Abstract
Background
Lobar emphysema in dogs and cats is caused by bronchial collapse during expiration and subsequent air trapping. Congenital causes such as bronchial cartilage defects or acquired causes such as compressive neoplastic lesions have been reported. Morbidity results from hyperinflation of the affected lung lobe and compression of adjacent thoracic structures.
Objective
To describe patient characteristics and imaging findings in dogs and cats with lobar emphysema.
Animals
Fourteen dogs and 3 cats with lobar emphysema diagnosed by imaging findings were retrospectively identified from veterinary referral hospital populations over a 10‐year period.
Methods
Cases that included thoracic radiography, thoracic computed tomography (CT), or both were included. All images were reviewed by a European College of Veterinary Diagnostic Imaging diplomate. Relevant case information included signalment, clinical findings, treatment, and histopathology where available.
Results
Ten of 17 (59%) patients were presented for evaluation of dyspnea and 6 (35%) for coughing. Eleven (65%) patients were <3 years of age. The right middle lung lobe was affected in 12 cases (71%) and multiple lobes were affected in 7 cases (41%). Congenital lobar emphysema was suspected in 14 cases (82%).
Conclusion and Clinical Importance
Lung lobe hyperinflation, atelectasis of nonaffected lung lobes, mediastinal shift, and thoracic wall and diaphragmatic wall deformation were common findings. Lobar or multilobar emphysema should be considered in patients with dyspnea or coughing, particularly younger patients. Although radiography is useful, CT provides better detail. In older patients, acquired causes of bronchial compression should be considered.
Keywords: anatomy and pathology, CLE, respiratory tract
Abbreviations
- CLE
congenital lobar emphysema
- CT
computed tomography
- ECVDI
European College of Veterinary Diagnostic Imaging
- HU
Hounsfield units
1. INTRODUCTION
Pulmonary lobar emphysema is rare in dogs and cats, and uncommon in humans, where it is most frequently identified during infancy and therefore often suspected to be congenital in nature. 1 , 2 , 3 Lobar emphysema is characterized by hyperinflation of ≥1 lung lobe because of bronchial obstruction and air trapping. During inspiration, negative airway pressure allows the affected bronchus to remain open. However, during expiration, the affected bronchus collapses, resulting in a 1‐way valve effect, with distension of the affected lobe because of accumulation of trapped air and subsequent compression of the adjacent lung lobes, heart, and mediastinal structures. 1 Progressive compression of these structures may result in rapid clinical deterioration because of cardiovascular and respiratory compromise or both.
Several cases of congenital lobar emphysema (CLE) are reported in the veterinary literature. Affected dogs and cats typically present as young animals with acute dyspnea. However, animals presenting with more chronic respiratory signs such as a cough, tachypnea, and exercise intolerance also have been reported. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 In animals, antemortem diagnosis is achieved using radiography, computed tomography (CT) or a combination of these techniques. Lung lobectomy has been described for management of CLE, with a good outcome reported in 8 of 8 cases reported. 4 , 5 , 6 , 9 , 10 , 11 , 14 , 15
Congenital defects of bronchial cartilage commonly are associated with development of CLE in human infants. 2 Extramural bronchial obstructions, including vascular developmental abnormalities such as a pulmonary artery sling anomaly, also have been reported as causes of CLE. 1 In addition, lesions such as bronchogenic cysts or lymphadenopathy have been reported as causes of acquired lobar emphysema in human medicine. 3 Bronchial cartilage defects have been described histologically in dogs with CLE, similar to human patients. 4 , 5 , 6 , 7 , 8 , 9 , 10 Histopathology is helpful in determining the etiology of CLE secondary to bronchial cartilage hypoplasia or aplasia. 4 , 5 , 6 , 7 , 8 , 9 , 17
Previous information about cases of lobar emphysema in dogs and cats is limited to single case reports. The purpose of our study was to complement the existing literature in terms of patient characteristics, pathophysiological causes, and diagnostic imaging findings.
2. MATERIAL AND METHODS
Medical records of dogs and cats with a diagnosis of lobar emphysema between January 2008 and October 2018 were reviewed from 6 referral veterinary hospitals: The Small Animal Teaching Hospital, University of Liverpool, Neston, UK; The Hospital for Small Animals, University of Edinburgh, Roslin, UK; Willows Veterinary Centre and Referral Service, Solihull, UK; North Carolina State Veterinary Hospital, Raleigh, North Carolina, USA; Vetivia Referral Service, Biarritz, France; and Istituto Veterinario di Novara, Novara, Italy.
Patients with thoracic radiography, thoracic CT, or both were included. Information regarding species, age, sex, body weight, breed, and presenting clinical signs were obtained from the medical records. A diagnosis of lobar emphysema was based on imaging findings including diffuse enlargement of ≥1 lung lobe with rounded margins, generalized hypoattenuation or radiolucency and any consequent compressive effect on surrounding structures using either thoracic radiography or CT. Absence of surgical treatment or pathological confirmation were not considered exclusion criteria. Other forms of emphysematous lung disease such as asthma in cats were excluded after review of the images if findings were not consistent with lobar emphysema. Additional information including clinicopathological findings, additional diagnostic imaging, surgical intervention, and histopathological diagnosis also were reported where available.
3. IMAGING STUDIES
Imaging studies were performed with the animal under sedation or general anesthesia. Diagnosis was based on radiography, CT, or a combination of both. Computed tomography was performed using a Toshiba Aquilion Prime 80‐slice, Siemens Somatom Volume Zoom 4‐slice, Siemens Definition AS 64‐slice, Siemens Symphony 64‐slice, GE Lightspeed 16‐slice helical, Siemens Somatom Emotion 16‐slice or General Electric Brightspeed 16‐slice systems. All CT scans were performed with the patients positioned in sternal recumbency. A range of image acquisition protocols were used with slice thicknesses between 1 and 1.25 mm.
All images were reviewed by a European College of Veterinary Diagnostic Imaging (ECVDI) board‐certified radiologist (J. M.) and an ECVDI resident in diagnostic imaging (J. G.) using digital imaging and communications in medicine viewer software, RadiAnt. Lung and soft tissue reconstructions were reviewed and multiplanar reconstruction was used when beneficial. Reviewers were not blinded to the final diagnosis of lobar emphysema (as part of the inclusion criteria) but were blinded to the surgical and pathological diagnosis when these were available. Imaging findings were recorded directly into descriptive sheets that were specifically designed by the investigators for both radiography and CT images.
On radiographs, the affected lobe or lobes were identified and the severity of the radiolucency of the emphysematous lobe was subjectively scored as mild, moderate, or severe. The bronchus supplying the affected lobe was identified and abnormalities (collapse, bronchial wall thickening, bronchiectasis) were noted where visible. In addition, the size of the pulmonary vessels of the affected lobe was subjectively assessed. Where CT was used, the affected lobe or lobes were identified and the number of Hounsfield units (HU) of the abnormal lung tissue was estimated on precontrast images by calculating the mean score from 3 regions of interest placed over the emphysematous lung lobe, taking care to avoid bronchovascular structures. These results then were compared with the average attenuation HU of an adjacent unaffected lobe that had no evidence of hyperinflation or atelectasis. The affected area of the emphysematous lobe was subjectively scored for the severity of hyperinflation (mild, moderate, or severe). Additional changes in the affected lobe such as soft tissue or cavitatory lesions were recorded. The affected bronchus was identified and recorded and presence of collapse, obstruction, bronchial wall thickening, or bronchiectasis was noted.
For both radiography and CT studies, changes in other lobes then were described, including the presence of abnormal lung patterns or atelectasis. Atelectasis was suspected when decreased volume and concurrent increased soft tissue opacity of the lung lobe were present. Mediastinal shift, tracheal compression, diaphragmatic deformation, thoracic wall deformation, cardiac silhouette compression, pleural effusion, and pneumothorax were subjectively graded on a 0 (absent) to 4 (severe) scale. Other congenital abnormalities, lymphadenomegaly, and any additional abnormalities also were noted if identified.
4. RESULTS
Seventeen animals including 14 dogs and 3 cats with lobar emphysema met the inclusion criteria. The breeds of dogs were Jack Russell Terrier (n = 2), Maltese (n = 2), and 1 each of Dachshund, Pekingese, Staffordshire Bull Terrier, Pomeranian, Rottweiler, Brittany Spaniel, Beagle, mixed breed, Weimaraner, and Shih Tzu. There were 6 males (3 neutered) and 9 females (5 spayed). The age of the dogs ranged from 7 weeks to 14 years old. Of the 14 dogs, 10 were <3 years of age and among those 6 were <1 year of age. The remaining 4 dogs were >10 years of age. The weights of dogs ranged from 2 to 35.7 kg (median, 4.7 kg).
The breeds of cats included 1 each of domestic shorthair, domestic longhair, and Abyssinian. There were 2 female cats (1 spayed) and 1 neutered male cat. The ages of the cats were 4 months old, 11 years old, and 12 years old, respectively. The weights of the cats ranged from 1.6 to 5.5 kg.
The most common presenting signs (cats and dogs) were dyspnea (10 of 17), coughing (6 of 17), dysphagia (3 of 17), vomiting (3 of 17), tachypnea (2 of 17), abdominal distension (2 of 17), increased respiratory noise (1 of 17), collapse (1 of 17), and anorexia (1 of 17). The clinical features are summarized in Table 1. In 1 case, lobar emphysema was identified as an incidental finding during investigation of co‐morbid conditions. The duration of clinical signs ranged from 1 day to 10 years.
TABLE 1.
Clinical features in 17 cases of lobar emphysema in dogs and cats
| Recorded parameter | Number of cases |
|---|---|
| Presenting sign | |
| Dypsnea | 10 |
| Coughing | 6 |
| Dysphagia | 3 |
| Vomiting | 3 |
| Tachypnea | 2 |
| Abdominal distension | 2 |
| Increased respiratory noise | 1 |
| Collapse | 1 |
| Anorexia | 1 |
| Imaging modality | |
| CT and radiography | 4 |
| CT only | 10 |
| Radiography only | 3 |
| Number of affected lung lobes on CT | |
| One | 9 |
| Two | 3 |
| Three | 1 |
| Four | 1 |
| Diagnosis | |
| Congenital lobar emphysema | 14 |
| Pulmonary neoplasia | 2 |
| Diaphragmatic hernia | 1 |
| Outcome | |
| Surgery | 10 |
| Survival to discharge | 8 |
| Perioperative death | 2 |
| Euthanasia | 2 |
| Died | 1 |
| Monitoring only | 2 |
| Lost to follow‐up | 2 |
Abbreviation: CT, computed tomography.
4.1. Clinicopathological findings
Hematology results were available for 13 of 17 patients (10 dogs, 3 cats). Abnormalities included thrombocytosis (3/13), neutrophilia (2/13), reticulocytosis (2/13), increased mean corpuscular hemoglobin concentration (2/13), increased hemoglobin concentration (1/13), and monocytosis (1/13). Biochemistry results were available for 13 of 17 patients (10 dogs, 3 cats). Increased preprandial and postprandial bile acid concentrations were detected in 1 dog that was diagnosed with a congenital portosystemic shunt at the time of investigations. Increased alanine aminotransferase (3/13) and increased alkaline phosphatase (3/13) activities were the only additional biochemical abnormalities in 3 dogs. No consistent clinicopathological abnormalities were detected in any animals.
4.2. Radiographic findings
Radiography of the thorax was performed in 7 patients (5 dogs and 2 cats) and the findings are summarized in Table 2. The affected lobe was readily identified in 3 cases. In the remaining 4 cases, it was more challenging to determine with certainty the affected lobe. In 3 of these cases, it was difficult to determine whether the right middle lobe, right cranial lobe, or both were affected (Figure 4).
TABLE 2.
Radiographic features of 7 dogs and cats with lobar emphysema
| Radiographic features of lobar emphysema | |
|---|---|
| Recorded parameter | Number of cases |
| Correct identification of the affected lung lobe possible (radiography) | |
| Yes | 3 |
| No | 4 |
| Radiography features | |
| Atelectasis of adjacent lobes | 6 |
| Mediastinal shift | 5 |
| Diaphragm deformation | 5 |
| Thoracic wall deformation | 5 |
FIGURE 4.
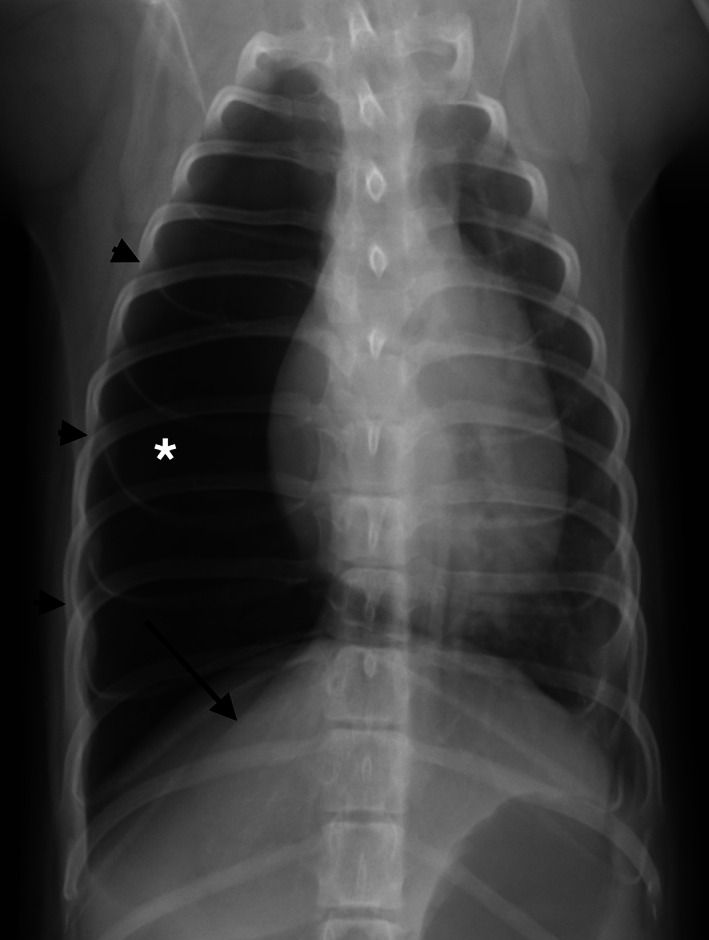
Dorsoventral radiograph of the thorax of a dog with congenital lobar emphysema (CLE) of the right middle lung lobe. Note the enlarged and hyperlucent lung lobe (*), expanded right side of the rib cage (black arrowheads) and compressed right diaphragmatic crus (black arrow). It was not possible to determine which lobe was emphysematous based on the radiographs alone
The radiolucency of the emphysematous lobe was graded as moderate in 6 cases and mild in 1 case. The affected lobe enlargement was scored as moderate in 6 cases and mild in 1 case. Cavitary lesions or soft tissue lesions within the affected lobes were not visualized on radiography. Collapse of the affected bronchus was visualized radiographically in 1 case. Bronchial obstruction, collapse, or both were not identified radiographically in the remaining 6 cases. The pulmonary vessels supplying the affected lung lobes were subjectively small in all cases. Bronchiectasis of the affected bronchus was identified in 3 cases (3 dogs).
An unstructured interstitial lung pattern was identified in the nonemphysematous caudo‐dorsal lung fields in 4 cases (2 dogs and 2 cats). Atelectasis of nonemphysematous lung lobes was visible in 6 cases (4 dogs and 2 cats). Mediastinal shift was detected in 5 of 7 cases (1 = score 4, 2 = score 2, 2 = score 1). Moderate tracheal compression was identified in 1 dog (1 = score 2). Mild pleural effusion was detected in 1 dog (1 = score 2). Pneumothorax was not evident in any case. The pulmonary vessels in the remainder of the nonemphysematous lung fields appeared either normal or were difficult to visualize (1 dog) and no congenital abnormalities were detected. Flattening of the diaphragm was identified in 5 cases (2 = score 2, 3 = score 1), and deformation of the thoracic wall was identified in 5 cases (2 = score 2, 3 = score 1). Thoracic lymphadenomegaly was not identified. Additional findings included a diaphragmatic hernia with herniation of abdominal viscera into the thorax in 1 cat. Hepatomegaly was noted in 1 dog and microhepatica in another dog.
4.3. Computed tomography findings
Most CT findings are summarized in Table 3. The affected lobes were identified in all 14 cases (12 dogs and 2 cats). The number of affected lobes was 1 in 9 cases, 2 in 3 cases, 3 in 1 case, and 4 in 1 case. The right middle lobe was most commonly involved (10/12) followed by the right cranial (4/12), accessory (2/12), and left cranial (2/12) lobes (Figure 3). The mean attenuations of the emphysematous lung and nonemphysematous normal lung are presented in Table 3.
TABLE 3.
CT features in 14 cases of lobar emphysema in dogs and cats
| CT features of lobar emphysema | |
|---|---|
| Recorded parameter | |
| Hounsfield units (HU) | Mean value |
| Affected lobe(s) | −949 |
| Nonemphysematous lung | −741 |
| Hyperinflation severity | Number of Cases |
| Mild | 5 |
| Moderate | 2 |
| Marked | 7 |
| CT features | Number of Cases |
| Atelectasis of adjacent lobes | 13 |
| Mediastinal shift | 9 |
| 1 | 2 |
| 2 | 2 |
| 3 | 2 |
| 4 | 3 |
| Diaphragmatic flattening | 9 |
| 1 | 1 |
| 2 | 4 |
| 3 | 4 |
| 4 | 0 |
| Thoracic wall deformation | 12 |
| 1 | 6 |
| 2 | 4 |
| 3 | 2 |
| 4 | 0 |
| Pleural effusion | 5 |
| 1 | 5 |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
| Pneumothorax | 4 |
| 1 | 1 |
| 2 | 2 |
| 3 | 0 |
| 4 | 4 |
| Cardiac compression | 5 |
| 1 | 4 |
| 2 | 1 |
| 3 | 0 |
| 4 | 0 |
Note: Severity scores from 1 (mild) to 4 (severe) are listed below the corresponding CT features where applicable with the frequency recorded.
Abbreviation: CT, computed tomography.
FIGURE 3.
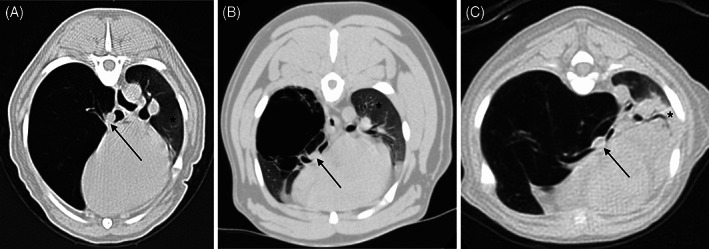
Transverse computed tomographic image in lung window (WL −500, WW 1400) of two dogs, A,B, and a cat, C, with congenital lobar emphysema (CLE) of the right middle lung lobe. Note the collapsed right middle bronchus (black arrows) and atelectasis of the remainder of the lung lobes (*)
Concurrent soft tissue lesions within the emphysematous lung lobes were identified in 5 cases including gas‐filled cavitary lung lesions (3/5), concurrent alveolar lung pattern within the inflated lobe (1/5), and soft tissue mass (1/5). In 13/14 cases, the bronchi supplying the emphysematous lobes were collapsed. In 3 cases, extramural lesions (2 neoplasia, 1 diaphragmatic defect with splenic herniation) compressing the bronchus supplying the emphysematous lobe and causing obstruction were identified. In 13/14 cases, the blood vessels supplying the emphysematous lung lobes were small.
Marked tracheobronchial lymphadenomegaly was noted in 1 cat diagnosed with pulmonary adenocarcinoma (Figure 1). Tracheal compression was seen in 1 dog with concurrent suspected tracheobronchomalacia. The pulmonary vessels of the nonaffected lobes were considered enlarged in 2/12 dogs and normal in the remaining dogs. Additional findings identified on CT included a left gastro‐caval shunt in 1 dog, a lung mass in 1 dog, and multifocal gas‐filled cavitary lesions consistent with bullae in 2 dogs and suspected hepatic cyst in 1 cat.
FIGURE 1.
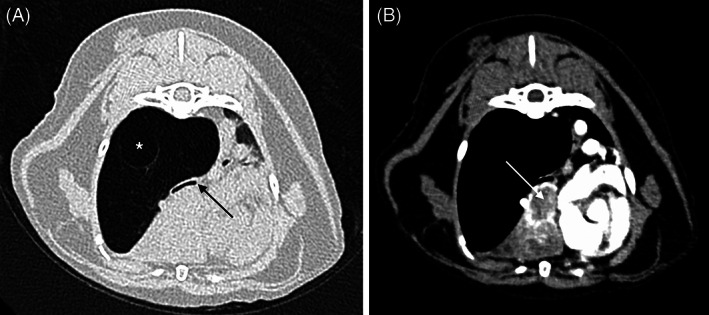
Transverse computed tomographic images in lung (WL −500, WW 1400, A) and soft tissue (WL 40, WW 400, B) windows of the thorax of a cat with acquired lobar emphysema of the right caudal lung lobe because of a pulmonary carcinoma within the right middle lung lobe (white arrow). There is interruption of the right caudal bronchus because of neoplastic infiltration or compression of the bronchus (black arrow). Note the large bulla within the emphysematous right caudal lung lobe (*)
4.4. Outcome
Ten of 17 patients underwent surgery (9 lung lobectomy and 1 diaphragmatic hernia repair). Of the patients that underwent lung lobectomy, 8 survived to discharge. The most frequent surgery performed was right middle lung lobectomy (7 patients). Combined right middle and right caudal lung lobectomy was performed in a cat and combined right middle and right cranial lung lobectomy was performed in a dog. The cat with the diaphragmatic hernia had surgery to reduce the hernia and repair the diaphragmatic defect without lobectomy of the emphysematous lung lobe.
Of the remaining 7 patients, 2 animals (1 cat and 1 dog) were euthanized at the time of diagnosis, 1 died 1 day after diagnosis and 2 were lost to follow‐up. One dog was monitored closely and underwent 3 subsequent CT scans over the course of a month. Images from each of the follow‐up CT scans documented stable appearance of the emphysematous lobe and no further imaging was performed. The final patient that presented as a 14‐week‐old puppy for evaluation of several episodes of dyspnea was treated medically before presenting again 10 years later with acute onset dyspnea and documented pulmonary hypertension.
Histopathological diagnosis was available for 9 of 17 patients. A diagnosis of CLE was made in 8 cases with bronchial cartilage hypoplasia recorded in 4 of these cases. Pulmonary adenocarcinoma was identified as a cause of lobar emphysema in the remaining case. The median age of animals at the time of diagnosis of suspected CLE was 1.26 years (range, 0.13‐14 years). The median age of animals at the time of diagnosis of acquired lobar emphysema was 11 years (range, 10.25‐12 years).
5. DISCUSSION
Our purpose was to describe the clinical and imaging features of pulmonary lobar emphysema. Lung lobe hyperinflation was a common feature in all cases of lobar emphysema in our study. Computed tomography readily identified the affected lung lobes, and all emphysematous lobes had lower attenuation than nonaffected lobes with no overlap in attenuation. Collapse of the bronchus supplying the emphysematous lobe was a commonly observed CT feature, and the blood vessels supplying the emphysematous lung lobes often were small. This finding was considered likely a result of hypoperfusion secondary to abnormal distension of the affected lobe, resulting in a compressive effect on the local pulmonary vasculature. Atelectasis of the adjacent lobes was a common feature, most frequently affecting the lung lobes within the same hemithorax as the emphysematous lung lobe.
Effects on the remaining thoracic structures were readily identified by CT and notable changes included flattening of the diaphragm, thoracic wall deformation and mediastinal shift (Figure 2). In addition, pleural effusion and pneumothorax were identified in several dogs. Pneumothorax is recognized as a possible complication of lobar emphysema in people and previously has been reported in a dog. 6 , 18 The potential for development of tension pneumothorax because of the valve effect of the collapsed bronchus may result in rapid decompensation.
FIGURE 2.
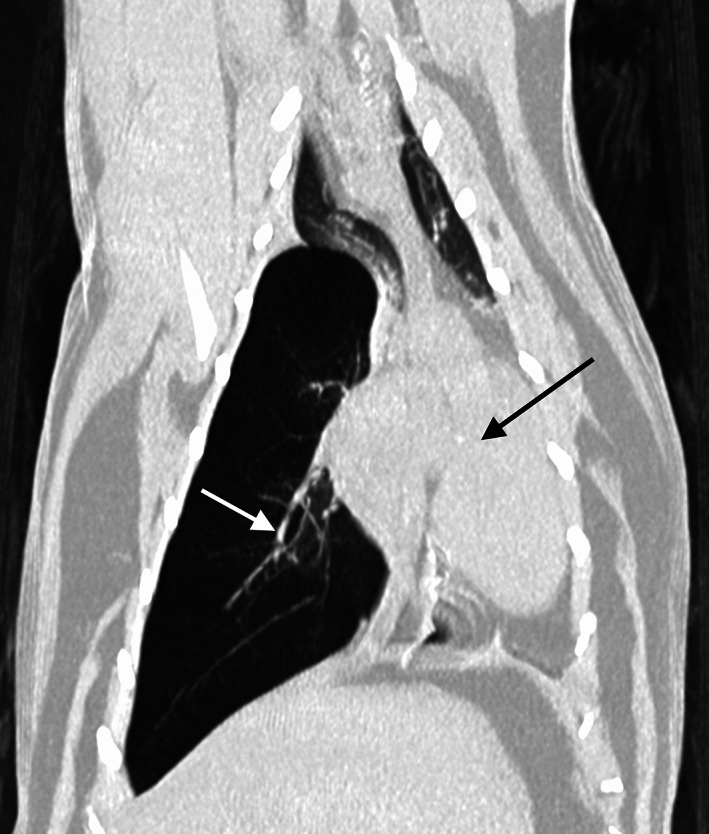
Dorsal computed tomographic image in lung window (WL −500, WW 1400) of the thorax of a cat with lobar emphysema of the right caudal lung lobe. Note the mediastinal shift towards the left (black arrow shows the displaced mediastinal structures). The right caudal pulmonary vessels are thin (white arrow)
Differentiating between pneumothorax and lobar emphysema is important clinically. In previous studies, cases of CLE in human infants initially have been mistaken for pneumothorax. 3 , 19 , 20 Cases with CLE were likely to show hyperlucency of the affected lobe with concurrent mediastinal shift, flattening of the diaphragm, widening of the rib space and atelectasis of the remaining lobes, features that may be misdiagnosed as tension pneumothorax. 19 The presence of bronchovesicular markings in the region of hyperlucency, however, is highly suggestive of CLE rather than tension pneumothorax, where no such markings are present. 19 These findings consistently were identified in our cases with superior accuracy using CT.
Images obtained using radiography allowed a diagnosis of lobar emphysema to be made in all cases, but specific lobe identification was inconsistent. In addition, it was not possible in most cases to identify the collapsed or obstructed bronchus leading into the emphysematous lung lobe. Atelectasis of an adjacent lung lobe was identified in most cases using radiography. Mediastinal shift, thoracic wall deformation, and flattening of the diaphragm also were readily identified using radiography.
As expected in the 4 cases of dual imaging (CT and radiography), images obtained using CT provided more information, particularly in determination of the affected lobe and identification of the collapsed or obstructed bronchus. In 1 case with lobar emphysema secondary to a diaphragmatic hernia, CT provided more detailed information about the nature of the bronchial compression and herniated viscera than did radiography alone. Based on our assessment, both imaging modalities would be considered suitable for the diagnosis of lobar emphysema. However, CT may provide more detailed information about the distribution of lesions and possible underlying etiology, which may be particularly useful for surgical planning. Additional CT considerations not discussed previously include the risk associated with sedation or general anesthesia and positive pressure ventilation. Positive pressure ventilation is not recommended in humans or dogs with suspected lobar emphysema because it may exacerbate the valve‐like effect of the collapsed bronchus, leading to further hyperinflation of the affected lobe or tension pneumothorax and progressive respiratory compromise. 21
In our study, dyspnea and coughing were the most common clinical signs, consistent with previously reported cases. 5 , 8 , 11 Clinical signs of lobar emphysema developed because of progressive hyperinflation and subsequent compression of adjacent structures leading to respiratory failure. Dysphagia and vomiting also were reported in a few patients. Dysphagia may have been the result of esophageal compression by the mass effect of the emphysematous lung lobe.
Clinical signs were variable in severity in this group of patients and in 1 case were detected as incidental findings at the time of imaging for unrelated investigations at the primary care practice. Most patients had a very short duration of clinical signs before presentation whereas 1 dog had a 10‐year history of intermittent coughing with acute onset of dyspnea.
In our small series of patients, no clear breed or sex predilection was identified. Clinical signs, however, were more common in young dogs, in keeping with previously reported cases of lobar emphysema in dog and cats. 5 , 6 , 7 , 8 , 9 , 10 , 14 , 15 , 16 , 17 In all 11 patients <3 years of age, no secondary cause of lobar emphysema was identified using either radiography or CT. In the 6 patients >3 years of age (median age, 11.5 years), 3/6 were found to have an underlying disease process leading to bronchial compression and subsequent lobar emphysema. The bimodal distribution of cases should prompt further consideration, depending on the presentation of cases in practice. In young patients, CLE would be considered more likely whereas in older patients with lobar emphysema, the attending clinician should consider concurrent diseases such as neoplasia.
In the 16 individual cases previously reported in the veterinary literature, the right middle lung lobe was most frequently affected. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Right middle lung lobe involvement also was a consistent finding in our study (Figure 3, Figure 5), all of which patients were diagnosed with presumed CLE. Four cases had involvement of the accessory lobe (1 combined with right caudal lung lobe emphysema). In 2 cats, involvement of the accessory lobe was secondary to external bronchial compression (1 diaphragmatic hernia, 1 pulmonary carcinoma). In cases involving lung lobes other than the right middle lobe, acquired causes of bronchial compression should be carefully excluded. In addition, considering the case of diaphragmatic hernia causing lobar emphysema, previous trauma (acute or chronic) should be considered as a possible etiology.
FIGURE 5.
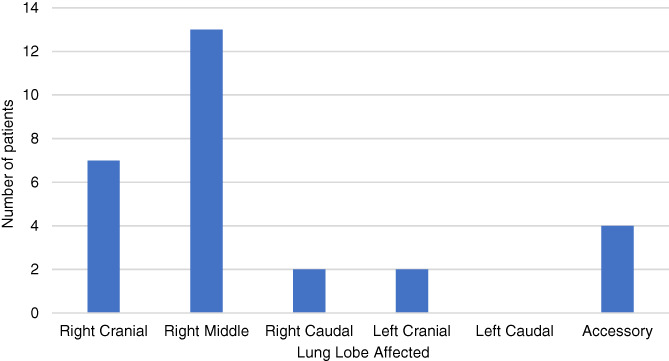
Graph to show frequency of hyperinflated lung lobes for dogs and cat with lobar emphysema (computed tomography and radiography cases combined)
Several cases featured involvement of multiple lung lobes, which also has been reported previously in human patients and in individual veterinary case reports. 13 , 16 , 22 This finding is not unexpected, because when a bronchial cartilage defect is suspected to be the cause of CLE, this malformation feasibly could affect multiple bronchi. Although most cases presented with involvement of the right middle lung lobe, it is possible that bronchial cartilage defects were present elsewhere, but did not result in the development of lobar emphysema. Histopathology, including both the emphysematous lobes and nonaffected bronchi, would be required to establish the validity of this hypothesis. It is unknown why the right middle lung lobe is the most commonly affected lobe. The right middle lung lobe, however, is also commonly affected by lung lobe torsion, which is thought to be caused by the narrow shape and high mobility of this lobe. 23 The increased mobility of the right middle lobe combined with cartilage hypoplasia may contribute to the predilection for CLE in this lobe.
Unsurprisingly, no specific clinicopathological findings were associated with the presence of lobar emphysema. Complete hematology, biochemistry, and urinalysis results, however, were not available for all patients, which limits the assessment of their utility.
In previous case reports, several patients underwent surgical treatment with lobectomy of the affected lobe. 5 , 9 , 10 , 11 Postoperative follow‐up of 15 months in a dog and 36 months in a cat without recurrence of clinical signs were reported. 3 , 8 However, in cases where surgery was not performed, the condition resulted in rapid deterioration and death or euthanasia. 5 , 7 , 8 , 12 , 13 , 22 Although long‐term outcomes were not available in our study, a similar proportion underwent surgical lobectomy with 80% (8/10) survival to discharge.
Limitations of our study include its retrospective design leading to bias in the recruitment of cases. Many cases had incomplete records with inconsistent availability of clinicopathological or histopathological information. Information regarding clinician selection of either radiography or CT was not available. Information regarding the use of breath holding during CT image acquisition was not available in most patients, and this technique may affect the severity of lung lobe hyperinflation and HU values. Only a small number of cases underwent both radiography and CT and therefore direct comparison of these modalities was not possible. A diagnosis of lobar emphysema was based on the imaging findings and where available was confirmed on histopathology. In some cases, however, histopathology was not available, and therefore the underlying pathology could not be determined. This factor may be particularly relevant in cases that underwent thoracic radiography, where more subtle lesions may not be identifiable.
6. CONCLUSION
Lobar or multilobar emphysema is a rare disease in both dogs and cats and should be considered as a differential diagnosis in patients presenting with dypsnea and or coughing, particularly young patients. Radiography is useful in diagnosing most cases, but CT is likely to provide additional details and allow for better surgical planning. In older patients with lobar emphysema, secondary extramural compressive causes should be considered, and CT should be favored over radiography whenever feasible. Although long‐term follow‐up was not possible in our study, it is likely that surgical treatment, where indicated, will be associated with a favorable outcome.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Retrospective collection and analysis of cases was approved by the Veterinary Research Ethics Committee of the Institute of Veterinary Science, Leahurst Campus, Neston, South Wirral, United Kingdom (reference VREC766).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. An abstract poster presentation of data from this study was presented at the European College of Veterinary Internal Medicine—Companion Animals Congress, September 2020, Virtual Congress.
Warwick H, Guillem J, Batchelor D, et al. Imaging findings in 14 dogs and 3 cats with lobar emphysema. J Vet Intern Med. 2021;35(4):1935–1942. 10.1111/jvim.16183
REFERENCES
- 1. Demir OF, Hangul M, Kose M. Congenital lobar emphysema. Int J Chron Obstruct Pulmon Dis. 2019;14:921‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamal YA. Management of congenital lobar emphysema: the current challenges. ARC JSurg. 2018;4:20‐25. [Google Scholar]
- 3. Cataneo DC, Rodrigues OR, Hasimoto EN, et al. Congenital lobar emphysema: 30‐year case series in two university hospitals. J Bras Pneumol. 2013;39:418‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orima H, Fujita M, Aoki S, et al. A case of lobar emphysema in a dog. J Vet Med Sci. 1992;54:797‐798. [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto H, Kakehata T, Hyodo T, et al. Surgical correction of congenital lobar emphysema in a dog. J Vet Med Sci. 2004;66:217‐219. [DOI] [PubMed] [Google Scholar]
- 6. Yun S, Lee H, Lim J, et al. Congenital lobar emphysema concurrent with pneumothorax and pneumomediastinum in a dog. J Vet Med Sci. 2016;78:909‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voorhout G, Goedegebuure SA, Nap RC. Congenital lobar emphysema caused by aplasia of bronchial cartilage in a Pekingese puppy. Vet Pathol. 1986;23:83‐84. [DOI] [PubMed] [Google Scholar]
- 8. Gopalakrishnan G, Stevenson GW. Congenital lobar emphysema and tension pneumothorax in a dog. J Vet Diagn Invest. 2007;19:322‐325. [DOI] [PubMed] [Google Scholar]
- 9. Early NF, Herrtage ME, Hall JL. Emergency diagnosis and treatment of congenital lobar emphysema in a puppy. Vet Rec Case Rep. 2018;6:e000592. [Google Scholar]
- 10. Ruth J, Rademacher N, Ogden D, et al. Imaging diagnosis—congenital lobar emphysema in a dog. Vet Radiol Ultrasound. 2011;52:79‐81. [PubMed] [Google Scholar]
- 11. Blonk M, Van De Maele I, Combes A, et al. Congenital lobar emphysema in a kitten. J Small Anim Pract. 2017;58:659‐663. [DOI] [PubMed] [Google Scholar]
- 12. Herrtage ME, Clarke DD. Congenital lobar emphysema in two dogs. J Small Anim Pract. 1985;26:453‐464. [Google Scholar]
- 13. Anderson WI, King JM, Flint TJ. Multifocal bullous emphysema with concurrent bronchial hypoplasia in two aged Afghan Hounds. J Comp Pathol. 1989;100:469‐473. [DOI] [PubMed] [Google Scholar]
- 14. Billet JPHG, Sharpe A. Surgical treatment of congenital lobar emphysema in a puppy. J Small Anim Pract. 2002;43:84‐87. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell C, Nykamp S. Imaging diagnosis: congenital lobar emphysema in an Old English Sheepdog Puppy. Vet Radiol Ultrasound. 2006;47:465‐467. [DOI] [PubMed] [Google Scholar]
- 16. Tennant BJ, Haywood S. Congenital bullous emphysema in a dog: a case report. J Small Anim Pract. 1987;28:109‐116. [Google Scholar]
- 17. Hoover JP, Henry GA, Panciera RJ. Bronchial cartilage dysplasia with multifocal lobar bullous emphysema and lung torsions in a pup. J Am Vet Med Assoc. 1992;201:599‐602. [PubMed] [Google Scholar]
- 18. Muramatsu T, Furuichi M, Nishii T, et al. Lobar emphysema with pneumothorax in an adult: report of a case. Surg Today. 2013;43:539‐541. [DOI] [PubMed] [Google Scholar]
- 19. Ulku R, Onat S, Ozcelik C. Congenital lobar emphysema. Differential diagnosis and therapeutic approach. Pediatr Int. 2008;50:658‐661. [DOI] [PubMed] [Google Scholar]
- 20. Aggarwal B, Gupta G, Maletha M, et al. Congenital lobar emphysema mimicking pneumothorax: a case report. J Evid Based Med Healthc. 2016;3:3033‐3035. [Google Scholar]
- 21. Prabhu M, Joseph TT. Congenital lobar emphysema: challenges in diagnosis and ventilation. Anesth Essays Res. 2012;6:203‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozçelik U, Göçmen A, Kiper N, et al. Congenital lobar emphysema: evaluation and long‐term follow‐up of thirty cases at a single center. Pediatr Pulmonol. 2003;35:384‐391. [DOI] [PubMed] [Google Scholar]
- 23. Park KM, Grimes JA, Wallace ML, et al. Lung lobe torsion in dogs: 52 cases (2005‐2017). Vet Surg. 2018;47:1002‐1008. [DOI] [PubMed] [Google Scholar]


