Abstract
Background
Interstitial glucose (IG) concentration measurement using a flash glucose monitoring system (FGMS) is a noninvasive, affordable, and informative method to regulate patients with diabetes mellitus (DM) but has not been fully validated in outpatient cats with DM.
Objectives
To further validate the FreeStyle Libre FGMS in outpatient diabetic cats.
Animals
Eight client‐owned cats with DM.
Methods
Prospective observational validation study. Tissue glue was used to attach the sensor to the cat. Lin's concordance correlation coefficient (ρ c) was used to compare IG concentrations measured by the FGMS to blood glucose concentrations measured using an automated biochemistry analyzer (ABA) and point‐of‐care glucometer (POCG).
Results
Data from 15 sensor placements in 8 cats were analyzed. Paired IG and ABA glucose concentrations (139 samples) had excellent correlation (ρ c = 0.96) as did IG and POCG glucose concentrations (142 samples, ρ c = 0.92). Sensor failure or displacement were recorded for 12/15 (80%) sensor placements. Median time of sensor activity was 7 days (range, 2‐13 days).
Conclusions and Clinical Importance
In outpatient cats with DM, the FGMS‐measured IG concentration correlated well with ABA‐measured blood glucose concentration, but a high rate of sensor failures was observed.
Keywords: continuous glucose monitoring, diabetes mellitus, feline, FreeStyle Libre, interstitial glucose, tissue glue
Abbreviations
- ABA
automated biochemistry analyzer
- DM
diabetes mellitus
- FGMS
flash glucose monitoring system
- IG
interstitial glucose
- POCG
point‐of‐care blood glucometer
1. INTRODUCTION
Diabetes mellitus (DM) is a common endocrinopathy in cats, and older, overweight, male, and certain pure bred cats are at increased risk for the disease. 1 , 2 , 3 The measurement of serial glucose concentrations is useful for veterinarians to determine the appropriate insulin dose to achieve optimal glycemic control. 4 However, diabetic monitoring in cats is complicated by the effects of stress on measured glucose concentrations, and many owners are unable to perform blood or urine glucose monitoring at home. 5 , 6 Measurement of serum fructosamine concentration is an alternative for cats that do not tolerate serial blood sampling and can provide a reflection of the average glucose concentration over the preceding 1 to 2 weeks. 7 , 8 , 9 However, serum fructosamine concentration does not provide information about fluctuations in blood glucose concentration and also is affected by serum albumin and thyroid hormone concentrations. 10
Continuous glucose monitoring devices are less invasive than serial venipuncture and provide substantially more data regarding glucose concentration fluctuations over time. Various continuous glucose monitoring systems previously have been investigated for use in diabetic cats, but the clinical use of these devices is limited because they are physically cumbersome, require at least once daily calibration with blood glucose concentration, and are expensive. 11 , 12 , 13 , 14 , 15 , 16 , 17
A newer flash glucose monitoring system (FGMS; FreeStyle Libre, Abbott, Alameda, California) is appealing for use in cats because of its small size, ease of use, affordability, intuitive computer software, and factory calibration which eliminates the need for venipuncture and calibration with blood glucose concentration. The FGMS has been validated in dogs with DM treated on an outpatient basis as well as in dogs hospitalized for diabetic ketoacidosis. 18 , 19 , 20 , 21 , 22 One study also has validated the FGMS in hospitalized diabetic cats. 23 In this study, the investigators used 8 sutures to attach the sensor to mostly nonsedated cats, a technique that could be unacceptable to some veterinarians and owners. 23 Another recent study described complications associated with the FGMS in diabetic cats, highlighting the commonplace use of this device and the need for further validation of its accuracy. 24
Our primary aim was to validate the use of the FGMS in outpatient diabetic cats using tissue glue to attach the sensor to the cat. A secondary aim was to assess the tolerability and adverse events associated with the sensor in cats.
2. MATERIALS AND METHODS
2.1. Study population
Client‐owned cats with naturally occurring DM that were examined at a university teaching hospital between April 2019 and July 2020 were prospectively enrolled in an observational validation study. Inclusion criteria included a diagnosis of DM, a body weight >3 kg, a body condition score ≥4/9, and a state of hydration (≤5% dehydrated). Owners also had to consent to three 10‐hour outpatient visits within the 13‐day period. Exclusion criteria included anemia (PCV < 27%) on the day of enrollment and recent exposure to drugs that can interfere with FGMS readings (acetaminophen, dopamine, icodextrin, salicylates, and ascorbic acid). 25 Additionally, cats could not undergo anesthesia, radiography, computed tomography, or magnetic resonance imaging for the duration of the 13‐day study period, because the FGMS sensor can be affected by these procedures. 25 Cats were included regardless of their insulin treatment regimen, which was dictated by the attending clinician. Cats with concurrent diseases and receiving various other medications were included. The University Privately Owned Animal Protocol Committee and the Institutional Animal Care and Use Committee approved the study and informed consent form, and owners signed the written informed consent form before enrollment.
2.2. Data collection
On the day of enrollment (day 0), a 14‐day FGMS sensor was placed in a standardized fashion by 1 of the authors (E.K. Shea) as previously described. 21 Briefly, an approximately 5 × 5 cm square area of skin on the dorsal neck was clipped and cleaned with dilute chlorhexidine followed by alcohol, and the area was allowed to dry fully for at least 1 minute. The sensor was placed according to the manufacturer's guidelines, with the addition of 3 to 4 drops of tissue glue on the adherent side of the sensor. 26 The sensor applicator was held firmly in place for 30 seconds after deployment of the sensor. No other adhesives or bandages were used, but some owners chose to place a stockinette on their cat to prevent removal of the sensor. Cats were fed and received their insulin and any other medications at home, before arrival at the hospital.
Data collection also was performed as previously described. 21 For each cat on day 0, the first blood sample was collected after a 1‐hour FGMS calibration period. A maximum of 2 mL was obtained from a peripheral or jugular vein for measurement of PCV, total protein concentration, point‐of‐care blood glucometer reading (POCG; Accu‐Chek Performa, Roche Diagnostics Corp, Indianapolis, Indiana), and blood glucose concentration measurement on the automated biochemistry analyzer (ABA; Vitros 4600 Chemistry System, Ortho‐Clinical Diagnostics, Rochester, New York). Subsequent blood samples were collected at 2‐hour intervals for a total of 5 samples per day per cat, for measurements of blood glucose concentration using the POCG and ABA. These 5 blood sample measurements at 2‐hour intervals also were performed during 2 follow‐up visits at approximately 1‐week intervals. Interstitial glucose (IG) concentration was measured by scanning the FGMS sensor using the cat's designated reader within 1 minute of blood collection. The POCG glucose concentration measurements were performed using 1 drop of whole blood immediately after venipuncture while ABA blood glucose concentration measurements were performed on serum. A single, study‐designated, POCG device was used throughout the study. For ABA glucose concentration measurements, blood samples were submitted immediately to the in‐house clinical pathology laboratory and centrifuged within 15 minutes of venipuncture. The ABA utilized in this study uses a colorimetric glucose oxidase method to quantify serum glucose concentration. The serum of each sample also was analyzed for evidence of hemolysis, lipemia, and icterus by trained laboratory technicians and subjectively graded as none, mild, moderate, or marked.
The sensor was left in place for the 13‐day study period, and owners were instructed to scan the sensor with the reader at least 3 times per day at 8‐hour intervals, because the sensor can store data for a maximum of 8 hours. Each cat returned for 2 follow‐up visits at approximately 1‐week intervals. During these visits, the same procedure as on the day of enrollment was followed, but the 1‐hour calibration period was not necessary because the sensor was already in place. If the sensor fell off or was removed before the intended 13‐day study duration, it was replaced by 1 of the authors (E.K. Shea) and the same protocol as for the day of enrollment was followed. At each of the visits, owners completed a standardized questionnaire about their cat's clinical signs, medications, times of meals and insulin administration, and any FGMS sensor‐related concerns. At the end of the final visit day, the sensor was removed by 1 of the authors (E.K. Shea).
2.3. Validation data analysis
All samples were analyzed together and in subgroups based on the ABA glucose concentration. Hypoglycemia, normoglycemia, hyperglycemia, and pronounced hyperglycemia were defined as an ABA measured blood glucose concentration of <67, 67‐168, >168, and >250 mg/dL, respectively. The detection limits of the FGMS sensor and POCG are 40 to 500 mg/dL and 10 to 600 mg/dL, respectively, and results outside of the detection limits were excluded from the validation analysis. Erroneous FGMS results and samples with insufficient volume also were excluded. For hemolysis, lipemia, and icterus, samples graded as “none” or “mild” were grouped together, and those graded as “moderate” or “marked” were grouped together.
2.4. Sample size calculation
Three sample size calculations were performed for the validation study. Two‐sided paired t tests were used to determine the number of samples required to detect a difference of at least 15 mg/dL between blood glucose concentration measured by the ABA and IG concentration measured by the FGMS. A difference of 15 mg/dL was chosen as the smallest clinically important difference that would be meaningful to detect. The calculation for samples with normoglycemia was made on the basis of the mean ± SD glucose concentration (99 ± 13 mg/dL) used to establish the reference interval for the ABA. The calculation for samples with hyperglycemia was made using a mean of 250 ± 13 mg/dL and the calculation for samples with hypoglycemia was made using a mean of 67 ± 13 mg/dL. It was assumed that the SD of glucose concentrations determined by the FGMS would be similar to that of the glucose concentrations determined by the ABA and that the SD of glucose concentration with hypoglycemia and hyperglycemia would be the same. Additional assumptions included a power of 0.8, type I error rate of 0.05, and a ratio of 1 between the ABA and FGMS sample sizes. The calculation resulted in a required sample size of 13 ABA and FGMS paired measurements for each of the 3 categories of glucose concentration for a total of 39 paired measurements. Each cat contributed 15 paired glucose measurements by providing 5 blood glucose and IG concentration measurements per day on 3 separate days over the 13‐day study period. Therefore, a minimum of 3 cats were required for the study. However, in dogs, approximately 30% of sensors were reported to fall out unintentionally, and preliminary personal experience of the investigators with the device indicated that the attrition rate in cats could be higher, and thus 15 sensor placements were included in the study. 18 , 21
2.5. Statistical analysis
Variables were assessed for normality visually and using skewness/kurtosis tests for normality. Most variables, including ABA, POCG, and FGMS glucose concentrations, were not normally distributed and therefore are reported as median (range) or count and percentage.
Lin's concordance correlation coefficient (ρ c) was used to compare the gold standard ABA to FGMS and POCG glucose concentrations, as previously reported. 21 Bland‐Altman plots were generated to visually evaluate the correlations. A multivariable linear regression model was used to evaluate the effects of ABA, PCV, TP, hemolysis, lipemia, and icterus on FGMS glucose concentration measurements.
Two mixed effects models were fitted to the data to account for glucose variability among cats, as previously reported in dogs. 21 Interstitial glucose concentration was modeled as a function of blood glucose concentration as follows:
with β 0 + β 1* (ABA measured blood glucose concentration) serving as the fixed effects of the model, the random effect occurring at the cat level, and an error term that is a function of undetermined factors such as within cat variability, sleep time, or mealtime. For the purpose of comparison, POCG glucose concentration measurements were similarly modeled as a function of blood glucose concentration as follows:
The normality of the residuals of the generalized linear models was confirmed graphically by plotting kernel density estimates and standardized normal probability (P‐P) plots. P‐values <.05 were considered significant for all comparisons. All statistical analyses were performed using a statistical software package (Stata, version 14.0 for Mac; Stata Corp, College Station, Texas).
3. RESULTS
3.1. Clinical findings
Eight individual diabetic cats were enrolled; 2 cats enrolled in the study 3 times, 3 cats enrolled in the study 2 times, and the remaining 3 cats enrolled once for a total of 15 sensor placements. Six cats were neutered males and 2 were neutered females. All cats were mixed breeds, with 7 domestic shorthair cats and 1 Maine Coon mixed cat. The median age of the 8 individual cats was 12.5 years (range, 5‐18 years), and the median weight at the time of initial enrollment was 5.3 kg (range, 3.9‐8.14 kg).
All cats received insulin injections twice daily, with a median dose of 0.43 units/kg (range, 0.13‐0.82 units/kg) at the time of initial enrollment. All cats were treated with glargine insulin (Lantus, Sanofi‐Aventis, Bridgewater, New Jersey) throughout the study. No cats were newly diagnosed diabetics nor did any have diabetic ketoacidosis or hyperosmolar hyperglycemia during the study.
The majority of cats (7/8, 87.5%) had concurrent illnesses, and 4/7 (57.1%) had >1 concurrent disease. Concurrent diseases included renal disease (diagnosed in 5 cats), gastrointestinal disease (3 cats), cardiac disease (2 cats), and 1 each of the following: chronic pancreatitis and cholangiohepatitis, feline immunodeficiency virus, dermatologic disease, ocular disease, hyperthyroidism, iatrogenic hypothyroidism, recent tail fracture, chronic nasal disease, and idiopathic vestibular events. Cats were treated with a variety of noninsulin medications including gabapentin (2; compounded formulation), maropitant (2; Cerenia, Zoetis, Kalamazoo, Michigan), prednisolone (2; generic formulation), and 1 each of the following: azithromycin (Zithromax, Pfizer Labs, New York, New York), chlorpheniramine (generic formulation), famciclovir (Famvir, Novartis, East Hanover, New Jersey), lactated ringer's administered SC (Baxter Healthcare Corporation, Deerfield, Illinois), levothyroxine (Soloxine, Virbac AH, Inc, Fort Worth, Texas), mirtazapine (Remeron, N.V. Organon, Oss, The Netherlands), methimazole (Tapazole, King Pharmaceuticals, Inc., Bristol, Tennessee), omeprazole (Prilosec, AstraZeneca Pharmaceuticals, Wilmington, Delaware), polyethylene glycol 3350 (MiraLax, Cardinal Healthy, Dublin, Ohio), ursodeoxycholic acid (compounded formulation), ocular medications, and fiber and probiotic supplementation.
One cat was assessed subjectively to be mildly dehydrated (<5%) on the first day of the study but this cat was assessed to be normally hydrated on the remaining days. All other cats were assessed to be subjectively well‐hydrated on examination on all 3 in‐hospital days. The median PCV for all examinations performed on days 0, 7, and 13 were 37% (range, 27%‐49%), 39% (range, 30%‐44%), and 37% (range, 25%‐42%), respectively. The median total protein concentrations for all examinations performed on days 0, 7, and 13 were 8.6 g/dL (range, 6.2‐9.4 g/dL), 8.6 g/dL (range, 6.4‐9 g/dL), and 8.1 g/dL (range, 6.2‐9.6 g/dL), respectively.
3.2. Validation data
Descriptive statistics for paired glucose concentrations measured by the FGMS, POCG, and ABA are presented in Table 1. These measurements were obtained from 15 sensor placements in 8 cats. Twenty‐three glucose concentration measurements were excluded from the analysis because of an erroneous FGMS reading (9 readings), a reading above the detection limits (9 on the FGMS and 1 on the POCG), or insufficient sample (3 ABA, 1 POCG). Results of the Lin's concordance correlation analyses are presented in Table 2. Only 6 paired samples with hypoglycemia were available for analysis, and the correlation coefficients between the FGMS and ABA and FGMS and POCG were not significantly different from 0 in this subgroup of glucose concentrations (P = .06 and P = .12, respectively). The correlation coefficient between the ABA and POCG for samples with hypoglycemia (n = 7) was significantly different from 0 (P < .001). All other correlation coefficients in the different subgroups of glucose concentrations were significantly different from 0 (P < .001 for all). A Bland‐Altman limits‐of‐agreement plot demonstrating these correlations is presented in Figure 1, and a Lin's concordance correlation graph is presented in Figure 2.
TABLE 1.
Descriptive statistics of glucose concentrations by method of measurement (analyzer) and by glucose concentration subgroup
| Type of sample | Analyzer | Number of samples | Mean ± SD (mg/dL) | Median (range) (mg/dL) |
|---|---|---|---|---|
| All samples | FGMS | 142 | 259.9 ± 112.7 | 281.5 (55‐468) |
| POCG | 158 | 254.5 ± 123.4 | 264.5 (40‐592) | |
| ABA | 154 | 275.3 ± 133.8 | 287.5 (45‐677) | |
| All samples with hyperglycemia | FGMS | 103 | 317.4 ± 71.1 | 310 (151‐468) |
| POCG | 117 | 310.7 ± 89.4 | 295 (141‐592) | |
| ABA | 113 | 336.5 ± 99.4 | 313 (170‐677) | |
| Samples with pronounced hyperglycemia | FGMS | 88 | 331.2 ± 66.8 | 321.5 (154‐468) |
| POCG | 102 | 326.3 ± 84.6 | 308 (141‐592) | |
| ABA | 98 | 354.5 ± 94.0 | 322.5 (255‐677) | |
| Samples with normoglycemia | FGMS | 33 | 114.9 ± 27.6 | 113 (62‐161) |
| POCG | 34 | 103.8 ± 25.7 | 108.5 (51‐161) | |
| ABA | 34 | 117.3 ± 23.7 | 125 (74‐153) | |
| Samples with hypoglycemia | FGMS | 6 | 70.3 ± 13.4 | 69 (55‐88) |
| POCG | 7 | 48 ± 6.6 | 48 (40‐55) | |
| ABA | 7 | 54.3 ± 7.5 | 57 (45‐63) |
Note: Hyperglycemia, pronounced hyperglycemia, normoglycemia, and hypoglycemia were defined based on automated biochemistry blood glucose concentrations as >168, >250, ≥67 and ≤168, and <67 mg/dL, respectively.
Abbreviations: ABA, automated biochemistry analyzer measurement (serum); FGMS, flash glucose monitoring system (interstitial glucose); POCG, point‐of‐care glucometer measurement (whole blood).
TABLE 2.
Lin's concordance correlation analyses of the differences among the gold standard automated biochemistry analyzer serum glucose concentration, interstitial glucose concentration measured by the flash glucose monitoring system, and blood glucose concentration measured by the point‐of‐care glucometer
| Type of sample | Examined methodology | Established methodology | Number of paired samples | ρ c (95% confidence interval) | Bias correction factor | Mean difference between methodologies ±SD (95% limits of agreement, mg/dL) |
|---|---|---|---|---|---|---|
| All samples | FGMS | ABA | 139 | 0.96 (0.95‐0.97) | 1.0 | 5.3 ± 31.6 (−56.6 to 67.2) |
| FGMS | POCG | 142 | 0.92 (0.89‐0.94) | 0.97 | 23.0 ± 37.6 (−50.6 to 96.6) | |
| POCG | ABA | 153 | 0.98 (0.97‐0.98) | 0.99 | 20.4 ± 19.7 (−18.2 to 59.0) | |
| All samples with hyperglycemia | FGMS | ABA | 100 | 0.86 (0.80‐0.91) | 0.99 | 7.0 ± 35.1 (−61.7 to 75.7) |
| FGMS | POCG | 103 | 0.74 (0.66‐0.82) | 0.92 | 27.3 ± 42.0 (−55.0 to 109.7) | |
| POCG | ABA | 112 | 0.94 (0.92‐0.96) | 0.96 | 23.4 ± 20.7 (−17.1 to 63.9) | |
| Samples with pronounced hyperglycemia | FGMS | ABA | 85 | 0.80 (0.72‐0.87) | 0.97 | 5.14 ± 37.2 (−67.7 to 78.0) |
| FGMS | POCG | 88 | 0.67 (0.56‐0.77) | 0.90 | 26.6 ± 45.0 (−61.6 to 114.8) | |
| POCG | ABA | 97 | 0.93 (0.90‐0.95) | 0.95 | 24.8 ± 21.6 (−17.7 to 67.2) | |
| Samples with normoglycemia | FGMS | ABA | 33 | 0.70 (0.52‐0.87) | 0.99 | −2.0 ± 20.1 (−41.4 to 37.4) |
| FGMS | POCG | 33 | 0.71 (0.54‐0.87) | 0.93 | 9.5 ± 18.2 (−26.1 to 45.2) | |
| POCG | ABA | 34 | 0.70 (0.55‐0.85) | 0.86 | 13.5 ± 15.2 (−16.2 to 43.3) | |
| Samples with hypoglycemia | FGMS | ABA | 6 | 0.33 (−0.02 to 0.68) | 0.38 | 16.5 ± 7.4 (2.0‐31.0) |
| FGMS | POCG | 6 | 0.20 (−0.05 to 0.45) | 0.23 | 22.3 ± 8.2 (6.3‐38.3) | |
| POCG | ABA | 7 | 0.66 (0.37‐0.95) | 0.68 | 6.3 ± 1.7 (2.9‐9.6) |
Note: Hyperglycemia, pronounced hyperglycemia, normoglycemia, and hypoglycemia were defined based on automated biochemistry blood glucose concentrations >168, >250, ≥67 and ≤168, and <67 mg/dL, respectively.
Abbreviations: ABA, automated biochemistry analyzer measurement (serum); FGMS, flash glucose monitoring system (interstitial glucose); POCG, point‐of‐care glucometer measurement (whole blood); ρ c, Pearson's correlation coefficient.
FIGURE 1.
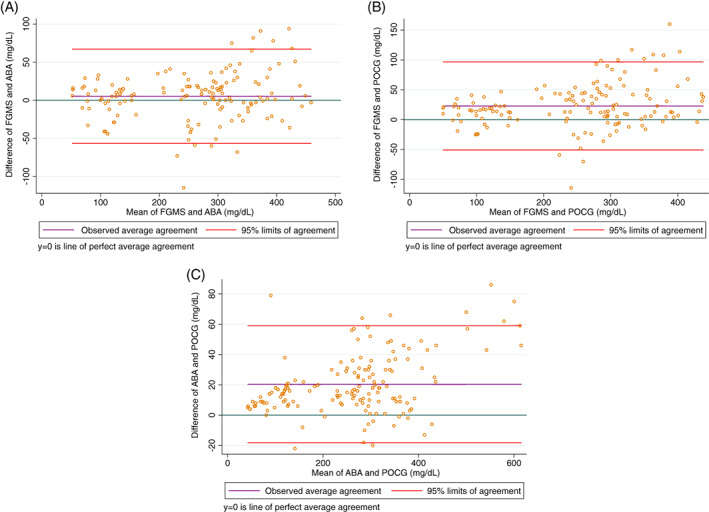
Bland‐Altman plot of the difference between the gold standard automated biochemistry analyzer serum glucose concentration and interstitial glucose concentration measured by the flash glucose monitoring system (A), between the blood glucose concentration measured by the point‐of‐care glucometer and interstitial glucose concentration measured by the flash glucose monitoring system (B), and between the gold standard automated biochemistry analyzer serum glucose concentration and the blood glucose concentration measured by the point‐of‐care glucometer (C) in all samples. For each plot, the green line represents a mean difference of 0 between the compared glucose concentrations, the purple line represents the actual mean difference between the compared glucose concentrations, and the red lines represent the 95% limits of agreement (mean difference ± 1.96 SD). The closer the purple line is to the green line, the better the agreement between the 2 compared glucose concentrations
FIGURE 2.
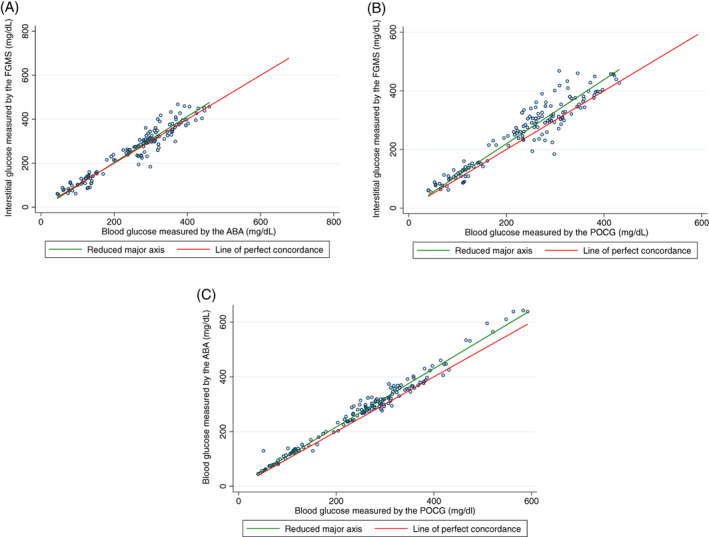
Lin's concordance correlation graphs of the gold standard automated biochemistry analyzer serum glucose concentration compared to interstitial glucose concentration measured by the flash glucose monitoring system (A), blood glucose concentration measured by the point‐of‐care glucometer compared to interstitial glucose concentration measured by the flash glucose monitoring system (B), and blood glucose concentration measured by the point‐of‐care glucometer compared to the gold standard automated biochemistry analyzer serum glucose concentration (C) in all samples. For each graph, the green line represents the reduced major axis and the red line represents the line of perfect concordance. The closer the green line is to the red line, the better the correlation between the 2 compared glucose concentrations
Results of the mixed effect models are presented in Table 3 and indicate that a significant positive correlation existed between the FGMS and ABA glucose concentrations as well as between POCG and ABA glucose concentrations (P < .001 for both). These models predict that for every 1 mg/dL increase in ABA measured blood glucose concentration, there is an increase in 0.97 mg/dL on the FGMS measured IG concentration and an increase in 0.94 mg/dL on the POCG measured blood glucose concentration. Both mixed effect models differed significantly from the ordinary linear regression models indicating that the random effects were significant in both models.
TABLE 3.
Results of mixed effects models modeled as: Interstitial glucose concentration measurements = β 0 + β 1* (ABA measured blood glucose concentration) + random effect on cat + error term and point‐of‐care glucometer glucose concentration measurements = β 0 + β 1* (ABA measured blood glucose concentration) + random effect on cat + error term
| Outcome variable (number of observations) | Mixed model term | Estimate | SE | P value |
|---|---|---|---|---|
| Interstitial glucose measured by the flash glucose monitoring system (139) | β 1 coefficient | 0.97 | 0.027 | <.001 |
| SD of random effects at cat level | 13.38 | 4.26 | N/A | |
| SD of error term | 28.42 | 1.75 | N/A | |
| Blood glucose measured by the point‐of‐care glucometer (153) | β 1 coefficient | 0.94 | 0.009 | <.001 |
| SD of random effects at cat level | 10.04 | 2.77 | N/A | |
| SD of error term | 13.84 | 0.81 | N/A |
The multivariable linear regression model identified a significant positive correlation between the ABA and FGMS glucose concentrations (P < .001). The coefficient for the ABA variable was 1.01. Therefore, for every 1 mg/dL increase in ABA blood glucose concentration there is an expected increase of 1.01 mg/dL on the FGMS measured IG concentration. Hemolysis, lipemia, and icterus were assessed as “moderate” or “marked” in 18/154 (12%), 9/154 (6%), and 0/154 (0%) of samples, respectively. Weak but significant positive correlations were found between PCV and FGMS‐measured IG concentration (P = .05) and between hemolysis and FGMS‐measured IG concentration (P = .05). For every 1% increase in PCV, there is a predicted increase of 0.98 mg/dL on the FGMS‐measured IG concentration, and for samples with moderate to severe hemolysis, there is a predicted increase of 17.5 mg/dL on the FGMS‐measured IG concentration compared to samples with no to mild hemolysis. No significant correlations were found between FGMS IG concentrations and total protein concentration, icterus, or lipemia. The combined contribution of ABA, PCV, and hemolysis to the variance in FGMS IG concentration was 93%.
3.3. Sensor tolerability and adverse events
In all cats, the sensor was placed on the dorsal neck in a standardized fashion as described with no complications. In 12/15 sensor placements (80%), the initial sensor fell off, was removed by the cat, or stopped working before the end of the 13‐day study period. Median time of sensor activity was 7 days (range, 2‐13 days). The 3 sensors that remained in place for the duration of the study were on 3 different cats, and 2 of these cats were enrolled in the study multiple times and experienced sensor failures during the other study periods. A t‐shirt made of stockinette was placed in 5/15 sensor placements in 5 individual cats; the sensor failed in 3 of these cats and remained functional throughout the study period in 2 of them. Of the 12 sensor failures, owners observed the cat scratching or chewing at the sensor site in 4 and the sensor was found off the cat in the remaining 8. The sensor was otherwise well tolerated in all cats, with only mild erythema noted at the sensor site after removal of 2 sensors in 2 different cats.
4. DISCUSSION
Our study further validates the use of an FGMS in diabetic cats, with excellent correlation between the FGMS and gold standard ABA glucose concentrations. Ours is the first study to validate the FGMS in a population of outpatient diabetic cats using tissue glue to adhere the sensor to the cat. The correlation coefficient between the FGMS and ABA glucose concentrations in all samples was 0.96, which is higher than the correlation coefficient of 0.90 reported in a previous study that used 8 sutures to adhere the FGMS sensor to the cats. 23 Potential reasons for this discrepancy include differences in the gold standard methodology for blood glucose concentration measurement (the hexokinase method was used in the previous study compared to the colorimetric glucose oxidase method used in our study), sensor application method of sutures versus glue, and patient populations. In addition, the previous study did not state if imaging, which could affect sensor accuracy, was performed while the sensor was in place, nor was it stated whether cats exposed to drugs that could influence sensor function were included in the study. 25
Our results are similar to those of previous studies validating the FGMS device in outpatient diabetic dogs, which reported correlation coefficients of 0.93 and 0.94 when comparing FGMS and ABA glucose concentrations. 18 , 21 The device also has been validated in dogs with diabetic ketoacidosis in 2 previous studies, with correlation coefficients of 0.88 and 0.89 when comparing FGMS IG and blood glucose concentrations. 19 , 20 The accuracy of the device in dogs in a diabetic crisis is likely decreased because of rapid insulin‐induced changes in blood glucose concentrations and the time required for glucose to equilibrate with the interstitial space, as well as dehydration, which was significantly correlated with decreased accuracy in 1 of these studies. 19 , 20 In a study utilizing a different continuous glucose monitor, the median time for blood glucose concentrations to equilibrate with IG concentrations in cats was 11.4 minutes, which is longer than the equilibration time in humans (4‐6 minutes) and dogs (5‐10 minutes). 12 , 27 , 28 , 29 Additional studies validating the use of the FGMS device in cats in diabetic crises are needed, because accuracy may be affected differently by rapid changes in glucose concentrations, hydration status, as well as electrolyte and acid‐base imbalances.
Correlation coefficients in the subgroups of hyperglycemia (0.86) and pronounced hyperglycemia (0.80) were lower than those identified when all samples were analyzed together, although they still indicated good correlation between FGMS and ABA glucose concentrations. These results are similar to those of the previous study in cats, which found a correlation coefficient of 0.88 in all hyperglycemic samples, 23 as well as previous studies in dogs, in which the correlation coefficients comparing FGMS and ABA glucose concentrations in samples with hyperglycemia were 0.85 18 and 0.91. 21 The decreased accuracy in these ranges may be the consequence of an inherent alteration in accuracy at higher glucose concentrations, a smaller number of samples in these groups, or subclinical changes in hydration and perfusion in poorly controlled diabetics. Clinicians should be aware of the decreased accuracy of the device in these glucose ranges, especially when using the device in animals with poorly controlled DM.
The correlation between the FGMS and the ABA in samples with normoglycemia was moderate and in samples with hypoglycemia was poor, but our study was underpowered in these subgroups. Despite a small number of samples with hypoglycemia, a significant correlation still was found between the ABA and POCG‐measured blood glucose concentrations, whereas the correlation coefficient between the ABA and FGMS‐measured glucose concentrations in samples with hypoglycemia was not significantly different from 0, indicating no correlation. Previous studies of this FGMS in cats and dogs also have not been powered to validate the device in hypoglycemic samples, but 1 study (reported in abstract form) found limited agreement between the FGMS and ABA during hypoglycemia in dogs with experimentally induced rapidly changing glucose concentrations. 30 One reason for a small number of samples with hypoglycemia in other studies could be that the FGMS does not report results below 40 mg/dL, although the lowest FGMS result in our study was 55 mg/dL. Additional studies examining the accuracy of this device in cats with hypoglycemia are needed.
The mixed effect models effectively predicted IG concentration and POCG glucose concentration. However, in the model predicting IG concentration, the between‐cat SD and error term SD were larger than in the model predicting POCG glucose concentrations. This observation could indicate a larger variation of between and within‐cat observations in the model in which the dependent variable is IG concentration compared to the model in which the dependent variable is POCG glucose concentration measurements.
The linear regression model showed that increased PCV and moderate to marked hemolysis were significantly correlated with increased IG concentrations. The FGMS measures glucose concentration in the interstitial space by the glucose oxidase method, which requires oxygen. Therefore, changes in PCV leading to alterations in perfusion and oxygenation of peripheral tissues could affect measurements of IG concentrations. Alternatively, changes in PCV could reflect subclinical changes in hydration status, which might affect IG concentrations. 20 , 25 A previous study of the FGMS in dogs found a correlation between total protein concentration and IG concentration, but no correlation between these 2 variables was found in our study. 21 Furthermore, the 0.98 mg/dL increase in IG concentration per 1% increase in PCV is not clinically important, but additional studies still are needed to confirm this finding and further evaluate its clinical relevance, especially in anemic cats and dogs. Moderate to marked hemolysis also was correlated with an increase in IG concentration by 17.5 mg/dL, which could be clinically relevant in the context of low glucose concentrations. The reason for this correlation, which was not found in a previous similarly designed study in dogs, is unknown. 21 It is also not known if the hemolysis was caused by in vivo or in vitro factors. Future studies are warranted to further investigate the effects of hemolysis on IG concentrations in both species.
No major adverse events were noted during the study period, but 80% of sensor placements failed because of premature detachment. A retrospective study of complications associated with the FGMS in cats found a much lower rate of early sensor detachment observed in only 15% of cats, although 2 cats in that study had major dermatologic complications including severe erosions in 1 cat and an abscess at the sensor site in another cat. 24 The authors of that study postulated that the use of cyanoacrylate tissue glue combined with the sensor adhesive may have contributed to the development of more severe skin reactions, and tissue glue therefore was not used in the majority of the cats in that study. 24 Interestingly, the same tissue glue utilized in that study was used for all cats in our study and only minor skin reactions were noted in 2/15 sensor placements. Only 3 to 4 drops of glue were used for sensor placement in our study, and thus the amount of glue utilized or other patient‐specific factors could have contributed to this difference. The higher rate of sensor detachment in our study could be a result of the location of sensor placement, which was the dorsal neck in our study compared to primarily the dorsolateral thorax in the previous study. 24 Another larger study of the same FGMS in hospitalized cats found a similar complication rate to the current study, with 70% of sensors failing after a median of 8.3 days despite fixing the sensor to the cat with 8 sutures. 23 Based on our results and the 2 previously described studies in cats, FGMS sensor failure appears to be more common in cats compared to dogs, in which sensor failure rates range from 30% to 44% in outpatients. 18 , 21 This higher rate of sensor failure in cats could be caused by species differences in skin thickness and elasticity, smaller body size of cats, and differences in behavior and activity. Future prospective studies are needed to confirm the optimal location and method of placement of the FGMS sensor in cats, and owners should be warned of a higher risk of sensor detachment or failure in this species.
Our study had several limitations. The small sample size precluded validation of the FGMS device in samples with normoglycemia and hypoglycemia, and assumptions made for sample size calculations could have resulted in miscalculation of the true sample size needed. Cats included in the study generally had poorly controlled DM as well as a variety of concurrent diseases and were on various noninsulin medications, which could have affected sensor application and tolerability, but this study population reflects the clinical population of diabetic cats at a tertiary referral hospital, and allows for generalizability of the findings to the true population of cats with DM.
In conclusion, we further validated the FGMS for use in outpatient cats with DM using tissue glue to adhere the sensor to the cat, but a higher rate of sensor detachment is expected in cats compared to dogs. Additional studies examining the optimal method and location of sensor application as well as validating the use of the device in the context of diabetic crises and hypoglycemia are needed.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Pennsylvania IACUC, protocol number 806686.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
The study was made possible by a gift from Ms. Catharine Adler.
Shea EK, Hess RS. Validation of a flash glucose monitoring system in outpatient diabetic cats. J Vet Intern Med. 2021;35(4):1703‐1712. 10.1111/jvim.16216
REFERENCES
- 1. Prahl A, Guptill L, Glickman NW, Tetrick M, Glickman LT. Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J Feline Med Surg. 2007;9(5):351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Öhlund M, Fall T, Ström Holst B, Hansson‐Hamlin H, Bonnett B, Egenvall A. Incidence of diabetes mellitus in insured Swedish cats in relation to age, breed and sex. J Vet Intern Med. 2015;29(5):1342‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Öhlund M, Egenvall A, Fall T, Hansson‐Hamlin H, Röcklinsberg H, Holst BS. Environmental risk factors for diabetes mellitus in cats. J Vet Intern Med. 2017;31(1):29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Behrend E, Holford A, Lathan P, Rucinsky R, Schulman R. 2018 AAHA diabetes management guidelines for dogs and cats. J Am Anim Hosp Assoc. 2018;54(1):1‐21. [DOI] [PubMed] [Google Scholar]
- 5. Nelson R. Stress hyperglycemia and diabetes mellitus in cats. J Vet Intern Med. 2002;16(2):121‐122. [DOI] [PubMed] [Google Scholar]
- 6. Rand JS, Kinnaird E, Baglioni A, Blackshaw J, Priest J. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med. 2002;16(2):123‐132. [DOI] [PubMed] [Google Scholar]
- 7. Lutz TA, Rand JS, Ryan E. Fructosamine concentrations in hyperglycemic cats. Can Vet J. 1995;36(3):155‐159. [PMC free article] [PubMed] [Google Scholar]
- 8. Crenshaw KL, Peterson ME, Heeb LA, Moroff SD, Nichols R. Serum fructosamine concentration as an index of glycemia in cats with diabetes mellitus and stress hyperglycemia. J Vet Intern Med. 1996;10(6):360‐364. [DOI] [PubMed] [Google Scholar]
- 9. Elliot DA, Nelson RW, Reusch CE, Feldman EC, Neal LA. Comparison of serum fructosamine and blood glycosylated hemoglobin concentrations for assessment of glycemic control in cats with diabetes mellitus. J Am Vet Med Assoc. 1999;214(12):1794‐1798. [PubMed] [Google Scholar]
- 10. Reusch CE, Tomsa K. Serum fructosamine concentration in cats with overt hyperthyroidism. J Am Vet Med Assoc. 1999;215(9):1297‐1300. [PubMed] [Google Scholar]
- 11. Ristic JME, Herrtage ME, Walti‐Lauger SMM, et al. Evaluation of a continuous glucose monitoring system in cats with diabetes mellitus. J Feline Med Surg. 2005;7(3):153‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moretti S, Tschuor F, Osto M, et al. Evaluation of a novel real‐time continuous glucose‐monitoring system for use in cats. J Vet Intern Med. 2010;24(1):120‐126. [DOI] [PubMed] [Google Scholar]
- 13. Dietiker‐Moretti S, Müller C, Sieber‐Ruckstuhl N, et al. Comparison of a continuous glucose monitoring system with a portable blood glucose meter to determine insulin dose in cats with diabetes mellitus. J Vet Intern Med. 2011;25(5):1084‐1088. [DOI] [PubMed] [Google Scholar]
- 14. Hoenig M, Pach N, Thomaseth K, De Vries F, Ferguson DC. Evaluation of long‐term glucose homeostasis in lean and obese cats by use of continuous glucose monitoring. Am J Vet Res. 2012;73(7):1100‐1106. [DOI] [PubMed] [Google Scholar]
- 15. Hafner M, Lutz TA, Reusch CE, Zini E. Evaluation of sensor sites for continuous glucose monitoring in cats with diabetes mellitus. J Feline Med Surg. 2013;15(2):117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wiedmeyer CE, Declue AE. Continuous glucose monitoring in dogs and cats. J Vet Intern Med. 2008;22(1):2‐8. [DOI] [PubMed] [Google Scholar]
- 17. Reineke EL, Fletcher DJ, King LG, Drobatz KJ. Accuracy of a continuous glucose monitoring system in dogs and cats with diabetic ketoacidosis. J Vet Emerg Crit Care. 2010;20(3):303‐312. [DOI] [PubMed] [Google Scholar]
- 18. Corradini S, Pilosio B, Dondi F, et al. Accuracy of a flash glucose monitoring system in diabetic dogs. J Vet Intern Med. 2016;30(4):983‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malerba E, Cattani C, Del Baldo F, et al. Accuracy of a flash glucose monitoring system in dogs with diabetic ketoacidosis. J Vet Intern Med. 2020;34(1):83‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silva DD, Cecci GRM, Biz G, Chiaro FN, Zanutto MS. Evaluation of a flash glucose monitoring system in dogs with diabetic ketoacidosis. Domest Anim Endocrinol. 2021;74:106525. [DOI] [PubMed] [Google Scholar]
- 21. Shea EK, Hess RS. Assessment of post‐prandial hyperglycemia and circadian fluctuation of glucose concentrations in diabetic dogs using a flash glucose monitoring system. J Vet Intern Med. 2021;35:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Del Baldo F, Canton C, Testa S, et al. Comparison between a flash glucose monitoring system and a portable blood glucose meter for monitoring dogs with diabetes mellitus. J Vet Intern Med. 2020;34(6):2296‐2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deiting V, Mischke R. Use of the “FreeStyle Libre” glucose monitoring system in diabetic cats. Res Vet Sci. 2021;135:253‐259. 10.1016/j.rvsc.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 24. Shoelson AM, Mahony OM, Pavlick M. Complications associated with a flash glucose monitoring system in diabetic cats. J Feline Med Surg. 2020;23:557‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. FreeStyle Libre Indications and Important Safety Information; 2020. https://www.freestylelibre.us/safety-information.html
- 26. FreeStyle Libre 14 day Flash Glucose Monitoring System User's Manual; 2018. https://freestyleserver.com/Payloads/IFU/2018/ART39764-001_rev-A-Web.pdf. Accessed August 7, 2020.
- 27. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes. 2013;62(12):4083‐4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory‐calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rebrin K, Steil GM, Van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol. 1999;277(3):561‐571. [DOI] [PubMed] [Google Scholar]
- 30. Patterson C, Howard L, Lidbury J, et al. Evaluation of a flash glucose monitoring system in dogs with rapidly changing glucose concentrations. 2020 ACVIM Forum On Demand Research Abstract Program. J Vet Intern Med. 2020;34:2884. 10.1111/jvim.15904. [DOI] [Google Scholar]


