Abstract
Propionibacterium acnes is a skin commensal bacterium that contributes to the development of acne vulgaris and other infections. Recent work revealed that P. acnes clinical isolates can be classified into distinct phylotypes, several of which have associations with healthy skin or acne. We sought to determine if these phylotypes induce different immunological responses and express protein factors that may contribute to their disease associations. We found that acne-associated P. acnes phylotypes induced 2- to 3-fold higher levels of IFN-γ and IL-17 in peripheral blood mononuclear cells compared with healthy phylotypes. On the other hand, P. acnes phylotypes associated with healthy skin induced 2- to 4-fold higher levels of IL-10. Comparative proteomic analysis of P. acnes phylotypes revealed a differential expression of several proteins, including an adhesion protein that was expressed at least 10-fold higher in acne-associated phylotypes and a cell surface hydrolase expressed in all phylotypes except those associated with healthy skin. Taken together, our data provide insight into how specific P. acnes phylotypes influence immune responses and the pathogenesis of acne.
INTRODUCTION
Acne vulgaris is a highly prevalent skin disease, affecting all ethnic groups with rates of up to 85% among 12- to 24-year-olds (Bhate and Williams, 2013). The gram-positive, anaerobic species Propionibacterium acnes is a commensal skin organism (Grice et al., 2009) traditionally implicated in the development of acne vulgaris (Beylot et al., 2014). Recently, typing of P. acnes has revealed associations of particular strains with different diseases (Yu et al., 2015a). Certain bacterial strains as identified by multilocus sequence typing were found to be associated with acne, such as type IA and IC strains (Lomholt and Kilian, 2010; McDowell et al., 2011, 2012, 2013). P. acnes types were further investigated using 16S ribotyping in conjunction with full genome sequencing (Fitz-Gibbon et al., 2013). Specifically, phylotype IB-1 was associated with acne, as were the ribotype 4 and 5 subgroups of phylotype IA-2. These acne-associated types were found in significant quantity in 30–40% of patients with acne, but rarely in individuals with healthy skin. The ribotype 1 subgroup of phylotype IA-2, together with phylotypes IA-1, IB-2, and IB-3, were found to be evenly distributed in patients with acne and individuals with healthy skin. Of note, the phylotype II, ribotype 6 (II-RT6) subgroup was found to be 99% associated with healthy skin. Additionally, P. acnes isolates belonging to phylotype III were not found in acne lesions, but composed approximately 20% of isolates from healthy skin (McDowell et al., 2013).
Studies of the ability of the different P. acnes phylotypes to trigger specific immune responses have focused on innate immunity. It appears that type II P. acnes induced higher levels of IL-8 in keratinocytes, and type IA induced higher involucrin (Nagy et al., 2005). Type IA and IB P. acnes induced a greater b-defensin response in sebocytes than type II (Nagy et al., 2006). Lysates from P. acnes may also have different effects in human skin explants (Jasson et al., 2013), and type I P. acnes were more readily endocytosed than type II (Furukawa et al., 2009). However, these studies investigated a limited number of phylotypes using less well-characterized strains and did not investigate the adaptive immune response. Although it is known that P. acnes induces T helper type 1 (Th1) (Mouser et al., 2003) and Th17 (Agak et al., 2014) responses, it is unclear whether these T-cell cytokines are differentially induced by P. acnes phylotypes. Here, we investigated whether the major P. acnes phylotypes induced different T-cell cytokine profiles to better understand their disease associations.
In addition to their modulation of the immune response, certain P. acnes strains may be associated with particular diseases because of differential expression of protein virulence factors. Only one comparative study, which did not involve the strains that were most strongly acne-associated, has examined the proteome of P. acnes (Holland et al., 2010), and only secreted proteins were assessed. Our previous study also examined the total and surface proteomes (Yu et al., 2015b), but only covered one strain of P. acnes. Continuing this work, we comprehensively investigated the proteome from several phylotypes of P. acnes to identify virulence factors that may be related to their different disease associations.
RESULTS
Growth phase of P. acnes affects host immune response
Several clinical isolates of P. acnes were selected (Supplementary Table S1 online) as representative of each of the identified phylotypes (Tomida et al., 2013). Also included were clinical isolates from phylotype II-RT6 due to its healthy skin association, and examples of phylotype IA-2 with (p+) and without (p−) a large plasmid associated with acne (Fitz-Gibbon et al., 2013). We assigned each phylotype group an acne association (“acne,” “healthy,” or “neutral,” with “neutral” referring to strains evenly distributed in both patients with acne and healthy individuals) based on the analysis of previous work (Fitz-Gibbon et al., 2013; McDowell et al., 2013).
All strains were harvested in both the exponential and stationary growth phases and used to stimulate peripheral blood mononuclear cells (PBMCs) isolated from healthy adult human donors. Exponential phase P. acnes induced 3- to 10-fold greater IFN-γ secretion across all phylotypes (Figure 1a), whereas stationary phase P. acnes induced 2- to 4-fold greater IL-17 secretion (Figure 1b). IL-10 secretion was consistently higher in the stationary phase only for cells treated with phylotype II (including RT 6 strains) and phylotype III (Figure 1c).
Figure 1. Exponential phase Propionibacterium acnes induces a greater Th1 response and stationary phase P. acnes induces a greater Th17 response.
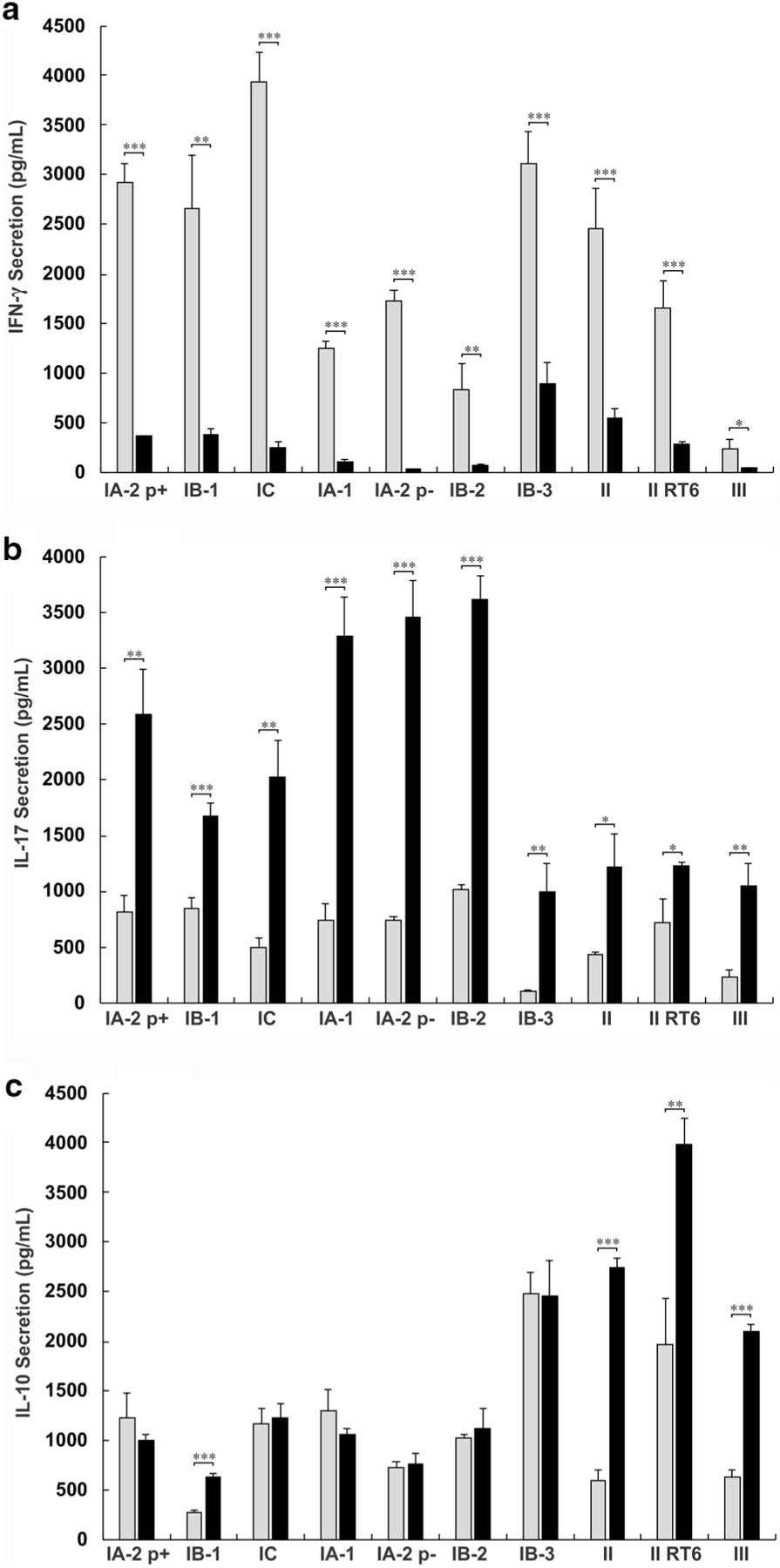
A total of 2.5 × 106/ml peripheral blood mononuclear cells were cultured with 2.5 × 106/ml live P. acnes. ELISA was conducted on the supernatant to determine the concentration of IFN-γ, IL-17, and IL-10. The level of cytokine secretion for exponential and stationary phase P. acnes is plotted for (a) IFN-γ, (b) IL-17, and (c) IL-10. Data are from one of five similar experiments. Statistics show t-test comparisons between exponential and stationary phase values. *P < 0.05, **P < 0.01, ***P < 0.001. p±, presence or absence of a large plasmid; RT, ribotype; Th1, T helper type 1; Th17, T helper type 17.
Distinct cytokine patterns are induced by different P. acnes phylotypes
P. acnes isolates of the phylotypes associated with acne (IA-2 p+, IB-1, and IC) and a subset of neutral phylotypes (IB-3 and II) induced 2- to 10-fold higher levels of IFN-γ in PBMCs compared with phylotypes associated with healthy skin (II-RT6 and III) and a second subset of neutral phylotypes (IA-1, IA-2 p−, and IB-2) (Figure 2). This second subset of neutral phylotypes, in contrast, induced nearly 2-fold higher IL-17 than acne-associated phylotypes, which in turn induced approximately 2-fold higher IL-17 than other phylotypes. Healthy skin-associated phylotypes together with the first subset of neutral phylotypes induced 2- to 4-fold more IL-10 than acne-associated phylotypes and the second subset of neutral phylotypes.
Figure 2. Phylotypes of Propionibacterium acnes induce distinct cytokine immune response patterns.
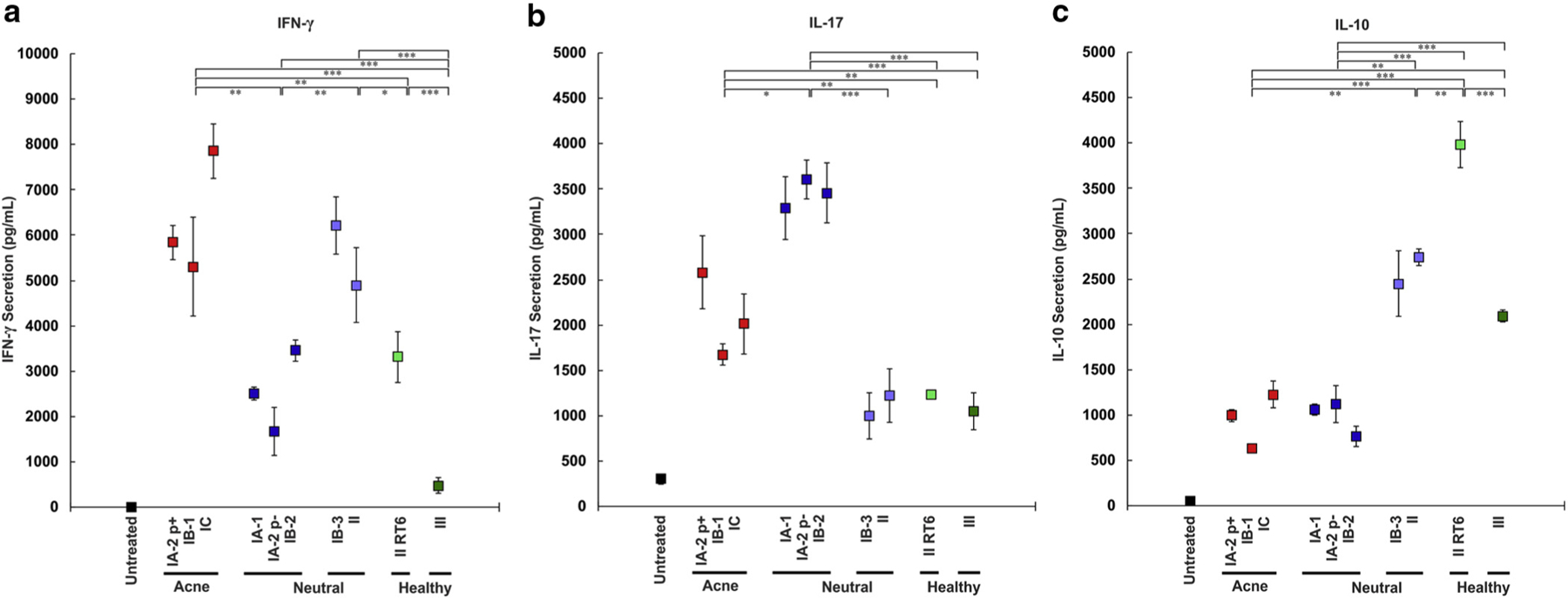
A total of 2.5 × 106/ml peripheral blood mononuclear cells were cultured with 2.5 × 106/ml live P. acnes in the exponential phase for (a) IFN-γ or the stationary phase for (b) IL-17 and (c) IL-10. ELISA was conducted on the supernatant to determine the concentration of IFN-γ, IL-17, and IL-10. Data are from one of five similar experiments. Statistics show t-test comparisons with the maximum two-way P value between the phylotype groups indicated. *P < 0.05, **P < 0.01, ***P < 0.001. p±, presence or absence of a large plasmid; RT, ribotype.
Cytokine induction patterns are similar across multiple donors
Because variability in the host immune response is an important component of acne pathogenesis, we assessed whether the patterns induced by the different P. acnes phylotypes were similar in multiple donors. Although the PBMCs from the five different donors exhibited high variability in the magnitude of their response to P. acnes (Supplementary Table S2 online), the relative response patterns to different phylotypes remained largely similar (Figure 3). Acne-associated phylotypes IA-2 p+, IB-1, and IC induced higher levels of IFN-γ and IL-17, but lower IL-10 across all donors. Healthy skin-associated phylotype II-RT6 and phylotype III induced lower IFN-γ and IL-17, but comparatively higher IL-10. Neutral phylotypes IB-3 and II induced lower IL-17 and higher IL-10 than the acne-associated strains. Neutral phylotypes IA-1, IA-2 p−, and IB-2 induced lower IFN-γ and higher IL-17 than the acne-associated strains in all donors. The magnitude of cytokine induction and difference between phylotypes was greatest in the exponential phase for IFN-γ and in the stationary phase for IL-17 and usually IL-10 (Supplementary Figure S1 online). Immunological cluster analysis indicates that the acne-associated phylotypes clustered together and the neutral phylotypes formed two distinct groups (Figure 4).
Figure 3. Cytokine induction patterns are similar across multiple donors.
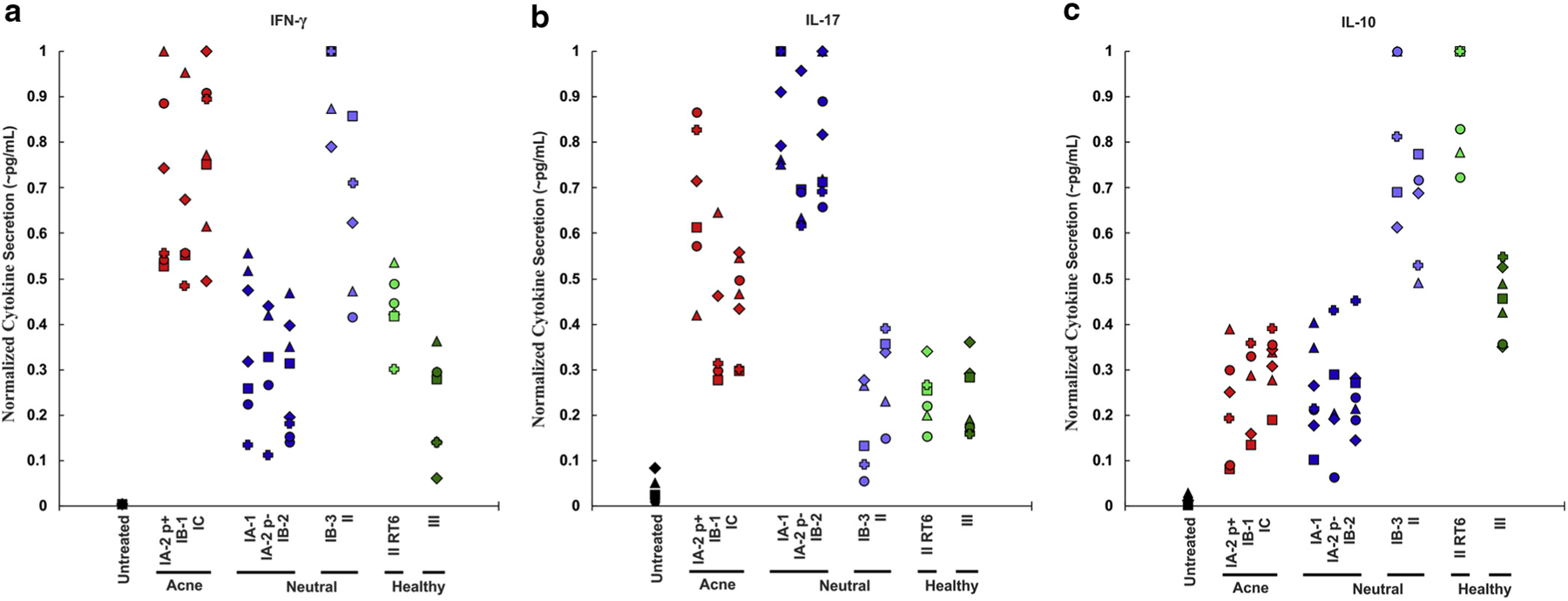
A total of 2.5 × 106/ml peripheral blood mononuclear cells were cultured with 2.5 × 106/ml live Propionibacterium acnes in the exponential phase for (a) IFN-γ or the stationary phase for (b) IL-17 and (c) IL-10. ELISA was conducted on the supernatant to determine the concentration of IFN-γ, IL-17, and IL-10. The cytokine concentrations normalized to the highest value induced by any P. acnes strain for each donor and cytokine. Each donor is represented by a different symbol. p±, presence or absence of a large plasmid; RT, ribotype.
Figure 4. Immunological clustering of phylotypes.
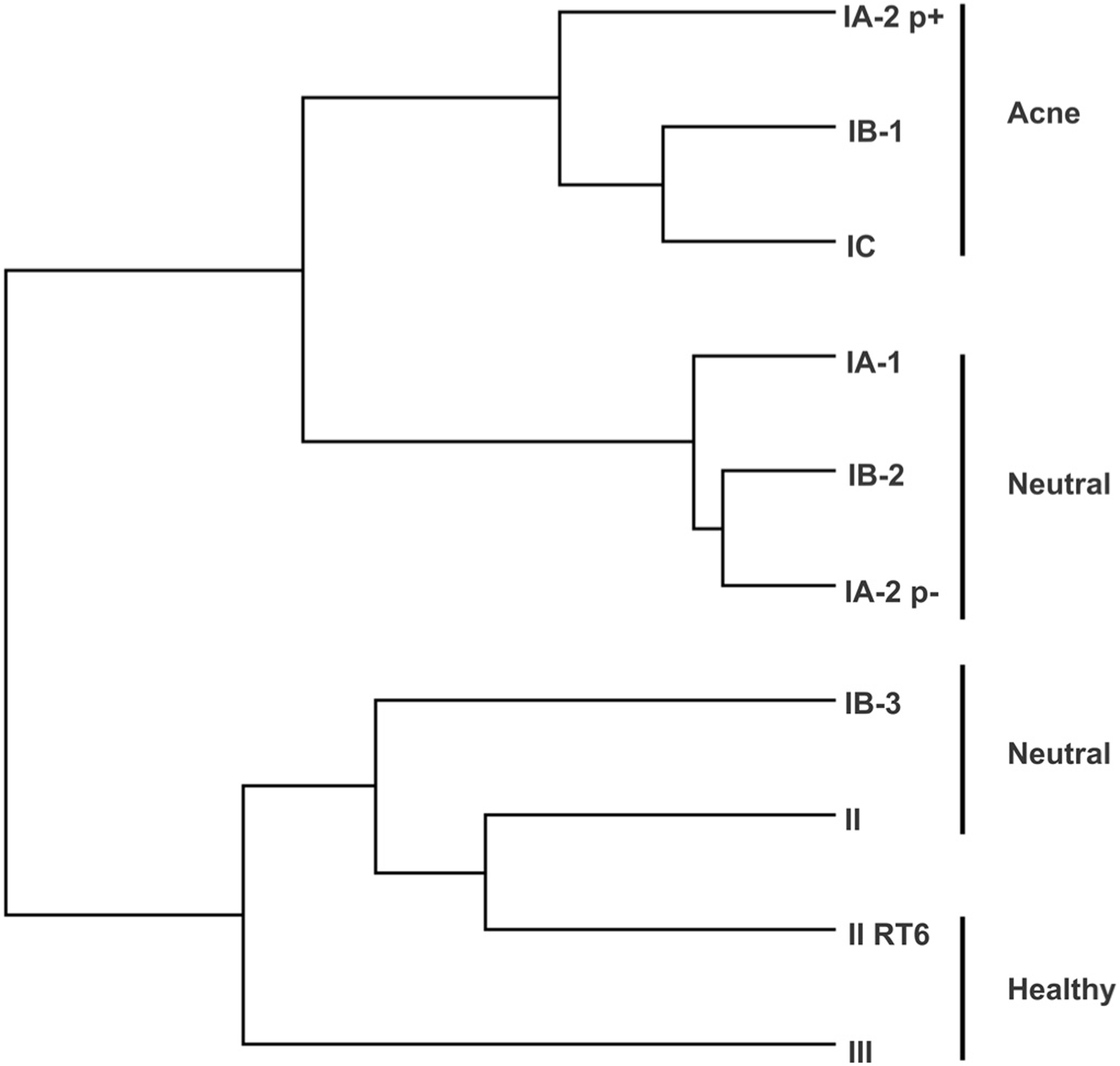
Propionibacterium acnes phylotypes were clustered with an average-distance city-block method using ELISA results for IFN-γ, IL-17, and IL-10. p±, presence or absence of a large plasmid; RT, ribotype.
Different P. acnes phylotypes have distinct proteomes
Because P. acnes strains may be associated with particular diseases due to variable expression of protein virulence factors, we investigated the proteomes of a selection of acne-associated (IA-2 p+ and IB-1), neutral (IA-1 and IB-2), and healthy skin-associated (II-RT6 and III) phylotypes. In total, 756 proteins were identified and quantified (Supplementary Table S3 online), representing approximately 30% of the total number of predicted proteins in the P. acnes genome. The cell secretion fractions together contained 168 identified proteins, and the cell wall fractions contained 35 proteins. Approximately half in each group had predicted non-cytoplasmic localization, which possessed predicted functions of lipid, carbohydrate, or protein digestion, and many of them had unknown function (Table 1). Each of the cytosolic and total cell extract fractions contained several hundred proteins. Many proteins, including several in the cell secretion and cell wall fractions, had markedly different levels of expression between phylotypes. These included one adhesion (50843565) that was 10-fold more abundant in the cell wall only in acne-associated phylotypes IA-2 p+ and IB-1 and in neutral phylotypes IA-1 and IB-2. Another adhesion (50842581) was detected only in healthy skin-associated phylotypes II-RT6 and III. Two Christie-Atkins-Munch-Peterson factors (CAMP) (50842175, 50842820) were secreted by all phylotypes, but another two (50842711, 50843546) were only identified to be secreted by phylotypes II-RT6 and III. These latter two phylotypes did not secrete a hydrolase (50843410) that was highly expressed in others. Other proteins, including several of unknown function, also had different expression patterns between phylotypes.
Table 1.
Selected surface and secreted proteins identified by mass spectrometry
| Protein name | Accession (gi) | MW (kDa) | Secreted protein quantity (fmol/µg total protein) |
Cell wall protein quantity (fmol/µg total protein) |
Protein function | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA-1 | IA-2p+ | IB-1 | IB-2 | II RT6 | III | IA-1 | IA-2p+ | IB-1 | IB-2 | II RT6 | III | ||||
| Adhesion | 50842581 | 48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1,777 | 8,963 | Adhesion |
| Adhesion | 50843565 | 42 | 627 | 33 | 0 | 0 | 0 | 0 | 3,408 | 4,607 | 2,257 | 5,489 | 205 | 0 | Adhesion |
| Adhesion | 50843645 | 48 | 400 | 184 | 0 | 0 | 0 | 0 | 1,712 | 0 | 0 | 5,958 | 0 | 0 | Adhesion |
| CAMP factor | 50842175 | 29 | 1,092 | 405 | 2,396 | 2,493 | 545 | 3,575 | 127 | 47 | 316 | 125 | 397 | 51 | Digestion |
| CAMP factor | 50842711 | 28 | 0 | 0 | 0 | 0 | 494 | 1,145 | 0 | 0 | 0 | 0 | 0 | 0 | Digestion |
| CAMP factor | 50842820 | 30 | 541 | 1,071 | 9,958 | 2,276 | 3,278 | 2,855 | 0 | 0 | 0 | 1,505 | 3,508 | 0 | Digestion |
| CAMP factor | 50843546 | 29 | 0 | 0 | 0 | 0 | 268 | 567 | 0 | 0 | 0 | 0 | 0 | 0 | Digestion |
| Cell wall hydrolase | 50843410 | 43 | 714 | 1,680 | 536 | 174 | 0 | 0 | 898 | 1,609 | 265 | 67 | 0 | 0 | Digestion |
| Endoglycoceramidase | 50842131 | 57 | 887 | 1,224 | 460 | 2,209 | 140 | 1,954 | 841 | 357 | 2,599 | 318 | 357 | 491 | Digestion |
| Iron transport lipoprotein | 50841911 | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 237 | 138 | 456 | 305 | 0 | 1,098 | Transporter |
| Lipase/acylhydrolase | 50843480 | 30 | 1,059 | 102 | 135 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Digestion |
| Lysozyme M1 | 50843125 | 32 | 396 | 165 | 0 | 0 | 105 | 748 | 0 | 0 | 0 | 0 | 0 | 0 | Cell wall |
| NPL/P60 protein | 50842209 | 41 | 567 | 1,216 | 3,034 | 1,511 | 2,782 | 879 | 0 | 0 | 0 | 0 | 0 | 0 | Digestion |
| Peptide ABC transporter | 50843590 | 61 | 313 | 150 | 0 | 0 | 0 | 0 | 1,694 | 1,603 | 1,697 | 206 | 70 | 954 | Transporter |
| Protein PAGK_2371 | 482891444 | 30 | 728 | 164 | 0 | 0 | 653 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Unknown |
| Protein PPA0532 | 50842016 | 46 | 546 | 40 | 0 | 0 | 640 | 362 | 0 | 0 | 0 | 0 | 0 | 0 | Unknown |
| Protein PPA0533 | 50842017 | 20 | 444 | 642 | 484 | 563 | 187 | 229 | 0 | 0 | 0 | 0 | 27 | 0 | Unknown |
| Protein PPA1197 | 50842677 | 27 | 0 | 43 | 0 | 0 | 68 | 0 | 2,453 | 1,403 | 4,515 | 3,420 | 0 | 0 | Unknown |
| Protein PPA1281 | 50842762 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 1,628 | 749 | 3,091 | 1,510 | 302 | 55 | Unknown |
| Protein PPA1498 | 50842976 | 64 | 2,615 | 89 | 0 | 0 | 37 | 272 | 0 | 0 | 0 | 0 | 0 | 0 | Digestion |
| Protein PPA1715 | 50843175 | 49 | 458 | 60 | 0 | 0 | 0 | 0 | 2,544 | 1,381 | 2,654 | 648 | 0 | 0 | Unknown |
| Protein PPA1939 | 50843388 | 17 | 266 | 733 | 7,484 | 3,717 | 533 | 222 | 166 | 46 | 747 | 4,327 | 1,984 | 0 | Unknown |
| Protein PPA2239 | 50843674 | 41 | 397 | 335 | 1,703 | 4,762 | 1,337 | 175 | 0 | 0 | 0 | 0 | 0 | 0 | Digestion |
| Rare lipoprotein A rlpa | 50843612 | 37 | 317 | 67 | 0 | 0 | 0 | 0 | 742 | 1,803 | 0 | 0 | 0 | 0 | Cell wall |
| Triacylglycerol lipase | 50843543 | 36 | 1,374 | 9,033 | 758 | 592 | 142 | 1,094 | 0 | 0 | 0 | 0 | 0 | 0 | Digestion |
Proteins are selected from the cell wall and secreted fractions analyzed by mass spectrometry. The table shows the quantity of each protein in femtomoles per microgram of total protein detected in each phylotype. Data from one of two similar experiments.
Abbreviations: CAMP, Christie-Atkins-Munch-Peterson; RT, ribotype.
DISCUSSION
Genomic analysis of P. acnes clinical isolates identified specific phylotypes associated with either acne, healthy skin, or found equally among patients with acne and individuals with healthy skin (“neutral” phylotypes) (Fitz-Gibbon et al., 2013; McDowell et al., 2013). Yet, it was unclear if these phylotypes were associated with different immune responses. Our data demonstrated that acne-associated phylotypes IA-2 p+, IB-1, and IC induce high levels of inflammatory IFN-γ and IL-17, and thus suggest that these specific P. acnes phylotypes may possess an increased propensity to induce acne due to induction of both Th1 and Th17 responses. This supports other recently published data showing high levels of both IFN-γ and IL-17 expression in acne lesions (Agak et al., 2014; Kistowska et al., 2015). Interestingly however, a large fraction of patients with acne do not harbor any known acne-associated phylotypes (Fitz-Gibbon et al., 2013). Our data suggest that acne in these patients may be associated with Th1 responses to neutral phylotypes IB-3 and II despite counterbalancing IL-10, or by mainly Th17 responses to neutral phylotypes IA-1, IA-2 p−, and IB-2. Overall, the differences between the immune responses to different P. acnes phylotypes are consistent with earlier studies that found correlations of phylotypes with disease (Fitz-Gibbon et al., 2013; McDowell et al., 2013). Thus, our data provide insight into the relationship between P. acnes strain populations and their association with healthy skin versus acne.
We found that IFN-γ induction was significantly increased in acne-associated phylotype IA-2 strains with a large plasmid (Fitz-Gibbon et al., 2013; Kasimatis et al., 2013) compared with neutral-associated phylotype IA-2 strains without the plasmid. While these disease associations are derived from their respective ribotypes, nearly all acne-associated ribotype 4 and 5 strains have the plasmid, and most of the neutral-association ribotype 1 strains do not (Fitz-Gibbon et al., 2013; Tomida et al., 2013). Thus, it is possible that this plasmid contains virulence factors that increase the propensity of a strain to induce acne (Kasimatis et al., 2013). Besides IA-2 p+ and IC strains, several members from phylotypes IA-1 and IB-2 also have this plasmid (Tomida et al., 2013), and future work assessing the immune response to these strains would help determine if the plasmid plays a role in acne pathogenesis.
The marked differences in IL-10 induction between P. acnes phylotype groups warrant further investigation. Our study showed that P. acnes phylotypes associated with acne induced low levels of IL-10, whereas P. acnes phylotypes II-RT6 and III associated with healthy skin and most associated with nonacne infections (Mak et al., 2013; McDowell et al., 2013; Rollason et al., 2013; Shannon et al., 2006) induced higher IL-10 production. IL-10 has the ability to reduce inflammation by downregulating IFN-γ and IL-17 (Ouyang et al., 2011). Because IL-10 can be secreted by regulatory T cells in response to P. acnes (Kopitar et al., 2006), investigating the expression patterns and regulation of regulatory T cells in acne lesions versus healthy skin is of high priority. Indeed, regulatory T cells play an important role in skin homeostasis via IL-10 (Eyerich and Zielinski, 2014; Pesenacker et al., 2015), which improves certain inflammatory skin diseases (Nomura et al., 2014; Weiss et al., 2004). Additionally, investigating IL-10 expression by local tissue macrophages as well as circulating monocytes in response to P. acnes phylotypes is also important for understanding its role in acne.
The variation in the adaptive immune responses to different P. acnes phylotypes may be accounted for by strain-specific expression of protein antigens. Also, it is possible that expression of various adhesion molecules may act as virulence factors in particular environments (Kostakioti et al., 2013). Thus, we studied the proteome of several P. acnes isolates. Our comparative proteomic analysis revealed that one adhesion (50843565), a hydrolase (50843410), and several proteins of unknown function (50842677, 50842762, and 50843175) have significantly higher expression in acne-associated phylotypes IA-2 p+ and IB-1 and in neutral phylotypes IA-1 and IB-2. In contrast, another adhesion (50842581) and two CAMP factors (50842711, 50843546) were more highly expressed in phylotypes II-RT6 and III associated with healthy skin (Table 1).
The potential for linking the proteome with specific immune responses may allow for the identification of protein vaccines candidates, a form of treatment that may be promising for acne (Kim, 2008; Simonart, 2013) and avoids antibiotic resistance (Lomholt and Kilian, 2014). Vaccination with P. acnes CAMP factor 2 (50842175), found in all phylotypes we examined, showed promise in animal models (Liu et al., 2011; Nakatsuji et al., 2011). Some of the cell surface proteins expressed only in acne or neutral strains such as the cell wall hydrolase (50843410), adhesion (50843565), or others may also be interesting to evaluate as vaccine candidates.
Despite numerous studies linking the presence of P. acnes to acne vulgaris, the exact role of the bacteria in acne pathogenesis remains unclear (Beylot et al., 2014; Shaheen and Gonzalez, 2011). Our finding of significant variation between the induced immune responses and proteomes of different P. acnes strains is complementary to earlier studies that found associations of phylotypes with disease (Fitz-Gibbon et al., 2013; McDowell et al., 2013) and provides insight into the pathogenesis of acne, suggesting that certain P. acnes phylotypes may play a more important role in acne pathogenesis. Future studies should, therefore, use well-characterized, phylotyped strains of P. acnes, due to potentially significant differences between strains. Studies are needed to determine whether different phylotype groups of P. acnes may cause varying clinical manifestations of acne. A greater understanding of how different P. acnes phylotypes induce distinct cytokine patterns may also provide new avenues for therapeutic intervention and prevention. A high priority for future work should be to determine whether strains associated with healthy skin can reduce Th1 or Th17 inflammation induced by other stains via IL-10 or other pathways, both in vitro and in vivo. Overall, our results suggest that modulating the immune response induced by specific P. acnes phylotypes may be useful for controlling the inflammatory response in acne pathogenesis and lead to new treatment alternatives to further explore.
METHODS
PBMC isolation
Blood was obtained from healthy donors after they signed written informed consent as approved by the Institutional Review Board at UCLA in accordance with the Declaration of Helsinki Principles. PBMCs were isolated from blood by Ficoll-Paque (GE Healthcare, Uppsala, Sweden) gradient and resuspended in RPMI 1640 with 10% fetal bovine serum (HyClone, South Logan, UT) before use. PBMCs were plated in each well in 48-well cell culture plates in a volume of 770 µl and a concentration of 2.5 × 106 cells/ml.
Bacterial culture
P. acnes strain ATCC 6919 was obtained from American Type Culture Collection (Manassas, VA). Other strains were obtained from the Biodefense and Emerging Infections Research Resources Repository (Manassas, VA) or were kindly donated by Huiying Li and István Nagy. Each day between 10 and 3 days before a PBMC isolation day, P. acnes strains representing all phylotypes were each inoculated from glycerol stocks into 3 ml of reinforced clostridial media (Oxoid, Cheshire, United Kingdom) and grown at 37 °C using AnaeroPack system sachets (Mitsubishi Gas Chemical Company, Tokyo, Japan). One day before PBMC isolation, one tube from each strain in the exponential phase as assessed by optical density at 600 nm with 1 cm path length (OD600) of 0.1–1.0, preferably 0.3–0.5, was selected and used to perform eight 1:2 serial dilutions into reinforced clostridial media.
Concurrently with PBMC isolation, one tube per strain was selected from the exponential phase (OD600 of 0.1–0.5, preferably 0.1–0.3) and the stationary phase (inoculated 1 day before a tube that reached maximum OD600 of approximately 1.5). P. acnes samples were transferred to 15 ml conical tubes and centrifuged at 3,000g for 10 minutes. The supernatant was discarded, and the pellet was resuspended in 10 ml of phosphate buffered saline (PBS). Tubes were centrifuged at 3,000g for 5 minutes, and the supernatant was discarded. Residual agar was removed with a pipette, and the pellet was resuspended in 1 ml of PBS and transferred to a microcentrifuge tube. Tubes were centrifuged at 3,000g for 2 minutes, and the supernatant was discarded. Stationary phase bacteria were resuspended in 1 ml of PBS, and exponential phase bacteria were resuspended in 0.5 ml of PBS. Bacteria were enumerated using a spectrophotometer with a conversion of 2.5 × 108 CFU/ml = 1 absorbance unit. Samples were processed as rapidly as possible after initial centrifugation, and P. acnes were immediately added to PBMCs on completion of processing at a concentration of 2.5 × 106 CFU/ml, which was found to have no effect on PBMC viability.
ELISA
Cytokine concentrations were assessed in PBMC culture supernatants by ELISA using the manufacturer’s protocols (R&D ELISA Development System, Minneapolis, MN). IFN-γ was measured using supernatants from 20 hours after P. acnes were added to PBMCs, and IL-17 and IL-10 were measured 68 hours after P. acnes were added to PBMCs. Differences in cytokine expression between phylotypes were assessed for statistical significance via the t-test. Cluster 3.0 (de Hoon et al., 2004) was used to cluster P. acnes phylotypes with an average-distance city-block method using ELISA results for IFN-γ, IL-17, and IL-10.
Protein fraction preparation
P. acnes strains HL005PA1, HL043PA1, HL110PA1, HL013PA1, HL110PA4, and ASN12 were inoculated into 3 ml of reinforced clostridial media and grown at 37 °C using AnaeroPack system sachets. After reaching the exponential phase (OD600 of 0.1–0.3), bacteria were collected by centrifugation transferred into 50 ml of reinforced clostridial media. Cultures were incubated for approximately 40 hours and collected in the late exponential phase (OD600 of approximately 1.0). Bacteria were centrifuged at 4,000g for 30 minutes, and the supernatant, containing secreted proteins, was filtered through 0.2 µm pores. The bacterial pellets were washed three times in PBS, and divided into four samples.
Lysozyme was used as described previously (Gallis et al., 1976) to digest the cell wall of P. acnes, creating protoplasts and releasing cell wall proteins. To accomplish this, one sample was resuspended in 200 µl of solution with 10 mM pH 7 phosphate buffer, 600 mM KCl, 10 mM MgCl2, and 1 mg/ml egg white lysozyme (Pierce Biotechnology, Waltham, MA). Another sample was resuspended in 200 µl of solution with 50 mM Tris-HCl, 250 mM sucrose, 10 mM MgCl2, 30 mM KCl, and 1 mg/ml egg white lysozyme. These two samples were incubated for 4 hours with rotation at 37 °C. Samples were then centrifuged at 1,000g for 5 minutes, and the supernatant was retained. Supernatants were centrifuged for an additional 5 minutes at 20,000g. The two supernatants containing cell wall proteins were combined and filtered through 0.2 µm pores.
The remaining two samples underwent beadbeating with a micro-MiniBeadbeater (Biospec Products, Bartlesville, OK) for 5 minutes. These samples were than sonicated to obtain the total cell extract fraction. One sample was further centrifuged first at 1,000g for 5 minutes and then at 8,000g for 5 minutes, retaining the supernatant. The sample was then centrifuged at 20,000g for 15 minutes, and the supernatant containing the cytosolic protein fraction was retained.
Mass spectrometry
Ten micrograms of protein from the cytosolic and total cell extract fractions, as quantified by Bradford assay, and all the other samples were adjusted to 20% trichloroacetic acid and incubated at 4 °C for 30 minutes. The samples were centrifuged at 20,000g for 5 minutes, and the pellets were washed with 200 µl of cold acetone. The pellet was resuspended in 50% 100 mM ammonium bicarbonate solution and 50% acetonitrile. The samples were reduced with 25 mM tris-(2-carboxyethyl)-phosphine for 30 minutes at 37 °C, alkylated with 75 mM iodoacetamide for 1 hour at room temperature in the dark, diluted to 5% acetonitrile in pH 8 100 mM ammonium bicarbonate buffer, and digested for 16 hours with 500 ng trypsin (Promega, Madison, WI). The samples were centrifuged at 20,000g for 10 minutes twice, with the supernatant retained. They were processed in a nano-ACQUITY 2D UHPLC system (Waters, Milford, MA) and analyzed with a Synapt G2 mass spectrometer (Waters) using elevated energy mass spectrometry, which is accurate for label-free protein quantification (Bond et al., 2013; Getie-Kebtie et al., 2013). The allowed mass range was 50–2,000 Da. Ultra-high performance liquid chromatography was performed with T3 C18 reversed-phase column by applying a linear solvent gradient over 90 minutes of 3–90% buffer B. Solvents were 0.1% formic acid for buffer A and 0.1% formic acid in acetonitile for buffer B. The collision energy was automatically ramped from 15 to 40 eV.
Mass spectrometry data were processed with IdentityE (Waters) within Protein Lynx Global Server (Waters). Search parameters included trypsin digest peptides with up to one missed cleavage. The mass tolerance was 0.025 Da for low energy ions and 0.01 Da for high energy ions. Identified peptides were matched to proteins in the P. acnes strain KPA171202 database with the addition of unique proteins from other strains. Protein quantification and identification was performed using Scaffold (Proteome Software, Portland, OR) with a false discovery rate of 5%, including only proteins with at least two unique peptides. Protein quantification was determined based on the peak intensities of the top three peptides of each protein. Protein localization was predicted using the PSORTb 3.0 tool (Yu et al., 2010). Protein signal peptides were predicted using SignalP 4.1 (Petersen et al., 2011). NCBI’s BLAST tool (Altschul et al., 1990) was used to further analyze proteins for function and clinical relevance.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank Huiying Li and István Nagy for generously providing P. acnes strains. This research was supported by NIH grant RO1-AR-053542 to JK, an Annenberg Foundation Grant to JK, NIH grant T32-AR-58921 to GA, an ADA Medical Student Fellowship to YY, an AARS Clinical Research Grant to YY, and a Carolyn L. Kuckein Student Research Fellowship to YY.
Abbreviations:
- P. acnes
Propionibacterium acnes
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate buffered saline
- RT
ribotype
- Th
T helper
Footnotes
CONFLICT OF INTEREST
YY, JC, and JK have filed for a patent related to this study.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.doi.org/10.1016/j.jid.2016.06.615.
REFERENCES
- Agak GW, Qin M, Nobe J, Kim MH, Krutzik SR, Tristan GR, et al. Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J Invest Dermatol 2014;134:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990;215:403–10. [DOI] [PubMed] [Google Scholar]
- Beylot C, Auffret N, Poli F, Claudel JP, Leccia MT, Del Giudice P, et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatol Venereol 2014;28:271–8. [DOI] [PubMed] [Google Scholar]
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol 2013;168:474–85. [DOI] [PubMed] [Google Scholar]
- Bond NJ, Shliaha PV, Lilley KS, Gatto L. Improving qualitative and quantitative performance for MS(E)-based label-free proteomics. J Proteome Res 2013;12:2340–53. [DOI] [PubMed] [Google Scholar]
- de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics 2004;20:1453–4. [DOI] [PubMed] [Google Scholar]
- Eyerich S, Zielinski CE. Defining Th-cell subsets in a classical and tissue-specific manner: examples from the skin. Eur J Immunol 2014;44: 3475–83. [DOI] [PubMed] [Google Scholar]
- Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol 2013;133:2152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Uchida K, Ishige Y, Ishige I, Kobayashi I, Takemura T, et al. Characterization of Propionibacterium acnes isolates from sarcoid and non-sarcoid tissues with special reference to cell invasiveness, serotype, and trigger factor gene polymorphism. Microb Pathog 2009;46:80–7. [DOI] [PubMed] [Google Scholar]
- Gallis HA, Miller SE, Wheat RW. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun 1976;13:1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getie-Kebtie M, Sultana I, Eichelberger M, Alterman M. Label-free mass spectrometry-based quantification of hemagglutinin and neuraminidase in influenza virus preparations and vaccines. Influenza Other Respir Viruses 2013;7:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science 2009;324:1190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C, Mak TN, Zimny-Arndt U, Schmid M, Meyer TF, Jungblut PR, et al. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol 2010;10:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasson F, Nagy I, Knol AC, Zuliani T, Khammari A, Dreno B. Different strains of Propionibacterium acnes modulate differently the cutaneous innate immunity. Exp Dermatol 2013;22:587–92. [DOI] [PubMed] [Google Scholar]
- Kasimatis G, Fitz-Gibbon S, Tomida S, Wong M, Li H. Analysis of complete genomes of Propionibacterium acnes reveals a novel plasmid and increased pseudogenes in an acne associated strain. Biomed Res Int 2013;2013:918320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J Acne vaccines: therapeutic option for the treatment of acne vulgaris? J Invest Dermatol 2008;128:2353–4. [DOI] [PubMed] [Google Scholar]
- Kistowska M, Meier B, Proust T, Feldmeyer L, Cozzio A, Kuendig T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol 2015;135:110–8. [DOI] [PubMed] [Google Scholar]
- Kopitar AN, Ihan Hren N, Ihan A. Commensal oral bacteria antigens prime human dendritic cells to induce Th1, Th2 or Treg differentiation. Oral Microbiol Immunol 2006;21:1–5. [DOI] [PubMed] [Google Scholar]
- Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 2013;3:a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PF, Nakatsuji T, Zhu W, Gallo RL, Huang CM. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine 2011;29:3230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS One 2010;5:e12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomholt HB, Kilian M. Clonality and anatomic distribution on the skin of antibiotic resistant and sensitive Propionibacterium acnes. Acta Derm Venereol 2014;94:534–8. [DOI] [PubMed] [Google Scholar]
- Mak TN, Yu SH, De Marzo AM, Bruggemann H, Sfanos KS. Multilocus sequence typing (MLST) analysis of Propionibacterium acnes isolates from radical prostatectomy specimens. Prostate 2013;73:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell A, Barnard E, Nagy I, Gao A, Tomida S, Li H, et al. An expanded multilocus sequence typing scheme for Propionibacterium acnes: investigation of ‘pathogenic’, ‘commensal’ and antibiotic resistant strains. PLoS One 2012;7:e41480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell A, Gao A, Barnard E, Fink C, Murray PI, Dowson CG, et al. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology 2011;157:1990–2003. [DOI] [PubMed] [Google Scholar]
- McDowell A, Nagy I, Magyari M, Barnard E, Patrick S. The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS One 2013;8:e70897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouser PE, Baker BS, Seaton ED, Chu AC. Propionibacterium acnes-reactive T helper-1 cells in the skin of patients with acne vulgaris. J Invest Dermatol 2003;121:1226–8. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect 2006;8:2195–205. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol 2005;124:931–8. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang CM. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One 2011;6:e14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Kabashima K, Miyachi Y. The panoply of alphabetaT cells in the skin. J Dermatol Sci 2014;76:3–9. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011;29:71–109. [DOI] [PubMed] [Google Scholar]
- Pesenacker AM, Broady R, Levings MK. Control of tissue-localized immune responses by human regulatory T cells. Eur J Immunol 2015;45:333–43. [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 2011;8:785–6. [DOI] [PubMed] [Google Scholar]
- Rollason J, McDowell A, Albert HB, Barnard E, Worthington T, Hilton AC, et al. Genotypic and antimicrobial characterisation of Propionibacterium acnes isolates from surgically excised lumbar disc herniations. Biomed Res Int 2013;2013:530382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen B, Gonzalez M. A microbial aetiology of acne: what is the evidence? Br J Dermatol 2011;165:474–85. [DOI] [PubMed] [Google Scholar]
- Shannon BA, Cohen RJ, Garrett KL. Polymerase chain reaction-based identification of Propionibacterium acnes types isolated from the male urinary tract: evaluation of adolescents, normal adults and men with prostatic pathology. BJU Int 2006;98:388–92. [DOI] [PubMed] [Google Scholar]
- Simonart T Immunotherapy for acne vulgaris: current status and future directions. Am J Clin Dermatol 2013;14:429–35. [DOI] [PubMed] [Google Scholar]
- Tomida S, Nguyen L, Chiu BH, Liu J, Sodergren E, Weinstock GM, et al. Pangenome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. MBio 2013;4:e00003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Mamelak AJ, La Morgia S, Wang B, Feliciani C, Tulli A, et al. The role of interleukin 10 in the pathogenesis and potential treatment of skin diseases. J Am Acad Dermatol 2004;50:657–75; quiz 76–8. [DOI] [PubMed] [Google Scholar]
- Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, et al. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010;26:1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Champer J, Garban H, Kim J. Typing of Propionibacterium acnes: a review of methods and comparative analysis. Br J Dermatol 2015a;172: 1204–9. [DOI] [PubMed] [Google Scholar]
- Yu Y, Champer J, Kim J. Analysis of the surface, secreted, and intracellular proteome of Propionibacterium acnes. EuPA Open Proteomics 2015b;9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


