Abstract
DNA methylation is the most important epigenetic modification involved in many essential biological processes. MET1 is one of DNA methyltransferases that affect the level of methylation in the entire genome. To explore the effect of MET1 gene silencing on gene expression profile of Chrysanthemum × morifolium 'Zijingling'. The stem section and leaves at the young stage were taken for transcriptome sequencing. MET1-RNAi leaves had 8 differentially expressed genes while 156 differentially expressed genes were observed in MET1-RNAi stem compared with control leaves and stem. These genes encode many key proteins in plant biological processes, such as transcription factors, signal transduction mechanisms, secondary metabolite synthesis, transport and catabolism and interaction. In general, 34.58% of the differentially expressed genes in leaves and stems were affected by the reduction of the MET1 gene. The differentially expressed genes in stem and leaves of transgenic plants went through significant changes. We found adequate amount of candidate genes associated with flowering, however, the number of genes with significant differences between transgenic and control lines was not too high. Several flowering related genes were screened out for gene expression verification and all of them were obseved as consistent with transcriptome data. These candidate genes may play important role in flowering variation of chrysanthemum. This study reveals the mechanism of CmMET1 interference on the growth and development of chrysanthemum at the transcriptional level, which provides the basis for further research on the epigenetic regulation mechanism in flower induction and development.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01022-1.
Keywords: Chrysanthemum, DNA methylation, Transcriptome sequencing, Flowering
Introduction
DNA methylation as the main form of epigenetic regulation where one of the S-adenosine methionine on methyl under the catalysis of DNA methyltransferase transferred to 5th carbon of cytosine ring in the DNA molecule, forming 5-methyl cytosine (5-mC) (Wu and Morris 2001). The DNA methylation modification classes are 5-methylcytosine, 6-methyladenine and 7-methylguanine, 5-mC is the main component in the plant (Baulcombe 2007). The epigenetic modification affects gene expression and adaptation significantly in the process of plant development. It has been extensively studied in a variety of plants. About 6 to 30% of the DNA in the plant genome is methylated (Zhang et al. 2006) with uneven distribution (Lu et al. 2006); based on sequence characteristics, it can be divided into CG, CHH and CHG sites, the CG sites with symmetric dinucleosides are predominant (Finnegan et al. 1998a, b; Bewick and Schmitz 2017). The methyltransferases involved in DNA methylation in plants are: (1) Domain rearranged methyltransferase (DRM), whose main function is to cause de novo DNA methylation and maintain CHH sites; (2) Methylatransferase 1(MET1), which plays a major role in maintaining methylation of CG sites, and also capable of de novo catalyze DNA methylation; (3) Chromomethylase (CMT), responsible for methylation of CHG sites (Matzke 2000).
The level of methylation of genes throughout the development of plants is spatially and temporally specific. For example, during tomato fruit ripening, CG methylation level drops down (Messeguer et al. 1991); during vegetative development, mature leaves have higher DNA methylation levels than seedlings in Arabidopsis thaliana, but the seeds have highest level of DNA methylation (Finnegan et al. 1998a, b). However, the methylation level of seedlings is higher than that of flag leaves in Oryza sativa (Xiong et al. 1999). DNA methylation is involved in plant individual development, such as seed germination (Lu et al. 2006), growth and development (King 1995), flowering induction (Li et al. 2016; Kang et al. 2019a), inflorescence morphology (Kang et al. 2019b), fruit ripening (Lang et al. 2017), embryo development (Wang et al. 2015), aging process (Smykal et al. 2007). Low or insufficient DNA methylation level in plants can lead to genetic abnormalities in plant's normal development and phenotype (King 1995). For example, induction of vernalization reduces methylation and promotes flowering (Sheldon et al. 1999), and reduction in methylation levels led to increase the activity of the transposable element (Brettell and Dennis 1991). The methylation level of genomic DNA of transgenic tobacco (MET1) was significantly lower than that of wild type, with small leaves, short internodes and abnormal flower morphology (Nakano et al. 2000). In addition, transcriptionally silenced transgenes can be reactivated by treatment with 5-azacytidine(5-azaC), especially in the promoter region (Fu et al. 2000). Therefore, treatment of plant material with the methyltransferase inhibitor 5-azaC produces a variety of phenotypic variations. For example: dwarf plant (Nie et al. 2009), smaller leaf (Zakrzewski et al. 2017), reduced seed setting rate (Lu et al. 2006), flowering time (Burn et al. 1993), clustered plant (Xiong et al. 1999), reduced regeneration (Eszter et al. 2003), vegetative organs(Gao et al. 2014) and reproduction of structural anomalies.
In view of the important role of DNA methylation in plant growth and development, many scholars have carried out transgenic research on DNA methylation-related enzymes. The MET1-antisense transgene of A. thaliana showed obvious developmental phenotypic abnormalities, such as small plants, changes in leaf size and shape, and changes in flowering time (Xiao et al. 2006; Shafiq et al. 2014); Arabidopsis drm1drm2cmt3 triple mutant showed growth, retardation and decreased plant size, partial infertility (Sako et al. 2012); Arabidopsis DME(DEMETER) had decreased seed activity, abnormal stamen growth, abnormal flowering development, etc. DNA demethylase ROS1 mutants also influenced small pods to become smaller (Cao and Jacobsen 2002; Gong et al. 2002). The CmMET1gene interfere lines of chrysanthemum showed dwarfing and early flowering phenotypes (Li et al. 2019).
Either DNA methyltransferase inhibitor treatment or overexpression or inhibition of DNA methylase results in methylation level disorder which causes various morphological variations and defects in plants; therefore, the dynamic balance of DNA methylation and demethylation level is very important.
C. morifolium originated in China and is the world's most famous flower with wide range of variability in ornamental plants. It possess many important values such as ornamental, edible, medicinal and tea use (Zhao et al. 2019). Previous research showed that DNA methylation has important regulatory effects on chrysanthemum growth and development, especially in flowering stage (Kang et al. 2019a).
Transcriptome sequencing is a rapid and comprehensive acquisition of the sum of RNA of a species under a certain state through a second-generation sequencing platform. It is widely used in plants to study gene expression, gene function, structure, alternative splicing, determination of metabolic pathways and gene differential expression analysis, etc. (Zhan et al. 2015; Wang et al. 2017). In recent years, several studies have reported the characteristics of chrysanthemum transcripts (Ke et al. 2018; Lu et al. 2018; Dong et al. 2020). However, at the molecular level, the mechanism by which DNA methylation regulates phenotype (especially flowering) variation has not been clarified.
In order to further elucidate the relationship between methylation of chrysanthemum and flowering stage, this study selected CmMET1 transgenic and control lines for transcriptome analysis. Based on RNA-seq analysis of transcriptome data, our results may improve the understanding of the effect of DNA methylation on gene expression in two important organs of chrysanthemum: stem and leaf, and to further study the breeding and improvement of the flowering period provides a preliminary basis.
Materials and methods
Experimental materials
The transgenic material 'Zijingling' used in this study was obtained by Li using RNAi technology and cultivated in the Laboratory of Plant Germplasm Resources and Genetic Engineering of Henan University. When the chrysanthemum developed 6–8 leaves, the top leaves (Fig. 1a, b) and the stem segments (Fig. 1c, d) were collected and immediately frozen in liquid nitrogen and stored in a -80 °C refrigerator for use. Three biological replicates were taken from both RNAi and control (CK) lines and used as transcriptome plant materials. The leaves (Fig. 1a, b), stem segments (Fig. 1c, d) and shoot tips (Fig. 1e, f) of the 'Zijingling' control and transgenic lines were used for expression verification of flowering-related genes.
Fig. 1.

Sample images. Left: control lines, right: MET1-RNAi lines, a, b leaf; c, d stem segments; e, f shoot tips. Note Transcriptome sequencing tissue material (a,b,c,d), materials for quantitative fluorescent expression analysis (a,b,c,d,e,f)
RNA isolation and transcriptome sequencing
Total RNA was extracted from the samples using the Trizol kit, RNA quality and concentration were detected by Nanodrop2000 and integrity was detected by agarose gel electrophoresis. After the RNA quality test was passed (that is, the RIN value of all samples was ≥ 8), a sequencing library [NEBNext mRNA Sample Prep Reagent Set for Illumina (NEB) was constructed, and 2 μg of each RNA was used for database sequencing. After the library was constructed, preliminary quantification was performed using Qubit2.0, and the library was diluted to 1 ng/μl. Then, the insert size of the library was detected using Agilent 2100. After the insert size determined, the qRT-PCR method was used. The effective concentration of the library was accurately quantified (effective library concentration > 2 nM) to ensure library quality. After the library was tested, high-throughput sequencing was performed by Beijing Baimaike Biotechnology Co., Ltd. using Illumina HiSeq 4000.
Transcriptome data assembly and gene function annotation
From the raw data, the sequencing adapters and primer sequences in Reads are truncated, and low-quality data is filtered to obtain high-quality data. Transcriptome data assembly was performed using Trinity. Unigenes sequence were combined with Nr (Deng et al. 2006), Swiss-Prot (Apweiler et al. 2004), GO (Gene Ontology) (Ashburner et al. 2000), COG (Tatusov et al. 2000), KOG (Koonin et al. 2004), eggNOG 4.5 (Jaime et al. 2016), KEGG (Kanehisa et al. 2004) database using Blast software. For comparison, the KEGG Orthology results of Unigene in KEGG were obtained using KOBAS 2.0 (Xie et al. 2011). After the amino acid sequence of Unigene was predicted, the HMMER (Eddy 1998) software was used to compare with the Pfam database to obtain the annotation information of Unigene (Finn et al. 2014).
Differential expression gene analysis
Differential expression gene analysis was performed using EBSeq to obtain a differentially expressed gene set between two samples (Leng et al. 2013). In the process of differential expression analysis, the effective Benjamini–Hochberg method is used to correct the p-value of the original hypothesis test, to finalize the corrected p-value, FDR (False Discovery Rate) was used. For the key indicators of differentially expressed gene screening, Fold Change ≥ 2 and FDR < 0.01 were used as screening criteria during the screening process.
Verification of differential genes
The differentially expressed genes were subjected to qRT-PCR and cDNA was used as a template. Quantitative PCR was performed using SYBR Premix Ex Taq (TaKaRa) with three biological replicates. The qRT-PCR procedure was as: the first step of 95 °C for 30 s. 95 °C 5 s, 60 °C 30 s, 40 cycles. Amplification was performed using a Roche 480 instrument. The qRT-PCR internal reference gene was constructed using the Chrysanthemum Internal Reference Gene Action (Wang et al. 2015) and other primers were designed with reference to the principle of fluorescent quantitative primer design (Table S1). The results were analyzed using the ΔΔCT method (Livak and Schmittgen 2002).
Results
Transcriptome sequencing and assembly
To study the transcriptome and gene expression profiles of the chrysanthemum cultivar 'Zijingling' under normal and RNAi interference comprehensively, cDNA samples from 12 leaves and stem segments of chrysanthemum young stage plants were sequenced using the Illumina HiSeq 4000 platform. Transcriptome sequencing of 12 samples from 12 pools is given in Table S2. After removing low-quality reads, a clean data of 82.28 Gb was obtained, with an average of 6.26 Gb (21.5 million) of reads per sample, and the percentage of Q30 base in each sample was not less than 90.12% (Table S2). In the Trinity program, 247,698 transcripts were obtained from clean reads, with a N50 length of 1383 bp and an average length of 929.31 bp. There were 100,800 unigenes with an average length of 746.96 bp (Table S3). The clean data for each sample was compared to a transcript or unigene library with a QC ratio between 42.87 and 44.85% (Table S2). The results show that after data quality control, a total of 100,800 unigenes were obtained, and the N50 of unigenes was 1281 bp, indicating that the assembly integrity was high.
Gene function annotation
To predict and analyze the function of unigenes, BLAST was used to functionally annotate gene sequences with multiple databases such as COG, GO, KEGG, KOG, Pfam, Swiss-Prot, eggNOG, and Nr. Out of 100,800 unigenes, 43,476 (43.13%) of unigenes successfully matched homologous sequences in at least one of the above databases. Among them, the unigenes in the COG, GO, KEGG, KOG, Pfam, Swiss-Prot, eggNOG, and Nr databases were 12,086 (11.99%), 16,088 (15.91%), 16,041 (15.91%), 23,296 (23.11%), 28,595 (28.37%), and 27,847 (27.63%), 38,003 (37.70%) and 41,507 (41.18%), respectively (Table S4). The Nr database has the largest number of genes annotated. In the Nr database, the chrysanthemum variety 'Zijingling' has the highest matching degree with Helianthus annuus, accounting for 44.39%, followed by Cynara cardunculus var. scolymus with a matching degree of 21.25%, followed by Citrus sinensis. The matching degree of A. thaliana and O. sativa Japonica Group was 3.83, 3.07 and 2.40%, respectively (Fig. 2a).
Fig. 2.
a Similarity of “Zijingling” sequences with those of other species. (b) Numbers of differentially expressed genes. Note (1 MET1-RNAi leaf and the control group leaf, 2 MET1-RNAi stem and control group stem, 3 The control group leaf and the control group stem, 4 MET1-RNAi leaf and MET1-RNAis stem)
Differential expression gene analysis with CK and RNAi line
During the analysis of differentially expressed genes, the data of MET1-RNAi-S-2 and CK-S-3 were discarded due to the poor correlation between T08 and T12 and other samples. The differentially expressed genes of the four groups were analyzed (FDR = 0.05 FC = 2): MET1-RNAi—leaves had 8 differentially expressed genes (8 up-regulated, 0 down-regulated) compared with control leaves (MET1-RNAi-L-1,2,3 Vs CK-L-1,2,3); MET1-RNAi stems (MET1-RNAi-S-1,3) had 156 differentially expressed genes (145 up-regulated and 11 down-regulated Compared with control stems (CK-S-1,2). Among them, 122 genes were clearly annotated, as detailed in Table S5.; Compared with control group stems (CK-L-1,2,3 Vs CK-S-1,2). A total of 8786 differentially expressed genes (3461 up-regulated, 5325 down-regulated) were observed in the control group of leaves and stems (Fig. 2b-3). Group of MET1-RNAi leaves and stems had 8832 differentially expressed genes (3195 up-regulated, 5128 down-regulated) (Fig. 2b-4).
Analysis of differentially expressed genes in MET1-RNAi leaves and control leaves
Compared with the leaves of the control group, the leaves of the transgenic material had eight differentially expressed genes, seven of which were annotated: 3 genes were viruses (Superfamily 1) RNA helicase; Two genes were RNA-dependent RNA polymerase; One was Carlavirus coat and one gene was a 7 kD viral coat protein. It is to be noted that the transcriptome sampling period was the seedling stage and probably that was the reason of having not many different genes in the leaf samples.
Analysis of differentially expressed genes between MET1-RNAi stem and control stem
KOG analysis
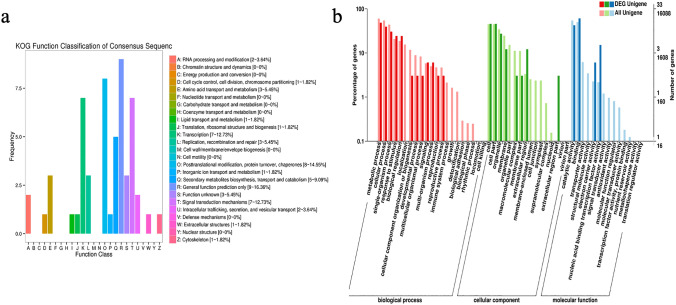
KOG analysis was performed on 156 differentially expressed genes. The results showed that 55 differentially expressed genes were annotated into 16 functional categories of the KOG classification. Among them, general functional prediction (R), post-translational modification/protein turnover partner (O), transcription factor (K) and signal transduction mechanism (T) were abundant and differentially expressed genes were 9, 8, 7 and 7 respectively. Synthesis/transportation/catabolism (Q), replication/recombination/repair (L), amino acid transport and metabolism (E), intracellular transport/secretion/vesicular transport (U) and RNA processing and modification of secondary metabolites (A) while 5, 3, 3, 2 and 2 were found as differentially expressed genes. Translation ribosome structure/biogenesis (J), cell cycle control/cell division/chromosomal division (D), lipid transport and metabolism (I) and transport and metabolism (P) of inorganic ions were the least (Fig. 3a).
Fig. 3.
a The KOG analysis of DEGs in MET1-RNAi stem and the control group stem. Note The abscissa is the classification content of KOG, and the ordinate is the number of genes. b The GO analysis of DEGs in MET1-RNAi stem and the control group stem. Note The abscissa is the classification content of GO, and the ordinate is the number of genes
GO analysis
GO functional annotation and classification statistics were performed on differentially expressed genes. A total of 33 differentially expressed genes were obtained for GO functional annotation. The GO database has three ontology that describe the molecular function, the cellular component and biological process of the gene, respectively. The three functional categories are 4, 8 and 12, respectively (Fig. 3b). In the molecular function classification, differentially expressed genes were mostly involved in binding and catalytic activity, followed by signal transducer activity and nucleic acid binding transcription factor (nucleic acid binding transcription factor). In the classification of cellular components, differentially expressed genes involved in cell parts and cells account for the most; Secondly, they are related to organelles, membranes, and extracellular regions. The differentially expressed genes involved in the extracellular matrix part, the collagen trimer, the membrane part and the macromolecular complex. In biological processes, differentially expressed genes are mostly involved in the metabolic and cellular processes while rare or few interfere with single-organism process, biological regulation and response to stimulus related differentially expressed genes and signaling, multi-organism processes, cellular component organization or biogenesis, reproductive processes and other related differentially expressed genes.
KEGG analysis
KEGG analysis of 156 differentially expressed genes was enriched into 17 KEGG pathways (Table 1). Among them, the differentially expressed genes were in the plant pathogen interaction (Plant-pathogen interaction, ko04626), C5-Branched dibasic acid metabolism (ko00660) and the phosphatidylinositol signaling system (ko04070). The level of enrichment on the pathway was most significant (Fig. 4a). Further analysis showed that the differentially expressed genes in the pathway of plant pathogen interaction were upregulated and the main metabolic pathways in the young stage were mainly plant pathogen interaction (ko04626) while biosynthesis of carotenoid and amino acids pathway was ko00906 and ko01230.
Table 1.
KEGG classification of DEGs in MET1-RNAi stem and the control group stem (FDR = 0.05)
| ID | Pathways | Annotated genes | Pathways ID |
|---|---|---|---|
| 1 | Plant-pathogen interaction | 4 (33.33%) | ko04626 |
| 2 | C5-Branched dibasic acid metabolism | 1 (8.83%) | ko00660 |
| 3 | Phosphatidylinositol signaling system | 2 (12.67%) | ko04070 |
| 4 | Valine, leucine and isoleucine biosynthesis | 1 (8.83%) | ko00290 |
| 5 | Carotenoid biosynthesis | 1 (8.83%) | ko00906 |
| 6 | Fatty acid elongation | 1 (8.83%) | ko00062 |
| 7 | Phenylalanine, tyrosine and tryptophan biosynthesis | 1 (8.83%) | ko00400 |
| 8 | Cyanoamino acid metabolism | 1 (8.83%) | ko00460 |
| 9 | 2-Oxocarboxylic acid metabolism | 1 (8.83%) | ko01210 |
| 10 | Aminoacyl-tRNA biosynthesis | 1 (8.83%) | ko00970 |
| 11 | RNA degradation | 1 (8.83%) | ko03018 |
| 12 | Biosynthesis of amino acids | 2 (12.67%) | ko01230 |
| 13 | Phenylpropanoid biosynthesis | 1 (8.83%) | ko00940 |
| 14 | Starch and sucrose metabolism | 1 (8.83%) | ko00500 |
| 15 | Endocytosis | 1 (8.83%) | ko04144 |
| 16 | Spliceosome | 1 (8.83%) | ko03040 |
| 17 | Protein processing in endoplasmic reticulum | 1 (8.83%) | ko04141 |
Fig. 4.
a Statistics of pathway enrichment of DEGs in MET1-RNAi stem and the control group stem. b G1 MET1-RNAi leaf and MET1-RNAi stem, G2 The control group leaf and control group stem. c G0 MET1-RNAi stem and control group stem, G1 MET1-RNAi leaf and MET1-RNAi stem, G2 the control group leaf and control group stem, G3 MET1-RNAi leaf and the control group leaf
Analysis of transcription factors
In the eggNOG database, a total of 24 genes were annotated as transcription factors (Table 2). As shown in Table 2, there were WRKY transcription factors (3), AP2/ERF transcription factors (3), AP2 (8) and MYB-like transcription factors (5). All these transcription factors were upregulated.
Table 2.
DEGs encoding transcription factors
| Gene ID | DEG | eggNOG annotation |
|---|---|---|
| c88538.graph_c0 | up | WRKY7 |
| c76883.graph_c0 | up | WRKY DNA -binding domain |
| c74652.graph_c0 | up | WRKY DNA -binding domain |
| c83600.graph_c0 | up | AP2/ERF domain-containing protein |
| c87026.graph_c2 | up | AP2/ERF domain-containing protein |
| c80833.graph_c0 | up | AP2/ERF domain-containing protein |
| c88237.graph_c0 | up | AP2 domain |
| c81105.graph_c0 | up | AP2 domain |
| c75954.graph_c0 | up | AP2 domain |
| c83779.graph_c0 | up | AP2 domain |
| c85945.graph_c0 | up | AP2 domain |
| c84147.graph_c0 | up | AP2 domain |
| c86655.graph_c0 | up | AP2 domain |
| c82953.graph_c0 | up | AP2 domain |
| c81836.graph_c0 | up | MYB-like DNA-binding domain |
| c88385.graph_c0 | up | MYB-like DNA-binding domain |
| c76003.graph_c0 | up | MYB-like DNA-binding domain |
| c82634.graph_c1 | up | MYB-like DNA-binding domain |
| c69244.graph_c0 | up | MYB-like DNA-binding domain |
| c83523.graph_c0 | up | Probable CCR4-associated factor 1 |
| c79767.graph_c0 | up | HD-ZIP protein, partial |
| c88892.graph_c0 | up | GRAS protein, partial |
| c79718.graph_c0 | up | GATA zinc finger |
Analysis of differentially expressed genes in leaf and stem transcriptome between MET1-RNAi and control lines
Venn diagram was used to analyze the differentially expressed gene sets in the control leaves and stems and MET1-RNAi leaves and MET1-RNAi stems. The results are shown in Fig. 4b, c. Among them, there were 6766 identical differentially expressed genes in the two groups, 1,557 differentially expressed genes were unique in MET1-RNAi leaves and stems, and 2020 differentially expressed genes were in control leaves and control stems (Fig. 4b). As shown in Fig. 4c, the differential genes were mainly concentrated between transgenic lines and different tissues of the control group. However, there were few different genes in the same tissue type as the sampling happened at young stage, and hence there was no significant difference on the phenotypic basis. The phenotypic variation of transgenic lines was mainly noticed in flowering stage and plant height, so transcriptomic sequencing in this stage with phenotypic variation may obtain more different genes. Based on this transcriptome sequencing, we have observed several differentially expressed genes. It is indicated that 65.42% of the differentially expressed genes in leaves and stems were not affected by the decrease of CmMET1 gene, while 34.58% of differentially expressed genes were affected by the decrease of CmMET1 gene.
In addition, the 6,676 differentially expressed genes in the KOG functional classification were mainly related to general functional prediction (R), post-translational modification, protein turnover, chaperone (O), secondary metabolite synthesis, transport and catabolism (Q), Carbohydrate transport and metabolism (G), signal transduction mechanism (T), lipid transport and metabolism (I), energy production and transformation (C) and inorganic ion transport and metabolism (P) (Fig. 5a). Differentially expressed genes unique to MET1-RNAi leaves and MET1-RNAi stems in the KOG functional classification, mainly with general functional prediction (R), signal transduction mechanism (T), replication/recombination/repair (L), cells Skeleton (Z), amino acid transport and metabolism (E), cell cycle control/cell division/chromosomal division (D), transcription factor (K), etc. (Fig. 5b). The differentially expressed genes were unique to the control group, the control group was classified into the KOG function, mainly with general functional prediction (R), post-translational modification/protein turnover/companion (O), signal transduction mechanism (T) and carbon water. Compound transport and metabolism (G), amino acid transport and metabolism (E), energy production and transformation (C) and replication/recombination/repair (L) were associated (Fig. 5c).
Fig. 5.
The KOG analysis of DEGs. a MET1-RNAi leaf and MET1-RNAi stem and the control group leaf and the control group stem, b MET1-RNAi leaf and MET1-RNAi stem; c. The control group leaf and the control group stem)
Differentially expressed genes transcripts annotated with chrysanthemum
As the expression of the CmMET1 gene is suppressed, the expression of its downstream regulatory genes is affected. The gene expression level is specific, some genes in the transcriptome of chrysanthemum were up-regulated and some were down-regulated. We performed a heat map analysis of all transcripts annotated in the transcriptome as chrysanthemums in 12 samples (Fig. 6). We observed that the trend of differences between stems and leaves was more obvious and genes were differentially expressed between the same tissue materials, but there was no significant differential expression trend between RNAi strains and control. As in transgenic lines, the expression level of CmMET1 was 52.5% lower compared to control plants (Li et al. 2019). That means CmMET1 gene expression was not completely silenced. Therefore, we believe that the existing screened differential genes are more sensitive to CmMET1 expression.
Fig. 6.

Clustered heat map comparing scaled expression values for the transcripts annotated with chrysanthemum
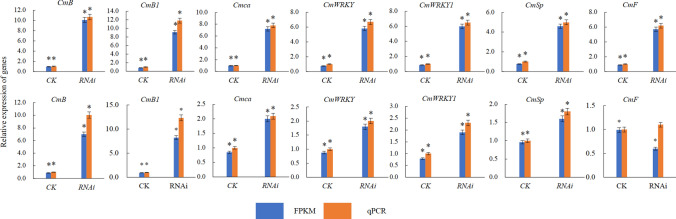
The qRT-PCR results of the differential genes in stem and leaf were verified
According to the results of differentially expressed genes, we screened out 14 candidate genes with significant differences. Fourteen differential genes were screened out from the differential genes of the MET1-RNAi stem and those of the control group for qRT-PCR verification; The results showed that the fluorescent quantitative PCR were consistent with the trend of the transcriptome expression profile (Fig. 7). Most of the 14 genes showed a trend of upregulated expression. It is speculated that the expression of 14 genes in transgenic lines is up-regulated due to decreased level of DNA methylation, which is likely to play an important role in the phenotype of transgenic lines.
Fig. 7.
Verification of differently expressed genes using qRT-PCR. Note The relative gene expression level was calculated by 2−ΔΔCT, and the expression level of ACTIN was the standard. Gene expression shows leaf tissue a gene expression shows stem tissue, b ‘*’ means significant difference, ‘**’ means highly significant difference, P < 0.05
Analyzed Expression differences of flower-related genes in leaves of RNAi interference strains
Ten differential genes related to flowering were screened out from differential genes of MET1-RNAi leaves and control leaves for qPCR verification. As shown in the Fig. 8, the RT-PCR expression pattern of 10 genes is consistent with the RNA-seq expression profile. CmCOL, CmGRAS, CmDOF, CmEMF2, CmCYC4, CmBBX24 and all other genes showed a downward regulation trend. It was speculated that 10 genes in transgenic lines were down-regulated due to reduction in DNA methylation after MET interference. These candidate genes may play an important role in the early flowering phenotype of transgenic lines (Fig. 8).
Fig. 8.
RNA-Seq and qRT-PCR were used to compare differentially expressed genes in leaves and stems. Note ‘*’ means significant difference, ‘**’ means highly significant difference, P < 0.05
qRT-PCR results of RNAi interference with differential genes in shoot tips
Six differential genes were screened from the differential genes of the MET1-RNAi leaves and the control leaves for qPCR verification. The results showed that the fluorescence quantitative PCR results were consistent with the transcriptome expression profile. The expression of 6 genes in transgenic lines was upregulated in leaf and stem tip. It was speculated that the decrease of DNA methylation level of 6 genes in transgenic lines led to the upregulation of expression levels, which was likely to play an important role in the early flowering phenotype of transgenic lines (Fig. 9).
Fig. 9.
RNA-Seq and qRT-PCR were used to compare differentially expressed genes in stems
Discussion
Transgene, as one of the important ways to study gene function and improve traits, has developed rapidly in the study of chrysanthemums, which aims to improve chrysanthemum flowering, color, plant height and resistance to diseases and insect pests. In this study, chrysanthemum cultivar 'Zijingling' wild type and MET1-RNAi strains were used as materials. The rate of DNA methylation in transgenic plants was 14.88% lower than non-transgenic plants, and CmMET1 gene expression level was 52.5% lower than non-transgenic plants (Li et al. 2019). The differential genes were analyzed in their stems and leaves using transcriptome technology.
Effect of changes in DNA methylation status on phenotype
Changes in DNA methylation rates in plants often lead to heritable mutations, with methylation at the CG site dominating the cytosine methylation pattern. In Arabidopsis, MET1 is the main DNA methylation transferase that maintains the methylation of CG sites in DNA. It is composed of MET1 gene mutation and MET1 antisense gene.
Plants with reduced CmMET1 activity caused by constitutive expression showed hypomethylation of the genome and abnormal plant development (embryo malformation, seed sterility). Changes in cytosine methylation patterns can also produce different phenotypic variations, especially in polyploid plants (Chen and Ni 2006).
MET1- RNAi induced gene methylation variation
RNA interference (RNAi) is a monitoring mechanism that exists in eukaryotes to resist foreign virus invasion, regulate gene expression and inhibit transposon activity (Baulcombe 2007). Studies have shown that RNA-dependent RNA polymerase (RdRP) plays important role in RNAi. In Arabidopsis, RdRP can amplify primary siRNA to form secondary siRNA, causing a strong gene silencing effect (Lipardi et al. 2001). In addition, RdRP can be involved in gene regulation through small RNAs (Lister et al. 2008). In Arabidopsis, compared to other site methylation, small RNAs have a 25-fold higher probability of site methylation modification (Cokus et al. 2008). In maize, RdRP can maintain silence by cooperating with transcriptase to generate siRNA (Arteaga-Vazquez et al. 2010). In the transgenic material of this study, the expression of RdRP gene in leaves and stems was upregulated about 200 times, which is consistent with the study in Arabidopsis. The decrease in DNA methylation promotes the gene expression of RdRP, which may be a mechanism for plants to maintain a balanced DNA methylation level in the genome, or it may be due to a decrease in DNA methylation level, which makes the virus lurking in the genome. The expression level increase and the defense mechanism of the plant activate to counter this response. This suggests that when epigenetic methods are used to regulate plant growth and development, we should consider its effect on the expression of potential viruses.
Relationship between plant flower formation and DNA methylation
Flower formation is an important transition stage of the plant life cycle, and flowering time is controlled by internal development and environmental signals. FLC and FT gene translation products act as repressors and activators to regulate the flowering time of Arabidopsis (Yang et al. 2012; Finnegan et al. 1998a, b). Therefore, the regulation of FLC and FT expression is a key factor in controlling flowering and epigenetic modification acts as a key to transcriptional regulation. Till date, studies have confirmed that vernalization is accompanied by DNA demethylation (Burn et al. 1993). The methyltransferase CmMET1 antisense genotype Arabidopsis compared with DNA methylation even without cold treatment. Arabidopsis mutants had lower rates of flowering predate compared to wild type, suggesting that DNA demethylation is associated with vernalization of plants (Finnegan et al. 1998a, b). The DNA methylation of chrysanthemum during the flowering process showed a downward trend, after short-day treatment, the methylation level also significantly decreased which led to the upregulation of FT expression (Zhu 2014). Its expression level rises. The methylation inhibitor 5-azaC prevents the transfer of methyl residues to DNA molecules by replacing cytosine during DNA replication. 5-azaC treatment of plants will cause DNA demethylation, which will cause the plant to show hypomethylation, partially instead of low temperature-induced early flowering. The method of using 5-azaC to regulate flowering has been applied to different species such as Chinese cabbage (Li et al. 2002), flax (Fieldes 1994), perilla (Kondo et al. 2010), spinach (Li et al. 2015), chrysanthemum (Kang et al. 2019a) and so on.
In conclusion, DNA methylation plays an important role in the development process of plants. Li et al. (2019) observed that MET1-RNAi strains of 'Zijingling' showed an early flowering phenotype. In order to screen the differentially expressed genes caused by MTE1 interference, transcriptomic sequencing was carried out to provide a research basis for further exploration of its possible mechanism.
Effect of gene expression in transgenic lines
Differential gene analysis of MET1-RNAi leaves and control leaves, MET1-RNAi stems and control stems, it is inferred that genes in stems are more likely to be regulated by DNA methylation, and related genes in leaves may be constitutively expressed. Reduction in DNA methylation has little effect on it. Decreased DNA methylation in stems of transgenic materials promotes transcription factors, signal transduction mechanisms, secondary metabolite synthesis, transport, and up-regulation of catabolic genes; With cellulose synthase family proteins, plant lipid transport downregulation of related genes such as protein, seed storage spiral domain protein and B3/B4 t-DNA binding domain protein. In previous studies, the CmMET1 gene was 46.51% was of the control expression level, the CmDRM2 gene expression was increased 31%, and the CmDME gene expression level was 73.58% of the control group (Li et al. 2019). However, in the analysis of differentially expressed genes, DNA methyltransferase and demethylase genes were not found. It may be in the analysis of transcriptome gene difference, the screening criteria was Fold Change ≥ 2 and FDR < 0.01, so no genes related to DNA methylation were screened. In addition, genes in plant are redundant in most cases and it is difficult for RNAi interference technology to interfere with all CmMET1 genes. The CmMET1 gene interference effect was 46.51%, which has caused significant changes in the expression of many genes. It has been inferred that these differentially expressed genes in leaves and stems are closely related to DNA methylation. The slight disturbance of DNA methylation will affect the expression of these genes. In addition, in order to further explore the gene expression in different tissues, the differentially expressed genes of MET1-RNAi leaves and MET1-RNAi stems were analyzed. There were 6766 identical differentially expressed genes in the two groups, 1557 differentially expressed genes were unique in MET1-RNAi leaves and MET1-RNAi stems, and 2020 differentially expressed genes were unique in control leaves and control stems. KOG analysis, 6766 differentially expressed genes are classified. The data analysis of the leaves and stems in control group also indirectly proved the reliability of the transcriptome data. However, after MET1 intervention, 65.42% of the differentially expressed genes inside of leaves and stems were not affected, and 34.58% of the differentially expressed genes were affected. Which also showed that CmMET1 interference had a great effect on gene expression in different tissues of chrysanthemum. Differential gene expression is the basis of cell and functional differentiation. Compared with the control, 34.58% of the differentially expressed genes in the leaves and stems have been affected, which will inevitably affect the function of these organs. What kind of changes have been brought about by growth and development needs further exploration.
Conclusions
The data indicate that CmMET1 interference promoted the expression of viral genes in leaves and stems of chrysanthemum. In addition, CmMET1 interference promoted the upregulation of genes related to signaling mechanisms, transcription factors, catalytic activities and nucleic acid-binding transcription factor activities in the stem. 65.42% of the differentially expressed genes in leaves and stems were not affected by the reduction of the CmMET1 gene, while 34.58% of the differentially expressed genes were affected by the reduction of the CmMET1gene.
It has been suggested that CmMET1 interference can cause the differential expression of many genes and the epigenetic regulation mechanism related to DNA methylation of specific genes needs further research.
Supplementary Information
Supplementary Materials: Table S1. Sequences for fluorescent quantitative. Table S2. Overview of the sequencing and assembly. Table S3. Length distribution of the transcripts and unigenes. Table S4. Functional annotation of unigenes. Table S5. Details of 122 different gene annotation results.
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by National Key Research and Development Program (2018YFD1000403), National Natural Science Foundation of China (31372090) and Science and Technology Development Project of Henan (182300410026).
Author contributions
L.S.L\ZY performed experiments and helped in data analysis. LSL and ZY analyzed data. KDR wrote the manuscript with contributions from all authors. WZC guided the design of the whole test scheme. All authors have read and approved the final manuscript.
Availability and data materials
File contains detailed descriptions of all supplemental files. Transcriptomic data are available at NCBI with the accession number: SUB7902302. Gene sequences are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures and tables.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dong-ru Kang and Yi Zhu contributed equally to this work and should be considered co-first authors.
Contributor Information
Dong-ru Kang, Email: kangdongru@henu.edu.cn.
Yi Zhu, Email: 467024502@qq.com.
Shuai-lei Li, Email: 1109569406@qq.com.
Peng-hui Ai, Email: aipenghui@henu.edu.cn.
Muhammad Ayoub Khan, Email: mak9129@yahoo.com.
Hong-xu Ding, Email: 2387446208@qq.com.
Ying Wang, Email: 2472283262@qq.com.
Zi-cheng Wang, Email: wzc@henu.edu.cn.
References
- Apweiler R, Bairoch A, Wu CH, et al. UniProt: the universal protein knowledgebase. Nucl Acids Res. 2004;32(90001):115–119. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Vazquez M, Sidorenko L, Rabanal FA, et al. RNA-mediated trans-communcation can establish paramutation at the bllocus in maize. Proc Natl Acad Sci USA. 2010;107(29):12986–12991. doi: 10.1073/pnas.1007972107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. Nat Genet Italic. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe DC. Molecular biology: Amplified silencing. Science. 2007;315(5809):199–200. doi: 10.1126/science.1138030. [DOI] [PubMed] [Google Scholar]
- Bewick AJ, Schmitz RJ. Gene body DNA methylation in plants. Curr Opin Plant Biol. 2017;36:103–110. doi: 10.1016/j.pbi.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettell RIS, Dennis ES. Reactivation of a silent Ac following tissue culture is associated with heritable alterations in its methylation pattern. MGG-Mol Gen Genet. 1991;229(3):365–372. doi: 10.1007/BF00267457. [DOI] [PubMed] [Google Scholar]
- Burn JE, Bagnall DJ, Metzger JD, et al. DNA methylation, vernalization, and the initiation of flowering. Proc Natl Acad Sci USA. 1993;90(1):287–291. doi: 10.1073/pnas.90.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XF, Jacobsen SE. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc Natl Acad Sci USA. 2002;99(4):16491–16498. doi: 10.1073/pnas.162371599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Ni Z. Mechanisms of genomic rearrangements and gene expression changes in plant polyploids. BioEssays. 2006;28(3):240–252. doi: 10.1002/bies.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokus SJ, Feng S, Zhang X, et al. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452(7184):215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YY, Li JQ, Wu SF, et al. Integrated NR database in protein annotation system and its localization. Comput Eng Ital. 2006;32(5):71–74. doi: 10.1109/INFOCOM.2006.241. [DOI] [Google Scholar]
- Dong W, Li M, Li Z, Li S, Zhu Y, Ding H, Wang Z. Transcriptome analysis of the molecular mechanism of Chrysanthemum flower color change under short-day photoperiod. Plant Physiol Biochem. 2020;146:315–328. doi: 10.1016/j.plaphy.2019.11.027. [DOI] [PubMed] [Google Scholar]
- Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14(9):755–763. doi: 10.1198/016214502388618870. [DOI] [PubMed] [Google Scholar]
- Eszter H, Szalai G, Janda T, Emil P, Ilona R, Demeter L. Effect of vernalisation and 5-azacytidine on the methylation level of DNA in wheat (Triticum aestivum L. cv. martonvásár) Plant Sci. 2003;165(4):692. doi: 10.1016/S0168-9452(03)00221-8. [DOI] [Google Scholar]
- Fieldes MA. Heritable effects of 5-azacytidine treatments on the growth and development of flax (Linum usitatissimum) genotrophs and genotypes. Genome. 1994;37(1):1–11. doi: 10.1139/g94-001. [DOI] [PubMed] [Google Scholar]
- Finn RD, Bateman A, Clements J, et al. Pfam: the protein families database. Nucl Acids Res. 2014;42:222–230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan EJ, Genger RK, Kovac K, et al. DNA methylation and the promotion of flowering by vernalization. Proc Natl Acad Sci USA. 1998;95(10):5824–5829. doi: 10.1073/pnas.95.10.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan EJ, Genger RK, Peacock WJ, et al. DNA methylation in plants. Ann Rev Plant Physiol Plant Mol Biol. 1998;49(1):223–247. doi: 10.1146/annurev.arplant.49.1.223. [DOI] [PubMed] [Google Scholar]
- Fu XD, Kohli A, Twyman RM, et al. Alternative silencing effects involve distinct types of non-spreading cytosine methylation at a three-gene, single-copy transgenic locus in rice. MGG—Mol Gen Genet. 2000;263(1):106–118. doi: 10.1007/PL00008669. [DOI] [PubMed] [Google Scholar]
- Gao W, Li S, Li Z, Huang Y, Deng C, Lu L. Detection of genome DNA methylation change in spinach induced by 5-azac. Mol Cell Probes. 2014 doi: 10.1016/j.mcp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Gong Z, Morales-Ruiz T, Ariza RR, et al. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylaselyase. Cell. 2002;111(6):803–814. doi: 10.1016/S0092-8674(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Jaime HC, Damian S, Kristoffer F, et al. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucl Acids Res. 2016;44:286–293. doi: 10.1093/nar/gkv1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Kawashima S, et al. The KEGG resource for deciphering the genome. Nucl Acids Res. 2004;32:277–280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DR, Dai SL, Gao K, Zhang F, Luo H. Morphological variation of Chrysanthemum lavandulifolium induced by 5-azaC treatment. Sci Hortic. 2019;257:108645. doi: 10.1016/j.scienta.2019.108645. [DOI] [Google Scholar]
- Kang DR, Dai SL, Gao K, Zhang F, Luo H. Morphological variation of Five Cut Chrysanthemum Cultivars Induced by 5-Azacytidine Treatment. Hortscience. 2019;54(7):1208–1216. doi: 10.21273/HORTSCI14012-18. [DOI] [Google Scholar]
- Ke W, Bai ZY, Liu QY, et al. Transcriptome analysis of chrysanthemum (Dendranthema grandiflorum) in response to low temperature stress. BMC Genom. 2018;19(1):319. doi: 10.1186/s12864-018-4706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GJ. Morphological development in Brassica oleracea is modulated by in vivo treatment with 5-azacytidine. J Pomol Horticult Sci. 1995;70(2):333–342. doi: 10.1080/14620316.1995.11515304. [DOI] [Google Scholar]
- Kondo H, Shiraya T, Wada KC, et al. Induction of flowering by DNA demethylation in Perilla frutescens and Silene armeria: heritability of 5-azacytidine-induced effects and alteration of the DNA methylation state by photoperiodic conditions. Plant Sci. 2010;178(3):321–326. doi: 10.1016/j.plantsci.2010.01.012. [DOI] [Google Scholar]
- Koonin EV, Fedorova ND, Jackson JD, et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004;5(2):1–28. doi: 10.1186/gb-2004-5-2-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Z, Wang Y, Tang K, Tang D, Datsenka T, Cheng J, et al. Critical roles of DNA demethylation in the activation of ripening-induced genes and inhibition of ripening-repressed genes in tomato fruit. Proc Natl Acad Sci. 2017 doi: 10.1073/pnas.1705233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng N, Dawson JA, Thomson JA, et al. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29(8):1035–1043. doi: 10.1093/bioinformatics/btt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Guang Z, Zhu Z (2002) Effects of 5-azacytidine(5-azaC) and gibberellic acid(GA_3) on flowering of Brassica campestris ssp. Chinensis L. Master’s thesis, Journal of Shanghai Jiaotong University (in Chinese)
- Li ZA, Li J, Liu YH, et al. DNA demethylation during chrysanthemum floral transition following short-day treatment. Electron J Biotechnol. 2016;21(3):77–81. doi: 10.1016/j.ejbt.2016.02.006. [DOI] [Google Scholar]
- Li SL, Li MM, Wang ZC, et al. Effects of the silencing of CmMET1 by RNA interference in chrysanthemum (Chrysanthemum morifolium) Plant Biotechnol Rep. 2019;13(1):63–72. doi: 10.1007/s11816-019-00516-5. [DOI] [Google Scholar]
- Li SF, Zhang GJ, Yuan JH, et al. Effect of 5-azaC on the growth, flowering time and sexual phenotype of spinach. Russ J Plant Physiol. 2015;62(5):670–675. doi: 10.1134/s1021443715050118. [DOI] [Google Scholar]
- Lipardi C, Wei Q, Paterson BM. RNAi as random degradative PCR: siRNA primers convert mRNA into dsRNAs that are degraded to generate new siRNAs. Cell. 2001 doi: 10.1016/s0092-8674(01)00537-2. [DOI] [PubMed] [Google Scholar]
- Lister RO, Mallwey RC, Tonti-Filippini J, et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133(3):523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using Real-Time quantitative PCR. Methods. 2002;75(4–5):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J, Bi H, Zhang A, et al. Comparative transcriptome analysis by RNA-Seq of the regulation of low temperature responses in Dendranthema morifolium. Hortic Environ Biotechnol. 2018;59:383–395. doi: 10.1007/s13580-018-0042-y. [DOI] [Google Scholar]
- Lu G, Wu X, Chen B, et al. Detection of DNA methylation changes during seed germination in rapeseed (Brassica napus) Chin Sci Bull. 2006;51(2):182–190. doi: 10.1007/s11434-005-1191-9. [DOI] [Google Scholar]
- Matzke MA. Transgene silencing by the host genome defense: implications for the evolution of epigenetic control mechanisms in plants and vertebrates. Plant Mol Biol. 2000;43(2/3):401–415. doi: 10.1023/A:1006484806925. [DOI] [PubMed] [Google Scholar]
- Messeguer R, Ganal MW, Steffens JC, et al. Characterization of the level, target sites and inheritance of cytosine methylation in tomato nuclear DNA. Plant Mol Biol. 1991;16(5):753–770. doi: 10.1007/BF00015069. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Steward N, Sekine M, et al. A tobacco NtMET1 cDNA encoding a DNA methyltransferase: molecular characterization and abnormal phenotypes of transgenic tobacco plants. Plant Cell Physiol. 2000;1(4):448–457. doi: 10.1093/pcp/41.4.448. [DOI] [PubMed] [Google Scholar]
- Nie LJ, He YX, Wang ZC, et al. The effect of 5-azacytidine to the DNA methylation and morphogenesis character of Chrysanthemum during in vitro growth. Acta Horticult Sin. 2009;36:1783–1790. [Google Scholar]
- Sako K, Maki Y, Kanai T, Kato E, Maekawa S, et al. Arabidopsis RPT2a 19S proteasome subunit, regulates gene silencing via DNA Methylation. PLoS One. 2012;7(5):e37086. doi: 10.1371/journal.pone.0037086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq S, Berr A, Shen WH. Combinatorial functions of diverse histone methylations in Arabidopsis thaliana flowering time regulation. New Phytol. 2014 doi: 10.1111/nph.12493. [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, et al. The FLF MADS box gene: a repressor of flowering in arabidopsis regulated by vernalization and methylation. Plant Cell. 1999;11(3):445–458. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smykal P, Valledor L, Rodríguez R. Assessment of genetic and epigenetic stability in long-term in vitro shoot culture of pea (Pisum sativum L.) Plant Cell Reports. 2007;26(11):1985–1998. doi: 10.1007/s00299-007-0413-9. [DOI] [PubMed] [Google Scholar]
- Tatusov RL, Galperin MY, Natale DA. The COG database: a tool for genome scale analysis of protein functions and evolution. Nucl Acids Res. 2000;28(1):33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Chen SM, Jiang JF, et al. Reference gene selection for cross-species and cross-ploidy level comparisons in Chrysanthemum spp. Sci Rep. 2015;5(1):8094. doi: 10.1038/srep08094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Wang HG, Liu XY, Lian S, Chen L, Qiao ZJ, McInerney CE, Wang L. Drought-induced transcription of resistant and sensitive common millet varieties. Anim Plant Sci. 2017;27(4):1303–1314. [Google Scholar]
- Wu CT, Morris JR. Genes genetics and epigenetics: a correspondence. Science. 2001;293(5532):1103–1105. doi: 10.1126/science.293.5532.1103. [DOI] [PubMed] [Google Scholar]
- Xiao W, Brown RC, Lemmon BE, Harada JJ, Fischer GRL. Regulation of seed size by hypomethylation of maternal and paternal genomes. Plant Physiol. 2006;142(3):1160–1168. doi: 10.1104/pp.106.088849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Mao X, Huang J, Ding Y, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucl Acids Res. 2011;39:316–322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong LZ, Xu CG, Maroof MAS, et al. Patterns of cytosine methylation in an elite rice hybrid and its parental lines, detected by a methylation-sensitive amplification polymorphism technique. Mol Gen Genet. 1999;261(3):439–446. doi: 10.1007/s004380050986. [DOI] [PubMed] [Google Scholar]
- Yang H, Han Z, Cao Y, et al. A companion cell-dominant and developmentally regulated H3K4 demethylase controls flowering time in arabidopsis via the repression of FLC expression. PLoS Genet. 2012;8(4):e1002664. doi: 10.1371/journal.pgen.1002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski F, Schmidt M, Van LM, et al. DNA methylation of retrotransposons, DNA transposons and genes in sugar beet (Beta vulgaris L.) Plant J Cell&Amp Mol Biol. 2017;90(6):1156–75. doi: 10.1111/tpj.13526. [DOI] [PubMed] [Google Scholar]
- Zhan J, Thakare D, Ma C, et al. RNA sequencing of laser-capture microdissected compartments of the maize kernel identifies regulatory modules associated with endosperm cell differentiation. Plant Cell. 2015;27(3):513–31. doi: 10.1105/tpc.114.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yazaki J, Sundaresan A, et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell. 2006;126(6):1189–1201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhang Q, Yan Y, Jia H, Han G. Antioxidant constituents of chrysanthemum 'jinsidaju' cultivated in kaifeng. Fitoterapia. 2019 doi: 10.1016/j.fitote.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Zhu QQ (2014) Effect of 5-azaC on DNA methylation and gene-expression of chrysanthemum. Master’s thesis, Henan University (in Chinese)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
File contains detailed descriptions of all supplemental files. Transcriptomic data are available at NCBI with the accession number: SUB7902302. Gene sequences are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures and tables.