Abstract
Two gene of class II photolyases, PiPhr1 (1833 bp) and PiPhr2 (1809 bp), from the Antarctic diatom Phaeodactylum tricornutum ICE-H were cloned, the recombinant proteins expressed and purified. The molecular weight of the recombinant photolyases were determined to be 68 kDa with a pI of 9.04 and 68.82 with a pI of 7.31, respectively. Activity studies showed that both the recombinant enzymes were involved in the repair DNA damaged by UV light, that is they were most likely photolyases involved in photorepair of DNA. Further confirmation of this function was demonstrated by the increased expression of PiPhr1 and PiPhr2 after exposure to UV radiation, blue light and dark conditions by qRT-PCR. In summary, PiPhr1 and PiPhr2 were up regulated by UVB irradiation and blue light at 0.5 h and 3 h. Longtime (3 h) exposure to dark also increased the expression of PiPhr1 and PiPhr2. In vitro photoreactivation assays showed that PiPhr1 and PiPhr2 could repair CPDs utilizing blue light. This is the first time CPD Class II photolyase has been reported from Antarctic diatom. These results will add to the knowledge of the diatom CPF family and assist in understanding the functional role of these genes in Antarctic diatoms.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02927-0.
Keywords: Antarctic diatom P. tricornutum ICE-H, CPD, CPD photolyase, Photoreactivation
Introduction
Solar ultraviolet (UV) radiation is the main cause of environmental damage suffered by DNA and triggers pyrimidine dimer in the double helix (Barlev and Sen 2018; Park et al. 1995). The predominant DNA damage induced by radiation is the cyclobutylpyrimidine dimer (CPD), with a second pyrimidine dimer, the pyrimidine–pyrimidone (6-4) photoproduct (6-4PP), also formed in a significant yield (Jepsen and Solov’yov 2017; Sancar 2003). If not repaired, DNA damage can cause abnormalities in gene expression and gradually organism degeneration, and finally death (Haber 2018; Jans et al. 2005; Kiselev et al. 2017). DNA photolyases can repair DNA damage by a photoinduced cyclic electron transfer reaction using the energy of blue-green light (λ = 300–500 nm) (Chinnapen and Sen 2004; Kao et al. 2005; Sancar 2003; Spampinato 2017). DNA photolyases recognize and bind to DNA lesions such as CPDs or 6-4PPs and, are usually classified as CPD photolyases or (6-4) photolyases (Brych et al. 2016; Zhang et al. 2017). Two kinds of photolyases share similar primary sequences and folding structures, but a photolyase repairs one photoproduct and cannot repair another (An et al. 2018). Five classes of photolyases have been reported: class I, II and III CPD photolyases, (6-4) photolyase and single-stranded DNA photolyase (DASH Crys) (Ozturk et al. 2007). Most photolyases are flavoproteins. Despite considerable structural deviations from the well-studied class I DNA photolyases, they share the main biological role, such as light-driven repair of the most common UV-induced DNA lesions.
Cryptochrome (Cry) is a kind of protein that is structurally homologous to DNA photolyase. Surprisingly though, Cry cannot repair UV-induced DNA lesions. The Crys and photolyases belong to cryptochrome/photolyase family (CPF), a class of flavoproteins that have flavin adenine dinucleotide (FAD) as the catalytic chromophore or a photoantenna such as 5, 10-methenyltetrahydrofolate (MTHF). CPF plays different roles in DNA repair, circadian rhythm and photoreception. The first diatom Cry (CPF1) was identified in Phaeodactylum tricornutum (Coesel et al. 2009). So far, one photolyase represents a canonical CPD class II photolyase, one CPD class II-like photolyase (Konig et al. 2017b), a plant-like Cry (CryP) (Juhas et al. 2014) and CPF1, with (6-4) photolyase activity and photoreceptor activity (Coesel et al. 2009), have been reported in P. tricornutum.
Diatoms are unicellular and phototrophic microalgae, which thrive in fresh water and marine environment. Compared to other eukaryotes, such as land plants or green algae, the metabolic pathways of diatoms are more complicated, with different compartmentalization sometimes observed (Konig et al. 2017a). Diatoms present unique genomic features, with genes derived from bacterial, plant and animal lineages (Bowler et al. 2008). Four diatoms, the pennate diatoms P. tricornutum (Bowler et al. 2008), Pseudonitzschia multiseries, and Fragilariopsis cylindrus, and the centric diatom Thalassiosira pseudonana (Armbrust et al. 2004) have been studied. These diatoms, spread from coastal to pelagic zones, adopt different survival strategies. The Antarctic environment is an important source of genetic material for identifying novel genes with potential biotechnological applications. Here, we present an Antarctic sea ice diatom, Phaeodactylum tricornutum ICE-H, which has been rarely studied (Li et al., 2020). In this study, we isolated two class II DNA photolyases, PiPhr1 and PiPhr2, from P. tricornutum ICE-H and performed protein activity assays in vitro. The PiPhr1and PiPhr2 displayed photorepair activity.
Materials and methods
Algal culture and growth conditions
The Antarctic ice algae, P. tricornutum ICE-H, were isolated from the floating ice near the Zhongshan Research Station of Antarctica (S 69°48′, E 77°48′) (Li et al. 2020). Cultivation was performed in 1000 mL closing membrane Erlenmeyer flasks containing 600–800 mL f/2 seawater medium at 5 ± 1 °C. Cells were routinely grown under a light density of 40 μmol photons m−2 s−1 with a daily cycle of 12 h light with 12 h dark (Qu et al. 2017).
Cloning of DNA photolyase gene and bioinformatics analysis
Total RNA of P. tricornutum ICE-H was extracted from liquid nitrogen ground algae powder using Trizol reagent (TransGen, China) as described in the instruction (An et al. 2018). The RNA was observed by agarose gel. The first strand of the complementary DNA (cDNA) for general PCR was synthesized from the RNA by the PrimeScript 1st strand cDNA Synthesis Kit (Takara, Dalian). The cDNAs were stored at − 20 °C. The full-length cDNA was PCR-amplified with PrimeSTAR Max DNA (Takara, Dalian) as follows (primers are in Table 1): 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min 30 s; and 10 min at 72 °C. The PCR fragments were cloned into PMD-18 T vector, transformed into Trans1-T1 Phage Resistant Chemically Competent Cells (TransGen Biotech) and then sequenced (Sangon).
Table 1.
Primers used in experiment
| Primers | Sequence (5′–3′) | Size of the PCR product |
|---|---|---|
| Primers for full length and expression | ||
| PiPhr1-F | ATGGCTCTACCGGAAGGC | 1830 bp |
| PiPh1r-R | CGTCTCGTGTTTGTGTGACTC | |
| PiPhr2-F | ATGCTCTTGTCCTCGCTGC | 1806 bp |
| PiPhr2-R | CTTTCTGGCAGTCTTTTGTT | |
| Primers for qRT-PCR | ||
| Rbcl-F | TCCTGGCGAAATACCCTGAC | 174 bp |
| Rbcl-R | CCTGGGAGAATGGACTGTGA | |
| PiPhr1-qF | AAGTGATGGGTGTTGTCAAGG | 187 bp |
| PiPhr1-qR | GCAATCCGCAATAGATGTAAGA | |
| PiPhr2-qF | CACAGCCTGGAACAGAAGC | 146 bp |
| PiPhr2-qR | CGAAATATGGCCGTGATTG | |
The sequence homology was detected by BLASX and UniProt. The nucleotide sequence of the photolyase was translated to protein sequence, which was matched with NCBI protein database. Multiple sequences were aligned by Clustal X program and analyzed by DNAMAN. The theoretical isoelectric point (pI) and molecular weight (Mw) were calculated by Expasy Compute pI/Mw tool. Based on the analysis of the photolyase protein sequences downloaded from GenBank, a phylogenetic tree was constructed with MEGA 6 by way of a neighbor-joining method.
Light treatment and quantitative real-time PCR
Through the daily cycle, P. tricornutum ICE-H was kept under 40 μmol photons m−2 s−1 light density. Two UVB lamps (Beijing Normal University, Beijing, wavelength 290–320 nm, 8 W) were used as the source of UVB irradiation at a rate of 100 μw cm−2. A suspension of algal cells was subjected to irradiance for 0.5 and 3 h to examine the mRNA expression of DNA photolyase gene from P. tricornutum ICE-H. Cells (50 mL) were harvested by centrifugation (4 °C, 5 min, 5000 g) at a certain time and RNA was extracted. At least 3 biological duplicates were performed.
Real-time PCR was performed on ABI StepOne Plus Real-Time PCR System (Applied Biosysytems, USA) with SYBR Premix Ex TaqTMII (TaKaRa Biotech Co., Dalian, China) for 40 cycles (95 °C for 5 s; 58 °C for 10 s; 72 °C for 40 s). Specific primers (Table 1) were designed with Primer Premier 5.0. A housekeeping gene, Rbcl (ribulose-1,5-bisphosphate carboxylase), was used as an internal control. The data were further analyzed using the comparative Ct (2−∆∆CT) method. To maintain consistency, the baseline was set automatically by the software.
Recombinant protein expression and purification
To clone a recombinant expression vector, the full-length photolyase gene was inserted into pEASY-Blunt E1 (Amp+) (TransGen Biotech). After being transformed into Transetta (DE3) Chemically Competent Cell (TransGen Biotech) and sequenced (Sangon), correct clones were stored with glycerol at − 80 °C (Li et al. 2015). The expression vector encodes for a protein with a C-terminal His-tag.
For protein expression, recombinant bacteria were grown in 300 mL LB (Amp+) with 1-L Erlenmeyer flask at 37 °C to an OD600 of 0.6. Expression was induced by adding IPTG to a final concentration of 0.5 mM and cells without IPTG were set as negative control. Then, the cells were shaken at 20 °C for 4 h. Cells were collected by centrifugation and washed with PBS buffer (50 mM Tris/HCl, 5 mM EDTA, 300 mM NaCl, 10% w/v glycerol, pH 7.4), centrifuged again and resuspended in the same buffer. Cells were, respectively, broken up with ultrasonic cell disruptor JY88-IIN (Ningbo scientz biotechnology, China) and then centrifuged at 4 °C and 15,000 g for 15 min. The supernatant and precipitate were stored at − 80 °C before SDS-PAGE and purification. The recombinant photolyase was purified with 5 mL Ni–NTA (Sangon). The binding buffer (25 mM Tris, 150 mM NaCl, pH 8.0) was used to clean the balance column at the flow rate of 5 mL/min. The supernatant sample was added to the column (the flow rate: 2 mL/min), and the column was washed with the binding buffer. Then the impurities were washed with the washing buffer (25 mM Tris, 150 mM NaCl, 20/50 mM Imidazole, pH 8.0), and the protein was washed with the elution buffer (25 mM Tris, 150 mM NaCl, 250 mM Imidazole, pH 8.0). The eluent was collected and confirmed by Western blot.
For SDS-PAGE detection, 12% SDS-PAGE was prepared. After gel electrophoresis (concentrate gel at 80 V for 20 min, separate gel at 120 V for 60 min), the gel was stained with Coomassie Brilliant Blue for 20 min and decolorized.
Western blot detection was carried out on the collected eluent. Preparation of polyacrylamide gel consists of 5% concentrated gel and 12% separated gel. After loading the sample, gel electrophoresis was performed: concentrated gel was kept at 80 V for 30 min and separation gel was kept at 120 V for 60 min. The transfer membrane was wet transferred at 250 mA for 90 min. Then the PVDF membrane was sealing with 5% skimmed milk powder and slowly oscillated at 37 °C for 2 h. The primary antibody is rabbit anti-his tag (Sangon, No. D110002), which was diluted at 1:500 and slowly oscillated at 37 °C for 60 min. The second antibody was goat anti-rabbit antibody (Sangon, No. D110058), which was diluted at 1: 8000 and slowly oscillated at 37 °C for 60 min. Color development was carried out by TMB kit.
In vitro assays for photolyase activity
We measured enzyme activity as reported (Li et al. 2015, 2010) with some adjustments. The repair activity of CPD photolyase was measured as following method: the CPD substrates were created by irradiation DNA oligomers 5′-TTTTTTTT TTTTTTTT-3′ (Sangon) for 4 h with UV (Beijing Normal University, Beijing, wavelength 290–320 nm, 8 W) on ice. The CPD was monitored photometrically by the absorbance of 260 nm by UV/Vis spectrophotometer (UV-2550). Before the repair assay, the purified protein had been photoreduced by blue light (450 nm, 10 nm FWHM, 50 μmol m−2 s−1) at 15 °C for 30 min. The repair buffer was prepared as follows: 50 mM Tris/Cl, 100 mM NaCl, 1 mM EDTA, 100 mg mL−1 BSA, 10% (w/v) glycerol, pH 7.6. The repair assay system contained 1 μM purified protein, 1 μM CPD and 10 μM DTT. The repair system (2 mL) was kept in the 2 mL sealed quartz cuvette. The mixtures were incubated in dark for 15 min and irradiated with a blue light (450 nm, 10 nm FWHM, 50 μmol m−2 s−1) for 2 h. During the blue light illumination, absorption changes of 260 nm were monitored using UV–Vis spectrophotometer (UV-2550 PC; Shimadzu, Neufahrn, Germany). The activity was evaluated from the increment of the absorbance. Bovine Serum Albumin (BSA) was used as negative control.
Statistical analysis
The experiments were conducted in three replicates and all data presented as the mean ± standard deviation (SD), which was analyzed by one-way analysis of variance (ANOVA). Significant statistical difference was analyzed using LSD with a threshold p value of 0.05.
Results
Cloning and sequencing of the photolyase genes
In the ice diatom P. tricornutum ICE-H, two potential DNA photolyase genes, PiPhr1 (MT512413) and PiPhr2 (MT534165), were isolated. The ORF of PiPhr1 is 1833 bp in length and encodes a 610 amino acid polypeptide, while PiPhr2 is 1809 bp in length and encodes a 602 amino acid polypeptide. The PiPhr1 protein has a deduced Mw of 68.0 kDa with a pI of 9.04, and the PiPhr2 protein has a deduced Mw of 68.82 kDa and a pI of 7.31. Both proteins are CPD photolyase proteins with an FAD-binding domain (Fig. S1). The amino acid sequences of PiPhr1 and PiPhr2 showed 79% and 84% similarities with those of Phaeodactylum tricornutum (Fig. 1).
Fig. 1.
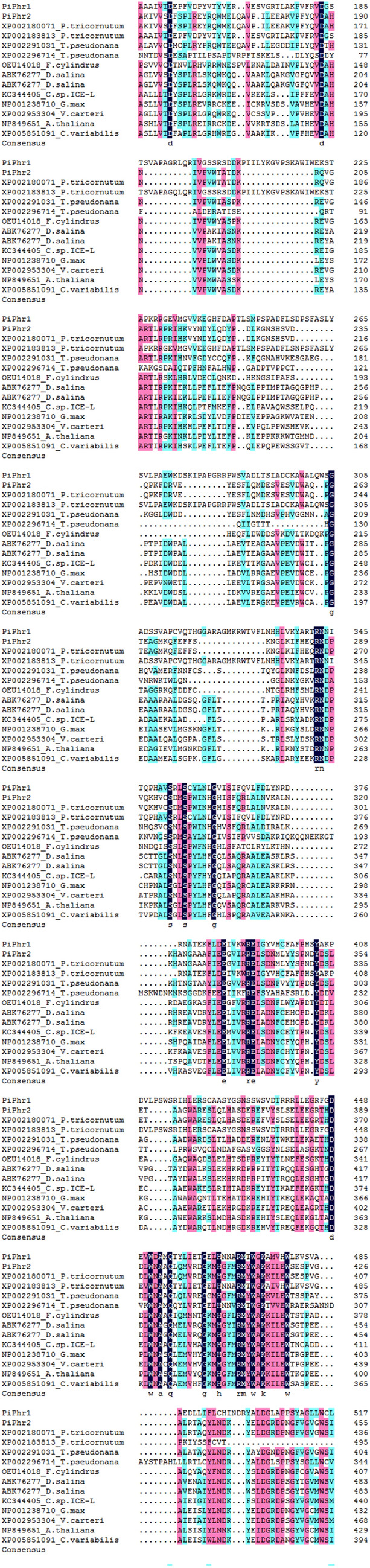
The similarity analysis and multiple sequence alignment of PiPhr1, PiPhr2 and CPD class II photolyase protein sequences
The phylogenetic tree presents most of the known major photolyase/Cry family: the animal Cry/6-4 photolyases, the Cry DASHs, the plant Crys, and the CPD class II photolyases (the CPD class I, CPD class III photolyases and bacterial cryptochromes and photolyases were excluded). From the phylogenetic tree (Fig. 2), it can be seen that all the proteins fell into four distinct subfamilies: the animal Cry/6-4 photolyases, the Cry DASHs, the plant Crys, and the CPD class II photolyases (Fig. 2). As shown in Fig. 2, the CPD class II photolyases clustered into a single monophyletic group. The result indicated that PiPhr1 and PiPhr2 were potential CPD class II photolyases, grouped with those from the diatoms, green algae and plants. In the CPD class II subfamily, the two photolyase genes from P. tricornutum ICE-H were clustered with those from the diatoms, apart from the subgroup that comprised genes from green algae and higher plants. Compare the functions and structure of the PiPh1, PiPhr2 with photolyase/cryptochrome family was showed in Table 2.
Fig. 2.
Phylogenetic analysis of photolyase proteins. A neighbor-joining (NJ) tree constructed based on analysis of the photolyase in P. tricornutum ICE-H and sequences downloaded from GenBank
Table 2.
Comparison of the functions and structure of the PiPh1, PiPhr2 with photolyase/cryptochrome family
| The photolyase/cryptochrome gene family | C-terminal extension | Photolyase activity | Function |
|---|---|---|---|
| CPD photolyase | No | Yes | Repair of CPD |
| (6-4) photolyase | No | Yes | Repair of 6-4PP |
| Plant cry | Yes | No | Circadian rhythm and photoreception |
| PiPh1 | No | Yes | Repair of CPD |
| PiPhr2 | No | Yes | Repair of CPD |
Real-time PCR analysis of PiPhr1 and PiPhr2 mRNA expression
Transcriptional expression levels of PiPhr1 and PiPhr2 under different conditions were studied by qRT-PCR. To find out the effects of different light conditions on the expression levels of PiPhr1 and PiPhr2, the samples were exposed to UVB irradiation, blue light and dark conditions for different time periods (0.5 and 3 h). A housekeeping gene, Rbcl, was used as an internal control. To better understand the changes in gene expression, the expression level of the gene under normal condition was set to 1 as a control (Fig.S3–Fig.S5). The results displayed that when exposed to UVB stress for 0.5 h, an increase in PiPhr1 and PiPhr2 expression (5.05 ± 0.40-fold and 3.48 ± 1.06-fold, respectively) was observed (Fig. 3). After UVB irradiation for 3 h, the expression of PiPhr1 dropped but was still higher than the control (3.38 ± 0.22-fold). However, that of PiPhr2 displayed an increase tend, up to 5.92 ± 0.42-fold at 3 h. The expression levels of both PiPhr1 and PiPhr2 under blue light followed a similar pattern to those observed under UVB radiation. PiPhr1 was increased 2.75 ± 0.64-fold at 0.5 h and decreased thereafter at 3 h to a level of about 1.26 ± 0.31-fold. PiPhr2 was 2.98 ± 0.31-fold higher than that of the control at 0.5 h and then increased slightly at 3 h (3.07 ± 0.53-fold). The results of the dark conditions were of special interest, where it was observed the expression level of PiPhr2 increased dramatically at 3 h. When exposed to dark stress, the expression levels of PiPhr1 and PiPhr2 were repressed at 0.5 h (0.63 ± 0.01-fold and 0.53 ± 0.04-fold, respectively). The transcript levels of both genes were enhanced at 3 h, reaching 4.41 ± 0.78-fold and 12.83 ± 1.32-fold, which might suggest that PiPhr1 and PiPhr2 were liable to induction under long periods of darkness.
Fig. 3.
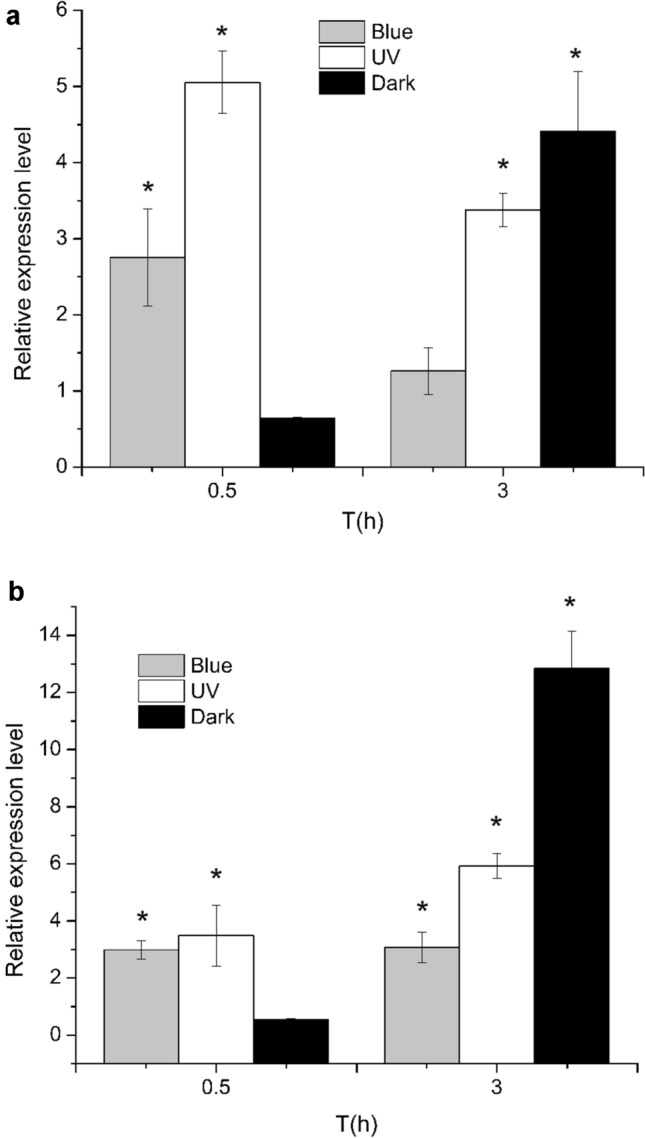
Relative expression level of the PiPhr1 and PiPhr2 exposed to UVB irradiation, blue light and dark condition. a: the expression level of PiPhr1 under different conditions; b: the expression level of PiPhr2 under different conditions (mean±SE,∗represented p < 0.05)
Activity assays in vitro
To test the activity of PiPhr1 and PiPhr2 to the photorepair of UV-induced DNA lesions, in vitro assays were performed. The PiPhr1 and PiPhr2 proteins were recombinantly produced and purified (Figs. 4 and 5). BSA, without any photorepair activity, was used as a negative control. The results of the assays are shown in Fig. 6 and Fig. S2. Under blue light, the absorbance at 260 nm began to increase (Fig. 6). The results showed that A260 of PiPhr1 increased continuously from 0.028 ± 0.0010 to 0.054 ± 0.0021 (60 min). And the A260 of PiPhr2 improved from 0.029 ± 0.0001 to 0.073 ± 0.0010 (40 min), then dropped to 0.068 ± 0.0015 (60 min). Increases of A260 revealed that purified PiPhr1 and PiPhr2 have photorepair activity. The results indicated that PiPhr1 and PiPhr2 played roles in photorepair in P. tricornutum ICE-H. Compared with PiPhr1, PiPhr2 displayed a higher activity in repairing CPD under lab conditions. When the activity assays was incubated for 60 min, the A260 of PiPhr2 was below the value at 40 min. This result demonstrated that, over a given time, the photolyase activity did not increase.
Fig. 4.

The SDS-PAGE analysis of purified PiPhr1 and PiPhr2. a: PiPhr1; b:PiPhr2
Fig. 5.

The Western blot analysis of purified PiPhr1 and PiPhr2. a: PiPhr1; b:PiPhr2
Fig. 6.
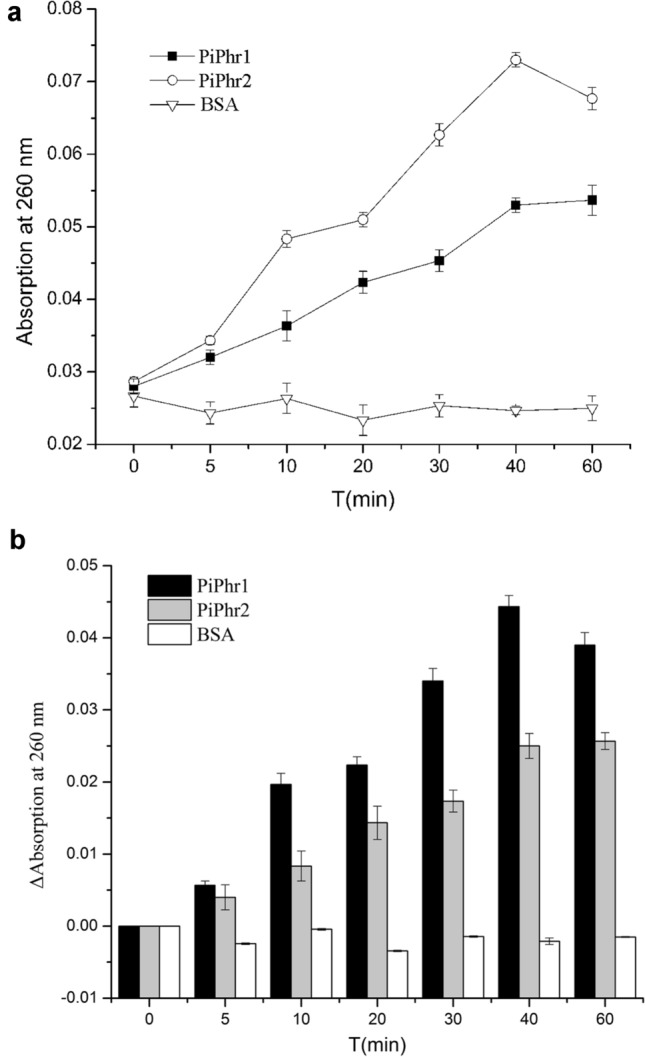
In vitro assay: changes of absorption at 260 nm when CPDs are repaired under blue light (mean ± SE). The repair assay system contained 0.5 μM purified protein and 0.5 µM CPD. A: changes of absorption at 260 nm. B: ΔAbsorbance at 260 nm
Discussion
The CPFs constitute an ancient family of the cryptochromes and the photolyases, which play significant roles in DNA repair (Oliveri et al. 2014). The photolyases have been well studied in many organisms, but only limited knowledge is available from the Antarctic diatom. Here, we present two photolyases from the Antarctic diatom P. tricornutum ICE-H, which provide more information on diatom photolyases and functional genes for biotechnological applications.
Photolyase proteins are 50–80 kDa proteins of 500–700 amino acids in length. The structures of the photolyases are characterized by two domains: an amino-terminal α/β domain and a carboxy-terminal α-helical domain. We isolated two class II DNA photolyases, PiPhr1 and PiPhr2, from the Antarctic ice diatom P. tricornutum ICE-H.
Phylogenetic studies were performed to probe the relationships of the photolyase proteins. It initially showed that photolyases were the common ancestor of the cryptochromes. Phylogenetic analyses with other known eukaryotic CPF sequences showed that these sequences fell into four subfamilies: the animal Cry/6-4 photolyases, the Cry DASHs, the plant Crys, and the CPD class II photolyases. The results revealed that plant Cry is more likely derived from CPD photolyase, whereas animal Cry is more closely correlated with 6-4 photolyase (Lucas-Lledo and Lynch 2009). PiPhr1 and PiPhr2 were grouped with the CPD class II subgroup that comprised proteins from diatoms, green algae and high plants. CPD class II is a sister class of all other photolyase/Cry superclasses and is more phylogenetically distant from others (Ozturk et al. 2007). In the cluster of the CPD class II subfamily, it can be speculated that PiPhr2 is a canonical CPD class II photolyase and PiPhr1 was a potential CPD class II-like photolyase, which was similar in P. tricornutum and T. pseudonana (Oliveri et al. 2014). For the class II photolyases, the lower amino acid sequence identities suggested they arose early in evolution (Zhang et al. 2017).
As photolyases repair DNA using near-UV/blue light photons as an energy source, the expression levels of PiPhr1 and PiPhr2 were determined after exposure to UVB irradiation, blue light and dark conditions. When exposed to blue light, organisms may survive via the NER (nucleotide excision repair) and photolyases repairing DNA, but the UV-protecting pigments also shield organisms from UV damage (Marizcurrena et al. 2017). Compared with the control, the PiPhr1 and PiPhr2 raised their expression levels under UV irradiation, revealing the key role they play in protecting the DNA of P. tricornutum ICE-H from UV irradiation. The expression of PiPhr2 was still upregulated after UV irradiation for more than 3 h. In contrast, the expression of PiPhr1 decreased after 3 h of UV irradiation, showing different expression patterns between the two CPD class II photolyases from P. tricornutum ICE-H. A similar trend took place when the algae were exposed to blue light, indicating these genes can be activated by blue light and UVB. Expression levels of PiPhr1 and PiPhr2 increased dramatically after exposure to dark condition for 3 h, revealing that PiPhr1 and PiPhr2 took part in dark adaptation of the cells. Under diurnal light/dark cycles, CPF genes of P. tricornutum expressed rhythmically, which increased in daytime and decreased in dark time (Oliveri et al. 2014). It may be related to DNA replication during diatom cell division (Oliveri et al. 2014). The increased expression levels of PiPhr1 and PiPhr2 may be in connection with cell division.
With the aim to better understand the role of photolyase activity of PiPhr1 and PiPhr2, recombinant protein expression and photoreactivation assays in vitro were performed. The results displayed CPD photolyase activity of PiPhr1 and PiPhr2. This indicated that PiPhr1 and PiPhr2 were CPD photolyases in P. tricornutum ICE-H. The gene that encoded a photolyase protein with CPD repair activity has been reported from higher plants and algae (Hirouchi et al. 2003; Okafuji et al. 2010). In Arabidopsis thaliana, CPD class II photolyases activated by UV/Vis could contribute to the electron transfer pathways (Kiselev et al. 2017; Okafuji et al. 2010). In the green algae Ostreococcus tauri, OtCPF2 is a CPD class II photolyases and showed CPD photolyase activity (Heijde et al. 2010). Reviews on the CPF protein family have been published (Konig et al. 2017b; Vechtomova et al. 2020). In the marine diatom P. tricornutum, CPF family members, including a canonical CPD class II photolyase and a CPD class II-like photolyase as well as several Cry-DASH cryptochromes, a plant-like cryptochrome and a protein of dual function acting as a (6-4) photolyase and blue light photoreceptor, have been found so far. Here, we present two CPD class II photolyases from the Antarctic diatom P. tricornutum ICE-H. Some work has shown that skin treated with liposomes containing CPD photolyase and exposure to light could reduce CPDs from the epidermis (Leccia et al. 2019). PiPhr1 and PiPhr2 are promising sunscreen additional ingredients. In a future study, we will search for and analyze other CPF family members in P. tricornutum ICE-H to enrich the diatom CPF family.
Conclusion
Two class II photolyase sequences, PiPhr1 and PiPhr2, were found in the Antarctic diatom P. tricornutum ICE-H. The ORFs of PiPhr1 and PiPhr2 are 1833 bp and 1809 bp in length, which encode class II photolyases. Expressions levels of PiPhr1 and PiPhr2 were upregulated by UVB irradiation, blue light and long periods (3 h) of dark condition. Photolyase repair activity of PiPhr1 and PiPhr2 was confirmed by in vitro assays. The results displayed that PiPhr1 and PiPhr2 could repair CPDs using blue light.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2018YFD0900705), Basic Scientific Fund for National Public Research Institutes of China (2020Q02), Natural Science Foundation of China (32000074), Natural Science Foundation of Shandong (ZR2019BD023), Tai Mountain Industry Leading Talent of Shan Dong (2019TSCYCX-06), China Postdoctoral Science Foundation (2019M662295), Postdoctoral Applied Research Projects of Qingdao (QD2019013).
Author contributions
MA performed the study and wrote this paper. CQ took part in the experiment. JM and ZS conducted the experiment and review.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Contributor Information
Meiling An, Email: rubyhawthorn@163.com.
Changfeng Qu, Email: cfqu@fio.org.cn.
Jinlai Miao, Email: miaojinlai@fio.org.cn.
Zhenxia Sha, Email: shazhenxia@163.com.
References
- An M, Zheng Z, Qu C, Wang X, Chen H, Shi C, Miao J. The first (6–4) photolyase with DNA damage repair activity from the Antarctic microalga Chlamydomonas sp ICE-I. Mutat Res. 2018;809:13–19. doi: 10.1016/j.mrfmmm.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, et al. The genome of the diatom Thalassiosirapseudonana: ecology, evolution, and metabolism. Science. 2004;306:79–86. doi: 10.1126/science.1101156. [DOI] [PubMed] [Google Scholar]
- Barlev A, Sen D. DNA's encounter with ultraviolet light: an instinct for self-preservation? Acc Chem Res. 2018;51:526–533. doi: 10.1021/acs.accounts.7b00582. [DOI] [PubMed] [Google Scholar]
- Bowler C, Allen AE, Badger JH, Grimwood J, Jabbari K, Kuo A, Maheswari U, Martens C, Maumus F, Otillar RP, et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature. 2008;456:239–244. doi: 10.1038/nature07410. [DOI] [PubMed] [Google Scholar]
- Brych A, Mascarenhas J, Jaeger E, Charkiewicz E, Pokorny R, Bolker M, Doehlemann G, Batschauer A. White collar 1-induced photolyase expression contributes to UV-tolerance of Ustilagomaydis. MicrobiologyOpen. 2016;5:224–243. doi: 10.1002/mbo3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnapen DJ, Sen D. A deoxyribozyme that harnesses light to repair thymine dimers in DNA. Proc Natl Acad Sci. 2004;101:65–69. doi: 10.1073/pnas.0305943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coesel S, Mangogna M, Ishikawa T, Heijde M, Rogato A, Finazzi G, Todo T, Bowler C, Falciatore A. Diatom PtCPF1 is a new cryptochrome/photolyase family member with DNA repair and transcription regulation activity. EMBO Rep. 2009;10:655–661. doi: 10.1038/embor.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JE. DNA repair. The search for homology. BioEssays. 2018 doi: 10.1002/bies.201700229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijde M, Zabulon G, Corellou F, Ishikawa T, Brazard J, Usman A, Sanchez F, Plaza P, Martin M, Falciatore A, et al. Characterization of two members of the cryptochrome/photolyase family from Ostreococcustauri provides insights into the origin and evolution of cryptochromes. Plant Cell Environ. 2010;33:1614–1626. doi: 10.1111/j.1365-3040.2010.02168.x. [DOI] [PubMed] [Google Scholar]
- Hirouchi T, Nakajima S, Najrana T, Tanaka M, Matsunaga T, Hidema J, Teranishi M, Fujino T, Kumagai T, Yamamoto K. A gene for a class II DNA photolyase from Oryza sativa: cloning of the cDNA by dilution-amplification. Mol Gen Genomics. 2003;269:508–516. doi: 10.1007/s00438-003-0856-9. [DOI] [PubMed] [Google Scholar]
- Jans J, Schul W, Sert YG, Rijksen Y, Rebel H, Eker AP, Nakajima S, van Steeg H, de Gruijl FR, Yasui A, et al. Powerful skin cancer protection by a CPD-photolyase transgene. Curr Biol. 2005;15:105–115. doi: 10.1016/j.cub.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Jepsen KA, Solov’yov IA. On binding specificity of (6-4) photolyase to a T(6-4)T DNA photoproduct. Eur Phys J D. 2017 doi: 10.1140/epjd/e2017-70818-2. [DOI] [Google Scholar]
- Juhas M, von Zadow A, Spexard M, Schmidt M, Kottke T, Buchel C. A novel cryptochrome in the diatom Phaeodactylumtricornutum influences the regulation of light-harvesting protein levels. FEBS J. 2014;281:2299–2311. doi: 10.1111/febs.12782. [DOI] [PubMed] [Google Scholar]
- Kao YT, Saxena C, Wang L, Sancar A, Zhong D. Direct observation of thymine dimer repair in DNA by photolyase. Proc Natl Acad Sci. 2005;102:16128–16132. doi: 10.1073/pnas.0506586102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev KV, Ogneva ZV, Dubrovina AS, Suprun AR, Tyunin AP. Altered somatic mutation level and DNA repair gene expression in Arabidopsis thaliana exposed to ultraviolet C, salt, and cadmium stresses. Acta Physiol Plantarum. 2017 doi: 10.1007/s11738-017-2600-9. [DOI] [Google Scholar]
- Konig S, Eisenhut M, Brautigam A, Kurz S, Weber APM, Buchel C. The influence of a cryptochrome on the gene expression profile in the diatom Phaeodactylumtricornutum under blue light and in darkness. Plant Cell Physiol. 2017;58:1914–1923. doi: 10.1093/pcp/pcx127. [DOI] [PubMed] [Google Scholar]
- Konig S, Juhas M, Jager S, Kottke T, Buchel C. The cryptochrome-photolyase protein family in diatoms. J Plant Physiol. 2017;217:15–19. doi: 10.1016/j.jplph.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Leccia MT, Lebbe C, Claudel JP, Narda M, Basset-Seguin N. New vision in photoprotection and photorepair. Dermatol Ther. 2019;9:103–115. doi: 10.1007/s13555-019-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu Z, Tan C, Guo X, Wang L, Sancar A, Zhong D. Dynamics and mechanism of repair of ultraviolet-induced (6-4) photoproduct by photolyase. Nature. 2010;466:887–890. doi: 10.1038/nature09192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ma L, Mou S, Wang Y, Zheng Z, Liu F, Qi X, An M, Chen H, Miao J. Cyclobutane pyrimidine dimers photolyase from extremophilic microalga: remarkable UVB resistance and efficient DNA damage repair. Mutat Res. 2015;773:37–42. doi: 10.1016/j.mrfmmm.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Li D, He Y, Zhang L, Wang F, Qu C, Miao J. The complete chloroplast genome of Phaeodactylumtricornutum ICE-H isolated from the Antarctic sea ice. Mitochondrial DNA Part B. 2020;5:1182–1183. doi: 10.1080/23802359.2020.1731340. [DOI] [Google Scholar]
- Lucas-Lledo JI, Lynch M. Evolution of mutation rates: phylogenomic analysis of the photolyase/cryptochrome family. Mol Biol Evol. 2009;26:1143–1153. doi: 10.1093/molbev/msp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marizcurrena JJ, Morel MA, Brana V, Morales D, Martinez-Lopez W, Castro-Sowinski S. Searching for novel photolyases in UVC-resistant Antarctic bacteria. Extremophiles. 2017;21:409–418. doi: 10.1007/s00792-016-0914-y. [DOI] [PubMed] [Google Scholar]
- Okafuji A, Biskup T, Hitomi K, Getzoff ED, Kaiser G, Batschauer A, Bacher A, Hidema J, Teranishi M, Yamamoto K, et al. Light-induced activation of class II cyclobutane pyrimidine dimer photolyases. DNA Repair. 2010;9:495–505. doi: 10.1016/j.dnarep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Oliveri P, Fortunato AE, Petrone L, Ishikawa-Fujiwara T, Kobayashi Y, Todo T, Antonova O, Arboleda E, Zantke J, Tessmar-Raible K, et al. The Cryptochrome/photolyase family in aquatic organisms. Mar Genomics. 2014;14:23–37. doi: 10.1016/j.margen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Ozturk N, Song SH, Ozgur S, Selby CP, Morrison L, Partch C, Zhong D, Sancar A. Structure and function of animal cryptochromes. Cold Spring Harb Symp Quant Biol. 2007;72:119–131. doi: 10.1101/sqb.2007.72.015. [DOI] [PubMed] [Google Scholar]
- Park HW, Kim ST, Sancar A, Deisenhofer J. Crystal structure of DNA photolyase from Escherichia coli. Science. 1995;268:1866–1872. doi: 10.1126/science.7604260. [DOI] [PubMed] [Google Scholar]
- Qu CF, Liu FM, Zheng Z, Wang YB, Li XG, Yuan HM, Li N, An ML, Wang XX, He YY, et al. Effects of ocean acidification on the physiological performance and carbon production of the Antarctic sea ice diatom Nitzschia sp. ICE-h. Mar Pollut Bull. 2017;120:184–191. doi: 10.1016/j.marpolbul.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev. 2003;103:2203–2237. doi: 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- Spampinato CP. Protecting DNA from errors and damage: an overview of DNA repair mechanisms in plants compared to mammals. Cell Mol Life Sci. 2017;74:1693–1709. doi: 10.1007/s00018-016-2436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vechtomova YL, Telegina TA, Kritsky MS. Evolution of proteins of the DNA photolyase/cryptochrome family. Biochemistry (mosc) 2020;85:131–153. doi: 10.1134/S0006297920140072. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang L, Zhong D. Photolyase: Dynamics and electron-transfer mechanisms of DNA repair. Arch Biochem Biophys. 2017;632:158–174. doi: 10.1016/j.abb.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.



