Abstract
Background
Hypochloremia is a strong negative prognostic factor in humans with congestive heart failure (CHF), but the implications of electrolyte abnormalities in small animals with acute CHF are unclear.
Objectives
To document electrolyte abnormalities present upon admission of small animals with acute CHF, and to assess the relationship between electrolyte concentrations and diuretic dose, duration of hospitalization and survival time.
Animals
Forty‐six dogs and 34 cats with first onset of acute CHF.
Methods
Retrospective study. The associations between electrolyte concentrations and diuretic doses were evaluated with Spearman rank correlation coefficients. Relationship with duration of hospitalization and survival were assessed by simple linear regression and Cox proportional hazard regression, respectively.
Results
The most commonly encountered electrolyte anomaly was hypochloremia observed in 24% (9/46 dogs and 10/34 cats) of cases. In dogs only, a significant negative correlation was identified between serum chloride concentrations at admission (median 113 mmol/L [97‐125]) and furosemide doses both at discharge (median 5.2 mg/kg/day [1.72‐9.57]; r = −0.59; P < .001) and at end‐stage heart failure (median 4.7 mg/kg/day [2.02‐7.28]; r = −0.62; P = .005). No significant hazard ratios were found for duration of hospitalization nor survival time for any of the electrolyte concentrations.
Conclusions and Clinical Importance
The observed association between serum chloride concentrations and diuretic doses suggests that hypochloremia could serve as a marker of disease severity and therapeutic response in dogs with acute CHF.
Keywords: chloride, diuretic, furosemide, potassium, sodium
Abbreviations
- AVS
arginine vasopressin system
- CHF
congestive heart failure
- Cl
chloride
- K
potassium
- Na
sodium
- RAAS
renin‐angiotensin‐aldosterone system
1. INTRODUCTION
In both human and veterinary medicine, electrolyte abnormalities occur in congestive heart failure (CHF) cases. This is thought to be due to neurohormonal activation by the renin‐angiotensin‐aldosterone (RAAS) and arginine vasopressin (AVS) systems, and because of the diuretic therapy initiated in such cases. 1 For decades, the fundamental role of sodium (Na) in fluid homeostasis and heart failure (HF) progression has been well established in human medicine. 2 Hyponatremia has sparked extensive interest as a strong predictor of adverse events, including worsened survival, rehospitalization and prolonged length of hospitalization in people with CHF. 3 , 4 , 5 A retrospective study performed in dogs with acute CHF demonstrated similar results, with significantly lower Na concentrations in nonsurvivors. 6 However, recent evidence suggests that serum chloride (Cl) concentrations, either at admission or at discharge, are more closely related to survival, renal function, and loop diuretic doses than Na in people with both acute or chronic HF. 7 , 8 , 9 , 10 The reason for this is suspected to be multifactorial, and related to Cl's unique neurohormonal and homeostatic roles. 11 While the fluid retention caused by maladaptive neurohormonal activation and arginine vasopressin release creates a “dilutional effect” decreasing both Na and Cl in equivalent proportions, 1 the formal perception of Cl operating only as a passive anion linked to Na concentrations is currently contested. It is now thought that Cl has a specific and critical role in regulating HF physiology in people. 12 Human patients with low serum Cl concentrations are suspected of having an excessive RAAS activation and potentially decreased diuretic efficacy, also commonly referred to as diuretic resistance. 8 , 12 , 13 , 14 In dogs with chronic CHF, serum Cl concentration is a strong differentiator between heart disease stages. 15 A cut‐off of <103.5 mmol/L accurately identifies stage D cases, which comprises dogs suspected of having or developing diuretic resistance. This unveils a potential connection between hypochloremia and diuretic resistance in dogs with chronic CHF. 15 However, data on the implications of electrolyte abnormalities on diuretic dosing, diuretic response and prognostic variables in small animals with acute presentation of CHF are lacking. Thus, the aims of this retrospective study in dogs and cats with acute CHF were 3‐fold: (a) to document the electrolyte abnormalities present upon admission, (b) to assess the association between serum electrolyte concentrations and diuretic dose, and (c) to evaluate the prognostic impact of serum electrolyte concentrations, as reflected by duration of hospitalization and survival time.
2. MATERIAL AND METHODS
2.1. Case selection
The medical records database of the Small Animal Clinic, Faculty of Veterinary Medicine of Ghent University were retrospectively reviewed for all dogs and cats presented with clinical signs of acute CHF, between January 2012 and August 2019. Only the first episode of acute CHF of each animal was recorded, and animals with a history of previous CHF episodes were excluded. The previous administration of any cardiac medication was not an exclusion criterion, as long as the animal was presented with its first clinically‐overt episode of acute CHF requiring hospitalization.
Cases were eligible for inclusion if the diagnosis of acute CHF was confirmed by medical imaging, and if a full serum electrolyte panel was available at the time of admission. The diagnosis was confirmed by a board‐certified cardiologist, a supervised cardiology resident or by experienced cardiology staff clinicians. Depending on the clinical stability of the animal at presentation, the diagnosis was confirmed by 1 of the following: (a) thoracic radiographs and complete echocardiographic examination or (b) point‐of‐care lung ultrasonography associated with emergency echocardiography for confirmation of left atrial enlargement with pulmonary edema, pleural effusion, or both for left‐sided CHF cases, or right atrial enlargement and ascites, pleural effusion, or both for right‐sided CHF cases.
In dogs and cats deemed clinically stable after initial stabilization, complete echocardiographic examinations were conducted, with transthoracic 2D, M‐mode, and conventional Doppler echocardiography, using a dedicated ultrasound unit (Vivid 7 or Vivid E95, General Electric Medical Systems, USA). Animals were placed in right and left lateral recumbency, and simultaneously, a 1‐lead electrocardiogram was recorded. Standard right parasternal (long and short axis) and left apical parasternal views were used for data acquisition. In cases remaining clinically unstable, exhibiting signs of respiratory distress incompatible with prolonged examinations, emergency echocardiography was limited to right parasternal long and short axis views. To confirm the diagnosis of CHF in unstable animals, point‐of care lung ultrasonography, abdominal ultrasonography, or both of the modalities were performed with a mobile ultrasound unit equipped with a microconvex transducer dedicated to emergency and intensive care (MyLab30CV, Esaote, Italy), as previously described. 16 , 17
Exclusion criteria were concurrent neoplasia, significant systemic disease (defined as any type of uncontrolled systemic disorder suspected to affect the animal's lifespan), interventional or surgical management, or the presence of missing data from the records. Cases suspected of having important renal impairment, defined by a creatinine value above 251 μmol/L, were excluded from the study. Given the potential of diuretic therapy to induce prerenal azotemia, evidence of increased creatinine between 125‐250 μmol/L in dogs and 140‐250 μmol/L in cats that already received diuretic therapy prior to admission was not considered an exclusion criterion. Cases suspected of reversible cardiomyopathy based on follow‐up examinations (eg, high suspicion of transient myocardial thickening in cats with evidence of improvement of the echocardiographic anomalies), or cases who died from noncardiac or mixed reasons were excluded from the survival analysis.
2.2. Data collection
Medical records were reviewed and the following data was tabulated in a digital spreadsheet (Microsoft Excel 16.30): signalment, clinical signs, ongoing medications, admission serum electrolyte concentrations including Na, potassium (K) and Cl, duration of hospitalization (defined as the number of hours spent by the patient in the clinic), doses of drugs prescribed at discharge (in mg/kg/day), loop diuretic doses at end‐stage disease (defined as the last diuretic dose available before death, in mg/kg/day), and date of death or date when the animal was lost to follow‐up. Primary care veterinarians and owners were contacted when follow up data was lacking from the records.
Serum electrolyte concentrations were measured by the use of an auto‐analyzer (Catalyst Dx Chemistry Analyzer, IDEXX). The respective reference intervals, as defined by IDEXX company, were 144‐160 mmol/L for Na, 3.5‐5.8 mmol/L for K and 109‐122 mmol/L for Cl in dogs, and 150‐165 mmol/L for Na, 3.5‐5.8 mmol/L for K and 112‐129 mmol/L for Cl in cats. Depending on the serum concentration of each electrolyte, cases were assigned a category using the lower and upper reference limits, defined as “hypo‐,” “normo‐,”or “hyper‐.”
2.3. Statistical analysis
All statistical analyses were conducted in R version 3.5.2 (“Eggshel Igloo”). General significance level was set at α ≤ 0.05. A Bonferroni correction was applied whenever a correction for multiple testing was necessary and the adapted significance threshold is mentioned between brackets. All analyses were ran in parallel for the 2 species. For the descriptive analysis, it was decided to report every variable as median and range as some variables were normally distributed in 1 species but not in the other. Electrolyte abnormalities observed upon admission were described in both the general sample, in the subgroup of dogs and cats who did not receive any loop diuretic prior to admission (defined as “furosemide‐naïve” dogs and cats respectively), and in left‐ and right‐sided CHF subgroups.
In the subset of dogs (n = 43) and cats (n = 32) that survived the hospitalization period, the association between electrolyte concentrations, loop‐diuretic doses and hospitalization duration were assessed. On account of the low number of animals included, cases receiving a loop‐diuretic other than furosemide (5 dogs and 2 cats) were excluded from the analyses focusing on diuretic doses. The correlations between Na, K, Cl and corrected Cl concentrations (calculated as previously described 18 ) on admission and the furosemide total dose at discharge and end‐stage, were calculated using Spearman rank correlation coefficients (α ≤ 0.05/4, n = 43 dogs and n = 32 cats at discharge, n = 27 dogs and n = 14 cats at end‐stage). Depending on the correlation coefficient (r) observed, the correlation was described as mild (0.30 < r < 0.50), moderate (0.50 < r < 0.70), or strong (>0.70). 19 Next, a Wilcoxon rank sum test was used to evaluate whether receiving furosemide before hospitalization influenced Cl concentrations on admission (α ≤ 0.05/4). Correlation analysis was repeated in the subset of dogs (n = 26 at discharge and n = 16 at end‐stage) and cats (n = 20 at discharge and n = 8 at end‐stage) that did not receive furosemide prior to admission (α ≤ 0.05/4) (defined as “furosemide‐naïve” animals). Finally, a simple linear regression model with electrolyte concentrations as independent variables and duration of hospitalization as dependent variable was made to evaluate the significance of the individual independent variables (α ≤ 0.05/4).
A survival analysis was conducted in all cats and dogs except those that died due to noncardiac reasons and cases suspected of reversible cardiomyopathies (n = 42 dogs and n = 30 cats), and censoring animals that were still alive at the end of the study period (6 dogs and 7 cats). Using a Cox proportional hazards regression model, the potential effect of electrolyte concentrations on time to cardiac mortality was assessed.
Since left‐ and right‐sided CHF have a different pathophysiology, treatment regimen and prognosis, the analyses mentioned above were also done in the subset of animals in left‐sided CHF (n = 41 dogs and 31 cats; α ≤ 0.05/4).
3. RESULTS
3.1. Study sample
In the predefined time span between January 2012 and August 2019, 434 medical records of small animals presented for acute CHF were reviewed. Three hundred and fifty‐four records were excluded with regards to the criteria listed above, and thus 80 cases (46 dogs and 34 cats) met the inclusion criteria. The median age of the dogs was 9.5 years (range, 1‐16 years) and the median weight was 12 kg (range, 2.35‐80 kg). The study sample consisted of 28 males and 18 females. The most common breed was Cavalier King Charles Spaniels (n = 7), followed by Chihuahuas, crossed breeds (n = 4 each) and German Shepherds (n = 3). The clinical data of the included dogs is presented in Table 1. Most dogs presented with at least 2 clinical signs (42/46, 91%), with the most common combination being tachypnea, dyspnea or both, and coughing (22/46, 48%). Forty‐one dogs were in left‐sided CHF and 5 dogs were in right‐sided CHF. The treatments received prior to admission are detailed in Table 2. Thirty‐three dogs were already on treatment at the time of admission. Sixteen dogs (35%) had already received furosemide at the time of admission. Four dogs received an injection of furosemide by the primary care veterinarian at the time of referral (exact dose unknown). Twelve dogs were receiving oral furosemide at home, without any concrete clinical evidence of a previous CHF episode (defined as lack of radiographic confirmation and absence of clinical signs described by the owners).
TABLE 1.
Clinical data of the dogs included in the retrospective study (n = 46)
| n | Percentage | |
|---|---|---|
| Cardiac diseases | ||
| MMVD | 25 | 54% |
| DCM | 14 | 30% |
| Tricuspid dysplasia | 3 | 7% |
| Mitral dysplasia | 2 | 4% |
| Aortic stenosis | 2 | 4% |
| Arrhythmias | ||
| Atrial fibrillation | 9 | 20% |
| SVT | 1 | 2% |
| Clinical signs | ||
| Tachypnea/dyspnea | 31 | 67% |
| Coughing | 24 | 52% |
| Exercise intolerance | 17 | 37% |
| Lethargy | 12 | 26% |
| Abdominal distension | 8 | 17% |
| Syncope | 7 | 15% |
| Cyanosis | 2 | 4% |
| Type of CHF | ||
| Pulmonary edema | 41 | 89% |
| Pleural effusion | 14 | 30% |
| Ascites | 10 | 22% |
Abbreviations: DCM, dilated cardiomyopathy; MMVD, myxomatous mitral valve disease; SVT, supraventricular tachycardia.
TABLE 2.
Treatment administered prior to admission in the included cases
| Treatment at admission | n | % | Median dose | Range of dose | |
|---|---|---|---|---|---|
| Dogs (n = 46) | Furosemide | 16 | 35% | 3.74 mg/kg/day a | 1.48‐5.20 |
| Pimobendan | 16 | 35% | 0.30 mg/kg BID | 0.16‐0.36 | |
| Benazepril | 14 | 30% | 0.39 mg/kg SID | 0.21‐0.54 | |
| Spironolactone | 8 | 17% | 2.86 mg/kg SID | 1.68‐3.92 | |
| Codeine | 3 | 7% | 1.89 mg/kg SID | 0.76‐6.90 | |
| Amoxicillin‐clavulanic acid | 2 | 4% | 16.40 mg/kg BID | 14.12‐18.75 | |
| Predisolone | 2 | 4% | 0.60 mg/kg BID | 0.4‐0.71 | |
| Atenolol | 2 | 4% | 1.0 mg/kg BID | 0.75‐1.33 | |
| Ramipril | 1 | 2% | 0.15 mg/kg SID | / | |
| Levothroxine | 1 | 2% | 9.1 μg/kg BID | / | |
| Cats (n = 34) | Furosemide | 9 | 26% | 1.98 mg/kg/day a | 1.58‐3.16 |
| Pimobendan | 2 | 6% | 0.28 mg/kg BID | 0.20‐0.36 | |
| Benazepril | 1 | 3% | 0.74 mg/kg SID | / | |
| Clopidogrel | 1 | 3% | 18.75 mg/cat | / | |
| Aspirin | 1 | 3% | 5.71 mg/kg every 3 days | / | |
| Mirtazapine | 1 | 3% | 1.10 mg/kg every 3 days | / | |
| Methimazole | 1 | 3% | 0.5 mg/kg BID | / |
Median calculated with the known dosages only (n = 12/16 for dogs and n = 4/9 for cats).
The cats included in this study had a median age of 7.1 years (range, 1.6‐15.1 years), and a median weight of 4.68 kg (range, 2.45‐7.20 kg). Twenty‐four were male and 10 were female. The most commonly represented breeds were European shorthair (n = 17), British Shorthair (n = 5), Sphynx (n = 4), and Maine Coon (n = 3). The clinical data of the included cats is disclosed in Table 3. Most cats presented with at least 2 clinical signs (23/34, 68%), with the most common combination being tachypnea, dyspnea or both, and lethargy (12/34, 35%). Thirty‐one cats were in left‐sided CHF and 3 cats were in right‐sided CHF. As described in Table 2, 11 cats were already under treatment at the time of admission, consisting mostly of furosemide either administered at home without concrete clinical evidence of a previous CHF episode (n = 4) or administered by the referring veterinarian at the time of referral (n = 5; exact dose unknown). One cat had well controlled hyperthyroidism under treatment with methimazole.
TABLE 3.
Clinical data of the cats included in the retrospective study (n = 34)
| n | Percentage | |
|---|---|---|
| Cardiac diseases | ||
| LVH | 21 | 61% |
| Mitral dysplasia | 6 | 18% |
| RCM | 3 | 9% |
| UCM | 2 | 6% |
| Tricuspid dysplasia | 1 | 3% |
| Cor triatriatum sinister | 1 | 3% |
| Arrhythmias | ||
| Atrial fibrillation | 2 | 6% |
| Isolated VPC | 1 | 3% |
| Clinical signs | ||
| Tachypnea/dyspnea | 32 | 94% |
| Lethargy | 16 | 47% |
| Abdominal distension | 4 | 12% |
| Syncope | 4 | 12% |
| Coughing | 3 | 9% |
| Exercise intolerance | 3 | 9% |
| Cyanosis | 1 | 3% |
| Type of CHF | ||
| Pulmonary edema | 28 | 82% |
| Pleural effusion | 18 | 53% |
| Ascites | 3 | 9% |
Abbreviations: LVH, left ventricular hypertrophy; RCM, restrictive cardiomyopathy; UCM, unclassified cardiomyopathy; VPC, ventricular premature complexes.
3.2. Serum electrolyte concentrations
The results of the electrolyte panels performed upon admission are summarized in Table 4. In the complete study sample, electrolyte abnormalities were identified in 47/80 cases, in 52% of dogs (24/46) and 68% of cats (23/34). Decreased electrolyte concentrations were identified in 48% of the cases (17/46 and 21/34 in dogs and cats respectively), and 11% showed increased electrolyte concentrations (7/46 and 2/34, respectively). In both dogs and cats, the most commonly encountered electrolyte anomaly upon admission was hypochloremia identified in 24% of all cases. The most commonly increased electrolyte was Na, with 9% of all cases showing hypernatremia (5/46 and 2/34, respectively).
TABLE 4.
Serum electrolyte concentrations at admission in the included dogs (n = 46) and cats (n = 34), in the subgroups of loop‐diuretic‐naïve dogs (n = 30) and cats (n = 24), and depending on the type of congestive heart failure
| Dogs | Furosemide‐naïve dogs | Dogs in left CHF | Dogs in right CHF | Cats | Furosemide‐naïve cats | Cats in left CHF | Cats in right CHF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | (N = 46) | (N = 30) | (N = 41) | (N = 5) | (N = 34) | (N = 24) | (N = 31) | (N = 3) | ||||||||
| electrolytes | Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | Median | Range |
| Na (mmol/L) | 153.5 | 134‐161 | 152 | 134‐161 | 153 | 134‐161 | 156 | 145‐158 | 157.5 | 148‐172 | 157 | 148‐172 | 157 | 148‐172 | 160 | 157‐162 |
| K (mmol/L) | 4.65 | 3.1‐6.0 | 4.7 | 3.1‐6.0 | 4.7 | 3.4‐6.0 | 4.5 | 3.1‐5.4 | 4.2 | 2.7‐4.8 | 4.4 | 3.1‐4.8 | 4.1 | 2.7‐4.8 | 4.6 | 4.3‐4.8 |
| Cl (mmol/L) | 113 | 97‐125 | 116 | 108‐125 | 112 | 97‐125 | 115 | 111‐121 | 117.5 | 96‐125 | 118 | 96‐125 | 117 | 96‐125 | 118 | 112‐123 |
| Electrolyte classes | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HypoNa | 4 | 9% | 1 | 3% | 4 | 10% | 0 | 0% | 4 | 12% | 3 | 13% | 4 | 13% | 0 | 0% |
| NormonNa | 37 | 80% | 26 | 87% | 32 | 78% | 5 | 100% | 28 | 82% | 19 | 79% | 25 | 81% | 3 | 100% |
| HyperNa | 5 | 11% | 3 | 10% | 5 | 12% | 0 | 0% | 2 | 6% | 2 | 8% | 2 | 6% | 0 | 0% |
| HypoK | 4 | 9% | 0 | 0% | 3 | 7% | 1 | 20% | 7 | 21% | 3 | 13% | 7 | 23% | 0 | 0% |
| NormoK | 41 | 89% | 29 | 97% | 37 | 90% | 4 | 80% | 27 | 79% | 21 | 87% | 24 | 77% | 3 | 100% |
| HyperK | 1 | 2% | 1 | 3% | 1 | 3% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| HypoCl | 9 | 20% | 3 | 10% | 9 | 22% | 0 | 0% | 10 | 29% | 6 | 25% | 9 | 29% | 1 | 33% |
| NormoCl | 36 | 78% | 26 | 87% | 31 | 76% | 5 | 100% | 24 | 71% | 18 | 75% | 22 | 71% | 2 | 67% |
| HyperCl | 1 | 2% | 1 | 3% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
Abbreviations: CHF, congestive heart failure; Cl, chloride; K, potassium; Na, sodium.
A similar pattern was observed in the “furosemide‐naïve” subgroup, with electrolyte abnormalities identified in 23/54 (43%) cases, in 30% of the dogs (9/30) and 58% of the cats (14/24), and the most commonly encountered electrolyte anomaly being hypochloremia, present in 17% of the cases (3/30 dogs and 6/24 cats). The furosemide‐naïve dogs had significantly higher serum chloride concentrations on admission compared to dogs that received furosemide prior to admission (Figure 1, P < .001). No such finding was made in cats (P = .863). Hypochloremia was also the most commonly observed electrolyte anomaly in the subgroup of animals in left‐sided CHF (in 9/41 dogs and 9/31 cats, 25% of the cases).
3.3. Duration of hospitalization
Three dogs and 2 cats did not survive hospitalization. One dog and the 2 cats succumbed to cardiopulmonary arrest refractory to resuscitation. The 2 other dogs were euthanized, 1 due to refractory CHF, and the other for mixed reasons (refractory CHF and acute kidney injury). In the subset of 43 dogs and 32 cats that survived until discharge, the median duration of hospitalization was 40 hours (range, 1‐146 hours) and 52 hours (range, 1‐115 hours), respectively.
After correction for multiple testing, there was no significant relationship between electrolyte concentrations on admission and duration of hospitalization (corrected P‐values of .29 for Na, 1 for K, .08 for Cl and 1 for corrected Cl in dogs, and 1 for all electrolytes in cats). A similar observation was made in the left‐sided CHF subgroup (corrected P‐values of .65 for Na, 1 for K, .1 for Cl and 1 for corrected Cl in dogs, and 1 for all electrolytes in cats).
3.4. Treatment
The details of the cardiac medications prescribed at discharge were available for all animals who survived hospitalization (n = 43 dogs and n = 32 cats) and are listed in Table 5. The median dose of furosemide at discharge prescribed was 5.2 mg/kg/day (range 1.72‐9.57 mg/kg/day) for dogs and 3.53 mg/kg/day (range 1.47‐7.58 mg/kg/day) in cats. Five dogs and 2 cats were discharged on torasemide instead of furosemide at a median dose of 0.28 mg/kg/day in dogs (range, 0.21‐0.50 mg/kg/day) and 0.23 mg/kg/day in cats (range, 0.21‐0.24 mg/kg/day). As described in the materials and methods, these animals were excluded from the statistical analyses focusing on diuretic dose.
TABLE 5.
Treatment prescribed at discharge in the included cases
| Treatment at discharge | n | % | Median dose | Range of dose | |
|---|---|---|---|---|---|
| Dogs (n = 43) | Furosemide | 38 | 88% | 5.20 mg/kg/day | 1.72‐9.57 |
| Torasemide | 5 | 12% | 0.28 mg/kg/day | 0.21‐0.50 | |
| Pimobendan | 43 | 100% | 0.28 mg/kg BID | 0.19‐0.36 | |
| Benazepril | 36 | 84% | 0.30 mg/kg SID | 0.19‐0.62 | |
| Spironolactone | 31 | 72% | 2.22 mg/kg SID | 1.20‐3.92 | |
| Digoxin | 7 | 16% | 0.0070 mg/kg BID | 0.004‐0.016 | |
| Atenolol | 3 | 7% | 1.33 mg/kg BID | 0.19‐1.49 | |
| L‐Carnitine supplement | 3 | 7% | Unknown | Unknown | |
| Diltiazem | 2 | 5% | / | 1.58‐1.85 mg/kg TID | |
| Taurine | 1 | 2% | 60.6 mg/kg BID | / | |
| Prednisolone | 1 | 2% | 0.36 mg/kg SID | / | |
| Omeprazole | 1 | 2% | 0.95 mg/kg BID | / | |
| Cats (n = 32) | Furosemide | 30 | 94% | 3.53 mg/kg/day | 1.47‐7.58 |
| Torasemide | 2 | 6% | 0.23 mg/kg/day | 0.21‐0.24 | |
| Clopidogrel | 18 | 56% | 18.75 mg/cat SID | / | |
| Benazepril | 13 | 41% | 0.29 mg/kg SID | 0.21‐0.74 | |
| Pimobendan | 12 | 38% | 0.26 mg/kg BID | 0.19‐0.36 | |
| Spironolactone | 9 | 28% | 2.17 mg/kg SID | 1.67‐3.57 | |
| Imidapril | 7 | 22% | 0.25 mg/kg SID | / | |
| Sotalol | 3 | 9% | 4.08 mg/kg BID | 3.74‐7.69 | |
| Atenolol | 3 | 9% | 1.39 mg/kg BID | 1.04‐1.84 | |
| Aspirin | 2 | 6% | / | 3.57‐5.71 mg/kg every 3 days | |
| Methimazole | 2 | 6% | / | 0.54‐0.86 mg/kg SID | |
| Amoxicillin‐clavulanic acid | 2 | 6% | / | 11.78‐13.90 mg/kg BID | |
| Mirtazapine | 2 | 6% | 3.75 mg/cat every 3 days | / |
Table 6 summarizes the correlation coefficients between each of the electrolytes and furosemide dose at discharge. A significant and moderate negative association was found between serum Cl concentration at admission and total furosemide dose at discharge in dogs (n = 38; r = −0.59; P < .001, Figure 1A). No such observation was made in cats (n = 30). No significant association was identified with Na or K in either dogs or cats. No significant correlation was observed with corrected Cl (P = .25 in dogs and P = 1 in cats).
TABLE 6.
Spearman correlation coefficients between electrolytes at admission and furosemide doses at discharge
| Electrolytes | All dogs (N = 38) | Furosemide‐naïve dogs (N = 26) | All cats (N = 30) | Furosemide‐naïve cats (N = 20) |
|---|---|---|---|---|
| Furosemide dose discharge | Furosemide dose discharge | Furosemide dose discharge | Furosemide dose discharge | |
| Na | −0.21 (P = .81) | −0.24 (P = .6) | −0.12 (P = 1) | −0.16 (P = 1) |
| K | −0.31 (P = .25) | −0.37 (P = .18) | 0.25 (P = .7) | 0.46 (P = .17) |
| Cl | −0.59 (P < .001) | −0.58 (P = .007) | −0.18 (P = 1) | −0.16 (P = 1) |
Notes: The P value presented are corrected for multiple testing with Bonferroni correction, and significant results obtained are bolded.
Abbreviations: Cl, chloride; K, potassium; Na, sodium.
FIGURE 1.
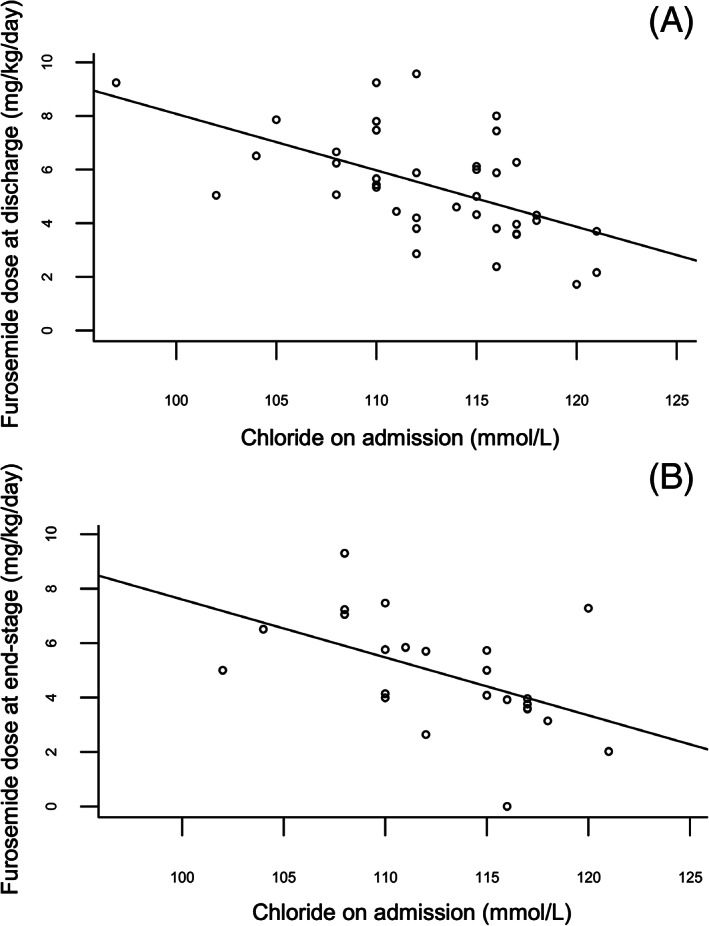
Scatter plot demonstrating correlation between serum chloride concentration on admission (mmol/L) and furosemide dose (mg/kg/day) in all dogs at discharge (A) and at end‐stage (B). The full line represents the linear association between chloride on admission (independent variable) and Y (depending on the graph, dependent variable) found with a simple linear model
Taking into account the significant difference in serum Cl concentrations between dogs who received furosemide prior to admission and dogs who never received furosemide before admission (ie, “furosemide‐naïve” dogs) (P < .001), a Spearman rank correlation coefficient was calculated to assess the association between Cl concentration and diuretic doses at discharge in the subgroup of furosemide‐naïve animals, which remained significant in dogs (r = −0.58; P = .007, Table 6, Figure 2A). Additionally, a similar significant association between Cl concentrations and furosemide dose at discharge was also observed in the subgroup of left‐sided CHF cases (r = −0.41; P = .004).
FIGURE 2.
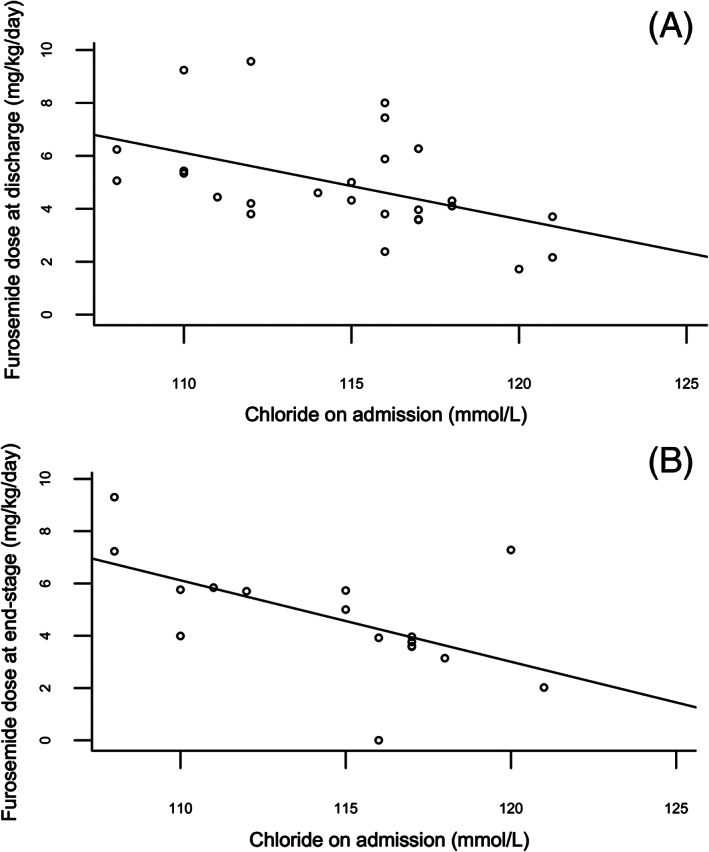
Scatter plot demonstrating correlation between serum chloride concentration on admission (mmol/L) and furosemide dose (mg/kg/day) in furosemide‐naïve dogs at discharge (A) and at end‐stage (B). The full line represents the linear association between chloride on admission (independent variable) and Y (depending on the graph, dependent variable) found with a simple linear model
Diuretic dose at end‐stage of disease was available for 27 dogs and 14 cats. The median dose of furosemide at end‐stage was 4.7 mg/kg/day (range 2.02‐7.28 mg/kg/day) in dogs and 3.34 mg/kg/day (range 1.61‐6.60 mg/kg/day) in cats. Four dogs and 4 cats were receiving torasemide instead of furosemide at end‐stage, at a median dose of 0.67 mg/kg/day in dogs (range, 0.2‐1.02 mg/kg/day) and 0.35 mg/kg/day (range, 0.30‐0.75 mg/kg/day) in cats and were excluded from the analyses focusing on diuretic doses.
A significant and moderate negative association was found between serum Cl concentration at admission and total furosemide dose at end‐stage in dogs (n = 23; r = −0.62; P = .005, Figure 1B), as illustrated in Table 7. Furthermore, this association remained significant in the subgroup of furosemide‐naïve dogs (n = 16; r = −0.62; P = .029, Table 7, Figure 2B), and in the subgroup of animals in left‐sided CHF (r = −0.51; P = .017). Comparably to discharge dosages, no significant association was identified with Cl concentrations in cats, and neither with Na or K concentrations in both species. No significant correlation was identified with corrected Cl concentrations (P = .11 in dogs and P = 1 in cats).
TABLE 7.
Spearman correlation coefficients between electrolytes at admission and furosemide doses at end‐stage
| Electrolytes | All dogs (N = 23) | Furosemide‐naïve dogs (N = 16) | All cats (N = 10) | Furosemide‐naïve cats (N = 8) |
|---|---|---|---|---|
| Furosemide dose end‐stage | Furosemide dose end‐stage | Furosemide dose end‐stage | Furosemide dose end‐stage | |
| Na | −0.04 (P = 1) | 0.03 (P = 1) | 0.29 (P = 1) | −0.02 (P = 1) |
| K | 0.10 (P = 1) | −0.09 (P = 1) | 0.35 (P = 1) | 0.45 (P = 1) |
| Cl | −0.62 (P = .005) | −0.62 (P = .029) | −0.18 (P = 1) | −0.19 (P = 1) |
Notes: The P value presented are corrected for multiple testing with Bonferroni correction, and significant results are bolded.
Abbreviations: Cl, chloride; K, potassium; Na, sodium.
3.5. Survival analysis
Follow‐up data was available for all dogs and 32 cats. Four dogs and 1 cat were excluded from the survival analysis due to euthanasia for noncardiac or mixed reasons. One cat was excluded based on a high suspicion of reversible myocarditis with resolution of the echocardiographic anomalies. The median survival time observed after first CHF episode was 125 days in dogs (n = 42; range, 3‐1217 days) and 141 days in cats (n = 30; range, 1‐1804 days). As illustrated by Table 8, no effect of electrolyte concentrations on time to cardiac mortality was identified, nor was there a correlation with corrected Cl concentrations (P = 1 in both dogs and cats). A similar observation was made in the subgroup of left‐sided CHF cases (P = 1 in both dogs and cats for all electrolytes).
TABLE 8.
Cox proportional hazards regression model to assess the potential effect of electrolyte concentration on time to cardiac mortality
| Dogs (N = 42) | Cats (N = 30) | |||||
|---|---|---|---|---|---|---|
| Electrolytes | Hazard ratio | 95% CI | Corrected P‐value | Hazard ratio | 95% CI | Corrected P‐value |
| Na | 0.976 | 0.923‐1.033 | 1 | 1.027 | 0.935‐1.129 | 1 |
| K | 0.602 | 0.322‐1.125 | .45 | 0.983 | 0.479‐2.017 | 1 |
| Cl | 0.958 | 0.903‐1.017 | .62 | 1.022 | 0.955‐1.094 | 1 |
Notes: The P value presented are corrected for multiple testing with Bonferroni correction.
Abbreviations: Cl, chloride; K, potassium; Na, sodium.
4. DISCUSSION
In this study, the admission serum electrolyte concentrations and their relationship with duration of hospitalization, diuretic doses both at discharge and at disease end‐stage, and survival time in 80 dogs and cats presented with acute CHF were documented. Electrolyte abnormalities are not uncommon in dogs and cats presented for a first episode of acute CHF, with hypochloremia being the most frequently encountered abnormality, independent of the prior administration of loop diuretics. To our knowledge, this is the first study documenting electrolyte abnormalities on admission in dogs and cats with a first episode of acute CHF. Our findings are consistent with a previous retrospective study including 145 dogs and cats with acute CHF, showing hypochloremia in 31% of the cases, though in that report not all animals included were in their first episode of CHF and some were included several times. 20 The observed prevalence in dogs and cats is higher than in people, where hypochloremia is observed in 7.4% to 13% of the cases. 8 , 14 , 21
A significant and moderate negative correlation was identified between serum Cl concentrations and furosemide doses both at discharge and at end‐stage disease in dogs. This association represents a clinically important finding of the present study. This finding advocates for assessment of serum Cl concentration in dogs with acute CHF, and suggests that, even during a first episode of CHF, this variable might help identify dogs who could require larger doses of diuretics, illustrating the potential of Cl as a marker for disease severity and response to diuretic therapy. Several large clinical studies have established this relationship in people, and have advanced that hypochloremia could serve as a marker of impaired diuretic response in HF patients. 7 , 13 , 14 , 21 Moreover, high diuretic doses have been significantly associated with poor outcome in people, 22 implying that hypochloremia could carry prognostic evidence by itself in small animals.
Diuretic resistance has no single‐accepted definition, but is most frequently known as “failure to decongest despite adequate doses of diuretics.” 23 It is well established that many human patients with acute CHF are or become resistant to loop diuretics. 24 From a pathophysiological perspective, the relationship between serum Cl and diuretic resistance is complex, and incompletely understood. In people, hypochloremia is suspected to be secondary to excess of angiotensin II, AVS‐mediated dilution, loop‐diuretic use, and altered acid‐base homeostasis, all of which are typically present in CHF. 12 , 25 Furthermore, hypochloremia might by itself drive central mechanisms contributing to diuretic resistance. Firstly, Cl seems to bind directly to the catalytic site of a family of serine‐threonine kinases, regulating their ability to influence important Na regulatory pathways, the RAAS, but also the transporters through which loop and thiazide diuretics mediate their effect. 26 , 27 , 28 , 29 Additionally, Cl plays a major role in acid‐base homeostasis, and its depletion takes part in the process driving metabolic alkalosis states. 11 , 30 Finally, the identification of genetic variants with a mutation in the code for a renal Cl channel associated with an increased risk of advanced HF provides further belief that Cl might occupy a more central role in HF than previously suspected. 31
Although diuretic resistance is suspected to occur in small animals with CHF as well, it is poorly defined and reports are scarce. Recently, a study compared electrolyte concentrations, indices of diuretic efficacy and RAAS activation markers in dogs with various stages of heart disease. 15 In that study, a few dogs in stage D HF with evidence of reduced diuretic efficacy had a more pronounced hypochloremia than the ones with adequate diuretic response. This supports the role of Cl as a marker or driver of diuretic resistance in dogs. However, chronic HF dogs were included, contrasting notably with our sample of dogs and cats in their first presentation of acute CHF, which were selected in accordance with several cohort‐based studies in people. 7 , 8 , 13 The mutual findings of both studies might refer to distinct aspects of diuretic resistance. While it was demonstrated that serum Cl concentrations strongly differentiates between stages C and D in dogs with HF, 15 the results of the present study suggest that Cl concentrations could pinpoint the presence of an intrinsic difference in response to loop‐diuretics at the time of the first episode of CHF. The pathophysiology behind this individual responsiveness is not known, but pharmacokinetic issues and additional compensatory Na reabsorption have been described in diuretic resistant people. 32 Our study focused solely on diuretic doses administered as a marker of response to diuretic therapy, and further studies in dogs and cats with acute CHF are required to assess the correlation in dogs and cats with acute CHF between serum Cl concentrations and markers of diuretic resistance validated in humans, such as diuretic efficacy (defined as urine output relative to the dose of furosemide administered), Na excretion fraction, urine Na‐to‐K ratio, and urinary Na concentration. 33
As mentioned above, considering that loop diuretic therapy is 1 of the main contributors of electrolyte depletion in CHF cases, and the retrospective nature of our study, the order of causality of the correlation between hypochloremia and administered furosemide doses remains unclear. It could be argued that hypochloremia is mainly a consequence of the administration of high doses of loop diuretics in our cases. However, our study identified a similar significant correlation between Cl concentrations and furosemide doses in the subgroup of furosemide‐naïve dogs. This suggests that, independently of previous loop‐diuretic administration, hypochloremia identified upon admission is significantly associated with the administration of higher diuretic doses, highlighting the potential of Cl as a clinically relevant marker in dogs with acute CHF.
No such correlation was identified with Na concentrations, which is consistent with the most recent literature available in people with CHF, where hypochloremia in the absence of hyponatremia remained prognostically significant. 7 , 9 Additionally, no significant findings were observed for corrected Cl concentrations, which per definition take Na concentrations into account. Whether this lack of significant P‐values is the result of our small sample size, the retrospective nature of our study, or the absence of direct effect from the Na concentration itself is not known at this time. Interestingly, no correlation with any electrolyte was identified in cats. This could be secondary to our relatively small sample group, but could also suggest a variability in the underlying mechanism driving diuretic response in the feline species.
No significant association between electrolyte concentrations and duration of hospitalization was identified in our study. However, the P‐value for Cl in dogs approached significance. Potentially, a larger and statistically more powerful study could disclose significantly longer hospitalization durations for hypochloremic dogs, as it has been described in people. 7
No significant hazard ratios were obtained after Cox proportional hazard regression for survival time. The absence of significant impact of hyponatremia is consistent with the most recent reports in the human literature. 7 , 8 , 14 Nonetheless, this finding contrasts with a previous retrospective study performed on 59 dogs with CHF disclosing significantly lower plasma Na concentrations in dogs failing to survive until discharge. 6 One explanation for this disparity resides in the number of dogs surviving until discharge (56% against 93% in our study). Follow‐up after discharge was also not recorded.
Likewise, serum K concentration did not impact survival in our study. This result contrasts with another retrospective study performed on 145 dogs showing greater survival to discharge for hypokalemic cases. 20 However, contrary to our study, the authors focused on electrolyte abnormalities during hospitalization as opposed to admission parameters, and did not include any follow‐up information.
Finally, no effect of Cl concentration on survival was noted in our study, in contrast to the body of literature available in people. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 21 , 25 However, differences in study design could account for this discrepancy. Many of these clinical studies were aiming at comparing the survival between classes of Cl concentrations. 7 , 13 , 14 , 21 However, our sample size was small compared to the large human clinical studies, which made it difficult to compare survival times between Cl classes, and it is possible that such an influence was not noticeable in our sample due to its size.
Our study has other limitations, mostly inherent to its retrospective design. Medical treatments administered prior to admission were not standardized, and the effect of the use of any other medication besides loop diuretics was not analyzed. Although belonging to the diuretics category, the effect of prior administration of spironolactone was not examined in our study, which could theoretically have altered the results. However, its mechanism of action and the previous reports available in the human literature disclosing a lack of association between spironolactone use and lower serum Cl concentrations 21 might support the validity of our results. Similarly, the animals' diet was not taken into account, which might have led to differences in dietary amounts of electrolytes and influenced our findings. Prospective studies with standardized treatment protocols are needed to completely rule out the potential influence of other medications and diet.
To maximize the number of cases included in the statistical analyses, our main study group comprised of both left‐sided and right‐sided CHF cases. The differences in pathophysiology between these 2 subgroups could lead to substantial variations in electrolyte concentrations, diuretic regimen, and prognosis. We tried to answer to this bias by repeating the analyses on the left‐sided CHF subgroup, showing similar results to those obtained in the complete study sample. However, the small number of right‐sided CHF cases included made meaningful statistical analysis in this subgroup impossible. Further studies focusing on animals with ascites, pleural effusion or both are needed to confirm our findings in right‐sided CHF cases.
As per definition in a retrospective design, treatment administered during hospitalization was also not standardized, and cases were supervised by different cardiologists. However, clinicians at our institution shared a relatively similar therapeutic management approach, based on clinical parameters such as respiratory rate and effort, limiting the subjectivity of the doses administered. The latter were selected on a case‐by‐case basis, using the published recommendations available at the time of inclusion, 34 , 35 with an average starting dose of furosemide of 2 mg/kg, and adapting the subsequent furosemide administrations according to evolution of the breathing status and frequency, and appetite. In case furosemide doses necessary to achieve clinical stabilization were deemed too extreme, the animal was switched to torasemide at the time of transfer from injectable to oral medications. The patient was discharged when his respiratory condition was deemed stable enough with oral medication for at least 12 hours. Additionally, as recommended in the literature, 34 , 35 the need for K‐supplementation was assessed based on the results of the serum electrolytes concentrations. The serum Cl concentration by itself was not taken into account in the diuretic dose decision‐making process.
The gold standard for confirmation of left‐sided CHF, thoracic radiography, was not performed in all cases. When radiography was unavailable, we attempted to ensure accurate inclusion of dogs and cats in CHF by relying on the combination of the history, clinical signs, physical examination findings, and point‐of‐care ultrasound assessments performed by experienced cardiologists, as previously described. 16 , 17 The reference intervals provided by the manufacturer of our auto‐analyzer for the electrolytes concentrations were not validated in a local sample. Finally, this study has a relatively small sample size, and was performed in a single referral center, and the results might thus not apply to all CHF cases.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Roche‐Catholy M, Van Cappellen I, Locquet L, Broeckx BJG, Paepe D, Smets P. Clinical relevance of serum electrolytes in dogs and cats with acute heart failure: A retrospective study. J Vet Intern Med. 2021;35(4):1652–1662. 10.1111/jvim.16187
REFERENCES
- 1. Sica DA. Sodium and water retention in heart failure and diuretic therapy: basic mechanisms. Cleve Clin J Med. 2006;73(suppl 2):S2‐S7. discussion S30‐3. [DOI] [PubMed] [Google Scholar]
- 2. O'Connor CM, Ahmad T. The role of sodium and chloride in heart failure: does it take two to tango? J Am Coll Cardiol. 2015;66(6):667‐669. [DOI] [PubMed] [Google Scholar]
- 3. Rusinaru D, Tribouilloy C, Berry C, et al. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: an individual patient data meta‐analysis: Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC). Eur J Heart Fail. 2012;14(10):1139‐1146. [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Abraham WT, Albert NM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry. Eur Heart J. 2007;28(8):980‐988. [DOI] [PubMed] [Google Scholar]
- 5. Corona G, Giuliani C, Parenti G, et al. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta‐analysis. PLoS One. 2013;8(12):80451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brady CA, Hughes D, Drobatz KJ. Association of hyponatremia and hyperglycemia with outcome in dogs with congestive heart failure. J Vet Emerg Crit Care (San Antonio). 2004;14(3):177‐182. [Google Scholar]
- 7. Grodin JL, Simon J, Hachamovitch R, et al. Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol. 2015;66(6):659‐666. [DOI] [PubMed] [Google Scholar]
- 8. Kondo T, Yamada T, Tamaki S, et al. Serial change in serum chloride during hospitalization could predict heart failure death in acute decompensated heart failure patients. Circ J. 2018;82(4):1041‐1050. [DOI] [PubMed] [Google Scholar]
- 9. Testani JM, Hanberg JS, Arroyo JP, et al. Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail. 2016;18(6):660‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Radulović B, Potočnjak I, Dokoza Terešak S, et al. Hypochloraemia as a predictor of developing hyponatraemia and poor outcome in acute heart failure patients. Int J Cardiol. 2016;212:237‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23(3):203‐211. [DOI] [PubMed] [Google Scholar]
- 12. Hanberg JS, Rao V, Ter Maaten JM, et al. Hypochloremia and diuretic resistance in heart failure: mechanistic insights. Circ Heart Fail. 2016;9(8):10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grodin JL, Sun JL, Anstrom KJ, et al. Implications of serum chloride homeostasis in acute heart failure (from ROSE‐AHF). Am J Cardiol. 2017;119(1):78‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ter Maaten JM, Damman K, Hanberg JS, et al. Hypochloremia, diuretic resistance, and outcome in patients with acute heart failure. Circ Heart Fail. 2016;9(8):003109. [DOI] [PubMed] [Google Scholar]
- 15. Adin D, Kurtz K, Atkins C, et al. Role of electrolyte concentrations and renin‐angiotensin‐aldosterone activation in the staging of canine heart disease. J Vet Intern Med. 2020;34(1):53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ward JL, Lisciandro GR, Keene BW, et al. Accuracy of point‐of‐care lung ultrasonography for the diagnosis of cardiogenic pulmonary edema in dogs and cats with acute dyspnea. J Am Vet Med Assoc. 2017;250(6):666‐675. [DOI] [PubMed] [Google Scholar]
- 17. Boysen SR, Lisciandro GR. The use of ultrasound for dogs and cats in the emergency room: AFAST and TFAST. Vet Clin North Am Small Anim Pract. 2013;43(4):773‐797. [DOI] [PubMed] [Google Scholar]
- 18. De Morais HA, Leisewitz AL. Mixed acid‐base disorders. In: SP DB, ed. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice. 4th ed. St. Louis, MO: Elsevier Saunders; 2012:302‐315. [Google Scholar]
- 19. Agokul H. User's guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goutal CM, Keir I, Kenney S, et al. Evaluation of acute congestive heart failure in dogs and cats: 145 cases (2007‐2008). J Vet Emerg Crit Care (San Antonio). 2010;20(3):330‐337. [DOI] [PubMed] [Google Scholar]
- 21. Grodin JL, Testani JM, Pandey A, et al. Perturbations in serum chloride homeostasis in heart failure with preserved ejection fraction: insights from TOPCAT. Eur J Heart Fail. 2018;20(10):1436‐1443. [DOI] [PubMed] [Google Scholar]
- 22. Okabe T, Yakushiji T, Kido T, et al. The association between high‐dose loop diuretic use at discharge and cardiovascular mortality in patients with heart failure. ESC Heart Fail. 2018;5(1):87‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96(3–4):132‐143. [DOI] [PubMed] [Google Scholar]
- 24. Ellison DH. Mechanistic insights into loop diuretic responsiveness in heart failure. Clin J Am Soc Nephrol. 2019;14(5):650‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaduganathan M, Pallais JC, Fenves AZ, et al. Serum chloride in heart failure: a salty prognosis. Eur J Heart Fail. 2016;18(6):669‐671. [DOI] [PubMed] [Google Scholar]
- 26. Kotchen TA, Luke RG, Ott CE, et al. Effect of chloride on renin and blood pressure responses to sodium chloride. Ann Intern Med. 1983;98:817‐822. [DOI] [PubMed] [Google Scholar]
- 27. Piala AT, Moon TM, Akella R, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7(324):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subramanya AR, Yang CL, McCormick JA, et al. WNK kinases regulate sodium chloride and potassium transport by the aldosterone‐sensitive distal nephron. Kidney Int. 2006;70(4):630‐634. [DOI] [PubMed] [Google Scholar]
- 29. Ponce‐Coria J, San‐Cristobal P, Kahle KT, et al. Regulation of NKCC2 by a chloride‐sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A. 2008;105(24):8458‐8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luke RG, Galla GH. It is chloride depletion alkalosis, not contraction alkalosis. JASN. 2012;23(2):204‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cappola TP, Matkovich SJ, Wang W, et al. Loss‐of‐function DNA sequence variant in the CLCNKA chloride channel implicates the cardio‐renal axis in interindividual heart failure risk variation. Proc Natl Acad Sci U S A. 2011;108(6):2456‐2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoorn EJ, Ellison DH. Diuretic Resistance. Am J Kidney Dis. 2017;69(1):136‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doering A, Jenkins CA, Storrow AB, et al. Markers of diuretic resistance in emergency department patients with acute heart failure. Int J Emerg Med. 2017;10(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keene BW, Atkins CE, Bonagura JD, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33(3):1127‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferasin L, DeFrancesco T. Management of acute heart failure in cats. J Vet Cardiol. 2015;17(suppl 1):173‐189. [DOI] [PubMed] [Google Scholar]


