adverse events such as acute kidney injury, gastrointestinal bleeding, stroke, cognitive decline, and falls.38,39 Excluding OTC medications may underestimate the effects of polypharmacy and/or PIM. In addition, OTC medications may interact with each other and with other prescription medications,38 including cancer therapies.8
Our study suggests that ≥8 medications is the optimal cut-off value associated with physical function impairments in older adults with cancer. A previous cross-sectional analysis of 385 older adults with various types of cancers suggested multiple cut-off values of polypharmacy (4.5-6.5) in relation to falls, frailty, physical function, and KPS.19 Another cross-sectional analysis of 1705 community dwelling men also identified several cut-points (3.5-6.5) of polypharmacy in relation to multiple adverse outcomes. In that study, polypharmacy was associated with falls, frailty, and mortality,36 but only prescription medications were counted. As the medication counts for our analysis included OTC and “self-prescribed” medications, a higher threshold is not an unexpected finding.
Our study indicated that polypharmacy (defined as the use of ≥8 medications) is associated with physical function impairments in older adults with cancer. A retrospective cohort of 837 patients with breast and colorectal cancers found that polypharmacy (≥5 medications) was associated with impairment in ADL and IADL.40 Associations between PP and physical function are likely multifactorial. Polypharmacy may reflect a high burden of comorbidities in this population. We included a measure of patient-reported comorbidity interference as a covariate, but this may not have captured the full impact of comorbidity on physical functioning. Polypharmacy may also contribute to adverse events and potential drug-drug interactions, which can worsen physical function. Finally, patients with poorer physical function may take more medications for symptoms, including OTC medications.
We demonstrated a high prevalence of PIM (62%) in this cohort. PIM prevalence ranged from 47% to 66% in a previously reported cohort of older adults with breast, colorectal and prostate cancers.41 PIM is associated with physical function impairments in our study. PIM may lead to adverse effects that impact physical function: for example, first generation antihistamines can cause drowsiness, delirium, and falls, which may precipitate physical and functional decline.42 Moreover, decreased physiologic resilience and altered drug metabolism in aging can make older adults more susceptible to medication adverse events.43 Findings on the association of PIM with other health outcomes are mixed in older adults with cancer.44,45 In patients with breast or colorectal cancer receiving adjuvant chemotherapy, there was no association between PIM and adverse health outcomes, including emergency room visits, hospitalizations, or death.46
Understanding the relationships between polypharmacy, PIM and outcomes of older patients with cancer could help guide interventions. For example, “deprescribing” (the planned discontinuation of medications) is an intervention strategy that has been investigated in community-dwelling older adults to optimize medication use.47 Data for deprescribing in older patients with cancer are very limited, and it is unclear how this strategy could best be implemented into oncology clinic workflows.48 Pharmacists have been effectively used in the clinic to identify polypharmacy and PIM,3 and a pharmacist-led deprescribing intervention in older adults with cancer has been shown to be feasible.49 Providing information to oncologists about patients’ medications increases in-clinic conversations about medication management,50 but it is unclear whether this is sufficient to drive deprescribing by oncologists. Randomized studies investigating deprescribing are needed to identify interventions that may improve adverse outcomes in older adults with cancer and polypharmacy and/or PIM.
Our study has several strengths. First, we included a large sample of older adults with advanced cancer receiving treatment in community oncology (i.e., “real world”) practices. We also utilized validated instruments to assess physical function. The study also has several limitations. First, the findings of this study should be interpreted carefully due to the cross-sectional design. We are unable to suggest causal hypotheses in our analyses since temporality of association is a strong criterion for causality. Second, medications were captured from a medication log, which did not take account into medication adherence. Third, this study used total count of all medications, which is a crude measure; however, it is easy to perform in a clinical setting, and it is not always feasible to tell which medications are prescribed versus over-the-counter. Fourth, we were not able to consider other inappropriate prescribing parameters such as indication, dosage, directions for use, and duration of usage. Fifth, medications were captured at one time point so we were not able to examine the potential changes over the course of cancer treatment; medications specifically initiated as supportive care medications for cancer treatment were not included in our analyses. Lastly, AUC results were only slightly better than chance to differentiate different functional outcomes by polypharmacy (AUC: 0.59, 0.58, and 0.59 for ADL, IADL, and PH respectively). This suggests that functional outcomes are influenced by other variables other than polypharmacy which could include cognition impairment51, malnutrition52, and comorbidities51.
In conclusion, we showed that polypharmacy, as defined by the use of ≥8 medications, and PIM, defined as the use of one or more medications included in the Beers criteria, were associated with physical function impairments in older adults with advanced cancer receiving cancer treatment. This highlights the importance of comprehensive evaluation of medication use, including the use of over-the-counter and “complementary” medication use, in older adults with cancer. Although causality was not addressed by this cross-sectional study, future work can determine whether optimizing medication use may reduce the risk of functional decline, thereby improving quality of life and survival.12 Future studies should prospectively evaluate the effect of polypharmacy and PIM on the longitudinal risk of physical functional decline in older adults with cancer and determine whether prospective interventions such as “deprescribing” can improve outcomes in these patients.
Acknowledgments
Funding
The work was funded through R01 CA177592 (Mohile), K24 AG056589 (Mohile), (NIA) R21/R33AG059206 (Mohile), NCI UG1CA189961 (Mohile), NCI R25CA102618 (Ramsdale), and a Wilmot Fellowship Award (Ramsdale and Loh). All statements in this report, including its findings and conclusions, are solely those of the authors, and do not necessarily represent the official views of the funding agencies.
References:
- 1.Siegel RL, Miller KD, Jemal A: Cancer Statistics, 2017. CA Cancer J Clin 67:7–30, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Sharma M, Loh KP, Nightingale G, et al. : Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol 7:346–53, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nightingale G, Hajjar E, Swartz K, et al. : Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J Clin Oncol 33:1453–9, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Maggiore RJ, Gross CP, Hurria A: Polypharmacy in older adults with cancer. Oncologist 15:507–22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochon PA, Gurwitz JH: The prescribing cascade revisited. Lancet 389:1778–1780, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Clyne B, Cooper JA, Hughes CM, et al. : ‘Potentially inappropriate or specifically appropriate?’ Qualitative evaluation of general practitioners views on prescribing, polypharmacy and potentially inappropriate prescribing in older people. BMC Fam Pract 17:109, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson MA, Sanders T, Palmer JL, et al. : Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol 18:2505–14, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Popa MA, Wallace KJ, Brunello A, et al. : Potential drug interactions and chemotoxicity in older patients with cancer receiving chemotherapy. J Geriatr Oncol 5:307–14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon KT, Choi MM, Zuniga MA: Potentially inappropriate medication use in elderly patients receiving home health care: a retrospective data analysis. Am J Geriatr Pharmacother 4:134–43, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Sarfati D, Koczwara B, Jackson C: The impact of comorbidity on cancer and its treatment. CA Cancer J Clin 66:337–50, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Extermann M, Aapro M, Bernabei R, et al. : Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 55:241–52, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Sia I, Ma HM, et al. : The synergistic effect of functional status and comorbidity burden on mortality: a 16-year survival analysis. PLoS One 9:e106248, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohile SG, Dale W, Somerfield MR, et al. : Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 36:2326–2347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried TR, O’Leary J, Towle V, et al. : Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc 62:2261–72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jyrkka J, Enlund H, Lavikainen P, et al. : Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf 20:514–22, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Turner JP, Shakib S, Singhal N, et al. : Prevalence and factors associated with polypharmacy in older people with cancer. Support Care Cancer 22:1727–34, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Mohamed MR, Ramsdale E, Loh KP, et al. : Associations of Polypharmacy and Inappropriate Medications with Adverse Outcomes in Older Adults with Cancer: A Systematic Review and Meta-Analysis. Oncologist, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muijrers PE, Knottnerus JA, Sijbrandij J, et al. : Changing relationships: attitudes and opinions of general practitioners and pharmacists regarding the role of the community pharmacist. Pharmacy World and Science 25:235–241, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Turner JP, Jamsen KM, Shakib S, et al. : Polypharmacy cut-points in older people with cancer: how many medications are too many? Support Care Cancer 24:1831–40, 2016 [DOI] [PubMed] [Google Scholar]
- 20.American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 63:2227–46, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Brorsson B, Asberg KH: Katz index of independence in ADL. Reliability and validity in short-term care. Scandinavian Journal of Rehabilitation Medicine 16:125–132, 1984 [PubMed] [Google Scholar]
- 22.Lawton MP, Brody EM: Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–86, 1969 [PubMed] [Google Scholar]
- 23.Whitelaw NA, Liang J: The structure of the OARS physical health measures. Med Care 29:332–47, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Fillenbaum GG, Smyer MA: The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol 36:428–34, 1981 [DOI] [PubMed] [Google Scholar]
- 25.Burholt V, Windle G, Ferring D, et al. : Reliability and validity of the Older Americans Resources and Services (OARS) social resources scale in six European countries. J Gerontol B Psychol Sci Soc Sci 62:S371–9, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Weintraub D, Oehlberg KA, Katz IR, et al. : Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry 14:169–75, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wild B, Eckl A, Herzog W, et al. : Assessing generalized anxiety disorder in elderly people using the GAD-7 and GAD-2 scales: results of a validation study. Am J Geriatr Psychiatry 22:1029–38, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Montejano Lozoya R, Martínez-Alzamora N, Clemente Marín G, et al. : Predictive ability of the Mini Nutritional Assessment Short Form (MNA-SF) in a free-living elderly population: a cross-sectional study. PeerJ 5:e3345, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruopp MD, Perkins NJ, Whitcomb BW, et al. : Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J 50:419–30, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owusu C, Schluchter M, Koroukian SM, et al. : Racial disparities in functional disability among older women with newly diagnosed nonmetastatic breast cancer. Cancer 119:3839–3846, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver KE, Leach CR, Leng X, et al. : Physical Functioning among Women 80 Years of Age and Older With and Without a Cancer History. J Gerontol A Biol Sci Med Sci 71 Suppl 1:S23–30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrick JL, Reeve BB, Kucharska-Newton AM, et al. : Functional status declines among cancer survivors: trajectory and contributing factors. J Geriatr Oncol 5:359–67, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.den Ouden ME, Schuurmans MJ, Mueller-Schotte S, et al. : Identification of high-risk individuals for the development of disability in activities of daily living. A ten-year follow-up study. Exp Gerontol 48:437–43, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Akaike H: Citation Classic - a New Look at the Statistical-Model Identification. Current Contents/Engineering Technology & Applied Sciences:22–22, 1981 [Google Scholar]
- 35.Masnoon N, Shakib S, Kalisch-Ellett L, et al. : What is polypharmacy? A systematic review of definitions. BMC geriatrics 17:230–230, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnjidic D, Hilmer SN, Blyth FM, et al. : Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 65:989–95, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Elliot K, Tooze JA, Geller R, et al. : The prognostic importance of polypharmacy in older adults treated for acute myelogenous leukemia (AML). Leuk Res 38:1184–90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roumie CL, Griffin MR: Over-the-counter analgesics in older adults: a call for improved labelling and consumer education. Drugs Aging 21:485–98, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Abraham O, Schleiden L, Albert SM: Over-the-counter medications containing diphenhydramine and doxylamine used by older adults to improve sleep. International journal of clinical pharmacy 39:808–817, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Abbema D, van Vuuren A, van den Berkmortel F, et al. : Functional status decline in older patients with breast and colorectal cancer after cancer treatment: A prospective cohort study. J Geriatr Oncol 8:176–184, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Feng X, Higa GM, Safarudin F, et al. : Potentially inappropriate medication use and associated healthcare utilization and Costs among older adults with colorectal, breast, and prostate cancers. J Geriatr Oncol, 2019 [DOI] [PubMed] [Google Scholar]
- 42.Kalpaklioglu F, Baccioglu A: Efficacy and safety of H1-antihistamines: an update. Antiinflamm Antiallergy Agents Med Chem 11:230–7, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Rosenthal RA, Kavic SM: Assessment and management of the geriatric patient. Crit Care Med 32:S92–105, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Maggiore RJ, Dale W, Gross CP, et al. : Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: effect on chemotherapy-related toxicity and hospitalization during treatment. J Am Geriatr Soc 62:1505–12, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin RJ, Ma H, Guo R, et al. : Potentially inappropriate medication use in elderly non-Hodgkin lymphoma patients is associated with reduced survival and increased toxicities. British journal of haematology 180:267–270, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karuturi MS, Holmes HM, Lei X, et al. : Potentially inappropriate medication use in older patients with breast and colorectal cancer. 124:3000–3007, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Page AT, Clifford RM, Potter K, et al. : The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol 82:583–623, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner JP, Shakib S, Bell JS: Is my older cancer patient on too many medications? J Geriatr Oncol 8:77–81, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Whitman A, DeGregory K, Morris A, et al. : Pharmacist-led medication assessment and deprescribing intervention for older adults with cancer and polypharmacy: a pilot study. Support Care Cancer 26:4105–4113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsdale E, Lemelman T, Loh K, et al. : Geriatric Assessment-Driven Polypharmacy Discussions Between Oncologists, Older Patients with Cancer, and Caregivers. Journal of the American Geriatrics Society 65:S110, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hajek A, König H-H: Longitudinal Predictors of Functional Impairment in Older Adults in Europe--Evidence from the Survey of Health, Ageing and Retirement in Europe. PloS one 11:e0146967–e0146967, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiesswetter E, Pohlhausen S, Uhlig K, et al. : Malnutrition is related to functional impairment in older adults receiving home care. J Nutr Health Aging 17:345–50, 2013 [DOI] [PubMed] [Google Scholar]


