Abstract
Background
Clinical use of gabapentin has increased; transdermal delivery in cats is incompletely studied.
Objective
To evaluate if gabapentin permeates feline skin in vitro and in vivo and to determine if pain scores improve after administration.
Animals
In vitro: cadaver skin from 6 cats; phase 1: 8 young, healthy client‐owned cats; phase 2: 15 client‐owned geriatric cats.
Methods
In vitro, gabapentin applied every q12h to ear or cervical skin in diffusion cells. Samples collected at 0, 2, 4, 12, and 24 hours after application. Phase 1: Cats assigned to 1 of 4 groups: 5 mg/kg or 10 mg/kg applied q8h for 5 days to either ear or cervical skin. Serum samples collected predose, and after 1 and 5 days. Phase 2: 10 mg/kg applied q8h for 5 days. Two validated pain scores recorded predose, and after days 1, 5, and 8. Serum samples collected predose, and after days 1 and 5. Samples were frozen at −80°C for concentration analysis utilizing a validated high‐performance liquid chromatography mass‐spectrometry method.
Results
Gabapentin was identified in all samples. Significant differences in gabapentin concentrations were observed from day 1 to day 5 (P < .02) and in pain scores from predose to day 5 (P < .05) and day 1 to day 5 (P < .05). No differences in pain scores were observed from predose to day 8 (P = .3).
Conclusions and Clinical Relevance
Gabapentin in a transdermal base penetrates feline skin in vitro, is absorbed systemically in cats, and may help decrease pain scores.
Keywords: feline analgesia, gabapentin, skin, transdermal
Abbreviations
- HPLC
high‐performance liquid chromatography
- PBS
phosphate‐buffered saline
1. INTRODUCTION
Gabapentin is a popular drug used in the treatment of several painful and stressful conditions. 1 , 2 , 3 Unfortunately, traditional PO drug administration can be difficult in domestic cats because of food selectivity and strong fight‐or‐flight response, which can negatively impact drug administration. Poor compliance with PO drug protocols can result in treatment failure and ongoing discomfort in cats. Therefore, a noninvasive option for long‐term drug delivery is needed for better management of cats with pain. Although several medications have been tested transdermally for use in cats to try and improve ease of administration and owner compliance, 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 only 2 are routinely used and only 1 is commercially available. 13 , 14 , 15 Transdermal delivery in veterinary medicine is challenging because of species differences in skin structure, barrier properties of skin, surface area required for optimal drug absorption, and lipid solubility of the medications used. 16 , 17 , 18 Transdermal administration of gabapentin recently was evaluated in humans, 3 but‐ transdermal absorption studies evaluating its use in cats are limited. 19
Our aim was to evaluate if gabapentin in a proprietary base (Lipoderm) permeates feline skin in vitro and in vivo and to determine if validated pain scores improve after its administration.
2. MATERIALS AND METHODS
2.1. In vitro skin
Approval was granted by the IACUC at Washington State University (ASAF # 6190). The skin from 6 fresh feline cadavers (3 cadavers per day, on 2 separate days) was collected. Samples were obtained from the Washington State University clinical skills laboratory. Skin (ear pinna and cervical area; 2 cm cranial to the scapula) was shaved and an approximately 2.5 × 2.5 cm section of inner ear pinna epidermis, dermis, and subcutaneous tissue (not including cartilage) was harvested and placed in phosphate‐buffered saline (PBS; Sigma‐Aldrich, St. Louis, Missouri). Skin samples were stored at −80°C for a maximum of 2 days until use.
2.2. Diffusion cells
Three Franz‐type diffusion cells (Permagear Inc, Hellertown, Pennsylvania) with a 2 cm donor compound opening were used and each experiment was performed in triplicate.
2.3. Transdermal gabapentin
Gabapentin in the proprietary base Lipoderm was obtained from local compounding pharmacies (Sid's Pharmacy, Pullman, Washington and Best Pet Rx, New York, NY), 1 pharmacy was utilized for the in vitro and in vivo phase 1 experiment (Sid's Pharmacy) and formulated the preparations to desired concentrations: 10% and 20%. Another pharmacy (Best Pet Rx) was utilized for the in vivo phase 2 portion of the study. Sample gabapentin specimens were submitted from the initial compounding pharmacy with study samples and quantified at 2.65 mg/0.05 mL and 11.7 mg/0.05 mL. A total of 0.1 mL of gabapentin in the proprietary base was applied to the inner ear pinna of the skin or to the formerly haired region of the skin for each experiment.
2.4. In vitro experiment
Three different feline cadavers were used for skin samples for each experiment (performed on 2 separate days). Skin from the ear pinna (full thickness not including cartilage) and cervical skin (full thickness) was harvested on the same day. Samples were placed in PBS in a secured plastic bag, and immediately frozen at −80°C. Samples were thawed in PBS at room temperature (21°C) for 12 hours before each experiment based on former studies of frozen animal tissue defrosted 20 and hydrated in PBS. Each sample then was mounted on a diffusion cell with 10 mL sterile PBS in its receptor chamber and placed in a warm water bath at 39°C as described in experiments evaluating topical gabapentin formulations. 3 Transdermal gabapentin (10% or 20% in a Lipoderm base) was applied using a gloved finger to the mounted cadaver skin at 0 and 12 hours. Samples were collected from the receptor chamber of the diffusion cell (100 μL) at 0, 2, 4, 12, and 24 hours after initial transdermal application and immediately frozen at −80°C. An equivalent volume of PBS was not added to the receptor chamber after each sample collection. Samples then were sent for gabapentin quantification using high‐performance liquid chromatography (HPLC) mass spectrometry analysis at The University of Tennessee Diagnostic Laboratory‐Pharmacology Laboratory. The limit of quantification for gabapentin at the reference laboratory was 25 ng/mL. Specific methods and validation of the gabapentin HPLC by the laboratory are described elsewhere. 21 , 22 All experiments were done in triplicate.
2.5. In vivo phase 1
Approval was granted by the ethical review board of Washington State University (IACUC ASAF # 6379). Eight client‐owned healthy cats (<6 years) were enrolled. Study cats had gabapentin (250 mg/mL) not to exceed 0.2 mL of volume per dose per cat applied transdermally to either the shaved (2 cm × 2 cm) dorsal cervical region (2 cm cranial to the scapula) or inner ear pinna by the owners at home for 5 days. Owners were given disposable rubber finger guards or gloves (owner preference) for use during topical gabapentin administration. Owners were instructed to apply the predetermined medication volume from a syringe directly to the assigned skin location (ear pinna or cervical area) and then gently rub the mixture onto the surface of the skin to fully cover the area. Owners were warned that systemic absorption of gabapentin could occur if they did not wear protective gloves when administering the medication. 3 No Elizabethan collars were used in this study, nor were the cats monitored for a specific amount of time after medication application.
Study cats were randomly assigned to 1 of the following groups: 5 mg/kg q8h applied to the ear pinna, 10 mg/kg q8h applied to the ear pinna, 5 mg/kg q8h applied to the cervical skin, and 10 mg/kg q8h applied to the cervical skin. Blood samples from each cat were obtained predose and at 1 and 5 days after applying the transdermal medication to the predetermined location (ear pinna or cervical skin). Blood samples were collected at the Washington State University teaching hospital. Blood was centrifuged at 2500 rpm for 10 minutes and serum samples immediately frozen at −80°C. Batched samples were sent for gabapentin concentration analysis to a laboratory utilizing a validated HPLC mass spectrometry method. 21 , 22
2.6. In vivo phase 2
Study approval was granted by the ethical review board at the Animal Medical Center (IACUC ASAF # F‐8‐19‐19‐G). Fifteen client‐owned cats (>8 years of age) were enrolled. The enrolled cats had been diagnosed with at least 1 of the following conditions: chronic kidney disease (International Renal Interest Society Stage 1 or 2), osteoarthritis, dental disease, or obesity. All cats were considered clinically stable based on physical examination and medical record review and were not receiving any medications. All cats received 10 mg/kg of transdermal gabapentin in a Lipoderm base (250 mg/mL), not to exceed 0.2 mL of volume per dose per cat q8h for 5 days to the inner ear pinna. Clients were instructed to alternate ears for each dose administration. The cats were not consistently monitored and were allowed to groom themselves. Pain scores using 2 validated pain assessment scales for cats 23 , 24 were recorded by the investigator (J.E. Slovak) before gabapentin application, and on days 1 and 5, and on day 8 (3 days after the last transdermal application). Blood sample collection for determination of serum gabapentin concentration was performed before gabapentin application, and on days 1 and 5, with serum samples processed as previously described.
2.7. Data analysis
Descriptive statistics and repeated measures analysis of variance using commercial software (SAS Institute Inc, Cary, North Carolina) were utilized for data analysis. Power analysis was not performed because of the exploratory nature of the study.
3. RESULTS
3.1. In vitro
Gabapentin was quantified in PBS samples at all time points (2, 4, 12, and 24 hours) for both the ear pinna and cervical skin experiments (Figure 1A,B).
FIGURE 1.
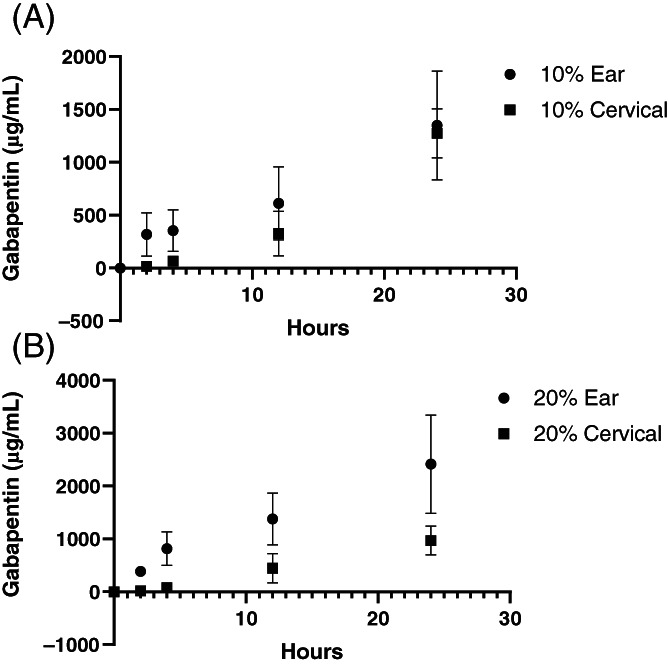
A, Gabapentin levels after 10% gabapentin applied to cadaver feline ear pinna and cervical skin. B, Gabapentin levels after 20% gabapentin applied to cadaver feline ear pinna and cervical skin
3.2. In vivo phase 1
Gabapentin was detected for all cats in all groups at day 1 (0.18‐1.3 μg/mL) and day 5 (0.11‐5.4 μg/mL; Figure 2A,B). No differences in gabapentin concentrations were found when comparing gabapentin dosing (5 mg/kg vs 10 mg/kg) or skin location (cervical skin vs ear pinna; P > .05).
FIGURE 2.
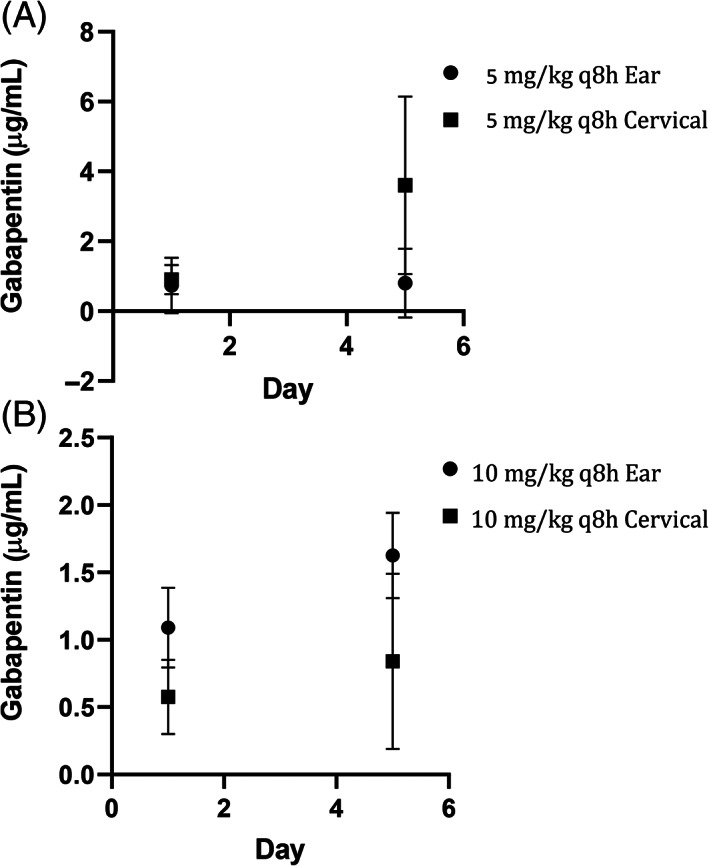
A, Gabapentin levels of healthy cats dosed at 5 mg/kg q8h of transdermal gabapentin to the ear pinna or cervical skin. B, Gabapentin levels of healthy cats dosed at 10 mg/kg q8h of transdermal gabapentin to the ear pinna or cervical skin
3.3. In vivo phase 2
Gabapentin was detected at day 1 (0.1‐1.47 μg/mL) and day 5 (0.31‐3.38 μg/mL) in all cats (Figure 3). The mean gabapentin concentration at day 1 was 0.6 ± 0.51 μg/mL and 1.32 ± 0.99 μg/mL at day 5. A significant difference in gabapentin concentration was observed from day 1 to day 5 (P < .02). Significant differences in pain scores were observed for both pain scales when comparing predose to day 5 (P < .05) and when comparing day 1 to day 5 (P < .05; Figures 4 and 5). No significant difference was observed in pain scores between predose and day 8 in any of the cats for either pain scale (P = .3).
FIGURE 3.
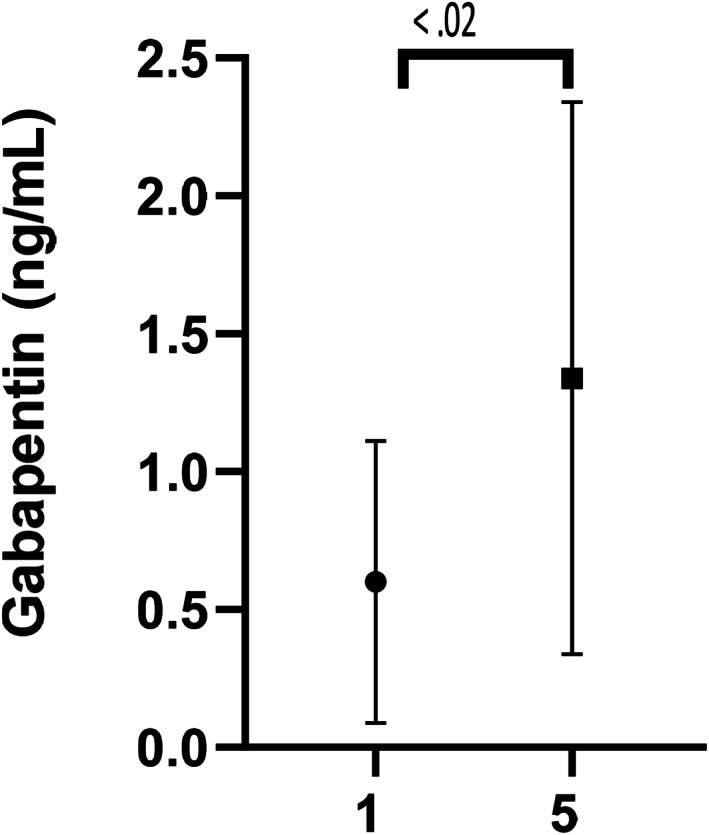
Gabapentin levels of 15 senior cats at day 1 and day 5 after transdermal gabapentin at 10 mg/kg q8h. Significant differences in gabapentin levels day 1 and day 5, P < .02
FIGURE 4.
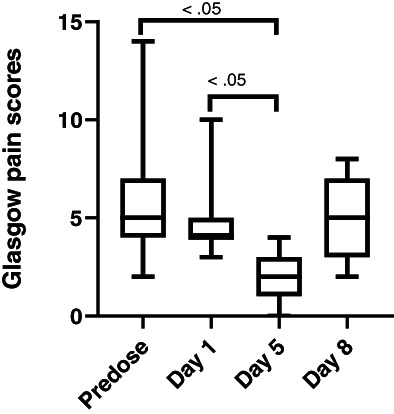
Glasgow pain scores for 15 cats administered transdermal gabapentin at 10 mg/kg q8h at predose, day 1, 5, and 8 (72 hours postdiscontinuing gabapentin) showing minimum, maximum, and median values. Maximum Glasgow pain score is 20
FIGURE 5.
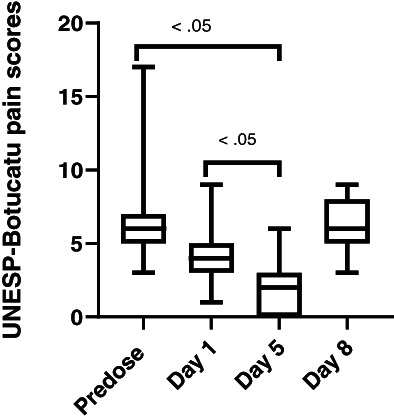
UNESP‐Botucatu scores for 15 cats administered transdermal gabapentin at 10 mg/kg q8h at predose, day 1, 5, and 8 (72 hours postdiscontinuing gabapentin) showing minimum, maximum, and median values. Maximum UNESP‐Botucatu pain score is 30
4. DISCUSSION
We investigated the permeation of transdermally administered gabapentin in the proprietary base Lipoderm across feline cadaver skin and in healthy cats at 2 sites (ear pinna and cervical skin). We also quantified gabapentin in geriatric cats undergoing treatment q8h for 5 days, and identified improvement of pain scores during administration and worsening of pain scores after discontinuation of the gabapentin. These findings suggest that transdermally administered gabapentin may be an analgesic option for some cats.
To date, only 1 study has investigated the pharmacokinetics of transdermal drug delivery of gabapentin in cats. 19 Although that study suggested poor transdermal absorption of gabapentin, minimal or no data was reported because many results were below the limit of detection and the duration of the transdermal application was unclear. 19 Additionally, gabapentin was applied solely to the ear pinna, and only 1 dose (10 mg/kg) was used in clinically healthy cats. Despite this information, our intent was to systematically investigate the use of transdermally administered gabapentin in cats by conducting both in vitro and in vivo experiments. Our initial in vitro experiments were designed to determine if permeation of gabapentin occurred using cadaver feline skin from different locations because transdermal absorption of medications can be impacted by skin location 25 , 26 utilizing Franz diffusion cells, a technique widely accepted in laboratory settings. 27 , 28 , 29 , 30 Lipoderm was chosen as our drug delivery vehicle based on published human and veterinary medical literature for its stability and consistency. 3 , 18 , 29 , 31 , 32 , 33 , 34 Based on our results, gabapentin does permeate both the feline ear pinna and cervical skin in vitro.
In vitro permeation studies have inherent limitations, including variability of the membrane used in the diffusion cells (synthetic vs natural), reproducibility, lack of simulation of physiologic conditions such as vascularity, metabolic reactions in the skin, temperature, stratum corneum thickness, and grooming behaviors of the cat. 16 , 17 , 29 , 30 , 35 For these reasons, we chose to use feline skin vs a synthetic membrane, controlled the water bath temperature to the average body temperature of cats, and shaved all skin samples to facilitate transdermal formulation exposure. Because ours was an in vitro study, dermal blood supply, grooming behavior, and body weight for dosing were not simulated. We utilized skin from 6 different cats which could have led to variable results because of inherent inter‐individual differences in feline skin. 16 , 17 Although each experiment was performed in triplicate to aid in validity of results, because of the skin sample size needed and availability of only 2 ear pinna samples per cat, experiments were performed in triplicate using skin from different cats. The small number of skin samples and replicates for the in vitro study could have increased the risk of type II error and should be kept in mind when interpreting these results. Permeation studies utilizing skin of the intended species is considered the gold standard, but alternative biological membrane sources may be useful to overcome some of the challenges of utilizing different individuals (although from the same species) in diffusion cell studies. 36 However, an advantage to utilizing skin from different cats is a more realistic model for future in vivo use. In addition, the gabapentin concentration reported in the in vitro study was cumulative, because an equivalent amount of PBS was not added after removing 100 μL of sample from the receptor chamber at each time point, which contributed to an increase in the gabapentin concentration in subsequent samples. Finally, the use of gabapentin compounded for transdermal use, especially from 2 different pharmacies, is a limitation of the study in that the concentrations of gabapentin may have been inconsistent, depending on the pharmacy used. 33 , 34
Because our in vitro permeation study indicated that gabapentin could be quantified in the receptor channel of the Franz diffusion cells, our next step was to use gabapentin in a Lipoderm base at predetermined doses and locations in healthy client‐owned cats. Performing preliminary in vivo experiments to determine if gabapentin can permeate feline skin in different skin locations is a clinically relevant and necessary step in clinical drug development and use. Skin permeation of medications has been investigated in several species and poses many challenges such as intra‐ and interpatient variability, 18 regional differences in individuals, 17 , 37 and other factors such as vascularity, sun exposure, hair, stratum corneum thickness, and pH. 17 , 18 , 25 , 26 , 35 We chose to use 2 skin sites in our in vivo study similar to our in vitro study because the ear pinna is a less haired region that owners can easily access, and cervical skin makes it more difficult for cats to ingest the drug by grooming, but also is an area to which owners could easily apply the medication, especially when shaved. Based on preliminary data, the serum concentrations of gabapentin obtained were comparable between sites of application in this small population of cats. Gabapentin was detected in all cats at all time points during the in vivo phase of the study. This result differs from results of a previously reported study. 19 Despite detection of gabapentin in the serum of all enrolled cats, the concentrations were variable and data were obtained from only a small number of cats, and results should be interpreted with caution.
One limitation of the in vivo study was that cats were not prevented from grooming during the study. For this reason, it is possible that gabapentin detected in the blood could have arisen from ingestion associated with grooming the pinna or cervical region, increasing serum gabapentin concentrations. Elizabethan collar use and continuous monitoring of the cats intentionally was avoided to mimic realistic circumstances in a home environment. We also relied on owners to topically administer the gabapentin at 8‐hour intervals to simulate a true patient experience. The owners may have been noncompliant, may not have maintained accurate dosing schedules, or the gabapentin may have been inadequately applied to the skin, despite the fact that in‐person demonstration and coaching were provided for each owner.
The phase 2 trial of transdermal administration of gabapentin in an aged population of cats allowed us to both quantify serum gabapentin concentrations and document pain scores before, during, and after discontinuation of treatment. A small group of older client‐owned cats was enrolled in the study. All cats were scored for pain using 2 validated pain scales that focus on both contact and noncontact observational assessments of cats. To determine if there was a clinical response to the gabapentin, all study cats were evaluated before administration, after 1 day, after 5 days, and 3 days after gabapentin was discontinued for a final assessment on day 8. This approach was used to compare predosing to 8‐day assessments and predosing to day 1 and day 1 to day 5 evaluations to see if scores improved. As in both previous studies, gabapentin was quantified in all samples tested and most importantly, no difference was observed in pain scores from predose to day 8, but a significant difference was identified when comparing predosing to day 5 and day 1 to day 5. The lack of difference between pain scores between predose and day 8 suggests that gabapentin's analgesic effect was not present 3 days after discontinuation. Our results indicate that gabapentin is absorbed by the transdermal route and that it changed the pain scores of the enrolled cats. Although 2 pain scales were used to evaluate the effect of transdermal gabapentin administration, because our study did not include control cats (or have a cross‐over design) and the evaluator was not blinded to the day of administration, these factors could have contributed to confirmation bias. The exploratory nature of our study is responsible for the lack of control cats or placebo medication, as well as the small number of cats enrolled. A large cross‐over study using blinded evaluation of the potential analgesic effect of gabapentin in a more homogeneous population of cats is recommended for a better understanding of gabapentin's role in the management of painful conditions in cats.
No reference interval is available for serum gabapentin concentrations in cats despite several studies investigating its pharmacokinetics and clinical use. 1 , 2 , 19 , 38 , 39 , 40 Data on optimal plasma gabapentin concentrations is only available in rats and humans. 19 , 41 , 42 Therapeutic and analgesic concentration ranges in cats may be different than those of other species, and extrapolation cannot be made. The concentrations measured in our cats transdermally treated with gabapentin are much lower than those in other published studies on the pharmacokinetics of gabapentin after IV and PO routes in cats, and these studies should not be directly compared. 19 , 38 Additional research is needed to advance the understanding of gabapentin's use as an analgesic in cats, including appropriate dosing and therapeutic drug concentrations. The primary intent of our study was to determine if transdermal permeation of gabapentin was possible in vitro and in vivo.
We only administered gabapentin for 5 days. Gabapentin often is used to treat chronic conditions, 2 , 43 , 44 and our pain scores could have been different with longer duration of treatment. However, studies evaluating gabapentin in acute painful or anxiety‐inducing situations have shown clinical effectiveness. 1 , 39 , 40 , 45 Only 2 pain scales were used in our study. At the time of our study, the feline grimace scale 46 had not yet been published and therefore the extended comprehensive composite pain scales were chosen for completeness and standardization. The use of pain scores and their validity is a debated topic. However, current research in analgesia, and guidelines in both veterinary and human medicine for individuals unable to self‐report, encourage use of a consistent observer utilizing validated methods, with subjective and objective measurements over a period of time for optimal pain assessment. 47 , 48 , 49 , 50
Our preliminary and small multistep study suggests that transdermally administered gabapentin in a Lipoderm base can permeate feline skin (cervical and ear pinna) and be detected in the serum of treated cats. These findings suggest transdermal application may be a reasonable route of administration in cats. Use of transdermally administered gabapentin at 10 mg/kg q8h decreased recorded pain scores after 5 days of treatment in our small study. Future studies evaluating the use of transdermally administered gabapentin in cats using the feline grimace scale, administration of gabapentin for a longer period of time, and comparing gabapentin concentrations using formulations from several compounding pharmacies should be performed.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All work was performed according to protocols IACUC ASAF #6379 and #F‐8‐19‐19‐G approved by Washington State University and Animal Medical Center IACUC. IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding for this study was provided by the Winn Feline Foundation, W19‐039. Research was supported by a 2018 WSU intra‐mural grant, College of Veterinary Medicine, Washington State University. The authors thank Dr Julianne Hwang and Dr Nicolas Villarino for their help with study design experiments. Some of the results of this study were presented as an oral abstract at the 2019 ACVIM Forum in Phoenix, Arizona and as an e‐poster presentation at the 2020 ACVIM Forum On Demand.
Slovak JE, Costa AP. A pilot study of transdermal gabapentin in cats. J Vet Intern Med. 2021;35:1981–1987. 10.1111/jvim.16137
Funding information Washington State University; College of Veterinary Medicine; 2018 WSU intra‐mural grant; Winn Feline Foundation, Grant/Award Number: W19‐039
REFERENCES
- 1. van Haaften KA, Forsythe LRE, Stelow EA, Bain MJ. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J Am Vet Med Assoc. 2017;251:1175‐1181. [DOI] [PubMed] [Google Scholar]
- 2. Guedes AGP, Meadows JM, Pypendop BH, Johnson EG, Zaffarano B. Assessment of the effects of gabapentin on activity levels and owner‐perceived mobility impairment and quality of life in osteoarthritic geriatric cats. J Am Vet Med Assoc. 2018;253:579‐585. [DOI] [PubMed] [Google Scholar]
- 3. Martin CJ, Alcock N, Hiom S, Birchall J. Development and evaluation of topical gabapentin formulations. Pharmaceutics. 2017;9(3):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eichstadt LR, Corriveau LA, Moore GE, Knipp GT, Cooper BR, Gwin WE. Absorption of transdermal fluoxetine compounded in a lipoderm base compared to oral fluoxetine in client‐owned cats. Int J Pharm Compd. 2017;21:242‐246. [PubMed] [Google Scholar]
- 5. Zajic LB, Herndon AK, Sieberg LG, et al. Assessment of absorption of transdermal ondansetron in normal research cats. J Feline Med Surg. 2017;19:1245‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helms SR. Treatment of feline hypertension with transdermal amlodipine: a pilot study. J Am Anim Hosp Assoc. 2007;43:149‐156. [DOI] [PubMed] [Google Scholar]
- 7. Egger CM, Glerum LE, Allen SW, Haag M. Plasma fentanyl concentrations in awake cats and cats undergoing anesthesia and ovariohysterectomy using transdermal administration. Vet Anaesth Analg. 2003;30:229‐236. [DOI] [PubMed] [Google Scholar]
- 8. Delamaide Gasper JA, Barnes Heller HL, Robertson M, Trepanier LA. Therapeutic serum phenobarbital concentrations obtained using chronic transdermal administration of phenobarbital in healthy cats. J Feline Med Surg. 2015;17:359‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith C, Barnes Heller HL, Reif N, van Hesteren M, Reinhart JM. Serum levetiracetam concentrations after transdermal levetiracetam administration, 3 times daily, to healthy cats. J Vet Intern Med. 2019;33:827‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnoski J, Lee‐Fowler TM, Boothe DM, Behrend EN. Serum theophylline after multiple dosing with transdermal gels in cats. J Feline Med Surg. 2019;21:329‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sleeper MM, O'Donnell P, Fitzgerald C, Papich MG. Pharmacokinetics of furosemide after intravenous, oral and transdermal administration to cats. J Feline Med Surg. 2019;21:882‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill KE, Gieseg MA, Kingsbury D, Lopez‐Villalobos N, Bridges J, Chambers P. The efficacy and safety of a novel lipophilic formulation of methimazole for the once daily transdermal treatment of cats with hyperthyroidism. J Vet Intern Med. 2011;25:1357‐1365. [DOI] [PubMed] [Google Scholar]
- 13. Benson KK, Zajic LB, Morgan PK, et al. Drug exposure and clinical effect of transdermal mirtazapine in healthy young cats: a pilot study. J Feline Med Surg. 2017;19:998‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sartor LL, Trepanier LA, Kroll MM, Rodan I, Challoner L. Efficacy and safety of transdermal methimazole in the treatment of cats with hyperthyroidism. J Vet Intern Med. 2004;18:651‐655. [DOI] [PubMed] [Google Scholar]
- 15. Buhles W, Quimby JM, Labelle D, Williams VS. Single and multiple dose pharmacokinetics of a novel mirtazapine transdermal ointment in cats. J Vet Pharmacol Ther. 2018;41:644‐651. [DOI] [PubMed] [Google Scholar]
- 16. Eichstadt LR. Compounding transdermal medication for feline patients. Int J Pharm Compd. 2016;20:271‐274. [PubMed] [Google Scholar]
- 17. Forsythe LE. Feline transdermal formulation considerations. Int J Pharm Compd. 2017;21:446‐452. [PubMed] [Google Scholar]
- 18. Riviere JE, Papich MG. Potential and problems of developing transdermal patches for veterinary applications. Adv Drug Deliv Rev. 2001;50:175‐203. [DOI] [PubMed] [Google Scholar]
- 19. Adrian D, Papich MG, Baynes R, Stafford E, Lascelles BDX. The pharmacokinetics of gabapentin in cats. J Vet Intern Med. 2018;32:1996‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stahl J, Braun M, Siebert J, Kietzmann M. The percutaneous permeation of a combination of 0.1% octenidine dihydrochloride and 2% 2‐phenoxyethanol (octenisept®) through skin of different species in vitro. BMC Vet Res. 2011;7:44‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baine K, Jones MP, Cox S, Martín‐Jiménez T. Pharmacokinetics of compounded intravenous and oral gabapentin in Hispaniolan Amazon parrots (Amazona ventralis). J Avian Med Surg. 2015;29:165‐173. [DOI] [PubMed] [Google Scholar]
- 22. Gold JR, Grubb TL, Green S, Cox S, Villarino NF. Plasma disposition of gabapentin after the intragastric administration of escalating doses to adult horses. J Vet Intern Med. 2020;34:933‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brondani JT, Mama KR, Luna SP, et al. Validation of the English version of the UNESP‐Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res. 2013;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reid J, Scott EM, Calvo G, Nolan AM. Definitive Glasgow acute pain scale for cats: validation and intervention level. Vet Rec. 2017;180:449. [DOI] [PubMed] [Google Scholar]
- 25. Mills PC, Magnusson BM, Cross SE. Investigation of in vitro transdermal absorption of fentanyl from patches placed on skin samples obtained from various anatomic regions of dogs. Am J Vet Res. 2004;65:1697‐1700. [DOI] [PubMed] [Google Scholar]
- 26. Mills PC, Cross SE. Regional differences in the in vitro penetration of hydrocortisone through equine skin. J Vet Pharmacol Ther. 2006;29:25‐30. [DOI] [PubMed] [Google Scholar]
- 27. Bartosova L, Bajgar J. Transdermal drug delivery in vitro using diffusion cells. Curr Med Chem. 2012;19:4671‐4677. [DOI] [PubMed] [Google Scholar]
- 28. Folzer E, Gonzalez D, Singh R, Derendorf H. Comparison of skin permeability for three diclofenac topical formulations: an in vitro study. Pharmazie. 2014;69:27‐31. [PubMed] [Google Scholar]
- 29. Heustess A, Spigener S, Sweitzer S, Romero‐Sandoval A, Asbill S. Analgesic efficacy and transdermal penetration of topical gabapentin creams: finding an optimal dose and pre‐treatment time. Int J Pharm Compd. 2015;19:167‐173. [PubMed] [Google Scholar]
- 30. Ng SF, Rouse JJ, Sanderson FD, Meidan V, Eccleston GM. Validation of a static Franz diffusion cell system for in vitro permeation studies. AAPS PharmSciTech. 2010;11:1432‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Q, Song Y, Page SW, Garg S. Evaluation of transdermal drug permeation as modulated by lipoderm and pluronic lecithin organogel. J Pharm Sci. 2018;107:587‐594. [DOI] [PubMed] [Google Scholar]
- 32. Carrer V, Alonso C, Pont M, et al. Effect of propylene glycol on the skin penetration of drugs. Arch Dermatol Res. 2020;312(5):337‐352. [DOI] [PubMed] [Google Scholar]
- 33. Shakshuki A, Yeung P, Agu RU. Compounded gabapentin for neuropathic pain: stability and beyond‐use date (BUD) in some commonly used bases. J Am Pharm Assoc. 2019;59:514‐520. [DOI] [PubMed] [Google Scholar]
- 34. Shakshuki A, Agu RU. Compounded topical gabapentin for neuropathic pain: does choice of base affect efficacy? Int J Pharm Compd. 2019;23:496‐503. [PubMed] [Google Scholar]
- 35. Gorzelanny C, Mess C, Schneider SW, Huck V, Brandner JM. Skin barriers in dermal drug delivery: which barriers have to be overcome and how can we measure them? Pharmaceutics. 2020;12:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neupane R, Boddu SHS, Renukuntla J, Babu RJ, Tiwari AK. Alternatives to biological skin in permeation studies: current trends and possibilities. Pharmaceutics. 2020;12:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mills PC, Cross SE. Transdermal drug delivery: basic principles for the veterinarian. Vet J. 2006;172:218‐233. [DOI] [PubMed] [Google Scholar]
- 38. Siao KT, Pypendop BH, Ilkiw JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res. 2010;71:817‐821. [DOI] [PubMed] [Google Scholar]
- 39. Pankratz KE, Ferris KK, Griffith EH, Sherman BL. Use of single‐dose oral gabapentin to attenuate fear responses in cage‐trap confined community cats: a double‐blind, placebo‐controlled field trial. J Feline Med Surg. 2018;20:535‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steagall PV, Benito J, Monteiro BP, Doodnaught GM, Beauchamp G, Evangelista MC. Analgesic effects of gabapentin and buprenorphine in cats undergoing ovariohysterectomy using two pain‐scoring systems: a randomized clinical trial. J Feline Med Surg. 2018;20:741‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lockwood PA, Cook JA, Ewy WE, Mandema JW. The use of clinical trial simulation to support dose selection: application to development of a new treatment for chronic neuropathic pain. Pharm Res. 2003;20:1752‐1759. [DOI] [PubMed] [Google Scholar]
- 42. Larsen MS, Keizer R, Munro G, et al. Pharmacokinetic/pharmacodynamic relationship of gabapentin in a CFA‐induced inflammatory hyperalgesia rat model. Pharm Res. 2016;33:1133‐1143. [DOI] [PubMed] [Google Scholar]
- 43. Lorenz ND, Comerford EJ, Iff I. Long‐term use of gabapentin for musculoskeletal disease and trauma in three cats. J Feline Med Surg. 2013;15:507‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muller G. Compounded gabapentin suspension for lower back pain in an older cat: a case report. Int J Pharm Compd. 2010;14:215‐217. [PubMed] [Google Scholar]
- 45. Hudec CP, Griffin CE. Changes in the stress markers cortisol and glucose before and during intradermal testing in cats after single administration of pre‐appointment gabapentin. J Feline Med Surg. 2020;22:138‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Evangelista MC, Watanabe R, Leung VSY, et al. Facial expressions of pain in cats: the development and validation of a feline grimace scale. Sci Rep. 2019;9:19128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Epstein ME, Rodanm I, Griffenhagen G, et al. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg. 2015;17:251‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manworren RC, Stinson J. Pediatric pain measurement, assessment, and evaluation. Semin Pediatr Neurol. 2016;23:189‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adrian D, Papich M, Baynes R, Murrell J, Lascelles BDX. Chronic maladaptive pain in cats: a review of current and future drug treatment options. Vet J. 2017;230:52‐61. [DOI] [PubMed] [Google Scholar]
- 50. Kunz M, Seuss D, Hassan T, et al. Problems of video‐based pain detection in patients with dementia: a road map to an interdisciplinary solution. BMC Geriatr. 2017;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]


