Abstract
The present investigation primarily focussed on evaluating the efficacy of exogenous proline on the flower longevity of Dianthus chinensis L. Floral buds were harvested at the paint brush stage (i.e., a day prior to anthesis) and divided into 6 sets, with one set of buds (i.e., control) held in distilled water and rest of the 5 sets were supplemented with various concentrations of proline, viz., 10 mM, 20 mM, 30 mM, 40 mM and 50 mM. The application of proline at 40 mM concentration proved out to be most effective in improving the longevity of the flowers by about 4 days as compared to the control. The ameliorated longevity coincided with enhanced floral diameter, fresh mass, dry mass and water content. The flowers with delayed senescence also maintained higher soluble proteins, sugars and phenols. The results suggest that exogenous proline effectively alleviates oxidative stress in the petal tissue, as evident by a relatively lower maloendialdehyde content, which is manifested in the form of reduced lipid peroxidation (LPO). Reduced LPO was commensurate with increased membrane stability, quantified by membrane stability index. Moreover, the flowers with improved longevity exhibited a decline in lipoxygenase activity and significant augmentation of antioxidant enzymes superoxide dismutase, catalase and ascorbate peroxidase.
Keywords: Antioxidant enzymes, Dianthus chinensis, Flower longevity, Lipid peroxidation, Lipoxygenase, Proline
Introduction
Proline, a low molecular weight and highly soluble α-imino acid, is an essential component of the non-enzymatic antioxidant defense system of plants under stress conditions. Plants under stress accumulate significantly high levels of proline i.e., nearly 80% of the amino acid pool as opposed to a meagre 5% in the absence of stress (Aspinal and Paleg 1981). Strikingly, it has been reported to accumulate in plant tissues even under non-stress conditions, especially during flower initiation and development (Mattioli et al. 2009). The various important roles of proline during plant growth and development have been extensively reviewed (Szabados and Savouré 2010; Hayat et al. 2012; Dar et al. 2016; Zouari et al. 2019). Proline being proteinogenic has a role in the primary metabolism but its ability to help the plant in combating stress conditions (Abdelaal et al. 2020; El-Shawa et al. 2020; Hanif et al. 2020; AlKahtani et al. 2021) rightfully makes it the quintessential sentinel of plants. The wide range of functions includes protein synthesis and stabilization (Hayat et al. 2012; Forlani et al. 2019), osmoprotection (Szabados and Savouré 2010; Ghaffari et al. 2021), signal transduction (Szabados and Savouré 2010; Hayat et al. 2012), redox balance (Shinde et al. 2016), protection of enzyme activity (Mishra and Dubey 2006) and scavenging of Reactive oxygen species (ROS) (Kumar et al. 2010; AlKahtani et al. 2021).
The generation of ROS is an inevitable phenomenon in the life of aerobic organisms. The imbalance arising in the concentration of ROS and antioxidative defense mechanisms (both enzymatic and nonenzymatic), with a subsequent overproduction of ROS, finally culminates in the production of oxidative stress. Oxidative stress, a hallmark of senescence, has been implicated to trigger senescence in flowers such as Chrysanthemum (Chakrabarty et al. 2007), Hemerocallis (Chakrabarty et al. 2009), Lilium (Arrom and Munné-Bosch 2010), Iris (Ahmad and Tahir 2016b) and also plays a crucial role in leaf senescence (Zimmermann and Zentgraf 2005). Furthermore, Wang et al. (2017) identified a ROS-responsive gene namely LcMCII – 1. The workers observed that ROS-dependent and natural senescence was promoted by the overexpression of this gene in Arabidopsis whereas gene silencing of the same resulted in delayed senescence in Litchi chinensis leaves, reflecting thereby, ROS can modulate the expression of senescence associated genes (SAGs) as well. Besides, a wide array of genes has been reported to be upregulated during senescence under oxidative stress (Kumar et al. 2019).
As the process of senescence progresses, ROS levels are amplified beyond plant-compatible limits, exposing the cellular components to the detrimental effects of these reactive species. Moreover, ROS also stimulates the production of ethylene (Arora et al. 2002). The damage ensued due to amplified ROS levels is such that even higher activity of antioxidative enzymes is unable to mitigate it (Chakrabarty et al. 2009). This necessitates the intervention of a non-enzymatic and potent antioxidant like proline, which not only quenches ROS but also enhances the activity of antioxidant enzymes and subsequently acts as a robust shield against oxidative damage. Kumar et al. (2009) observed a significant increase in the endogenous proline content of rose cut flowers at the open stage followed by a dramatic decline as the petals senesced. This study suggests that exogenous proline can be helpful in enhancing flower longevity. Moreover, the information pertaining to the effect of proline in postharvest senescence is very scanty.
The flower senescence in Dianthus chinensis L. is marked by a climacteric-like upsurge of ethylene (Trippi and Paulin 1984). An important experimental study by Bartoli et al. (1996) confirmed a close association between ethylene upsurge and generation of ROS during the petal senescence of Dianthus caryophyllus. Isolated or cut flowers provide an ideal experimental system to study the complex mechanism of senescence. Dianthus, a model flower system for senescence studies (Ahmad and Tahir 2016a), is also an important ornamental with considerable potential in the global floricultural market. Therefore, chemical manipulations in the form of proline treatment in this beautiful flower can not only provide an insight in the understanding of the intricate mechanism of senescence but also help in devising an efficient protocol to facilitate the postharvest handling of cut flowers in general and Dianthus in particular. Keeping in view the important role of proline in the mitigation of oxidative stress, osmoprotection and enhancement of antioxidant enzyme activity, the objectives of the present study were undertaken to explore the potential of this α-imino acid in extending the longevity of Dianthus cut flowers by analysing the changes in various physiological and biochemical parameters of the petal tissue in response to exogenous proline treatment. Besides, the study also focuses on understanding the role of exogenous proline in modulating postharvest senescence of isolated flowers.
Material and methods
Plant material
Healthy and uniform floral buds at the paint brush stage (a day prior to anthesis) were carefully harvested at 9:00 am from the flower stems of Dianthus chinensis, growing in the experimental plots of Kashmir University botanical garden (KUBG). The cut ends of the buds were immediately dipped in Distilled water (DW) and carried to the laboratory. The pedicels of buds were recut to maintain a uniform length of about 3 cm and divided into 6 sets (representing 6 treatments), with each set comprising of 30 buds. Before the transfer of buds to vials, the mouth of all the vials was sealed by aluminium foil to avoid any loss of solution due to evaporation. A small hole was punched in the foil cover of each vial to hold one bud per vial. The first set of buds designated as control was transferred to vials containing DW. The other 5 sets were transferred to vials containing different concentrations of proline viz., 10 mM, 20 mM, 30 mM, 40 mM and 50 mM. The treatment effect was determined by keeping the experimental setup at a temperature of 26 ± 2 °C in the laboratory with a relative humidity of 65 ± 5% and a light of 12 h period per day. Day of transfer of buds to vials was labelled as day 0 and comprehensive analysis of various parameters was done on day 2 (D2) and day 6 (D6) of the experiment, followed by careful visual monitoring until flowers from all the treatments senesced.
Flower longevity and floral diameter
Flower longevity was assessed by careful visual inspection and was calculated as the number of days from day 1 of the experiment (after anthesis) up to the day the flowers began exhibiting visible symptoms of senescence like petal inrolling, colour change and wilting due to reduction of turgidity. Therefore, in the present study, flower longevity essentially indicates the time taken by an open flower to get senesced. The diameter of flower heads was assessed on days 2 and 6 of the experiment by calculating the average of two perpendicular measurements of the top surface of the flower.
Fresh mass, dry mass and water content
Petal tissue from fully open flowers was taken and weighed for the determination of fresh mass. For dry mass, the same samples were oven dried at 70 °C for 48 h and weighed again. Water content was measured by calculating the difference between fresh mass and dry mass.
Estimation of sugar fractions and phenols
Petal tissue weighed about 1 g was taken randomly from flowers of each treatment on the day of analysis. It was chopped and fixed in hot 70% ethanol. The fixed petal tissue was later macerated and centrifuged thrice. Suitable aliquots were taken from the supernatant for the estimation of total phenols, reducing, non-reducing and total sugars. Total phenols were assessed by Swain and Hillis method (1959) using gallic acid as standard whereas Nelson’s method (1944) was adopted for the quantification of reducing sugars with glucose taken as standard. For the estimation of total sugars, the fraction of non-reducing sugars was first converted to reducing sugars by the invertase enzyme, and then the overall concentration of sugars was determined by Nelson’s method (1944). Non-reducing sugars were estimated by calculating the difference in the quantity of total and non-reducing sugars.
Protein estimation
Petal tissue (1 g) samples were taken randomly from each treatment and macerated in 1 mL of 100 mM phosphate buffer (pH 7.2) comprising of 150 mM NaCl, 1 mM EDTA, 1% (v/v) Triton X-100, 10% (v/v) glycerol, 10% (w/v) PVP and 1 mM Dithiothreitol. The centrifugation of the homogenate was carried out at 12,000 × g for 15 min at 4 °C. The supernatant was collected and used to quantify soluble proteins by following the procedure of Lowry et al. (1951) with BSA acting as standard.
Membrane stability index (MSI)
MSI was assessed indirectly by determining solute leakage or conductivity of the petal tissues by suitably adopting the method of Sairam (1994). 100 mg petal tissue was placed in 20 mL double distilled water (DDW) in two separate sets. The first set was incubated at 25 ˚C for 30 min and the conductivity of the samples was designated as C1. The second set was incubated at 100˚C for 15 min and conductivity in this case was designated as C2. The values of conductivity were determined by using Elico CM180 Conductivity meter. MSI was calculated by using the formula
Lipoxygenase activity (LOX)
The protocol elaborated by Axelrod et al. (1981) was used to assay the activity LOX in the samples. 1 g of petal tissue was macerated in 1 ml extraction buffer comprising 50 mM potassium phosphate buffer (pH 6.5), 10% polyvinylpyrrolidone (PVP), 0.25% Triton X-100, and 1 mM phenylmethanesulfonyl fluoride (PMSF). 1 mL of reaction mixture containing 50 mM Tris–HCl buffer (pH 6.5) and 0.4 mM linoleic acid was allowed to react with 10 µl of crude petal extract and absorbance was recorded at 234 nm for 5 min. The activity of LOX was expressed as µmol min−1 mg−1 protein.
Lipid peroxidation (LPO)
Lipid peroxidation in the petal tissues was determined by Heath and Packer (1968) method and expressed as µM MDA g−1 fm. Petal tissue (0.5 g) was macerated in 15 ml of 0.1% trichloroacetic acid (TCA) and centrifuged at 15,000 × g for 10 min under refrigeration. 1 mL of supernatant was taken and mixed with 4 mL of 0.5% TBA diluted in TCA (20%). The reaction was started by incubating the mixture at 95˚C in a water bath for 25 min and the reaction was ended by placing it in ice. Absorbance was taken at 532 and 600 nm. Non-specific absorbance at 600 nm was subtracted from the value observed at 532 nm.
Antioxidant enzyme activity
The activity of superoxide dismutase (SOD) was assayed by the method of Dhindsa et al. (1981). 0.5 g of petal tissue was macerated and homogenized with 500 µL of 0.1 mM potassium phosphate buffer (pH = 7.8) containing 0.1 mM EDTA, 1% PVP and 0.5% (v/v) Triton X-100. The homogenate was centrifuged at 15,000 × g for 10 min in a refrigerated centrifuge and the supernatant obtained was used for the enzyme assay. SOD activity was measured by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT). The reaction mixture comprised of 50 mM sodium carbonate, 75 µM nitroblue tetrazolium (NBT), 0.1 mM EDTA, 13 mM methionine in 50 mM phosphate buffer (pH = 7.8) and 0.1 mL of the enzyme extract in a final volume of 3 mL. Absorbance was measured at 560 nm and one unit of SOD activity was defined as the quantity of the enzyme which inhibits the photoreduction of NBT to blue formazan by 50% as compared to the reaction mixture devoid of the enzyme extract. The SOD activity was expressed as units min−1 mg−1 protein.
Catalase (CAT) activity was determined by the method of Aebi (1984). Petal tissue (0.5 g) was macerated and homogenized in 500 µL of 100 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA, followed by centrifugation at 15,000 × g for 10 min at 4 °C. The reaction mixture, with a final volume of 3 mL, contained 50 mM potassium phosphate buffer (pH 7.0), 12.5 mM H2O2, 50 µL enzyme extract and distilled water. The reaction was started by adding H2O2, and the CAT activity was assayed by the consumption of H2O2 for 3 min at 240 nm and was expressed as µM H2O2 red. min−1 mg−1 protein.
The protocol elaborated by Chen and Asada (1989) was used to assay ascorbate peroxidase (APX) activity. 0.5 g of petal tissue was macerated in 500 µL sodium phosphate buffer (100 mM) containing 5 mM ascorbate, 10% glycerol and 1 mM EDTA. The homogenized mixture was centrifuged at 15,000 × g for 10 min under refrigeration and the supernatant containing the enzyme extract was utilized for assaying the enzyme activity. The APX activity was determined in 1 ml reaction mixture containing 50 mM potassium phosphate buffer (pH = 7.0), 0.1 mM ascorbate, 0.3 mM H2O2 and 50 µL enzyme extract. The decrease in the absorbance was recorded for 3 min at 290 nm and the enzyme activity was expressed as µmol min−1 mg−1 protein.
Experimental design and statistical analysis
The experimental design followed was completely randomized. IBM SPSS® statistics V21.0 software was used to compare treatment means. The statistical significance of the differences between individual treatments was determined by Duncan’s multiple range test (DMRT) at P < 0.05.
Results
The average longevity of Dianthus chinensis flowers after anthesis in the field and that of the detached flower maintained in DW was about 4 days. Senescence in this beautiful flower is marked by the inrolling of petals followed by wilting and a prominent change in colour from pink to yellow. During the present investigation, all the flower buds, i.e., both control and buds treated with proline opened fully on day 1. Exogenous proline at 40 mM concentration exhibited an ameliorative effect on the postharvest attributes of isolated flowers of Dianthus as compared to the control (Fig. 1).
Fig. 1.

Vials in triplicates arranged from left to right represent the effect of various concentrations of proline on the senescence of isolated flowers of Dianthus chinensis on the day of transfer to holding solution (day 0), 2nd day (day 2), 6th day (day 6) and 9th day of experiment (day 9). Control includes the buds transferred to distilled water
Flower longevity and floral diameter
The transfer of cut flowers of Dianthus to holding solution containing 40 mM proline showed significant improvement in longevity, which was about 4 days more than the control. The average longevity increased with an increase in the concentration of proline, i.e., 5, 6 and 7 days, respectively at 10 mM, 20 mM and 30 mM concentration and decreased at 50 mM, where it was just about 6 days. The least value of longevity was recorded for the flowers held in DW (Fig. 2).
Fig. 2.
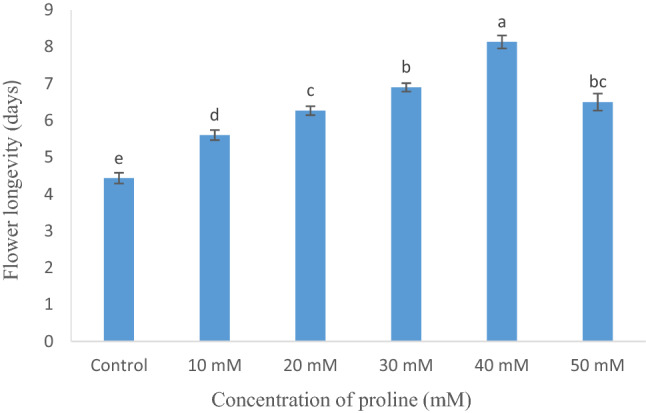
Flower longevity of isolated flowers of Dianthus chinensis held in distilled water (control) and treated with different concentrations of proline. Each value is the mean of 6 replicates and error bars represent ± SE (standard error). Bars with different letters differ significantly at P < 0.05 by DMRT
The diameter of flowers supplemented with 40 mM proline was higher than the control. However, it remained more or less the same from day 2 to 6 (Table 1).
Table 1.
Floral diameter, fresh mass, dry mass, water content, total sugars, reducing and non-reducing sugars in the petal tissues of isolated flowers of Dianthus chinensis treated with different concentrations of proline
| Days after transfer | Control | Concentration of proline | ||||
|---|---|---|---|---|---|---|
| 10 mM | 20 mM | 30 mM | 40 mM | 50 mM | ||
| Floral diameter (cm) | ||||||
| 2 | 2.79 ± 0.05e | 3.09 ± .01d | 3.47 ± .06c | 3.91 ± .03b | 4.08 ± .01a | 3.44 ± .01c |
| 6 | – | 3.22 ± .01e' | 3.62 ± 0.05d' | 4.23 ± 0.05c' | 5.28 ± .03a' | 4.48 ± .09b' |
| Fresh mass (mg) | ||||||
| 2 | 0.154 ± 0.01e | 0.245 ± 0.027d | 0.331 ± 0.012c | 0.379 ± 0.01b | 0.568 ± 0.012a | 0.343 ± 0.003bc |
| 6 | – | 0.182 ± 0.007d' | 0.237 ± 0.018c' | 0.296 ± 0.009b' | 0.396 ± 0.013a' | 0.322 ± 0.01b' |
| Dry mass (mg) | ||||||
| 2 | 0.076 ± 0.007e | 0.12 ± 0.012d | 0.158 ± 0.005c | 0.182 ± 0.002b | 0.228 ± 0.002a | 0.163 ± 0.003bc |
| 6 | – | 0.076 ± 0.007d' | 0.103 ± 0.009c' | 0.145 ± 0.004b' | 0.198 ± 0.007a' | 0.147 ± 0.012b' |
| Water content (mg) | ||||||
| 2 | 0.079 ± 0.003d | 0.125 ± 0.018c | 0.172 ± 0.007b | 0.198 ± 0.008b | 0.34 ± 0.009a | 0.18 ± 0.003b |
| 6 | – | 0.106 ± 0.009d' | 0.134 ± 0.021c'd' | 0.152 ± 0.011b'c' | 0.199 ± 0.006a' | 0.174 ± 0.002a'b' |
| Total sugars (mg g−1 fm) | ||||||
| 2 | 16.24 ± 0.97e | 25.11 ± 1.06d | 29.98 ± 0.79c | 36.3 ± 1.01b | 48.87 ± 1.98a | 34.33 ± 1.19b |
| 6 | – | 17.76 ± 1.32c' | 22.15 ± 1.09b' | 24.97 ± 0.93b' | 32.47 ± 0.53a' | 25.33 ± 1.68b' |
| Reducing sugars (mg g−1 fm) | ||||||
| 2 | 8.52 ± 0.86e | 13.26 ± 0.98d | 17.91 ± 1.05c | 25.26 ± 0.87b | 36.76 ± 1.28a | 25.8 ± 0.79b |
| 6 | – | 9.61 ± 0.43d' | 14.40 ± 0.92c' | 18.31 ± 0.68b' | 24.85 ± 0.95a' | 21.36 ± 1.97a'b' |
| Non-reducing sugars (mg g−1 fm) | ||||||
| 2 | 7.72 ± 0.34d | 11.85 ± 0.83b | 12.07 ± 1.79a | 11.04 ± 1.74b | 12.11 ± 3.26a | 8.53 ± 1.15c |
| 6 | – | 8.15 ± 0.90a' | 7.75 ± 0.20a' | 6.66 ± 1.60a'b' | 7.62 ± 1.46a' | 3.97 ± 0.54b' |
Each value in the table denotes mean of 6 replicates ± SE. The letters a–e and a'–e' (floral diameter); a–e and a'–d' (fresh mass); a–e and a'–d' (dry mass); a–d and a'–d' (water content); a–e and a'–c' (total sugars); a–e and a'–d' (reducing sugars); a–d and a'–b' (non-reducing sugars) represent the statistically significant differences between individual treatments and values with similar letters do not differ significantly at P < 0.05 by DMRT
Fresh mass, dry mass, water content and sugar fractions
Exogenous proline at 40 mM concentration maintained maximum fresh mass, dry mass and water content i.e., 268%, 200% and 330%, respectively, more than the control. Further, all these parameters showed a decreasing trend with the progression in time from day 2 to 6.
The content of sugar fractions (reducing, non-reducing and total sugars) was significantly higher as compared to the control. However, the progression in time from day 2 to 6 resulted in reduced content of sugar fractions (Table 1).
Soluble proteins and total phenols
In the present study, the soluble protein content increased gradually with an increase in proline concentration, with maximum protein content registered in flowers treated with 40 mM proline (Fig. 3a). Maximum phenolic content was recorded in flowers treated with 40 mM proline and the least was observed in the flowers held in DW, i.e., control (Fig. 3b). Moreover, both proteins and phenols decreased with the passage of time from day 2 to 6 (Fig. 3a–b).
Fig. 3.
Effect of different concentrations of proline on (a) Soluble proteins (b) Total phenols (c) Lipid peroxidation (LPO) and (d) Membrane stability index (MSI) in the petal tissue of isolated flowers of Dianthus chinensis. Each value is the mean of 6 replicates and error bars represent ± SE (standard error). Bars with different letters differ significantly at P < 0.05 by DMRT
Lipid peroxidation and membrane stability index
LPO, measured by estimating the MDA content in petal tissues, was recorded lowest in flowers with extended longevity. Flowers held in solutions containing 40 mM proline exhibited significantly lower LPO, i.e., 57% lesser than the control. Reduced levels of LPO were also found to be associated with higher MSI. Flowers with maximum longevity registered an increment of near about 92% in the MSI of petal tissue as compared to the control. LPO increased whereas MSI decreased in all the treatments with time from day 2 to 6 (Fig. 3c–d).
Lipoxygenase activity
The petal samples from the flowers treated with 40 mM proline showed a marked decrease in the activity of the LOX enzyme. On the contrary, the activity of LOX increased with the progression of time from day 2 to 6 (Fig. 4a).
Fig. 4.
Effect of different concentrations of proline on the activities of (a) Lipoxygenase (LOX) (b) Superoxide dismutase (SOD) (c) Catalase (CAT) and (d) Ascorbate peroxidase (APX) in the petal tissue of isolated flowers of Dianthus chinensis. Each value is the mean of 6 replicates and error bars represent ± SE (standard error). Bars with different letters differ significantly at P < 0.05 by DMRT
Antioxidant enzyme activity
All the three antioxidant enzymes, i.e., SOD, CAT and APX showed enhanced activities in flowers treated with proline, with maximum activity recorded in flowers treated with 40 mM proline. The increase in the activity of SOD, CAT and APX was about 263%, 316% and 231%, respectively than the control. Moreover, with the progression in time from day 2 to 6, the activity of all the three enzymes declined (Fig. 4b–d).
Discussion
Application of exogenous proline at 40 mM concentration significantly improved floral longevity by alleviating senescence symptoms. Proline is a potent free radical scavenger and its role in improving the longevity of cut flowers has been reported earlier by Kumar et al. (2010) in rose. A low proline turnover during senescence was also observed in rose (Kumar et al. 2009), suggesting the role of proline during petal senescence. Proline effectively quenches ROS (Kumar et al. 2010; Hayat et al. 2012; El-Shawa et al. 2020; AlKahtani et al. 2021) and enhances the antioxidant enzyme activity (Hayat et al. 2012; Hanif et al. 2020; AlKahtani et al. 2021), besides maintaining the integrity of membranes and stability of proteins (Szabados and Savouré 2010). Moreover, Kumar et al. (2010) observed proline induced activity of proline dehydrogenase, an important enzyme involved in proline catabolism. Since higher proline catabolism is associated with high energy output, therefore proline might act as an energy source and delay ageing in petals. All these factors contribute cumulatively towards retarding senescence, as manifested by the extended longevity of Dianthus flowers. Furthermore, the relatively enhanced floral diameter of treated flowers can be attributed to the fact that proline being an important compatible osmolyte helps in maintaining the turgidity of petal tissue by improving water uptake. Exogenous proline has been shown to improve the degree of cell hydration in plants, preferably by lowering water potential and enhancing water uptake, which subsequently postpones dehydration and maintains turgor (Ahmed et al. 2011; Zouari et al. 2019). Delayed senescence in Dianthus has been found to be associated with a higher fresh and dry mass of petal tissues (Dar et al. 2015), as senescence is characterized by deterioration of cellular components followed by remobilization of essential nutrients to developing parts. El-Shawa et al. (2020) have reported a significant improvement of various floral attributes of Calendula plants like floral quality, fresh weight, dry weight, floral diameter as well as vase life on the application of exogenous proline.
As observed from the results, the flowers treated with exogenous proline maintained higher soluble proteins as compared to control. Hanif et al. (2020) reported in a pot experiment conducted on rice that the exogenous application of proline improved the content of total soluble proteins both under stress as well as non-stress conditions, suggesting the possible role of proline in maintaining higher levels of soluble proteins. It can be argued that alleviated oxidative stress by proline treatment protects membrane proteins and lipids, which could have inhibited the release of vacuolar proteases, thereby shielding the cytosolic proteins from degradation. Moreover, proline acts as a molecular chaperone and is capable of maintaining the stability of proteins (Szabados and Savouré 2010). A mechanism for protein protection by proline proposed by Forlani et al. (2019) suggests that proline at high concentration stabilizes macromolecules, especially proteins by exerting a kosmotropic effect, enabling the molecules to form protective hydration shells.
Phenols represent one of the most common and widespread secondary plant metabolites with a well-documented role in the adaptive response of plants towards biotic and abiotic stress. The regulatory role of phenols has been reported earlier in the flower senescence of Iris (Ahmad and Tahir 2017). Furthermore, Dar et al. (2015) have observed elevated levels of total phenols at the fully open flower stage in Dianthus chinensis and reduced levels during senescence. Such observations are further substantiated by the research findings on Iris versicolor and Iris japonica (Ahmad and Tahir 2017). In the present study, flowers supplemented with 40 mM proline, exhibiting extended longevity, reported higher total phenolic content, possibly due to the effect of proline on the phenol biosynthetic pathways. Kwok and Shetty (1998) demonstrated that endogenous proline and its analogs increased phenolic content by stimulating pentose, shikimate and phenylpropanoid pathways. Elevated phenolic levels in response to exogenous proline treatment were observed in Citrus (Mohammadrezakhani et al. 2019) and Aloe vera (Nakhaie et al. 2020). Besides maintaining the elevated content of phenols, flowers with delayed senescence also showed higher content of various soluble sugars. Proline treatment has been found to increase the content of soluble sugars in Arabidopsis (Moustakas et al. 2011) and Lupinus (Rady et al. 2016). Dar et al. (2015) have reported reduced levels of various sugars during senescence in both Dianthus chinensis and Dianthus barbatus as compared to fully open fresh flowers.
In Dianthus, senescence is characterized by an ethylene upsurge which induces the generation of ROS besides other senescence-related events (Bartoli et al. 1996; Rogers 2012). ROS moieties include O2•−, 1O2, OH•, HO2•− and H2O2, which have the potential of oxidizing a wide range of cellular macromolecules (Rogers 2012). Rogers (2012) suggests that elevated ROS levels are highly correlated with senescence of cut flowers than with natural flowers. Plants have mechanisms to quench all types of ROS, however, as senescence progresses, an imbalance is inevitable (Kumar et al. 2019). An important target of superoxide radicals is the membrane lipids, causing their peroxidation, a key event in the initiation of senescence (Rogers 2012). LPO is manifested in the form of loss of membrane integrity and an escalation of ion leakage or reduced MSI, which is a key marker for petal and leaf senescence (Rogers 2012). The damage ensued by LPO is further aggravated by its tendency to undergo autocatalytic amplification (Rogers 2012; Kumar et al. 2019). Treatment with proline in the present study reduced LPO (estimated by MDA content in the petal tissue) by effectively quenching the highly toxic ROS, which corroborates with the findings of Kumar et al. (2010) in rose. Ozden et al. (2009); Mohammadrezakhani et al. (2019); Abdelaal et al. (2020); AlKahtani et al. (2021) and Hanif et al. (2020) also reported reduced LPO whereas Seleiman et al. (2020) observed improved MSI in response to exogenous proline treatment. Furthermore, alleviation of oxidative stress has been implicated in the retarded senescence of cut flowers like Chrysanthemum (Chakrabarty et al. 2007), Hemerocallis (Chakrabarty et al. 2009, Gladiolus (Saeed et al. 2016), Lisianthus (Ataii et al. 2015) and Nicotiana (Nisar et al. 2021).
Generally, LPO is preceded by the activity of LOX during senescence (Rogers 2012). Membrane lipids are oxidized enzymatically by LOX, generating oxide, alkoxy and peroxy radicals, which further accelerate LPO (Rogers and Munné-Bosch 2016). Consequently, membrane deterioration is exacerbated as senescence progresses. Although LOX is not the primary trigger for LPO, it has been implicated in the petal senescence of Gladiolus (Peary and Prince 1990), Dianthus (Rouet-Mayer et al. 1992), rose (Fukuchi-Mizutani et al. 2000) and Hemerocallis (Rubinstein 2000). The data from the present investigation reveal a significant alleviation of LOX activity in response to exogenous proline. These observations are in conformity with earlier studies on rice (Hasanuzzaman et al. 2014) and Citrus (Mohammadrezakhani et al. 2019).
The toxicity of ROS makes its elimination imperative for cell survival. Since the generation of oxygen free radicals and H2O2 is inevitable, therefore, plants have evolved various mechanisms to maintain ROS homeostasis within cells (Halliwell 2006). An effective strategy to eliminate ROS is by the enzymatic activity of SOD, CAT and APX. SOD primarily catalyzes the dismutation of O2•− radicals while CAT and APX help in the detoxification of H2O2 via the Halliwell–Asada pathway (Rogers 2012). Various workers have elucidated the role of these enzymes in flower senescence, viz., Gladiolus (Hossain et al. 2006), Dianthus (Zhang et al. 2007), Chrysanthemum (Chakrabarty et al. 2007) and Iris (Ahmad and Tahir 2016b). Exogenous proline at 40 mM concentration significantly enhanced the activity of SOD, CAT and APX. Proline has been reported to act as an enzyme protectant (Mishra and Dubey 2006) and treatment with antioxidants in general (Seleiman et al. 2020) and proline in particular (Hanif et al. 2020) has been shown to reinforce the activity of SOD and CAT with a concomitant rectification of ion imbalance. Also, in rose flowers treated with proline, Kumar et al. (2010) observed considerably higher SOD activity which sustained for 3.3 days more than the control. A significant improvement of CAT activity was also observed in response to proline treatment in barley plants (Abdelaal et al. 2020) Moreover, APX activity was also enhanced by exogenous proline in the plant species, such as Vitis (Ozden et al. 2009) and Citrus (Mohammadrezakhani et al. 2019).
Conclusion and future perspectives
The present study revealed that the isolated flowers of Dianthus chinensis harvested at the paint brush stage and treated with 40 mM proline significantly improved the longevity of this beautiful ornamental by ameliorating various postharvest attributes. Proline acts like an efficient sentinel to impede various detrimental effects associated with petal senescence. According to the authors, the enhanced longevity was a consequence of alleviated oxidative stress, decreased lipid peroxidation, improved water content, proteins, phenols and increased activity of antioxidant enzymes in the petal tissue. Moreover, the biochemical analysis substantiated the complex interplay of various biomolecules in the modulation of senescence. The role of proline in imparting stress tolerance remains well established in plants and proline being a potent antioxidant provides substantial scope for further studies on flower senescence. Future studies could be targeted at deciphering the metabolic pathways of proline during flower senescence and the corresponding molecular mechanisms and crosstalks involved, besides various associated signalling cascades need to be undertaken in detail. A comprehensive understanding of these mechanisms can not only provide an insight into the intricate mechanism of petal senescence but also help in devising non-toxic, environment-friendly and economical techniques to improve the postharvest performance of various ornamentals, which has far-reaching implications on floricultural trade and commerce.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelaal KAA, Attia KA, Alamery SF, El-Afry MM, Ghazy AI, Tantawy DS, Al-Doss AA, El-Shawy ESE, Abu-Elsaoud AM, Hafez YM. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustain. 2020;12:1736. doi: 10.3390/su12051736. [DOI] [Google Scholar]
- Aebi H. Catalase in Vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahmad SS, Tahir I. How and why of flower senescence: understanding from models to ornamentals. Indian J Plant Physiol. 2016;21:446–456. doi: 10.1007/s40502-016-0267-7. [DOI] [Google Scholar]
- Ahmad SS, Tahir I. Increased oxidative stress, lipid peroxidation and protein degradation trigger senescence in Iris versicolor L. flowers. Physiol Mol Biol Plants. 2016;22:507–514. doi: 10.1007/s12298-016-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SS, Tahir I. Regulatory role of phenols in flower development and senescence in the genus Iris. Indian J Plant Physiol. 2017;22:135–140. doi: 10.1007/s40502-016-0278-4. [DOI] [Google Scholar]
- Ahmed CB, Magdich S, Rouina BB, Sensoy S, Boukhris M, Abdullah FB. Exogenous proline effects on water relations ions contents in leaves and roots of young olive. Amino Acids. 2011;40:565–573. doi: 10.1007/s00726-010-0677-1. [DOI] [PubMed] [Google Scholar]
- AlKahtani MD, Hafez YM, Attia K, Rashwan E, Husnain LA, AlGwaiz HI, Abdelaal KA. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxid. 2021;10:398. doi: 10.3390/antiox10030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Sairam RK, Srivastava GC. Oxidative stress and antioxidative system in plants. Curr Sci. 2002;25:1227–1238. [Google Scholar]
- Arrom L, Munné-Bosch S. Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci. 2010;179:289–295. doi: 10.1016/j.plantsci.2010.05.002. [DOI] [Google Scholar]
- Aspinal D, Paleg G. Proline accumulation: physiological aspects. In: Paleg LG, Aspinall D, editors. The Physiology and Biochemistry of Drought Resistance in Plants. Australia: Academic Press, Sydney; 1981. pp. 280–295. [Google Scholar]
- Ataii D, Naderi R, Khandan-Mirkohi A. Exogenous putrescine delays senescence of Lisianthus cut flowers. J Ornam Plants. 2015;5:167–174. [Google Scholar]
- Axerold B, Chesbrough TM, Laakso S. Lipoxygenase from soybean. In: Lowenstein JM, editor. Meth Enzymol. New York: Academic press; 1981. pp. 441–451. [Google Scholar]
- Bartoli CG, Simontacchi M, Montaldi E, Puntarulo S. Oxidative stress, antioxidant capacity and ethylene production during ageing of cut carnation (Dianthus caryophyllus) petals. J Exp Bot. 1996;47:595–601. doi: 10.1093/jxb/47.4.595. [DOI] [Google Scholar]
- Chakrabarty D, Chatterjee J, Datta SK. Oxidative stress and antioxidant activity as the basis of senescence in chrysanthemum florets. Plant Growth Regul. 2007;53:107–115. doi: 10.1007/s10725-007-9208-9. [DOI] [Google Scholar]
- Chakrabarty D, Verma AK, Datta SK. Oxidative stress and antioxidant activity as the basis of senescence in Hemerocallis (day lily) flowers. J Hortic. 2009;1:113–119. [Google Scholar]
- Chen GX, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. doi: 10.1093/oxfordjournals.pcp.a077713. [DOI] [Google Scholar]
- Dar RA, Tahir I, Ahmad SS. Is the biochemical mechanism of petal senescence similar within a genus? A case study of Dianthus. Hortic Environ Biotechnol. 2015;56:654–661. doi: 10.1007/s13580-015-1068-z. [DOI] [Google Scholar]
- Dar MI, Naikoo MI, Rehman F, Naushin F, Khan FA (2016) Proline accumulation in plants: roles in stress tolerance and plant development. In: Osmolytes and plants acclimation to changing environment: emerging omics technologies. Springer, New Delhi p 155-166
- Dhindsa RS, Plumb-Dhindsa PA, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32:93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- El-Shawa GM, Rashwan EM, Abdelaal KA. Mitigating salt stress effects by exogenous application of proline and yeast extract on morpho-physiological, biochemical and anatomical characters of calendula plants. Sci J Flowers Ornam Plants. 2020;7:461–482. doi: 10.21608/sjfop.2020.135166. [DOI] [Google Scholar]
- Forlani G, Trovato M, Funck D, Signorelli S (2019) Regulation of proline accumulation and its molecular and physiological functions in stress defence. In: Osmoprotectant-mediated abiotic stress tolerance in plants Springer, Cham p. 73–97
- Fukuchi-Mizutani M, Ishiguro K, Nakayama T, Utsunomiya Y, Tanaka Y, Kusumi T, Ueda T. Molecular and functional characterization of a rose lipoxygenase cDNA related to flower senescence. Plant Sci. 2000;160:129–137. doi: 10.1016/S0168-9452(00)00373-3. [DOI] [PubMed] [Google Scholar]
- Ghaffari H, Tadayon MR, Bahador M, Razmjoo J (2021) Investigation of the proline role in controlling traits related to sugar and root yield of sugar beet under water deficit conditions. Agric Water Manag 243:106448
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physio. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif S, Saleem MF, Sarwar M, Irshad M, Shakoor A, Wahid MA, Khan HZ. Biochemically triggered heat and drought stress tolerance in rice by proline application. J Plant Growth Regul. 2020 doi: 10.1007/s00344-020-10095-3. [DOI] [Google Scholar]
- Hasanuzzaman M, Alam M, Rahman A, Hasanuzzaman M, Nahar K, Fujita M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res Int. 2014;2014:17. doi: 10.1155/2014/757219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7:1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hossain Z, Mandal AKA, Datta SK, Biswas AK. Decline in ascorbate peroxidase activity–A prerequisite factor for tepal senescence in gladiolus. J Plant Physiol. 2006;163:186–194. doi: 10.1016/j.jplph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kumar N, Pal M, Srivastava GC. Proline metabolism in senescing rose petals (Rosa hybrida L. ‘First Red’) J Hortic Sci Biotechnol. 2009;84:536–540. doi: 10.1080/14620316.2009.11512562. [DOI] [Google Scholar]
- Kumar N, Pal M, Singh A, SaiRam RK, Srivastava GC. Exogenous proline alleviates oxidative stress and increase vase life in rose (Rosa hybrida L. ‘Grand Gala’) Sci Hortic. 2010;127:79–85. doi: 10.1016/j.scienta.2010.09.009. [DOI] [Google Scholar]
- Kumar V, Khare T, Srivastav A, Surekha C, Shriram V, Wani SH (2019) Oxidative stress and leaf senescence: important insights. In: Senescence Signalling and Control in Plants Academic Press p. 139–163
- Kwok D, Shetty K. Effects of proline and proline analogs on total phenolic and rosmarinic acid levels in shoot clones of thyme (Thymus vulgaris L.) J Food Biochem. 1998;22:37–51. doi: 10.1111/j.1745-4514.1998.tb00229.x. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Mattioli R, Costantino P, Trovato M. Proline accumulation in plants: not only stress. Plant Signal Behav. 2009;4:1016–1018. doi: 10.4161/psb.4.11.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Dubey RS. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: role of proline as enzyme protectant. J Plant Physiol. 2006;163:927–936. doi: 10.1016/j.jplph.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Mohammadrezakhani S, Hajilou J, Rezanejad F, Zaare-Nahandi F. Assessment of exogenous application of proline on antioxidant compounds in three Citrus species under low temperature stress. J Plant Interact. 2019;14:347–358. doi: 10.1080/17429145.2019.1629033. [DOI] [Google Scholar]
- Moustakas M, Sperdouli I, Kouna T, Antonopoulou CI, Therios I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011;65:315–325. doi: 10.1007/s10725-011-9604-z. [DOI] [Google Scholar]
- Nakhaie A, Habibi G, Vaziri A. Exogenous proline enhances salt tolerance in acclimated Aloe vera by modulating photosystem II efficiency and antioxidant defense. S Afr J Bot. 2020 doi: 10.1016/j.sajb.2020.06.005. [DOI] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. doi: 10.1016/S0021-9258(18)71980-7. [DOI] [Google Scholar]
- Nisar S, Dar RA, Tahir I. Salicylic acid retards senescence and makes flowers last longer in Nicotiana plumbaginifolia (Viv) Plant Physiol Rep. 2021;26:128–136. doi: 10.1007/s40502-021-00569-1. [DOI] [Google Scholar]
- Ozden M, Demirel U, Kahraman A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci Hortic. 2009;119:163–168. doi: 10.1016/j.scienta.2008.07.031. [DOI] [Google Scholar]
- Peary JS, Prince TA. Floral lipoxygenase: activity during senescence and inhibition by phenidone. J Am Soc Hortic. 1990;115:455–457. doi: 10.21273/JASHS.115.3.455. [DOI] [Google Scholar]
- Rady Taha Mahdi nMRSAH. Proline enhances growth, productivity and anatomy of two varieties of Lupinus termis L. grown under salt stress. S Afr J Bot. 2016;102:221–227. doi: 10.1016/j.sajb.2015.07.007. [DOI] [Google Scholar]
- Rogers HJ. Is there an important role for reactive oxygen species and redox regulation during floral senescence? Plant Cell Environ. 2012;35:217–233. doi: 10.1111/j.1365-3040.2011.02373.x. [DOI] [PubMed] [Google Scholar]
- Rogers H, Munné-Bosch S. Production and scavenging of reactive oxygen species and redox signaling during leaf and flower senescence: similar but different. Plant Physiol. 2016;171:1560–1568. doi: 10.1104/pp.16.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet-Mayer MA, Bureau JM, Lauriere C. Identification and characterization of lipoxygenase isoforms in senescing carnation petals. Plant Physiol. 1992;98:971–978. doi: 10.1104/pp.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Regulation of cell death in flower petals. In: Lam E, Fukuda H, Greenberg J, editors. Programmed Cell Death in Higher Plants. Dordrecht: Springer; 2000. pp. 59–74. [Google Scholar]
- Saeed T, Hassan I, Abbasi NA, Jilani G. Antioxidative activities and qualitative changes in gladiolus cut flowers in response to salicylic acid application. Sci Hortic. 2016;210:236–241. doi: 10.1016/j.scienta.2016.07.034. [DOI] [Google Scholar]
- Sairam RK. Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Indian J Exp Biol. 1994;32:594–594. [Google Scholar]
- Seleiman MF, Semida WM, Rady nM, Mohamed GF, Hemida KA, Alhammad BA, Shami A. Sequential application of antioxidants rectifies ion imbalance and strengthens antioxidant systems in salt-stressed cucumber. Plants. 2020;9:1783. doi: 10.3390/plants9121783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde S, Villamor JG, Lin W, Sharma S, Verslues PE. Proline coordination with fatty acid synthesis and redox metabolism of chloroplast and mitochondria. Plant Physiol. 2016;172:1074–1088. doi: 10.1104/pp.16.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Trippi V, Paulin A. The senescence of cut carnations: a phasic phenomenon. Physiol Plant. 1984;60:221–226. doi: 10.1111/j.1399-3054.1984.tb04568.x. [DOI] [Google Scholar]
- Wang C, Lü P, Zhong S, Chen H, Zhou B. LcMCII-1 is involved in the ROS-dependent senescence of the rudimentary leaves of Litchi chinensis. Plant Cell Rep. 2017;36:89–102. doi: 10.1007/s00299-016-2059-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo WM, Chen SM, Han L, Li ZM. The role of N-lauroylethanolamine in the regulation of senescence of cut carnations (Dianthus caryophyllus) J Plant Physiol. 2007;164:993–1001. doi: 10.1016/j.jplph.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Zentgraf U. The correlation between oxidative stress and leaf senescence during plant development. Cell Mol Biol Lett. 2005;10:515. [PubMed] [Google Scholar]
- Zouari M, Hassena AB, Trabelsi L, Rouina BB, Decou R, Labrousse P. Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants. Cham: Springer; 2019. Exogenous proline-mediated abiotic stress tolerance in plants: Possible mechanisms; pp. 99–121. [Google Scholar]




