Abstract
Physicians use perioperative decision-support tools to mitigate risks and maximize benefits to achieve the most successful outcome for patients. Contemporary risk-assessment practices augment surgeon’s judgement and experience with decision-support algorithms driven by big data and machine learning. These algorithms accurately assess risk for a wide range of postoperative complications by parsing large datasets and performing complex calculations that would be cumbersome for busy clinicians. Even with these advancements, large gaps in perioperative risk assessment remain; decision-support algorithms often cannot account for risk-reduction therapies applied during a patient’s perioperative course, and do not quantify tradeoffs between competing goals of care (e.g., balancing postoperative pain control with the risk of respiratory depression or balancing intraoperative volume resuscitation with risk for complications from pulmonary edema). Multi-objective optimization solutions have been applied to similar problems successfully, but have not yet been applied to perioperative decision-support. Given the large volume of data available via electronic medical records, including intraoperative data, it is now feasible to successfully apply multi-objective optimization in perioperative care. Clinical application of multi-objective optimization would require semiautomated pipelines for analytics and reporting model outputs and a careful development and validation process. Under these circumstances, multi-objective optimization has the potential to support personalized, patient-centered, shared decision-making with precision and balance.
Article summary:
Risk calculators and decision-support tools estimate the probability of individual or composite outcomes, yet approaches and techniques in multi-objective optimization are unknown to most clinicians. Here we describe the potential for multi-objective optimization methods to quantify tradeoffs among competing outcomes in perioperative medicine.
Single objectives versus reality
Historically, assessing the risks of undergoing surgery relied solely on surgeon experience and intuition. Preoperative risk assessments have evolved into an evidence-based practice using objective clinical data and validated risk-assessment tools. Risk-scoring systems such as the American College of Surgeons National Surgical Quality Improvement Program Risk Calculator, the Surgical Risk Preoperative Assessment System, MySurgeryRisk, Predictive OpTimal Trees in Emergency Surgery Risk Calculator, and others aim to provide patients and clinicians with estimates of risk for postoperative complications.1–4 These calculators leverage large volumes of data, regression modeling, and machine-learning techniques to accurately predict postoperative complications. These predictions can inform shared decision-making processes involving patients, their caregivers, and clinicians.
One major weakness of most existing surgical risk calculators is that they fail to incorporate a dynamic mathematical adjustment for change in the probability of outcomes or complications that is expected to occur when various treatments are applied. Instead, the calculators estimate static risk for individual complications or composite outcomes. Risk estimates for composite outcomes better represent aggregate risk for a larger, more comprehensive set of complications, but they often group rare events with common events and group severe complications with minor complications; the resulting predictions lack granularity and interpretability.5 Even when risk estimates are accurate, granular, and interpretable, they often do not identify the specific clinical decisions that are the primary drivers of clinical outcomes, much less suggest an optimal management strategy to optimize outcomes.
Perioperative decision-support tools predicated on numerical rating scales present numerous challenges. The definition of pain as a fifth vital sign by the American Pain Society and subsequent emphasis by the Joint Commission led to an implicit administrative goal to minimize patient-reported pain.6 This single-objective optimization is easily accomplished by administering high-dose fentanyl in the postanesthesia care unit, although this may not lead to improvement in overall patient outcome. Thoughtful clinicians also seek to avoid respiratory depression, nausea, emesis, pruritus, constipation, opioid dependence, and adverse opioid-related events.7–11 These other important outcomes may be monitored, reported, and valued separately, inappropriately, or not at all, leading to a treatment approach that fails to achieve the ultimate goal of restoring function (e.g., breathing, return of bowel function, and ambulation). In a sentinel investigation by Vila et al.,12 an opioid-based quality improvement program was associated with a 6% improvement in patient satisfaction with pain management and a 223% increase in postoperative safety events, demonstrating the potential consequences of single-objective optimization.
Principles of multi-objective optimization
Many real-life decision-making situations involve multiple goals. For example, a stock broker designing a portfolio seeks a high return on investment and low risk, a mechanical engineer building an engine seeks high horsepower and low fuel consumption, and an anesthesiologist caring for a patient in the postoperative period seeks low pain severity and early return to function. The common thread across these decision problems is conflict between the goals cited. Financial portfolios that have high returns tend to be risky, powerful cars use more gasoline, and high-dose opioids can impair postoperative function. In addition, these problems typically have a set of good solutions rather than a single best solution. Often, to improve one goal, the decision-maker must sacrifice another goal. Only two conflicting goals are considered in these examples; tradeoffs can incorporate many goals or objectives.
It is possible to form complex decision-making scenarios as mathematical optimization problems with multiple objectives. These formulations are called multi-objective optimization problems. A decision (or a solution) is represented by assigning values to variables, for example the amount of intravenous fluid administered during surgery. Let xi represent the ith decision variable and x = (x1,…,xn) the collection of decision variables. An objective, in this context, is a function that maps decisions to a goal. Let fj(x) represent the jth goal as a function of a decision. Formally, the associated optimization problem is stated as
where x is a decision contained within X, which represents the decision space, or the set of all feasible decisions. This example arbitrarily minimizes the objectives; it is possible to minimize some objectives while maximizing others. The set of all possible outcomes that can result from all possible decisions is called the objective space. The goal is to find a solution that optimizes all functions.
Consider the portfolio optimization problem described by Harry Markowitz, in which one is interested in creating a portfolio of stocks with high expected return and low risk. Assume that an investor wants to invest a total of $1, distributed among 3 available stocks. Her decision space, X, is represented by the amount she invests in each stock. A solution, x, might look like, x=(0,0.25,0.75), representing no investment in stock 1, 25 cents in stock 2, and 75 cents in stock 3. Expected portfolio return is a weighted sum of each stock’s expected return, which can be written as f1(x)=x1μ1+x2μ2+x3μ3, where μi is the expected return of stock i. Similarly, objective 2, portfolio-wide risk, can be written as f2(x)=x12σ12+x22σ22+x32σ32, where σi2 is the variance of stock i’s return (for simplicity, we assume that stock returns are independent of one another). The associated optimization problem is maxf1(x),minf2(x),subject tox1+x2+x3=1,x1,x2,x3≥0.
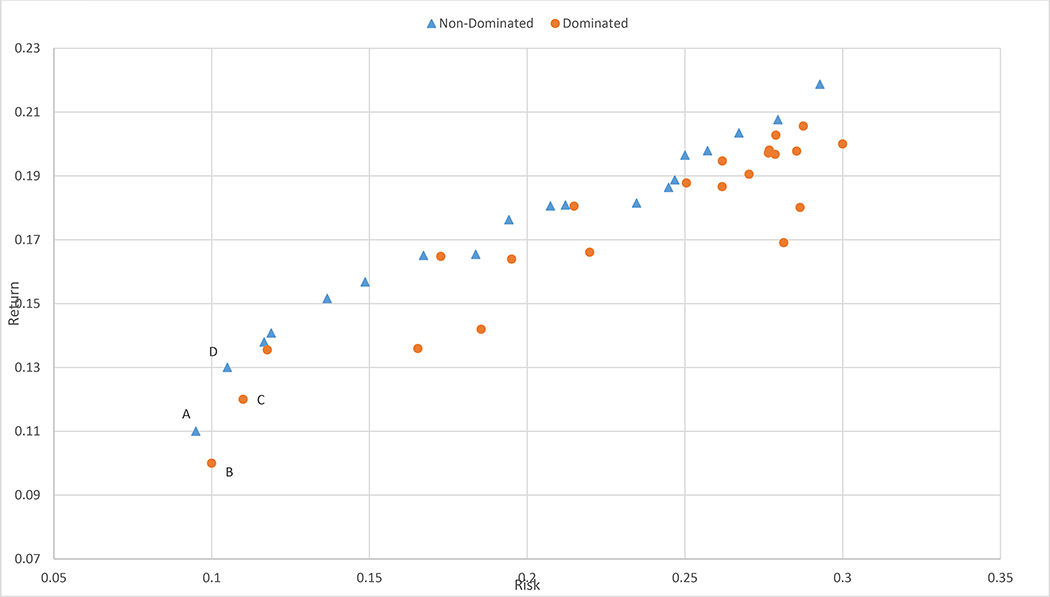
The solution to this problem is a set of points known as the efficient frontier. The solutions in the frontier are called efficient solutions. One solution is said to dominate another solution if it is strictly better in 1 objective and not worse in others. The efficient frontier is composed of solutions that are not dominated by any other solution. Figure 1 represents a sampling of portfolios in the objective space (i.e., risk versus return). The solution corresponding to point A is nondominated because there is no portfolio that has lower risk than A. Portfolio A dominates B because it has both higher returns and lower risk, but A does not dominate C. C is dominated by D. This example depicts multiobjective optimization problems as having sets of solutions rather than a single solution. Given this set of solutions, if there are additional preferences not previously included in the model criteria, then such preferences may help decision-makers choose among available solutions for a given multiobjective optimization problem.13
Fig. 1.
Risk vs. return profiles of stock portfolios.
Multi-objective optimization in clinical practice
Multi-objective optimization challenges abound in surgery and perioperative medicine. Perhaps one of the simplest rubrics is in postoperative pain management, which has the goals of minimizing pain while minimizing opioids and maximizing indicators of surgical recovery, such as breathing, return of bowel function, and ambulation. Tradeoffs among these goals are illustrated in Figure 2.
Fig. 2.
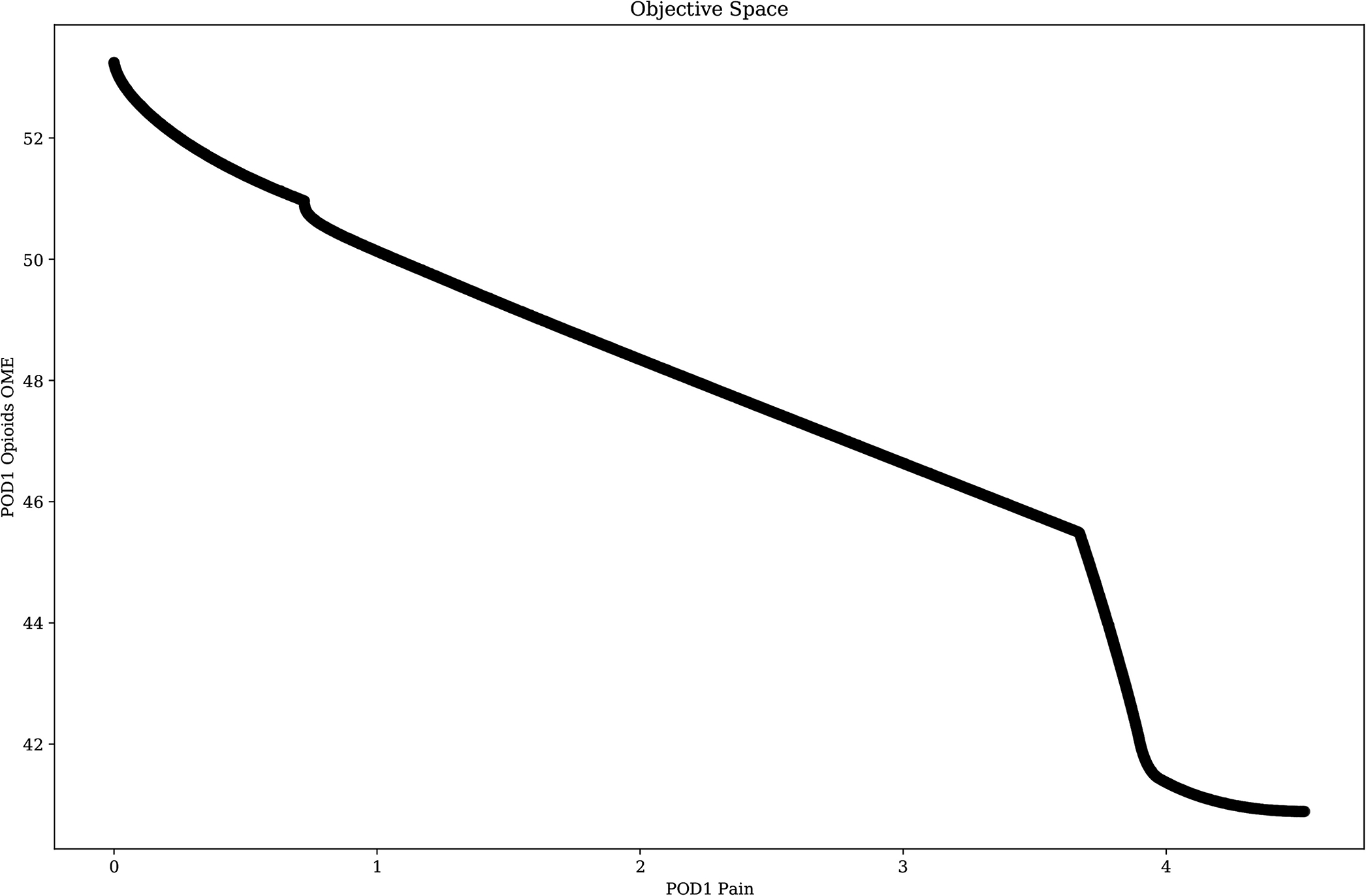
Postoperative day one (POD1) mean pain intensity vs. oral morphine milligram equivalents.
Decisions on perioperative fluid management, vasopressor and inotropic support, ventilation management, antibiotic selection, and blood glucose management typify those hourly decisions, which may impart multiple and imbalanced consequences.13–20 Notably, while decision heuristics commonly aim to restore “normal” parameters, many patients suffer comorbid factors that may transiently or permanently challenge this underlying strategy.21 From a broader perspective, decisions on the timing of surgery are common in older adults with hip fractures, with data supporting early repair as well as correcting reversible clinical abnormalities, which may conflict with early repair.22–24 Perioperative neurocognitive disorders (e.g., delirium and postoperative cognitive change), which are the most common postoperative complications in older adults, may be exacerbated by pain intensity and opioids, suggesting further opportunities for optimization across competing endpoints.25–31
Prior limited experience for clinical multi-objective optimization
The authors are unaware of any published applications of multi-objective optimization in perioperative decision-making. However, similar concepts have been applied in performing partial hepatectomy for multifocal hepatocellular carcinoma. Liver surgeons seek to maximize complete resection or destruction of the target lesions and the size and function of the liver remnant. Multi-objective optimization has been formally deployed in exploring the distribution of Level 1, 2, and 3 trauma centers in Colorado, minimizing total system access time and the number of casualties who could not reach the desired level of care.32 Similar work has been performed regarding the Scottish trauma system.33 Multi-objective optimization has been used in surgical device design and development for cochlear implants, coronary stents, and numerous orthopedic implants.34–36 Generally, these designs attempt to minimize the risk of insertion and device-specific complications while maximizing device longevity and functionality. Multi-objective optimization has also been applied to simulated radiation therapy for brain lesions, seeking to maximize normal neuroanatomy around the lesion while delivering a theoretically sufficient dose of radiation to destroy the lesion.37 Similar methods have been applied to electrostimulation for acetabular bone reformation.38 This work focused on arranging electrodes to achieve a homogenous field distribution and optimal simulation interval, objectives that occasionally conflict.
Challenges and future directions in clinical implementation of multi-objective optimization
The increasing availability of large volumes of data from electronic health records offers opportunities to apply advanced data science techniques to frame clinical challenges as multi-objective optimization problems that balance tradeoffs among competing goals. Conceptually, this approach seems preferable to traditional decision-support tools that estimate probabilities for individual outcomes in isolation or estimate probabilities for composite outcomes that lack granularity and interpretability. However, evidence supporting the clinical efficacy of multi-objective optimization is lacking. Future investigations should seek retrospective validation followed by prospective clinical application with comparison to standard decision-making and traditional decision-support tools. This would require semiautomated pipelines for analytics and reporting model outputs. If successful, multi-objective optimization has the potential to support personalized, patient-centered, shared decision-making with precision and balance.
Highlights.
Topic:
Multi-objective optimization in perioperative decision-making
Purpose:
Describe a framework for achieving balance among several competing goals of care
State-of-the-Art:
Multi-objective optimization can quantify tradeoffs among several outcomes of interest, offering advantages over traditional, single-objective optimization
Knowledge Gaps:
The feasibility and efficacy of multi-objective optimization in augmenting surgical decision-making have not been reported
Technology Gaps:
Clinical application of multi-objective optimization would require semiautomated pipelines for analytics and reporting model outputs
Future Directions:
Retrospective development and validation of multi-objective optimization decision-support tools, followed by clinical application in a prospective, observational setting
Acknowledgments
Funding/Financial support: This work was supported by the Donn M. Dennis, MD, Endowed Professorship (P.J.T.). T.J.L. was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number K23 GM140268.
Footnotes
Conflict of interest/Disclosure: The authors of this research have no conflict of interest to report.
References
- 1.Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013;217:833–42 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meguid RA, Bronsert MR, Juarez-Colunga E, Hammermeister KE, Henderson WG. Surgical Risk Preoperative Assessment System (SURPAS): III. Accurate preoperative prediction of 8 adverse outcomes using 8 predictor variables. Ann Surg 2016;264:23–31. [DOI] [PubMed] [Google Scholar]
- 3.Bihorac A, Ozrazgat-Baslanti T, Ebadi A, Motaei A, Madkour M, Pardalos PM, et al. MySurgeryRisk: development and validation of a machine-learning risk algorithm for major complications and death after surgery. Ann Surg 2018;269:652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertsimas D, Dunn J, Velmahos GC, Kaafarani HMA. Surgical risk is not linear: derivation and validation of a novel, user-friendly, and machine-learning-based Predictive OpTimal Trees in Emergency Surgery Risk (POTTER) calculator. Ann Surg 2018;268:574–83. [DOI] [PubMed] [Google Scholar]
- 5.Myles PS, Devereaux PJ. Pros and cons of composite endpoints in anesthesia trials. Anesthesiology 2010;113:776–8. [DOI] [PubMed] [Google Scholar]
- 6.Max MB, Donovan M, Miaskowski CA, Ward SE, Gordon D, Bookbinder M, et al. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 1995;274:1874–80. [DOI] [PubMed] [Google Scholar]
- 7.Waljee JF, Zhong L, Hou H, Sears E, Brummett C, Chung KC. The use of opioid analgesics following common upper extremity surgical procedures: a national, population-based study. Plast Reconstr Surg 2016;137:355e–64e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic opioid dependence in the United States. Ann Surg 2017;265:728–30. [DOI] [PubMed] [Google Scholar]
- 9.Shafi S, Collinsworth AW, Copeland LA, Ogola GO, Qiu T, Kouznetsova M, et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surgery 2018;153:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, Jackson K, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother 2007;41:400–6. [DOI] [PubMed] [Google Scholar]
- 11.Oderda GM, Evans RS, Lloyd J, Lipman A, Chen C, Ashburn M, et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage 2003;25:276–83. [DOI] [PubMed] [Google Scholar]
- 12.Vila H Jr., Smith RA, Augustyniak MJ, Nagi PA, Soto RG, Ross TW, et al. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg 2005;101:474–80, table of contents. [DOI] [PubMed] [Google Scholar]
- 13.Miller TE, Myles PS. Perioperative fluid therapy for major surgery. Anesthesiology 2019;130:825–32. [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Byrne L, van Haren F. Fluid resuscitation in sepsis: the great 30 mL per kg hoax. J Thorac Dis 2020;12:S37–s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty M, Buggy DJ. Intraoperative fluids: how much is too much? Br J Anaesth 2012;109:69–79. [DOI] [PubMed] [Google Scholar]
- 16.Bowater RJ, Stirling SA, Lilford RJ. Is antibiotic prophylaxis in surgery a generally effective intervention? Testing a generic hypothesis over a set of meta-analyses. Ann Surg 2009;249:551–6. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- 18.Lesur O, Delile E, Asfar P, Radermacher P. Hemodynamic support in the early phase of septic shock: a review of challenges and unanswered questions. Ann Intensive Care 2018;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care 2019;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy P, Duggar B, Butterworth J. Blood glucose management in the patient undergoing cardiac surgery: A review. World J Cardiol 2014;6:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazmuri RJ, de Gomez CA. From a pressure-guided to a perfusion-centered resuscitation strategy in septic shock: Critical literature review and illustrative case. J Crit Care 2020;56:294–304. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari M, Swiontkowski M. Management of acute hip fracture. N Engl J Med 2017;377:2053–62. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin MA, Orosz GM, Magaziner J, Hannan EL, McGinn T, Morrison RS, et al. Preoperative status and risk of complications in patients with hip fracture. J Gen Intern Med 2006;21:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simunovic N, Devereaux PJ, Sprague S, Guyatt GH, Schemitsch E, Debeer J, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ 2010;182:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, Browndyke JN, Wright CB, Culley DJ, et al. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth 2019;123:464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg 2011;112:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand 2001;45:275–89. [DOI] [PubMed] [Google Scholar]
- 28.Eckenhoff RG, Maze M, Xie Z, Culley DJ, Goodlin SJ, Zuo Z, et al. Perioperative neurocognitive disorder: state of the preclinical science. Anesthesiology 2020;132:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Med Sci 2003;58:M76–81. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein SM, Poultsides L, Baaklini LR, Mörwald EE, Cozowicz C, Saleh JN, et al. Postoperative delirium in total knee and hip arthroplasty patients: a study of perioperative modifiable risk factors. Br J Anaesth 2018;120:999–1008. [DOI] [PubMed] [Google Scholar]
- 31.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative Delirium: The Importance of Pain and Pain Management. Anesth Analg 2006;102:1267–73. [DOI] [PubMed] [Google Scholar]
- 32.Jansen JO, Moore EE, Wang H, Morrison JJ, Hutchison JD, Campbell MK, et al. Maximizing geographical efficiency: An analysis of the configuration of Colorado’s trauma system. J Trauma Acute Care Surg 2018;84:762–70. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Jin Y, Jansen JO. Data-driven surrogate-assisted multiobjective evolutionary optimization of a trauma system. IEEE Transactions on Evolutionary Computation 2016;20:939–52. [Google Scholar]
- 34.Xiang S, Hong Y, Zhong-hua N. Multi-objective design optimization of coronary stent mechanical properties. Chinese Journal of Dialysis and Artificial Organs. 2012:6. [Google Scholar]
- 35.Zheng K, Scholes CJ, Chen J, Parker D, Li Q. Multiobjective optimization of cartilage stress for non-invasive, patient-specific recommendations of high tibial osteotomy correction angle–a novel method to investigate alignment correction. Med Eng Phys 2017;42:26–34. [DOI] [PubMed] [Google Scholar]
- 36.Ramos‐de‐Miguel Á, Escobar JM, Greiner D, Ramos‐Macías Á. A multiobjective optimization procedure for the electrode design of cochlear implants. Int J Numer Method Biomed Eng 2018;34:e2992. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Zhang JB, Cheng G, Schell MC, Okunieff P. Multi-objective optimization in radiotherapy: applications to stereotactic radiosurgery and prostate brachytherapy. Artif Intell Med 2000;19:39–51. [DOI] [PubMed] [Google Scholar]
- 38.Potratz C, Kluess D, Ewald H, van Rienen U. Multiobjective optimization of an electrostimulative acetabular revision system. IEEE Trans Biomed Eng 2010;57:460–8. [DOI] [PubMed] [Google Scholar]