Abstract
Background
Many studies of epilepsy in veterinary medicine use subjective data (eg, caregiver‐derived histories) to determine seizure frequency. Conversely, in people, objective data from electroencephalography (EEG) are mainly used to diagnose epilepsy, measure seizure frequency and evaluate efficacy of antiseizure drugs. These EEG data minimize the possibility of the underreporting of seizures, a known phenomenon in human epileptology.
Objective
To evaluate the correlation between reported seizure frequency and EEG frequency of ictal paroxysmal discharges (PDs) and to determine whether seizure underreporting phenomenon exists in veterinary epileptology.
Animals
Thirty‐three ambulatory video‐EEG recordings in dogs showing ≥1 ictal PD, excluding dogs with status epilepticus.
Methods
Retrospective observational study. Ictal PDs were counted manually over the entire recording to obtain the frequency of EEG seizures. Caregiver‐reported seizure frequency from the medical record was categorized into weekly, daily, hourly, and per minute seizure groupings. The Spearman rank test was used for correlation analysis.
Results
The coefficient value (r s) comparing reported seizure to EEG‐confirmed ictal PD frequencies was 0.39 (95% confidence interval [CI] = 0.048‐0.64, P = .03). Other r s values comparing history against various seizure types were: 0.36 for motor seizures and 0.37 for nonmotor (absence) seizures.
Conclusions and Clinical Importance
A weak correlation was found between the frequency of reported seizures from caregivers (subjective data) and ictal PDs on EEG (objective data). Subjective data may not be reliable enough to determine true seizure frequency given the discrepancy with EEG‐confirmed seizure frequency. Confirmation of the seizure underreporting phenomenon in dogs by prospective study should be carried out.
Keywords: electroencephalography, ictal PDs, paroxysmal discharges, seizure underreporting phenomenon
Abbreviations
- AEEG
ambulatory video‐EEG
- ASD
antiseizure drugs
- EEG
electroencephalography
- PDs
paroxysmal discharges
1. INTRODUCTION
Epilepsy is the most common chronic neurological disorder in veterinary as well as human medicine. 1 , 2 Moreover, many epilepsy features (eg, pathophysiology, physical manifestations, or semiology) and therapeutic options are common to both fields. However, a decisive test for diagnosis and monitoring typically used in people, electroencephalography (EEG), is underutilized in dogs. Electroencephalography is the standard test to confirm seizures, categorize epilepsy conditions and optimize treatment in people. It has a long history of use in veterinary medicine as well, showing evolution in the technique over the years, but with low uptake at last evaluation. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 The International Veterinary Epilepsy Task Force recommends EEG to achieve the highest level of confidence in the diagnosis. 11 Verifying its utility in dogs with epilepsy should help further improve the management of epilepsy in veterinary medicine.
When EEG is used to diagnose seizures and epilepsy in human and veterinary medicine, the abnormalities are similar between the species. Paroxysmal discharge (PD) is a collective term indicating waveforms that have abrupt onset, rapid attainment of a maximum amplitude, and sudden termination. 12 Paroxysmal discharges typically are used to describe epileptiform and seizure patterns. 12 , 13
In people, objective data (ie, EEG) mainly are used to diagnose epilepsy and evaluate the efficacy of antiseizure drugs (ASD). This diagnostic tool can prevent the occurrence of the seizure underreporting phenomenon, the poor ability of patients to describe their seizures. A large body of literature supports this phenomenon in human epileptology. Human patients documented fewer than 50% of their seizures on average compared to EEG, possibly because of seizure‐induced seizure unawareness. 14 , 15 , 16 Generally, one‐third of daytime and two thirds of nocturnal seizures failed to be documented. 15 The descriptive support of patients' relatives, colleagues, or caregivers could somewhat improve the phenomenon, but this support is often only helpful in daytime. Those seizures generally are considered to occur in daytime (55%) and at night (45%) at similar proportions make this support less valuable. 15 In contrast, many studies do not use objective information in veterinary neurology. Instead, subjective data (eg, caregiver‐based questionnaires) are used. 17 Thus, we hypothesized that seizure frequency may be underreported in veterinary medicine. Electroencephalography can be used to evaluate brain function objectively, with detection of physiological and pathological electrical discharges. However, EEG has yet to be used to examine whether or not the seizure underreporting phenomenon exists in dogs with epilepsy.
To generate better awareness of the validity of EEG to assess if seizures can be missed in dogs, we evaluated the correlation between reported seizure frequency and EEG seizure frequency of epileptogenic PDs.
2. MATERIALS AND METHODS
Ours was an observational retrospective study. A search was performed of the medical records of 5 academic and private veterinary referral hospitals: the Ontario Veterinary College (OVC), Seattle Veterinary Specialists (SVS), VCA West Los Angeles Animal Hospital, the University of Helsinki, and LMU Munich.
Inclusion criteria for the dogs were: a complete medical record and ambulatory video‐EEG (AEEG) showing ≥1 ictal PD regardless of ASD treatment regimen. To be considered complete, medical records had to include breed, sex, age, ASD treatment regimen, a description of seizure semiology, and reported seizure frequency. All AEEG recordings were performed in the referral hospitals with owner consent to clinically diagnose and monitor seizures. When sedation or general anesthesia was used for EEG electrode placement, AEEG extended beyond recovery to a normal mentation state. In other words, entirely sedated EEG was excluded. Electroencephalography also was excluded when muscle or movement artifact obscured the cortical signal, precluding identification of PDs. Dogs with status epilepticus also were excluded.
Wireless AEEG with synchronized video was recorded using a Trackit MK3 AEEG/Polygraphy recorder (Lifelines Neurodiagnostic Systems, Troy, Illinois), with scalp EEG using 13 channels (both hemispheres symmetrically in the frontal [F3, F4, F7, F8], parietal [C3, C4], temporal [T3, T4] and occipital [O1, O2] regions and 3 midline anterior‐posterior locations [Fz, Cz, Pz]) in addition to a reference electrode (between the medial canthi) and a ground electrode (dorsal cervical midline, 2‐5 cm caudal to the nuchal crest). 18 Ambulatory EEG was recorded using current standard minimally invasive SC (needle or wire) electrodes. 19 , 20 The duration of AEEG recordings was not standardized for various reasons, including dogs removing electrodes during the recording, sufficient episodes of interest obtained, or time of hospital discharge.
The number of ictal PDs was counted manually throughout the recording and then divided by the duration of the recording (minutes) to obtain seizure frequency (number/min). The starting point of manual counting was when EEG instrumentation was completed and recovery from sedation or general anesthesia was confirmed, if used. Ictal PDs were defined as waveforms that stood out against the background, were of cortical origin (ie, displaying a cortical gradient, electrical field, and possibly lag), and that were associated with a motor or behavioral manifestation suggestive of a seizure on synchronized video. They included spike, polyspike, and spike‐and‐wave patterns. Any single waveform PDs without movement or behavioral manifestation on video were classified as interictal PDs. Furthermore, vigilance levels were taken into consideration. During sleep, any abnormal waveforms (eg, 3 Hz spike‐and‐slow‐wave, not just isolated spikes) without accompanying motor activity (confirmed by synchronized video) were considered interictal PDs, because it would not be possible to ascertain nonmotor seizures during sleep. 21 If associated motor activity was present, events were defined as ictal PDs. Once confirmed to show behavioral manifestation on video, the PDs were classified as focal or generalized according to electroencephalographic ictal onset. Interpretation beyond observation was necessary for classification because some manifestations occurred in >1 seizure type. 1 For example, when the earliest prominent feature was myoclonus, these events were classified as myoclonic seizures. Conversely, seizures in which behavioral arrest (discontinuation of movement and decreased responsiveness to the environment) was the earliest prominent feature were classified as absence seizures when they showed generalized spike‐wave PDs. 1 , 22 For the purposes of our study, the definitions of seizure types were EEG‐based rather than based on the owner's description.
Reported seizure frequency was extracted from the caregiver description in the medical record. This variable was categorized into 4 groups: weekly, daily, hourly, and per minute seizures to minimize recall bias. For example, if the seizure frequency was once per week, it was categorized into the weekly group. If the owner reported the seizure was likely to reoccur within 24 hours, it was categorized into the daily group. If a dog manifested seizures 5‐7 times per week, it would be classified into the weekly group unless the seizures occur every day, in which case the dog would be classified into the daily group.
The correlation between frequency of ictal PDs and reported seizure frequency was determined using the Spearman rank test, because the data were expected to be dependent and not normally distributed. The correlation also was evaluated separately for different types of seizure semiology as grouped by the International League Against Epilepsy and International Veterinary Epilepsy Task Force, investigating motor seizures as well as nonmotor seizures (absence seizures with and without myoclonus). 1 The motor seizure category included focal motor, myoclonic and generalized tonic‐clonic seizures. Statistical significance was defined as P < .05. Statistical tests were performed using RStudio version 1.1.463 (RStudio, PBC, Boston, Massachusetts).
A subset of AEEG data and medical records of these dogs was documented in previous studies, of which 5 Rhodesian Ridgebacks also were included in the present study. 18 , 23 , 24 , 25 However, the correlation between reported seizure frequency and ictal PD frequency was not evaluated.
3. RESULTS
Thirty‐three AEEG recordings of dogs were included. Twenty‐two different breeds were identified, including Alaskan Klee Kai, Beagle, Boxer, Cavalier King Charles Spaniel, Chinese Hairless Crested dog, Dachshund, English Bulldog, French Bulldog, German Shepherd, Golden Retriever, Jack Russell Terrier, Labrador Retriever, Lapponian Herder, Papillon, Pomeranian, Pug, Rhodesian Ridgeback, Siberian Husky, Shetland Sheepdog, Shih Tzu, Yorkshire Terrier, and mixed breed.
There were 16 females (14 spayed) and 17 males (15 castrated). Ages ranged from 1 to 15 years (mean, 7.2 years; median, 6.0 years). In terms of the cause of seizures, 24 suspected idiopathic epilepsy and 9 suspected structural epilepsy (neoplasia, inflammation, infection, and degenerative) cases were identified. With respect to seizure types, 11 absence seizures (33%), 16 myoclonic seizures (49%), 2 generalized tonic‐clonic seizures (6%), and 4 focal motor seizures (12%) were identified based on behavioral manifestations and EEG findings. 1 Of the 11 absence seizures, 2 showed no myoclonus. Examples of typical semiology reported by the caregivers for each seizure type are summarized in Table 1.
TABLE 1.
Examples of typical semiology reported by the caregivers for each seizure type
| Type of seizures | Typical semiology reported by the caregivers |
|---|---|
| Absence seizure with/without myoclonus | Unresponsive abnormal behavior, zoning out with/without eye, face or body twitches, or head bobbing |
| Myoclonic seizures | Twitches on face, neck, or whole body or head bobbing |
| Generalized tonic‐clonic seizures | Stiff legs followed by jerky paddling movements |
| Focal motor seizures | Rhythmic muscle twitches in 1 leg |
Note: There are similarities in semiology among certain seizure types.
The duration of all AEEG recordings ranged from 5 to 1320 minutes (median, 100 minutes; mean, 214.1 minutes). Eight AEEG recordings were made after use of sedation or general anesthesia for electrode placement, but manual count of ictal PDs was performed after complete recovery from sedation or anesthesia. Ictal PD frequency during AEEG ranged from 0.002 to 2.54 (number/min; median, 0.30/min; mean, 0.61/min). Duration of ictal PDs typically was 1‐30 seconds, but intra‐dog variability was identified. Therefore, we did not describe the duration of ictal PDs statistically (ie, mean, median).
Reported seizure frequency (weekly, daily, hourly, or per minute) and ictal PDs frequency (number/min) are shown in Figure 1. The coefficient value (r s) comparing reported seizure and ictal PD frequency was 0.39 with 95% confidence interval (CI) = 0.048‐0.64 and P = .03, indicating weak correlation between them (Table 2).
FIGURE 1.
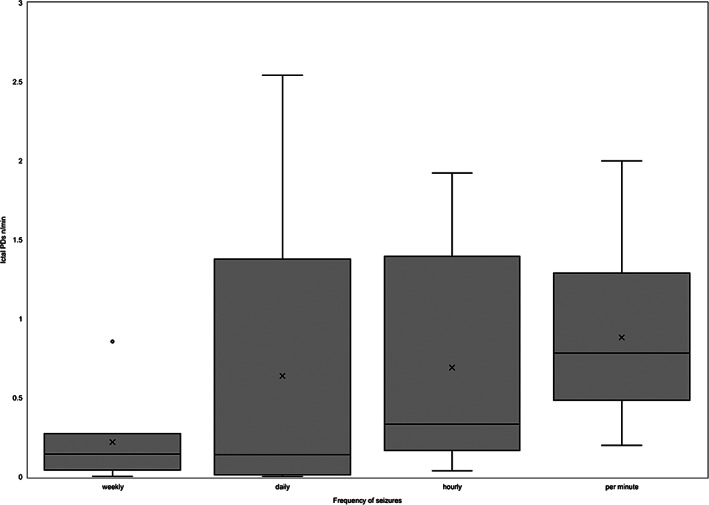
Boxplot for frequency of reported seizure (subjective) and ictal paroxysmal discharges (PDs; objective) from ambulatory video‐EEG (AEEG) recordings of 33 dogs. The number of ictal PDs was counted manually through the AEEG recording and then divided by the duration of the recording (minutes) in order to obtain frequency of ictal PDs (n/min). Reported seizure frequency from caregivers was categorized into 4 groups: weekly, daily, hourly, and per minute seizures. O, outlier; X, mean marker
TABLE 2.
Comparison of the reported seizures and ictal paroxysmal discharge (PD) frequency, Spearman's rank correlation test for each seizure type
| Correlation | ||||||
|---|---|---|---|---|---|---|
| Type of seizures | Number of cases | Coefficient values (r s) | 95% CI | P value | Positive/Negative | Meaning |
| All types | 33 | 0.39 | 0.048 to 0.64 | .03 | Positive | Weak correlation |
| Motor seizures | 22 | 0.36 | −0.075 to 0.68 | .1 | – | Nonsignificant |
| Myoclonic seizures | 16 | 0.21 | −0.32 to 0.64 | .44 | – | Nonsignificant |
| All absence seizures | 11 | 0.37 | −0.29 to 0.80 | .26 | – | Nonsignificant |
| Absence with myoclonus | 9 | 0.64 | −0.035 to 0.92 | .06 | – | Nonsignificant |
Abbreviation: CI, confidence interval.
The motor seizures category included myoclonic, focal motor, and generalized tonic‐clonic cases, based on clinical manifestations. 26 The r s values comparing history against various seizure types thus were: 0.36 (95% CI = −0.075 to 0.68; P = .1) for motor seizures and 0.21 (95% CI = −0.32 to 0.64; P = .44) for myoclonic seizures. Because of low case numbers, correlations were not calculated for focal motor and generalized tonic‐clonic seizure types. The nonmotor seizures category included absence seizures with and without myoclonus based on clinical manifestations. 26 The correlation was 0.37 (95% CI = −0.29 to 0.80; P = .26) for absence seizures with and without myoclonus and 0.64 (95% CI = −0.035 to 0.92; P = .06) for absence seizures with myoclonus. Because there were only 2 cases of absence seizures without myoclonus, correlation was not calculated.
Furthermore, r s was calculated in 2 groups (with ASD and without ASD) to determine if ASD treatment affected the correlation between reported seizure frequency and ictal PD frequency. Dogs without ASD treatment (n = 17) showed r s = 0.53 (moderate correlation, 95% CI = 0.067‐0.81; P = .03), whereas dogs receiving ASD treatment (n = 16) had r s = 0.35 (95% CI = −0.18 to 0.72; P = .19), which suggested a difference between those with and without ASD treatment. However, this difference failed to reach statistical significance (P = .55: Z test). Dogs in the ASD treatment group were receiving 1 or more of the following drugs: gabapentin, imepitoin, levetiracetam, phenobarbital, primidone, and zonisamide.
4. DISCUSSION
We found a weak correlation (r s = 0.39) between seizure frequency reported by caregivers (subjective data) and ictal PD frequency on AEEG (objective data), making it likely that the seizure underreporting phenomenon occurs in veterinary patients and that reported seizure frequency does not match EEG seizure frequency. Importantly, subjective data may not be sufficiently reliable to determine actual seizure frequency, given the discrepancy with EEG‐confirmed seizure frequency. Therefore, our data suggest that EEG should be performed to confirm actual seizure frequency when seizures occur on at least a weekly basis.
Correlations for history and seizure types did not reach statistical significance. However, some observations suggest that additional prospective studies with larger sample sizes may be warranted. Within the motor seizures category, the correlation for myoclonic seizures was low (r s = 0.21). Dogs with myoclonic seizures were categorized into all groups of reported seizure frequency (1, weekly; 9, daily; 2, hourly; and 4, per minute). Characteristics of seizures included muscle twitches (focal facial muscles) and muscle jerks (head, neck, limb, or whole body). It might be expected that myoclonic seizures would have strong correlation with reported seizure frequency whereas absence seizures would have weak correlation, because not all absence seizures are manifested by myoclonus, but such was not the case. One reason for this finding might be that myoclonic episodes may vary in intensity and happen during the night, meaning that caregivers could easily overlook them. Similarly, the weak correlation for all absence seizures (r s = 0.37) and strong correlation for absence seizures with myoclonus (r s = 0.64) was expected. Absence seizures with myoclonus are more conspicuous, leading to a longer episode, and enabling caregivers to detect episodes more accurately. On the other hand, seizures without myoclonus are more challenging to detect because their manifestations may not be marked enough to be discerned. None of the seizure types had more than a moderate correlation, which again suggests that EEG should be utilized to accurately document seizure frequency.
The r s of 0.53 for dogs without ASDs (n = 17) was larger than that of dogs receiving ASDs (r s = 0.35; n = 16), but no statistically significant difference was identified (P = .55; Z test). This finding warrants future research, because ASD treatment might have decreased the severity of motor manifestations, which in turn could have made their detection by caregivers more challenging. This possibility suggests that ASD administration may mask some subclinical seizures, and treatment should be taken into account when evaluating the seizure frequency from the caregiver's observations only. Additional research using large case numbers would be needed to elucidate this issue.
We calculated the number of ictal PDs over the duration of AEEG recording, but we acknowledge there may be controversy over the definition of an ictal PD. Detection is easier for dogs manifesting motor seizures. By contrast, ictal PDs may be more difficult to identify when affected dogs show no motor manifestations, such as during absence seizures. 24 , 27 Thus, the human medical literature 21 was used to determine the definition of ictal PDs in our study. During sleep, any abnormal waveforms, even 3 Hz spike‐and‐slow‐waves without motor manifestations (confirmed using synchronized video) were considered to be interictal PDs, otherwise we defined them as ictal PDs. On the other hand, while the patient is awake, any abnormal waveforms that do not constitute background activity would be considered ictal PDs as long as the waveforms have cortical origin. These ictal PDs did not include single waveform PDs without movement because these were classified as interictal. Additional uncertainty may be introduced by not counting those PDs. Therefore, it was not possible to count all myoclonic episodes because sometimes muscle artifact prevented confirmation of the cortical origin of the waveform. Furthermore, in humans, many reported physiological patterns resemble ictal PDs (eg, hypnagogic or hypnopompic hypersynchrony and positive occipital sharp transients of sleep). 28 , 29 , 30 These EEG patterns have yet to be reported in dogs. Finally, EEG interpretation agreement within and among veterinary reviewers has yet to be reported. Thus, we recognize the limitations of EEG interpretation in dogs that could lead to overinterpretation of ictal PDs.
Given these considerations, the frequency of myoclonic and absence episodes on EEG in our study may be different from the true event frequency. However, in our study population, EEG recordings showing either nonmotor sleep discharges or myoclonic episodes with obfuscating muscle artifact did not account for the majority, and it is unlikely that they would substantially alter our results. Furthermore, not counting these episodes on EEG would predispose to undercounting, strengthening the correlation with caregiver‐reported seizure frequency. This could be considered a practical limitation of AEEG in veterinary medicine and thus our standardized calculation method is reasonable to detect ictal PDs.
Our study had some other limitations. Selection and information bias could have occurred because of the retrospective nature of our study (ie, very few EEG studies were requested for dogs manifesting generalized tonic‐clonic seizures, perhaps because there was less doubt that these were seizures). As indicated, the seizures reported by caregivers were classified into 4 groups to minimize information bias, but recall bias still may have occurred. In addition, dogs that underwent AEEG monitoring may not be representative of the general population. Indeed, absence seizures represented 33.3% of all seizure types in our study, whereas their incidence in all seizure types is approximately 2.2%‐6% in the human population with epilepsy. 31 , 32 The fact that 15% of our study population consisted of Rhodesian Ridgebacks suffering from a distinct genetic epilepsy characterized by myoclonic and absence seizures may have influenced our results.
There is poor agreement among veterinary observers on the visual identification of seizure types other than generalized tonic‐clonic seizures, and thus EEG was more likely to be ordered for patients with nongeneralized tonic‐clonic seizures. 33 Unfortunately, this meant that in our study there were few cases of generalized motor (tonic‐clonic) and focal to bilateral tonic‐clonic seizures, frequently encountered seizure types in the general population. 31 , 34 , 35 A prospective multicenter investigation of the seizure underreporting phenomenon still is needed to address these limitations and provide more accurate estimates of the incidence of seizure types in the canine population. Lastly, blinded EEG interpretation was not performed while reviewing the AEEG recording data. It would be challenging to blind the AEEG evaluators because the recordings were reviewed using video synchronization to confirm behavioral ictal manifestations. We acknowledge that this lack of blinded EEG interpretation also may have introduced bias.
Accurate seizure control has an impact on dogs with epilepsy with regard to their quality of life and survival time. 36 The common goal in all types of epilepsy is to control seizure activity by preventing spread of aberrant electrical discharges from the epileptogenic focus and increasing the seizure threshold using ASDs. 37 , 38 Electroencephalographic PDs are used in human medicine as objective data to evaluate frequency, severity, and location of onset of seizures as well as a measure of ASD efficacy. 39 , 40 , 41 , 42 , 43 , 44 , 45 However, in veterinary medicine, subjective data (eg, caregiver‐based questionnaires) mainly have been used. 17 The possible inaccuracy of caregiver seizure frequency estimates increases the likelihood that the efficacy of ASDs may be overestimated. Utilizing AEEG in seizure management could decrease the overestimation of ASD efficacy. A prospective study is needed to clarify this possibility.
In conclusion, our study indicates that only a weak correlation exists between caregiver‐reported seizure frequency (subjective data) and ictal PD frequency on EEG (objective data), that is, caregiver‐reported seizure frequency does not match EEG seizure frequency. Importantly, this result points out that subjective data may not be sufficiently reliable to determine true seizure frequency, given the discrepancy with confirmed seizure frequency on AEEG. Therefore, we believe AEEG should be performed to determine objective seizure frequency, especially when dogs are suspected to manifest myoclonic seizures or behavioral arrest. These findings cast doubt on the ease of clinical management of some seizure types. Confirmation of the seizure underreporting phenomenon in veterinary medicine by prospective studies would be ideal.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All ambulatory video‐electroencephalography recordings were performed with owner consent for clinical reasons.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by the Ontario Veterinary College Pet Trust Fund (#054488), the Ontario graduate scholarship, the Jane and Aatos Erkko Foundation (HL), and the Canada Foundation for Innovation (#30953). The support of Dr William Sears, University of Guelph, Canada, for help with statistical analysis is gratefully acknowledged.
Ukai M, Parmentier T, Cortez MA, et al. Seizure frequency discrepancy between subjective and objective ictal electroencephalography data in dogs. J Vet Intern Med. 2021;35(4):1819–1825. 10.1111/jvim.16158
Funding information Canada Foundation for Innovation, Grant/Award Number: #30953; Jane ja Aatos Erkon Säätiö; Ontario Veterinary College Pet Trust Fund, Grant/Award Number: #054488
REFERENCES
- 1. Fisher RS, Cross JH, D'Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58(4):531‐542. [DOI] [PubMed] [Google Scholar]
- 2. Thomas WB. Idiopathic epilepsy in dogs and cats. Vet Clin North Am Small Anim Pract. 2010;40(1):161‐179. [DOI] [PubMed] [Google Scholar]
- 3. Klemm WR. Technical aspects of electroencephalography in animal research. Am J Vet Res. 1965;26(115):1237‐1248. [PubMed] [Google Scholar]
- 4. Fox MW. Postnatal development of the EEG in the dog‐III. J Small Anim Pract. 1967;8(2):109‐112. [DOI] [PubMed] [Google Scholar]
- 5. Steiss JE. A survey of current techniques in veterinary electrodiagnostics: EEG, spinal evoked and brainstem auditory evoked potential recording. Vet Res Commun. 1988;12(4):281‐288. [DOI] [PubMed] [Google Scholar]
- 6. Croft PG. The EEG as an aid to diagnosis of nervous diseases in the dog and cat. J Small Anim Pract. 1962;3(4):205‐213. [Google Scholar]
- 7. Redding RW, Knecht CE. Atlas of Electroencephalography in Dog and Cat. New York, NY: Praeger Publishers; 1984. [Google Scholar]
- 8. Holliday TA, Williams DC. Advantages of Digital Electroencephalography in Clinical Veterinary Medicine Part 1. Vet Neurol Neurosurg J. 2001;3(1):11. [Google Scholar]
- 9. Holliday TA, Williams DC. Advantages of Digital Electroencephalography in Clinical Veterinary Medicine‐2. Vet Neurol Neurosurg J. 2003;5(1):16. [Google Scholar]
- 10. Utsugi S, Saito M, Sato T, et al. Relationship between interictal epileptiform discharges under medetomidine sedation and clinical seizures in canine idiopathic epilepsy. Vet Rec. 2020;187(2):67‐67. [DOI] [PubMed] [Google Scholar]
- 11. De Risio L, Bhatti S, Muñana K, et al. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kane N, Acharya J, Beniczky S, et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract. 2017;2:170‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Risio L, Platt S. Canine and Feline Epilepsy: Diagnosis and Management. Wallingford, UK: CABI; 2014. [Google Scholar]
- 14. Elger CE, Hoppe C. Diagnostic challenges in epilepsy: seizure under‐reporting and seizure detection. Lancet Neurol. 2018;17(3):279‐288. [DOI] [PubMed] [Google Scholar]
- 15. Blachut B, Hoppe C, Surges R, et al. Counting seizures: the primary outcome measure in epileptology from the patients' perspective. Seizure. 2015;29:97‐103. [DOI] [PubMed] [Google Scholar]
- 16. Hoppe C, Poepel A, Elger CE. Epilepsy – accuracy of patient seizure counts. Arch Neurol. 2007;64(11):1595‐1599. [DOI] [PubMed] [Google Scholar]
- 17. Charalambous M, Brodbelt D, Volk HA. Treatment in canine epilepsy—a systematic review. BMC Vet Res. 2014;22(10):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. James FMK, Cortez MA, Monteith G, et al. Diagnostic utility of wireless video‐electroencephalography in unsedated dogs. J Vet Intern Med. 2017;31(5):1469‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. James FMK, Allen DG, Bersenas AME, et al. Investigation of the use of three electroencephalographic electrodes for long‐term electroencephalographic recording in awake and sedated dogs. Am J Vet Res. 2011;72(3):384‐390. [DOI] [PubMed] [Google Scholar]
- 20. Musteata M, Borcea DG, Ștefănescu R, et al. Influence of stainless needle electrodes and silver disk electrodes over the interhemispheric cerebral coherence value in vigil dogs. Sensors. 2018;18(11):3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hrachovy RA, Frost JD. The EEG in selected generalized seizures. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2006;23(4):312‐332. [DOI] [PubMed] [Google Scholar]
- 22. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522‐530. [DOI] [PubMed] [Google Scholar]
- 23. Parmentier T, Monteith G, Cortez MA, et al. Effect of prior general anesthesia or sedation and antiseizure drugs on the diagnostic utility of wireless video electroencephalography in dogs. J Vet Intern Med. 2020;34(5):1967‐1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wielaender F, James FMK, Cortez MA, et al. Absence seizures as a feature of juvenile myoclonic epilepsy in Rhodesian Ridgeback dogs. J Vet Intern Med. 2018;32(1):428‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wielaender F, Sarviaho R, James F, et al. Generalized myoclonic epilepsy with photosensitivity in juvenile dogs caused by a defective DIRAS family GTPase 1. Proc Natl Acad Sci U S A. 2017;114(10):2669‐2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berendt M, Farquhar RG, Mandigers PJJ, et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poma R, Ochi A, Cortez MA. Absence seizures with myoclonic features in a juvenile Chihuahua dog. Epileptic Disord. 2010;12(2):138‐141. [DOI] [PubMed] [Google Scholar]
- 28. Azzam R, Azar NJ. Marked seizure reduction after MCT supplementation. Case Rep Neurol Med. 2013;2013:809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asadi‐Pooya AA, Sperling MR. Normal awake, drowsy, and sleep EEG patterns that might be overinterpreted as abnormal. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2019;36(4):250‐256. [DOI] [PubMed] [Google Scholar]
- 30. Mizrahi EM. Avoiding the pitfalls of EEG interpretation in childhood epilepsy. Epilepsia. 1996;37(1):S41‐S51. [DOI] [PubMed] [Google Scholar]
- 31. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935‐1984. Epilepsia. 1993;34(3):453‐468. [DOI] [PubMed] [Google Scholar]
- 32. Engel J, Pedley TA, Aicardi J. Incidence and prevalence. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia, PA: Wolters Kluwer; 2008:45‐56. [Google Scholar]
- 33. Packer RMA, Berendt M, Bhatti S, et al. Inter‐observer agreement of canine and feline paroxysmal event semiology and classification by veterinary neurology specialists and non‐specialists. BMC Vet Res. 2015;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berendt M, Gram L. Epilepsy and seizure classification in 63 dogs: a reappraisal of veterinary epilepsy terminology. J Vet Intern Med. 1999;13(1):14‐20. [PubMed] [Google Scholar]
- 35. Licht BG, Licht MH, Harper KM, et al. Clinical presentations of naturally occurring canine seizures: similarities to human seizures. Epilepsy Behav. 2002;3(5):460‐470. [DOI] [PubMed] [Google Scholar]
- 36. Fredsø N, Koch BC, Toft N, et al. Risk factors for survival in a university hospital population of dogs with epilepsy. J Vet Intern Med. 2014;28(6):1782‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Husna M, Kurniawan SN. Biomolecular mechanism of antiepileptic drugs. Malang Neurol J. 2018;4(1):38‐45. [Google Scholar]
- 38. Podell M, Volk HA, Berendt M, et al. 2015 ACVIM small animal consensus statement on seizure management in dogs. J Vet Intern Med. 2016;30(2):477‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benbadis SR, O'Neill E, Tatum WO, et al. Outcome of prolonged video‐EEG monitoring at a typical referral epilepsy center. Epilepsia. 2004;45(9):1150‐1153. [DOI] [PubMed] [Google Scholar]
- 40. Esmail EH, Nawito AM, Labib DM, et al. Focal interictal epileptiform discharges in idiopathic generalized epilepsy. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2016;37(7):1071‐1077. [DOI] [PubMed] [Google Scholar]
- 41. Sagi V, Kim I, Bhatt AB, et al. Generalized paroxysmal fast activity in EEG: an unrecognized finding in genetic generalized epilepsy. Epilepsy Behav. 2017;76:101‐104. [DOI] [PubMed] [Google Scholar]
- 42. Scheuer ML, Continuous EEG. Monitoring in the intensive care unit. Epilepsia. 2002;43(3):114‐127. [DOI] [PubMed] [Google Scholar]
- 43. Shih JJ, Fountain NB, Herman ST, et al. Indications and methodology for video‐electroencephalographic studies in the epilepsy monitoring unit. Epilepsia. 2018;59(1):27‐36. [DOI] [PubMed] [Google Scholar]
- 44. Szaflarski JP, Lindsell CJ, Zakaria T, et al. Seizure control in patients with idiopathic generalized epilepsies: EEG determinants of medication response. Epilepsy Behav. 2010;17(4):525‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tatum WO, Rubboli G, Kaplan PW, et al. Clinical utility of EEG in diagnosing and monitoring epilepsy in adults. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2018;129(5):1056‐1082. [DOI] [PubMed] [Google Scholar]


