Abstract
The present study was carried out to silence the transcription factor genes ZCT1, ZCT2 and ZCT3 via lipofectamine based antisense LNA GapmeRs transfection into the protoplasts of established photomixotrophic cell suspensions. The photomixotrophic cell suspensions with a threshold of 0.5% sucrose were raised and established using two-tiered CO2 providing flasks kept under high light intensity. The photomixotrophic cell suspensions showed morphologically different thick-walled cells under scanning electron microscopic analysis in comparison to the simple thin-walled parenchymatous control cell suspensions. The LC–MS analysis registered the vindoline production (0.0004 ± 0.0001 mg/g dry wt.) in photomixotrophic cell suspensions which was found to be absent in control cell suspensions. The protoplasts were isolated from the photomixotrophic cell suspensions and subjected to antisense LNA GapmeRs silencing. Three lines, viz. Z1A, Z2C and Z3G were obtained where complete silencing of ZCT1, ZCT2 and ZCT3 genes, respectively, was observed. The Z3G line was found to show maximum production of vindoline (0.038 ± 0.001 mg/g dry wt.), catharanthine (0.165 ± 0.008 mg/g dry wt.) and vinblastine (0.0036 ± 0.0003 mg/g dry wt.). This was supported by the multifold increment in the gene expression of TDC, SLS, STR, SGD, d4h, dat, CrT16H and Crprx. The present work indicates the master regulation of ZCT3 knockdown among all three ZCTs transcription factors in C. roseus to enhance the terpenoid indole alkaloids production. The successful silencing of transcription repressor genes has been achieved in C. roseus plant system by using photomixotrophic cell cultures through GapmeR based silencing. The present study is a step towards metabolic engineering of the TIAs pathway using protoplast transformation in C. roseus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01017-y.
Keywords: Catharanthine, LC–MS, Protoplast, TIAs, Vinblastine, Vindoline
Introduction
The terpenoid indole alkaloids (TIAs) pathway operating in the anti-neoplastic herb Catharanthus roseus is of enormous significance in the ongoing metabolic engineering-based research advancements in this medicinal plant. This is primarily because of two major reasons. Firstly, the two-dimeric alkaloids vincristine and vinblastine (found in C. roseus) are important components used in the chemotherapeutic treatment of Hodgkin’s/non-Hodgkin’s lymphomas and other metastatic lymphoblastic leukemia (Verma et al. 2015, 2017a). The insufficient concentrations of these two alkaloids in C. roseus plants is considered to be a critical limitation that needs to be immediately addressed since the demand for these molecules is increasing exponentially, leading to their escalating higher market price as a consequence. C. roseus as a medicinal plant and its effect in the treatment of various ailments is comprehensive. Recently a new compounds namely catharoseumine, 17- deacetoxycyclovinblastine and 14, 15- didehydrocyclovinblastine and 17- deacetoxyvinamidine have been reported. These newly isolated indole alkaloids showed effective activity against human cancer cell under in vitro conditions. The other important alkaloids as vinodoline, vindolicine, vindolidine and vindolinine have also been cited as important antidiabetic activity containing metabolites that were extracted from leaves of C. roseus plants (Pham et al. 2020).
The recent study showed the epigenetic regulation level of genes that could play a role in the monoterpenoid indole alkaloids (MIA) accumulation at the developmental, organ or at the environmental level. It was correlated that gene expression at the tissue level was related to specific methylation signatures which depended upon gene parts and cytosine contexts. Apart from it, the same study found DNA methylation variations were very much compliant with the transcription factors. These recent developments direct the flow of information towards epigenetic regulation of specialized metabolism (Bernonville et al. 2020). Henceforth, considering such immense possibility of gaining important metabolites of this plant system, there is a requirement to be more focused on producing these metabolites through specific approaches. Amongst it, the metabolic engineering approaches are of uttermost importance as it will help in understanding the whole pathway of the plant system which will help in diverting the flux of metabolite synthesis in the desired direction. However, it will also help in over or under expressing certain genes at in planta level and at heterologous levels also, in other expression systems. Secondly, the basic research carried in the area of TIAs biogenesis in C. roseus plants in the last 30 years had shown that multi-step biosynthesis TIAs are very rigidly regulated, spatially as well as temporally, at the level of cellular/tissue differentiation and environmental signals. At least 35 enzymatic steps of the pathway are known today to operate in four different types of cell/tissue types (epidermis, internal phloem parenchyma, idioblasts, and laticifers) in five subcellular compartments, viz. thylakoid membrane, nucleus, cytosol, vacuole, and endoplasmic reticulum (De Luca and Cutler 1987; St-Pierre et al. 1999; Facchini 2001; El-Sayed and Verpoorte 2007; Facchini and De Luca 2008; Verma et al. 2012, 2017a).
Diversion of this flux from cathenamine-derived intermediate tabersonine to vindoline involves the seven-step enzymatic process, which takes place in aerial parts of the plant (Qu et al. 2015). Firstly, tabersonine undergoes hydroxylation to form 16 hydroxytabersonine by a cytochrome P450 monooxygenase enzyme (Besseau et al. 2013) tabersonine-16-hydroxylase (T16H) followed by its methylation by 16-hydroxy tabersonine-16-O-methyl transferase (16OMT) (Levac et al. 2008). Further, the two enzymes tabersonine 3-oxygenase (T3O) (Kellner et al. 2015; Qu et al. 2015) and tabersonine 3-reductase (T3R) (Qu et al. 2015) catalyse a concerted two-step conversion of tabersonine or 16-methoxytabersonine to 3-hydroxy–2,3-dihydrotabersonine or 3-hydroxy-16-methoxy-2,3-dihydrotabersonine. The obtained 16-methoxy-2,3-dihydro-3hydroxytabersonine is further converted to deacetoxyvindoline on the action of N-methyl transferase (NMT) (De Luca et al. 1987). The last two steps in vindoline synthesis involve the participation of two cytosolic enzymes including desacetoxyvindoline-4-hydroxylase (D4H) (Vazquez-Flota et al. 1997) and deacetylvindoline-4-O-acetyltransferase (DAT) (St. Pierre et al. 1998). Finally, vindoline and catharanthine are transported to vacuoles for their dimerization into vinblastine and then to vincristine to mark the completion of the MIA pathway in C. roseus. The production of alkaloids in C. roseus cell lines and in vitro plants are majorly affected by flux diversion at intermediate points, their inability of the final conversion of a specific reaction and transcription factor (ORCA, ZCTs, etc.) expressions.
The three members of the Cys2/Hys2-type (TF IIIA-type) Zinc finger C. roseus protein family namely, ZCT1, ZCT2 and ZCT3, are known to down regulate the transcriptional activities of the two enzymes of the monoterpene indole alkaloid biosynthetic pathway namely, tryptophan decarboxylase (TDC) and strictosidine synthase (STR) by acting on their promoters (Memelink and Gantet 2007). The ZCTs activation in C. roseus partially mediates the rapid defense response controlling mechanism for terpenoid indole alkaloid production. Apart from terpenoid indole alkaloid biosynthesis, ZCTs are also involved in other defense responses like cold drought, salt and oxidative stress (Pauw et al. 2004).
One of the major drawbacks of the heterotrophic cell culture of C. roseus was found to be the absence of vinblastine (VLB) and vincristine (VCR). The possible reason for this failure is associated with the inability of the usually heterotrophic cultured cells to synthesize the monomeric alkaloid vindoline that is required for the dimerization with another monomeric moiety catharanthine to synthesize VRC and VLB. The biogenesis of vindoline involves the expression of an enzyme (N-methyltransferase) on the biochemically active thylakoid membrane of a functional chloroplast, which is a significant limitation in the heterotrophic cultures (St. Pierre et al. 1998).
Past studies have indicated that while limitations in hyper synthesis and accumulation of TIA can appear in both the primary branches (terpenoid as well indole moieties) that lead to the formation of strictosidine, the advancement of the flux towards dimeric VLB and VCR is primarily restricted due to organelle and organ differentiation in the cultured tissues that are necessary for vindoline synthesis and its dimerisation step with catharanthine towards the terminal steps of the pathway (Verma et al. 2012). Autotrophic to photomixotrophic tissues with functional chloroplastic enzyme machinery have to be screened to allow vindoline production in cultured tissue.
The rapid progress of “omics” technologies in the last decade has been associated with the emergence of protoplast system as an alternative for non-Agrobacterium based transformation protocols. Such technologies have been proven to be better options for rapid screening of gene silencing and genome–editing targets for siRNA, miRNA and CRISPR technologies. As the protoplasts are devoid of cell walls, they provide an uncomplicated system for gene delivery materials during plant genetic engineering approaches (Burris et al. 2016).
A GapmeR (14–16 nucleotide length chimeric antisense nucleotide) can induce RNase-H cleavage since it holds a central block sequence of monomer deoxynucleotides and importantly this GapmeR’s central block is flanked by 2’O ribonucleotides. In another way, a bridged nucleic acid (BNAs), an artificial modified ribonuclease monomer also covers and protects the nuclease degradation of the internal blocks of GapmeRs (Exiqon Catalogue-2016). Specifically for RNase-H mediated cleavage of selected and targeted RNAs, GapmeRs have been used with an added advantage of reduction of numbers of phosphothioate linkages. The mechanism of action of GapmeR antisense oligonucleotide is the recruitment of RNase H that promotes selective cleavage of a specific nucleotide sequence. This cleavage results in the initiation of an antisense effect in the given strand of nucleotide. In recent times, inhibition of selected gene functions has been performed by this GapmeR based antisense technique.
Based on this backdrop, the present work was carried out to address and overcome such limitation by selecting photomixotrophic in vitro cell suspension cultures of C. roseus along with the down regulation of ZCTs to enhance maximum flux towards the dimeric alkaloid production. Photomixotrophy in cell cultures was also thought to make the cultured tissues self-nourishing that would impart hormone autotrophy in them as well. This would additionally supplement the biosynthetic capability of these cells (Emara et al. 2018). Eventually, the present investigation dealt with the isolation of protoplasts from photomixotrophic cell suspension cultures of C. roseus, their ZCTs silencing via lipofectamine-based GapmeR transformation and the establishment of transgenic cell suspensions with higher TIAs content.
Material and methods
Raising and the establishment of photomixotrophic cell suspensions
The seeds of C. roseus (CV Nirmal, National Gene Bank accession number 0865) were used for the study. The plants were established in the CSIR-NCL glasshouse using seeds. Leaf explants were cut and washed with detergent and kept under running tap water for 1 h. Washed leaves were treated with Savlon® antiseptic liquid (2–3 min) and subsequently with absolute alcohol for 30 s. This was followed by 0.1% (w/v) mercuric chloride-based surface sterilization for the duration of 3 min. The sterilized leaves were thoroughly washed with sterile water and inoculated on the basal medium (Murashige and Skoog 1962). After 10 days of inoculation in MS basal medium, aseptic leaves were subjected to Agrobacterium rhizogenes (strain A4) mediated genetic transformations. The bacterial suspension was raised in liquid (Yeast Mannitol Broth) YMB medium (Hooykaas et al. 1977) supplemented with 50 mg/l kanamycin to co-cultivate the sterile explants. The bacterial culture in their exponential phase of growth with OD of 0.5 at 600 nm (24 h old) was used to co-cultivate the explants via wounding them with sterile needles dipped in the bacterial suspension. Explants wounded without bacterial suspension were plated to serve as controls. The explants were placed on a semi-solid hormone-free MS medium for five days followed by shifting to MS basal medium supplemented with cephalexin and ampicillin (500 mg/l each) for 15–20 days for bacterial elimination and root induction. The resultant root clones were shifted to one-fourth strength of Gamborg’s B5 semi-solid medium (Gamborg 1968) for growth and multiplication. The root cultures were incubated under a 16 h photoperiod. The highly proliferating root clone was subjected to callus induction. Fresh roots were placed on semi-solid MS medium fortified with 1.0 mg/l 2, 4-dichlorophenoxy acetic acid (2,4-D) + 0.5 mg/l benzylaminopurine (BAP). The emerging hard green callus was frequently subcultured to obtain the fragile loose callus. To raise photomixotrophic callus and maintain the continuous CO2-enriched gaseous phase around the growing tissues, a two-tier culture flask was used (Verma et al. 2014). The lower flask contained 60 ml of carbonate buffer consisting of 0.2 M KHCO3 and 0.2 M K2CO3 in 3:1 (pH = 9.2) for continuous release of CO2 into the gaseous head space in the upper flask (approx. 2% CO2 level). The explants from the stock culture [Grown hetrotrophically at 3 (w/v) level of sucrose in the medium] were transferred to the media supplemented either with 3, 2, 1, or 0% sucrose. After the end of each four-week culture period, the surviving tissue at each level of sucrose were transferred to fresh medium in such a way that one portion of each of tissue exposure to the medium with either the same levels of sucrose or a level higher or two level lower than that in their previous culture period. Ultimately, the surviving tissues were multiplied at the lowest threshold level of sucrose to obtain the photomixotrophic callus (Fig S1). To raise cell suspensions, 10 g of freshly induced fragile callus (Growing hetrotrophically at 3% sucrose as well as photomixotrophically on 0.5% sucrose level) was inoculated in 50 ml MS basal medium supplemented with 2.0 mg/l α-naphthalene acetic acid (NAA) + 0.2 mg/l Kinetin (Kn). The cultures were incubated on a rotary shaker (120 rpm) at 24 ± 2 °C under 3000 lx intensity illumination. They were observed under ZEISS OBSERVER.Z1 microscope (Carl Zeiss, Germany) to observe the morphological differences. The Rubisco assay of the photomixotrophic cell suspension was done by Sci-Fi Biologicals, Pune, India following the method of Verma et al. (2017b).
Scanning Electron Microscopic analysis of the photomixotrophic and control cell suspensions
To structurally characterize and differentiate the photomixotrophic cell suspensions from heterotrophic cell suspensions, Scanning Electron Microscope (SEM) analysis was performed. Samples were prepared by using one-month-old cell suspensions. One ml of the respective cell suspension was pelleted in the Eppendorf tube. The supernatant was discarded and one ml of 2.0% glutaraldehyde was added to the same Eppendorf tube. The dispensed cell suspensions were then incubated for two h at 4 °C which was followed by centrifugation and discarding of the supernatant. The retained pellet was dispensed in 200 µl of 1X PBS. After 5 min the centrifugation was done and the supernatant was discarded. The pellet was dispensed gradually in 30, 50 and 80% ethanol for 5 min and centrifuged to remove the supernatant. Finally, absolute ethanol was added to the sample and kept for drying. A drop from each sample was transferred to the silicon wafer chip, which was kept in a hydrated chamber for 30 min so that the cells can adhere. Following this with the help of sputter coater, they were coated with gold, palladium (Au–Pd). The chips were viewed at 15 kV accelerating voltage in Quanta 200 3D (FEI).
Protoplast isolation and the lipofectamine based ZCTs antisense LNA GapmeR transfection of the photomixotrophic cell suspensions
As antisense LNA GapmeR (to knockdown ZCT proteins) can be introduced to cell suspensions via lipofectamine transfection and protoplasts cultures are a prerequisite for such transfection. Therefore, protoplasts were isolated from the photomixotrophic cell suspensions growing on 0.5% sucrose solution. The suspension cell clusters of the photomixotrophic cell suspensions were allowed for settling down in the flask. The medium was decanted slowly and replaced with CPW 13 M solutions (Table S1). It was left for 1 h for pre-plasmolysis. Twenty ml of enzyme solution (consisted of Cellulase R 10–1%; Hemicellulose 0.5% and Maceroenzyme 4% in 2.0 mM MES, 13% Mannitol with CPW salts at pH of 5.8) per 250 ml flask were added and incubated overnight (15 h) at 28 °C. The digested contents were poured on to a 75 μ sieve. These were agitated and washed with CPW 13 M. The filtrate was collected in screw-capped sterilized centrifuge tubes. The tubes were spun at 80 g for 5 min (thrice by replacing CPW 13 M) to sediment the protoplasts and to remove unused enzymes. The CPW 13 M was removed with the help of a pipette and replaced with 12 ml of CPW21S solution. These tubes were spun at 100 g for 10 min. The protoplasts collected in the form of a band at the surface of CPW 21S solution in the centrifuge tube. The protoplasts were transferred to a fresh centrifuge tube in CPW 13 M solution. Once again, tubes were spun at 80 g for 5 min and CPW 13 M solution was replaced by protoplast culture medium (PCM) (Kao and Michayluk1975) consisting of macro- and micronutrients, 40.0 g/l sucrose, 5.0 g/l myo-inositol, 0.5 g/l MES, 0.05 g/l ascorbic acid, 90.0 g/l mannitol, pH 5.8). The protoplast counting is important to adjust suitable density for transformation. For this, a Haemocytometer (Fuchs-Rosenthal, Country marking with chamber depth of 0.2 mm and volume of each small subunit as 1/16 mm3) was used. The protoplast suspension was brought to a final density of 1 × 104 protoplast/ml and cultured in the 90 mm petri dishes. The petri dishes were sealed with parafilm and incubated at 25 ± 2 °C under dark conditions.
Antisense LNA GapmeRs for all the three genes (ZCT1, ZCT2 and ZCT3) were designed from EXIQON (Denmark). For each gene two antisense LNA GapmeRs have been designed (Table 1) and tested for efficiency. The received oligonucleotide was spun down to pellet and resuspended in nuclease-free water, and then it was mixed by vortexing and subsequently used in aliquots avoiding freeze–thaw cycles. Protoplasts were then plated in the 24-welled plate in PCM followed by lipofectamine (3000) transfection. Next, 10.0 µl of antisense LNA GapmeR DNA was mixed with 1.0 µl of P3000 reagent in 25.0 µl Opti-MEM I, which was then diluted with 1.5 µl of lipofectamine 3000 reagent. Both the mixtures were combined and incubated at room temperature (25 °C) for 5 min. The incubated complex (50 µl) after 5 min was added to protoplasts plated in PCM (24-welled plate). After 2 h, the PCM was replaced and protoplasts were further cultured to observe under ZEISS OBSERVER.Z1 microscope for cell wall regeneration and callus formation. Once the calli were obtained, the transfected lines were subjected to Real time-PCR studies.
Table 1.
Sequence of Antisense LNA GapmerR used to knockdown ZCT proteins in C. roseus
| No. | Antisense LNA GapmerR in vitro standard | Sequence |
|---|---|---|
| 1 | ZCT1-1 | 5’ C*C*G*C*T*A*A*A*G*A*T*T*G*A*T*G 3’ |
| 2 | ZCT1-2 | 5’ C*G*C*C*T*A*A*A*G*C*C*T*G*A*A*A 3’ |
| 3 | ZCT2-1 | 5’ T*T*A*A*T*C*C*G*T*G*C*T*T*G*A*T 3’ |
| 4 | ZCT2-2 | 5’ T*C*G*A*A*C*A*T*T*C*G*T*G*A*G*T 3’ |
| 5 | ZCT3-1 | 5’ C*G*C*G*A*G*C*A*A*G*C*A*T*A*A*T 3’ |
| 6 | ZCT3-2 | 5’ A*G*A*G*T*A*G*T*A*G*T*T*G*A*T*G 3’ |
| 7 | NEGATIVE CONTROL | 5’ A*A*C*A*C*G*T*C*T*A*T*A*C*G*C 3’ |
LC–MS analysis of the raised tissue
LC/MS analysis of the cell suspensions at different levels was conducted by the Center for Applications of Mass Spectrometry (CAMS), NCL-Innovation Centre, Pune, India. Alkaloid analysis was performed on Agilent Binary LC 1260 system equipped with Agilent (3.0 × 75 mm) C4 column. The column was used as the stationary phase at 40 °C. The mobile phase consisted of water with formic acid as solvent A (0.1 ml/100 ml water) and Acetonitrile (LC–MS grade) as solvent B. The flow rate was kept at 0.3 ml/min. The gradient elution started with 90% A/10% B for 0–4.9 min followed by 40% A/60% B for 5 min. This was successively followed with 5% A/95% B 5 for 1–7.0 min and finally completed with 90% A/10% B 7.1–12 min. the total sample run time was 12 min. The injection volume was 1.0 μl. Peak identification was obtained by comparing the retention time and the UV spectra of the fraction alkaloid chromatogram with vindoline, catharanthine and vinblastine standards which were purchased from Sigma Aldrich. Mass spectrometric analysis was performed on an Agilent 6540 UHD QTOF MS mass spectrometer. Mass spectra data were recorded on an ionization mode for a mass range of m/z 140–1200. Other mass spectrometer conditions were as follows: Nebulizing gas pressure: 30 psi; drying gas flow: 5 l/min; drying gas temperature: 325 °C; nebulizing gas flow: 5 l/min. For analysis purpose Masshunter workstation software v.B.05.01 was used.
Real-time PCR (qPCR) analysis
Real-time PCR analysis of cell suspensions at different stages was conducted by Sci-Fi Biologicals, Pune Maharashtra India. The analysis was performed on the QUANTSTUDIO 5 real-time PCR system (Thermo Fisher Scientific, USA); TRIZOL based RNA isolation was followed by c-DNA synthesis via Verso cDNA synthesis Kit (Thermo Scientific, USA). The PCR was performed for each sample in triplicate with negative control. The reaction was performed using 2X Power SYBR™ Green PCR Master Mix in a 20 µl final volume reaction. Melting curve analysis was done to ensure amplification of the specific amplicon. All real-time PCR quantifications were performed with a non-template control and the endogenous control actin. The gene expression levels were interpolated from standard curves for relative expression and normalized to actin in the same tissue. The real-time transcript analysis was performed with thirteen candidate genes specific to TIAs biosynthesis (Table S2). The cycling program consisted of 10-min incubation at 95 °C followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s. The melt curve study was performed by the increment of 0.05 s at 95 °C to 60 °C and quantification cycle (Ct) values were acquired for each sample with the Quant Studio software (Applied Biosystems, USA). The RNA isolation was conducted with TRIzol method and the real time PCR was run with triplicate sampling. For each 1.5 g of plant samples, 1.0 ml of TRIzol was used. For each of the sample 500 ng of RNA was used for c DNA synthesis. Verso c DNA synthesis kit (Thermo Scientific) was used to prepare a complete c DNA pool. The reaction parameter for c DNA synthesis was 42 °C and 30 min time with the number of cycles restricted to 1 and inactivation was performed at 95 °C with 2 min time duration. The PCR was conducted for each sample in duplicate with the negative controls. The reaction was performed in a 20 µl reaction consisting of 50 ng of the template.
Results and discussion
Establishment of the photomixotrophic cell suspensions and their characterization on the basis of morphology, chemical analysis and gene expression
The mercuric chloride-treated fresh leaves of C. roseus showed 80% survival on MS basal medium after the10th day of inoculation. The susceptibility of the aseptic leaf explants towards A. rhizogenes strains A4 for hairy root induction was tested and 7 independent hairy root clones were obtained after 20 days of co-cultivation. On transfer to one-fourth strength of Gamborg’s B5 semi-solid medium, the highly proliferated root clone p6 was selected for callus induction. The callus induction was noticed on the 15th day on transfer to callusing medium. The induced callus was found to be green but extremely hard (Fig. 1) and frequent sub-culturing on same medium for five-six times lead to the generation of loose fragile callus (Fig. 1). This fragile callus was subjected to the selection scheme to raise the photomixotrophic cultures (Fig. S1). Major prerequisites for establishing photomixotrophic cell cultures are the presence and maintenance of high chlorophyll content and photosynthetic competence of the cells even in the active dividing phase. Therefore, rapidly growing and highly chlorophyllous cell culture should be available for the selection procedure (Perez et al. 2015). This is the reason behind selecting the hairy root clone p6 for raising the photomixotrophic cultures. Following this selection scheme, the maximum threshold level of 0.5% sucrose was obtained, wherein the steadily growing the photomixotrophic line was selected after six months of the rigorous selection procedure (Fig. 1). Generally, lowering the sugar content in the nutrient medium and simultaneous supplementation of CO2 partial pressure above the ambient air level stimulates a photosynthetic development. Under the selection pressure, cells with higher photosynthetic capabilities survive and take up the photomixotrophic growth. Usually, the conversion from the photoheterotrophic to photomixotrophic conditions is accompanied by the drastic reduction in growth rate, chlorophyll content and viability of cells (Kozai 2010). This was well evident in selecting the stable photomixotrophic line at the threshold level of 0.5% sucrose in the present study. For initiating cell suspensions, the control and photomixotrophic cells were shifted to liquid cell suspension medium, fortified with respective sucrose levels. Repeated sieving and frequent sub culturing every 10th day in cell suspension medium resulted in the establishment of fine suspensions within 50–60 days. These suspensions were served as control cell suspensions (Fig. 2) and photomixotrophic cell suspensions (Fig. 3), respectively. The resultant fine cell suspensions were differing in their morphology. The photomixotrophic cell suspensions were slightly brownish and much granular in comparison to cream color control cell suspensions. Microscopic examination of these cultures revealed that while the cells of control cell suspensions were oval with a thin smooth cell wall (Fig. 2), the photomixotrophic cells were found to be highly differentiated, with thick wall and variable shapes (Fig. 3). The SEM analysis also showed the smooth surface of control cell suspensions while trachiedial appearance of photomixotrophic cell suspensions. The average size of smooth-walled cell suspensions (control) and of trachiedial appearance cell suspensions (photomixorophic) was found to be 119 × 71 µm and 178 × 39 µm, respectively. The photomixotrophic cell suspensions recorded the 1.5-fold increase in Rubisco activity in comparison to the control cell suspensions.
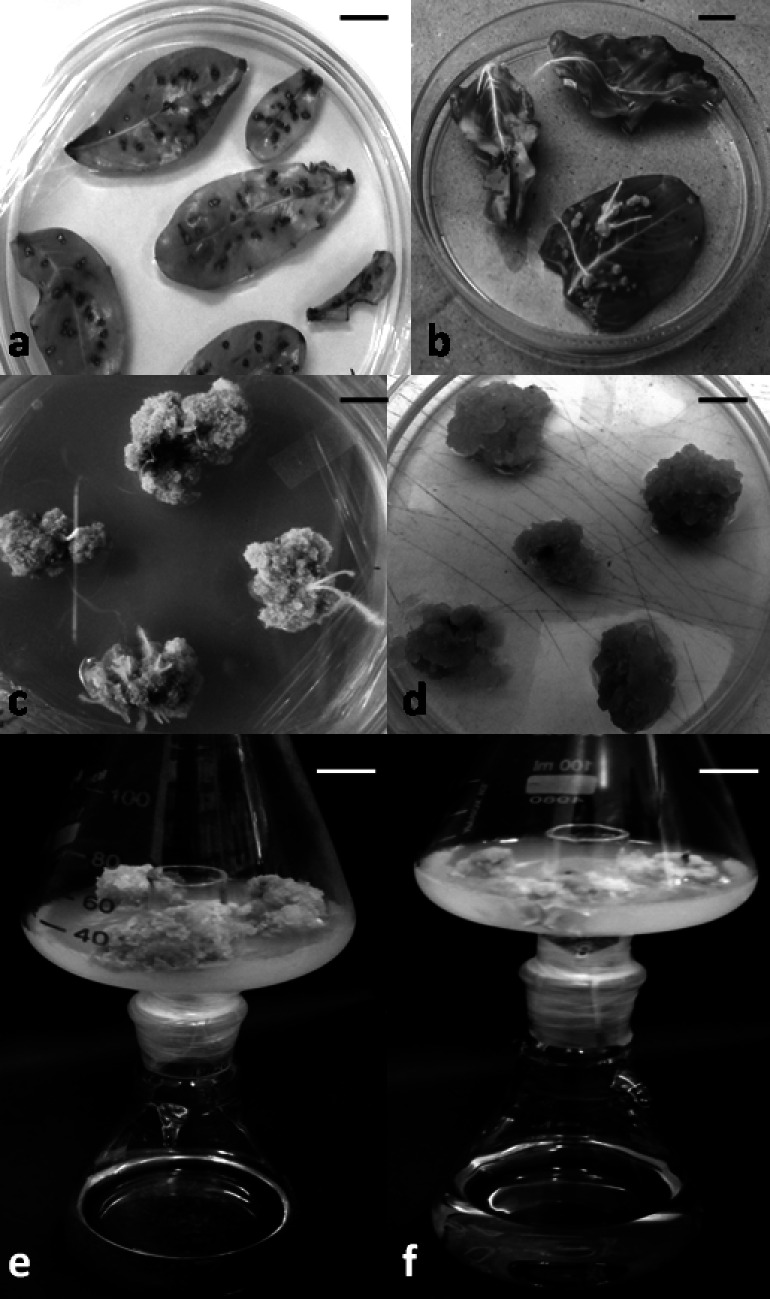
Fig. 1.
Hairy root induction, subsequent callus and suspension raising under photoautotrophic conditions in C. roseus. a leaf explants co-cultivated with A4 strain; b hairy root emergence from the co-cultivated leaf explants; c compact green callus obtained from hairy roots; d fragile callus after subculturing; e callus grown on 3.0% sucrose as control and f callus grown on 0.5% sucrose as maximum threshold under CO2 enriched two tier flask. Scale bar = 1.0 cm
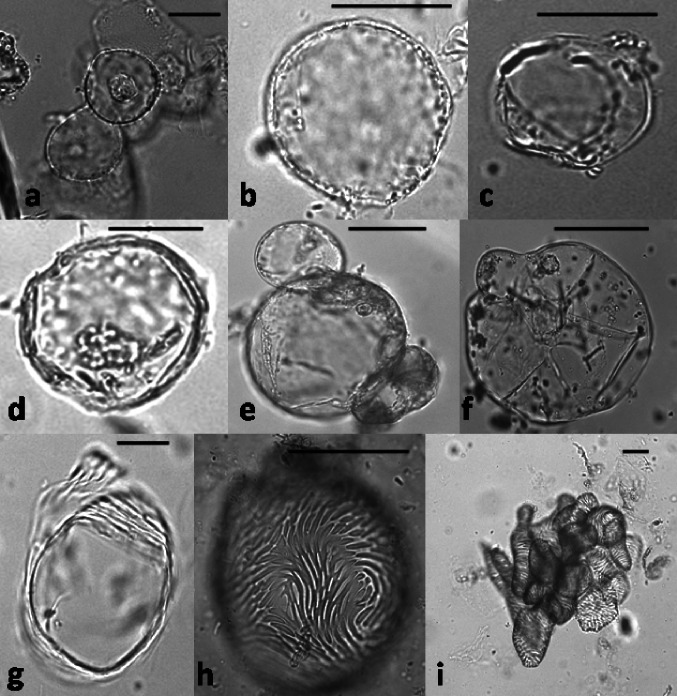
Fig. 2.
Established control cell suspensions of C. roseus. a thirty days old control cell suspension, scale bar = 1 cm; b control cell suspension shown under microscope with scale bar = 10 µm; c-j globular to oval smooth walled cells observed under scanning electron microscope with scale bar = 10 µm
Fig. 3.
Established photomixotrophic cell suspensions of C. roseus. a thirty-days old control cell suspension, scale bar = 1 cm; b photomixotrophic cell suspension shown under microscope with scale bar = 20 µm; c-g elongated thick walled cells observed under scanning electron microscope with scale bar = 20 µm
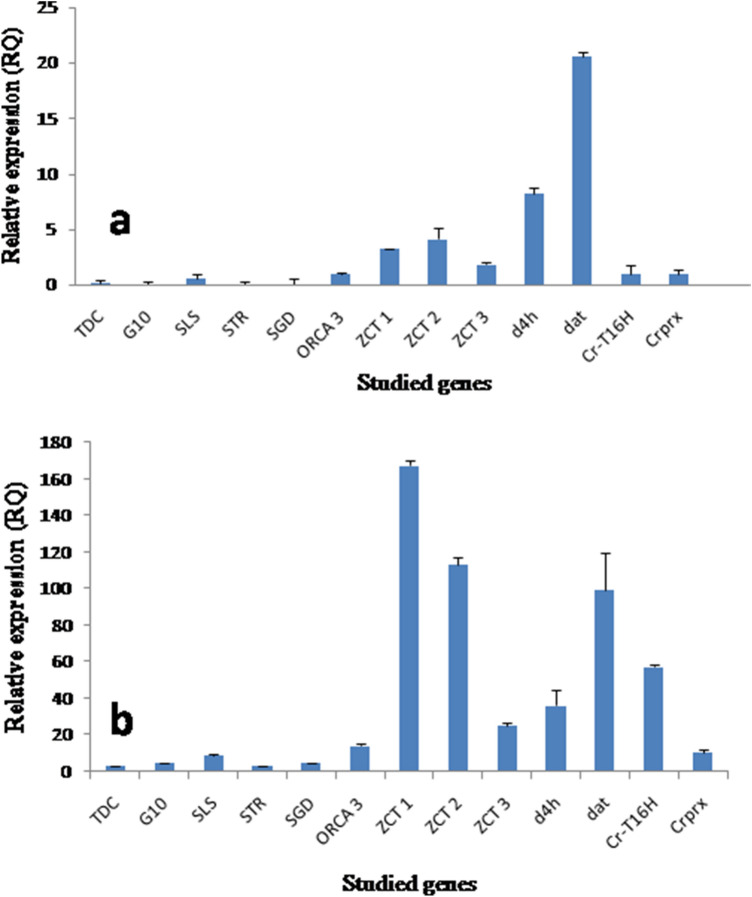
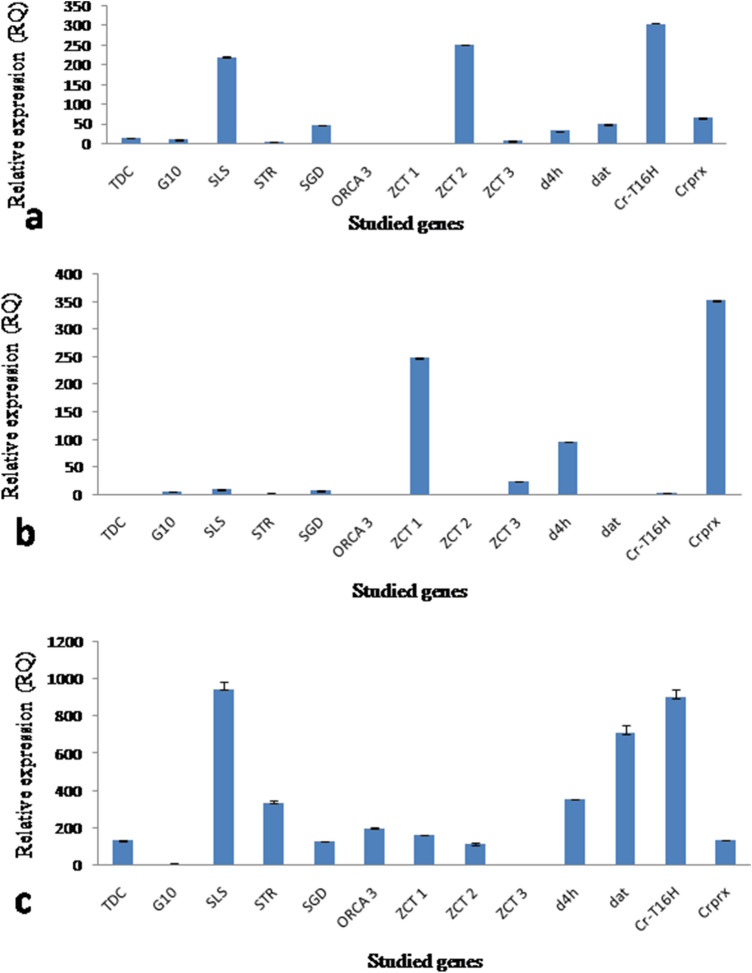
The LC–MS analysis showed the presence of vindoline in hairy root clone p6 (0.0002 ± 0.0001 mg/g dry wt.) and photomixotrophic cell suspensions (0.0004 ± 0.0001 mg/g dry wt.) while catharanthine was reported only in the photomixotrophic cell suspensions (0.002 ± 0.001 mg/g dry wt.) (Fig. 4). The control cell suspensions did not showed the presence of any of the monomeric alkaloid tested. The real time PCR analysis of thirteen candidate genes (Table S2) belonging to the TIAs pathway revealed higher expression in photomixotrophic cell cultures in comparison to the control cell suspensions (Fig. 5). The photomixotrophic cell suspensions showed more than the 5–15-fold increase in d4h, dat, crprx, TDC, SLS, ZCT3 and ORCA3 and the 30–80-fold increase in ZCT2, STR, CrT16H, ZCT1, G10H and SGD gene expression level. Khan et al. (2002) reported the red coloration and folded leaves in the photomixotrophic cultures of E. tereticornis, which is a sign of stress. Similarly, in the present study, to combat stress conditions inferred by photomixotrophic environment, cells started differentiation and developed thick walls. This might have lead to improved catharanthine production and related higher gene expression as stress stimulates alkaloid production. In a similar kind of study in other plant system, Geipal et al. (2014) registered 230% increase in alpha-tocopherol production in the photomixotrophic cell cultures in comparison to heterotrophic cell cultures.
Fig. 4.
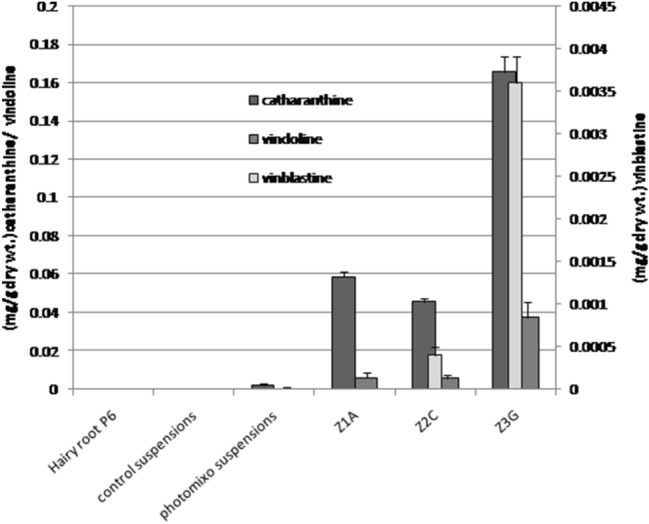
LC–MS analysis of all the tissue types of C. roseus (Z1A, Z2C, and Z3G are ZCTs knockdown photomixotrophic cell suspensions)
Fig. 5.
Real-time PCR analysis of the TIAs genes of C. roseus. a In control cell suspensions, b and photomixotrophic cell suspensions
Lipofectamine based antisense LNA GapmeR transfection into the photomixotrophic cell suspension protoplasts and the generation of ZCTs knockdown transgenic lines
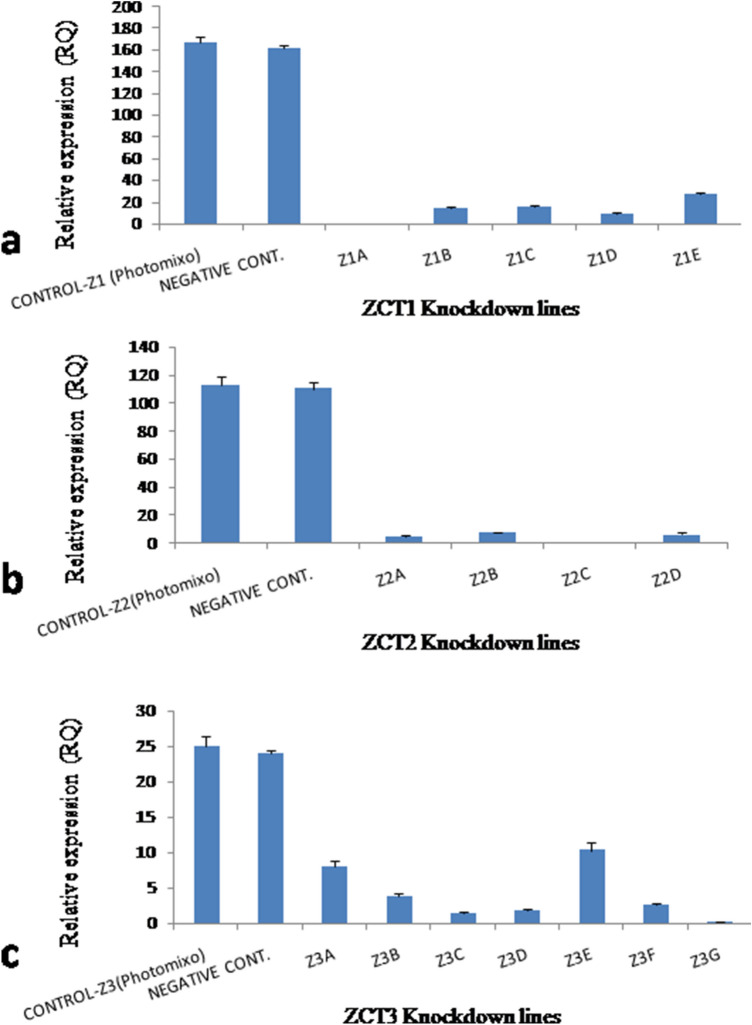
Protoplasts were isolated successfully from the photomixotrophic cell suspensions with a yield of 4 × 104 protoplast/ml (Fig. 6). The lipofactamine transfection associated introduction of antisense LNA GapmeR, which was aimed at knocking down the selected ZCT proteins in suspension cultures of C. roseus showed prominent results. The transfected protoplasts were showing the generation of cell wall within 3 days of transfection followed by division and growth of cell mass within 15 days (Fig. 6). The cell mass obtained was subjected to frequent subculture and further establishment of the cell suspensions. This establishment duration was found to be two to three months to maintain each line separately. In the case of ZCT1 knockdown, three lines namely Z1A, Z1B and Z1C were obtained by transfection with antisense LNA GapmeR ZCT1-1, while Z1D and Z1E were recovered by transfection with antisense LNA GapmeR ZCT1-2. Out of five lines generated, Z1A was showing complete silencing of ZCT1 in comparison to other four lines which showed partial silencing (Fig. 7a). Similarly, ZCT2 knockdown showed the development of two lines each from antisense LNA GapmeR ZCT2-1 (Z2A and Z2B) and antisense LNA GapmeR ZCT2-2 (Z2C and Z2D). Out of these four lines Z2C showed complete silencing of ZCT2 (Fig. 7b). The maximum of seven lines were obtained by ZCT3 knockdown. Three lines (Z3A, Z3B and Z3C) belong to antisense LNA GapmeR ZCT3-1 while four lines (Z3D, Z3E, Z3F and Z3G) belong to antisense LNA GapmeR ZCT3-2. In this case, complete silencing of ZCT3 was observed in Z3G line (Fig. 7c). On the basis of the above results, Z1A, Z2C and Z3G were established as ZCT1, ZCT2 and ZCT3 knockdown lines, respectively, and were further subjected to LC–MS and gene expression studies. Keeping a note of the real-time expression analysis, it was pertinent to check the TIAs content in these knocked down cell lines to show any indication of diversion of metabolite flux towards dimeric alkaloids. Interestingly the selected lines clearly state and confirm the presence of monomeric catharanthine and vindoline (all three lines) and dimeric VLB in the Z2C and Z3G cell lines. Compared to the control photomixotrophic suspension cultures, all three transgenic cell lines namely, Z1A, Z2C and Z3G showed multilevel increments in catharanthine and vindoline content (Fig. 4). The maximum catharanthine content was found in Z3G cell lines (0.165 ± 0.008 mg/g dry wt.) followed by Z1A (0.059 ± 0.003 mg/g dry wt.) and Z2C (0.046 ± 0.002 mg/g dry wt.). The Z3G line also showed maximum vindoline production (0.038 ± 0.001 mg/g dry wt.) which was 90-fold more than the control photomixotrophic suspension cultures and six-fold more than the Z1A and Z2C lines. The Z2C and Z3G lines showed the presence of vinblastine. The Z3G line showed the nine-fold more VLB in comparison to Z2C. It is important to note here that line Z1A did not show any presence of VLB (Fig. 4). The LC–MS analysis results were abiding to the earlier discussed hypothesis, which states that knocking down of certain ZCT genes could play an important role in moving the flux towards monoterpenoid indole alkaloid biosynthetic pathway as ZCT genes were known to be down regulating the transcriptional activities of major enzymes of the MIA pathways i.e. TDC and STR. To further strengthen the role of these knockdown ZCT genes in cell lines, a complete relative analysis of all the major TIAs genes were performed through real-time expression analysis in Z1A, Z2C and Z3G. To the expectations all the three cell lines (where expression of ZCTs were blocked) showed the multiple fold enhancement in the expression of other TIAs genes (Fig. 8).
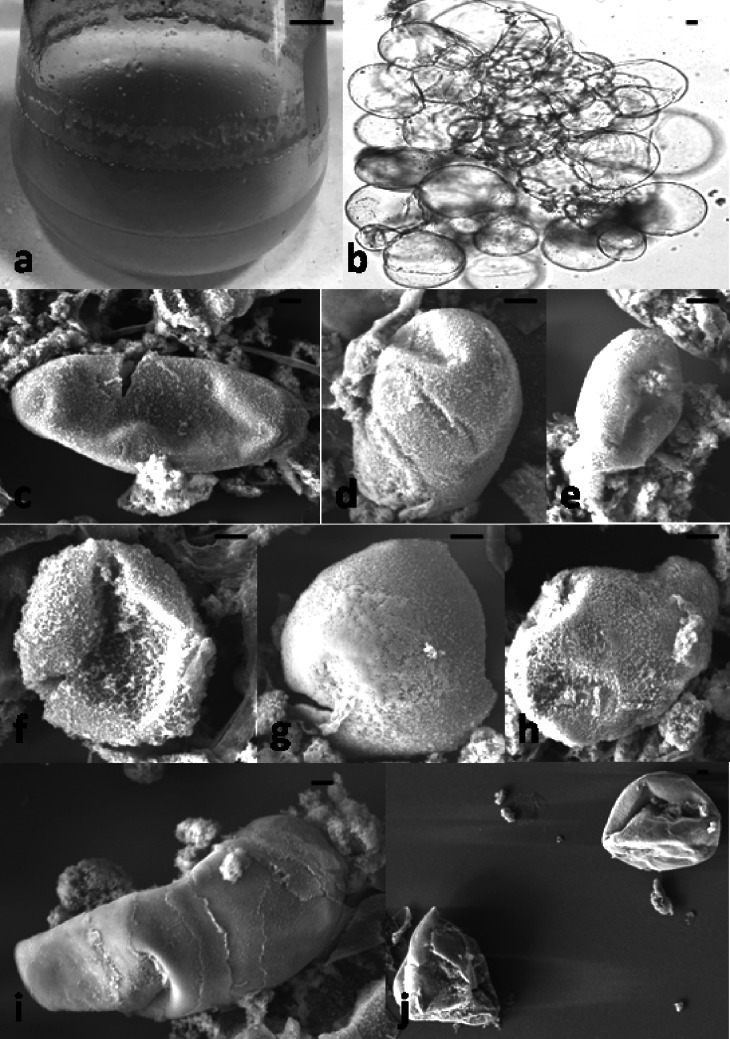
Fig. 6.
Protoplast isolation and transformation in photomixotrophic cell suspensions of C. roseus. a-c protoplast isolated from photomixotrophic cell suspensions; d protoplast after transfection with ZCT3-2 antisense LNA GapmeR; e–h cell wall formation stages and division after transfection; (i) micro calli formation in Z3G transgenic line. Scale bar = 50 µm
Fig. 7.
Real time PCR analysis of a ZCT1; b ZCT2 and c ZCT3 genes in different transgenic lines obtained after transfection with antisense LNA GapmeR in C. roseus photomixotrophic cell suspensions
Fig. 8.
Real time PCR analysis of TIAs genes of C. roseus in established transgenic lines obtained after transfection with antisense LNA GapmeR in C. roseus photomixotrophic cell suspensions. a ZCT1 knockdown line Z1A; b ZCT2 knockdown line Z2C and c ZCT1 knockdown line Z3G
The Z1A line, the expression of ZCT1 was found to be negligible, but due to higher expression of ZCT2, no significant change in STR and TDC expression was observed, while more than the ten-fold increase in SGD, the 20-fold increase in SLS and the five-fold increase in Crprx gene expression was observed that may lead to enhanced catharanthine and vindoline production. Similarly, in the case of Z2C, a significant two-fold increase in d4h and the 30-fold increase in Crprx gene expression lead to enhanced catharanthine, vindoline and vinblastine content. Here, again the higher expression of ZCT1 was observed that might interfere with the expression of TDC and STR and in turn other TIAs genes. The gene expression studies of Z3G was found to be interesting as in this case another transcription factor ORCA3 associated with C. roseus that is known to enhance TIAs production was observed. It showed the 20-fold increase in comparison to control photomixotrophic cell suspensions while it was not expressed in Z1A and Z2C. The Z3G line showed the significant multi-fold enhancement in expression of TDC, SLS, STR, SGD, d4h, dat, CrT16H and Crprx that might lead to the higher production of catharanthine, vindoline and vinblastine. The over-expression of ORCA3 genes and its effect on the suspension lines was previously performed by van-der Fits and Memelink (2000) in which genes such as TDC, STR, SLS, CPR, AS, DXS and D4H were found to be up- regulated. In addition, in this particular study it was concluded that DAT genes were unaffected in their expression by ORCA gene overexpression and SGD gene expression was also enhanced. In addition to this discussion, a similar study by Peeble et al. (2009) have revealed that overexpression of ORCA3 have also resulted in enhancement of the transcript numbers of ZCT1 and ZCT2 in the given samples and it was assumed that transcriptional repressors may have attributed to a counter-response which could have led to an increase in the transcript of the TIA pathway-related genes.
In the present study, the ZCT3 knockdown led to enhanced ORCA3 expression in Z3G line. The results obtained here are coherent with the previous reports, which suggest that this may be one of the reasons for the multifold increase in the TIAs production in Z3G line. A study by Peebles et al. (2009) reveals that the samples overexpressing ORCA3 showed the increase in the transcripts of ZCT1 and ZCT2. This counter-response of transcriptional repressors was said to be responsible for the transcripts of TIA pathway genes. But in the present study, ORCA3 expression was enhanced due to silencing of ZCT3; therefore, an increase in other TIAs gene expression was observed.
Through this photomixotrophic cell culture targeted ZCTs knockdown study, a major breakthrough can be achieved as vinblastine was absent in previously reported heterotrophic cell cultures. The presence of vindoline in the knockdown cell lines could be a promising aspect of this study as vindoline is responsible for dimerization with catharanthine which further results in the synthesis of vinblastine. This is quite evident in the present study as once the ZCT genes showed lower expression, the other related genes were amplified and led to the enhanced production of the TIAs.
C. roseus is under global attention for applying metabolic engineering efforts to enhance the production of its anti-neoplastic tertiary indole alkaloids vincristine and vinblastine. The callus and cell suspension cultures of this important medicinal herb have been found to lack the capacity to produce these dimeric alkaloids due to the absence of one of their monomeric moiety vindoline that combines with another monomeric moiety catharanthine through a peroxidation reaction in the leaves in planta. Studies carried out so far have indicated the absence of the expression of a key enzyme N-methyl transferase responsible for vindoline synthesis, in the undifferentiated heterotrophic cultured cell as they lack functional chloroplasts. The present study was carried out to explore the possibility of inducing photomixotrophy in the cell suspension cultures with functional chloroplasts and advancing the feasibility of vindoline synthesis in them to extend the TIA pathway up to the dimerization step.
Photomixorophy is a suitable method of micro propagation in which photosynthesis and inorganic nutrient uptake is utilized in partial amount with minimal sugar use in culture media for growth of the cells and carbohydrate accumulation.
Cells growing in conventional tissue culture systems usually encounter physiological and anatomical abnormalities including the inability of photosynthesis, low chlorophyll content, parenchymatous growth etc. (Xiao et al. 2011). Photomixotrophic systems could reduce these problems. It is a nutritional system, where cells use both endogenous and exogenous carbohydrates as the energy source. In the present study photomixotrophy leads to the generation of thick-walled specialized cells which has enhanced TIAs accumulating ability along with higher associated gene expression levels. Photomixotrophic cell suspensions in the present study proved ideal starting tissue for ZCTs knocking down in C. roseus.
There are plethora of evidences, which have revealed that biosynthesis of the TIAs is governed by transcriptional factors that in turn works on the structural genes in the given plant system i.e. C. roseus. Successful characterization of many of the transcriptional factors has been achieved in C. roseus with the identification of their regulatory response. More importantly many of these characterized transcriptional factors have been applied and assessed for their role in TIA biosynthesis (Liu et al. 2017). Three of the Cys2/ His 2–type zinc finger transcription factors namely ZCT1, ZCT2 and ZCT3 were shown to have abilities to repress the STR and TDC promoter genes when they were isolated by a yeast one- hybrid system. This repression of STR and TDC genes was attributed to the presence of LxLxL motif, which is a known repression domain located in the C-terminal region of ZCTs. It is noteworthy to mention here that yeast extract and methyl jasmonate were also reported to play an important role in inducing transcriptional activating activation of ORCAs. It is pertinent to address that ZCTs have different structural differences as well as they have different STR and TDC promoter binding sites as well. Pauw et al. (2004) concluded in their study ZCT1 and ZCT2 have more common approach towards STR and TDC promoter binding sites but in the case of ZCT3 the sites were different. Apart from structural and binding to STR and TDC promoters differences, ZCTs have functional differences also as was mentioned by Rizvi et al. (2016) which states that ZCT3 cause no repression of Hydroxymethylbutenyl-4-diphosphate synthase (HDS) gene but ZCT1 and ZCT2 are known to be the repressor of this particular gene. In the same case study, it was proposed that though ZCTs may have a close association with each other but functionally these genes have a distinct effect on TIA biosynthesis. A study by Chebbi et al. (2014) showed that ZCT1 and ZCT2 but not ZCT3 repress the HDS promoter activity. Elaborating this point, it was revealed that no single ZCT may have the sufficient role in the TIA biosynthesis as it was found that even the silencing of ZCT1 could not result in the TIA biosynthesis increment. Furthermore, it was supported by the role of the remaining ZCT3 gene, whose possible enhanced expression could have compromised the silencing effect of ZCT1 gene.
Conclusion
The present study constitutes the first report of the establishment of the photomixotrophic C. roseus cell cultures followed by successful silencing of the transcription repressor genes ZCT1, ZCT2 and ZCT3 using protoplast- GapmeR transformation technology. The ZCT1 (Z1A) and ZCT2 (Z2C) silenced lines were showing enhancement of the TIAs and associated gene expression up to a limit due to elevated expression levels of the ZCT3. However, the ZCT3 silenced (Z3G) line where ZCT3 expression level was found to be nil, showed the multifold increase in the TIAs production independent to the expression levels of ZCT1 and ZCT2. According to the present studies, ZCT3 silencing is most crucial among all the three to enhance the TIAs production in C. roseus. The successful silencing of transcription repressors genes was highly desirable in C. roseus plant system and this is the first report of utilization of the photomixotrophic cell cultures of C. roseus used for GapmeR based silencing of transcription factor. The present work is a step towards providing a better understanding of the ZCT transcription factor and will lead to the increment in efforts to enhance terpenoid indole alkaloids production by rerouting the flux.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The work presented here has been supported by DST-FAST TRACK YSS/2015/001417. Grateful appreciation also goes to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the financial support in the form of CSIR-SRA (Pool Scientist) at CSIR-NCL to the senior author. Valuable inputs given by Prof. S. Gantait for manuscript improvement have been highly acknowledged.
Author contribution
PV and SAK conceptualized the problem and carried out experiments. VP assisted in interpretation part. AKM has given the concept of introducing photoautotrophy in C. roseus cell cultures for experimentation. Manuscript has been written by PV, SAK and VP.
Declarations
Conflict of interests
Author declares that there is no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Priyanka Verma, Email: priyankaurbest@gmail.com.
Shamshad Ahmad Khan, Email: shamshadkhanptc@gmail.com.
Varsha Parasharami, Email: varshaparasharami@gmail.com.
Ajay Kumar Mathur, Email: akmcath@gmail.com.
References
- Bernonville TD, Maury S, Delaunay A, Daviaud C, Chaparro C, Tost J, O'Connor SE, Courdavault V. Developmental methylome of the medicinal plant Catharanthus roseus unravels the tissue-specific control of the monoterpene indole alkaloid pathway by DNA methylation. International J Mol Sci. 2020;21:6028. doi: 10.3390/ijms21176028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseau S, Kellner F, Lanoue A, Thamm AM, Salim V, Schneider B, Geu-Flores F, Höfer R, Guirimand G, Guihur A, Oudin A, Glevarec G, Foureau E, Papon N, Clastre M, Giglioli-Guivarc'h N, St-Pierre B, Werck-Reichhart D, Burlat V, De Luca V, O'Connor SE, Courdavault V. A pair of tabersonine 16-hydroxylases initiates the synthesis of vindoline in an organ-dependent manner in Catharanthus roseus. Plant Physiol. 2013;163:1792–1803. doi: 10.1104/pp.113.222828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris KP, Dlugosz EM, Collins AG, Stewart N Jr, Lenaghan SC (2016) Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L) Plant Cell Rep 35: 693–704 [DOI] [PMC free article] [PubMed]
- Chebbi M, Ginis O, Courdavault V, Glevarec G, Lanoue A, Clastre M, Papon N, Gaillard C, Atanassova R, St-Pierre B, et al. ZCT1 and ZCT2 transcription factors repress the activity of a gene promoter from the methyl erythritol phosphate pathway in Madagascar periwinkle cells. J Plant Physiol. 2014;171:1510–1513. doi: 10.1016/j.jplph.2014.07.004. [DOI] [PubMed] [Google Scholar]
- De Luca V, Cutler AJ. Subcellular localization of enzymes involved in indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 1987;85:1099–1102. doi: 10.1104/pp.85.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Balsevich J, Tyler RT, Kurz WGW. Characterisation of a novel N-methyl transferase (NMT) from Catharanthus roseus plants: Detection of NMT and other enzymes of indole alkaloid biosynthetic pathway in different cell suspension culture systems. Plant Cell Rep. 1987;6:458–461. doi: 10.1007/BF00272782. [DOI] [PubMed] [Google Scholar]
- El-Sayed M, Verpoorte R. Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev. 2007;6:277–305. doi: 10.1007/s11101-006-9047-8. [DOI] [Google Scholar]
- Emara HA, Nower AA, Hamza EM, Shaib ELF. Evaluation of photomixotrophic technique and several carbohydrate sources as affecting banana micropropagation. Int J Curr Microbiol App Sci. 2018;7:788–804. doi: 10.20546/ijcmas.2018.710.088. [DOI] [Google Scholar]
- Facchini PJ. Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Mol Biol. 2001;52:29–66. doi: 10.1146/annurev.arplant.52.1.29. [DOI] [PubMed] [Google Scholar]
- Facchini PJ, De Luca V. Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants. Plant. 2008;J54:763–784. doi: 10.1111/j.1365-313X.2008.03438.x. [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soyabean root cells. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Geipel K, Song X, Socher ML, et al. Induction of a photomixotrophic plant cell culture of Helianthus annuus and optimization of culture conditions for improved α-tocopherol production. Appl Microbiol Biotechnol. 2014;98:2029–2040. doi: 10.1007/s00253-013-5431-7. [DOI] [PubMed] [Google Scholar]
- Hooykaas PJJ, Klapwijk PM, Nuti MP, Schilperoort RA, Rorsch A. Transfer of the Agrobacterium tumefaciens Ti-plasmid to avirulent Agrobacteria and to Rhizobium explanta. J Gen Microbiol. 1977;98:477–482. doi: 10.1099/00221287-98-2-477. [DOI] [Google Scholar]
- Kao KN, Michayluk MR. Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta. 1975;126:105–110. doi: 10.1007/BF00380613. [DOI] [PubMed] [Google Scholar]
- Kellner F, Geu-Flores F, Sherden NH, Brown S, Foureau E, Courdavault V, O’Connor SE. Discovery of a P450-catalyzed step in vindoline biosynthesis: a link between the aspidosperma and eburnamine alkaloids. Chem Commun (camb) 2015;51:7626–7628. doi: 10.1039/C5CC01309G. [DOI] [PubMed] [Google Scholar]
- Khan PSSV, Kozai T, Nguyen QT, Kubota C, Dhawan V. Growth and net photosynthetic rates of Eucalyptus tereticornis. Smith under photomixotrophic and various photoautotrophic micropropagation conditions. Plant Cell Tiss Org Cult. 2002;71:141–146. doi: 10.1023/A:1019935208418. [DOI] [Google Scholar]
- Kozai T. Photoautotrophic micropropagation-environmental control for promoting photosynthesis. Propag Ornam Plants. 2010;10:188–204. [Google Scholar]
- Levac D, Murata J, Kim WS, De Luca V. Application of carborundum abrasion for investigating leaf epidermis: molecular cloning of Catharanthus roseus 16 -hydroxy- tabersonine-16-O- methyltransferase. Plant J. 2008;53:225–323. doi: 10.1111/j.1365-313X.2007.03337.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Cai J, Wang R, Ynag S. Transcriptional regulation and transport of terpenoid indole alkaloid in Catharanthus roseus: exploration of new research directions. Int J Mol Sci. 2017;18:53. doi: 10.3390/ijms18010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memelink J, Gantet P. Transcription factors involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Phytochem Rev. 2007;6:353–362. doi: 10.1007/s11101-006-9051-z. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Pauw B, Hilliou FA, Martin VS, Chatel G, de Wolf CJ, Champion A, Pré M, van Duijn B, Kijne JW, van der Fits L, et al. Zinc finger proteins act as transcriptional repressors of alkaloid biosynthesis genes in Catharanthus roseus. J Biol Chem. 2004;279:52940–52948. doi: 10.1074/jbc.M404391200. [DOI] [PubMed] [Google Scholar]
- Peebles CA, Hughes EH, Shanks JV, San KY. Transcriptional response of the terpenoid indole alkaloid pathway to the overexpression of ORCA3 along with jasmonic acid elicitation of Catharanthus roseus hairy roots over time. Metabolic Eng. 2009;11:76–86. doi: 10.1016/j.ymben.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Perez LP, Montesinos YP, Olmedo JG, Sanchez RR, Montenegro ON, Rodriguez RB, Ribalta OH, Escriba RCR, Daniels D, Gomez-Kosky R. Effects of different culture conditions (photoautotrophic, photomixotrophic) and the auxin indole-butyric acid on the in vitro acclimatization of papaya (Carica papaya L. var. Red Maradol) plants using zeolite as support. African J Biotech. 2015;14:2622–2635. doi: 10.5897/AJB2015.14814. [DOI] [Google Scholar]
- Pham HNT, Vuong QV, Bowyer MC, Scarlett CJ. Phytochemicals derived from Catharanthus roseus and their health benefits. Technologies. 2020;8:80. doi: 10.3390/technologies8040080. [DOI] [Google Scholar]
- Qu Y, Easson MLAE, Froese J, Simionescu R, Hudlicky T, De Luca V. Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc Natl Acad Sci USA. 2015;112:6224–6229. doi: 10.1073/pnas.1501821112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NF, Weaver JD, Cram EJ, Lee-Parsons CW. Silencing the transcriptional repressor, ZCT1, illustrates the tight regulation of terpenoid indole alkaloid biosynthesis in Catharanthus roseus hairy roots. PLoSONE. 2016 doi: 10.1371/journal.pone.0159712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre B, Laflamme P, Alarco AM, LucaV De. The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J. 1998;14:703–713. doi: 10.1046/j.1365-313x.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- St-Pierre B, Vazquez-Flota FA, De Luca V. Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell. 1999;11:887–900. doi: 10.1105/tpc.11.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Memelink J. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science. 2000;289:295–297. doi: 10.1126/science.289.5477.295. [DOI] [PubMed] [Google Scholar]
- Vazquez-Flota F, De Carolis E, Alarco A-M, De Luca V. Molecular cloning and characterization of desacetoxyvindoline 4-hydroxylase, a 2-oxoglutarate dependent dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don Plant Mol Biol. 1997;34:935–948. doi: 10.1023/A:1005894001516. [DOI] [PubMed] [Google Scholar]
- Verma P, Mathur AK, Srivastava A, Mathur A. Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloids pathway in Catharanthus roseus: A literature up-date. Protoplasma. 2012;249:255–268. doi: 10.1007/s00709-011-0291-4. [DOI] [PubMed] [Google Scholar]
- Verma P, Khan SA, Mathur AK, Ghosh S, Shanker K, Kalra A. Improved sanguinarine production via biotic and abiotic elicitations and precursor feeding in cell suspensions of latex-less variety of Papaver somniferum with their gene expression studies and upscaling in bioreactor. Protoplasma. 2014;251:1359–1371. doi: 10.1007/s00709-014-0638-8. [DOI] [PubMed] [Google Scholar]
- Verma P, Mathur AK, Khan SA, Verma N, Sharma A. Transgenic studies for modulating terpenoid indole alkaloids pathway in Catharanthus roseus: present status and future options. Phytochem Rev. 2015;16:19–54. doi: 10.1007/s11101-015-9447-8. [DOI] [Google Scholar]
- Verma P, Khan SA, Parasharami VA, Mathur AK. Biotechnological interventions to modulate terpenoidindole alkaloid pathway in Catharanthus roseus using in vitro tools and approaches. In Catharanthus roseus: Current Research and Future Prospects. In: Naeem M, Aftab T, Khan M, editors. Catharanthus roseus. Champp: Springer; 2017. pp. 247–275. [Google Scholar]
- Verma P, Khan SA, Masood N, Manika N, Sharma A, Verma N, Mathur AK. Differential rubisco content and photosynthetic efficiency of rol gene integrated Vinca minor transgenic plant: Correlating factors associated with morpho-anatomical changes, gene expression and alkaloid productivity. J Plant Physiol. 2017;219:12–21. doi: 10.1016/j.jplph.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Niu G, Kozai T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult. 2011;105:149–158. doi: 10.1007/s11240-010-9863-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.