Abstract
Background
Persistent fontanelles (PFs) are, in Chihuahuas, almost ubiquitous. Furthermore, Chihuahuas are predisposed to other craniomorphological abnormalities, including syringomyelia (SM), ventriculomegaly, and craniocervical junction (CCJ) overcrowding resulting in neural tissue deviation. It is, however, undetermined if PFs are more common in dogs with these structural abnormalities, and their etiology is unknown.
Hypothesis/Objectives
Persistent fontanelles are more numerous and larger in Chihuahuas with low body weight, older age, SM, dilated fourth ventricle, ventriculomegaly, and CCJ overcrowding.
Animals
Fifty client‐owned Chihuahuas.
Methods
Cross‐sectional study evaluating the association of both the number of cranial sutures affected by PFs (NAS) and total fontanelle area (TFA), based on computed tomography with SM, fourth ventricle dilatation, lateral ventricle volume, and extent of neural tissue compression at the CCJ based on magnetic resonance images.
Results
The NASs was higher and TFA larger in dogs with low body weight (NAS: P = .007; 95% confidence interval [CI] = 0.384‐0.861; TFA: P = .002; 95% CI = −1.91 to −0.478), larger lateral ventricles (NAS: P ≤ .001; 95% CI = 1.04‐1.15; TFA: P ≤ .001; 95% CI = 0.099‐0.363), and more severe neural tissue compression at the CCJ (NAS: P ≤ .001; 95% CI = 1.26‐2.06; TFA: P = .03; 95% CI = 0.066‐1.13). Similarly, dogs with SM (NAS: P = .004; 95% CI = 1.26‐3.32; TFA: mean ± SD, 130 ± 217 mm2; P = .05) had higher NAS and larger TFA than did dogs without SM (43.7 ± 61.0 mm2). Age was not associated with NAS (P = .81; 95% CI = 0.989‐1.01) or TFA (P = .33; 95% CI = −0.269 to 0.092).
Conclusions and Clinical Importance
Persistent fontanelles are associated with small size, SM, ventriculomegaly, and CCJ overcrowding.
Keywords: Chiari‐like malformation, craniocervical junction, syringomyelia, ventriculomegaly
Abbreviations
- CCJ
craniocervical junction
- CKCS
Cavalier King Charles Spaniel
- CM
Chiari‐like malformation
- CSF
cerebrospinal fluid
- CT
computed tomography
- MRI
magnetic resonance imaging
- NAS
number of cranial sutures affected by PFs
- PF
persistent fontanelle
- SM
syringomyelia
- TFA
total fontanelle area
1. INTRODUCTION
Persistent fontanelles (PFs) are characterized by osseous defects at cranial sutures or at their intersections. They are prevalent in Chihuahuas, the smallest dog breed in the world, with 92% of these dogs having either 1 or several of them. A molera, a dorsal PF between the paired frontal and parietal bones, is in some countries even included in the Chihuahua breed standards. 2 , 3 Most PFs in Chihuahuas are at locations comparable of fontanelles in children, but 38% occur at other cranial sutures. 1
In children, bone‐deficient lesions of the skull can occur because of congenital defects of bone ossification (lacunar skull), bone erosion (copper‐beaten appearance), or delayed cranial suture closure (sutural diastasis). These lesions are associated with premature cranial suture closure restricting normal growth of the cranium. 4 The pathomechanism of PFs in Chihuahuas is unknown.
In addition to PFs, Chihuahuas also are predisposed to other craniomorphological abnormalities such as short cranial base and close proximity of the atlas to the occiput. These abnormalities possibly occur because of premature cranial base synchondrosis closure. 5 , 6 This phenomenon causes neural tissue deviation at the craniocervical junction (CCJ), which is evident as medullary elevation and dorsal spinal cord compression at C1‐2. 7 , 8 , 9 Overcrowding of the CCJ disturbs cerebrospinal fluid (CSF) flow at the foramen magnum and predisposes to syringomyelia (SM). 10 In addition to CCJ overcrowding, SM commonly occurs in Chihuahuas. It is more likely in dogs with more severe neural tissue deviation at the CCJ. 6 , 9
Although PFs are common in Chihuahuas and can be identified in dogs affected by SM and CCJ overcrowding, no study has evaluated any potential association between PFs and these other malformations. Of these malformations, both SM and CCJ overcrowding are associated with clinical and behavioral signs of pain, but it is commonly thought that PFs in Chihuahuas are clinically irrelevant. 9 Our aim was to assess any association between the PFs—evaluated as number of affected sutures (NAS) and total fontanelle area (TFA) on computed tomography (CT) images —and the following:
the dog's signalment: breed type (long‐haired or smooth‐haired), sex, age, and weight;
SM, SM maximum transverse width, fourth ventricle dilatation, and lateral ventricular volume; and
extent of CCJ overcrowding (concomitant medullary kinking and dorsal spinal cord compression).
Our hypotheses were that PFs would be more numerous (higher NAS) and larger (increased TFA):
in smaller and older dogs, but with no difference between Chihuahua breed types or sex;
in dogs with SM, fourth ventricle dilatation, or enlarged lateral ventricles; and
in dogs with CCJ abnormalities such as concomitant medullary kinking and dorsal spinal cord compression.
2. MATERIALS AND METHODS
2.1. Case selection
Our data included 50 Chihuahuas that were part of the same cohort including 53 Chihuahuas in another study published earlier. 9 Our study included dogs that underwent both CT and magnetic resonance imaging (MRI) and did or did not have Chiari‐like malformation (CM) or SM‐related clinical signs. The dogs were recruited from the caseload of the Veterinary Teaching Hospital of the University of Helsinki. We used similar recruitment methods, inclusion, and exclusion criteria as in an earlier study assessing CM and SM‐affected Chihuahuas. 9 The dogs with CM/SM‐related clinical signs could be of any age, they had to have at least 1 clinical sign typical of CM or SM, and no findings on brain and cervical spinal MRI other than CCJ overcrowding, SM, or ventriculomegaly. The dogs without CM/SM‐related clinical signs had to be at least 3 years of age and showing no signs of illness. Imaging of the dogs with CM/SM‐related clinical signs was a diagnostic procedure, and dogs without CM/SM‐related clinical signs underwent imaging for breeding selection purposes to detect CM or SM.
2.2. Diagnostic imaging procedures
Under general anesthesia, all dogs underwent CT and MRI. For head and cervical spine (to the level of the caudal C3 vertebra) CT images, we used a helical dual‐slice CT scanner (Somatom Emotion Duo, Siemens AG, Forcheim, Germany), with a bone algorithm and a slice thickness of 1 mm (feed/rotation, 2 mm; reconstruction increment, 0.5 mm; 110 kV). We positioned the dogs so that the base of the skull was aligned perpendicular to the ventral vertebral canal in the cranial cervical spine.
For MR images, we used a 0.2 Tesla MR scanner (Esaote S.p.A, Genova, Italy) and positioned all dogs in sternal recumbency with the base of the skull aligned perpendicular to the ventral vertebral canal at the first 2 cervical vertebrae. In dogs without CM/SM‐related clinical signs, MRI consisted of sagittal T1‐ and T2‐weighted sequences of the brain and cervical spine (with slice thickness ranging from 3.5 to 4.5 mm), T1‐ and T2‐weighted transverse sequences of the brain, and T1‐weighted transverse sequences of the spinal cord between C1 and C4/5. Transverse slices were adjusted to center the syrinx, if visible. Dogs with CM/SM‐related clinical signs underwent a similar protocol with the addition of T2‐weighted transverse images throughout the entire cervical spine.
2.3. Image analysis
Image analysis included both CT and MR images. We used OsiriX Medical Imaging Software (Pixmeo SARL, Bernex, Switzerland) to analyze CT images for the NASs and TFAs. For MR image analysis, each evaluator used imaging software available to them and included determination of whether SM or fourth ventricle dilatation was present. In the instance of SM, we also measured the maximum transverse width of the syrinx. Furthermore, we measured lateral ventricular volumes and the severity of neural parenchymal deviation at the CCJ (caused by dorsal spinal cord compression and medullary elevation) as detailed below. Evaluators were, in all image evaluations, blinded to each other's findings and to other imaging modality (CT/MRI) analysis results.
2.3.1. Number of affected sutures and TFA
A PF was defined as full‐thickness loss of bone at a cranial suture (ie, at junctions between the membrane‐derived bones on the cranial vault; Figure 1). 1 Two board‐certified neurologists (A.‐M. Kiviranta and T.S. Jokinen) independently evaluated the CT images and hence were unaware of the dogs' clinical status and MRI findings. The evaluators first assessed the presence, number, and location of all PFs. If, after their individual assessment, the evaluators disagreed about the presence or number of PFs, or about the sutures affected, the evaluators reassessed the CT images together to reach consensus. After consensus, each evaluator independently measured the area of each PF, as described earlier, using the closed polygon tool of the software. 1
FIGURE 1.
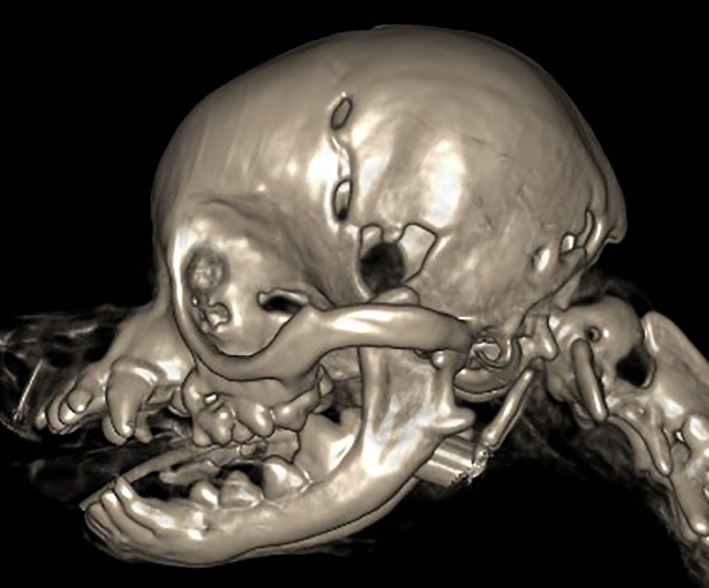
Volume‐rendering‐technique computed tomography image of a Chihuahua skull in left lateral view, showing 2 sharply demarcated persistent fontanelles at the left frontoparietal suture and 1 at the intersection of the left frontal, sphenoid, and parietal bones, hence at a location similar to that of a sphenoidal fontanelle in children
2.3.2. Syringomyelia
The dogs were grouped, according to the British Veterinary Association/Kennel Club CM and SM Health Scheme, as either having or not having SM. Two board‐certified neurologists (C. Rusbridge and T.S. Jokinen) assessed the T1‐weighted sagittal and transverse MR images for SM, defined as a central canal dilatation or a separate syrinx that was at least 2 mm in diameter. For syrinx width assessment, evaluators used T1‐weighted transverse images of the spinal cord.
2.3.3. Sum index
To provide an objective assessment of the severity of the neural parenchymal deviation at the CCJ, a board‐certified neurologist (A.‐M. Kiviranta) measured dorsal spinal cord compression, medullary elevation, and their sum index (dorsal spinal cord compression index + medullary kinking index) on the sagittal T2‐weighted MR images, utilizing a methodology similar to that described previously. 9
2.3.4. Dilatation of the fourth ventricle
Two board‐certified neurologists (C. Rusbridge and T.S. Jokinen) independently evaluated the midsagittal T1‐ and T2‐weighted brain MR images for any enlargement of the fourth ventricle. These evaluators subjectively classified the fourth ventricle as enlarged if the “slit‐like” ventricle appeared triangular, and the neural tissue appeared rostrodorsally deviated (Figure 2). In cases with different decisions, the evaluators reached a consensus decision.
FIGURE 2.
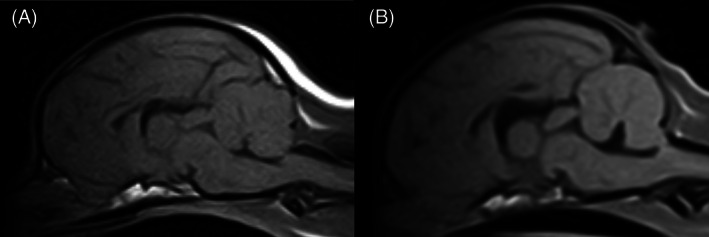
Sagittal T1‐weighted, magnetic resonance image of a Chihuahua with (B) and without (A) fourth ventricle dilatation. A, Chihuahua with a slit‐like fourth ventricle grouped as having no fourth ventricle dilatation. B, Chihuahua with a triangular shaped fourth ventricle and mild deviation of the rostroventral cerebellar tissue grouped as having fourth ventricle dilatation
2.3.5. Lateral ventricular volume assessment
One board‐certified neurologist (A.‐M. Kiviranta) measured the lateral ventricular volumes using the OsiriX Medical Imaging Software polygonal area tool. The borders of the left and right lateral ventricles were traced, in a manner similar to that described previously for measuring the cranial volumes in each T1‐weighted transverse brain MR image slice. 11 After measurement of all slices, the volume measurement software calculated the left and right lateral ventricular volumes and recorded them in mm3.
2.4. Statistical analysis
The total NASs were analyzed using univariate Poisson regression: the total NAS served as the response, with each explanatory factor assessed separately as the fixed term. Explanatory factors were age, breed (1 Chihuahua mix was excluded from the breed analysis), weight, sex (1 castrated male was included in the total male population), presence of SM (and if present its maximum width), fourth ventricle dilatation, lateral ventricular volume, and sum index.
The TFA was first log‐transformed for analysis to satisfy the normality assumptions of the statistical modeling. Because of some zero observations (4 dogs lacking a fontanelle), the transformation was conducted as log (total PF area + 1). The mean TFA, measured by the 2 evaluators, was used for the analysis. The analysis utilized either a 1‐way analysis of variance model or linear regression (depending on the type of explanatory variable). The log‐transformed TFA served as the response, and each explanatory factor was assessed separately as the fixed term. The normality of the model residuals was investigated using Shapiro‐Wilks tests and normal QQ‐plots. P‐values of <.05 were considered statistically significant. All P‐values were 2‐sided and not adjusted for multiple testing. All statistical analyses were done using the SAS System for Windows, version 9.4 (SAS Institute Inc, Cary, North Carolina).
3. RESULTS
3.1. Association of PFs with signalment
The mean ± SD age of the dogs was 58 ± 28 months (range, 7‐139 months) and their mean ± SD weight 2.8 ± 0.6 kg (range, 1.4‐4.3 kg). Furthermore, the mean ± SD age of dogs without CM/SM‐related clinical signs was 63 ± 18 months, and in dogs with CM/SM‐related clinical signs was 55 ± 36 months.
When evaluating any possible association with the NAS or the TFA and breed, age, sex, and weight, the NAS was significantly higher (P = .01) and TFA larger (P = .002) in dogs with lower body weight. No difference, however, was associated with NAS or TFA and breed type, age, or sex (Tables 1, 2, 3).
TABLE 1.
Association of number of affected sutures and signalment and structural variables (Univariate Poisson regression)
| Variable | Estimate | 95% CI | P‐value |
|---|---|---|---|
| Breed | 1.07 | 0.623‐1.83 | .81 |
| Age | 0.999 | 0.989‐1.01 | .81 |
| Sex | 1.03 | 0.602‐1.78 | .90 |
| Weight | 0.575 | 0.384‐0.861 | .01 |
| Syringomyelia | 2.04 | 1.26‐3.32 | .004 |
| Syrinx width | 0.938 | 0.734‐1.20 | .61 |
| Dilated fourth ventricle | 1.14 | 0.661‐1.97 | .64 |
| Lateral ventricle volume | 1.10 | 1.04‐1.15 | <.001 |
| Sum index | 1.61 | 1.26‐2.06 | <.001 |
Note: Variables with P < .05 are bolded.
Abbreviation: CI, confidence interval.
TABLE 2.
Association of total persistent fontanelle area and signalment and structural variables (1‐way ANOVA analyses)
| Variable | Result | Number | % of all dogs | Mean (mm2) | SD | P‐value |
|---|---|---|---|---|---|---|
| Breed | Smooth haired | 23 | 46 | 98.3 | 201 | .83 |
| Longhaired | 26 | 52 | 63.1 | 85.4 | ||
| Sex | Male | 23 | 46 | 59.4 | 91.3 | .17 |
| Female | 27 | 54 | 94.1 | 185 | ||
| Syringomyelia | No | 30 | 60 | 43.7 | 61.0 | .05 |
| Yes | 20 | 40 | 130 | 217 | ||
| Dilated fourth ventricle | No | 32 | 64 | 59.0 | 72.4 | .49 |
| Yes | 18 | 36 | 112 | 230 |
Note: Variables with P < .05 are bolded.
TABLE 3.
Association of total persistent fontanelle area and severity of clinical signs, weight, and age (linear regression analyses)
| Variable | Estimate (log‐scale) | 95% CI (log scale) | P‐value |
|---|---|---|---|
| Age (increase 10 months) | −0.089 | −0.269‐0.092 | .33 |
| Weight (increase of 1 kg) | −1.19 | −1.91‐(−0.478) | .002 |
| Syrinx width | 0.159 | −0.400‐0.718 | .56 |
| Lateral ventricle volume (sum increase 1 unit) | 0.231 | 0.099‐0.363 | <.001 |
| Sum index (increase 0.1 unit) | 0.598 | 0.066‐1.13 | .03 |
Note: Variables with P < .05 are bolded.
Abbreviation: CI, confidence interval.
3.2. Association of PFs with SM and ventricular size
Of 50 dogs, 20 (40%) had SM and 18 (36%) had a dilated fourth ventricle. Because 1 dog lacked T1‐weighted transverse brain MR images, lateral ventricular volume was measured in 49 dogs, and mean ± SD volume was 3.1 ± 3.5 mm3 (range, 0.09‐14.8 mm3). When evaluating any possible association with NAS or TFA and SM, maximum syrinx width, dilated fourth ventricle, and lateral ventricular volume, NAS was significantly higher (P = .004) and TFA larger (P = .05) in dogs with SM. Furthermore, the NAS was significantly higher and TFA larger (both P < .001) in dogs with an increase in lateral ventricular volume (Tables 1, 2, 3; Figures 3 and 4).
FIGURE 3.
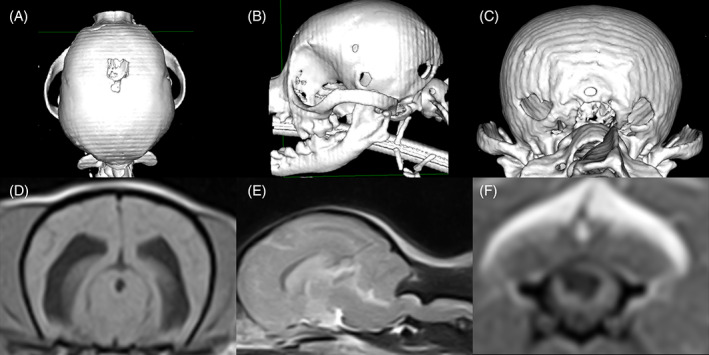
A Chihuahua (weight 3.5 kg) with multiple persistent fontanelles, ventriculomegaly, syringomyelia, and craniocervical junction overcrowding. A‐C, Volume‐rendering‐technique computed tomography image of a Chihuahua skull in dorsal (A), left lateral (B), and caudal (C) views, showing multiple, persistent fontanelles (total fontanelle area of 214 mm2). D, T1‐weighted transverse brain magnetic resonance image of the same dog in (A‐C), showing moderate ventriculomegaly (ventricular volume 2.9 mm3). E, T2‐weighted sagittal brain and spinal cord (C1‐C5) magnetic resonance image of the same dog in (A‐C), showing concomitant medullary elevation and dorsal spinal cord compression at C1‐C2 (sum index 0.68) and syringomyelia at C2‐C3. F, T1‐weighted, transverse spinal cord magnetic resonance image of the same dog from (A‐C), showing an asymmetrical syringomyelia (maximum width 4.7 mm) at the level of C3 and extending into the left dorsal horn
FIGURE 4.
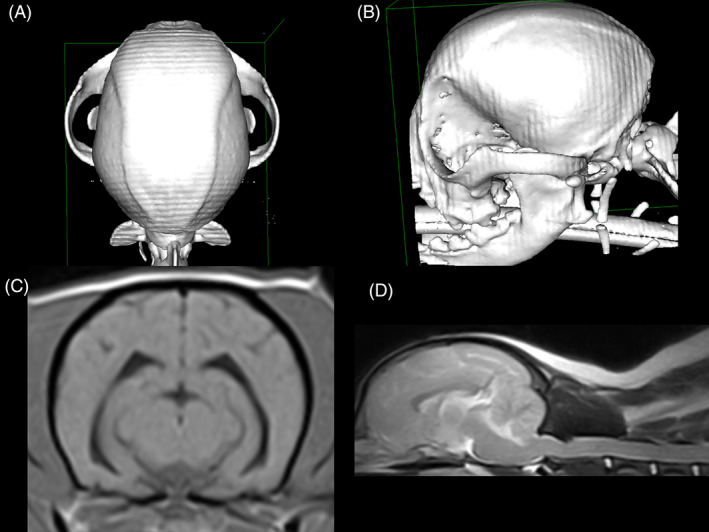
A Chihuahua (weight 4.3 kg) without persistent fontanelles. A,B, Volume‐rendering‐technique computed tomography image of a Chihuahua skull in dorsal (A) and left lateral views (B), showing no persistent fontanelles. C, T1‐weighted transverse brain magnetic resonance image of the same dog from (A, B), lacking ventriculomegaly (ventricular volume 0.57 mm3). D, T2‐weighted sagittal brain and spinal cord (C1‐C5) magnetic resonance image of the same dog from (A, B), showing a very mild medullary elevation, moderate dorsal spinal cord compression at C1‐C2 (sum index 0.43), and lacking syringomyelia
3.3. Association of PFs with CCJ structures
Of the 50 dogs, in 3, the sum index was not measurable because of suboptimal positioning in T2‐weighted sagittal MR images, which left 47 dogs for evaluation of the sum index (dorsal spinal cord compression index + medullary kinking index). The mean ± SD sum index was 47% ± 10% (range, 29%‐68%). The NAS was significantly higher (P < .001) and TFA larger (P = .03) in dogs with an increase in the sum index (Tables 1 and 3; Figures 3 and 4).
4. DISCUSSION
Our study shows that, according to our hypotheses, higher number and larger size of PFs are associated with lower body weight, SM, ventriculomegaly, and overcrowding of the CCJ appearing as concomitant medullary elevation and dorsal spinal cord compression at the level of C1‐2. Although their concurrent occurrence does not prove causality, our findings suggest that PFs are associated with the occurrence of these structural abnormalities. Thus, they may share the same pathophysiology. Because SM and CCJ overcrowding may cause neuropathic pain and motor deficits, our findings challenge the current conception that PFs are a clinically irrelevant finding not associated with other structural abnormalities.
4.1. Association of PFs with dogs' signalment
The number of cranial sutures affected by PFs was higher and TFAs larger in Chihuahuas with lower body weight. The dogs in our study had a median body weight of 2.8 kg, exceeding the American and British breed standard guidelines for body weight (2.7 kg), and at the upper reference limit of the Federation Cynologique Internationale (3.0 kg, preferably 1.5‐2.5 kg). 2 , 12 , 13 The Chihuahua is the world's smallest dog breed. In dogs, body size is genetically regulated, with 17 quantitative trait loci explaining, in purebred dogs, 80% to 88% of their variation in weight and height. 14 Among the major determinants for very small size in dogs are insulin‐like growth factor 1 and its receptor, because the expression of insulin‐like growth factor 1 affects embryonic and postnatal skeletal development. 15 , 16 , 17 That lower body weight was associated with more numerous and larger PFs in these Chihuahuas may indicate that factors responsible for their extremely small size also may be associated with their cranial growth and ossification.
Cranial growth occurs as long as the cranial synchondroses on the cranial base and cranial sutures on the cranial vault remain open. 18 In children, craniosynostosis is a disorder that affects the developing cranium because of premature cranial suture closure. It restricts normal cranial growth and leads to abnormal cranial shape. 19 In addition to this abnormal head shape, craniosynostotic children also are predisposed to congenital defects of cranial vault bone ossification (lacunar skull), or signs of active remodeling of bone (seen as a diffuse copper‐beaten appearance of the cranial vault bones in cranial radiography). Both forms of cranial bone defects are associated with craniosynostosis. Although they represent different methods of development (congenital vs acquired) they may occur concurrently. 4 Hence, because small body size appears to be associated with the occurrence and severity of PFs, restricted growth of these miniature‐sized dogs may share common pathomechanisms with development of their PFs. Additional studies however are necessary to evaluate if PF occur only in dogs with very small body size, if they are associated with craniosynostosis (including premature synchondrosis closure), and if they share similar pathophysiology with the bony defects observed in craniosynostotic children.
Contradicting our hypothesis, NAS was not higher nor was TFA larger in older Chihuahuas. This finding could suggest that the PFs, or the majority of them in the dogs of our study, arise either during embryological development of the cranial vault or at an early age. Because of the lack of repeated scanning, it is beyond the scope of our study to assess whether the lesions initially developed because of some embryological dysfunction of skull ossification or as a result of resorption of normally developed bone at an early age. Additional studies are necessary to evaluate if the bony defects described in our study are congenital or acquired defects or possibly a combination of these 2, similar to findings in craniosynostotic children. 4
4.2. Association of PFs with SM and ventricular size
Higher NAS and larger TFA occurred in Chihuahuas with SM and ventricular enlargement. Dogs with SM commonly show ventricular enlargement (ventriculomegaly). Because maximum syrinx width correlates with ventricular volume, this correlation suggests a common etiology between them. 20 Cranial sutures develop at the site of dural reflections. The dura controls skull growth by mechanosensory capacity that responds to the expanding brain mass. Therefore, a possible explanation for the association between ventricular enlargement and more numerous and larger PFs may be that ventriculomegaly causes expansion of the cranial content and results in failure of the ossification of the fibrous membrane between the separate skull bones. 21 In addition to possible expansion of the cranial content, ventriculomegaly has intraparenchymal effects. In dogs lacking clinical signs related to hydrocephalus, periventricular blood flow and cerebral white matter volumes are decreased when compared to dogs lacking ventriculomegaly. 22 , 23 These findings suggest that even in clinically nonaffected dogs with ventriculomegaly intraventricular pressure may be increased. Although concurrent occurrence of enlarged lateral ventricles and PFs does not prove causality, we suggest that ventriculomegaly may affect cranial ossification.
Although both SM and ventricular enlargement were associated with higher NASs and larger TFAs, no association was found with maximum syrinx transverse width. In a study describing the distribution of SM along the spinal cord in cavalier King Charles spaniels (CKCSs), SM were most common in the cranial cervical spinal cord between C1 and C4. Although maximum syrinx width was most common at the same location, in 46% of those dogs, maximum syrinx transverse width occurred caudal to C4, and in 38% it occurred between T2 and L2. 24 Among our dogs, the cervical MR images extended in clinically nonaffected Chihuahuas from C1 to C4/5 and in clinically affected Chihuahuas from C1 to C7. This finding means that, if the distribution of the maximum syrinx transverse width is similar to that described in CKCSs, in some dogs the maximum syrinx width was not imaged. This possibility could be an explanation for the lack of association between the NAS and TFA with syrinx width.
Syringomyelia is associated with several cranial bony morphological changes such as small frontal sinuses, shortening of the skull base at the caudal cranial fossa, increased cranial width and height, and rostro‐cranial doming (higher degree of brachycephaly), and decreased occipital crest. 6 , 25 , 26 , 27 , 28 These bony changes are thought to occur because of premature cranial base spheno‐occipital synchondrosis closure reported in CKCSs and other brachycephalic toy breed dogs. 5
Although studies evaluating PFs in non‐Chihuahua breeds also affected by SM are lacking, studies in those dogs describe other, possibly acquired cranial defects. A longitudinal study in CKCS affected with SM described that, during repeated imaging, the foramen magnum enlarges. This change can occur because of active occipital bone resorption, presumably occurring because of pressure of the cerebellum against the occipital bone. 29 Furthermore, another report described copper‐beaten appearance in a Brussels Griffon with CM, SM, and ventriculomegaly. 30 These findings suggest that active bone resorption may occur in SM‐affected animals. Because several morphological abnormalities are associated with SM and consequently ventriculomegaly (disorders of CSF flow), and because enlargement of the cranial CSF content may affect cranial development and bone remodeling, PFs may share common pathomechanisms with SM and ventriculomegaly.
Conversely, in our study, fourth ventricle enlargement was not associated with higher NAS or larger TFA. Because in normal dogs the CSF content of the fourth ventricle is proportionally much smaller than that of the lateral ventricles, in the dogs of our study dilatation of the fourth ventricle may not have been large enough to disturb the ossification process of the membranous skull. 31
4.3. Association of PFs with CCJ structures
The number of cranial sutures affected by PFs was higher and the TFAs larger in Chihuahuas with CCJ overcrowding. Such overcrowding can cause severe neural tissue deviation or compression and is associated with SM. 9 A study evaluating the morphology of the CCJ in Chihuahuas with or without SM found that in SM‐affected dogs, the cranial base at the caudal cranial fossa was shorter and the atlas closer to the basiocciput. 6 Cranial base shortening and cervical spine morphological abnormalities may occur because of premature cranial base synchondrosis closure. 5 In addition to CCJ overcrowding, premature cranial base synchondrosis closure may predispose to narrowing of the jugular foramina and may compromise venous outflow from the cranium, because CKCSs with SM have smaller jugular foramina and venous sinuses in the caudal cranial fossa than do CKCSs without SM. 32 , 33 This disturbance in venous outflow from the cranium may increase cranial venous pressure, as occurs in children. 34
Venous hypertension, along with hydrocephalus and airway obstruction, in children with craniosynostosis is important cause of increased intracranial pressure. 35 Furthermore, increased intracranial pressure is associated with narrowing of the jugular foramina in craniosynostotic children. 34 An association with PF severity and with degree of neural tissue deviation at the CCJ may occur as a result of the cranial base shortening observed in Chihuahuas. We thus suggest that overcrowding of the CCJ, by interfering with cranial CSF and possibly venous outflow, consequently may disturb cranial ossification.
4.4. Limitations of the study
Our study had some limitations. During the analysis, we used total PF areas, rather than using ratios of total PF areas to cranial volumes, because we had a homogenous, single‐breed population and the PF area comparisons were consistent with the results obtained from the number of cranial sutures, which is not weight or size dependent. Furthermore, because of time constraints related to the use of low‐field MRI, we were unable to image the entire spine. Failure to image the thoracic and lumbar spine may have led us to miss some of the syrinxes and to miss a possible association with the maximum syrinx width and PFs. Furthermore, the grading of fourth ventricle dilatation was subjective (yes/no) with no attempt to grade lesion severity. This may have led to underestimation of the association of these lesions with the PFs. We adopted a previously used method to describe lateral ventricular volumes. Rather than describe the true volume of the lateral ventricles, because validation between the measurement and true lateral ventricular volume was lacking, we used the method as an indicator of lateral ventricular volume to enable comparison with the PFs. Finally, almost all of the Chihuahuas, although from many different breeders and owners, originated from the same country. International, multicontinental studies will be necessary to confirm these findings in a larger Chihuahua population.
In conclusion, because both the number of cranial sutures affected by PFs and the total area of the PFs were associated with SM, ventriculomegaly, and CCJ overcrowding, our findings suggest that these structures may share similar pathophysiology. Our findings hence challenge the current concept that PFs are a clinically irrelevant finding not associated with other structural abnormalities. Furthermore, the finding of an association between lower body weight and a higher number of and larger PFs questions the ethics of selective breeding of Chihuahuas with very low body weights. Additional studies will be necessary to evaluate the pathophysiology of PFs and determine whether PFs develop because of embryological dysfunction of skull ossification, because of resorption of normally developed bone at an early age, because of disturbances of suture closure, or a combination of these mechanisms occurring simultaneously.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The Finnish Animal Experimental Board (ESAVI/5794/04.10.03/2011 and ESAVI/9184/04.10.07/2014) approved obtaining the CT and MR images for diagnostic purposes.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
We thank the Finnish Chihuahua Club and Chihuahua owners for their participation in the study and The Finnish Veterinary Foundation, Agria/Svenska Kennelklubben Forskningsfond, and The Veterinary Teaching Hospital of Helsinki University for financial support of the study. Furthermore, we want to thank all the Veterinary Teaching Hospital's anesthesiologists in addition to veterinary and radiology technicians for their expertise in providing the anesthesia and CT and MRI images of the dogs.
Kiviranta A‐M, Rusbridge C, Lappalainen AK, Junnila JJT, Jokinen TS. Persistent fontanelles in Chihuahuas. Part II: Association with craniocervical junction abnormalities, syringomyelia, and ventricular volume. J Vet Intern Med. 2021;35:1848–1856. 10.1111/jvim.16123
Funding information Agria/Svenska Kennelklubben Forskningsfond, Grant/Award Number: N2018‐0024; The Finnish Veterinary Foundation
REFERENCES
- 1. Kiviranta AM, Rusbridge C, Lappalainen AK, et al. Persistent fontanelles in Chihuahuas. Part I: Distribution and clinical significance. J Vet Intern Med. 2021. (Forthcoming). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Kennel Club ; 2019. http://images.akc.org/pdf/breeds/standards/Chihuahua.pdf
- 3. Canadian Kennel Club ; 2019. https://www.ckc.ca/CanadianKennelClub/media/Breed-Standards/Group%205/Chihuahua-Long-Short-Coat.pdf
- 4. Tuite GF, Evanson J, Chong WK, et al. The beaten copper cranium: a correlation between intracranial pressure, cranial radiographs, and computed tomographic scans in children with craniosynostosis. Neurosurgery. 1996;39:691‐699. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt MJ, Volk H, Klingler M, Failing K, Kramer M, Ondreka N. Comparison of closure times for cranial base synchondroses in mesaticephalic, brachycephalic, and Cavalier King Charles Spaniel dogs. Vet Radiol Ultrasound. 2013;54:497‐503. [DOI] [PubMed] [Google Scholar]
- 6. Knowler SP, Kiviranta AM, McFadyen AK, et al. Craniometric analysis of the hindbrain and Craniocervical junction of Chihuahua, Affenpinscher and Cavalier King Charles Spaniel dogs with and without syringomyelia secondary to Chiari‐like malformation. PLoS One. 2017;12(1):e0169898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cerda‐Gonzalez S, Olby NJ, Griffith EH. Medullary position at the craniocervical junction in mature cavalier King Charles spaniels: relationship with neurologic signs and syringomyelia. J Vet Intern Med. 2015;29:882‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cerda‐Gonzalez S, Olby NJ, Griffith EH. Dorsal compressive atlantoaxial bands and the craniocervical junction syndrome: association with clinical signs and syringomyelia in mature cavalier King Charles spaniels. J Vet Intern Med. 2015;29:887‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiviranta AM, Rusbridge C, Laitinen‐Vapaavuori O, et al. Syringomyelia and Craniocervical junction abnormalities in Chihuahuas. J Vet Intern Med. 2017;31:1771‐1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cerda‐Gonzalez S, Olby NJ, Broadstone R. Characteristics of cerebrospinal fluid flow in Cavalier King Charles Spaniels analyzed using phase velocity cine magnetic resonance imaging. Vet Radiol Ultrasound. 2009;50:467‐476. [DOI] [PubMed] [Google Scholar]
- 11. Cerda‐Gonzalez S, Olby NJ, McCullough S, et al. Morphology of the caudal fossa in Cavalier King Charles Spaniels. Vet Radiol Ultrasound. 2009;50:37‐46. [DOI] [PubMed] [Google Scholar]
- 12. The Kennel Club ; 2019. https://www.thekennelclub.org.uk/services/public/breed/standard.aspx?id=6150
- 13. Federation Cynologicue Internationale ; 2019. http://www.fci.be/Nomenclature/Standards/218g09-en.pdf
- 14. Hayward J, Castelhano M, Oliveira K, et al. Complex disease and phenotype mapping in the domestic dog. Nat Commun. 2016;7:10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sutter NB, Bustamante CD, Chase K, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoopes BC, Rimbault M, Liebers D, Ostrander EA, Sutter NB. The insulin‐like growth factor 1 receptor (IGF1R) contributes to reduced size in dogs. Mamm Genome. 2012;23:780‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF‐1 signaling on the skeleton. Front Endocrinol (Lausanne). 2013;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472‐485. [DOI] [PubMed] [Google Scholar]
- 19. Governale LS. Craniosynostosis. Pediatr Neurol. 2015;53:394‐401. [DOI] [PubMed] [Google Scholar]
- 20. Driver CJ, Rusbridge C, Cross HR, McGonnell I, Volk HA. Relationship of brain parenchyma within the caudal cranial fossa and ventricle size to syringomyelia in cavalier King Charles spaniels. J Small Anim Pract. 2010;51:382‐386. [DOI] [PubMed] [Google Scholar]
- 21. Rusbridge C, Knowler P. The need for head space: brachycephaly and cerebrospinal fluid disorders. Life. 2021;11(2):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt MJ, Laubner S, Kolecka M, et al. Comparison of the relationship between cerebral white matter and grey matter in normal dogs and dogs with lateral ventricular enlargement. PLoS One. 2015;10(5):e0124174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt MJ, Kolecka M, Kirberger R, Hartmann A. Dynamic susceptibility contrast perfusion magnetic resonance imaging demonstrates reduced periventricular cerebral blood flow in dogs with ventriculomegaly. Front Vet Sci. 2017;4:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loderstedt S, Benigni L, Chandler K, et al. Distribution of syringomyelia along the entire spinal cord in clinically affected Cavalier King Charles Spaniels. Vet J. 2011;190:359‐363. [DOI] [PubMed] [Google Scholar]
- 25. Scrivani PV, Thompson MS, Winegardner KR, Dewey CW, Scarlett JM. Association between frontal‐sinus size and syringohydromyelia in small‐breed dogs. Am J Vet Res. 2007;68:610‐613. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell TJ, Knowler SP, van den Berg H, Sykes J, Rusbridge C. Syringomyelia: determining risk and protective factors in the conformation of the Cavalier King Charles Spaniel dog. Canine Genet Epidemiol. 2014;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knowler SP, McFadyen AK, Freeman C, et al. Quantitative analysis of Chiari‐like malformation and syringomyelia in the Griffon Bruxellois dog. PLoS One. 2014;9(2):e88120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knowler SP, Cross C, Griffiths S, et al. Use of morphometric mapping to characterise symptomatic Chiari‐like malformation, secondary syringomyelia and associated brachycephaly in the Cavalier King Charles Spaniel. PLoS One. 2017;12(1):e0170315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Driver CJ, De Risio L, Hamilton S, et al. Changes over time in craniocerebral morphology and syringomyelia in cavalier King Charles spaniels with Chiari‐like malformation. BMC Vet Res. 2012;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rusbridge C, Stringer F, Knowler SP. Clinical application of diagnostic imaging of Chiari‐like malformation and syringomyelia. Front Vet Sci. 2018;5:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reinitz LZ, Bajzik G, Garamvölgyi R, et al. Linear relationship found by magnetic resonance imaging between cerebrospinal fluid volume and body weight in dogs. Acta Vet Hung. 2017;65:1‐12. [DOI] [PubMed] [Google Scholar]
- 32. Schmidt MJ, Ondreka N, Sauerbrey M, Volk H, Rummel C, Kramer M. Volume reduction of the jugular foramina in Cavalier King Charles Spaniels with syringomyelia. BMC Vet Res. 2012;8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fenn J, Schmidt MJ, Simpson H, Driver CJ, Volk HA. Venous sinus volume in the caudal cranial fossa in Cavalier King Charles spaniels with syringomyelia. Vet J. 2013;197:896‐897. [DOI] [PubMed] [Google Scholar]
- 34. Rich PM, Cox TC, Hayward RD. The jugular foramen in complex and syndromic craniosynostosis and its relationship to raised intracranial pressure. AJNR Am J Neuroradiol. 2003;24:45‐51. [PMC free article] [PubMed] [Google Scholar]
- 35. Abu‐Sittah GS, Jeelani O, Dunaway D, Hayward R. Raised intracranial pressure in Crouzon syndrome: incidence, causes, and management. J Neurosurg Pediatr. 2016;17:469‐475. [DOI] [PubMed] [Google Scholar]


