Abstract
The amino acid, proline, is utilized by different organisms to offset cellular imbalances caused by environmental stresses. The wide use of proline as a stress adaptor molecule indicates that proline has a fundamental biological role in stress response. A comprehensive analysis of the transcript abundance of proline metabolizing genes is fundamental for the assessment of function and regulation of each gene. Using available microarray data and quantitative real-time RT-PCR, the expression profiles of gene encoding key proline biosynthesis and degradation enzymes i.e., OAT, P5CS, P5CR and PDH were examined. Interestingly, validation of candidate genes in rice using in-silico data provided strong evidence for their involvement in stress response. Note that, OsOAT, OsP5CS1, OsP5CS2, OsP5CR showed similar expression pattern in quantitative real-time RT-PCR results as compared to microarray data. However, OsPDH showed a different expression pattern which may be due to the genotypic variation. Furthermore, a biochemical assay measuring proline content gave us a proper indication of the accumulation of proline under stressed conditions. Identification of key proline metabolizing genes from rice and Arabidopsis provides insights on the molecular regulation of proline homeostasis, to initiate metabolic engineering to develop stress-resilient plants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01023-0.
Keywords: Proline metabolism, RT-PCR, Rice, Arabidopsis, Transcriptome, Proline
Introduction
Plants are frequently exposed to inescapable environmental factors such as, high light intensity, UV, fluctuation of temperature, freezing, drought, salinity, heavy metal toxicity, and hypoxia that lead to abiotic stresses (Hirayama et al. 2010). As plants cannot move and physically avoid the exposure to these stressors, they try to increase their cellular osmotic potential through the accumulation of various intracellular organic osmolytes such as proline, glycine betaine, mannitol and trehalose (Khaleghi et al. 2019; Pérez-Llano et al. 2020) to stabilize the intracellular environment as well as the structures of proteins, membranes, and subcellular organelles (Verslues et al. 2006). Among them, proline is an important osmoprotectant molecule that accumulates in many organisms including bacteria, fungi, and plants upon exposure to external stress (Chun et al. 2018; Ma et al. 2020). One of the main adaptive mechanisms is the accumulation of proline in the cytoplasm under salinity (Islam et al. 2016). Proline is frequently accumulated under several harmful stimuli like oxidative damage by reactive oxygen species to protect cells (Gupta et al. 2010). Plant cells maintain water status in their tissues through osmotic adjustment during salinity and drought stresses which involve the biosynthesis of proline (Hossain et al. 2021). Although transgenic plants over-accumulating proline enhanced resistance to drought and salinity stress (Kumar et al. 2015), the mechanism of action behind this observation is still a matter of debate. It is not clear whether the accumulation of proline itself or the enzyme activity controlling its homeostasis played a major role in withstanding the deleterious effect of stress (Kavi Kishor and Sreenivasulu 2014). Therefore, metabolic engineering of proline biosynthesis pathway for stress tolerance remains an open question (Verslues and Sharma 2010; Bhaskara et al. 2015).
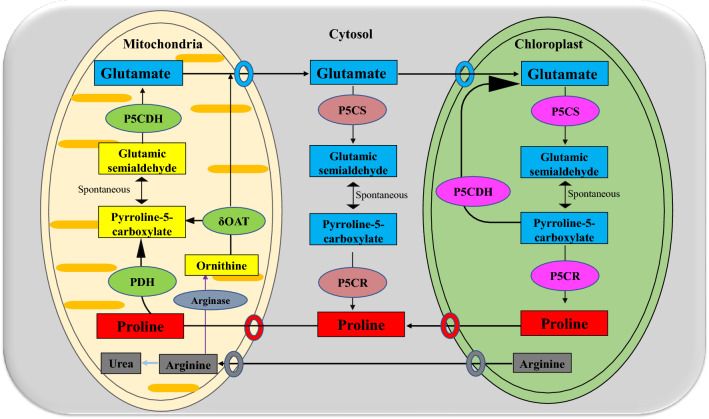
Proline is synthesized through two different pathways (Khanna-Chopra et al. 2019). Firstly, glutamate is converted to proline by two successive reduction steps catalyzed by pyrroline-5-carboxylate synthetase (P5CS) and pyrroline-5-carboxylate reductase (P5CR); and secondly, proline can be synthesized from ornithine, which is transaminated to Δ1 pyrroline-5- carboxylate (P5C) by a mitochondrion located ornithine-δ-aminotransferase (δ-OAT) enzyme (Fig. 1). Proline synthesis occurs in the cytosol and chloroplast; while its degradation occurs in the mitochondria (Khanna-Chopra et al. 2019). In most plant species, P5CS is encoded by two genes and P5CR is encoded by one. Proline catabolism occurs in mitochondria via the sequential action of proline dehydrogenase or proline oxidase (PDH or POX) producing P5C from proline, and P5C dehydrogenase (P5CDH), which converts P5C to glutamate. The biosynthetic enzymes (P5CS1, P5CS2 and P5CR) are predicted to be localized in the cytosol, whereas a mitochondrial localization is predicted for the enzymes involved in proline catabolism (such as PDH1, PDH2, P5CDH and OAT) (Szabados and Savouré 2010). The prominent hypothesis is that ornithine results from arginine degradation by OAT converts to P5C/GSA (Glutamic semialdehyde), which acts as a substrate for proline production by P5CR (Verbruggen and Hermans, 2008). This model is controversial because OAT is in mitochondria whereas P5CR is localized in cytosol in Arabidopsis (Winter et al. 2015). Hence, further research is needed to understand whether produced GSA by OAT is directly used for proline synthesis or converts to glutamate by P5CDH and enters the proline production cycle through P5CS (Funck et al. 2008, 2012). The glutamate pathway accounts for the major proline accumulation during osmotic stress (Shamsul et al. 2012). The ornithine pathway also contributes to proline accumulation during salt stress as the free proline content, P5CS mRNA, δ-OAT mRNA, and δ-OAT enzyme activity were all found to be enhanced in young Arabidopsis plants by salt treatment (Roosens et al. 1998). Moreover, in-vivo application of a powerful and irreversible inhibitor of δ-OAT, gabaculine at a very low concentration of 1 mmolm−3 to radish cotyledons considerably reduced salt‐stress induced proline accumulation; demonstrating the contribution of the ornithine pathway to proline synthesis (Hervieu et al. 1995). On the contrary, proline is catabolized by the sequential action of proline dehydrogenase (PDH) and P5C dehydrogenase (P5CDH) (Fig. 1).
Fig. 1.
Proline biosynthesis and degradation pathway. Proline biosynthesis occurs in the cytosol and chloroplast, while its degradation occurs in mitochondria. P5C (δ-pyrroline-5-carboxylate), P5CR -pyrroline-5-carboxylate reductase, P5CS -pyrroline-5-carboxylate synthase, GSA -glutamic semialdehyde, PDH -Proline dehydrogenase, OAT -Ornithine aminotransferase
P5CS (EC 2.7.2.11/1.2.1.41) is a bifunctional enzyme with γ-glutamyl kinase and glutamic γ-semialdehyde dehydrogenase activities that catalyzes the first two steps in proline biosynthesis. In eubacteria (gene proB) and yeast (gene PRO1) (Li and Brandriss 1992), it is a monofunctional protein, while in plants and mammals, it is a bifunctional enzyme (Hu et al. 1992) that consists of two domains: a N-terminal GK domain and a C-terminal γ-glutamyl phosphate reductase domain (1.2.1.41). There are two different forms of P5CS—P5CS1 and P5CS2; which in plants resulted from an evolutionary duplication event (Turchetto Zolet et al. 2009). These two duplicated enzymes perform non-redundant functions and their genes showed different expression patterns (Ginzberg et al. 1998). Knockout of P5CS1 causes a drastic reduction of stress-induced proline synthesis in Arabidopsis, hypersensitivity to salt stress and an excess accumulation of reactive oxygen species (ROS) (Szekely et al. 2008). In contrast to P5CS1, there is no significant transcriptional upregulation of P5CS2 under stress and thus, it might have a minor role in stress-induced proline accumulation (Strizhov et al. 1997; Szekely et al. 2008). Besides, overexpression of δ-OAT resulted in enhanced proline accumulation in Arabidopsis and rice under salinity stress (Roosens et al. 2002; Wu et al. 2003). However, the level of proline under stress is maintained by the upregulation of P5CS genes and down-regulation of PDH genes (Lei et al. 2016; Mansour and Ali 2017).
Given the important role of P5CS, P5CR, δ-OAT and PDH enzymes for the maintenance of proline homeostasis in response to various abiotic stresses, we sought to characterize their corresponding transcripts in detail from Arabidopsis and rice. A genome-wide investigation of Arabidopsis and rice was performed to analyze their sequence homology, evolutionary relationship, subcellular localization, and presence of conserved motifs/domains. Furthermore, transcript profiling of these proline biosynthesis and degradation genes was analyzed under key developmental stages, anatomical tissues and in response to various unfavorable stress conditions using publicly available microarray datasets. Expression of proline metabolizing genes was functionally validated in one of the local Bangladeshi rice variety against six abiotic stresses and abscisic acid treatment through quantitative real-time PCR (qRT-PCR) and found to be positively correlated with their respective total proline content as compared to the untreated control condition.
Materials and methods
Data retrieval from Arabidopsis and Rice genome databases, and construction of gene structure and domain architecture
To identify the proteins involved in proline biosynthesis and degradation pathway, blastP search had been employed using already characterized known protein sequence as a query in TAIR database (https://www.Arabidopsis.org/) for Arabidopsis and MSU Rice Genome Annotation Project Database (http://rice.plantbiology.msu.edu/) for rice. After identification of the genes, chromosomal location, coordinates of genes and protein data such as polypeptide length (AA), isoelectric point (pI) and molecular mass (kDa) had been collected from the same databases and verified with the other protein databases like Uniprot (https://www.uniprot.org/) and ProtParam (https://web.expasy.org/protparam/). The alternative splice forms were named as the gene name followed by the Arabic number after the dot “.”. The exon–intron position of each gene was analyzed based on the genomic and transcript sequence available from TAIR and MSU Rice Genome Annotation Project database for Arabidopsis and rice, respectively. Protein sequences were examined to determine the presence of conserved domains using the Pfam database (http://pfam.xfam.org/) with default parameters. The subcellular localization of the proteins was predicted from CELLO v.2.5: subCELlular LOcalization predictor (http://cello.life.nctu.edu.tw/) (Yu et al. 2006) and WoLF PSORT (https://www.genscript.com/wolf-psort.html) software (Horton et al. 2007). ChloroP (http://www.cbs.dtu.dk/services/ChloroP/) was used to verify the chloroplast localization (Emanuelsson et al. 1999).
Phylogenetic relationship analysis
The phylogenetic tree was formulated applying the neighbor-joining method (Saitou and Nei 1987) in MEGA version X (Kumar et al. 2018) with 1000 bootstrap replicates. The other parameters were set as default. Phylogenetic tree of OAT, P5CS, P5CR, PDH proteins was generated using sequences from different species including bacteria, archaea and plant downloaded from NCBI.
Identification of Cis-regulatory element in the putative promoter region
To reveal the correlation of transcriptional regulation amongst the genes involved in proline biosynthesis and degradation pathway from Arabidopsis and rice, the putative 5′ upstream genomic sequence (approximately 1.5 kb) from the transcription start site, were retrieved from the TAIR and TIGR databases, respectively for Arabidopsis and rice, and analyzed using PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Lescot et al. 2002).
Construction of homology-based protein modelling
Homology based modelling of the OsOAT, OsP5CS, OsP5CR and OsPDH proteins were built based on the highly similar PDB template structure using the SWISS-MODEL program (https://swissmodel.expasy.org/) (Biasini et al. 2014). All the structures including the active site residues were visualized using Chimera (Pettersen et al. 2004). The accuracy and conformation angle of the predicted protein models were evaluated by the Ramachandran plot analysis using the RAMPAGE server (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php) (Biasini et al. 2014).
Expression analysis using microarray database
The expression data of Arabidopsis and rice proline metabolizing genes were retrieved from genevestigator (https://genevestigator.com/gv/index.jsp) for different developmental stages, anatomical tissues and in response to stress conditions (Hruz, 2008). The log2-transformed normalized values were used to generate heat maps with hierarchical clustering through the MeV software package (Eisen et al. 1998).
Plant material, RNA extraction, and Reverse transcriptase PCR
Rice seeds of BRRI-53 variety were grown in the controlled environmental condition of 28 ± 2 ºC temperature and 16 h photoperiod in a growth chamber. Fifteen days’ old seedlings were then used for various abiotic stress treatment such as salt (200 mM NaCl), drought (150 mM Mannitol), oxidative (30% H2O2), cold (4 °C), heat (42 °C), and dehydration (air-dried) (Islam et al. 2015), for 16 h. The untreated seedlings were considered as a control to all these stresses. The tissues were harvested, and total plant RNA was isolated from rice shoots using TRIzol reagent (Invitrogen, USA) as per the manufacturer’s protocol.
First-strand cDNA was synthesized using Superscript III First Strand cDNA Synthesis Kit (Invitrogen, USA) according to the manufacturer’s instructions. A reaction mix (12 µl) was prepared in a PCR tube containing 10 µl of DNase treated RNA, 1 µl of DEPC-H2O and 1 µl oligo dT primer. The reaction mixture was incubated at 65 °C for 5 min and chilled down on ice immediately for 5 min. Then, a master mix of 4 µl containing 5X reaction buffer, 1 µl of Riboblock RNAse inhibitor, 1 µl of Revertaid and 2 µl of 10 mM dNTPs mix was added to the tubes. The mixture was incubated at 42 °C for 60 min. After the first strand synthesis, the reaction was terminated by heat inactivation at 70 °C for 5 min. Gene-specific primers were designed using primer blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) with customized parameters (Table S1) and eEF primer is used as an internal primer (Islam et al. 2015).
Estimation of proline content
Proline was determined as described by Bates et al. (1973) (Anna et al. 2010). Fresh leaf tissues were homogenized in 10 ml of 3% sulphosalicylic acid. Homogenate was centrifuged at 10,000 g for 15 min and subsequently, 2 ml filtrate was mixed with 2 ml of acid ninhydrin and 2 ml of glacial acetic acid. 2.5 g ninhydrin is successively added to 60 ml glacial acetic acid and 40 ml 6 M orthophosphate and kept at stirring and warm it until it dissolved. The mixture was incubated in a water bath at 100 ºC for 1 h until the colored complex is developed and the reaction was terminated by cooling in ice. Reaction mixture was vortexed with 4 ml of toluene for 15–20 s. The optical density of the layer with chromophore was read at 520 nm. The proline concentration can be determined using a standard curve and calculated on a fresh weight basis (usually expressed as microgram per gram FW or micromole per gram FW).
Results
Identification and chromosomal distribution of genes involved in the proline biosynthesis and degradation pathway
Six genes encoding thirteen proteins were involved in proline metabolism in Arabidopsis (Table 1). Among them, only AtP5CS1 had four alternate splice variants. Transcripts of AtP5CS2, AtP5CR, AtProDH1, AtProDH2 had two alternate splice variants each. On the contrary, five genes encoded six proline metabolizing proteins in rice, as one of the members, i.e., OsP5CS1 had two splice forms (Table 2). The proline metabolizing genes were distributed randomly on different chromosomes of Arabidopsis and rice (Fig. S1).
Table 1.
List of genes involved in the biosynthesis and degradation of proline in Arabidopsis thaliana L. along with their chromosomal locations, alternative spliced forms, CDS and polypeptide length, localization and co-expressed genes
| Gene | Chr no | Locus | CDS coordinate (5′to 3′) | CDS (bp) | Amino acids | Mass (kDa) | pI | Location | Co-expressed genes |
|---|---|---|---|---|---|---|---|---|---|
| AtOAT | 5 | AT5G46180.1 | 18,718,475–18,721,375 | 1428 | 475 | 52.18 | 7.15 | Chl1,2, Mt1,2 | AAE16 (At3g23790), enoyl CoA hydratase2 (At1g76150), acyl-CoA oxidase2 (At5g65110), glycoprotein (At1g04970), PPDK (At4g15530), zinc finger (At4g31240), aspartate aminotransferase 3(At5g11520) |
| AtP5CS1 | 2 | AT2G39800.1 | 16,598,164–16,603,319 | 2154 | 717 | 77.70 | 5.89 | ER1, Chl1, Nu1, Cy2, Plas1, Mt2 | AT3G55610.1, BRADI2G23507, BRADI5G26390, GLYMA01G24530, GLYMA02G41850, GLYMA03G12240, GLYMA07G16510, GLYMA14G07120, GLYMA14G13346, GLYMA18G40770, P5CS, POPTR_0008S06060G, POPTR_0010S20590G |
| 2 | AT2G39800.2 | 16,598,167–16,603,059 | 1845 | 614 | 66.76 | 5.60 | Chl1, Plas1, Nu1, Vac1, Cy2 | ||
| 2 | AT2G39800.3 | 16,598,072–16,603,061 | 2145 | 714 | 77.44 | 5.85 | Chl1, ER1, Nu1, Cy2, Mt2 | ||
| 2 | AT2G39800.4 | 16,598,072–16,603,061 | 2154 | 717 | 77.70 | 5.89 | ER1, Chl1, Nu1, Cy2, Plas1, Mt2 | ||
| AtP5CS2 | 3 | AT3G55610.1 | 20,624,020–20,629,093 | 2181 | 726 | 78.87 | 6.80 | ER1, Chl1, Nu1, Plas1, Mt2, Cy2 | AT2G39800.1, AT2G39800.4, BRADI2G23507, BRADI5G26390, GSD1, GLYMA01G24530, GLYMA02G41850, GLYMA03G12240, GLYMA07G16510, GLYMA14G07120, GLYMA14G13346, GLYMA18G40770, P5CS, PHYPADRAFT_117839, POPTR_0008S06060G |
| 3 | AT3G55610.2 | 20,624,970–20,629,093 | 1869 | 622 | 67.69 | 7.43 | ER1, Chl1, Nu1, Plas1, Mt2, Cy2 | ||
| AtP5CR | 5 | AT5G14800.1 | 4,785,960–4,787,839 | 831 | 276 | 28.62 | 7.81 | Cy2, Chl1, Mt1, Pero1 | MPPN (At3g16310), kinase (At1g19600), L7Ae/L30e/S12e/Gadd45 (At1g15930), Ribosomal S3 (At5g35530), RPS10B (At5g41520), L14p/L23e (At1g04480), fibrillarin 1 (At5g52470), drought-induced 21 (At4g15910), RRN3 (At2g34750), TIF3E1(At3g57290), Nug2(At1g52980) |
| 5 | AT5G14800.2 | 4,785,962–4,787,839 | 672 | 223 | 23.24 | 5.33 | Cy2, Pero1, Nu1, Chl1, Mt1, Vac1 | ||
| AtPDH1 | 3 | AT3G30775.1 | 12,448,636–12,451,248 | 1500t / 2086e,n | 499 | 54.96 | 6.87 | Mt2, Chl1, Cy1, Plas1 | myo-inositol oxygenase 2 (At2g19800), GDPD2 (At5g41080), seedimbibition 2(At3g57520), glutamate dehydrogenase2 (At5g07440), TMAC2(At3g02140), RUP1(At5g52250), F-box family protein (At5g27920), PYR1-like 6 (At2g40330), Zinc finger (At5g60710) |
| 3 | AT3G30775.2 | 12,448,636–12,451,556 | 2174 | 499 | 54.96 | 6.87 | Mt2, Chl1, Cy1, Plas1 | ||
| AtPDH2 | 5 | AT5G38710.1 | 15,501,126–15,504,269 | 1431 | 476 | 53.07 | 7.56 | Mt2, Chl1 | 2OG(At5g05600), RELA/SPOT homolog 2 (At3g14050), peroxisomal adenine nucleotide carrier 2 (At5g27520), xylogalacturonan deficient 1 (At5g33290), F-box family protein (At2g27310), STP13 (At5g26340), alpha/beta-Hydrolases (At2g39420), kinase (At1g74360), Thioredoxin superfamily protein (At1g28480) |
| 5 | AT5G38710.2 | 15,501,184–15,503,744 | 1605 | 466 | 51.78e | 6.89 | Mt2, Chl1 |
Chl Chloroplast; Mt Mitochondria; ER Endoplasmic reticulum; Nu Nucleus; Cy cytosol; Plas plasma membrane; Vac vacular; Pero peroxisome
Databases: 1WoLF pSORT; 2CELLO; tTAIR; eEnsemble Plants; nNCBI
Table 2.
List of genes involved in the biosynthesis and degradation of proline in rice (Oryza sativa L.) along with their chromosomal locations, alternative spliced forms, CDS and polypeptide length, localization and co-expressed genes
| Gene | Chr. No | Locus | CDS coordinate 5′to 3′ | CDS (bp) | Amino acids | Mass (kDa) | pI | Location | Co-expressed genes |
|---|---|---|---|---|---|---|---|---|---|
| OsOAT | 3 | LOC_Os03g44150.1 | 24,818,067—24,810,051 | 1422 | 473 | 51.44 | 7.54 | Chl1, Mt2, ER1 | AT5G46180, Bradi1g13830, GRMZM2G08082, GSVIVG00021525001, LOC_Os03g44150, POPTR_0011s02390, Sb01g013600 |
| OsP5CS1 | 5 |
LOC_Os05g38150.1 LOC_Os05g38150.2 |
22,381,039—22,374,029 | 2151 | 716 | 77.74 | 6.37 | Chl1, ER1, Nu1, Cy2, Mt2, Plas1 | AT2G39800, AT3G55610, Bradi2g54920, GRMZM2G028535, GRMZM2G375504, GSVIVG00016467001, LOC_Os01g62900, LOC_Os05g38150, POPTR_0008s06060, POPTR_0010s20590, Sb03g039820, Sb09g022290 |
| OsP5CS2 | 1 | LOC_Os01g62900.1 | 36,430,978—36,440,843 | 2208 | 735 | 79.50 | 6.10 | Chl1, Nu1, Cy2, Mt2, Vac1 | AT2G39800, AT3G55610, Bradi2g54920, GRMZM2G028535, GRMZM2G375504, GSVIVG00016467001, LOC_Os01g62900, LOC_Os05g38150, POPTR_0008s06060, POPTR_0010s20590, Sb03g039820, Sb09g022290 |
| OsP5CR | 1 | LOC_Os01g71990.1 | 41,721,574—41,718,016 | 855 | 284 | 29.67 | 6.92 | Chl2, Vac1, Cy1, Mt1 | AT5G14800, Bradi2g60730, GRMZM2G068665, GSVIVG00033588001, LOC_Os01g71990, POPTR_0006s04100, Sb03g045690 |
| OsPDH | 10 | LOC_Os10g40360.1 | 21,617,952—21,620,589 | 1473 | 490 | 51.63 | 6.49 | Chl1,2, Mt1,2 | AT3G30775, AT5G38710, Bradi3g32880, GRMZM2G053720, GSVIVG00036483001, LOC_Os10g40360, POPTR_0004s10610, POPTR_0017s14250, Sb01g029660 |
Chl Chloroplast; Mt Mitochondria; ER Endoplasmic reticulum; Nu Nucleus; Cy cytosol; Plas plasma membrane; Vac vacular; Pero peroxisome
Detailed information of each gene such as locus number, CDS and protein length, chromosomal coordinates, molecular mass (kDa), isoelectric point (pI) along with the subcellular localization and co-expressed genes were analyzed (Tables 1 and 2). The length of coding DNA sequence (CDS) of the members in Arabidopsis varied from 672 bp (AtP5CR) to 2180 bp (AtP5CS1), while that in rice varied from 855 bp (OsP5CR) to 2208 bp (OsP5CS2). The length of the candidate proteins ranged from 223aa (AtP5CR) to 726aa (AtP5CS2) in Arabidopsis and 284aa (OsP5CR) to 735aa (OsP5CS2) in rice. The molecular weight also varied between 23.24 kDa (AtP5R) to 78.87 kDa (AtP5CS2) in Arabidopsis and 29.67 kDa (OsP5CR) to 79.51 kDa (OsP5CS2) in rice. The predicted pI values were ranged from 5.89 (AtP5SC1) to 7.81 (AtP5CR) and 6.10 (OsP5CS2) to 7.54 (OsOAT) in Arabidopsis and rice, respectively. Most of the Arabidopsis proline metabolizing proteins were mainly predicted to be localized in chloroplast and mitochondria. Some of the proteins were predicted in endoplasmic reticulum (AtP5CS1, AtP5CS2), plasma membrane (AtP5CS1, AtP5CS2), cytosol (AtP5CS1, AtP5CS2, AtP5CR and AtProDH1) and nucleus (AtP5CS1, AtP5CS2 and AtP5CR) (Table 1). Similarly, localization of rice proline metabolism proteins was mainly predicted in mitochondria and chloroplast (Table 2). Apart from that, some of the proteins were predicted to localize in the cytosol (OsP5CS1, OsP5CS2 and OsP5CR), endoplasmic reticulum (OsOAT, OsP5CS1), nucleus (OsP5CS1, OsP5CS2) and plasma membrane (OsP5CS1). Co-expression network analysis (Tables 1 and 2) indicated that various stress-responsive proteins were co-expressed along with the proline metabolizing proteins such as protein kinase family, thioredoxin superfamily, F-box family protein and various other stress-responsive proteins.
Gene and protein structures of proline metabolizing members
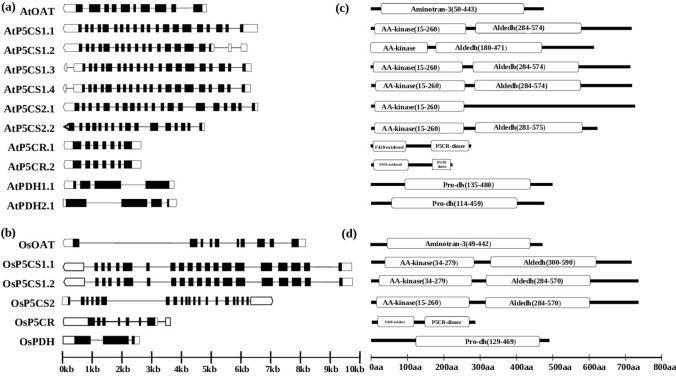
Detailed analysis of the exon–intron structure of proline metabolizing genes along with their splice variants in Arabidopsis (Fig. 2a) and rice (Fig. 2b) showed a high degree of variation among themselves. The number of introns varied from 2 to 20 in the open reading frames (ORFs) of different genes (Fig. 2a and b). Three introns were found in AtPDH, while OsPDH contained two introns in their respective ORF. The highest numbers of 20 introns were found in the AtP5CS1.3 and AtP5CS1.4 transcripts, followed by OsP5CS1 with 19 introns. An interesting pattern of intron distribution was observed among the putative paralogous members. Most of the paralogs showed the presence of the same number of introns with few exceptions. Longer introns are selectively advantageous that could counterbalance the mutational bias and improve the recombination frequency.
Fig. 2.
Gene and protein structures of proline metabolizing members. Gene structure of the proline metabolizing transcripts from a Arabidopsis; b rice including the alternative spliced forms were drawn. All the exons are shown in filled black boxes and the introns are indicated by black lines. The 5’-UTR regions are shown using empty boxes and the 3’-UTR regions are shown in empty arrows which also indicate the direction of the gene. Left to right direction of the transcript indicates “ + ” strand, while the right to left one indicates “-” strand, relative to the annotation of the genome sequence. The size of the introns, exons, and UTRs could be estimated from the scale at the bottom. Domain architecture of all the proline biosynthesis and catabolic proteins from Arabidopsis (c); rice (d) were analyzed using Pfam (http://pfam.xfam.org/). All the domains were represented by boxes. The position of the domain(s) is indicated by the amino acid number inside the box. The length of full proteins is indicated by exact amino acid numbers and relative position of the domains could be interpreted by the scale given below
Domain architecture analysis showed that AtP5CS1, AtP5CS2 and AtP5CR have two functional domains (Fig. 2c). Similarly, OsP5CS1, OsP5CS2 and OsP5CR proteins contain two functional domains, while the rest have only a single domain (Fig. 2d). OsOAT and AtOAT contain aminotransferase class-III (aminotran_3) domain (PF00202) which includes acetylornithine aminotransferase (EC: 2.6.1.11), that catalyzes the transfer of an amino group from acetylornithine to alpha-ketoglutarate, yielding N-acetyl-glutamic-5-semialdehyde and glutamic acid. OsP5CS1, OsP5CS2, AtP5CS1, AtP5CS2 have two functional domains i.e., amino acid kinase family (PF00696) and aldehyde dehydrogenase family (PF00171). Furthermore, OsP5CR and AtP5CR protein contain the dimerization domain, P5CR_dimer along with another domain called F420_oxidored. Analysis of conserved motifs of proline metabolizing proteins identified ten distinct conserved motifs (Table S2 and S3).
All the candidate proteins such as OAT, P5CS, P5CR, PDH from Arabidopsis and rice were aligned along with member from other organisms to have an evolutionary linkage and descended from a common ancestor (Fig. S2, S3, S4, S5). The proteins from different plant species were clustered together and belonged to the same class in the phylogenetic study of OAT (Fig. S6), P5CS (Fig. S7), P5CR (Fig. S8) and PDH (Fig. S9). Existence of each class of this gene family indicated that OAT, P5CS, P5CR and PDH proteins were diversified after the split of monocot and dicot.
Homology based 3D structure modelling of proline metabolizing proteins
To know the arrangement of active site residues and the overall 3D- coordination, a homology-based modelling of proline metabolizing proteins was built based on the closely related template structure of acetylornithine aminotransferase (PDB ID: 5VIU), Medicago truncatula (delta)1-Pyrroline-5-Carboxylate Reductase (MtP5CR) (PDB ID: 5BSE), human Pyrroline-5-carboxylate synthetase (PDB ID: 2H5G) and Proline utilization A (PDB ID: 5KF6) for OsOAT, OsP5CS, OsP5CR and OsPDH, respectively. OsOAT and OsP5CR proteins are homodimer and homo-decamer enzyme, respectively, while the other enzymes exist as a monomer in the solution. OsOAT monomer structure consisted of sixteen α-helical loops and twenty-one β sheets (Fig. 3a) followed by OsP5CR monomer (chain B) structure consisted of sixteen α-helical loops and eight-β sheets (Fig. 3b). On the other side, OsP5CS monomer (chain B) structure consisted of nineteen α-helical loops and seventeen β- sheets (Fig. 3c), and OsPDH monomer (chain B) structure consisted of 47% α-helical loops and 14% β- sheets (Fig. 3d). The active site of OsOAT was coordinated with Tyr 84, Ser 85, Ser 111, Phe 176, Arg 179 and Glu 231 residues. OSP5CR active site was coordinated with Arg 129, Ser 154, Phe 158, Gln 208, Leu 218, His 219, Ser 220 and Glu 221 residues. OsP5CR active site was coordinated to the Gln 347, Ser 372, Val 591, Cys 602 and His 710 residues. The active site of OsPDH was coordinated with Asp 146, Arg 203, Leu 255, Tyr 274 and Tyr 283 residues.
Fig. 3.
Homology based structural modelling. Three-dimensional homology-based structure of a OsOAT; b OsP5CR; c OsP5CS; d OsPDH proteins were built using Swiss-model server based on the available closest PDB templates of 5VIU, 5BSE, 2H5G, and 5KF6; respectively. All the α-helices were marked with purple colour, while β-sheets were marked with orange. The active sites residues were identified based on the alignment with the template structure and shown by red ball-stick model. The structure and active residues were visualized and generated using chimera program
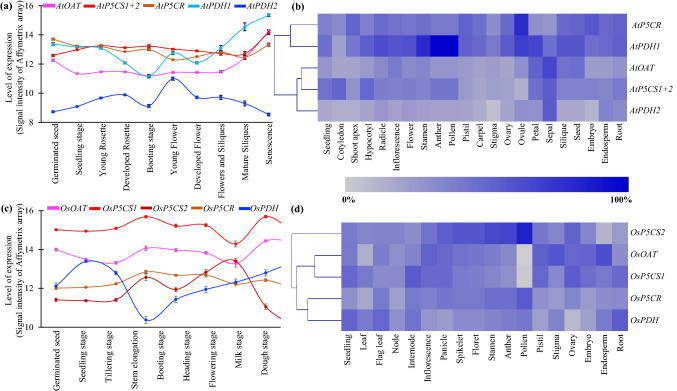
Expression profiling of Arabidopsis and rice proline metabolizing genes at different developmental stages and anatomical tissues
Expression analysis of all the proline metabolizing genes exhibited high levels of transcript abundance at all the developmental stages including germinated seed, seedling stage, Young rosette, developed rosette, booting stage, young flower, developed flower, flowers and siliques, mature siliques and senescence (Fig. 4a); and anatomical tissues including seedling, cotyledon, shoot apex, hypocotyl, radicle, inflorescence, flower, stamen, anther, pollen, pistil, carpel, stigma, ovary, ovule, petal, sepal, silique, seed, embryo, endosperm, root etc. (Fig. 4b). Arabidopsis proline metabolizing transcripts showed low to medium level of expression in all the development stages (Fig. 4a). AtPDH2 showed negligible expression in all the developmental stages. Interestingly, AtPDH1 and AtPDH2 showed an opposite pattern of expression after the followed development stage. Similarly, AtPDH2 maintained low level of expression in all the analyzed tissues except for sepal only (Fig. 4b). However, AtPDH1 showed a high level of expression in the reproductive tissues- stamen, anther and pollen.
Fig. 4.
Expression profiling of proline metabolizing genes in different developmental stages and tissues. Expression of proline metabolizing genes were analyzed with hierarchical clustering in developmental stages (a and c) and various anatomical tissues (b and d) from Arabidopsis (a and b) and rice (c and d). The normalized expression value was retrieved from genevestigator (https://genevestigator.com/) and used to generate heatmaps with hierarchical clustering using MeV software package. The colour intensity of the scale below the heat map indicates the intensity of the expression
Similarly, the expression of rice proline metabolizing genes was examined at different developmental stages including germinated seed, seedling stage, tillering stage, stem elongation, booting stage, heading stage, flowering stage, milk stage and dough stage (Fig. 4c); and anatomical tissues including seedling, leaf, flag leaf, node, internode, inflorescence, panicle, spikelet, floret, stamen, anther, pollen, pistil, stigma, ovary, embryo, endosperm, root etc. (Fig. 4d). Here, OsP5CS1 was highly expressed and OsPDH showed low level of expression in all the development stages (Fig. 4c). This indicated the imperative role proline synthesizing and degradation enzymes in the plant growth and development. Likewise, OsP5CS1 showed the medium to high level of transcript abundance in all tissues except pollen (Fig. 4d). Transcript of OsOAT also expressed significantly in most of the analyzed tissues except pollen. A high transcript abundance of OsP5CS2 was observed in pollen (Fig. 4d), that indicates the tissue specific functional distribution of alternative spliced forms. The fluctuation of proline metabolizing genes in various developmental stages under normal conditions leads to investigate their expression patterns during stress.
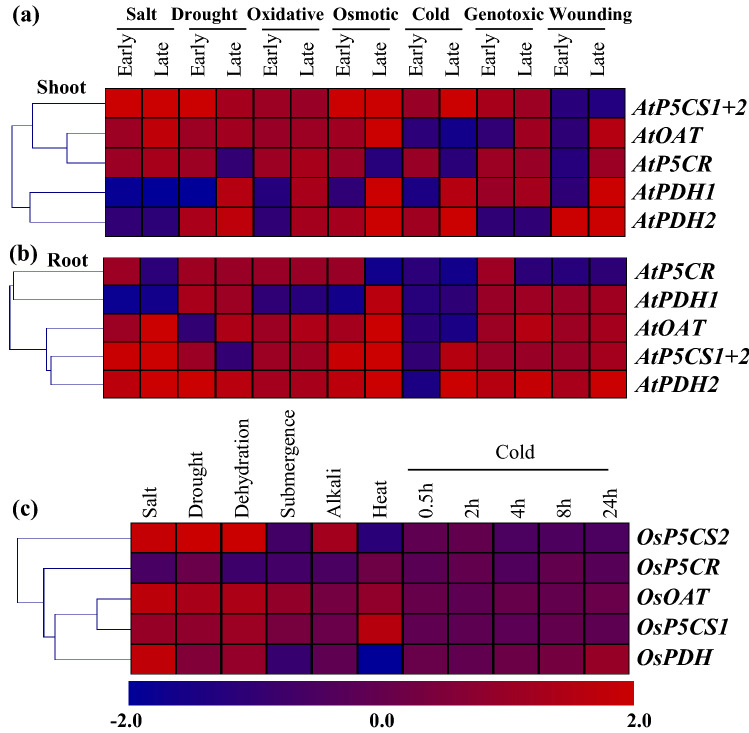
Expression analysis of Arabidopsis and rice proline metabolizing genes at different abiotic stress conditions
Expression of proline biosynthesis and degradation pathway genes were analyzed in both roots and shoot tissues under different stress conditions. A divergent pattern of expression was observed depending on the type and period of abiotic stress exposure in Arabidopsis and rice (Fig. 5). Most of the Arabidopsis genes showed medium to high level of upregulation in response to salt, drought, oxidative, osmotic, cold, genotoxic, and wounding at both shoot and root tissues (Fig. 5a and b). Transcripts for AtP5CS1 and AtP5CS2 showed upregulation in response to all these stresses except wounding (Fig. 5a). Similar level of upregulation was observed for OsP5CS1 and OsP5CS2 transcripts in response to salt, drought, and dehydration stresses, but mainly downregulated against cold stress (Fig. 5c). Interestingly, AtOAT transcript showed upregulation in response to all abiotic stresses in both shoot and root tissues, except cold. However, transcripts of AtP5CR, AtPDH1 and AtPDH2 showed a mixed pattern of transcriptional alteration.
Fig. 5.
Expression profiling of proline metabolizing genes in response to various abiotic stresses. Expression profiling of proline biosynthesis and degrading pathway genes with hierarchical clustering were performed in response to different abiotic stresses (indicated at the top of each lane) in Arabidopsis (a and b) and rice (b). The normalized fold change in expression value was downloaded from genevestigator (https://genevestigator.com/) and used to generate heatmaps with hierarchical clustering using MeV software package. The colour scale below the heat map indicates expression values; blue indicates downregulation while red indicates upregulation of individual transcript
Likewise, the expression of OsOAT was mainly upregulated in response to different abiotic stresses including salt, drought, dehydration, and submergence. It is noted that all the proline metabolizing genes are mainly downregulated in response to 0.5 h to 24 h of cold exposure (Fig. 5c). However, expression of OsPDH transcript was also upregulated in response to salt and cold stress and downregulated in heat, and submergence stress.
In silico analysis of the putative promoter regions
To reveal the correlation of transcriptional regulation amongst the proline biosynthesis and degradation pathway genes, the ~ 1.5 kb upstream putative promoter sequences of each gene were analyzed. Several important cis-regulatory elements and stress-responsive motifs were found to be present in the promoter region (Fig. 6). Several well-known stress-related cis-regulatory elements such as MYB-binding site (MBS), ABA-response element (ABRE), anoxia-response element (ARE), heat shock element (HSE), fungal elicitor responsive element (Box-W1), defence and stress-responsive elements (TC-rich repeats) etc. which were present in the promoters of the genes involved in proline metabolism. Promoters of OsP5CR and OsPDH genes showed the presence of W1-Box elements (Fig. 6b). MYB regulatory cis-elements (TAACTG) were present in AtP5CS1, AtP5CS2, AtP5CR and OsOAT, OsP5CS1, OsP5CS2 and OsPDH (Fig. 6a and b). Presence of these stress-related motifs in the promoters of these genes could be directly correlated to the altered gene expression under various abiotic stresses (Fig. 5).
Fig. 6.
Identification of putative cis-regulatory elements. Putative cis-regulatory elements were identified in the putative promoter region of all proline metabolizing genes from a Arabidopsis; b rice. The approximate positions of putative cis-regulatory elements were shown in the diagram, present in the ~ 1.5 kb upstream region of various genes predicted by the PlantCARE database. Various elements such as MYB, ABRE, ARE, HSE. LTR, GARE motifs etc. are represented
Validation of stress-specific transcript alteration of proline metabolizing genes
Expression profiles of all proline biosynthesis and degradation genes were further validated, in response to salt, drought, cold, heat, dehydration and oxidative stresses (Fig. 7) using qRT-PCR analysis. Expression profiles of proline metabolizing genes showed an almost similar pattern in real-time PCR as observed in the microarray data. Expression of all four proline synthesizing genes OsOAT, OsP5CS1, OsP5CS2 and OsP5CR showed consistent upregulation in response to salt, drought, and oxidative stresses; while the proline degrading, OsPDH showed downregulation in these conditions (Fig. 7). This indicates the putative mechanism behind the maintenance of proline homeostasis in these stress conditions. Interestingly, heat treatment induces the upregulation, while cold and dehydration cause most downregulation of all the analyzed transcripts. This suggested that proline metabolizing genes might be regulated differently during stresses.
Fig. 7.
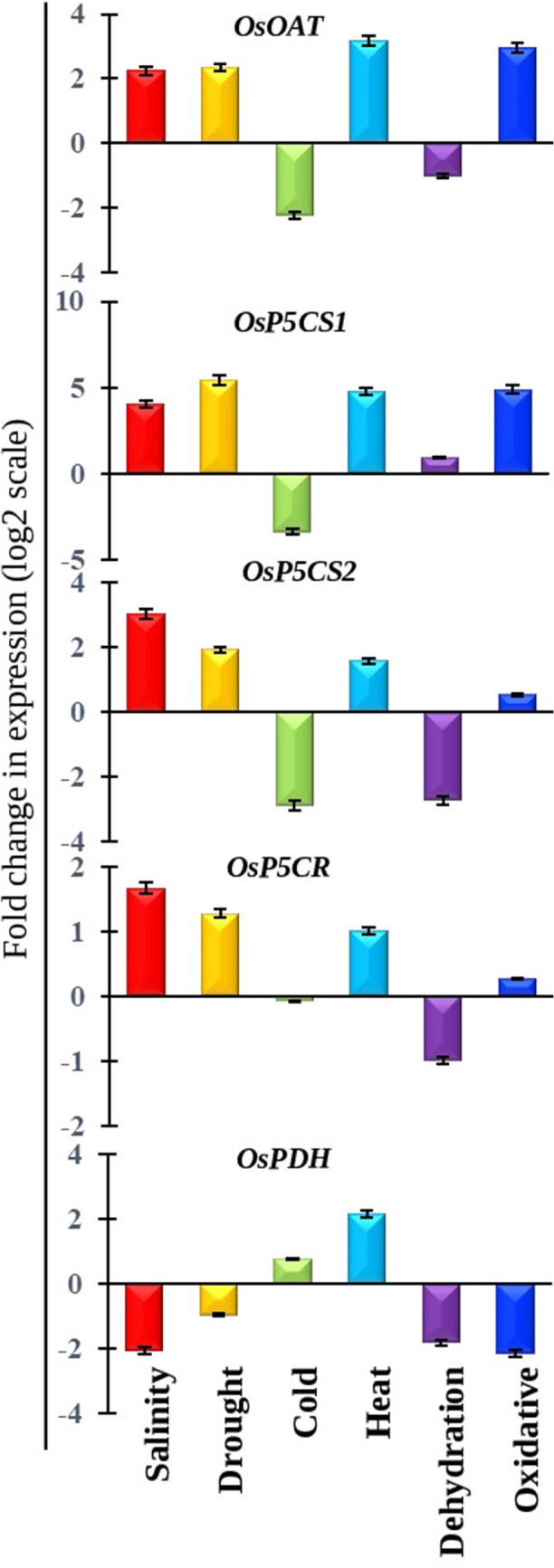
Functional validation of proline metabolizing gene’s expression in response to various abiotic stresses. Expression profiles of proline metabolizing genes were performed in response to salt, drought, cold, heat, dehydration, and oxidative stresses at a Bangladeshi rice variety. Quantitative RT-PCR was used to investigate the expression levels of each gene. The log2 scale of fold change in each gene under each condition was presented. The results were represented by the mean value of fold change ± standard deviation using eEF gene as a reference gene
Functional correlation of the expression data with the metabolic outcome
The changes in the transcript abundance of different proline metabolizing genes in response to different abiotic stresses will directly influence the cellular proline content. The proline content was measured in response to salt, drought, cold, heat, dehydration, and oxidative stresses from the same 15 days old rice seedlings and compared with the corresponding untreated control samples. The accumulation of proline was found to be remarkably induced by 1.2 to 1.5 -fold higher than control in response to all the stress conditions (Fig. 8). Thus, the upregulation of proline biosynthesis genes such as OsOAT, OsP5CS1, OsP5CS2 and the downregulation of proline depredating genes such as OsPDH in response to salt, drought, and oxidative stresses, could be directly correlated with the enhanced proline content. The transcript upregulation of OsPDH in response to heat stress (Fig. 7) could be corelated with the minimal enhancement of proline content as compared with control (Fig. 8).
Fig. 8.

Measurement of proline contents in rice in response to abiotic stress. Proline content in rice seedlings under various stress conditions e.g., Salt (200 mM NaCl), Drought (150 mM Mannitol), Heat (42 °C), Cold (4 °C), Dehydration (without water), and oxidative stress (30% H2O2) were measured along with respective control samples. The results were represented by the mean value of fold change ± standard deviation (n = 3). *, *** represents the significance level of paired student's two-tailed t-test with a p value less than 0.05 and 0.001; respectively
Discussion
The present study focused on the exploration of the complete genome for the presence of proline biosynthesis and degradation genes in two model plants- Arabidopsis, and rice. Dramatic accumulation of proline is a common physiological response in plants exposed to various abiotic stresses (Kaur et al. 2015). Proline has chemical properties, including high solubility and zwitterionic structure, common to protective compatible solutes (Bhaskara et al. 2015). A total of six proline metabolizing genes were identified in Arabidopsis (Table 1) and five in rice (Table 2). Two isozymes of P5CS genes are present in Arabidopsis and rice as consistent with the previous report in Medicago truncatula, Medicago sativa and Lycopersicon esculentum (Armengaud et al. 2004). When plant cells are exposed to different abiotic stresses like salt or osmotic, proline biosynthesis is regulated by the P5CS1 enzyme and its transcript is induced by these stresses in the chloroplasts (Székely et al. 2008). Current data suggest that proline biosynthesis and degradation pathway genes play an important role in various signal transduction and abiotic stress response pathways. There are many reports about proline accumulation in peanut, sunflower, pepper, wheat, and potato plants under drought conditions (Fiasconaro et al. 2019; Furlan et al. 2020; Liu et al. 2019; Shehzad et al. 2020; Wang et al. 2019). Accumulation of proline under salinity stress has been widely reported in many plant species including maize (Molazem and Bashirzadeh 2015) and soybean (Çelik et al. 2011). There was no uniform gene expression pattern for plant proline biosynthesis and degradation pathway genes. According to the microarray data AtOAT, AtP5CS1, AtP5CS2, AtPDH1, AtPDH2 and AtP5CR as well as OsOAT, OsP5CS1, OsP5CS2, OsP5CR and OsPDH genes, exhibited incongruous expression patterns in various anatomical tissues. Expression levels of AtPDH2 are generally low but increased in senescent leaves and floral organs (Funck et al. 2010). Expression of AtPDH1 and AtP5CDH is repressed by osmotic stress and upregulated by proline, with the changes being more pronounced for PDH1 (Verbruggen et al. 2008). A strong expression of PDH1 transcript was observed in the stigma and pollen, while P5CDH is only upregulated in pollen (Deuschle et al. 2004; Nakashima et al 1998). Four genes, AtP5CS1, AtP5CS2, AtP5CR and AtPDH1 were highly and indiscriminately expressed in all the investigated tissues under normal conditions (Fig. 4b). Similarly, OsOAT and OsP5CS1 were highly expressed in all the anatomical tissues and developmental stages (Fig. 4). Various cis-regulatory elements (CREs) play crucial roles in controlling the gene expressions in specific cell types, conditions, and developmental stages (Yifeng et al. 2015). Analysis of the putative promoter region of proline metabolizing genes from Arabidopsis and rice reveals the presence of different stress-inducible elements (Fig. 6). Their presence could be directly correlated with the transcription alteration of proline metabolism genes in adverse stress conditions.
Expression of the proline biosynthesis and degradation genes were found to be closely associated with salinity, drought, dehydration, submergence, heat, and cold stresses. The key gene for proline biosynthesis, OsP5CS1 and OsP5CS2 transcripts were found to be upregulated (Fig. 7) which added the importance for proline accumulation under salinity, drought, heat, and oxidative stresses, similar with their microarray profile (Fig. 5c). In response to freezing stress, P5CS showed enhanced transcription as compared to non-stressed Fritillaria imperialis plants (Hajihashemi et al. 2020). Noted that, both the transcripts (OsP5CS1and OsP5CS2) showed downregulation in cold stress. Besides, microarray data suggested that the increasing duration of cold treatment doesn’t show any remarkable change in the expression of OsP5CS1 and OsP5SC2 transcripts as compared with control sample (Fig. 5c). The increase in proline content directly accompanied by a significant increase in the expression of P5CS gene. Upregulation of proline biosynthetic genes like P5CS after cold stress has been reported previously (Hur et al. 2004). Over expression of P5CS gene in petunia plant enhanced tolerance against cold stress (Jamshidnia et al. 2018). As OAT is the key enzyme in ornithine pathway, enhance the expression of OAT leads to accumulation of proline in stressed condition (Anwar et al. 2020). Microarray data showed upregulation of OsOAT and AtOAT transcripts in stressed condition. Transcript of AtOAT is found to be downregulated in cold stress (Fig. 5a and b), while OsOAT showed no significant change in cold stress (Fig. 5c). Moreover, overexpression of AtOAT in wheat provided tolerance against drought, salinity, and heat stress conditions (Anwar et al. 2020). It was also demonstrated that overexpression of OsOAT enhanced osmotic tolerance in transgenic rice (You et al. 2012). Another key gene in proline biosynthesis pathway, P5CR, helps plant to accumulate proline (Anwar et al. 2020). In this study both AtP5CR and OsP5CR showed upregulation under multiple abiotic stress conditions (Fig. 5). On the contrary, OsPDH, a proline degrading gene showed upregulation in 24 h of cold stress (Fig. 5c) which also validated by qRT-PCR (Fig. 6) that could be directly corelated with no significant change in the accumulation of proline content in cold stress condition (Fig. 8).
The enhanced synthesis or decreased degradation of proline are supposed to result in the accumulation under stress conditions through overexpressing P5CS gene or suppression of PDH gene expression have been widely studied in the model plant Arabidopsis thaliana, tobacco as well as many other plants (Wang et al. 2015). Besides, an earlier study suggested that exogenous H2O2 treatment can lead to a rapid accumulation of proline in maize seedlings (Yang et al. 2009). A similar level of proline accumulation was observed in various abiotic stress treatment in the present study (Fig. 8). Increase in proline synthesis through overexpression of OAT in Nicotiana plumbaginifolia supported the hypothesis of a direct role of OAT in proline accumulation (Adamipour et al. 2020). The upregulated expression of OsOAT and OsP5CS1 in response to salt, drought, and oxidative stresses (Fig. 7) could be directly correlated with the increased proline content in rice seedlings (Fig. 8). Rasel et al. 2020 reported that proline content was higher in Nonabokra and Ghunsi genotypes and lower in salt-sensitive check BINA dhan-17 and suggested that proline content could be considered as important determinants for the identification of salt-tolerant rice genotypes.
In the present study, an epigrammatic analysis of proline biosynthesis and degradation pathway genes of Arabidopsis and rice was carried out to enhance our knowledge about the regulation of the individual genes and their elementary traits in stress responses. All the proline biosynthesis and degradation pathway genes were analyzed in terms of their genomic architecture, exon–intron distribution, domain organization, phylogenetic relationship and protein structure. In addition, expression profiles of the candidate genes in different distinct developmental stages and tissues representing their imperative role through the growth and development of rice and Arabidopsis. Overall, this study provides a comprehensive transcript profiling of the plant proline metabolizing genes under various adverse conditions is imperative to understand the underlying mechanisms of their stress pensiveness and responsiveness. This study will aid further analysis of various functional aspects of OAT, P5CS, P5CR and PDH genes to validate its potential candidature for crop improvement. Proline content measurement provides insights into stress responsiveness under salinity, drought, cold, heat, dehydration, and oxidative stress. Studying the behaviour of these plants in response to several stresses can provide us valuable information for future genetic manipulation. The main goal of this study was to investigate the comprehensive analysis of proline biosynthesis and degradation pathway genes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
SA acknowledge the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh for providing NST fellowship. TI and SA acknowledge Plant Breeding and Biotechnology Laboratory, Department of Botany, University of Dhaka the logistic support and laboratory facilities. TI designed the experiment and SA retrieved the data and prepared the manuscript. TI and SA analyzed the data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adamipour N, Khosh-Khui M, Salehi H, Razi H, Karami A, Moghadam A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol Biochem. 2020;155:105–113. doi: 10.1016/j.plaphy.2020.07.028. [DOI] [PubMed] [Google Scholar]
- Anna J, Sylva Z, Miroslav K, Miroslava V, Ilja TP. Freezing tolerance and proline content of in vitro selected hydroxyproline resistant winter oilseed rape. Czech J Genet Plant Breed. 2010;46(1):35–40. doi: 10.17221/52/2009-CJGPB. [DOI] [Google Scholar]
- Anwar A, Wang K, Wang J, et al. Expression of Arabidopsis Ornithine Aminotransferase (AtOAT) encoded gene enhances multiple abiotic stress tolerances in wheat. Plant Cell Rep. 2020 doi: 10.1007/s00299-021-02699-0. [DOI] [PubMed] [Google Scholar]
- Armengaud P, Thiery L, Buhot N, March GG, Savoure A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant. 2004;120(3):442–450. doi: 10.1111/j.0031-9317.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bhaskara GB, Yang T-H, Verslues PE. Dynamic proline metabolism: importance and regulation in water limited environments. Front Plant Sci. 2015;6:484. doi: 10.3389/fpls.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo CT, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çelik Ö, Atak C. Evaluation of proline accumulation and Δ1-pyrroline-5-carboxylate Synthetase (P5CS) Gene expression during salinity stress in two soybean (Glycine max L. Merr.) varieties. Pol J Environ Stud. 2011;21(3):559–564. [Google Scholar]
- Chun SC, Paramasivan M, Chandrasekaran M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front Microbiol. 2018;9:2525. doi: 10.3389/fmicb.2018.02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle K, Funck D, Forlani G, Stransky H, Biehl A, Leister D, van der Graaff E, Kunze R, Frommera WB. The role of D1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell. 2004;16(12):3413–3425. doi: 10.1105/tpc.104.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Heijne G. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 1999;8(5):978–984. doi: 10.1110/ps.8.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiasconaro ML, Lovato M, Antolín M, Clementi L, Torres N, Gervasio S, Martín C. Role of proline accumulation on fruit quality of pepper (Capsicum annuum L.) grown with a K-rich compost under drought conditions. Sci Hortic. 2019;249:280–288. doi: 10.1016/j.scienta.2019.02.002. [DOI] [Google Scholar]
- Funck D, Stadelhofer B, Koch W. Ornithine-δ-aminotransferase is essential for Arginine Catabolism but not for Proline Biosynthesis. BMC Plant Biol. 2008;8:40. doi: 10.1186/1471-2229-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funck D, Eckard S, Müller G. Non- redundant functions of two proline dehydrogenase isoforms in Arabidopsis. BMC Plant Biol. 2010;10:70. doi: 10.1186/1471-2229-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funck D, Winter G, Baumgarten L, et al. Requirement of proline synthesis during Arabidopsis reproductive development. BMC Plant Biol. 2012;12:191. doi: 10.1186/1471-2229-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan AL, Bianucci E, Giordano W, Castro S, Becker DF. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol Biochem. 2020;151:566–578. doi: 10.1016/j.plaphy.2020.04.010. [DOI] [PubMed] [Google Scholar]
- Ginzberg I, Stein H, Kapulnik Y, Szabados L, Strizhov N, Schell J, Koncz C, Zilberstein A. Isolation and characterization of two different DNAs of delta1-pyrroline-5-carboxylate synthase in alfalfa, transcriptionally induced upon salt stress. Plant Mol Biol. 1998;38(5):755–764. doi: 10.1023/A:1006015212391. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK. Heat shock proteins in toxicology: how close and how far? Life Sci. 2010;86(11–12):377–384. doi: 10.1016/j.lfs.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Hajihashemi S, Brestic M, Landi M, et al. Resistance of Fritillaria imperialis to freezing stress through gene expression, osmotic adjustment and antioxidants. Sci Rep. 2020;10:10427. doi: 10.1038/s41598-020-63006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu F, Ledily F, Huault C, Billard JP. Contribution of ornithine aminotransferase to proline accumulation in NaCl-treated radish cotyledons. Plant Cell and Environ. 1995;18(2):205–210. doi: 10.1111/j.1365-3040.1995.tb00354.x. [DOI] [Google Scholar]
- Hirayama T, Shinozaki K. Research on plant abiotic stress responses in the post-genome era: past, present and future. The Plant J. 2010;61:1041–1052. doi: 10.1111/j.1365-313X.2010.04124.x. [DOI] [PubMed] [Google Scholar]
- Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucl Acids Res. 2007 doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A, Skalicky M, Brestic M, Maitra S, Ashraful Alam M, Syed MA, Hossain J, Sarkar S, Saha S, Bhadra P, et al. Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy. 2021;11:241. doi: 10.3390/agronomy11020241. [DOI] [Google Scholar]
- Hu CA, Delauney AJ, Verma DP. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. PNAS. 1992;89(19):9354–9358. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, et al. Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinform. 2008;2008:5. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Jung K-H, Lee C-H, An G. Stress-inducible OsP5CS2 gene is essential for salt and cold tolerance in rice. Plant Sci. 2004;167:417–426. doi: 10.1016/j.plantsci.2004.04.009. [DOI] [Google Scholar]
- Islam T, Manna M, Kaul T. Genome-wide dissection of Arabidopsis and rice for the identification and expression analysis of glutathione peroxidases reveals their stress-specific and overlapping response patterns. Plant Mol Biol Rep. 2015;33:1413–1427. doi: 10.1007/s11105-014-0846-6. [DOI] [Google Scholar]
- Islam F, Yasmeen T, Arif MS, Ali S, Ali B, Hameed S, Zhou W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. J Plant Growth Regul. 2016;80:23–36. doi: 10.1007/s10725-015-0142-y. [DOI] [Google Scholar]
- Kaur G, Asthir B. Proline: a key player in plant abiotic stress tolerance. Biol Plant. 2015;59:609–619. doi: 10.1007/s10535-015-0549-3. [DOI] [Google Scholar]
- Kavi Kishor PB, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014;37(2):300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- Khaleghi A, Naderi R, Brunetti C, Maserti BE, Salami SA, Babalar M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci Rep. 2019;9(1):19250. doi: 10.1038/s41598-019-55889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna-Chopra R, Semwal V, Lakra N, Pareek A. Plant tolerance to environmental stress. 1. UK: Taylor & Francis group; 2019. Proline – A key regulator conferring plant tolerance to salinity and drought; pp. 59–80. [Google Scholar]
- Kumar V, Shriram V, Hossain MA, Kavi Kishor PB. Engineering proline metabolism for enhanced plant salt stress tolerance. In: Wani SH, Hussain MA, editors. Managing Salt Tolerance in Plants: Molecular and Genomic Perspectives. 1. Boca Raton FL, USA: CRC Press/Taylor & Francis Group; 2015. pp. 353–372. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Xu Z, Liang J, Luo X, Zhang Y, Feng X, et al. Poly (g-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul. 2016;78:233–241. doi: 10.1007/s10725-015-0088-0. [DOI] [Google Scholar]
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucl Acids Res. 2002;30(1):325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Brandriss MC. Proline biosynthesis in Saccharomyces cerevisiae: molecular analysis of the PRO1 gene, which encodes gamma-glutamyl kinase. J Bacteriol. 1992;174:4148–4156. doi: 10.1128/jb.174.12.4148-4156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang L, Li Y, Li X, Zhang J. Proline metabolism-related gene expression in four potato genotypes in response to drought stress. Biol Plant. 2019;63:757–764. doi: 10.32615/bp.2019.153. [DOI] [Google Scholar]
- Ma Y, Dias MC, Freitas H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front Plant Sci. 2020;11:591911. doi: 10.3389/fpls.2020.591911Molazem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molazem D, Bashirzadeh A. Impact of salinity stress on proline reaction, peroxide activity and antioxidant enzymes in maize (Zea mays L.) Pol J Environ Stud. 2015;24(2):597–603. [Google Scholar]
- Mansour MMF, Ali EF. Evaluation of proline functions in saline conditions. Phytochemistry. 2017;140:52–68. doi: 10.1016/j.phytochem.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Satoh R, Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K. A gene encoding proline dehydrogenase is not only induced by proline and hypo-osmolarity, but is also developmentally regulated in the reproductive organs of Arabidopsis. Plant Physiol. 1998;118(4):1233–1241. doi: 10.1104/pp.118.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Llano Y, Rodríguez-Pupo EC, Druzhinina IS, Chenthamara K, Cai F, Gunde-Cimerman N, Zalar P, Gostinčar C, Kostanjšek R, Folch-Mallol JL, Batista-García RA, Sánchez-Carbente MDR. Stress reshapes the physiological response of halophile fungi to salinity. Cells. 2020;9(3):525. doi: 10.3390/cells9030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera-A visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Rasel M, Tahjib-Ul-Arif M, Hossain MA, et al. Screening of salt-tolerant rice landraces by seedling stage phenotyping and dissecting biochemical determinants of tolerance mechanism. J Plant Growth Regul. 2020 doi: 10.1007/s00344-020-10235-9. [DOI] [Google Scholar]
- Roosens NH, Thu TT, Iskandar HM, Jacobs M. Isolation of the ornithine-d-aminotransferase cDNA and effect of salt stress on its expression in Arabidopsis thaliana. Plant Physiol. 1998;117(1):263–271. doi: 10.1104/pp.117.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosens NH, Bitar F, Loenders K, Angenon G, Jacobs M. Overexpression of ornithine-delta-aminotransferase increases proline biosynthesis and confers osmotolerance in transgenic plants. Mol Breeding. 2002;9:73–80. doi: 10.1023/A:1026791932238. [DOI] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shamsul H, Qaiser H, Mohammed N, Arif SW, John P, Aqil A. Role of proline under changing environments. Plant Signal Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad MA, Nawaz F, Ahmad F, Ahmad N, Masood S. Protective effect of potassium and chitosan supply on growth, physiological processes and antioxidative machinery in sunflower (Helianthus annuus L.) under drought stress. Ecotoxicol Environ Saf. 2020;187:109841. doi: 10.1016/j.ecoenv.2019.109841. [DOI] [PubMed] [Google Scholar]
- Strizhov N, Abraham E, Okresz L, Blickling S, Zilberstein A, Schell J, Koncz C, Szabados L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997;12(3):557–569. doi: 10.1046/j.1365-313x.1997.00557.x. [DOI] [PubMed] [Google Scholar]
- Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Turchetto-Zolet AC, Margis-Pinheiro M, Margis R. The evolution of pyrroline-5-carboxylate synthase in plants: a key enzyme in proline synthesis. Mol Genet Genomics. 2009;281(1):87–97. doi: 10.1007/s00438-008-0396-4. [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- Verslues PE, Sharma S. Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book. 2010;8:e0140. doi: 10.1199/tab.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu JH, Zhu JK. Methods and concepts in quantifying resistance to drought, salt and freezing abiotic stresses that affect plant water status. Plant J. 2006;45(4):523–539. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Tang X, Hon W, Shao HB. Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front Plant Sci. 2015;6:792. doi: 10.3389/fpls.2015.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mao Z, Zhang J, Hemat M, Huang M, Cai J, Zhou Q, Dai T, Jiang D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post anthesis drought stress in winter wheat (Triticum aestivum L.) Environ Exp Bot. 2019;166:103804. doi: 10.1016/j.envexpbot.2019.103804. [DOI] [Google Scholar]
- Winter G, Todd CD, Trovato M, Forlani G, Funck D. Physiological implications of arginine metabolism in plants. Front Plant Sci. 2015;6:534. doi: 10.3389/fpls.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LQ, Fan ZM, Guo L, Li YQ, Zhang WJ, Qu LJ, Chen ZL. Over-expression of an Arabidopsis delta-OAT gene enhances salt and drought tolerance in transgenic rice. Chin Sci Bull. 2003;48:2594–2600. doi: 10.1360/03wc0218. [DOI] [Google Scholar]
- Yang SL, Lan SS, Gong M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol. 2009;166(15):1694–1699. doi: 10.1016/j.jplph.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Yifeng L, Chih-yu C, Alice MK, Wyeth WW. The identification of cis-regulatory elements: a review from a machine learning perspective. BioSystems. 2015;138:6–17. doi: 10.1016/j.biosystems.2015.10.002. [DOI] [PubMed] [Google Scholar]
- You J, Hu H, Xiong L. An ornithine δ-aminotransferase gene OsOAT confers drought and oxidative stress tolerance in rice. Plant Sci. 2012;197:59–69. doi: 10.1016/j.plantsci.2012.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.