Abstract
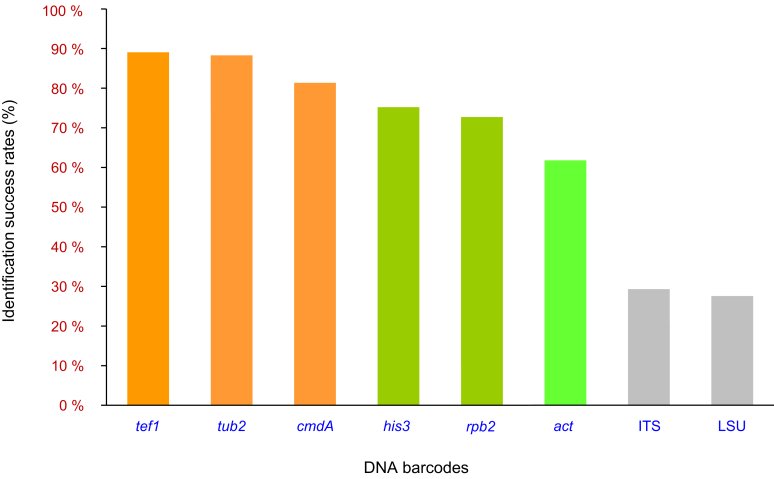
Calonectria represents a genus of phytopathogenic ascomycetous fungi with a worldwide distribution. In recent years, there has been an increase in the number of taxonomic studies on these fungi. Currently, there are 169 described species of Calonectria based on comparisons of DNA sequence data, combined with morphological characteristics. However, for some of these species, the sequence data utilised at the time of their description were relatively limited. This has justified an urgent need to reconsider the species boundaries for Calonectria based on robust genus-wide phylogenetic analyses. In this study, we utilised 240 available isolates including the ex-types of 128 Calonectria species, and re-sequenced eight gene regions (act, cmdA, his3, ITS, LSU, rpb2, tef1 and tub2) for them. Sequences for 44 Calonectria species, for which cultures could not be obtained, were downloaded from GenBank. DNA sequence data of all the 169 Calonectria species were then used to determine their phylogenetic relationships. As a consequence, 51 species were reduced to synonymy, two new species were identified, and the name Ca. lauri was validated. This resulted in the acceptance of 120 clearly defined Calonectria spp. The overall data revealed that the genus includes 11 species complexes, distributed across the Prolate and Sphaero-Naviculate Groups known to divide Calonectria. The results also made it possible to develop a robust set of DNA barcodes for Calonectria spp. To accomplish this goal, we evaluated the outcomes of each of the eight candidate DNA barcodes for the genus, as well as for each of the 11 species complexes. No single gene region provided a clear identity for all Calonectria species. Sequences of the tef1 and tub2 genes were the most reliable markers; those for the cmdA, his3, rpb2 and act gene regions also provided a relatively effective resolution for Calonectria spp., while the ITS and LSU failed to produce useful barcodes for species discrimination. At the species complex level, results showed that the most informative barcodes were inconsistent, but that a combination of six candidate barcodes (tef1, tub2, cmdA, his3, rpb2 and act) provided stable and reliable resolution for all 11 species complexes. A six-gene combined phylogeny resolved all 120 Calonectria species, and revealed that tef1, tub2, cmdA, his3, rpb2 and act gene regions are effective DNA barcodes for Calonectria.
Key words: Cylindrocladium, DNA barcoding, Multi-gene phylogeny, Plant pathogens, Taxonomy
Introduction
The genus Calonectria (Hypocreales, Nectriaceae) includes many aggressive plant pathogens and species that are broadly distributed in sub-tropical and tropical regions of the world. These species have a wide host range that includes more than 335 plant species (Crous 2002) and they are commonly collected from soils, leaves, stems, roots and fruits (Lombard et al. 2010a, 2015a, Chen et al. 2011, Alfenas et al. 2015, Gehesquiere et al. 2015, Li et al. 2017, Lopes et al. 2017, Marin-Felix et al. 2017, Jiang et al. 2019, Pham et al. 2019). A total of 169 species have been described in Calonectria (Ca.) using DNA sequence-based phylogenetic inference and morphological comparisons (Li et al. 2017, Marin-Felix et al. 2017, Pham et al. 2019).
Many species of Calonectria are well-known causal agents of important diseases on various agricultural, horticultural and forestry crops. Species such as Ca. pseudonaviculata and Ca. henricotiae are important agents of boxwood (Buxus spp.) blight in Germany, the Netherlands, Slovenia, UK and USA (Henricot & Culham 2002, Brand 2005, Gehesquiere et al. 2015, Daughtrey 2019, Freitas et al. 2019). Many species such as Ca. pentaseptata (in China and Vietnam, Crous et al. 2012, Li et al. 2017), Ca. pteridis (in Brazil, Freitas et al. 2019), Ca. reteaudii (in Australia, India and Vietnam, Old et al. 2003) and Ca. spathulata (in Colombia, Rodas et al. 2005) are important causal agents of Calonectria leaf blight (CLB) in Eucalyptus plantations. Calonectria ilicicola is known as a common pathogen responsible for the red crown rot of soybeans in China, Japan and USA (Gai et al. 2017, Yamamoto et al. 2017, Kleczewski et al. 2019), causing significant yield losses.
The importance of Calonectria spp. has justified many taxonomic studies on these fungi in recent years. Lombard et al. (2010a) provided a genus-wide phylogeny for the 66 available Calonectria spp. That study included sequence data for seven gene regions including act (actin), cmdA (calmodulin), his3 (histone H3), ITS (the internal transcribed spacer regions 1 and 2 and the 5.8S gene of the ribosomal RNA), LSU (28S large subunit RNA gene), tef1 (translation elongation factor 1-alpha) and tub2 (β-tubulin). Subsequently, a polyphasic approach, including combined DNA sequence data for the cmdA, tef1 and tub2 gene regions together with morphological comparisons was used to resolve the taxonomy of 141 Calonectria species (Lombard et al. 2016). A year later, Marin-Felix et al. (2017) provided rpb2 sequence data for 68 Calonectria species and Jayawardena et al. (2019) reconstructed the phylogeny of Calonectria based on sequence data for cmdA, his3, tef1 and tub2 gene regions. A problem has, however, arisen due to a lack of uniformity of sequence data in different studies, and particularly the his3 and rpb2. Futhermore, some other gene regions are not consistently available for all of 169 species of Calonectria. This calls to question the strength and relevance of the phylogenetic backbone for an important group of plant pathogens as well as the reliability of species boundaries that must define plant disease diagnoses and management.
DNA barcoding provides an effective and widely used tool for fungal species identification (Schoch et al. 2012, Stielow et al. 2015, Vu et al. 2019). Various molecular markers have been utilised for species recognition in Calonectria (Lombard et al. 2010a, 2016, Li et al. 2017, Marin-Felix et al. 2017, Jayawardena et al. 2019). But there is a lack of consistency in the markers used in different studies. There remains a need for a standard suite of DNA barcodes to distinguish among all species in Calonectria. This can be a complex goal given that barcodes for a particular species complex in a genus is not necessarily best suited to other species complexes, as has been seen in Fusarium (Lombard et al. 2019, Maryani et al. 2019, Sandoval-Denis et al. 2019, Wang et al. 2019, Xia et al. 2019). Under these circumstances, it has become necessary to explore whether a similar situation also applies to Calonectria.
The aim of this study was to produce a comprehensive suite of DNA sequence data for all 169 currently recognised species of Calonectria using eight gene regions. These data were then utilised to construct a genus-wide phylogeny and to reconsider the phylogenetic relationships and species boundaries for Calonectria. Furthermore, the resolution power of each of these eight gene regions was evaluated for the genus as a whole and the species complexes that define it. This made it possible to identify an optimal suite of DNA barcodes that can be used to reliably identify Calonectria species.
Materials and methods
Isolates
Sequence data for all the 169 described Calonectria species (including data for the ex-type isolates) were included in the phylogenetic analyses. Of these, 240 isolates representing 128 species (Supplementary Table S1) were obtained from culture collections, including those of the Westerdijk Fungal Biodiversity Institute (CBS), Utrecht, The Netherlands, the culture collection of the Forestry Agricultural and Biotechnology Institute, University of Pretoria, Pretoria (CMW), South Africa, and the Culture Collection of the China Eucalypt Research Centre (CERC), Chinese Academy of Forestry (CAF), ZhanJiang, GuangDong Province, China. Isolates from these culture collections were plated onto 2 % malt extract agar (MEA: 20 g malt extract and 20 g agar per litre water) and subsequently single hyphal-tips were transferred to fresh MEA plates and incubated at 25 °C for 7 d. It was not possible to obtain cultures for 76 isolates representing 44 of the described Calonectria species (Supplementary Table S2). In this case, sequences were downloaded from GenBank (http://www.ncbi.nlm.nih.gov) and included in the analyses. For three species (Ca. angustata, Ca. pini and Ca. metrosideri), isolates were sequenced and data were also downloaded from GenBank. They are consequently included in both Supplementary Tables S1 and S2. The fungarium specimens for the novel taxa represented by dried sporulating cultures were deposited in the Herbarium Mycologicum, Academiae Sinicae (HMAS), Beijing, China.
DNA extraction, PCR and sequencing
For the 240 Calonectria isolates representing 128 species obtained from culture collections (Supplementary Table S1) and used in this study, mycelium was harvested from 7-d-old cultures on MEA using a sterile scalpel, and genomic DNA was extracted using the CTAB method “5” described by Van Burik et al. (1998). Eight different loci were amplified and sequenced, including act, cmdA, his3, ITS, LSU, rpb2, tef1 and tub2. Primers ACT-512F and ACT-783R (Carbone & Kohn 1999) were used to amplify the act gene region; CAL-228F and CAL-2Rd (Carbone & Kohn 1999, Quaedvlieg et al. 2011) for the cmdA gene region; CYLH3F and CYLH3R (Crous et al. 2004) for the his3 gene region; V9G (De Hoog & van den Ende 1998) and ITS4 (White et al. 1990) for the ITS region; LR0R (Moncalvo et al. 1995) and LR5 (Vilgalys & Hester 1990) for the LSU region; fRpb2-5F and fRpb2-7cR (Liu et al. 1999, Reeb et al. 2004) for the rpb2 gene region; EF1-728F (Carbone & Kohn 1999) and EF2 (O’Donnell et al. 1998) for the tef1 gene region and primers pairs T1 (O’Donnell & Cigelnik 1997) and CYLTUB1R (Crous et al. 2004) were used to amplify the tub2 gene region.
The PCR reaction mixtures consisted of 17.5 μL TopTaq™ Master Mix, 1 μL of each primer, 2 μL DNA samples and nuclease-free H2O were made up to the final volume of 35 μL. The PCR conditions for the seven regions (with the exception of rpb2) were as follows: an initial denaturation step at 95 °C for 5 min; then 35 amplification cycles at [95 °C for 30 s; annealing 61 °C (act) / 55 °C (cmdA, his3, ITS, LSU) / 52 °C (tef1 and tub2) for 30 s; 72 °C for 1 min], and the final extension step at 72 °C for 10 min. For the rpb2 gene region, a touchdown PCR protocol was used: an initial denaturation step at 95 °C for 5 min, then (95 °C for 30 s, 58 °C for 30 s, 72 °C for 90 s) × 10 cycles, (95 °C for 30 s, 58 °C for 45 s, 72 °C for 90 s + 5 s/cycle increase) × 30 cycles, and final extension step at 72 °C for 10 min.
All the PCR products were sequenced in both directions using the same primers used for amplification. Raw sequences for each gene region were edited and consensus sequences were generated using Geneious v. 9.1.4 (Kearse et al. 2012).
Phylogenetic analyses
Sequence data for eight gene regions of 316 Calonectria isolates were used in the phylogenetic analyses. These were derived from 240 isolates representing 128 species obtained from culture collections and sequenced in this study (Supplementary Table S1). For the 76 isolates representing 44 species that we were unable to obtain, available sequence data were downloaded from GenBank (Supplementary Table S2). Sequence data for Curvicladiella cignea (CBS 109167 and CBS 109168) were used as the outgroup taxa in the analyses following the example of Pham et al. (2019). Multiple sequences were aligned using the online version of MAFFT v. 7 (Katoh & Standley 2013) and the alignment was trimmed at both ends where necessary with MEGA v. 6.0.5 (Tamura et al. 2013). For some of the 316 isolates, not all the eight gene regions were available, and here all of the available sequences were used in the individual analyses. In the case of the eight gene region combined analyses, missing sequence data were replaced with “N”.
Two phylogenetic approaches were used in this study. These included Maximum Parsimony (MP) analyses performed with PAUP v. 4.0 b10 (Swofford 2003) and Maximum Likelihood (ML) analyses performed with PhyML v. 3.0 (Guindon & Gascuel 2003). The sequence datasets for the eight individual gene regions and a concatenated dataset for those regions were used to determine the phylogenetic relationships among species. A partition homogeneity test (PHT) (Farris et al. 1994) was conducted to determine whether the datasets for the eight gene regions could be combined.
For the MP analyses, all characters were unordered and equally weighted. Gaps were regarded as a fifth character, phylogenetic trees were obtained using a heuristic tree search criterion, including 1 000 random stepwise additions and tree-bisection-reconstruction (TBR) branch swapping. Branches of zero-length were collapsed. Statistical supports for tree-branch points were determined using bootstrap analyses with 1 000 replicates (Felsenstein 1985). Tree length (TL), retention index (RI), consistency index (CI), rescaled consistency index (RC) and homoplasy index (HI) (Supplementary Table S3) were calculated for parsimony trees.
For the ML analyses, the best substitution model for each dataset was selected by JModeltest v. 2.1.7 (Posada 2008). All sequences generated in this study were submitted to GenBank (www.ncbi.nlm.nih.gov) (Supplementary Table S1) and final alignments were deposited in TreeBASE (http://treebase.org).
Individual Calonectria species were recognised based on concordance of multiple gene genealogies. Two criteria were applied in determining species boundaries based on phylogenetic analyses and sequence comparisons. These were (i) when isolate(s) formed a distinct lineage that differentiated them from other isolates in at least two of the eight individual gene regions sequenced and where these groupings did not contradict those for other loci; (ii) when the isolates formed independent lineages supported by high bootstrap values in the combined tree based on the eight-gene concatenated dataset, and where they had fixed Single Nucleotide Polymorphisms (SNPs) that differentiated them from their phylogenetically closest relatives. These phylogenetically defined taxa were then also considered in terms of their morphology and mating type. To avoid confusion, in Supplementary Tables S1 and S2, the code “A” was used to represent the Calonectria species before the taxonomic reconsideration of species boundaries in the present study. The code “B” was used for the 120 species names that have been accepted after revision and as presented in the Taxonomy section of this study.
Morphology, mating system and geographic distribution
Depending on their availability, isolates representing the novel Calonectria spp. were selected for morphological study. Synthetic nutrient-poor agar (SNA, Nirenburg 1981) was used to induce the asexual morphs in culture. Agar plugs from axenic cultures were transferred to five replicate plates of SNA and incubated at 25 °C for 7 d. Fungal structures were transferred to a drop of 85 % lactic acid on microscope slides. Gross morphological characteristics were examined with a Zeiss Axio Imager A1 microscope (Carl Zeiss Ltd., Germany).
For isolates selected as the holotype of novel taxa, each characteristic morphological structure was measured with 50 replicates, and 30 replicate measurements were made for additional isolates. Minimum, maximum and average (mean) measurements were recorded as (minimum–) (average–standard deviation)–(aver+standard deviation) (–maximum).
Optimal growth temperatures for each of the novel Calonectria spp. were determined on MEA. Agar plugs were transferred from the actively growing edges of 7-d-old cultures with a 5 mm diam cork borer and inoculated at the centres of 90 mm Petri dishes containing MEA. Cultures were grown at seven different temperatures ranging from 5 °C to 35 °C, at 5 °C intervals (each isolate with five replicates). Colony diameters were measured after seven days. Colony colours were described based on the colour charts of Rayner (1970) and using 7-d-old cultures on MEA incubated at 25 °C.
Morphological characteristics of all Calonectria species were re-evaluated in comparison to multilocus DNA sequence phylogenetic analyses. The important morphological features include vesicle shape and diameter, macroconidial septation and dimensions, perithecial colour, number of ascospores in the asci, ascospore septation and dimensions. In addition, ex-type isolate location and the mating system (heterothallic or homothallic) were also considered where this was known.
Selection of DNA barcodes for species recognition
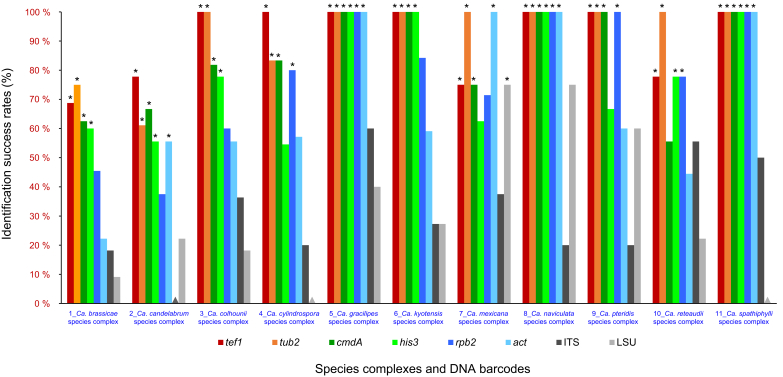
The analyses of DNA sequences for eight gene regions made it possible to identify clear boundaries between putative Calonectria species. These data formed the basis to test the success of each of these gene regions to distinguish species. This Identification Success Rate (ISR) was calculated by dividing the number of identified species for each gene region by the total number of species of Calonectria recognised in the phylogenetic analyses of eight gene regions.
The ISR of each candidate DNA barcode was computed based on the eight individual gene phylogenetic trees and the eight-gene combined phylogenetic tree. For each candidate DNA barcode, the ISRs were calculated both for the entire genus Calonectria, as well for each of the recognised species complexes.
Results
Phylogenetic analyses
Sequence data generated for the eight gene regions of the 240 isolates representing 128 of the 169 described species of Calonectria were deposited in GenBank (Supplementary Table S1). Amplicons generated for act, cmdA, his3, ITS, LSU, rpb2, tef1 and tub2 gene regions were approximately 300, 750, 500, 700, 870, 860, 560, 650 bp, respectively. Sequence data for the 76 isolates representing 44 species for which cultures could not be obtained were downloaded from GenBank and included in the final datasets (Supplementary Table S2).
Alignments for each of the gene regions and for the combined datasets were as follows: act (246 isolates, 286 characters), cmdA (314 isolates, 721 characters), his3 (305 isolates, 498 characters), ITS (268 isolates, 705 characters), LSU (267 isolates, 866 characters), rpb2 (234 isolates, 863 characters), tef1 (316 isolates, 563 characters), tub2 (285 isolates, 652 characters) and combined (318 isolates, 5 154 characters). The partition homogeneity test (PHT) performed on the concatenated dataset of eight gene regions yielded a P-value of 0.01. This suggested some incongruence in the datasets for the eight regions, and the accuracy of the combined data could have suffered relative to the individual partitions (Cunningham 1997). Although the P-value was low, the datasets for multiple gene regions were combined for phylogenetic analyses, as has been done in a number of previous studies (Lombard et al. 2016, Marin-Felix et al. 2017, Pham et al. 2019).
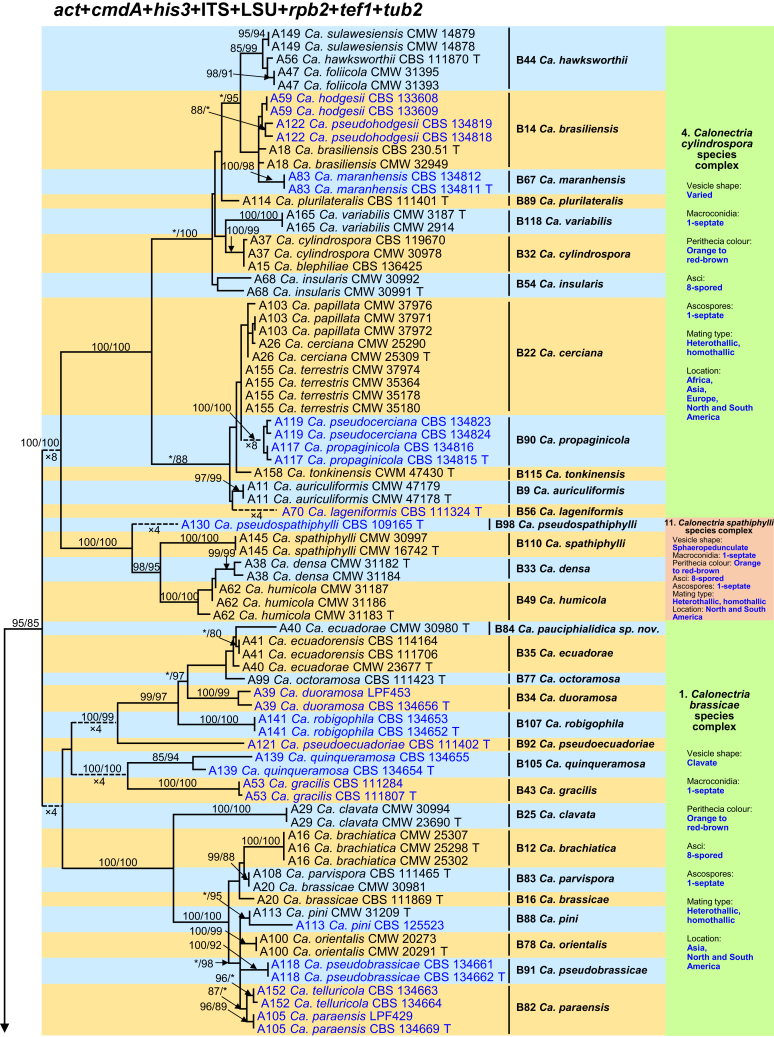
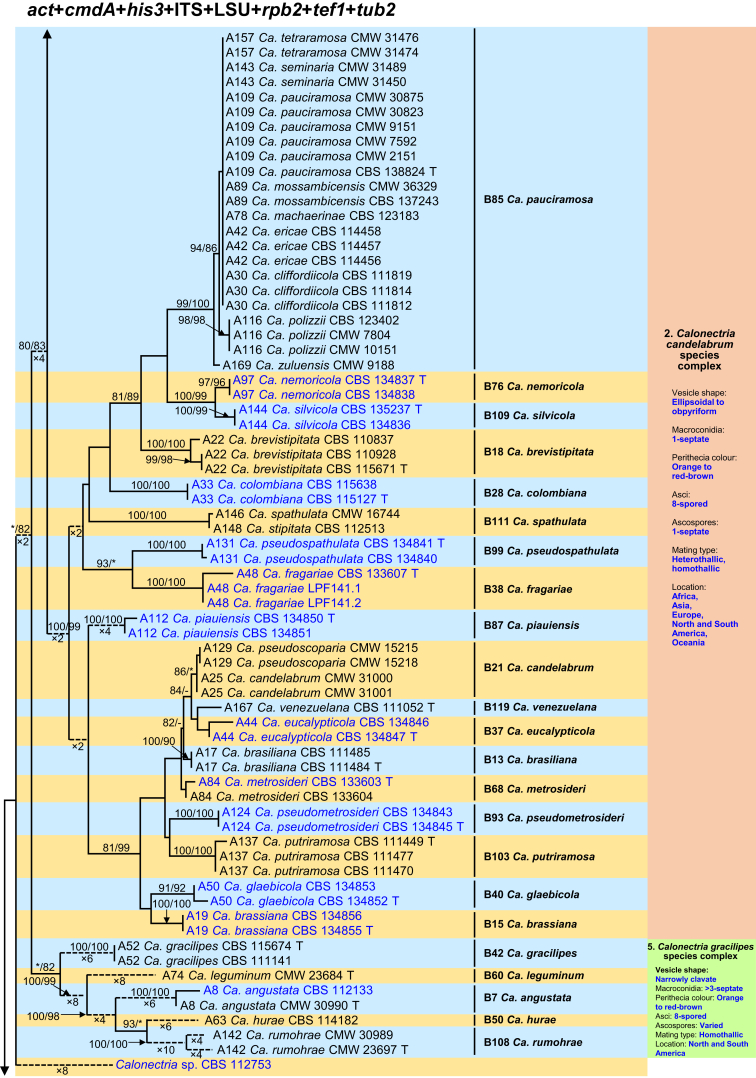
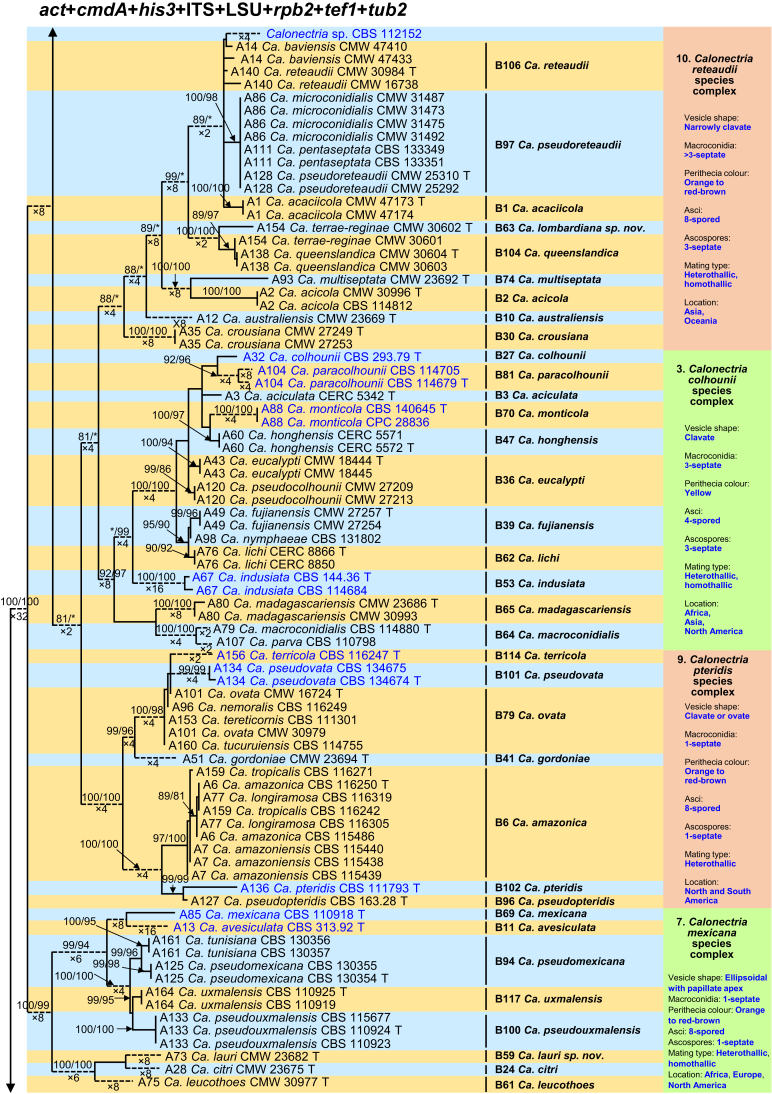
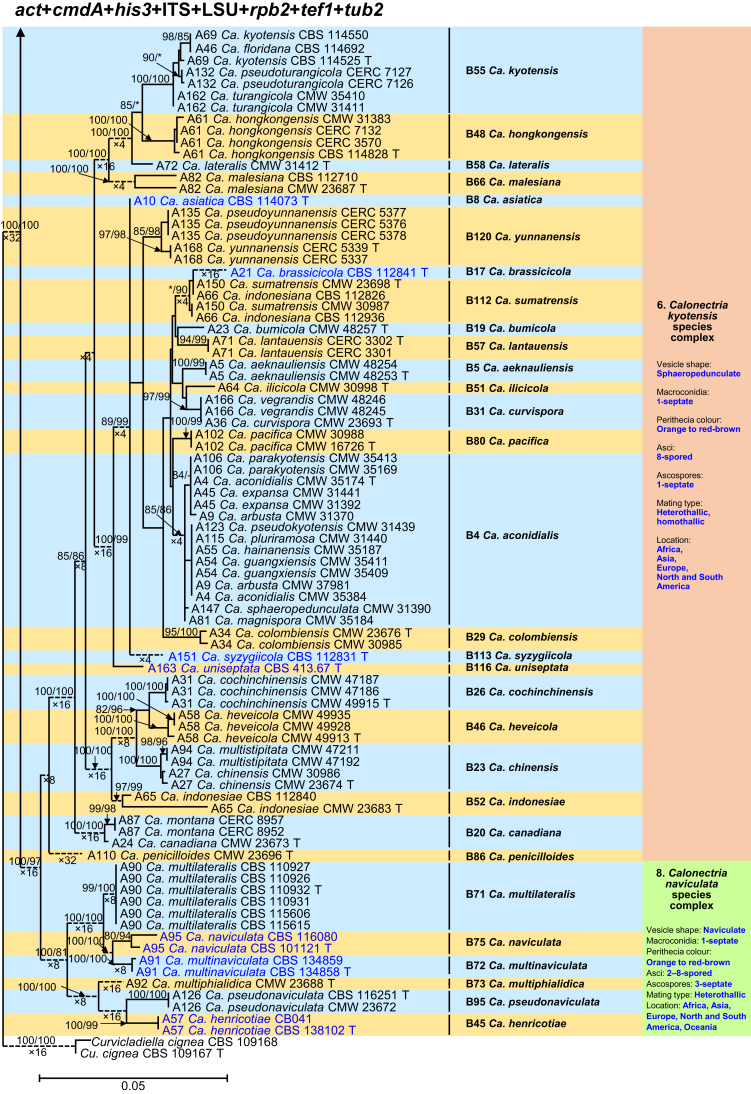
Phylogenetic analyses based on the eight individual gene regions and the combined sequence datasets were conducted using both the ML and MP methods (TreeBASE No. 26083). Tree topologies derived from the ML and MP analyses of the six individual gene regions (act, cmdA, his3, rpb2, tef1 and tub2) and the combined datasets were essentially congruent with each other and they formed well-supported lineages generally matching the morphological features of the purported species. The non-coding gene regions (ITS and LSU) are known to have poor ability to discriminate between Calonectria species (Lombard et al. 2010a) and several species resided in a single clade even though they had clearly different morphological characteristics (Supplementary Figs S4, S5). Only the ML trees are presented in this study, and bootstrap support values from ML and MP analyses are indicated above the tree branches (Fig. 1, Supplementary Figs S1–8). Statistical results and important parameters emerging from the phylogenetic analyses were provided in Supplementary Table S3.
Fig. 1.
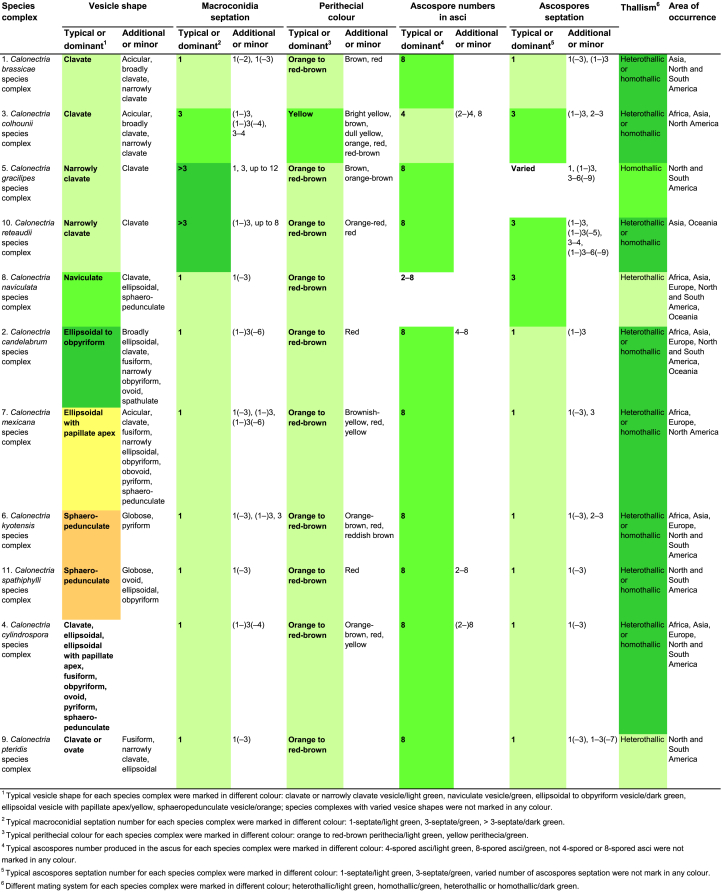
Phylogenetic tree of the genus Calonectria based on maximum likelihood (ML) analyses of combined DNA dataset of act, cmdA, his3, ITS, LSU, rpb2, tef1 and tub2 gene sequences. Codes A1 to A169 showed the 169 Calonectria species before their taxonomic reconsideration in this study, codes B1 to B120 indicated the 120 accepted Calonectria species after the revision emerging from this study. Seventy-six isolates unable to obtain in this study were marked in blue, their sequences were downloaded from NCBI. Bootstrap values ≥ 80 % for ML and MP analyses are presented at the branches. Bootstrap values lower than 80 % are marked with “∗”, and absent analyses values are marked with “-”. Ex-type isolates of Calonectria species after revision are marked with “T”. The tree is rooted to Curvicladiella cignea (CBS 109167, and CBS 109168). Calonectria species grouped in 11 species complexes. Typical morphological characteristics, mating type and location (geographic regions where isolates have been identified) of the 11 species complexes are shown at the right side of the phylogenetic tree.
The combined datasets for the eight sequenced gene regions comprised 5 154 characters, including alignment gaps. Of these, 3 472 were parsimony-uninformative and 1 682 were parsimony-informative. The eight-gene phylogenetic tree (Fig. 1) divided the Calonectria spp. into 11 well-supported clades and these reside in two major groups, the Prolate Group and the Sphaero-Naviculate Group. In the Prolate Group, species are characterised by their clavate to pyriform to ellipsoidal vesicles. Nine well-supported clades reside in the Prolate Group. This group includes nine species complexes including the Ca. brassicae, Ca. candelabrum, Ca. colhounii, Ca. cylindrospora, Ca. gracilipes, Ca. mexicana, Ca. pteridis, Ca. reteaudii and Ca. spathiphylli species complexes (Fig. 1). The remaining two well-supported clades reside in the Sphaero-Naviculate Group. The Sphaero-Naviculate Group is defined by vesicles having sphaeropedunculate or naviculate morphology. The two clades in this group accommodate two species complexes, those defined by Ca. kyotensis and Ca. naviculata (Fig. 1).
The Calonectria species before their taxonomic reconsideration in this study (codes A1 to A169) and after the revision emerging from this study (codes B1 to B120) are presented (Supplementary Tables S1 and S2, Fig. 1, Supplementary Figs S1–8). Based on the results of this study and a reconsideration of species boundaries, all accepted species are listed in Table 1. These species are also presented in the Taxonomy section below together with the taxonomic synonyms.
Table 1.
Accepted Calonectria species emerging from the results of this study.
| Code B1 | Accepted species name2 | Isolates representing the species 3,4,5 | Other collection number5 | Hosts | Area of occurrence | Collector | GenBank accession Numbers6 |
References or source of data |
|---|---|---|---|---|---|---|---|---|
| act; cmdA; his3; ITS; LSU; rpb2; tef1; tub2 | ||||||||
| B1 | Calonectria acaciicola | CMW 47173T | CBS 143557 | Soil (Acacia auriculiformis plantation) | Do Luong, Nghe An, Vietnam | N.Q. Pham & T.Q. Pham | MT3349337; MT335160; MT335399; MT359620; MT359380; MT412474; MT412690; MT412930 | Pham et al. (2019), this study |
| CMW 47174 | CBS 143558 | Soil (A. auriculiformis plantation) | Do Luong, Nghe An, Vietnam | N.Q. Pham & T.Q. Pham | MT334934; MT335161; MT335400; MT359621; MT359381; MT412475; MT412691; MT412931 | Pham et al. (2019), this study | ||
| B2 | Ca. acicola | CMW 30996T | – | Phoenix canariensis | Northland, New Zealand | H. Pearson | MT334935; MT335162; MT335401; MT359622; MT359382; MT412476; MT412692; MT412932 | Gadgil & Dick (2004), Lombard et al. (2010a), this study |
| CBS 114812 | CMW 51216 | P. canariensis | Northland, New Zealand | H. Pearson | MT334936; MT335163; MT335402; MT359623; MT359383; MT412477; MT412693; MT412933 | Gadgil & Dick (2004), Lombard et al. (2010a), this study | ||
| B3 | Ca. aciculata | CERC 5342T | CBS 142883; CMW 47645 | Eucalyptus urophylla × E. grandis | YunNan, China | S.F. Chen & J.Q. Li | MT334937; MT335164; MT335403; MT359624; MT359384; MT412478; MT412694; MT412934 | Li et al. (2017), this study |
| B4 | Ca. aconidialis | CMW 35174T | CBS 136086; CERC 1850 | Soil (Eucalyptus plantation) | HaiNan, China | X. Mou & S.F. Chen | MT334938; MT335165; MT335404; MT359625; MT359385; MT412479; MT412695; N/A8 | Lombard et al. (2015a), this study |
| CMW 35384 | CBS 136091; CERC 1886 | Soil (Eucalyptus plantation) | HaiNan, China | X. Mou & S.F. Chen | MT334939; MT335166; MT335405; MT359626; MT359386; N/A; MT412696; N/A | Lombard et al. (2015a), this study | ||
| CMW 31370 | CBS 136079; CERC 1705 | Soil (Eucalyptus plantation) | GuangXi, China | X. Zhou, G. Zhao & F. Han | MT334940; MT335167; MT335406; MT359627; MT359387; MT412480; MT412697; N/A | Lombard et al. (2015a), this study | ||
| CMW 31390 | CBS 136081; CERC 1725 | Soil (Eucalyptus plantation) | GuangXi, China | X. Zhou, G. Zhao & F. Han | MT334952; MT335179; MT335418; MT359639; MT359399; MT412485; MT412709; N/A | Lombard et al. (2015a), this study | ||
| CMW 31392 | CBS 136247; CERC 1727 | Soil (Eucalyptus plantation) | GuangXi, China | X. Zhou, G. Zhao & F. Han | MT334942; MT335169; MT335408; MT359629; MT359389; MT412481; MT412699; N/A | Lombard et al. (2015a), this study | ||
| CMW 31439 | CBS 137332; CERC 1774 | Soil (Eucalyptus plantation) | GuangXi, China | X. Zhou, G. Zhao & F. Han | MT334951; MT335178; MT335417; MT359638; MT359398; N/A; MT412708; N/A | Lombard et al. (2015a), this study | ||
| CMW 31440 | CBS 136976; CERC 1775 | Soil (Eucalyptus plantation) | GuangXi, China | X. Zhou, G. Zhao & F. Han | MT334950; MT335177; MT335416; MT359637; MT359397; N/A; MT412707; N/A | Lombard et al. (2015a), this study | ||
| CMW 35169 | CBS 136085; CERC 1845 | Soil (Eucalyptus plantation) | GuangDong, China | X. Mou & R. Chang | MT334948; MT335175; MT335414; MT359635; MT359395; MT412483; MT412705; N/A | Lombard et al. (2015a), this study | ||
| CMW 35184 | CBS 136249; CERC 1860 | Soil (Eucalyptus plantation) | GuangXi, China | X. Mou & R. Chang | MT334947; MT335174; MT335413; MT359634; MT359394; N/A; MT412704; N/A | Lombard et al. (2015a), this study | ||
| CMW 35187 | CBS 136248; CERC 1863 | Soil (Eucalyptus plantation) | HaiNan, China | X. Mou & S.F. Chen | MT334946; MT335173; MT335412; MT359633; MT359393; N/A; MT412703; N/A | Lombard et al. (2015a), this study | ||
| CMW 35409 | CBS 136092; CERC 1900 | Soil (Eucalyptus plantation) | GuangXi, China | X. Mou & R. Chang | MT334944; MT335171; MT335410; MT359631; MT359391; N/A; MT412701; N/A | Lombard et al. (2015a), this study | ||
| B5 | Ca. aeknauliensis | CMW 48253T | CBS 143559 | Soil (Eucalyptus plantation) | Aek Nauli, North Sumatra, Indonesia | M.J. Wingfield | MT334953; MT335180; MT335419; MT359640; MT359400; MT412486; MT412710; N/A | Pham et al. (2019), this study |
| CMW 48254 | CBS 143560 | Soil (Eucalyptus plantation) | Aek Nauli, North Sumatra, Indonesia | M.J. Wingfield | MT334954; MT335181; MT335420; MT359641; MT359401; MT412487; MT412711; N/A | Pham et al. (2019), this study | ||
| B6 | Ca. amazonica | CBS116250T | CMW 51234; CPC 3534 | E. tereticornis | Amazon, Brazil | P.W. Crous & A.C. Alfenas | MT334955; MT335182; MT335421; MT359642; MT359402; MT412488; MT412712; MT412935 | Lombard et al. (2016), this study |
| CBS 115486 | CMW 51223; CPC 3894 | E. tereticornis | Amazon, Brazil | P.W. Crous & A.C. Alfenas | MT334956; MT335183; MT335422; MT359643; MT359403; MT412489; MT412713; MT412936 | Lombard et al. (2016), this study | ||
| CBS 115440 | CMW 51222; CPC 3885 | E. tereticornis | Amazon, Brazil | P.W. Crous & A.C. Alfenas | MT334957; MT335184; MT335423; MT359644; MT359404; N/A; MT412714; MT412937 | Lombard et al. (2016), this study | ||
| CBS 116271 | CMW 51236; CPC 3559 | Eucalyptus sp. | Amazon, Brazil | P.W. Crous & A.C. Alfenas | MT335148; MT335385; MT335625; MT359846; MT359606; MT412677; MT412916; MT413123 | Lombard et al. (2016), this study | ||
| CBS 116319 | CMW 51832; CPC 3761 | Eucalyptus sp. | Amazon, Brazil | P.W. Crous & A.C. Alfenas | MT334960; MT335187; MT335426; MT359647; MT359407; MT412490; MT412717; MT412940 | Marin-Felix et al. (2017), this study | ||
| B7 | Ca. angustata | CMW 30990T | CBS 114544; CPC 2347; P99-0454 | Tillandsia capitata | Sarasota nursery, Florida, USA | R.M. Leahy | MT334963; N/A; MT335429; MT359650; MT359410; MT412493; MT412720; MT412943 | Crous et al. (2000, 2006), Lombard et al. (2010a), this study |
| CBS 112133 | CMW 30983; CPC 3152; P99-1321 | Tillandsia capitata | USA | R.M. Leahy | GQ280427; GQ267362; DQ190695; GQ280549; GQ280670; KY653360; FJ918552; DQ190593 | Crous et al. (2000, 2006), Lombard et al. (2010a), Marin-Felix et al. (2017) | ||
| B8 | Ca. asiatica | CBS 114073T | CMW 23782; CPC 3900 | Debris (leaf litter) | Prathet Thai, Thailand | N.L. Hywel-Jones | GQ280428; AY725741; AY725658; GQ280550; GQ280672; N/A; AY725705; AY725616 | Crous et al. (2004), Lombard et al. (2010a) |
| B9 | Ca. auriculiformis | CMW 47178T | CBS 143561 | Soil (A. auriculiformis plantation) | Hau Loc, Thanh Hoa, Vietnam | N.Q. Pham & T.Q. Pham | MT334964; MT335190; MT335430; MT359651; MT359411; MT412494; MT412721; MT412944 | Pham et al. (2019), this study |
| CMW 47179 | CBS 143562 | Soil (A. auriculiformis plantation) | Hau Loc, Thanh Hoa, Vietnam | N.Q. Pham & T.Q. Pham | N/A; MT335191; MT335431; MT359652; MT359412; MT412495; MT412722; MT412945 | Pham et al. (2019), this study | ||
| B10 | Ca. australiensis | CMW 23669T | CBS 112954; CPC 4714 | Ficus pleurocarpa | Queensland, Australia | C. Pearce & B. Paulus | MT334965; MT335192; MT335432; MT359653; MT359413; MT412496; MT412723; MT412946 | Crous et al. (2006), Lombard et al. (2010a), this study |
| B11 | Ca. avesiculata | CBS 313.92T | ATCC 38226; CMW 23670; CPC 2373 | Ilex vomitoria | Cairo, Georgia, USA | S.A. Alfieri | GQ280431; GQ267364; DQ190620; GQ280553; GQ280675; N/A; GQ267294; AF333392 | Schubert et al. (1989), Crous (2002), Lombard et al. (2010a) |
| B12 | Ca. brachiatica | CMW 25298T | CBS 123700 | Pinus maximinoi | Buga, Colombia | M.J. Wingfield | N/A; MT335195; MT335435; MT359656; MT359416; MT412499; MT412726; MT412948 | Lombard et al. (2009), this study |
| CMW 25302 | – | Pi. tecunumanii | Buga, Colombia | M.J. Wingfield | N/A; MT335196; MT335436; MT359657; MT359417; MT412500; MT412727; MT412949 | Lombard et al. (2009), this study | ||
| CMW 25307 | – | Pi. tecunumanii | Buga, Colombia | M.J. Wingfield | N/A; MT335197; MT335437; MT359658; MT359418; MT412501; MT412728; MT412950 | Lombard et al. (2009), this study | ||
| B13 | Ca. brasiliana | CBS 111484T | CMW 51187; CPC 1924 | Soil | Brazil | A.C. Alfenas | MT334968; MT335198; MT335438; MT359659; MT359419; MT412502; MT412729; MT412951 | Lombard et al. (2016), this study |
| CBS 111485 | CMW 51188; CPC 1929 | Soil | Brazil | A.C. Alfenas | MT334969; MT335199; MT335439; MT359660; MT359420; MT412503; MT412730; MT412952 | Lombard et al. (2016), this study | ||
| B14 | Ca. brasiliensis | CBS 230.51T | IMI 299576 | Eucalyptus sp. | Ceara state, Brazil | T.R. Ciferri | MT334970; MT335200; MT335440; MT359661; MT359421; MT412504; MT412731; MT412953 | Batista (1951), Crous (2002), Lombard et al. (2010b), this study |
| CMW 32949 | CBS 114257; CPC 1944 | Eucalyptus sp. | Aracruz, Brazil | A.C. Alfenas | MT334971; MT335201; MT335441; MT359662; MT359422; MT412505; MT412732; MT412954 | Lombard et al. (2010a), this study | ||
| CBS 133609 | LPF245 | Anadenanthera peregrina | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | N/A; KC491222; N/A; N/A; N/A; N/A; KC491225; KC491228 | Alfenas et al. (2013b, 2015) | ||
| CBS 134818 | LPF262 | Azadirachta indica (leaf) | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | N/A; KM395991; KM396079; N/A; N/A; N/A; KM395817; KM395905 | Alfenas et al. (2015) | ||
| B15 | Ca. brassiana | CBS 134855T | LPF378 | Soil (Eucalyptus brassiana plantation) | Teresina, Piauí, Brazil | R.F. Alfenas | N/A; KM396056; KM396139; N/A; N/A; N/A; KM395882; KM395969 | Alfenas et al. (2015) |
| CBS 134856 | LPF379 | Soil (E. brassiana plantation) | Teresina, Piauí, Brazil | R.F. Alfenas | N/A; KM396057; KM396140; N/A; N/A; N/A; KM395883; KM395970 | Alfenas et al. (2015) | ||
| B16 | Ca. brassicae | CBS 111869T | CPC 2409 | Argyreia splendens | Indonesia | F. Bugnicourt | MT334972; MT335202; MT335442; MT359663; MT359423; MT412506; MT412733; MT412955 | Crous (2002), Lombard et al. (2010a, 2016), this study |
| B17 | Ca. brassicicola | CBS 112841T | CMW 51206; CPC 4552 | Soil (Brassica sp.) | Indonesia | M.J. Wingfield | N/A; KX784561; N/A; N/A; N/A; N/A; KX784689; KX784619 | Lombard et al. (2016) |
| B18 | Ca. brevistipitata | CBS 115671T | CMW 51226; CPC 949 | Soil | Mexico | P.W. Crous | MT334973; MT335203; MT335443; MT359664; MT359424; MT412507; MT412734; MT412956 | Lombard et al. (2016), this study |
| CBS 110928 | CMW 51170; CPC 951 | Soil | Mexico | P.W. Crous | MT334974; MT335204; MT335444; MT359665; MT359425; MT412508; MT412735; MT412957 | Lombard et al. (2016), this study | ||
| CBS 110837 | CMW 51163; CPC 913 | Soil | Mexico | P.W. Crous | MT335057; MT335289; MT335529; MT359750; MT359510; MT412586; MT412820; MT413034 | Lombard et al. (2016), this study | ||
| B19 | Ca. bumicola | CMW 48257T | CBS 143575 | Soil (Eucalyptus plantation) | Aek Nauli, North Sumatra, Indonesia | M.J. Wingfield | MT334975; MT335205; MT335445; MT359666; MT359426; MT412509; MT412736; N/A | Pham et al. (2019), this study |
| B20 | Ca. canadiana | CMW 23673T | CBS 110817; STE-U 499 | Picea sp. | Canada | S. Greifenhagen | MT334976; MT335206; MT335446; MT359667; MT359427; MT412510; MT412737; MT412958 | Kang et al. (2001b), Crous (2002), Lechat et al. (2010), this study |
| CERC 8952 | – | Soil | HeNan, China | S.F. Chen | MT335058; MT335290; MT335530; MT359751; MT359511; MT412587; MT412821; MT413035 | Liu & Chen (2017), this study | ||
| B21 | Ca. candelabrum | CMW 31000 | CPC 1675; UFV 117 | Eucalyptus sp. | Amazonas, Brazil | A.C. Alfenas | MT334977; MT335207; MT335447; MT359668; MT359428; MT412511; MT412738; MT412959 | Crous (2002), Lombard et al. (2010a, 2015b), this study |
| CMW 31001 | STE-U 1679; UFV 126 | Eucalyptus sp. | Amazonas, Brazil | A.C. Alfenas | MT334978; MT335208; MT335448; MT359669; MT359429; MT412512; MT412739; MT412960 | Crous (2002), Lombard et al. (2010a, 2015b), this study | ||
| CMW 15218 | CBS 125257 | E. grandis | Las Golondrinas, Pichincha, Ecuador | M.J. Wingfield | MT334979; MT335209; MT335449; MT359670; MT359430; MT412513; MT412740; MT412961 | Lombard et al. (2010a), this study | ||
| B22 | Ca. cerciana | CMW 25309T | CBS 123693 | E. urophylla × E. grandis hybrid cutting | CERC nursery, GuangDong, China | M.J. Wingfield & X.D. Zhou | MT334981; MT335211; MT335451; MT359672; MT359432; MT412515; MT412742; MT412963 | Lombard et al. (2010c), this study |
| CMW 25290 | CBS 123695 | E. urophylla × E. grandis hybrid cutting | CERC nursery, GuangDong, China | M.J. Wingfield & X.D. Zhou | MT334982; MT335212; MT335452; MT359673; MT359433; MT412516; MT412743; MT412964 | Lombard et al. (2010c), this study | ||
| CMW 35180 | CBS 136642; CERC 1856 | Soil (Eucalyptus plantation) | GuangDong, China | X. Mou & R. Chang | MT334986; MT335216; MT335456; MT359677; MT359437; MT412520; MT412747; MT412968 | Lombard et al. (2015a), this study | ||
| CMW 37976 | CBS 136097; CERC 1939; CPC 23517 | Soil (Eucalyptus plantation) | GuangDong, China | X. Mou & R. Chang | MT334983; MT335213; MT335453; MT359674; MT359434; MT412517; MT412744; MT412965 | Lombard et al. (2015a), this study | ||
| B23 | Ca. chinensis | CMW 23674T | CBS 114827; CPC 4101 | Soil | Hong Kong, China | E.C.Y. Liew | MT334990; MT335220; MT335460; MT359681; MT359441; MT412524; MT412751; MT412972 | Crous et al. (2004), Lombard et al. (2010a), this study |
| CMW 30986 | CBS 112744; CPC 4104 | Soil | Hong Kong, China | E.C.Y. Liew | MT334991; MT335221; MT335461; MT359682; MT359442; MT412525; MT412752; MT412973 | Crous et al. (2004), Lombard et al. (2010a), this study | ||
| CMW 47192 | CBS 143573 | Soil (Acacia hybrid plantation) | Tuyen Quang, Vietnam | N.Q. Pham & T.Q. Pham | MT335068; MT335300; MT335540; MT359761; MT359521; MT412597; MT412831; MT413045 | Pham et al. (2019), this study | ||
| B24 | Ca. citri | CMW 23675T | CBS 186.36 | Citrus sinensis | Florida, USA | H.S. Fawcett | MT334992; MT335222; MT335462; MT359683; MT359443; MT412526; MT412753; MT412974 | Fawcett & Klotz (1937), Crous (2002), this study |
| B25 | Ca. clavata | CMW 23690T | ATCC 66389; CBS 114557; CPC 2536; P078-1543 | Callistemon viminalis | Lake Placid, Florida, USA | C.P. Seymour & E.L. Barnard | MT334993; MT335223; MT335463; MT359684; MT359444; MT412527; MT412754; MT412975 | El-Gholl et al. (1993b), Crous (2002), Lombard et al. (2010a), this study |
| CMW 30994 | CBS 114666; CPC 2537; P078-1261 | Root debris in peat | Lee County, Florida, USA | D. Ferrin | MT334994; MT335224; MT335464; MT359685; MT359445; MT412528; MT412755; MT412976 | El-Gholl et al. (1993b), Crous (2002), Lombard et al. (2010a), this study | ||
| B26 | Ca. cochinchinensis | CMW 49915T | CBS 143567 | Soil (Hevea brasiliensis plantation) | Duong Minh Chau, Tay Ninh, Vietnam | N.Q. Pham, Q.N. Dang & T.Q. Pham | MT334995; MT335225; MT335465; MT359686; MT359446; MT412529; MT412756; MT412977 | Pham et al. (2019), this study |
| CMW 47186 | CBS 143568 | Soil (A. auriculiformis plantation) | Song May, Dong Nai, Vietnam | N.Q. Pham & T.Q. Pham | MT334996; MT335226; MT335466; MT359687; MT359447; MT412530; MT412757; MT412978 | Pham et al. (2019), this study | ||
| CMW 47187 | CBS 143569 | Soil (A. auriculiformis plantation) | Song May, Dong Nai, Vietnam | N.Q. Pham & T.Q. Pham | MT334997; MT335227; MT335467; MT359688; MT359448; MT412531; MT412758; MT412979 | Pham et al. (2019), this study | ||
| B27 | Ca. colhounii | CBS 293.79T | CMW 30999 | Camellia sinensis | Mauritius | A. Peerally | GQ280443; GQ267373; DQ190639; GQ280565; GQ280687; KY653376; GQ267301; DQ190564 | Peerally (1973), Crous (2002), Crous et al. (2006), Lombard et al. (2010a) |
| B28 | Ca. colombiana | CBS 115127T | CMW 30871; CPC 1160 | Soil | La Selva, Colombia | M.J. Wingfield | GQ280538; GQ267455; FJ972442; GQ280660; GQ280782; N/A; FJ972492; FJ972423 | Schoch et al. (1999), Crous (2002), Lombard et al. (2010a, b) |
| CBS 115638 | CMW 30766; CPC 1161 | Soil | La Selva, Colombia | M.J. Wingfield | GQ280539; GQ267456; FJ972441; GQ280661; GQ280783; N/A; FJ972491; FJ972422 | Schoch et al. (1999), Crous (2002), Lombard et al. (2010a, b) | ||
| B29 | Ca. colombiensis | CMW 23676T | CBS 112220; CPC 723 | Soil (E. grandis trees) | La Selva, Colombia | M.J. Wingfield | MT334998; MT335228; MT335468; MT359689; MT359449; MT412532; MT412759; MT412980 | Crous et al. (2004), this study |
| CMW 30985 | CBS 112221; CPC 724 | Soil (E. grandis trees) | La Selva, Colombia | M.J. Wingfield | MT334999; MT335229; MT335469; MT359690; MT359450; MT412533; MT412760; MT412981 | Crous et al. (2004), this study | ||
| B30 | Ca. crousiana | CMW 27249T | CBS 127198 | E. grandis | FuJian, China | M.J. Wingfield | MT335000; MT335230; MT335470; MT359691; MT359451; MT412534; MT412761; MT412982 | Chen et al. (2011), this study |
| CMW 27253 | CBS 127199 | E. grandis | FuJian, China | M.J. Wingfield | MT335001; MT335231; MT335471; MT359692; MT359452; MT412535; MT412762; MT412983 | Chen et al. (2011), this study | ||
| B31 | Ca. curvispora | CMW 23693T | CBS 116159; CPC 765 | Soil | Tamatave, Madagascar | P.W. Crous | MT335002; MT335232; MT335472; MT359693; MT359453; MT412536; MT412763; N/A | Victor et al. (1997), Crous (2002), Lombard et al.(2010a, 2015a), this study |
| CMW 48245 | CBS 143565 | Soil (Eucalyptus plantation) | Aek Nauli, North Sumatra, Indonesia | M.J. Wingfield | MT335003; MT335233; MT335473; MT359694; MT359454; MT412537; MT412764; N/A | Pham et al. (2019), this study | ||
| B32 | Ca. cylindrospora | CBS 119670 | CMW 51310; CPC 12766 | Pistacia lentiscus | Italy | N/A | MT335006; MT335236; MT335476; MT359697; MT359457; MT412540; MT412767; MT412985 | Lombard et al. (2015a,b, 2016), this study |
| CMW 30978 | CBS 110666; P90.1479; STE-U 497 | Ilex vomitoria | Florida, USA | N.E. El-Gholl | MT335007; MT335237; MT335477; MT359698; MT359458; MT412541; MT412768; MT412986 | Crous (2002), Lombard et al. (2010a, 2015b), this study | ||
| CBS 136425 | CMW 51321; CPC 21859 | Blephilia ciliata | Ellerbe, North Carolina, USA | T. Sharp | MT335005; MT335235; MT335475; MT359696; MT359456; MT412539; MT412766; MT412984 | Crous et al. (2013), this study | ||
| B33 | Ca. densa | CMW 31182T | CBS 125261 | Soil | Las Golondrinas, Pichincha, Ecuador | M.J. Wingfield | MT335008; MT335238; MT335478; MT359699; MT359459; N/A; MT412769; MT412987 | Lombard et al. (2010a), this study |
| CMW 31184 | CBS 125249 | Soil | Las Golondrinas, Pichincha, Ecuador | M.J. Wingfield | MT335009; MT335239; MT335479; MT359700; MT359460; N/A; MT412770; MT412988 | Lombard et al. (2010a), this study | ||
| B34 | Ca. duoramosa | CBS 134656T | LPF434 | Soil (tropical rainforest) | Monte Dourado, Pará, Brazil | R.F. Alfenas | N/A; KM396027; KM396110; N/A; N/A; N/A; KM395853; KM395940 | Alfenas et al. (2015) |
| LPF453 | – | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | N/A; KM396028; KM396111; N/A; N/A; N/A; KM395854; KM395941 | Alfenas et al. (2015) | ||
| B35 | Ca. ecuadorae | CMW 23677T | CBS 111406; CPC 1635 | Soil | Ecuador | M.J. Wingfield | MT335012; MT335242; MT335482; MT359703; MT359463; MT412544; MT412773; MT412991 | Crous et al. (2006), Lombard et al. (2010a), this study |
| CBS 111706 | CMW 51821; CPC 1636 | Soil | Ecuador | M.J. Wingfield | MT335010; MT335240; MT335480; MT359701; MT359461; MT412542; MT412771; MT412989 | Marin-Felix et al. (2017), this study | ||
| B36 | Ca. eucalypti | CMW 18444T | CBS 125275 | E. grandis | Aek Nauli, Sumatra Utara, Indonesia | M.J. Wingfield | MT335013; MT335243; MT335483; MT359704; MT359464; MT412545; MT412774; MT412992 | Lombard et al. (2010a), this study |
| CMW 18445 | CBS 125276 | E. grandis | Aek Nauli, Sumatra Utara, Indonesia | M.J. Wingfield | MT335014; MT335244; MT335484; MT359705; MT359465; MT412546; MT412775; MT412993 | Lombard et al. (2010a), this study | ||
| CMW 27209 | CBS 127195 | E. dunnii | FuJian, China | M.J. Wingfield | MT335108; MT335341; MT335581; MT359802; MT359562; MT412634; MT412872; MT413084 | Chen et al. (2011), this study | ||
| B37 | Ca. eucalypticola | CBS 134847T | LPF124 | Eucalyptus sp. (seedling) | Santa Barbara, Minas Gerais state, Brazil | A.C. Alfenas | N/A; KM396051; KM396134; N/A; N/A; N/A; KM395877; KM395964 | Alfenas et al. (2015) |
| CBS 134846 | LPF121 | Eucalyptus sp. (leaf) | Eunapolis, Bahia, Brazil | A.C. Alfenas | N/A; KM396050; KM396133; N/A; N/A; N/A; KM395876; KM395963 | Alfenas et al. (2015) | ||
| B38 | Ca. fragariae | CBS 133607T | LPP040 | Fragaria × ananassa | Santa Maria do Jetibá, Espírito Santo, Brazil | U.P. Lopes | N/A; KM998966; KM998964; N/A; N/A; N/A; KM998963; KM998965 | Lopes et al. (2017) |
| LPF141.1 | – | Fragaria × ananassa | Santa Maria do Jetibá, Espírito Santo, Brazil | U.P. Lopes | N/A; KX500191; KX500194; N/A; N/A; N/A; KX500197; KX500195 | Lopes et al. (2017) | ||
| LPF141.2 | – | Fragaria × ananassa | Santa Maria do Jetibá, Espírito Santo, Brazil | U.P. Lopes | N/A; KX500192; KX500193; N/A; N/A; N/A; KX500198; KX500196 | Lopes et al. (2017) | ||
| B39 | Ca. fujianensis | CMW 27257T | CBS 127201 | E. grandis | FuJian, China | M.J. Wingfield | MT335019; MT335249; MT335489; MT359710; MT359470; MT412551; MT412780; MT412998 | Chen et al. (2011), this study |
| CMW 27254 | CBS 127200 | E. grandis | FuJian, China | M.J. Wingfield | MT335020; MT335250; MT335490; MT359711; MT359471; MT412552; MT412781; MT412999 | Chen et al. (2011), this study | ||
| CBS 131802 | CMW 51317; HGUP 100003 | Nymphaea tetragona | Guiyang, Guizhou, China | S.Y. Qin | MT335070; MT335302; MT335542; MT359763; MT359523; MT412599; MT412833; MT413047 | Xu et al. (2012), this study | ||
| B40 | Ca. glaebicola | CBS 134852T | LPF406 | Soil (Eucalyptus plantation) | Martinho Campos, Minas Gerais, Brazil | A.C. Alfenas | N/A; KM396053; KM396136; N/A; N/A; N/A; KM395879; KM395966 | Alfenas et al. (2015) |
| CBS 134853 | LPF407 | Eucalyptus sp. (leaf) | Tocantins, Bico do Papagaio, Brazil | R.F. Alfenas | N/A; KM396054; KM396137; N/A; N/A; N/A; KM395880; KM395967 | Alfenas et al. (2015) | ||
| B41 | Ca. gordoniae | CMW 23694T | ATCC 201837; CBS 112142; P97-2567; STE-U 3136 | Gordonia lasianthus | Florida, USA | D. Chiappini | MT335021; MT335251; MT335491; MT359712; MT359472; MT412553; MT412782; MT413000 | Leahy et al. (2000), Crous (2002), Lombard et al. (2010a), this study |
| B42 | Ca. gracilipes | CBS 115674T | CMW 51227; STE-U 1153 | Soil | La Selva, Colombia | M.J. Wingfield | MT335022; MT335252; MT335492; MT359713; MT359473; MT412554; MT412783; MT413001 | Crous et al. (1997a, 2006), Crous (2002), this study |
| CBS 111141 | CMW 51174; CPC 1211 | Soil | La Selva, Colombia | M.J. Wingfield | MT335023; MT335253; MT335493; MT359714; MT359474; MT412555; MT412784; MT413002 | Crous (2002), Crous et al. (2006), this study | ||
| B43 | Ca. gracilis | CBS 111807T | AR2677; CMW 51189; PPRI 4176; STE-U 2634 | Manilkara zapota | Pará, Brazil | F. Carneiro de Albuquerque | GQ280488; GQ267407; DQ190646; GQ280610; GQ280732; KY653390; GQ267323; AF232858 | Crous et al. (1993c, 2006), Crous (2002), Lombard et al. (2016), Marin-Felix et al. (2017) |
| CBS 111284 | CMW 51175; CPC 1483 | Soil | Imbrapa, Brazil | P.W. Crous | GQ280489; GQ267408; DQ190647; GQ280611; GQ280733; KY653389; GQ267324; DQ190567 | Crous et al. (1993c, 2006), Crous (2002), Lombard et al. (2016), Marin-Felix et al. (2017) | ||
| B44 | Ca. hawksworthii | CBS 111870T | CMW 51194; CPC 2405 | Nelumbo nucifera | Pamplemousses garden, Mauritius | A. Peerally | MT335024; MT335254; MT335494; MT359715; MT359475; MT412556; MT412785; MT413003 | Crous (2002), this study |
| CMW 14878 | CBS 125277 | Eucalyptus sp. | Sulawesi, Indonesia | M.J. Wingfield | MT335141; MT335378; MT335618; MT359839; MT359599; MT412670; MT412909; MT413119 | Lombard et al. (2010a), this study | ||
| CMW 31393 | CBS 136641 | E. urophylla × E. grandis | GuangXi, China | X. Zhou & G. Zhao | MT335017; MT335247; MT335487; MT359708; MT359468; MT412549; MT412778; MT412996 | Lombard et al. (2015a), this study | ||
| B45 | Ca. henricotiae | CBS 138102T | CB045 | Buxus sempervirens | Lokeren, East Flanders, Belgium | B. Gehesquiere & K. Heungens | N/A; KF815157; KF815185; JX535322; N/A; N/A; N/A; JX535308 | Gehesquiere et al. (2015) |
| CB041 | – | B. sempervirens | Lokeren, East Flanders, Belgium | B. Gehesquiere & K. Heungens | N/A; KF815156; KF815184; N/A; N/A; N/A; N/A; KF815129 | Gehesquiere et al. (2015) | ||
| B46 | Ca. heveicola | CMW 49913T | CBS 143570 | Soil (Hevea brasiliensis plantation) | Bau Bang, Binh Duong, Vietnam | N.Q. Pham, Q.N. Dang & T.Q. Pham | MT335025; MT335255; MT335495; MT359716; MT359476; N/A; MT412786; MT413004 | Pham et al. (2019), this study |
| CMW 49928 | CBS 143571 | Soil | Bu Gia Map National Park, Binh Phuoc, Vietnam | N.Q. Pham, Q.N. Dang & T.Q. Pham | MT335048; MT335280; MT335520; MT359741; MT359501; MT412577; MT412811; MT413025 | Pham et al. (2019), this study | ||
| CMW 49935 | CBS 143572 | Soil | Bu Gia Map National Park, Binh Phuoc, Vietnam | N.Q. Pham, Q.N. Dang & T.Q. Pham | MT335049; MT335281; MT335521; MT359742; MT359502; MT412578; MT412812; MT413026 | Pham et al. (2019), this study | ||
| B47 | Ca. honghensis | CERC 5572T | CBS 142885; CMW 47669 | Soil (Eucalyptus plantation) | HongHe, YunNan, China | S.F. Chen & J.Q. Li | MT335026; MT335256; MT335496; MT359717; MT359477; MT412557; MT412787; MT413005 | Li et al. (2017), this study |
| CERC 5571 | CBS 142884; CMW 47668 | Soil (Eucalyptus plantation) | HongHe, YunNan, China | S.F. Chen & J.Q. Li | MT335027; MT335257; MT335497; MT359718; MT359478; MT412558; MT412788; MT413006 | Li et al. (2017), this study | ||
| B48 | Ca. hongkongensis | CBS 114828T | CMW 51217; CPC 4670 | Soil | Hong Kong, China | M.J. Wingfield | MT335028; MT335258; MT335498; MT359719; MT359479; MT412559; MT412789; MT413007 | Crous et al. (2004), this study |
| CERC 7132 | CMW 47499 | Soil | FuJian, China | S.F. Chen | MT335031; MT335261; MT335501; MT359722; MT359482; MT412562; MT412792; MT413010 | Li et al. (2017), this study | ||
| B49 | Ca. humicola | CMW 31183T | CBS 125251 | Soil | Las Golondrinas, Pichincha, Ecuador | M.J. Wingfield | MT335032; MT335262; MT335502; MT359723; MT359483; N/A; MT412793; MT413011 | Lombard et al. (2010a), this study |
| CMW 31186 | CBS 125252 | Soil | Las Golondrinas, Pichincha, Ecuador | L. Lombard | MT335033; MT335263; MT335503; MT359724; MT359484; N/A; MT412794; MT413012 | Lombard et al. (2010a), this study | ||
| CMW 31187 | CBS 125269 | Soil | Las Golondrinas, Pichincha, Ecuador | L. Lombard | MT335034; MT335264; MT335504; MT359725; MT359485; N/A; MT412795; MT413013 | Lombard et al. (2010a), this study | ||
| B50 | Ca. hurae | CBS 114182 | CMW 51823; CPC 1714; UFV 216 | Rumohra adiantiformis | Brazil | A.C. Alfenas | MT335035; MT335265; MT335505; MT359726; MT359486; MT412563; MT412796; MT413014 | Crous (2002), Crous et al. (2006), this study |
| B51 | Ca. ilicicola | CMW 30998T | CBS 190.50; IMI 299389; STE-U 2482 | Solanum tuberosum | Bogor, Java, Indonesia | K.B. Boedijn & J. Reitsma | MT335036; MT335266; MT335506; MT359727; MT359487; MT412564; MT412797; N/A | Boedijn & Reitsma (1950), Crous (2002), Lombard et al. (2010a), this study |
| B52 | Ca. indonesiae | CMW 23683T | CBS 112823; CPC 4508 | Syzygium aromaticum | Warambunga, Indonesia | M.J. Wingfield | MT335037; MT335267; MT335507; MT359728; MT359488; MT412565; MT412798; MT413015 | Crous et al. (2004), this study |
| CBS 112840 | CMW 51205; CPC 4554 | S. aromaticum | Warambunga, Indonesia | M.J. Wingfield | MT335038; MT335268; MT335508; MT359729; MT359489; MT412566; MT412799; MT413016 | Crous et al. (2004), this study | ||
| B53 | Ca. indusiata | CBS 144.36T | CMW 23699 | Camellia sinensis | Sri lanka | N/A | GQ280536; GQ267453; GQ267262; GQ280658; GQ280780; KY653396; GQ267332; GQ267239 | Crous (2002), Lombard et al. (2010a, 2016), Marin-Felix et al. (2017) |
| CBS 114684 | CMW 51213; CPC 2446; UFV16 | Rhododendron sp. | Florida, USA | N.E. El-Gholl | GQ280537; GQ267454; DQ190653; GQ280659; GQ280781; N/A; GQ267333; AF232862 | Crous et al. (1999, 2006), Crous (2002) | ||
| B54 | Ca. insularis | CMW 30991T | CBS 114558; CPC 768 | Soil | Tamatave, Madagascar | P.W. Crous | N/A; MT335269; MT335509; MT359730; MT359490; MT412567; MT412800; MT413017 | Schoch et al. (1999), Lombard et al. (2010a, 2016), this study |
| CMW 30992 | CBS 114559; CPC 954 | Soil | Conejos, Veracruz, Mexico | M.J. Wingfield | N/A; MT335270; MT335510; MT359731; MT359491; MT412568; MT412801; MT413018 | Lombard et al. (2010a, 2016), this study | ||
| B55 | Ca. kyotensis | CBS 114525T | ATCC 18834; CMW 51824; CPC 2367 | Robinia pseudoacacia | Japan | T. Terashita | MT335039; MT335271; MT335511; MT359732; MT359492; MT412569; MT412802; MT413019 | Terashita (1968), Crous (2002), Lombard et al. (2016), this study |
| CBS 114550 | CMW 51825; CPC 2351 | Soil | China | M.J. Wingfield | MT335016; MT335246; MT335486; MT359707; MT359467; MT412548; MT412777; MT412995 | Lombard et al. (2016), this study | ||
| CBS 114692 | ATCC18882; CMW 51826 | Prunus persica | Georgia, USA | N/A | MT335015; MT335245; MT335485; MT359706; MT359466; MT412547; MT412776; MT412994 | Sobers (1969), Crous (2002), Marin-Felix et al. (2017), this study | ||
| CERC 7126 | CBS 142890; CMW 47496 | Soil | FuZhou, FuJian, China | S.F. Chen | MT335121; MT335356; MT335596; MT359817; MT359577; MT412649; MT412887; MT413098 | Li et al. (2017), this study | ||
| CMW 31411 | CBS 136077 | Soil (Eucalyptus plantation) | GuangXi, China | X. Zhou, G. Zhao & F. Han | MT335151; MT335388; MT335628; MT359849; MT359609; N/A; MT412919; MT413126 | Lombard et al. (2015a), this study | ||
| B56 | Ca. lageniformis | CBS 111324T | CMW 51177; CPC 1473 | Eucalyptus sp. (leaf) | Rivière Noire, Mauritius | H. Smith | N/A; KX784574; N/A; KY653256; KY653312; KY653400; KX784702; KX784632 | Lombard et al. (2016), Marin-Felix et al. (2017) |
| B57 | Ca. lantauensis | CERC 3302T | CBS 142888; CMW 47252 | Soil | LiDao, Hong Kong, China | M.J. Wingfield & S.F. Chen | MT335040; MT335272; MT335512; MT359733; MT359493; MT412570; MT412803; N/A | Li et al. (2017), this study |
| CERC 3301 | CBS 142887; CMW 47251 | Soil | LiDao, Hong Kong, China | M.J. Wingfield & S.F. Chen | MT335041; MT335273; MT335513; MT359734; MT359494; N/A; MT412804; N/A | Li et al. (2017), this study | ||
| B58 | Ca. lateralis | CMW 31412T | CBS 136629 | Soil (Eucalyptus plantation) | GuangXi, China | X. Zhou, G. Zhao & F. Han | MT335042; MT335274; MT335514; MT359735; MT359495; MT412571; MT412805; MT413020 | Lombard et al. (2015a), this study |
| B59 | Ca. lauri sp. nov. | CMW 23682T | CBS 749.70 | Ilex aquifolium | Vijlen, Vijlenerbos, South-East Limburg, Netherlands | H.A. van der Aa | MT335043; MT335275; MT335515; MT359736; MT359496; MT412572; MT412806; MT413021 | Lechat et al. (2010), Lombard et al. (2010a), this study |
| B60 | Ca. leguminum | CMW 23684T | CBS 728.68 | Annona squamosa | Sao Paulo, Brazil | M.B. Figueiredo | MT335044; MT335276; MT335516; MT359737; MT359497; MT412573; MT412807; MT413022 | Figueiredo & Namekata (1967), Crous (2002), Lombard et al. (2010a), this study |
| B61 | Ca. leucothoes | CMW 30977T | ATCC 64824; CBS 109166; CPC 2385; P88-490 | Leucothoe axillaris | Florida, USA | N.E. El-Gholl | MT335045; MT335277; MT335517; MT359738; MT359498; MT412574; MT412808; N/A | El-Gholl et al. (1989), Crous (2002), Lombard et al. (2015a), this study |
| B62 | Ca. lichi | CERC 8866T | – | Soil | HeNan, China | S.F. Chen | MT335046; MT335278; MT335518; MT359739; MT359499; MT412575; MT412809; MT413023 | Liu & Chen (2017), this study |
| CERC 8850 | – | Soil | HeNan, China | S.F. Chen | MT335047; MT335279; MT335519; MT359740; MT359500; MT412576; MT412810; MT413024 | Liu & Chen (2017), this study | ||
| B63 | Ca. lombardiana sp. nov. | CMW 30602T | CBS 112634; CPC 4233; Lynfield 417 | Xanthorrhoea australis | Victoria, Australia | T. Baigent | MT335156; MT335395; MT335635; MT359856; MT359616; MT412686; MT412926; MT413133 | Crous (2002), Crous et al. (2006), Lombard et al. (2010c), this study |
| B64 | Ca. macroconidialis | CBS 114880T | CMW 51219; CPC 307; PPRI 4000 | E. grandis | Sabie, Mpumalanga, South Africa | P.W. Crous | MT335050; MT335282; MT335522; MT359743; MT359503; MT412579; MT412813; MT413027 | Crous et al. (1993c), Crous (2002), Lombard et al. (2010a), this study |
| CBS 110798 | CMW 51817; CPC 410 | E. grandis (roots) | Sabie, Mpumalanga, South Africa | P.W. Crous | MT335051; MT335283; MT335523; MT359744; MT359504; MT412580; MT412814; MT413028 | Lombard et al. (2016), this study | ||
| B65 | Ca. madagascariensis | CMW 23686T | CBS 114572; CPC 2252 | Soil | Rona, Madagascar | J.E. Taylor | MT335052; MT335284; MT335524; MT359745; MT359505; MT412581; MT412815; MT413029 | Crous (2002), Crous et al. (2006), Lombard et al. (2010a), this study |
| CMW 30993 | CBS 114571; CPC 2253 | Soil | Rona, Madagascar | J.E. Taylor | MT335053; MT335285; MT335525; MT359746; MT359506; MT412582; MT412816; MT413030 | Crous (2002), Crous et al. (2006), Lombard et al. (2010a), this study | ||
| B66 | Ca. malesiana | CMW 23687T | CBS 112752; CPC 4223 | Soil | Northern Sumatra, Indonesia | M.J. Wingfield | MT335054; MT335286; MT335526; MT359747; MT359507; MT412583; MT412817; MT413031 | Crous et al. (2004), this study |
| CBS 112710 | CMW 51199; CPC 3899 | Leaf litter | Prathet, Thailand | N.L. Hywel-Jones | MT335055; MT335287; MT335527; MT359748; MT359508; MT412584; MT412818; MT413032 | Crous et al. (2004), this study | ||
| B67 | Ca. maranhensis | CBS 134811T | LPF142 | Eucalyptus sp. (leaf) | Açailandia, Maranhao, Brazil | A.C. Alfenas | N/A; KM396035; KM396118; N/A; N/A; N/A; KM395861; KM395948 | Alfenas et al. (2015) |
| CBS 134812 | LPF143 | Eucalyptus sp. (leaf) | Açailandia, Maranhao, Brazil | A.C. Alfenas | N/A; KM396036; KM396119; N/A; N/A; N/A; KM395862; KM395949 | Alfenas et al. (2015) | ||
| B68 | Ca. metrosideri | CBS 133603T | LPF101 | Metrosideros polymorpha | Viçosa, Minas Gerais state, Brazil | R.F. Alfenas | N/A; KC294304; KC294307; N/A; N/A; N/A; KC294310; KC294313 | Alfenas et al. (2013a, 2015) |
| CBS 133604 | CMW 51320; LPF103 | Metrosideros polymorpha | Viçosa, Minas Gerais state, Brazil | R.F. Alfenas | MT335056; MT335288; MT335528; MT359749; MT359509; MT412585; MT412819; MT413033 | Alfenas et al. (2013a, 2015), this study | ||
| B69 | Ca. mexicana | CBS 110918T | CMW 9055; STE-U 927 | Soil | Uxmal, Yucatan, Mexico | M.J. Wingfield | GQ280474; GQ267396; FJ972460; GQ280596; GQ280718; KY653412; FJ972526; AF210863 | Schoch et al. (1999), Crous (2002), Lombard et al. (2010a), Marin-Felix et al. (2017) |
| B70 | Ca. monticola | CBS 140645T | CPC 28835 | Soil | Chiang Mai, Thailand | P.W. Crous | N/A; KT964771; N/A; KT964775; KT983443; N/A; KT964773; KT964769 | Crous et al. (2015) |
| CPC 28836 | – | Soil | Chiang Mai, Thailand | P.W. Crous | N/A; KT964772; N/A; KT964776; KT983444; N/A; KT964774; KT964770 | Crous et al. (2015) | ||
| B71 | Ca. multilateralis | CBS 110932T | CMW 51171; CPC 957 | Soil | Uxmal, Mexico | P.W. Crous | MT335060; MT335292; MT335532; MT359753; MT359513; MT412589; MT412823; MT413037 | Lombard et al. (2016), this study |
| CBS 110926 | CMW 51168; CPC 947 | Soil | Uxmal, Mexico | P.W. Crous | MT335061; MT335293; MT335533; MT359754; MT359514; MT412590; MT412824; MT413038 | Lombard et al. (2016), this study | ||
| CBS 110927 | CMW 51169; CPC 948 | Soil | Uxmal, Mexico | P.W. Crous | MT335062; MT335294; MT335534; MT359755; MT359515; MT412591; MT412825; MT413039 | Lombard et al. (2016), this study | ||
| B72 | Ca. multinaviculata | CBS 134858T | LPF233 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | N/A; KM396072; KM396155; N/A; N/A; N/A; KM395898; KM395985 | Alfenas et al. (2015) |
| CBS 134859 | LPF418 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | N/A; KM396073; KM396156; N/A; N/A; N/A; KM395899; KM395986 | Alfenas et al. (2015) | ||
| B73 | Ca. multiphialidica | CMW 23688T | Cam 13; CBS 112678 | Soil (roots of Musa sp.) | Cameroon | Abadie | MT335066; MT335298; MT335538; MT359759; MT359519; MT412595; MT412829; MT413043 | Crous et al. (2004), Lombard et al. (2010a), this study |
| B74 | Ca. multiseptata | CMW 23692T | CBS 112682; CPC 1589 | E. grandis | North Sumatra, Indonesia | M.J. Wingfield | MT335067; MT335299; MT335539; MT359760; MT359520; MT412596; MT412830; MT413044 | Crous et al. (1998, 2006), Crous (2002), this study |
| B75 | Ca. naviculata | CBS 101121T | CMW 30974 | Leaf litter | Joao Pessoa, Brazil | R.F. Castaneda | GQ280478; GQ267399; GQ267252; GQ280600; GQ280722; KM232309; GQ267317; GQ267211 | Lombard et al. (2010a, 2015b) |
| CBS 116080 | CMW 16723; STE-U 627 | Soil | Manaus, Amazonas, Brazil | M.J. Wingfield | GQ280477; GQ267398; GQ267251; GQ280599; GQ280721; KY653417; GQ267316; AF333409 | Crous et al. (1997a), Crous (2002), Lombard et al. (2010a), Marin-Felix et al. (2017) | ||
| B76 | Ca. nemoricola | CBS 134837T | LPF085 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | N/A; KM396066; KM396149; N/A; N/A; N/A; KM395892; KM395979 | Alfenas et al. (2015) |
| CBS 134838 | LPF090 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | N/A; KM396067; KM396150; N/A; N/A; N/A; KM395893; KM395980 | Alfenas et al. (2015) | ||
| B77 | Ca. octoramosa | CBS 111423T | CMW 51819; CPC 1650 | Soil | Ecuador | M.J. Wingfield | MT335071; MT335303; MT335543; MT359764; MT359524; MT412600; MT412834; MT413048 | Marin-Felix et al. (2017), this study |
| B78 | Ca. orientalis | CMW 20291T | CBS 125260 | Soil | Langam, Indonesia | M.J. Wingfield | MT335072; MT335304; MT335544; MT359765; MT359525; MT412601; MT412835; MT413049 | Lombard et al. (2010a), this study |
| CMW 20273 | CBS 125259 | Soil | Teso East, Indonesia | M.J. Wingfield | MT335073; MT335305; MT335545; MT359766; MT359526; MT412602; MT412836; MT413050 | Lombard et al. (2010a), this study | ||
| B79 | Ca. ovata | CMW 16724T | CBS 111299; ATCC 76225; UFV 89 | E. urophylla | Monte Dourado, Pará, Brazil | N.E. El-Gholl | MT335075; MT335307; MT335547; MT359768; MT359528; N/A; MT412838; MT413052 | El-Gholl et al. (1993a), Crous (2002), Marin-Felix et al. (2017), this study |
| CMW 30979 | CBS 111307; UFV 90 | E. tereticornis | Tucuruí, Pará, Brazil | P.W. Crous | MT335076; MT335308; MT335548; MT359769; MT359529; N/A; MT412839; MT413053 | Crous (2002), Lombard et al. (2010a), this study | ||
| CBS 111301 | CMW 51176; CPC 1429 | E. tereticornis | Tucuruí, Pará, Brazil | P.W. Crous | MT335077; MT335309; MT335549; MT359770; MT359530; N/A; MT412840; MT413054 | Lombard et al. (2016), this study | ||
| CBS 114755 | CMW 51827; CPC 1403 | E. tereticornis | Tucuruí, Pará, Brazil | P.W. Crous | MT335078; MT335310; MT335550; MT359771; MT359531; N/A; MT412841; MT413055 | Marin-Felix et al. (2017), this study | ||
| CBS 116249 | CMW 51829; CPC 3533 | Soil (Eucalyptus plantation) | Brazil | P.W. Crous | MT335074; MT335306; MT335546; MT359767; MT359527; MT412603; MT412837; MT413051 | Marin-Felix et al. (2017), this study | ||
| B80 | Ca. pacifica | CMW 16726T | A1568; CBS 109063; IMI 354528; STE-U 2534 | Araucaria heterophylla | Hawaii, USA | M. Aragaki | MT335079; MT335311; MT335551; MT359772; MT359532; MT412604; MT412842; N/A | Kang et al. (2001b), Crous (2002), Crous et al. (2004), this study |
| CMW 30988 | CBS 114038 | Ipomoea aquatica | Auckland, New Zealand | C.F. Hill | MT335080; MT335312; MT335552; MT359773; MT359533; MT412605; MT412843; N/A | Crous (2002), Crous et al. (2004), Lombard et al. (2010a), this study | ||
| B81 | Ca. paracolhounii | CBS 114679T | CMW 51212; CPC 2445 | N/A | USA | A.Y. Rossman | N/A; KX784582; N/A; KY653268; KY653324; KY653423; KX784714; KX784644 | Lombard et al. (2016), Marin-Felix et al. (2017) |
| CBS 114705 | CMW 51215; CPC 2423 | Annona reticulata (fruit) | Australia | D. Hutton | N/A; N/A; N/A; KY653269; KY653325; KY653424; KX784715; KX784645 | Lombard et al. (2016), Marin-Felix et al. (2017) | ||
| B82 | Ca. paraensis | CBS 134669T | LPF430 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | N/A; KM396011; KM396094; N/A; N/A; N/A; KM395837; KM395924 | Alfenas et al. (2015) |
| LPF429 | – | Soil (tropical rainforest) | Monte Dourado, Pará, Brazil | R.F. Alfenas | N/A; KM396015; KM396098; N/A; N/A; N/A; KM395841; KM395928 | Alfenas et al. (2015) | ||
| CBS 134664 | LPF217 | Soil (tropical rainforest) | Mucuri, Bahia, Brazil | E. Zauza | N/A; KM396017; KM396100; N/A; N/A; N/A; KM395843; KM395930 | Alfenas et al. (2015) | ||
| B83 | Ca. parvispora | CBS 111465T | CPC 1902 | Soil | Brazil | A.C. Alfenas | MT335082; MT335314; MT335554; MT359775; MT359535; MT412607; MT412845; MT413057 | Marin-Felix et al. (2017), this study |
| CMW 30981 | CBS 111478; CPC 1921 | Soil | Brazil | A.C. Alfenas | MT335081; MT335313; MT335553; MT359774; MT359534; MT412606; MT412844; MT413056 | Lombard et al. (2010a, 2016), this study | ||
| B84 | Ca. pauciphialidica sp. nov. | CMW 30980T | CBS 111394; CPC 1628 | Soil | Ecuador | M.J. Wingfield | MT335083; MT335315; MT335555; MT359776; MT359536; MT412608; MT412846; MT413058 | Crous et al. (2006), Lombard et al. (2010a), this study |
| B85 | Ca. pauciramosa | CBS 138824T | CMW 5683; CPC 971 | Soil | Knysna, South Africa | P.W. Crous | MT335093; MT335325; MT335565; MT359786; MT359546; MT412618; MT412856; MT413068 | Schoch et al. (1999), Crous (2002), Lombard et al. (2010a), this study |
| CMW 9151 | – | A. mearnsii | South Africa | L. Lombard | MT335096; MT335328; MT335568; MT359789; MT359549; MT412621; MT412859; MT413071 | Lombard et al. (2010b), this study | ||
| CBS 111812 | CMW 51190; CPC 2631 | Cliffordia feruginea | George, Western Cape Province, South Africa | P.W. Crous | MT335084; MT335316; MT335556; MT359777; MT359537; MT412609; MT412847; MT413059 | Lombard et al. (2016), this study | ||
| CBS 114458 | CMW 51211; CPC 2019 | Erica capensis | California, USA | S.T. Koike | MT335087; MT335319; MT335559; MT359780; MT359540; MT412612; MT412850; MT413062 | Lombard et al. (2016), this study | ||
| CBS 123183 | CMW 51311; CPC 15378 | Machaerina sinclairii | Auckland University Campus, Auckland, New Zealand | C.F. Hill | MT335090; MT335322; MT335562; MT359783; MT359543; MT412615; MT412853; MT413065 | Lombard et al. (2016), this study | ||
| CBS 123402 | CMW 30872 | Arbutus unedo | Carrubba, Sicily, Italy | G. Polizzi | MT335099; MT335331; MT335571; MT359792; MT359552; MT412624; MT412862; MT413074 | Lombard et al. (2010b), this study | ||
| CBS 137243 | CMW 36327 | E. grandis × E. camaldulensis | Bandula, Manica, Mozambique | J. Roux & S. Maússe-Sitoe | MT335091; MT335323; MT335563; MT359784; MT359544; MT412616; MT412854; MT413066 | Crous et al. (2013), Lombard et al. (2016), this study | ||
| CMW 9188 | CBS 125268 | E. grandis | Kwambonambi, KwaZulu-Natal, South Africa | L. Lombard | MT335159; MT335398; MT335638; MT359859; MT359619; MT412689; MT412929; MT413136 | Lombard et al. (2010b), this study | ||
| CMW 31450 | CBS 136632; CERC 1785 | E. urophylla × E. grandis | ZhanJiang, GuangDong, China | G. Zhao | MT335102; MT335334; MT335574; MT359795; MT359555; MT412627; MT412865; MT413077 | Lombard et al. (2015a), this study | ||
| CMW 31474 | CBS 136635; CERC 1809 | E. urophylla × E. grandis | ZhanJiang, GuangDong, China | G. Zhao | MT335104; MT335336; MT335576; MT359797; MT359557; MT412629; MT412867; MT413079 | Lombard et al. (2015a), this study | ||
| B86 | Ca. penicilloides | CMW 23696T | CBS 174.55; STE-U 2388 | Prunus sp. | Hatizyo Island, Japan | M. Ookubu | MT335106; MT335338; MT335578; MT359799; MT359559; MT412631; MT412869; MT413081 | Tubaki (1958), Crous (2002), this study |
| B87 | Ca. piauiensis | CBS 134850T | LPF377 | Soil (Eucalyptus plantation) | Teresina, Piauí, Brazil | R.F. Alfenas | N/A; KM396060; KM396143; N/A; N/A; N/A; KM395886; KM395973 | Alfenas et al. (2015) |
| CBS 134851 | LPF381 | Soil (tropical rainforest) | Teresina, Piauí, Brazil | R.F. Alfenas | N/A; KM396061; KM396144; N/A; N/A; N/A; KM395887; KM395974 | Alfenas et al. (2015) | ||
| B88 | Ca. pini | CMW 31209T | CBS 123698 | Pinus patula | Buga, Valle del Cauca, Colombia | C.A. Rodas | MT335107; MT335339; MT335579; MT359800; MT359560; MT412632; MT412870; MT413082 | Lombard et al. (2010a), this study |
| CBS 125523 | CMW 31210 | Pinus patula | Buga, Valle del Cauca, Colombia | C.A. Rodas | GQ280518; GQ267437; GQ267274; GQ280640; GQ280762; N/A; GQ267345; GQ267225 | Lombard et al. (2010a) | ||
| B89 | Ca. plurilateralis | CBS 111401T | CMW 51178; CPC 1637 | Soil | Ecuador | M.J. Wingfield | N/A; MT335340; MT335580; MT359801; MT359561; MT412633; MT412871; MT413083 | Lombard et al. (2016), this study |
| B90 | Ca. propaginicola | CBS 134815T | LPF220 | Eucalyptus sp. (seedling) | Santana, Pará, Brazil | A.C. Alfenas | N/A; KM396040; KM396123; N/A; N/A; N/A; KM395866; KM395953 | Alfenas et al. (2015) |
| CBS 134816 | LPF222 | Eucalyptus sp. (seedling) | Santana, Pará, Brazil | A.C. Alfenas | N/A; KM396041; KM396124; N/A; N/A; N/A; KM395867; KM395954 | Alfenas et al. (2015) | ||
| CBS 134824 | LPF367 | Eucalyptus sp. (seedling) | Santana, Pará, Brazil | A.C. Alfenas | N/A; KM396049; KM396132; N/A; N/A; N/A; KM395875; KM395962 | Alfenas et al. (2015) | ||
| B91 | Ca. pseudobrassicae | CBS 134662T | LPF280 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | N/A; KM396023; KM396106; N/A; N/A; N/A; KM395849; KM395936 | Alfenas et al. (2015) |
| CBS 134661 | LPF260 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | N/A; KM396022; KM396105; N/A; N/A; N/A; KM395848; KM395935 | Alfenas et al. (2015) | ||
| B92 | Ca. pseudoecuadoriae | CBS 111402T | CMW 51179; CPC 1639 | Soil | Ecuador | M.J. Wingfield | N/A; KX784589; N/A; KY653273; KY653329; KY653432; KX784723; KX784652 | Lombard et al. (2016), Marin-Felix et al. (2017) |
| B93 | Ca. pseudometrosideri | CBS 134845T | LPF210 | Soil (Eucalyptus plantation) | Maceió, Alagoas, Brazil | M.M. Coutinho | N/A; KM395995; KM396083; N/A; N/A; N/A; KM395821; KM395909 | Alfenas et al. (2015) |
| CBS 134843 | LPF100 | Metrosideros polymorpha | Viçosa, Minas Gerais, Brazil | A.C. Alfenas | N/A; KM395993; KM396081; N/A; N/A; N/A; KM395819; KM395907 | Alfenas et al. (2015) | ||
| B94 | Ca. pseudomexicana | CBS 130354T | CMW 51313; DISTEF-TCROU1 | Callistemon sp. | Tunis, Carthage, Tunisia | G. Polizzi | MT335110; MT335343; MT335583; MT359804; MT359564; MT412636; MT412874; MT413086 | Lombard et al. (2011), this study |
| CBS 130355 | CMW 51314; DISTEF-TCROU3 | Callistemon sp. | Tunis, Carthage, Tunisia | G. Polizzi | MT335111; MT335344; MT335584; MT359805; MT359565; MT412637; MT412875; MT413087 | Lombard et al. (2011), this study | ||
| CBS 130357 | CMW 51316; DISTEF-TCL1 | Ca. laevis | Tunis, Carthage, Tunisia | G. Polizzi | MT335149; MT335386; MT335626; MT359847; MT359607; MT412678; MT412917; MT413124 | Lombard et al. (2011), this study | ||
| B95 | Ca. pseudonaviculata | CBS 116251T | CMW 51235; CPC 3399; Lynfield 824 | Buxus sempervirens | Kumeu, West Auckland, New Zealand | R. MacDiarmid | N/A; MT335345; MT335585; MT359806; MT359566; MT412638; MT412876; MT413088 | Crous et al. (2002), Lombard et al. (2016), this study |
| CMW 23672 | CBS 114417; CPC 10926 | B. sempervirens | New Zealand | C. Crepel | N/A; MT335346; MT335586; MT359807; MT359567; MT412639; MT412877; MT413089 | Lombard et al. (2010a, 2016), this study | ||
| B96 | Ca. pseudopteridis | CBS 163.28T | CMW 51159; IMI 299579 | Washingtonia robusta | USA | C.D. Sherbakoff | MT335112; MT335347; MT335587; MT359808; MT359568; MT412640; MT412878; N/A | Sherbakoff (1928), Alfenas et al. (2015), Lombard et al. (2016), this study |
| B97 | Ca. pseudoreteaudii | CMW 25310T | CBS 123694 | E. urophylla × E. grandis | GuangDong, China | M.J. Wingfield & X.D. Zhou | MT335119; MT335354; MT335594; MT359815; MT359575; MT412647; MT412885; MT413096 | Lombard et al. (2010c), this study |
| CMW 25292 | CBS 123696 | E. urophylla × E. grandis | GuangDong, China | M.J. Wingfield & X.D. Zhou | MT335120; MT335355; MT335595; MT359816; MT359576; MT412648; MT412886; MT413097 | Lombard et al. (2010c), this study | ||
| CMW 31487 | CBS 136638; CERC 1822 | E. urophylla × E. grandis | ZhanJiang, GuangDong, China | G. Zhao | MT335113; MT335348; MT335588; MT359809; MT359569; MT412641; MT412879; MT413090 | Lombard et al. (2015a), this study | ||
| CBS 133349 | CMW 51318 | Eucalyptus hybrid | Hanoi, Bavi, Vietnam | P.Q. Thu | MT335117; MT335352; MT335592; MT359813; MT359573; MT412645; MT412883; MT413094 | Crous et al. (2012), this study | ||
| B98 | Ca. pseudospathiphylli | CBS 109165T | CMW 30976; CPC 1623 | Soil | Ecuador | M.J. Wingfield | GQ280493; GQ267412; AF348241; GQ280615; GQ280737; KY653435; FJ918562; FJ918513 | Kang et al. (2001b), Crous (2002), Lombard et al. (2010a, c), Marin-Felix et al. (2017) |
| B99 | Ca. pseudospathulata | CBS 134841T | LPF072 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | N/A; KM396070; KM396153; N/A; N/A; N/A; KM395896; KM395983 | Alfenas et al. (2015) |
| CBS 134840 | LPF066 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | N/A; KM396069; KM396152; N/A; N/A; N/A; KM395895; KM395982 | Alfenas et al. (2015) | ||
| B100 | Ca. pseudouxmalensis | CBS 110924T | CMW 51166; CPC 942 | Soil | Mexico | P.W. Crous | MT335123; MT335358; MT335598; MT359819; MT359579; MT412651; MT412889; MT413100 | Lombard et al. (2016), this study |
| CBS 110923 | CMW 51165; CPC 941 | Soil | Mexico | P.W. Crous | MT335124; MT335359; MT335599; MT359820; MT359580; MT412652; MT412890; MT413101 | Lombard et al. (2016), this study | ||
| CBS 115677 | CMW 51228; CPC 943 | Soil | Mexico | P.W. Crous | MT335125; MT335360; MT335600; MT359821; MT359581; MT412653; MT412891; MT413102 | Lombard et al. (2016), this study | ||
| B101 | Ca. pseudovata | CBS 134674T | LPF267 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | N/A; KM396032; KM396115; N/A; N/A; N/A; KM395858; KM395945 | Alfenas et al. (2015) |
| CBS 134675 | LPF285 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | N/A; KM396033; KM396116; N/A; N/A; N/A; KM395859; KM395946 | Alfenas et al. (2015) | ||
| B102 | Ca. pteridis | CBS 111793T | ATCC 34395; CMW 16736; CPC 2372 | Arachniodes adiantiformis | USA | F. Schickedanz | GQ280494; GQ267413; DQ190679; GQ280616; GQ280738; KY653438; FJ918563; DQ190578 | Crous et al. (1993c, 2006), Crous (2002), Lombard et al. (2010a), Marin-Felix et al. (2017) |
| B103 | Ca. putriramosa | CBS 111449T | CMW 51181; CPC 1951 | Eucalyptus cutting | Brazil | A.C. Alfenas | MT335129; MT335364; MT335604; MT359825; MT359585; MT412657; MT412895; MT413105 | Lombard et al. (2016), this study |
| CBS 111470 | CMW 51182; CPC 1940 | Soil | Brazil | A.C. Alfenas | MT335130; MT335365; MT335605; MT359826; MT359586; MT412658; MT412896; MT413106 | Lombard et al. (2016), this study | ||
| CBS 111477 | CMW 51183; CPC 1928 | Soil | Brazil | A.C. Alfenas | MT335131; MT335366; MT335606; MT359827; MT359587; MT412659; MT412897; MT413107 | Lombard et al. (2016), this study | ||
| B104 | Ca. queenslandica | CMW 30604T | CBS 112146; CPC 3213 | E. urophylla | Lannercost, Queensland, Australia | B. Brown | MT335132; MT335367; MT335607; MT359828; MT359588; MT412660; MT412898; MT413108 | Kang et al. (2001a), Lombard et al. (2010c), this study |
| CMW 30603 | CBS 112155; CPC 3210 | E. pellita | Lannercost, Queensland, Australia | P.Q Thu & K.M. Old | MT335133; MT335368; MT335608; MT359829; MT359589; MT412661; MT412899; MT413109 | Kang et al. (2001a), Lombard et al. (2010c), this study | ||
| CMW 30601 | CBS 112151; CPC 3202; DFRI00150 | E. urophylla | Cardwell, Queensland, Australia | C. Hanwood | MT335134; MT335369; MT335609; MT359830; MT359590; MT412662; MT412900; MT413110 | Crous (2002), Crous et al. (2006), Lombard et al. (2010c), this study | ||
| B105 | Ca. quinqueramosa | CBS 134654T | LPF065 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | N/A; KM396029; KM396112; N/A; N/A; N/A; KM395855; KM395942 | Alfenas et al. (2015) |
| CBS 134655 | LPF281 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | N/A; KM396030; KM396113; N/A; N/A; N/A; KM395856; KM395943 | Alfenas et al. (2015) | ||
| B106 | Ca. reteaudii | CMW 30984T | CBS 112144; CPC 3201 | E. camaldulensis | Chon Thanh, Binh Phuoc, Vietnam | M.J. Dudzinski & P.Q. Thu | MT335135; MT335370; MT335610; MT359831; MT359591; MT412663; MT412901; MT413111 | Kang et al. (2001a), Crous (2002), Crous et al. (2006), this study |
| CMW 16738 | CBS 112143; CPC 3200 | Eucalyptus sp. (leaves) | Binh Phuoc, Vietnam | M.J. Dudzinski & P.Q. Thu | MT335136; MT335371; MT335611; MT359832; MT359592; MT412664; MT412902; MT413112 | Kang et al. (2001a), Crous (2002), Crous et al. (2006), this study | ||
| CMW 47410 | CBS 143563 | E. urophylla (leaf) | Bavi, Hanoi, Vietnam | N.Q. Pham & T.Q. Pham | MT334966; MT335193; MT335433; MT359654; MT359414; MT412497; MT412724; N/A | Pham et al. (2019), this study | ||
| B107 | Ca. robigophila | CBS 134652T | LPF192 | Eucalyptus sp. (leaf) | Açailandia, Maranhao, Brazil | R.F. Alfenas | N/A; KM396024; KM396107; N/A; N/A; N/A; KM395850; KM395937 | Alfenas et al. (2015) |
| CBS 134653 | LPF193 | Eucalyptus sp. (leaf) | Açailandia, Maranhao, Brazil | R.F. Alfenas | N/A; KM396025; KM396108; N/A; N/A; N/A; KM395851; KM395938 | Alfenas et al. (2015) | ||
| B108 | Ca. rumohrae | CMW 23697T | CBS 111431; CPC 1716; UFV 218 | Rumohra adiantiformis | Volkan, Panama | J.W. Miller & R.M. leahy | MT335137; MT335372; MT335612; MT359833; MT359593; N/A; MT412903; MT413113 | El-Gholl et al. (1997), Crous (2002), Crous et al. (2006), this study |
| CMW 30989 | CBS 109062; CPC 1603 | Adiantum sp. | The Netherlands | R. Pieters | MT335138; MT335373; MT335613; MT359834; MT359594; MT412665; MT412904; MT413114 | El-Gholl et al. (1997), Crous (2002), Crous et al. (2006), this study | ||
| B109 | Ca. silvicola | CBS 135237T | LPF081 | Soil (tropical rainforest) | Mucuri, Bahia, Brazil | A.C. Alfenas & P.W. Crous | N/A; KM396065; KM396148; N/A; N/A; N/A; KM395891; KM395978 | Alfenas et al. (2015) |
| CBS 134836 | LPF079 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | N/A; KM396062; KM396145; N/A; N/A; N/A; KM395888; KM395975 | Alfenas et al. (2015) | ||
| Calonectria sp. | CBS 112152 | CPC 3203 | E. camaldulensis | Vietnam | N/A | N/A; KX784602; N/A; KY653291; KY653347; KY653463; KX784745; KX784672 | Lombard et al. (2016), Marin-Felix et al. (2017) | |
| Calonectria sp. | CBS 112753 | CPC 4225 | N/A | Indonesia | N/A | N/A; KX784598; N/A; KY653292; KY653348; KY653464; KX784740; KX784667 | Lombard et al. (2016), Marin-Felix et al. (2017) | |
| B110 | Ca. spathiphylli | CMW 16742T | ATCC 44730; CBS 114540; STE-U 2185 | Spathiphyllum sp. | Florida, USA | C.L. Schoulties | N/A; MT335374; MT335614; MT359835; MT359595; MT412666; MT412905; MT413115 | El-Gholl et al. (1992), Crous (2002), Lombard et al. (2010a, 2016), this study |
| CMW 30997 | CBS 116168; CPC 789 | Spathiphyllum sp. | Switzerland | L. Petrini | N/A; MT335375; MT335615; MT359836; MT359596; MT412667; MT412906; MT413116 | El-Gholl et al. (1992), Crous (2002), Lombard et al. (2016), this study | ||
| B111 | Ca. spathulata | CMW 16744T | CBS 555.92 | E. viminalis | Brazil | N.E. El-Gholl | MT335139; MT335376; MT335616; MT359837; MT359597; MT412668; MT412907; MT413117 | Crous & Kang (2001), Crous (2002), Lombard et al. (2016), this study |
| CBS 112513 | CMW 51197; CPC 3851 | Eucalyptus sp. | Colombia | M.J. Wingfield | MT335140; MT335377; MT335617; MT359838; MT359598; MT412669; MT412908; MT413118 | Lombard et al. (2016), this study | ||
| B112 | Ca. sumatrensis | CMW 23698T | CBS 112829; CPC 4518 | Soil | Northern Sumatra, Indonesia | M.J. Wingfield | MT335145; MT335382; MT335622; MT359843; MT359603; MT412674; MT412913; N/A | Crous et al. (2004), this study |
| CMW 30987 | CBS 112934; CPC 4516 | Soil | Northern Sumatra, Indonesia | M.J. Wingfield | MT335146; MT335383; MT335623; MT359844; MT359604; MT412675; MT412914; N/A | Crous et al. (2004), this study | ||
| CBS 112936 | CMW 51207; CPC 4504 | Soil | Northern Sumatra, Indonesia | M.J. Wingfield | MT335143; MT335380; MT335620; MT359841; MT359601; MT412672; MT412911; N/A | Lombard et al. (2016), this study | ||
| B113 | Ca. syzygiicola | CBS 112831T | CMW 51204; CPC 4511 | Syzygium aromaticum | Sumatra, Indonesia | M.J. Wingfield | N/A; N/A; N/A; N/A; N/A; N/A; KX784736; KX784663 | Lombard et al. (2016) |
| B114 | Ca. terricola | CBS 116247T | CMW 51232; CPC 3583 | Soil (Eucalyptus plantation) | Brazil | P.W. Crous | N/A; N/A; N/A; N/A; N/A; N/A; KX784738; KX784665 | Lombard et al. (2016) |
| B115 | Ca. tonkinensis | CMW 47430T | CBS 143576 | Soil (Eucalyptus plantation) | Bavi, Hanoi, Vietnam | N.Q. Pham & T.Q. Pham | MT335147; MT335384; MT335624; MT359845; MT359605; MT412676; MT412915; MT413122 | Pham et al. (2019), this study |
| B116 | Ca. uniseptata | CBS 413.67T | CMW 23678; CPC 2391; IMI 299577 | Paphiopedilum callosum | Celle, Germany | W. Gerlach | GQ280451; GQ267379; GQ267248; GQ280573; GQ280695; N/A; GQ267307; GQ267208 | Lombard et al. (2016) |
| B117 | Ca. uxmalensis | CBS 110925T | CMW 51167; CPC 945 | Soil | Uxmal, Mexico | P.W. Crous | MT335153; MT335390; MT335630; MT359851; MT359611; MT412681; MT412921; MT413128 | Lombard et al. (2016), this study |
| CBS 110919 | CMW 51164; CPC 928 | Soil | Uxmal, Mexico | P.W. Crous | MT335154; MT335391; MT335631; MT359852; MT359612; MT412682; MT412922; MT413129 | Lombard et al. (2016), this study | ||
| B118 | Ca. variabilis | CMW 3187T | AR2675; CBS 114677; CPC 2436 | Schefflera morototoni | Pará, Brazil | F.C. de Albuquerque | N/A; MT335392; MT335632; MT359853; MT359613; MT412683; MT412923; MT413130 | Crous et al. (1993b), Crous (2002), Lombard et al. (2010a, 2016), this study |
| CMW 2914 | CBS 112691; CPC 2506 | Theobroma grandiflorum | Pará, Brazil | F. Carneiro | N/A; MT335393; MT335633; MT359854; MT359614; MT412684; MT412924; MT413131 | Crous et al. (1993b), Crous (2002), Lombard et al. (2010a, 2016), this study | ||
| B119 | Ca. venezuelana | CBS 111052T | CMW 51173; CPC 1183 | Soil | Acarigua, Venezuela | M.J. Wingfield | MT335155; MT335394; MT335634; MT359855; MT359615; MT412685; MT412925; MT413132 | Lombard et al. (2016), this study |
| B120 | Ca. yunnanensis | CERC 5339T | CBS 142897; CMW 47644 | Soil (Eucalyptus plantation) | YunNan, China | S.F. Chen & J.Q. Li | MT335157; MT335396; MT335636; MT359857; MT359617; MT412687; MT412927; MT413134 | Li et al. (2017), this study |
| CERC 5337 | CBS 142895; CMW 47642 | Soil (Eucalyptus plantation) | YunNan, China | S.F. Chen & J.Q. Li | MT335158; MT335397; MT335637; MT359858; MT359618; MT412688; MT412928; MT413135 | Li et al. (2017), this study | ||
| CERC 5376 | CBS 142892; CMW 47655 | Soil (Eucalyptus plantation) | PuEr, YunNan, China | S.F. Chen & J.Q. Li | MT335126; MT335361; MT335601; MT359822; MT359582; MT412654; MT412892; MT413103 | Li et al. (2017), this study | ||
| Curvicladiella cignea | CBS 109167T | CPC 1595; MUCL 40269 | Decaying leaf | French Guiana | C. Decock | KM231122; KM231287; KM231461; AF220973; AY793431; KM232311; KM231867; KM232002 | Decock & Crous (1998), Crous et al. (2006), Lombard et al. (2015b) | |
| CBS 109168 | CPC 1594; MUCL 40268 | Decaying seed | French Guiana | C. Decock | KM231121; KM231286; KM231460; KM231745; JQ666074; KM232312; KM231868; KM232003 | Decock & Crous (1998), Crous et al. (2006), Lombard et al. (2015b) |
Codes (B1 to B120) of the 120 accepted Calonectria species resulting from the present study.
One hundred and twenty accepted species resulting from the present study.
Key isolates of the 120 Calonectria species after revision.
T: ex-type isolates of the Calonectria species after revision.
AR: Amy Y. Rossman working collection; ATCC: American Type Culture Collection, Virginia, USA; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CERC: China Eucalypt Research Centre, ZhanJiang, GuangDong Province, China; CMW: Culture collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; CPC: Pedro Crous working collection housed at Westerdijk Fungal Biodiversity Institute; HGUP: Plant Pathology Herbarium of Gui Zhou University, GuiYang 550025, China; IMI: International Mycological Institute, CABI Bioscience, Egham, Bakeham Lane, UK; LPF: Laboratorio de Patologia Florestal, Universidade Federal de Viçsa, Viçsa, Brazil; MUCL: Mycotheque, Laboratoire de Mycologie Systematique st Appliqee, I’Universite, Louvian-la-Neuve, Belgium; PPRI: Plant Protection Research Institute, Pretoria, South Africa; STE-U: Department of Plant Pathology, University of Stellenbosch, South Africa; UFV: Universidade Federal de Viçsa, Viçsa, Brazil.
act: actin; cmdA: calmodulin; his3: histone H3; ITS: the internal transcribed spacer regions 1 and 2 and the 5.8S gene of the ribosomal RNA; LSU: 28S large subunit RNA gene; rpb2: the second largest subunit of RNA polymerase; tef1: translation elongation factor 1-alpha; tub2: β-tubulin.
GenBank accession number obtained in this study are in bold.
N/A: information are not available.
Morphology, mating system and geographic distribution
The key morphological characteristics of all Calonectria species were summarised, listing all species in the 11 species complexes (Supplementary Table S4). Based on the typical/dominant vesicle shape of species in each species complex, the 11 species complexes were separated into six groups: (1) clavate or narrowly clavate vesicle group (Ca. brassicae, Ca. colhounii, Ca. gracilipes and Ca. reteaudii species complexes); (2) naviculate vesicle group (Ca. naviculata species complex); (3) ellipsoidal to obpyriform vesicle group (Ca. candelabrum species complex); (4) ellipsoidal vesicle with papillate apex group (Ca. mexicana species complex); (5) sphaeropedunculate vesicle group (Ca. kyotensis and Ca. spathiphylli species complexes); (6) varied shapes vesicle group (Ca. cylindrospora and Ca. pteridis species complexes) (Table 2, Fig. 1). Other than vesicle shape, differences in other key morphological features including macroconidial septation, perithecial colour, number of ascospores in the asci and ascospore septation are presented in Table 2, Supplementary Table S4 and Fig. 1. Differences in mating type and geographic distribution for the species in all 11 species complexes are documented in Table 2, Supplementary Table S4 and Fig. 1.
Table 2.
Morphological characteristics, thallism and area of occurrence of the 11 Calonectria species complexes.
Species in four species complexes (Ca. brassicae, Ca. colhounii, Ca. gracilipes and Ca. reteaudii) have vesicles that are clavate or narrowly clavate. The Ca. brassicae species complex generally produces 1-septate macroconidia and 1-septate ascospores, distinguishing them from taxa in the Ca. colhounii, Ca. gracilipes and Ca. reteaudii species complexes. The Ca. colhounii species complex typically has yellow perithecia and 4-spored asci, rare in all the other species complexes. Species in both the Ca. gracilipes and Ca. reteaudii species complexes generally produce macroconidia with more than three septa (Table 2, Supplementary Table S4). Species in the Ca. naviculata species complex typically have naviculate vesicles and those in the Ca. mexicana species complex generally produces ellipsoidal vesicles with papillate apices, morphological features rarely found in other species complexes (Table 2, Supplementary Table S4). Ellipsoidal to obpyriform vesicles are typical of species in the Ca. candelabrum species complex (Table 2, Supplementary Table S4) and those in the Ca. kyotensis and Ca. spathiphylli species complexes generally produce sphaeropedunculate vesicles and have 1-septate macroconidia. Perithecial colour, and number of ascospores in the asci of Ca. kyotensis species complex taxa are generally similar to those in the Ca. spathiphylli species complex (Table 2, Supplementary Table S4). Species in the Ca. cylindrospora and Ca. pteridis species complexes do not have vesicles that are morphologically characteristic, those the former have vesicles of variable shape, while those in the latter are generally clavate or ovate (Table 2, Supplementary Table S4).
Morphological comparisons showed that most of the Calonectria species can be distinguished based on vesicle shape and diameter, macroconidial septation and dimensions, perithecial colour, number of ascospores in the asci, ascospore septation and dimensions (Supplementary Table S4). Some species were difficult to distinguish solely based on morphological characteristics. For example, the Ca. pseudospathulata (Ca. candelabrum species complex) and Ca. auriculiformis (Ca. cylindrospora species complex) all produce obpyriform vesicles, and 1-septate macroconidia with overlapping dimensions (Supplementary Table S4). Some species in the same species complex shared a similar morphology; for example, Ca. pseudobrassicae and Ca. paraensis (both in the Ca. brassicae species complex) both produce clavate vesicles, and 1-septate macroconidia with overlapping dimensions (Supplementary Table S4).
Species in the Ca. naviculata and Ca. pteridis species complexes are heterothallic, while those in the Ca. gracilipes species complex are homothallic. Calonectria species in the remaining eight complexes include both heterothallic and homothallic mating systems (Table 2, Supplementary Table S4).
A summary of species distribution globally, showed that species in the Ca. candelabrum and Ca. naviculata species complexes occur on all the continents other than Antarctica. In contrast, species in the Ca. gracilipes, Ca. pteridis and Ca. spathiphylli species complexes were from North and South America and those in the Ca. reteaudii species complex were from Asia and Oceania. Species in other species complexes occurred on between three and five continents (Table 2, Supplementary Table S4).
Species reduced to synonymy
Based on the criteria decided upon for species delimitation, 51 previously identified species were found to represent synonyms of other known species (Supplementary Tables S1 and S2). Justification for reducing these 51 previously accepted taxa to synonymy with earlier names is provided in the taxonomy section.
Novel taxa
Based on phylogenetic placement in the individual gene phylogenies as well as in the multi-gene phylogenetic trees (Fig. 1, Supplementary Figs S1–8), two isolates (CMW 30602 and CMW 30980) formed two distinct lineages. These lineages were clearly distinct from those representing any of the other described species and they are recognised here as two novel taxa described in the Taxonomy section. In the Ca. brassicae species complex, a single isolate (CMW 30980), treated as Ca. ecuadorae in the earlier studies (Crous et al. 2006), formed a distinct lineage in the rpb2, tef1, tub2 and in the combined gene tree. This fungus clustered close to but separate from Ca. ecuadorae (ex-type CMW 23677, CBS 111706, and CBS 114164) and Ca. octoramosa (ex-type CBS 111423) (Fig. 1, Supplementary Figs S6–8). Isolate CMW 30980 is thus considered to represent a novel species. Isolate CMW 30602 in the Ca. reteaudii species complex was identified as Ca. terrae-reginae by Lombard et al. (2010c), and formed a distinct lineage in the ITS, tef1 and tub2 analyses as well as in the combined phylogenetic tree (Fig. 1, Supplementary Figs S4, S7, S8). This isolate represents an undescribed species closely related to but separate from Ca. queenslandica (ex-type CMW 30604, CMW 30601, and CMW 30603) (Fig. 1, Supplementary Figs S4, S7).
Calonectria lauri (Vanderw.) Lechat & Crous was proposed as the sexual morph of Cylindrocladium ilicicola by Lechat in 2010. The basionym Tetracytum lauri is invalid and thus the name Ca. lauri is invalid. The results of the present study have shown that Ca. lauri represents a well-defined species and its name is consequently validated in the taxonomy section.
Taxonomy (Species listed in alphabetical order)
Following the results of the multi-gene phylogenetic analyses and consideration of the morphological characteristics of the 169 described Calonectria spp. (codes A1 to A169 in Supplementary Tables S1 and S2), 51 species are reduced to synonymy and three novel Calonectria species (Calonectria lauri was validated and it is treated as a valid species in the current study) are recognised. This results in 120 Calonectria spp. now accepted and these are listed in Table 1 (codes B1 to B120 in Supplementary Tables S1 and S2). Morphological characteristics of the Calonectria spp. identified in this study and their closest relatives are summarised (Supplementary Table S4). The 120 accepted Calonectria species emerging from this study and their synonymies are presented below.
acaciicola Calonectria N.Q. Pham et al., Mycologia 111: 88. 2019.
In: Calonectria reteaudii species complex.
Typus: PREM 62105 holotype.
Ex-type culture: CBS 143557 = CMW 47173.
Type locality: Vietnam, Nghe An, Do Luong.
Type substrate: Soil in Acacia auriculiformis plantation.
Barcodes: act = MT334933; cmdA = MT335160; his3 = MT335399; rpb2 = MT412474; tef1 = MT412690; tub2 = MT412930 (alternative markers: ITS = MT359620; LSU = MT359380).
Notes: Calonectria acaciicola is phylogenetically closely related to Ca. pseudoreteaudii and Ca. reteaudii. The sequences of cmdA, his3, rpb2, tef1 and tub2 gene regions can differentiate Ca. acaciicola from these two species. The macroconidia of Ca. acaciicola [(av. 94 × 7 μm); Pham et al. 2019] are smaller than those of Ca. pseudoreteaudii [(av. 104 × 8 μm); Lombard et al. 2010c] but larger than those of Ca. reteaudii [(av. 84 × 6.5 μm); Kang et al. 2001a] (Supplementary Table S4).
acicola Calonectria Gadgil & M.A. Dick, N.Z. Jl For. Sci. 34: 316. 2004.
Synonym: Cylindrocladium acicola Gadgil & M.A. Dick, N.Z. Jl For. Sci. 34: 316. 2004.
In: Calonectria reteaudii species complex.
Typus: NZFRI-M 5213 holotype.
Ex-type culture: CMW 30996.
Type locality: New Zealand, Northland.
Type substrate: Phoenix canariensis.
Barcodes: act = MT334935; cmdA = MT335162; his3 = MT335401; rpb2 = MT412476; tef1 = MT412692; tub2 = MT412932 (alternative markers: ITS = MT359622; LSU = MT359382).
Notes: Calonectria acicola is phylogenetically closely related to Ca. multiseptata. They can be distinguished from each other by the formation of megaconidiophores and megaconidia, a feature not observed in Ca. acicola but typical in Ca. multiseptata (Crous 2002, Gadgil & Dick 2004). Calonectria acicola is homothallic (Gadgil & Dick 2004) (Supplementary Table S4).
aciculata Calonectria J.Q. Li et al., IMA Fungus 8: 273. 2017.
In: Calonectria colhounii species complex.
Typus: PREM 61941 holotype.
Ex-type culture: CBS 142883 = CMW 47645 = CERC 5342.
Type locality: China, YunNan Province.
Type substrate: Eucalyptus urophylla × E. grandis.
Barcodes: act = MT334937; cmdA = MT335164; his3 = MT335403; rpb2 = MT412478; tef1 = MT412694; tub2 = MT412934 (alternative markers: ITS = MT359624; LSU = MT359384).
Notes: Calonectria aciculata is closely related to Ca. colhounii and Ca. honghensis, but can be distinguished from these two species by its macroconidial dimensions. The macroconidia of Ca. aciculata [(av. 69 × 5.5 μm); Li et al. 2017] are longer than those of Ca. colhounii [(av. 65 × 5 μm); Peerally 1973] and Ca. honghensis [(av. 54 × 5.5 μm); Li et al. 2017]. Calonectria aciculata is homothallic (Li et al. 2020) (Supplementary Table S4).
aconidialis Calonectria L. Lombard et al., Stud. Mycol. 80: 162. 2015.
Synonyms: Calonectria arbusta L. Lombard et al., Stud. Mycol. 80: 162. 2015.
Calonectria expansa L. Lombard et al., Stud. Mycol. 80: 167. 2015.
Calonectria guangxiensis L. Lombard et al., Stud. Mycol. 80: 169. 2015.
Calonectria hainanensis L. Lombard et al., Stud. Mycol. 80: 170. 2015.
Calonectria magnispora L. Lombard et al., Stud. Mycol. 80: 174. 2015.
Calonectria parakyotensis L. Lombard et al., Stud. Mycol. 80: 176. 2015.
Calonectria pluriramosa L. Lombard et al., Stud. Mycol. 80: 178. 2015.
Calonectria pseudokyotensis L. Lombard et al., Stud. Mycol. 80: 178. 2015.
Calonectria sphaeropedunculata L. Lombard et al., Stud. Mycol. 80: 180. 2015.
In: Calonectria kyotensis species complex.
Typus: CBS H-21481 holotype.
Ex-type culture: CBS 136086 = CMW 35174 = CERC 1850.
Type locality: China, HaiNan Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334938; cmdA = MT335165; his3 = MT335404; rpb2 = MT412479; tef1 = MT412695 (alternative markers: ITS = MT359625; LSU = MT359385).
Notes: Calonectria aconidialis is phylogenetically closely related to Ca. pacifica and can be differentiated from that species by the sequences of act, cmdA, his3, rpb2, ITS, LSU and tef1 gene regions. The asexual structures of Ca. aconidialis failed to develop (Lombard et al. 2015a). This species is homothallic (Lombard et al. 2015a) (Supplementary Table S4).
aeknauliensis Calonectria N.Q. Pham & M.J. Wingf., Mycologia 111: 93. 2019.
In: Calonectria kyotensis species complex.
Typus: PREM 62107 holotype.
Ex-type culture: CBS 143559 = CMW 48253.
Type locality: Indonesia, Northern Sumatra, Aek Nauli.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334953; cmdA = MT335180; his3 = MT335419; rpb2 = MT412486; tef1 = MT412710; (alternative markers: ITS = MT359640; LSU = MT359400).
Notes: Calonectria aeknauliensis is closely related to Ca. curvispora and Ca. ilicicola, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. aeknauliensis [(av. 47 × 5 μm); Pham et al. 2019] are shorter than those of Ca. curvispora [(av. 60 × 5 μm); Victor et al. 1997] and smaller than those of Ca. ilicicola [(av. 62 × 6 μm); Boedijn & Reitsma 1950] (Supplementary Table S4).
amazonica Calonectria L. Lombard & Crous, Stud. Mycol. 85: 171. 2016.
Synonyms: Calonectria amazoniensis L. Lombard & Crous, Stud. Mycol. 85: 173. 2016.
Calonectria tropicalis L. Lombard & Crous, Stud. Mycol. 85: 192. 2016.
Calonectria longiramosa L. Lombard & Crous, Stud. Mycol. 86: 114. 2017.
In: Calonectria pteridis species complex.
Typus: CBS H-22750 holotype.
Ex-type culture: CBS 116250 = CMW 51234 = CPC 3534.
Type locality: Brazil, Amazon.
Type substrate: Eucalyptus tereticornis.
Barcodes: act = MT334955; cmdA = MT335182; his3 = MT335421; rpb2 = MT412488; tef1 = MT412712; tub2 = MT412935 (alternative markers: ITS = MT359642; LSU = MT359402).
Notes: Calonectria amazonica is closely related to Ca. pteridis and Ca. pseudopteridis, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. amazonica [(av. 79 × 5 μm); Lombard et al. 2016] are smaller than those of Ca. pteridis [(av. 82 × 5.5 μm); Crous et al. 1993c] and Ca. pseudopteridis [(av. 87.5 × 5.7 μm); Sobers 1968, Alfenas et al. 2015]. Calonectria amazonica is heterothallic (Li et al. 2020) (Supplementary Table S4).
amazoniensis Calonectria L. Lombard & Crous, Stud. Mycol. 85: 173. 2016.
(see Calonectria amazonica)
In: Calonectria pteridis species complex.
Typus: CBS H-22751 holotype.
Ex-type culture: CBS 115440 = CMW 51222 = CPC 3885.
Type locality: Brazil, Amazon.
Type substrate: Eucalyptus tereticornis.
Barcodes: act = MT334957; cmdA = MT335184; his3 = MT335423; tef1 = MT412714; tub2 = MT412937 (alternative markers: ITS = MT359644; LSU = MT359404).
Notes: Calonectria amazoniensis was treated as a synonym of Ca. amazonica in this study. In comparisons of DNA sequences for seven available gene regions, there were only three base differences in the tub2 gene sequences between ex-type isolate of Ca. amazoniensis (CBS 115440) and the ex-type isolate of Ca. amazonica (CBS 116250). Both species produce clavate vesicles of similar dimensions (Ca. amazoniensis: 5–7 μm; Ca. amazonica: 5–6 μm) and 1-septate macroconidia. The macroconidia of Ca. amazoniensis (av. 69 × 4 μm) are smaller than those of Ca. amazonica (av. 79 × 5 μm) (Lombard et al. 2016), which was considered to represent intraspecific variation (Supplementary Table S4).
angustata Calonectria (Crous & El-Gholl) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium angustatum Crous & El-Gholl, Mycoscience 41: 522. 2000.
In: Calonectria gracilipes species complex.
Typus: PREM 56546 holotype.
Ex-type culture: CBS 114544 = CMW 30990 = CPC 2347 = P99-0454.
Type locality: USA, Florida, Sarasota nursery.
Type substrate: Tillandsia capitata.
Barcodes: act = MT334963; his3 = MT335429; rpb2 = MT412493; tef1 = MT412720; tub2 = MT412943 (alternative markers: ITS = MT359650; LSU = MT359410).
Notes: Calonectria angustata is phylogenetically closely related to Ca. hurae and Ca. leguminum, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. angustata [(av. 110 × 10 μm); Crous et al. 2000] are shorter than those of Ca. hurae [(av. 120 × 7.5 μm); Crous 2002] but larger than those of Ca. leguminum [(av. 60 × 5 μm); Figueiredo & Namekata 1967, Crous 2002] (Supplementary Table S4).
arbusta Calonectria L. Lombard et al., Stud. Mycol. 80: 162. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21482 holotype.
Ex-type culture: CBS 136079 = CMW 31370 = CERC 1705.
Type locality: China, GuangXi Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334940; cmdA = MT335167; his3 = MT335406; rpb2 = MT412480; tef1 = MT412697 (alternative markers: ITS = MT359627; LSU = MT359387).
Notes: Calonectria arbusta was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for seven available gene regions, the only differences between the ex-type isolate of Ca. arbusta (CMW 31370) and the ex-type isolate of Ca. aconidialis (CMW 35174) was in the his3 and tef1 sequences, where there were two base differences in the his3 and one base difference in the tef1 sequences. Both species are homothallic and produce orange to orange-brown perithecia, 8-spored asci and 1-septate ascospores, and share the similar ascospores dimensions (Ca. arbusta: av. 38 × 7 μm; Ca. aconidialis: av. 36 × 6 μm) (Lombard et al. 2015a, Li et al. 2020; Supplementary Table S4).
asiatica Calonectria Crous & Hywel-Jones, Stud. Mycol. 50: 419. 2004.
Synonym: Cylindrocladium asiaticum Crous & Hywel-Jones, Stud. Mycol. 50: 419. 2004.
In: Calonectria kyotensis species complex.
Typus: CBS 9889 holotype.
Ex-type culture: CBS 114073 = CMW 23782 = CPC 3900.
Type locality: Thailand, Prathet Thai.
Type substrate: Leaf litter.
Barcodes: act = GQ280428; cmdA = AY725741; his3 = AY725658; tef1 = AY725705; tub2 = AY725616 (alternative markers: ITS = GQ280550; LSU = GQ280672).
Notes: Calonectria asiatica is closely related to Ca. yunnanensis, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. asiatica [(av. 53 × 5 μm); Crous et al. 2004] are larger than those of Ca. yunnanensis [(av. 43 × 4.5 μm); Li et al. 2017], and the vesicles of Ca. asiatica (12–17 μm) are wider than those of Ca. yunnanensis (2–4.5 μm). Calonectria asiatica is homothallic (Crous et al. 2004) (Supplementary Table S4).
auriculiformis Calonectria N.Q. Pham et al., Mycologia 111: 85. 2019.
In: Calonectria cylindrospora species complex.
Typus: PREM 62109 holotype.
Ex-type culture: CBS 143561 = CMW 47178.
Type locality: Vietnam, Thanh Hoa, Hau Loc.
Type substrate: Soil in Acacia auriculiformis plantation.
Barcodes: act = MT334964; cmdA = MT335190; his3 = MT335430; rpb2 = MT412494; tef1 = MT412721; tub2 = MT412944 (alternative markers: ITS = MT359651; LSU = MT359411).
Notes: Calonectria auriculiformis is closely related to Ca. lageniformis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. auriculiformis (av. 43 × 4 μm) are longer than those of Ca. lageniformis (av. 40 × 5 μm). Furthermore, Ca. auriculiformis has five tiers of branches in its conidiogenous apparatus in comparison to three in Ca. lageniformis (Lombard et al. 2016, Pham et al. 2019).
australiensis Calonectria (Crous & K.D. Hyde) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium australiense Crous & K.D. Hyde, Stud. Mycol. 55: 221. 2006.
In: Calonectria reteaudii species complex.
Typus: CBS H-17872 holotype.
Ex-type culture: CBS 112954 = CMW 23669 = CPC 4714.
Type locality: Australia, Queensland.
Type substrate: Ficus pleurocarpa.
Barcodes: act = MT334965; cmdA = MT335192; his3 = MT335432; rpb2 = MT412496; tef1 = MT412723; tub2 = MT412946 (alternative markers: ITS = MT359653; LSU = MT359413).
Notes: Phylogenetically, Ca. australiensis forms a distinct lineage separate from other species in this species complex.
avesiculata Calonectria T.S. Schubert et al., Canad. J. Bot. 67: 2415. 1989.
Synonym: Cylindrocladium avesiculatum D.L. Gill et al., Phytopathology 61: 60. 1971.
In: Calonectria mexicana species complex.
Typus: FLAS F55193 (holotype of Ca. avesiculata; Schubert et al. 1989), BPI 414546 (isosyntype of Cy. avesiculatum; Gill et al. 1971).
Ex-type culture: CBS 313.92 = CMW 23670 = CPC 2373 = ATCC 38226 (Lombard et al. 2010a).
Type locality: USA, Georgia, Cairo.
Type substrate: Ilex vomitoria.
Barcodes: act = GQ280431; cmdA = GQ267364; his3 = DQ190620; tef1 = GQ267294; tub2 = AF333392 (alternative markers: ITS = GQ280553; LSU = GQ280675).
Notes: Calonectria avesiculata is closely related to Ca. mexicana, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. avesiculata [(av. 64 × 5 μm); Schubert et al. 1989] are larger than those of Ca. mexicana [(av. 45 × 4 μm); Schoch et al. 1999], and the vesicles of Ca. avesiculata (1–4 μm) are narrower than those of Ca. mexicana (7–12 μm). Furthermore, Ca. avesiculata is characterised by producing a thick-walled stipe extension (Crous 2002), a feature not observed for Ca. mexicana. Calonectria avesiculata is heterothallic (Schubert et al. 1989) (Supplementary Table S4).
baviensis Calonectria N.Q. Pham et al., Mycologia 111: 90. 2019.
(see Calonectria reteaudii)
In: Calonectria reteaudii species complex.
Typus: PREM 62111 holotype.
Ex-type culture: CBS 143563 = CMW 47410.
Type locality: Vietnam, Hanoi, Bavi.
Type substrate: Eucalyptus urophylla.
Barcodes: act = MT334966; cmdA = MT335193; his3 = MT335433; rpb2 = MT412497; tef1 = MT412724; (alternative markers: ITS = MT359654; LSU = MT359414).
Notes: Calonectria baviensis was treated as a synonym of Ca. reteaudii in this study. In comparisons of DNA sequences for the seven available gene regions, a single base difference in each of the cmdA, rpb2 and tef1 sequences was found between the ex-type isolate of Ca. baviensis (CMW 47410) and the ex-type isolate of Ca. reteaudii (CMW 30984). Both species produce clavate vesicles with similar dimensions (Ca. baviensis: 3–6 μm; Ca. reteaudii: 3–6 μm) and 5-septate macroconidia. Macroconidia of Ca. baviensis (av. 96 × 6.5 μm) are longer than those of Ca. reteaudii (av. 84 × 6.5 μm) (Kang et al. 2001a, Pham et al. 2019), which was considered to represent intraspecific variation (Supplementary Table S4).
blephiliae Calonectria Crous & Hodges, Persoonia 31: 225. 2013.
(see Calonectria cylindrospora)
In: Calonectria cylindrospora species complex.
Typus: CBS H-21434 holotype.
Ex-type culture: CBS 136425 = CMW 51321 = CPC 21859.
Type locality: USA, North Carolina, Ellerbe.
Type substrate: Blephilia ciliata.
Barcodes: act = MT335005; cmdA = MT335235; his3 = MT335475; rpb2 = MT412539; tef1 = MT412766; tub2 = MT412984 (alternative markers: ITS = MT359696; LSU = MT359456).
Notes: Calonectria blephiliae was treated as a synonym of Ca. cylindrospora in this study. In comparisons of DNA sequences for eight gene regions, all sequences for the ex-type isolate of Ca. blephiliae (CBS 136425) were 100 % identical to those for the isolate of Ca. cylindrospora (CMW 30978); the only differences between the ex-type isolate of Ca. blephiliae (CBS 136425) and the isolate of Ca. cylindrospora (CBS 119670) was found in the act gene sequences where only one base difference was observed. Calonectria cylindrospora and Ca. blephiliae both produce clavate to ellipsoidal vesicles with overlapping dimensions (Ca. blephiliae: 7–10 μm; Ca. cylindrospora: 6–8 μm) and 1-septate macroconidia. The macroconidia of Ca. blephiliae (av. 50 × 4.5 μm) are larger than those of Ca. cylindrospora (av. 45 × 4 μm) (Crous 2002, Crous et al. 2013, Lombard et al. 2015b), which was considered to represent intraspecific variation (Supplementary Table S4).
brachiatica Calonectria L. Lombard et al., Persoonia 23: 44. 2009.
In: Calonectria brassicae species complex.
Typus: PREM 60197 holotype.
Ex-type culture: CBS 123700 = CMW 25298.
Type locality: Colombia, Buga.
Type substrate: Pinus maximinoi.
Barcodes: cmdA = MT335195; his3 = MT335435; rpb2 = MT412499; tef1 = MT412726; tub2 = MT412948 (alternative markers: ITS = MT359656; LSU = MT359416).
Notes: Calonectria brachiatica is closely related to Ca. parvispora, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. brachiatica [(av. 44 × 5 μm); Lombard et al. 2009] are larger than those of Ca. parvispora [(av. 29 × 4 μm); Marin-Felix et al. 2017] (Supplementary Table S4).
brasiliana Calonectria L. Lombard & Crous, Stud. Mycol. 85: 174. 2016.
In: Calonectria candelabrum species complex.
Typus: CBS H-22752 holotype.
Ex-type culture: CBS 111484 = CMW 51187 = CPC 1924.
Type locality: Brazil.
Type substrate: Soil.
Barcodes: act = MT334968; cmdA = MT335198; his3 = MT335438; rpb2 = MT412502; tef1 = MT412729; tub2 = MT412951 (alternative markers: ITS = MT359659; LSU = MT359419).
Notes: Calonectria brasiliana is closely related to Ca. metrosideri, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. brasiliana [(av. 53 × 4 μm); Lombard et al. 2016] are longer than those of Ca. metrosideri [(av. 45 × 4 μm); Alfenas et al. 2013a] (Supplementary Table S4).
brasiliensis Calonectria (Bat. & Cif.) L. Lombard et al., Stud. Mycol. 66: 19. 2010.
Basionym: Cylindrocladium scoparium var. brasiliensis Bat. & Cif., (as brasiliense) Boletim de SA.I.C. Pernambuco 18: 188. 1952.
Synonyms: Cylindrocladium brasiliense (Bat. & Cif.) Peerally, (as braziliensis) CMI Descriptions of Pathogenic Fungi and Bacteria 427. 1974.
Calonectria hodgesii R.F Alfenas et al., Trop. Plant. Pathol. 38: 517. 2013.
Calonectria pseudohodgesii R.F. Alfenas et al., Stud. Mycol. 80: 118. 2015.
In: Calonectria cylindrospora species complex.
Typus: IMI 43688 holotype.
Ex-type culture: CBS 230.51 = IMI 299576.
Type locality: Brazil, Ceara State.
Type substrate: Eucalyptus spp.
Barcodes: act = MT334970; cmdA = MT335200; his3 = MT335440; rpb2 = MT412504; tef1 = MT412731; tub2 = MT412953 (alternative markers: ITS = MT359661; LSU = MT359421).
Notes: Calonectria brasiliensis is closely related to Ca. maranhensis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. brasiliensis [(av. 38 × 3.5 μm); Batista 1951] are smaller than those of Ca. maranhensis [(av. 57 × 5 μm); Alfenas et al. 2015] (Supplementary Table S4).
brassiana Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 100. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS H-21376 holotype.
Ex-type culture: CBS 134855 = LPF378.
Type locality: Brazil, Piauí state, Teresina.
Type substrate: Soil in Eucalyptus brassiana plantation.
Barcodes: cmdA = KM396056; his3 = KM396139; tef1 = KM395882; tub2 = KM395969.
Notes: Calonectria brassiana is closely related to Ca. glaebicola, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. brassiana (av. 53 × 4 μm) are longer than those of Ca. glaebicola (av. 50 × 4 μm) (Alfenas et al. 2015) (Supplementary Table S4).
brassicae Calonectria (Pamwar & Bohra) L. Lombard et al., Persoonia 23: 45. 2009.
Basionym: Cylindrocladium brassicae Panwar & Bohra, Indian Phytopathol. 27: 425. 1974.
Synonyms: Cylindrocarpon gracile Bugnic., Encycl. Mycologique 11: 162. 1939.
Cylindrocladium gracile (Bugnic.) Boesew., Trans. Brit. Mycol. Soc. 78: 554. 1982.
Cylindrocladium clavatum Hodges & L.C. May, Phytopathology 62: 900. 1972.
In: Calonectria brassicae species complex.
Typus: PREM 51032 holotype.
Ex-type culture: CBS 111869 = CPC 2409.
Type locality: Indonesia.
Type substrate: Argyreia splendens.
Barcodes: act = MT334972; cmdA = MT335202; his3 = MT335442; rpb2 = MT412506; tef1 = MT412733; tub2 = MT412955 (alternative markers: ITS = MT359663; LSU = MT359423).
Notes: Calonectria brassicae is closely related to Ca. brachiatica and Ca. parvispora, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. brassicae [(av. 53 × 4.5 μm); Crous 2002] are longer than those of Ca. brachiatica [(av. 44 × 5 μm); Lombard et al. 2009] and Ca. parvispora [(av. 29 × 4 μm); Marin-Felix et al. 2017] (Supplementary Table S4).
brassicicola Calonectria L. Lombard & Crous, Stud. Mycol. 85: 174. 2016.
In: Calonectria kyotensis species complex.
Typus: CBS H-22753 holotype.
Ex-type culture: CBS 112841 = CMW 51206 = CPC 4552.
Type locality: Indonesia.
Type substrate: Soil under Brassica sp.
Barcodes: cmdA = KX784561; tef1 = KX784689; tub2 = KX784619.
Notes: Calonectria brassicicola is closely related to Ca. sumatrensis, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. brassicicola [(av. 42 × 5 μm); Lombard et al. 2016] are shorter than those of Ca. sumatrensis [(av. 58 × 5 μm); Crous et al. 2004], and the vesicles of Ca. brassicicola (3−5 μm) are narrower than those of Ca. sumatrensis (8–13 μm) (Crous et al. 2004, Lombard et al. 2016) (Supplementary Table S4).
brevistipitata Calonectria L. Lombard & Crous, Stud. Mycol. 85: 175. 2016.
In: Calonectria candelabrum species complex.
Typus: CBS H-22754 holotype.
Ex-type culture: CBS 115671 = CMW 51226 = CPC 949.
Type locality: Mexico.
Type substrate: Soil.
Barcodes: act = MT334973; cmdA = MT335203; his3 = MT335443; rpb2 = MT412507; tef1 = MT412734; tub2 = MT412956 (alternative markers: ITS = MT359664; LSU = MT359424).
Notes: Calonectria brevistipitata is closely related to Ca. nemoricola, Ca. pauciramosa and Ca. silvicola, and can be distinguished from these three species by the dimensions of its macroconidia. The macroconidia of Ca. brevistipitata [(av. 31 × 3.5 μm); Lombard et al. 2016] are smaller than those of Ca. nemoricola [(av. 45 × 4 μm); Alfenas et al. 2015], Ca. pauciramosa [(av. 50 × 4.5 μm); Crous 2002] and Ca. silvicola [(av. 41 × 4.5 μm); Alfenas et al. 2015]. Calonectria brevistipitata is heterothallic (Li et al. 2020) (Supplementary Table S4).
bumicola Calonectria N.Q. Pham & M.J. Wingf., Mycologia 111: 94. 2019.
In: Calonectria kyotensis species complex.
Typus: PREM 62123 holotype.
Ex-type culture: CBS 143575 = CMW 48257.
Type locality: Indonesia, Northern Sumatra, Aek Nauli.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334975; cmdA = MT335205; his3 = MT335445; rpb2 = MT412509; tef1 = MT412736 (alternative markers: ITS = MT359666; LSU = MT359426).
Notes: Calonectria bumicola is phylogenetically closely related to Ca. lantauensis and can be differentiated from that species by the sequences of act, cmdA, his3, rpb2 and tef1 gene regions. The asexual and sexual morphs of Ca. bumicola could not be induced by Pham et al. (2019). This species was shown to be homothallic by Li et al. (2020).
canadiana Calonectria (J.C. Kang et al.) L. Lombard et al., IMA Fungus 1: 106. 2010.
Basionym: Cylindrocladium canadense J.C. Kang et al., Syst. Appl. Microbiol. 24: 210. 2001.
Synonyms: Calonectria canadensis (J.C. Kang et al.) L. Lombard et al., Stud. Mycol. 66: 56. 2010. Nom. inval., Art. 53.1.
Calonectria montana Q.L. Liu & S.F. Chen, Mycokeys 26: 48. 2017.
In: Calonectria kyotensis species complex.
Typus: PREM 57195 holotype.
Ex-type culture: CBS 110817 = CMW 23673 = STE-U 499.
Type locality: Canada.
Type substrate: Picea sp.
Barcodes: act = MT334976; cmdA = MT335206; his3 = MT335446; rpb2 = MT412510; tef1 = MT412737; tub2 = MT412958 (alternative markers: ITS = MT359667; LSU = MT359427).
Notes: Phylogenetically, Ca. canadiana forms a distinct lineage separate from other species in this species complex.
candelabrum Calonectria (Viégas) Rossman et al., (as candelabra), Stud. Mycol. 80: 210. 2015.
Basionym: Cylindrocladium candelabrum Viégas, Bragantia 6: 370. 1946.
Synonyms: Calonectria scoparia Ribeiro & Matsuoka, In: Ribeiro, M.Sc. Thesis, Heterotalismo em C. scoparium Morgan: 28. 1978 (nom. inval., Art. 29).
Calonectria scoparia Peerally, Mycotaxon 40: 341. 1991.
Calonectria pseudoscoparia L. Lombard et al., Stud. Mycol. 66: 53. 2010.
In: Calonectria candelabrum species complex.
Typus: PREM 51044 (neotype of Cy. candelabrum; Crous 2002).
Ex-type culture: Not available.
Key cultures: CMW 31000 = CPC 1675 = UFV 117, CMW 31001 = STE-U 1679 = UFV 126 (Alfenas et al. 2015).
Key culture locality: Brazil, Amazonas (locality of CMW 31000 and CMW 31001; Alfenas et al. 2015).
Key culture substrate: Eucalyptus sp. (substrate of CMW 31000 and CMW 31001; Alfenas et al. 2015).
Barcodes: Culture CMW 31000: act = MT334977; cmdA = MT335207; his3 = MT335447; rpb2 = MT412511; tef1 = MT412738; tub2 = MT412959 (alternative markers: ITS = MT359668; LSU = MT359428); culture CMW 31001: act = MT334978; cmdA = MT335208; his3 = MT335448; rpb2 = MT412512; tef1 = MT412739; tub2 = MT412960 (alternative markers: ITS = MT359669; LSU = MT359429)
Notes: Calonectria candelabrum is closely related to Ca. eucalypticola and Ca. venezuelana, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. candelabrum [(av. 60 × 4.5 μm); Crous 2002] are longer than those of Ca. eucalypticola [(av. 50 × 4 μm); Alfenas et al. 2015] and Ca. venezuelana [(av. 58 × 5 μm); Lombard et al. 2016]. Calonectria candelabrum is heterothallic (Crous 2002, Li et al. 2020) (Supplementary Table S4).
cerciana Calonectria L. Lombard et al., Persoonia 24: 7. 2010.
Synonyms: Calonectria papillata L. Lombard et al., Stud. Mycol. 80: 175. 2015.
Calonectria terrestris L. Lombard et al., Stud. Mycol. 80: 182. 2015.
In: Calonectria cylindrospora species complex.
Typus: PREM 60241 holotype.
Ex-type culture: CBS 123693 = CMW 25309.
Type locality: China, GuangDong, CERC nursery.
Type substrate: E. urophylla × E. grandis hybrid cutting.
Barcodes: act = MT334981; cmdA = MT335211; his3 = MT335451; rpb2 = MT412515; tef1 = MT412742; tub2 = MT412963 (alternative markers: ITS = MT359672; LSU = MT359432).
Notes: Calonectria cerciana is closely related to Ca. tonkinensis, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. cerciana (av. 44 × 5 μm) are larger than those of Ca. tonkinensis (av. 41.5 × 4 μm), and the vesicles of Ca. cerciana (8–13 μm) are wider than those of Ca. tonkinensis (3–7 μm) (Lombard et al. 2010c, Pham et al. 2019) (Supplementary Table S4).
chinensis Calonectria (Crous) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium chinense Crous, Stud. Mycol. 50: 420. 2004.
Synonym: Calonectria multistipitata N.Q. Pham et al., Mycologia 111: 98. 2019.
In: Calonectria kyotensis species complex.
Typus: PREM 62118 holotype.
Ex-type culture: CBS 114827 = CMW 23674 = CPC 4101.
Type locality: China, Hong Kong.
Type substrate: Soil.
Barcodes: act = MT334990; cmdA = MT335220; his3 = MT335460; rpb2 = MT412524; tef1 = MT412751; tub2 = MT412972 (alternative markers: ITS = MT359681; LSU = MT359441).
Notes: Calonectria chinensis is phylogenetically closely related to Ca. cochinchinensis and Ca. heveicola, it can be distinguished from these two species by the sequences of cmdA, his3, rpb2, tef1 and tub2 gene regions.
citri Calonectria (H.S. Fawc. & Klotz) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Candelospora citri H.S. Fawc. & Klotz, Mycologia 29: 213. 1937.
Synonym: Cylindrocladium citri (H.S. Fawc. & Klotz) Boedijn & Reitsma, Reinwardtia 1: 57. 1950.
In: Calonectria mexicana species complex.
Typus: K (isotype; Crous 2002).
Ex-type culture: CBS 186.36 = CMW 23675 (Lombard et al. 2010a).
Type locality: USA, Florida.
Type substrate: Citrus sinensis.
Barcodes: act = MT334992; cmdA = MT335222; his3 = MT335462; rpb2 = MT412526; tef1 = MT412753; tub2 = MT412974 (alternative markers: ITS = MT359683; LSU = MT359443).
Notes: Calonectria citri is closely related to Ca. lauri, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. citri [(av. 58 × 4 μm); Crous 2002] are smaller than those of Ca. lauri [(av. 60 × 5.5 μm); Lechat et al. 2010] (Supplementary Table S4).
clavata Calonectria Alfieri et al., Mycotaxon 48: 206. 1993.
Synonym: Cylindrocladium flexuosum Crous, Syst. Appl. Microbiol. 18: 248. 1995.
In: Calonectria brassicae species complex.
Typus: FLAS F55430 [holotype of Ca. clavata; El-Gholl et al. 1993b], PREM 51721 (holotype of Cy. flexuosum; Crous et al. 1995).
Ex-type culture: CBS 114557 = CMW 23690 = CPC 2536 = P078-1543 = ATCC 66389 (Crous 2002, Lombard et al. 2010a).
Type locality: USA, Florida, Lake Placid.
Type substrate: Callistemon viminalis.
Barcodes: act = MT334993; cmdA = MT335223; his3 = MT335463; rpb2 = MT412527; tef1 = MT412754; tub2 = MT412975 (alternative markers: ITS = MT359684; LSU = MT359444).
Notes: Phylogenetically, Ca. clavata forms a distinct lineage separate from other species in this species complex. Calonectria clavata is heterothallic (El-Gholl et al. 1993b, Li et al. 2020).
cliffordiicola Calonectria L. Lombard & Crous, Stud. Mycol. 85: 177. 2016.
(see Calonectria pauciramosa)
In: Calonectria candelabrum species complex.
Typus: CBS H-22755 holotype.
Ex-type culture: CBS 111812 = CMW 51190 = CPC 2631.
Type locality: South Africa, Western Cape Province, George.
Type substrate: Cliffordia feruginea.
Barcodes: act = MT335084; cmdA = MT335316; his3 = MT335556; rpb2 = MT412609; tef1 = MT412847; tub2 = MT413059 (alternative markers: ITS = MT359777; LSU = MT359537).
Notes: Calonectria cliffordiicola was treated as a synonym of Ca. pauciramosa in this study. In comparisons of DNA sequences for eight gene regions, all the sequences of the isolates of Ca. cliffordiicola (ex-type CBS 111812, CBS 111814, and CBS 111819) were 100 % identical to those of the isolates of Ca. pauciramosa (ex-type CBS 138824, CMW 2151, CMW 7592, CMW 9151, CMW 30823, and CMW 30875). Both species produce obpyriform to ellipsoidal vesicles with overlapping dimensions (Ca. cliffordiicola: 7–9 μm; Ca. pauciramosa: 5–11 μm) and 1-septate macroconidia, the macroconidia of Ca. cliffordiicola (av. 40 × 4 μm) are smaller than those of Ca. pauciramosa (av. 50 × 4.5 μm) (Crous 2002, Lombard et al. 2016), which was considered to represent intraspecific variation (Supplementary Table S4).
cochinchinensis Calonectria N.Q. Pham et al., Mycologia 111: 95. 2019.
In: Calonectria kyotensis species complex.
Typus: PREM 62115 holotype.
Ex-type culture: CBS 143567 = CMW 49915.
Type locality: Vietnam, Tay Ninh, Duong Minh Chau.
Type substrate: Soil in Hevea brasiliensis plantation.
Barcodes: act = MT334995; cmdA = MT335225; his3 = MT335465; rpb2 = MT412529; tef1 = MT412756; tub2 = MT412977 (alternative markers: ITS = MT359686; LSU = MT359446).
Notes: Calonectria cochinchinensis is phylogenetically closely related to Ca. heveicola and can be differentiated from Ca. heveicola by sequence data of act, cmdA, his3, ITS, tef1 and tub2 gene regions. In addition, Ca. cochinchinensis produces longer stipe extensions [(147–208 μm); Pham et al. 2019] than those of Ca. heveicola [(138–189 μm); Pham et al. 2019] (Supplementary Table S4).
colhounii Calonectria Peerally, Mycol. Res. 61: 92. 1973.
Synonym: Cylindrocladium colhounii Peerally, Mycol. Res. 61: 92. 1973.
In: Calonectria colhounii species complex.
Typus: IMI 167581 holotype.
Ex-type culture: CBS 293.79 = CMW 30999.
Type locality: Mauritius.
Type substrate: Camellia sinensis.
Barcodes: act = GQ280443; cmdA = GQ267373; his3 = DQ190639; rpb2 = KY653376; tef1 = GQ267301; tub2 = DQ190564 (alternative markers: ITS = GQ280565; LSU = GQ280687).
Notes: Calonectria colhounii is phylogenetically closely related to Ca. aciculata and Ca. honghensis, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. colhounii [(av. 65 × 5 μm); Peerally 1973] are smaller than those of Ca. aciculata [(av. 69 × 5.5 μm); Li et al. 2017], and longer than those of Ca. honghensis [(av. 54 × 5.5 μm); Li et al. 2017]. In addition, the ascospores of Ca. colhounii (av. 55 × 6 μm) are longer than those of Ca. honghensis (av. 49 × 6 μm) (Peerally 1973, Li et al. 2017). Calonectria colhounii is homothallic (Peerally 1973) (Supplementary Table S4).
colombiana Calonectria L. Lombard et al., Stud. Mycol. 66: 23. 2010.
In: Calonectria candelabrum species complex.
Typus: PREM 60295 holotype.
Ex-type culture: CBS 115127 = CMW 30871 = CPC 1160.
Type locality: Colombia, La Selva.
Type substrate: Soil.
Barcodes: act = GQ280538; cmdA = GQ267455; his3 = FJ972442; tef1 = FJ972492; tub2 = FJ972423 (alternative markers: ITS = GQ280660; LSU = GQ280782).
Notes: Calonectria colombiana is closely related to Ca. brevistipitata, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. colombiana (av. 37 × 3 μm) are longer than those of Ca. brevistipitata (av. 31 × 3.5 μm), and the vesicles of Ca. colombiana (8–12 μm) are wider than those of Ca. brevistipitata (5–8 μm) (Lombard et al. 2010b, 2016). Calonectria colombiana is characterised as being homothallic, while Ca. brevistipitata is heterothallic (Lombard et al. 2010b, Li et al. 2020) (Supplementary Table S4).
colombiensis Calonectria Crous, Stud. Mycol. 50: 421. 2004.
Synonym: Cylindrocladium colombiense Crous, Stud. Mycol. 50: 421. 2004.
In: Calonectria kyotensis species complex.
Typus: CBS 9890 holotype.
Ex-type culture: CBS 112220 = CMW 23676 = CPC 723.
Type locality: Colombia, La Selva.
Type substrate: Soil under Eucalyptus grandis.
Barcodes: act = MT334998; cmdA = MT335228; his3 = MT335468; rpb2 = MT412532; tef1 = MT412759; tub2 = MT412980 (alternative markers: ITS = MT359689; LSU = MT359449).
Notes: Phylogenetically, Ca. colombiensis forms a distinct lineage separate from other species in this species complex. Ca. colombiensis is homothallic (Li et al. 2020).
crousiana Calonectria S.F. Chen et al., Persoonia 26: 6. 2011.
In: Calonectria reteaudii species complex.
Typus: PREM 60453 holotype.
Ex-type culture: CBS 127198 = CMW 27249.
Type locality: China, FuJian Province.
Type substrate: Eucalyptus grandis.
Barcodes: act = MT335000; cmdA = MT335230; his3 = MT335470; rpb2 = MT412534; tef1 = MT412761; tub2 = MT412982 (alternative markers: ITS = MT359691; LSU = MT359451).
Notes: Phylogenetically, Ca. crousiana forms a distinct lineage separate from other species in this species complex. Calonectria crousiana is homothallic (Chen et al. 2011, Li et al. 2020).
curvispora Calonectria (Crous & D. Victor) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium curvisporum Crous & D. Victor, Syst. Appl. Microbiol. 20: 283. 1997.
Synonym: Calonectria vegrandis N.Q. Pham & M.J. Wingfield, Mycologia 111: 99. 2019.
In: Calonectria kyotensis species complex.
Typus: PREM 51751 holotype.
Ex-type culture: CBS 116159 = CMW 23693 = CPC 765.
Type locality: Madagascar, Tamatave.
Type substrate: Soil.
Barcodes: act = MT335002; cmdA = MT335232; his3 = MT335472; rpb2 = MT412536; tef1 = MT412763 (alternative markers: ITS = MT359693; LSU = MT359453).
Notes: Calonectria curvispora is closely related to Ca. aeknauliensis and Ca. ilicicola, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. curvispora [(av. 60 × 5 μm); Victor et al. 1997] are smaller than those of Ca. ilicicola [(av. 62 × 6 μm); Boedijn & Reitsma 1950] but longer than those of Ca. aeknauliensis [(av. 47 × 5 μm); Pham et al. 2019] (Supplementary Table S4).
cylindrospora Calonectria (Ellis & Everh.) Rossman et al., Stud. Mycol. 80: 210. 2015.
Basionym: Diplocladium cylindrosporum Ellis & Everh., Bull. Torrey Bot. Club 27: 58. 1900.
Synonyms: Cylindrocladium scoparium Morgan, Bot. Gaz. 17: 191. 1892.
Cylindrocladium pithecolobii Petch, Ann. Roy. Bot. Gard. (Peradeniya) 6: 244. 1917.
Cylindrocladium ellipticum Alfieri et al., Phytopathology 60: 1213. 1970.
Calonectria morganii Crous et al., Mycol. Res. 97: 706. 1993.
Calonectria blephiliae Crous & Hodges, Persoonia 31: 225. 2013.
In: Calonectria cylindrospora species complex.
Typus: PREM 51042 (holotype of Ca. morganii; Crous et al. 1993a), BPI 414576 (neotype of Cy. scoparium; Crous et al. 1993a).
Ex-type culture: Not available.
Key cultures: CBS 119670 = CMW 51310 = CPC 12766, CBS 110666 = CMW 30978 = STE-U 497 = P90.1479 (Crous 2002, Lombard et al. 2015a).
Key culture locality: Italy (locality of CBS 119670; Lombard et al. 2015a), USA, Florida (locality of CBS 110666; Lombard et al. 2015a).
Key culture substrate: Pistacia lentiscus (substrate of CBS 119670; Lombard et al. 2015a), Ilex vomitoria (substrate of CBS 110666; Lombard et al. 2015a).
Barcodes: Culture CBS 119670: act = MT335006; cmdA = MT335236; his3 = MT335476; rpb2 = MT412540; tef1 = MT412767; tub2 = MT412985 (alternative markers: ITS = MT359697; LSU = MT359457); culture CBS 110666: act = MT335007; cmdA = MT335237; his3 = MT335477; rpb2 = MT412541; tef1 = MT412768; tub2 = MT412986 (alternative markers: ITS = MT359698; LSU = MT359458).
Notes: Calonectria cylindrospora is phylogenetically closely related to Ca. variabilis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. cylindrospora [(av. 45 × 4 μm); Crous et al. 1993a, Lombard et al. 2015b] are smaller than those of Ca. variabilis [(av. 73 × 5 μm); Crous et al. 1993b], Ca. variabilis readily produces a microconidial morph in culture on CLA (Crous et al. 1993b), which is not observed in Ca. cylindrospora (Crous 2002). Calonectria cylindrospora is characterised as being heterothallic, while Ca. variabilis is homothallic (Crous et al. 1993b).
densa Calonectria L. Lombard et al., Stud. Mycol. 66: 46. 2010.
In: Calonectria spathiphylli species complex.
Typus: PREM 60302 holotype.
Ex-type culture: CBS 125261 = CMW 31182.
Type locality: Ecuador, Pichincha Province, Las Golondrinas.
Type substrate: Soil.
Barcodes: act = MT335008; cmdA = MT335238; his3 = MT335478; tef1 = MT412769; tub2 = MT412987 (alternative markers: ITS = MT359699; LSU = MT359459).
Notes: Calonectria densa is closely related to Ca. humicola and it can be distinguished from that species by the formation of lateral stipe extensions, a characteristic not known for Ca. humicola. The macroconidia of Ca. densa [(av. 54 × 6 μm); Lombard et al. 2010a] are larger than those of Ca. humicola [(av. 51 × 5 μm); Lombard et al. 2010a] (Supplementary Table S4).
duoramosa Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 100. 2015.
In: Calonectria brassicae species complex.
Typus: CBS H-21380 holotype.
Ex-type culture: CBS 134656 = LPF434.
Type locality: Brazil, Pará state, Monte Dourado.
Type substrate: Soil.
Barcodes: cmdA = KM396027; his3 = KM396110; tef1 = KM395853; tub2 = KM395940.
Notes: Calonectria duoramosa forms a single lineage closely related to Ca. octoramosa, and the smaller numbers (–2) of fertile branches in its conidiogenous apparatus can be distinguished from Ca. octoramosa (–8). Furthermore, the macroconidia of Ca. duoramosa [(av. 46 × 4 μm); Alfenas et al. 2015] are longer than those of Ca. octoramosa [(av. 36 × 4 μm); Marin-Felix et al. 2017] (Supplementary Table S4).
ecuadorae Calonectria (Crous & M.J. Wingf.) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium ecuadorae Crous & M.J. Wingf. (as ecuadoriae), Stud. Mycol. 55: 222. 2006.
Synonym: Calonectria ecuadorensis L. Lombard & Crous, Stud. Mycol. 86: 112. 2017.
In: Calonectria brassicae species complex.
Typus: CBS H-17871 holotype.
Ex-type culture: CBS 111406 = CMW 23677 = CPC 1635.
Type locality: Ecuador.
Type substrate: Soil.
Barcodes: act = MT335012; cmdA = MT335242; his3 = MT335482; rpb2 = MT412544; tef1 = MT412773; tub2 = MT412991 (alternative markers: ITS = MT359703; LSU = MT359463).
Notes: Calonectria ecuadorae is closely related to Ca. octoramosa and Ca. pauciphialidica, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. ecuadorae [(av. 51 × 4.5 μm); Crous et al. 2006] are larger than those of Ca. octoramosa [(av. 36 × 4 μm); Marin-Felix et al. 2017] and Ca. pauciphialidica (av. 29.5 × 3 μm) (Supplementary Table S4).
ecuadorensis Calonectria L. Lombard & Crous, Stud. Mycol. 86: 112. 2017
(see Calonectria ecuadorae)
In: Calonectria brassicae species complex.
Typus: CBS H-23134 holotype.
Ex-type culture: CBS 111706 = CMW 51821 = CPC 1636.
Type locality: Ecuador.
Type substrate: Soil.
Barcodes: act = MT335010; cmdA = MT335240; his3 = MT335480; rpb2 = MT412542; tef1 = MT412771; tub2 = MT412989 (alternative markers: ITS = MT359701; LSU = MT359461).
Notes: Calonectria ecuadorensis was treated as a synonym of Ca. ecuadorae in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. ecuadorensis (CBS 111706) and the ex-type isolate of Ca. ecuadorae (CMW 23677) was in the act, cmdA and rpb2 sequences, where there was one base difference in the act, two base differences in the cmdA and one base difference in the rpb2 sequences. Both species produce clavate vesicles of similar dimensions (Ca. ecuadorensis: 4–6 μm; Ca. ecuadorae: 3–5 μm). The macroconidia of Ca. ecuadorensis (av. 37 × 4 μm) are smaller than those of Ca. ecuadorae (av. 51 × 4.5 μm) (Crous et al. 2006, Marin-Felix et al. 2017), which was considered to represent intraspecific variation (Supplementary Table S4).
ericae Calonectria L. Lombard & Crous, Stud. Mycol. 85: 178. 2016.
(see Calonectria pauciramosa)
In: Calonectria candelabrum species complex.
Typus: CBS H-22756 holotype.
Ex-type culture: CBS 114458 = CMW 51211 = CPC 2019.
Type locality: USA, California.
Type substrate: Erica capensis.
Barcodes: act = MT335087; cmdA = MT335319; his3 = MT335559; rpb2 = MT412612; tef1 = MT412850; tub2 = MT413062 (alternative markers: ITS = MT359780; LSU = MT359540).
Notes: Calonectria ericae was treated as a synonym of Ca. pauciramosa in this study. In comparisons of DNA sequences for eight gene regions, all the sequences of the isolates of Ca. ericae (ex-type CBS 114458, CBS 114456, and CBS 114457) were 100 % identical to those of the isolates of Ca. pauciramosa (ex-type CBS 138824, CMW 2151, CMW 7592, CMW 9151, CMW 30823, and CMW 30875). Both species produce obpyriform to ellipsoidal vesicles with similar dimensions (Ca. ericae: 6–10 μm; Ca. pauciramosa: 5–11 μm) and 1-septate macroconidia. The macroconidia of Ca. ericae (av. 37 × 4 μm) are smaller than those of Ca. pauciramosa (av. 50 × 4.5 μm) (Crous 2002, Lombard et al. 2016), which was considered to represent intraspecific variation (Supplementary Table S4).
eucalypti Calonectria L. Lombard et al., Stud. Mycol. 66: 47. 2010.
Synonym: Calonectria pseudocolhounii S.F. Chen et al., Persoonia 26: 7. 2011.
In: Calonectria colhounii species complex.
Typus: PREM 60298 holotype.
Ex-type culture: CBS 125275 = CMW 18444.
Type locality: Indonesia, Sumatra Utara, Aek Nauli.
Type substrate: Eucalyptus grandis.
Barcodes: act = MT335013; cmdA = MT335243; his3 = MT335483; rpb2 = MT412545; tef1 = MT412774; tub2 = MT412992 (alternative markers: ITS = MT359704; LSU = MT359464).
Notes: Calonectria eucalypti is closely related to Ca. aciculata and Ca. honghensis, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. eucalypti [(av. 72 × 6 μm); Lombard et al. 2010a] are larger than those of Ca. aciculata [(av. 69 × 5.5 μm); Li et al. 2017] and Ca. honghensis [(av. 54 × 5.5 μm); Li et al. 2017]. Calonectria eucalypti is homothallic (Li et al. 2020) (Supplementary Table S4).
eucalypticola Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 105. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS H-21359 holotype.
Ex-type culture: CBS 134847 = LPF124.
Type locality: Brazil, Minas Gerais state, Santa Barbara.
Type substrate: Eucalyptus seedling.
Barcodes: cmdA = KM396051; his3 = KM396134; tef1 = KM395877; tub2 = KM395964.
Notes: Calonectria eucalypticola is closely related to Ca. candelabrum and Ca. venezuelana, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. eucalypticola [(av. 50 × 4 μm); Alfenas et al. 2015] are smaller than those of Ca. candelabrum [(av. 60 × 4.5μm); Crous 2002] and Ca. venezuelana [(av. 58 × 5 μm); Lombard et al. 2016] (Supplementary Table S4).
expansa Calonectria L. Lombard et al., Stud. Mycol. 80: 167. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21483 holotype.
Ex-type culture: CBS 136247 = CMW 31392 = CERC 1727.
Type locality: China, GuangXi Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334942; cmdA = MT335169; his3 = MT335408; rpb2 = MT412481; tef1 = MT412699 (alternative markers: ITS = MT359629; LSU = MT359389).
Notes: Calonectria expansa was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for seven available gene regions, only a single base difference in the tef1 gene sequence was found between the ex-type isolate of Ca. expansa (CMW 31392) and the ex-type isolate of Ca. aconidialis (CMW 35174). Both species are homothallic and produce orange to orange-brown perithecia, 8-spored asci and 1-septate ascospores, and share the similar ascospores dimensions (Ca. expansa: av. 39 × 6 μm; Ca. aconidialis: av. 36 × 6 μm) (Lombard et al. 2015a, Li et al. 2020; Supplementary Table S4).
floridana Calonectria Sobers, Phytopathology 59: 366. 1969.
(see Calonectria kyotensis)
In: Calonectria kyotensis species complex.
Typus: BPI 414553 holotype.
Ex-type culture: CBS 114692 = CMW 51826 = ATCC 18882.
Type locality: USA, Georgia.
Type substrate: Prunus persica.
Barcodes: act = MT335015; cmdA = MT335245; his3 = MT335485; rpb2 = MT412547; tef1 = MT412776; tub2 = MT412994 (alternative markers: ITS = MT359706; LSU = MT359466).
Notes: Calonectria floridana was treated as a synonym of Ca. kyotensis in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. floridana (CBS 114692) and the ex-type isolate of Ca. kyotensis (CBS 114525) was in the cmdA, his3 and LSU sequences, where there was one base difference in the cmdA, two base differences in the his3 and one base difference in the LSU sequences. Both species are homothallic and produce globose vesicles of similar dimensions (Ca. floridana: 8.3–17.9 μm; Ca. kyotensis: 8.8–19 μm) and 1-septate macroconidia. The macroconidia of Ca. floridana (av. 46 × 4.3 μm) are longer than those of Ca. kyotensis (av. 41 × 4 μm) (Terashita 1968, Sobers 1969, Crous 2002), which was considered to represent intraspecific variation (Supplementary Table S4).
foliicola Calonectria L. Lombard et al., Stud. Mycol. 80: 167. 2015.
(see Calonectria hawksworthii)
In: Calonectria cylindrospora species complex.
Typus: CBS H-21472 holotype.
Ex-type culture: CBS 136641 = CMW 31393.
Type locality: China, GuangXi Province.
Type substrate: Eucalyptus urophylla × E. grandis clone leaf.
Barcodes: act = MT335017; cmdA = MT335247; his3 = MT335487; rpb2 = MT412549; tef1 = MT412778; tub2 = MT412996 (alternative markers: ITS = MT359708; LSU = MT359468).
Notes: Calonectria foliicola was treated as a synonym of Ca. hawksworthiii in this study. In comparisons of DNA sequences for eight gene regions, the only differences between the ex-type isolate Ca. foliicola (CMW 31393) and the ex-type isolate Ca. hawksworthii (ex-type CBS 111870) was in the act, his3 and tub2 sequences, where there was one base difference in the act, three base differences in the his3, and three base differences in the tub2 sequences. Both species produce ellipsoidal vesicles with overlapping dimensions (Ca. foliicola: 6–13 μm; Ca. hawksworthii: 6–9 μm) and 1-septate macroconidia. The macroconidia of Ca. foliicola (av. 47 × 5 μm) are shorter than those of Ca. hawksworthii (av. 56 × 4 μm) (Crous 2002, Lombard et al. 2015a), which was considered to represent intraspecific variation (Supplementary Table S4).
fragariae Calonectria R.F. Alfenas et al., Australas. Plant. Pathol. 47: 6. 2017.
In: Calonectria candelabrum species complex.
Typus: VIC 42833 holotype.
Ex-type culture: CBS 133607 = LPP040.
Type locality: Brazil, Espírito Santo State, Santa Maria do Jetibá.
Type substrate: Fragaria × ananassa.
Barcodes: cmdA = KM998966; his3 = KM998964; tef1 = KM998963; tub2 = KM998965.
Notes: Calonectria fragariae is closely related to Ca. pseudospathulata, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. fragariae [(av. 39 × 4 μm); Lopes et al. 2017] are shorter than those of Ca. pseudospathulata [(av. 43 × 4 μm); Alfenas et al. 2015] (Supplementary Table S4).
fujianensis Calonectria S.F. Chen et al., Persoonia 26: 8. 2011.
Synonym: Calonectria nymphaeae Yong Wang bis et al., Mycotaxon 122: 181. 2013.
In: Calonectria colhounii species complex.
Typus: PREM 60460 holotype.
Ex-type culture: CBS 127201 = CMW 27257.
Type locality: China, FuJian Province.
Type substrate: Eucalyptus grandis.
Barcodes: act = MT335019; cmdA = MT335249; his3 = MT335489; rpb2 = MT412551; tef1 = MT412780; tub2 = MT412998 (alternative markers: ITS = MT359710; LSU = MT359470).
Notes: Calonectria fujianensis is phylogenetically closely related to Ca. lichi. The sequences of ITS, tef1 and tub2 gene regions can differentiate Ca. fujianensis from that species. The macroconidia of Ca. fujianensis [(av. 52.5 × 4 μm); Chen et al. 2011] are smaller than those of Ca. lichi [(av. 65.7 × 6 μm); Liu & Chen 2017]. Calonectria fujianensis is homothallic (Chen et al. 2011) (Supplementary Table S4).
glaebicola Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 106. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS H-21378 holotype.
Ex-type culture: CBS 134852 = LPF406.
Type locality: Brazil, Minas Gerais state, Martinho Campos.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: cmdA = KM396053; his3 = KM396136; tef1 = KM395879; tub2 = KM395966.
Notes: Calonectria glaebicola is closely related to Ca. brassiana, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. glaebicola (av. 50 × 4 μm) are shorter than those of Ca. brassiana (av. 53 × 4 μm) (Alfenas et al. 2015) (Supplementary Table S4).
gordoniae Calonectria (Leahy et al.) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium gordoniae Leahy et al., Mycotaxon 76: 80. 2000.
In: Calonectria pteridis species complex.
Typus: FLAS F56790 holotype.
Ex-type culture: CBS 112142 = CMW 23694 = STE-U 3136 = ATCC 201837 = P97-2567.
Type locality: USA, Florida.
Type substrate: Gordonia lasianthus.
Barcodes: act = MT335021; cmdA = MT335251; his3 = MT335491; rpb2 = MT412553; tef1 = MT412782; tub2 = MT413000 (alternative markers: ITS = MT359712; LSU = MT359472).
Notes: Calonectria gordoniae is closely related to Ca. ovata, Ca. pseudovata and Ca. terricola, and can be distinguished from these three species by the dimensions of its macroconidia. The macroconidia of Ca. gordoniae [(av. 50 × 4.5 μm); Leahy et al. 2000] are smaller than those of Ca. ovata [(av. 70 × 5 μm); El-Gholl et al. 1993a] and Ca. pseudovata [(av. 69 × 5 μm); Alfenas et al. 2015], but longer than those of Ca. terricola [(av. 46 × 4.5 μm); Lombard et al. 2016] (Supplementary Table S4).
gracilipes Calonectria Crous & Mchau, Mycologia 89: 654. 1997.
Synonym: Cylindrocladium graciloideum Crous & Mchau, Mycologia 89: 654. 1997.
In: Calonectria gracilipes species complex.
Typus: PREM 54417 (holotype of Ca. gracilipes; Crous et al. 1997a), PREM 55299 (holotype of Cy. graciloideum; Crous et al. 1997a).
Ex-type culture: CBS 115674 = CMW 51227 = STE-U 1153 (Crous 2002, Crous et al. 2006).
Type locality: Colombia, La Selva.
Type substrate: Soil.
Barcodes: act = MT335022; cmdA = MT335252; his3 = MT335492; rpb2 = MT412554; tef1 = MT412783; tub2 = MT413001 (alternative markers: ITS = MT359713; LSU = MT359473).
Notes: Calonectria gracilipes is closely related to Ca. angustata, Ca. hurae, Ca. leguminum and Ca. rumohrae, and can be distinguished from these four species by the dimensions of its macroconidia. The macroconidia of Ca. gracilipes [(av. 45 × 4.5 μm); Crous et al. 1997a] are smaller than those of Ca. angustata [(av. 110 × 10 μm); Crous et al. 2000], Ca. hurae [(av. 120 × 7.5 μm); Crous 2002], Ca. leguminum [(av. 60 × 5 μm); Figueiredo & Namekata 1967, Crous 2002] and Ca. rumohrae [(av. 110 × 9 μm); El-Gholl et al. 1997]. Calonectria gracilipes is homothallic (Crous et al. 1997a) (Supplementary Table S4).
gracilis Calonectria Crous et al., Mycotaxon 46: 224. 1993.
Synonym: Cylindrocladium pseudogracile Crous, Mycol. Res. 101: 213. 1997.
In: Calonectria brassicae species complex.
Typus: PREM 51031a (holotype of Ca. gracilis; Crous et al. 1993c), PREM 51031b (holotype of Cy. pseudogracile; Crous et al. 1997b).
Ex-type culture: CBS 111807 = CMW 51189 = STE-U 2634 = AR2677 = PPRI 4176. (Crous 2002, Lombard et al. 2016)
Type locality: Brazil, Pará state.
Type substrate: Manilkara zapota.
Barcodes: act = GQ280488; cmdA = GQ267407; his3 = DQ190646; rpb2 = KY653390; tef1 = GQ267323; tub2 = AF232858 (alternative markers: ITS = GQ280610; LSU = GQ280732).
Notes: Calonectria gracilis is closely related to Ca. quinqueramosa, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. gracilis (av. 56 × 4.5 μm) are smaller than those of Ca. quinqueramosa (av. 59 × 5 μm), while the vesicles of Ca. gracilis [(2–11 μm); Crous et al. 1993c] are wider than those of Ca. quinqueramosa [(3–5 μm); Alfenas et al. 2015]. Calonectria gracilis is homothallic (Li et al. 2020) (Supplementary Table S4).
guangxiensis Calonectria L. Lombard et al., Stud. Mycol. 80: 169. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21484 holotype.
Ex-type culture: CBS 136092 = CMW 35409 = CERC 1900.
Type locality: China, GuangXi Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334944; cmdA = MT335171; his3 = MT335410; tef1 = MT412701 (alternative markers: ITS = MT359631; LSU = MT359391).
Notes: Calonectria guangxiensis was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for six gene regions (rpb2 and tub2 are not available for Ca. guangxiensis, tub2 not available for Ca. aconidialis), all six gene sequences for isolates of Ca. guangxiensis (ex-type isolate CMW 35409, and CMW 35411) were 100 % identical to the ex-type isolate of Ca. aconidialis (CMW 35174). Both species are homothallic and produce orange to orange-brown perithecia, 8-spored asci and 1-septate ascospores, and share the similar ascospores dimensions (Ca. guangxiensis: av. 36 × 6 μm; Ca. aconidialis: av. 36 × 6 μm) (Lombard et al. 2015a, Li et al. 2020; Supplementary Table S4).
hainanensis Calonectria L. Lombard et al., Stud. Mycol. 80: 170. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21480 holotype.
Ex-type culture: CBS 136248 = CMW 35187 = CERC 1863.
Type locality: China, HaiNan Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334946; cmdA = MT335173; his3 = MT335412; tef1 = MT412703 (alternative markers: ITS = MT359633; LSU = MT359393).
Notes: Calonectria hainanensis was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for six available gene regions (rpb2 and tub2 are not available for Ca. hainanensis, tub2 not available for Ca. aconidialis), all six gene sequences for the ex-type isolate of Ca. hainanensis (CMW 35187) were 100 % identical to the ex-type isolate of Ca. aconidialis (CMW 35174). Both species are homothallic and produce orange to orange-brown perithecia, 8-spored asci and 1-septate ascospores, and share the similar ascospores dimensions (Ca. hainanensis: av. 34 × 6 μm; Ca. aconidialis: av. 36 × 6 μm) (Lombard et al. 2015a; Supplementary Table S4).
hawksworthii Calonectria (Peerally) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium hawksworthii Peerally, Mycotaxon 40: 375. 1991.
Synonyms: Calonectria sulawesiensis L. Lombard et al., Stud. Mycol. 66: 53. 2010.
Calonectria foliicola L. Lombard et al., Stud. Mycol. 80: 167. 2015.
In: Calonectria cylindrospora species complex.
Typus: MUCL 30866 holotype.
Ex-type culture: CBS 111870 = CMW 51194 = CPC 2405.
Type locality: Mauritius, Pamplemousses garden.
Type substrate: Leaves of Nelumbo nucifera.
Barcodes: act = MT335024; cmdA = MT335254; his3 = MT335494; rpb2 = MT412556; tef1 = MT412785; tub2 = MT413003 (alternative markers: ITS = MT359715; LSU = MT359475).
Notes: Calonectria hawksworthii is closely related to Ca. brasiliensis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. hawksworthii [(av. 56 × 4 μm); Crous 2002] are larger than those of Ca. brasiliensis [(av. 38 × 3.5 μm); Batista 1951] (Supplementary Table S4).
henricotiae Calonectria Gehesquière et al., Pl. Pathol. 65: 47. 2015.
In: Calonectria naviculata species complex.
Typus: BPI 892910 holotype.
Ex-type culture: CBS 138102 = CB045.
Type locality: Belgium, East Flanders, Lokeren.
Type substrate: Buxus sempervirens.
Barcodes: cmdA = KF815157; his3 = KF815185; tub2 = JX535308 (alternative markers: ITS = JX535322).
Notes: Calonectria henricotiae is closely related to Ca. pseudonaviculata, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. henricotiae [(av. 56 × 5.6 μm); Gehesquiere et al. 2015] are shorter than those of Ca. pseudonaviculata [(av. 60 × 5 μm); Crous et al. 2002]. Calonectria henricotiae is heterothallic (Li et al. 2020) (Supplementary Table S4).
heveicola Calonectria N.Q. Pham et al., Mycologia 111: 95. 2019.
In: Calonectria kyotensis species complex.
Typus: PREM 62118 holotype.
Ex-type culture: CBS 143570 = CMW 49913.
Type locality: Vietnam, Binh Duong, Bau Bang.
Type substrate: Soil in Hevea brasiliensis plantation.
Barcodes: act = MT335025; cmdA = MT335255; his3 = MT335495; tef1 = MT412786; tub2 = MT413004 (alternative markers: ITS = MT359716; LSU = MT359476).
Notes: Calonectria heveicola is phylogenetically closely related to Ca. cochinchinensis and can be differentiated from that species by sequences of act, cmdA, his3, ITS, tef1 and tub2 gene regions. In addition, Ca. heveicola [(138–189 μm); Pham et al. 2019] produces shorter stipe extensions than those of Ca. cochinchinensis [(147–208 μm); Pham et al. 2019]. Calonectria heveicola is heterothallic (Li et al. 2020) (Supplementary Table S4).
hodgesii Calonectria R.F Alfenas et al., Trop. Plant. Pathol. 38: 517. 2013.
(see Calonectria brasiliensis)
In: Calonectria cylindrospora species complex.
Typus: CBS H-21147 holotype.
Ex-type culture: CBS 133609 = LPF245.
Type locality: Brazil, Minas Gerais state, Viçosa.
Type substrate: Anadenanthera peregrina.
Barcodes: cmdA = KC491222; tef1 = KC491225; tub2 = KC491228.
Notes: Calonectria hodgesii was treated as a synonym of Ca. brasiliensis in this study. In comparisons of DNA sequences for three available gene regions, the only difference between the ex-type isolate of Ca. hodgesii (CBS 133609) and the ex-type isolate of Ca. brasiliensis (CBS 230.51) was in the cmdA, tef1 and tub2 sequences, where there were three base differences in the cmdA, one base difference in the tef1 and one base difference in the tub2 sequences. Both species produce ellipsoidal vesicles of similar dimensions (Ca. hodgesii: 6–11 μm; Ca. brasiliensis: 7–11 μm) and 1-septate macroconidia. The macroconidia of Ca. hodgesii (av. 50 × 4.5 μm) are larger than those of Ca. brasiliensis (av. 38 × 3.5 μm) (Batista 1951, Lombard et al. 2010b, Alfenas et al. 2013b), which was considered to represent intraspecific variation (Supplementary Table S4).
honghensis Calonectria J.Q. Li et al., IMA Fungus 8: 273. 2017.
In: Calonectria colhounii species complex.
Typus: PREM 61943 holotype.
Ex-type culture: CBS 142885 = CMW 47669 = CERC 5572.
Type locality: China, YunNan Province, HongHe Region.
Type substrate: Soil in a Eucalyptus plantation.
Barcodes: act = MT335026; cmdA = MT335256; his3 = MT335496; rpb2 = MT412557; tef1 = MT412787; tub2 = MT413005 (alternative markers: ITS = MT359717; LSU = MT359477).
Notes: Calonectria honghensis is closely related to Ca. aciculata and Ca. colhounii, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. honghensis [(av. 54 × 5.5 μm); Li et al. 2017] are shorter than those of Ca. aciculata [(av. 69 × 5.5 μm); Li et al. 2017] and Ca. colhounii [(av. 65 × 5 μm); Peerally 1973]. In addition, the ascospores of Ca. honghensis (av. 49 × 6 μm) are shorter than those of Ca. colhounii (av. 55 × 6 μm) (Peerally 1973, Li et al. 2017). Calonectria honghensis is homothallic (Li et al. 2020) (Supplementary Table S4).
hongkongensis Calonectria Crous, Stud. Mycol. 50: 422. 2004.
Synonym: Cylindrocladium hongkongense Crous, Stud. Mycol. 50: 422. 2004.
In: Calonectria kyotensis species complex.
Typus: CBS 9886 holotype.
Ex-type culture: CBS 114828 = CMW 51217 = CPC 4670.
Type locality: China, Hong Kong.
Type substrate: Soil.
Barcodes: act = MT335028; cmdA = MT335258; his3 = MT335498; rpb2 = MT412559; tef1 = MT412789; tub2 = MT413007 (alternative markers: ITS = MT359719; LSU = MT359479).
Notes: Calonectria hongkongensis is closely related to Ca. kyotensis, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. hongkongensis [(av. 46.5 × 4 μm); Crous et al. 2004] are longer than those of Ca. kyotensis [(av. 41 × 4 μm); Terashita 1968], whereas the vesicles of Ca. hongkongensis (8–14 μm) are narrower than those of Ca. kyotensis (8.8–19 μm) (Sobers 1972, Crous et al. 2004). Calonectria hongkongensis is homothallic (Crous et al. 2004) (Supplementary Table S4).
humicola Calonectria L. Lombard et al., Stud. Mycol. 66: 49. 2010
In: Calonectria spathiphylli species complex.
Typus: PREM 60369 holotype.
Ex-type culture: CBS 125251 = CMW 31183.
Type locality: Ecuador, Pichincha Province, Las Golondrinas.
Type substrate: Soil.
Barcodes: act = MT335032; cmdA = MT335262; his3 = MT335502; tef1 = MT412793; tub2 = MT413011 (alternative markers: ITS = MT359723; LSU = MT359483).
Notes: Calonectria humicola is phylogenetically closely related to Ca. densa, and can be distinguished from that species by its lack of lateral stipe extensions, a feature that is common in Ca. densa. The macroconidia of Ca. humicola [(av. 51 × 5 μm); Lombard et al. 2010a] are smaller than those of Ca. densa [(av. 54 × 6 μm); Lombard et al. 2010a] (Supplementary Table S4).
hurae Calonectria (Linder & Whetzel) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cercosporella hurae Linder & Whetzel, Mycologia 29: 656. 1937.
Synonyms: Cylindrocladiopsis hurae (Linder & Whetzel) U. Braun, Mycotaxon 51: 40. 1994.
Cylindrocladium hurae (Linder & Whetzel) Crous, In: Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera: 185. 2002.
Cylindrocladium heptaseptatum Sobers et al., Phytopathology 65: 333. 1975.
Cylindrocladiopsis lagerstroemiae J.M. Yen, Mycotaxon 8: 236. 1979.
In: Calonectria gracilipes species complex.
Typus: Chardon No. 363 (lectotype, Crous 2002).
Ex-type culture: Not available.
Key culture: CBS 114182 = CMW 51823 = CPC 1714 = UFV 216 (Lombard et al. 2016).
Key culture locality: Brazil (Lombard et al. 2016).
Key culture substrate: Rumohra adiantiformis (Lombard et al. 2016).
Barcodes: Culture CBS 114182: act = MT335035; cmdA = MT335265; his3 = MT335505; rpb2 = MT412563; tef1 = MT412796; tub2 = MT413014 (alternative markers: ITS = MT359726; LSU = MT359486).
Notes: Calonectria hurae is phylogenetically closely related to Ca. angustata and Ca. rumohrae, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. hurae [(av. 120 × 7.5 μm); Crous 2002] are longer and narrower than those of Ca. angustata [(av. 110 × 10 μm); Crous et al. 2000] and Ca. rumohrae [(av. 110 × 9 μm); El-Gholl et al. 1997] (Supplementary Table S4).
ilicicola Calonectria Boedijn & Reitsma, Reinwardtia 1: 58. 1950.
Synonyms: Calonectria theae var. crotalariae Loos, Trans. Brit. Mycol. Soc. 33: 18. 1950.
Calonectria crotalariae (Loos) D.K. Bell & Sobers, Phytopathology 56: 1364. 1966.
Cylindrocladium crotalariae (Loos) D.K. Bell & Sobers, Phytopathology 56: 1364. 1966. (nom. inval.!, Art.36)
Cylindrocladium parasiticum Crous, M.J. Wingf. & Alfenas, Mycol. Res. 97: 892. 1993.
In: Calonectria kyotensis species complex.
Typus: IMI 264540 holotype.
Ex-type culture: CBS 190.50 = CMW 30998 = STE-U 2482 = IMI 299389.
Type locality: Indonesia, Java, Bogor.
Type substrate: Solanum tuberosum.
Barcodes: act = MT335036; cmdA = MT335266; his3 = MT335506; rpb2 = MT412564; tef1 = MT412797 (alternative markers: ITS = MT359727; LSU = MT359487).
Notes: Calonectria ilicicola is closely related to Ca. aeknauliensis and Ca. curvispora, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. ilicicola [(av. 62 × 6 μm); Boedijn & Reitsma 1950] are larger than those of Ca. aeknauliensis [(av. 47 × 5 μm); Pham et al. 2019] and Ca. curvispora [(av. 60 × 5 μm); Victor et al. 1997]. Calonectria ilicicola is homothallic (Li et al. 2020) (Supplementary Table S4).
indonesiae Calonectria (Crous) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium indonesiae Crous, Stud. Mycol. 50: 424. 2004.
In: Calonectria kyotensis species complex.
Typus: CBS 9891 holotype.
Ex-type culture: CBS 112823 = CMW 23683 = CPC 4508.
Type locality: Indonesia, Warambunga.
Type substrate: Soil.
Barcodes: act = MT335037; cmdA = MT335267; his3 = MT335507; rpb2 = MT412565; tef1 = MT412798; tub2 = MT413015 (alternative markers: ITS = MT359728; LSU = MT359488).
Notes: Calonectria indonesiae is phylogenetically closely related to Ca. chinensis, Ca. cochinchinensis and Ca. heveicola, and can be distinguished from these three species by the dimensions of its macroconidia. The macroconidia of Ca. indonesiae [(av. 50.5 × 4 μm); Crous et al. 2004] are longer than those of Ca. chinensis [(av. 45 × 4 μm); Crous et al. 2004], Ca. cochinchinensis [(av. 46 × 4 μm); Pham et al. 2019] and Ca. heveicola [(av. 44.5 × 4 μm); Pham et al. 2019] (Supplementary Table S4).
indonesiana Calonectria L. Lombard & Crous, Stud. Mycol. 85: 179. 2016.
(see Calonectria sumatrensis)
In: Calonectria kyotensis species complex.
Typus: CBS H-22757 holotype.
Ex-type culture: CBS 112936 = CMW 51207 = CPC 4504.
Type locality: Indonesia, Northern Sumatra.
Type substrate: Soil.
Barcodes: act = MT335143; cmdA = MT335380; his3 = MT335620; rpb2 = MT412672; tef1 = MT412911 (alternative markers: ITS = MT359841; LSU = MT359601).
Notes: Calonectria indonesiana was treated as a synonym of Ca. sumatrensis in this study. In comparisons of DNA sequences for seven available gene regions, the only differences between the ex-type isolate of Ca. indonesiana (CBS 112936) and the ex-type isolate of Ca. sumatrensis (CMW 23698) were two base differences in the his3 gene region. Both species produce sphaeropedunculate vesicles with overlapping dimensions (Ca. indonesiana: 8–10 μm; Ca. sumatrensis: 8–13 μm) and 1-septate macroconidia. Macroconidia of Ca. indonesiana (av. 43 × 5 μm) are shorter than those of Ca. sumatrensis (av. 58 × 5 μm), which was considered to represent intraspecific variation (Crous et al. 2004, Lombard et al. 2016) (Supplementary Table S4).
indusiata Calonectria (Seaver) Crous, Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera: 94. 2002.
Basionym: Nectria indusiata Seaver, Mycologia 20: 58. 1928.
Synonyms: Cercosporella theae Petch, Ann. Roy. Bot. Gard. Peradeniya. 6: 246. 1917.
Candelospora theae (Petch) Wakef. ex Gadd, Monographs on Tea Production in Ceylon: 59. 1949.
Cylindrocladium theae (Petch) Subram., In: Subramnian, Hyphomycetes, an account of Indian species, except Cercosporae: 731. 1971.
Cylindrocladium theae (Petch) Alfieri & sobers, Phytopathology 62: 650. 1972 (homonym).
Calonectria theae Loos, Trans. Brit. Mycol. Soc. 33: 17. 1950.
In: Calonectria colhounii species complex.
Typus: No. 3176 holotype.
Ex-type culture: CBS 144.36 = CMW 23699.
Type locality: Sri Lanka.
Type substrate: Camellia sinensis.
Barcodes: act = GQ280536; cmdA = GQ267453; his3 = GQ267262; rpb2 = KY653396; tef1 = GQ267332; tub2 = GQ267239 (alternative markers: ITS = GQ280658; LSU = GQ280780).
Notes: Phylogenetically, Ca. indusiata forms a distinct lineage separate from other species in the Ca. colhounii species complex. The macroconidia of Ca. indusiata (av. 81 × 6 μm) are smaller than those of Ca. macroconidialis (av. 90 × 6.5 μm), and longer than other species in the Ca. colhounii species complex (Supplementary Table S4).
insularis Calonectria C.L. Schoch & Crous, Mycologia 91: 293. 1999.
Synonym: Cylindrocladium insulare C.L. Schoch & Crous, Mycologia 91: 293. 1999.
In: Calonectria cylindrospora species complex.
Typus: PREM 55760 [holotype of Ca. insularis; Schoch et al. 1999], PREM 55758 (holotype of Cy. insulare; Schoch et al. 1999).
Ex-type culture: CBS 114558 = CMW 30991 = CPC 768 (Crous 2002, Lombard et al. 2015a)
Type locality: Madagascar, Tamatave.
Type substrate: Soil.
Barcodes: cmdA = MT335269; his3 = MT335509; rpb2 = MT412567; tef1 = MT412800; tub2 = MT413017 (alternative markers: ITS = MT359730; LSU = MT359490).
Notes: Calonectria insularis is closely related to Ca. cylindrospora and Ca. variabilis, and can be distinguished from these two species by the dimensions of its ascospores and macroconidia. The ascospores of Ca. insularis [(av. 33 × 6 μm); Schoch et al. 1999] are shorter than those of Ca. cylindrospora [(av. 37 × 6 μm); Crous et al. 1993a, Lombard et al. 2015b] and Ca. variabilis [(av. 42 × 5 μm); Crous et al. 1993b]. The macroconidia of Ca. insularis [(av. 45 × 4 μm); Schoch et al. 1999] are smaller than those of Ca. variabilis [(av. 73 × 5 μm); Crous et al. 1993b]. Calonectria insularis and Ca. cylindrospora are characterised as being heterothallic, while Ca. variabilis is homothallic (Crous et al. 1993b, Schoch et al. 1999).
kyotensis Calonectria Terash., Trans. Mycol. Soc. Japan. 8: 124. 1968.
Synonyms: Calonectria floridana Sobers, Phytopathology 59: 366. 1969.
Calonectria turangicola L. Lombard et al., Stud. Mycol. 80: 184. 2015.
Calonectria pseudoturangicola J.Q. Li et al., IMA Fungus 8: 279. 2017.
In: Calonectria kyotensis species complex.
Typus: IFO No. 11597 holotype.
Ex-type culture: CBS 114525 = CMW 51824 = CPC 2367 = ATCC 18834.
Type locality: Japan.
Type substrate: Robinia pseudoacacia.
Barcodes: act = MT335039; cmdA = MT335271; his3 = MT335511; rpb2 = MT412569; tef1 = MT412802; tub2 = MT413019 (alternative markers: ITS = MT359732; LSU = MT359492).
Notes: Calonectria kyotensis is closely related to Ca. hongkongensis, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. kyotensis [(av. 41 × 4 μm); Terashita 1968] are shorter than those of Ca. hongkongensis [(av. 46.5 × 4 μm); Crous et al. 2004], whereas the vesicles of Ca. kyotensis (8.8–19 μm) are wider than those of Ca. hongkongensis (8–14 μm) (Sobers 1972, Crous et al. 2004). Calonectria kyotensis is homothallic (Terashita 1968) (Supplementary Table S4).
lageniformis Calonectria L. Lombard & Crous, Stud. Mycol. 85: 181. 2016.
In: Calonectria cylindrospora species complex.
Typus: CBS H-22758 holotype.
Ex-type culture: CBS 111324 = CMW 51177 = CPC 1473.
Type locality: Mauritius, Rivière Noire.
Type substrate: Eucalyptus sp.
Barcodes: cmdA = KX784574; rpb2 = KY653400; tef1 = KX784702; tub2 = KX784632 (alternative markers: ITS = KY653256; LSU = KY653312).
Notes: Calonectria lageniformis is closely related to Ca. auriculiformis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. lageniformis (av. 40 × 5 μm) are shorter than those of Ca. auriculiformis (av. 43 × 4 μm). Calonectria lageniformis only produces up to three branches per conidiophore, whereas Ca. auriculiformis can have up to five branches per conidiophore (Lombard et al. 2016, Pham et al. 2019).
lantauensis Calonectria J.Q. Li et al., IMA Fungus 8: 277. 2017.
In: Calonectria kyotensis species complex.
Typus: PREM 61946 holotype.
Ex-type culture: CBS 142888 = CMW 47252 = CERC 3302.
Type locality: China, Hong Kong Region, LiDao Distict.
Type substrate: Soil.
Barcodes: act = MT335040; cmdA = MT335272; his3 = MT335512; rpb2 = MT412570; tef1 = MT412803 (alternative markers: ITS = MT359733; LSU = MT359493).
Notes: Calonectria lantauensis is phylogenetically closely related to Ca. bumicola. This species can be differentiated from Ca. bumicola by the sequences of act, cmdA, his3, rpb2 and tef1 gene regions.
lateralis Calonectria L. Lombard et al., Stud. Mycol. 80: 173. 2015.
In: Calonectria kyotensis species complex.
Typus: CBS H-21469 holotype.
Ex-type culture: CBS 136629 = CMW 31412.
Type locality: China, GuangXi Province.
Type substrate: Soil in a Eucalyptus plantation.
Barcodes: act = MT335042; cmdA = MT335274; his3 = MT335514; rpb2 = MT412571; tef1 = MT412805; tub2 = MT413020 (alternative markers: ITS = MT359735; LSU = MT359495).
Notes: Calonectria lateralis is closely related to Ca. hongkongensis and Ca. kyotensis, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. lateralis [(av. 39 × 4 μm); Lombard et al. 2015a] are shorter than those of Ca. hongkongensis [(av. 46.5 × 4 μm); Crous et al. 2004] and Ca. kyotensis [(av. 41 × 4 μm); Terashita 1968]. Calonectria lateralis is homothallic (Lombard et al. 2015a) (Supplementary Table S4).
lauri Calonectria Lechat & Crous, sp. nov. MycoBank MB836241.
Synonyms: Candelospora ilicicola Hawley, Proc. Roy. Irish Acad. 31: 11. 1912.
Cylindrocladium ilicicola (Hawley) Boedijn & Reitsma, Reinwardtia 1: 57. 1950.
Tetracytum lauri Vanderw., Parasitica 1: 145. 1945. (as laurii). Nom. inval., Arts 35.1, 39.1 (Melbourne)
Calonectria lauri (Vanderw.) Lechat & Crous, IMA Fungus 1: 103. 2010. Nom. inval., Art. 39.1 (Melbourne)
In: Calonectria mexicana species complex.
Description and illustrations: Lechat et al. (2010).
Typus: Netherlands, South-East Limburg, Vijlenerbos, Vijlen, Ilex aquifolium, Aug. 1970, H.A van der Aa (holotype CBS H-15110, ex-type culture CBS 749.70 = CMW 23682).
Barcodes: act = MT335043; cmdA = MT335275; his3 = MT335515; rpb2 = MT412572; tef1 = MT412806; tub2 = MT413021 (alternative markers: ITS = MT359736; LSU = MT359496).
Notes: The original description of Ca. lauri (Lechat et al. 2010) is invalid, as its basionym Tetracytum lauri is invalid. This issue is now addressed, and the name Ca. lauri validated here. Calonectria lauri is closely related to Ca. citri, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. lauri [(av. 60 × 5.5 μm); Lechat et al. 2010] are larger than those of Ca. citri [(av. 58 × 4 μm); Crous 2002]. Calonectria lauri is homothallic (Li et al. 2020) (Supplementary Table S4).
leguminum Calonectria (Rehm) Crous, Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera: 107. 2002.
Basionym: Nectria leguminum Rehm, Hedwigia 39: 221. 1900.
Synonyms: Calonectria quinqueseptata Figueiredo & Namek., Arch. Inst. Biol. (São Paulo). 34: 91. 1967.
Cylindrocladium leguminum Crous, Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera: 107. 2002.
In: Calonectria gracilipes species complex.
Typus: Ule 2282 (holotype of Nectria leguminum; Crous 2002), PREM 57208 (holotype of Cy. leguminum; Crous 2002).
Ex-type culture: CBS 728.68 = CMW 23684 (Crous 2002, Lombard et al. 2010a).
Type locality: Brazil, Sao Paulo.
Type substrate: Annona squamosa.
Barcodes: act = MT335044; cmdA = MT335276; his3 = MT335516; rpb2 = MT412573; tef1 = MT412807; tub2 = MT413022 (alternative markers: ITS = MT359737; LSU = MT359497).
Notes: Calonectria leguminum is phylogenetically closely related to Ca. angustata, Ca. gracilipes, Ca. hurae and Ca. rumohrae, and can be distinguished from these four species by the dimensions of its macroconidia. The macroconidia of Ca. leguminum [(av. 60 × 5 μm); Figueiredo & Namekata 1967, Crous 2002] are smaller than those of Ca. angustata [(av. 110 × 10 μm); Crous et al. 2000], Ca. hurae [(av. 120 × 7.5 μm); Crous 2002] and Ca. rumohrae [(av. 110 × 9 μm); El-Gholl et al. 1997], larger than those of Ca. gracilipes [(av. 45 × 4.5 μm); Crous et al. 1997a]. Calonectria leguminum is homothallic (Crous 2002) (Supplementary Table S4).
leucothoes Calonectria (El-Gholl et al.) L. Lombard et al. (as leucothoës), Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium leucothoes El-Gholl et al., Canad. J. Bot. 67: 2530. 1989.
Synonym: Cylindrocladium perseae T.S. Schub. et al., Mycotaxon 73: 474. 1999.
In: Calonectria mexicana species complex.
Typus: FLAS F55387 holotype.
Ex-type culture: CBS 109166 = CMW 30977 = CPC 2385 = ATCC 64824 = P88-490.
Type locality: USA, Florida.
Type substrate: Leucothoe axillaris.
Barcodes: act = MT335045; cmdA = MT335277; his3 = MT335517; rpb2 = MT412574; tef1 = MT412808 (alternative markers: ITS = MT359738; LSU = MT359498).
Notes: Calonectria leucothoes is closely related to Ca. citri and Ca. lauri, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. leucothoes [(av. 73 × 5 μm); El-Gholl et al. 1989] are larger than those of Ca. citri [(av. 58 × 4 μm); Crous 2002] and longer than those of Ca. lauri [(av. 60 × 5.5 μm); Lechat et al. 2010]. Calonectria leucothoes is characterised as being heterothallic, while Ca. lauri is homothallic (Li et al. 2020) (Supplementary Table S4).
lichi Calonectria Q.L. Liu & S.F. Chen, Mycokeys 26: 45. 2017.
In: Calonectria colhounii species complex.
Typus: CSFF 2019 holotype.
Ex-type culture: CERC 8866.
Type locality: China, HeNan Province.
Type substrate: Soil.
Barcodes: act = MT335046; cmdA = MT335278; his3 = MT335518; rpb2 = MT412575; tef1 = MT412809; tub2 = MT413023 (alternative markers: ITS = MT359739; LSU = MT359499).
Notes: Calonectria lichi is phylogenetically closely related to Ca. fujianensis. The sequences of the ITS, tef1 and tub2 gene regions can differentiate Ca. lichi from that species. The macroconidia of Ca. lichi [(av. 65.7 × 6 μm); Liu & Chen 2017] are larger than those of Ca. fujianensis [(av. 52.5 × 4 μm); Chen et al. 2011]. Calonectria lichi is homothallic (Li et al. 2020) (Supplementary Table S4).
lombardiana Calonectria Q.L. Liu & S.F. Chen, sp. nov. MycoBank MB835285. Fig. 2.
Fig. 2.
Calonectria lombardiana. A–C. Macroconidiophore. D–G. Narrowly clavate vesicles. H, I. Conidiogenous apparatus with conidiophore branches and cylindrical to allantiod phialides. J, K. Macroconidia. Scale bars: A–C = 50 μm, D–I = 10 μm, J, K = 20 μm.
In: Calonectria reteaudii species complex.
Etymology: Named for Dr Lorenzo Lombard, recognising his significant contribution to the taxonomy of Calonectria.
Sexual morph unknown. Macroconidiophores consisting of a stipe, a suite of penicillate arranged fertile branches, a stipe extension, and a terminal vesicle; stipe septate, hyaline, smooth, 43–139 × 4–7 μm; stipe extensions septate, straight to flexuous 158–216 μm long, 2–5 μm wide at the apical septum, terminating in a narrowly clavate vesicle, 2–4 μm diam; lateral stipe extensions (90° to main axis) absent. Conidiogenous apparatus 34–80 μm wide, and 35–76 μm long; primary branches aseptate, 13–29 × 3–7 μm; secondary branches aseptate, 11–21 × 3–5 μm; tertiary branches aseptate, 9–19 × 2–4 μm, each terminal branch producing 3–4 phialides; phialides cylindrical to allantoid, hyaline, aseptate, 9−20 × 2–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (64–)74–86(–98) × (5–)5.5–6.5(–7.5) μm, (av. 80 × 6 μm), 5-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies forming abundant white aerial mycelium at 25 °C on MEA, moderate sporulation; reverse cinnamon after 7 d. Chlamydospores extensive throughout the medium, forming microsclerotia. Optimal growth temperature 25 °C, no growth at 5 °C and 35 °C, after 7 d, colonies at 10 °C, 15 °C, 20 °C, 25 °C and 30 °C reached 11.1 mm, 20.4 mm, 41.2 mm, 59.5 mm and 55.3 mm, respectively.
Typus: Australia, Victoria, on Xanthorrhoea australis, T. Baigent (holotype HMAS 255718, ex-type culture CBS 112634 = CMW 30602 = CPC 4233 = Lynfield 417).
Barcodes: act = MT335156; cmdA = MT335395; his3 = MT335635; rpb2 = MT412686; tef1 = MT412926; tub2 = MT413133 (alternative markers: ITS = MT359856; LSU = MT359616).
Notes: Calonectria lombardiana is a new species in the Ca. reteaudii species complex, closely related to Ca. queenslandica (Fig. 1). The sequences of ITS, tef1 and tub2 gene regions can differentiate Ca. lombardiana from that species. In addition, the macroconidia of Ca. lombardiana (av. 80 × 6 μm) are longer than those of Ca. queenslandica [(av. 69 × 6 μm); Lombard et al. 2010c] (Supplementary Table S4).
longiramosa Calonectria L. Lombard & Crous, Stud. Mycol. 86: 114. 2017.
(see Calonectria amazonica)
In: Calonectria pteridis species complex.
Typus: CBS H-22759 holotype.
Ex-type culture: CBS 116319 = CMW 51832 = CPC 3761.
Type locality: Brazil, Amazon.
Type substrate: Eucalyptus sp.
Barcodes: act = MT334960; cmdA = MT335187; his3 = MT335426; rpb2 = MT412490; tef1 = MT412717; tub2 = MT412940 (alternative markers: ITS = MT359647; LSU = MT359407).
Notes: Calonectria longiramosa was treated as a synonym of Ca. amazonica in this study. In comparisons of DNA sequences for eight gene regions, the ex-type isolate of Ca. longiramosa (CBS 116319) were 100 % identical to the ex-type isolate of Ca. amazonica (CBS 116250). Both of the species produce clavate vesicles with overlapping dimensions (Ca. longiramosa: 5–8 μm; Ca. amazonica: 5–6 μm) and 1-septate macroconidia. The macroconidia of Ca. longiramosa (av. 71 × 5 μm) are shorter than those of Ca. amazonica (av. 79 × 5 μm) (Lombard et al. 2016), which was considered to represent intraspecific variation (Supplementary Table S4).
machaerinae Calonectria L. Lombard & Crous, Stud. Mycol. 85: 181. 2016.
(see Calonectria pauciramosa)
In: Calonectria candelabrum species complex.
Typus: CBS H-22760 holotype.
Ex-type culture: CBS 123183 = CMW 51311 = CPC 15378.
Type locality: New Zealand, Auckland, Auckland University Campus.
Type substrate: Foliar lesion of Machaerina sinclairii.
Barcodes: act = MT335090; cmdA = MT335322; his3 = MT335562; rpb2 = MT412615; tef1 = MT412853; tub2 = MT413065 (alternative markers: ITS = MT359783; LSU = MT359543).
Notes: Calonectria machaerinae was treated as a synonym of Ca. pauciramosa in this study. In comparisons of DNA sequences for eight gene regions, all the sequences of Ca. machaerinae (ex-type CBS 123183) were 100 % identical to those of Ca. pauciramosa (ex-type CBS 138824, CMW 2151, CMW 7592, CMW 9151, CMW 30823, and CMW 30875). Both species produce obpyriform to ellipsoidal vesicles with overlapping dimensions (Ca. machaerinae: 6–9 μm; Ca. pauciramosa: 5–11 μm) and 1-septate macroconidia. The macroconidia of Ca. machaerinae (av. 38 × 4 μm) are smaller than those of Ca. pauciramosa (av. 50 × 4.5 μm) (Crous 2002, Lombard et al. 2016), which was considered to represent intraspecific variation (Supplementary Table S4).
macroconidialis Calonectria (Crous et al.) Crous, Canad. J. Bot. 77: 1818. 1999.
Basionym: Calonectria colhounii var. macroconidialis Crous et al., Mycotaxon 46: 222. 1993.
Synonyms: Cylindrocladium colhounii var. macroconidiale Crous et al., Mycotaxon 46: 222. 1993.
Cylindrocladium macroconidiale (Crous et al.) Crous, Canad. J. Bot. 77: 1818. 1999.
Calonectria parva L. Lombard & Crous, Stud. Mycol. 85: 183. 2016.
In: Calonectria colhounii species complex.
Typus: PREM 51036 (holotype of Ca. macroconidialis; Crous et al. 1999), PREM 51035 (holotype of Cy. macroconidiale; Crous et al. 1999).
Ex-type culture: CBS 114880 = CMW 51219 = CPC 307 = PPRI 4000 (Crous 2002, Crous et al. 2006).
Type locality: South Africa, Mpumalanga, Sabie.
Type substrate: Eucalyptus grandis.
Barcodes: act = MT335050; cmdA = MT335282; his3 = MT335522; rpb2 = MT412579; tef1 = MT412813; tub2 = MT413027 (alternative markers: ITS = MT359743; LSU = MT359503).
Notes: Calonectria macroconidialis is closely related to Ca. madagascariensis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. macroconidialis [(av. 90 × 6.5 μm); Crous et al. 1999] are larger than those of Ca. madagascariensis [(av. 55 × 4.5 μm); Crous 2002]. Furthermore, Ca. macroconidialis is characterised as being heterothallic, while Ca. madagascariensis is homothallic (Crous et al. 1999, Crous 2002) (Supplementary Table S4).
madagascariensis Calonectria Crous, Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera: 112. 2002.
Synonym: Cylindrocladium madagascariense Crous, Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera: 112. 2002.
In: Calonectria colhounii species complex.
Typus: PREM 57198 (holotype of Ca. madagascariensis; Crous 2002), PREM 57199 (holotype of Cy. madagascariense; Crous 2002).
Ex-type culture: CBS 114572 = CMW 23686 = CPC 2252 (Crous et al. 2006).
Type locality: Madagascar, Rona.
Type substrate: Soil.
Barcodes: act = MT335052; cmdA = MT335284; his3 = MT335524; rpb2 = MT412581; tef1 = MT412815; tub2 = MT413029 (alternative markers: ITS = MT359745; LSU = MT359505).
Notes: Calonectria madagascariensis is closely related to Ca. macroconidialis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. madagascariensis [(av. 55 × 4.5 μm); Crous 2002] are smaller than those of Ca. macroconidialis [(av. 90 × 6.5 μm); Crous et al. 1999]. Calonectria madagascariensis is homothallic (Crous 2002) (Supplementary Table S4).
magnispora Calonectria L. Lombard et al., Stud. Mycol. 80: 174. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21471 holotype.
Ex-type culture: CBS 136249 = CMW 35184 = CERC 1860.
Type locality: China, GuangXi Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334947; cmdA = MT335174; his3 = MT335413; tef1 = MT412704 (alternative markers: ITS = MT359634; LSU = MT359394).
Notes: Calonectria magnispora was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for six available gene regions, one base difference in each of the cmdA, LSU and tef1 sequences was found between the ex-type isolate of Ca. magnispora (CMW 35184) and the ex-type isolate of Ca. aconidialis (CMW 35174). Both species are homothallic and produce orange to orange-brown perithecia, 8-spored asci and 1-septate ascospores, the ascospores of Ca. magnispora (av. 40 × 6 μm) are longer than those of Ca. aconidialis (av. 36 × 6 μm) (Lombard et al. 2015a), which was considered to represent intraspecific variation (Supplementary Table S4).
malesiana Calonectria (Crous) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium malesianum Crous, Stud. Mycol. 50: 425. 2004.
In: Calonectria kyotensis species complex.
Typus: CBS 9885 holotype.
Ex-type culture: CBS 112752 = CMW 23687 = CPC 4223.
Type locality: Indonesia, Northern Sumatra.
Type substrate: Soil.
Barcodes: act = MT335054; cmdA = MT335286; his3 = MT335526; rpb2 = MT412583; tef1 = MT412817; tub2 = MT413031 (alternative markers: ITS = MT359747; LSU = MT359507).
Notes: Calonectria malesiana is closely related to Ca. lateralis, and can be distinguished by dimensions of its macroconidia. The macroconidia of Ca. malesiana [(av. 47.5 × 4 μm); Crous et al. 2004] are longer than those of Ca. lateralis [(av. 39 × 4 μm); Lombard et al. 2015a] (Supplementary Table S4).
maranhensis Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 107. 2015.
In: Calonectria cylindrospora species complex.
Typus: CBS H-21360 holotype.
Ex-type culture: CBS 134811 = LPF142.
Type locality: Brazil, Maranhao state, Açailandia.
Type substrate: Eucalyptus leaf.
Barcodes: cmdA = KM396035; his3 = KM396118; tef1 = KM395861; tub2 = KM395948.
Notes: Calonectria maranhensis is closely related to Ca. brasiliensis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. maranhensis [(av. 57 × 5 μm); Alfenas et al. 2015] are larger than those of Ca. brasiliensis [(av. 38 × 3.5 μm); Batista 1951] (Supplementary Table S4).
metrosideri Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 108. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS H-21146 holotype.
Ex-type culture: CBS 133603 = LPF101.
Type locality: Brazil, Minas Gerais state, Viçosa.
Type substrate: Metrosideros polymorpha.
Barcodes: cmdA = KC294304; his3 = KC294307; tef1 = KC294310; tub2 = KC294313.
Notes: Calonectria metrosideri is closely related to Ca. brasiliana, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. metrosideri [(av. 45 × 4 μm); Alfenas et al. 2013a] are shorter than those of Ca. brasiliana [(av. 53 × 4 μm); Lombard et al. 2016] (Supplementary Table S4).
mexicana Calonectria C.L. Schoch & Crous, Mycologia 91: 294. 1999.
Synonym: Cylindrocladium mexicanum C.L. Schoch & Crous, Mycologia 91: 294. 1999.
In: Calonectria mexicana species complex.
Typus: PREM 55763 (holotype of Ca. mexicana; Schoch et al. 1999); PREM 55761 (holotype of Cy. mexicanum; Schoch et al. 1999).
Ex-type culture: CBS 110918 = CMW 9055 = STE-U 927 (Lombard et al. 2016).
Type locality: Mexico, Yucatan, Uxmal.
Type substrate: Soil.
Barcodes: act = GQ280474; cmdA = GQ267396; his3 = FJ972460; rpb2 = KY653412; tef1 = FJ972526; tub2 = AF210863 (alternative markers: ITS = GQ280596; LSU = GQ280718).
Notes: Calonectria mexicana is closely related to Ca. avesiculata, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. mexicana [(av. 45 × 4 μm); Schoch et al. 1999] are smaller than those of Ca. avesiculata [(av. 64 × 5 μm); Schubert et al. 1989], and the vesicles of Ca. mexicana (7–12 μm) are wider than those of Ca. avesiculata (1–4 μm). Calonectria mexicana is heterothallic (Schoch et al. 1999) (Supplementary Table S4).
microconidialis Calonectria L. Lombard et al., Stud. Mycol. 80: 175. 2015.
(see Calonectria pseudoreteaudii)
In: Calonectria reteaudii species complex.
Typus: CBS H-21473 holotype.
Ex-type culture: CBS 136638 = CMW 31487 = CERC 1822.
Type locality: China, GuangDong Province, ZhanJiang.
Type substrate: Eucalyptus urophylla × grandis clone seedling.
Barcodes: act = MT335113; cmdA = MT335348; his3 = MT335588; rpb2 = MT412641; tef1 = MT412879; tub2 = MT413090 (alternative markers: ITS = MT359809; LSU = MT359569).
Notes: Calonectria microconidialis was treated as a synonym of Ca. pseudoreteaudii in this study. In comparisons of DNA sequences, all eight gene sequences for the isolates of Ca. microconidialis (ex-type CMW 31487, CMW 31473, CMW 31475, and CMW 31492) were 100 % identical to the isolates of Ca. pseudoreteaudii (ex-type CMW 25310, and CMW 25292). Both species produce narrowly clavate vesicles with overlapping dimensions (Ca. microconidialis: 3–7 μm; Ca. pseudoreteaudii: 3–5 μm), they also produce multiple-septate (> 3) macroconidia and 1–3-septate microconidia. The macroconidia of Ca. microconidialis (av. 88 × 8 μm) are shorter than those of Ca. pseudoreteaudii (av. 104 × 8 μm) (Lombard et al. 2010c, 2015a), which was considered to represent intraspecific variation (Supplementary Table S4).
montana Calonectria Q.L. Liu & S.F. Chen, Mycokeys 26: 48. 2017.
(see Calonectria canadiana)
In: Calonectria kyotensis species complex.
Typus: CSFF 2022 holotype.
Ex-type culture: CERC 8952.
Type locality: China, HeNan.
Type substrate: Soil under natural forest.
Barcodes: act = MT335058; cmdA = MT335290; his3 = MT335530; rpb2 = MT412587; tef1 = MT412821; tub2 = MT413035 (alternative markers: ITS = MT359751; LSU = MT359511).
Notes: Calonectria montana was treated as a synonym of Ca. canadiana in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. montana (CERC 8952) and the ex-type isolate of Ca. canadiana (CMW 23673) was in the ITS, rpb2 and tub2 sequences, where there was one base difference in the ITS, one base difference in the rpb2 and seven base differences in the tub2 sequences. Both species produce pyriform to sphaeropedunculate vesicles with overlapping dimensions (Ca. montana: 4–12.5 μm; Liu & Chen 2017, Ca. canadiana: 6–10 μm; Kang et al. 2001b) and 1-septate macroconidia. The macroconidia of Ca. montana [(av. 43.2 × 4.6 μm); Liu & Chen 2017] are shorter than those of Ca. canadiana [(av. 50 × 4 μm); Kang et al. 2001b], which was considered to represent intraspecific variation (Supplementary Table S4).
monticola Calonectria L. Lombard & Crous, Persoonia 35: 293. 2015.
In: Calonectria colhounii species complex.
Typus: CBS H-22376 holotype.
Ex-type culture: CBS 140645 = CPC 28835.
Type locality: Thailand, Chiang Mai.
Type substrate: Soil.
Barcodes: cmdA = KT964771; tef1 = KT964773; tub2 = KT964769 (alternative markers: ITS = KT964775; LSU = KT983443).
Notes: Calonectria monticola is closely related to Ca. honghensis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. monticola [(av. 49 × 5 μm); Crous et al. 2015] are smaller than those of Ca. honghensis [(av. 54 × 5.5 μm); Li et al. 2017] (Supplementary Table S4).
mossambicensis Calonectria S. Maússe-Sitoe et al., Persoonia 31: 291. 2013.
(see Calonectria pauciramosa)
In: Calonectria candelabrum species complex.
Typus: PREM 60821 holotype.
Ex-type culture: CBS 137243 = CMW 36327.
Type locality: Mozambique, Manica, Bandula.
Type substrate: Cutting clones of Eucalyptus grandis × E. camaldulensis.
Barcodes: act = MT335091; cmdA = MT335323; his3 = MT335563; rpb2 = MT412616; tef1 = MT412854; tub2 = MT413066 (alternative markers: ITS = MT359784; LSU = MT359544).
Notes: Calonectria mossambicensis was treated as a synonym of Ca. pauciramosa in this study. In comparisons of DNA sequences for eight gene regions, all the sequences of the isolates of Ca. mossambicensis (ex-type CBS 137243, and CMW 36329) were 100 % identical to those of the isolates of Ca. pauciramosa (ex-type CBS 138824, CMW 2151, CMW 7592, CMW 9151, CMW 30823, and CMW 30875). Both species produce obpyriform to ellipsoidal vesicles with overlapping dimensions (Ca. mossambicensis: 2–8 μm; Ca. pauciramosa: 5–11 μm) and 1-septate macroconidia. The macroconidia of Ca. mossambicensis (av. 42 × 4 μm) are smaller than those of Ca. pauciramosa (av. 50 × 4.5 μm) (Crous 2002, Crous et al. 2013), which was considered to represent intraspecific variation (Supplementary Table S4).
multilateralis Calonectria L. Lombard et al., Stud. Mycol. 85: 182. 2016.
In: Calonectria naviculata species complex.
Typus: CBS H-22762 holotype.
Ex-type culture: CBS 110932 = CMW 51171 = CPC 957.
Type locality: Mexico, Uxmal.
Type substrate: Soil.
Barcodes: act = MT335060; cmdA = MT335292; his3 = MT335532; rpb2 = MT412589; tef1 = MT412823; tub2 = MT413037 (alternative markers: ITS = MT359753; LSU = MT359513).
Notes: Calonectria multilateralis is closely related to Ca. multinaviculata and Ca. naviculata, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. multilateralis [(av. 33 × 3 μm); Lombard et al. 2016] are shorter than those of Ca. multinaviculata [(av. 46 × 3.5 μm); Alfenas et al. 2015] and Ca. naviculata [(av. 45 × 3 μm); Crous et al. 1997a] (Supplementary Table S4).
multinaviculata Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 108. 2015.
In: Calonectria naviculata species complex.
Typus: CBS 134858 holotype.
Ex-type culture: CBS 134858 = LPF233.
Type locality: Brazil, Bahia state, Mucuri.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: cmdA = KM396072; his3 = KM396155; tef1 = KM395898; tub2 = KM395985.
Notes: Calonectria multinaviculata is phylogenetically closely related to Ca. naviculata, Ca. multinaviculata can be distinguished from Ca. naviculata by its lateral stipe extensions, a feature not observed for the Ca. naviculata (Crous et al. 1997a, Alfenas et al. 2015) (Supplementary Table S4).
multiphialidica Calonectria (Crous et al.) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium multiphialidicum Crous et al., Stud. Mycol. 50: 425. 2004.
In: Calonectria naviculata species complex.
Typus: CBS 9887 holotype.
Ex-type culture: CBS 112678 = CMW 23688 = Cam 13.
Type locality: Cameroon.
Type substrate: Soil surrounding roots of Musa sp.
Barcodes: act = MT335066; cmdA = MT335298; his3 = MT335538; rpb2 = MT412595; tef1 = MT412829; tub2 = MT413043 (alternative markers: ITS = MT359759; LSU = MT359519).
Notes: Calonectria multiphialidica is closely related to Ca. henricotiae and Ca. pseudonaviculata, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. multiphialidica [(av. 53 × 4.5 μm); Crous et al. 2004] are smaller than those of Ca. henricotiae [(av. 56 × 5.6 μm); Gehesquiere et al. 2015] and Ca. pseudonaviculata [(av. 60 × 5 μm); Crous et al. 2002] (Supplementary Table S4). Furthermore, Ca. multiphialidica has eight tiers of branches in its conidiogenous apparatus in comparison to the two in Ca. henricotiae and four in Ca. pseudonaviculata (Crous et al. 2002, 2004, Gehesquiere et al. 2015).
multiseptata Calonectria Crous & M.J. Wingfield, Mycol. Res. 102: 530. 1998.
Synonym: Cylindrocladium multiseptatum Crous & M.J. Wingf., Mycol. Res. 102: 530. 1998.
In: Calonectria reteaudii species complex.
Typus: PREM 55343 (holotype of Ca. multiseptata; Crous et al. 1998), PREM 55344 (holotype of Cy. multiseptatum; Crous et al. 1998)
Ex-type culture: CBS 112682 = CMW 23692 = CPC 1589 (Crous 2002, Crous et al. 2006).
Type locality: Indonesia, North Sumatra.
Type substrate: Eucalyptus grandis.
Barcodes: act = MT335067; cmdA = MT335299; his3 = MT335539; rpb2 = MT412596; tef1 = MT412830; tub2 = MT413044 (alternative markers: ITS = MT359760; LSU = MT359520).
Notes: Calonectria multiseptata is phylogenetically closely related to Ca. acicola. The ability of Ca. multiseptata to produce megaconidiophores and megaconidia in culture distinguishes it from Ca. acicola (Crous 2002, Gadgil & Dick 2004). Calonectria multiseptata is homothallic (Crous 2002) (Supplementary Table S4).
multistipitata Calonectria N.Q. Pham et al., Mycologia 111: 98. 2019.
(see Calonectria chinensis)
In: Calonectria kyotensis species complex.
Typus: PREM 62121 holotype.
Ex-type culture: CBS 143573 = CMW 47192.
Type locality: Vietnam, Tuyen Quang.
Type substrate: Soil in Acacia hybrid plantation.
Barcodes: act = MT335068; cmdA = MT335300; his3 = MT335540; rpb2 = MT412597; tef1 = MT412831; tub2 = MT413045 (alternative markers: ITS = MT359761; LSU = MT359521).
Notes: Calonectria multistipitata was treated as a synonym of Ca. chinensis in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. multistipitata (CMW 47192) and the ex-type isolate of Ca. chinensis (CMW 23674) was in the his3, ITS, tef1 and tub2 sequences, where there was one base difference in the his3, one base difference in the ITS, one base difference in the tef1 and four base differences in the tub2 sequences. Both species produce sphaeropedunculate vesicles with overlapping dimensions (Ca. multistipitata: 5–10 μm; Ca. chinensis: 6–9 μm) and 1-septate macroconidia. The macroconidia of Ca. multistipitata (av. 32 × 3.5 μm) are smaller than those of Ca. chinensis (av. 45 × 4 μm) (Crous et al. 2004, Pham et al. 2019), which was considered to represent intraspecific variation (Supplementary Table S4).
naviculata Calonectria Crous & M.J. Wingf., Mycologia 89: 654. 1997.
Synonym: Cylindrocladium naviculatum Crous & M.J. Wingf., Mycotaxon 50: 443. 1994.
In: Calonectria naviculata species complex.
Typus: PREM 54418 (holotype of Ca. naviculata; Crous et al. 1997a), PREM 51542 (holotype of Cy. naviculatum; Crous et al. 1994).
Ex-type culture: CBS 101121 = CMW 30974 (Lombard et al. 2010a).
Type locality: Brazil, Joao Pessoa.
Type substrate: Leaf litter.
Barcodes: act = GQ280478; cmdA = GQ267399; his3 = GQ267252; rpb2 = KM232309; tef1 = GQ267317; tub2 = GQ267211 (alternative markers: ITS = GQ280600; LSU = GQ280722).
Notes: Calonectria naviculata is phylogenetically closely related to Ca. multinaviculata, it can be distinguished from Ca. multinaviculata by its lack of lateral stipe extensions, a feature that is common in Ca. multinaviculata (Crous et al. 1997a, Alfenas et al. 2015). Calonectria naviculata is heterothallic (Crous et al. 1997a) (Supplementary Table S4).
nemoralis Calonectria L. Lombard & Crous, Stud. Mycol. 86: 114. 2017.
(see Calonectria ovata)
In: Calonectria pteridis species complex.
Typus: CBS H-23135 holotype.
Ex-type culture: CBS 116249 = CMW 51829 = CPC 3533.
Type locality: Brazil.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT335074; cmdA = MT335306; his3 = MT335546; rpb2 = MT412603; tef1 = MT412837; tub2 = MT413051 (alternative markers: ITS = MT359767; LSU = MT359527).
Notes: Calonectria nemoralis was treated as a synonym of Ca. ovata in this study. In comparisons of DNA sequences for seven available gene regions, the only difference between the ex-type isolate of Ca. nemoralis (CBS 116249) and the ex-type isolate of Ca. ovata (CMW 16724) was found in the tub2 gene sequences where one base difference occurred. Both of the species produce ovoid vesicles and 1-septate macroconidia. The macroconidia of Ca. nemoralis [(av. 53 × 4 μm); Marin-Felix et al. 2017] are smaller than those of Ca. ovata [(av. 70 × 5 μm); Victor et al. 1997], which was considered to represent intraspecific variation (Supplementary Table S4).
nemoricola Calonectria R.F. Alfenas et al., (as nemuricola) Stud. Mycol. 80: 109. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS H-21358 holotype.
Ex-type culture: CBS 134837 = LPF085.
Type locality: Brazil, Minas Gerais state, Araponga.
Type substrate: Soil in tropical rainforest.
Barcodes: cmdA = KM396066; his3 = KM396149; tef1 = KM395892; tub2 = KM395979.
Notes: Calonectria nemoricola is closely related to Ca. silvicola, and can be distinguished from Ca. silvicola by the dimensions of its macroconidia. The macroconidia of Ca. nemoricola [(av. 45 × 4 μm); Alfenas et al. 2015] are longer than those of Ca. silvicola [(av. 41 × 4.5 μm); Alfenas et al. 2015] (Supplementary Table S4).
nymphaeae Calonectria Yong Wang bis et al., Mycotaxon 122: 181. 2013.
(see Calonectria fujianensis)
In: Calonectria colhounii species complex.
Typus: HGUPd100003 holotype.
Ex-type culture: CBS 131802 = CMW 51317 = HGUP 100003.
Type locality: China, Guizhou, Guiyang.
Type substrate: Nymphaea tetragona.
Barcodes: act = MT335070; cmdA = MT335302; his3 = MT335542; rpb2 = MT412599; tef1 = MT412833; tub2 = MT413047 (alternative markers: ITS = MT359763; LSU = MT359523).
Notes: Calonectria nymphaeae was treated as a synonym of Ca. fujianensis in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. nymphaeae (CBS 131802) and the ex-type isolate of Ca. fujianensis (CMW 27257) was in the ITS, tef1 and tub2 sequences, where there was one base difference in the ITS, three base differences in the tef1 and three base differences in the tub2 sequences. Both species are homothallic and produce clavate vesicles with similar dimensions (Ca. nymphaeae: 3–5 μm; Ca. fujianensis: 3–5 μm). The macroconidia of Ca. nymphaeae (av. 61 × 5.9 μm) are larger than those of Ca. fujianensis (av. 52.5 × 4 μm) (Chen et al. 2011, Xu et al. 2012, Li et al. 2020), which was considered to represent intraspecific variation (Supplementary Table S4).
octoramosa Calonectria L. Lombard & Crous, Stud. Mycol. 86: 120. 2017.
In: Calonectria brassicae species complex.
Typus: CBS H-23136 holotype.
Ex-type culture: CBS 111423 = CMW 51819 = CPC 1650.
Type locality: Ecuador.
Type substrate: Soil.
Barcodes: act = MT335071; cmdA = MT335303; his3 = MT335543; rpb2 = MT412600; tef1 = MT412834; tub2 = MT413048 (alternative markers: ITS = MT359764; LSU = MT359524).
Notes: Calonectria octoramosa is closely related to Ca. ecuadorae, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. octoramosa [(av. 36 × 4 μm); Marin-Felix et al. 2017] are smaller than those of Ca. ecuadorae [(av. 51 × 4.5 μm); Crous et al. 2006] (Supplementary Table S4).
orientalis Calonectria L. Lombard et al., Stud. Mycol. 66: 49. 2010.
In: Calonectria brassicae species complex.
Typus: PREM 60303 holotype.
Ex-type culture: CBS 125260 = CMW 20291.
Type locality: Indonesia, Langam.
Type substrate: Soil.
Barcodes: act = MT335072; cmdA = MT335304; his3 = MT335544; rpb2 = MT412601; tef1 = MT412835; tub2 = MT413049 (alternative markers: ITS = MT359765; LSU = MT359525).
Notes: Calonectria orientalis is closely related to Ca. pini, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. orientalis (av. 48 × 4 μm) are longer than those of Ca. pini (av. 44 × 5 μm) (Lombard et al. 2010a) (Supplementary Table S4). Furthermore, Ca. orientalis produces five tiers of branches in its conidiogenous apparatus in comparison to the three in Ca. pini (Lombard et al. 2010a).
ovata Calonectria D. Victor & Crous, Syst. Appl. Microbiol. 20: 282. 1997
Synonyms: Cylindrocladium ovatum El-Gholl et al., Canad. J. Bot. 71: 469. 1993.
Calonectria tereticornis L. Lombard & Crous, Stud. Mycol. 85: 190. 2016.
Calonectria nemoralis L. Lombard & Crous, Stud. Mycol. 86: 114. 2017.
Calonectria tucuruiensis L. Lombard & Crous, Stud. Mycol. 86: 123. 2017.
In: Calonectria pteridis species complex.
Typus: PREM 51726 (holotype of Ca. ovata; Victor et al. 1997), FLAS F55638 (holotype of Cy. ovatum; El-Gholl et al. 1993a).
Ex-type culture: CBS 111299 = CMW 16724 = ATCC 76225 = UFV 89 (Lombard et al. 2010a).
Type locality: Brazil, Pará, Monte Dourado.
Type substrate: Eucalyptus urophylla.
Barcodes: act = MT335075; cmdA = MT335307; his3 = MT335547; tef1 = MT412838; tub2 = MT413052 (alternative markers: ITS = MT359768; LSU = MT359528).
Notes: Calonectria ovata is phylogenetically closely related to Ca. pseudovata and Ca. terricola. The macroconidia of Ca. ovata [(av. 70 × 5 μm); Victor et al. 1997] are larger than those of Ca. terricola [(av. 46 × 4.5 μm); Lombard et al. 2016]. Furthermore, the ability of Ca. ovata to produce miconidiophores and microconidia in culture distinguishes it from Ca. terricola (Victor et al. 1997, Lombard et al. 2016). Morphologically, Ca. ovata shows some overlap with Ca. pseudovata, while the sequences of cmdA, his3, tef1 and tub2 gene regions can differentiate Ca. ovata from Ca. pseudovata (Victor et al. 1997, Alfenas et al. 2015). Calonectria ovata is heterothallic (Li et al. 2020).
pacifica Calonectria (J.C. Kang et al.) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium pacificum J.C. Kang et al., Syst. Appl. Microbiol. 24: 213. 2001.
In: Calonectria kyotensis species complex.
Typus: PREM 57209 holotype.
Ex-type culture: CBS 109063 = CMW 16726 = IMI 354528 = STE-U 2534 = A1568.
Type locality: USA, Hawaii.
Type substrate: Araucaria heterophylla.
Barcodes: act = MT335079; cmdA = MT335311; his3 = MT335551; rpb2 = MT412604; tef1 = MT412842 (alternative markers: ITS = MT359772; LSU = MT359532).
Notes: Calonectria pacifica is phylogenetically closely related to Ca. aconidialis and can be differentiated from that species by the sequences of act, cmdA, his3, rpb2, ITS, LSU and tef1 gene regions.
papillata Calonectria L. Lombard et al., Stud. Mycol. 80: 175. 2015.
(see Calonectria cerciana)
In: Calonectria cylindrospora species complex.
Typus: CBS H-21487 holotype.
Ex-type culture: CBS 136097 = CMW 37976 = CPC 23517 = CERC 1939.
Type locality: China, GuangDong.
Type substrate: Soil in a Eucalyptus plantation.
Barcodes: act = MT334983; cmdA = MT335213; his3 = MT335453; rpb2 = MT412517; tef1 = MT412744; tub2 = MT412965 (alternative markers: ITS = MT359674; LSU = MT359434).
Notes: Calonectria papillata was treated as a synonym of Ca. cerciana in this study. In comparisons of DNA sequences for eight gene regions, there were differences between the ex-type isolate of Ca. papillata (CMW 37976) and the ex-type isolate of Ca. cerciana (CMW 25309) in the ITS, rpb2 and tub2 sequences. These included one base difference in the ITS sequences, one base difference in the rpb2 and two base differences in the tub2 sequences. Both species produce obpyriform vesicles and 1-septate macroconidia, and have similar vesicle dimensions (Ca. papillata: 8–14 μm; Ca. cerciana: 8–13 μm) and macroconidia (Ca. papillata: av. 45 × 4 μm; Ca. cerciana: av. 44 × 5 μm) (Lombard et al. 2010c, 2015a) (Supplementary Table S4).
paracolhounii Calonectria L. Lombard & Crous, Stud. Mycol. 85: 183. 2016.
In: Calonectria colhounii species complex.
Typus: CBS H-22763 holotype.
Ex-type culture: CBS 114679 = CMW 51212 = CPC 2445.
Type locality: USA.
Type substrate: Unknown.
Barcodes: cmdA = KX784582; rpb2 = KY653423; tef1 = KX784714; tub2 = KX784644 (alternative markers: ITS = KY653268; LSU = KY653324).
Notes: Calonectria paracolhounii is closely related to Ca. colhounii, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. colhounii [(av. 65 × 5 μm); Peerally 1973] are longer than those of Ca. paracolhounii [(av. 41 × 5 μm); Lombard et al. 2016] (Supplementary Table S4).
paraensis Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 111. 2015.
Synonym: Calonectria telluricola R.F. Alfenas et al., Stud. Mycol. 80: 125. 2015.
In: Calonectria brassicae species complex.
Typus: CBS H-21379 holotype.
Ex-type culture: CBS 134669 = LPF430.
Type locality: Brazil, Pará state, Monte Dourado.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: cmdA = KM396011; his3 = KM396094; tef1 = KM395837; tub2 = KM395924.
Notes: Calonectria paraensis is closely related to Ca. orientalis, Ca. pini and Ca. pseudobrassicae. The macroconidia of Ca. paraensis [(av. 42 × 5 μm); Alfenas et al. 2015] are shorter than those of Ca. orientalis [(av. 48 × 4 μm); Lombard et al. 2010a] and Ca. pini [(av. 44 × 5 μm); Lombard et al. 2010a]. Calonectria paraensis has two tiers of branches in its conidiogenous apparatus in comparison to the five in Ca. orientalis, three in Ca. pini and Ca. pseudobrassicae (Lombard et al. 2010a, Alfenas et al. 2015) (Supplementary Table S4).
parakyotensis Calonectria L. Lombard et al., Stud. Mycol. 80: 176. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21470 holotype.
Ex-type culture: CBS 136085 = CMW 35169 = CERC 1845.
Type locality: China, GuangDong Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334948; cmdA = MT335175; his3 = MT335414; rpb2 = MT412483; tef1 = MT412705 (alternative markers: ITS = MT359635; LSU = MT359395).
Notes: Calonectria parakyotensis was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for seven available gene regions, all seven gene sequences for the isolates of Ca. parakyotensis (ex-type CMW 35169, and CMW 35413) were 100 % identical to those of the ex-type isolate of Ca. aconidialis (CMW 35174). Both species are homothallic but no sexual morph was observed for Ca. parakyotensis (Lombard et al. 2015a, Li et al. 2020; Supplementary Table S4).
parva Calonectria L. Lombard & Crous, Stud. Mycol. 85: 183. 2016.
(see Calonectria macroconidialis)
In: Calonectria colhounii species complex.
Typus: CBS H-22764 holotype.
Ex-type culture: CBS 110798 = CMW 51817 = CPC 410.
Type locality: South Africa, Mpumalanga, Sabie.
Type substrate: Eucalyptus grandis (roots).
Barcodes: act = MT335051; cmdA = MT335283; his3 = MT335523; rpb2 = MT412580; tef1 = MT412814; tub2 = MT413028 (alternative markers: ITS = MT359744; LSU = MT359504).
Notes: Calonectria parva was treated as a synonym of Ca. macroconidialis in this study. In comparisons of DNA sequences for eight gene regions, the only differences between the ex-type isolate of Ca. parva (CBS 110798) and the ex-type isolate of Ca. macroconidialis (CBS 114880) was found in the tef1 gene sequences. Both species produce clavate vesicles of similar dimensions (Ca. parva: 3–5 μm; Ca. macroconidialis: 3–5 μm) and 3-septate macroconidia (Crous et al. 1999, Lombard et al. 2016). The macroconidia of Ca. parva (av. 72 × 6 μm) are smaller than those of Ca. macroconidialis (av. 90 × 6.5 μm) (Crous et al. 1999, Lombard et al. 2016), which was considered to represent intraspecific variation (Supplementary Table S4).
parvispora Calonectria L. Lombard & Crous, Stud. Mycol. 86: 120. 2017.
In: Calonectria brassicae species complex.
Typus: CBS H-22765 holotype.
Ex-type culture: CBS 111465 = CPC 1902.
Type locality: Brazil.
Type substrate: Soil.
Barcodes: act = MT335082; cmdA = MT335314; his3 = MT335554; rpb2 = MT412607; tef1 = MT412845; tub2 = MT413057 (alternative markers: ITS = MT359775; LSU = MT359535).
Notes: Calonectria parvispora is closely related to Ca. brachiatica, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. parvispora [(av. 29 × 4 μm); Marin-Felix et al. 2017] are smaller than those of Ca. brachiatica [(av. 44 × 5 μm); Lombard et al. 2009] (Supplementary Table S4).
pauciphialidica Calonectria Q.L. Liu & S.F. Chen, sp. nov. MycoBank MB835284. Fig. 3.
Fig. 3.
Calonectria pauciphialidica. A–C. Macroconidiophore. D–G. Macroconidia. Scale bars: A–C = 20 μm, D–G = 10 μm.
In: Calonectria brassicae species complex.
Etymology: Name refers to the macroconidiophores that produce few phialides.
Sexual morph unknown. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, stipe extension and vesicle not observed; stipe septate, hyaline, smooth, 40–80 × 3–4 μm; Conidiogenous apparatus 8.6–20 μm wide, and 14.3–25 μm long; primary branches aseptate, 11–18 × 2–3 μm, each terminal produces two to four phialides, phialides doliiform to reniform, hyaline, aseptate, 6−9 × 2–3 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (25–)27–32(–36) × (2–)2.5–3.5(–4) μm, (av. 29.5 × 3 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies white on the surface and buff in reverse after 7 d at 25 °C on MEA; abundant wooly aerial mycelium with moderate sporulation on the medium surface; chlamydospores not observed. Optimal growth temperature 25 °C, no growth at 5 °C and 35 °C, after 7 d, colonies at 10 °C, 15 °C, 20 °C, 25 °C and 30 °C reached 15.1 mm, 21.4 mm, 45.1 mm, 58.2 mm and 42.1 mm, respectively.
Typus: Ecuador, soil, 20 Jun. 1997, M.J. Wingfield (holotype HMAS 255717, ex-type culture CBS 111394 = CMW 30980 = CPC 1628).
Barcodes: act = MT335083; cmdA = MT335315; his3 = MT335555; rpb2 = MT412608; tef1 = MT412846; tub2 = MT413058 (alternative markers: ITS = MT359776; LSU = MT359536).
Notes: Calonectria pauciphialidica is a novel species in the Ca. brassicae species complex, closely related to Ca. ecuadorae (Fig. 1, Supplementary Table S4). The sequences of cmdA, his3, rpb2, tef1 and tub2 gene regions can differentiate Ca. pauciphialidica from that species. The smaller number of branches and phialides of Ca. pauciphialidica distinguish it from Ca. ecuadorae; in addition, macroconidia of Ca. pauciphialidica (av. 29.5 × 3 μm) are smaller than those of Ca. ecuadorae [(av. 51 × 4.5 μm); Crous et al. 2006] (Supplementary Table S4).
pauciramosa Calonectria C.L. Schoch & Crous, Mycologia 91: 289. 1999.
Synonyms: Cylindrocladium pauciramosum C.L. Schoch & Crous, Mycologia 91: 289. 1999.
Calonectria polizzii L. Lombard et al., Stud. Mycol. 66: 25. 2010.
Calonectria zuluensis L. Lombard & Crous, Stud. Mycol. 66: 25. 2010.
Calonectria mossambicensis S. Maússe-Sitoe et al., Persoonia 31: 291. 2013.
Calonectria seminaria L. Lombard et al., Stud. Mycol. 80: 179. 2015.
Calonectria tetraramosa L. Lombard et al., Stud. Mycol. 80: 183. 2015.
Calonectria cliffordiicola L. Lombard & Crous, Stud. Mycol. 85: 177. 2016.
Calonectria ericae L. Lombard & Crous, Stud. Mycol. 85: 178. 2016.
Calonectria machaerinae L. Lombard & Crous, Stud. Mycol. 85: 181. 2016.
In: Calonectria candelabrum species complex.
Typus: PREM 55754 [holotype of Ca. pauciramosa; Schoch et al. 1999], PREM 55752 (holotype of Cy. pauciramosum; Schoch et al. 1999).
Ex-type culture: CBS 138824 = CMW 5683 = CPC 971 (Crous 2002, Lombard et al. 2010a).
Type locality: South Africa, Knysna.
Type substrate: Soil/Eucalyptus grandis (Crous 2002, Lombard et al. 2015a)
Barcodes: act = MT335093; cmdA = MT335325; his3 = MT335565; rpb2 = MT412618; tef1 = MT412856; tub2 = MT413068 (alternative markers: ITS = MT359786; LSU = MT359546).
Notes: Calonectria pauciramosa is closely related to Ca. nemoricola and Ca. silvicola, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. pauciramosa [(av. 50 × 4.5 μm); Crous 2002] are longer than those of Ca. nemoricola [(av. 45 × 4 μm); Alfenas et al. 2015] and Ca. silvicola [(av. 41 × 4.5 μm); Alfenas et al. 2015] (Supplementary Table S4). Calonectria pauciramosa is heterothallic (Crous 2002, Li et al. 2020). Calonectria pauciramosa is an important plant pathogen that has been reported as the dominant species in many countries such as Australia, Brazil, Italy, South Africa and the USA (Koike et al. 1999, Polizzi & Crous 1999, Schoch et al. 1999, Crous 2002), while Lombard et al. (2010c) and Chen et al. (2011) also confirmed its occurrence in China. In this study, the eight species reduced to synonymy with Ca. pauciramosa were originally collected in different climatic zones on five continents (Africa, Asia, Europe, North America and Oceania), supporting the view that Ca. pauciramosa is a cosmopolitan species with the ability to exist in areas with a range of climatic conditions.
penicilloides Calonectria (Tubaki) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Candelospora penicilloides Tubaki, Nogaoa 2: 58. 1952.
Synonym: Cylindrocladium penicilloides (Tubaki) Tubaki, J. Hattori Bot. Lab. 20: 154. 1958.
In: Calonectria kyotensis species complex.
Typus: type specimen lost (Tubaki 1958).
Ex-type culture: CBS 174.55 = CMW 23696 = STE-U 2388.
Type locality: Japan, Hatizyo Island.
Type substrate: Prunus sp.
Barcodes: act = MT335106; cmdA = MT335338; his3 = MT335578; rpb2 = MT412631; tef1 = MT412869; tub2 = MT413081 (alternative markers: ITS = MT359799; LSU = MT359559).
Notes: Phylogenetically, Ca. penicilloides forms a distinct lineage separate from other species in this species complex.
pentaseptata Calonectria L. Lombard et al., Persoonia 29: 157. 2012.
(see Calonectria pseudoreteaudii)
In: Calonectria reteaudii species complex.
Typus: CBS H-21062 holotype.
Ex-type culture: CBS 133349 = CMW 51318.
Type locality: Vietnam, Bavi, Hanoi.
Type substrate: Eucalyptus hybrid.
Barcodes: act = MT335117; cmdA = MT335352; his3 = MT335592; rpb2 = MT412645; tef1 = MT412883; tub2 = MT413094 (alternative markers: ITS = MT359813; LSU = MT359573).
Notes: Calonectria pentaseptata was treated as a synonym of Ca. pseudoreteaudii in this study. In comparison of DNA sequences, all eight gene sequences for the isolates of Ca. pentaseptata (ex-type CBS 133349, and CBS 133351) were 100 % identical to the isolates of Ca. pseudoreteaudii (ex-type CMW 25310, and CMW 25292). Both species produce narrowly clavate vesicles with similar dimensions (Ca. pentaseptata: 2–6 μm; Ca. pseudoreteaudii: 3–5 μm) and multiple-septate (> 3) macroconidia. The macroconidia of Ca. pentaseptata (av. 98 × 7 μm) are smaller than those of Ca. pseudoreteaudii (av. 104 × 8 μm) (Lombard et al. 2010c, Crous et al. 2012), which was considered to represent intraspecific variation (Supplementary Table S4).
piauiensis Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 112. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS H-21375 holotype.
Ex-type culture: CBS 134850 = LPF377.
Type locality: Brazil, Piauí state, Teresina.
Type substrate: Soil in Eucalyptus brassiana plantation.
Barcodes: cmdA = KM396060; his3 = KM396143; tef1 = KM395886; tub2 = KM395973.
Notes: Phylogenetically, Ca. piauiensis forms a distinct lineage separate from other species in this species complex.
pini Calonectria L. Lombard et al., Stud. Mycol. 66: 52. 2010.
In: Calonectria brassicae species complex.
Typus: PREM 60304 holotype.
Ex-type culture: CBS 123698 = CMW 31209.
Type locality: Colombia, Valle del Cauca, Buga.
Type substrate: Pinus patula.
Barcodes: act = MT335107; cmdA = MT335339; his3 = MT335579; rpb2 = MT412632; tef1 = MT412870; tub2 = MT413082 (alternative markers: ITS = MT359800; LSU = MT359560).
Notes: Calonectria pini is closely related to Ca. orientalis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. pini [(av. 44 × 5 μm); Lombard et al. 2010a] are shorter than those of Ca. orientalis [(av. 48 × 4 μm); Lombard et al. 2010a] (Supplementary Table S4). Furthermore, the small number of branches (−3) in the conidiogenous apparatus also distinguishes it from Ca. orientalis (−5) (Lombard et al. 2010a).
plurilateralis Calonectria L. Lombard & Crous, Stud. Mycol. 85: 184. 2016.
In: Calonectria cylindrospora species complex.
Typus: CBS H-22766 holotype.
Ex-type culture: CBS 111401 = CMW 51178 = CPC 1637.
Type locality: Ecuador.
Type substrate: Soil.
Barcodes: cmdA = MT335340; his3 = MT335580; rpb2 = MT412633; tef1 = MT412871; tub2 = MT413083 (alternative markers: ITS = MT359801; LSU = MT359561).
Notes: Calonectria plurilateralis is phylogenetically closely related to Ca. brasiliensis, Ca. hawksworthii and Ca. maranhensis, and can be distinguished from these species by the dimensions of its macroconidia. The macroconidia of Ca. plurilateralis [(av. 34 × 4 μm); Lombard et al. 2016] are shorter than those of Ca. brasiliensis [(av. 38 × 3.5 μm); Batista 1951], Ca. hawksworthii [(av. 56 × 4 μm); Lombard et al. 2010a] and Ca. maranhensis [(av. 57 × 5 μm); Alfenas et al. 2015] (Supplementary Table S4).
pluriramosa Calonectria L. Lombard et al., Stud. Mycol. 80: 178. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21485 holotype.
Ex-type culture: CBS 136976 = CMW 31440 = CERC 1775.
Type locality: China, GuangXi Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334950; cmdA = MT335177; his3 = MT335416; tef1 = MT412707 (alternative markers: ITS = MT359637; LSU = MT359397).
Notes: Calonectria pluriramosa was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for six available gene regions (rpb2 and tub2 are not available for Ca. pluriramosa, tub2 not available for Ca. aconidialis), all six gene sequences for the ex-type isolates of Ca. pluriramosa were 100 % identical to the ex-type isolate of Ca. aconidialis (CMW 35174). No sexual morph has been observed for Ca. pluriramosa (Lombard et al. 2015a, Supplementary Table S4).
polizzii Calonectria L. Lombard et al., Stud. Mycol. 66: 25. 2010.
(see Calonectria pauciramosa)
In: Calonectria candelabrum species complex.
Typus: PREM 60297 holotype.
Ex-type culture: CBS 123402 = CMW 30872.
Type locality: Italy, Sicily, Carrubba.
Type substrate: Arbutus unedo.
Barcodes: act = MT335099; cmdA = MT335331; his3 = MT335571; rpb2 = MT412624; tef1 = MT412862; tub2 = MT413074 (alternative markers: ITS = MT359792; LSU = MT359552).
Notes: Calonectria polizzii was treated as a synonym of Ca. pauciramosa in this study. In comparisons of DNA sequences for eight gene regions, the only differences between the ex-type isolate of Ca. polizzii (CBS 123402) and the ex-type isolate of Ca. pauciramosa (CBS 138824) were five base differences in the tub2 sequence. Both species are heterothallic and produce obpyriform to ellipsoidal vesicles with overlapping dimensions (Ca. polizzii: 6–9 μm; Ca. pauciramosa: 5–11 μm) and 1-septate macroconidia. The macroconidia of Ca. polizzii (av. 37 × 4 μm) are shorter than those of Ca. pauciramosa (av. 50 × 4.5 μm) (Crous 2002, Lombard et al. 2010b, Li et al. 2020), which was considered to represent intraspecific variation (Supplementary Table S4).
propaginicola Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 115. 2015.
Synonym: Calonectria pseudocerciana R.F. Alfenas et al., Stud. Mycol. 80: 117. 2015.
In: Calonectria cylindrospora species complex.
Typus: CBS H-21366 holotype.
Ex-type culture: CBS 134815 = LPF220.
Type locality: Brazil, Pará state, Santana.
Type substrate: Eucalyptus seedling.
Barcodes: cmdA = KM396040; his3 = KM396123; tef1 = KM395866; tub2 = KM395953.
Notes: Calonectria propaginicola is closely related to Ca. cerciana and Ca. tonkinensis, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. propaginicola [(av. 49 × 4 μm); Alfenas et al. 2015] are longer than those of Ca. cerciana [(av. 44 × 5 μm); Lombard et al. 2010c] and Ca. tonkinensis [(av. 41.5 × 4 μm); Pham et al. 2019] (Supplementary Table S4).
pseudobrassicae Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 116. 2015.
In: Calonectria brassicae species complex.
Typus: CBS H-21371 holotype.
Ex-type culture: CBS 134662 = LPF280.
Type locality: Brazil, Pará state, Santana.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: cmdA = KM396023; his3 = KM396106; tef1 = KM395849; tub2 = KM395936.
Notes: Calonectria pseudobrassicae is closely related to Ca. orientalis, Ca. paraensis and Ca. pini. The macroconidia of Ca. pseudobrassicae [(av. 41 × 5 μm); Alfenas et al. 2015] are shorter than those of Ca. orientalis [(av. 48 × 4 μm); Lombard et al. 2010a] and Ca. pini [(av. 44 × 5 μm); Lombard et al. 2010a]. Calonectria pseudobrassicae has three tiers of branches in its conidiogenous apparatus in comparison to the two in Ca. paraensis (Alfenas et al. 2015) (Supplementary Table S4).
pseudocerciana Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 117. 2015.
(see Calonectria propaginicola)
In: Calonectria cylindrospora species complex.
Typus: CBS H-21366 holotype.
Ex-type culture: CBS 134824 = LPF367.
Type locality: Brazil, Pará state, Santana.
Type substrate: Eucalyptus seedling.
Barcodes: cmdA = KM396049; his3 = KM396132; tef1 = KM395875; tub2 = KM395962.
Notes: Calonectria pseudocerciana was treated as a synonym of Ca. propaginicola in this study. In comparisons of DNA sequences for four available gene regions, the only differences between the ex-type isolate of Ca. pseudocerciana (CBS 134824) and the ex-type isolate of Ca. propaginicola (CBS 134815) was in the cmdA, his3 and tub2 sequences, where there were eight base differences in the cmdA, one base difference in the his3 and one base difference in the tub2 sequences. Both species produce obpyriform to sphaeropedunculate vesicles with similar dimensions (Ca. pseudocerciana: 7–12 μm; Ca. propaginicola: 5–12 μm) and 1-septate macroconidia, the macroconidia of Ca. pseudocerciana (av. 45 × 4 μm) are shorter than those of Ca. propaginicola (av. 49 × 4 μm) (Alfenas et al. 2015), which was considered to represent intraspecific variation (Supplementary Table S4).
pseudocolhounii Calonectria S.F. Chen et al., Persoonia 26: 7. 2011.
(see Calonectria eucalypti)
In: Calonectria colhounii species complex.
Typus: PREM 60456 holotype.
Ex-type culture: CBS 127195 = CMW 27209.
Type locality: China, FuJian Province.
Type substrate: Eucalyptus dunnii.
Barcodes: act = MT335108; cmdA = MT335341; his3 = MT335581; rpb2 = MT412634; tef1 = MT412872; tub2 = MT413084 (alternative markers: ITS = MT359802; LSU = MT359562).
Notes: Calonectria pseudocolhounii was treated as a synonym of Ca. eucalypti in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. pseudocolhounii (CMW 27209) and the ex-type isolate of Ca. eucalypti (CMW 18444) was in the his3, ITS and tub2 sequences, where there were three base differences in the his3, one base difference in the ITS and four base differences in the tub2 sequences. Both species are homothallic and produce clavate vesicles with similar dimensions (Ca. pseudocolhounii: 3.5–6 μm; Ca. eucalypti: 4–6 μm) and 3-septate macroconidia (Lombard et al. 2010a, Chen et al. 2011, Li et al. 2020). The macroconidia of Ca. pseudocolhounii (av. 60 × 4.5 μm) are smaller than those of Ca. eucalypti (av. 72 × 6 μm) (Lombard et al. 2010a, Chen et al. 2011), which was considered to represent intraspecific variation (Supplementary Table S4).
pseudoecuadoriae Calonectria L. Lombard & Crous, Stud. Mycol. 85: 185. 2016.
In: Calonectria brassicae species complex.
Typus: CBS H-22768 holotype.
Ex-type culture: CBS 111402 = CMW 51179 = CPC 1639.
Type locality: Ecuador.
Type substrate: Soil.
Barcodes: cmdA = KX784589; rpb2 = KY653432; tef1 = KX784723; tub2 = KX784652 (alternative markers: ITS = KY653273; LSU = KY653329).
Notes: Calonectria pseudoecuadoriae forms a single lineage closely related to Ca. robigophila, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. pseudoecuadoriae [(av. 38 × 3.5μm); Lombard et al. 2016] are smaller than those of Ca. robigophila [(av. 50 × 4 μm); Alfenas et al. 2015] (Supplementary Table S4).
pseudohodgesii Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 118. 2015.
(see Calonectria brasiliensis)
In: Calonectria cylindrospora species complex.
Typus: CBS H-21368 holotype.
Ex-type culture: CBS 134818 = LPF262.
Type locality: Brazil, Minas Gerais state, Viçosa.
Type substrate: Leaf of rooted Azadirachta indica cutting.
Barcodes: cmdA = KM395991; his3 = KM396079; tef1 = KM395817; tub2 = KM395905.
Notes: Calonectria pseudohodgesii was treated as a synonym of Ca. brasiliensis in this study. In comparisons of DNA sequences for four available gene regions, the only difference between the ex-type isolate of Ca. pseudohodgesii (CBS 134818) and the ex-type isolate of Ca. brasiliensis (CBS 230.51) was in the cmdA, his3, tef1 and tub2 sequences, where there were three base differences in the cmdA, two base differences in the his3, two base differences in the tef1 and one base difference in the tub2 sequences. Both species produce ellipsoidal to obpyriform vesicles of similar dimensions (Ca. pseudohodgesii: 4–10 μm; Ca. brasiliensis: 7–11 μm) and 1-septate macroconidia, the macroconidia of Ca. pseudohodgesii (av. 54 × 4.5 μm) are larger than those of Ca. brasiliensis (av. 38 × 3.5 μm) (Batista 1951, Lombard et al. 2010b, Alfenas et al. 2015), which was considered to represent intraspecific variation (Supplementary Table S4).
pseudokyotensis Calonectria L. Lombard et al., Stud. Mycol. 80: 178. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21774 holotype.
Ex-type culture: CBS 137332 = CMW 31439 = CERC 1774.
Type locality: China, GuangXi Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334951; cmdA = MT335178; his3 = MT335417; tef1 = MT412708 (alternative markers: ITS = MT359638; LSU = MT359398).
Notes: Calonectria pseudokyotensis was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for six available gene regions (rpb2 and tub2 are not available for Ca. pseudokyotensis, tub2 not available for Ca. aconidialis), all six gene sequences for the ex-type isolate of Ca. pseudokyotensis (CMW 31439) were 100 % identical to the ex-type isolate of Ca. aconidialis (CMW 35174). No sexual morph was observed for Ca. pseudokyotensis (Lombard et al. 2015a, Supplementary Table S4).
pseudometrosideri Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 118. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS 134845 (preserved as metabolically inactive culture, Alfenas et al. 2015).
Ex-type culture: CBS 134845 = LPF210.
Type locality: Brazil, Alagoas state, Maceió.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: cmdA = KM395995; his3 = KM396083; tef1 = KM395821; tub2 = KM395909.
Notes: Calonectria pseudometrosideri is closely related to Ca. putriramosa, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. pseudometrosideri (av. 51 × 4.5 μm) are longer than those of Ca. putriramosa (av. 43 × 5 μm), while the vesicles of Ca. pseudometrosideri (5–7 μm) are narrower than those of Ca. putriramosa (7–10 μm) (Alfenas et al. 2015, Lombard et al. 2016) (Supplementary Table S4).
pseudomexicana Calonectria L. Lombard et al., Persoonia 27: 76. 2011.
Synonym: Calonectria tunisiana L. Lombard et al., Persoonia 27: 77. 2011.
In: Calonectria mexicana species complex.
Typus: CBS H-20685 holotype.
Ex-type culture: CBS 130354 = CMW 51313 = DISTEF-TCROU1.
Type locality: Tunisia, Carthage, Tunis.
Type substrate: Callistemon sp.
Barcodes: act = MT335110; cmdA = MT335343; his3 = MT335583; rpb2 = MT412636; tef1 = MT412874; tub2 = MT413086 (alternative markers: ITS = MT359804; LSU = MT359564).
Notes: Calonectria pseudomexicana is closely related to Ca. pseudouxmalensis and Ca. uxmalensis, and can be distinguished from these two species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. pseudomexicana (av. 45 × 5 μm) are larger than those of Ca. pseudouxmalensis (av. 29 × 3 μm) and Ca. uxmalensis (av. 30 × 3 μm), and vesicles of Ca. pseudomexicana (9–14 μm) are wider than those of Ca. pseudouxmalensis (5–9 μm) and Ca. uxmalensis (5–8 μm) (Lombard et al. 2011, 2016) (Supplementary Table S4).
pseudonaviculata Calonectria (Crous et al.) L. Lombard et al., Stud. Mycol. 66: 56. 2010.
Basionym: Cylindrocladium pseudonaviculatum Crous et al., Sydowia 54: 26. 2002.
Synonym: Cylindrocladium buxicola Henricot, Mycologia 94: 993. 2002.
In: Calonectria naviculata species complex.
Typus: PREM 57313 holotype.
Ex-type culture: CBS 116251 = CMW 51235 = CPC 3399 = Lynfield 824.
Type locality: New Zealand.
Type substrate: Buxus sempervirens.
Barcodes: cmdA = MT335345; his3 = MT335585; rpb2 = MT412638; tef1 = MT412876; tub2 = MT413088 (alternative markers: ITS = MT359806; LSU = MT359566).
Notes: Calonectria pseudonaviculata is closely related to Ca. henricotiae, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. pseudonaviculata [(av. 60 × 5 μm); Crous et al. 2002] are longer than those of Ca. henricotiae [(av. 56 × 5.6 μm); Gehesquiere et al. 2015]. Calonectria pseudonaviculata is heterothallic (Li et al. 2020) (Supplementary Table S4).
pseudopteridis Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 118. 2015.
Basionym: Cylindrocladium macrosporum Sherb., Phytopathology 18: 219. 1928.
In: Calonectria pteridis species complex.
Typus: BPI 414558 holotype.
Ex-type culture: CBS 163.28 = CMW 51159 = IMI 299579.
Type locality: USA.
Type substrate: Washingtonia robusta.
Barcodes: act = MT335112; cmdA = MT335347; his3 = MT335587; rpb2 = MT412640; tef1 = MT412878; (alternative markers: ITS = MT359808; LSU = MT359568).
Notes: Calonectria pseudopteridis is closely related to Ca. pteridis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. pseudopteridis [(av. 87.5 × 5.7 μm) are longer than those of Ca. pteridis [(av. 82 × 5.5 μm); Sobers 1968, Crous et al. 1993c, Alfenas et al. 2015] (Supplementary Table S4).
pseudoreteaudii Calonectria L. Lombard et al., Persoonia 24: 8. 2010.
Synonyms: Calonectria pentaseptata L. Lombard et al., Persoonia 29: 157. 2012.
Calonectria microconidialis L. Lombard et al., Stud. Mycol. 80: 175. 2015.
In: Calonectria reteaudii species complex.
Typus: PREM 60290 holotype.
Ex-type culture: CBS 123694 = CMW 25310.
Type locality: China, GuangDong.
Type substrate: Eucalyptus urophylla × E. grandis hybrid cutting.
Barcodes: act = MT335119; cmdA = MT335354; his3 = MT335594; rpb2 = MT412647; tef1 = MT412885; tub2 = MT413096 (alternative markers: ITS = MT359815; LSU = MT359575).
Notes: Calonectria pseudoreteaudii is phylogenetically closely related to Ca. acaciicola and Ca. reteaudii. The sequences of his3, ITS, rpb2, tef1 and tub2 gene regions can differentiate Ca. pseudoreteaudii from these two species. The macroconidia of Ca. pseudoreteaudii [(av. 104 × 8 μm); Lombard et al. 2010c] are larger than those of Ca. acaciicola [(av. 94 × 7 μm); Pham et al. 2019] and Ca. reteaudii [(av. 84 × 6.5 μm); Kang et al. 2001a]. Calonectria pseudoreteaudii is heterothallic (Li et al. 2020) (Supplementary Table S4).
pseudoscoparia Calonectria L. Lombard et al., Stud. Mycol. 66: 53. 2010.
(see Calonectria candelabrum)
In: Calonectria candelabrum species complex.
Typus: PREM 60305 holotype.
Ex-type culture: CBS 125257 = CMW 15218.
Type locality: Ecuador, Pichincha Province, Las Golondrinas.
Type substrate: Eucalyptus grandis cutting.
Barcodes: act = MT334979; cmdA = MT335209; his3 = MT335449; rpb2 = MT412513; tef1 = MT412740; tub2 = MT412961 (alternative markers: ITS = MT359670; LSU = MT359430).
Notes: Calonectria pseudoscoparia was treated as a synonym of Ca. candelabrum in this study. In comparisons of DNA sequences for eight gene regions, only one base difference in the tub2 gene sequences was found between the ex-type isolate of Ca. pseudoscoparia (CMW 15218) and the isolates of Ca. candelabrum (CMW 31000, and CMW 31001). Both species produce ellipsoidal to obpyriform vesicles with overlapping dimensions (Ca. pseudoscoparia: 6–10 μm; Ca. candelabrum: 5–8 μm) and 1-septate macroconidia. The macroconidia of Ca. pseudoscoparia (av. 48 × 4 μm) are smaller than those of Ca. candelabrum (av. 60 × 4.5 μm) (Viégas 1946, Crous 2002, Lombard et al. 2010a, 2015b), which was considered to represent intraspecific variation (Supplementary Table S4).
pseudospathiphylli Calonectria J.C. Kang et al., Syst. Appl. Microbiol. 24: 215. 2001.
Synonym: Cylindrocladium pseudospathiphylli J.C. Kang et al., Syst. Appl. Microbiol. 24: 215. 2001.
In: Calonectria spathiphylli species complex.
Typus: PREM 57196 (holotype of Ca. pseudospathiphylli; Kang et al. 2001b), PREM 57197 (holotype of Cy. pseudospathiphylli; Kang et al. 2001b).
Ex-type culture: CBS 109165 = CMW 30976 = CPC 1623 (Alfenas et al. 2015, Lombard et al. 2016).
Type locality: Ecuador.
Type substrate: Soil.
Barcodes: act = GQ280493; cmdA = GQ267412; his3 = AF348241; rpb2 = KY653435; tef1 = FJ918562; tub2 = FJ918513 (alternative markers: ITS = GQ280615; LSU = GQ280737).
Notes: Calonectria pseudospathiphylli forms a single lineage closely related to Ca. densa, Ca. humicola and Ca. spathiphylli. No lateral stipe extension was observed in Ca. pseudospathiphylli, whereas these are common in Ca. densa. Calonectria pseudospathiphylli produce stipe extensions (up to 250 μm) longer than those of Ca. humicola (up to 157 μm). The macroconidia of Ca. pseudospathiphylli (av. 52 × 4 μm) obviously smaller than those of Ca. spathiphylli (av. 70 × 6μm) (El-Gholl et al. 1992, Kang et al. 2001b, Lombard et al. 2010a). Calonectria pseudospathiphylli is characterised as being homothallic, while Ca. spathiphylli is heterothallic (El-Gholl et al. 1992, Kang et al. 2001b) (Supplementary Table S4).
pseudospathulata Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 118. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS H-21356 holotype.
Ex-type culture: CBS 134841 = LPF072.
Type locality: Brazil, Minas Gerais state, Araponga.
Type substrate: Soil in tropical rainforest.
Barcodes: cmdA = KM396070; his3 = KM396153; tef1 = KM395896; tub2 = KM395983.
Notes: Calonectria pseudospathulata is closely related to Ca. fragariae, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. pseudospathulata [(av. 43 × 4 μm); Alfenas et al. 2015] are longer than those of Ca. fragariae [(av. 39 × 4 μm); Lopes et al. 2017] (Supplementary Table S4).
pseudoturangicola Calonectria J.Q. Li et al., IMA Fungus 8: 279. 2017.
(see Calonectria kyotensis)
In: Calonectria kyotensis species complex.
Typus: PREM 61948 holotype.
Ex-type culture: CBS 142890 = CMW 47496 = CERC 7126.
Type locality: China, FuJian Province, FuZhou City.
Type substrate: Soil.
Barcodes: act = MT335121; cmdA = MT335356; his3 = MT335596; rpb2 = MT412649; tef1 = MT412887; tub2 = MT413098 (alternative markers: ITS = MT359817; LSU = MT359577).
Notes: Calonectria pseudoturangicola was treated as a synonym of Ca. kyotensis in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. pseudoturangicola (CERC 7126) and the ex-type isolate of Ca. kyotensis (CBS 114525) was in the cmdA, ITS, LSU and tub2 sequences, where there was one base difference in the cmdA, one base difference in the ITS, one base difference in the LSU and three base differences in the tub2 sequences. Both species are homothallic and produce sphaeropedunculate (globose) vesicles and 1-septate macroconidia of similar dimensions (Ca. pseudoturangicola: av. 40 × 3.5 μm, Ca. kyotensis: av. 41 × 4 μm) (Terashita 1968, Li et al. 2017, 2020; Supplementary Table S4).
pseudovata Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 120. 2015.
In: Calonectria pteridis species complex.
Typus: CBS H-21370 holotype.
Ex-type culture: CBS 134674 = LPF267.
Type locality: Brazil, Pará state, Santana.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: cmdA = KM396032; his3 = KM396115; tef1 = KM395858; tub2 = KM395945.
Notes: Calonectria pseudovata is phylogenetically closely related to Ca. ovata and Ca. terricola. Morphologically, Ca. pseudovata shows some overlap with Ca. ovata, while the sequences of cmdA, his3, tef1 and tub2 gene regions can differentiate Ca. pseudovata from Ca. ovata. The macroconidia of Ca. pseudovata [(av. 69 × 5 μm); Alfenas et al. 2015] are larger than those of Ca. terricola [(av. 46 × 4.5 μm); Lombard et al. 2016]. Furthermore, the ability of Ca. pseudovata and Ca. ovata to produce miconidiophores and microconidia in culture distinguishes it from Ca. terricola.
pseudouxmalensis Calonectria L. Lombard & Crous, Stud. Mycol. 85: 187. 2016.
In: Calonectria mexicana species complex.
Typus: CBS H-22769 holotype.
Ex-type culture: CBS 110924 = CMW 51166 = CPC 942.
Type locality: Mexico.
Type substrate: Soil.
Barcodes: act = MT335123; cmdA = MT335358; his3 = MT335598; rpb2 = MT412651; tef1 = MT412889; tub2 = MT413100 (alternative markers: ITS = MT359819; LSU = MT359579).
Notes: Calonectria pseudouxmalensis is closely related to Ca. uxmalensis, the sequences of cmdA, his3, tef1 and tub2 gene regions can distinguish Ca. pseudouxmalensis from Ca. uxmalensis. Morphologically, these two species are similar to each other. However, no lateral stipe extensions were observed in Ca. pseudouxmalensis, a feature that has been reported in Ca. uxmalensis (Lombard et al. 2016) (Supplementary Table S4).
pseudoyunnanensis Calonectria J.Q. Li et al., IMA Fungus 8: 279. 2017.
(see Calonectria yunnanensis)
In: Calonectria kyotensis species complex.
Typus: PREM 61950 holotype.
Ex-type culture: CBS 142892 = CMW 47655 = CERC 5376.
Type locality: China, YunNan, PuEr Region.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT335126; cmdA = MT335361; his3 = MT335601; rpb2 = MT412654; tef1 = MT412892; tub2 = MT413103 (alternative markers: ITS = MT359822; LSU = MT359582).
Notes: Calonectria pseudoyunnanensis was treated as a synonym of Ca. yunnanensis in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. pseudoyunnanensis (CERC 5376) and the ex-type isolate of Ca. yunnanensis (CERC 5339) was in the his3 and tub2 sequences, where there were three base differences in the his3 and four base differences in the tub2 sequences. Both species are homothallic and produce sphaeropedunculate vesicles and 1-septate macroconidia (Li et al. 2017, 2020), and share vesicles of similar dimensions (Ca. pseudoyunnanensis: 2.5–5 μm; Ca. yunnanensis: 2–4.5 μm). The macroconidia of Ca. pseudoyunnanensis [(av. 47.5 × 5 μm); Li et al. 2017] are larger than those of Ca. yunnanensis [(av. 43 × 4.5 μm); Li et al. 2017] which was considered to represent intraspecific variation (Supplementary Table S4).
pteridis Calonectria Crous et al., Mycotaxon 46: 228. 1993.
Synonym: Cylindrocladium pteridis F.A. Wolf, J. Elisha Mitchell Sci. Soc. 42: 59. 1926.
In: Calonectria pteridis species complex.
Typus: PREM 51033 (holotype of Ca. pteridis; Crous et al. 1993c), BPI 414564 (holotype of Cy. pteridis; Wolf 1926).
Ex-type culture: CBS 111793 = CMW 16736 = CPC 2372 = ATCC 34395 (Crous et al. 2006).
Type locality: USA.
Type substrate: Arachniodes adiantiformis.
Barcodes: act = GQ280494; cmdA = GQ267413; his3 = DQ190679; rpb2 = KY653438; tef1 = FJ918563; tub2 = DQ190578 (alternative markers: ITS = GQ280616; LSU = GQ280738).
Notes: Calonectria pteridis is closely related to Ca. pseudopteridis, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. pteridis [(av. 82 × 5.5 μm); Crous et al. 1993c] are shorter than those of Ca. pseudopteridis [(av. 87.5 × 5.7 μm); Sobers 1968, Alfenas et al. 2015]. (Supplementary Table S4).
putriramosa Calonectria L. Lombard & Crous, Stud. Mycol. 85: 188. 2016.
In: Calonectria candelabrum species complex.
Typus: CBS H-22770 holotype.
Ex-type culture: CBS 111449 = CMW 51181 = CPC 1951.
Type locality: Brazil.
Type substrate: Eucalyptus cuttings.
Barcodes: act = MT335129; cmdA = MT335364; his3 = MT335604; rpb2 = MT412657; tef1 = MT412895; tub2 = MT413105 (alternative markers: ITS = MT359825; LSU = MT359585).
Notes: Calonectria putriramosa is closely related to Ca. pseudometrosideri, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. putriramosa (av. 43 × 5 μm) are shorter than those of Ca. pseudometrosideri (av. 51 × 4.5 μm), while the vesicles of Ca. putriramosa (7–10 μm) are wider than those of Ca. pseudometrosideri (5–7 μm) (Alfenas et al. 2015, Lombard et al. 2016) (Supplementary Table S4).
queenslandica Calonectria L. Lombard et al., Persoonia 24: 8. 2010.
Synonym: Calonectria terrae-reginae L. Lombard et al., Persoonia 24: 8. 2010.
In: Calonectria reteaudii species complex.
Typus: PREM 60243 holotype.
Ex-type culture: CBS 112146 = CMW 30604 = CPC 3213.
Type locality: Australia, Queensland, Lannercost.
Type substrate: Eucalyptus urophylla.
Barcodes: act = MT335132; cmdA = MT335367; his3 = MT335607; rpb2 = MT412660; tef1 = MT412898; tub2 = MT413108 (alternative markers: ITS = MT359828; LSU = MT359588).
Notes: Calonectria queenslandica is phylogenetically closely related to Ca. lombardiana (Fig. 1). Sequences of ITS, tef1 and tub2 gene regions can differentiate Ca. queenslandica from Ca. lombardiana. In addition, the macroconidia of Ca. queenslandica [(av. 69 × 6 μm); Lombard et al. 2010c] are shorter than those of Ca. lombardiana (av. 80 × 6 μm) (Supplementary Table S4).
quinqueramosa Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 123. 2015.
In: Calonectria brassicae species complex.
Typus: CBSH-21355 holotype.
Ex-type culture: CBS 134654 = LPF065.
Type locality: Brazil, Pará state, Monte Dourado.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: cmdA = KM396029; his3 = KM396112; tef1 = KM395855; tub2 = KM395942.
Notes: Calonectria quinqueramosa is closely related to Ca. gracilis, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. quinqueramosa (av. 59 × 5 μm) are larger than those of Ca. gracilis (av. 56 × 4.5 μm), while the vesicles of Ca. quinqueramosa [(3–5 μm); Alfenas et al. 2015] are narrower than those of Ca. gracilis [(2–11 μm); Crous et al. 1993c]. Calonectria quinqueramosa is homothallic (Alfenas et al. 2015) (Supplementary Table S4).
reteaudii Calonectria (Bugnic.) C. Booth, Mycol. Pap. 104: 41. 1966.
Basionym: Neonectria reteaudii Bugnic., Encycl. Mycol. 11: 189. 1939.
Synonyms: Cylindrocarpon reteaudii Bugnic., Encycl. Mycol. 11: 189. 1939.
Cylindrocladium reteaudii (Bugnic.) Boesew., Trans. Brit. Mycol. Soc. 78: 553. 1982.
Cylindrocladium quinqueseptatum Boedijn & Reitsma, Reinwardtia 1: 59. 1950.
Calonectria baviensis N.Q. Pham et al., Mycologia 111: 90. 2019.
In: Calonectria reteaudii species complex.
Typus: PREM 57211 (neotype of Ca. reteaudii; Crous 2002), PREM 57212 (neotype of Cy. reteaudii; Crous 2002).
Ex-type culture: CBS 112144 = CMW 30984 = CPC 3201 (Crous 2002, Crous et al. 2006).
Type locality: Vietnam, Binh Phuoc, Chon Thanh.
Type substrate: Eucalyptus camaldulensis.
Barcodes: act = MT335135; cmdA = MT335370; his3 = MT335610; rpb2 = MT412663; tef1 = MT412901; tub2 = MT413111 (alternative markers: ITS = MT359831; LSU = MT359591).
Notes: Calonectria reteaudii is phylogenetically closely related to Ca. acaciicola and Ca. pseudoreteaudii. The sequences of the his3, rpb2, tef1 and tub2 gene regions can differentiate Ca. reteaudii from these two species. The macroconidia of Ca. reteaudii [(av. 84 × 6.5 μm); Kang et al. 2001a] are smaller than those of Ca. acaciicola [(av. 94 × 7 μm); Pham et al. 2019] and Ca. pseudoreteaudii [(av. 104 × 8 μm); Lombard et al. 2010c]. Calonectria reteaudii is heterothallic (Kang et al. 2001a) (Supplementary Table S4).
robigophila Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 123. 2015.
In: Calonectria brassicae species complex.
Typus: CBS H-21361 holotype.
Ex-type culture: CBS 134652 = LPF192.
Type locality: Brazil, Maranhao state, Açailandia.
Type substrate: Eucalyptus sp.
Barcodes: cmdA = KM396024; his3 = KM396107; tef1 = KM395850; tub2 = KM395937.
Notes: Calonectria robigophila forms a single lineage closely related to Ca. duoramosa, and it produce six tiers of branches in its conidiogenous apparatus in comparison to the two in Ca. duoramosa. The macroconidia of Ca. robigophila [(av. 50 × 4 μm); Alfenas et al. 2015] are longer than those of Ca. duoramosa [(av. 46 × 4 μm); Alfenas et al. 2015] (Supplementary Table S4).
rumohrae Calonectria El-Gholl & Alfenas, Mycotaxon 64: 478. 1997.
Synonym: Cylindrocladium rumohrae El-Gholl & Alfenas, Mycotaxon 64: 478. 1997.
In: Calonectria gracilipes species complex.
Typus: FLAS F56116a & b (holotypes of Ca. rumohrae and Cy. rumohrae; El-Gholl et al. 1997).
Ex-type culture: CBS 111431 = CMW 23697 = CPC 1716 = UFV 218 (Crous 2002, Crous et al. 2006).
Type locality: Panama, Volkan.
Type substrate: Rumohra adiantiformis.
Barcodes: act = MT335137; cmdA = MT335372; his3 = MT335612; tef1 = MT412903; tub2 = MT413113 (alternative markers: ITS = MT359833; LSU = MT359593).
Notes: Calonectria rumohrae is phylogenetically closely related to Ca. hurae, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. rumohrae [(av. 110 × 9 μm); El-Gholl et al. 1997] are shorter but wider than those of Ca. hurae [(av. 120 × 7.5 μm); Crous 2002]. Calonectria rumohrae is homothallic (El-Gholl et al. 1997) (Supplementary Table S4).
seminaria Calonectria L. Lombard et al., Stud. Mycol. 80: 179. 2015.
(see Calonectria pauciramosa)
In: Calonectria candelabrum species complex.
Typus: CBS H-21475 holotype.
Ex-type culture: CBS 136632 = CMW 31450 = CERC 1785.
Type locality: China, GuangDong Province, ZhanJiang.
Type substrate: Eucalyptus urophylla × E. grandis clone seedling leaf.
Barcodes: act = MT335102; cmdA = MT335334; his3 = MT335574; rpb2 = MT412627; tef1 = MT412865; tub2 = MT413077 (alternative markers: ITS = MT359795; LSU = MT359555).
Notes: Calonectria seminaria was treated as a synonym of Ca. pauciramosa in this study. In comparisons of DNA sequences for eight gene regions, all the sequences of the isolates of Ca. seminaria (ex-type CMW 31450, and CMW 31489) were 100 % identical to those of the isolates of Ca. pauciramosa (ex-type CBS 138824, CMW 2151, CMW 7592, CMW 9151, CMW 30823, and CMW 30875). Both species produce obpyriform to ellipsoidal vesicles and 1-septate macroconidia, and shared the similar vesicles (Ca. seminaria: 6–11 μm; Ca. pauciramosa: 5–11 μm) and macroconidial dimensions (Ca. seminaria: av. 47 × 5 μm; Ca. pauciramosa: av. 50 × 4.5 μm) (Crous 2002, Lombard et al. 2015a) (Supplementary Table S4).
silvicola Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 123. 2015.
In: Calonectria candelabrum species complex.
Typus: CBS H-21357 holotype.
Ex-type culture: CBS 135237 = LPF081.
Type locality: Brazil, Bahia state, Mucuri.
Type substrate: Soil in tropical rainforest.
Barcodes: cmdA = KM396065; his3 = KM396148; tef1 = KM395891; tub2 = KM395978.
Notes: Calonectria silvicola is closely related to Ca. nemoricola, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. silvicola [(av. 41 × 4.5 μm); Alfenas et al. 2015] are shorter than those of Ca. nemoricola [(av. 45 × 4 μm); Alfenas et al. 2015] (Supplementary Table S4).
spathiphylli Calonectria El-Gholl et al., Mycotaxon 45: 296. 1992.
Synonym: Cylindrocladium spathiphylli Schoult., et al., Mycotaxon 16: 268. 1982.
In: Calonectria spathiphylli species complex.
Typus: FLAS 55655 [holotype of Ca. spathiphylli; El-Gholl et al. 1992), ATCC 44730 (holotype of Cy. spathiphylli; Schoulties et al. 1982, Crous 2002).
Ex-type culture: CBS 114540 = CMW 16742 = ATCC 44730 = STE-U 2185 (Crous 2002, Alfenas et al. 2015).
Type locality: USA, Florida.
Type substrate: Spathiphyllum sp.
Barcodes: cmdA = MT335374; his3 = MT335614; rpb2 = MT412666; tef1 = MT412905; tub2 = MT413115 (alternative markers: ITS = MT359835; LSU = MT359595).
Notes: Calonectria spathiphylli is closely related to Ca. densa and Ca. humicola, and can be distinguished from these two species by the dimensions of its macroconidia. Macroconidia of Ca. spathiphylli [(av. 70 × 6 μm); El-Gholl et al. 1992] are longer than those of Ca. densa [(av. 54 × 6 μm); Lombard et al. 2010a] and larger than those of Ca. humicola [(av. 51 × 5 μm); Lombard et al. 2010a]. Calonectria spathiphylli is characterised as being heterothallic (El-Gholl et al. 1992) (Supplementary Table S4).
spathulata Calonectria El-Gholl et al., Mycotaxon 26: 159. 1986 emend. Crous, Mycoscience 42: 55. 2001.
Synonyms: Cylindrocladium spathulatum El-Gholl et al., Mycotaxon 26: 159. 1986 emend. Crous, Mycoscience 42: 55. 2001.
Calonectria stipitata L. Lombard & Crous, Stud. Mycol. 85: 188. 2016.
In: Calonectria candelabrum species complex.
Typus: FLAS F54257a (holotype of Ca. spathulata; Crous & Kang 2001), FLAS F54257b (holotype of Cy. spathulatum; Crous & Kang 2001).
Ex-type culture: CBS 555.92 = CMW 16744 (Lombard et al. 2010a).
Type locality: Brazil.
Type substrate: Eucalyptus viminalis.
Barcodes: act = MT335139; cmdA = MT335376; his3 = MT335616; rpb2 = MT412668; tef1 = MT412907; tub2 = MT413117 (alternative markers: ITS = MT359837; LSU = MT359597).
Notes: Calonectria spathulata is closely related to Ca. colombiana, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. spathulata (av. 80 × 6 μm) are larger than those of Ca. colombiana (av. 37 × 3 μm). Furthermore, Ca. spathulata can produce both microconidiophores and microconidia, a feature not observed for Ca. colombiana (Crous & Kang 2001, Lombard et al. 2010b). Calonectria spathulata is homothallic (Crous & Kang 2001) (Supplementary Table S4). This species is commonly associated with leaf spots and cutting rot of Eucalyptus cultivated in plantations and nurseries, and has a wide geographic distribution in South America (Argentina, Brazil, Colombia and Ecuador) (El-Gholl et al. 1986, Crous & Kang 2001, Rodas et al. 2005).
sphaeropedunculata Calonectria L. Lombard et al., Stud. Mycol. 80: 180. 2015.
(see Calonectria aconidialis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21486 holotype.
Ex-type culture: CBS 136081 = CMW 31390 = CERC 1725.
Type locality: China, GuangXi Province.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT334952; cmdA = MT335179; his3 = MT335418; rpb2 = MT412485; tef1 = MT412709 (alternative markers: ITS = MT359639; LSU = MT359399).
Notes: Calonectria sphaeropedunculata was treated as a synonym of Ca. aconidialis in this study. In comparisons of DNA sequences for seven available gene regions, a single base difference in the cmdA and two base differences in the LSU sequences occurred between the ex-type isolate of Ca. sphaeropedunculata (CMW 31390) and the ex-type isolate of Ca. aconidialis (CMW 35174). Both species are homothallic and produce orange to orange-brown perithecia, 8-spored asci and 1-septate ascospores, and share the similar ascospores dimensions (Ca. sphaeropedunculata: av. 37 × 6 μm; Ca. aconidialis: av. 36 × 6 μm) (Lombard et al. 2015a, Li et al. 2020; Supplementary Table S4).
stipitata Calonectria L. Lombard & Crous, Stud. Mycol. 85: 188. 2016.
(see Calonectria spathulata)
In: Calonectria candelabrum species complex.
Typus: CBS H-22771 holotype.
Ex-type culture: CBS 112513 = CMW 51197 = CPC 3851.
Type locality: Colombia.
Type substrate: Eucalyptus sp.
Barcodes: act = MT335140; cmdA = MT335377; his3 = MT335617; rpb2 = MT412669; tef1 = MT412908; tub2 = MT413118 (alternative markers: ITS = MT359838; LSU = MT359598).
Notes: Calonectria stipitata was treated as a synonym of Ca. spathulata in this study. In comparisons of DNA sequences for eight gene regions, the ex-type isolate of Ca. stipitata (CBS 112513) and the isolate of Ca. spathulata (CMW 16744) only had one base difference in the his3 gene sequence. Both species produce ellipsoidal to obpyriform vesicles of similar dimensions (Ca. stipitata: 7–11 μm; Ca. spathulata: 6–10 μm). The macroconidia of Ca. stipitata (av. 32 × 4 μm) are smaller than those of Ca. spathulata (av. 80 × 6 μm) (El-Gholl et al. 1986, Crous & Kang 2001, Lombard et al. 2016), which was considered to represent intraspecific variation.
sulawesiensis Calonectria L. Lombard et al., Stud. Mycol. 66: 53.2010.
(see Calonectria hawksworthii)
In: Calonectria cylindrospora species complex.
Typus: PREM 60300 holotype.
Ex-type culture: CBS 125277 = CMW 14878.
Type locality: Indonesia, Sulawesi.
Type substrate: Leaf of Eucalyptus sp.
Barcodes: act = MT335141; cmdA = MT335378; his3 = MT335618; rpb2 = MT412670; tef1 = MT412909; tub2 = MT413119 (alternative markers: ITS = MT359839; LSU = MT359599).
Notes: Calonectria sulawesiensis was treated as a synonym of Ca. hawksworthii in this study. In comparisons of DNA sequences for eight gene regions, the only difference between the ex-type isolate of Ca. sulawesiensis (CMW 14878) and the ex-type isolate of Ca. hawksworthii (CBS 111870) was in the act, tef1 and tub2 sequences, where there were four base differences in the act, one base difference in the tef1 and four base differences in the tub2 sequences. Both species produce ellipsoidal vesicles with overlapping dimensions (Ca. sulawesiensis: 5–7 μm; Ca. hawksworthii: 6–9 μm) and 1-septate macroconidia. The macroconidia of Ca. sulawesiensis (av. 48 × 4 μm) are shorter than those of Ca. hawksworthii (av. 56 × 4 μm) (Crous 2002, Lombard et al. 2010a), which was considered to represent intraspecific variation (Supplementary Table S4).
sumatrensis Calonectria (Crous) L. Lombard et al., Stud. Mycol. 66: 56. 2010
Basionym: Cylindrocladium sumatrense Crous, Stud. Mycol. 50: 426. 2004.
Synonym: Calonectria indonesiana L. Lombard & Crous, Stud. Mycol. 85: 179. 2016.
In: Calonectria kyotensis species complex.
Typus: CBS 9888 holotype.
Ex-type culture: CBS 112829 = CMW 23698 = CPC 4518.
Type locality: Indonesia, Northern Sumatra.
Type substrate: Soil.
Barcodes: act = MT335145; cmdA = MT335382; his3 = MT335622; rpb2 = MT412674; tef1 = MT412913 (alternative markers: ITS = MT359843; LSU = MT359603).
Notes: Calonectria sumatrensis is closely related to Ca. brassicicola, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. sumatrensis [(av. 58 × 5 μm); Crous et al. 2004] are longer than those of Ca. brassicicola [(av. 42 × 5 μm); Lombard et al. 2016], and the vesicles of Ca. sumatrensis (8–13 μm) are wider than those of Ca. brassicicola (3−5 μm) (Supplementary Table S4).
syzygiicola Calonectria L. Lombard & Crous, Stud. Mycol. 85: 190. 2016.
In: Calonectria kyotensis species complex.
Typus: CBS H-22772 holotype.
Ex-type culture: CBS 112831 = CMW 51204 = CPC 4511.
Type locality: Indonesia, Sumatra.
Type substrate: Soil under Syzygium aromaticum.
Barcodes: ITS = KX784736; LSU = KX784663.
Notes: Calonectria syzygiicola is closely related to Ca. asiatica, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. syzygiicola [(av. 45 × 5 μm); Lombard et al. 2016] are shorter than those of Ca. asiatica [(av. 53 × 5 μm); Crous et al. 2004], and the vesicles of Ca. syzygiicola (3–6 μm) are narrower than those of Ca. asiatica (12–17 μm) (Crous et al. 2004, Lombard et al. 2016) (Supplementary Table S4).
telluricola Calonectria R.F. Alfenas et al., Stud. Mycol. 80: 125. 2015.
(see Calonectria paraensis)
In: Calonectria brassicae species complex.
Typus: CBS H-21365 holotype.
Ex-type culture: CBS 134664 = LPF217.
Type locality: Brazil, Bahia state, Mucuri.
Type substrate: Soil in tropical rainforest.
Barcodes: cmdA = KM396017; his3 = KM396100; tef1 = KM395843; tub2 = KM395930.
Notes: Calonectria telluricola was treated as a synonym of Ca. paraensis in this study. In comparisons of DNA sequences for the four available gene regions, the only difference between the ex-type isolate of Ca. telluricola (CBS 134664) and the ex-type isolate of Ca. paraensis (CBS 134669) was in the cmdA, his3, tef1 and tub2 sequences, where there was one base difference in the cmdA, two base differences in the his3, one base difference in the tef1 and two base differences in the tub2 sequences. Both species produce clavate vesicles and 1-septate macroconidia, and shared the similar vesicle (Ca. telluricola: 3–6 μm; Ca. paraensis: 4–6 μm) and macroconidial dimensions (Ca. telluricola: av. 41 × 5 μm; Ca. paraensis: av. 42 × 5 μm) (Alfenas et al. 2015) (Supplementary Table S4).
tereticornis Calonectria L. Lombard & Crous, Stud. Mycol. 85: 190. 2016.
(see Calonectria ovata)
In: Calonectria pteridis species complex.
Typus: CBS H-22773 holotype.
Ex-type culture: CBS 111301 = CMW 51176 = CPC 1429.
Type locality: Brazil, Pará, Tucuruí.
Type substrate: Eucalyptus tereticornis.
Barcodes: act = MT335077; cmdA = MT335309; his3 = MT335549; tef1 = MT412840; tub2 = MT413054 (alternative markers: ITS = MT359770; LSU = MT359530).
Notes: Calonectria tereticornis was treated as a synonym of Ca. ovata in this study. In comparisons of DNA sequences for seven available gene regions, the only differences between the ex-type isolate of Ca. tereticornis (CBS 111301) and the ex-type isolate of Ca. ovata (CMW 16724) was in the LSU and tub2 sequences where there was one base difference in the LSU and one base difference in the tub2 sequences. Both of the species produce ovoid vesicles of similar dimensions (Ca. tereticornis: 8–14 μm; Ca. ovata: 8–14 μm) and 1-septate macroconidia. The macroconidia of Ca. tereticornis [(av. 59 × 5 μm); Lombard et al. 2016] are shorter than those of Ca. ovata [(av. 70 × 5 μm); Victor et al. 1997], which was considered to represent intraspecific variation (Supplementary Table S4).
terrae-reginae Calonectria L. Lombard et al., Persoonia 24: 8. 2010.
(see Calonectria queenslandica)
In: Calonectria reteaudii species complex.
Typus: PREM 60239 holotype.
Ex-type culture: CBS 112151 = CMW 30601 = CPC 3202 = DFRI00150.
Type locality: Australia, Queensland, Cardwell.
Type substrate: Eucalyptus urophylla.
Barcodes: act = MT335134; cmdA = MT335369; his3 = MT335609; rpb2 = MT412662; tef1 = MT412900; tub2 = MT413110 (alternative markers: ITS = MT359830; LSU = MT359590).
Notes: Calonectria terrae-reginae was treated as a synonym of Ca. queenslandica in this study. In comparisons of DNA sequences for eight gene regions, only a single base difference in the tub2 gene sequence was found between the ex-type isolate of Ca. terrae-reginae (CMW 30601) and the ex-type isolate of Ca. queenslandica (CMW 30604). Both species produce narrowly clavate vesicles of similar dimensions (Ca. terrae-reginae: 3–5 μm; Ca. queenslandica: 3–4 μm) and 4–6-septate macroconidia. The macroconidia of Ca. terrae-reginae (av. 76 × 6 μm) are longer than those of Ca. queenslandica (av. 69 × 6 μm) (Lombard et al. 2010c), which was considered to represent intraspecific variation (Supplementary Table S4).
terrestris Calonectria L. Lombard et al., Stud. Mycol. 80: 182. 2015.
(see Calonectria cerciana)
In: Calonectria cylindrospora species complex.
Typus: CBS H-21478 holotype.
Ex-type culture: CBS 136642 = CMW 35180 = CERC 1856.
Type locality: China, GuangDong.
Type substrate: Soil in a Eucalyptus plantation.
Barcodes: act = MT334986; cmdA = MT335216; his3 = MT335456; rpb2 = MT412520; tef1 = MT412747; tub2 = MT412968 (alternative markers: ITS = MT359677; LSU = MT359437).
Notes: Calonectria terrestris was treated as a synonym of Ca. cerciana in this study. In comparisons of DNA sequences for eight gene regions, the only differences between the ex-type isolate Ca. terrestris (CMW 35180) and the ex-type isolate of Ca. cerciana (CMW 25309) was in the ITS and tef1 sequences, where there was one base difference in the ITS and three base differences in the tef1 sequences. Both species produce obpyriform vesicles with overlapping dimensions (Ca. terrestris: 5–12 μm; Ca. cerciana: 8–13 μm) and 1-septate macroconidia. The macroconidia of Ca. terrestris (av. 38.5 × 4.5 μm) are smaller than those of Ca. cerciana (av. 44 × 5 μm) (Lombard et al. 2010c, 2015a), which was considered to represent intraspecific variation (Supplementary Table S4).
terricola Calonectria L. Lombard & Crous, Stud. Mycol. 85: 191. 2016.
In: Calonectria pteridis species complex.
Typus: CBS H-22774 holotype.
Ex-type culture: CBS 116247 = CMW 51232 = CPC 3583.
Type locality: Brazil.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: tef1 = KX784738; tub2 = KX784665.
Notes: Calonectria terricola is closely related to Ca. ovata and Ca. pseudovata, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. terricola [(av. 46 × 4.5 μm); Lombard et al. 2016] are smaller than those of Ca. ovata [(av. 70 × 5 μm); Victor et al. 1997] and Ca. pseudovata [(av. 69 × 5 μm); Alfenas et al. 2015]. In addition, no microconidiophores and microconidia were reported in Ca. terricola, a feature that is common in Ca. ovata and Ca. pseudovata (Victor et al. 1997, Alfenas et al. 2015, Lombard et al. 2016) (Supplementary Table S4).
tetraramosa Calonectria L. Lombard et al., Stud. Mycol. 80: 183. 2015.
(see Calonectria pauciramosa)
In: Calonectria candelabrum species complex.
Typus: CBS H-21477 holotype.
Ex-type culture: CBS 136635 = CMW 31474 = CERC 1809.
Type locality: China, GuangDong Province, ZhanJiang.
Type substrate: Eucalyptus urophylla × E. grandis clone seedling leaf.
Barcodes: act = MT335104; cmdA = MT335336; his3 = MT335576; rpb2 = MT412629; tef1 = MT412867; tub2 = MT413079 (alternative markers: ITS = MT359797; LSU = MT359557).
Notes: Calonectria tetraramosa was treated as a synonym of Ca. pauciramosa in this study. In comparisons of DNA sequences for eight gene regions, all the sequences of the isolates of Ca. tetraramosa (ex-type CMW 31474, and CMW 31476) were 100 % identical to those of the isolates of Ca. pauciramosa (ex-type CBS 138824, CMW 2151, CMW 7592, CMW 9151, CMW 30823, and CMW 30875). Both species produce obpyriform vesicles and 1-septate macroconidia, and shared similar vesicle (Ca. tetraramosa: 4–10 μm; Ca. pauciramosa: 5–11 μm) and macroconidial dimensions (Ca. tetraramosa: av. 48 × 5 μm; Ca. pauciramosa: av. 50 × 4.5 μm) (Crous 2002, Lombard et al. 2015a) (Supplementary Table S4).
tonkinensis Calonectria N.Q. Pham et al., Mycologia 111: 87. 2019.
In: Calonectria cylindrospora species complex.
Typus: PREM 62124 holotype.
Ex-type culture: CBS 143576 = CMW 47430.
Type locality: Vietnam, Hanoi, Bavi.
Type substrate: Soil in a Eucalyptus plantation.
Barcodes: act = MT335147; cmdA = MT335384; his3 = MT335624; rpb2 = MT412676; tef1 = MT412915; tub2 = MT413122 (alternative markers: ITS = MT359845; LSU = MT359605).
Notes: Calonectria tonkinensis is closely related to Ca. cerciana, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. tonkinensis (av. 41.5 × 4 μm) are smaller than those of Ca. cerciana (av. 44 × 5 μm), and the vesicles of Ca. tonkinensis (3–7 μm) are narrower than those of Ca. cerciana (8–13 μm) (Lombard et al. 2010c, Pham et al. 2019) (Supplementary Table S4).
tropicalis Calonectria L. Lombard & Crous, Stud. Mycol. 85: 192. 2016.
(see Calonectria amazonica)
In: Calonectria pteridis species complex.
Typus: CBS H-22776 holotype.
Ex-type culture: CBS 116271 = CMW 51236 = CPC 3559.
Type locality: Brazil, Amazon.
Type substrate: Eucalyptus sp.
Barcodes: act = MT335148; cmdA = MT335385; his3 = MT335625; rpb2 = MT412677; tef1 = MT412916; tub2 = MT413123 (alternative markers: ITS = MT359846; LSU = MT359606).
Notes: Calonectria tropicalis was treated as a synonym of Ca. amazonica in this study. In comparisons of DNA sequences for eight gene regions, the only differences between the ex-type isolate of Ca. tropicalis (CBS 116271) and the ex-type isolate of Ca. amazonica (CBS 116250) was in the ITS, rpb2 and tub2 sequences, where there was one base difference in the ITS, one base difference in the rpb2 and three base differences in the tub2 sequences. Both species produce clavate vesicles and 1(–3)-septate macroconidia, and shared similar vesicle (Ca. tropicalis: 5–6 μm; Ca. amazonica: 5–6 μm) and macroconidial dimensions (Ca. tropicalis: av. 80 × 5 μm; Ca. amazonica: av. 79 × 5 μm) (Lombard et al. 2016) (Supplementary Table S4).
tucuruiensis Calonectria L. Lombard & Crous, Stud. Mycol. 86: 123. 2017.
(see Calonectria ovata)
In: Calonectria pteridis species complex.
Typus: CBS H-22777 holotype.
Ex-type culture: CBS 114755 = CMW 51827 = CPC 1403.
Type locality: Brazil, Pará, Tucuruí.
Type substrate: Eucalyptus tereticornis.
Barcodes: act = MT335078; cmdA = MT335310; his3 = MT335550; tef1 = MT412841; tub2 = MT413055 (alternative markers: ITS = MT359771; LSU = MT359531).
Notes: Calonectria tucuruiensis was treated as a synonym of Ca. ovata in this study. In comparisons of DNA sequences for seven available gene regions, there was one base difference between the ex-type isolate of Ca. tucuruiensis (CBS 114755) and the ex-type isolate of Ca. ovata (CMW 16724) in each of the LSU and tub2 sequences. Both of the species produce ovoid vesicles with overlapping dimensions (Ca. tucuruiensis: 9–12 μm; Ca. ovata: 8–14 μm) and 1-septate macroconidia. The macroconidia of Ca. tucuruiensis [(av. 63 × 5 μm); Marin-Felix et al. 2017] are shorter than those of Ca. ovata [(av. 70 × 5 μm); Victor et al. 1997], which was considered to represent intraspecific variation (Supplementary Table S4).
tunisiana Calonectria L. Lombard et al., Persoonia 27: 77. 2011.
(see Calonectria pseudomexicana)
In: Calonectria mexicana species complex.
Typus: CBS H-20684 holotype.
Ex-type culture: CBS 130357 = CMW 51316 = DISTEF-TCL1.
Type locality: Tunisia, Carthage, Tunis.
Type substrate: Callistemon laevis.
Barcodes: act = MT335149; cmdA = MT335386; his3 = MT335626; rpb2 = MT412678; tef1 = MT412917; tub2 = MT413124 (alternative markers: ITS = MT359847; LSU = MT359607).
Notes: Calonectria tunisiana was treated as a synonym of Ca. pseudomexicana in this study. In comparisons of DNA sequences for eight gene regions, the only differences between the ex-type isolate of Ca. tunisiana (CBS 130357) and the ex-type isolate of Ca. pseudomexicana (CBS 130354) was in the LSU and tef1 sequences, where there was one base difference in the LSU and six base differences in the tef1 sequences. Both species produce fusiform to broadly ellipsoidal vesicles with papillate apex and 1-septate macroconidia, and similar vesicles (Ca. tunisiana: 8–14 μm; Ca. pseudomexicana: 9–14 μm). The macroconidia of Ca. tunisiana (av. 49 × 5 μm) are longer than those of Ca. pseudomexicana (av. 45 × 5 μm) (Lombard et al. 2011), which was considered to represent intraspecific variation (Supplementary Table S4).
turangicola Calonectria L. Lombard et al., Stud. Mycol. 80: 184. 2015.
(see Calonectria kyotensis)
In: Calonectria kyotensis species complex.
Typus: CBS H-21488 holotype.
Ex-type culture: CBS 136077 = CMW 31411.
Type locality: China, GuangXi Province.
Type substrate: Soil in a Eucalyptus plantation.
Barcodes: act = MT335151; cmdA = MT335388; his3 = MT335628; tef1 = MT412919; tub2 = MT413126 (alternative markers: ITS = MT359849; LSU = MT359609).
Notes: Calonectria turangicola was treated as a synonym of Ca. kyotensis in this study. In comparison of DNA sequences for seven available gene regions, the only difference between the ex-type isolate of Ca. turangicola (CMW 31411) and the ex-type isolate of Ca. kyotensis (CBS 114525) was in the LSU, tef1 and tub2 sequences, where there was one base difference in the LSU, two base differences in the tef1 and two base differences in the tub2 sequences. Both species are homothallic and produce sphaeropedunculate (globose) vesicles and 1-septate macroconidia of similar dimensions (Ca. turangicola: av. 44 × 4 μm, Ca. kyotensis: av. 41 × 4 μm) (Terashita 1968, Lombard et al. 2015a, Li et al. 2020; Supplementary Table S4).
uniseptata Calonectria Gerlach, Phytopathol. Z. 61: 379. 1968.
In: Calonectria kyotensis species complex.
Typus: No. 10759 holotype.
Ex-type culture: CBS 413.67 = CMW 23678 = CPC 2391 = IMI 299577.
Type locality: Germany, Celle.
Type substrate: Paphiopedilum callosum.
Barcodes: act = GQ280451; cmdA = GQ267379; his3 = GQ267248; tef1 = GQ267307; tub2 = GQ267208 (alternative markers: ITS = GQ280573; LSU = GQ280695).
Notes: Calonectria uniseptata is closely related to Ca. asiatica and Ca. syzygiicola, and can be distinguished from these two species by the dimensions of its ascospores and vesicles. The ascospores of Ca. uniseptata [(av. 41.5 × 6.3 μm); Gerlach 1968] are longer than those of Ca. asiatica [(av. 33 × 6 μm); Crous et al. 2004], and the vesicles of Ca. uniseptata (8.2–20.4 μm) are wider than those of Ca. syzygiicola (3–6 μm) (Gerlach 1968, Lombard et al. 2016) (Supplementary Table S4).
uxmalensis Calonectria L. Lombard & Crous, Stud. Mycol. 85: 193. 2016.
In: Calonectria mexicana species complex.
Typus: CBS H-22761 holotype.
Ex-type culture: CBS 110925 = CMW 51167 = CPC 945.
Type locality: Mexico, Uxmal.
Type substrate: Soil.
Barcodes: act = MT335153; cmdA = MT335390; his3 = MT335630; rpb2 = MT412681; tef1 = MT412921; tub2 = MT413128 (alternative markers: ITS = MT359851; LSU = MT359611).
Notes: Calonectria uxmalensis is closely related to Ca. pseudouxmalensis, and sequences of cmdA, his3, tef1 and tub2 gene regions can distinguish Ca. uxmalensis from Ca. pseudouxmalensis. Morphologically, these two species are similar to each other, while the ability of Ca. uxmalensis to produce lateral stipe extensions can differentiate Ca. uxmalensis from Ca. pseudouxmalensis (Lombard et al. 2016) (Supplementary Table S4).
variabilis Calonectria Crous et al., Syst. Appl. Microbiol. 16: 270. 1993.
Synonym: Cylindrocladium variabile Crous et al., Syst. Appl. Microbiol. 16: 270. 1993.
In: Calonectria cylindrospora species complex.
Typus: PREM 51039 (holotype of Ca. variabilis; Crous et al. 1993b), PREM 51041 (holotype of Cy. variabile; Crous et al. 1993b).
Ex-type culture: CBS 114677 = CMW 3187 = CPC 2436 = AR2675 (Crous et al. 1993b, Crous 2002)
Type locality: Brazil, Pará.
Type substrate: Schefflera morototoni.
Barcodes: cmdA = MT335392; his3 = MT335632; rpb2 = MT412683; tef1 = MT412923; tub2 = MT413130 (alternative markers: ITS = MT359853; LSU = MT359613).
Notes: Calonectria variabilis is phylogenetically closely related to Ca. cylindrospora, and can be distinguished from that species by the dimensions of its macroconidia. The macroconidia of Ca. variabilis [(av. 73 × 5 μm); Crous et al. 1993b] are larger than those of Ca. cylindrospora [(av. 45 × 4 μm); Crous et al. 1993a, Lombard et al. 2015b]. Calonectria variabilis readily produces a microconidial morph in culture on CLA (Crous et al. 1993b), a feature not observed for Ca. cylindrospora (Crous et al. 1993a). Calonectria variabilis is characterised as being homothallic, while Ca. cylindrospora is heterothallic (Crous et al. 1993b).
vegrandis Calonectria N.Q. Pham & M.J. Wingfield, Mycologia 111: 99. 2019.
(see Calonectria curvispora)
In: Calonectria kyotensis species complex.
Typus: PREM 62113 holotype.
Ex-type culture: CBS 143565 = CMW 48245.
Type locality: Indonesia, North Sumatra, Aek Nauli.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT335003; cmdA = MT335233; his3 = MT335473; rpb2 = MT412537; tef1 = MT412764 (alternative markers: ITS = MT359694; LSU = MT359454).
Notes: Calonectria vegrandis was treated as a synonym of Ca. curvispora in this study. In comparisons of DNA sequences for seven available gene regions, all seven gene sequences for the isolates of Ca. vegrandis (ex-type CMW 48245, and CMW 48246) were 100 % identical to the ex-type isolate of Ca. curvispora (CMW 23693). Both species produce sphaeropedunculate vesicles and 1-septate macroconidia, whereas Ca. curvispora typically produce curved macroconidia which are straight in Ca. vegrandis (Victor et al. 1997, Lombard et al. 2010a, Pham et al. 2019), suggesting that curved or straight macroconidia may be not a reliable feature to distinguish between these species. The macroconidia of Ca. vegrandis (av. 41 × 4.5 μm) are smaller than those of Ca. curvispora (av. 60 × 5 μm) (Victor et al. 1997, Lombard et al. 2010a, Pham et al. 2019), which was considered to represent intraspecific variation (Supplementary Table S4).
venezuelana Calonectria L. Lombard et al., Stud. Mycol. 85: 195. 2016.
In: Calonectria candelabrum species complex.
Typus: CBS H-22778 holotype.
Ex-type culture: CBS 111052 = CMW 51173 = CPC 1183.
Type locality: Venezuela, Acarigua.
Type substrate: Soil.
Barcodes: act = MT335155; cmdA = MT335394; his3 = MT335634; rpb2 = MT412685; tef1 = MT412925; tub2 = MT413132 (alternative markers: ITS = MT359855; LSU = MT359615).
Notes: Calonectria venezuelana is closely related to Ca. candelabrum and Ca. eucalypticola, and can be distinguished from these two species by the dimensions of its macroconidia. The macroconidia of Ca. venezuelana [(av. 58 × 5 μm); Lombard et al. 2016] are shorter than those of Ca. candelabrum [(av. 60 × 4.5 μm); Crous 2002], and larger than those of Ca. eucalypticola [(av. 50 × 4 μm); Alfenas et al. 2015] (Supplementary Table S4).
yunnanensis Calonectria J.Q. Li et al., IMA Fungus 8: 282. 2017.
Synonym: Calonectria pseudoyunnanensis J.Q. Li et al., IMA Fungus 8: 279. 2017.
In: Calonectria kyotensis species complex.
Typus: PREM 61955 holotype.
Ex-type culture: CBS 142897 = CMW 47644 = CERC 5339.
Type locality: China, YunNan.
Type substrate: Soil in Eucalyptus plantation.
Barcodes: act = MT335157; cmdA = MT335396; his3 = MT335636; rpb2 = MT412687; tef1 = MT412927; tub2 = MT413134 (alternative markers: ITS = MT359857; LSU = MT359617).
Notes: Calonectria yunnanensis is closely related to Ca. asiatica, and can be distinguished from that species by the dimensions of its macroconidia and vesicles. The macroconidia of Ca. yunnanensis [(av. 43 × 4.5 μm); Li et al. 2017] are smaller than those of Ca. asiatica [(av. 53 × 5 μm); Crous et al. 2004], and the vesicles of Ca. yunnanensis (2–4.5 μm) are narrower than those of Ca. asiatica (12–17 μm). Calonectria yunnanensis is homothallic (Li et al. 2020) (Supplementary Table S4).
zuluensis Calonectria L. Lombard & Crous, Stud. Mycol. 66: 25. 2010.
(see Calonectria pauciramosa)
In: Calonectria candelabrum species complex.
Typus: PREM 60292 holotype.
Ex-type culture: CBS 125268 = CMW 9188.
Type locality: South Africa, KwaZulu-Natal, Kwambonambi.
Type substrate: Eucalyptus grandis clonal cutting.
Barcodes: act = MT335159; cmdA = MT335398; his3 = MT335638; rpb2 = MT412689; tef1 = MT412929; tub2 = MT413136 (alternative markers: ITS = MT359859; LSU = MT359619).
Notes: Calonectria zuluensis was treated as a synonym of Ca. pauciramosa in this study. In comparisons of DNA sequences for eight gene regions, the only differences between the ex-type isolate of Ca. zuluensis (CMW 9188) and the ex-type isolate of Ca. pauciramosa (CBS 138824) was in the act and tub2 sequences, where there were two base differences in the act and three base differences in the tub2 sequences. Both species are heterothallic and produce obpyriform to ellipsoidal vesicles of similar dimensions (Ca. zuluensis: 6–10 μm; Ca. pauciramosa: 5–11 μm) and 1-septate macroconidia. The macroconidia of Ca. zuluensis (av. 36 × 4 μm) are smaller than those of Ca. pauciramosa (av. 50 × 4.5 μm) (Crous 2002, Lombard et al. 2010b, Li et al. 2020), which was considered to represent intraspecific variation (Supplementary Table S4).
Selection of DNA barcodes for species recognition
Phylogenetic analyses of sequences for eight gene regions resulted in the delineation of 120 clearly defined Calonectria species. These eight gene regions thus serve as putative DNA barcodes. As with most other fungi, it is not possible to use a single DNA barcode to identify species of Calonectria. Consequently, the Identification Success Rate (ISR) was calculated for each of the eight putative barcodes for all 120 species of Calonectria and also for species residing in each of the 11 well-defined species complexes (Supplementary Table S5).
For the genus as a whole, the results showed that sequence data for tef1 and tub2 had the strongest ability to correctly identify species (ISRs: 89.1 % and 88.3 %, respectively; Supplementary Table S5, Fig. 4). This was followed by cmdA, his3, rpb2 and act gene regions which also provided relatively effective resolution of the Calonectria spp. (Supplementary Table S5, Fig. 4). The efficacy of the ITS and LSU regions to identify species was relatively low (ISRs: 29.3 % and 27.6 %, respectively; Supplementary Table S5, Fig. 4).
Fig. 4.
The identification success rates of the eight candidate DNA barcodes for the genus Calonectria.
For the 11 species complexes, the results showed that the most effective barcodes could not be employed across all species complexes. The tef1 sequences provided resolution power in the Ca. candelabrum and Ca. cylindrospora species complexes (Supplementary Table S5, Fig. 5). In contrast, tub2 was the best DNA barcode for the Ca. brassicae and Ca. reteaudii species complexes (Supplementary Table S5, Fig. 5), act and tub2 both the best for the Ca. mexicana species complex. The frequency of successful identification using the eight gene region data in the 11 species complexes also differed. Collectively, the six regions (tef1, tub2, cmdA, his3, rpb2 and act) provided stable and reliable resolution power for species in each of the 11 species complexes. Our results suggested that all of the 120 Calonectria species accepted in this study could be distinguished based on the six-region (tef1, tub2, cmdA, his3, rpb2, and act) combined phylogeny, and 118 of the 120 (98 %) Calonectria species could be recognised based on the tef1 and tub2 gene region combined phylogeny.
Fig. 5.
The identification success rates of the eight candidate DNA barcodes for each of the 11 species complex; “∗” above the histogram indicate four of the barcodes with highest identification success rates within each of the 11 species complexes; Triangle indicate the identification success rate of the candidate DNA barcodes is zero.
Discussion
This study represents the most comprehensive molecular phylogenetic overview of Calonectria, a genus that includes many important plant pathogens. Sequence data for eight gene regions of 240 isolates representing 128 species were newly generated. In the case of 44 species for which cultures could not be obtained, sequence data were sourced from GenBank so as to consider all 169 previously described species. Phylogenetic analyses were conducted for each of the eight gene regions individually as well as for a combined dataset. The results provided clear evidence that 51 species are indistinguishable from previously described taxa and synonymies have been provided for these. Two novel taxa were recognised and described as Ca. lombardiana and Ca. pauciphialidica. Furthermore, the name Ca. lauri was validated. The overall result of the study is the acceptance of 120 clearly defined species of Calonectria. The eight sequenced gene regions were then evaluated individually to identify the most useful DNA barcodes to accurately identify these species. These analyses showed that six gene regions (tef1, tub2, cmdA, his3, rpb2 and act) provide effective barcodes for the genus.
The combined eight-gene phylogenetic tree emerging from this study placed the 120 accepted Calonectria species in the two major groups; the Prolate Group and the Sphaero-Naviculate Group. This is consistent with the results of previous studies (Lombard et al. 2016, Jayawardena et al. 2019), showing a clear phylogenetic distinction between these two groups in Calonectria. The previously recognised 10 species complexes (Lombard et al. 2016) were also well resolved. Interestingly, the results of this study also revealed a new species complex, that we have defined as the Calonectria gracilipes species complex. Delimitation of this new species complex was supported by both phylogenetic placement and morphological characteristics.
Species in the Ca. brassicae species complex typically have clavate vesicles and 1-septate macroconidia, producing orange to red-brown perithecia, 8-spored asci and 1-septate ascospores. The species complex also includes both heterothallic and homothallic species, and these are primarily distributed in South America (Crous 2002, Crous et al. 2006, Lombard et al. 2009, 2010a, 2016, Alfenas et al. 2015, Marin-Felix et al. 2017, Li et al. 2020). In the present study, two species were reduced to synonymy based on phylogenetic inference; Ca. ecuadorensis reduced to synonymy with Ca. ecuadorae, and Ca. telluricola with Ca. paraensis. The description of Ca. pauciphialidica adds a novel species to this species complex, and it is closely related to, but morphologically distinct from Ca. ecuadorae. Sixteen species are now recognised in the Ca. brassicae species complex.
Species in the Ca. candelabrum species complex are characterised by ellipsoidal to obpyriform vesicles and 1-septate macroconidia, producing orange to red-brown perithecia, 8-spored asci and 1-septate ascospores. The species complex includes both heterothallic and homothallic species that are known from Africa, Asia, Europe, North and South America, Oceania (Viégas 1946, Schoch et al. 1999, Crous & Kang 2001, Crous 2002, Lombard et al. 2010a, b, 2015a, b, 2016, Alfenas et al. 2013a, 2015, Lopes et al. 2017, Li et al. 2020). The Ca. candelabrum species complex includes important plant pathogens some of which pose a serious threat to global forestry and agricultural industries (Schoch et al. 1999, Crous 2002, Lombard et al. 2010b, 2015a, Crous et al. 2013, Alfenas et al. 2015). In the present study, 10 species were reduced to synonymy based on phylogenetic inference, eight (Ca. cliffordiicola, Ca. ericae, Ca. machaerinae, Ca. mossambicensis, Ca. polizzii, Ca. seminaria, Ca. tetraramosa and Ca. zuluensis) of which were reduced to synonymy with Ca. pauciramosa. In addition, one species (Ca. pseudoscoparia) was reduced to synonymy with Ca. candelabrum, and one species (Ca. stipitata) with Ca. spathulata. Eighteen species are now included in the Ca. candelabrum species complex.
Species in the Ca. colhounii species complex typically have clavate vesicles and 3-septate macroconidia, producing yellow perithecia, which is considered as the most characteristic feature of the species complex. They include 4-spored asci and 3-septate ascospores and both heterothallic and homothallic species. Homothallism is the dominant mating system (Crous 2002, Lombard et al. 2010a, Chen et al. 2011, Li et al. 2017, 2020) and the majority of species have been collected from Asia (Peerally 1973, Crous et al. 1999, Crous 2002, Lombard et al. 2010a, 2016, Chen et al. 2011, Xu et al. 2012, Li et al. 2017, 2020, Liu & Chen 2017). In the present study, three species were reduced to synonymy based on phylogenetic inference. These include Ca. pseudocolhounii reduced to synonymy with Ca. eucalypti, Ca. nymphaeae was synonymised with Ca. fujianensis, and Ca. parva with Ca. macroconidialis. In addition, Ca. madagascariensis with typical clavate vesicles was shown to reside in the Ca. colhounii species complex, consistent with the findings of Lombard et al. (2010a). This result is in contrast to the phylogeny presented by Jayawardena et al. (2019) who suggested that the species formed a distinct lineage apart from species in the 11 species complexes. Based on our results, 11 species reside in the Ca. colhounii species complex, including Ca. madagascariensis.
In the Ca. cylindrospora species complex, species have variable vesicle shapes, including clavate, ellipsoidal, fusiform, obpyriform to sphaeropedunculate, and they typically produce 1-septate macroconidia, orange to red-brown perithecia, 8-spored asci and 1-septate ascospores. The species complex includes both heterothallic and homothallic species (Crous et al. 1993b, Schoch et al. 1999, Crous 2002, Li et al. 2020). Species in the Ca. cylindrospora species complex exhibit a broad geographic distribution including Africa, Asia, Europe, North and South America (Crous et al. 1993c, 2013, Crous 2002, Lombard et al. 2010a, b, c, 2015a, 2016, Alfenas et al. 2013b, 2015, Pham et al. 2019, Li et al. 2020). In the present study, eight species were reduced to synonymy based on phylogenetic inference including two species (Ca. hodgesii and Ca. pseudohodgesii) as synonyms of Ca. brasiliensis, two species (Ca. papillata and Ca. terrestris) as synonyms of Ca. cerciana, two species (Ca. foliicola and Ca. sulawesiensis) as synonyms of Ca. hawksworthii, one species (Ca. blephiliae) as a synonym of Ca. cylindrospora and one species (Ca. pseudocerciana) as a synonym of Ca. propaginicola. As a consequence, 12 species are now accepted in the Ca. cylindrospora species complex.
In the Ca. gracilipes species complex, species are characterised by narrowly clavate vesicles and multi-septate (> 3) macroconidia, producing orange to red-brown perithecia and 8-spored asci. Species in this species complex have a homothallic mating system (Crous et al. 1997a, El-Gholl et al. 1997, Crous 2002) and they have been reported from both North and South America (Figueiredo & Namekata 1967, Crous et al. 1997a, 2000, El-Gholl et al. 1997, Crous 2002). Five species are now included in the Ca. gracilipes species complex.
Species in the Ca. kyotensis species complex typically have sphaeropedunculate vesicles and 1-septate macroconidia, producing orange to red-brown perithecia, 8-spored asci and 1-septate ascospores. Both heterothallic and homothallic species are known in the species complex and a majority of the members have been described from Asia (Boedijn & Reitsma 1950, Tubaki 1958, Sobers & Seymour 1967, Gerlach 1968, Terashita 1968, Sobers 1969, Alfieri et al. 1982, Victor et al. 1997, Kang et al. 2001b, Crous 2002, Crous et al. 2004, Lombard et al. 2015a, 2016, Li et al. 2017, 2020, Liu & Chen 2017, Pham et al. 2019). Results of the present study have shown that 17 species in this complex are the same as earlier described taxa. Of these, nine species (Ca. arbusta, Ca. expansa, Ca. guangxiensis, Ca. hainanensis, Ca. magnispora, Ca. parakyotensis, Ca. pluriramosa, Ca. pseudokyotensis and Ca. sphaeropedunculata) were reduced to synonymy with Ca. aconidialis, three (Ca. floridana, Ca. pseudoturangicola and Ca. turangicola) with Ca. kyotensis, one (Ca. montana) with Ca. canadiana, one (Ca. multistipitata) with Ca. chinensis, one (Ca. vegrandis) with Ca. curvispora, one (Ca. indonesiana) with Ca. sumatrensis and one (Ca. pseudoyunnanensis) with Ca. yunnanensis. Consequently, 24 species are now accommodated in the Ca. kyotensis species complex, making it the largest Calonectria species complex.
Species in the Ca. mexicana species complex typically have ellipsoidal vesicles with a papillate apex and 1-septate macroconidia. The species complex includes both heterothallic and homothallic species, although the heterothallic mating system is dominant (El-Gholl et al. 1989, Schubert et al. 1989, Schoch et al. 1999, Crous 2002). Species in the Ca. mexicana complex have been collected from many continents, including Africa, Europe and North America (El-Gholl et al. 1989, Schubert et al. 1989, Schoch et al. 1999, Crous 2002, Lechat et al. 2010, Lombard et al. 2011, 2016, Li et al. 2020). In the present study, Ca. tunisiana was reduced to synonymy with Ca. pseudomexicana. Consequently, eight species are now accommodated in this species complex.
In the Ca. naviculata species complex, species typically have naviculate vesicles and 1-septate macroconidia. Species in the species complex have a heterothallic mating system (Crous et al. 1997a, 2002, Gehesquiere et al. 2015, Li et al. 2020) and they have been collected from Africa, Asia, Europe, North and South America and Oceania (Crous et al. 1997a, 2002, 2004, Crous 2002, Alfenas et al. 2015, Gehesquiere et al. 2015, Lombard et al. 2016, LeBlanc et al. 2019, Li et al. 2020). In the study of Jayawardena et al. (2019), Ca. pseudonaviculata formed a distinct lineage and did not reside in any of the previously defined species complexes. Results of the present study have shown that this species resides comfortably in the Ca. naviculata species complex, consistent with the results of Lombard et al. (2010a, 2016). Currently, six species are included in the Ca. naviculata species complex.
In the Ca. pteridis species complex, species typically have clavate or ovate vesicles and 1-septate macroconidia. Species have a heterothallic mating system (El-Gholl et al. 1993a, Li et al. 2020) and have commonly been collected in North and South America (Sobers 1968, Crous et al. 1993c, El-Gholl et al. 1993a, Leahy et al. 2000, Crous 2002, Alfenas et al. 2015, Lombard et al. 2016, Marin-Felix et al. 2017, Li et al. 2020). Results of the present study have shown that six species in this complex are synonyms of earlier described taxa. Three species (Ca. amazoniensis, Ca. longiramosa and Ca. tropicalis) have thus been reduced to synonymy with Ca. amazonica and three species (Ca. nemoralis, Ca. tereticornis and Ca. tucuruiensis) with Ca. ovata. Consequently, seven species are now accommodated in the Ca. pteridis species complex.
In the Ca. reteaudii species complex, species are characterised by having narrowly clavate vesicles, multi-septate (> 3) macroconidia and they produce orange to red-brown perithecia, 8-spored asci and multi-septate (> 3) ascospores. Both heterothallic and homothallic Calonectria species have been described in this species complex (Kang et al. 2001a, Crous 2002, Gadgil & Dick 2004, Lombard et al. 2010c, Chen et al. 2011, Li et al. 2020) and species have been reported in Asia and Oceania (Kang et al. 2001a, Crous 2002, Gadgil & Dick 2004, Crous et al. 2006, 2012, Lombard et al. 2010c, 2015a, 2016, Chen et al. 2011, Li et al. 2017, 2020, Pham et al. 2019). Four species in this species complex were reduced to synonymy, including two species (Ca. microconidialis and Ca. pentaseptata) with Ca. pseudoreteaudii, one species (Ca. terrae-reginae) with Ca. queenslandica and one species (Ca. baviensis) with Ca. reteaudii. The description of Ca. lombardiana adds a new member to the Ca. reteaudii species complex and its larger macroconidial dimensions easily distinguish it from Ca. queenslandica, its closest phylogenetic relative. Nine species are now accepted as residing in the Ca. reteaudii species complex.
Calonectria species in the Ca. reteaudii species complex incorporate many important causal agents of plant disease, resulting in mainly leaf blights on a wide range of hosts in several countries including Australia, China, India, Laos and Vietnam (Old et al. 2003, Chen et al. 2011, Pham et al. 2019). Calonectria pentaseptata, initially described from Vietnam, has been reported from the GuangDong, GuangXi and HaiNan provinces of China (Crous et al. 2012, Lombard et al. 2015a, Li et al. 2017), causing severe leaf blight diseases on Eucalyptus in plantations and nurseries. In the present study, Ca. pentaseptata and Ca. microconidialis (Crous et al. 2012, Lombard et al. 2015a) were reduced to synonymy with Ca. pseudoreteaudii. Calonectria pseudorateaudii was originally described by Lombard et al. (2010c) from stems of E. urophylla × E. grandis hybrid cuttings in GuangDong Province, China. The results of the present study suggest that this species has been in China at least for 10 yr and continues to pose a serious threat to the Eucalyptus industry.
In the Ca. spathiphylli species complex, species typically have sphaeropedunculate vesicles and 1-spetate macroconidia and produce orange to red-brown perithecia, 8-spored asci and 1-septate ascospores. Both heterothallic and homothallic Calonectria species have been described in this species complex (El-Gholl et al. 1992, Kang et al. 2001b) and species are commonly collected in North and South America (El-Gholl et al. 1992, Kang et al. 2001b, Crous 2002, Lombard et al. 2010a). Four species are now accommodated in the Ca. spathiphylli species complex.
Results of this study have revealed interesting patterns regarding the geographic distribution of various groups of Calonectria spp. For example, more than 75 % of the species in the Ca. brassicae and Ca. candelabrum species complexes have been described from South American countries such as Brazil, Colombia, Ecuador and Venezuela (Alfenas et al. 2013a, b, 2015). Likewise, species in the Ca. kyotensis species complex appear to represent an Asian assemblage, with 19 of the 24 species collected in Asian countries such as China, Indonesia, Japan, Malaysia and Vietnam (Crous 2002, Crous et al. 2004, Lombard et al. 2015a, Li et al. 2017, Liu & Chen 2017, Pham et al. 2019). Of those species, 14 were isolated from soil collected in Asia, suggesting this geographic region could be their centre of origin. There are, however, few studies that have considered the centres of origin of Calonectria species (Wright et al. 2010, Freitas et al. 2019, LeBlanc et al. 2019, Malapi-Wight et al. 2019), emphasizing a need for population biology studies on these fungi in the future.
The synonymised species in this study were generally from regions adjacent to those from which the validated taxa were originally collected. For example, Ca. cerciana, together with its now recognised synonyms Ca. papillata and Ca. terrestris were isolated from adjacent sites in GuangDong Province in southern China (Lombard et al. 2010c, 2015a). However, there were also some cases where species reduced to synonymy were from different continents where the climates differed significantly. For example, Ca. pauciramosa and the eight species (Ca. cliffordiicola, Ca. ericae, Ca. machaerinae, Ca. mossambicensis, Ca. polizzii, Ca. seminaria, Ca. tetraramosa and Ca. zuluensis) reduced to synonymy with it, thus were isolated from Africa, Asia, Europe and North America (Schoch et al. 1999, Crous & Kang 2001, Crous et al. 2013, Lombard et al. 2010b, 2015a, 2016, Alfenas et al. 2015). The present study further supports the fact that Ca. pauciramosa has a worldwide distribution and that it is able to exist under various climatic conditions (Schoch et al. 1999, Crous 2002, Lombard et al. 2010b, 2015a, 2016, Crous et al. 2013).
Results of this study showed that six loci (act, cmdA, his3, rpb2, tef1 and tub2) can serve as reliable DNA barcodes for species delimitation in Calonectria. A similar six gene approach has been established for Colletotrichum, where the act, chs-1, gapdh, his3, ITS and tub2 regions have been employed to reveal the phylogenetic relationships of species with curved conidia from herbaceous hosts (Damm et al. 2009). Likewise, six markers (act, cmdA, gapdh, gs, ITS and tub2) were utilised to characterise the species of Colletotrichum associated with coffee berries in northern Thailand (Prihastuti et al. 2009). A six gene (act, ITS, LSU, rpb1, tef1 and tub2) approach has also been used for the phylogenetic analyses of Allantonectria, Nectria, and Pleonectria (Hirooka et al. 2012). The present study has revealed that optimal barcodes for taxon delimitation differs for the various Calonectria species complexes. This is similar to the case in Fusarium, where five barcodes (cmdA, IGS, rpb2, tef1 and tub2) were applied to stabilise the taxonomic position of species in the Fusarium oxysporum species complex (Lombard et al. 2019). Similarly, Wang et al. (2019) utilised five loci (cmdA, ITS, rpb1, rpb2 and tef1) to evaluate and clarify the phylogenetic relationships of the F. incarnatum-equiseti species complex. Although the ideal set of barcodes differed among the various species complexes in Calonectria, six loci (act, cmdA, his3, rpb2, tef1 and tub2) served as the most phylogenetically informative markers. These are consequently recommended as the universal DNA barcodes for species delimitation in Calonectria.
Multi-gene phylogenetic analyses combined with morphological features provides a robust means to delimit fungal species boundaries (James et al. 2006, Hibbett et al. 2007, Liu et al. 2015, Chen et al. 2017, Woudenberg et al. 2017). This polyphasic approach has been widely employed in the taxonomy of Calonectria species (Lombard et al. 2010a, Chen et al. 2011, Crous et al. 2012, Alfenas et al. 2015, Lombard et al. 2016, Marin-Felix et al. 2017, Pham et al. 2019). However, a problem pertaining to the taxonomy of this group is that studies have used different markers and gene regions to identify species. This lack of consistency has resulted in confusion, and the many synonyms identified in the present study also arise, at least to some extent, from an inconsistency of taxonomic approaches.
In an attempt to provide a unified taxonomic system for Calonectria, we have evaluated the sequence data for eight gene regions that are available for all 120 species. These results showed that sequences of tef1 and tub2 provide markers that can recognise the majority of Calonectria species. The tef1 and tub2 combined phylogeny made it possible to recognise 118 of the 120 (98 %) species, indicating that combinations of tef1 and tub2 gene sequences are suitable for preliminary identification of Calonectria species. Results of this study indicated that the tef1 region provided the best barcode, which is in contrast to the study of Lombard et al. (2010a), who suggested that the cmdA region provides the best signal for species recognition in these fungi. Results of the present study also indicated that the rpb2 region provides stable and reliable taxonomic signal for Calonectria identification and this is the first study where that gene region has been sequenced for a large collection of species in this genus.
Overall, the results of this study have provided a robust overview of the species boundaries in Calonectria. This is based largely on phylogenetic inference using DNA sequences for eight gene regions. It is evident that, over a period of approximately 10 yr (Lombard et al. 2010a, 2015a, 2016, Li et al. 2017, Marin-Felix et al. 2017, Pham et al. 2019), numerous species have been described in Calonectria that are synonyms of existing species. This is a common problem in many groups of fungi where species were described based on relatively limited DNA sequence data. As time has passed, new and more informative gene regions have become available to define phylogenetic species boundaries more clearly. The DNA sequence backbone emerging and reliable DNA barcodes resulting from the present study provides a robust foundation for future taxonomic studies on the group. It is recognised that in many cases, species identity in this study is based on only a single or a small number of isolates. Clearly, larger collections of isolates will help to further confirm species boundaries and provide a better understanding of species delineation. However, the framework presented here will provide an ideal basis on which to explore this further. Furthermore, the genomes of numerous Calonectria spp. have now been sequenced (Malapi-Wight et al. 2016a, b, Ye et al. 2018, Liu et al. 2019). In time, it is likely that phylogenomic studies will increase the clarity of species boundaries and provide even more robust DNA barcodes for Calonectria.
Acknowledgements
This study was provided with financial support by the National Key R&D Program of China, China (project no. 2017YFD0600103), the Fundamental Research Funds for the Central Non-Profit Research Institution of CAF, China (Project No. CAFYBB2018QC003), the National Natural Science Foundation of China, China (NSFC) (project no. 31622019), the National Ten-thousand Talents Program, China (project No. W03070115) and the GuangDong Top Young Talents Program, China (project No. 20171172).
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.simyco.2020.08.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alfenas R.F., Lombard L., Pereira O.L., et al. Diversity and potential impact of Calonectria species in Eucalyptus plantations in Brazil. Studies in Mycology. 2015;80:89–130. doi: 10.1016/j.simyco.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfenas R.F., Pereira O.L., Ferreira M.A., et al. Calonectria metrosideri, a highly aggressive pathogen causing leaf blight, root rot, and wilt of Metrosideros spp. in Brazil. Forest Pathology. 2013;43:257–265. [Google Scholar]
- Alfenas R.F., Pereira O.L., Jorge V.L., et al. A new species of Calonectria causing leaf blight and cutting rot of three forest tree species in Brazil. Tropical Plant Pathology. 2013;38:513–521. [Google Scholar]
- Alfieri S.A., El-Gholl N.E., Schoulties C.L. Homothallism in Calonectria ilicicola. Mycologia. 1982;74:513–514. [Google Scholar]
- Batista A.C. Cylindrocladium scoparium Morgan var. brasiliensis Batista & Ciferri, a new fungus on Eucalyptus. Boletim da Secretaria de agricultura, industria e comercio do Estado de Pernambuco. 1951;18:188–191. [Google Scholar]
- Boedijn K.B., Reitsma J. Notes on the genus Cylindrocladium (Fungi: Mucedineae) Reinwardtia. 1950;1:51–60. [Google Scholar]
- Brand T. Auftreten von Cylindrocladium buxicola B. henricot an Buchsbaum in Nordwest-Deutschland. Nachrichtenblatt des deutschen Pflanzenschutzdienstes. 2005;57:237–240. [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Chen Q., Hou L.W., Duan W.J., et al. Didymellaceae revisited. Studies in Mycology. 2017;87:105–159. doi: 10.1016/j.simyco.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.F., Lombard L., Roux J., et al. Novel species of Calonectria associated with Eucalyptus leaf blight in Southeast China. Persoonia. 2011;26:1–12. doi: 10.3767/003158511X555236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W. APS Press; St. Paul, Minnesota, USA: 2002. Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. [Google Scholar]
- Crous P.W., Alfenas A.C., Wingfield M.J. Calonectria scoparia and Calonectria morganii sp. nov., and variation among isolates of their Cylindrocladium anamorphs. Mycological Research. 1993;97:701–708. [Google Scholar]
- Crous P.W., Groenewald J.Z., Hill C.F. Cylindrocladium pseudonaviculatum sp. nov. from New Zealand, and new Cylindrocladium records from Vietnam. Sydowia. 2002;54:23–34. [Google Scholar]
- Crous P.W., Groenewald J.Z., Risède J.M., et al. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology. 2004;50:415–430. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z., Risède J.M., et al. Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology. 2006;55:213–226. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Janse B.J.H., Victor D., et al. Characterization of some Cylindrocladium species with three-septate conidia using morphology, isozyme banding patterns and DNA polymorphisms. Systematic and Applied Microbiology. 1993;16:266–273. [Google Scholar]
- Crous P.W., Kang J.C. Phylogenetic confirmation of Calonectria spathulata and Cylindrocladium leucothoes based on morphology, and sequence data of the β-tubulin and ITS rRNA genes. Mycoscience. 2001;42:51–57. [Google Scholar]
- Crous P.W., Kang J.C., Schoch C.L., et al. Phylogenetic relationships of Cylindrocladium pseudogracile and Cylindrocladium rumohrae with morphologically similar taxa, based on morphology and DNA sequences of internal transcribed spacers and β-tubulin. Canadian Journal of Botany. 1999;77:1813–1820. [Google Scholar]
- Crous P.W., Korf A., Zyl vanWH. Nuclear DNA polymorphisms of Cylindrocladium species with 1-septate conidia and clavate vesicles. Systematic and Applied Microbiology. 1995;18:244–250. [Google Scholar]
- Crous P.W., Mchau G.R., Zyl W.H.V., et al. New species of Calonectria and Cylindrocladium isolated from soil in the tropics. Mycologia. 1997;89:653–660. [Google Scholar]
- Crous P.W., Schoch C.L., El-Gholl N., et al. Cylindrocladium angustatum sp. nov., a new leaf spot pathogen of Tillandsia capitata from Florida, USA. Mycoscience. 2000;41:521–526. [Google Scholar]
- Crous P.W., Shivas R.G., Wingfield M.J., et al. Fungal Planet description sheets: 128–153. Persoonia. 2012;29:146–201. doi: 10.3767/003158512X661589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Theron L., Van Zyl W.H. Delineation of Cylindrocladium species with 1-3-septate conidia and clavate vesicles based on morphology and rDNA RFLPs. Mycological Research. 1997;101:210–214. [Google Scholar]
- Crous P.W., Wingfield M.J., Alfenas A.C. Additions to Calonectria. Mycotaxon. 1993;46:217–234. [Google Scholar]
- Crous P.W., Wingfield M.J., Alfenas A.C., et al. Cylindrocladium naviculatum sp. nov., and two new vesiculate hyphomycete genera, Falcocladium and Vesicladiella. Mycotaxon. 1994;50:441–458. [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J., et al. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Mohammed C., et al. New foliar pathogens of Eucalyptus from Australia and Indonesia. Mycological Research. 1998;102:527–532. [Google Scholar]
- Crous P.W., Wingfield M.J., Roux J.J., et al. Fungal Planet description sheets: 371–399. Persoonia. 2015;35:264–327. doi: 10.3767/003158515X690269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C.W. Can three incongruence tests predict when data should be combined? Molecular Biology and Evolution. 1997;14:733–740. doi: 10.1093/oxfordjournals.molbev.a025813. [DOI] [PubMed] [Google Scholar]
- Damm U., Woudenberg J.H.C., Cannon P.F., et al. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Diversity. 2009;39:45–87. [Google Scholar]
- Daughtrey M.L. Boxwood blight: threat to ornamentals. Annual Review of Phytopathology. 2019;57:189–209. doi: 10.1146/annurev-phyto-082718-100156. [DOI] [PubMed] [Google Scholar]
- De Hoog G., Van den Ende A.H.G.G. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Decock C., Crous P.W. Curvicladium gen. nov., a new hyphomycete genus from French Guiana. Mycologia. 1998;90:276–281. [Google Scholar]
- El-Gholl N.E., Alfenas A.C., Crous P.W., et al. Description and pathogenicity of Cylindrocladium ovatum sp. nov. Canadian Journal of Botany. 1993;71:466–470. [Google Scholar]
- El-Gholl N.E., Alfenas A.C., Junghans D.T., et al. Description of Calonectria rumohrae sp. nov. (anamorph = Cylindrocladium rumohrae sp. nov.) Mycotaxon. 1997;64:467–484. [Google Scholar]
- El-Gholl N.E., Alfieri S.A., Jr., Barnard E.L. Description and pathogenicity of Calonectria clavata sp. nov. Mycotaxon. 1993;48:201–216. [Google Scholar]
- El-Gholl N.E., Kimbrough J.W., Barnard E.L., et al. Calonectria spathulata sp. nov. Mycotaxon. 1986;XXVI:151–164. [Google Scholar]
- El-Gholl N.E., Leahy R.M., Schubert T.S. Cylindrocladium leucothoeae sp. nov. Canadian Journal of Botany. 1989;67:2529–2532. [Google Scholar]
- El-Gholl N.E., Uchida J.Y., Alfenas A.C., et al. Induction and description of perithecia of Calonectria spathiphylli sp. nov. Mycotaxon. 1992;45:285–300. [Google Scholar]
- Farris J.S., Kallersjo M., Kluge A.G., et al. Testing significance of incongruence. Cladistics. 1994;10:315–319. [Google Scholar]
- Fawcett H.S., Klotz L.J. A new species of Candelospora causing decay of citrus fruits. Mycologia. 1937;29:207–215. [Google Scholar]
- Felsenstein J. Confidence intervals on phylogenetics: an approach using bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Figueiredo M.B., Namekata T. Record on the occurrence of Calonectria quinqueseptata n. sp., the perfect form of Cylindrocladium quinqueseptatum, on Annona squamosa and Eucalyptus sp. Archivos do Instituto Biologico, Sao Paulo. 1967;34:91–96. [Google Scholar]
- Freitas R.G., Alfenas R.F., Guimaraes L.M.S., et al. Genetic diversity and aggressiveness of Calonectria pteridis in Eucalyptus spp. Plant Pathology. 2019;68:869–877. [Google Scholar]
- Gadgil P., Dick M. Fungi silvicolae novazelandiae: 10. New Zealand Journal of Forestry Science. 2004;44:1–7. [Google Scholar]
- Gai Y.P., Deng Q.W., Chen X.L., et al. Phylogenetic diversity of Calonectria ilicicola causing Cylindrocladium black rot of peanut and red crown rot of soybean in southern China. Journal of General Plant Pathology. 2017;83:273–282. [Google Scholar]
- Gehesquiere B., Crouch J.A., Marra R.E., et al. Characterization and taxonomic reassessment of the box blight pathogen Calonectria pseudonaviculata, introducing Calonectria henricotiae sp. nov. Plant Pathology. 2015;65:37–52. [Google Scholar]
- Gerlach V.W. Calonectria uniseptata n. sp. die bisher unbekannte hauptfruchtform von Cylindrocladium scoparium Morgan. Journal of Phytopathology. 1968;61:372–381. [Google Scholar]
- Gill D.L., Alfieri S.A., Sobers E.K. A new disease of Ilex spp. caused by Cylindrocladium avesiculatum sp. nov. Phytopathology. 1971;61:58–60. [Google Scholar]
- Guindon S., Gascuel O. A simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Systematic Biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Henricot B., Culham A. Cylindrocladium buxicola, a new species affecting Buxus spp., and its phylogenetic status. Mycologia. 2002;94:980–997. [PubMed] [Google Scholar]
- Hibbett D.S., Binder M., Bischoff J.F., et al. A higher-level phylogenetic classification of the Fungi. Mycological Research. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Hirooka Y., Rossman A.Y., Samuels G.J., et al. A monograph of Allantonectria, Nectria, and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Studies in Mycology. 2012;71:1–210. doi: 10.3114/sim0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T.Y., Kauff F., Schoch C.L., et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- Jayawardena R.S., Hyde K.D., Jeewon R., et al. One stop shop II: taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50 (2019) Fungal Diversity. 2019;94:41–129. [Google Scholar]
- Jiang G., Gao F., Yue H., et al. First report of a fruit spot of Macadamia caused by Calonectria pentaseptata in Yunnan, China. Plant Disease. 2019;104:575. [Google Scholar]
- Kang J.C., Crous P.W., Old K.M., et al. Non-conspecificity of Cylindrocladium quinqueseptatum and Calonectria quinqueseptata based on a β-tubulin gene phylogeny and morphology. Canadian Journal of Botany. 2001;79:1241–1247. [Google Scholar]
- Kang J.C., Crous P.W., Schoch C.L. Species concepts in the Cylindrocladium floridanum and Cy. spathiphylli complexes (Hypocreaceae) based on multi-allelic sequence data, sexual compatibility and morphology. Systematic and Applied Microbiology. 2001;24:206–217. doi: 10.1078/0723-2020-00026. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczewski N., Plewa D., Kangas C., et al. First report of red crown rot of Soybeans caused by Calonectria ilicicola (Anamorph: Cylindrocladium parasiticum) in Illinois. Plant Disease. 2019;103:1777. [Google Scholar]
- Koike S.T., Henderson D.M., Crous P.W., et al. A new root and crown rot disease of heath in California caused by Cylindrocladium pauciramosum. Plant Disease. 1999;83:589. doi: 10.1094/PDIS.1999.83.6.589D. [DOI] [PubMed] [Google Scholar]
- Leahy R.M., Schubert T.S., El-Gholl N.E. Cylindrocladium gordoniae sp. nov. Mycotaxon. 2000;76:77–83. [Google Scholar]
- LeBlanc N., Gehesquière B., Salgado-Salazar C., et al. Limited genetic diversity across pathogen populations responsible for the global emergence of boxwood blight identified using SSRs. Plant Pathology. 2019;68:861–868. [Google Scholar]
- Lechat C., Crous P.W., Groenewald J.Z. The enigma of Calonectria species occurring on leaves of Ilex aquifolium in Europe. IMA Fungus. 2010;1:101–108. doi: 10.5598/imafungus.2010.01.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Q., Wingfield B.D., Wingfield M.J., et al. Mating genes in Calonectria and evidence for a heterothallic ancestral state. Persoonia. 2020;45:163–176. doi: 10.3767/persoonia.2020.45.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Q., Wingfield M.J., Liu Q.L., et al. Calonectria species isolated from Eucalyptus plantations and nurseries in South China. IMA Fungus. 2017;8:259–294. doi: 10.5598/imafungus.2017.08.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.F., Chen S.F., Ferreira M.A., et al. Draft genome sequences of five Calonectria species from Eucalyptus plantations in China, Celoporthe dispersa, Sporothrix phasma and Alectoria sarmentosa. IMA Fungus. 2019;10:1–13. doi: 10.1186/s43008-019-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.L., Chen S.F. Two novel species of Calonectria isolated from soil in a natural forest in China. MycoKeys. 2017;26:25–60. [Google Scholar]
- Liu X.Z., Wang Q.M., Göker M., et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Studies in Mycology. 2015;81:85–147. doi: 10.1016/j.simyco.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Lombard L., Chen S.F., Mou X., et al. New species, hyper-diversity and potential importance of Calonectria spp. from Eucalyptus in South China. Studies in Mycology. 2015;80:151–188. doi: 10.1016/j.simyco.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D., et al. Phylogeny and systematics of the genus Calonectria. Studies in Mycology. 2010;66:31–69. doi: 10.3114/sim.2010.66.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D., et al. Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Studies in Mycology. 2010;66:15–30. doi: 10.3114/sim.2010.66.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Merwe van der N.A., Groenewald J.Z., et al. Generic concepts in Nectriaceae. Studies in Mycology. 2015;80:189–245. doi: 10.1016/j.simyco.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Polizzi G., Guarnaccia V., et al. Calonectria spp. causing leaf spot, crown and root rot of ornamental plants in Tunisia. Persoonia. 2011;27:73–79. doi: 10.3767/003158511X615086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Rodas C.A., Crous P.W., et al. Calonectria (Cylindrocladium) species associated with dying Pinus cuttings. Persoonia. 2009;23:41–47. doi: 10.3767/003158509X471052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Sandoval-Denis M., Lamprecht S.C., et al. Epitypification of Fusarium oxysporum–clearing the taxonomic chaos. Persoonia. 2019;43:1–47. doi: 10.3767/persoonia.2019.43.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Wingfield M.J., Alfenas A.C., et al. The forgotten Calonectria collection: pouring old wine into new bags. Studies in Mycology. 2016;85:159–198. doi: 10.1016/j.simyco.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Zhou X.D., Crous P.W., et al. Calonectria species associated with cutting rot of Eucalyptus. Persoonia. 2010;24:1–11. doi: 10.3767/003158510X486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes U.P., Alfenas R.F., Zambolim L., et al. A new species of Calonectria causing rot on ripe strawberry fruit in Brazil. Australasian Plant Pathology. 2017;47:1–11. [Google Scholar]
- Malapi-Wight M., Demers J.E., Veltri D., et al. LAMP detection assays for boxwood blight pathogens: a comparative genomics approach. Scientific Reports. 2016;6:26140. doi: 10.1038/srep26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapi-Wight M., Salgado-Salazar C., Demers J.E., et al. Sarcococca blight: use of whole-genome sequencing for fungal plant disease diagnosis. Plant Disease. 2016;100:1093–1100. doi: 10.1094/PDIS-10-15-1159-RE. [DOI] [PubMed] [Google Scholar]
- Malapi-Wight M., Veltri D., Gehesquière B., et al. Global distribution of mating types shows limited opportunities for mating across populations of fungi causing boxwood blight disease. Fungal Genetics and Biology. 2019;27:103246. doi: 10.1016/j.fgb.2019.103246. [DOI] [PubMed] [Google Scholar]
- Marin-Felix Y., Groenewald J.Z., Cai L., et al. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology. 2017;86:99–216. doi: 10.1016/j.simyco.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryani N., Sandoval-Denis M., Lombard L., et al. New endemic Fusarium species hitch-hiking with pathogenic Fusarium strains causing Panama disease in small-holder banana plots in Indonesia. Persoonia. 2019;43:48–69. doi: 10.3767/persoonia.2019.43.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncalvo J.M., Wang H.H., Hseu R.S. Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacers and 25S ribosomal DNA sequences. Mycologia. 1995;87:223–238. [Google Scholar]
- Nirenburg H.I. A simplified method for identifying Fusarium spp. occurring on wheat. Canadian Journal of Botany. 1981;59:1599–1609. [Google Scholar]
- O’Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- O’Donnell K., Kistler H.C., Cigelnik E., et al. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the USA. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old K.M., Wingfield M.J., Yuan Z.Q. Center for International Forestry Research; Jakarta, Indonesia: 2003. A manual of diseases of Eucalyptus in South-East Asia. [Google Scholar]
- Peerally M.A. Calonectria colhounii sp. nov., a common parasite of tea in Mauritius. Transactions of the British Mycological Society. 1973;61:89–93. [Google Scholar]
- Pham N.Q., Barnes I., Chen S.F., et al. Ten new species of Calonectria from Indonesia and Vietnam. Mycologia. 2019;111:78–102. doi: 10.1080/00275514.2018.1522179. [DOI] [PubMed] [Google Scholar]
- Polizzi G., Crous P.W. Root and collar rot of milkwort caused by Cylindrocladium pauciramosum, a new record for Europe. European Journal of Plant Pathology. 1999;105:407–411. [Google Scholar]
- Posada D. JModelTest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Prihastuti H., Cai L., Chen H., et al. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity. 2009;39:89–109. [Google Scholar]
- Quaedvlieg W., Kema G.H.J., Groenewald J.Z., et al. Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia. 2011;26:57–69. doi: 10.3767/003158511X571841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute and British Mycological Society; Kew, Surrey, UK: 1970. A mycological colour chart. [Google Scholar]
- Reeb V., Lutzoni F., Roux C., et al. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution. 2004;32:1036–1060. doi: 10.1016/j.ympev.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Rodas C.A., Lombard L., Gryzenhoinf M., et al. Cylindrocladium blight of Eucalyptus grandis in Colombia. Australasian Plant Pathology. 2005;34:143–149. [Google Scholar]
- Sandoval-Denis M., Lombard L., Crous P.W. Back to the roots: a reappraisal of Neocosmospora. Persoonia. 2019;43:90–185. doi: 10.3767/persoonia.2019.43.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Crous P.W., Wingfield B.D., et al. The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia. 1999;91:286–298. [Google Scholar]
- Schoch C.L., Seifert K.A., Huhndorf S., et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoulties C.L., El-Gholl N.E., Alfieri JrSA. Cylindrocladium spathiphylli sp. nov. Mycotaxon. 1982;16:265–272. [Google Scholar]
- Schubert T.S., El-Gholl N.E., Alfieri JrSA., et al. Calonectria avesiculata sp. nov. Canadian Journal of Botany. 1989;67:2414–2419. [Google Scholar]
- Sherbakoff C.D. Washington palm leaf spot due to Cylindrocladium macrosporum n. sp. Phytopathology. 1928;18:219–225. [Google Scholar]
- Sobers E.K. Morphology and host range of Cylindrocladium pteridis. Phytopathology. 1968;58:1265–1270. [Google Scholar]
- Sobers E.K. Calonectria floridana sp. nov., the perfect stage of Cylindrocladium floridanum. Phytopathology. 1969;59:364–366. [Google Scholar]
- Sobers E.K. Morphology and pathogenicity of Calonectria floridana, Calonectria kyotensis and Calonectria uniseptata. Phytopathology. 1972;62:485–487. [Google Scholar]
- Sobers E.K., Seymour C.P. Cylindrocladium floridanum sp. n. associated with decline of peach trees in Florida. Phytopathology. 1967;57:389–393. [Google Scholar]
- Stielow J.B., Levesque C.A., Seifert K.A., et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia. 2015;35:242–263. doi: 10.3767/003158515X689135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts, USA: 2003. PAUP∗. Phylogenetic Analysis Using Parsimony (∗and other methods) [Google Scholar]
- Tamura K., Stecher G., Peterson D., et al. MEGA6: Molecular evolutionary genetics analysis v. 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashita T. A new species of Calonectria and its conidial state. Transactions of the Mycological Society of Japan. 1968;8:124–129. [Google Scholar]
- Tubaki K. Studies on the Japanese Hyphomycetes. V. Leaf & stem group with a discussion of the classification of Hyphomycetes and their perfect stages. The Journal of the Hattori Botanical Laboratory. 1958;20:142–244. [Google Scholar]
- Van Burik J.A.H., Schreckhise R.W., White T.C., et al. Comparison of six extraction techniques for isolation of DNA from filamentous fungi. Medical Mycology. 1998;36:299–303. [PubMed] [Google Scholar]
- Victor D., Crous P.W., Janse B.J.H., et al. Genetic variation in Cylindrocladium floridanum and other morphologically similar Cylindrocladium species. Systematic and Applied Microbiology. 1997;20:268–285. [Google Scholar]
- Viégas A.P. Alguns fungos do Brasil: XIII - Hifomicetos. Bragantia. 1946;6:353–442. [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D., Groenewald M., De Vries M., et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology. 2019;92:135–154. doi: 10.1016/j.simyco.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.M., Chen Q., Diao Y.Z., et al. Fusarium incarnatum-equiseti complex from China. Persoonia. 2019;43:70–89. doi: 10.3767/persoonia.2019.43.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.J., Bruns T., Lee S., et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications. 1990;18:315–322. [Google Scholar]
- Wolf F.A. Brown leaf spot of leather leaf fern. Journal of the Elisha Mitchell Scientific Society. 1926;42:55–62. [Google Scholar]
- Woudenberg J.H.C., Hanse B., Van Leeuwen G.C.M., et al. Stemphylium revisited. Studies in Mycology. 2017;87:77–103. doi: 10.1016/j.simyco.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L.P., Davis A.J., Wingfield B.D., et al. Population structure of Cylindrocladium parasiticum infecting peanuts (Arachis hypogaea) in Georgia, USA. European Journal of Plant Pathology. 2010;127:199–206. [Google Scholar]
- Xia J.W., Sandoval-Denis M., Crous P.W., et al. Numbers to names–restyling the Fusarium incarnatum-equiseti species complex. Persoonia. 2019;43:186–221. doi: 10.3767/persoonia.2019.43.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.J., Qin S.Y., Hao Y.Y., et al. A new species of Calonectria causing leaf disease of water lily in China. Mycotaxon. 2012;122:177–185. [Google Scholar]
- Yamamoto R., Nakagawa A., Shimada S., et al. Histopathology of red crown rot of soybean. Journal of General Plant Pathology. 2017;83:23–32. [Google Scholar]
- Ye X., Zhong Z., Liu H., et al. Whole genome and transcriptome analysis reveal adaptive strategies and pathogenesis of Calonectria pseudoreteaudii to Eucalyptus. BMC Genomics. 2018;19:358. doi: 10.1186/s12864-018-4739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.