Abstract
Background
Endothelial dysfunction might contribute to the development of leptospiral pulmonary hemorrhage syndrome (LPHS).
Hypothesis
Serum concentrations of markers of endothelial activation and dysfunction are higher in dogs with leptospirosis and correlate with the occurrence of LPHS and a higher case fatality rate.
Animals
Clinically healthy dogs (n = 31; 10/31 dogs confirmed healthy based on no detected abnormalities on blood work), dogs with leptospirosis with LPHS (n = 17) and without LPHS (n = 15), dogs with acute kidney injury not due to leptospirosis (AKI‐nL, n = 34).
Methods
Observational study. Serum concentrations of soluble intercellular adhesion molecule 1 (sICAM‐1), vascular endothelial growth factor (VEGF), and angiopoietin‐2 (Ang‐2) at admission were compared between groups. Correlations with outcome and the accuracy to predict LPHS were examined.
Results
Soluble intercellular adhesion molecule (sICAM‐1), VEGF, and Ang‐2 concentrations were higher in dogs with AKI‐nL (sICAM‐1 34.7 ng/mL, interquartile range [IQR] = 24.4‐75.5; VEGF 43.1 pg/mL, IQR = 12.3‐79.2; Ang‐2 8.5 ng/mL, IQR = 6.2‐12.3), leptospirosis without LPHS (sICAM‐1 45.1 ng/mL, IQR = 30.6‐59.0; VEGF 32.4 pg/mL, IQR = 12.5‐62.6; Ang‐2 9.6 ng/mL, IQR = 6.9‐19.3), and LPHS (sICAM‐1 69.7 ng/mL, IQR = 42.1‐89.1; VEGF 51.8 pg/mL, IQR = 26.3‐96.7; Ang‐2 8.0 ng/mL, IQR = 5.6‐12.2) compared to controls (P < .001). In dogs with leptospirosis, VEGF and sICAM‐1 were higher in nonsurvivors (sICAM‐1 89.4 ng/mL, IQR = 76.5‐101.0; VEGF 117.0 pg/mL, IQR = 90.3‐232.4) than survivors (P = .004) and sICAM‐1 predicted the development of LPHS.
Conclusions
Soluble intercellular adhesion molecule 1, VEGF, and Ang‐2 do not discriminate leptospirosis from AKI‐nL. In dogs with leptospirosis, sICAM‐1 and VEGF predict outcome and sICAM‐1 might identify dogs at risk for LPHS.
Keywords: acute kidney injury, bleeding, canine, endothelial marker, Leptospira, respiratory
Abbreviations
- AKI
acute kidney injury
- AKI‐nL
acute kidney injury not due to leptospirosis
- Ang‐2
angiopoietin‐2
- AUC
area under the curve
- CRP
C‐reactive protein
- CV
coefficient of variation
- ICAM
intercellular adhesion molecule
- IQR
interquartile range
- LLD
lower limit of detection
- LPHS
leptospiral pulmonary hemorrhage syndrome
- MAT
microscopic agglutination test
- ROC
receiver‐operating characteristics
- sICAM
soluble intercellular adhesion molecule
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Leptospiral pulmonary hemorrhage syndrome (LPHS) is a severe form of leptospirosis. It has been increasingly recognized in humans and dogs and has become a major cause of death. 1 , 2 The pathogenesis of LPHS is poorly understood, hampering the application of effective therapies. Leptospiral pulmonary hemorrhage syndrome is characterized by rapidly progressive intra‐alveolar hemorrhage in the absence of pulmonary inflammatory cell infiltration. 3 , 4 , 5 In contrast to liver and kidney, relatively few leptospires can be found in lung tissue of affected humans and animals. 3 , 5 , 6 While leptospirosis might lead to both hyper‐ or hypocoagulable states, there is no clear association between hypocoagulation and LPHS. 5 , 7 , 8 Evidence has emerged suggesting a role of the endothelium in many of the disease manifestations of leptospirosis, including LPHS. 9 , 10
Direct contact of endothelial cells with live Leptospira spp. or leptospiral proteins in vitro leads to endothelial cell activation with overexpression of ICAM‐1 and E‐selectin, 11 , 12 , 13 loss of adherens junction proteins, 14 and loss of monolayer integrity. 10 Furthermore, ICAM‐1 and vascular cell adhesion molecule are overexpressed in lung tissue from human patients with LPHS compared to patients with pulmonary hemorrhage due to other causes, supporting the hypothesis that endothelial activation plays a role in the pathogenesis of LPHS. 6
While direct determination of the functional and structural state of the endothelium is difficult in vivo, serum markers of endothelial activation and dysfunction are well described in human patients with leptospirosis. Serum concentrations of soluble ICAM‐1, 15 , 16 E‐selectin, 15 , 17 angiopoietin‐2 (Ang‐2), 18 vascular endothelial growth factor (VEGF), 19 and syndecan‐1 16 are increased in human patients with leptospirosis compared to healthy controls, and increased serum concentrations of sICAM‐1, 16 syndecan‐1, 16 VEGF, 19 and Ang‐2 18 are associated with a more severe clinical course of disease as defined by the presence of acute kidney injury (AKI), sepsis, and intensive care admission. Soluble E‐selectin serum concentrations are higher in nonsurvivors, but E‐selectin serum concentrations are not associated with bleeding in this cohort. 20 Increased concentration of endothelial activation markers could be caused by shedding of cell surface ICAM‐1 by leptospiral proteases, 15 , 21 , 22 endothelial activation, or endothelial damage. 16 However, a control group of patients with AKI of other etiologies is not included in any human study, therefore precluding any conclusions with regards to the specific pathogenic mechanism of leptospirosis compared to other causes of AKI. Furthermore, their association with the occurrence of pulmonary hemorrhage has not been examined in detail.
The aims of this study were to explore serum markers of endothelial activation (sICAM‐1) and dysfunction (VEGF, Ang‐2) in order to better understand the role of the endothelium in the pathogenesis of LPHS and to identify suitable biomarkers predicting the occurrence of LPHS and outcome.
We hypothesized that serum concentrations of sICAM‐1, VEGF, and Ang‐2 are increased in dogs with leptospirosis compared to healthy dogs and dogs with AKI not due to leptospirosis (AKI‐nL) and that in dogs with leptospirosis, serum concentrations of these markers are positively correlated with the occurrence of LPHS and a poorer outcome.
2. MATERIALS AND METHODS
2.1. Study design
Dogs with leptospirosis with LPHS (LPHS group) and without LPHS (Leptospirosis group) and dogs with AKI due to other causes (AKI‐nL) which were presented to the Vetsuisse Faculty Bern (Switzerland) between 2013 and 2017 were included in this observational study. Clinically healthy staff‐owned dogs and healthy control dogs from a genetic screening project were included as controls. 23 Prospectively collected samples (n = 17) from dogs with leptospirosis, LPHS and AKI‐nL as well as fresh aliquots of samples from the same cohorts collected for previous studies (n = 49) were used. Owner consent for the collection of blood samples from healthy dogs and the use of clinical data and surplus samples from dogs was obtained before enrolment.
2.2. Animals
All dogs in the control group were deemed clinically healthy based on history and no detected abnormalities on physical examination. CBC and biochemistry profile to support their good health and to calculate correlations with endothelial cell activation or dysfunction and markers of inflammation such as neutrophil and band neutrophil counts and C‐reactive protein (CRP) was performed in 10/31 control dogs.
All sick dogs underwent clinical evaluation including physical examination, CBC, routine biochemistry, and urine analysis if they were not anuric, and abdominal ultrasound. Further diagnostic testing was directed by the specific problems of the dogs and included thoracic radiography, urine culture as well as testing for other potential infectious agents such as serologic testing against Angiostrongylus vasorum, Ehrlichia canis, Anaplasma phagocytophilum, or detection of Babesia canis via blood PCR.
In dogs with AKI or a suspicion of leptospirosis, microagglutination testing (MAT) was performed according to OIE standards at the national reference laboratory for animal leptospirosis against a panel of pathogenic Leptospira including serovars Grippotyphosa, Australis, Bratislava, Canicola, Icterohemorrhagiae, Copenhageni, Pomona, Tarassovi, Hardjo, Bataviae, Autumnalis, and Sejroe. 24
2.3. Case definitions
2.3.1. Group 1: Acute leptospirosis without pulmonary hemorrhage (Leptospirosis group)
A diagnosis of leptospirosis was based on clinical findings and the presence of at least 1 of: a positive urine or tissue real time‐PCR, 25 MAT seroconversion with 4‐fold rise in titer, a single positive MAT titer of ≥1 : 800, a single positive IgM lateral flow assay (Witness Lepto, Canine Leptospira Antibody Test Kit, Zoetis Deutschland GmbH, Germany), or a strong clinical evidence with at least 2 classical organ manifestations of leptospirosis (renal, hepatic, or hemorrhagic) in the absence of another etiology. The last criteria was only considered in cases where leptospirosis could not be confirmed serologically due to early death. Leptospiral pulmonary hemorrhage syndrome was excluded by the absence of clinical signs and pulmonary infiltrates on thoracic radiographs.
2.3.2. Group 2: Acute leptospirosis with pulmonary hemorrhage (LPHS group)
The diagnosis of acute leptospirosis was made as described for group 1. The diagnosis of LPHS was based on clinical signs and radiographic evidence of multifocal alveolar infiltrates. This approach is limited by the fact that alveolar patterns can also be caused by other pathologies such as pneumonia or fluid overload. However, more aggressive diagnostics to confirm the presence of intra‐alveolar bleeding such as bronchoscopy and bronchoalveolar lavage were not performed because of the anesthetic risk and the risks of respiratory complications due to the lavage procedure.
2.3.3. Group 3: AKI‐nL group
Dogs were included in this group if they presented with acute renal azotemia and leptospirosis was excluded in this cohort based on the absence of seroconversion on paired serum samples or a single reciprocal MAT titer of ≤1 : 200 in conjunction with a convincing alternative diagnosis. Acute kidney injury was diagnosed in the context of consistent historical, clinical, or laboratory findings if at least 2 of the following criteria were fulfilled: (a) presence of renal azotemia with a serum creatinine concentration ≥ 1.7 mg/dL, persisting at least 24 hours after correction of prerenal factors; (b) increase in serum creatinine ≥ 0.3 mg/dL during a 48 hours interval in the absence of prerenal factors; (c) persistent pathological oligo/anuria (<1 mL/kg/min over 6 hours) after volume repletion; and (d) evidence of tubular injury with renal glucosuria or granular casts on urinalysis. Dogs with evidence of underlying chronic kidney disease were not excluded from the study.
2.3.4. Group 4: Healthy dogs
This group contained clinically healthy dogs from a genetic study and clinically healthy staff‐owned dogs. 23 Dogs in this group had no abnormalities on history and physical examination. CBC and biochemistry panels, confirming the health of control dogs, were only available for 10 of 31 animals.
2.4. Sample collection and handling
Blood was collected from the Vena saphena lateralis, Vena cephalica, or Vena jugularis using a 21 gauge needle into serum or lithium heparin tubes (Microvette 200 uL; Sarstedt AG, Nümbrecht, Germany), or both. Heparinized blood was centrifuged at 4000 rpm for 10 minutes at 4°C to separate plasma from cellular components. Plasma was used for biochemical analysis. For collection of serum, blood was left to clot for 30 minutes at room temperature. After centrifugation at ambient temperature, serum was separated and frozen at −80°C until further analysis. No proteolytic inhibitors were added to the serum before refrigeration.
Samples were collected between January 2016 and December 2017 on day 1 and, if possible, also on day 3 after admission. In addition, serum samples stored at −80°C from dogs with leptospirosis with and without LPHS as well as AKI‐nL and healthy dogs presented between January 2013 and December 2015 were used Associations between storage time and the concentrations of sICAM‐1, Ang‐2, and VEGF were examined via multiple linear regression analysis (sICAM‐1) or ordinale regression analysis (Ang‐2, VEGF) using disease group and sampling date as covariables and did not show any association between serum concentrations and storage time (data not shown).
2.5. Sample analysis
For all ELISA assays, samples were measured in duplicate by the same person and the measurements were repeated if the coefficient of variation (CV) between duplicates was >10%.
Serum sICAM‐1 was measured using a commercial canine‐specific ELISA‐kit (SEA548Ca; Cloud‐Clone Corp., Houston, Texas) according to the manufacturer's instructions. This ELISA‐kit was previously used for the measurement of canine soluble ICAM‐1 in serum of dogs with babesiosis. 26 Samples were diluted 1 : 3 in phosphate buffered saline (PBS). Samples from 3 dogs were used to calculate interassay variability and aliquots of these samples were measured on all plates. In our experiments, the mean intra‐assay variability was 4.2% and the lower limit of detection (LLD) 1 ng/mL. As the interassay variability was very high (CV = 57%), sICAM‐1 concentrations were first compared between groups for each plate (Table S1, Figure S1A‐F) and subsequently across plates after normalization of the values via a correction factor. The correction factor was calculated as the average sICAM‐1 value of the 3 controls on the reference plate divided by the average value of these controls on the test plate (Table S2). Plate 2 was taken as reference as the measured values lay in‐between those of plates 1 and 3. Selection of plate 1 or plate 3 as reference would have resulted in different correction factors but would have equally allowed to examine the relative differences between groups.
Serum VEGF concentrations were measured using a commercial canine VEGF ELISA kit (Canine VEGF Quantikine ELISA Kit, R&D Systems, Inc, Minneapolis, Minnesota; product datasheet available at: https://www.rndsystems.com/products/canine-vegf-quantikine-elisa-kit_cave00) as previously described. This assay has been validated for measurement of canine VEGF and was previously used by our group to detect VEGF concentrations in dogs with systemic inflammatory response syndrome or sepsis and in healthy dogs. 27 Inter‐ and intra‐assay variability were assessed in a previous study in our laboratory and were 4.6% and 4.9%, respectively. 27 The LLD of the assay was set at 15 pg/mL, which corresponds to the concentration of the lowest standard.
Serum Ang‐2 concentration was measured according to the manufacturer's instructions using a commercial ELISA test kit (Human Angiopoietin‐2 Immunoassay; R&D Systems, Inc; product datasheet available at: https://www.rnsystems.com/products/human-angiopoietin-2-quantikine-elisa-kit_dang20) for use in humans that was validated for use in dogs. 28 Samples were diluted 1 : 5 with calibrator d iluent. If the measured Ang‐2 concentration was outside the range of the standard curve (standard curve: 0.05‐3 ng/mL) samples were retested at a dilution of 1 : 10. If these samples were still above the detection limit at the 1 : 10 dilution, they were retested once more at a dilution of 1 : 20 in order to obtain concentrations within the standard curve.
The respective coefficients of variation reflecting intra‐ and interassay variability were assessed in a previous study in our laboratory and were 5.6% for samples with high or low Ang‐2 concentrations, and 6.7% (low concentrations) and 4.0% (high concentrations), respectively. 28
2.6. Statistical analysis
Statistical analysis was performed using a commercial software package (NCSS Statistical Software, 2016; NCSS; LLC, Kaysville, Utah, available at: ncss.com/software/ncss). Descriptive statistics were performed for dog characteristics, including sex, neuter status, age, body weight, and baseline blood variables. Shapiro‐Wilk testing indicated non‐normal distribution for several continuous variables, which were therefore reported as median and interquartile range (IQR).
As 52% of VEGF measurements were below the lower level of detection of 15 pg/mL, the frequency of VEGF concentrations below the LLD was compared between disease groups and between survivors and nonsurvivors among dogs with leptospirosis (Leptospirosis and LPHS groups) using Fisher's exact test. Differences in serum concentrations of sICAM‐1, VEGF, and Ang‐2 and of markers of inflammation, including neutrophil count, band neutrophils, albumin, and CRP, were compared between disease groups and among dogs with leptospirosis between survivors and nonsurvivors using the Kruskal‐Wallis Multiple Comparison Z‐Value test (which corrects for multiple comparisons). For this analysis, VEGF measurements below the LLD were substituted with an LLD/2 (7.5 pg/mL). 29
Outliers were generally not excluded from the analysis. Dogs euthanized for financial reasons rather than intractable disease were excluded from the outcome analysis.
Because of non‐normal distribution of the data, the correlation between sICAM‐1, VEGF, and Ang‐2 and the associations of these 3 markers with clinical and laboratory variables and outcome were assessed using the Spearman rank correlation test.
The performance of sICAM‐1, VEGF, and Ang‐2 to discriminate healthy from diseased dogs, dogs with leptospirosis from dogs with AKI‐nL and healthy dogs and dogs with LPHS from the other groups and to predict a negative outcome was assessed using receiver‐operating characteristics (ROC) analysis. Optimal cutoff points for sensitivity and specificity were determined using the most favorable cost‐benefit ratios to obtain the largest possible proportion of correctly classified dogs and were reported if the area under the curve (AUC) was >0.5. Statistical significance for all statistical tests was set at P < .05.
3. RESULTS
3.1. Study sample
Samples and clinical data from 97 dogs were used for this study: 15 dogs with leptospirosis without LPHS (Leptospirosis group), 17 dogs with LPHS (LPHS group), 34 dogs were diagnosed with AKI due to other causes than leptospirosis (AKI‐nL group), and 31 dogs were clinically healthy. Twelve dogs were crossbreeds and 85 were purebreds belonging to 38 different breeds, the most common breeds being Labrador Retriever (n = 16), Malinois (n = 15), Golden retriever (n = 5), Siberian Husky (n = 5), German shepherd (n = 4), and Bernese Mountain dog (n = 4). Malinois dogs (14/31; 45%) were overrepresented in the group of healthy dogs, as these dogs were primarily recruited to participate as healthy controls in a genetic survey. 23 There were 54 males (entire n = 23; neutered n = 31) and 43 females (entire n = 8; spayed n = 35) included in the study. There were no significant differences regarding age, body weight, sex, and neuter status across the 4 groups. Characteristics of healthy dogs, dogs with AKI, and dogs with leptospirosis with and without LPHS are shown in Table 1.
TABLE 1.
Characteristics of healthy dogs, dogs with acute kidney injury due to other causes (AKI‐nL), dogs with leptospirosis without and with leptospiral pulmonary hemorrhage syndrome (LPHS)
| Variable | Reference range | Healthy | AKI‐nL | Leptospirosis without LPHS | Leptospirosis with LPHS | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | ||
| Age (y) | 20 | 7.4 (5.3‐9.0) | 34 | 4.5 (1.0‐9.0) | 15 | 5.0 (1.0‐8.0) | 16 | 6.0 (2.3‐8.8) | |
| Body weight (kg) | 31 | 24.8 (21.0‐28.0) | 34 | 27.2 (20.5‐32.1) | 15 | 25.0 (17.5‐30.6) | 17 | 26.0 (12.9‐33.0) | |
| Sex | 31 | 34 | 15 | 17 | |||||
| Male (n; intact/neutered) | 6/11 | 5/13 | 4/2 | 8/5 | |||||
| Female (n; intact/ neutered) | 3/11 | 3/13 | 2/7 | 0/4 | |||||
| Hematocrit (L/L) | 0.39‐0.57 | 10 | 0.47 (0.45‐0.51) | 34 | 0.39 (0.32‐0.43) a | 14 | 0.38 (0.34‐0.44) c | 17 | 0.30 (0.26‐0.35) a , b , d |
| Thrombocytes (103/μL) | 150‐400 | 11 | 336 (279‐434) | 33 | 209 (132‐311) a | 14 | 161 (35‐315) a | 17 | 138 (88‐208) a |
| Leucocyte count (103/μL) | 6‐12 | 10 | 6.6 (6.0‐8.7) | 33 | 12.5 (8.0‐19.5) | 14 | 14.0 (10.9‐19.6) | 17 | 13.7 (9.6‐15.8) |
| Mature neutrophils (103/μL) | 3‐11.5 | 11 | 4.6 (3.7‐5.0) | 33 | 11.0 (6.6‐15.9) a | 14 | 11.1(8.4‐15.8) a | 17 | 10.7 (7.9‐13.1) a |
| Band neutrophils (103/μL) | 0‐0.3 | 10 | 0.06 (0‐0.13) | 33 | 0.11 (0‐0.57) | 14 | 0.14 (0.07‐0.54) | 17 | 0.14 (0‐0.4) |
| Urea (mg/dL) | 19.8‐64.7 | 11 | 34.2 (32.4‐54.7) | 34 | 300.3 (200.6‐372.4) a | 15 | 309.3 (120.1‐368.2) a | 17 | 343.5 (255.3‐409.0) a |
| Creatinine (mg/dL) | 0.6‐1.3 | 11 | 0.8 (0.7‐0.9) | 34 | 8.0 (5.2‐12.7) a | 15 | 7.4 (3.5‐10.2) a | 17 | 9.7 (8.0‐13.6) a |
| Bilirubin (mg/dL) | 0.03‐0.23 | 11 | 0.02 (0.01‐0.04) | 32 | 0.20 (0.15‐0.33) a | 15 | 0.22 (0.16‐0.44) a | 17 | 0.21 (0.17‐2.23) a |
| Albumin (g/dL) | 3.0‐4.1 | 11 | 3.36 (3.07‐3.52) | 34 | 2.85 (2.2‐3.14) a | 15 | 2.75 (2.58‐3.04) a | 17 | 2.31 (2.05‐2.65) a |
| CRP (mg/L) | 0‐10.7 | 11 | 1.1 (0‐6.2) c , d | 29 | 14.1 (2.6‐50.2) c , d | 8 | 44.5 (38.5‐141.3) a , b | 11 | 53.1 (32.0‐89.8) a , b |
| Outcome | |||||||||
| Alive to discharge n (%) | — | — | 25 | 25 (73.5) | 14 | 14 (93.3) | 13 | 13 (76.5) | |
| Euthanized/died n (%) | — | — | 9 | 9 (26.5) | 1 | 1 (6.7) | 4 | 4 (23.5) | |
Note: Data are shown as medians and interquartile ranges unless stated otherwise. Significance was defined as P ≤ .05 after Bonferroni correction.
Abbreviations: CRP, C‐reactive protein; IQR, interquartile range; n, number of samples.
Significant difference compared to healthy.
Significant difference compared to AKI due to other causes.
Significant difference compared to leptospirosis with LPHS.
Significant difference compared to leptospirosis without LPHS.
Of the 31 dogs included as healthy controls, 21 were deemed healthy based on no abnormalities on history and physical examination and 10 had additional CBC and biochemistry panels performed and no abnormalities were detected.
In dogs with leptospirosis, the diagnosis was confirmed in 12 dogs via a single MAT of ≥1 : 800 and in 17 dogs by a ≥4‐fold rise in MAT titer in paired serum samples to 1 or several serovars. Three dogs had a single positive rapid diagnostic test. Leptospirosis was excluded in dogs with AKI‐nL by a paired MAT titer (n = 9) or a single titer below 1 : 800 (n = 11) and a convincing alternative diagnosis (n = 14). Of the 34 dogs with AKI, 10 had a history of ingestion of nephrotoxic substances (grapes n = 6; non‐steroidal anti‐inflammatory drugs n = 2; ethylene glycol n = 1; maleic acid n = 1). Acute on chronic kidney disease was present in 6 dogs, pyelonephritis in 2, AKI following heat stroke in 2 dogs, and 1 each of the following: trauma, renal amyloidosis, glomerulonephritis, snake bite, and hemolytic uremic syndrome. The origin of AKI remained unknown in 9 dogs.
3.2. Baseline laboratory variables
All 3 disease groups (AKI‐nL, leptospirosis, LPHS) showed significant differences in median thrombocyte count, urea, creatinine, bilirubin, and albumin compared to healthy controls. Hematocrit was significantly lower in the LPHS group compared to the AKI‐nL and leptospirosis groups. C‐reactive protein was significantly lower in healthy dogs and AKI‐nL group compared to leptospirosis and LPHS groups (Table 1). Among dogs with leptospirosis (with and without LPHS) median bilirubin concentrations were significantly higher in nonsurvivors compared to survivors (Table 2).
TABLE 2.
Differences between survivors and nonsurvivors among dogs with leptospirosis with and without LPHS
| Variable | n | Survivors | n | Nonsurvivors | P value |
|---|---|---|---|---|---|
| Age (y) | 27 | 5 (1‐8) | 4 | 8 (3.5‐8.6) | .26 |
| Body weight (kg) | 27 | 26 (16‐34) | 5 | 25 (10.4‐28.9) | .42 |
| Thrombocytes (103/μL) | 26 | 155 (126‐269) | 5 | 70 (29‐79) & | .007 |
| Leucocyte count (103/μL) | 26 | 13.3 (9.8‐15.3) | 5 | 19.5 (12.0‐21.7) | .1 |
| Mature neutrophils (103/μL) | 26 | 10.6 (8.0‐12.5) | 5 | 15.5 (10.4‐17.7) | .07 |
| Band neutrophils (103/μL) | 26 | 0.1 (0‐0.3) | 5 | 0.49 (0.32‐0.75) & | .02 |
| Total protein (g/dL) | 27 | 5.72 (5.19‐6.41) | 5 | 4.67 (3.67‐5.17) & | .01 |
| Albumin (g/dL) | 27 | 2.67 (2.31‐2.94) | 5 | 2.1 (1.66‐2.51) & | .02 |
| Bilirubin (mg/dL) | 27 | 0.20 (0.16‐0.26) | 5 | 3.7 (1.10‐13.82) * | .001 |
| CRP (mg/L) | 15 | 42 (32‐126) | 4 | 65 (48‐107) | .48 |
Abbreviations: CRP, C‐reactive protein; LPHS, leptospiral pulmonary hemorrhage syndrome; n, number of samples.
P ≤ .05 after Bonferroni correction.
P ≤ .05 Kruskal‐Wallis Multiple Comparison Z‐test.
3.3. Serum sICAM‐1 concentrations
Comparison of day 1 serum sICAM‐1 concentrations between groups within ELISA plates showed consistently higher concentrations for leptospirosis, LPHS, and AKI‐nL groups compared to healthy controls (Table S1, Figure S1). Median corrected day 1 sICAM‐1 concentrations were significantly higher in dogs with AKI‐nL (n = 16; 34.7 ng/mL; IQR, 24.4‐75.5), leptospirosis (n = 9; 45.1 ng/mL; IQR, 30.6‐59.0), and LPHS groups (n = 14; 69.7 ng/mL; IQR, 42.1‐89.1) compared to healthy controls (n = 31; 18.6 ng/mL; IQR, 9.9‐23.6; P < .001; Figure 1A). There were no significant differences between the 3 disease groups. Median corrected serum sICAM‐1 was significantly higher in dogs with leptospirosis with or without LPHS which died or were euthanized due to intractable disease (n = 4; 89.4 ng/mL; IQR, 76.5‐101.0) compared to dogs that survived to discharge (n = 19; 50.9 ng/mL; IQR, 30.7‐65.4; P = .004; Figure 2A).
FIGURE 1.
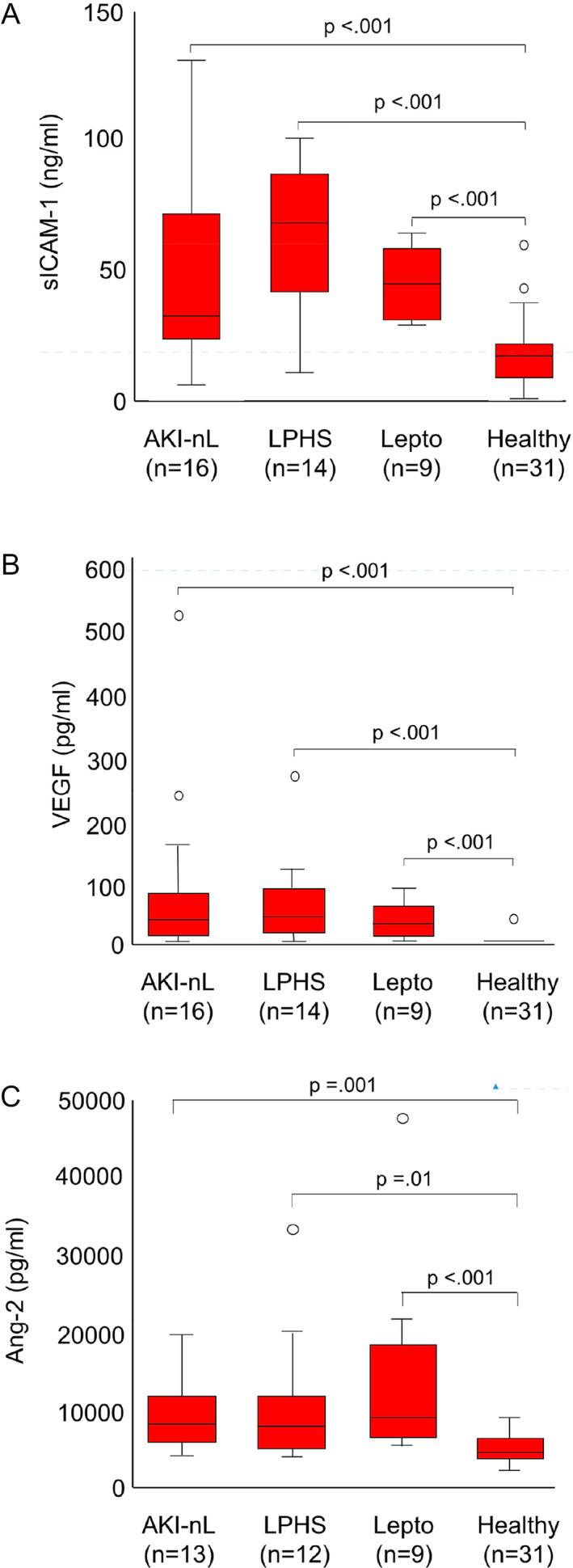
A‐C, Day 1 serum soluble intercellular adhesion molecule (sICAM‐1; A), vascular endothelial growth factor (VEGF; B), and angiopoietin‐2 serum concentrations (Ang‐2; C) in healthy dogs and dogs with acute kidney injury due to other etiologies (AKI‐nL), leptospirosis without pulmonary hemorrhage (Lepto), and dogs with leptospiral pulmonary hemorrhage syndrome (LPHS). Median sICAM‐1, VEGF, and Ang‐2 concentrations are significantly higher in the 3 disease groups compared to healthy controls, but there is no significant difference between disease groups. The central lines in the boxes represent the median values, and the top and bottom of the boxes represent the 75th and 25th percentiles, respectively
FIGURE 2.
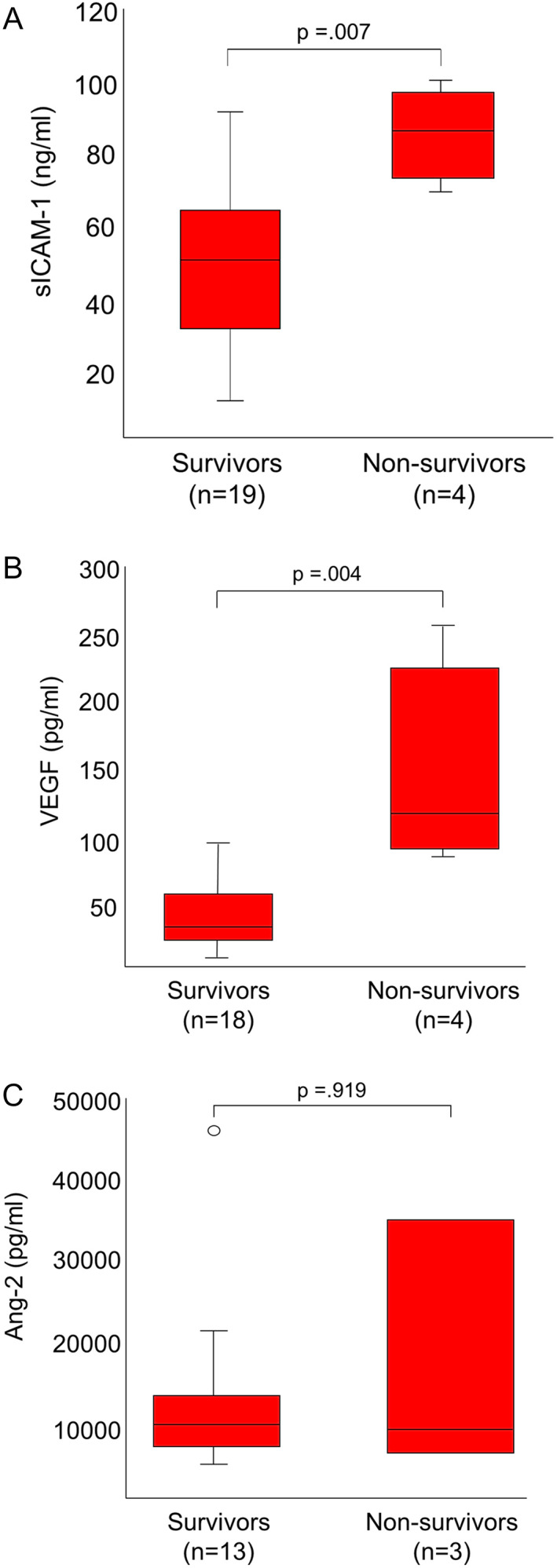
A‐C, Day 1 serum soluble intercellular adhesion molecule (sICAM‐1; A), vascular endothelial growth factor (VEGF; B), and angiopoietin‐2 serum concentrations (Ang‐2; C) in surviving and nonsurviving dogs with leptospirosis with or without pulmonary hemorrhage. Median sICAM‐1 and VEGF serum concentrations are significantly higher in nonsurvivors compared to survivors. The central lines in the boxes represent the median values, and the top and bottom of the boxes represent the 75th and 25th percentiles, respectively
Median day 3 corrected serum sICAM‐1 in dogs with LPHS (n = 5; 87.5 ng/mL; IQR, 52.3‐101.7) was 3 times that of dogs with AKI‐nL (n = 5; 32.3 ng/mL; IQR, 10.9‐80.3) or leptospirosis without LPHS (n = 2; 31.6 ng/mL; IQR, 29.3‐34.0), but the numbers of available samples were small and this difference not statistically significant (P = .16).
3.4. Serum VEGF concentrations
Serum day 1 VEGF concentrations were below the LLD (15 pg/mL) in 96% (n = 30/31) of healthy dogs, 27% (n = 3/18) of dogs with AKI‐nL, 25% (n = 2/8) of dogs with leptospirosis without LPHS, and 14% (n = 2/14) of dogs with LPHS. The frequency of day 1 VEGF concentrations below the LLD was significantly higher in healthy dogs (P < .001) compared to the 3 disease groups but was not significantly different between the 3 disease groups.
Median serum day 1 VEGF was significantly higher in dogs with AKI‐nL (n = 18; 43.1 pg/mL; IQR, 12.3‐79.2), leptospirosis (n = 8; 32.4 pg/mL; IQR, 12.5‐62.6), and LPHS groups (n = 14; 51.8 pg/mL; IQR, 26.3‐96.7) compared to healthy controls (P < .001; Figure 1B). There was no significant difference between disease groups.
Median day 1 VEGF was significantly higher in dogs with leptospirosis with or without LPHS, which died or were euthanized due to intractable disease (n = 4; 117.0 pg/mL; IQR, 90.3‐232.4) compared to dogs that survived to discharge (n = 18; 32.4 pg/mL; IQR, 21.2‐57.9; P = .004; Figure 2B).
Day 3 serum VEGF concentrations were below the limit of detection in the only 2 samples available from dogs with leptospirosis without LPHS, while median day 3 concentrations were 20.5 ng/mL (IQR, 15‐121; n = 5) in dogs with AKI and 58.1 ng/mL (IQR, 21‐111.4; n = 4) in dogs with LPHS. The difference between groups was not statistically significant (P = .09).
3.5. Serum Ang‐2 concentrations
Median day 1 serum Ang‐2 concentrations were significantly higher in dogs with AKI‐nL (n = 13; 8.5 ng/mL; IQR, 6.2‐12.3), leptospirosis (n = 9; 9.6 ng/mL; IQR, 6.9‐19.3), and LPHS groups (n = 12; 8.0 ng/mL; IQR, 5.6‐12.2) compared to healthy controls (n = 31; 5.4 ng/mL; IQR, 4.6‐7.3; P < .001). There was no statistically significant difference in Ang‐2 serum concentrations between disease groups (Figure 1C).
There was no significant difference in day 1 Ang‐2 concentrations among dogs with leptospirosis with or without LPHS between survivors (n = 18; 9.5 ng/mL; IQR, 6.5‐13.3) and nonsurvivors (n = 3; 8.7 ng/mL; IQR, 5.9‐34.1; Figure 2C). The small number of day 3 samples precluded statistical analysis of this time point.
3.6. Correlation analysis
Serum sICAM‐1 and VEGF concentrations were moderately but significantly positively correlated with segmented neutrophil count, bilirubin concentration and CRP, and negatively correlated with plasma albumin. There was a significant moderate positive correlation of sICAM‐1 and VEGF. Both sICAM‐1 and VEGF were weakly positively correlated with Ang‐2 (Table 3). Serum Ang‐2 was not significantly correlated with most markers of inflammation, except for a very weak correlation with CRP.
TABLE 3.
Correlation of day 1 serum soluble intercellular adhesion molecule‐1 (sICAM‐1), vascular endothelial growth factor (VEGF), and Angiopoietin‐2 (Ang‐2) with markers of inflammation
| Variable | sICAM‐1 | VEGF | Ang‐2 | |||
|---|---|---|---|---|---|---|
| Spearman ρ | P value | Spearman ρ | P value | Spearman ρ | P value | |
| Leucocyte count | 0.48 | <.001 | 0.36 | .01 | 0.18 | .26 |
| Mature neutrophils | 0.51 | <.001 | 0.44 | .002 | 0.26 | .09 |
| Band neutrophils | 0.38 | .009 | 0.36 | .01 | 0.18 | .22 |
| Albumin | −0.52 | .001 | −0.6 | <.001 | −0.24 | .11 |
| Bilirubin | 0.52 | <.001 | 0.64 | <.001 | 0.38 | .01 |
| CRP | 0.65 | <.001 | 0.7 | <.001 | 0.38 | .04 |
| D1 Ang‐2 | 0.26 | .04 | 0.47 | <.001 | — | — |
| D1 VEGF | 0.68 | <.001 | — | — | .47 | <.001 |
| D1 sICAM‐1 | — | — | 0.68 | <.001 | 0.26 | .04 |
Abbreviation: CRP, C‐reactive protein.
3.7. ROC analysis
Optimum cutoff values, sensitivities, specificities and AUC to discriminate healthy from diseased dogs (AKI‐nL, leptospirosis, LPHS), dogs with leptospirosis with and without LPHS from dogs with AKI‐nL, dogs with LPHS from dogs without LPHS and to predict a negative outcome among dogs with leptospirosis with or without LPHS for sICAM‐1, VEGF, and Ang‐2 are shown in Table 4.
TABLE 4.
Sensitivities, specificities, and areas under the curve (AUC) for optimum cutoff concentrations to predict disease (AKI due to other causes, leptospirosis without or with LPHS) and a negative outcome in dogs with leptospirosis without LPHS or with leptospiral pulmonary hemorrhage syndrome
| Outcome | AUC (95% CI) | Cutoff | Sensitivity (95% CI) | Specificity (95% CI) | Z‐value (AUC > 0.5) | P value |
|---|---|---|---|---|---|---|
| Healthy vs diseased (AKI‐nL/Lepto/LPHS) | ||||||
| sICAM‐1 (ng/mL) | 0.86 (0.74‐0.93) | 25.1 | 0.87 (0.73‐0.96) | 0.81 (0.63‐0.93) | 8.0 | .0 |
| VEGF (pg/mL) | 0.88 (0.77‐0.93) | 13.9 | 0.80 (0.64‐0.91) | 0.97 (0.83‐1.0) | 9.4 | .0 |
| Ang‐2 (ng/mL) | 0.81 (0.68‐0.89) | 8.3 | 0.53 (0.35‐0.7) | 0.90 (0.74‐0.98) | 5.8 | .0 |
| Leptospirosis vs AKI‐nL | ||||||
| sICAM‐1 (ng/mL) | 0.61 (0.38‐0.77) | 43.5 | 0.7 (0.47‐0.87) | 0.63 (0.34‐0.85) | 1.1 | .14 |
| VEGF (pg/mL) | 0.51 (0.3‐0.67) | 25.8 | 0.82 (0.6‐0.95) | 0.3 (0.13‐0.59) | 0.1 | .47 |
| Ang‐2 (ng/mL) | 0.55 (0.32‐0.73) | 5.2 | 0.95 (0.76‐1.0) | 0.23 (0.05‐0.54) | 0.5 | .31 |
| LPHS vs Lepto | ||||||
| sICAM‐1 (ng/mL) | 0.72 (0.43‐0.88) | 68.4 | 0.57 (0.29‐0.82) | 1.0 (0.66‐1.0) | 2.0 | .02 |
| VEGF (pg/mL) | 0.62 (0.32‐0.81) | 49.6 | 0.57 (0.29‐0.82) | 0.75 (0.35‐0.97) | 1.0 | .17 |
| Ang‐2 (ng/mL) | 0.35 (0.09‐0.57) | — | — | — | −1.2 | .88 |
| Death or euthanasia in dogs with Lepto or LPHS | ||||||
| sICAM‐1 (ng/mL) | 0.93 (0.71‐0.99) | 73.4 | 1.0 (0.4‐1.0) | 0.9 (0.67‐1.0) | 8.3 | .0 |
| VEGF (pg/mL) | 0.97 (0.73‐1.0) | 87.1 | 1.0 (0.4‐1.0) | 0.9 (0.66‐1.0) | 14.0 | .0 |
| Ang‐2 (ng/mL) | 0.52 (0.06‐0.84) | 34.1 | 0.33 (0.01‐0.91) | 0.94 (0.73‐1.0) | 0.1 | .47 |
Abbreviations: AKI, acute kidney injury; AKI‐nL, acute kidney injury due to other etiologies; CI, confidence interval; Lepto, leptospirosis without leptospiral pulmonary hemorrhage syndrome; LPHS, leptospiral pulmonary hemorrhage syndrome; sICAM‐1, soluble intercellular adhesion molecule‐1; VEGF, vascular endothelial growth factor.
All 3 markers discriminated between healthy and diseased dogs with reasonable accuracy, although the sensitivity of serum Ang‐2 was limited. Among dogs with leptospirosis, sICAM‐1 was the only 1 of the 4 markers which was associated with the occurrence of LPHS, albeit with low sensitivity (57%), but high specificity (100%).
In contrast to Ang‐2, sICAM‐1 and VEGF predicted a negative outcome (death or euthanasia) with high accuracy.
4. DISCUSSION
The results of this study show that concentrations of serum markers of endothelial cell activation (sICAM‐1) and dysfunction (VEGF and Ang‐2) are significantly increased in day 1 samples of dogs with leptospirosis with or without LPHS compared to clinically healthy control dogs. However, a significant elevation was also observed in dogs with AKI due to other etiologies. These findings suggest that the 3 disease groups have a similar degree of endothelial activation and dysfunction upon admission and therefore do not support the hypothesis that infection with Leptospira spp. leads to a specifically high degree of endothelial activation and dysfunction as suggested in in vitro models. 9 , 10 , 30 , 31 , 32 Median day 3 concentrations of sICAM‐1 and VEGF suggested further activation or dysregulation in dogs with LPHS compared to AKI‐nL and leptospirosis without LPHS groups, but the available numbers of samples were too small to draw a more general conclusion.
Endothelial activation, increased endothelial permeability, and inflammation are closely interlinked. Increased concentration of endothelial markers might therefore be a consequence of a systemic inflammatory response to infection or uremic inflammation. 9 , 10 , 30 , 31 , 32 In this cohort, soluble ICAM‐1 and VEGF were positively correlated with CRP and neutrophil count and negatively correlated with albumin, a negative acute phase protein. In contrast, Ang‐2 was only weakly correlated with CRP. Increases in sICAM‐1 and VEGF are therefore closely related to the systemic inflammatory response in dogs with AKI (due to leptospirosis or other etiologies), while Ang‐2 might be less inflammation driven in this setting.
Upon renal injury, renal endothelial cells and proximal tubular epithelial cells produce pro‐inflammatory cytokines and chemokines leading to systemic inflammation. 31 Cytokine profiles including IL‐1a, IL‐1b, TGF‐b and the enzyme 5‐lipoxygenase in dogs with renal injury due to leptospirosis are indistinguishable from those with AKI of noninfectious causes. 32 In addition, serum CRP concentrations are not significantly different between dogs with leptospirosis‐associated AKI and AKI due to other causes. Together these findings suggest that dogs with leptospirosis, LPHS, and AKI‐nL have a similar degree of systemic inflammation and likely inflammatory‐associated endothelial activation.
Soluble ICAM‐1 and VEGF concentrations at admission were significantly higher in nonsurvivors than survivors and increased serum concentrations predicted a negative outcome with high accuracy. Furthermore, increased sICAM‐1 serum concentrations correctly predicted LPHS in 72% of dogs with leptospirosis. Beyond a better understanding of the pathogenic mechanisms of leptospirosis and LPHS, sICAM‐1 might therefore represent a useful biomarker to identify dogs at risk of LPHS.
In contrast, Ang‐2 had no discriminatory power to predict the occurrence of LPHS or death in dogs with leptospirosis. In dogs with sepsis or SIRS, Ang‐2 is significantly higher in nonsurviving compared to surviving dogs and Ang‐2 serum concentrations of >25.3 ng/mL are highly predictive of death. 27 The lack of association of Ang‐2 serum concentrations with outcome in dogs with leptospirosis might be due to particularities of the pathogenic mechanisms of leptospirosis‐associated sepsis. Leptospiral infection triggers an increase of pro‐inflammatory cytokines comparable to other Gram‐negative infections. 32 , 33 However, compared to other Gram‐negative bacteria, leptospiral lipopolysaccharide is of low endotoxicity and is recognized by the TLR‐2 rather than the TLR‐4 receptor, suggesting different mechanisms of cell immune response. 34 , 35 Differences in the pathogenic mechanism are likely also reflected by the fact that commonly used outcome prediction severity scoring systems used in intensive care settings, such as the sequential organ failure assessment score or the Acute Physiology and Chronic Health Evaluation II (APACHE II) score overestimate death from sepsis in humans with severe leptospirosis. 36
Limitations of this study include the limited number of dogs per study group, and the relative heterogenicity in the AKI‐nL group. The separation between leptospirosis without LPHS and leptospirosis with LPHS groups was based on the presence of clinical signs and infiltrates on thoracic radiographs and was not confirmed by bronchoalveolar lavage, which could have led to misclassification of individual dogs. Furthermore, only 10/31 dogs in the control group had CBC and biochemistry panels performed to support their good health and to be included in the correlation analysis of endothelial cell activation or dysfunction markers with other blood markers of inflammation such as neutrophilia, left shift, and CRP.
Finally, while the results of this study strongly suggest that dogs with leptospirosis and AKI‐nL have significantly higher sICAM‐1 concentrations compared to healthy dogs, these results have to be treated with caution, due to the high interassay variability observed for the sICAM‐1 ELISA.
5. CONCLUSION
Serum concentration of markers of endothelial activation and dysfunction are increased in dogs with AKI due to leptospirosis and other causes compared to healthy dogs. The correlations with other markers of inflammation suggest that endothelial activation is related to systemic inflammatory response to either infection or uremic inflammation. Among dogs with leptospirosis VEGF and ICAM‐1 serum concentrations predicted death with high accuracy. Soluble ICAM‐1 was the only of the 4 markers which was associated with the occurrence of LPHS in this study sample, but this result has to be interpreted with caution as the canine sICAM‐1 ELISA used had high interassay variability.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study protocol was approved by the Swiss Federal Food Safety and Veterinary Office (BE 38/15).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Table 1 Handling of high inter‐assay variability of soluble intercellular adhesion molecule‐1 (sICAM‐1). A Significantly different from healthy control. Abbreviations: AKI‐nL, acute kidney injury due to other etiologies; D1, day 1; Lepto, leptospirosis without LPHS; LPHS, leptospirosis with LPHS; n, number of samples
Supplemental Table 2 Calculation of the correction factor
Supplementakl Figure
ACKNOWLEDGMENT
Funding for this study was provided by an institutional grant and a grant of the Commission for Veterinary specialization of the Vetsuisse Faculty University of Bern. Parts of this study were presented as a poster at the 2018 American College of Veterinary Internal Medicine Forum, Seattle, Washington. The authors acknowledge Dr Beatriz Vidondo from the Department of Clinical Research and Public Health (DCR‐VPH) for her assistance in the statistical data analysis.
Sonderegger F, Nentwig A, Schweighauser A, et al. Association of markers of endothelial activation and dysfunction with occurrence and outcome of pulmonary hemorrhage in dogs with leptospirosis. J Vet Intern Med. 2021;35(4):1789–1799. 10.1111/jvim.16163
Funding information Commission for Veterinary specialization of the Vetsuisse Faculty University of Bern
REFERENCES
- 1. Major A, Schweighauser A, Francey T. Increasing incidence of canine leptospirosis in Switzerland. Int J Environ Res Public Health. 2014;11(7):7242‐7260. 10.3390/ijerph110707242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marotto PCF, Ko AI, Murta‐Nascimento C, et al. Early identification of leptospirosis‐associated pulmonary hemorrhage syndrome by use of a validated prediction model. J Infect. 2010;60(3):218‐223. 10.1016/j.jinf.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schuller S, Callanan JJ, Worrall S, et al. Immunohistochemical detection of IgM and IgG in lung tissue of dogs with leptospiral pulmonary haemorrhage syndrome (LPHS). Comp Immunol Microbiol Infect Dis. 2015;40:47‐53. 10.1016/j.cimid.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 4. Kohn B, Steinicke K, Arndt G, et al. Pulmonary abnormalities in dogs with leptospirosis. J Vet Intern Med. 2010;24(6):1277‐1282. 10.1111/j.1939-1676.2010.0585.x. [DOI] [PubMed] [Google Scholar]
- 5. Nally JE, Chantranuwat C, Wu XY, et al. Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am J Pathol. 2004;164(3):1115‐1127. 10.1016/S0002-9440(10)63198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Carlo Bernardi F, Ctenas B, da Silva LFF, et al. Immune receptors and adhesion molecules in human pulmonary leptospirosis. Hum Pathol. 2012;43(10):1601‐1610. 10.1016/J.HUMPATH.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 7. Barthélemy A, Magnin M, Pouzot‐Nevoret C, et al. Hemorrhagic, hemostatic, and thromboelastometric disorders in 35 dogs with a clinical diagnosis of leptospirosis: a prospective study. J Vet Intern Med. 2017;31(1):69‐80. 10.1111/jvim.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mastrorilli C, Dondi F, Agnoli C, et al. Clinicopathologic features and outcome predictors of Leptospira interrogans Australis serogroup infection in dogs: a retrospective study of 20 cases (2001–2004). J Vet Intern Med. 2007;21(1):3‐10. 10.1111/j.1939-1676.2007.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 9. Evangelista K, Franco R, Schwab A, et al. Leptospira interrogans binds to cadherins. PLoS Negl Trop Dis. 2014;8(1):1‐11. 10.1371/journal.pntd.0002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinez‐Lopez DG, Fahey M, Coburn J. Responses of human endothelial cells to pathogenic and non‐pathogenic Leptospira species. PLoS Negl Trop Dis. 2010;4(12):1‐11. 10.1371/journal.pntd.0000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atzingen MV, Gómez RM, Schattner M, et al. Lp95, a novel leptospiral protein that binds extracellular matrix components and activates e‐selectin on endothelial cells. J Infect. 2009;59(4):264‐276. 10.1016/j.jinf.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 12. Vieira ML, D'Atri LP, Schattner M, et al. A novel leptospiral protein increases ICAM‐1 and E‐selectin expression in human umbilical vein endothelial cells. FEMS Microbiol Lett. 2007;276(2):172‐180. 10.1111/j.1574-6968.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- 13. Gómez RM, Vieira ML, Schattner M, et al. Putative outer membrane proteins of Leptospira interrogans stimulate human umbilical vein endothelial cells (HUVECS) and express during infection. Microb Pathog. 2008;45(5):315‐322. 10.1016/j.micpath.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 14. Sato H, Coburn J. Leptospira interrogans causes quantitative and morphological disturbances in adherens junctions and other biological groups of proteins in human endothelial cells. PLoS Negl Trop Dis. 2017;11(7):1‐27. 10.1371/journal.pntd.0005830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raffray L, Giry C, Thirapathi Y, et al. Increased levels of soluble forms of E‐selectin and ICAM‐1 adhesion molecules during human leptospirosis. PLoS One. 2017;12(7):1‐11. 10.1371/journal.pone.0180474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Libório AB, Braz MBM, Seguro AC, et al. Endothelial glycocalyx damage is associated with leptospirosis acute kidney injury. Am J Trop Med Hyg. 2015;92(3):611‐616. 10.4269/ajtmh.14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paris DH, Jenjaroen K, Blacksell SD, et al. Differential patterns of endothelial and leucocyte activation in “typhus‐like” illnesses in Laos and Thailand. Clin Exp Immunol. 2008;153(1):63‐67. 10.1111/j.1365-2249.2008.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lukasz A, Hoffmeister B, Graf B, et al. Association of Angiopoietin‐2 and dimethylarginines with complicated course in patients with leptospirosis. PLoS One. 2014;9(1):5‐9. 10.1371/journal.pone.0087490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papa A, Kotrotsiou T. Cytokines in human leptospirosis. Trans R Soc Trop Med Hyg. 2015;109(12):749‐754. 10.1093/trstmh/trv095. [DOI] [PubMed] [Google Scholar]
- 20. Goeijenbier M, Gasem MH, Meijers JCM, et al. Markers of endothelial cell activation and immune activation are increased in patients with severe leptospirosis and associated with disease severity. J Infect. 2015;71(4):437‐446. 10.1016/j.jinf.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 21. Fraga TR, Dos Santos Courrol D, Castiblanco‐Valencia MM, et al. Immune evasion by pathogenic Leptospira strains: the secretion of proteases that directly cleave complement proteins. J Infect Dis. 2013;209(6):876‐886. 10.1093/infdis/jit569. [DOI] [PubMed] [Google Scholar]
- 22. Hashimoto VL, Abreu PAE, Carvalho E, et al. Evaluation of the elastinolytic activity and protective effect of Leptallo I, a protein composed by metalloprotease and FA5/8C domains, from Leptospira interrogans Copenhageni. Microb Pathog. 2013;61–62:29‐36. 10.1016/j.micpath.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 23. Mauri N, Kleiter M, Leschnik M, et al. A missense variant in KCNJ10 in Belgian shepherd dogs affected by spongy degeneration with cerebellar ataxia (SDCA1). G3. 2017;7(2):663‐669. 10.1534/g3.116.038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leptospirosis. OIE Terrestrial Manual. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.12_LEPTO.pdf. Published 2018.
- 25. Rojas P, Monahan AM, Schuller S, et al. Detection and quantification of leptospires in urine of dogs: a maintenance host for the zoonotic disease leptospirosis. Eur J Clin Microbiol Infect Dis. 2010;29(10):1305‐1309. 10.1007/s10096-010-0991-2. [DOI] [PubMed] [Google Scholar]
- 26. Barić Rafaj R, Kuleš J, Selanec J, et al. Markers of coagulation activation, endothelial stimulation, and inflammation in dogs with babesiosis. J Vet Intern Med. 2013;27(5):1172‐1178. 10.1111/jvim.12146. [DOI] [PubMed] [Google Scholar]
- 27. König M, Nentwig A, Marti E, et al. Evaluation of plasma angiopoietin‐2 and vascular endothelial growth factor in healthy dogs and dogs with systemic inflammatory response syndrome or sepsis. J Vet Intern Med. 2019;33(2):569‐577. 10.1111/jvim.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. König ML, Lettry SC, Marti E, et al. Validation of a human angiopoietin‐2 ELISA for measurement of angiopoietin‐2 concentrations in canine plasma samples and supernatant of primary canine aortic endothelial cell cultures. Am J Vet Res. 2018;79(8):803‐810. 10.2460/ajvr.79.8.803. [DOI] [PubMed] [Google Scholar]
- 29. Ogden TL. Handling results below the level of detection. Ann Occup Hyg. 2010;54(3):255‐256. 10.1093/annhyg/mep099. [DOI] [PubMed] [Google Scholar]
- 30. de Pablo R, Monserrat J, Reyes E, et al. Circulating sICAM‐1 and sE‐Selectin as biomarker of infection and prognosis in patients with systemic inflammatory response syndrome. Eur J Intern Med. 2013;24(2):132‐138. 10.1016/j.ejim.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 31. Edelstein CL, Akcay A, Nguyen Q. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:1‐12. 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nentwig A, Schweighauser A, Maissen‐Villiger C, et al. Assessment of the expression of biomarkers of uremic inflammation in dogs with renal disease. Am J Vet Res. 2016;77(2):218‐224. 10.2460/ajvr.77.2.218. [DOI] [PubMed] [Google Scholar]
- 33. Reis EAG, Hagan JE, Ribeiro GS, et al. Cytokine response signatures in disease progression and development of severe clinical outcomes for leptospirosis. PLoS Negl Trop Dis. 2013;7(9):1‐7. 10.1371/journal.pntd.0002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nascimento ALTO, Verjovski‐Almeida S, Van Sluys MA, et al. Genome features of Leptospira interrogans serovar Copenhageni. Braz J Med Biol Res. 2004;37:459‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Werts C, Tapping RI, Mathison JC, et al. Leptospiral lipopolysaccharide activates cells through a TLR2‐dependent mechanism. Nat Immunol. 2001;2(4):346‐352. 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 36. Velissaris D, Karanikolas M, Flaris N, et al. Commonly used severity scores are not good predictors of mortality in sepsis from severe leptospirosis: a series of ten patients. Crit Care Res Pract. 2012;2012:1‐6. 10.1155/2012/532376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Handling of high inter‐assay variability of soluble intercellular adhesion molecule‐1 (sICAM‐1). A Significantly different from healthy control. Abbreviations: AKI‐nL, acute kidney injury due to other etiologies; D1, day 1; Lepto, leptospirosis without LPHS; LPHS, leptospirosis with LPHS; n, number of samples
Supplemental Table 2 Calculation of the correction factor
Supplementakl Figure


