Abstract
Background
The renin‐angiotensin‐aldosterone system (RAAS), when chronically activated, is harmful and RAAS‐suppressive drugs are beneficial in the treatment of congestive heart failure (CHF). Mineralocorticoid receptor antagonists are widely used in the treatment of CHF in people.
Hypothesis/Objectives
To determine if a mineralocorticoid receptor antagonist (spironolactone) is beneficial and safe in CHF due to myxomatous mitral valve disease (MMVD) of varying severity, we hypothesized that, when combined with furosemide, a combination product (S+BNZ) containing the ACE inhibitor (ACE‐I), benazepril, and spironolactone, would be superior to benazepril alone.
Animals
Five hundred and sixty‐nine client‐owned dogs, with MMVD and CHF (ACVIM Stage C) of ≤10‐days' duration.
Methods
After initial stabilization, dogs were randomized into a positive‐controlled, double‐blind, multicenter trial, to receive furosemide plus S+BNZ or furosemide plus benazepril. The primary outcome variable was the percentage of dogs reaching cardiac endpoint before Day 360. Cardiac endpoint was defined as cardiac death or euthanasia, recurrence of pulmonary edema, necessity for nonauthorized cardiac drug(s) or a furosemide dosage >8 mg/kg/d.
Results
A significantly lower percentage of dogs treated with S+BNZ reached the primary outcome variable by Day 360 (OR = 0.56; 95% CI, 0.32‐0.98; P = .04) and risk of dying or worsening from cardiac causes, was significantly reduced (HR = 0.73; 95% CI = 0.59‐0.89, P = .002) vs benazepril alone. Adverse events, potentially associated with treatment, were rare and equal between groups.
Conclusion and Clinical Importance
The combination of S+BNZ is effective, safe, and superior to benazepril alone, when used with furosemide for the management of mild, moderate or severe CHF caused by MMVD in dogs.
Keywords: ACE inhibitor, aldosterone breakthrough, mitral regurgitation, MRA, RAAS
Abbreviations
- ABT
aldosterone breakthrough
- ACE
angiotensin converting‐enzyme
- ACE‐I
ACE inhibitor
- ACVIM
American College of Veterinary Internal Medicine
- AE
adverse event
- Ang II
angiotensin II
- ARB
angiotensin II receptor blocker
- ARNI
angiotensin receptor neprilysin inhibitor
- BCS
body condition score
- BESST
BEnazepril Spironolactone STudy
- BNZ
benazepril
- BP
blood pressure
- BUN
blood urea nitrogen
- CHF
congestive heart failure
- FDA‐CVM
Food & Drug Administration‐Center for Veterinary Medicine
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- MMVD
myxomatous mitral valve disease
- MRA
mineralocorticoid receptor antagonist
- No
Number
- RAAS
renin angiotensin aldosterone system
- S+BNZ
combination of spironolactone and benazepril, CARDALIS®
- SAE
serious adverse event
- Sample
cohort or subpopulation of dogs studied
- SCD
sudden cardiac death
- sCr
serum creatinine
1. INTRODUCTION
The renin‐angiotensin‐aldosterone system (RAAS), when chronically activated, is harmful to multiple organ systems, promoting cardiac deterioration and failure. 1 , 2 Current treatment of cardiovascular disease relies heavily upon RAAS‐suppressive drugs, which include angiotensin converting enzyme inhibitors (ACE‐I), angiotensin II receptor blockers (ARB), and mineralocorticoid receptor antagonists (MRA). 1
There are 4 ACE‐I (enalapril, benazepril, imidapril, and ramipril) approved and marketed internationally for veterinary use, although none are currently marketed in the United States. The ARBs have gained acceptance for use in human cardiovascular disease, but no ARB is currently FDA‐approved for congestive heart failure (CHF) treatment in animals in the United States or globally.
The MRA class of RAAS inhibitors became a cornerstone in human CHF management because of recognition of aldosterone breakthrough (ABT) 2 and the demonstration of beneficial effects from the addition of spironolactone to standard CHF therapy in the landmark RALES study. 3 This, and subsequent studies, propelled the MRA (spironolactone and eplerenone) to wide acceptance as an adjunctive therapy along with ACE‐I, ARB, beta‐blockers, and angiotensin receptor‐neprilysin inhibitor (ARNI) in human CHF. 4 , 5 , 6 , 7 In a randomized clinical trial, the ACE‐I, benazepril, and the MRA, spironolactone, plus standard therapy provided a survival benefit, compared to a similar protocol without spironolactone in dogs with relatively mild CHF due to myxomatous mitral valve disease (MMVD). 8
Subsequently, we have gained a better understanding of the RAAS; the pharmaceutical causes and frequency of ABT in ACVIM Stages B2, C, and D MMVD; the early onset of ABT in dogs being treated with loop diuretics; and the difficulties and cost in demonstrating ABT in individual dogs. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 This understanding has moved some to advocate that an MRA should universally accompany other RAAS‐suppressant therapies. 17 , 18 A once‐daily, safe, and effective, multimodal RAAS suppressant is an attractive for management of canine cardiac disease. While there are no US‐licensed ACE‐I, MRA, or ARB marketed for use in dogs, the 2019 ACVIM MMVD Consensus Committee advocates spironolactone with an ACE‐I for treatment of chronic Stages C and D CHF, due to MMVD. 19
We hypothesized that the combination of spironolactone and benazepril would be superior in terms of clinical efficacy to benazepril when each was combined with furosemide and other measures in managing CHF in dogs with MMVD.
2. MATERIALS AND METHODS
2.1. Trial design
The BEnazepril Spironolactone STudy (BESST) was a multicenter, double‐blind, randomized, Good Clinical Practice clinical trial, with superiority analysis, comparing benazepril and spironolactone (CARDALIS®, Ceva Santé Animale, Libourne, France) vs benazepril hydrochloride (Fortekor, Elanco, Cuxhaven, Germany) in the management of CHF in dogs with MMVD. The North Carolina State University Institutional Animal Care and Use Committee approved this study (protocol 12‐058‐O).
2.2. Enrollment criteria
With client informed consent, dogs ≥2.5 kg of any age, breed, or sex, with MMVD and CHF (ACVIM Stage C) 19 , 20 of up to 10 days' duration were eligible. Requirements included moderate to severe mitral regurgitation, with a ≥ 3/6 left‐sided systolic murmur; with either exercise intolerance or dyspnea at Day 0 (D0), or within the preceding 10 days; cardiomegaly and radiographic pulmonary edema (score ≥ 2; Table 1) at D0 or within 10 days prior; and left atrium‐to aorta ratio (LA : Ao) ≥1.6, with moderate to severe mitral regurgitation by Doppler echocardiography. In the stabilization period (≤10 days prior to D0), the protocol allowed pulmonary edema to be treated with all necessary therapy (eg, diuretics [all routes], pimobendan, oxygen, dobutamine, hydralazine, nitroprusside, etc). The duration of furosemide administered IV was limited to ≤48 hours. At D0, pulmonary edema must have been stabilized, as evidenced by a radiographic score ≤2. All cardiac drugs (except furosemide, the study drugs, and rate‐control drugs [digoxin or calcium channel blockers]) were discontinued by D0. The use of all other cardiac drugs, including pimobendan, was restricted to ensure the outcome of the study was related solely to the tested treatment and to avoid the potential confounding effect of administering an additional medication.
TABLE 1.
Description of the ordinal clinical and radiographic scoring system
| Variable | Score | Clinical description |
|---|---|---|
| Appetite | 0 | Normal |
| 1 | Mild: Slight decrease compared to normal | |
| 2 | Small: Marked decreased compared to normal | |
| 3 | Anorexia | |
| Cough | 0 | None |
| 1 | Occasional, (few times a week) | |
| 2 | Frequent (a few times a day) | |
| 3 | Persistent (hourly or more frequent) | |
| Dyspnea | 0 | None |
| 1 | Somewhat labored, breathing deeper or faster than normal | |
| 2 | Marked labored breathing, able to lie in lateral recumbency | |
| 3 | Respiratory distress, remains in sternal recumbency | |
| Exercise intolerance | 0 | None: Walks distance without difficulty |
| 1 | Moderate: Walks short distance without difficulty but fatigue evident | |
| 2 | Severe: Walks short distance with difficulty and severe fatigue | |
| 3 | Exercise impossible | |
| Syncope | 0 | None |
| 1 | Less than or equal to 4 per month | |
| 2 | More than 4 episodes per month | |
| Pulmonary edema | 1 | Radiographs within normal limits |
| 2 | Interstitial lung pattern | |
| 3 | Interstitial with widespread and prominent bronchiolar cuffing | |
| 4 | Alveolar pattern with air bronchograms | |
| 5 | Severe pulmonary edema such that the cardiac silhouette and pulmonary vasculature are obscured |
Dogs were excluded if intended for breeding, pregnant or lactating; if they had noncardiogenic syncope, congenital or other acquired heart disease; severe/uncontrolled concomitant disorders; or were treated prior to Day‐10 with a cardiac medication (except ACE‐I, which were allowed as early as 30 days prior to D0); or required cardiac therapy at D0, other than a diuretic, ACE‐I, digoxin and diltiazem.
2.3. Removal from study
Removal from study, without endpoint attainment, occurred for the following reasons: development of systemic disease or a noncardiac adverse event (AE) that might have interfered with the ability to continue or the ability to assess endpoints; major protocol deviations; owner/dog noncompliance; or withdrawal by owner.
2.4. Study samples
For statistical analysis, the dogs were divided into 2 overlapping safety and efficacy subpopulations, termed samples (Figure 1A). The safety sample (all dogs receiving ≥1 dose of either treatment) was used to evaluate field safety variables (number of AE and biochemical and hematology variables). It was also used for the time‐to‐event analyses, including the time‐to‐cardiac endpoint and time to all‐cause withdrawal analyses.
FIGURE 1.
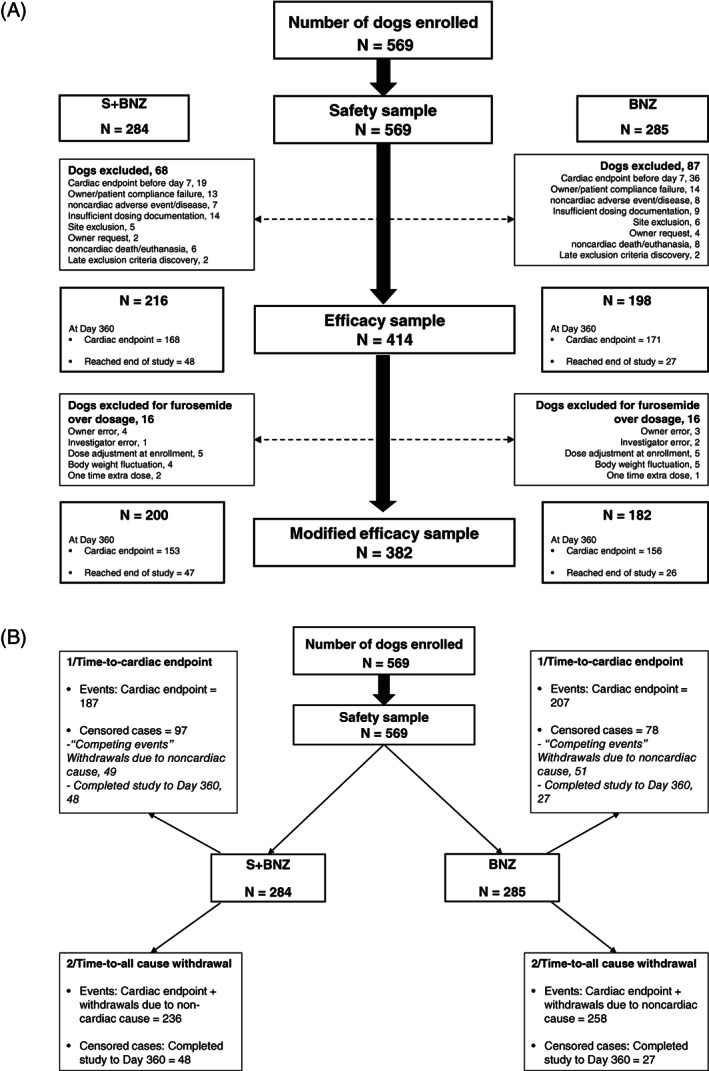
A, Study sample flowchart: Efficacy sample (for cardiac endpoint at Day 360) and modified efficacy sample (for cardiac endpoint at Day 30, 90, 180, 270, 360). B, Study sample flowchart: Safety sample for time‐to‐event analysis, event being defined as: (1) cardiac endpoint; (2) all cause withdrawal
The efficacy sample, devoid dogs having major protocol deviations, was used to assess the percentage of Day 360 cardiac endpoints and scores for quality of life, clinical signs, body condition, FETCH, 21 and pulmonary edema. More specifically, this sample included all cases, except those having reached cardiac endpoint before Day 7; dogs removed from study for a noncardiac event (AE, injury, noncardiac death/euthanasia); noncompliance with dosing, follow‐up, or procedures; owner electing to remove the dog; post enrollment discovery of dogs having exclusion criteria; or sites enrolling ≤2 dogs per treatment group.
A modified efficacy sample, assessing percentage of cardiac endpoints at each visit, included all efficacy sample dogs, except those receiving a short‐term increase over the allowed furosemide dosage >6 mg/kg/d at D0 or 8 mg/kg/d after D0. This was considered as a deviation from protocol by the FDA‐CVM, which requested their exclusion for the calculation of percentages of cardiac endpoints at each visit.
The efficacy and modified efficacy samples, defined as requested by the FDA‐CVM, were made to analyze the primary and secondary outcome variables in dogs which either reached the cardiac endpoint after Day 7 visit or completed the follow‐up.
2.5. Randomization, allocation, and treatment
Randomization to treatment groups utilized a computer‐generated table (PLAN procedure in SAS/STAT 9.4, SAS Institute, Cary, North Carolina). As dogs were enrolled, a unique identification number and treatment code was assigned, based on order of presentation and the randomization table for each site, in a 1 : 1 ratio of S+BNZ (test) to BNZ (control).
Group S+BNZ dogs received 0.25 mg/kg benazepril and 2 mg/kg spironolactone once daily in a fixed‐dosage tablet (CARDALIS 2.5/20 mg, 5/40 mg, 10/80 mg, benazepril/spironolactone). Group BNZ dogs received 0.25 mg/kg benazepril once daily (Fortekor 2.5 mg, 5 mg, 20 mg). The number of tablets was adjusted to the nearest half tablet, to achieve the target dosage.
Oral furosemide at ≤6 mg/kg/d was mandatory at D0. This post‐stabilization dosage was chosen to allow flexibility in the management of clinical signs. The permissible relatively high dosage was aimed at avoiding excessively rapid or drastic reduction in diuretic dosage on D0, thereby minimizing the risk of CHF recurrence. After D0, the dosage could be adjusted at the veterinarians' discretion (≤8 mg/kg/d) for cough or tachypnea (if radiographic evidence of pulmonary edema was absent or had not worsened since the last visit), weight loss/gain, dehydration, or azotemia. Dogs requiring a furosemide dosage higher than 8 mg/kg/d were considered refractory, 20 thereby having reached the cardiac endpoint.
2.6. Blinding
Primary packaging consisted of either a bottle or a blister pack marked only with a generic study label. Test products were packaged and dispensed in a secondary box, identical for both treatments, to ensure masking and prevent visualization of the primary containers. However, that the active control and the test article were different in appearance was not revealed to clients. The investigator assessed historical, physical and diagnostic data, while a treatment dispenser at each site was responsible for allocating/dispensing treatment, receiving and accounting for returned medication; and assessing owners' dosing compliance. Therefore, the owner, as well as the investigator, were blinded to treatment.
At each visit the number of tablets dispensed and returned was reconciled with the owner's records, to determine if doses had been given appropriately. Neither the investigators, the monitor, nor the study sponsor had access to the randomization list. Predefined procedures were in place for instances when there might be a need for unblinding, at which time the dog would be withdrawn from the study.
2.7. Clinical evaluation and monitoring schedule
Study visits were performed at Day 0, 7, 30, 90, 180, 270, and 360 (Table 2). A medical history, heartworm test, murmur grade, and heart failure classification were obtained at D0. A physical examination, clinical signs scoring (Table 1), FETCH quality of life questionnaire, 21 hematology (except Day 7), serum chemistry, thoracic radiographs, and systolic blood pressure were obtained at each visit. Urinalysis, an ECG, and an echocardiogram were assessed on Day 0, 180, and 360. At each visit, owner observations, dosing compliance and AE were tracked.
TABLE 2.
Scheduled visit for each dog and diagnostic procedures performed during various study time points (enrollment visit (V1): Day 0 (first day of treatment))
| Scheduled visit number | V1 | V2 | V3 | V4 | V5 | V6 | V7 |
|---|---|---|---|---|---|---|---|
| Day number | D0 | D7 ± 2 | D30 ± 4 | D90 ± 7 | D180 ± 7 | D270 ± 7 | D360 ± 7 |
| Physical examination | X | X | X | X | X | X | X |
| FETCH questionnaire | X | X | X | X | X | X | X |
| Thoracic radiographs | X | X | X | X | X | X | X |
| Systolic arterial BP | X | X | X | X | X | X | X |
| ECG | X | X | X | ||||
| Echocardiogram | X | X | X | ||||
| Hematology | X | X | X | X | X | X | |
| Serum chemistry | X | X | X | X | X | X | X |
| Urinalysis | X | X | X | ||||
| Heartworm antigen | X | ||||||
| Heart murmur intensity | X | ||||||
| Heart failure classification | X |
Abbreviations: BP, blood pressure.
2.8. Primary outcome variable
The primary outcome variable was the percentage of dogs having reached the cardiac endpoint and removed from study by Day 360. The cardiac endpoint was defined as: death/euthanasia from cardiac cause (including sudden cardiac death [SCD], defined as sudden death not specifically attributable to a noncardiac cause); recurrence/worsening of pulmonary edema by radiography; newly‐documented cardiogenic ascites; or worsening or new occurrence of cardiac clinical signs, necessitating therapy with a nonauthorized cardiac drug or furosemide dosage >8 mg/kg/d.
If the cardiac endpoint was reached between D0 and D7, the case was considered to be clinically unstable, determined nonevaluable for the purpose of efficacy assessment and was removed from the efficacy/modified efficacy samples. However, these data were retained and included in determination of time‐to‐cardiac endpoint and to all‐cause withdrawal analyses, and for the safety assessment.
2.9. Secondary outcome variables
Secondary outcome variables were analyzed and compared to baseline, to provide full evaluation of the efficacy of S+BNZ. Secondary outcome variables included the percentage of dogs reaching the cardiac endpoint at each visit (Day 30, 90, 180, 270, and 360); the time‐to‐cardiac endpoint; the time‐to‐death/euthanasia from cardiac cause; the clinical signs score including pulmonary edema score (Table 1); body condition score; and quality of life (FETCH) score at each visit.
2.10. Post hoc analyses
Post hoc analyses included time‐to‐withdrawal for all causes, percentage of dogs reaching cardiac endpoint on or before Day 7, and additional analyses of cardiac death/euthanasia data.
2.11. Safety
Safety evaluation included assessment of AE and serious adverse events (SAE), incidence of newly appearing clinical signs of CHF, and laboratory values between groups over the course of the study.
2.12. Statistics
The study was based on an expected Day 360 treatment failure rate for the BNZ group of 60% and for the S+BNZ group of 45%, with a statistical power of 80% and risk alpha of 5%. In Bernay et al., 8 the primary endpoint was reached by 10.8% in the spironolactone group compared to 25.5% in the control group. Thus, a 15% difference was observed between the groups. In the BENCH study, 33 50 to 60% of dogs reached the endpoint in the benazepril group after a 6‐12 months follow‐up. The necessary sample size, calculated with a Fisher's exact test for 2 proportions, estimated 186 evaluable dogs per treatment group. The goal was to achieve 250 evaluable cases in each group, with the additional cases to account for an ~20% dropout rate.
The percentage reaching cardiac endpoint at Day 360 was evaluated using the efficacy sample and percentage reaching the cardiac endpoint at each visit was performed on the modified efficacy sample (Figure 1A).
For the time‐to‐cardiac endpoint analysis, cardiac endpoint was considered as the event whereas premature withdrawals for noncardiac causes and dogs which completed the study were considered as censored cases. For the time to all‐cause of withdrawal, events were defined as either cardiac or noncardiac premature terminations, whereas dogs which completed the study were considered as censored cases (Figure 1B).
Demographics and diagnostic findings in study dogs were visually inspected for normality, summarized, and displayed as mean (SD), median (interquartile range [IQR]), or count (percentage). Comparisons between groups were made using 2 sample t‐tests, Wilcoxon rank sum tests, chi‐squared, or Fisher's exact tests, as appropriate.
Cardiac endpoint by Day 360 and at each visit was evaluated using a logistic mixed model with a logit link (the GLIMMIX procedure in SAS, SAS Institute, Cary, North Carolina; version 9.4). Treatment was included in the statistical model as a fixed effect, while clinic and the clinic‐by‐treatment interactions were included as random effects. Results were evaluated at each visit and not as repeated measures given the cumulative nature of the results (once a dog has a cardiac endpoint, it remained a cardiac endpoint).
At Day 360, the odds ratio of cardiac endpoint and its 95% confidence interval were derived to summarize study results. The percentages reaching cardiac endpoint and a 95% confidence interval were provided for each visit. Treatment effects were evaluated, using a 2‐sided test at alpha = .05.
Time‐to‐event analyses utilized the nonparametric Kaplan‐Meier method with the log‐rank test to compare survival functions between treatment groups. A frailty regression model was utilized to detect covariate effects on time‐to‐event, taking into account the heterogeneous nature of the study sample by using a parametric approach with the investigation center as a random effect. This was done first by univariable analyses followed by a multivariable analysis of variables with a P‐value of <.2 in the univariable analysis with backwards selection (Table S1). Sensitivity testing, performed on the final model, included treatment as a fixed‐effect, then forward selection, starting with the variables with the lowest P‐value. Quantitative variables were dichotomized using their median to solve convergence issues. Goodness‐of‐fit and the proportional hazards assumption were assessed using the previously described Martingale residuals. 22 P‐values <.05 were considered as statistically significant.
The incidence of SCD and CHF was estimated using a cumulative incidence function. Dogs which had a premature termination without having SCD or CHF were considered to have a competing event and such dogs which completed the study were censored. The Gray K sample test was used to compare the curves. 23 A diagram summarizing statistical methodology with the different tests used at each step is included (Figure 2).
FIGURE 2.
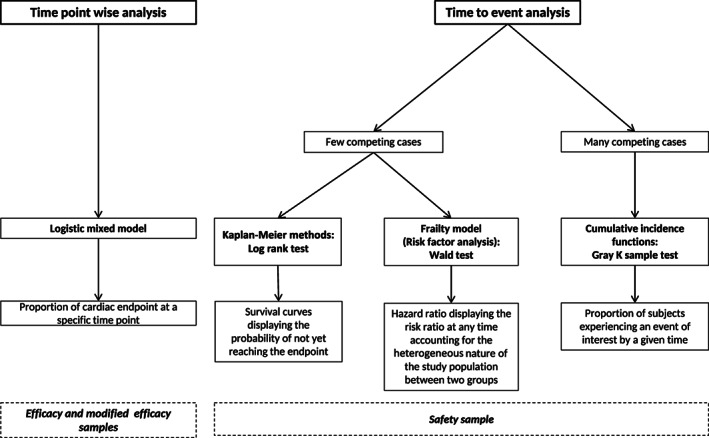
Diagram summarizing statistical methodology related to results from the BEnazepril Spironolactone STudy
For each variable (QOL, clinical, body condition, and pulmonary edema scores) and each dog, an area under the curve, averaged by the number of days in the study, was calculated. This provided an average value for the variable, incorporating all observations made, independent of the time in the study. 24 These areas were compared using a Wilcoxon rank‐sum test.
Blood variables were analyzed using a Student test at each time point, as there were no noticeable patterns or trending. Expectedly, the number of observations decayed substantially over the course of the study.
3. RESULTS
3.1. Characteristics of defined study dogs subpopulations (samples)
This study involved 27 US specialty practices, in which ACVIM‐boarded specialists recruited 569 client‐owned dogs from February 2012 through July 2016. Dogs enrolled in the study were randomized to receive either the combination of spironolactone and benazepril (n = 284) or benazepril (n = 285) (Figure 1A). All 569 dogs were included in the safety sample. As directed by the FDA‐CVM, dogs which were prematurely withdrawn either for cardiac reason before or at the Day 7 visit or for noncardiac reasons at any point in the study (155 total, 68 S+BNZ and 87 BNZ) were excluded from efficacy analyses (Table 3). The remaining 414 dogs constituted the efficacy sample. However, in the posttreatment evaluation, 32 dogs (16 dogs in each group) had exceeded either the 6 mg/kg/d at D0 or the 8 mg/kg/d after D0 furosemide dosage. This was typically a brief and modest excursion for reasons such as dosage adjustment at enrollment, owner/investigator error, change in body weight between visits, or 1 extra dose in an emergency room setting or by the owner without consulting the investigator. These dogs were removed from the modified efficacy sample (n = 382 dogs; Figure 1A).
TABLE 3.
Comparison of endpoint attainment and premature withdrawals between treatment groups
| S+BNZ (N = 284) | BNZ (N = 285) | ||||
|---|---|---|---|---|---|
| Dog No. | Dog % | Dog No. | Dog % | P | |
| Completed study to Day 360 | 48 | 16.9 | 27 | 9.5 | |
| Withdrawals prior to Day 360 | 236 | 83.1 | 258 | 90.5 | |
| Cardiac endpoints (before/after Day 7) | 187 (19/168) | 65.8 (6.7/59.1) | 207 (36/171) | 72.6 (12.6/60.0) | .02 |
| Recurrence of pulmonary edema and/or necessity of disallowed cardiac treatment | 149 | 52.5 | 159 | 55.8 | |
| Cardiac deaths | 38 | 13.4 | 48 | 16.8 | |
| Sudden death | 16 | 5.6 | 8 | 2.8 | .09 |
| Total CHF related deaths | 22 | 7.7 | 40 | 14.1 | .01 |
| Cardiac death | 10 | 3.5 | 19 | 6.6 | |
| Euthanasia | 12 | 4.2 | 21 | 7.4 | |
| Withdrawals due to noncardiac cause (noncompliance, adverse event, owner request) | 49 | 17.3 | 51 | 17.9 | |
| Owner/patient non‐compliance | 13 | 4.6 | 14 | 4.9 | |
| Non cardiac AE | 7 | 2.5 | 8 | 2.8 | |
| Dosing documentation insufficient | 14 | 4.9 | 9 | 3.2 | |
| Site exclusion | 5 | 1.8 | 6 | 2.1 | |
| Owner request | 2 | 0.7 | 4 | 1.4 | |
| Non‐cardiac death/euthanasia | 6 | 2.1 | 8 | 2.8 | |
| Late discovery of exclusion criteria | 2 | 0.7 | 2 | 0.7 | |
Notes: Data listed as count (%).
Abbreviations: AE, adverse event; BNZ, benazepril group; No., number; S+BNZ, spironolactone and benazepril group.
The sex breakdown was 212 females and 202 males and mean age of dogs at inclusion was 11.0 years (range, 3.4‐19.2 years). Dogs represented primarily small breeds and had a median (IQR) body weight of 6.9 kg (4.8‐10.2 kg). Cavalier King Charles spaniels and Chihuahuas each represented nearly 10% of the study sample with Shih Tzu, Maltese, and Dachshund breeds each representing more than 5%. The study groups were well‐balanced at baseline with respect to demographic and diagnostic findings, except for appetite score where there was a significant difference (P = .02), being poorer in the S+BNZ group (Table 4).
TABLE 4.
Baseline characteristics of the efficacy sample
| Variables | S+BNZ (n = 216) | BNZ (n = 198) | P |
|---|---|---|---|
| Age (years) | 11.1 (2.3) | 10.9 (2.5) | .5 |
| Weight (kg) | 7.1 (4.9‐10.3) | 6.7 (4.7‐10.0) | .84 |
| Sex (male/female) | 109/107 | 93/105 | .48 |
| Neutered/entire | 195/21 | 178/20 | .9 |
| Mixed breed/CKCS/other breeds | 58/20/138 | 45/20/133 | .62 |
| Appetite (0/1/2/3) | 128/51/32/5 | 137/43/18/0 | .02 |
| Cough (0/1/2/3) | 29/57/102/28 | 26/58/83/31 | .68 |
| Dyspnea (0/1/2/3) | 69/100/42/5 | 68/95/32/3 | .75 |
| Exercise intolerance (0/1/2/3) | 72/123/17/4 | 63/119/14/2 | .84 |
| Syncope (0/1/2) | 183/31/2 | 174/22/2 | .61 |
| BCS score | 5 (4‐5) | 5 (4‐6) | .88 |
| FETCH score | 23 (12‐39) | 22 (11‐35) | .32 |
| Pulmonary edema score (1/2) | 54/162 | 52/145 | .74 |
| ECG normal (Yes/No) | 130/86 | 110/88 | .37 |
| Heart rate (bpm) | 140 (124‐160) | 144 (130‐160) | .16 |
| Systolic blood pressure (mm Hg) | 134 (28) | 133 (26) | .6 |
| VHS | 11.9 (1.1) | 11.9 (1.0) | .64 |
| Mitral regurgitation (moderate/severe) | 57/159 | 43/155 | .32 |
| LA/Ao | 2.3 (0.5) | 2.3 (0.5) | .58 |
| LVIDDn (mm) | 2.1 (0.3) | 2.1 (0.2) | .66 |
| LVIDSn (mm) | 1.0 (0.3) | 1.0 (0.3) | .39 |
| Creatinine (mg/dL) | 1.1 (0.5) | 1.1 (0.4) | .71 |
| Urea nitrogen (BUN, mg/dL) | 30 (16) | 30 (15) | .88 |
| Potassium (mEq/L) | 4.5 (0.5) | 4.5 (0.5) | .45 |
| Urine specific gravity | 1.016 (0.010) | 1.015 (0.010) | .45 |
| Phosphorus (mg/dL) | 4.4 (1.4) | 4.4 (1.3) | .61 |
| Chloride (mEq/L) | 109 (6) | 109 (6) | .27 |
| Sodium (mEq/L) | 149 (3) | 150 (3) | .39 |
| Pretrial cardiac medications | |||
| Pretrial cardiac medications (Yes/No) | (187/29) | (167/31) | .52 |
| Furosemide | 186 (86.1) | 164 (82.8) | |
| Furosemide dosage (mg/kg) | 4.1 (3.3‐5.0) | 4.3 (3.4‐5.1) | .26 |
| ACE‐I | 50 (23.1) | 54 (27.3) | |
| ACE‐I + pimobendan | 29 (13.4) | 23 (11.6) | |
| ACE‐I + pimobendan + spironolactone | 1 (0.5) | 1 (0.5) | |
| ACE‐I + spironolactone | 1 (0.5) | 1 (0.5) | |
| Pimobendan | 23 (10.6) | 15 (7.6) | |
| Pimobendan + spironolactone | 0 (0) | 0 (0) | |
| Pretrial ACE‐I (Yes/No) | 27/189 | 28/170 | .62 |
Notes: Data listed as mean (SD), median (interquartile range) or count (%).
Abbreviations: ACE‐I, angiotensin converting enzyme inhibitors; BNZ, benazepril group; BUN, blood urea nitrogen; CKCS, cavalier King Charles spaniel; FETCH, functional evaluation of cardiac health; ISACHC, International Small Animal Cardiac Health Council; LA : Ao, left atrium to aortic root diameter ratio; LVIDDn, normalized left ventricular dimension at end‐diastole; LVIDSn, normalized left ventricular dimension at end‐systole; S+BNZ, spironolactone and benazepril group; VHS, vertebral heart size.
The median daily dosages of benazepril and spironolactone were respectively 0.37 mg/kg (IQR, 0.31‐.44) and 2.97 mg/kg (IQR, 2.46‐3.49) in the S+BNZ group and the median daily doses of benazepril in the BNZ group was 0.36 mg/kg (IQR, 0.31‐0.43). Prior to D0, 84% of dogs received furosemide (Day −10 to D0), 39% received an ACE‐I (Day −30 to D0), 22% received pimobendan (Day −10 to D0), and 1.0% received spironolactone (Day −10 to D0), in various combinations (Table 4).
3.2. Primary outcome variable
3.2.1. Cardiac endpoint at Day 360
Of the 414 dogs included in the evaluation of efficacy, 216 received the test product and 198 the control drug. A significantly lower percentage of S+BNZ dogs reached the cardiac endpoint by Day 360 than did those in the BNZ group (168/216, 77.8% in the S+BNZ group vs 171/198, 86.4% in the BNZ group). The odds of reaching cardiac endpoint were 44% less in the S+BNZ group than in the BNZ group (OR = 0.56; 95% CI, 0.32‐0.98; P = .04; Figure 3).
FIGURE 3.
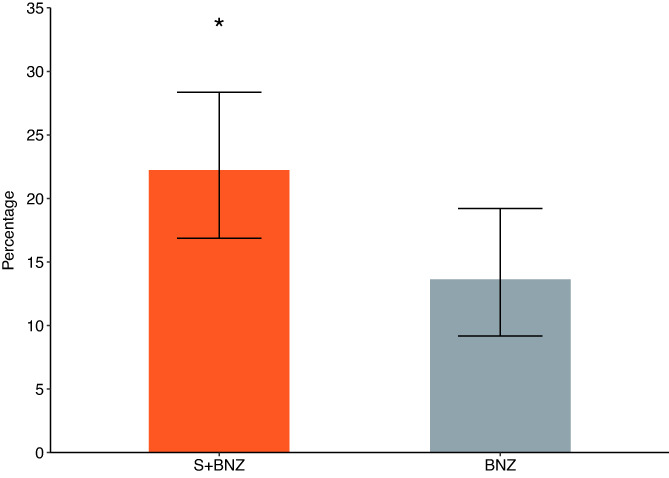
Percentage of dogs with 95% CI remaining in study at Day 360 (*P = .04). BNZ, benazepril group; S+BNZ, spironolactone plus benazepril group
3.3. Secondary outcome variables
3.3.1. Cardiac endpoint by visit
In the modified efficacy sample, at each time point evaluated, the percentage reaching the cardiac endpoint in the S+BNZ group was lower than in the BNZ group, and this difference was statistically significant at each evaluation after Day 30 (Visit 3; Figure 4).
FIGURE 4.
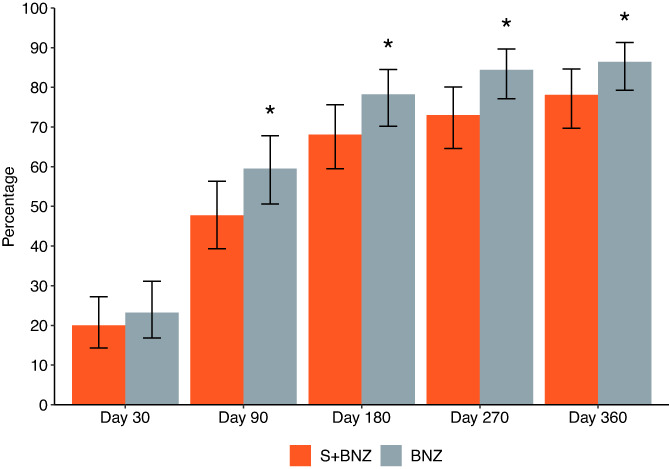
Percentages of dogs with 95% CI reaching the cardiac endpoint at each study visit (*Day 90, P = .03; Day 180, P = .04; Day 270, P = .01; Day 360, P = .04). BNZ, benazepril group; S+BNZ, spironolactone plus benazepril group
3.3.2. Time‐to‐cardiac endpoint
Dogs in the S+BNZ group exhibited a statistically significantly lower probability of reaching the cardiac endpoint (log‐rank test, P = .002; Figure 5). The difference in median time‐to‐endpoint was 36 days (S+BNZ, 105 days, 95% CI [87‐139 days] vs. BNZ, 69 days, 95% CI [54‐89 days]). Visual inspection reveals that the curves start to separate at Day 25.
FIGURE 5.
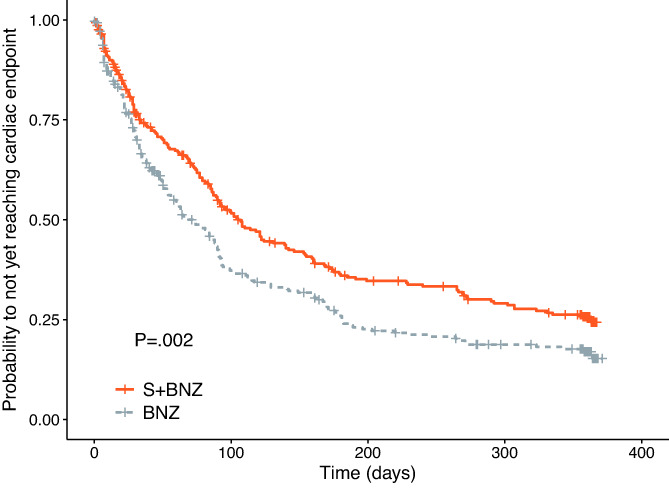
Kaplan‐Meier survival curves displaying the probability of not yet reaching the cardiac endpoint in the S+BNZ group (orange solid line) vs the BNZ group (gray dotted line). There were 284 dogs in the S+BNZ group and 285 dogs in the BNZ group at the outset (safety sample). Cross marks represent censored observations
The final multivariable model included treatment and the following variables at baseline: LA : Ao, LVIDDn, furosemide dosage, heart rate, VHS, serum chloride concentration, and systolic blood pressure. After adjusting the treatment effect on these variables, there was a 27% reduction in risk of dying or worsening from cardiac causes in the S+BNZ vs the BNZ group (HR = 0.73; 95% CI, 0.59‐0.89; P = .002; Table 5).
TABLE 5.
Multivariable frailty model displaying the hazard ratio (HR) and 95% confidence interval (CI) (time‐to‐cardiac endpoint)
| Variable (baseline) | Comparison | HR | 95% CI | P |
|---|---|---|---|---|
| Treatment | S+BNZ vs BNZ | 0.73 | 0.59‐0.89 | .002 |
| Heart rate (bpm) | >140 vs ≤140 | 1.44 | 1.17‐1.79 | .001 |
| LA : Ao | >2.2 vs ≤2.2 | 1.44 | 1.14‐1.83 | .003 |
| LVIDDn | >2.1 vs ≤2.1 | 1.57 | 1.25‐1.97 | <.001 |
| Furosemide (mg/kg) | >4.12 vs ≤4.12 | 1.58 | 1.26‐1.98 | <.001 |
| VHS | >11.9 vs ≤11.9 | 1.28 | 1.03‐1.59 | .03 |
| Chloride (mEq/L) | >110 vs ≤110 | 0.73 | 0.59‐0.92 | .006 |
| Systolic blood pressure (mm Hg) | >131 vs ≤131 | 0.79 | 0.64‐0.98 | .04 |
Notes: Mean site variance of the study site, 0.12 (SE, 0.07, P = .03).
Abbreviations: BNZ, benazepril group; LA : Ao, left atrium to aortic root diameter ratio; LVIDDn, normalized left ventricular dimension at end‐diastole; S+BNZ, spironolactone and benazepril group; VHS, vertebral heart size.
3.3.3. Time‐to‐cardiac death or euthanasia
The overall survival probability was significantly better in the S+BNZ group vs the BNZ group (log‐rank test, P = .04, Figure S1). Median time‐to‐event could not be calculated for either treatment group as <50% of at risk dogs experienced cardiac death/euthanasia in the 360‐day study.
3.3.4. Progression of clinical signs, pulmonary edema, and quality of life
The progression of clinical scores for all clinical signs (Table S2) was evaluated as a secondary outcome. Analysis did not reveal statistical difference between the 2 groups over time, although a trend for a higher percentage of dogs showing improvement in Day 360 clinical and pulmonary edema scores was observed in the S+BNZ group.
3.4. Post hoc analyses
3.4.1. Time‐to‐all cause withdrawal
All causes of withdrawal include cardiac origin before Day 7, cardiac origin after Day 7 (ie, cardiac endpoint), and noncardiac origin, including noncompliance, AE, euthanasia, and owner requests (Table 3 and Figure 1B). Group S+BNZ dogs exhibited a significantly lower probability of being withdrawn from the study for all causes (log‐rank test, P < .001; Figure 6). The final multivariable model includes treatment, heart rate, LA : Ao, LVIDDn, serum chloride concentration, VHS, baseline furosemide dosage, and systolic blood pressure. After adjusting the treatment effect on these variables, there was a 26% reduction in risk of being withdrawn for all causes in the S+BNZ group vs the BNZ group (HR = 0.74; 95% CI, 0.61‐0.89; P = .001; Table 6).
FIGURE 6.
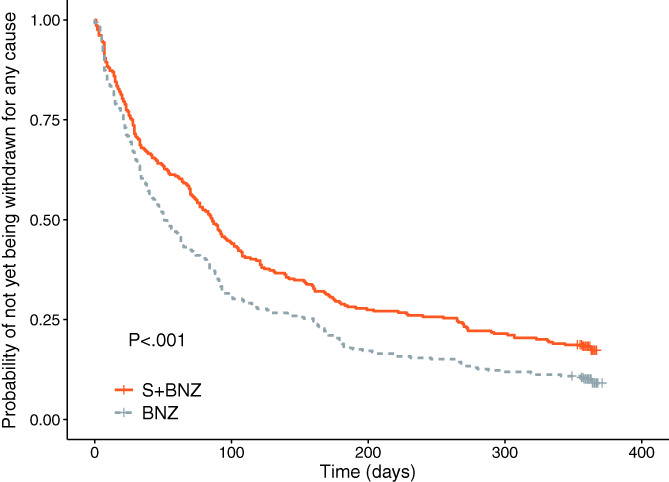
Kaplan‐Meier survival curves displaying the probability of not being withdrawn for any cause in the S+BNZ group (orange solid line) vs the BNZ group (gray dotted line). There were 284 dogs in the S+BNZ group and 285 dogs in the BNZ group at the outset (safety sample). Cross marks represent censored observations
TABLE 6.
Multivariable frailty model displaying the hazard ratio (HR) and 95% confidence interval (CI) (time to all causes of withdrawal)
| Variable (baseline) | Comparison | HR | 95% CI | P |
|---|---|---|---|---|
| Treatment | S+BNZ vs BNZ | 0.74 | 0.61‐0.89 | .001 |
| Heart rate (bpm) | >140 vs ≤140 | 1.40 | 1.15‐1.70 | .001 |
| LA : Ao | >2.2 vs ≤2.2 | 1.45 | 1.17‐1.79 | .001 |
| LVIDDn | >2.1 vs ≤2.1 | 1.61 | 1.31‐1.98 | <.001 |
| Furosemide (mg/kg) | >4.12 vs ≤4.12 | 1.54 | 1.26‐1.89 | <.001 |
| VHS | >11.9 vs ≤11.9 | 1.23 | 1.01‐1.50 | .04 |
| Chloride (mEq/L) | >110 vs ≤110 | 0.79 | 0.64‐0.96 | .02 |
| Systolic blood pressure (mm Hg) | >131 vs ≤131 | 0.82 | 0.68‐0.99 | .05 |
Notes: Mean site variance of the study site, 0.10 (SE, 0.05, P = .02).
Abbreviations: BNZ, benazepril group; BUN, blood urea nitrogen; LA : Ao, left atrium to aortic root diameter ratio; LVIDDn, normalized left ventricular dimension at end‐diastole; S+BNZ, spironolactone and benazepril group; VHS, vertebral heart size.
3.4.2. Cardiac endpoint in first 7 days
Of the 569 dogs entering the study, 55 dogs reached the cardiac endpoint before or at the Day 7 visit (19 S+BNZ dogs and 36 BNZ dogs, 6.7% and 12.6%, respectively). This 2‐fold greater occurrence in dogs not receiving spironolactone was statistically significant (P = .02).
3.5. Safety
3.5.1. Cardiac death or euthanasia
The number of deaths in the BESST was small (100 of 569 dogs [17.6%] entering study). Not surprisingly, the majority of these (86%) had a cardiac cause (Table 3). All noncardiac deaths were owner‐requested euthanasia for various diseases associated with old age (e.g., arthritis, neoplasia, etc.). Cardiac deaths were classified as either SCD or spontaneous death/euthanasia related to cardiac disease progression. All dogs dying suddenly or found dead were considered to have experienced cardiac death. Sudden death, while uncommon, was twice as frequently observed, though not significantly different, in group S+BNZ vs group BNZ (16 of 284 vs 8 of 285, P = .09). However, death due to cardiac decompensation was significantly greater in group BNZ (P = .01).
3.5.2. Adverse events
The incidence of AE was compared between the 2 treatment groups (Figure 7). The majority of observed AE were associated with the dogs' cardiac condition (dyspnea, anorexia, cough, exercise intolerance, nonspecified circulatory disorder, cardiac insufficiency, and tachypnea) and therefore, were expected. The percentage of animals demonstrating these signs was similar between groups, not statistically significantly different, never varying by >5%.
FIGURE 7.
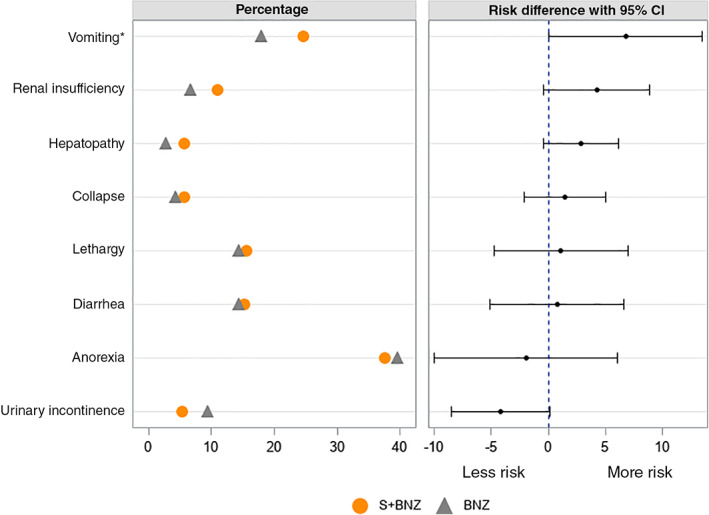
Percentage, risk difference, and the 95% CI for dog shaving at least 1 adverse event during the study, which was unrelated to cardiac condition (*P = .05). BNZ, benazepril group; S+BNZ, spironolactone plus benazepril group
The difference in incidence of AE, not specifically related to cardiac condition and occurring in more than 5% of the total number of study dogs, was not statistically significant, with the 1 exception of vomiting (24.6% in the S+BNZ group vs 17.9% in the BNZ group, P = .049; Figure 7). The incidence of SAE was also not different between BNZ and S+BNZ groups (Table 7). Importantly, SAE potentially related to treatment, were rare (2.1%), affecting 6 dogs in each group, as were fatal treatment‐related AE (3 dogs total, 0.4% and 0.7% in S+BNZ and BNZ groups, respectively).
TABLE 7.
Adverse events and serious adverse events (which result in death, life‐threatening, persistent disability) documented in dogs in the S+BNZ and the BNZ (dogs could have more than one adverse event or more than one clinical sign at the time of the adverse event)
| Variables | S+BNZ (n = 284) | BNZ (n = 285) | P |
|---|---|---|---|
| Adverse events | |||
| Dogs with at least 1 AE | 265 (93.3) | 260 (91.2) | .35 |
| Number of AE | 1409 (52.9) | 1257 (47.1) | NA |
| Dogs with at least 1 treatment‐related AE a | 45 (15.8) | 34 (11.9) | .18 |
| Number of treatment related AE a | 90 (57.7) | 66 (42.3) | NA |
| Serious adverse events | |||
| Dogs with at least 1 SAE | 139 (48.9) | 134 (47.0) | .68 |
| Number of SAE | 387 (54.0) | 330 (46.0) | NA |
| Dogs with at least 1 treatment‐related SAE a | 6 (2.1) | 6 (2.1) | 1 |
| Number of treatment related SAE a | 17 (56.7) | 13 (43.3) | NA |
| Death/euthanasia due to SAE | |||
| Dogs with fatal SAE | 36 (12.7) | 42 (14.7) | .48 |
| Dogs with fatal treatment‐related SAE | 1 (0.4) | 2 (0.7) | .56 |
Notes: Data listed as count (%).
Abbreviations: AE, adverse event; BNZ, benazepril group; NA, not applicable; S+BNZ, spironolactone and benazepril group; SAE, serious adverse event.
Treatment‐related AE/SAE = AE considered as related, possibly related, or probably related.
3.5.3. Renal function and electrolytes
There were no statistically significant differences in baseline values or follow‐up values between the 2 groups for BUN, sCr, or serum potassium. Furthermore, serum potassium and sCr concentrations remained stable during the 360 days of the study, with median values never approaching the high end of the reference range (Figure S2).
4. DISCUSSION
The BESST is the largest veterinary cardiology clinical trial, to date, representing the strongest type of clinical investigation as a multicentric prospective, randomized, adequately‐powered, double‐blind trial, carried out under FDA‐CVM standards by specialists. The results are similar to those of human CHF MRA clinical trials, 3 , 25 , 26 as well as a European study of canine CHF. 8
The combination of spironolactone and benazepril fixed‐dose tablet, given concurrently with furosemide, was safe, providing superior results to benazepril alone in several important ways, including time‐to‐cardiac endpoint and time to all‐cause withdrawal. The percentage of dogs reaching cardiac endpoint before Day 360 was significantly lower in the S+BNZ group, with risk of dying or worsening from cardiac causes vs the BNZ group being reduced by 27%.
Advantages were also seen in secondary outcome variables, with percentage of dogs reaching the cardiac endpoint in the S+BNZ group being significantly lower than in the BNZ group at each time point after 30 days (P = .02), demonstrating early, persistent treatment benefit during the course of CHF due to MMVD. Inspection of the Kaplan‐Meier curve indicates benefit, with curve separation apparent at 25 days, mimicking human MRA studies. 25 , 26 The use of S+BNZ provided significant benefit within 50 days, which was sustained through the 1‐year study. Additionally, S+BNZ group dogs exhibited a longer median time‐to‐cardiac endpoint vs the BNZ group (41.1% longer; 105 days vs 69 days, respectively).
Time‐to‐cardiac death or euthanasia was also significantly prolonged in the S+BNZ group vs the control. Specifically, S+BNZ dogs, while experiencing a greater number of sudden deaths than BNZ dogs (P = .09, Table 7), had significantly fewer spontaneous and euthanasia deaths due to CHF‐progression (P = .01).
Unexpectedly, twice the number of dogs in the BNZ group (36 vs 19; 12.6% vs 6.6%; P = .07) reached the cardiac endpoint in first 7 days. We hypothesize that, as CHF was stabilized and drugs were tapered or stopped per protocol, some less stable dogs, randomized to benazepril without the benefit of spironolactone, experienced recrudescence of clinical signs. This finding has practical and clinically important ramifications, emphasizing the value in early multimodal, RAAS suppression, as shown in EMPHASIS‐HF and EPHESUS Trials, and now in the BESST. 25 , 26
There was a significant (P < .001), 26% overall risk reduction for dogs being withdrawn from the study for any cause in the S+BNZ group. Multivariable analysis showed S+BNZ‐treatment to be a contibuting factor significantly reducing all‐cause withdrawal. This implies that the benefit provided by S+BNZ on cardiac endpoint is not diminished by poor compliance or dog withdrawal because of adverse effects.
4.1. Safety
The safety results in the BESST reflect the long history of ACE‐I and spironolactone safety in veterinary medicine. 8 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 The incidence of serious and nonserious AE was similar between groups and potentially treatment‐related SAE were rare, while treatment‐related AE, resulting in spontaneous death or euthanasia, were exceedingly rare. All‐cause withdrawal was 25% less in the S+BNZ group, reflecting positive outcome and paucity of AE.
In CHF, renal function can be negatively impacted by age, reduced renal perfusion, diuretics, and RAAS‐suppressants. Overall, the latter have been shown to be safe. 8 , 27 , 28 In the current study, BUN and sCr revealed no significant differences between study groups and the percentage of dogs with “renal insufficiency” AE was identical (2.4%). As expected with CHF and off‐loading therapies, indicators of reduced renal function rose over time in both groups.
Use of RAAS‐suppressive therapies, particularly MRA with ARB or ACE‐I, has been associated with hyperkalemia, sometimes fatal, in humans, 3 , 25 , 26 , 37 , 38 although this has not been observed with any frequency in the dog. 8 , 27 , 28 , 29 In the BESST, there was no significant difference in potassium concentrations between groups, with median values never approaching hyperkalemia. Hyperkalemia was noted in both groups, but only resulted in 4 AE, all in the S+BNZ group, with 1 (potassium, 6.3 mEq/L) resulting in withdrawal from the study. Potassium concentrations >6.0 mEq/L were rare (overall incidence 0.35%), with values greater than the upper reference range documented in 24 dogs (4.2%; 16 S+BNZ, 8 BNZ; P = .09), confirming hyperkalemia not to be a major risk in CHF‐dogs, treated with MRA and ACE‐I.
The benefits of ACE‐inhibition have been demonstrated in multiple, controlled clinical trials in dogs with CHF due to both MMVD and dilated cardiomyopathy. 30 , 31 , 32 , 33 , 34 , 35 , 36 As mentioned, 3 large human studies have shown clear benefit with inclusion of broader RAAS‐suppressive therapy in managing CHF with reduced ejection fraction (HFrEF), when a MRA was added to standard therapy, including an ACE‐I or ARB. The RALES 3 (ischemic and nonischemic HF, NYHA III‐IV), EPHESUS 25 (postacute myocardial infarction, with HF), and EMPHASIS‐HF 26 (ischemic and nonischemic HF, NYHA II‐IV) evaluated a MRA (spironolactone or eplerenone) added to optimal HF therapy, including RAAS‐suppression with ACE‐I (RALES) or either an ACE‐I or ARB (EPHESUS and EMPHASIS‐HF). Each demonstrated a reduction in death rate and hospitalization associated with cardiac disease. These study results led to recommendation in multiple human CHF treatment guidelines for MRA use in therapy of HF with reduced left ventricular ejection fraction. 4 , 5 , 6 , 7 Importantly, MRA therapy was shown collectively to be effective in mild to severe CHF. In veterinary medicine, the BESST represents the second clinical trial to show superior results with inclusion of spironolactone in the management CHF in dogs.
4.2. Aldosterone breakthrough
Mineralocorticoid receptor antagonist's greatest benefit is thought to produce blockade of the mineralocorticoid receptor, thereby countering aldosterone's damaging effects in ABT. 1 Use of RAAS‐suppression, with ACE‐I, ARB, or MRA, alone or in combination, is therefore a key strategy in treating chronic cardiovascular and renal disease with RAAS‐activation. 1 , 2
The findings of early ABT in studies of normal dogs, dogs with cardiac disease, and in CHF human patients suggest that early MRA employment in Stage C (CHF) is advisable, whenever an ACE‐I is prescribed. 1 , 2 , 16 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 We hypothesize that the clinical benefits observed with the combination of spironolactone and benazepril in the BESST are due to the existence of ABT. Further studies of RAAS activation in dogs with cardiac disease are needed.
4.3. Limitations
The study design of the BESST is of the highest rigor. There was, however, no placebo arm in this study, as this was considered to be unethical because ACE‐I have proven effective and are FDA‐approved for the management of CHF. It would have been interesting to include pimobendan in both treatment groups; however, this study sought to demonstrate the specific contribution of spironolactone to the efficacy of the combination. Study designers grappled with the ethics of a CHF study not including pimobendan, which had, at the time, been recently shown to benefit dogs in CHF with MMVD. 48 A panel of 10 cardiologists, representatives from the Sponsor and the FDA‐CVM discussed this and concluded that a short‐term study (1‐year) of early CHF without pimobendan was ethical. This decision was made because the owners were informed participants; investigators had 10 days to stabilize dogs, enrolling them only if they felt it was safe; and dogs were carefully monitored for CHF recurrence, providing the opportunity for prompt removal from the study and unrestricted therapy. It is possible that the observed benefit of spironolactone may be modified when it is administered with other medication and future studies would be required to quantify the effect of spironolactone administered in conjunction with other cardiac pharmaceutical agents (e.g., pimobendan). Lastly, while it would have been of interest to further define mechanisms of MRA efficacy by measuring RAAS biomarkers, it was beyond the scope of the hypothesis we investigated.
5. CONCLUSIONS
A combination of the MRA, spironolactone, and the ACE‐I, benazepril, in a fixed 8 : 1 ratio, is more effective than benazepril alone when included in the medical management of new‐onset CHF, due to MMVD. The use of the combination of spironolactone and benazepril results in reduced or delayed recurrence of CHF and its associated clinical signs, while reducing death rate over the 12‐month study. Furthermore, under clinical field conditions, the safety profile of the combination product is indistinguishable from that of benazepril alone.
CONFLICT OF INTEREST DECLARATION
Melissa Coffman, Emilie Guillot, Thomas Blondel, Catherine Garelli‐Paar, Shuo Feng and Susanne Heartsill are employees of Ceva Santé Animale. During the past 5 years, Clarke E. Atkins has received research funding, reimbursement for travel, honoraria for speaking and preparation of educational materials, and consulting fees from Ceva Santé Animale, Boehringer Ingelheim Animal Health GmbH, Vetoquinol, and Virbac, all of whom market cardiac drugs. This manuscript describes the effects of a drug manufactured by Ceva Santé Animale.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the IACUC of North Carolina State University Veterinary Hospital, protocol 12‐058‐O.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1 Kaplan‐Meier survival curves displaying the probability of not yet experiencing cardiac death in the S+BNZ group (orange solid line) vs the BNZ group (gray dotted line). There were 284 dogs in the S+BNZ group and 285 dogs in the BNZ group at the outset (safety population). Cross marks represent censored observations.
Figure S2 Boxplots of renal functions and electrolytes (safety population). A, Creatinine (mg/dL). B, Potassium (mEq/L). C, Urea nitrogen (BUN) (mg/dL)
Appendix S1. Tables.
ACKNOWLEDGMENT
Funding provided by Ceva Santé Animale for the registration of CARDALIS by FDA‐CVM (Freedom of Information Summary, NADA #141‐538 [July 27, 2020], CARDALIS). The authors thank William Tyrell (Chesapeake Veterinary Cardiology Associates/The Life Centre), Jess Weidman (Chesapeake Veterinary Cardiology Associates/Dogwood Veterinary Emergency and Specialty Center), Eva Sikorska (Pittsburgh Veterinary Specialty & Emergency Center), William Rausch (Portland Cardiology Associates/Dove Lewis Emergency Animal Hospital), Tacy Rupp (Veterinary Cardiopulmonary Care Center), Linda Lehmkuhl (MedVet Medical and Cancer Center for Pets), Jonathan Goodwin (Garden State Veterinary Specialists), Philip Fox (The Animal Medical Center), Clarke Atkins and Teresa DeFrancesco (North Carolina State College of Veterinary Medicine), Richard Kienle (VCA Bay Area Veterinary Specialists), Karen Sanderson (Rocky Mountain Veterinary Cardiology/Veterinary Referral Center of Colorado), Allison Heaney (Rocky Mountain Veterinary Cardiology/Alpenglow Veterinary Specialty and Emergency Center), Emily Olson (Veterinary Referral and Critical Care), Ryan Fries and Kathy Wright (MedVet Medical & Cancer Center for Pets), Michael Luethy (Chicago Veterinary Emergency & Specialty Center), Mitchell Crystal (North Florida Veterinary Specialists), Jeffrey Dennis (Blue Pearl Veterinary Partners), Eryn Leffers (Veterinary Medical Specialists of Houston), Kristin Lavely (VCA Animal Center of Sonoma County), Kirstie Barrett (VCA West Los Angeles Animal Hospital), Brian MacKie (VCA Sacramento Veterinary Referral Center), Justin Williams (VCA San Francisco Veterinary Specialists), Sharon Huston (VCA Emergency Animal Hospital & Referral Center), Dan Hall (South Carolina Veterinary Specialists and Emergency Care), Dewey Carpenter (Coral Springs Animal Hospital), Jonathan Abbott (Virginia Tech College of Veterinary Medicine), Bryan Bottorff (Seattle Veterinary Specialists). The authors also thank all technicians and assistants involved in this study, as well as the dogs, and their owners, who participated in the study.
Coffman M, Guillot E, Blondel T, et al. Clinical efficacy of a benazepril and spironolactone combination in dogs with congestive heart failure due to myxomatous mitral valve disease: The BEnazepril Spironolactone STudy (BESST). J Vet Intern Med. 2021;35(4):1673–1687. 10.1111/jvim.16155
Funding information Ceva Santé Animale
REFERENCES
- 1. Ames MK, Atkins CE, Pitt B. The renin‐angiotensin‐aldosterone system and its suppression. J Vet Intern Med. 2019;33:363‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486‐492. [DOI] [PubMed] [Google Scholar]
- 3. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709‐717. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur Heart J. 2012;33(14):1787‐1847. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guidelines for the management of heart failure. Circulation. 2013;128:e240‐e327. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Euro J Cardiol. 2016;8:891‐975. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137‐e161. [DOI] [PubMed] [Google Scholar]
- 8. Bernay F, Bland JM, Haggstrom J, et al. Efficacy of spironolactone on survival in dogs with naturally occurring mitral regurgitation caused by myxomatous mitral valve disease. J Vet Intern Med. 2010;24:331‐341. [DOI] [PubMed] [Google Scholar]
- 9. Lantis AC, Ames MK, Atkins CE, et al. Aldosterone breakthrough with benazepril in furosemide‐activated renin‐angiotensin‐aldosterone system in normal dogs. J Vet Pharmacol Therap. 2015;38:65‐73. [DOI] [PubMed] [Google Scholar]
- 10. Lantis AC, Ames MK, Werre S, et al. The effect of enalapril on furosemide‐activated renin‐angiotensin‐aldosterone system in healthy dogs. J Vet Pharmacol Therap. 2015;38:513‐517. [DOI] [PubMed] [Google Scholar]
- 11. Atkins CE, Rausch WP, Gardner SY, et al. The effect of amlodipine and the combination of amlodipine and enalapril on the renin‐angiotensin‐aldosterone system in the dog. J Vet Pharmacol Therap. 2007;30:394‐400. [DOI] [PubMed] [Google Scholar]
- 12. Sayer MB, Atkins CE, Fujii Y, et al. Acute effects of pimobendan and furosemide on the circulating renin‐angiotensin‐aldosterone system in healthy dogs. J Vet Intern Med. 2009;23:1003‐1006. [DOI] [PubMed] [Google Scholar]
- 13. Ames MK, Atkins CE, Lantis AC, et al. Effect of furosemide and high‐dosage pimobendan administration on the renin‐angiotensin‐aldosterone system in dogs. Am J Vet Res. 2013;74:1084‐1090. [DOI] [PubMed] [Google Scholar]
- 14. Ames MK, Atkins CE, Lee S, et al. Effects of high doses of enalapril and benazepril on the pharmacologically activated renin‐angiotensin‐aldosterone system in clinically normal dogs. Am J Vet Res. 2015;76:1041‐1050. [DOI] [PubMed] [Google Scholar]
- 15. Lantis AC, Atkins CE, DeFrancesco TC. Effects of furosemide and the combination of furosemide and the labeled dosage of pimobendan on the circulating renin‐angiotensin‐aldosterone system in clinically normal dogs. Am J Vet Res. 2011;72:1646‐1651. [DOI] [PubMed] [Google Scholar]
- 16. Ames MK, Atkins CE, Lantis AC, et al. Evaluation of subacute change in RAAS activity (as indicated by urinary aldosterone: creatinine, after pharmacologic provocation) and the response to ACE inhibition. J Renin Angiotensin Aldosterone Syst. 2016;17(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atkins CE, Haggstrom J. Pharmacologic management of myxomatous mitral valvular disease in dogs. J Vet Cardiol. 2012;14:165‐184. [DOI] [PubMed] [Google Scholar]
- 18. Ames MK, Atkins CE. Beyond furosemide: the role of diuretics in congestive heart failure part 2 – spironolactone. Today's Vet Pract. 2016;6:87‐92. [Google Scholar]
- 19. Keene BW, Atkins CE, Bonagura J, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2019;33:1127‐1140. [DOI] [PubMed] [Google Scholar]
- 20. Atkins CE, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23:1142‐1150. [DOI] [PubMed] [Google Scholar]
- 21. Freeman LM, Rush JE, Farabaugh AE, et al. Development and evaluation of a questionnaire for assessing health‐related quality of life in dogs with cardiac disease. J Am Vet Med Assoc. 2005;226:1864‐1868. [DOI] [PubMed] [Google Scholar]
- 22. Therneau TM, Grambsch PM, Fleming TR. Martingale‐based residuals for survival models. Biometrika. 1990;77:147‐160. [Google Scholar]
- 23. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141‐1154. [Google Scholar]
- 24. Matthews JN, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. Br Med J. 1990;300:230‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309‐1321. [DOI] [PubMed] [Google Scholar]
- 26. Zannad F, McMurray J, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11‐21. [DOI] [PubMed] [Google Scholar]
- 27. Atkins CE, Brown WA, Coats JR, et al. Effects of long‐term administration of enalapril on clinical indicators of renal function in dogs with compensated mitral regurgitation. J Am Vet Med Assoc. 2002;221:654‐658. [DOI] [PubMed] [Google Scholar]
- 28. Lefebvre HP, Ollivier E, Atkins CE, et al. Safety of spironolactone in dogs with chronic heart failure because of degenerative valvular disease: a population‐based, longitudinal study. J Vet Intern Med. 2013;27:1083‐1091. [DOI] [PubMed] [Google Scholar]
- 29. Thomason JD, Rockwell JE, Fallaw TK, et al. Influence of combined angiotensin‐converting enzyme inhibitors and spironolactone on serum K+, Mg++, and Na+ concentrations in small dogs with degenerative mitral valve disease. J Vet Cardiol. 2007;9:103‐108. [DOI] [PubMed] [Google Scholar]
- 30. The IMPROVE Study Group . Acute and short‐term hemodynamic, echocardiographic clinical effects of enalapril maleate in dogs with naturally acquired heart failure: results of the invasive multicenter PROspective veterinary evaluation of enalapril study. J Vet Intern Med. 1995;9:234‐242. [DOI] [PubMed] [Google Scholar]
- 31. The COVE Study Group . Controlled clinical evaluation of enalapril in dogs with heart failure: results of the Cooperative Veterinary Enalapril study group. J Vet Intern Med. 1995;9:243‐252. [DOI] [PubMed] [Google Scholar]
- 32. Ettinger S, Benitz A, Ericsson G, et al. Effects of enalapril maleate on survival of dogs with naturally occurring acquired heart failure. J Am Vet Med Assoc. 1998;213:1573‐1577. [PubMed] [Google Scholar]
- 33. The BENCH BENazepril in Canine Heart Disease Study Group . The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: results of a multi‐center, prospective, randomized, double‐blinded, placebo‐controlled, long‐term clinical trial. J Vet Cardiol. 1999;1:7‐18. [DOI] [PubMed] [Google Scholar]
- 34. Sent U, Haarer‐Kindler M, Huttig A, et al. Prospective multi‐centre, long‐term ramipril (Vasotop®) treatment of dogs with naturally acquired heart failure. Kleintierpraxis. 2000;45:123‐131. [Google Scholar]
- 35. Amberger C, Chetboul V, Bomassi E, et al. Comparison of the effects of imidapril and enalapril in a prospective, multicentric randomized trial in dogs with naturally acquired heart failure. J Vet Cardiol. 2004;6:9‐16. [DOI] [PubMed] [Google Scholar]
- 36. Besche B, Chetboul V, Lefay M‐P, et al. Clinical evaluation of imidapril in congestive heart failure in dogs: results of the EFFIC study. J Small Anim Pract. 2007;48:265‐270. [DOI] [PubMed] [Google Scholar]
- 37. Pitt B, Bakris G, Ruilope LM, et al. Serum potassium and clinical outcomes in the eplerenone post‐acute myocardial infarction heart failure efficacy and survival study (EPHESUS). Circulation. 2008;118:1643‐1650. [DOI] [PubMed] [Google Scholar]
- 38. Montford JR, Linas S. How dangerous is hyperkalemia. J Am Soc Nephrol. 2017;28:3155‐3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mochel JP, Fink M, Peyrou M, et al. Pharmacokinetic/pharmacodynamic modeling of renin‐angiotensin aldosterone biomarkers following angiotensin‐converting enzyme (ACE) inhibition therapy with benazepril in dogs. Pharm Res. 2015;32(6):1931‐1946. [DOI] [PubMed] [Google Scholar]
- 40. Mochel JP, Teng CH, Peyrou M, et al. Sacubitril/valsartan (LCZ696) significantly reduces aldosterone and increases cGMP circulating levels in a canine model of RAAS activation. Eur J Pharm Sci. 2019;128:103‐111. [DOI] [PubMed] [Google Scholar]
- 41. Konta M, Nagakawa M, Sakatani A, et al. Evaluation of the inhibitory effects of telmisartan on drug‐induced renin‐angiotensin‐aldosterone system activation in normal dogs. J Vet Cardiol. 2018;20:376‐383. [DOI] [PubMed] [Google Scholar]
- 42. Haggstrom J, Hansson K, Karlberg BE, et al. Effects of long‐term treatment with enalapril or hydralazine on the renin‐angiotensin‐aldosterone system and fluid balance in dogs with naturally acquired mitral valve regurgitation. Am J Vet Res. 1996;57:1645‐1652. [PubMed] [Google Scholar]
- 43. Lovern CS, Swecker WS, Lee JC, et al. Additive effects of a sodium chloride restricted diet and furosemide administration in healthy dogs. Am J Vet Res. 2001;62(11):1793‐1796. [DOI] [PubMed] [Google Scholar]
- 44. Ames MK, Atkins CE, Eriksson A, et al. Aldosterone breakthrough in dogs with naturally occurring myxomatous mitral valve disease. J Vet Cardiol. 2017;19:218‐227. [DOI] [PubMed] [Google Scholar]
- 45. Palmer BR, Pilbrow AP, Frampton CM, et al. Plasma aldosterone levels during hospitalization are predictive of survival post‐myocardial infarction. Eur Heart J. 2008;29:2489‐2496. [DOI] [PubMed] [Google Scholar]
- 46. Girerd N, Pang PS, Swedberg K, et al. Serum aldosterone is associated with mortality and re‐hospitalization in patients with reduced ejection fraction hospitalized for acute heart failure: analysis from the EVEREST trial. Eur J Heart Fail. 2014;15:1228‐1235. [DOI] [PubMed] [Google Scholar]
- 47. Häggström J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22:1124‐1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Kaplan‐Meier survival curves displaying the probability of not yet experiencing cardiac death in the S+BNZ group (orange solid line) vs the BNZ group (gray dotted line). There were 284 dogs in the S+BNZ group and 285 dogs in the BNZ group at the outset (safety population). Cross marks represent censored observations.
Figure S2 Boxplots of renal functions and electrolytes (safety population). A, Creatinine (mg/dL). B, Potassium (mEq/L). C, Urea nitrogen (BUN) (mg/dL)
Appendix S1. Tables.


